Spatial extent of riverine flood plumes and exposure of marine ecosystems in the Tully coastal region, Great Barrier Reef
Michelle Devlin A C and Britta Schaffelke BA Catchment to Reef Research Group, ACTFR, James Cook University, Townsville, Qld 4811, Australia.
B Australian Institute of Marine Science, PMB 3, Townsville, MC, Qld 4810, Australia.
C Corresponding author. Email: michelle.devlin@jcu.edu.au
Marine and Freshwater Research 60(11) 1109-1122 https://doi.org/10.1071/MF08343
Submitted: 15 December 2008 Accepted: 15 September 2009 Published: 17 November 2009
Abstract
Tully River flood plume monitoring data for 11 events (1994–2008) were used to determine what physical characteristics of the floods (size of flood, direction of plume movement, shape of hydrograph) most influence the flood plume water quality and areal extent. During some events, the maximum area influenced by the Tully flood plumes extended into the Coral Sea. Areal extents depended on wind direction and discharge volume, with large extents more likely during light or northerly winds. Strong gradients in water quality existed away from the Tully mouth during the wet season and the adjacent marine ecosystems were regularly exposed to land-derived material. Flood plumes were grouped into three plume types: primary, secondary and tertiary plumes, based on water-quality characteristics (suspended solids, coloured dissolved organic matter and chlorophyll). The number of reefs and seagrasses exposed to plume waters varied from year to year, and was dependent on the characteristics of the event. Over the 11 years, out of the major 37 reefs and 13 seagrass meadows identified in the Tully marine area, between 11 (30%) and 37 coral reefs (100%) and most of the seagrass meadows were inundated by either a primary or secondary plume every year.
Introduction
River run-off is the principal carrier of eroded soil (sediment), nutrients, pesticides and chemical pollutants from the land into the coastal and inshore waters of the Great Barrier Reef (GBR) lagoon (Furnas 2003). On average, ~70 km3 of freshwater is discharged each year by rivers and streams into the GBR lagoon (Furnas 2003). Most of this run-off is delivered in discrete, short-lived flood events during the 5-month summer wet season, forming distinct flood plumes in the coastal zone that sometimes reach far out into the lagoon. In the wet season, the estuaries of the GBR coast are dominated by river run-off, and the ‘estuarine’ mixing zone, where the salinities range from 0 to 36, is located in the marine environment (Dagg et al. 2004), which is quite different to many temperate rivers (Eyre 1998).
Riverine plumes and the materials they carry have always had an impact on the GBR during these short-term events. However, elevated concentrations of nutrients, suspended sediments and pesticides, owing to changes in land use over the past 200 years of European settlement, are now potentially affecting the health of coastal and inshore ecosystems (Furnas 2003; Brodie and Mitchell 2005; Fabricius 2005; Schaffelke et al. 2005). The large quantities of sediment, nitrogen, phosphorus and significant amounts of pesticides lost from agricultural systems are easily measurable in rivers as they discharge into the GBR in flood conditions (Devlin and Brodie 2005; Lewis et al. 2009). The flood waters of rivers draining catchments dominated by agriculture typically have, for example, up to 30-fold higher concentrations of dissolved inorganic nitrogen (nitrate and ammonium) than rivers with undeveloped catchments.
Land run-off in many systems is seen as a source of contaminants that can have a negative impact on coastal ecosystem health and productivity. Increased turbidity and herbicide concentrations can negatively affect the growth and abundance of coastal and inshore seagrasses (Schaffelke et al. 2005; Waycott et al. 2005). In addition to physical disturbance, water quality is an important driver of coral reef health at local (reviewed in Fabricius 2005), regional (van Woesik et al. 1999; Fabricius et al. 2005), and GBR-wide scales (De’ath and Fabricius 2008). The effects of various water-quality constituents are manifold, including disturbance by sedimentation, light reduction by increased turbidity, reduced calcification rates by excess inorganic nutrients and inhibition of photosynthesis by herbicide exposure, and generally affect early life-history stages more than adult corals (e.g. Fabricius 2005; Negri et al. 2005; Cantin et al. 2007). Increases in freshwater discharge, sediment load and nutrients have been linked with a decline in live coral cover (Restrepo et al. 2006) and an increase in the areas of deoxygenated water in summer (Malakoff 1998; McKee et al. 2004). Corals are phototrophic organisms and reduced light availability as a result of high turbidity or sedimentation leads to resource limitation (Fabricius 2005; Cooper et al. 2008). In addition, exposure of corals to elevated levels of nutrients, sedimentation and turbidity may affect certain species that are sensitive or vulnerable to these environmental conditions. This may lead, in the medium to long term, to reduced densities of juvenile corals, subsequent changes in the community composition, decreased species richness and shifts to communities that are dominated by more resilient coral species and macroalgae (van Woesik et al. 1999; Fabricius et al. 2005; DeVantier et al. 2006).
The impact of flood plumes, in terms of their extent, duration and biogeochemical processes, is intrinsically linked to catchment management and reef health; however, our understanding of the drivers and consequences of plume waters is limited for the GBR. The aim of the present study was to analyse flood plume monitoring data from one GBR catchment and its associated marine area over a period of 14 years to determine what physical characteristics of the floods (size of flood, direction of plume movement, shape of hydrograph) most influence the flood plume water quality and areal extent, assuming that no major land-use changes occurred that caused changes in material loads and delivery.
Materials and methods
Data collection
Riverine plume monitoring is an essential component of the long-term monitoring of marine water quality in the GBR. Flood plume monitoring was conducted by the Great Barrier Reef Marine Park Authority (GBRMPA) from 1994 to 2002 (Devlin and Brodie 2005). Recent plume monitoring (2007 to present) has been undertaken as part of the current Reef Plan Marine Monitoring Program (Prange et al. 2007). This programme has monitored water quality, seagrass and coral reef status in the inshore GBR lagoon (along ~1000 km of coastline) since 2005 as part of a government initiative ‘to halt and reverse the decline in water quality entering the GBR’.
Study area
The Tully and Murray catchments are located within the Wet Tropics Region of North Queensland and drain wet tropical rainforest in the upper reaches, beef grazing along the mid reaches and a large coastal floodplain with a series of interconnected wetlands that have been extensively modified to support sugarcane and banana production as well as urban centres (Armour et al. 2009; Kroon 2009). The considerable floodplain network transports sediments, nutrients and pesticides into the GBR, either directly through these wetlands or via the larger Tully and Murray Rivers (Bainbridge et al. 2009). During the wet season, the coastal and inshore areas adjacent to the Tully catchment are regularly exposed to flood waters from the Tully River, and to a lesser extent from the Herbert River via the Hinchinbrook Channel.
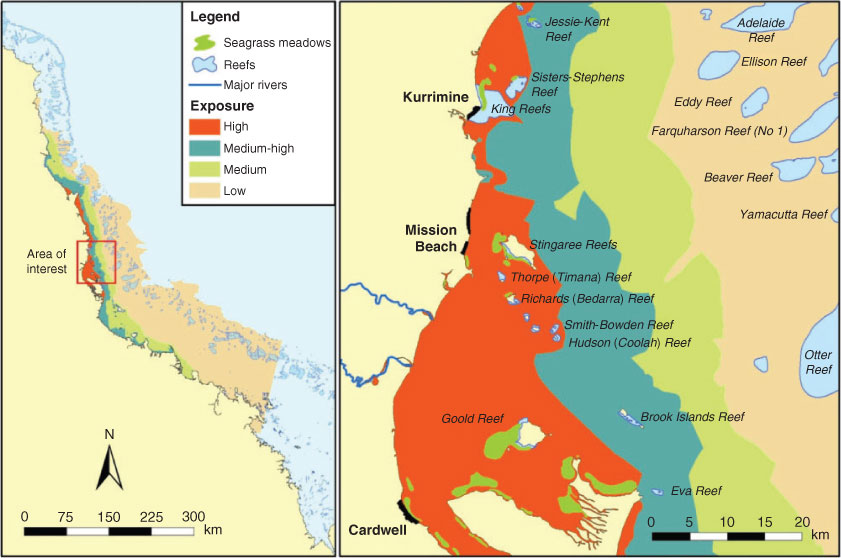
The Tully River is one of Australia’s least variable rivers, representing the generally wet tropical climate of the region. It floods regularly, one to four times per year, with riverine discharge extending into the adjacent marine waters. The marine environment adjacent to the Tully catchment has several continental islands with well-developed fringing reefs, which are of public and economic importance for the tourism industry and recreational activities including camping and fishing (GBRMPA 2009). The coastal and inshore zone also supports intertidal and subtidal seagrass beds. The area has several inshore Marine National Park Zones (‘no-take’ zones that allow non-extractive recreational use) and a large Conservation Park Zone (very limited extraction of marine resources permitted) around the greater Dunk Island area. Key benthic habitats in this area include 37 coral reefs (including coastal and inshore fringing reefs and inner midshelf platform reefs) and 14 seagrass meadows (coastal and inshore around islands).
Description of the plume events
Hydrograph and weather records for the Tully area were investigated for 11 flood events over the period 1994–2008. The information collated for each event included the hydrograph trajectory, total discharge volume, nutrient and sediment loads and prevailing wind strength and direction (Fig. 1). Flow volumes from all floods from 1972 were combined and percentiles were calculated for small, average and large flood events. We rated a flood event as ‘average’ when the annual discharge was within the inter-quartile range of the long-term data set, that is, from 2 122 424 to 3 607 342 mL. Small floods had a discharge less than the 25th percentile and large floods had a discharge greater than the 75th percentile (Fig. 1).
Plume extent and the environmental drivers of the extent and duration of the plume
Aerial images from 1994 to 1999 were combined with remote sensing images from 2002 to 2008 to describe the full extent of riverine plumes from the Tully River during 11 events over that period. River plumes monitored from 1994 to 1999 were mapped using aerial survey techniques. Over the monsoonal season, weather reports were closely monitored and when plumes formed, aerial surveys were conducted once or twice during the event. Plumes were readily observable as brown turbid water masses contrasting with the clearer seawater. The visible edge of the plume was followed at an altitude of 1000–2000 m in a light aircraft and mapped using a global positioning system (GPS). Where individual rivers flooded simultaneously, as often happens in the wet tropics, adjacent plumes merged into a continuous area. In these cases, efforts were made to distinguish the edge of the individual river plumes through colour differences (these efforts were only successful during 1998 and 2000). In all other years, the extents of the combined plumes were mapped. Spatial analyses using GIS techniques were applied to the aerial survey results. Flood plumes associated with Cyclone Sadie (1994), Cyclone Violet (1995), Cyclone Ethel (1996), Cyclone Justin (1997), Cyclone Sid (1998) and Cyclone Rona (1999) were plotted.
Moderate Resolution Imaging Spectroradiometer (MODIS) remote sensing Level-0 data were acquired from the NASA Ocean Colour website (http://oceancolor.gsfc.nasa.gov). SeaWiFS Data Analysis System (SeaDAS) routines were used to process MODIS Aqua and Terra data, producing quasi-true colour images and Level-2 products for periods corresponding to high flow rates in the Tully River from 2003 to 2008 with little or no cloud cover. Chlorophyll a and coloured dissolved organic matter (CDOM) absorption at 412 nm were estimated using the GSM01 algorithm at 250-m resolution (Maritorena et al. 2002).
The highly turbid nature of the study region and the close proximity to the coastal zone mean that standard near-infrared (NIR) atmospheric corrections are inaccurate and the quality of the retrieved product may be reduced (Wang and Shi 2007). To alleviate this effect, the atmospheric correction described by Wang and Shi (2007) was implemented in SeaDAS. Processing filters, such as cloud and stray light masks, were not used because they may result in regions of interest containing high sediment loads being masked. Suitable images for quantifying flood plume extent were often difficult to identify because of dense cloud cover during flood periods; thus, only four events were identified between 2003 and 2008.
Single images were selected on the basis of their image quality and transposed from geo-referenced true colour images and/or CDOM measurements into GIS shape files.
The MODIS imagery was re-referenced to conform to Geocentric Datum of Australia (GDA), map grid of Australia (MGA) projection. This was simply done by applying the imagery geographic coordinate values to the MGA-94 projected values (metres) until a simple bilinear solution (i.e. universal transverse mercartor (UTM)) was achieved. If a more rigorous algorithm (i.e. cubic) was applied over the image then the true spherical projection was achieved.
The derived CDOM absorption at 412 nm combined with careful examination of the quasi-true colour and chlorophyll a images provided the information used to define river plume ‘type’ and extent. A combination of high CDOM absorption and high sediment concentrations apparent in the quasi-true colour imagery defined the boundaries of ‘primary plumes’. Regions with high CDOM absorption and high chlorophyll a concentrations, but reduced sediment loads, were identified as ‘secondary plumes’. ‘Tertiary plumes’ were defined by low chlorophyll a concentrations and low CDOM absorption values. These are simple qualitative indices for separating the different stages of plume movement and extent, and further work on threshold definition is required.
For the final imagery classification and interpretation, two products were used. The initial classification method, as described above, allowed us to map the three main plume mapping densities (e.g. primary, secondary and tertiary) on the basis of CDOM absorption, and the second, the true colour images, allowed for a visual correlation of the classified values. By using both of these products, it was possible to delineate the three recognised plume classifications with a suitable degree of confidence. In areas where cloud had completely obscured the plume, an estimation of the plume extents was achieved by correlating the plume patterns from consecutive imagery periods in the following days.
A qualitative analysis was applied to each plume using the characteristics of the plume (discharge volume, certain wind conditions) to interpret the extent and direction of the plume relative to the size of the event (small, average or large based on total flow). Aerial surveys or remote sensing images of the plume extent represented only 1 day during a plume event, thus providing a single snapshot in time. However, by focusing on one catchment and combining all available plume surveys, an estimate of the overall extent of the riverine plume could be identified, driven by wind and flow patterns. A plume exposure map was calculated from the intersection of the plume image and type from both the aerial surveys (1995–2000) and remote sensing images (2003–2008) for the Tully marine area.
Water-quality sampling inside the plumes
Water samples were collected from multiple sites within the plume waters. The sampling locations were dependent on which rivers were flooding and the areal extent of the plume, but generally samples were collected in a series of transects heading out from the mouth of the Tully River. The timing of the sampling also depended on the type of event and the logistics of vessel deployment. Most samples were collected inside the visible area of the plume, although some samples were taken outside the edge of the plume for comparison. Salinity profiles were taken at all sites. Surface samples were collected at 0.5 m below the surface, with either a Niskin bottle or a plastic sampling container. Samples taken at depth were collected with Niskin bottles. The volumes filtered for all analyses depended on the turbidity of the water. Subsamples were filtered onto glass-fibre (GF/F) filters for an analysis of chlorophyll; the filter and retained algal cells were wrapped in aluminium foil and frozen. A second subsample was filtered onto a pre-weighed 0.45-μm membrane filter to determine the amount of suspended solids. Nutrient samples were collected using sterile 50-mL syringes and pre-rinsed three times with the seawater to be sampled. A 0.45-μm disposable membrane filter was then fitted to the syringe and 10-mL samples were collected in polypropylene screw-top sample tubes, pre-rinsed with filtered water. The tubes were then stored either on ice in an insulated container or in a freezer, depending on the sampling vessel. Separate samples for silicate analysis were stored at room temperature.
Analytical methods
Processing of the water samples occurred in different laboratories with comparable methods and quality-assurance techniques. The samples were analysed for concentrations of dissolved inorganic nutrients (NH4, NO2, NO3, NO2+, NO3, PO4 and Si) by standard procedures (Ryle et al. 1982) implemented on a Skalar 20/40 autoanalyser (Skalar Analytical, Breda, The Netherlands), with baselines run against artificial seawater. Analyses of the total dissolved nutrients (total dissolved nitrogen and total dissolved phosphate) were carried using persulfate digestion of the water samples (Valderrama 1981), and were then analysed for inorganic nutrients, as above. Dissolved organic nitrogen and dissolved organic phosphate were calculated by subtracting the separately measured inorganic nutrient concentrations (above) from the TDN and TDP values. Particulate nitrogen concentrations of the particulate matter collected on the GF/F filters were determined by high-temperature combustion using an ANTEK Model 707 Nitrogen Analyser (Antek Instruments Inc., Houston, TX, USA). The filters were freeze-dried before analysis. Following primary (650°C) and secondary combustion (1050°C), the nitrogen oxides produced were quantified by chemiluminescence.
Particulate phosphorus was determined colourimetrically (Parsons et al. 1984) following acid-persulfate digestion of the organic matter retained on the glass fibre filters. Acid-washed glass mini-scintillation vials were used as reaction vessels. Filters were placed in the vials with 5 mL of 5% w/v potassium persulfate and refluxed to dryness on an aluminium block heater using acid-washed marbles as stoppers for the vials. Following digestion, 5 mL of deionized water was added to each vial and the filter and salt residue was resuspended and pulverized to dissolve all soluble material. The residue in the vials was compressed by centrifugation at 3500 rev min–1 and the inorganic P determined colourimetrically in aliquots of the supernatant. Inorganic and organic P standards were run with the batch of samples.
Chlorophyll a concentrations were determined by fluorescence following maceration of algal cells and pigment extraction in acetone (Parsons et al. 1984). A Turner 10-005R fluorometer was used for the analysis and was periodically calibrated against diluted chlorophyll extracts prepared from log-phase diatom cultures (Jeffrey and Humphrey 1975). The concentrations of suspended solids were determined gravimetrically from the difference between loaded and unloaded membrane filter weights after drying the filters overnight at 60°C. Wet filter salt blanks were subtracted from the resulting weight.
Assessment of exposure for key marine habitats (seagrass beds and coral reefs)
For a detailed description of the flood and non-flood (ambient) water-quality conditions in the Tully marine area, high-frequency instrument records were obtained from one site, Dunk Island (5 m depth, from October 2007 to October 2008. The Eco FLNTUSB Combination instruments (Wet Laboratories, Philomath, OR, USA) simultaneously measure in situ chlorophyll fluorescence and turbidity and are designed for long deployments. The data were converted from raw instrumental records into actual measurement units (μg L–1 for chlorophyll fluorescence, NTU for turbidity) according to standard procedures of the manufacturer. The records were quality checked using a time-series data-editing software (WISKI-TV, Kisters, Aachen, Germany). Turbidity readings were converted to total suspended solids (TSS) concentrations using an equation derived from a correlation of instrument data and TSS concentrations from concurrently collected water samples: (TSS (mg L–1) = 1.3 × FLNTUSB Turbidity (NTU)) (Schaffelke et al. 2009).
Data analysis
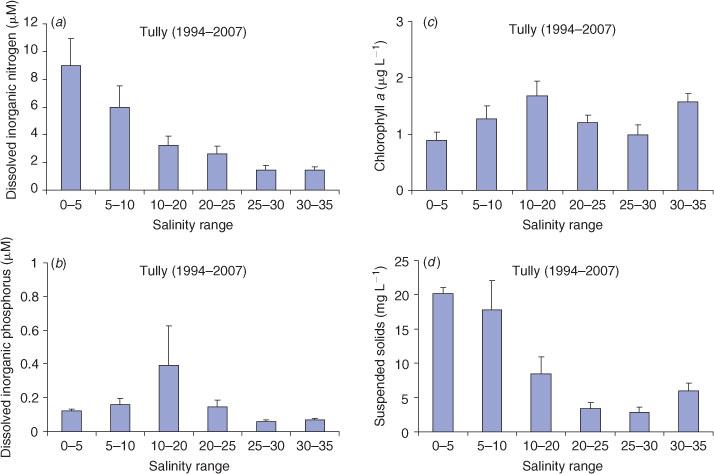
Transport of the materials in the plume was investigated by mixing profiles, which relate concentrations of water-quality constituents to salinity. These profiles are commonly used to analyse processes in flood plumes, such as estimating conservative or non-conservative mixing processes (Eyre 2000). However, problems with the interpretation of these relationships may arise when the concentrations of the parameters change rapidly in the river/plume interface, such as rapid deposition of particulate matter in the lower salinity zones. Mixing profiles for dissolved inorganic nitrogen (DIN), dissolved inorganic phosphorus (DIP), TSS and chlorophyll a were selected to represent the movement of dissolved and particulate matter through the plumes, the uptake of dissolved inorganic nutrients and the growth of phytoplankton biomass through the plume.
Composite plume mapping
Plume exposure maps were produced using a combination of plume indices and ArcMap geoprocessing. Using the plume indices described above, each polygon was assigned a numeric (short integer) value, that is, primary plume = 3, secondary plume = 2 and tertiary plume = 1, into an ‘index’ field. A combined data set was then produced by applying a UNION function (geoprocessing function in ArcMap) to all plume data sets, which produced a composite table of each plume index and an ‘exposure’ value was calculated by summing all the ‘index’ values for each polygon. The polygons were then aggregated on the basis of their new exposure value. The plume exposure value was overlaid on the selected Tully marine area to calculate the frequency of exposure for key benthic habitats.
Results
Extent of the plumes
Over the 11-year study period, the spatial extents of the flood plumes from the Tully River were highly variable from year to year (Fig. 1a–c). Small flood events, calculated as being below the 25th percentile of the long-term discharge record, occurred in 1995 and 2003, with limited offshore movement of the plume water. In 1995, SE winds constrained the small volume of water to the coast, whereas in 2003 the winds varied from S to SW, resulting in a larger offshore plume off the Tully marine area. There was a well-defined tertiary plume south of the Tully, most likely influenced by the southern flooding rivers Herbert and Burdekin (Fig. 1a, c).
Average floods occurred in 1994, 1996, 1997, 1998, 2004 and 2008. The 1994 and 1997 plumes covered a very large area as a result of the northerly winds. In contrast, the 1996 and 1998 floods, which had similar flows, covered a much smaller area owing to the prevailing SE winds. The W/SW winds in the 2004 flood period moved the primary plume further offshore. The 2008 flow event had a very large spatial extent with the secondary and tertiary plumes reaching into the Coral Sea. This was partly because of the prevailing SW winds, but also a result of the very large flow event of the Burdekin River in January/February 2008, leading to a combined flood plume from several rivers.
Large events, calculated as being above the 75th percentile of the long-term discharge record, occurred in 1999, 2000 and 2007. Prevailing south-easterly winds during the flood periods in 1999 and 2000 constrained the plume extents to the coast. In contrast, the large flow volume of the 2007 flood event coupled with the S/SW winds resulted in a large plume extent for both the primary and secondary plumes, which almost reached the midshelf reefs.
Water-quality gradients
The concentrations of water-quality constituents were highly variable within and between flood events (Table 1). These values are not only influenced by the size of the event and the wind direction influencing the plume extent, but are also highly dependent on the time of sampling relative to the hydrograph. For example, the 1994 flood was an average-sized event in terms of flow, but the plume was dispersed over a large area (Fig. 1a), resulting in only marginally elevated water-quality constituent concentrations compared with the non-flood values (Table 1). In contrast, the 1995 event was a small flood, but the plume was constrained to the coast (Fig. 1a) and had high concentrations of TSS, DIN and high chlorophyll at the time of sampling (Table 1). The plume of the large 1999 event, which had a total sediment export of 150 000 tonnes over that wet season, was sampled 5 days after peak flow, by which time the river flow had decreased, but the flood plume was constrained to the coast by winds (Fig. 1b). The TSS values reached as high as 15 mg L–1 (Table 1), exceeding summer water-quality guideline trigger values by approximately fivefold (GBRMPA 2009).
The transport and dilution of water-quality constituents within the plumes were analysed using the mixing profiles of DIN, DIP, TSS and chlorophyll a against salinity (Fig. 2). Overall, DIN decreased along an increasing salinity gradient, controlled by conservative (dilution) and non-conservative (biogeochemical uptake) processes. There was, on average, a reduction of 10–20% in the DIN freshwater end-member through the salinity range, with DIN concentrations in the higher salinities (above 30) clearly elevated (1–5 μM) in comparison with non-flood levels (Furnas 2003; De’ath and Fabricius 2008). Concentrations at the freshwater end varied between events, with initial concentrations exceeding 15 μM in 1995 and 2007, in comparison to all other years where the initial concentrations ranged from 5 μM to just under 10 μM. Sampling in both 1995 and 2007 captured the ‘first flush’ events carrying high concentrations of newly mobilised DIN from the fertilised agriculture lands on the adjacent catchment (Bainbridge et al. 2009; Mitchell et al. 2009). The DIP showed an increase from the lower to middle salinity ranges, reflecting desorption of dissolved inorganic phosphorus from suspended particles and dilution in higher salinities.
The TSS concentrations throughout the events ranged from 0.8 to 39.1 mg L–1 (Fig. 2). Chlorophyll concentrations were variable, ranging from just below the detection limit to 4.6 μg L–1. The average chlorophyll values at the freshwater end were low (0.2–2 μg L–1), reflecting limitation of growth as a result of corresponding high TSS values and light-limiting conditions. The chlorophyll a maxima were measured in the 10–20 salinity range, suggesting that phytoplankton growth was optimal in the middle salinity range with low TSS concentrations, high nutrients and adequate light conditions (Fig. 2). Chlorophyll also increased slightly in the 30–35 salinity range, indicating that uptake of available inorganic nutrients and increased phytoplankton growth were still occurring in the secondary/tertiary plume areas.
Exposure of key marine habitats (seagrass beds and coral reefs) to flood plumes
In total, 147 water-quality samples were taken in the Tully marine area during flood events. Of these, 85% of chlorophyll measurements (n = 101) exceeded the chlorophyll water quality trigger value of 0.63 μg L–1 and 32% exceeded the TSS (n = 63) trigger value of 2.4 mg L–1 (GBRMPA 2009) set for the summer period. Less frequent sampling occurred for particulate nitrogen (PN) and particulate phosphate (PP), but all 31 measurements exceeded both of the water quality trigger values for PN (1.8 μM) and PP (0.11 μM).
Instrumental records from 2007 to 2008 gave a detailed picture of the water-quality conditions during both flood and non-flood conditions at Dunk Island (Table 2). During the main flood period of the Tully River (December 2007 to March 2008), the water at the Dunk Island station had a mean chlorophyll concentration of 0.59 μg L–1 and 38% of the mean daily chlorophyll values exceeded the summer chlorophyll trigger value for the GBR (0.63 μg L–1; GBRMPA 2009). The mean chlorophyll concentration during the non-flood period was 0.34 μg L–1, which is close to the winter chlorophyll trigger value (0.32 μg L–1; GBRMPA 2009). The suspended solids concentrations around Dunk Island were slightly elevated during the flood period (Table 2), but were very variable all year round. The mean TSS concentration during the flood period was 3.4 mg L–1, with a maximum daily mean of 23 mg L–1, reached during the March flood peak. The mean concentration during the non-flood period was 2.4 mg L–1, above the mean annual trigger value for the GBR (2.0 mg L–1; GBRMPA 2009). Approximately 30% of the daily values exceeded this guideline trigger value in both flood and non-flood conditions.

|
The number of reefs and seagrasses exposed to the plume waters varied from year to year, and depended on the type of plume. Over the 11 years, a minimum of 11 reefs (30%) and a maximum of 37 reefs (100%) were inundated by either a primary or secondary plume (Fig. 3; Table 3), indicating that it is likely that at least one-third of the reefs is exposed to plume waters every year. For the years with remote sensing data available to validate plume type (1998, 2003–2008), we estimated that 6–15 reefs were inundated by primary plumes carrying high sediment loads, which is up to 41% of the inshore reefs in the Tully marine area and that 5–16 reefs (43%) were inundated by secondary plumes with elevated nutrient and chlorophyll concentrations. A smaller number of inshore reefs were inundated by a tertiary flood plume in three flood events (Table 3). It is important to note that tertiary plume extents and the associated exposure of reefs may have been underestimated in the years when the plume extent was estimated from aerial images only (1995–2000) on the basis of a colour change between the fresh and marine waters. Out of the 14 seagrass beds within the Tully marine area, at least 13 were inundated by either a primary or secondary plume in 10 of the 11 analysed events (Fig. 1), with the exception of 2000, when only seven seagrass beds were affected (Fig. 3; Table 3).
|
Discussion
Riverine flood plumes regularly inundate the marine environment of the Tully area, sometimes several times per year. Using both aerial and remote sensing images, we identified that riverine plumes can extend, in certain years, much further offshore and at more frequent intervals than previously reported (Fig. 1; Devlin et al. 2003; Devlin and Brodie 2005; Maughan et al. 2008). Previously, the small number of water-quality measurements in flood plumes indicated that there was an inshore–offshore gradient for many water-quality constituents; however, the frequency and intensity of the inundation and the concentration were unknown (Devlin et al. 2001; Brodie and Mitchell 2005).
Classification of the riverine plumes into distinct types (primary, secondary and tertiary plumes) helps elucidate more clearly the transport of different water-quality constituents in Tully River plumes by defining the spatial movement of the suspended sediments, dissolved nutrients and chlorophyll by the extent of the specific plume type.
Spatio-temporal patterns of plume water are difficult to resolve using only traditional biogeochemical methods owing to the constraints of direct sampling. This problem can be addressed by using satellite observations of visible spectral radiance regularly collected by NASA imagery Although suitable remote sensing images were only available for a limited number of days during the analysed high flow events for the Tully marine area, mainly because of heavy cloud cover (Rakwatin et al. 2007), the use of the remote sensing images gave far more detailed information about the plume type and the main constituents associated with that type than a composite aerial plume image, which can only be assessed visually.
The prevailing wind at any point during the high flow event was the dominant factor controlling the movement, extent and direction of the Tully plume. It has been previously reported that the prevailing and often strong SE winds constrain plume waters to the coast with a northwards movement, whereas at low wind speeds plumes move in a northerly direction from the river mouth as a result of Coriolis forcing and can spread well offshore (Chao 1988a; Wolanski 1994; Devlin et al. 2003; Devlin and Brodie 2005). If the wind forcing is opposed to the Coriolis forcing in direction, that is, northerly or north-easterly winds, the overall plume movement may be to the south. The extent of the transport of dissolved and particulate nutrients is also related to the intensity and duration of the rainfall event and the flow during the different stages over the river discharge hydrograph (rising, peak, falling waters). For example, a large first flush event in a wet season in the Tully catchment, such as those sampled in 1998, 2003 and 2007, would export very high loads of dissolved and particulate nutrients into the GBR lagoon owing to the mobilisation of the inorganic material stored in the agricultural soils.
Tully flood plumes move in response to prevailing weather conditions over the coastal shelf with the plume itself constituting an estuary with mixing processes from the freshwater end (mouth of the river) to the seawater end (end of plume). Constituents act differently within the plume water. For some constituents, the plume water is a simple mixing interface between the rivers and the lagoon. For others, the river and the corresponding plume act as an open-ended system in which biological and chemical removal takes place, substantially reducing the amount of constituent that reaches the reef (Dagg et al. 2004). Cycling processes within plumes for different constituents are markedly different and hence plume cycling can not only change total nutrient loads, but can also modify the ratios of one nutrient to another, which holds implications for the biological responses to plume waters.
The transport of dissolved inorganic nitrogen was controlled primarily by dilution, with elevated concentrations moving large distances (>20 km) offshore. The removal of DIN appears to be dominated by conservative mixing, indicating that the physical processes (dilution) are operating over shorter time frames than the biogeochemical processes Although there was a substantial decrease in the DIN concentrations through the salinity gradient, our within-plume sampling data indicate that dissolved nitrogen moved further offshore than suspended solids and at elevated concentrations compared with baseline values. Thus, there is a greater potential for the uptake of DIN by phytoplankton over large areas of the Tully marine area.
In contrast to the movement of DIN, average concentrations of DIP increased in the mid salinity range, suggesting that desorption of inorganic P from particulate P is occurring at these salinities. Davies and Eyre (2005) report on a similar process in the Daintree estuary, with low concentrations of DIP at low salinities, most likely assimilated by phytoplankton and increasing in the middle estuary, originating from desorption of inorganic P from suspended sediments as the pH increases through the estuary. This can be an important mechanism for the transport of phosphate to the ocean in other rivers; for example, in the Amazon River more than half of the phosphate reaching the ocean is released from particulate matter during plume mixing (DeMaster and Pope 1996).
The highest values of TSS were measured in the freshest parts of the plumes, with values close to ambient in the higher salinities, suggesting deposition of the heavier particulate matter close to the coast. In the initial mixing zone, water velocity is reduced and most of the river-derived particulate matter settles from the plume.
The non-conservative profile of chlorophyll along salinity gradients within plumes reflected the complex relationship between phytoplankton growth and nutrient and light availability (Cloern 2001). Pelagic and benthic algal and microbial communities rapidly take up the nutrients exported by flood plumes into the GBR lagoon waters (Alongi and McKinnon 2005), leading to short-lived phytoplankton blooms and transient events of higher level organic production (McKinnon and Thorrold 1993; Furnas et al. 2005). This is shown in the salinity range between 10 and 25, where the highest chlorophyll concentrations were measured, with suspended sediment levels being sufficiently low to allow enhanced phytoplankton productivity, fuelled by the elevated nutrients from the plume waters (McKinnon and Thorrold 1993). Removal of inorganic nutrients across the plume-water fronts at a salinity of ~26 has also been noted in the Yantze River plume (Tian et al. 1993) and the Annan River (Davies and Eyre 2005).
A risk assessment based on the prevailing movement of river plumes from all major GBR rivers identifies coral reefs at high risk of exposure to flood plumes; these coral reefs are mainly located to the north/north-east of rivers draining catchments with a high proportion of fertilised agriculture (Maughan et al. 2008). During the northerly and/or offshore winds in 1994, 1997 and 2008 (Fig. 1a–c), riverine plumes moved far offshore, reaching the mid and outer shelf reefs and even the Coral Sea, potentially exposing offshore marine ecosystems to materials transported by the flood plumes. In contrast, the prevailing south-easterly winds keep plumes confined to the coastal and inshore areas, but, owing to limited dilution and dispersal, expose ecosystems in these areas to elevated concentrations of nutrients, suspended solids and other material transported in the run-off.
Instrumental water quality records at Dunk Island, which has seagrass and coral reef habitats, showed that over the course of 1 year, the concentrations of suspended solids (measured as turbidity) were often elevated, whereas chlorophyll concentrations were relatively low for most of the year, but showed a clear flood signal. Elevated sediment and nutrient levels decrease in a matter of weeks after a flood event by sedimentation, biological uptake, dilution and dispersal. Material in the GBR inshore waters remains in the coastal zone until transported out of the GBR lagoon over weeks to months, primarily via the northern and southern ends of the reef (Luick et al. 2007; Wang et al. 2007) or after being assimilated into the inshore food web through biological uptake until it is eventually removed from the system by remineralisation or burial (Alongi and McKinnon 2005). Wind and tide-driven turbidity events are common in the GBR lagoon and are important drivers of the underwater light climate that shapes coastal benthic ecosystems such as seagrass meadows and coral reefs (Larcombe et al. 1995; Alongi and McKinnon 2005; Cooper et al. 2008). Terrestrial fine sediment transported into the Tully coastal area by flood plumes may be easily resuspended for prolonged periods of time (Wolanski et al. 2008), especially after large flood events, leading to frequent spikes in turbidity.
A comparison of plume data to water-quality guidelines (GBRMPA 2009) shows that a large proportion of the measured data exceeds trigger values for TSS, chlorophyll, PN and PP. The inshore coral reefs and seagrass beds adjacent to the Tully catchment are likely to be affected by these elevated concentrations, at least during the weeks of exposure. The longer-term impacts of flood plumes are currently not well understood, but are the subject of ongoing research. These impacts include, for example, recurrent resuspension of settled material leading to periodically elevated TSS concentrations over long time periods or ongoing high nutrient availability from foodweb cycling. Our estimates of the exposure of marine ecosystems to flood plumes showed that coastal and inshore coral reefs and seagrass beds in the Tully marine area were inundated every year by primary plumes and were exposed to intermittently high sediment and high nutrient concentrations during flood plumes, and potentially high loads of sedimenting particles.
The major adverse effect on corals is decreased light availability as a result of high water turbidity and short-term or intermediate smothering by high sedimentation during flood events or because of resuspension of terrigenous fine sediments by wind and waves.
Our assessment of 11 flood events from 1994 to 2008 showed that as a result of the regular high rainfall and associated flooding, the marine ecosystems adjacent to the Tully catchment are regularly exposed to elevated concentrations of nutrients, suspended sediments and other land-derived materials, such as herbicides. Knowledge about the overall catchment loads and sources of land-derived materials as well as the relationships to various land uses in the Tully area is continually improving (e.g. Armour et al. 2009; Bainbridge et al. 2009; Mitchell et al. 2009; Wallace et al. 2009). The effects of excess nutrients and sediments in the marine environment are also increasingly understood (e.g. De’ath and Fabricius 2008). However, less well known are the physical and biogeochemical processes transporting and transforming land-derived materials in the marine environment, as well as the hydrodynamics of the GBR inshore area that control, for example, residence times. The missing links between catchment and marine processes hamper the implementation of management options for specific water-quality constituents. A primary use for the results of the present study will be to set targets connecting end-of-river loads of particular materials to an intermediate end-point target, such as chlorophyll (Brodie et al. 2009), and, in the future, to an ecological end-point target, such as a composite indicator for coral reef health (Fabricius et al. 2005).
Acknowledgements
The authors would like to thank Lachlan McKinna, Greg Nelson-White, Matt Shirving and others for their excellent work in helping to process and produce the remote sensing images and GIS shape files. We also acknowledge funding by the Australian Department for Environment, Heritage, Water and the Arts, the Great Barrier Reef Marine Park Authority and the Reef Plan Marine Monitoring Program (2007–current), which was also the main source of data for this paper.
Alongi, D. M. , and McKinnon, A. D. (2005). The cycling and fate of terrestrially-derived sediments and nutrients in the coastal zone of the Great Barrier Reef shelf. Marine Pollution Bulletin 51, 239–252.
| Crossref | GoogleScholarGoogle Scholar | CAS | PubMed |
DeMaster, D. J. , and Pope, R. (1996). Nutrient dynamics in Amazon shelf waters: results from AMASSEDS. Continental Shelf Research 16, 263–289.
| Crossref | GoogleScholarGoogle Scholar |
Eyre, B. D. (1998). Transport, retention and transformation of material in Australian estuaries. Estuaries 21, 540–551.
| Crossref | GoogleScholarGoogle Scholar | CAS |
Furnas, M. , Mitchell, A. , Skuza, M. , and Brodie, J. E. (2005). In the other 90%: phytoplankton responses to enhanced nutrient availability in the GBR lagoon. Marine Pollution Bulletin 51, 253–265.
| Crossref | GoogleScholarGoogle Scholar | CAS | PubMed |
Jeffrey, S. W. , and Humphrey, G. W. (1975). New spectrophotometric equations for determining chlorophylls a, b, c and c2 in higher plants, algae and natural phytoplankton. Biochemie und Physiologie der Pflanzen 167, 191–198.
| CAS |
Maritorena, S. , Siegel, D. A. , and Peterson, A. (2002). Optimization of a semi-analytical ocean color model for global scale applications. Applied Optics 41, 2705–2714.
| Crossref | GoogleScholarGoogle Scholar | PubMed |
Rakwatin, P. , Takeuchi, W. , and Yasuoka, Y. (2007). Stripe noise reduction in MODros. Inf. Serv. data by combining histogram matching with facet filter. IEEE Transactions on Geoscience and Remote Sensing 45, 1844–1856.
| Crossref | GoogleScholarGoogle Scholar |
Schaffelke, B. , Mellors, J. , and Duke, N. C. (2005). Water quality in the Great Barrier Reef region: responses of mangrove, seagrass and macroalgal communities. Marine Pollution Bulletin 51, 279–296.
| Crossref | GoogleScholarGoogle Scholar | CAS | PubMed |
Tian, R. C. , Hu, F. X. , and Saliot, A. (1993). Biogeochemical processes controlling nutrients at the turbidity maximum and the plume water fronts in the Changjiang Estuary. Biogeochemistry 19, 83–102.
| Crossref | GoogleScholarGoogle Scholar |
Wolanski, E. , Fabricius, K. E. , Cooper, T. F. , and Humphrey, C. (2008). Wet season fine sediment dynamics on the inner shelf of the Great Barrier Reef. Estuarine, Coastal and Shelf Science 77, 755–762.
| Crossref | GoogleScholarGoogle Scholar |