Systematics and biology of the iconic Australian scribbly gum moths Ogmograptis Meyrick (Lepidoptera : Bucculatricidae) and their unique insect–plant interaction
M. Horak A D , M. F. Day A , C. Barlow B , E. D. Edwards A , Y. N. Su A and S. L. Cameron CA CSIRO Ecosystem Sciences, Canberra, ACT 2601, Australia.
B CSIRO Plant Industry, Canberra, ACT 2601, Australia.
C School of Earth, Environment & Biological Sciences, Science and Engineering Faculty, Queensland University of Technology, Brisbane, Qld 4001, Australia.
D Corresponding author. Email: Marianne.Horak@csiro.au
Invertebrate Systematics 26(4) 357-398 https://doi.org/10.1071/IS12022
Submitted: 6 April 2012 Accepted: 31 July 2012 Published: 27 November 2012
Abstract
Many smooth-barked Eucalyptus spp.in south-eastern Australia bear distinctive scribbles caused by the larva of some Ogmograptis spp. However, although these scribbles are conspicuous, the systematics and biology of the genus is poorly known. This has been addressed through detailed field and laboratory studies of the biology of three species (O. racemosa Horak, sp. nov., O. fraxinoides Horak, sp. nov., O. scribula Meyrick) in conjunction with a comprehensive taxonomic revision supported by a molecular phylogeny utilising the mitochondrial Cox1 and nuclear 18S genes. In brief, eggs are laid in bark depressions and the first-instar larvae bore into the bark to the level where the future cork cambium forms (the phellogen). Early-instar larvae bore wide, arcing tracks in this layer before forming a tighter zig-zag-shaped pattern. The second-last instar turns and bores either closely parallel to the initial mine or doubles its width, along the zig-zag-shaped mine. The final instar possesses legs and a spinneret (unlike the earlier instars) and feeds exclusively on callus tissue that forms within the zig-zag-shaped mine formed by the previous instar, before emerging from the bark to pupate at the base of the tree. The scars of mines then become visible scribbles following the shedding of the outer bark. Sequence data confirm the placement of Ogmograptis within the Bucculatricidae, suggest that the larvae responsible for the ‘ghost scribbles’ (raised scars found on smooth-barked eucalypts) are members of the related genus Tritymba Meyrick, and support the morphology-based species groups proposed for Ogmograptis. The formerly monotypic genus Ogmograptis Meyrick is revised and divided into three species groups. Eleven new species are described: Ogmograptis fraxinoides Horak, sp. nov., Ogmograptis racemosa Horak, sp. nov., and Ogmograptis pilularis Horak, sp. nov., forming the scribula group with Ogmograptis scribula Meyrick; Ogmograptis maxdayi Horak, sp. nov., Ogmograptis barloworum Horak, sp. nov., Ogmograptis paucidentatus Horak, sp. nov., Ogmograptis rodens Horak, sp. nov., Ogmograptis bignathifer Horak, sp. nov., and Ogmograptis inornatus Horak, sp. nov., as the maxdayi group; Ogmograptis bipunctatus Horak, sp. nov., Ogmograptis pulcher Horak, sp. nov., Ogmograptis triradiata (Turner), comb. nov., and Ogmograptis centrospila (Turner), comb. nov., as the triradiata group. Ogmograptis notosema (Meyrick) cannot be assigned to a species group as the holotype has not been located. Three unique synapomorphies, all derived from immatures, redefine the family Bucculatricidae, uniting Ogmograptis, Tritymba (both Australian) and Leucoedemia Scoble & Scholtz (African) with Bucculatrix Zeller, which is the sister group of the Southern Hemisphere genera. The systematic history of Ogmograptis and the Bucculatricidae is discussed.
Additional keywords: callus, Eucalyptus, Leucoedemia, mine, phellogen, phylogeny, Tritymba.
Introduction
The gum-tree stands by the spring
I peeled its splitting bark
And found the written track
Of a life I could not read
(Judith Wright 1955)
The ‘scribbles’ on the bark of some smooth-barked Eucalyptus species in south-eastern Australia are quintessentially Australian and gained an iconic status as a result of the classic children’s books by May Gibbs (1918 and later) and the poem ‘Scribbly-Gum’ by Judith Wright (1955). These scribbles were first thought to be caused by beetle larvae, a misconception still alive today (e.g. Flannery 2010). Upton (1997) detailed the discovery by Greaves in 1934 that a very small moth is responsible. Meyrick (1935) described the moth, reared from larvae emerging from Eucalyptus pauciflora Sieber ex Spreng, in the mountains west of Canberra, as Ogmograptis scribula Meyrick, but he found it difficult to assign the new genus to a family. He included it in the Elachistidae but stated that the longitudinally ribbed cocoon ‘suggests the cocoon of a Bucculatrix, but there is no real relationship’. The position of Ogmograptis remained so enigmatic that Common (1990) omitted the genus in his authoritative Moths of Australia.
In 1958, I. F. B. Common collected several pupae of O. scribula from E. pauciflora at the type locality west of Canberra, but elucidation of the life history producing these unique tracks was never attempted as they are generated beneath the bark and therefore not easily observable while the larva is present. Cooke and Edwards (2007) analysed the pattern of scribbles on three species of Eucalyptus in the Australian Capital Territory, and concluded that there was more than one species of Ogmograptis. Obvious differences in wing pattern among the specimens recognised as Ogmograptis in the Australian National Insect Collection (ANIC) supported this supposition.
This study is the result of collaboration between entomologists and botanists, guided over several years by one of us (M. F. Day), and aimed at elucidating the phylogeny, taxonomy and biology of the scribbly moth system, as described by Whitten (2012). We describe the interaction between Ogmograptis larva and the tree that produces bark scribbles and we show that the related genus Tritymba is responsible for ‘ghost scribbles’, mines in the vascular cambium that result in raised scars visible on smooth-barked Eucalyptus spp. Our studies show that Ogmograptis comprises numerous species that are newly accommodated in three species groups, the scribula group (which produces the bark scribbles) and the maxdayi and triradiata groups (whose larval biology is not known). Finally, the systematics of Ogmograptis and the Bucculatricidae are discussed in the light of synapomorphic behaviours and structures identified in the course of this study.
Material and methods
Material studied and preparation
The taxonomic revision was largely based on specimens in the ANIC or reared during the present study. Because differences among species are often subtle, type series were usually restricted to a single population. Genitalia preparations were made following Robinson (1976) and Common (1990), and wing preparations followed the method developed by Common (1990).
Scanning electron microscopy
Cryo-scanning electron micrographs of the larvae and the surrounding tissue were produced by CB. In the field, sections of bark were removed from the trunk to expose Ogmograptis larvae in their tracks. The part of the track containing a larva was excised from the tree trunk with a razor blade, flash frozen in a dry shipper previously cooled with liquid N2 to −196°C and stored at −196°C until examined. Scanning electron microscopy was performed on a Cambridge 360 SEM equipped with a cryo-stage and cryo-transfer unit. Samples were removed from the vials mounted onto brass stubs using colloidal graphite paste (Agar Aids) and transferred via the cryo-transfer unit into the column of the SEM. Under observation, samples were slowly warmed to, and held at, −90°C to sublime surface frost, returned to the preparation chamber of the cryo-transfer unit for sputter coating with gold and then placed back into the SEM column for observation. Micrographs of larval legs and abdominal structures were taken using conventional SEM. Larvae were fixed in 70% ethanol, dehydrated through a graded ethanol series, critical-point dried, mounted on stubs and sputter coated with gold. Images were captured with a JEOL 6400 SEM.
Light microscopy
Cross-sections of eucalypt bark for light microscopy were produced by CB. In the field small pieces of bark from Eucalyptus racemosa ssp. rossii R. Baker & H.G. Smith (~4 cm2) were removed from trees, and, from the centre of each, small strips (~2 mm × ~4 mm) were cut using single-edged razor blades. The strips were placed in a fixative solution of 3% glutaraldehyde in 25 mm sodium phosphate buffer pH 7.2 and stored at 4°C. In the laboratory the fixative solution was discarded, the samples washed with several changes of the phosphate buffer, dehydrated through an ethanol series and infiltrated with LRWhite resin (London Resin Co.). The samples were infiltrated for 1–2 weeks in pure resin and polymerised in flat aluminium foil dishes in a 70°C oven under nitrogen. Sections 1–2 µM thick were cut with glass and diamond knives on a Reichert ultracut microtome, dried onto glass slides, stained with a solution of 0.1% Toluidine Blue O and examined under an optical microscope.
Molecular methods
Whole genomic DNA was extracted from thoracic muscle tissue with the DNeasy Blood and Tissue kit (QIAGEN). Two genes were amplified, a portion of the mitochondrial cytochrome c oxidase subunit 1 (= cox1) and the nuclear small subunit rRNA gene (18S). A 659-bp portion of the 5′ end of Cox1 was amplified with the LCO/HCO primers (Folmer et al. 1994). Almost the entire 18S gene was amplified (1798 bp) using the primers 1.2F, b3.0, a0.7, bi, a2.0 and 9R (Whiting 2002). PCRs and sequencing reactions were conducted as in our previous studies (Cameron et al. 2009; Dowton et al. 2009); sequencing trace files were generated on an ABI3730 capillary sequencer (Applied Biosystems) at the John Curtin Medical Centre, Biomolecular Resource Facility (Australian National University). Raw sequence files were edited and assembled into contigs in Sequencher ver. 4.9 (GeneCodes Corporation).
Alignments of each gene were undertaken in Sequencher ver 4.9 by eye using Bucculatrix as an outgroup. Alignments were trivial as Cox1 included no indels and 18S only 5 single-base indels. Alignments of each gene were concatenated in MacClade ver 4.06 (Maddison and Maddison 2003). Models for each partition were chosen using AIC as implemented in ModelTest (Posada and Crandall 1998). Phylogenetic analysis was performed using parsimony (MP) and likelihood (ML) methods using PAUP 4.0b10 (Swofford 2002) and Bayesian analysis (BA) using MrBayes ver 3.1.2 (Huelsenbeck and Ronquist 2001). Bootstrap supports for MP and ML trees were calculated with PAUP 4.0b10 with 1000 replicates. Phylogenetic analyses were performed using either a single (MP, ML) or two (BA) data partitions based on each gene region. All Bayesian analyses were run with unlinked partitions using two independent runs, each run consisting of four chains (three hot and one cold chain), for 3 million generations with sampling every 1000 generations; convergence was achieved by all analyses within 3 million generations, as determined using Tracer ver. 1.4 (Rambaut and Drummond 2007). Completed Bayesian analyses were examined for asymptotic behaviour of each parameter and of total tree likelihood; trees collected before this asymptotic point were treated as burn-in and discarded (generally the first 30 000–60 000 generations). Bayesian and ML run files are available for each analysis from SLC upon request.
Abbreviations
AMSA Australian Museum, Sydney, Australia
ANIC Australian National Insect Collection, CSIRO, Canberra
BMNH The Natural History Museum, London (British Museum of Natural History)
DEMV Department of Entomology, Museum of Victoria, Melbourne
GS genitalia slide
NP National Park
SAMA South Australian Museum, Adelaide
SEM scanning electron micrograph
Biology
Life history of the Ogmograptis scribula group
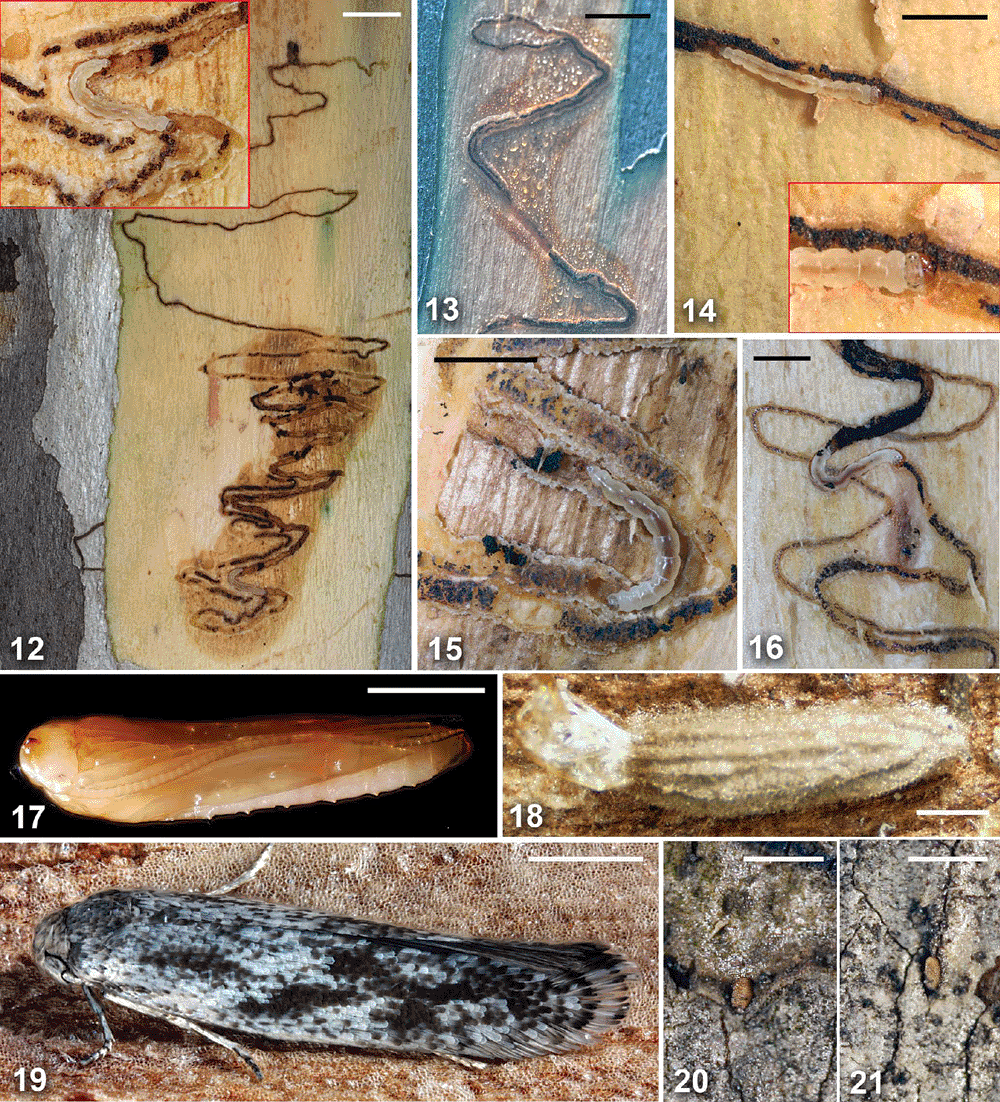
The life history of the scribula group was studied in O. racemosa Horak, sp. nov., and, in lesser detail, in O. scribula Meyrick, 1935, and O. fraxinoides Horak, sp. nov. The layout of larval tracks in the scribula group follows a general pattern that is summarised in Table 1, with crucial points in the track marked from A to D as explained in Table 1 and indicated in the relevant illustrations (Figs 4, 9). There are, however, differences in the arrangement of the track components between different species, as discussed below. In a one-year life cycle, in autumn, rarely in winter, eggs are laid singly on the bark surface in small depressions or crevices (Figs 20, 21). Eggs often occur in the narrow ledge along the edges of the most recently shed outer bark. The first-instar larva chews through the underside of the egg directly into the bark, filling the empty egg shell with its droppings. When the larva reaches the depth at which next year’s cork cambium (phellogen) will be formed, it makes a 90° turn (Table 1: A), usually associated with a widening of the track to about double its previous width. From there the larva bores tangentially near the future phellogen layer. The initial entry track is much narrower than its continuation beyond the turn, suggesting that the larva may be moulting into the second instar at this point. No head capsule has, however, been found among the droppings in this section of the track in the 10 samples examined. The tangential track (Table 1: A–B) is typically at an angle between 90° and 120° to the axis of the trunk, usually forming a few long, quite loose zig-zags, always beneath bark that will be shed in the following year. Each time the track reaches the margin of the bark to be shed in the next year, it turns back and remains within the confines of the bark to be abscised (Fig. 9). The track is distinctly wider in the second half of this section, suggesting that the larva moults into the third instar about halfway.

|

|

|
At some point, usually after making a particularly long, possibly ‘exploratory’ loop, the larva moults into what is likely to be its fourth and penultimate instar, and the track changes into a more regular pattern of shorter zig-zags within the confines of the width of the last loop (points B–C). This set of shorter and more regular zig-zags varies in two ways between taxa. First, this final set of zig-zags either follows in the same direction (either downward or upward) as the narrow, initial zig-zags (Figs 1–4), or, as in O. pilularis Horak, sp. nov., it changes direction and is superimposed over the second half of the earlier track (Figs 5, 6). Second, there are two different patterns with regard to the return track from the turning loop (Table 1: C–D). In O. scribula, O. fraxinoides, O. pilularis and other unidentified Ogmograptis species of the scribula group, the returning larva follows the first track in a closely parallel but separate track (Figs 1–3, 12, 15). In contrast, the larva of O. racemosa re-enters the first track after a small turning loop, enlarging the track to double width on its return passage (Figs 4, 9).
Observations of scribbles on various eucalypt species suggest that the track of O. racemosa is the exception, with most larvae of the scribula group returning in a closely parallel track rather than in the same track after the turning loop. Up to this point the larva is extremely long and slender, lacks legs, has a particularly shaped prothoracic shield, lacks a spinneret and lives as a true borer, feeding on the bark tissue it excavates to form its mine (Fig. 7). In O. racemosa the return track ends in a second turning loop (Point D) at the opposite end of the set of shorter, more regular loops. In this second turning loop the caterpillar moults into the last, probably fifth, instar with well developed legs and a spinneret. The location of this turning point varies from track to track, with the larva turning before reaching the beginning of the set of tight loops (Fig. 4) or extending the track beyond the onset of the tight loops before turning (Fig. 9). The synchronisation of larval development within a given side of a eucalypt trunk as well as the feeding behaviour of the last-instar larva suggest that the stimulus for this last turn and for moulting may be growth activity of the cork cambium rather than the larva having reached a certain spatial position in its track. Evidence from tracks of O. scribula, O. fraxinoides and O. pilularis is ambiguous as to whether the larva, either before or after moulting into the last instar, returns in the same track or sometimes crosses over into the parallel track.
While the larva makes the final turn and moults into the fully legged last-instar caterpillar, the set of double tracks fills with callus tissue, and the bark in the vicinity usually becomes discoloured (Figs 12, 13). The final-instar larva feeds exclusively on this turgid callus (Figs 12, 14–16, 28) which includes the frass left by the larva while boring the track. In O. scribula, O. fraxinoides and O. pilularis, where the return track is parallel but separate, there is only one frass line embedded in the callus (Fig. 15), in contrast to O. racemosa which returns in the same track and thus produces two frass lines (Fig. 14) (Table 1: D–E). The final instar lasts only a few weeks with the larva growing rapidly, eating callus along the track until it has reached full size, and boring to the outside of the bark, producing a narrow, slit-like exit hole (Table 1: E) not visible in exposed scribbles as the outer bark has abscised. The larva descends to the bottom of the tree and pupates at its base behind loose bark or in the topmost layer of the surrounding soil. The longitudinally ribbed cocoon (Fig. 18), characteristic of bucculatricids, is usually attached to a firm substrate, either the base of the tree or a stone or piece of bark in the soil adjacent to it. The caterpillar first spins a larger, loose outer layer of silk, which is then pulled in, forming longitudinal folds (the ribs of the finished cocoon) fixed to the dense inner layer. The cocoon has a preformed exit flap, and the pupa pushes itself partly out of the cocoon before the adult emerges from the pupal shell. Cocoon formation is surprisingly fast, with a caterpillar of either O. scribula or O. fraxinoides collected whilst boring out of its track found within a perfect cocoon six hours later in the laboratory.
In addition to O. racemosa, we collected the two morphologically distinct larval forms of both O. scribula and O. fraxinoides. For both species we found a penultimate legless instar which is a genuine borer feeding on bark tissue and a final instar with legs feeding exclusively on the callus produced within the gallery excavated by the penultimate instar. Furthermore, the similarities in the complex pattern of surface scribbles on other smooth-barked eucalypts suggest that this life history applies to all Ogmograptis species feeding in the cork cambium layer.
Histological results
SEM images of early- and last-instar larvae of O. racemosa and their tracks show the different tissues the larva is feeding on during the early borer and the later callus feeder phases. Whereas the last-instar larva is surrounded by callus tissue of large, spherical, thin-walled cells (Figs 28, 29), the track of the legless larva exposes normal bark tissue without callus cells (Figs 26, 27). Cross-sections of preserved eucalypt bark across recent O. racemosa tracks show in detail the mass of large, spherical, thin-walled callus cells at the site of the larval track, in contrast to the normal cork layer with a basal row of thick-walled phellem cells followed by numerous rows of flat phellem cells (Figs 22, 23). The stacks of cells are contiguous across the phelloderm and phellogen, into the orderly columns of the phellem as well as into the proliferating mass of callus cells (Fig. 22), suggesting that the callus is produced by the phellogen. If this observation is confirmed, it means that within the area of the double track the phellogen produces callus instead of cork tissue at the site of larval activity. The tracks become filled with callus which remains attached to the tree after the outer bark is removed at the level of the newly formed cork cells produced by the phellogen elsewhere (Figs 13, 15, 22). Particularly obvious is the termination of the row of thick-walled phellem cells at the edge of the callus (Fig. 22). These observations provide strong support for the hypothesis that the callus within the double track is generated by the phellogen, but more detailed studies are required to confirm this.
Phenology
The duration of the various life stages has to be inferred as the entire development takes place beneath the surface of the bark. The bark may be forcibly split off at the cork cambium only during later stages of larval development, when the larva has started the set of double tracks. This probably coincides with the onset of activity in the cork cambium providing a discontinuous layer that allows the forced removal of the outer bark. Eventually, once a new cork layer is fully established, the outer bark falls off and the track is exposed. At this point the larva has already left and pupated. O. racemosa is the only species for which we have sufficient data to roughly time the life history. Adults emerged from collected larvae or pupae or were attracted to light from 28 March to 15 May in the wider Canberra region, Jervis Bay and Wedderburn, New South Wales, with most on the wing during April. We found legless larvae of the penultimate instar on the southern, cooler side of E. racemosa spp. rossii near Gunning, New South Wales, on 1 December 2008. On the northern side of the same trees the larvae were already in their final instar with legs, feeding on callus tissue. Several of the legless larvae were still near the beginning of the double track, not far beyond the first turning loop (Fig. 7) whilst several were found at the crucial point just after the second turning loop (D in Fig. 9), in the penultimate instar and about to moult into the final instar with legs, which were clearly visible beneath the soon-to-be-shed larval skin. Pupae were collected at the base of E. racemosa spp. rossii in Canberra and Gunning from the end of December through to January. These dates allow no exact timing, but suggest that the larva grows only slowly over many months, feeding on the bark tissue in the widely looping narrow track that precedes the set of double tracks. The fact that on 1 December 2008 on the same tree the least developed larvae on the southern side of the tree were still at the beginning of boring the return track whilst those on the northern side were already last-instar larvae, suggests that the last two phases, the boring of the set of double tracks and the last instar callus feeding, must occur rapidly, probably in weeks rather than months. Pupation time varies, with pupae of O. racemosa collected in mid-January emerging from late March to mid-April, spanning the summer months. On the other hand, six mature larvae of O. scribula extracted from their tracks on 6 and 10 February 2010 pupated in 6–48 h and emerged 26–33 days after the collection date. They were all females, which, given that in over 50% of the tracks the larva had already left, suggests that the males may have emerged earlier. However, eight pupae of O. fraxinoides collected on 8 March 2010 yielded five males which emerged between 12 March and 4 April 2010 and three females between 21 and 24 March 2010. The emergence of these O. scribula and O. fraxinoides specimens coincided with the passage of low-pressure weather systems.
Biology of Tritymba
Observations of fresh Tritymba tracks suggest that this genus has a similar biology to that of Ogmograptis. The track of a Tritymba larva also forms a closed loop, with the larva re-entering the terminal part of its track (Fig. 11). The raised contour of fresh tracks (Fig. 11) and the strong welts of their scars when they reach the surface of the bark (Fig. 10) both suggest that the tracks of Tritymba also become filled with callus on which the last-instar larva feeds. Whereas the tracks of the O. scribula group are located in the cork cambial layer, those of Tritymba are in the deeper vascular cambium. Their scars become visible only after years of growth have brought their impression to the surface of the bark. Unlike the bark scribbles where one sees the scribbles themselves, in the Tritymba scribbles it is only the reaction to mining, the displaced tissue, which becomes visible. The vascular cambium is persistent and keeps producing tissue, pushing the scar inward and outward.
Molecular systematics
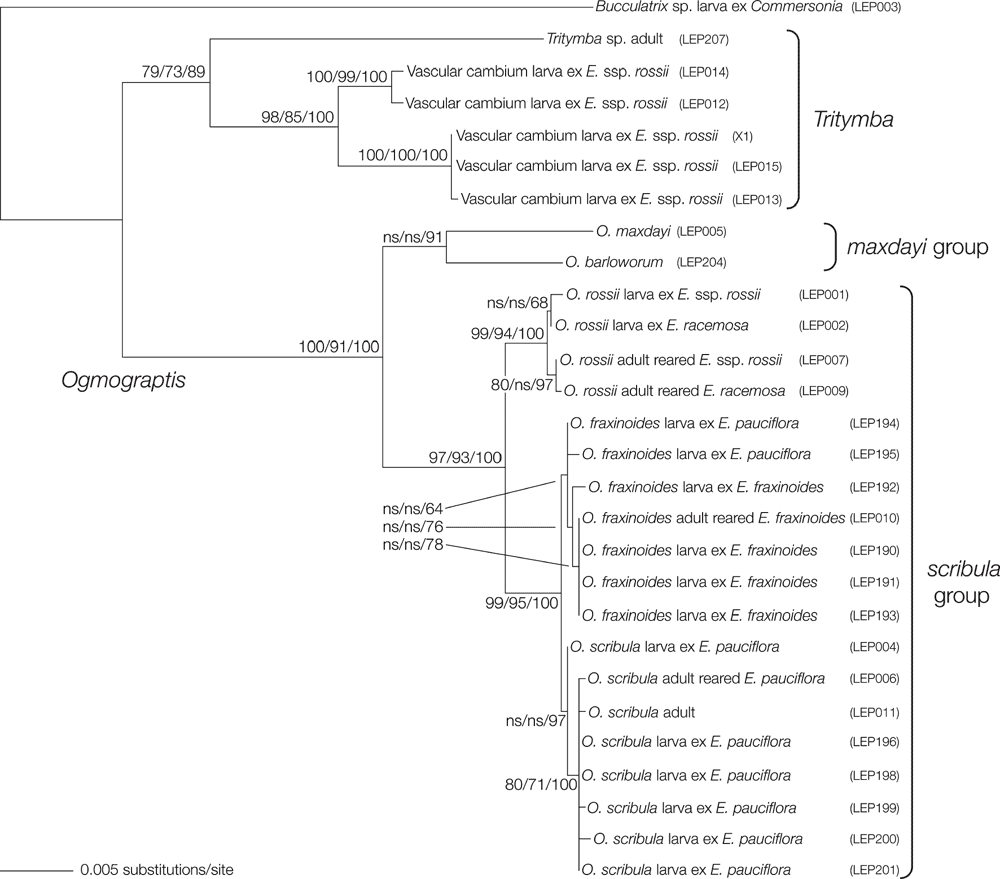
A molecular systematic study (Table 2) was conducted in concert with the morphological investigations to match larval and adult forms in the absence of rearing, to test species-group assignments based on morphology (see below) and to help identify the vascular cambium–mining larvae responsible for the ‘ghost scribbles’. Preliminary trees (not shown) supported the inclusion of Ogmograptis plus the vascular cambium–mining species within Gracillarioidea and as sister to Bucculatrix, results later confirmed by Mutanen et al. (2010), who referred Tritymba to the Bucculatricidae. Bucculatrix was thus chosen as the outgroup for subsequent analyses. These results (Fig. 30) suggest that the ‘ghost scribble’-producing species are members of the large genus Tritymba, although there is a significant molecular divergence between the confirmed adult specimens and the larval specimens, comparable to that between the maxdayi and scribula species groups within Ogmograptis. While sequences were obtainable from only two of the three Ogmograptis species groups, the phylogenetic results support the morphology-derived species group assignments (see below). For both O. fraxinoides and O. scribula, molecular sequences match for larvae and adults, thus confirming their utility for identifications. While both O. fraxinoides and O. scribula were found to be monophyletic, nodal support for their monophyly was weak due to the limited molecular divergence between the two species.
|
Taxonomy
Genus Ogmograptis Meyrick, 1935
Type species: Ogmograptis scribula Meyrick, 1935.
Diagnosis
Head. Scaling smoothly appressed; frons short; antennal scape with strong anterior extension by broad scales to form a partial ‘eye cap’; labial palpus with 3 segments, straight; maxillary palpus with 1 segment.
Wings. Venation reduced; forewing with M-stem weak, costa and Sc fused into marginal vein, radius 4-branched, all ending anterior to apex, R(4+5) and M1 stalked in wingtip with M1 posterior to apex, altogether three M and CuA veins; CuP weak, 1A+2A without basal loop; hindwing with Sc+R1 as marginal vein, Rs and two M-branches on weakly developed vein along middle of wing, cell open, CuA well developed, unbranched.
Abdomen. Apodemes of sternum II long, slender.
Male genitalia. Tegumen hood-shaped, fused with band-shaped vinculum; uncus deeply bifid, pointed; socii at most an elongate patch of bristles; gnathos arms fused into distally fringed process; saccus long to very long, slender; valva long, slender, simple; transtilla two slender lateral processes; juxta a sclerotised band usually with a dorsally strongly projecting central portion; aedeagus long and slender, with a slightly bulbous base, straight or with a bent base or with a large basal loop.
Female genitalia. Ovipositor with slender apophyses; ovipositor lobes usually membranous, closely appressed to each other, with scattered bristles on outer surface, rarely sclerotised, spinulose and posteriorly oriented. Ostium at base of S8, without sclerotised sterigma; ductus bursae slender, with sclerotised, funnel-shaped distal portion or with a minute sclerite somewhat below ostium and a lightly sclerotised bursa neck; ductus seminalis originating from neck of corpus bursae, with large, diverticulate bulla seminalis close to corpus bursae; usually with scobination across part of corpus bursae.
Larva. Strongly polymorphic: last instar with thoracic and abdominal legs and spinneret present, but anal prolegs reduced and without crochets; earlier instars without legs and spinneret. Larva with last abdominal segment modified with posterolaterally strongly protruding lobes supported by internal rod-shaped skeleton. Last-instar larva with polymorphic tarsi: prothoracic tarsus with distal setae unmodified but slender tarsal claw, meso- and metathoracic tarsi with two distal setae greatly enlarged, spatulate to cone-shaped but with strong normal claw.
Pupa. Adecticous, protruded from longitudinally ribbed cocoon on emergence.
Description
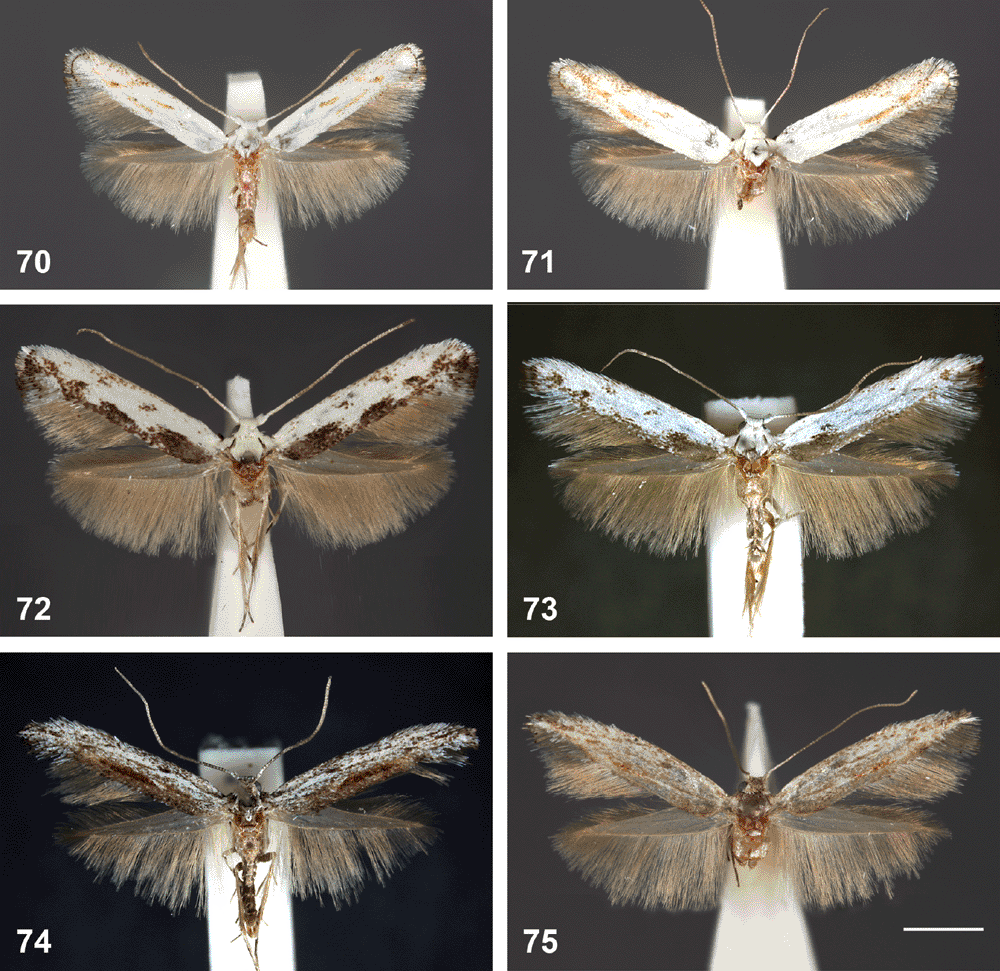
Head (Figs 58–61). Scales smoothly appressed; frons short, ventrally narrowing, triangular, scaling not extending below eyes; lateral tufts on vertex flattened, appressed, their tips meeting medially. Eye large, naked, with interocular index ~0.85. Ocelli and chaetosema absent. Antennae filiform, 0.75–0.95× length of forewing, entirely scaled with two rings of scales per segment; scape expanded into a dense ‘eye-cap’ by large broad scales extending anteriorly far beyond scape; first segment not ‘notched’. Pilifers well developed. Labial palpus 3-segmented, slender, straight, drooping, as long as vertical diameter of eye, ratio of segments from base 1 : 0.7 : 1.4, third segment with apical vom Rath’s organ. Proboscis short, 1.5× length of labial palpus, naked, with small, 1-segmented, knob-shaped maxillary palpus with few large scales.
Wingspan. 8–13 mm.
Thorax. Scaling smooth. Metafurca with posterior apophyses elongate, straight, slender, free.
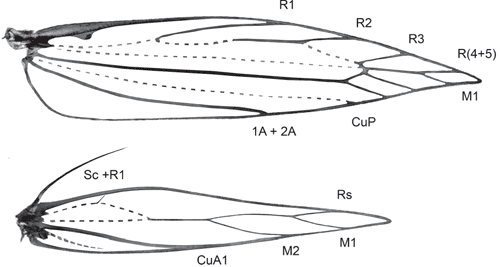
Forewing (Fig. 36). Lanceolate, pointed; W/L index 0.20–0.21; cell closed; M-stem weak, to base of R(4+5) and M1; costa and subcosta fused into strong marginal vein, retinaculum a hook from its base; R 4-branched, weak up to R1; R1 from below middle; R2 from 3/5, R3 from just before end of cell, R(4+5) fused into single vein (stalked in Tritymba Meyrick) and stalked with M1 from end of cell; R(4+5) to before and M1 to behind apex in tip of wing; M2 closely approximated to stalk of R(4+5) and M1, CuA1 more distant; CuP weak; 1A+2A to about middle of wing, without basal loop, no trace of 3A.
Hindwing (Fig. 36). Lanceolate, widest just before middle; ~2/3 width of forewing; W/L index 0.16–0.17; frenulum in male a single strong bristle, in female two bristles (rarely one only); venation reduced with Sc+R1 as marginal vein, Rs and two M-branches on weakly developed vein along middle of wing which anastomoses at 1/3 wing length with strongly curved weak vein remnant (?base of Rs) running to near base parallel to costa and with trace of a crossvein to costa (?R1); cell open; CuA well developed, unbranched; A hardly discernible trace.
Legs. Forelegs with epiphysis well developed, approximately half length of foretibia; tibial spurs 0–2-4; hindtibia in both sexes with very long loose hair scales along dorsal and ventral margins.
Abdomen. Unmodified in both sexes, without scale tufts. Paired sternal apodemes of A2 long, slender; sternal rods indicated as pigmented traces across S2.
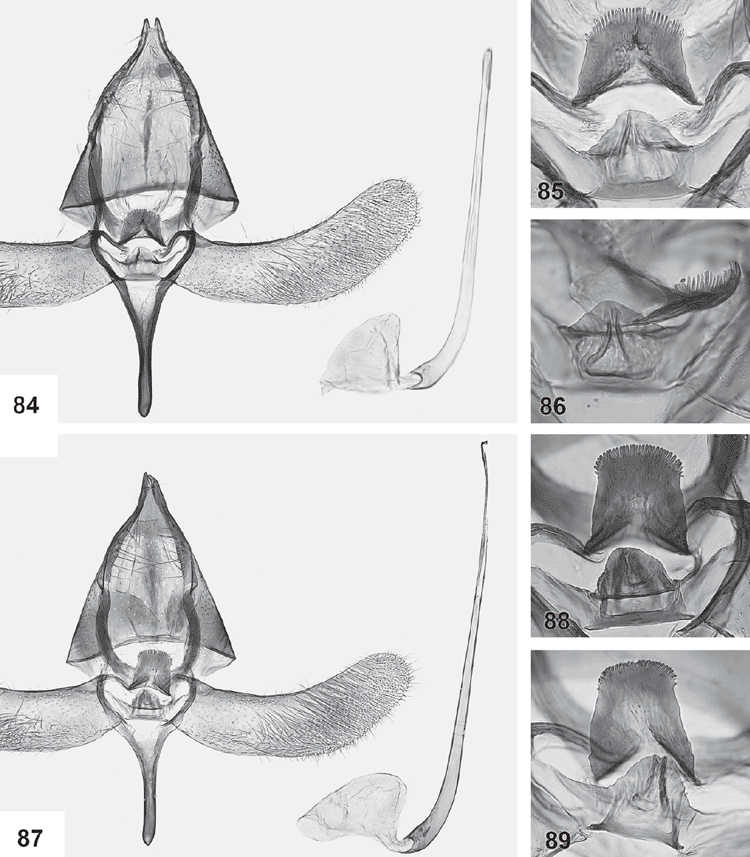
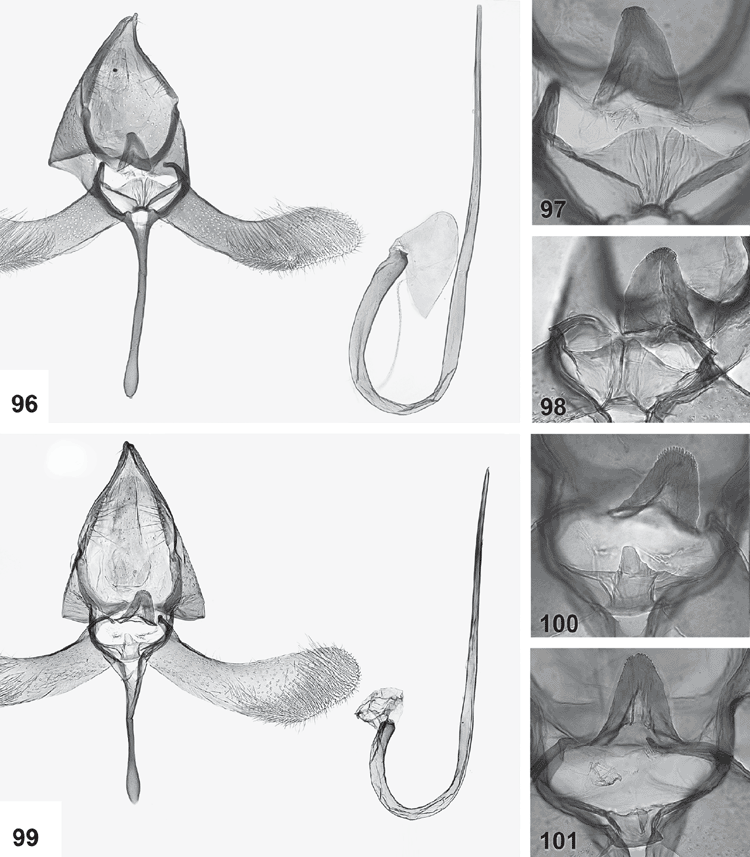
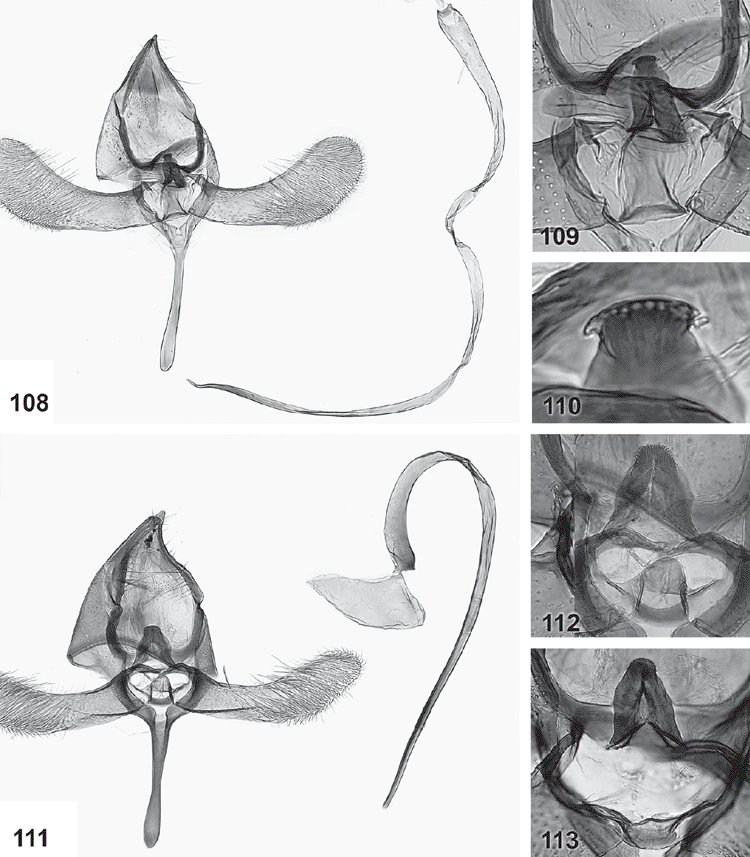
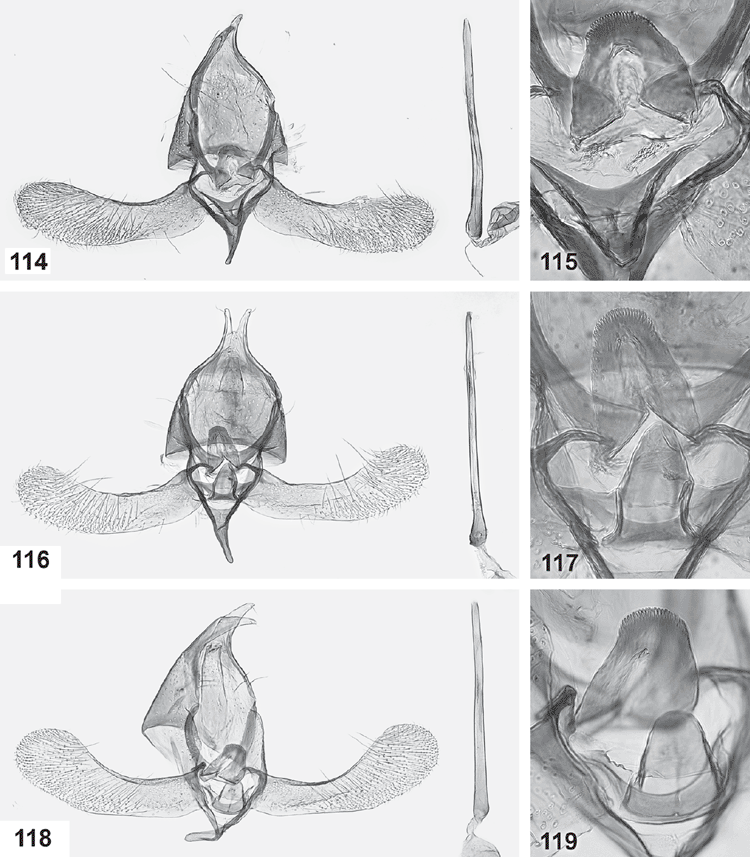
Male genitalia (Figs 84–119). Tegumen well sclerotised, broad, hood-shaped, fused with band-shaped vinculum. Uncus well developed, two parallel, short, pointed processes. Socii elongate lateral patches of bristles, sometimes only 2–3 bristles. Gnathos paired arms, apically fused into a paddle-shaped to pointed process, with distal fringe or teeth. Saccus a moderately to very long, slender, straight process. Valva long, slender, simple; transtilla incomplete, two slender, curved processes from base of costa. Anellus membranous, very flimsy; juxta well developed, sclerotised, a transverse band usually with a central shield-shaped and dorsally protruding portion, often diagnostic. Aedeagus long and slender, with a slightly bulbous base, either entirely straight, with a bent base or with a large basal loop; sometimes 1–2 indistinct needle-shaped structures in vesica.
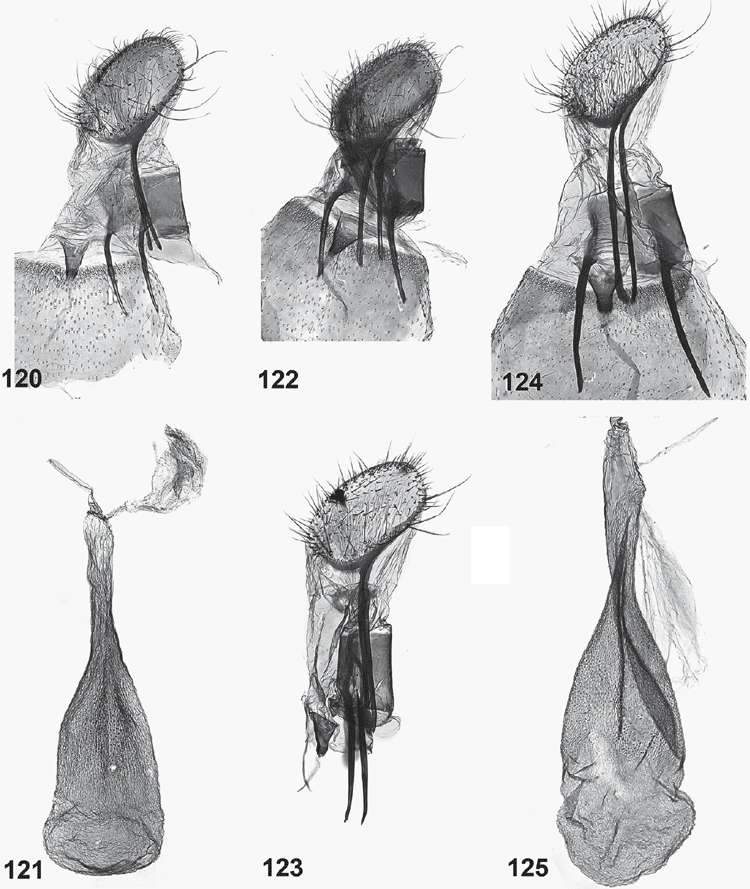
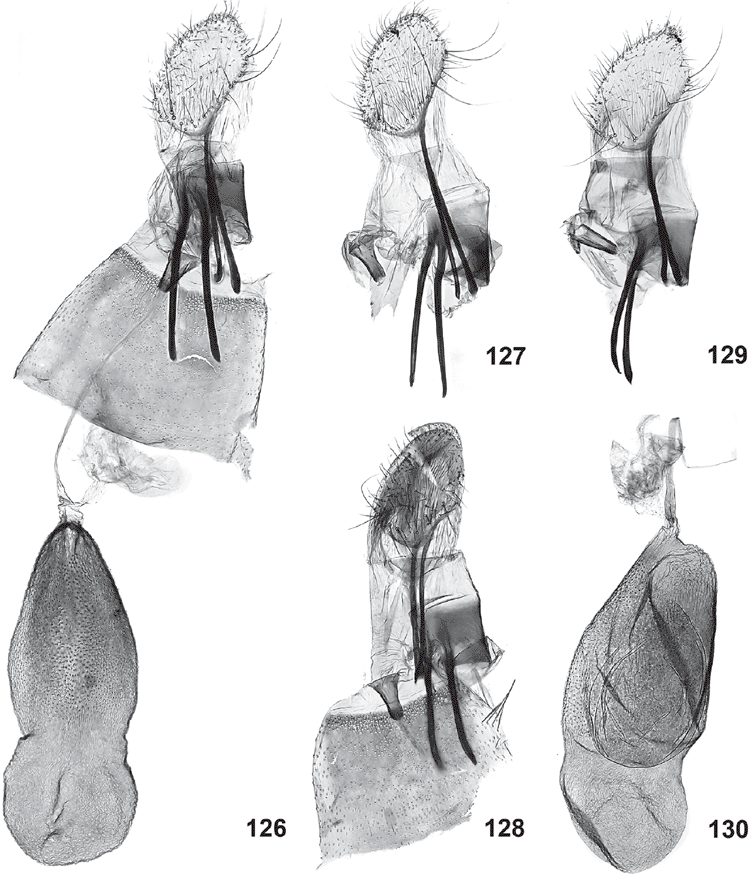
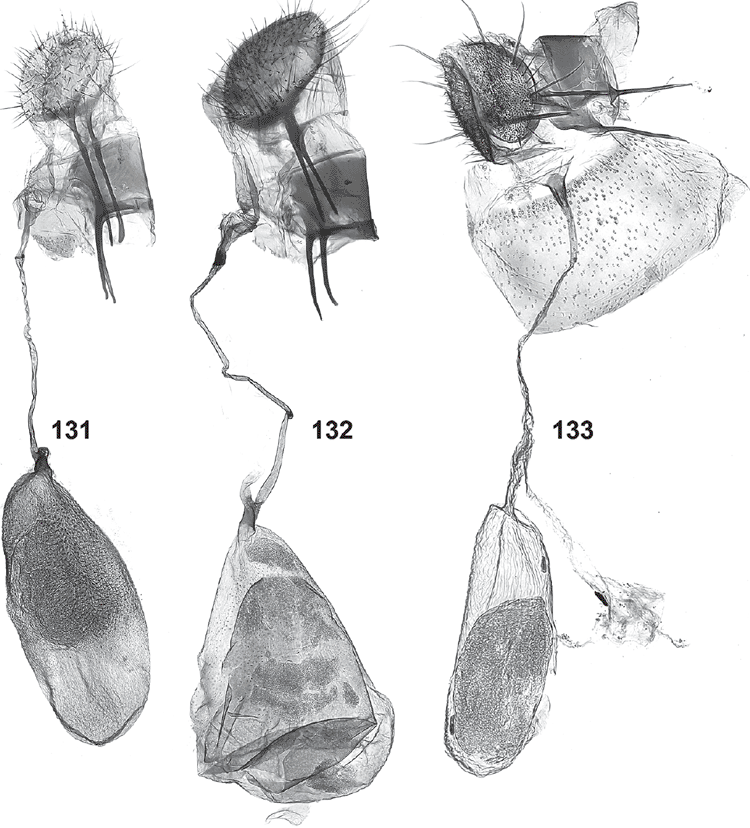
Female genitalia (Figs 120–133). Ovipositor with both apophyses well developed, slender; ovipositor lobes usually membranous, ovate, leaf-like, parallel and closely appressed to each other, with scattered bristles on outer surface, rarely spinulose and sclerotised, posteriorly oriented (in triradiata group). Ostium at base of S8, without sclerotised sterigma. Bursa copulatrix differentiated into ductus bursae and corpus bursae, with ductus seminalis originating from neck of corpus bursae, with a large, diverticulate bulla seminalis close to corpus bursae; ductus bursae very slender, with either a sclerotised, funnel-shaped distal portion or with a minute sclerite somewhat below ostium and a lightly sclerotised bursa neck; corpus bursae with scobination across part of corpus bursae except in triradiata group.
Egg (Figs 20, 21)
Length: 0.41–0.50 mm, width 0.17–0.25 mm; elongate-ovate, narrower at one end, usually somewhat apple-pip-shaped, but exact shape depending on depression in which the egg has been deposited; chorion weakly reticulate.
|
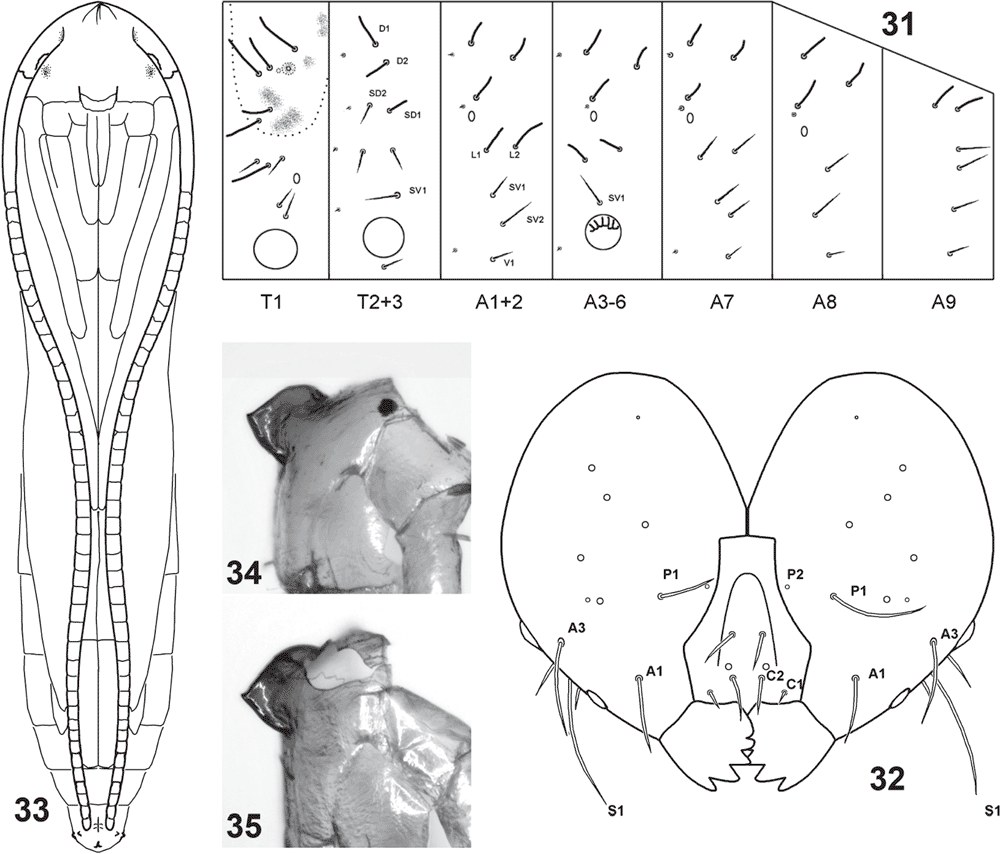
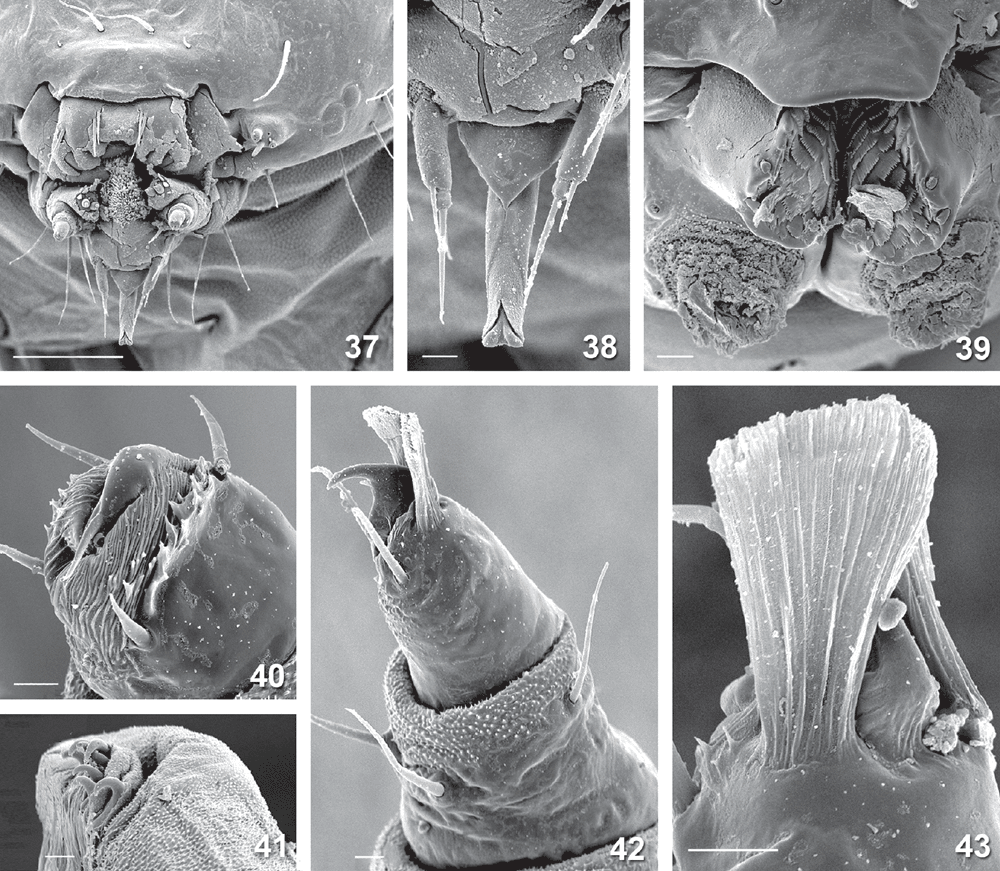
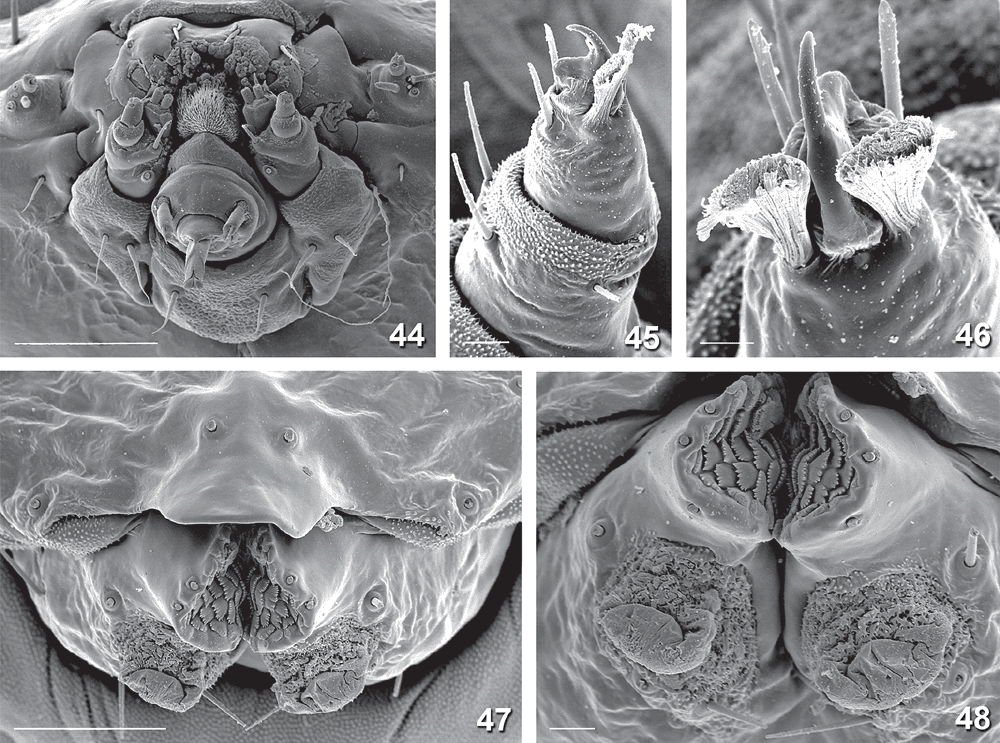
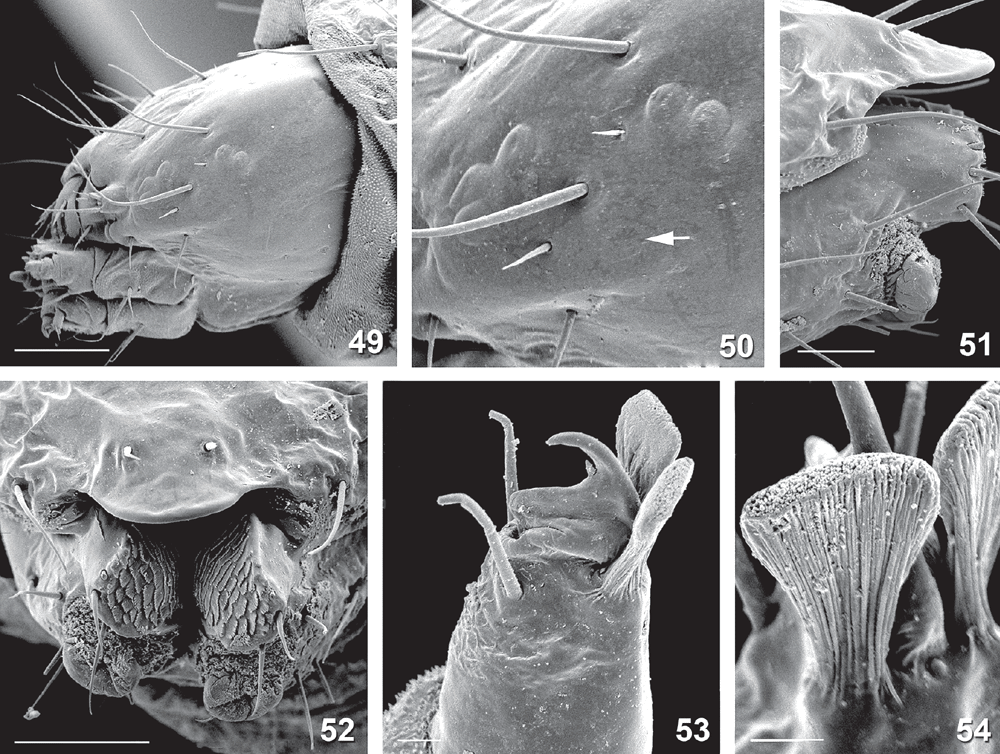
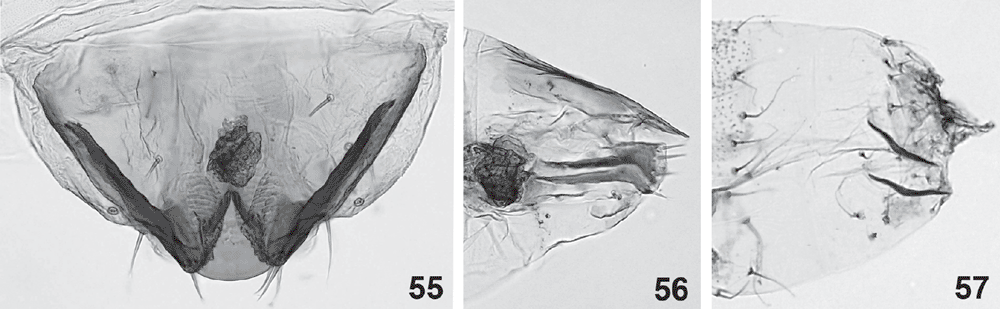
Final-instar larva (Figs 12, 14–16, 28, 31, 32, 37–55). Maximum length 11 mm. Head prognathous, dorso-ventrally flattened, reddish ochreous to reddish brown; epicranial notch extending 1/3 length of head; frontoclypeus subtriangular, apex not quite reaching epicranial notch; 6 stemmata on each side, with the 6th not visible except in macerated head capsule: 1 and 2 close to touching each other, 3 close to usually partially fused with fused 4 and 5, and 6 strongly reduced, not protruding, narrow and posteriorly attenuated (Figs 37, 49, 50); length of many cranial setae reduced to that typical of proprioceptors and their interpretation questionable, with one of the anterior group (A) absent and only one long setae of the posterior group (P) present (Fig. 32). Thoracic legs well developed, with polymorphic tarsus, prothoracic tarsus with two unmodified distal setae and a long, slender claw (base obliterated by fluid in all SEM preparations) (Fig. 40), meso- and metathoracic tarsus with the two distal setae strongly modified, spatulate to cone-shaped, with a strong, distally curved claw (Figs 42, 43, 45, 46, 53, 54). Prothoracic shield pale, with some greyish marks, with five setae and one pore; T1 with L series trisetose, SV bisetose, no V setae; T2+3 with D, SD and L series all bisetose, SV and V unisetose; A1+2 with L and SV series bisetose, V unisetose; A3–6 with L series bisetose, SV unisetose, no V seta; A7 with L and SV series usually bisetose (SV sometimes unisetose), V unisetose; A8 with L, SV and V all unisetose; A9 with 4–6 setae on each side, with position even of the two dorsal ones very variable (Fig. 31). Prolegs present on A3–6, 10; ventral prolegs rather short, with a semicircle of 5–6 crochets along the inner side of each proleg (Fig. 41); anal prolegs reduced, without crochets (Figs 39, 47, 48, 52). A10 modified (Figs 39, 47, 48, 51, 52), with distally strongly protruding lateral lobes crowned by 4 setae each along vertical posterior margin, covered by flat, hand-shaped protuberances on inner surface (anal region); each lobe supported by an internal skeleton of two rods: one much larger, horizontally along lateral margin, widening into a vertical, flat, triangular lobe in apical tip, ventrally joined by a sharply inwardly and downwardly angled, much shorter, thinner and lightly sinuate second rod, with both these ventral rods meeting in a V-shaped connection below anal opening (Fig. 55).
Penultimate larval instar (Figs 7, 8, 24–27, 56). Maximum length 4.5 mm. Extremely long and slender, without legs; head prognathous, dorsoventrally strongly flattened, without spinneret (Fig. 24), mandibles with blunt teeth; prothorax with well developed setae, dorsal shield with a narrow dark band along lateral and posterior margin, with an antero-median extension, forming a transverse, rounded E-shape (Fig. 25); except on head capsule, prothorax and last segment no setae visible; anal segment modified (Fig. 26), with internal skeleton of two longitudinal, posteriorly widened rods (Fig. 56).
Length: 3.8–4.4 mm. Adecticous, with appendages free. Frontal process pointed and blade-shaped (Figs 34, 35). Labial palpi visible. Antennae to tip of abdomen, forewings nearly concealing hindwings, to middle of A7. Abdomen with dorsum of segments 3–7 in female and 3–8 in male with a transverse band of scattered spines in anterior half; A10 with 6 wart-like projections around its base.
Cocoon (Fig. 18)
Length: 5–8 mm. Elongate-ovate to spindle-shaped, wider and more domed at one end, longitudinally ribbed; higher end with a preformed, roughly semicircular exit flap, usually beneath a projecting, loosely spun apron.
Remarks
Genitalia morphology in both sexes suggests three discrete species groups within Ogmograptis, two of which are also supported by the molecular data. The scribula group, O. scribula and all other species reared from bark scribbles, i.e. with tracks in the cork cambium layer, have the base of the aedeagus bent at up to 90°, a moderately long saccus, a sclerotised funnel at the entrance to the ductus bursae and a pale and dark mottled wing pattern. The maxdayi group of six species including O. maxdayi, with unknown larval biology, has the base of the aedeagus forming a nearly complete loop, a very long, slender and distally lightly clubbed sacculus, a ductus bursae with a small sclerite somewhat below the ostium and a sclerite at the entrance to the corpus bursa. Both these groups have modified, leaf-like ovipositor lobes appressed to each other. O. triradiata (Turner), O. centrospila (Turner) and two newly described species, O. bipunctatus Horak, sp. nov., and O. pulcher Horak, sp. nov., all with snow white ground colour, few black-sprinkled yellowish streaks and a black line around the termen, form the apparently more plesiomorphic triradiata group. Their larval biology is unknown. They have a straight aedeagus, shorter saccus, an unmodified ovipositor with posteriorly oriented, spinulose lobes, and a sclerotised funnel at the entrance to the ductus seminalis. The modified ovipositor lobes of the maxdayi and scribula groups suggest a different biology from the triradiata group. The level of divergence in the DNA data between the scribula group and the two species sequenced from the maxdayi group would justify different subgenera, but formalising this is premature until molecular data for the triradiata group and biological information for all three subsets of Ogmograptis are available.
Checklist of species
scribula group
Ogmograptis scribula Meyrick, 1935.
Ogmograptis fraxinoides Horak, sp. nov.
Ogmograptis racemosa Horak, sp. nov.
Ogmograptis pilularis Horak, sp. nov.
maxdayi group
Ogmograptis maxdayi Horak, sp. nov.
Ogmograptis barloworum Horak, sp. nov.
Ogmograptis paucidentatus Horak, sp. nov.
Ogmograptis rodens Horak, sp. nov.
Ogmograptis bignathifer Horak, sp. nov.
Ogmograptis inornatus Horak, sp. nov.
triradiata group
Ogmograptis triradiata (Turner, 1926) (Cateristis), comb. nov.
Ogmograptis centrospila (Turner, 1923) (Opostega), comb. nov.
Ogmograptis bipunctatus Horak, sp. nov.
Unassigned to species group
Ogmograptis notosema (Meyrick, 1922) (Cryphioxena).
The scribula group
This group is characterised by a moderately long saccus and a moderately long aedeagus with a bent base, by appressed, leaf-shaped ovipositor lobes, and a sclerotised funnel below the ostium. All species have speckled wings with five roughly longitudinal dark streaks, and they are so similar that they cannot be distinguished externally. Interspecific genitalia differences also are subtle, confined to the relative width of the teeth on the gnathos fringe, the ribs of the juxta, the relative length of the apophyses and the shape of the sclerotised funnel below the ostium and of the corpus bursae. This group comprises the scribbly moths feeding in the cork cambium layer, with the shape of the scribble often taxonomically indicative. There is considerable correlation between Ogmograptis species and eucalypt host species, but it is not exclusive, with two of the four species studied sometimes found on the same eucalypt species (see Discussion).
Ogmograptis scribula Meyrick
(Figs 1, 2, 12, 15, 20, 21, 37–43, 62, 63, 84–86, 120–122)
Material examined
Diagnosis
Forewings evenly speckled, somewhat darker along dorsum, with five well defined longitudinal dark streaks, the two distal streaks usually connected. Male genitalia: base of aedeagus lightly curved and saccus rather short and stout, with tip of gnathos broadly rounded and with long, slender fringe; central juxta plate dorsally triangularly projecting, with dorsally converging ribs. Female genitalia: appressed, leaf-like, elongate-ovate ovipositor lobes, with short posterior apophyses and with sclerotised narrow funnel below ostium less wide than long; corpus bursae bottle-shaped.
Description
Wingspan: (7.5 mm) 10–12 mm.
Head and thorax. Grey-brown, speckled with white scales; upper frons and anterior part of eye cap white.
Forewings. White, finely and evenly speckled with grey-brown scales, somewhat denser near dorsum; with five well defined, longitudinal, slender grey-brown streaks, one along midline in basal third, two subparallel and distally slightly converging between 1/3 and middle of wing, the more dorsal of these along fold, the fourth at 3/5 of wing and 1/4 of wing width below costa and the fifth at 2/3 along midline, the last two usually connected in the middle, sometimes a less well defined grey-brown spot at base of apical cilia; terminal cilia white with grey-brown tips, forming two parallel dark bands around apex and termen, dorsal cilia grey.
Hindwings. Pale grey, cilia concolorous.
Legs. White, variably speckled with grey, anterior pair mostly grey, middle pair with tarsi ringed with grey.
Abdomen. Silvery grey, darker dorsally.
Male genitalia (Figs 84–86). Gnathos tip broadly rounded, with full apical fringe of long, slender teeth, 4–5 times as long as wide; valva long and sides nearly parallel, widest near apex; saccus rather short; juxta a dorsally deeply sinuate band with a projecting dorsally triangular, shield-shaped median plate with a prominent ventral bar and 2–3 strongly converging ribs running towards dorsal tip of central plate; aedeagus moderately long, straight except for smoothly curved, short, lightly bulbous base; vesica with an indistinct needle-shaped cornutus.
Female genitalia (Figs 120–122). Ovipositor lobes membranous, appressed; elongate-ovate; posterior apophyses, short (1.2–1.3× length of sclerotised dorsal margin of ovipositor lobe). Ductus bursae membranous, with narrow sclerotised funnel below ostium; corpus bursae bottle-shaped, with posterior 1/3 much narrower and parallel-sided, with large central area of scobination.
Biology
Snow gum, E. pauciflora Sieber ex Spreng., is the host of O. scribula, with scribbles on the trunk and large branches. According to our observations, scribbles are found only up to an altitude of 1400 m in the Brindabella Ranges and up to the same altitude in the Kosciuszko National Park, suggesting that O. scribula might be restricted to ssp. pauciflora. The track of O. pauciflora (Figs 1, 2, 12, 15) consists of initial slender, long and rather irregular loops followed by the final set of thicker, shorter and moderately closely approximated zig-zags, with the terminal turns often shorter and/or more widely spaced, giving the doubled track set a somewhat triangular outline. The returning track is parallel but rather distant, with the space between the two tracks usually wider than the width of each track. In some localities (e.g. Sawpit Creek, Kosciuszko NP), E. pauciflora is host also to O. fraxinoides, with the two species often occurring on the same tree.
Adults reared from mature larvae collected on 12 February 2010 at Bull’s Head, Brindabella Range, emerged after 26–30 days. Specimens reared from pupae or fully grown larvae have a wingspan from 11–12 mm, smaller larvae extracted from their track produce much smaller adults, from 7.5 mm upwards, if they survive at all.
Remarks
According to Meyrick’s description, there are three paratypes with the same data as the holotype in the BMNH. There are also five males with the same handwritten label as the holotype in the ANIC, but they were not included in the type series. The labels of the type series give E. coriacea as the host plant, which is a synonym of E. pauciflora.
Ogmograptis scribula and O. fraxinoides both have the forewing sprinkled darker along dorsum, a juxta with dorsally converging ribs and a bottle-shaped corpus bursae. However, the male of O. scribula has a longer gnathos fringe with narrower teeth, the juxta is dorsally triangular rather than rounded as in O. fraxinoides, and the female has much shorter posterior apophyses than O. fraxinoides.
Ogmograptis fraxinoides Horak, sp. nov.
(Figs 3, 35, 44–48, 58, 59, 64, 65, 87–89, 123–125)
Material examined
Diagnosis
Forewings with dark speckling concentrated along dorsum, with five longitudinal streaks narrow and all separate, the two costal ones often weak or absent. Male genitalia: with base of aedeagus lightly curved and saccus rather short and stout, with broadly rounded gnathos tip with short fringe, with dorsally projecting margin of central juxta plate semicircular, not triangular as in O. scribula, with dorsally converging ribs. Female genitalia: with appressed, leaf-like, elongate-ovate ovipositor lobes, with long posterior apophyses, with sclerotised funnel below ostium as wide as long, and with bottle-shaped corpus bursae.
Biology
White ash, Eucalyptus fraxinoides H.Deane & Maiden, and E. pauciflora are both hosts of O. fraxinoides, but only material from the former host at Brown Mt is included in the type series. On E. fraxinoides, scribbles are on the trunk above the rough bark at the base of the tree and on large branches, often at high density. The track of O. fraxinoides (Fig. 3) consists of initial slender, rather irregular and sometimes long zig-zags followed by the final set of thicker, shorter and closely approximated zig-zags, with the returning track separate but usually very closely parallel to the initial track so that the distance between the tracks is hardly more than the width of each track. On mature trees there are often large numbers of scribbles, with the doubled part of the track compressed and usually with a rectangular outline. On E. pauciflora scribbles of O. fraxinoides were nearly all on the southern face of the trunk and hardly ever in the bottom 1–2 m.
At Pipers Lookout, Brown Mt, Southern Forests NP, larvae were found boring out of their track on 8 February 2010, with about half the larvae having already left the mine. Pupae collected on 9 March 2010 behind bark at the foot of the tree or on leaves in nearby leaf litter emerged between 12 March and 4 April, but mainly 21–24 March. On the slope above Sawpit Creek, Kosciuszko NP, larvae found boring out of their tracks on E. pauciflora pupated on 7 February 2010. The first cocoon was found 6 h after the larva was collected, several more formed their cocoons in the next 48 h, and two adults emerged after 33 days.
Remarks
This species occurs together with O. scribula on E. pauciflora on the slope above Sawpit Creek in Kosciuszko NP, with no E. fraxinoides nearby, and photos of scribbles on snow gum on Mt. Kaputar suggest that it is present there as well. O. fraxinoides can be separated from the very similar O. scribula by a shorter gnathos fringe with wider teeth, a dorsally more rounded juxta in the male, and by much longer posterior apophyses in the female. While the two species have very modest molecular divergences, resulting in the limited nodal support for their reciprocal monophyly (Fig. 30), genitalic and scribble structure differences support their separation into two species. It is possible that the limited molecular divergences between these species, and their sharing of E. pauciflora as a host in some regions, is evidence of their recent speciation.
Etymology
The species name refers to the host tree.
Ogmograptis racemosa Horak, sp. nov.
(Figs 4, 7–9, 13, 14, 16–19, 22, 24–28, 31–34, 36, 49–56, 66, 67, 90–92, 126, 127)
Material examined
Diagnosis
Forewings evenly speckled, usually three dark longitudinal marks in basal half of wing and an irregular larger dark mark in distal half. Male genitalia: base of aedeagus lightly curved and saccus rather short and stout, with rounded-truncate gnathos tip with long fringe of slender teeth, with dorsally projecting pentagonal central juxta plate with vertical ribs, the two flanking the central plate on each side pronounced and with tips often projecting beyond dorsal margin. Female genitalia: appressed, leaf-like, elongate-ovate ovipositor lobes, with long posterior apophyses and with sclerotised narrow funnel below ostium, less wide than long, and with ovate to slightly hourglass-shaped corpus bursae.
Biology
O. racemosa occurs on both inland scribbly gum or white gum, E. racemosa ssp. rossii R. Baker & H.G. Smith, and narrow-leaved scribbly gum or snappy gum, E. racemosa spp. racemosa Cav., supporting the recent decision that the two taxa are merely subspecies (Pfeil and Henwood 2004). Scribble tracks on E. haemastoma Smith look exactly like those on E. racemosa, and given the close relationship between the two species it would not be surprising if E. haemastoma also serves as host for O. racemosa. Most of our observations and the type series are based on material from E. racemosa ssp. rossii. Scribbles are present on the trunk and large branches. The track of O. racemosa (Figs 4, 9, 13, 14, 16) is unique as the return track is not parallel to but adjoining the initial track, enlarging it to double width. This results in initial slender, long and rather irregular zig-zags followed by a final set of much thicker, shorter, single zig-zags with a turning loop at each end.
Remarks
O. racemosa and O. pilularis are superficially similar and share a juxta with vertical rather than dorsally converging ribs and an ovate to hourglass-shaped corpus bursae. However, O. racemosa has a gnathos fringe with longer, narrower teeth.
Etymology
The species name refers to the host tree.
Ogmograptis pilularis Horak, sp. nov.
(Figs 5, 6, 68, 69, 93–95, 128–130)
Material examined
Diagnosis
Forewings lightly speckled, usually three dark longitudinal marks in basal half of wing and an irregular oblique dark mark in distal half. Male genitalia: base of aedeagus lightly curved and saccus rather short and stout, with rounded-truncate gnathos tip with moderately long fringe of broad teeth, with dorsally projecting pentagonal central juxta plate with vertical ribs, the two flanking the central plate on each side pronounced and with tips projecting beyond dorsal margin. Female genitalia: appressed, leaf-like, elongate-ovate ovipositor lobes, with long posterior apophyses and with narrow sclerotised funnel below ostium, less wide than long, and with ovate to hourglass-shaped corpus bursae.
Biology
Blackbutt, E. pilularis Sm., is the host of O. pilularis, with scribbles only observed high on the larger smooth branches above the entirely rough-barked trunk. The track of O. pilularis (Figs 5, 6) is unlike other scribbles examined, having the terminal set of thick zig-zags produced in the reverse direction, superimposed over the initial, slender zig-zags. The initial, widely spaced zig-zag track ends in a sharp turning loop with the conspicuously wider returning track usually closely following back to the previous turning point, then proceeding as the final set of thick, short and closely spaced zig-zags across the zig-zags of the initial track. After a second turning loop at the end of this final set the track returns closely parallel for several zig-zags before joining the original track.
Remarks
O. pilularis can be separated from the closely related O. racemosa by the shorter, wider teeth of the gnathos fringe.
Etymology
The species name refers to the host tree.
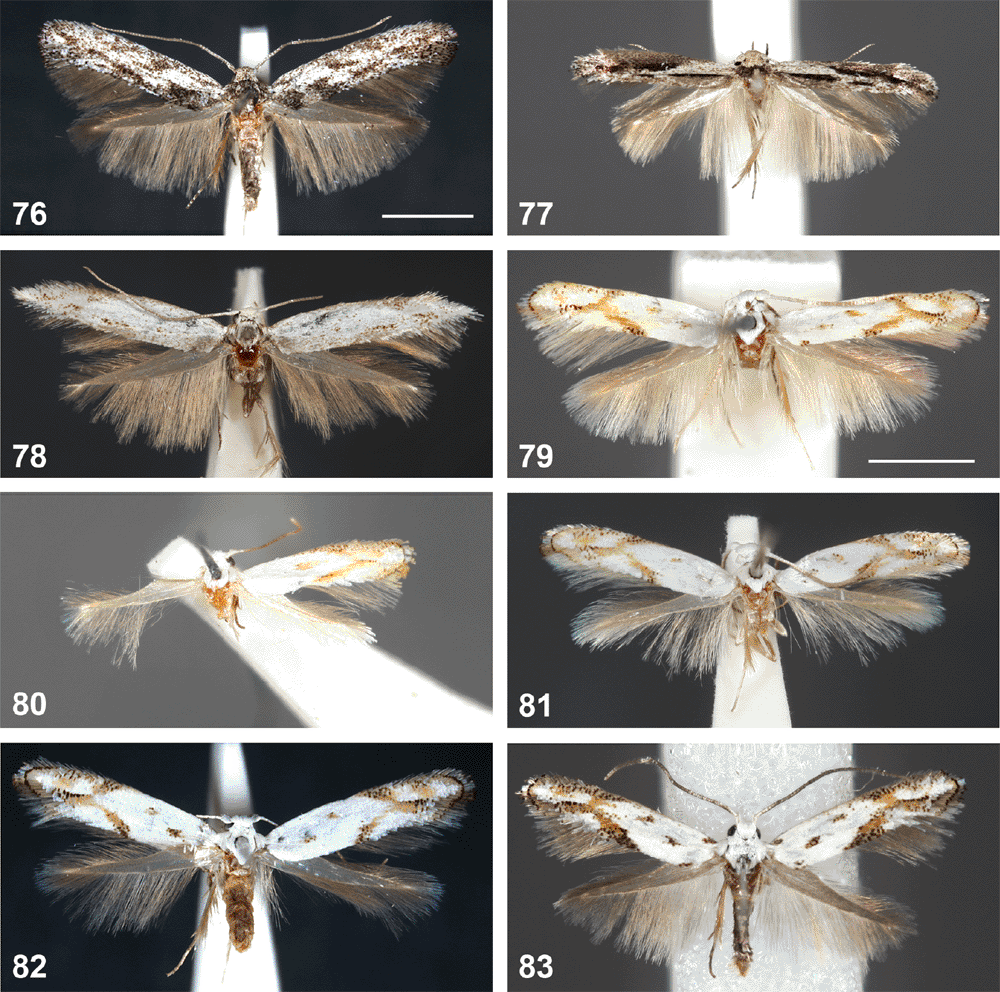
The maxdayi group
This group is characterised by a very long slender saccus, a long crosier-shaped aedeagus, appressed, leaf-shaped ovipositor lobes, and a sclerotised ring somewhat below the ostium. The wing pattern in this group is much more diverse than in the other species groups of Ogmograptis. Interspecific genitalia differences also are usually obvious, especially in the shape of the gnathos tip and the juxta. Nothing is known about the larval biology of this group.
Ogmograptis maxdayi Horak, sp. nov.
(Figs 60, 61, 70, 71, 96–98, 131)
Material examined
Diagnosis
Forewings white, with a few scattered dark-tipped scales in distal half and in a double row around termen, with five small roughly longitudinal yellow streaks, some sprinkled with dark grey. Male genitalia: very long, crosier-shaped aedeagus; club-shaped saccus; gnathos tip fringed only along central 2/3; valva with costa convex in distal half and apex tapering, narrowly rounded; juxta wide, band-shaped, with a median crease. Female genitalia: appressed, leaf-like, broadly ovate ovipositor lobes; ductus bursae narrow, sclerotised, with a narrow membranous funnel below ostium ending in a small sclerotised ring; corpus bursae ovate with a short, triangular, partly sclerotised neck.
Description
Wingspan: 8–12 mm.
Head and thorax (Figs 60, 61). White except for some pale grey scales on lower part of face and anterior edge of eye cap, and a silvery grey antennal flagellum.
Forewings. White, with widely scattered off-white scales; scales with narrow grey-brown apex in a narrow band along costa from 1/4 to apex, expanded into an irregular wider band beyond middle; far fewer off-white scales scattered along dorsum beyond 1/4, reaching to middle of wing in distal third; four or five longitudinal, deep yellow streaks, the first faint and often absent, a slender line along midline of wing in basal third, the next two subparallel and distally slightly converging near middle of wing, the shorter one just below middle of wing and 1/3 from costa, the longer dorsal one along fold from just below middle to 2/3, the fourth a short, indistinct dash at 2/3, ~1/4 below costa, and the fifth at 3/4 of wing near midline, an oblique dash, distally sometimes extended as a narrow line towards termen; yellow streaks often sprinkled with a few dark-tipped off-white scales, especially the fifth one; terminal cilia white with short dark grey tips, forming two narrow dark bands around apex and termen in perfect specimens, the basal one darker; dorsal cilia pale grey.
Hindwings. Pale grey, cilia concolorous.
Legs. White, variably touched with grey, darker on more anterior legs.
Abdomen. Silvery grey, darker dorsally.
Male genitalia (Figs 96–98). Gnathos with rounded tip with moderately long apical fringe only around central 2/3 of apical curvature; valva moderately long, much wider (1.4–1.6×) in distal half with costa lightly convex and apex tapering, sharply rounded; saccus long, slender, club-shaped; juxta a wide band with a strong ventral bar, with dorsal margin long and slightly sinuate, with a weakly beak-shaped vertical median crease; aedeagus very long, with a large basal loop, crosier-shaped, broadest at base, gradually tapering to narrow apex; vesica without obvious cornuti.
Female genitalia (Fig. 131). Ovipositor lobes membranous, appressed, broadly ovate, with scattered bristles; posterior apophyses 1.7 × as long as anterior apophyses. Ductus bursae with a long, narrow, membranous funnel below ostium ending in a narrow, strongly sclerotised ring, remainder of ductus narrow and lightly sclerotised; corpus bursae ovate, with large area of very sharp and long spinules in anterior 2/3, and with a short, triangular, partially sclerotised neck leading to ductus bursae and ductus seminalis.
Remarks
One male and two females from Warrandyte, Victoria, are conspecific but have not been included in the type series from Black Mt, ACT. Superficially, O. maxdayi resembles a species of the triradiata group, but the yellow marks in the distal half of the forewing are never joined into a three-branched structure. The band-shaped juxta with a prominent median crease is diagnostic for O. maxdayi.
Etymology
This species is named for Max Day who inspired and guided this study.
Ogmograptis barloworum Horak, sp. nov.
Material examined
Diagnosis
Forewings mostly white with few scattered dark scales along costa and an irregularly wide dark grey band along most of dorsum, any dark marks in costal half of wing very small. Male genitalia: with crosier-shaped aedeagus and very long, club-shaped saccus, with gnathos tip strongly tapering and fringed around its entire rounded apex, with distally broadly rounded valva tip, and juxta with a dorsally sharply projecting median plate.
Remarks
Among the described species, O. barloworum is characteristic with its bipartite forewing, largely white in the costal half and largely grey-black along the dorsum, but there are somewhat similar undescribed species with additional black marks on the costa. The combination of a strongly tapering gnathos tip and a juxta with a dorsally irregularly or weakly projecting median plate is characteristic of the species. A single female from Gembrook, Victoria, seems to be conspecific and is tentatively described and figured, but not included in the type series.
Etymology
This species is named for Peter and Celia Barlow who both played a crucial role in unravelling the biology of Ogmograptis.
Ogmograptis paucidentatus Horak, sp. nov.
Material examined
Diagnosis
Forewings white with scattered dark brown scales and usually three brown short streaks in costal half, and largely brown, often partially red-brown in dorsal half, with a long dark mark along fold. Male genitalia: crosier-shaped aedeagus and very long, club-shaped saccus, uncus tips short and blunt, gnathos with acute apex with only a few teeth, valva distally only moderately wider, apex broadly rounded, juxta in slide preparation folded and projecting as a narrow beak. Female genitalia: appressed, leaf-like, broadly ovate ovipositor lobes, ductus bursae narrow, sclerotised, slightly widening near corpus bursae, with a short, sclerotised tube below ostium, corpus bursae ovate.
Remarks
Among seven males of otherwise similar size there is one much smaller specimen with identical genitalia. The red-brown streak along the dorsum of the forewing and the unique gnathos tip readily characterise O. paucidentatus.
Etymology
The species name refers to the few teeth on the gnathos tip.
Ogmograptis rodens Horak, sp. nov.
|
Material examined
Diagnosis
Forewings white, speckled with dark brown scales, with five longitudinal dark grey-brown marks as obvious but ill-defined streaks or smudges, and with basal third of dorsum dark grey-brown and a grey-brown streak to apex. Male genitalia: crosier-shaped aedeagus and very long, club-shaped saccus; gnathos with broadly rounded apex with fringe of a few teeth only in centre of distal margin; valva rather short and apex tapering, narrowly rounded; juxta in slide preparation folded and projecting as a long, narrow beak.
Remarks
Superficially, O. rodens might be confused with other undescribed black and white mottled species, but the combination of the unique gnathos tip and the very large dorsally projecting median shield of the juxta are diagnostic.
Etymology
The species name refers to the gnathos tip which recalls a rodent’s teeth.
Ogmograptis bignathifer Horak, sp. nov.
|
Material examined
Diagnosis
Forewings with speckled white band running diagonally from base of costa to tornus, bordered by an interrupted black line on each side, outwardly followed by speckled grey. Male genitalia: crosier-shaped aedeagus with a sharply pointed apex and long, weakly club-shaped saccus; gnathos arms branched into upper paddle-shaped processes and ventral branches combining to form the twice narrowed gnathos tip with a widened and weakly curved apex with a fringe along its entire margin; juxta with a long and narrow central plate.
Remarks
O. bignathifer is unmistakable due to its unique wing pattern and its highly modified gnathos with duplicated tips.
Etymology
The species name refers to the distally branched gnathos arms.
Ogmograptis inornatus Horak, sp. nov.
Material examined
Diagnosis
Forewings white with scattered scales with grey-brown tips mainly in a band along costa and along dorsum, with irregular sprinklings of dark brown-tipped scales at 2/5 costa, near end of fold and in wing tip. Male genitalia: crosier-shaped aedeagus and long, club-shaped saccus, with gnathos tip complex with laterally projecting flanges and a small, inset, rounded-truncate apex with a fringe around its margin, and juxta with a long, dorsally roundly projecting median plate.
Remarks
O. inornatus is the species with by far the least wing markings and is characterised by lateral flanges to the gnathos tip.
Etymology
The species name refers to the lack of a well-defined forewing pattern.
The triradiata group
This group is characterised by a very short saccus, a short, straight aedeagus and unmodified ovipositor lobes. The species are all very small and superficially similar, with the number of spots in the basal half of the forewing and the degree of dark speckling in its distal half providing differences between species. Interspecific genitalia differences are subtle, confined to the shapes of the uncus and gnathos tips, the juxta and possibly the aedeagus shape. A single female, of O. bipunctatus, is known. Specimens of this group are mostly represented as single specimens in the collection, and nothing is known about their biology.
Ogmograptis triradiata (Turner) comb. nov.
|
Material examined
Diagnosis
Forewings white with three small spots in line across basal half of wing and the distal markings forming a three-branched yellowish ochreous structure with black-tipped scales towards wing margin, with scattered black-tipped scales along distal 2/5 costa, a subapical yellowish mark and a conspicuous black line around termen. Hindwings pale grey. Male genitalia: faintly sinuate aedeagus with weakly bulbous base; short saccus strongly tapering to truncate apex; broad gnathos gradually tapering to weakly rounded tip and with a slight concavity in lateral margin below apical fringe; juxta (folded forward and strongly foreshortened in slide) apparently with a long, dorsally rounded-pointed strongly projecting shield-shaped median plate.
Description
Adult male (Fig. 79)
Wingspan: 9 mm.
Head and thorax. White except for a silvery grey antennal flagellum.
Forewings. White with well defined marks of yellowish ochreous and few black-tipped scales; three small spots in a line across wing in basal half: a few black-tipped scales on bend at base of dorsum, a yellowish ochrous spot with few black-tipped scales near fold at 1/5 of wing and a spot near costa below middle; the three typical Ogmograptis markings in distal half of wing yellowish ochreous, long and slender, confluent in middle of wing, forming a three-branched mark, sprinkled with black-tipped grey scales where the yellow streaks reach costa and dorsum and along the third streak extending towards termen; a sprinkling of black-tipped grey scales along distal 2/5 of costa; a preapical spot of yellowish ochreous scales; terminal cilia white, basal row black-tipped forming a black row around apex.
Hindwings. Pale grey, cilia concolorous.
Legs. White, only foreleg touched with grey.
Abdomen. Silvery grey, darker dorsally.
Male genitalia (Figs 114, 115). Uncus tips long, slender, pointed with three or four socii bristles on each side; gnathos tip broad, gradually tapering to weakly rounded apex, with suggestion of a concavity in lateral margin below apical fringe; valva 1.5× as wide in distal third as at narrowest point at 1/3; saccus very short, subtriangular, gradually tapering to narrow, truncate tip; juxta (folded forward and strongly foreshortened as mounted on slide) apparently with a long, dorsally rounded-pointed strongly projecting shield-shaped median plate; aedeagus faintly sinuate, slender, with weakly bulbous base and tapering tip.
Remarks
O. triradiata was described in the New Zealand genus Cateristis Meyrick, 1889, in the Lyonetiidae, and listed as such in the lyonetiid part of the ‘Checklist of the Lepidoptera of Australia’ (Nielsen 1996a). The type species of Cateristis, Cateristis eustyla Meyrick, 1889, is known from a single male collected in the Riccarton Bush, Christchurch, New Zealand, 23 December 1882, and one specimen from Hobart, Tasmania, collected 31 January 1882, both in The Natural History Museum, London. Dugdale (1988) designated the male from New Zealand as the lectotype. Cateristis is quite distinct from Ogmograptis with a loosely scaled vertex and a pecten of long, slender scales. Neither Cateristis eustyla nor any congeneric material has ever been collected in New Zealand, apart from the lectotype, but the genus is clearly widely distributed in Australia with material from an unidentified species very close to, if not conspecific with, E. eustyla from the mountains near Canberra. The taxonomic position of Cateristis is unresolved, but it is clearly not a bucculatricid.
Ogmograptis centrospila (Turner) comb. nov.
(Fig. 80)
Material examined
Diagnosis
Forewings white with a small yellowish ochreous mark near centre of wing and the distal markings forming a pale, three-branched yellowish ochreous structure with few black-tipped scales, with scattered black-tipped scales along distal 2/5 costa, an indistinct subapical yellowish mark and a narrow black line around termen. Hindwings very pale grey.
Remarks
Both male syntypes in the ANIC lack the abdomen. The specimen labelled ‘type’ by Turner is here designated as the lectotype for taxonomic stability. The paralectotype from Brisbane has much darker scaling on the underside of the forewing, similar to O. triradiata, and is probably not conspecific with the lectotype.
Ogmograptis bipunctatus Horak, sp. nov.
Material examined
Diagnosis
Forewings white with two small spots in basal half of wing and distal markings forming a three-branched yellowish ochreous structure with conspicuous black-tipped scales towards wing margin, with scattered black-tipped scales along distal 2/5 costa; a subapical yellowish mark and a conspicuous black line around termen. Hindwings pale grey. Male genitalia: straight slender aedeagus with bulbous base; short saccus strongly tapering to rounded apex; a rather narrow gnathos tapering to rounded-truncate tip with fringe extending onto lateral margin; juxta with a very long, dorsally rounded-triangular and strongly projecting shield-shaped median plate with prominent lateral ribs. Female genitalia: unmodified, spinulose, posteriorly directed ovipositor lobes, ductus bursae membranous, short, with sclerotised funnel below ostium, corpus bursae bottle-shaped, membranous.
Etymology
The species name refers to the two dots in the basal half of the forewing.
Ogmograptis pulcher Horak, sp. nov.
Material examined
Diagnosis
Forewings white with four small spots in basal half of wing and the distal markings forming a three-branched yellowish ochreous structure with patches of black-tipped scales towards wing margin, with scattered black-tipped scales along distal 2/5 costa, an indistinct subapical yellowish mark and a conspicuous black line around termen. Hindwings leaden grey. Male genitalia: straight gradually tapering aedeagus; very short saccus with distal half not tapering; broad gnathos gradually tapering to rounded-truncate apex with fringe along entire apex; juxta (folded forward and strongly foreshortened in slide) apparently with a long, dorsally rounded-truncate shield-shaped median plate.
Etymology
The species name refers to the beautiful forewing pattern.
Unassigned to species group
Ogmograptis notosema (Meyrick, 1922)
Original description
‘♂. 13 mm. Head, thorax white, tongue obsolete. Palpi porrected, grey-whitish. Forewings elongate-lanceolate, acute; white; an oblique-triangular dark fuscous blotch on dorsum near base, a trapezoidal spot beyond middle, and an erect-triangular spot of irroration at tornus, these connected by an irregular dorsal line; two or three scattered dark fuscous scales towards costa, and a small group towards apex; cilia light grey, on costa white, on upper part of termen mixed white, at apex with a patch of black irroration. Hindwings 3 absent; grey; cilia light ochreous-grey. VICTORIA, Gisborne; 1 ex. (Coll. Lower).’.
Remarks
The holotype of O. notosema has not been found. The species was based on material sent by Lower to Meyrick for description. Meyrick eventually returned the material to Lower with a numbered list of his identifications, including species he described in 1922 from the material. The specimens bore corresponding numbers but were otherwise unlabelled, and the types were not labelled. It is possible that the holotype is still unrecognised in the SAMA.
Nielsen (1996b) included Cryphioxena notosema in its original combination in the Bucculatricidae, a decision in line with Meyrick’s remark that Cryphioxena Meyrick ‘is possibly allied to the Australian Paraphyllis’, which is a junior synonym of the bucculatricid Tritymba Lower. Meyrick’s (1935) decision to include the species in Ogmograptis is here followed though the description of the wing pattern does not tally with any of the known species of Ogmograptis. The type species of Cryphioxena is C. haplomorpha Meyrick from Mozambique.
Discussion
Insect–plant interactions between Ogmograptis and Eucalyptus
Ogmograptis comprises three species groups; however, in the present study detailed biological information was obtained only for members of the O. scribula group, the classical ‘scribbly moths’ that produce the well known bark scribbles on smooth-barked Eucalyptus species (Figs 1–6). Immature stages of the closely related genus Tritymba also have been collected from Eucalyptus, with several larvae extracted from tracks in the vascular cambium of E. racemosa spp. rossii (Fig. 11). Such tracks in the cambium are known not only from smooth-barked species like E. racemosa spp. rossii where their scars appear on the surface as ‘ghost scribbles’ (Fig. 10), but also from rough-barked eucalypt species where their scars appear on the sapwood of bark-stripped logs. Ghost scribbles have not been observed on other tree genera except for Angophora floribunda (Sm.) Sweet (Moore 1972), suggesting that Tritymba is restricted to Eucalyptus and possibly close relatives. Hence, we suspect that the larvae of the other two species groups of Ogmograptis also feed on Eucalyptus or at least on members of the Myrtaceae. Females of the O. maxdayi group share modified leaf-like ovipositor lobes with the scribula group whilst those of the triradiata group are less derived, similar to those of Tritymba. These differences in ovipositor morphology suggest different egg-laying behaviour. Two species of the maxdayi group have been collected at Warrandyte in Victoria, a locality with eucalypts but without any bark scribbles. Further investigations of the associations between larvae of these other Ogmograptis species groups and their host trees will likely greatly expand our knowledge of this mutualism.
The tracks of the O. scribula group are characterised by three very unusual traits: (1) the entire track is positioned at the level of the phellogen layer (cork cambium) and becomes exposed when the outer bark is shed (Figs 1–6), (2) the later part of the track becomes filled with callus tissue that is contiguous with the phellogen (Fig. 22), and (3) the last-instar larva re-enters the callus-filled track and feeds exclusively on callus. This obligate behavioural switch, from a legless borer feeding on bark tissue to a last-instar larva with legs feeding on callus tissue, is reliant on the appropriate reaction of the host tree to the larval activity to produce the necessary callus tissue. The formation of these distinctive tracks therefore depends on an intimate interaction between the Ogmograptis larva and its eucalypt host tree. Development of the larva and the associated track is synchronised, within a narrow window, for each species. It seems to be correlated with host phenology, in particular with the cycle of bark shedding, though this may be an epiphenomenon. Ogmograptis track formation here is primarily considered from an entomological angle, in relationship to the life history of Ogmograptis. A companion paper addressing the tissue reaction of the eucalypt host to the larval activity of Ogmograptis, in conjunction with bark phenology, is currently in preparation.
At present we can only point to the synchronicity of Ogmograptis larval activities with eucalypt phenology and tissue developments without providing any causal explanations. The close fit between host tree physiology and Ogmograptis life history suggests, however, a finely tuned system of host/herbivore interactions. The main questions to be addressed are (1) the role of the phellogen with regard to the location of the larval track, and (2) the mechanism leading to the callus production within the section of doubled tracks. Mining close to or within a cambial layer is a highly unusual biology for a lepidopteran larva, confirmed only for three species of Opostegidae (Davis 1989). Opostegid biology is known for only a few species, which are either leaf miners, stem miners or produce very long mines within the vascular cambium (Grossenbacher 1910; Kumata 1984; Davis 1989; Davis and Stonis 2007). In one Hawaiian leaf-mining species the mine terminates in a circular callus-like structure that is formed by the larva feeding in a spiral pattern and the upper epidermis of this area proliferating. According to Davis and Stonis (2007), ‘the larva [then] feeds beneath it until maturity’. In the O. scribula group, this behaviour is obligate and highly refined, with the eucalypt host usually shedding only part of its outer bark in any given year, and the areas shed from year to year not congruent. Active Ogmograptis tracks are restricted to bark due to be shed at the next bark dehiscence, and the larvae consistently turn back just before they reach the edge of the bark that will slough off (Fig. 9). According to our cursory observations the phellogen layer in Eucalyptus bark is morphologically not discernible when the Ogmograptis larva bores its early mine, yet the larva bores at the correct level. Once the outer bark falls off the entire track is revealed at exactly this level (Figs 1–6). We have not investigated whether the female oviposits randomly, resulting in death of all larvae from eggs laid on bark that will not be shed in the next season, whether the female moth lays her eggs only on areas of bark that will be shed in the next summer, or if the first-instar larvae can orient on the tree to such areas. The latter is unlikely as we have never found an early track crossing the boundary between patches of bark shed in different years. If the second option applies, then the adult can determine, at a time when we were not able to morphologically locate the phellogen, where it will develop.
The synchronicity of larval development and tree phenology is very obvious when the larva switches from being a borer to a callus feeder. As described in the Biology section, the final moult to the last-instar larva and the growth of callus in the second part of the track coincide, with a few weeks’ delay on the cooler southern side of the tree trunk. The second turning point is the location of the final moult, and examination of old scribbles shows that its position varies, from along the zig-zag track (Fig. 4) to well beyond it (Fig. 9), suggesting that external factors provide the cue for the final moult. The final moult also coincides with the moment when it becomes possible to easily remove the outer bark layer, along the friable abscission layer of cork starting to be produced by the phellogen. The timing of the final moult at the moment when the track becomes filled with callus tissue ensures that the necessary food is available for the last-instar larva.
The most remarkable and unique aspect of the life history of the O. scribula group is that the final-instar larva returns to feeding on the callus filling the mine it has previously excavated. At this point the larva exclusively consumes callus tissue, together with the embedded droppings from the earlier boring phase, and it grows very quickly. While feeding on callus forming around the entrance to the larval tunnel is widespread among lepidopteran larvae, especially in xyloryctids, cossids and hepialids, there are very few examples where a mining insect returns to feed on the callus growing within its mine (Hering 1951). Examples include Liriomyza strigata Meigen (Diptera : Agromyzidae), which produces a forked track along the mid-rib of the leaf, and species of the Nepticula argyropeza group (Lepidoptera : Nepticulidae), which produce a thickening of the leaf petiole. In both cases, however, the larvae feed as conventional leaf miners in the leaf blade between consuming callus tissue in their central mine channel. Two Phytomyza (Diptera : Agromyzidae) species on thistles also mine in the mid-rib of leaves, which becomes considerably swollen and appears like a gall, but both species also mine lateral tracks outside the thickened area (Hering 1951). The Hawaiian opostegid Paralopostega callosa Swezey, with its larva feeding under a callus-like structure (Davis 1989), is the only known case of obligate callus-feeding within a mine by a lepidopteran. Among all these examples none have the obligatory switch of feeding mode observed in the O. scribula group, or the significant morphological changes between relevant larval instars.
The obvious question is what triggers callus growth in the second half of the mine. Callus growth is a common wound reaction and is known to occur within insect mines, particularly in association with vascular bundles, and is often more pronounced around the excrements of the miner (Hering 1951). In the Ogmograptis track, however, callus growth is restricted to the final, doubled-up zig-zags, usually within an area of stained bark, suggesting physiological change (Figs 12, 13). Narrow early tracks may become filled with callus only if they are close to a doubled-up zig-zag track, and then only in the portion close to the double track. Interestingly, in the drought summer of 2006–07 the E. racemosa ssp. rossii at Gunning produced hardly any callus and most larvae died in their track before reaching maturity. Given that the last-instar larva relies, for its survival, on the presence of callus, the question arises whether callus growth is a by-product of a wound reaction, or whether larval activity induces callus growth. Two aspects of its behaviour indicate that the Ogmograptis larva may maximise production of callus in the latter part of its mine. First, the double track means a concentration of larval activity, which could enhance any effect on the host, whether through simple mechanical damage alone or also through chemical stimuli from droppings or feeding activity. The physiology of the tissue including and surrounding these double-tracked zig-zags appears modified, as suggested by discolouration (Figs 4, 12, 13). This discolouration is not yet visible when the penultimate instar is ready to moult (Fig. 9). Second, the life history of the larva is timed so that completion of the double track coincides with the activity of the cork cambium.
The obligatory switch by the last-instar larva of the O. scribula group from feeding as a borer in the bark at the phellogen layer to feeding on callus growing within its own mine represents a unique and highly adapted life history. In the nutritionally poor substrate of the outer bark the penultimate instar larva sets up a system that produces highly nutritious food for the last-instar larva. A planned study on host tissue reaction to larval activity will hopefully shed some light on the stimuli needed for callus growth, and to answer the question of whether it is induced by larval activity beyond simple wounding and the presence of frass.
Host specificity of the Ogmograptis scribula group
Both Ogmograptis and Tritymba are probably confined to the genus Eucalyptus and possibly close relatives within the Myrtaceae. Ogmograptis has been observed only on smooth-barked eucalypts of the subgenus E. (Eucalyptus), and then only on the smooth-barked parts of the tree; however, we have not tried to investigate whether Ogmograptis tracks are present within rough eucalypt bark where they never become exposed. The life history of both the O. maxdayi and triradiata groups are unknown and their larvae could possibly mine in rough-barked eucalypts. Tritymba definitely feeds also on rough-barked eucalypt species where the scars of the tracks made in the vascular cambium do not become visible on the surface but may be seen when the bark is stripped. Moore (1972) described and figured tracks of an unidentified insect, likely to be Tritymba, from many species of Eucalyptus and also from Angophora floribunda (Sm.) Sweet in New South Wales. On the basis of molecular data (Fig. 30), possibly two Tritymba species have been found together on E. racemosa spp. rossii, and the very diverse tracks suggest that there may be even more species on this eucalypt species. The question whether each Tritymba species is restricted to a single host while eucalypt species can host several Tritymba species has to be left to a future study of this large genus.
Eucalypts and Ogmograptis species of the scribula group, on the other hand, seem to have a more exclusive host relationship. Cooke and Edwards (2007) surveyed bark scribbles from the scribula group on three eucalypt species (E. pauciflora, E. racemosa ssp. rossii and E. delegatensis R.T. Baker) in the Australian Capital Territory, and demonstrated that ‘scribble measurements differed significantly between all three host species’. Whilst the initial mining direction of the larva, i.e. burrowing left versus right or up versus down, appeared random, there were clear differences in the density of scribbles depending on trunk aspect, with the southern face favoured in E. racemosa ssp. rossii and both the eastern and southern faces in E. pauciflora. Scribble shape was analysed on the basis of 10 parameters, including various measurements and directional changes of the two track parts, and found to differ significantly between all three host species. Our morphological studies of four species of the scribula group revealed diagnostic differences in the genitalia among the four species and, more surprisingly, differences also in larval morphology among the three species examined.
Ogmograptis bark scribbles have been reported from 26 species of Eucalyptus (Brooker et al. 2002), listed in Table 3 according to the classification by Brooker (2000). All 26 species belong to the monophyletic subgenus E. (Eucalyptus) which is endemic to eastern and south-western Australia (Ladiges et al. 2010). However, bark scribbles are present only in part of the range of the subgenus Eucalyptus, namely in the south-eastern corner of Australia from Fraser Island to Tasmania, as far west as the Pilliga scrub in New South Wales and the Grampians in Victoria. Scribble tree species range from mallees to tall trees, some on good soils, but most on skeletal and poor soils. Scribble density may vary greatly, from extremely crowded to sparse, even between neighbouring trees. In some species they seem to be generally infrequent. In species such as E. pilularis and E. delegatensis with a rough-barked trunk, scribbles are apparent only on the smooth-barked upper branches, though the rough bark has not been investigated. Fourteen of the 26 eucalypt species reported to have scribbles in their normal habitat are cultivated in the Australian National Botanic Gardens in Canberra, but there scribbles are found only on the two subspecies of E. racemosa. The resident population of O. racemosa on the local E. racemosa ssp. rossii uses ssp. racemosa as a host but does not colonise any of the other 13 species. This strengthens the hypothesis that species of the scribula group are restricted largely to a single eucalypt species; however, this is not an exclusive association. Of the four species studied, O. racemosa is found on both subspecies of E. racemosa, with the closely related E. haemastoma a possible second host species. O. fraxinoides has been reared from both E. fraxinoides and E. pauciflora. On the latter it may coexist with O. scribula on the same tree, albeit in a differently shaped track predominantly on the southern face of the trunk. Very rarely, oviposition occurs on the ‘wrong’ tree, and larval development apparently can take place. On Fraser Island, in the close vicinity of large and densely scribbled specimens of E. racemosa ssp. racemosa, a few isolated scribbles were found on adjacent Angophora costata (Gaertn.) Britten. However, their rather irregular shape suggests that conditions on Angophora were maybe suboptimal for the larva, and such scribbles were never found at any distance from stands of E. racemosa.
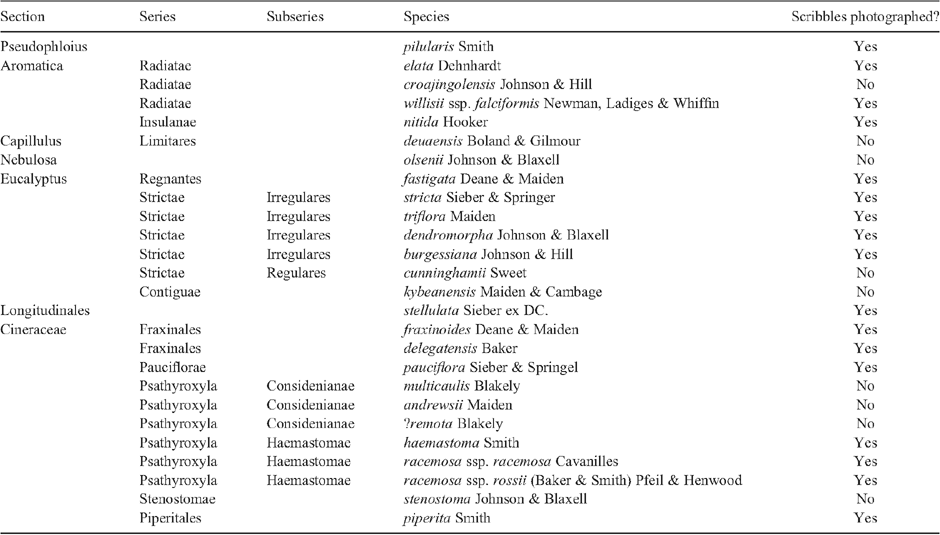
|
Taxonomic position of Ogmograptis
The four previously named Ogmograptis species were described in four different genera assigned to three different families: Opostega centrospila Turner as a lyonetiid (Turner 1923), Cateristis triradiata Turner as a tineid (Turner 1926), and Cryphioxena notosema Meyrick (Meyrick 1922) and O. scribula Meyrick (Meyrick 1935) as elachistids. Nielsen and Common (1991) tentatively included Ogmograptis in the Bucculatricidae on the strength of its ribbed cocoon, as foreshadowed by Meyrick in his description. Both O. centrospila and O. triradiata were referred to the Lyonetiidae as species of Cateristis in the ‘Checklist of the Lepidoptera of Australia’ (Nielsen 1996a). The identity of Cryphioxena notosema Meyrick cannot be conclusively resolved as the holotype has not been located in the South Australian Museum. Meyrick (1922) initially described it as an elachistid in the genus Cryphioxena Meyrick, which is based on a type species from Mozambique (Meyrick 1921), but later transferred it to Ogmograptis (Meyrick 1935). Nielsen (1996b) restored its original combination but transferred the genus Cryphioxena to the Bucculatricidae, and considered it closely related to Ogmograptis. However, the fact that Meyrick (1935) had referred this species to Ogmograptis when describing that genus justifies including it in Ogmograptis, in the absence of further information.
The initially diverse family assignments of Ogmograptis species were prompted by the greatly enlarged antennal scape, the common denominator of all the groups concerned. An antennal scape with a strong pecten or an eye cap is found in several lepidopteran superfamilies, invariably in groups of very small body size, probably to cover the eyes. A particularly large version, a so-called eye cap, occurs in the monotrysian Nepticuloidea and somewhat less prominent developments are found in two lower ditrysian goups, the gracillarioid Bucculatricidae and the yponomeutoid Lyonetiidae, as well as in several gelechioid groups. Independent developments of such an obvious trait has long caused taxonomic confusion, and species of Ogmograptis have been described in, or assigned to, all the above groups. For similar reasons, the Bucculatricidae have long been incorrectly associated with the Lyonetiidae.
The large, widely distributed and highly derived genus Bucculatrix Zeller was an isolated taxon of uncertain affinity. Description of a second bucculatricine genus, the monotypic Leucoedemia Scoble & Scholtz from southern Africa, rich in plesiomorphies, did not provide any obvious apomorphies for the group beyond the longitudinally ribbed cocoon (Scoble and Scholtz 1984). Nielsen and Common (1991) tentatively included Ogmograptis in the Bucculatricidae on the strength of such a cocoon. With its life history better known it is now evident that Ogmograptis also shares the other three attributes that Scoble and Scholtz (1984) listed as relating Leucoedemia and Bucculatrix, namely a larval character and two life-history traits. Setae L1 and L2 on the abdominal larval segments are widely separated in all three taxa, a configuration Mackay (1972) listed as a possible bucculatricid family character, in contrast to the lyonetiid condition with L1 and L2 closer on the abdominal segments. The three groups share the same type of heteromorphosis between the penultimate and the final larval instar, from a legless larva to one with well developed thoracic legs, abdominal prolegs and a spinneret. Detailed study of the larva of Ogmograptis has identified two additional unique synapomorphies also shared with Bucculatrix. The first is a modified, slender tarsal claw on the prothoracic leg (Fig. 40) together with a pair of hugely enlarged, spatulate, dorso-distal setae on the meso- and metathoracic legs (Figs 42, 43); the second is a highly modified last abdominal segment (Figs 55–57). None of these modifications are mentioned in the description of the larva of Leucoedemia, but the internal skeleton of the last abdominal segment is figured (Scoble and Scholtz 1984: fig. 16). The tarsal modifications are apparent only if investigated at very high magnification and without a SEM could well have escaped attention. Significantly, the dorsal pair of tarsal setae is missing in the drawing of the metathoracic larval leg of Leucoedemia (Scoble and Scholtz 1984: fig. 13). Davis et al. (2002) provided excellent figures of these tarsal modifications for Bucculatrix caribbea Davis & Landry, and suggested that the slender prothoracic claw might assist in cocoon construction. Our observation of the initial construction of a loose outer cocoon layer in Ogmograptis, longitudinal folds of which are then pulled inward and spun together with the inner layer to form the characteristic ribs, would support such an hypothesis. Absence of the paddle-shaped modified setae could be important to the function of the hook-like slender prothoracic claw.
In parallel to the present study, a recent phylogenetic analysis of lepidopteran relationships based on molecular evidence (Mutanen et al. 2010) indicated that Ogmograptis and Tritymba are closely related and linked to Bucculatrix Zeller. This finding came as a surprise as Tritymba had been included in the Plutellidae because the antennae are held forward when the moth is at rest, unlike Ogmograptis, in which the antennae rest along the costa (Zborowski and Edwards 2007). Morphology of adults and immatures of Tritymba confirm that it is a rather generalised taxon within the Bucculatricidae, and possesses the newly identified synapomorphies of this family – the leg structures and modified last abdominal segment, described above. The similarity of the larval biology of Tritymba and Ogmograptis further reinforces their close relationship.
With the Bucculatricidae now comprising four genera it is timely to re-evaluate this family. Adult morphology of these genera reveals a deep split between Bucculatrix, present on all continents, and the other three genera which are restricted to southern Africa and Australia. The adults of Ogmograptis, Tritymba and Leucoedemia are structurally much more generalised than the highly derived Bucculatrix. All three share a short frons not extending below the eye, appressed vertex scaling and well developed, 3-segmented labial palpi, in contrast to the strongly lengthened frons, the tufted vertex scaling and the reduced, 1-segmented labial palpi of Bucculatrix. Unlike Bucculatrix, Ogmograptis has a metafurca with elongate, slender posterior apophyses and shares rather plesiomorphic male genitalia with well developed uncus and gnathos with Tritymba and Leucoedemia, but the socii are present only as a line of setae in Ogmograptis and are apparently absent in Leucoedemia. The gnathos of Tritymba shares unique and conspicuous apomorphies with both Leucoedemia and Ogmograptis, a hairy medial plate with the former and a fringe along its apical margin with the latter. This strongly suggests that the three genera are monophyletic. They also share several plesiomorphies such as a well developed juxta and a pronounced saccus. The valva is simple in Ogmograptis but has a hairy ventral ridge in Tritymba and a free ventral lobe covered with modified, hand-shaped setae in Leucoedemia, structures that could be interpreted as a sacculus. The female genitalia of all three genera have the apophyses anteriores present and, except for one subordinate species group within Ogmograptis, they all have the distal end of the ductus bursae forming a sclerotised funnel below the ostium. Only Leucoedemia has a signum, a spinulose plate. The genitalia of Bucculatrix are very different and highly derived in both sexes. Those of the male are especially varied and indicate a long period of diversification, corroborated by the wide distribution of the genus, which is found on all continents (Braun 1963). Prominent, usually dorsally projecting setose socii are characteristic for Bucculatrix, with the uncus and gnathos nearly always absent. Discussing evolutionary trends of the male genitalia within Bucculatrix, Braun (1963) states that ‘the most complex are considered the most specialised’; however, the description by Puplesis et al. (1992) of a species with a well developed gnathos and a large saccus disproves this assessment. In this case complexity represents an ancestral, not yet reduced, configuration. Seksjaeva (1995) acknowledges this and suggests that a valva differentiated into a sacculus and cucullus also represents an ancestral state, preceding the simple digit-shaped valva of most Bucculatrix species. The female genitalia are characterised by a unique signum, a ring of spined longitudinal ribs, and by the loss of the anterior apophyses except in one species with mostly generalised male genitalia (Puplesis et al. 1992).
Immature morphology of the four bucculatricid genera also reflects the deep split between Bucculatrix and the three southern hemisphere genera. Leucoedemia, Ogmograptis and Tritymba share a strongly modified last abdominal segment with an internal skeleton and reduced anal prolegs whilst Bucculatrix has highly developed, long and slender abdominal prolegs. However, whilst the last abdominal segment is unmodified in the last-instar Bucculatrix larva, two sclerotised rods are present in the penultimate instar of an Australian Bucculatrix species (Fig. 57), presumably remnants of the synapomorphic bucculatricid interior skeleton. Immatures of Tritymba have not been studied in detail, but larval and pupal morphology of Ogmograptis agree very well with that of Leucoedemia (Scoble and Scholtz 1984), apart from the following differences. The larva of Leucoedemia lacks the puncture on the prothoracic shield, V on T2+3 and some setae on A7–9, presumably of the L and/or SV series. The pupa of Leucoedemia has a band of spinules also on the second abdominal segment and its wings reach to the tip of the abdomen. The relevant figure (Scoble and Scholtz 1984: fig. 17) suggests that labial palpi are present, as in Ogmograptis. In contrast, the pupa of Bucculatrix has no visible labial palpi and only a single row of small abdominal spines (Mosher 1969; Davis et al. 2002).
The molecular phylogeny (Fig. 30, Table 2), based on Cox1 and 18S, confirms and refines the bucculatricid relationships derived from morphology as outlined above. Within Ogmograptis there is a deep split between the scribula and the maxdayi groups, with the species of the latter group much more deeply divided than those of the scribula group. In our samples, O. scribula and O. fraxinoides, while monophyletic, are poorly supported, which might indicate comparatively recent speciation between the two, further supported by their sharing of E. pauciflora as a host. The Tritymba cluster comprises three deeply divided taxa, one a single adult and two based on five larvae extracted from E. racemosa ssp. rossii. The very deep splits within Tritymba indicate that more than one genus may be involved, suggesting that a revision of the group may reinstate Paraphyllis Meyrick, which was synonymised in the Checklist of the Lepidoptera of Australia (Edwards 1996).
In summary, the molecular data and the morphology of larva, pupa and adults all indicate that Bucculatrix is the sister group of Ogmograptis, Tritymba and Leucoedemia. A bucculatricid phylogeny based on such a basal split makes sense in every respect. The Leucoedemia group has, at best, vestigial socii but retains a well developed uncus and gnathos, while in Bucculatrix the socii have become prominent and the uncus and gnathos have been lost except in the most generalised genitalia. Seksjaeva’s (1995) assessment that a valva differentiated into sacculus and cucullus is an early stage in the evolution of Bucculatrix could well be paralleled within the Leucoedemia group, with the simple, reduced valva of Ogmograptis being the derived condition. In the female, the apophyses anteriores are retained in the Leucoedemia group but present in Bucculatrix only in B. formosa Puplesis, Seksjaeva & Puplesiene, the species with the least derived male genitalia (Puplesis et al. 1992). The hairy gnathos shared by the African Leucoedemia and the Australian Tritymba is such an astonishing and unique synapomorphy that it can only be explained by a sister group relationship between the two genera, pointing to a shared Gondwanan ancestry. Gentry (1982) suggested a Gondwanan origin for the family Anacardiaceae, which includes the host of Leucoedemia, Ozara paniculosa (Sond.). Eucalyptus, the host of Tritymba and Ogmograptis, has a long history on parts of Gondwana including Patagonia and Australia (Ladiges et al. 2003; Gandolfo et al. 2011).
Evolution of feeding modes within the Bucculatricidae can only be assessed once the insect–plant interactions between Ogmograptis and its eucalypt host are fully understood. The feeding mode of the larvae of Tritymba and the Ogmograptis scribula group is, as far as known, unique: boring a tunnel along a cambium layer which then becomes filled with callus tissue on which the last-instar larva feeds. Leucoedemia and several species of Bucculatrix produce stem galls, but most Bucculatrix species feed externally after an initial mining phase. Nearly all gall-producing Bucculatrix species feed on Asteraceae and their genitalia are much more derived than those of some externally feeding species (Braun 1963).
Acknowledgements
This study would never have been possible without the generous advice, help and support from numerous people across many disciplines. Particular thanks go to Peter Barlow for his invaluable hands-on help on his property over many years. Darren Lewis, Royal National Park, kindly provided material that confirmed, early on, that Ogmograptis is no simple borer. Laurence Mound, Peter Macnicol, Len Willan and Jeremy and Pippa Holloway generously helped with field work and Dave Britton (Australian Museum, Sydney) donated valuable reared material to the ANIC. Unique material collected by Doug Hilton and Axel Kallies (Walter and Eliza Hall Institute) for this revision is gratefully acknowledged, as well as Doug’s discovery of the types of two Ogmograptis species among the Lyonetiidae. Cecilia Melano provided bark of E. pilularis. We are indebted to Margaret McCully and Ian Brooker from CSIRO Plant Industry for advice on plant morphology and eucalypt taxonomy, and to Pauline Ladiges, University of Melbourne, for support and advice. We thank Cate Smith for molecular work, Eric Hines for SEMs of larvae and Natalie Barnett for photos of adults, all former CSIRO Entomology. Collecting permits from NSW National Parks and Wildlife Service, ACT National Parks, and the Australian National Botanic Gardens, Canberra, made this study possible, and are gratefully acknowledged. Anantanarayanan Raman kindly commented on an early draft of the insect–plant interaction discussion and Doug Hilton generously gave his time to thoroughly edit the final manuscript. We thank ETT IMPRINT for permission to quote from Judith Wright’s poem ‘Scribbly-Gum’.
References
Braun, A. F. (1963). The genus Bucculatrix in America north of Mexico (Microlepidoptera). Memoir of the American Entomological Society 18(i–iii), 1–208, pls I–XLV.Brooker, M. I. H. (2000). A new classification of the genus Eucalyptus L’Hér. (Myrtaceae). Australian Systematic Botany 13, 79–148.
| A new classification of the genus Eucalyptus L’Hér. (Myrtaceae).Crossref | GoogleScholarGoogle Scholar |
Brooker, M. I. H., Slee, A. V., Connors, J. R., and Duffy, S. M. (2002). ‘EUCLID: Eucalypts of Southern Australia.’ (CD ROM) 2nd edn. (CSIRO Publishing: Melbourne.)
Cameron, S. L., Sullivan, J., Song, H., Miller, K. B., and Whiting, M. F. (2009). A mitochondrial genome phylogeny of the Neuropterida (lace-wings, alderflies and snakeflies) and their relationship to the other holometabolous insect orders. Zoologica Scripta 38, 575–590.
| A mitochondrial genome phylogeny of the Neuropterida (lace-wings, alderflies and snakeflies) and their relationship to the other holometabolous insect orders.Crossref | GoogleScholarGoogle Scholar |
Common, I. F. B. (1990). ‘Moths of Australia.’ (Melbourne University Press: Melbourne.)
Cooke, J., and Edwards, T. (2007). The behaviour of scribbly gum moth larvae Ogmograptis sp. Meyrick (Lepidoptera: Bucculatricidae) in the Australian Capital Territory. Australian Journal of Entomology 46, 269–275.
| The behaviour of scribbly gum moth larvae Ogmograptis sp. Meyrick (Lepidoptera: Bucculatricidae) in the Australian Capital Territory.Crossref | GoogleScholarGoogle Scholar |
Davis, D. R. (1989). Generic revision of the Opostegidae, with a synoptic catalog of the world’s species (Lepidoptera: Nepticuloidea). Smithsonian Contributions to Zoology 478, 1–97.
| Generic revision of the Opostegidae, with a synoptic catalog of the world’s species (Lepidoptera: Nepticuloidea).Crossref | GoogleScholarGoogle Scholar |
Davis, D. R., and Stonis, J. R. (2007). A revision of the New World plant-mining moths of the family Opostegidae (Lepidoptera: Nepticuloidea). Smithsonian Contributions to Zoology 625, 1–212.
| A revision of the New World plant-mining moths of the family Opostegidae (Lepidoptera: Nepticuloidea).Crossref | GoogleScholarGoogle Scholar |
Davis, D. R., Landry, B., and Roque-Albelo, L. (2002). Two new Neotropical species of Bucculatrix leaf miners (Lepidoptera: Bucculatricidae) reared from Cordia (Boraginaceae). Revue Suisse de Zoologie 109, 277–294.
Dowton, M., Cameron, S. L., Austin, A. D., and Whiting, M. F. (2009). Phylogenetic approaches for the analysis of mitochondrial genome sequence data in the Hymenoptera – a lineage with both rapidly and slowly evolving mitochondrial genomes. Molecular Phylogenetics and Evolution 52, 512–519.
| Phylogenetic approaches for the analysis of mitochondrial genome sequence data in the Hymenoptera – a lineage with both rapidly and slowly evolving mitochondrial genomes.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXms12htr4%3D&md5=cacdf78ceb2c6ecdcabf5785c5e55675CAS |
Dugdale, J. S. (1988). Lepidoptera – annotated catalogue, and keys to family group taxa. Fauna of New Zealand 14, 1–262.
Edwards, E. D. (1996). Plutellidae. In ‘Checklist of the Lepidoptera of Australia. Vol. 4. Monographs on Australian Lepidoptera’. (Eds E. S. Nielsen, E. D. Edwards and T. V. Rangsi.) p. 53. (CSIRO Publishing: Melbourne.)
Flannery, T. (2010). Here on Earth. ‘An Argument for Hope’. (The Text Publishing Company: Melbourne.)
Folmer, O., Black, M., Hoeh, W., Lutz, R., and Vrijenhoek, R. (1994). DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Molecular Marine Biology and Biotechnology 3, 294–299.
| 1:CAS:528:DyaK2MXjt12gtLs%3D&md5=0a6e1d841a9ef8fafdf936427c59141bCAS |
Gandolfo, M. A., Hermsen, E. J., Zamaloa, M. C., Nixon, K. C., González, C. C., Wilf, P., Cúneo, N. R., and Johnson, K. R. (2011). Oldest known Eucalyptus macrofossils are from South America. PLoS ONE 6, e21084.
| Oldest known Eucalyptus macrofossils are from South America.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXot1yqtLg%3D&md5=d39eaaa2ffe626d4afcde079c56e0686CAS |
Gentry, A. H. (1982). Neotropical floristic diversity: phytogeographical connections between Central and South America, Pleistocene climatic functions, or an accident of the Andean orogeny? Annals of the Missouri Botanical Garden 69, 557–593.
| Neotropical floristic diversity: phytogeographical connections between Central and South America, Pleistocene climatic functions, or an accident of the Andean orogeny?Crossref | GoogleScholarGoogle Scholar |
Gibbs, M. (1918). ‘Snugglepot and Cuddlepie, their Adventures Wonderful.’ (Angus & Robertson: Sydney.)
Grossenbacher, J. G. (1910). Medullary spots: a contribution to the life history of some cambium miners. New York Agricultural Experiment Station Technical Bulletin 15, 49–65.
Hering, M. (1951). ‘Biology of the Leaf Miners.’ (Dr W. Junk: The Hague, Netherlands.)
Huelsenbeck, J. P., and Ronquist, F. R. (2001). MrBayes: Bayesian inference of phylogeny. Biometrics 17, 754–755.
| 1:STN:280:DC%2BD3MvotV2isw%3D%3D&md5=89aaec60163cef8dac4fe0ecf046c64cCAS |
Kumata, T. (1984). Cambium miners making pith flecks in broad-leaved trees – a review of cambium miners (in Japan). Hoppô Ringyô 36, 6–15.
Ladiges, P. Y., Udovicic, F., and Nelson, G. (2003). Australian biogeographical connections and the phylogeny of large genera in the plant family Myrtaceae. Journal of Biogeography 30, 989–998.
| Australian biogeographical connections and the phylogeny of large genera in the plant family Myrtaceae.Crossref | GoogleScholarGoogle Scholar |
Ladiges, P. Y., Bayly, M. J., and Nelson, G. J. (2010). East–west continental vicariance in Eucalyptus subgenus Eucalyptus. In ‘Beyond Cladistics. The Branching of a Paradigm’. (Eds D. M. Williams and S. Knapp.) pp. 267–302. (University of California Press: Berkeley and Los Angeles.)
Mackay, M. R. (1972). Larval sketches of some microlepidoptera, chiefly North American. Memoirs of the Entomological Society of Canada 88, 5–83.
Maddison, W., and Maddison, D. (2003). ‘MacClade ver. 4.06.’ (Sinauer Associates: Sunderland, MA.)
Meyrick, E. (1921). Descriptions of South African Micro-Lepidoptera. Annals of the Transvaal Museum 8, 49–148.
Meyrick, E. (1922). ‘Exotic Microlepidoptera 2.’ pp. 481–512. (Taylor and Francis: London.)
Meyrick, E. (1935). ‘Exotic Microlepidoptera 4.’ pp. 593–608. (Taylor and Francis: London.)
Moore, K. M. (1972). Observations on some Australian forest insects. Australian Zoologist 17, 30–39.
Mosher, E. (1969). ‘Lepidoptera Pupae. Five Collected Works on the Pupae of North American Lepidoptera’. (Entomological Reprint Specialists: East Lansing, MI.)
Mutanen, M., Wahlberg, N., and Kaila, L. (2010). Comprehensive gene and taxon coverage elucidates radiation patterns in moths and butterflies. Proceedings of the Royal Society Biological Sciences Series B 277, 2839–2848.
| Comprehensive gene and taxon coverage elucidates radiation patterns in moths and butterflies.Crossref | GoogleScholarGoogle Scholar |
Nielsen, E. S. (1996a). Lyonetiidae. In ‘Checklist of the Lepidoptera of Australia. Vol. 4. Monographs on Australian Lepidoptera’. (Eds E. S. Nielsen, E. D. Edwards and T. V. Rangsi.) pp. 57–58. (CSIRO Publishing: Melbourne.)
Nielsen, E. S. (1996b). Bucculatricidae. In ‘Checklist of the Lepidoptera of Australia. Vol. 4. Monographs on Australian Lepidoptera’. (Eds E. S. Nielsen, E. D. Edwards and T. V. Rangsi.) p. 45. (CSIRO Publishing: Melbourne.)
Nielsen, E. S., and Common, I. F. B. (1991). Lepidoptera (Moths and Butterflies). In ‘The Insects of Australia. Vol. 2’. (Ed. CSIRO.) pp. 817–915. (Melbourne University Press: Melbourne.)
Pfeil, B. E., and Henwood, M. J. (2004). Multivariate analysis of morphological variation in Eucalyptus series Psathyroxyla Blakely (Myrtaceae): taxonomic implications. Telopea 10, 711–724.
Posada, D., and Crandall, K. A. (1998). ModelTest: testing the best-fit model of nucleotide substitution. Bioinformatics (Oxford, England) 14, 817–818.
| ModelTest: testing the best-fit model of nucleotide substitution.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1MXktlCltw%3D%3D&md5=96deade1c75c4e555f1a98ea1a9c9435CAS |
Puplesis, R., Seksjaeva, S., and Puplesiene, J. (1992). Bucculatrix formosa sp. n., a remarkable species from the Kugitangtau Mountains (Central Asia) (Lepidoptera: Bucculatricidae). Nota lepidopterologica 15, 41–46.
Rambaut, A., and Drummond, A. J. (2007). Tracer v1.4. http://beast.bio.ed.ac.uk/Tracer
Robinson, G. S. (1976). The preparation of slides of Lepidoptera genitalia with special reference to the Microlepidoptera. Entomologist’s Gazette 27, 127–132.
Scoble, M. J., and Scholtz, C. H. (1984). A new, gall-feeding moth (Lyonetiidae: Bucculatricinae) from South Africa with comments on larval habits and phylogenetic relationships. Systematic Entomology 9, 83–94.
| A new, gall-feeding moth (Lyonetiidae: Bucculatricinae) from South Africa with comments on larval habits and phylogenetic relationships.Crossref | GoogleScholarGoogle Scholar |
Seksjaeva, S. V. (1995). New intrageneric groups within the genus Bucculatrix (Lepidoptera, Bucculatricidae) designated on the basis of the morphology of the male genitalia. Entomological Review 74, 111–119.
Swofford, D. L. (2002). ‘PAUP* Phylogenetic Analysis using Parsimony (*and Other Methods) Ver. 4.’ (Sinauer Associates: Sunderland, MA.)
Turner, A. J. (1923). New Australian Micro-Lepidoptera. Transactions of the Royal Society of South Australia 47, 165–194.
Turner, A. J. (1926). Studies in Australian Lepidoptera. Transactions of the Royal Society of South Australia 50, 120–155.
Upton, M. S. (1997). ‘A Rich and Diverse Fauna. The History of the Australian National Insect Collection 1926–1991’. (CSIRO Publishing: Melbourne.)
Whiting, M. F. (2002). Mecoptera is paraphyletic: multiple genes and phylogeny of Mecoptera and Siphonaptera. Zoologica Scripta 31, 93–104.
| Mecoptera is paraphyletic: multiple genes and phylogeny of Mecoptera and Siphonaptera.Crossref | GoogleScholarGoogle Scholar |
Whitten, M. (2012). Deciphering nature’s message stick. Meanjin 71, 30–38.
Wright, J. (1955). Scribbly-Gum. In ‘The Two Fires’. (Angus and Robertson: Sydney.)
Zborowski, P., and Edwards, T. (2007). ‘A Guide to Australian Moths.’ (CSIRO Publishing: Melbourne.)