Contribution of climate change to degradation and loss of critical fish habitats in Australian marine and freshwater environments
Morgan S. Pratchett A F , Line K. Bay B , Peter C. Gehrke C , John D. Koehn D , Kate Osborne B , Robert L. Pressey A , Hugh P. A. Sweatman B and David Wachenfeld EA ARC Centre of Excellence for Coral Reef Studies, James Cook University, Townsville, Qld 4811, Australia.
B Australian Institute of Marine Science, PMB 3, Townsville MC, Qld 4810, Australia.
C Snowy Mountains Engineering Corporation, Level 1, 154 Melbourne Street, South Brisbane, Qld 4101, Australia.
D Arthur Rylah Institute for Environmental Research, Department of Sustainability and Environment, 123 Brown Street, Heidelberg, Vic. 3084, Australia.
E Great Barrier Reef Marine Park Authority, PO Box 1379, Townsville, Qld 4810, Australia.
F Corresponding author. Email: morgan.pratchett@jcu.edu.au
Marine and Freshwater Research 62(9) 1062-1081 https://doi.org/10.1071/MF10303
Submitted: 3 December 2010 Accepted: 10 May 2011 Published: 21 September 2011
Journal Compilation © CSIRO Publishing 2011 Open Access CC BY-NC-ND
Abstract
Australia’s aquatic ecosystems are unique, supporting a high diversity of species and high levels of endemism; however, they are also extremely vulnerable to climate change. The present review assesses climate-induced changes to structural habitats that have occurred in different aquatic ecosystems. Climatic impacts are often difficult to discern against the background of habitat degradation caused by more direct anthropogenic impacts. However, climate impacts will become more pronounced with ongoing changes in temperature, water chemistry, sea level, rainfall patterns and ocean currents. Each of these factors is likely to have specific effects on ecosystems, communities or species, and their relative importance varies across different marine and freshwater habitats. In the Murray–Darling Basin, the greatest concern relates to declines in surface water availability and riverine flow, owing to declining rainfall and increased evaporative loss. On the Great Barrier Reef, increasing temperatures and ocean acidification contribute to sustained and ongoing loss of habitat-forming corals. Despite the marked differences in major drivers and consequences of climate change, the solution is always the same. Greenhouse-gas emissions need to be reduced as a matter of urgency, while also minimising non-climatic disturbances. Together, these actions will maximise opportunities for adaptation by species and increase ecosystem resilience.
Additional keywords: biodiversity, disturbance, fishes, Great Barrier Reef, Murray–Darling Basin.
Introduction
In aquatic habitats, as on land, anthropogenic climate change is having significant and appreciable effects on ecosystems, communities and species (Walther et al. 2002; Parmesan and Yohe 2003; Hoegh-Guldberg and Bruno 2010). Major effects include declines in biodiversity and productivity (O’Reilly et al. 2003), changes in taxonomic composition and community dynamics (Hughes et al. 2003; Xenopoulos et al. 2005; Ficke et al. 2007), and shifts in the geographic ranges, distribution and abundance of species (Perry et al. 2005; Balston 2009; Pitt et al. 2010) mediated by increases in climate-related temperature and changes in many other physical characteristics. Aquatic habitats may be even more vulnerable, and changing more rapidly as a consequence of recent climate change, than are terrestrial ecosystems (Richardson and Poloczanska 2008). However, research on the effects of climate change in aquatic ecosystems lags behind that of terrestrial ecosystems. The number of marine and freshwater studies included in the Fourth Assessment Report of the IPCC (Rosenzweig et al. 2007) was <0.3% of land-based studies, reflecting a general imbalance in levels of scientific research undertaken in aquatic versus terrestrial systems (Richardson and Poloczanska 2008). Understanding of the effects of climate change in aquatic ecosystems is also difficult because of (1) their size or complexity, (2) inherent variability in environmental conditions and (3) limited long-term records of environmental variables (Richardson and Poloczanska 2008).
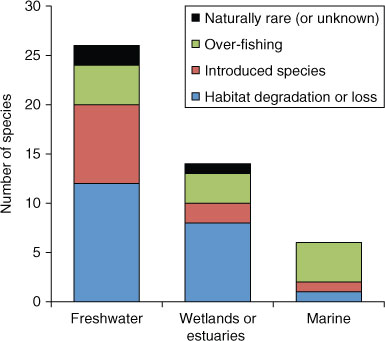
Although there is a strong terrestrial bias in documented effects of climate change on natural ecosystems and organisms (Parmesan 2007; Richardson and Poloczanska 2008), climate change has already had significant, and often spectacular, impacts in marine and freshwater ecosystems. In 1998, for example, warm ocean waters caused extensive bleaching and widespread mortality of scleractinian corals throughout the world (Goreau et al. 2000; Wilkinson 2000). Links between increasing ocean temperatures and regional-scale bleaching of scleractinian corals are incontrovertible (Hughes et al. 2003; Hoegh-Guldberg et al. 2007), and climate-induced coral bleaching has greatly exacerbated ongoing declines in the abundance of habitat-forming scleractinian corals at many locations (Hoegh-Guldberg 1999; Wilkinson 2004). In freshwater ecosystems, climate change poses a direct threat to the existence of many habitats, thereby exacerbating already very high rates of species extinction and contraction of species ranges. Habitat degradation and loss is the major threat for threatened fish species in freshwater ecosystems, wetlands and estuaries (Fig. 1) and this will be exacerbated by changes in the quality and quantity of habitat as a result of increasing temperatures, altered rainfall and sea-level rise. In coastal freshwater habitats, for example, sea-level rise and reduced river flow will lead to increased saline intrusion (Bayliss et al. 1997; Mulrennan and Woodroffe 1998). Extinction rates of freshwater fishes are equal or higher than those of many terrestrial taxa (Heino et al. 2009), and are expected to increase in coming decades because of projected effects of global climate change (Xenopoulos et al. 2005).
|
The vulnerability of aquatic ecosystems to global climate change is due to the profound influence of environmental conditions (especially temperature, water flow or nutrient availability) on biological and ecological processes, and the sensitivities of species, within these systems. Coral reefs are considered to be among the most vulnerable ecosystems to climate change (Walther et al. 2002), owing to the temperature sensitivities of corals, which may bleach and die when sea temperatures exceed normal local limits by as little as 1.0°C (Jokiel and Coles 1990). There are few other ecosystems in which the major habitat-forming organisms function so close to their upper thermal limit. Vulnerability of aquatic habitats to climate change is further heightened by the degraded state of many aquatic habitats (e.g. Schindler 2001; Valiela et al. 2001; Hughes et al. 2003; Worm et al. 2006). For the most part, effects of climate change compound on habitat degradation and losses that have occurred as a result of more localised anthropogenic disturbances, such as overfishing, pollution and habitat modification (e.g. Schindler 2001; Hughes et al. 2003). The cumulative effects of exploitation, pollution, and habitat modification and fragmentation on individual species may also make them much more vulnerable to climate change (e.g. Schindler 2001; Wooldridge 2009; Davies 2010). This is particularly so for freshwater and estuarine ecosystems, which are disproportionately affected by increasing development, land and water use.
Climate change may have an impact on any number of the ecological processes (e.g. nutrient cycling, energy transfer, sediment transport, recruitment, or dispersal of animals), and structural or biological components (e.g. communities or individual species) of aquatic habitats. For fishes living in marine and freshwater ecosystems, climate change will have both direct and indirect effects (Roessig et al. 2004; Munday et al. 2008; Pratchett et al. 2009b). Being ectotherms, changes in temperature will have direct effects on fish physiological condition, oxygen consumption, developmental rate, growth, swimming ability, reproductive performance and behaviour (e.g. Van der Kraak and Pankhurst 1997). Changes to water chemistry (especially reductions in pH), which are expected to occur by 2100 with current levels of greenhouse-gas emissions, have also been shown to interfere with sensory abilities of some marine fishes (e.g. Munday et al. 2009), which may have important ramifications for settlement and survivorship (Dixson et al. 2010). However, the most immediate effects of climate change on fishes are likely to be indirect, caused by changes in availability, structure and connections of critical marine and freshwater habitats. Climate-induced changes to habitat structure have already been recorded in many aquatic ecosystems, where they have had significant effects on both abundance and diversity of fishes (Swales et al. 1999; Wilson et al. 2006; Pratchett et al. 2008).
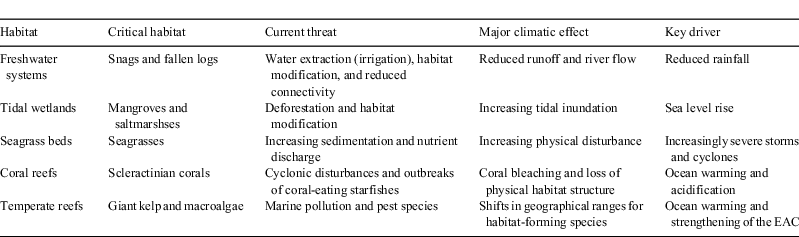
The purpose of the present review is to consider effects of climate change on critical fish habitats in Australia, comparing the major drivers of climatic impacts across freshwater ecosystems, tidal wetlands, seagrass beds, tropical coral reefs and temperate rocky reefs (Table 1). Australia’s aquatic ecosystems are unique, supporting a high diversity of species and high levels of endemism (Unmack 2001; Poloczanska et al. 2007), and are extremely vulnerable to the major environmental changes expected to occur in some locations (Poloczanska et al. 2007). In general, there are very limited data available with which to assess the effects of climate change on aquatic ecosystems in Australia, especially compared with Europe and North America (Hughes 2003; Poloczanska et al. 2007). Two notable exceptions are the Great Barrier Reef (GBR) and the Murray–Darling Basin (MDB), for which anthropogenic threats and changes in ecosystem state have been documented for >100 years (e.g. Murray–Darling Basin Commission 2004a, 2004b; Daley and Griggs 2006, 2008; Davies et al. 2010). Within each of these distinct and iconic systems, the recent and observable effects of climate change will be compared with degradation and loss of critical fish habitats caused by other more direct anthropogenic disturbances. The projected effects of climate change will also be considered to assess their likely impacts in coming decades.
Major drivers of climatic impacts in different aquatic ecosystems
The fundamental cause of anthropogenic climate change is increases in atmospheric concentrations of greenhouse gases (mainly CO2), which leads to increases in average atmospheric temperatures. In aquatic ecosystems, increasing atmospheric temperatures will warm surface waters of oceans, lakes and rivers (Levitus et al. 2000). However, because water has a higher heat capacity than air, this means that increases in water temperatures will lag behind those of atmospheric temperatures (Lough 2007). A significant component of anthropogenically produced CO2 (approximately one-third, thus far) is also dissolved in the world’s oceans, lakes and rivers (Roessig et al. 2004). Additional CO2 dissolved in the ocean reacts with seawater to form weak carbonic acid, causing pH to decline and reducing the availability of dissolved carbonate ions required by many marine calcifying organisms (e.g. corals, other invertebrates and coralline algae) to build their shells or skeletons (Orr et al. 2005).
Other environmental changes linked to global climate change that will affect aquatic habitats include reduced rainfall, more extreme rainfall events, or altered rainfall patterns and especially long dry spells (CSIRO 2008), increased severity of tropical cyclones (Madin and Connolly 2006), sea-level rise (Short and Neckles 1999) and changes to ocean circulation and current patterns (Munday et al. 2008, 2009). Tropical cyclones may become more intense in a warmer world (Webster et al. 2005), which would cause greater damage to key habitat-forming species, such as corals and seagrasses (Madin and Connolly 2006). Sea-level rise will lead to a redistribution (where possible) of intertidal and shallow coastal habitats, as well as increased seawater intrusion into estuaries and the lower reaches of rivers (Short and Neckles 1999). Changes to ocean circulation and current patterns will alter dispersal of marine larvae (Munday et al. 2008, 2009) and, combined with reductions in vertical water mixing, will limit the supply of nutrients and subsequent productivity. Changes to temperatures and flows in freshwater ecosystems are also likely to alter processes such as productivity and the downstream drift of larvae (Lake 2003). A complete loss of freshwater may occur for some habitats such as wetlands. Each of these factors is likely to have specific effects on ecosystems, communities or species; however, their relative importance will vary greatly among habitats (Table 1).
Freshwater ecosystems
For many freshwater systems (especially lotic systems), the most critical factor affecting habitat structure, as well as nutrient loading, transport of materials and organisms, is the amount of flow (Walker et al. 1995). Variation in river flow over time scales from days to decades is very important in setting the dynamic equilibrium among habitat patches (Thorp et al. 2006). Changes in flow dynamics resulting from climate change will therefore have profound effects on riverine habitats and the fishes that depend on them (Walker et al. 1995; Meyer et al. 1999). Declines in inflows, combined with increasing evaporation will also reduce lake levels (Carpenter et al. 1992; Hughes 2003). Xenopoulos et al. (2005) suggested that 75% of freshwater fish throughout the world will become extinct through part of their range by 2070 because of reduced river discharges. Australia has large areas of dryland rivers and highly variable flow regimes that are prone to periods of low or zero flow (Walker et al. 1995). Hence, reductions in river flows under a drier climate regime (CSIRO 2008) are likely to exacerbate the existing declines in fish species richness attributable to reduced flows.
Reductions to river flows because of water extraction have already been shown to affect native fishes in lowland reaches of the Murray–Darling river system (Gehrke and Harris 2000). Despite predictions of reduced total rainfall, increased intensity of rainfall may also result in increased turbidity from suspended sediments transported from surrounding catchments. The resultant reduced light penetration for photosynthesis by macrophytes, phytoplankton and benthic algae may potentially lead to shifts in primary production in aquatic food webs (Bunn et al. 2003). Decreased rainfall and higher temperatures will increase the risk of fires within the catchment and subsequent erosion of sediments can lead to fish kills and the smothering of habitats (Lyon and O’Connor 2008).
Increased river temperatures are likely to affect the distribution of freshwater species, leading to increased elevation of cold-water species, accompanied by an potential upstream range extension of warm-water species (Buisson et al. 2008). Negative effects of increasing temperature are also likely to be exacerbated by loss of riparian vegetation, which reduces shading and increases evaporative water loss (McKergow et al. 2006; Rayne et al. 2008; Davies 2010). Warmer temperatures and increased evaporation rates, combined with a drier climate across much of Australia, will result in increased drying of wetlands and waterholes in ephemeral rivers, and may result in some permanent habitats becoming intermittent (Kennard et al. 2010). Some intermittent freshwater habitats may also cease to exist, have extended dry periods or will become much rarer leading to smaller geographic ranges of certain species or reduced connectivity among populations. Whereas increases in temperatures may allow southerly extensions to the range of some marine species (Booth et al. 2011), the discontinuous nature of freshwater catchments can prevent similar range changes and, indeed, may lead to range contractions for many freshwater species (Bond et al. 2011). For the most part, these effects will compound on to pre-existing threats associated with water extraction and aquifer draw-down, especially in dry inland areas (e.g. central Queensland).
Of the 46 fish species listed under the Australian Environment Protection and Biodiversity Conservation (EPBC) Act 1999 (www.environment.gov.au/biodiversity/threatened/index.html; July 2010), most (56%) are from freshwater habitats (Fig. 1). The primary cause of declines in the geographic range or overall abundance of these freshwater fishes is habitat degradation or loss (Fig. 1; Pollard et al. 1990). The other major contributor to increased extinction risk of freshwater fishes in Australia is the introduction of exotic species or translocation of Australian native species, which prey on or compete with native species (e.g. Ingram et al. 1990). In 1990, an independent analysis of threatened species (Pollard et al. 1990) suggested that 34% (65 of 192 species) of Australia’s freshwater fishes were already threatened by habitat loss, invasive species or overfishing.
Coastal wetlands
Coastal wetlands are critical intertidal habitats dominated by plant species (e.g. salt marshes and mangroves) and occupy very specific and often narrow zones, structured by the interactions between sea level, land elevation and sediment accretion (Morris et al. 2002). Coastal wetlands are therefore very sensitive to climate-related changes in atmospheric temperature, CO2 concentrations, rainfall and freshwater input (Michener et al. 1997; Morris et al. 2002; Lovelock and Ellison 2007). However, the greatest effect of climate change on coastal wetlands (including mangroves and salt marshes) is expected to arise as a result of sea-level rise (Michener et al. 1997; Morris et al. 2002; Gilman et al. 2008).
Mangrove trees are well adapted to being inundated with saltwater; however, growth of trees declines or forests retreat landward with increasing frequency and duration of inundation. Previous episodes of sea-level rise have led to proliferation of mangroves in northern Australia (e.g. Woodroffe 1990), and increasing atmospheric CO2 may actually lead to increased growth and productivity of mangroves (Lovelock and Ellison 2007). However, development and modification of coastal habitats have resulted in loss of wetlands, disruption to connectivity, enhanced nutrients, and generated physical barriers that reduce the potential for landward migration of wetlands along much of Australia’s coastline (Lovelock and Ellison 2007), referred to as ‘coastal squeeze’ (Doody 2004). In Australia, current rates of sea-level rise are estimated to be 0.3–0.5 mm per year, which are much lower than most global estimates. However, mangroves are already progressing landward and encroaching on salt-marsh environments at many locations along Australia’s south-eastern coast (e.g. Saintilan and Williams 1999), which may be partly due to sea-level rise. Since 1983, 14% of Australia’s mangrove habitat has been lost (Valiela et al. 2001), and a further 231 km2 is destroyed each year (Valiela et al. 2009), mostly as a result of land reclamation and deforestation. Declines in the areal extent of mangrove forests directly affect the diversity of mangrove species as well as ecosystem function (Duke et al. 2007). Globally, Nicholls et al. (1999) calculated that 13–31% of coastal wetlands have already been lost; however, only 0–2% of this habitat loss was attributable to sea-level rise. With current rates of deforestation, Valiela et al. (2009) suggested that mangroves will be lost before major impacts from projected sea-level rise become fully apparent.
Coastal wetlands are an important component of coastal ecosystems in providing a buffer zone between freshwater and marine environments. Mangroves and salt marshes are capable of high rates of denitrification and intercept land-based nitrates, thereby protecting coastal seagrass meadows and coral reefs from excessive eutrophication (Valiela et al. 2009). Salt-marsh and mangrove sediments also retain many industrial contaminants (e.g. heavy metals), which would otherwise affect in marine ecosystems (Twilley 1995). There is also a strong positive relationship between the spatial extent of wetlands and the productivity of adjacent seagrass meadows (Valiela et al. 2009). Coastal wetlands (including associated estuarine areas) are also important habitats in their own right, supporting up to 300 species of fishes (mainly in tropical environments) and up to 290 kg of fish biomass per hectare (Blaber 2002). The nutrient-rich water within, and flowing from, coastal wetlands supports many commercially important fish species. Along the Queensland coast, Manson et al. (2005) demonstrated that fishery catches of barramundi (Lates calcarifer) were positively correlated with the local extent of mangroves. Coastal wetlands provide significant subsidies (both in terms of nutrients and larval production) to other coastal habitats, whereby strong connectivity between mangroves and seagrasses, or mangroves and coral reefs leads to increases in fishery productivity (Mumby et al. 2004; Meynecke et al. 2008).
Increasing loss of coastal wetlands has significant ecological and geophysical impacts, including destabilisation of coastal shorelines and sediments, increased contamination and eutrophication of coastal ecosystems leading to lower productivity, and loss of habitat for fishes, as well as for many other important species, such as migratory shorebirds (Valiela et al. 2009). There are currently 14 fish species from Australian coastal wetlands that are considered threatened, and the major threats to these species are habitat loss and overfishing (Fig. 1). Increasing coastal modification and fragmentation also poses a major threat to species within these vulnerable ecosystems by limiting potential for autonomous adaptation to climate change, whereby natural landward migration of key habitat types might otherwise keep pace with the projected sea-level rise. Changes in the elevation of mangroves are also constrained by several processes (e.g. groundwater inputs, local productivity, decomposition and compression of organic material) that control mangrove sediment surface elevation (Gilman et al. 2008). Consequently, there are some locations (e.g. sediment-deficit locations) where landward migration of mangroves will be unable to keep pace with sea-level rise, irrespective of anthropogenic modification of coastal habitats (Gilman et al. 2008).
Seagrass beds
Seagrass beds are a unique and important coastal habitat, formed by the growth of flowering plants (as distinct from algae or seaweed) in shallow sandy habitats throughout tropical and temperate biomes (Dennison 2009). Seagrasses as a whole are particularly sensitive to a wide range of environmental changes resulting from climate change, including changes in temperature, salinity, water clarity and nutrient loads, elevated CO2 concentrations and ocean acidification, sea-level rise, as well as the frequency or severity of cyclones (e.g. Short and Neckles 1999; Waycott et al. 2007). In part, increases in CO2 concentrations may actually increase growth and productivity of seagrasses. The major effect of climate change is expected to be a shift in the relative abundance of seagrass species, with increased abundance of more ephemeral species (e.g. Waycott et al. 2007). Complete loss of seagrasses is expected to be localised (e.g. at the mouths of rivers) and may be offset by increases in the extent of seagrasses in areas where seagrass growth is currently limited by nutrient availability (Waycott et al. 2007). However, the global extent of seagrass beds is declining at 1.5% (or 110 km2 per year) as a result of direct disturbances (Waycott et al. 2009), mostly excessive eutrophication, sedimentation and disease (Orth et al. 2006; Waycott et al. 2009).
Australia has some of the most extensive and diverse seagrass beds in the world. On the Great Barrier Reef, the total area of seagrass beds is twice that of coral-dominated habitat (Dennison 2009). Moreover, extensive seagrass beds in south-eastern Australia support one-third of all seagrass species (Carruthers et al. 2007). However, Kirkman (1997) estimated that the total area of seagrass beds in Australia declined by 1450 km2 (or >20%) between 1986 and 1996, mostly in the tropics and mostly as a result of cyclonic disturbances. Recovery of tropical seagrasses subject to anthropogenic disturbances or cyclones is generally very rapid, although recovery has been hampered in some areas by habitat degradation (increasing sedimentation) and declining water quality. In temperate waters (e.g. Western Port in Victoria), where direct anthropogenic disturbances have killed off large areas of seagrass, there is limited prospect of recovery (Dennison 2009).
Seagrass habitats are an important component of coastal ecosystems, contributing greatly to their productivity. Although very sensitive to excessive nutrient loads, seagrasses also trap considerable amounts of nutrients arising from terrestrial and estuarine habitats (Orth et al. 2006). Seagrasses represent an important source of food for several threatened species, including dugongs and some sea turtles (Waycott et al. 2009). They may also serve as nursery grounds for economically or functionally important fishes that otherwise reside on coral reefs or other inter-connected habitats (Mumby et al. 2004). Localised declines in some fishes have been observed following the reduction in seagrass areas (e.g. Butler and Jernakoff 1999). It remains to be tested, however, what proportion of coastal fishes are specifically reliant on non-reef habitats such as seagrass beds, and whether these fishes could utilise alternative habitat types if seagrass beds were unavailable (e.g. Mumby et al. 2004).
Coral reefs
Climate change is widely recognised as the most important emerging threat to coral reef ecosystems and there are many ways that climate change will affect coral-reef species, communities and ecosystems (Hughes et al. 2003; West and Salm 2003; Hoegh-Guldberg et al. 2007; Wachenfeld et al. 2007). The most devastating effects of climate change, so far, have been large-scale and severe episodes of coral bleaching, resulting from increasing sea-surface temperatures (Goreau et al. 2000). In 1998, coral bleaching occurred in >50 countries throughout the world, killing up to 90% of corals, which then lead to marked changes in the taxonomic composition of coral assemblages (e.g. Reigl and Purkis 2009). In Australia, the effects of coral bleaching have so far been moderate or very localised (Wilkinson 2004). This is particularly true on the Great Barrier Reef, as will be discussed later; however, the most devastating effects of coral bleaching on Australian coral reefs were recorded at Scott Reef, on the north-western shelf (Smith et al. 2008). In 1998, very hot and calm conditions led to extensive bleaching of corals to a depth of 30 m, and many of these corals (up to 90%) subsequently died (Smith et al. 2008). Extensive coral loss was further implicated in localised declines in the abundance of coral reef fishes, especially butterfly fishes and damselfishes (Halford and Caley 2009). Although there was significant recovery in the aftermath of the bleaching, neither coral nor fish assemblages had returned to their pre-bleaching composition 6 years after the bleaching event (Smith et al. 2008; Halford and Caley 2009).
Coral loss, and associated changes in the biological and physical structure of coral-reef habitats, has an important influence on the abundance and diversity of coral-reef fishes (Wilson et al. 2006; Pratchett et al. 2008). Declines in coral cover have direct effects on the abundance of reef fishes, especially among coral-dependent species (e.g. Kokita and Nakazono 2001; Munday 2004; Pratchett et al. 2004). Extensive coral loss may also result in declines in habitat and topographical complexity (Sheppard et al. 2002; Graham et al. 2007), which are critical for sustaining high diversity of reef fishes and other reef-associated organisms (Wilson et al. 2006; Pratchett et al. 2009b). When coral loss is combined with structural collapse of reef habitats, up to 65% of reef fishes may experience declines in abundance (e.g. Jones et al. 2004). Increasing frequency and severity of mass-bleaching events are clearly linked to climate change (Walther et al. 2002; Hughes et al. 2003) and the longer-term future is potentially catastrophic, not just for corals, but for coral-reef fishes as well.
Globally, coral reefs are facing significant and accelerating coral loss. Wilkinson (2004) estimated that 20% of the world’s coral reefs have already been destroyed, whereby coral cover has declined by >90% and there is limited prospect of recovery. Coral cover has declined by 20–90% on a further 50% of the world’s coral reefs, and these reefs may be destroyed by 2050 (Wilkinson 2004). The 1998 global mass-bleaching event contributed greatly to coral-reef degradation, especially in the Indian Ocean (e.g. Graham et al. 2008). However, coral-reef degradation is mostly concentrated in eastern Africa, South-east Asia, and the central and southern Caribbean (Wilkinson 1999). All these areas have relatively large human populations living adjacent to coral-reef habitats, and have also had a long history and high levels of exploitation (e.g. Pandolfi et al. 2003), indicating that coral loss is caused, or at least precipitated, by direct anthropogenic disturbances.
Whereas effects of climate change are already apparent on corals, it is also likely that the contribution of climate change to overall degradation of reef ecosystems will increase in the coming decades (Hoegh-Guldberg 1999; Wilkinson 1999). Given projected increases in sea-surface temperatures, and limited capacity for corals to acclimatise or adapt, coral-bleaching events will become more frequent and more severe through time (Hoegh-Guldberg 1999; Donner et al. 2005). By 2050, most coral reefs are expected to be subject to annual thermal anomalies equivalent to those experienced in 1998 (Hoegh-Guldberg 1999), suggesting that mass bleaching will occur at intervals much less than the time required for corals (populations and communities) to recover from successive major bleaching events (Donner et al. 2005). Effects of increasing temperature will also be further exacerbated by ocean acidification (Hoegh-Guldberg et al. 2007), which reduces coral growth and increases susceptibility to coral bleaching. Furthermore, the long history of anthropogenic degradation and the increased fragmentation of coral-reef habitats have partly eroded reef resilience, making coral-reef habitats much more susceptible to future climate change (Hughes et al. 2003).
Temperate rocky reefs
Australia has high biodiversity and endemicity of temperate marine flora (e.g. Kerswell 2006), which is considered to be very vulnerable to increasing ocean warming. Marine algae with latitudinal ranges centred in temperate Australia are generally cold-water specialists and cannot survive in warmer waters. Accordingly, the geographic ranges of some of these habitat-forming algae have contracted southwards over the past few decades (Wernberg et al. 2009), exhibiting an adaptation strategy for temperate species to avoid increasing temperatures. However, given the lack of landmasses and associated shallow coastal habitats to the south of Australia, most species will have limited opportunity for further pole-ward shifts in their latitudinal ranges to accommodate further increases in ocean temperatures. Increasing ocean temperatures may also lead to southern range extensions for potentially devastating marine invasive species, such as the black sea urchin, Centrostephanus rodgersii (Thresher et al. 2003; Ling et al. 2009), which may be further enhanced by strengthening of the East Australian Current (EAC). Invasive species are one of the greatest contemporary threats to global biodiversity and climate change is expected to facilitate establishment and expansion of many new species on temperate reefs (Wernberg et al. 2009).
Climate change (specifically, increasing temperatures and strengthening of the EAC) has been directly linked to changes in distribution and abundance of herbivorous species along the south-eastern coast, which have contributed to declines in the abundance of giant kelps (Edgar 1999; Ling et al. 2009). The abundance of giant kelp (Macrocysitis pyrifera) has declined dramatically over the past 30 years, especially in south-eastern Tasmania (Edgar 1997). In the 1950s, the total area of kelp forests in Australia was estimated to be 120 km2; however, more recent estimates of 8 km2 in 1986, and 0.5 km2 in 1988–1989, point to a major loss of giant kelp (Edgar 1997). Several factors may account for these dramatic losses, including increased southward penetration and influence of the EAC along the eastern coast of Tasmania, which has increased the water temperature by 1.5°C since 1940 (Edgar 1999). The currents and increased temperatures facilitated local increases of Centrostephanus rodgersii, which feeds on giant kelp (Edgar 1999). Further contributors to declines in abundance of giant kelp are marine pollution and the introduction of the Japanese kelp (Undaria pinnatifida), which has colonised many areas formerly occupied by M. pyrifera (Wernberg et al. 2009).
Globally, loss of kelp and macroalgal environments has resulted from increased disease, herbivory, physiological stress, and/or interactions among these processes (Steneck et al. 2002). At lower latitudes, periodic deforestations result from anomalies in temperature, salinity or nutrients that either kill kelps directly or trigger diseases that may kill physiologically stressed plants. At higher latitudes (40–60°), herbivory by sea urchins is the most common and most important agent of kelp deforestation. Both of these effects may be linked to sustained and ongoing climate change, although loss of kelp forests and changes in habitat conditions of temperate rocky reefs have been most pronounced in areas adjacent to urban centres, caused by coastal development, oil spills, fishery depletion of apex predators and invasions of introduced species. In heavily urbanised areas of Japan, for example, terrestrial deforestation and damming of rivers reduced availability of iron and humic substances necessary for kelp development (e.g. Suzuki et al. 1995). Loss of habitat-forming algae has important consequences for local biodiversity and ecological function (Steneck et al. 2002). Most notably, kelp forests concentrate and magnify secondary production, thereby supporting complex food webs in coastal zones. Kelp forests also provide habitat to high diversity and abundance of marine organisms, including mammals, fishes, crabs, sea urchins and molluscs (Mann 1973). Losses of kelp forests and temperate macroalgal habitats are expected to continue, if not accelerate, as a result of climate change and human population growth (Steneck et al. 2002), contributing to widespread degradation of coastal habitats.
Observed v. predicted climate impacts
Global climate change is being caused by anthropogenic forcing of the climate system (Houghton et al. 2001), and not only are atmospheric concentrations of greenhouse gases rising, but the rate of increase is accelerating (e.g. Canadell et al. 2007). Similarly, increases in atmospheric temperatures are expected to continue throughout the next century and are likely to accelerate over the next two decades because of continued increases in greenhouse-gas emissions (Houghton et al. 2001). As a consequence, even if climatic impacts are not yet apparent (or have had minor influence compared with other, more direct anthropogenic disturbances), the effects of global climate change on ecosystems, communities and species will become greater over time. Accordingly, this section explores both current (observed) and projected effects of climate change in two specific aquatic systems, namely the Murray–Darling Basin and the Great Barrier Reef. These are both large, relatively well studied systems, of considerable ecological and economic importance to Australia.
The Murray–Darling Basin
The Murray–Darling Basin (MDB) is one of the world’s largest catchments, covering more than 1 million km2 and one-seventh of the area of mainland Australia (Crabb 1997). Ranging over 13 degrees of latitude (from 24°S to 37°S) and up to 2000 m in altitude, the MDB experiences a wide range of climatic conditions. The MDB consists of 24 major river catchments grouped into the northern Darling and southern Murray regions. The rivers are fed mainly by rainfall on the western slopes of the Great Dividing Range. The average annual river flow of the MDB (10 000–22 000 GL) is low by world standards (Maheshwari et al. 1995), although inter-annual variability in flow is extremely high, especially within the drier and temperate regions of the basin (Walker et al. 1995). Major riverbeds increasingly become completely dry, which causes extensive mortality of resident fishes (Fig. 2). Drought is a recurrent, natural event in the Murray–Darling Basin, and the native fish have evolved to cope with periodic reductions in habitat and river flow (Lintermans and Cottingham 2007; Bond et al. 2008). However, water extraction and flow regulation, for the purposes of agriculture, have greatly intensified the effects of droughts (e.g. Bond et al. 2008).

|
The MDB catchment yields AU$10 billion of agricultural produce annually, accounting for 40% of the agricultural production of Australia. Approximately 80% of the MDB has been cleared for agriculture, mostly for dryland grazing, although there are also small areas of intensive horticulture, cotton and cereal crops, which are heavily reliant on irrigation (CSIRO 2008). Current water use accounts for 48% of surface water availability across the entire MDB, representing an increase of >500% since 1925 (CSIRO 2008). Groundwater extraction has also increased (doubling in just the past 10–20 years), although records of groundwater use are very limited (CSIRO 2008). River flows are also highly regulated and there is intense and increasing competition between water-resource development and environmental needs (Walker 1985, 1992; Gehrke and Harris 2000).
The MDB is inhabited by 46 species of native fish (Lintermans 2007), most of which have declined in abundance or become highly restricted in their spatial distribution throughout the past century (Murray–Darling Basin Commission 2004a, 2004b; Lintermans and Cottingham 2007). In total, 57% of these species are now listed as threatened under either Federal or State legislation (Lintermans 2007). In 2005–2007, fishes of the MDB were comprehensively sampled as part of the Sustainable Rivers Audit (Davies et al. 2010). Native fishes were not caught in many (43%) of the sample sites and zones where they were predicted to occur, and the biomass of exotic species often exceeded that of native species (Davies et al. 2010). The overall abundance of native fishes in the MDB is suggested to have declined to ~10% of pre-European levels (Murray–Darling Basin Commission 2004b). These declines are linked to a range of threats, including declining river flows, an overall scarcity of surface water habitats and limited flows during severe droughts. During droughts, the habitats available for fish are reduced and isolated, and their quality (including water quality) deteriorates through time (Lintermans and Cottingham 2007; Fig. 2). Moreover, crowding of fish in refugia may increase exposure to alien species, and increases competition, predation and diseases (Lintermans and Cottingham 2007). It is during these periods when agricultural demands for water are at their peak that environmental flows are critical to reduce damage to important aquatic ecosystems, communities and species (Bond et al. 2008).
Observed climate impacts
Surface water availability is a major limiting factor for freshwater habitats in the MDB, and is influenced by several different climatic drivers, including atmospheric temperatures and seasonal patterns of rainfall. Average annual rainfall in the MDB in the 10 years to 2006 (440 mm) was 4% lower than the annual average for the period 1985–2006 (CSIRO 2008). These data are consistent with projected declines in average annual rainfall throughout Australia as a result of global climate change. However, there is high inter-annual and inter-decadal variability in rainfall for the MDB, and similar periods of low rainfall occurred in the 1890s, 1940s, 1960s and 1980s. Recent declines in average rainfall during the ‘millenium’ drought may or may not reflect global climate change, whereas average annual runoff recorded from 1997 to 2006 is 21% below the long-term average, and unprecedented in the historical (>100 years) record (CSIRO 2008). As a dryland river system, average evaporation is very high (94% of precipitation) and runoff averages only 4% of precipitation (Davies et al. 2010). CSIRO (2008) attributed this reduction in runoff to disproportionate declines in autumn and early winter rainfall in the southern MDB. As a consequence, the soils are less saturated, and together with moderate declines in winter rainfall, this greatly reduces runoff. Increasing atmospheric temperatures also exacerbate evaporative loss.
Non-climatic threats
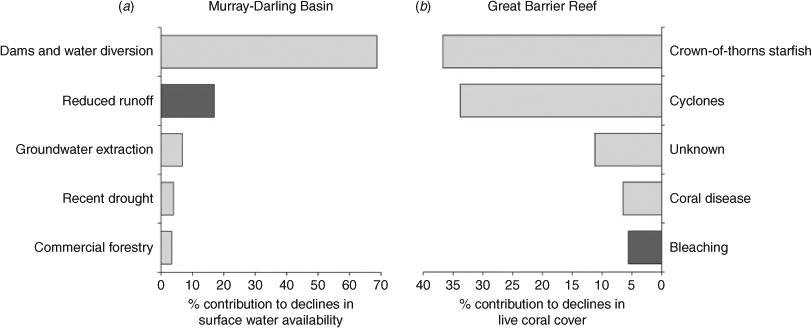
Declines in runoff attributable to recent climate change (and specifically, temporal shifts in major rainfall periods) contribute significantly (up to 17%) to observed declines in surface water availability (Fig. 3; Van Dijk et al. 2006). However, recent and readily detectable changes in surface water availability are mostly attributable to the large number of dams and extensive diversion of flows (Fig. 3), especially in the upper Darling where 178% of average annual runoff can be stored and diverted (Kingsford 2000). Reduced runoff and surface water availability directly affect riverine flow throughout the MDB (Van Dijk et al. 2006), such that many rivers no longer flood and significant areas of floodplains are now permanent water storages (Kingsford 2000). Across the entire MDB, water retention in public and private dams, as well as direct extraction, reduces surface water availability by as much as 68% (CSIRO 2008). By comparison, the recent drought (1996–2006) contributed <5% to recent declines in surface water availability (Fig. 3). In all likelihood, recent reductions in river flow attributable to climate change (specifically, the reduced runoff as a result of increased evaporative loss) would have had negligible impacts on freshwater habitats were it not for pre-existing levels of water extraction, retention and diversion across the MDB (CSIRO 2008).
|
Major threats to the freshwater habitats and fishes in the MDB, and the 57 species of freshwater fishes that utilise these habitats, have been well documented (e.g. Cadwallader 1978; Koehn and O’Connor 1990; Murray–Darling Basin Commission 2004a; Davies et al. 2010). Foremost among these threats are (1) changes to flow regimes, including reduced flow as a result of water retention and diversion, and reduced seasonal variability in flow rates, (2) habitat degradation, including removal of riparian vegetation, removal of structural woody habitat (snags) and increasing sedimentation, (3) declines in water quality, resulting from addition of cold water (from dam releases), land clearing, pesticides, nutrients, sediment and other contaminants, (4) barriers to flow and fish migration (dams, weirs and culverts), (5) historical over-fishing, and (6) high densities of non-native species. The relative importance of these different threats varies among locations and among different species of native fish; however, it is regulation of water flows that is considered to be the most critical change, with significant effects across a wide range of species and major impacts on the functioning and resilience of freshwater habitats (Gehrke and Harris 2000; Kingsford 2011). Gehrke and Harris (2000), for example, showed that regulated rivers contained a higher proportion of non-native fishes than did unregulated rivers, and also had lower than expected diversity of fishes in lower reaches. The effects of regulated flows are further compounded by overall reductions in riverine flow because of increasing retention and diversion of surface water (Kingsford 2011).
Degradation and loss of freshwater habitats in the MDB has been occurring for more than a century, and must be substantially reversed to re-establish viable populations for most native fishes (Murray–Darling Basin Commission 2004b). The MDB Ministerial Council Native Fish Strategy (Murray–Darling Basin Commission 2004b) aims to rehabilitate native-fish communities to 60% of their pre-European abundance and distribution. The major interventions required to achieve this aim are restoration of riverine and floodplain habitats (e.g. Davies 2010), as well as restoration of environmental flows. These two actions are expected to increase the abundance of native fishes in MDB by at least 45%. It is clear, however, that restoration and maintenance of historical flow rates will be increasingly challenged by ongoing climate change and competition with other water users (Van Dijk et al. 2006). To account for the declines in surface water availability attributed to recent climate change, current water storage and diversion would need to be reduced by 20–35% across the MDB. Moreover, water storage and diversion would need to be further reduced with ongoing climate change, whereas water-storage capacity is actually increasing at ~6% per year (CSIRO 2008) and there are existing plans for many new dams across the MDB (Kingsford 2000).
Future climatic impacts
Recent declines in rainfall in the MDB cannot be unambiguously linked to climate change (e.g. Lough et al. 2011), although it is expected that large parts of Australia will become progressively drier in the coming decades. On the basis of projected declines in total rainfall over the next 20–50 years (especially in the south-eastern section of the Basin), river flows in the MDB are expected to further decline by 5–15% (Van Dijk et al. 2006). However, even if rainfall is unchanged, increasing atmospheric temperatures will reduce inflows to freshwater environments because of increasing evaporative water loss (Cai and Cowan 2008). The impact of climate change on water use is also likely to be significant (Van Dijk et al. 2006). Reduced rainfall and increased temperatures will increase the demand for irrigation water and reduce the amount of water in storage. Coupled with increasing water demands by human societies, climate change will put increasing pressure on environmental flows critical for sustaining freshwater habitats in the MDB. Other potential effects include decreases in the frequency and magnitude of floodplain inundation, leading to increased salinity, altered sediment and nutrient delivery, anoxic episodes, and increased incidence of algal blooms (Van Dijk et al. 2006). Poor water quality can lead to an increased incidence of fish kills (Koehn 2005).
The overall effects of climate change on the MDB will largely depend on other activities being undertaken to reduce water use. Ironically, strategies to mitigate the effects of climate change may exacerbate some impacts on freshwater systems. Tree plantations established to sequester CO2 emissions will reduce runoff from forested catchments and have the potential to further reduce stream flows (Hafi et al. 2010).
The Great Barrier Reef
Australia’s Great Barrier Reef (GBR) is the largest coral reef system in the world, consisting of ~2900 individual reefs spanning 14 degrees of latitude and stretching 2100 km from north to south. The GBR Marine Park was established in 1975 under the GBR Marine Park Act, and was declared the world’s largest World Heritage Area in 1981 (Wachenfeld et al. 2007). On July 1st 2004, 33% of the area encompassed within the GBR Marine Park was closed to fishing (up from 4.5% previously), including at least 20% protection for each of 70 distinct bioregions (Fernandes et al. 2005). This increased level of protection was partly motivated by increased recognition of threats posed to the GBR from declining water quality, commercial and recreational fishing, and global climate change (Olsson et al. 2008). The new network of no-take areas was a key step towards increasing the resilience of the GBR, while maintaining important goods and services that are provided by this system (Fernandes et al. 2005). In contrast to the MDB, the GBR contains a total of at least 2000 species of fish.
The GBR provides significant social and economic benefits to Australia. Tourism activities in the GBR and its catchment contribute AU$6.1 billion per year directly to the Australian economy, and employ an estimated 63 000 people (Wachenfeld et al. 2007). Commercial fishing in the GBR generates a further AU$119 million per year, and employs 3600 people (Wachenfeld et al. 2007). There is also a significant value associated with intangible benefits (i.e. non-use values) associated with maintaining the ‘near-pristine’ status of the GBR (Oxford Economics 2009). However, increasing use of the GBR places considerable pressure on the overriding conservation objective of the GBR Marine Park Authority to ensure its ecological sustainability. The ecological status, as well as the economic value, of the GBR is also likely to be undermined by sustained and ongoing global climate change. However, climate change also provides additional incentive to improve current management of the GBR, whereby local actions (e.g. improving the water quality and reducing threats to reef habitats) could greatly increase coral-reef resilience (Marshall and Schuttenberg 2006). For example, Wooldridge (2009) estimated that significant improvements in water quality on the inshore GBR would enable local corals to withstand a further 2.0–2.5°C of temperature rise.
Observed climate impacts
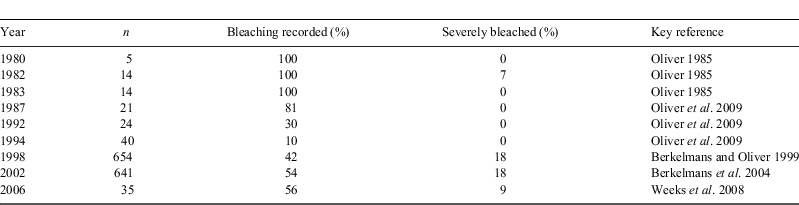
As for coral reefs globally, the most obvious sign of climatic impacts on the GBR are recent increases in the incidence and severity of coral bleaching (Table 2). While coral-reef habitats account for <7% of the area encompassed within the GBR Marine Park, they are an ecologically and economically important component of this system (e.g. Pratchett et al. 2008). Moreover, coral-reef habitats are extremely vulnerable to increasing changes in temperature and ocean acidification (Walther et al. 2002; Hoegh-Guldberg et al. 2007). Major instances of coral bleaching have been recorded at fairly regular intervals on the GBR, extending back to 1980, when conspicuous bleaching of common corals (mostly Acropora and Montipora) was first noted at several isolated reefs between Townsville and Cairns (Oliver 1985). Since then, successive bleaching episodes have tended to occur over increasing geographic scales, affecting greater number of reefs, and greater numbers of coral species and colonies, with increased severity (Table 2; Oliver et al. 2009). Australia was largely spared during the 1998 global mass-bleaching, which caused extensive bleaching in virtually every region of the world, and killed up to 90% of corals, especially in the Indian Ocean (Wilkinson 2004). On the GBR, bleaching was conspicuous and widespread in 1998; however, bleached corals mostly recovered well (Wilkinson 2004) and overall mortality rates were generally low (Maynard et al. 2008; Anthony and Marshall 2009).
The most extensive and most severe bleaching episode to affect the GBR occurred in 2002 (Berkelmans et al. 2004; Maynard et al. 2008), corresponding with the highest sea-surface temperatures (often >33°C) recorded on the GBR. During this event, bleaching was recorded at 54% of reefs surveyed across the length and breadth of the GBR (Berkelmans et al. 2004). As with previous bleaching events, bleaching was more extensive and more severe on inshore reefs compared with offshore reefs (Berkelmans et al. 2004), but there is little data on mortality v. recovery of bleached corals (Baird and Marshall 2002; Maynard et al. 2008). The most recent bleaching event, in 2006, was mostly restricted to the southern GBR (south of 20°S), and effects were highly localised (Weeks et al. 2008). At the Keppel Islands, >90% of corals bleached in 2006 and 40% of corals subsequently died (Diaz-Pulido et al. 2009). Elsewhere (e.g. Heron Island), coral bleaching was relatively minor, owing to the frequent intrusions of cooler oceanic waters (Weeks et al. 2008). Effects of coral bleaching are typically very patchy within and among reefs of the GBR, which may promote persistence of species and also enhances recovery and resilience of coral communities (Oliver et al. 2009).
Non-climatic threats
The GBR has been subject to a long history of exploitation and anthropogenic degradation, especially on near-shore reefs. Coastal populations of Indigenous Australians have harvested food from the GBR for millennia; however, exploitation of many species (e.g. turtles, dugongs, sea cucumbers) and degradation of reef habitats increased significantly following European settlement (Daley et al. 2008). Importantly, introduction of sheep and cattle in many major catchments adjacent to the GBR was initiated in the 1860s, after which, there was a doubling of sediment loads introduced to the GBR lagoon during major flood events (McCulloch et al. 2003). Although no longer pristine, the GBR has much lower levels of depletion for carnivores, herbivores and architectural species than do other major reef ecosystems (Pandolfi et al. 2003).
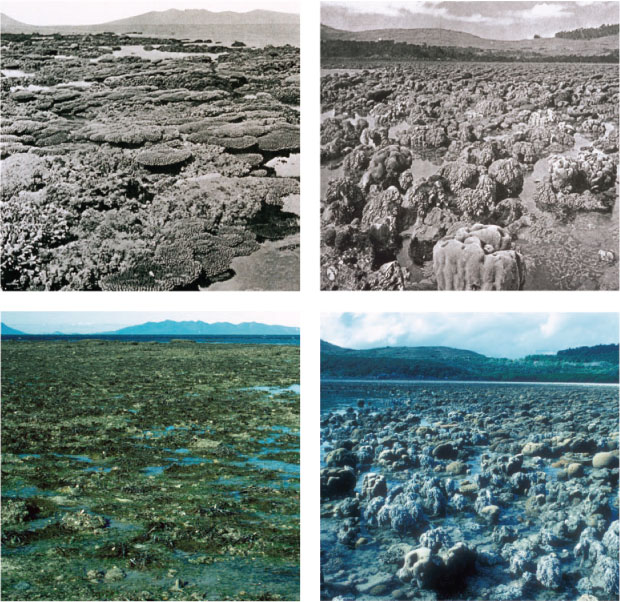
Inshore reefs of the GBR are considered to be much more degraded (Pandolfi et al. 2003), having less coral and more macroalgae than do offshore reefs (e.g. Wismer et al. 2009). To some extent, these cross-shelf differences are to be expected given natural gradients in terrestrial v. ocean influences, as well as differences in the composition of fish assemblages, especially reduced abundance of major herbivores on inshore reefs (Hoey and Bellwood 2008). However, inshore reefs are highly variable, and coral cover is extremely high (>80%) on some inshore reefs (Sweatman et al. 2007). However, inshore reefs are frequently exposed to flood plumes and to sediment that is resuspended from the shallow bottom by wave action (Larcombe and Woolfe 1999), and the lack of corals on some inshore reefs may have been exacerbated by anthropogenic activities (agriculture and development) in adjacent catchments. Photographic records of inshore reefs spanning >100 years reveal long-term changes in habitat structure at some sites (Wachenfeld 1997). At Stone Island, for example, photographs taken by Saville-Kent in 1890 reveal extensive cover of branching corals on the exposed reef flat. Comparable photographs of the same reef flat taken in 1994 do not show any corals, but patches of macroalgae interspersed across a mud flat (Fig. 4a). These long-term changes in habitat structure are largely attributed to chronic stresses associated with declining water quality (Hughes et al. 2010). However, detailed analyses of historical photographs by Wachenfeld (1997) revealed that these changes were not consistent across all locations, and many inshore reefs retain a habitat structure similar to that recorded pre-1950s (Fig. 4b).
|
More recently (mostly since 1960), coral reefs in the GBR have been subject to increasing large-scale disturbances. Bellwood et al. (2004) suggested that average coral cover declined >40% from 1960 to 2000, and attributed these losses to cumulative effects of increasing outbreaks of the corallivorous crown-of-thorns starfish (Acanthaster planci) and coral bleaching. Broad-scale and ongoing monitoring by the Australian Institute of Marine Science (e.g. Sweatman et al. 2008) shows that coral cover on the GBR is highly dynamic, caused by inter-reef variation in the effects of (and recovery from) several different types of disturbance, including cyclones, outbreaks of A. planci, bleaching and coral disease. Reef-wide coral cover declined very little between 1995 and 2009, and declines that were observed were mainly attributable to the effects of cyclones and outbreaks of A. planci rather than to those of climate-related disturbances (Fig. 3; Osborne et al. 2011). Similarly, Bruno and Selig (2007) showed that climate change played a minimal part in protracted coral declines apparent across much of the Pacific. It is incontrovertible that regional-scale incidences of mass-bleaching are caused by climate-related increases in sea-surface temperatures (Hughes et al. 2003); however, bleaching currently accounts for <6% of coral loss recorded on the GBR between 1995 and 2009 (Fig. 3).
Climate change may have also exacerbated coral loss attributable to coral disease (Bruno et al. 2007) or cyclones (Webster et al. 2005); however, it is difficult to establish the contribution of climate change to these effects, although both have contributed greatly to recent coral loss (Fig. 3). Bruno et al. (2007) found a significant relationship between the occurrence of positive temperature anomalies and incidence of white syndrome, but only on reefs with high (>50%) coral cover. Moreover, white syndrome is only one of several different coral diseases that occurs on the GBR, and the links between global warming and these diseases (e.g. black-band and brown-band disease) is unclear. In terms of cyclones, the intensity (if not occurrence) of severe tropical storms is influenced by sea-surface temperatures, and is expected to increase with ocean warming (Landsea et al. 2006). However, Klotzbach (2006) found no change in the frequency or intensity of tropical cyclones from 1986 until 2005, despite a 0.25°C increase in sea-surface temperature. Further, apparent long-term increases in cyclone intensity (e.g. Webster et al. 2005) are attributed to improved methods for quantifying cyclone intensity after 1984 (Landsea et al. 2006).
Future climatic impacts
So far, climate impacts on Australia’s coral reefs have been relatively minor compared with the extent of coral loss caused by non-climatic disturbances (Fig. 3). Climate change-related increases in sea-surface temperatures and declines in ocean pH are, however, expected to increasingly affect coral reefs in the future (Hoegh-Guldberg 1999; Hoegh-Guldberg et al. 2007). Climate projections suggest that mass bleaching will become a biannual phenomenon by 2030 if corals do not acclimatise or adapt (Donner et al. 2005). Increased thermal tolerance by 0.5–1.0°C by 2050 could delay projected bleaching events by 5 years, possibly allowing affected reefs some recovery between major disturbances. The future cover and species composition of scleractinian corals and the ecosystems they support will depend on their ability to adjust to current rates of ocean warming and acidification. Despite this, very few data exist on which to evaluate the adaptive capacity of coral communities, species or populations to these trends.
Communities can adapt to climate change through shifts in community composition towards more tolerant species (Hughes et al. 2003). Shifts in community species composition following bleaching have been widely documented (e.g. Edwards et al. 2001; Loya et al. 2001). Recovery of reefs may occur through the growth of more resilient survivors (Loya et al. 2001)) or through recruitment and recovery of fast-growing but often more sensitive species (Edwards et al. 2001; Pratchett et al. 2009a). Therefore, although climate change is expected to change the community composition of reef corals, it is not yet clear whether a shift towards more tolerant species will occur.
Coral populations can acclimatise or adapt by increasing the frequency of more tolerant individuals (genotypes) within populations. Although increases in the thermal tolerance of some coral populations have been observed following major bleaching events (e.g. Maynard et al. 2008), no study has directly linked differences in allelic frequencies to thermal tolerance in coral (Maynard et al. 2008). D’Croz and Mate (2004) found genetic divergence between Caribbean populations of Pocillopora damicornis in cooler and warmer reef areas with corresponding thermal tolerances. Edmunds (1994) found that rates of natural bleaching differed among genotypes in Montastrea anularis; however, this author did not associate tolerance with specific alleles. Corals may also gain increased thermal tolerance by changing their dominant symbionts towards types with greater thermal tolerances, a process termed ‘symbiont shuffling’ (e.g. Berkelmans and van Oppen 2006). Although there are limits to thermal acclimatisation through symbiont shuffling (i.e. 1.5°C when dominated by GBR D type instead of C2 type), field evidence suggests that reef-wide changes in dominant Symbiodinium type can offer corals some short-term protection against predicted temperature increases (Jones et al. 2008). However, increases in the proportion of thermally tolerant Symbiodinium can lead to reduced growth of the host coral (Jones and Berkelmans 2010), an effect which may exacerbate reduced calcification rates caused by ocean acidification.
The potential for adaptation of corals, and the time scales over which this can occur, can be modelled if quantitative genetic estimates of heritability are known. Heritability describes the amount of genetic variation in a population which natural selection can act on (i.e. additive genetic variation results in offspring with intermediate appearance to parents). At present, only two studies have estimated heritability in any coral trait (Meyer et al. 2009; Császár et al. 2010). Both studies found significant heritability in molecular and physiological traits of both host and symbionts, suggesting the potential for genetic adaptation in these traits. Estimates of heritability of more coral traits, as well as links between these traits and fitness (e.g. growth and survival) in a variety of temperature and pH scenarios, are required before effective modelling of coral’s responses to climate-change effects can occur.
Managing effects of climate change
Clearly, urgent action is required to minimise global greenhouse-gas emissions and thereby reduce longer-term climatic impacts on Australia’s aquatic ecosystems. However, drastic reductions in emissions, even if they are implemented immediately, will not guarantee the persistence of ecosystems, communities and species that are already being affected by global climate change and will be affected for some time into the future. It is predicted, for example, that global atmospheric temperatures will increase by >1°C by the end of the century, regardless of future emission scenarios, and Australia’s climate is currently warming faster than the global average (Lough et al. 2011). Reductions in global greenhouse-gas emissions may limit extreme changes in environmental conditions and reduce rates of change to which species must adapt. In the short-term, however, management must attempt to facilitate the adaptation of species and ecosystems to inevitable climate change, however extreme that turns out to be.
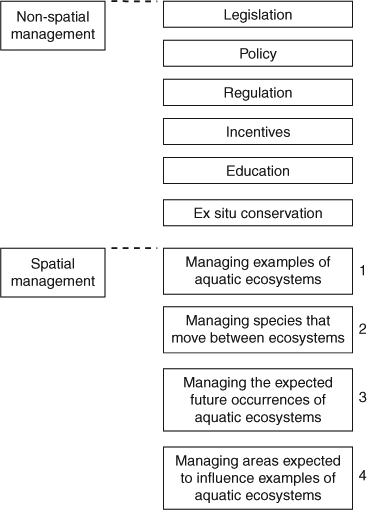
Management will need to be both non-spatial and spatial (Fig. 5), although this section focuses principally on spatial management interventions. Non-spatial management approaches are often (although not always) applied without being area-specific. Some examples for aquatic ecosystems include regulation for sustainable harvest of species, reduced by-catch, quarantine arrangements to prevent the arrival of invasive species, environmental water allocations, and strategies to reduce water extraction from streams and wetlands for agricultural and urban uses. As a last resort, ex situ conservation might be necessary to avoid extinction of some species (e.g. translocations) for which spatial management options have been exhausted (L. Shoo, A. Hoffman, S. Williams, R. Pressey, and Y. Williams, unpubl. data).
|
Four kinds of spatial management are distinguished here that can help promote adaptation of aquatic ecosystems and species to climate change (Fig. 5). In practice, these would be applied in various combinations, and often with the support of policy, regulation and other non-spatial approaches. The conventional, and still important, approach to conservation has been to establish protected areas to represent examples of ecosystems (Fig. 5: Box 1), given that few ecosystems can be managed for conservation in their entirety (Margules et al. 2002). Protected areas are not particularly applicable to habitats such as rivers, which are long and linear and subjected to upstream and downstream influences (Koehn 2003). More recently, however, conservation planning has begun to address a variety of spatially explicit management actions in addition to protected areas (Joseph et al. 2009). The protection of specific areas remains an important tool in a changing climate, to ensure that refugia, or areas that might be buffered from the effects of climate change, are effectively managed. Examples of this may be occurrences of mangroves that might undergo relatively mild effects of climate change (McLeod and Salm 2006) and coral reefs that are buffered from rising sea-surface temperatures by cool upwellings and other physical factors (Marshall and Johnson 2007). An additional reason is to enhance the resilience of ecosystems to the effects of climate change. For coral reefs, spatial management can promote recovery of corals after bleaching events by maintaining healthy populations of herbivorous fish to reduce algal growth and by grouping protected reefs, so that bleached reefs are well supplied with coral propagules from unaffected reefs (Obura 2005). The protection of refuge pools in rivers or wetlands can allow opportunities for recolonisation under higher flows.
Resilience to climate change is likely to be an important goal of spatial management for aquatic ecosystems generally. Among the factors determining resilience are the attributes of the species (Crook et al. 2010) and the functional integrity of ecosystems. This can be expected to minimise the impacts of climate change on ecosystems or confer the ability to recover from those impacts. Stressors that can be directly managed, such as water extraction, sedimentation and eutrophication, should therefore be limited or reduced (Hughes et al. 2003) and fishing mortalities should be minimised (Bellwood et al. 2004; Marshall and Schuttenberg 2006). A related trend towards managing for resilience is the increasing attention given to spatial planning for the persistence of processes (Pressey et al. 2007). Recent work, for example, has begun to address time-series data and quantitative conservation objectives related to the spatio-temporal dynamics of the anomalies of sea-surface temperature and the risk of coral bleaching (Game et al. 2008; N. Ban, R. Pressey, and S. Weeks, unpubl. data).
Direct spatial management of aquatic ecosystems (Fig. 5: Box 1) also involves restoration. Spatial priorities can be identified, for example, to replant mangroves (McLeod and Salm 2006), to reverse the impacts of engineering works such as drainage and floodgates in coastal wetlands (Pressey and Middleton 1982) or enhance structural woody habitats (Nicol et al. 2004). In deciding whether restoration is warranted, managers must consider the historical condition of these ecosystems, the feasibility of returning areas to their pre-disturbance states, the cost of the interventions, and proximity to spatial management actions for other ecosystems. The success of some recovery actions such as revegetation could be compromised under climate-change scenarios.
Given that ecosystems are not self-contained units, a complementary management approach is to consider the requirements of species with life histories that involve moving among ecosystems (Fig. 5: Box 2). This is important for freshwater systems (Dudgeon et al. 2006) where diadromous fishes undertake extensive longitudinal migrations between headwaters and the estuaries or the open ocean (Beger et al. 2010). Functional riverine connectivity is essential to ensure that movements can continue. It is also important for the marine realm, where proximity of different ecosystems can determine the composition and abundance of species in each (Skilleter et al. 2005; Mumby 2006). Fully integrated management of aquatic ecosystems would also recognise the dependence of many terrestrial species on freshwater and marine systems (Dudgeon et al. 2006; Beger et al. 2010). Essentially, this approach to management requires knowledge of the complementary nature of particular ecosystems and appropriate conservation actions in each. Under climate change, it will also require some understanding of how each of the ecosystems, and the biological connections between them, might change.
Climate change implies changes in the distribution of species and ecosystems; so, management for climate change must anticipate these changes and consider the areas to which aquatic ecosystems and species might move (Fig. 5: Box 3). One application of this approach would be to identify intertidal wetlands with the potential to move landward because of lack of adjacent development or seawalls, and to protect both of these wetlands and the inland areas that they will occupy with higher sea levels (McLeod and Salm 2006). A related application is the translocation of species. As problematic as this might appear, this may be the only prospect for the survival of species that are unable to disperse unassisted in responding to future suitable climates. Some progress has been made towards rigorous assessment of options and risks for translocation (Hoegh-Guldberg et al. 2008).
Even more so than in the terrestrial realm, aquatic ecosystems can be adversely affected by distant anthropogenic threats that may be compounded by climate change. The impacts of distant threats are particularly obvious in large catchments from which sediment, nutrients and toxins can affect freshwater, estuarine and marine ecosystems downstream (Brodie et al. 2009). Management approaches are therefore needed for areas expected to influence aquatic ecosystems, often from afar (Stoms et al. 2005), and these will be more effective with understanding of the influence of climate change on land use and runoff (Fig. 5: Box 4). In some regions, climate change will increase human needs for water and perhaps exacerbate the effects of reduced rainfall and runoff on freshwater ecosystems (Abell 2002). In other regions, the distributions and types of land uses will be adjusted to altered climates, with implications for surrounding ecosystems (Dudgeon et al. 2006). In this context, management of activities in catchments could reduce nutrient runoff to inshore marine waters and lessen the effects of high sea temperatures on coral bleaching (Marshall and Johnson 2007; Wooldridge and Done 2009). Ideally, management actions in catchments would integrate diverse objectives, including conservation of terrestrial ecosystems, reduced disturbance of freshwater ecosystems, and appropriate water quality of near-shore marine ecosystems. This would allow managers to more cost-effectively address multiple objectives by identifying areas that contributed to all of them.
Conclusions
The present review has revealed conspicuous evidence of recent climatic impacts across Australian aquatic habitats. In coastal wetlands, for example, mangroves are already progressing landward and encroaching on salt-marsh environments at many locations along Australia’s south-eastern coast (e.g. Saintilan and Williams 1999). These changes will have significant effects on fishes that live within these habitats (e.g. Gillanders et al. 2011), and are expected to worsen with ongoing changes in temperature, water chemistry, sea level, rainfall patterns and ocean currents (Hobday and Lough 2011). Thus far, the effects of climate change have been relatively minor compared with the extended history and often-extensive habitat degradation caused by more direct anthropogenic impacts (e.g. Fig. 3). However, effects of climate change compound on habitat degradation and losses that have already occurred as a result of more localised anthropogenic disturbances (e.g. Schindler 2001; Hughes et al. 2003). Moreover, the cumulative effects of exploitation, pollution, and habitat modification and fragmentation make aquatic populations and communities much more vulnerable to climate change (e.g. Schindler 2001; Wooldridge 2009; Davies 2010). Despite marked differences in the major drivers of climatic impacts in different aquatic ecosystems (Table 1), there are strong similarities in the management actions required to address effects of climate change. Minimising global greenhouse-gas emissions is important to reduce longer-term and more extreme impacts of climatic change in Australia’s aquatic ecosystems, and immediate action is also required to reverse the effects of direct anthropogenic disturbances and restore ecosystem function and resilience to these ecosystems.
Acknowledgements
This manuscript was written as part of the Australian Society for Fish Biology (ASFB) Climate change and Australian aquatic environments: the future for fish and fisheries symposium (Melbourne, July 2010). We thank the ASFB for this opportunity, their assistance and support. We also thank Andrew Boulton, Alistair Hobday, Paul Reich, and the two anonymous reviewers for their comments that greatly improved this manuscript.
References
Abell, R. (2002). Conservation biology for the biodiversity crisis: a freshwater follow-up. Conservation Biology 16, 1435–1437.| Conservation biology for the biodiversity crisis: a freshwater follow-up.Crossref | GoogleScholarGoogle Scholar |
Anthony, K. R. N., and Marshall, P. (2009). Coral reefs. In ‘Report Card of Marine Climate Change for Australia: Detailed Scientific Assessment’. (Eds E. S. Poloczanska, A. J., Hobday and A. J. Richardson.) pp. 171–185. (NCCARF Publication, Southport, Queensland.)
Baird, A. H., and Marshall, P. A. (2002). Mortality, growth and reproduction in scleractinian corals following bleaching on the Great Barrier Reef. Marine Ecology Progress Series 237, 133–141.
| Mortality, growth and reproduction in scleractinian corals following bleaching on the Great Barrier Reef.Crossref | GoogleScholarGoogle Scholar |
Balston, J. (2009). An analysis of the impacts of long-term climate variability on the commercial barramundi (Lates calcarifer) fishery of north-east Queensland, Australia. Fisheries Research 99, 83–89.
| An analysis of the impacts of long-term climate variability on the commercial barramundi (Lates calcarifer) fishery of north-east Queensland, Australia.Crossref | GoogleScholarGoogle Scholar |
Bayliss, P., Ellis-Evans, J. C., and Laybourn-Parry, J. (1997). Temporal patterns of primary production in a large ultra-oligotrophic Antarctic freshwater lake. Polar Biology 18, 363–370.
| Temporal patterns of primary production in a large ultra-oligotrophic Antarctic freshwater lake.Crossref | GoogleScholarGoogle Scholar |
Beger, M., Grantham, H. S., Pressey, R. L., Wilson, K. A., Peterson, E. L., Dorfman, D., Mumby, P. J., Lourival, R., Brumbaugh, D. R., and Possingham, H. P. (2010). Conservation planning for connectivity across marine, freshwater, and terrestrial realms. Biological Conservation 143, 565–575.
| Conservation planning for connectivity across marine, freshwater, and terrestrial realms.Crossref | GoogleScholarGoogle Scholar |
Bellwood, D. R., Hughes, T. P., Folke, C., and Nystrom, M. (2004). Confronting the coral reef crisis. Nature 429, 827–833.
| Confronting the coral reef crisis.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXltVKltb8%3D&md5=4161ce76cca64ed6d1b5a7e203add990CAS |
Berkelmans, R., and Oliver, J. K. (1999). Large scale bleaching of corals on the Great Barrier Reef. Coral Reefs 18, 55–60.
| Large scale bleaching of corals on the Great Barrier Reef.Crossref | GoogleScholarGoogle Scholar |
Berkelmans, R., and van Oppen, M. J. H. (2006). The role of zooxanthellae in the thermal tolerance of corals: a ‘nugget of hope’ for coral reefs in an era of climate change. Proceedings. Biological Sciences 273, 2305–2312.
| The role of zooxanthellae in the thermal tolerance of corals: a ‘nugget of hope’ for coral reefs in an era of climate change.Crossref | GoogleScholarGoogle Scholar |
Berkelmans, R., De’ath, G., Kininmonth, S., and Skirving, W. J. (2004). A comparison of the 1998 and 2002 coral bleaching events on the Great Barrier Reef: spatial correlation, patterns, and predictions. Coral Reefs 23, 74–83.
| A comparison of the 1998 and 2002 coral bleaching events on the Great Barrier Reef: spatial correlation, patterns, and predictions.Crossref | GoogleScholarGoogle Scholar |
Blaber, S. J. M. (2002). Fish in hot water: the challenge facing fish and fisheries in tropical estuaries. Journal of Fish Biology 61, 1–20.
Bond, N. R., Lake, P. S., and Arthington, A. H. (2008). The impacts of drought on freshwater ecosystems: an Australian perspective. Hydrobiologia 600, 3–16.
| The impacts of drought on freshwater ecosystems: an Australian perspective.Crossref | GoogleScholarGoogle Scholar |
Bond, N., Thomson, J., Reich, P., and Stein, J. (2011). Using species distribution models to infer potential climate change-induced range shifts of freshwater fish in south-eastern Australia. Marine and Freshwater Research 62, 1043–1061.
| Using species distribution models to infer potential climate change-induced range shifts of freshwater fish in south-eastern Australia.Crossref | GoogleScholarGoogle Scholar |
Booth, D. J., Bond, N., and Macreadie, P. (2011). Detecting range shifts among Australian fishes in response to climate change. Marine and Freshwater Research 62, 1027–1042.
| Detecting range shifts among Australian fishes in response to climate change.Crossref | GoogleScholarGoogle Scholar |
Brodie, J., Lewis, S., Bainbridge, Z., Mitchell, A., Waterhouse, J., and Kroon, F. (2009). Target setting for pollutant discharge management of rivers in the Great Barrier Reef catchment area. Marine and Freshwater Research 60, 1141–1149.
| Target setting for pollutant discharge management of rivers in the Great Barrier Reef catchment area.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXhsVWqtLzP&md5=9c807f60905650182096715fa5fba19aCAS |
Bruno, J. F., and Selig, E. R. (2007). Regional decline of coral cover in the Indo-Pacific: timing, extent, and subregional comparisons. PLoS ONE 2, e711.
| Regional decline of coral cover in the Indo-Pacific: timing, extent, and subregional comparisons.Crossref | GoogleScholarGoogle Scholar |
Bruno, J. F., Selig, E. R., Casey, K. S., Page, C. A., Willis, B. L., Harvell, C. D., Sweatman, H., and Melendy, A. M. (2007). Thermal stress and coral cover as drivers of coral disease outbreaks. PLoS ONE 5, e124.
Buisson, L., Thuiller, W., Lek, S., Lim, P., and Grenouillet, G. (2008). Climate change hastens the turnover of stream fish assemblages. Global Change Biology 14, 2232–2248.
| Climate change hastens the turnover of stream fish assemblages.Crossref | GoogleScholarGoogle Scholar |
Bunn, S. E., Davies, P. M., and Winning, M. (2003). Sources of organic carbon supporting the food web of an arid zone floodplain river. Freshwater Biology 48, 619–635.
Butler, A., and Jernakoff, P. (1999). ‘Seagrass in Australia: Strategic Review and Development of an R and D Plan.’ (CSIRO: Victoria)
Cadwallader, P. L. (1978). Some causes of the decline in range and abundance of native fish in the Murray–Darling river system. Proceedings of the Royal Society of Victoria 90, 211–224.
Cai, W., and Cowan, T. (2008). Evidence of impacts from rising temperature on inflows to the Murray–Darling Basin. Geophysical Research Letters 35, L07701.
| Evidence of impacts from rising temperature on inflows to the Murray–Darling Basin.Crossref | GoogleScholarGoogle Scholar |
Canadell, J. G., Kirschbaum, M. U. F., Kurz, W. A., Sanz, M.-J., Schlamadinger, B., and Yamagata, Y. (2007). Factoring out natural and indirect human effects on terrestrial carbon sources and sinks. Environmental Science & Policy 10, 370–384.
| Factoring out natural and indirect human effects on terrestrial carbon sources and sinks.Crossref | GoogleScholarGoogle Scholar |
Carpenter, S. R., Fisher, S. G., Grimm, N. B., and Kitchell, J. F. (1992). Global change and freshwater ecosystems. Annual Review of Ecology and Systematics 23, 119–139.
| Global change and freshwater ecosystems.Crossref | GoogleScholarGoogle Scholar |
Carruthers, T. J. B., Dennison, W. C., Kendrick, G. A., Waycott, M., Walker, D. I., and Cambridge, M. L. (2007). Seagrasses of south-west Australia: a conceptual synthesis of the world’s most diverse and extensive seagrass meadows. Journal of Experimental Marine Biology and Ecology 350, 21–45.
| Seagrasses of south-west Australia: a conceptual synthesis of the world’s most diverse and extensive seagrass meadows.Crossref | GoogleScholarGoogle Scholar |
Crabb, P. (1997). Impacts of anthropogenic activities, water use and consumption on water resources and flooding, Australia state of the environment technical paper series (inland waters). Department of the Environment, Canberra.
Crook, D. A., Reich, P., Bond, N. R., McMaster, D., Koehn, J. D., and Lake, P. S. (2010). Using biological information to support proactive strategies for managing freshwater fish during drought. Marine and Freshwater Research 61, 379–387.
| Using biological information to support proactive strategies for managing freshwater fish during drought.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXjvFSjs7g%3D&md5=80d269acaf21f989f18f33c88f071d64CAS |
Császár, N. B. M., Ralph, P. J., Frankham, R., Berkelmans, R., and van Oppen, M. J. H. (2010). Estimating the potential for adaptation of corals to climate warming. PLoS ONE 5, e9751.
| Estimating the potential for adaptation of corals to climate warming.Crossref | GoogleScholarGoogle Scholar |
CSIRO (2008). Water availability in the Murray–Darling Basin. A report to the Australian Government from the CSIRO Murray–Darling Basin Sustainable Yields Project. (CSIRO: Canberra.)
D’Croz, L., and Mate, J. L. (2004). Experimental responses to elevated water temperature in genotypes of the reef coral Pocillopora damicornis from upwelling and non-upwelling environments in Panama. Coral Reefs 23, 473–483.
| Experimental responses to elevated water temperature in genotypes of the reef coral Pocillopora damicornis from upwelling and non-upwelling environments in Panama.Crossref | GoogleScholarGoogle Scholar |
Daley, B., and Griggs, P. (2006). Mining the reefs and cays: coral, guano and rock phosphate extraction in the Great Barrier Reef, Australia, 1844–1940. Environmental History 12, 395–433.
| Mining the reefs and cays: coral, guano and rock phosphate extraction in the Great Barrier Reef, Australia, 1844–1940.Crossref | GoogleScholarGoogle Scholar |
Daley, B., and Griggs, P. (2008). Exploiting marine wildlife in Queensland: the commercial dugong and marine turtle fisheries, 1847–1969. Australian Economic History Review 48, 227–265.
| Exploiting marine wildlife in Queensland: the commercial dugong and marine turtle fisheries, 1847–1969.Crossref | GoogleScholarGoogle Scholar |
Daley, B., Griggs, P., and Marsh, H. (2008). Reconstructing reefs: qualitative research and the environmental history of the Great Barrier Reef, Australia. Qualitative Research 8, 584–615.
| Reconstructing reefs: qualitative research and the environmental history of the Great Barrier Reef, Australia.Crossref | GoogleScholarGoogle Scholar |
Davies, P. M. (2010). Climate change implications for river restoration in global biodiversity hotspots. Restoration Ecology 18, 261–268.
| Climate change implications for river restoration in global biodiversity hotspots.Crossref | GoogleScholarGoogle Scholar |
Davies, P. E., Harris, J. H., Hillamn, T. J., and Walker, K. F. (2010). The sustainable rivers audit: assessing river ecosystem health in the Murray–Darling Basin, Australia. Marine and Freshwater Research 61, 764–777.
| The sustainable rivers audit: assessing river ecosystem health in the Murray–Darling Basin, Australia.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXptFGrs7Y%3D&md5=4f28a4e5622032c57e03c810de587d26CAS |
Dennison, W. C. (2009). Global trajectories of seagrasses, the biological sentinels of coastal ecosystems. In ‘Global Loss of Coastal Habitats: Rates, Causes and Consequences’. (Ed. C. M. Duarte.) pp. 89–106. (Fundación BBVA: Bilbao.)
Diaz-Pulido, G., McCook, L. J., Dove, S., Berkelmans, R., Roff, G., Kline, D. I., Weeks, S., Evans, R. D., Williamson, D. H., and Hoegh-Guldberg, O. (2009). Doom and boom on a resilient reef: climate change, algal overgrowth and coral recovery. PLoS ONE 4, e5239.
| Doom and boom on a resilient reef: climate change, algal overgrowth and coral recovery.Crossref | GoogleScholarGoogle Scholar |
Dixson, D. L., Munday, P. L., and Jones, G. P. (2010). Ocean acidification disrupts the innate ability of fish to detect predator olfactory cues. Ecology Letters 13, 68–75.
| Ocean acidification disrupts the innate ability of fish to detect predator olfactory cues.Crossref | GoogleScholarGoogle Scholar |
Donner, S. D., Skirving, W. J., Little, C. M., Oppenheimer, M., and Hoegh-Guldberg, O. (2005). Global assessment of coral bleaching and required rates of adaptation under climate change. Global Change Biology 11, 2251–2265.
| Global assessment of coral bleaching and required rates of adaptation under climate change.Crossref | GoogleScholarGoogle Scholar |
Doody, J. P. (2004). ‘Coastal squeeze’ – an historical perspective. Journal of Coastal Conservation 10, 129–138.
| ‘Coastal squeeze’ – an historical perspective.Crossref | GoogleScholarGoogle Scholar |
Dudgeon, D., Arthington, A. H., Gessner, M. O., Kawabata, Z., Knowler, D. J., Leveque, C., Naiman, R. J., Prieur-Richard, A.-H., Soto, D., Staissny, M. L. J., and Sullivan, C. A. (2006). Freshwater biodiversity: importance, threats, status and conservation challenges. Biological Reviews of the Cambridge Philosophical Society 81, 163–182.
| Freshwater biodiversity: importance, threats, status and conservation challenges.Crossref | GoogleScholarGoogle Scholar |
Duke, N. C., Meyneck, J.-O., Dittmann, S., Ellison, A. M., Anger, K., Berger, U., Cannicci, S., Diele, K., Ewel, K. C., Field, C. D., Kpedam, N., Lee, S. Y., Marchand, C., Nordhaus, I., and Dahdouh-Guebas, F. (2007). A world without mangroves? Science 317, 41.
| A world without mangroves?Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXns1Cruro%3D&md5=d2a750e735a4642fdd4a16a8166f36c6CAS |
Edgar, G. J. (1997). ‘Australian Marine Life.’ (Reed Books: Melbourne.)
Edgar, G. J. (1999). Experimental analysis of structural versus trophic importance of seagrass beds. I. Effects on macrofaunal and meiofaunal invertebrates. Vie et Milieu 49, 239–248.
Edmunds, P. J. (1994). Evidence that reef-wide patterns of coral bleaching may be the result of the distribution of bleaching-susceptible clones. Marine Biology 121, 137–142.
| Evidence that reef-wide patterns of coral bleaching may be the result of the distribution of bleaching-susceptible clones.Crossref | GoogleScholarGoogle Scholar |
Edwards, A. J., Clarke, S., Zahir, H., Rajasuriya, A., Naseer, A., and Rubens, J. (2001). Coral bleaching and mortality on artificial and natural reefs in Maldives in 1998, sea surface temperature anomalies and initial recovery. Marine Pollution Bulletin 42, 7–15.
| Coral bleaching and mortality on artificial and natural reefs in Maldives in 1998, sea surface temperature anomalies and initial recovery.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXhtlWnt7s%3D&md5=571efdf3110e4ea7136ea034a54a2178CAS |
Fernandes, L., Day, J., Lewis, A., Slegers, S., Kerrigan, B., Breen, D., Cameron, D., Jago, B., Hall, J., Lowe, D., Innes, J., Tanzer, J., Chadwick, V., Thompson, L., Gorman, K., Simmons, M., Barnett, B., Sampson, K., De’ath, G., Mapstone, B., Marsh, H., Possingham, H., Ball, I., Wards, T., Dobbs, K., Aumend, J., Slater, D., and Stapleton, K. (2005). Establishing representative no-take areas in the Great Barrier Reef: Large-scale implementation of theory on marine protected areas. Conservation Biology 19, 1733–1744.
| Establishing representative no-take areas in the Great Barrier Reef: Large-scale implementation of theory on marine protected areas.Crossref | GoogleScholarGoogle Scholar |
Ficke, A. D., Myrick, C. A., and Hansen, L. J. (2007). Potential impacts of global climate change on freshwater fishes. Reviews in Fish Biology and Fisheries 17, 581–613.
| Potential impacts of global climate change on freshwater fishes.Crossref | GoogleScholarGoogle Scholar |
Game, E. T., Watts, M. E., Wooldridge, S., and Possingham, H. P. (2008). Planning for persistence in marine reserves: a question of catastrophic importance. Ecological Applications 18, 670–680.
| Planning for persistence in marine reserves: a question of catastrophic importance.Crossref | GoogleScholarGoogle Scholar |
Gehrke, P. C., and Harris, J. H. (2000). Large-scale patterns in species richness and composition of temperate riverine fish communities. Marine and Freshwater Research 51, 165–182.
| Large-scale patterns in species richness and composition of temperate riverine fish communities.Crossref | GoogleScholarGoogle Scholar |
Gillanders, B. M., Elsdon, T. S., Halliday, I. A., Jenkins, G. P., Robins, J. B., and Valesini, F. J. (2011). Potential effects of climate change on Australian estuaries and fish-utilising estuaries: a review. Marine and Freshwater Research 62, 1115–1131.
| Potential effects of climate change on Australian estuaries and fish-utilising estuaries: a review.Crossref | GoogleScholarGoogle Scholar |
Gilman, E. L., Ellison, J., Duke, N. C., and Field, C. (2008). Threats to mangroves from climate change and adaptation options: a review. Aquatic Botany 89, 237–250.
| Threats to mangroves from climate change and adaptation options: a review.Crossref | GoogleScholarGoogle Scholar |
Goreau, T., McClanahan, T., Hayes, R., and Strong, A. (2000). Conservation of coral reefs after the 1998 global bleaching event. Conservation Biology 14, 5–15.
| Conservation of coral reefs after the 1998 global bleaching event.Crossref | GoogleScholarGoogle Scholar |
Graham, N. A. J., Wilson, S. K., Jennings, S., Polunin, N. V. C., Robinson, J., Bijoux, J. P., and Daw, T. M. (2007). Lag effects in the impacts of mass coral bleaching on coral reef fish, fisheries, and ecosystems. Conservation Biology 21, 1291–1300.
| Lag effects in the impacts of mass coral bleaching on coral reef fish, fisheries, and ecosystems.Crossref | GoogleScholarGoogle Scholar |
Graham, N. A. J., McClanahan, T. R., MacNeil, M. A., Wilson, S. K., Polunin, N. V. C., Jennings, S., Chabanet, P., Clark, S., Spalding, M. D., Letourneur, Y., Bigot, L., Galzin, R., Ohman, M. C., Garpe, K. C., Edwards, A. J., and Sheppard, C. R. C. (2008). Climate warming, marine protected areas and the ocean-scale integrity of coral reef ecosystems. PLoS ONE 3, e3039.
| Climate warming, marine protected areas and the ocean-scale integrity of coral reef ecosystems.Crossref | GoogleScholarGoogle Scholar |
Hafi, A., Thorpe, S., and Foster, A. (2010). The impact of climate change on the irrigated agricultural industries in the Murray–Darling Basin. In ‘ABARE Conference’. Paper 09.3. Australian Agricultural and Resource Economics Society, 11–13 February, Cairns, Qld.
Halford, A. R., and Caley, M. J. (2009). Towards an understanding of resilience in isolated coral reefs. Global Change Biology 15, 3031–3045.
| Towards an understanding of resilience in isolated coral reefs.Crossref | GoogleScholarGoogle Scholar |
Heino, J., Virkkala, R., and Toivonen, H. (2009). Climate change and freshwater biodiversity: detected patterns, future trends and adaptations in northern regions. Biological Reviews of the Cambridge Philosophical Society 84, 39–54.
| Climate change and freshwater biodiversity: detected patterns, future trends and adaptations in northern regions.Crossref | GoogleScholarGoogle Scholar |
Hobday, A. J., and Lough, J. (2011). Projected climate change in Australian marine and freshwater environments. Marine and Freshwater Research 62, 1000–1014.
| Projected climate change in Australian marine and freshwater environments.Crossref | GoogleScholarGoogle Scholar |
Hoegh-Guldberg, O. (1999). Climate change, coral bleaching and the future of the world’s coral reefs. Marine and Freshwater Research 50, 839–866.
| Climate change, coral bleaching and the future of the world’s coral reefs.Crossref | GoogleScholarGoogle Scholar |
Hoegh-Guldberg, O., and Bruno, J. F. (2010). The impact of climate change on the world’s marine ecosystems. Science 328, 1523–1528.
| The impact of climate change on the world’s marine ecosystems.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXnsVWnt7Y%3D&md5=a2e5b8b5397c5584684eafb902fb3e95CAS |
Hoegh-Guldberg, O., Mumby, P. J., Hooten, A. J., Steneck, R. S., and Greenfield, P. (2007). Coral reefs under rapid climate change and ocean acidification. Science 318, 1737–1742.
| Coral reefs under rapid climate change and ocean acidification.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXhsVWhu7fN&md5=b074c829a513c1a5f7a4dd1596f39ea3CAS |
Hoegh-Guldberg, O., Hughes, L., McIntyre, S., Lindenmayer, B. D., Parmesan, C., Possingham, H. P., and Thomas, C. D. (2008). Assisted colonization and rapid climate change. Science 321, 345–346.
| Assisted colonization and rapid climate change.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD1cvlvFWqsg%3D%3D&md5=2fd0e496e29dfd4ac47d0598881a15e6CAS |
Hoey, A. S., and Bellwood, D. R. (2008). Cross-shelf variation in the role of parrotfishes on the Great Barrier Reef. Coral Reefs 27, 37–47.
| Cross-shelf variation in the role of parrotfishes on the Great Barrier Reef.Crossref | GoogleScholarGoogle Scholar |
Houghton, J. T., Ding, Y., Griggs, D. J., Noguer, M., van der Linden, P. J., Dai, X., Maskell, K., and Johnson, C. A. (2001). ‘IPCC Third Assessment Report: Climate Change 2001.’ (Cambridge University Press: Cambridge, UK.)
Hughes, L. (2003). Climate change and Australia: trends, projections and impacts. Austral Ecology 28, 423–443.
| Climate change and Australia: trends, projections and impacts.Crossref | GoogleScholarGoogle Scholar |
Hughes, T. P., Baird, A. H., Bellwood, D. R., Card, M., Connolly, S. R., Folke, C., Grosberg, R., Hoegh-Guldberg, O., Jackson, J. B. C., Kleypas, J., Lough, J. M., Marshall, P., Nystrom, M., Palumbi, S. R., Pandolfi, J. M., Rosen, B., and Roughgarden, J. (2003). Climate change, human impacts, and the resilience of coral reefs. Science 301, 929–933.
| Climate change, human impacts, and the resilience of coral reefs.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXmt1elsb4%3D&md5=51509a1cdf062823fc9b4614ec8c4f8fCAS |
Hughes, T. P., Graham, N. A. J., Jackson, J. B. C., Mumby, P. J., and Steneck, R. S. (2010). Rising to the challenge of sustaining coral reef resilience. Trends in Ecology & Evolution 25, 633–642.
| Rising to the challenge of sustaining coral reef resilience.Crossref | GoogleScholarGoogle Scholar |
Ingram, B. A., Barlow, C. G., Burchmore, J. J., Gooley, G. J., Rowland, S. J., and Sanger, A. C. (1990). Threatened native freshwater fishes in Australia – some case histories. Journal of Fish Biology 37, 175–182.
| Threatened native freshwater fishes in Australia – some case histories.Crossref | GoogleScholarGoogle Scholar |
Jokiel, P. L., and Coles, S. L. (1990). Response of Hawaiian and other Indo-Pacific reef corals to elevated temperature. Coral Reefs 8, 155–162.
| Response of Hawaiian and other Indo-Pacific reef corals to elevated temperature.Crossref | GoogleScholarGoogle Scholar |
Jones, A., and Berkelmans, R. (2010). Potential costs of acclimatization to a warmer climate: growth of a reef coral with heat tolerant vs. sensitive symbiont types. PLoS ONE 5, e10437.
| Potential costs of acclimatization to a warmer climate: growth of a reef coral with heat tolerant vs. sensitive symbiont types.Crossref | GoogleScholarGoogle Scholar |
Jones, G. P., McCormick, M. I., Srinivasan, M., and Eagle, J. V. (2004). Coral decline threatens fish biodiversity in marine reserves. Proceedings of the National Academy of Sciences of the United States of America 101, 8251–8253.
| Coral decline threatens fish biodiversity in marine reserves.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXkslCitbw%3D&md5=3a1b1e419efbbede725edf981d66c044CAS |
Jones, A. M., Berkelmans, R., van Oppen, M. J. H., Mieog, J. C., and Sinclair, W. (2008). A community change in the algal endosymbionts of a scleractinian coral following a natural bleaching event: field evidence of acclimatization. Proceedings. Biological Sciences 275, 1359–1365.
| A community change in the algal endosymbionts of a scleractinian coral following a natural bleaching event: field evidence of acclimatization.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD1c3ptFChsg%3D%3D&md5=79a9a8d83f9d5c5e4ad165cd06b70fc1CAS |
Joseph, L. N., Maloney, R. F., and Possingham, H. P. (2009). Optimal allocation of resources among threatened species: a project prioritization protocol. Conservation Biology 23, 328–338.
| Optimal allocation of resources among threatened species: a project prioritization protocol.Crossref | GoogleScholarGoogle Scholar |
Kennard, M. J., Pusey, B. J., Olden, J. D., Mackay, S. J., Stein, J., and Marsh, N. (2010). Classification of natural flow regimes in Australia to support environmental flow management. Freshwater Biology 55, 171–193.
| Classification of natural flow regimes in Australia to support environmental flow management.Crossref | GoogleScholarGoogle Scholar |
Kerswell, A. P. (2006). Global biodiversity patterns of benthic marine algae. Ecology 87, 2479–2488.
| Global biodiversity patterns of benthic marine algae.Crossref | GoogleScholarGoogle Scholar |
Kingsford, R. T. (2000). Ecological impacts of dams, water diversions and river management on floodplain wetlands in Australia. Austral Ecology 25, 109–127.
| Ecological impacts of dams, water diversions and river management on floodplain wetlands in Australia.Crossref | GoogleScholarGoogle Scholar |
Kingsford, R. T. (2011). Conservation management of rivers and wetlands under climate change – a synthesis. Marine and Freshwater Research 62, 217–222.
| Conservation management of rivers and wetlands under climate change – a synthesis.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXjsVKksb0%3D&md5=f827938094a01b252709717279509058CAS |
Kirkman, H. (1997). Seagrasses of Australia. Australia state of the environment technical paper eeries (estuaries and the sea). Department of the Environment, Canberra.
Klotzbach, P. J. (2006). Trends in global tropical cyclone activity over the past twenty years (1986–2005). Geophysical Research Letters 33, L10805.
| Trends in global tropical cyclone activity over the past twenty years (1986–2005).Crossref | GoogleScholarGoogle Scholar |
Koehn, J. D. (2003). Riverine aquatic protected areas: protecting species, communities or ecosystem processes? In ‘Aquatic Protected Areas – What Works Best and How do we Know? Proceedings of the World Congress on Aquatic Protected Areas, Cairns, Qld, August 2002. (Eds J. P. Beumer, A. Grant and D.C. Smith.) pp. 614–624. (University of Queensland: St Lucia, Qld.)
Koehn, J. (2005). The loss of valuable Murray cod in fish kills: a science and management perspective. In ‘Management of Murray Cod in the Murray–Darling Basin: Statement, Recommendations and Supporting Papers’. Proceedings of a workshop held in Canberra, ACT, 3–4 June 2004. (Eds M. Lintermans and B. Phillips.) pp. 73–82. (Murray–Darling Basin Commission and Cooperative Research Centre for Freshwater Ecology: Canberra.)
Koehn, J. D., and O’Connor, W. G. (1990). Threats to Victorian freshwater fish. Victorian Naturalist 107, 5–12.
Kokita, T., and Nakazono, A. (2001). Rapid response of an obligately corallivorous filefish Oxymonacanthus longirostris (Monacanthidae) to a mass coral bleaching event. Coral Reefs 20, 155–158.
| Rapid response of an obligately corallivorous filefish Oxymonacanthus longirostris (Monacanthidae) to a mass coral bleaching event.Crossref | GoogleScholarGoogle Scholar |
Lake, P. S. (2003). Ecological effects of perturbation by drought in flowing waters. Freshwater Biology 48, 1161–1172.
| Ecological effects of perturbation by drought in flowing waters.Crossref | GoogleScholarGoogle Scholar |
Landsea, C. W., Harper, B. A., Hoarau, K., and Knaff, J. A. (2006). Can we detect trends in extreme tropical cyclones? Science 313, 452–454.
| Can we detect trends in extreme tropical cyclones?Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28Xns1agt7Y%3D&md5=7b280097c911a187bf2a5cc0bbfcde86CAS |
Larcombe, P., and Woolfe, K. J. (1999). Increased sediment supply to the Great Barrier Reef will not increase sediment accumulation at most coral reefs. Coral Reefs 18, 163–169.
| Increased sediment supply to the Great Barrier Reef will not increase sediment accumulation at most coral reefs.Crossref | GoogleScholarGoogle Scholar |
Levitus, S., Antonov, J. I., Boyer, T. P., and Stephens, C. (2000). Warming of the world ocean. Science 287, 2225–2229.
| Warming of the world ocean.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3cXitlSns7w%3D&md5=8fce1d2f0d98b586046a21ab011bd2dbCAS |
Ling, S. D., Johnson, C. R., Ridgway, K., Hobday, A. J., and Haddon, M. (2009). Climate-driven range extension of a sea urchin: inferring future trends by analysis of recent population dynamics. Global Change Biology 15, 719–731.
| Climate-driven range extension of a sea urchin: inferring future trends by analysis of recent population dynamics.Crossref | GoogleScholarGoogle Scholar |
Lintermans, M. (2007). ‘Fishes of the Murray–Darling Basin: An Introductory Guide.’ (Murray–Darling Basin Commission: Canberra.)
Lintermans, M., and Cottingham, P. (2007). Fish out of water – lessons for managing native fish during drought. Final report of the Drought Expert Panel. Murray–Darling Basin Commission, Canberra.
Lough, J. (2007). Climate and climate change on the Great Barrier Reef. In ‘Climate Change and the Great Barrier Reef: A Vulnerability Assessment’. (Eds J. E. Johnson and P. A. Marshall.) pp. 15–50. (Great Barrier Reef Marine Park Authority and the Australian Greenhouse Office: Townsville, Qld.)
Lough, J., Hobday, A. J., and Jones, D. (2011). Observed climate change in Australian marine and freshwater environments. Marine and Freshwater Research 62, 984–999.
| Observed climate change in Australian marine and freshwater environments.Crossref | GoogleScholarGoogle Scholar |
Lovelock, C. E., and Ellison, J. C. (2007). Vulnerability of mangroves and tidal wetlands of the Great Barrier Reef to climate change. In ‘Climate Change and the Great Barrier Reef: A Vulnerability Assessment’. (Eds J. E. Johnson and P. A. Marshall.) pp. 237–269. (Great Barrier Reef Marine Park Authority and Australian Greenhouse Office: Townsville, Qld.)
Loya, Y., Sakai, K., Yamazota, K., Nakano, Y., Sambali, H., and van Woesik, R. (2001). Coral bleaching: the winners and the losers. Ecology Letters 4, 122–131.
| Coral bleaching: the winners and the losers.Crossref | GoogleScholarGoogle Scholar |
Lyon, J. P., and O’Connor, J. P. (2008). Smoke on the water: Can riverine fish populations recover following a catastrophic fire-related sediment slug? Austral Ecology 33, 794–806.
Madin, J. S., and Connolly, S. R. (2006). Ecological consequences of major hydrological disturbances on coral reefs. Nature 444, 477–480.
| Ecological consequences of major hydrological disturbances on coral reefs.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28Xht1aku7zP&md5=04736d1b2272d19b660781f637a700f4CAS |
Maheshwari, B. L., Walker, K. F., and McMahon, T. A. (1995). Effects of regulation on the flow regime of the river Murray, Australia. Regulated Rivers: Research and Management 10, 15–38.
| Effects of regulation on the flow regime of the river Murray, Australia.Crossref | GoogleScholarGoogle Scholar |
Mann, K. H. (1973). Seaweeds: their productivity and strategy for growth. Science 182, 975–981.
| Seaweeds: their productivity and strategy for growth.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC3cvlsFymtg%3D%3D&md5=8f6fdd7f4ea22e76313f805555c15eedCAS |
Manson, F. J., Loneragan, N. R., Harch, B. D., Skilleter, G. A., and Williams, L. (2005). A broadscale analysis of links between coastal fisheries production and mangrove extent; a case study for northeastern Australia. Fisheries Research 74, 69–85.
| A broadscale analysis of links between coastal fisheries production and mangrove extent; a case study for northeastern Australia.Crossref | GoogleScholarGoogle Scholar |
Margules, C. R., Pressey, R. L., and Williams, P. H. (2002). Representing biodiversity: data and procedures for identifying priority areas for conservation. Journal of Biosciences 27, 309–326.
| Representing biodiversity: data and procedures for identifying priority areas for conservation.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD38vit1Sguw%3D%3D&md5=5609d98f2f6df971a444f0fd769a06cbCAS |
Marshall, P. A., and Johnson, J. E. (2007). The Great Barrier Reef and climate change: vulnerability and management implications. In ‘Climate Change and the Great Barrier Reef: A Vulnerability Assessment’. (Eds J. E. Johnson and P. A. Marshall.) pp. 773–801. (Great Barrier Reef Marine Park Authority and Australian Greenhouse Office: Townsville, Qld.)
Marshall, P. A., and Schuttenberg, H. (2006). ‘A Reef Manager’s Guide to Coral Bleaching.’ (Great Barrier Reef Marine Park Authority: Townsville, Qld.)
Maynard, J. A., Anthony, K. R. N., Marshall, P. A., and Masiri, I. (2008). Major bleaching events can lead to increased thermal tolerance in corals. Marine Biology 155, 173–182.
| Major bleaching events can lead to increased thermal tolerance in corals.Crossref | GoogleScholarGoogle Scholar |
McCulloch, M., Fallon, S., Wyndham, T., Hendy, E., Lough, J., and Barnes, D. (2003). Coral record of increased sediment flux to the inner Great Barrier Reef since European settlement. Nature 421, 727–730.
| Coral record of increased sediment flux to the inner Great Barrier Reef since European settlement.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXhsV2rsbg%3D&md5=402158e9653a3f30a3ec22ecca1558edCAS |
McKergow, L. A., Prosser, I. P., Weaver, D. M., Grayson, R. B., and Reed, A. E. G. (2006). Performance of grass and eucalyptus riparian buffers in a pasture catchment, Western Australia, part 2: water quality. Hydrological Processes 20, 2327–2346.
| Performance of grass and eucalyptus riparian buffers in a pasture catchment, Western Australia, part 2: water quality.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XotVWqsL0%3D&md5=7c6ad218a245d8afe44029b9088b91c4CAS |
McLeod, E., and Salm, R. V. (2006). ‘Managing Mangroves for Resilience to Climate Change.’ (IUCN: Gland, Switzerland.)
Meyer, J. L., Sale, M. J., Mulholland, P. J., and Poff, N. L. (1999). Impacts of climate change on aquatic ecosystem functioning and health. Journal of the American Water Resources Association 35, 1373–1386.
| Impacts of climate change on aquatic ecosystem functioning and health.Crossref | GoogleScholarGoogle Scholar |
Meyer, E., Davies, S., Wang, S., Willis, B. L., Abrego, D., Juenger, T. E., and Matz, M. V. (2009). Genetic variation in responses to a settlement cue and elevated temperature in the reef-building coral Acropora millepora. Marine Ecology Progress Series 392, 81–92.
| Genetic variation in responses to a settlement cue and elevated temperature in the reef-building coral Acropora millepora.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXhsFaiu7vO&md5=a65c88d12c804c11752bd5668b19c846CAS |
Meynecke, J.-O., Lee, S. Y., and Duke, N. C. (2008). Linking spatial metrics and fish catch reveals the importance of coastal wetland connectivity to inshore fisheries in Queensland, Australia. Biological Conservation 141, 981–996.
| Linking spatial metrics and fish catch reveals the importance of coastal wetland connectivity to inshore fisheries in Queensland, Australia.Crossref | GoogleScholarGoogle Scholar |
Michener, W. K., Blood, E. R., Bildstein, K. L., Brinson, M. M., and Gardner, L. R. (1997). Climate change, hurricanes and tropical storms, and rising sea level in coastal wetlands. Ecological Applications 7, 770–801.
| Climate change, hurricanes and tropical storms, and rising sea level in coastal wetlands.Crossref | GoogleScholarGoogle Scholar |
Morris, J. T., Sundareshwar, P. V., Nietch, C. T., Kjerfve, B., and Cahoon, D. R. (2002). Responses of coastal wetlands to rising sea level. Ecology 83, 2869–2877.
| Responses of coastal wetlands to rising sea level.Crossref | GoogleScholarGoogle Scholar |
Mulrennan, M. E., and Woodroffe, C. D. (1998). Saltwater intrusion into the coastal plains of the Lower Mary River, Northen Territory, Australia. Journal of Environmental Management 54, 169–188.
| Saltwater intrusion into the coastal plains of the Lower Mary River, Northen Territory, Australia.Crossref | GoogleScholarGoogle Scholar |
Mumby, P. J. (2006). Connectivity of reef fish between mangroves and coral reefs: algorithms for the design of marine reserves at seascape scales. Biological Conservation 128, 215–222.
| Connectivity of reef fish between mangroves and coral reefs: algorithms for the design of marine reserves at seascape scales.Crossref | GoogleScholarGoogle Scholar |
Mumby, P. J., Edwards, A. J., Arias-Gonzalez, J. E., Lindeman, K. C., Blackwell, P. G., Gall, A., Gorczynska, M. I., Harborne, A. R., Pescod, C. L., Renken, H., Wabnitz, C. C. C., and Llewellyn, G. (2004). Mangroves enhance the biomass of coral reef fish communities in the Caribbean. Nature 427, 533–536.
| Mangroves enhance the biomass of coral reef fish communities in the Caribbean.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXpsFWgtw%3D%3D&md5=4bf730e4308d8df8a948b78cbd147219CAS |
Munday, P. L. (2004). Habitat loss, resource specialisation, and extinction on coral reefs. Global Change Biology 10, 1642–1647.
| Habitat loss, resource specialisation, and extinction on coral reefs.Crossref | GoogleScholarGoogle Scholar |
Munday, P. L., Jones, G. P., Pratchett, M. S., and Williams, A. (2008). Climate change and the future for coral reef fishes. Fish and Fisheries 9, 261–285.
| Climate change and the future for coral reef fishes.Crossref | GoogleScholarGoogle Scholar |
Munday, P. L., Dixson, D. L., Donelson, J. M., Jones, G. P., Pratchett, M. S., Dvitsina, G. V., and Doving, K. B. (2009). Ocean acidification impairs olfactory discrimination and homing ability of a marine fish. Proceedings of the National Academy of Sciences of the United States of America 106, 1848–1852.
| Ocean acidification impairs olfactory discrimination and homing ability of a marine fish.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXitV2isrg%3D&md5=b393a4ea34b06c3afc4a75c959e7efeeCAS |
Murray–Darling Basin Commission (2004a). Native Fish Strategy for the Murray–Darling Basin 2003–2013. (Murray–Darling Basin Commission, Canberra.)
Murray–Darling Basin Commission (2004b). Fish theme pilot audit technical report – sustainable rivers audit. MDBC Publication 06/04. (Murray–Darling Basin Commission, Canberra.)
Nicholls, R. F., Hoozemans, F. M. J., and Marchand, M. (1999). Increasing flood risk and wetland losses due to global se-level rise: regional and global analyses. Global Environmental Change 9, S69–S87.
| Increasing flood risk and wetland losses due to global se-level rise: regional and global analyses.Crossref | GoogleScholarGoogle Scholar |
Nicol, S. J., Lieschke, J. A., Lyon, J. P., and Koehn, J. D. (2004). Observations on the distribution and abundance of carp and and native fish, and their reponses to a habitat restoration trial in the Murray River, Australia. New Zealand Journal of Marine and Freshwater Research 38, 541–551.
| Observations on the distribution and abundance of carp and and native fish, and their reponses to a habitat restoration trial in the Murray River, Australia.Crossref | GoogleScholarGoogle Scholar |
O’Reilly, C. M., Alin, S. R., Plisnier, P., Cohen, A. S., and McKee, B. A. (2003). Climate change decreases aquatic ecosystem productivity of Lake Tanganyika, Africa. Nature 424, 766–768.
| Climate change decreases aquatic ecosystem productivity of Lake Tanganyika, Africa.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXmt1antr8%3D&md5=51a9088ad674501b5eb3f3ce0309e4feCAS |
Obura, D. O. (2005). Resilience and climate change: lessons from coral reefs and bleaching in the Western Indian Ocean. Estuarine, Coastal and Shelf Science 63, 353–372.
| Resilience and climate change: lessons from coral reefs and bleaching in the Western Indian Ocean.Crossref | GoogleScholarGoogle Scholar |
Oliver J. 1985 Recurrent seasonal bleaching and the mortality of corals on the Great Barrier Reef. Proceedings of the Fifth International Coral Reef Congress 4 , 201–206
Oliver, J., Berkelmans, R., and Eakin, C. M. (2009). Coral bleaching in space and time. In ‘Coral Bleaching: Patterns and Processes, Causes and Consequences’. (Eds M. van Oppen and J. Lough.) pp. 21–40. (Springer-Verlag: Heidelberg, Germany.)
Olsson, P., Folke, C., and Hughes, T. P. (2008). Navigating the transition to ecosystem-based management of the Great Barrier Reef, Australia. Proceedings of the National Academy of Sciences of the United States of America 105, 9489–9494.
| Navigating the transition to ecosystem-based management of the Great Barrier Reef, Australia.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXovVOjsbw%3D&md5=9c791d59bd044e3cecd33a111a37a34aCAS |
Orr, J. C., Fabry, V. J., Aumont, O., Bopp, L., Doney, S. C., Feely, R. A., Gnanadesikan, A., Gruber, N., Ishida, A., Joos, F., Key, R. M., Lindsay, K., Maier-Reimer, E., Matear, R. J., Monfray, P., Mouchet, A., Najjar, R. G., Plattner, G.-K., Rodgers, K. B., Sabine, C. L., Sarmiento, J. L., Schlitzer, R., Slater, R. D., Totterdell, I. J., Weirig, M.-F., Yamanaka, Y., and Yool, A. (2005). Anthropogenic ocean acidification over the twenty-first century and its impact on calcifying organisms. Nature 437, 681–686.
| Anthropogenic ocean acidification over the twenty-first century and its impact on calcifying organisms.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXhtVCjsL%2FE&md5=9390bc0fada8743ec20f80a7ffe47e99CAS |
Orth, R. J., Carruthers, T. J. B., Dennison, W. C., Duarte, C. M., Fourqurean, J. W., Heck, K. L., Hughes, A. R., Kendrick, G. A., Kenworthy, W. J., Olyarnik, S., Short, F. T., Waycott, M., and Williams, S. L. (2006). A global crisis of seagrass ecosystems. Bioscience 56, 987–996.
| A global crisis of seagrass ecosystems.Crossref | GoogleScholarGoogle Scholar |
Osborne, K., Dolman, A. M., Burgess, S. C., and Johns, K. A. (2011). Disturbance and the dynamics of coral cover on the Great Barrier Reef (1995–2009). PLoS ONE 6, e17516.
| Disturbance and the dynamics of coral cover on the Great Barrier Reef (1995–2009).Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXjslKmurY%3D&md5=4e2ad6cf75812c0de9fd09b77b2a9d51CAS |
Osmond, M., Airame, S., Caldwell, M., and Day, J. (2010). Lessons for marine conservation planning: A comparison of three marine protected area planning processes. Ocean and Coastal Management 53, 41–51.
Oxford Economics (2009). ‘Valuing the Effects of the Great Barrier Reef Bleaching.’ (Great Barrier Reef Foundation: Newstead, Qld.)
Pandolfi, J. M., Bradbury, R. H., Sala, E., Hughes, T. P., Bjorndal, K. A., Cooke, R. G., McArdle, D., McClenachan, L., Newman, M. J. H., Parades, G., Warner, R. R., and Jackson, J. B. C. (2003). Global trajectories of the long-term decline of coral reef ecosystems. Science 301, 955–958.
| Global trajectories of the long-term decline of coral reef ecosystems.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXmt1elsLw%3D&md5=773db5266e939527174dfbc9263e0c68CAS |
Parmesan, C. (2007). Influences of species, latitudes and methodologies on estimates of phenological response to global warming. Global Change Biology 13, 1860–1872.
| Influences of species, latitudes and methodologies on estimates of phenological response to global warming.Crossref | GoogleScholarGoogle Scholar |
Parmesan, C., and Yohe, G. (2003). A globally coherent footprint of climate change impacts across natural systems. Nature 421, 37–42.
| A globally coherent footprint of climate change impacts across natural systems.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXoslM%3D&md5=881f3bd5bf4de5383d00e613bb3bd47fCAS |
Perry, A. L., Low, P. J., Ellis, J. R., and Reynolds, J. D. (2005). Climate change and distribution shifts in marine fishes. Science 308, 1912–1915.
| Climate change and distribution shifts in marine fishes.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXlsVWmtbg%3D&md5=b99cf337ddaccb32659fd2b7ab3c4576CAS |
Pitt, N. R., Poloczanska, E. S., and Hobday, A. J. (2010). Climate-driven range changes in Tasmanian intertidal fauna. Marine and Freshwater Research 61, 963–970.
| Climate-driven range changes in Tasmanian intertidal fauna.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXht1Snt7nO&md5=32e05d9dd0bf87c8ad1bf412ba6f57bbCAS |
Pollard, D. A., Ingrah, B. A., Harris, J. H., and Reynolds, L. F. (1990). Threatened fishes in Australia – an overview. Journal of Fish Biology 37, 67–78.
| Threatened fishes in Australia – an overview.Crossref | GoogleScholarGoogle Scholar |
Poloczanska, E. S., Babcock, R. C., Butler, A., Hobday, A. J., Hoegh-Guldberg, O., Kunz, T. J., Matear, R., Milton, D., Okey, T. A., and Richardson, A. J. (2007). Climate change and Australian marine life. Oceanography and Marine Biology: An Annual Review 45, 409–480.
Pratchett, M. S., Wilson, S. K., Berumen, M. L., and McCormick, M. I. (2004). Sub-lethal effects of coral bleaching on an obligate coral feeding butterflyfish. Coral Reefs 23, 352–356.
| Sub-lethal effects of coral bleaching on an obligate coral feeding butterflyfish.Crossref | GoogleScholarGoogle Scholar |
Pratchett, M. S., Munday, P. L., Wilson, S. K., Graham, N. A. J., Cinner, J. E., Bellwood, D. R., Jones, G. P., Polunin, N. V. C., and McClanahan, T. R. (2008). Effects of climate-induced coral bleaching on coral-reef fishes: ecological and economic consequences. Oceanography and Marine Biology: An Annual Review 46, 251–296.
| Effects of climate-induced coral bleaching on coral-reef fishes: ecological and economic consequences.Crossref | GoogleScholarGoogle Scholar |
Pratchett, M. S., Baird, A. H., McCowan, D. M., Coker, D. J., Cole, A. J., and Wilson, S. K. (2009a). Protracted declines in coral cover and fish abundance following climate-induced coral bleaching on the Great Barrier Reef. In ‘Proceedings of the 11th International Coral Reef Symposium’. (Ed B. Riegl) Ft Lauderdale, Florida. pp. 1042–1046. (Ft Lauderdale)
Pratchett, M. S., Wilson, S. K., Graham, N. A. J., Munday, M. S., Jones, G. P., and Polunin, N. V. C. (2009b). Multi-scale temporal effects of climate-induced coral bleaching on motile reef organisms. In ‘Coral Bleaching: Patterns and Processes, Causes and Consequences’. (Eds M. van Oppen and J. Lough.) pp. 139–158. (Springer-Verlag: Heidelberg, Germany.)
Pressey, R. L., and Middleton, M. J. (1982). Impacts of flood mitigation works on coastal wetlands in New South Wales. Wetlands (Australia) 2, 27–44.
Pressey, R. L., Cabeza, M., Watts, M. E., Cowling, R. M., and Wilson, K. A. (2007). Conservation planning in a changing world. Trends in Ecology & Evolution 22, 583–592.
| Conservation planning in a changing world.Crossref | GoogleScholarGoogle Scholar |
Rayne, S., Henderson, G., Gill, P., and Forest, K. (2008). Riparian forest harvesting effects on maximum water temperatures in wetland-sourced headwater streams from the Nicola River Watershed, British Columbia, Canada. Water Resources Management 22, 565–578.
| Riparian forest harvesting effects on maximum water temperatures in wetland-sourced headwater streams from the Nicola River Watershed, British Columbia, Canada.Crossref | GoogleScholarGoogle Scholar |
Reigl, B., and Purkis, S. (2009). Spatial and temporal dynamics of Arabian Gulf coral assemblages quantified from remote-sensing and in situ monitoring data. Marine Ecology Progress Series 287, 99–113.
Richardson, A. J., and Poloczanska, E. S. (2008). Ocean science: under-resourced and under threat. Science 320, 1294–1295.
| Ocean science: under-resourced and under threat.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXnt1yrsr4%3D&md5=ff4a7c1b419f6bbca5186134885fd5d9CAS |
Roessig, J. M., Woodley, C. M., Cech, J. J., and Hansen, L. J. (2004). Effects of global climate change on marine and estuarine fishes and fisheries. Reviews in Fish Biology and Fisheries 14, 251–275.
| Effects of global climate change on marine and estuarine fishes and fisheries.Crossref | GoogleScholarGoogle Scholar |
Rogers, K., and Saintilan, N. (2002). Remapping of SEPP 14 wetlands in the Shoalhaven District. Wetlands Australia 20, 55–65.
Rosenzweig, C., Casassa, G., Karoly, D. J., Imeson, A., Liu, C., Menzel, A., Rawlins, S., Root, T. L., Seguin, B., and Tryjanowski, P. (2007). Assessment of observed changes and responses in natural and managed systems. In ‘Climate Change 2007: Impacts, Adaptation and Vulnerability. Contribution of Working Group II to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change’. (Eds M. L. Parry, O. F. Canziani, J. P. Palutikof, P. J. van der Linden and C. E. Hanson.) pp. 79–131. (Cambridge University Press: Cambridge, UK.)
Saintilan, N., and Williams, R. J. (1999). Mangrove transgression in to saltmarsh environments in south-east Australia. Global Ecology and Biogeography 8, 117–124.
| Mangrove transgression in to saltmarsh environments in south-east Australia.Crossref | GoogleScholarGoogle Scholar |
Schindler, D. W. (2001). The cumulative effects of climate warming and other human stresses on Canadian freshwaters in the new millennium. Canadian Journal of Fisheries and Aquatic Sciences 58, 18–29.
| The cumulative effects of climate warming and other human stresses on Canadian freshwaters in the new millennium.Crossref | GoogleScholarGoogle Scholar |
Sheppard, C. R. C., Spalding, S., Bradshaw, C., and Wilson, S. (2002). Erosion vs. recovery of coral reefs after 1998 El Niño: Chagos reefs, Indian Ocean. Ambio 31, 40–48.
Short, F. T., and Neckles, H. A. (1999). The effects of global climate changes on seagrasses. Aquatic Botany 63, 169–196.
| The effects of global climate changes on seagrasses.Crossref | GoogleScholarGoogle Scholar |
Skilleter, G. A., Olds, A., Loneragan, N. R., and Zharikov, Y. (2005). The value of patches of intertidal seagrass to prawns depends on their proximity to mangroves. Marine Biology 147, 353–365.
| The value of patches of intertidal seagrass to prawns depends on their proximity to mangroves.Crossref | GoogleScholarGoogle Scholar |
Smith, L. D., Gilmour, J. P., and Heyward, A. J. (2008). Resilience of coral communities on an isolated system of reefs following catastrophic mass-bleaching. Coral Reefs 27, 197–205.
| Resilience of coral communities on an isolated system of reefs following catastrophic mass-bleaching.Crossref | GoogleScholarGoogle Scholar |
Steneck, R. S., Graham, M. H., Bourque, B. J., Corbett, D., Erlandson, J. M., Estes, J. A., and Tegner, M. J. (2002). Kelp forest ecosystems: biodiversity, stability, resilience and future. Environmental Conservation 29, 436–459.
| Kelp forest ecosystems: biodiversity, stability, resilience and future.Crossref | GoogleScholarGoogle Scholar |
Stoms, D. M., Davis, F. W., Andelman, S. J., Carr, M. H., Gaines, S. D., Halpern, B. S., Hoenicke, R., Leibowitz, S. G., Leydecker, A., Madin, E. M. P., Tallis, H., and Warner, R. R. (2005). Integrated coastal reserve planning: making the land-sea connection. Frontiers in Ecology and the Environment 3, 429–436.
Suzuki, T., Kuma, K., Kudo, I., and Matsunaga, K. (1995). Iron requirement of the brown macroalgae Laminaria japonica, Undaria pinnatifida and the crustose coralline algae, and their competition in the northern Japan Sea. Phycologia 34, 201–205.
| Iron requirement of the brown macroalgae Laminaria japonica, Undaria pinnatifida and the crustose coralline algae, and their competition in the northern Japan Sea.Crossref | GoogleScholarGoogle Scholar |
Swales, S., Storey, A. W., Roderick, I. D., and Figa, B. S. (1999). Fishes of floodplain habitats of the Fly River system, Papua New Guinea, and changes associated with El Nino droughts and algal blooms. Environmental Biology of Fishes 54, 389–404.
| Fishes of floodplain habitats of the Fly River system, Papua New Guinea, and changes associated with El Nino droughts and algal blooms.Crossref | GoogleScholarGoogle Scholar |
Sweatman, H. P. A., Thompson, A., Delean, S., Davidson, J., and Neale, S. (2007). Status of near-shore reefs of the Great Barrier Reef 2004. Marine and Tropical Sciences Research Facility Research Report Series. (Reef and Rainforest Research Centre Limited, Cairns, Qld.)
Sweatman, H. P. A., Cheal, A. J., Coleman, G. J., Emslie, M. J., Johns, K., Jonker, M., Miller, I., and Osborne, K. (2008). Long-term monitoring of the Great Barrier Reef, Status Report. 8. Australian Institute of Marine Science, Townsville, Qld.
Thorp, J. H., Thoms, M. C., and Delong, M. D. (2006). The riverine ecosystem synthesis: biocomplexity in river networks across space and time. River Research and Applications 22, 123–147.
| The riverine ecosystem synthesis: biocomplexity in river networks across space and time.Crossref | GoogleScholarGoogle Scholar |
Thresher, R., Proctor, C., Ruiz, G., MacKinnon, C., Walton, W., Rodriguez, L., and Bax, N. (2003). Invasion dynamics of the European shore crab, Carcinus maenas, in Australia. Marine Biology 142, 867–876.
Twilley, R. R. (1995). Properties of mangrove ecosystems related to the energy signature of coastal environments. In ‘Maximum Power: The Ideas and Applications of H. T. Odum’. (Ed. C. A. S. Hall.) pp. 43–62. (University Press of Colorado: Niwot, CO.)
Unmack, P. J. (2001). Biogeography of Australian freshwater fishes. Journal of Biogeography 28, 1053–1089.
| Biogeography of Australian freshwater fishes.Crossref | GoogleScholarGoogle Scholar |
Valiela, I., Bowen, J. L., and York, J. K. (2001). Mangrove forests: one of the world's threatened major tropical environments. Bioscience 51, 807–815.
| Mangrove forests: one of the world's threatened major tropical environments.Crossref | GoogleScholarGoogle Scholar |
Valiela, I., Kinney, E., Bulbertson, J., Peacock, E., and Smith, S. (2009). Global losses of mangroves and salt marshes. In ‘Global Loss of Coastal Habitats: Rates, Causes and Consequences’. (Ed. C. M. Duarte.) pp. 107–138. (Fundación BBVA: Bilbao)
Van Der Kraak, G., and Pankhurst, N. W. (1997). Temperature effects on the reproductive performance of fish. In ‘Global Warming: Implications for Freshwater and Marine Fish’. (Eds C. M. Wood and D. G. McDonald.) pp. 159–176. (Cambridge University Press: Cambridge, UK.)
Van Dijk, A., Evans, R., Hairsine, P., Khan, S., Nathan, R., Paydar, Z., Viney, N., and Zhang, L. (2006). ‘Risks to the Shared Water Resources of the Murray–Darling Basin.’ (Murray-Darling Basin Commission: Canberra.)
Wachenfeld, D. R. (1997) Long-term trends in the status of coral reef-flat benthos – the use of historical photographs. In ‘State of the Great Barrier Reef World Heritage Area Workshop: GBRMPA Workshop Series 23’. (Eds D. Wachenfeld, J., Oliver and K. Davis.) pp. 134–148. (Great Barrier Reef Marine Park Authority: Townsville, Qld.)
Wachenfeld, D., Johnson, J., Skeat, A., Kenchington, R., Marshall, P., and Innes, J. (2007). Introduction to the Great Barrier Reef and climate change. In ‘Climate Change and the Great Barrier Reef: A Vulnerability Assessment’. (Eds J. E Johnson and P. A. Marshall.) pp. 1–13. (Great Barrier Reef Marine Park Authority and Australian Greenhouse Office: Townsville, Qld.)
Walker, K. F. (1985). A review of the ecological effects of river regulation in Australia. Hydrobiologia 125, 111–129.
| A review of the ecological effects of river regulation in Australia.Crossref | GoogleScholarGoogle Scholar |
Walker, K. F. (1992) The River Murray, Australia: a semiarid low-land river. In ‘The Rivers Handbook. Vol. 1’. (Eds P. Calow and G. E. Petts.) pp. 472–492. (Blackwell Scientific Publications: Oxford, UK.)
Walker, K. F., Sheldon, F., and Puckridge, J. T. (1995). A perspective on dryland river ecosystems. Regulated Rivers: Research and Management 11, 85–104.
| A perspective on dryland river ecosystems.Crossref | GoogleScholarGoogle Scholar |
Walther, G., Post, E., Convey, P., Menzel, A., Parmesan, C., Beebee, T. J. C., Fromentin, J., Hoegh-Guldberg, O., and Bairlein, F. (2002). Ecological response to recent climate change. Nature 416, 389–395.
| Ecological response to recent climate change.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38XislantL8%3D&md5=585bf263e6cdaf7f555072db8bf3257dCAS |
Waycott, M., Collier, C., McMahon, K., Ralph, P., McKenzie, L., Udy, J., and Grech, A. (2007). Vulnerability of seagrasses in the Great Barrier Reef to climate change. In ‘Climate Change and the Great Barrier Reef: A Vulnerability Assessment’. (Eds J. E. Johnson and P. A. Marshall.) pp. 193–235. (Great Barrier Reef Marine Park Authority and Australian Greenhouse Office: Townsville, Qld.)
Waycott, M., Duarte, C. M., Carruthers, T. J. B., Orth, R. J., Dennison, W. C., Olyarnik, S., Calladine, A., Fourqurean, J. W., Heck, K. L., Hughes, A. R., Kendrick, G. A., Kenworthy, W. J., Short, F. T., and Williams, S. L. (2009). Accelerating loss of seagrasses across the globe threatens coastal ecosystems. Proceedings of the National Academy of Sciences of the United States of America 106, 12 377–12 381.
| Accelerating loss of seagrasses across the globe threatens coastal ecosystems.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXpslGjsbo%3D&md5=34dbad507adea00cecbd4b5134a0e888CAS |
Webster, P. J., Holland, G. J., Curry, J. A., and Chang, H. R. (2005). Changes in tropical cyclone number and intensity in a warming environment. Science 309, 1844–1846.
| Changes in tropical cyclone number and intensity in a warming environment.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXpvFCisLY%3D&md5=f82efb4bd2d817dc681c1c46b0be03fbCAS |
Weeks, S. J., Anthony, K. R. N., Bakun, A., Feldman, G. C., and Hoegh-Guldberg, O. (2008). Improved predictions of coral bleaching using seasonal baselines and higher spatial resolution. Limnology and Oceanography 53, 1369–1375.
| Improved predictions of coral bleaching using seasonal baselines and higher spatial resolution.Crossref | GoogleScholarGoogle Scholar |
Wernberg, T., Campbell, A., Coleman, M. A., Connell, S. D., Kendrick, G. A., Moore, P. J., Russell, B. D., Smale, D. A., and Steinberg, P. D. (2009). Macroalgae and temperate rocky reefs. In ‘Report Card of Marine Climate Change for Australia; Detailed Scientific Assessment’. (Eds E. S. Poloczanska, A. J., Hobday and A. J. Richardson.) pp. 128–146. (NCCARF Publication: Southport, Qld)
West, J. M., and Salm, R. V. (2003). Resistance and resilience to coral bleaching: implications for coral reef conservation and management. Conservation Biology 17, 956–967.
| Resistance and resilience to coral bleaching: implications for coral reef conservation and management.Crossref | GoogleScholarGoogle Scholar |
Wilkinson, C. (1999). Global and local threats to coral reef functioning and existence: review and predictions. Marine and Freshwater Research 50, 867–878.
| Global and local threats to coral reef functioning and existence: review and predictions.Crossref | GoogleScholarGoogle Scholar |
Wilkinson, C. R. (2000). World-wide coral reef bleaching and mortality during 1998: a global climate change warning for the new millennium? In ‘Seas at the Millennium: An Environmental Evaluation. 3. Global Issues and Processes’. (Ed. C. R. C. Sheppard.) pp. 43–57. (Elsevier Science: Amsterdam.)
Wilkinson, C. (2004). ‘Status of Coral Reefs of the World: 2004.’ (Australian Institute of Marine Science: Townsville, Qld.)
Wilson, S. K., Graham, N. A. J., Pratchett, M. S., Jones, G. P., and Polunin, N. V. C. (2006). Multiple disturbances and the global degradation of coral reefs: are reef fishes at risk or resilient? Global Change Biology 12, 2220–2234.
| Multiple disturbances and the global degradation of coral reefs: are reef fishes at risk or resilient?Crossref | GoogleScholarGoogle Scholar |
Wismer, S., Hoey, A. S., and Bellwood, D. R. (2009). Cross-shelf benthic community structure on the Great Barrier Reef: relationships between macroalgal cover and herbivore biomass. Marine Ecology Progress Series 376, 45–54.
| Cross-shelf benthic community structure on the Great Barrier Reef: relationships between macroalgal cover and herbivore biomass.Crossref | GoogleScholarGoogle Scholar |
Woodroffe, C. D. (1990). The impacts of sea-level rise on mangrove shorelines. Progress in Physical Geography 14, 483–520.
| The impacts of sea-level rise on mangrove shorelines.Crossref | GoogleScholarGoogle Scholar |
Wooldridge, S. A. (2009). Water quality and coral bleaching thresholds: formalising the linkage for the inshore reefs of the Great Barrier Reef, Australia. Marine Pollution Bulletin 58, 745–751.
| Water quality and coral bleaching thresholds: formalising the linkage for the inshore reefs of the Great Barrier Reef, Australia.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXltFWgtL4%3D&md5=354b158ca6baf31b16f1636cd4dc5f4bCAS |
Wooldridge, S. A., and Done, T. J. (2009). Improved water quality can ameliorate effects of climate change on corals. Ecological Applications 19, 1492–1499.
| Improved water quality can ameliorate effects of climate change on corals.Crossref | GoogleScholarGoogle Scholar |
Worm, B., Barbier, E. B., Beaumont, N., Duffy, J. E., Folke, C., Halpern, B. S., Jackson, J. B. C., Lotze, H. K., Micheli, F., Palumbi, S. R., Sala, E., Selkoe, K. A., Stachowicz, J. J., and Watson, R. (2006). Impacts of biodiversity loss on ocean ecosystem services. Science 314, 787–790.
| Impacts of biodiversity loss on ocean ecosystem services.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XhtFKit73J&md5=4106f1f8a48fe17715cc2eba3ad22778CAS |
Xenopoulos, M. A., Lodge, D. M., Alcamo, J., Märker, M., Shulze, K., and Van Vuuren, D. P. (2005). Scenarios of freshwater fish extinctions from climate change and water withdrawl. Global Change Biology 11, 1557–1564.
| Scenarios of freshwater fish extinctions from climate change and water withdrawl.Crossref | GoogleScholarGoogle Scholar |