Evolutionary phenomics and the emerging enlightenment of arthropod systematics
Andrew R. Deans A D , István Mikó A , Benjamin Wipfler B and Frank Friedrich CA Department of Entomology, Pennsylvania State University, 501 ASI Building, University Park, PA 16802, USA.
B Entomology Group, Institut für Spezielle Zoologie und Evolutionsbiologie, Friedrich-Schiller-Universität Jena, Jena 07743, Germany.
C Biocenter Grindel and Zoological Museum Hamburg, Hamburg University, Hamburg 20146, Germany.
D Corresponding author. Email: adeans@psu.edu
Invertebrate Systematics 26(3) 323-330 https://doi.org/10.1071/IS12063
Submitted: 14 August 2012 Accepted: 6 September 2012 Published: 21 September 2012
Abstract
Published research on the diversity and evolutionary history of Arthropoda sets a high standard for data collection and the integration of novel methods. New phylogenetic estimation algorithms, divergence time approaches, collaborative tools and publishing standards, to name a few, were brought to the broader scientific audience in the context of arthropod systematics. The treatment of morphology in these studies, however, has largely escaped innovation. Lodes rich in characters too often go unexplored, phenotype concepts are published with inadequate documentation and the way observations are textualised leaves them inaccessible to a majority of biologists. We discuss these issues, using data from recent arthropod systematics publications, and offer several that stand to restore the broad utility of morphological data. Specifically, we focus on: (1) the potential of internal soft-part characters and how to integrate their observation into arthropod systematics projects through dissection and serial sectioning; (2) the importance of capturing observations in images, especially using relatively new approaches, like laser scanning confocal microscopy and three-dimensional reconstruction; and (3) the untapped potential of established knowledge representation methods, which may help make the descriptive components of arthropod systematics research more accessible to other domains.
Introduction
Systematists arguably benefit from the most diverse array of data sources and technological approaches in the life sciences. We use information from genomes, transcriptomes, proteomes, phenomes and whole biomes to elucidate niche restrictions, discover cryptic diversity, delimit and diagnose taxa, estimate relationships and divergence times, discover characters and to more fully understand evolution. The systematist’s toolkit, therefore, is correspondingly large and continually expanding. The integration of new tools, however, appears to be uneven. Johnson (2011) recently described the latest collaborative environments, specimen data sharing resources and advances in publishing, which are percolating through the community. Molecular advances, likewise, are rapidly finding their way into systematists’ workflows and will soon become mainstream (e.g. next generation sequencing; Bybee et al. 2011; Lemmon and Moriarty Lemmon 2012). Breakthroughs in the realm of morphology, however, are admired but rarely used outside a handful of laboratories. The promise of a ‘renaissance of morphology’, triggered by the proliferation of new tools (e.g. Popper and Schaffner 1959; Stuessy et al. 2003; Budd and Olsson 2007; Friedrich and Beutel 2008), has yet to be broadly realised in arthropod systematics.
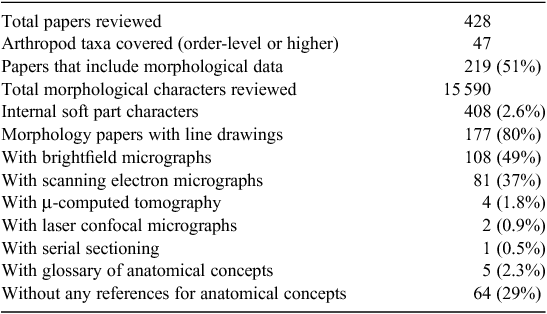
To explore this predicament in a more structured (if crude) way, we surveyed 428 arthropod systematics (sensu lato) papers, published from 2007 to early 2012 in seven journals and scored them for several variables relevant to morphology-based systematics (Table 1; complete dataset at http://dx.doi.org/10.6084/m9.figshare.94214). The results show, unsurprisingly perhaps, that morphology remains the primary data source for arthropod systematists. Our survey also uncovers three aspects of arthropod systematics research that we predict will benefit from the adoption of contemporary methods: (1) internal soft-part characters remain largely unexplored despite demonstrable utility for phylogenetics and diagnosis; (2) new imaging technology is vastly underutilised despite increasing accessibility; and (3) phenotypic concepts, especially anatomical entities, used in character descriptions too often remain undocumented. We discuss these deficiencies below and offer some thoughts on how to bring arthropod systematics through a renaissance and into enlightenment.
|
Prospecting for relevant internal phenotypes
Methodological inertia guides most arthropod systematists to digest muscles, glands, ganglia and other soft parts, so that we can cleanly prospect for characters on sclerites. The resulting soft-tissue soup is poured down the drain, so to speak, with potassium hydroxide or captured in vials after treatment with proteinase. We read >15 500 character descriptions in our literature review and found a paltry 408 that referred to internal soft parts. Yet our understanding of arthropodean phenotypes, in the context of evolutionary history (a core goal of systematics), would benefit from deeper observation of these structures. The soft structures themselves serve as evidence for phylogenetics (e.g. Friedrich and Beutel 2010), but they also inform us about the hard parts, including external features.
Muscle characters, for example, are usually ignored in phylogenetic analyses but, most problematically, also in descriptions and interpretations of skeletal characters. Homology hypotheses are often built around the overall similarity of the ‘topography’ of two characters (here the shape and biospatial relationship of the structure with other anatomical entities; see discussion in Seltmann et al. 2012). Although a sclerite can be described based on its relationships to other sclerites, its relationship with muscles is often crucial for generating well founded homology hypotheses. The mesoscutum in Hemiptera (Insecta) looks remarkably similar to the mesoscutum in Hymenoptera; in both taxa, the median area of the mesoscutum is delimited submedially by two longitudinal sulci. These areas could easily be interpreted as homologous anatomical structures. Observations of the thoracic intima, however, reveal that the sulci are defined by different muscles and hence are most likely not homologous (Mikó et al. 2012). Similar examples can be found in characters that involve antennal sensilla, which are too often classified based on superficial external morphology (Romani et al. 2010). The functional mechanisms that underlie these cuticular modifications, common characters for species diagnosis in many insects, are impossible to understand without observing the innervations of the sensory cell, as well as their corresponding exocrine glands (Romani et al. 2010). The internal phenome, in these cases muscle morphology and sensillar neuroanatomy, yields critical evidence that shapes our understanding of superficially equivalent structures.
Mining the internal phenome in most arthropods can be as simple (and inexpensive) as preparing a specimen and dissecting it under a microscope. The process also requires that one be willing to divide a specimen into many smaller parts, a process to which many systematists seem have an aversion (especially if the species is rare). Dissections can be made on either wet specimens in alcohol or glycerine or on a specimen that has been put through a regimented drying process (e.g. Quicke et al. 1999), with more-or-less well preserved internal soft structures. The cardinal difference between wet and dried specimens is the physical relationships between anatomical structures. In wet specimens, muscles, glands and nerves are more strongly connected to each other and to the integument, whereas in dried specimens they are easy to detach. Dried specimens, therefore, are feasible for the description of the spatial position of anatomical structures, while wet specimens can be used for checking muscle insertions and origins. Since dissections are inherently dissociative (not inevitably destructive), visualisation and imaging of the specimens are highly recommended in different phases of the mining process. Vilhelmsen et al. (2010) and Mikó et al. (2007, 2012), provide examples of how dissections and subsequent observations of internal soft-tissue features can inform character concepts.
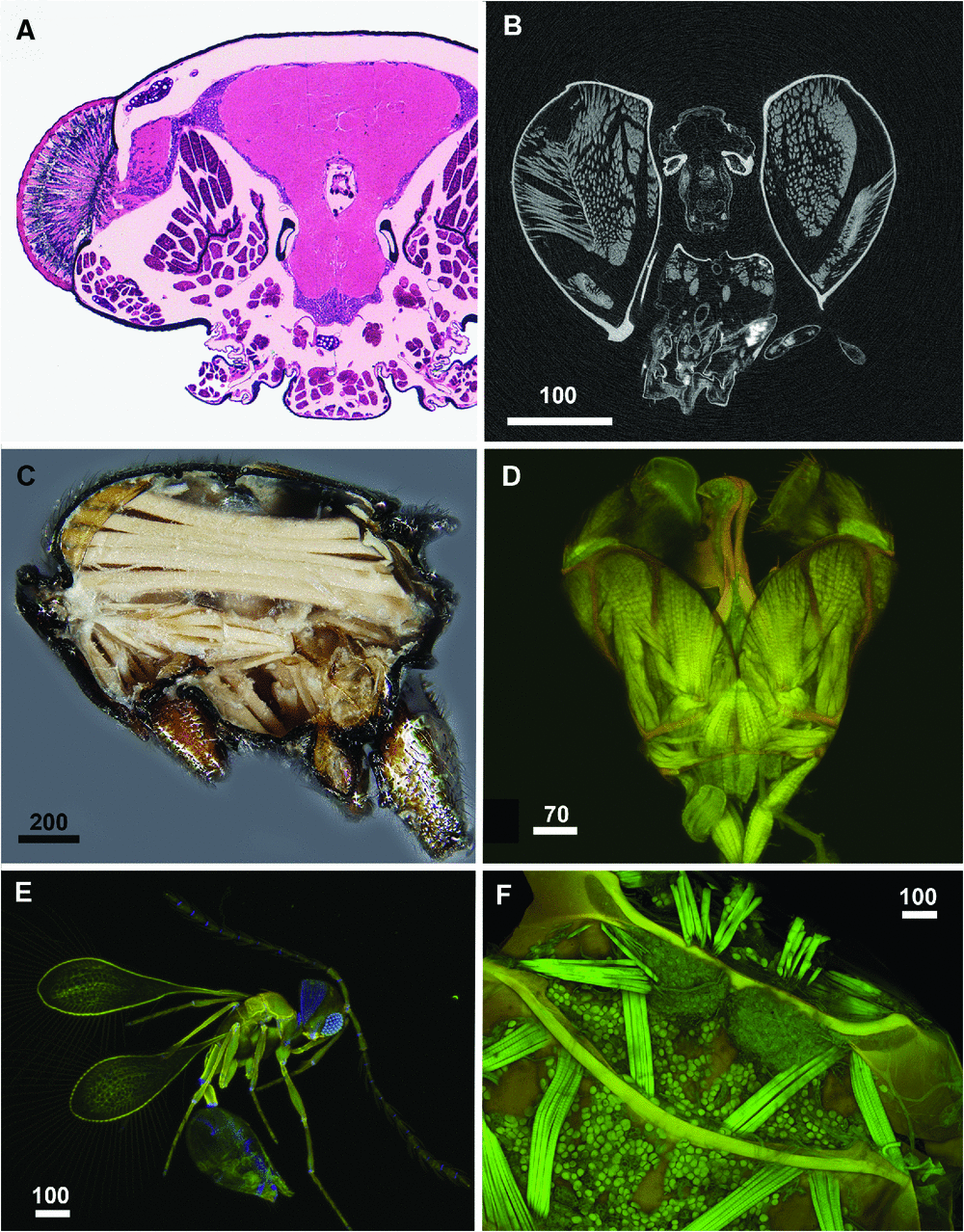
A more involved, precise and highly dissociative way of mining the internal phenome is to serially section a specimen, either using histological or X-ray-based techniques. Histological sectioning (Fig. 1A ) is common in comparative morphology studies and some physiological research but rarely used by systematists, possibly due to problems presented by heavily sclerotised cuticle, although advances in epoxy resin embedding media and microtome technology have solved most of these problems. Fixation of freshly collected specimens is required for subcellular histological observations, but semi-thin sections of alcohol-preserved specimens can provide important and useful information about the gross morphology of arthropods. Great examples of histological sectioning in the context of arthropod systematics include Wirkner and Richter (2007), Edgecombe and Koch (2008), Beutel et al. (2009a , 2009b , 2010), Friedrich and Beutel (2010), Camacho (2011), Szucsich et al. (2011) and Koch and Edgecombe (2012).
A more recent advancement in serial sectioning is to use X-rays to virtually slice the arthropod specimen in a process called microtomography (µCT) (Fig. 1B ). This method is non-dissociative but usually offers lower-resolution and much weaker tissue contrast images than histology. Stained or unstained specimens can be scanned in a pipette tip filled with water, alcohol or glycerine or embedded in Araldite or Canada balsam. Critical point drying is the best preparation technique, when staining is not an option, since it maximises the contrast between the biological structures and the surrounding medium (air). There are two primary µCT resources used for arthropods: desktop machines (DTµCT; e.g. machines by Phoenix, SkyScan), which are more widely accessible and relatively inexpensive, and synchrotron-radiation-based µCT (SRµCT), which yields much higher resolution but typically requires careful planning and access to relatively high-demand facilities. With SRµCT, one has the opportunity to use the absorption contrasting technique (Beckmann et al. 2008, Friedrich and Beutel 2008), whereas only phase contrasting (Wilkins et al. 1996, Hörnschemeyer et al. 2002, Dunlop et al. 2012) is available with DTµCT. Although phase contrast scans allow strong delimitation of the specimen from the surrounding media, the tissue-contrast of the resulting micrographs is very low. The tissue contrast of pure absorption contrast data are, however, very high, and therefore SRµCT might be considered an excellent alternative to histological sections (Friedrich and Beutel 2008). Muscles, nervous system, glands and different grades of skeletal sclerotisation can easily be distinguished. However, for detailed information on thin peripheral nerves or on the cellular aspects of tissues, the data from whole-specimen scans are not yet as informative as those from histological sections. Hardware improvements (larger sensors, stable beams, etc), advances in X-ray-sensitive staining (Metscher 2009), and new developments in corrosion casting (Wirkner and Richter 2007) will help surmount these issues.
Examples of articles using SRµCT to infer arthropod systematics include Beutel et al. (2009a), Friedrich and Beutel (2010), Huckstorf and Wirkner (2011), Talarico et al. (2011), Wipfler et al. (2011) and Friedemann et al. (2012). Researchers have also used SRµCT to explore the characteristics of obscured fossil insects in amber (Tafforeau et al. 2006; Lak et al. 2008) and to partially reconstruct the internal phenomes of fossil insects (Pohl et al. 2010). Microtomography of fossil vertebrate tissues is also a possible future application of SRµCT in arthropod systematics, for example to examine pigment composition (Wogelius et al. 2011).
Documenting observed phenotypes
The arthropod systematics papers we read reported >15 000 characters, and most of these publications included at least some token illustrations, almost exclusively as line drawings or light or traditional scanning electron micrographs (SEMs). While these methods are convenient, given their general accessibility, low cost, and long history, they are also limited in their efficiency. None of the studies we read (2007–2012) illustrated every character state for every taxon, despite the existence of tools that facilitate this level of documentation (Liljeblad et al. 2008). Yet imaging and annotating characters is crucial to the repeatability of our research and the repurposing of our observations. Refinements in brightfield methods (e.g. Buffington and Gates 2008), another common approach to illustrating observations, are easy to implement and should increase the utility of the resulting images (Fig. 1C ). Two emerging classes of imaging methods, however, are poised to facilitate broader discovery and richer documentation of phenotypes for arthropod systematists: (1) Confocal Laser Scanning Microscopy (CLSM); and (2) three-dimensional reconstruction.
Confocal Laser Scanning Microscopy
Confocal Laser Scanning Microscopy, used extensively since the late 1980s by cellular and molecular biologists, has only recently been applied to arthropod systematics (Galassi 1997, Klaus et al. 2003, Klaus and Schawaroch 2006). CLSM has several advantages over traditional light microscopy and SEM. (1) depending on the CLSM strategy used and the size of the specimen, one can produce and annotate three-dimensional models of the object of interest, which results in a highly efficient representation strategy (see below); and (2) unlike scanning electron microscopy, or even light microscopy for some specimens, one can readily differentiate tissues – for example, skeletal structures (Michels 2007) (Fig. 1D, F ), muscles, resilin-rich areas (Fig. 1E ) of the integument (Neff et al. 2000; Andersen 2004; Burrows et al. 2011; Michels and Gorb 2012), or even calcified regions (Haug et al. 2011) can be differentiated by lasers of different wavelengths (Fig. 1E ). Specimens also do not require exotic media: Canada balsam, euparal, glycerine jelly (Michels 2007; Michels and Gorb 2012) or agarose are the usual embedding media. CLSM can also be used for visualisation of internal structures through the cuticle in transparent specimens, which rapidly yields information about the site of origin of muscles corresponding with external cuticle modifications (Fig. 1D ). Specimens with strongly melanised cuticle can be made transparent with hydrogen peroxide (Stüben and Linsenmair 2008), without affecting the autofluorescence of insect anatomical structures and destroying soft structures (A. R. Deans, pers. obs.)
Three-dimensional reconstruction
Most anatomical structures are readily visualised in two-dimensions, but rendering these structures as three-dimensional interactive models is a more realistic and efficient representation. Indeed, three-dimensional reconstruction has a relatively long history in biology (Gaunt and Gaunt 1978) and even among some arthropod systematists, especially when personal computers enabled a switch from a tedious manual process to computer-assisted reconstruction (Huijsmans et al. 1986; Winslow et al. 1987; Verbeek et al. 1995).
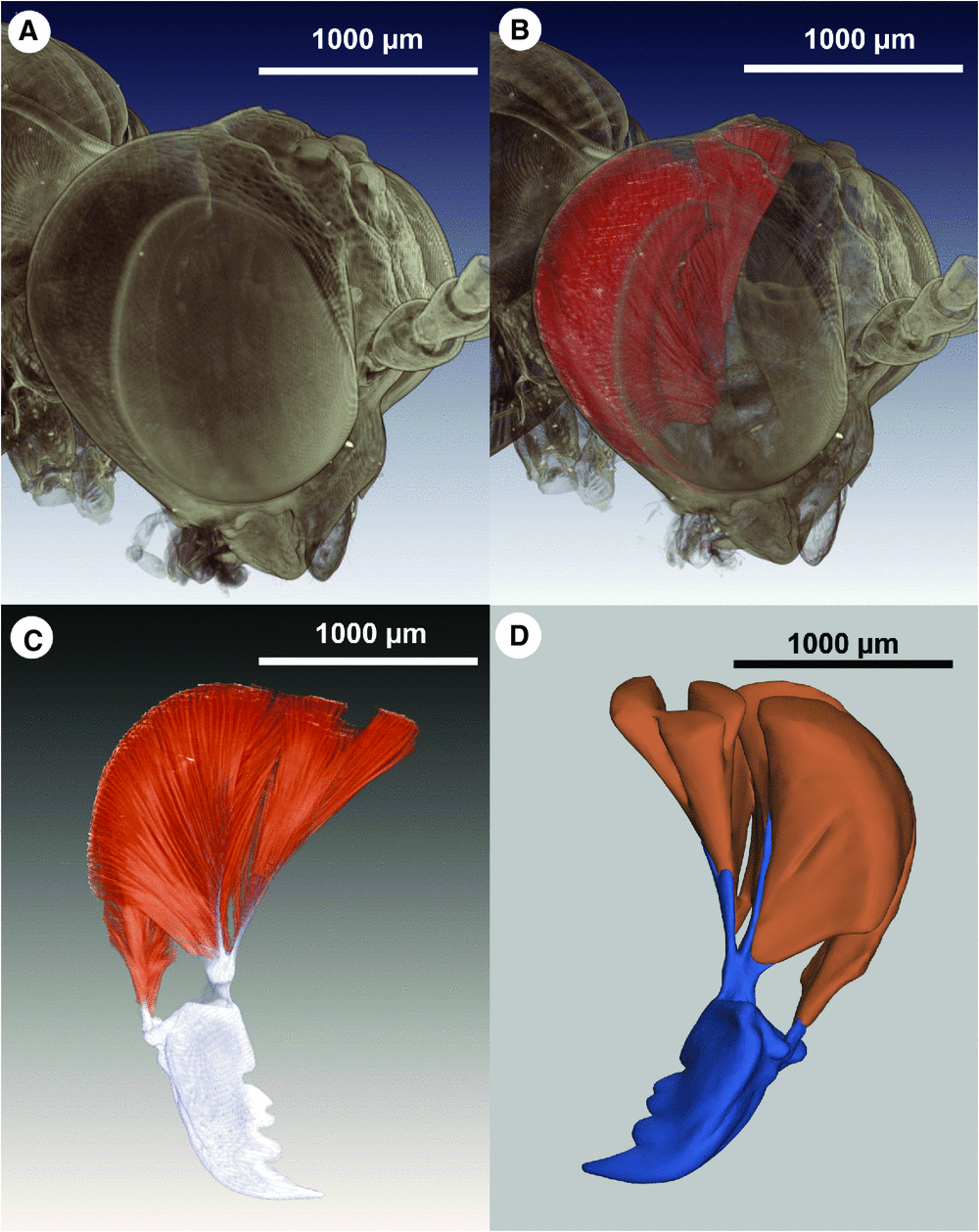
Aligned image sets from CLSM, histological sectioning or µCT can be rendered as three-dimensional models using one of several software packages (e.g. Imaris Bitplane (http://www.bitplane.com/go/products/imaris), Amira (http://www.amira.com/), Zeiss LSM Image browser (http://www.embl.de/eamnet/html/body_image_browser.html)) and either the surface (SR) or volume rendering (VR) technique (Kaufman and Mueller 2006). Surface rendering provides information exclusively about the surface of selected structures of the specimen (Fig. 2D ), whereas VR captures both internal and external features of the structures (Fig. 2A–C ). Models rendered straight from the raw image data are immediately useful for illustrating external characters (Fig. 2A ) and dissected parts (Fig. 1D ). However, annotating different structures on individual image sections, an admittedly chronophagous process with current technology (Ruthensteiner 2008), facilitates the rendering of interactive models, whereby structures can be added or removed to facilitate viewing of obscured phenotypes (i.e. virtual dissection; see example in Fig. 2B, C ).
The Portable Document Format (PDF) can now integrate SR three-dimensional models (supplementary file available at http://dx.doi.org/10.6084/m9.figshare.94272) as embedded, interactive figures (Ruthensteiner and Heß 2008), which makes distribution of these illustrations a relatively trivial exercise; only common, free software (namely Adobe Acrobat Reader) is required. Volume rendered models currently can only be published as supplementary files, but the ability to incorporate them into PDFs will likely happen in the near future (Ruthensteiner et al. 2010).
Explicit textualisation of concepts
Phenomic data cannot be represented by images alone, as they are difficult to datamine and require context and a history of interpretation that can only be communicated in words. Virtually every named organism is associated with a textual diagnosis, and each morphology-based paper we read in our review included blocks of prosaic natural language that attempted to communicate knowledge about phenotypes. We feel strongly, as communicated by Deans et al. (2012), that phenomic data derived from the systematic process, including the observations described in textual descriptions, should be availed broadly to other domains. Unfortunately, we have no standardised way of publishing phenotype data (Vogt et al. 2010; Deans et al. 2012). Nucleotide data, for example, are represented by symbols (A, G, C, etc.) designated by the International Union of Pure and Applied Chemistry (IUPAC) and universally accepted by biologists. Strings of these symbols are submitted to an accessible, highly functional and robust database, like the National Center for Biotechnology Information’s GenBank (Benson et al. 2012). The purpose of these standards, in part, was to ‘facilitate comparisons ... as in the search for homologies’ (IUPAC-IUB Commission on Biochemical Nomenclature 1970). There is no equivalent yet for arthropod morphology. Homonymy is rampant (Yoder et al. 2010), and phenotypes are often painstakingly qualified (‘somewhat reddish’) or too vague to be meaningful (‘labrum narrow’ versus ‘labrum broad’ versus ‘labrum very broad’).
Emerging informatics standards stand to dramatically improve the way we share phenotype data. Deans et al. (2012) alluded to one method that was developed initially for representing mutant model organism phenotypes, the Entity–Quality (EQ) formalism (Mabee et al. 2007; Mungall et al. 2010), but which could be adapted to systematics. In the EQ approach, anatomical and phenotype quality concepts are explicitly defined and organised as ontologies (see Washington and Lewis 2008); the concepts from the ontologies are then composed in a way that makes the phenotype data (i.e. the character states) queryable. The most successful deployment of this approach, at least in an evolutionary biology context, is found in the world of ichthyology (Phenoscape project; see Mabee et al. 2012). Phenoscape combines morphological data from fish phylogenetic matrices with data from mutant model organisms (primarily Danio rerio; http://zfin.org) and uses the logic inherent in ontologies to inform, in part, hypotheses about genotype–phenotype interactions. Examples of fine-grained EQs, composed in Web Ontology Language (OWL; http://www.w3.org/TR/owl-features/) and applied to species descriptions, are also emerging in arthropod research (Mullins et al. 2012).
While it is too early to apply the EQ approach broadly in arthropod systematics, we do think systematists should more rigorously document their concepts, especially for anatomy. Almost one-third of the papers we reviewed provided no references for anatomical concepts, and only 2.3% provided a glossary. In most cases, the intended readership, i.e. other specialist taxonomists, will understand which ‘paramere’ ( = five different structures in Hymenoptera) or ‘forearm’ ( = part of the male genitalia of some Lepidoptera) the authors mean in their character description, but homonymy could induce confusion in anyone attempting to repurpose those observations. A simple solution is to append one’s publication with a glossary of terms, explicitly defined using genus differentia (Smith 2005). Some insect systematists, Talamas et al. (2011) and Mikó et al. (2012) for example, have started doing this in the form of a table that also includes links (Uniform Resource Identifiers or URIs) to anatomical concepts in the Hymenoptera Anatomy Ontology (Yoder et al. 2010) and, in some cases, to phenotypic qualities in the Phenotype Quality Ontology (PATO; Mungall et al. 2010) (see Seltmann et al. 2012 for a description of the process).
Conclusion
Arthropod systematists are charged with researching more than half of all known life forms, a process that requires extraordinary levels of data collection and methods innovation. The majority of data and hypotheses generated by our science – nucleotide sequences, specimen collecting events, taxonomic names, phylogenetic trees, etc. – are already availed broadly using community-developed standards, and we continue to lead the field in mechanisms of collaboration and dissemination. In our view, at least three aspects of the systematic process stand to benefit from a shift in strategy or the incorporation of emerging technology. (1) Internal anatomy remains a largely untapped resource for evidence of taxonomic association and evolutionary history. We encourage arthropod systematists to expand their character-harvesting repertoire to include more dissociative methods (dissection, sectioning) and to explore X-ray-based sectioning methods, which are increasingly accessible. (2) Phenomic observations would be more robustly documented if we utilise CLSM and three-dimensional reconstruction of serial sections, especially now that annotated and interactive three-dimensional models can be easily published. (3) We should also invest in the development of new standards (and testing of emerging standards) that facilitate sharing of textual phenotype data. Prosaic natural language is by far the most expressive way to communicate one’s interpretations of phenotypes, but the resulting summary remains largely inaccessible to researchers outside of the community of domain experts (i.e. taxonomist specialists). However, biology is increasingly integrative, and arthropod systematists can no longer afford to keep these observations within their circle of experts. Of course we acknowledge that resources are limited and that wholesale adoption of our suggestions is currently impossible. We hope, however, that our viewpoint stimulates discussion about how we take the next steps to dramatically improve and extend our science.
Acknowledgements
The ideas presented herein were developed during meetings funded by the US National Science Foundation (NSF; grants DBI-0850223, DEB-0956049) and the Biodiversity Synthesis Center of the Encyclopedia of Life, and in rigorous conversation with participants of the Phenotype RCN (http://www.phenotypercn.org). We thank the NSF-funded National Evolutionary Synthesis Center (EF-0905606) for providing the support for synergistic interactions. The opinions expressed herein are of the authors and do not necessarily reflect those of the US NSF. We are indebted to the anonymous reviewers, who provided invaluable critical feedback and insights that greatly improved the manuscript. Eva Johannes (Cellular and Molecular Imaging Facility, North Carolina State University) provided much guidance and discussion of CLSM methods. Rolf Beutel (Friedrich-Schiller-Universität Jena, Germany) inspired us to broaden our approaches to insect morphology with much of this new technology. Finally, we thank Andy Austin for the inviting us to contribute this viewpoint.
References
Andersen, S. O. (2004). Regional differences in degree of resilin cross-linking in the desert locust, Schistocerca gregaria. Insect Biochemistry and Molecular Biology 34, 459–466.| Regional differences in degree of resilin cross-linking in the desert locust, Schistocerca gregaria.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXjsVWnsr0%3D&md5=1056c2365e6a7b2fadffcbb20c7a00e9CAS |
Beckmann, F., Herzen, J., Haibel, A., Müller, B., and Schreyer, A. (2008). High density resolution in synchrotron-radiation-based attenuation-contrast microtomography. Proceedings – Society of Photo-Optical Instrumentation Engineers 7078, 70781D.
| High density resolution in synchrotron-radiation-based attenuation-contrast microtomography.Crossref | GoogleScholarGoogle Scholar |
Benson, D. A., Karsch-Mizrachi, I., Clark, K., Lipman, D. J., Ostell, J., and Sayers, E. W. (2012). GeneBank. Nucleic Acids Research 40, D48–D53.
| GeneBank.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXhs12hur3P&md5=230d09e8990e3e1e06e1d26c3efe1844CAS |
Beutel, R. G., Friedrich, F., and Whiting, M. F. (2009a). Head morphology of Caurinus (Boreidae, Mecoptera) and its phylogenetic implications. Arthropod Structure & Development 37, 418–433.
| Head morphology of Caurinus (Boreidae, Mecoptera) and its phylogenetic implications.Crossref | GoogleScholarGoogle Scholar |
Beutel, R. G., Kristensen, N. P., and Pohl, H. (2009b). Resolving insect phylogeny: the significance of cephalic structures of the Nannomecoptera in understanding endopterygote relationships. Arthropod Structure & Development 38, 427–460.
| Resolving insect phylogeny: the significance of cephalic structures of the Nannomecoptera in understanding endopterygote relationships.Crossref | GoogleScholarGoogle Scholar |
Beutel, R. G., Friedrich, F., and Aspök, U. (2010). The larval head of Nevrorthidae and the phylogeny of Neuroptera (Insecta). Zoological Journal of the Linnean Society 158, 533–562.
| The larval head of Nevrorthidae and the phylogeny of Neuroptera (Insecta).Crossref | GoogleScholarGoogle Scholar |
Budd, G. E., and Olsson, L. (2007). Editorial: a renaissance for evolutionary morphology. Acta Zoologica 88, 1.
| Editorial: a renaissance for evolutionary morphology.Crossref | GoogleScholarGoogle Scholar |
Buffington, M. L., and Gates, M. (2008). Advanced imaging techniques II: using a compound microscope for photographing point-mount specimens. American Entomologist 54, 222–224.
Burrows, M., Borycz, J. A., Shaw, S. R., Elvin, C. M., and Meinertzhagen, I. A. (2011). Antibody labelling of resilin in energy stores for jumping in plant sucking insects. PLoS ONE 6, e28456.
| Antibody labelling of resilin in energy stores for jumping in plant sucking insects.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXhs1KhurvN&md5=8d1490cb6cb48159b3e10987d3ae16b2CAS |
Bybee, S. M., Bracken-Grissom, H. D., Hermansen, R. A., Clement, M. J., Crandall, K. A., and Felder, D. L. (2011). Directed next generation sequencing for phylogenetics: an example using Decapoda (Crustacea). Zoologischer Anzeiger – A Journal of Comparative Zoology 250, 497–506.
| Directed next generation sequencing for phylogenetics: an example using Decapoda (Crustacea).Crossref | GoogleScholarGoogle Scholar |
Camacho, M. D. (2011). Cephalic musculature in five genera of Symphyla (Myriapoda). Arthropod Structure & Development 40, 159–185.
| Cephalic musculature in five genera of Symphyla (Myriapoda).Crossref | GoogleScholarGoogle Scholar |
Deans, A. R., Yoder, M. J., and Balhoff, J. P. (2012). Time to change how we describe biodiversity. Trends in Ecology & Evolution 27, 78–84.
| Time to change how we describe biodiversity.Crossref | GoogleScholarGoogle Scholar |
Dunlop, J. A., Wirth, S., Penney, D., McNeil, A., Bradley, R. S., Withers, P. J., and Preziosi, R. F. (2012). A minute fossil phoretic mite recovered by phase-contrast X-ray computed tomography. Biology Letters 8, 457–460.
| A minute fossil phoretic mite recovered by phase-contrast X-ray computed tomography.Crossref | GoogleScholarGoogle Scholar |
Edgecombe, G. D., and Koch, M. (2008). Phylogeny of scolopendromorph centipedes (Chilopoda): morphological analysis featuring characters from the peristomatic area. Cladistics 24, 872–901.
| Phylogeny of scolopendromorph centipedes (Chilopoda): morphological analysis featuring characters from the peristomatic area.Crossref | GoogleScholarGoogle Scholar |
Friedemann, K., Wipfler, B., Bradler, S., and Beutel, R. (2012). On the head morphology of Phyllium and the phylogenetic relationships of Phasmatodea (Insecta). Acta Zoologica 93, 184–199.
| On the head morphology of Phyllium and the phylogenetic relationships of Phasmatodea (Insecta).Crossref | GoogleScholarGoogle Scholar |
Friedrich, F., and Beutel, R. G. (2008). Micro-computer tomography and a renaissance of insect morphology. Proceedings – Society of Photo-Optical Instrumentation Engineers 7078, 70781U.
| Micro-computer tomography and a renaissance of insect morphology.Crossref | GoogleScholarGoogle Scholar |
Friedrich, F., and Beutel, R. G. (2010). Goodbye Halteria? The thoracic morphology of Endopterygota (Insecta) and its phylogenetic implications. Cladistics 26, 579–612.
| Goodbye Halteria? The thoracic morphology of Endopterygota (Insecta) and its phylogenetic implications.Crossref | GoogleScholarGoogle Scholar |
Galassi, D. M. P. (1997). The genus Pseudectinosoma Kunz, 1935: an update, and description of Pseudectinosoma kunzi sp. n. from Italy (Crustacea: Copepoda: Ectinosomatidae). Archiv fuer Hydrobiologie 139, 277–287.
Gaunt, W. A., and Gaunt, O. N. (1978). ‘Three-dimensional Reconstruction in Biology.’ (University Park Press: Baltimore, MD, USA.)
Haug, J. T., Haug, C., Kutschera, V., Mayer, G., Maas, A., Liebau, S., Castellani, C., Wolfram, U., Clarkson, E. N. K., and Waloszek, D. (2011). Autofluorescence imaging, an excellent tool for comparative morphology. Journal of Microscopy 244, 259–272.
| Autofluorescence imaging, an excellent tool for comparative morphology.Crossref | GoogleScholarGoogle Scholar |
Hörnschemeyer, T., Beutel, R. G., and Pasop, F. (2002). Head structures of Priacma serrata LeConte (Coleoptera, Archostemata) inferred from X-ray tomography. Journal of Morphology 252, 298–314.
| Head structures of Priacma serrata LeConte (Coleoptera, Archostemata) inferred from X-ray tomography.Crossref | GoogleScholarGoogle Scholar |
Huckstorf, K., and Wirkner, C. S. (2011). Comparative morphology of the hemolymph vascular system in krill (Euphausiacea; Crustacea). Arthropod Structure & Development 40, 39–53.
| Comparative morphology of the hemolymph vascular system in krill (Euphausiacea; Crustacea).Crossref | GoogleScholarGoogle Scholar |
Huijsmans, D. B., Lamers, W. H., Los, J. A., and Strackee, J. (1986). Toward computerized morphometric facilities: a review of 58 software packages for computer-aided 4D reconstruction, quantification and picture generation from parallel serial sections. The Anatomical Record 216, 449–470.
| Toward computerized morphometric facilities: a review of 58 software packages for computer-aided 4D reconstruction, quantification and picture generation from parallel serial sections.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaL2s%2Fpsl2ksA%3D%3D&md5=1b8ef5cfca750e28eea7f1ebf2b30b84CAS |
IUPAC-IUB Commission on Biochemical Nomenclature (CBN) (1970). Abbreviations and symbols for nucleic acids, polynucleotides, and their constituents. Biochemistry 9, 4022–4027.
| Abbreviations and symbols for nucleic acids, polynucleotides, and their constituents.Crossref | GoogleScholarGoogle Scholar |
Johnson, N. F. (2011). A collaborative, integrated and electronic future for taxonomy. Invertebrate Systematics 25, 471–475.
| A collaborative, integrated and electronic future for taxonomy.Crossref | GoogleScholarGoogle Scholar |
Kaufmann, A., and Mueller, K. (2006). Overview of volume rendering. In ‘The Visualization Handbook’. (Eds C. Hansen and C. R. Johnson.) pp. 127–174. (Academic Press: London, UK.)
Klaus, A. V., and Schawaroch, V. (2006). Novel methodology utilizing confocal laser scanning microscopy for systematic analysis in arthropods (Insecta). Integrative and Comparative Biology 46, 207–214.
| Novel methodology utilizing confocal laser scanning microscopy for systematic analysis in arthropods (Insecta).Crossref | GoogleScholarGoogle Scholar |
Klaus, A. V., Kulasekera, V. L., and Schawaroch, V. (2003). Three-dimensional visualization of insect morphology using confocal laser scanning microscopy. Journal of Microscopy 212, 107–121.
| Three-dimensional visualization of insect morphology using confocal laser scanning microscopy.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD3srlsFCjsA%3D%3D&md5=057a01e2230e927e6f357f23f5ed554cCAS |
Koch, M., and Edgecombe, G. D. (2012). The preoral chamber in geophilomorph centipedes: comparative morphology, phylogeny, and the evolution of centipede feeding structures. Zoological Journal of the Linnean Society 165, 1–62.
| The preoral chamber in geophilomorph centipedes: comparative morphology, phylogeny, and the evolution of centipede feeding structures.Crossref | GoogleScholarGoogle Scholar |
Lak, M., Néraudeau, D., Nel, A., Cloetens, P., Perrichot, V., and Tafforeau, P. (2008). Phase contrast Xray synchrotron imaging: opening access to fossil inclusions in opaque amber. Microscopy and Microanalysis 14, 251–259.
| Phase contrast Xray synchrotron imaging: opening access to fossil inclusions in opaque amber.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXlvF2rtL0%3D&md5=ae293671f8e616b725eb0d8da34dbb92CAS |
Lemmon, A. R., and Moriarty Lemmon, E. (2012). High-throughput identification of informative nuclear loci for shallow-scale phylogenetics and phylogeography. Systematic Biology , .
| High-throughput identification of informative nuclear loci for shallow-scale phylogenetics and phylogeography.Crossref | GoogleScholarGoogle Scholar |
Liljeblad, J., Ronquist, F., Nieves-Aldrey, J.-L., Fontal-Cazalla, F., Ros-Farre, P., Gaitros, D., and Pujade-Villar, J. (2008). A fully web-illustrated morphological phylogenetic study of relationships among oak gall wasps and their closest relatives (Hymenoptera: Cynipidae). Zootaxa 1796, 1–73.
Mabee, P. M., Ashburner, M., Cronk, Q., Gkoutos, G. V., Haendel, M., Segerdell, E., Mungall, C., and Westerfield, M. (2007). Phenotype ontologies: the bridge between genomics and evolution. Trends in Ecology & Evolution 22, 345–350.
| Phenotype ontologies: the bridge between genomics and evolution.Crossref | GoogleScholarGoogle Scholar |
Mabee, P. M., Balhoff, J. P., Dahdul, W. M., Lapp, H., Midford, P. E., Vision, T. J., and Westerfield, M. (2012). 500,000 fish phenotypes: The new informatics landscape for evolutionary and developmental biology of the vertebrate skeleton. Journal of Applied Ichthyology 28, 300–305.
| 500,000 fish phenotypes: The new informatics landscape for evolutionary and developmental biology of the vertebrate skeleton.Crossref | GoogleScholarGoogle Scholar |
Metscher, B. D. (2009). MicroCT for comparative morphology: simple staining methods allow high-contrast 3D imaging of diverse non-mineralized animal tissues. BMC Physiology 9, 11.
| MicroCT for comparative morphology: simple staining methods allow high-contrast 3D imaging of diverse non-mineralized animal tissues.Crossref | GoogleScholarGoogle Scholar |
Michels, J. (2007). Confocal laser scanning microscopy: using cuticular autofluorescence for high resolution morphological imaging in small crustaceans. Journal of Microscopy 227, 1–7.
| Confocal laser scanning microscopy: using cuticular autofluorescence for high resolution morphological imaging in small crustaceans.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD2svhvV2gsA%3D%3D&md5=911f5d33aae2059f5ab522b4af9d192bCAS |
Michels, J., and Gorb, S. N. (2012). Detailed three-dimensional visualization of resilin in the exoskeleton of arthropods using confocal laser scanning microscopy. Journal of Microscopy 245, 1–16.
| Detailed three-dimensional visualization of resilin in the exoskeleton of arthropods using confocal laser scanning microscopy.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38Xhs1Cgsrc%3D&md5=6b0ad7907f359448d1aae42781beb5abCAS |
Mikó, I., Vilhelmsen, L., Johnson, N. F., Masner, L., and Pénzes, Z. (2007). Morphology of Scelionidae (Hymenoptera: Platygastroidea): head and mesosoma. Zootaxa 1571, 1–78.
Mikó, I., Friedrich, F., Yoder, M. J., Hines, H. M., Deitz, L. L., Bertone, M. A., Seltmann, K. C., Wallace, M. S., and Deans, A. R. (2012). On dorsal prothoracic appendages in treehoppers (Hemiptera: Membracidae) and the nature of morphological evidence. PLoS ONE 7, e30137.
| On dorsal prothoracic appendages in treehoppers (Hemiptera: Membracidae) and the nature of morphological evidence.Crossref | GoogleScholarGoogle Scholar |
Mullins, P. L., Kawada, R., Balhoff, J. P., and Deans, A. R. (2012). A revision of Evaniscus (Hymenoptera, Evaniidae) using ontology-based semantic phenotype annotation. ZooKeys , .
Mungall, C. J., Gkoutos, G. V., Smith, C. L., Haendel, M. A., Lewis, S. E., and Ashburner, M. (2010). Integrating phenotype ontologies across multiple species. Genome Biology 11, R2.
| Integrating phenotype ontologies across multiple species.Crossref | GoogleScholarGoogle Scholar |
Neff, D., Frazier, S. F., Quimby, L., Wang, R.-T., and Zill, S. (2000). Identification of resilin in the leg of cockroach, Periplaneta americana: confirmation by a simple method using pH dependence of UV fluorescence. Arthropod Structure & Development 29, 75–83.
| Identification of resilin in the leg of cockroach, Periplaneta americana: confirmation by a simple method using pH dependence of UV fluorescence.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD2sjltFGrsQ%3D%3D&md5=23144fef4e089569b9c23ba9188d94c3CAS |
Pohl, H., Wipfler, B., Grimaldi, D., Beckmann, F., and Beutel, R. G. (2010). Reconstructing the anatomy of the 42-million-year-old fossil †Mengeatertiaria (Insecta, Strepsiptera). Naturwissenschaften 97, 855–859.
| Reconstructing the anatomy of the 42-million-year-old fossil †Mengeatertiaria (Insecta, Strepsiptera).Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXhtVyhurjE&md5=19e20614b3d3a7006a64f07b661e8872CAS |
Popper, H., and Schaffner, F. (1959). The renaissance of morphology. Gastroenterology 37, 689–692.
| 1:STN:280:DyaF3c7os12itA%3D%3D&md5=7ce34ee4995d8c9acaebb3a78b29e1a2CAS |
Quicke, D. L. J., Lopez-Vaamonde, C., and Belshaw, R. (1999). Preservation of hymenopteran specimens for subsequent molecular and morphological study. Zoologica Scripta 28, 261–267.
| Preservation of hymenopteran specimens for subsequent molecular and morphological study.Crossref | GoogleScholarGoogle Scholar |
Romani, R., Isidoro, N., and Bin, F. (2010). Antennal structures used in communication by egg parasitoids. Progress in Biological Control 9, 57–96.
| Antennal structures used in communication by egg parasitoids.Crossref | GoogleScholarGoogle Scholar |
Ruthensteiner, B. (2008). Soft part 3D visualization by serial sectioning and computer reconstruction. Zoosymposia 1, 63–100.
Ruthensteiner, B., and Heß, M. (2008). Embedding 3D models of biological specimens in PDF publications. Microscopy Research and Technique 71, 778–786.
| Embedding 3D models of biological specimens in PDF publications.Crossref | GoogleScholarGoogle Scholar |
Ruthensteiner, B., Baeumler, N., and Barnes, D. G. (2010). Interactive 3D volume rendering in biomedical publications. Micron (Oxford, England) 41, 886–898.
| Interactive 3D volume rendering in biomedical publications.Crossref | GoogleScholarGoogle Scholar |
Seltmann, K. C., Yoder, M. J., Mikó, I., Forshage, M., Bertone, M. A., Agosti, D., Austin, A. D., Balhoff, J. P., Borowiec, M. L., Brady, S. G., Broad, G. R., Brothers, D. J., Burks, R. A., Buffington, M. L., Campbel, H. M., Dew, K. J., Ernst, A. F., Fernández-Triana, J. L., Gates, M. W., Gibson, G. A. P., Jennings, J. T., Johnson, N. F., Karlsson, D., Kawada, R., Krogmann, L., Kula, R. R., Mullins, P. L., Ohl, M., Rasmussen, C., Ronquist, F., Schulmeister, S., Sharkey, M. J., Talamas, E., Tucker, E., Vilhelmsen, L., Ward, P. S., Wharton, R. A., and Deans, A. R. (2012). A hymenopterists’ guide to the Hymenoptera Anatomy Ontology: utility, clarification, and future directions. Journal of Hymenoptera Research 27, 67–88.
| A hymenopterists’ guide to the Hymenoptera Anatomy Ontology: utility, clarification, and future directions.Crossref | GoogleScholarGoogle Scholar |
Smith, B. (2005). The logic of biological classification and the foundations of biomedical ontology. In ‘Invited Papers from the 10th International Conference in Logic Methodology and Philosophy of Science (Oviedo, Spain, 2003)’. (Ed. D. Westerståhl.) pp. 505–520. (King’s College Publications: London, UK).
Stüben, M., and Linsenmair, E. (2008). Advances in insect preparation: bleaching, clearing and relaxing ants (Hymenoptera: Formicidae). Myrmecological news 12, 15–21.
Stuessy, T. F., Mayer, V., and Hörandl, E. (2003). Deep morphology: toward a renaissance of morphology in plant systematics. Regnum Vegetabile 141, 1–326.
Szucsich, N. U., Pennerstorfer, M., and Wirkner, C. S. (2011). The mouthparts of Scutigerella immaculata: Correspondences and variation among serially homologous head appendages. Arthropod Structure & Development 40, 105–121.
| The mouthparts of Scutigerella immaculata: Correspondences and variation among serially homologous head appendages.Crossref | GoogleScholarGoogle Scholar |
Tafforeau, P., Boistel, R., Boller, E., Bravin, A., Brunet, M., Chaimanee, Y., Cloetens, P., Feist, M., Hoszowska, J., Jaeger, J. J., Kay, R. F., Lazzari, V., Marivaux, L., Nel, A., Nemoz, C., Thibault, X., Vignaud, P., and Zabler, S. (2006). Applications of X-ray synchrotron microtomography for non-destructive 3D studies of paleontological specimens. Applied Physics. A, Materials Science & Processing 83, 195–202.
| Applications of X-ray synchrotron microtomography for non-destructive 3D studies of paleontological specimens.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XivVOgtLs%3D&md5=7fbc7e2765639717a5934684a9b0c4feCAS |
Talamas, E., Masner, L., and Johnson, N. F. (2011). Revision of the Paridris nephta species group (Hymenoptera, Platygastroidea, Platygastridae). Zookeys 133, 49–94.
| Revision of the Paridris nephta species group (Hymenoptera, Platygastroidea, Platygastridae).Crossref | GoogleScholarGoogle Scholar |
Talarico, G., Lipke, E., and Alberti, G. (2011). Gross morphology, histology, and ultrastructure of the alimentary system of Ricinulei (Arachnida) with emphasis on functional and phylogenetic implications. Journal of Morphology 272, 89–117.
| Gross morphology, histology, and ultrastructure of the alimentary system of Ricinulei (Arachnida) with emphasis on functional and phylogenetic implications.Crossref | GoogleScholarGoogle Scholar |
Verbeek, F. J., Huijsmans, D. B., Baeten, R. J. A. M., Schoutsen, N. J. C., and Lamers, W. H. (1995). Design and implementation of a database and program for 3D reconstruction from serial sections: a data-driven approach. Microscopy Research and Technique 30, 496–512.
| Design and implementation of a database and program for 3D reconstruction from serial sections: a data-driven approach.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK2Mzit1Knsw%3D%3D&md5=2f15de7872f34410a7c1c29400bbdb52CAS |
Vilhelmsen, L., Mikó, I., and Krogmann, L. (2010). Beyond the wasp-waist: structural diversity and phylogenetic significance of the mesosoma in apocritan wasps (Insecta: Hymenoptera). Zoological Journal of the Linnean Society 159, 22–194.
| Beyond the wasp-waist: structural diversity and phylogenetic significance of the mesosoma in apocritan wasps (Insecta: Hymenoptera).Crossref | GoogleScholarGoogle Scholar |
Vogt, L., Bartolomaeus, T., and Giribet, G. (2010). The linguistic problem of morphology: Structure versus homology and the standardization of morphological data. Cladistics 26, 301–325.
| The linguistic problem of morphology: Structure versus homology and the standardization of morphological data.Crossref | GoogleScholarGoogle Scholar |
Washington, N., and Lewis, S. (2008). Ontologies: scientific data sharing made easy. Nature Education 1, .
Wilkins, S. W., Gureyev, T. E., Gao, D., Pogany, A., and Stevenson, A. W. (1996). Phase-contrast imaging using polychromatic hard X-rays. Nature 384, 335–338.
| Phase-contrast imaging using polychromatic hard X-rays.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK28Xnt1ajtLg%3D&md5=ef199b87d292341881bfbd72b90f79bcCAS |
Winslow, J. L., Bjerknes, M., and Cheng, H. (1987). Three-dimensional reconstruction of biological objects using a graphics engine. Computers and Biomedical Research, an International Journal 20, 583–602.
| Three-dimensional reconstruction of biological objects using a graphics engine.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaL1c%2FotFGhtA%3D%3D&md5=453207991d4f0f9161a32993e4ab6cc3CAS |
Wipfler, B., Machida, R., Müller, B., and Beutel, R. G. (2011). On the head morphology of Grylloblattodea (Insecta) and the systematic position of the order, with a new nomenclature for the head muscles of Dicondylia. Systematic Entomology 36, 241–266.
| On the head morphology of Grylloblattodea (Insecta) and the systematic position of the order, with a new nomenclature for the head muscles of Dicondylia.Crossref | GoogleScholarGoogle Scholar |
Wirkner, C. S., and Richter, S. (2007). The circulatory system in Mysidacea—implications for the phylogenetic position of Lophogastrida and Mysida (Malacostraca, Crustacea). Journal of Morphology 268, 311–328.
| The circulatory system in Mysidacea—implications for the phylogenetic position of Lophogastrida and Mysida (Malacostraca, Crustacea).Crossref | GoogleScholarGoogle Scholar |
Wogelius, R. A., Manning, P. L., Barden, H. E., Edwards, N. P., Webb, S. M., Sellers, W. I., Taylor, K. G., Larson, P. L., Dodson, P., You, H., Da-qing, L., and Bergmann, U. (2011). Trace Metals as Biomarkers for Eumelanin Pigment in the Fossil Record. Science 333, 1622–1626.
| Trace Metals as Biomarkers for Eumelanin Pigment in the Fossil Record.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXhtFGitb%2FJ&md5=2f591c1cf483edc50069c016b4c64a54CAS |
Yoder, M. J., Mikó, I., Seltmann, K. C., Bertone, M. A., and Deans, A. R. (2010). A gross anatomy ontology for Hymenoptera. PLoS ONE 5, e15991.
| A gross anatomy ontology for Hymenoptera.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXltVaqsg%3D%3D&md5=14a90bea6957c05a2395a3c0a9c32d58CAS |