Image-enhanced burnt otoliths, bomb radiocarbon and the growth dynamics of redfish (Sebastes mentella and S. fasciatus) off the eastern coast of Canada
Steven E. Campana A D , Alexandra E. Valentin B , Shayne E. MacLellan C and Joanne B. Groot CA Bedford Institute of Oceanography, Fisheries and Oceans Canada, PO Box 1006, Dartmouth, NS, B2Y 4A2, Canada.
B Institut Maurice-Lamontagne, Fisheries and Oceans Canada, 850 route la Mer, Mont-Joli, QC, G5H 3Z4, Canada.
C Pacific Biological Station, Fisheries and Oceans Canada, 3190 Hammond Bay Road, Nanaimo, BC, V9T 6N7, Canada.
D Corresponding author. Present address: Life and Environmental Sciences, University of Iceland, IS-101 Reykjavik, Iceland. Email: steven.e.campana@gmail.com
Marine and Freshwater Research 67(7) 925-936 https://doi.org/10.1071/MF15002
Submitted: 3 January 2015 Accepted: 11 June 2015 Published: 22 October 2015
Journal Compilation © CSIRO Publishing 2016 Open Access CC BY-NC-ND
Abstract
Many past attempts to age deep-water redfish (Sebastes mentella) and Acadian redfish (S. fasciatus) in the north-west Atlantic have been stymied by inappropriate ageing methods, the absence of age validation and the failure to differentiate among species. Herein we report substantial improvements in methods for ageing Sebastes spp. by linking the established ‘crack and burn’ method to modern sectioning and image-enhancement protocols. Bomb radiocarbon assays of the otolith core and monitoring of year-class progression confirmed the accuracy of the resulting age determinations to an age of 46 years. The use of microsatellite DNA to confirm species identity eliminated past confusion caused by species mixtures. Age determinations of 1252 redfish from the eastern coast of Canada demonstrated the presence of significant differences in growth rate and longevity both between the two redfish species and among populations and stocks, with a maximum observed longevity of 70 years. Even within species and stocks, an individual fish with a fork length of 38 cm could be anywhere between 15 and 50 years of age, highlighting a near cessation of somatic growth after sexual maturation. In keeping with other deep-water species, sustainable management will require more attention to the low productivity expected of redfish stocks, rather than the high initial biomass that can support short-term but high catch rates.
Additional keywords: age validation, annuli, break and burn, 14C, false check.
Introduction
Although most deep-water fisheries have a shorter history of exploitation than those in shallower waters, their record shows a disproportionately high level of overexploitation (Roberts 2002). It has been recognised only recently that deep-sea fishes tend to be slow growing, long lived and characterised by a late age of sexual maturation, making them particularly sensitive to overfishing and slow to recover from depletion (Clarke et al. 2003). Some high-profile examples of serious overfishing have occurred, at least in part, because of overly optimistic impressions of growth and productivity brought on by age underestimation. For example, the orange roughy (Hoplostethus atlanticus) off New Zealand was fished intensively on the basis of a presumed longevity of 20–30 years (Tracey and Horn 1999). By the time it was realised that the species grew extremely slowly and could live to an age of more than 100 years (Smith et al. 1995; Andrews et al. 2009), the damage had been done and many populations had already been fished almost to the point of collapse. It is possible that roundnose grenadier (Coryphaenoides rupestris) in the north-west Atlantic will never recover from its fishery, in part because of slow growth rates and longevities of 60 years, which are much greater than previously suspected (Clarke et al. 2003). Indeed, longevities exceeding 100 years become increasingly frequent as habitat depth increases (Cailliet et al. 2001; Roberts 2002), an observation that would have been ridiculed some 30 years ago (Campana 2001).
The speciose genus Sebastes supports commercial fisheries in both the Pacific and Atlantic oceans, where they are known as rockfish and redfish respectively. Many of the species are deep-water species and some are now known to reach ages of over 75 (in the Atlantic) and 200 years (in the Pacific) (Campana et al. 1990; Munk 2001), making them less capable of supporting an intensive fishery. Indeed, overexploitation leading to fisheries closures has already occurred in some areas (Department of Fisheries and Oceans Canada, DFO 2012). The problem has been exacerbated by difficulties in age determination and controversy over the appropriate ageing method (Campana et al. 1990; Nedreaas 1990; Stransky et al. 2005a). Early attempts at redfish age determination using scales (Perlmutter and Clarke 1949) persisted until recently in some regions of the north-east Atlantic, but appear to grossly underestimate the ages of older fish (Nedreaas 1990). Most redfish are now aged using either the ‘crack and burn’ (C&B) method or with otolith thin sections, two methods that appear to give roughly comparable results (Stransky et al. 2005b). The accuracy of the C&B method has been validated in two independent radiometric studies (Campana et al. 1990; Stransky et al. 2005a). Nevertheless, growth parameters reported in different studies have varied widely, in part because of the difficulty of distinguishing among the three redfish species Sebastes marinus, S. mentella and S. fasciatus (Saborido-Rey et al. 2004). As a result, it has been difficult to know whether there are real differences in growth trajectories among regions and species, or whether the reported differences are due to differences in otolith interpretation or errors in species identification.
The objectives of the present study were to: (1) improve the accuracy and precision of existing methods of redfish age determination and age validation; (2) remove uncertainty in species identification by using microsatellite DNA to identify individual fish; and (3) apply standardised ageing methods and interpretation criteria to large sample sizes of S. mentella and S. fasciatus across a broad region of the north-west Atlantic in order to test current perceptions of redfish growth.
Material and methods
Samples of redfish were collected with otter trawls aboard research vessels in 2001 and 2002, mostly during routine annual groundfish surveys. Specimens were sampled from large aggregations of redfish (i.e. >100 kg by set) in the Gulf of St Lawrence and along the Laurentian Channel (North Atlantic Fisheries Organization (NAFO) Units 1 and 2), on the southern slope of the Grand Banks (NAFO 3O), the eastern Grand Banks (NAFO 3LN) and in the Labrador Sea (NAFO 2J3K). Individuals were also sampled in the Saguenay fjord (far western Gulf of St Lawrence) using handlines during ice-fishing in winter 2003, as well as in the east arm of the Bonne Bay fjord (west coast of Newfoundland) in spring 2002 (Fig. 1). The specimens were frozen immediately after capture. After thawing in the laboratory, fork length, sex and maturity were recorded, and a piece of muscle tissue was preserved in 95% ethanol awaiting genetic analyses. The sagittal otolith pair was then removed from each fish and stored dry in paper envelopes until the time of processing for age determination. Additional otoliths (stored dry in paper envelopes) were sampled from the archived otolith collection housed at the Northwest Fisheries Science Centre in St John’s (Newfoundland, Canada). These additional otoliths belonged to redfish sampled during routine multispecies trawl surveys conducted in 2011 in the Labrador Sea and for which fork length, sex and maturity were recorded at sea. In all cases, the species identity of individual fish was unknown at the time of collection, but was subsequently assigned on the basis of the genotype at 13 microsatellite loci. DNA extraction and microsatellite amplification were performed as described by Valentin et al. (2014). DNA was extracted from the muscle tissue preserved in ethanol or, for the 2011 sample, from the material adhering to the archived otoliths. The extraction procedure was slightly modified for the otolith samples, adding a step where the extracted DNA (eluted in a volume of 400 µL) was concentrated to 50 µL using a Microcon centrifugal filter YM-100 (EMD Millipore, Billerica, MA, USA) and the standard Microcon protocol. Specimens with an incomplete genotype at more than three loci were discarded from the database. After species identification was completed, individuals were assigned to population based on genotype (Valentin et al. 2014).
Samples of Unit 3 (see Fig. 1) redfish were collected in the summer of 2013 as part of routine annual groundfish surveys of the Scotian Shelf. Otoliths were collected as described above, but muscle tissue was not collected for subsequent genetic identification. Rather, it was assumed that most or all of the samples were from S. fasciatus based on previous genetic and meristic surveys of the region (Roques et al. 2001; Valentin et al. 2006, 2014).
In order to compare recent growth rates with those collected and aged several decades earlier, historic samples of what were probably S. mentella (based on depth of collection) were collected from Unit 2 in 1982 and 1986, and then aged using traditional C&B methods for use in another study (Campana et al. 1990). Those lengths at age were then compared with those of other samples from Unit 2, collected and aged in 2002.
Protocol for age determination
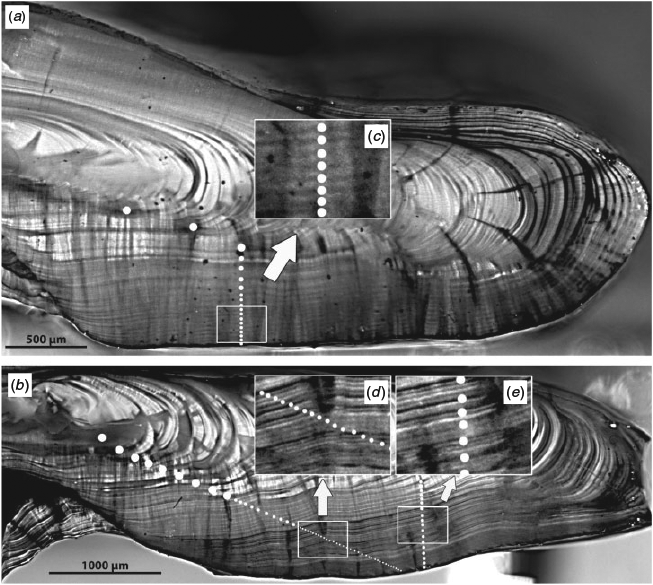
One sagittal otolith from each fish was cut transversely through the core (perpendicular to the longest axis of the otolith) with a single blade of an Isomet saw so as to ensure a flat, smooth surface for the subsequent burning (Charles et al. 2013). The otolith was not embedded in epoxy, but was firmly pressed into plasticine (modelling clay) on the IsoMet chuck (Buehler World Wide Headquarters, Lake Bluff, IL, USA) during sectioning. To ensure that the saw blade was precisely aligned with the otolith core, the blade was first lowered so as to press lightly into the putty on the saw chuck, leaving an impression against which the pencil-marked otolith core could be aligned when the otolith was pressed into the putty. Cuts were made at slow–medium speed with relatively little weight on the cutting arm to avoid breakage. Using forceps, the cut surface of the otolith half that best showed the core and annuli was held perpendicular to (and over) the flame of an alcohol burner for 2–10 s, or until the surface was chestnut brown in colour. The sulcus side was left facing up so as to avoid excessive charring along the sulcus edge. Otoliths with a particularly thin dorsal edge were tilted with the edge up and away from the flame. After cooling, the charred otolith half was pressed into putty such that the cut surface was exactly parallel to the surface of the microscope lens and thus parfocal across the otolith surface. After coating the cut surface with a light layer of mineral oil, the surface was imaged with a digital video camera (Olympus DP-72, see http://www.olympus-ims.com/en/microscope/dp72/) working at a 14-bit colour depth per channel through a dissecting microscope under reflected light at a resolution of 4000 × 3000 pixels. The resulting image was then digitally enhanced with Adobe Photoshop CS2 (see http://www.adobe.com/products/photoshop.html) through grey level expansion to fill the entire grey level space with the levels spanning that of the otolith, followed by an unsharp mask filter using a radius of 10–20 pixels and amounts of 100%–200%. Two images were captured: one using a microscope objective magnification of ~13× to view the entire cut surface and a second zoomed into the dorsal region between the core and the proximal surface where older growth bands were most likely to form. Images were captured within 1 h of burning, before the burn clarity had decreased.
Comparison between ageing methods
In order to determine the optimal method for otolith interpretation, a matched pair comparison was made between ageing methods, whereby one sagittal otolith from each fish was cut transversely through the core with a single blade of an Isomet saw. One-half of each otolith was subsequently sent to a laboratory (Pacific Biological Station (PBS), Nanaimo, BC, Canada) with expertise in ‘break and burn’ ageing (MacLellan 1997) for burning and ageing under reflected light, whereas the remaining unburnt half of the same otolith was imaged under reflected light and aged by a second laboratory (Bedford Institute of Oceanography (BIO), Dartmouth, NS, Canada) with expertise in image enhancement. Therefore, each otolith was aged using two methods and two laboratories. As a second part of the study, the image-enhanced, unburnt half of the aged otolith was subsequently and independently burned and re-aged by the second laboratory (BIO), thus providing a matched-pair comparison of image-enhanced and burnt otoliths by the same laboratory.
Age interpretations at both PBS and BIO were made without knowledge of fish length or of the age estimates of the other laboratory, with both using a 1 January birth date. Systematic ageing differences between the methods and between the laboratories were evaluated with age bias plots, whereas precision was quantified using the CV.
Samples from 51 Sebastes otoliths were used in the comparison study. The fork lengths of S. mentella (n = 30) ranged from 30 to 40 cm (mean 35.8 cm), whereas the fork lengths of S. fasciatus (n = 21) ranged from 23 to 36 cm (mean 29.6 cm). Both sexes were equally represented in the samples.
Bomb radiocarbon assays for age validation
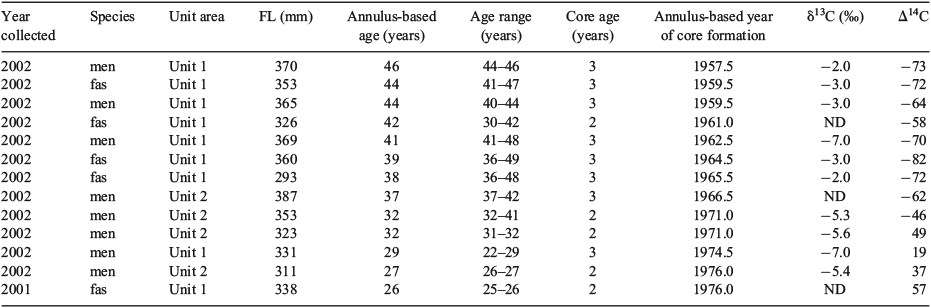
Otolith cores (n = 13) for bomb radiocarbon age validation were isolated from a 1-mm transverse section through the otolith core made using paired blades on the Isomet saw. Otolith cores representing the first 2–3 years of life were isolated from the section as a solid piece with a Merchantek computer-controlled micromilling machine (ESI, Portland, OR, USA) using 300-µm diameter steel cutting bits and burrs. The date of sample formation was calculated as the year of fish collection minus the number of growth increments from the edge of the otolith to the midpoint of the range of growth increments present in the extracted core. After sonification in ultrapure water and drying, the sample was weighed to the nearest 0.1 mg in preparation for the 14C assay with accelerator mass spectrometry (AMS). AMS assays also provided δ13C (‰) values, which were used to correct for isotopic fractionation effects and provide information on the source of the carbon. Where there was insufficient sample material for the δ13C assay, mean values were used for correction of Δ14C values. Radiocarbon values were subsequently reported as Δ14C, which is the per mille (‰) deviation of the sample from the radiocarbon concentration of 19th century wood, corrected for sample decay before 1950 according to methods outlined by Stuiver and Polach (1977). The mean s.d. of individual radiocarbon assays was ~3‰.
The reference Δ14C carbonate chronology for the north-west Atlantic (NWA) was derived from 73 otoliths of young, known-age fish of various species (including Sebastes spp.) whose cores were formed between 1949 and 2000 (Campana et al. 2008). Differences in Δ14C among species are neither expected nor observed if the species inhabit marine waters of similar water mass characteristics.
The feature of a bomb radiocarbon chronology that best serves as a stable dated reference mark is the year of initial increase above pre-bomb levels in response to the period of atmospheric testing of nuclear weapons. Comparison of this year of initial increase in the reference chronology with that of the species being tested (in this case redfish) provides a good measure of the accuracy of age estimation, because consistent over- or underestimation of age will shift the calculated year of initial increase in the test chronology to earlier or more recent years (Francis et al. 2010). The year of initial appearance of bomb Δ14C (YT) was defined as described by Campana et al. (2008).
Growth analyses
The von Bertalanffy growth function was fitted to the length-at-age data by species, stock and decade:
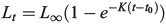
where Lt is the fork length of redfish (cm) at age t (years), L∞ is the asymptotic length, K is a growth coefficient (year–1) and t0 is the age at zero length. Non-linear regression was used to estimate the parameters of the model using SPSS software (ver. 23, IBM Corporation, Armonk, NY, USA). Growth model comparisons were made between stocks, species and sexes by using the sum of squares reduction test, calculating the F statistic from a full model that estimates model parameters for each group and a reduced model that combines groups in a single set of model parameters (Haddon 2001). The same growth comparisons were subsequently made between genetic populations of each species.
Results
Presumed annual growth bands were visible in all otoliths, although interpretability varied among otoliths. In general, otoliths were clear and relatively easy to read (for Sebastes), although the presence of multiple checks formed during the first few years of life sometimes complicated the interpretation of young fish. Growth bands formed after ~20 years of age were sometimes extremely narrow or faint, making them challenging to identify.
Comparison between ageing methods
The visibility and interpretability of growth bands differed noticeably with the mode of sample preparation (Fig. 2). For any given otolith, the visibility of the growth increment sequence improved markedly after either image enhancement or burning. However, the combination of burning followed by image enhancement almost always produced the clearest growth pattern. In contrast, the growth sequence in otolith sections that were neither burned nor enhanced tended to be indistinct in all but young fish.
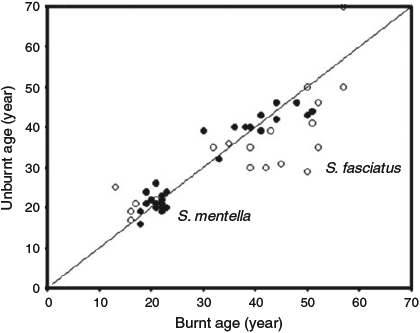
The matched-pair age comparisons for S. mentella demonstrated that comparable ages were obtained independent of the method or the laboratory. Ages ranged from 16–43 years using unburnt image enhancement to 18–50 years with burning by either laboratory. There was no obvious bias across the methods or the laboratories for S. mentella (Fig. 3). The CV between burnt and unburnt images within a laboratory was 6.5%. The CV between unburnt (BIO) and burnt (PBS) was 5.9%, whereas the CV between laboratories for burnt preparations was 7.3%.
|
The matched-pair age comparisons for S. fasciatus indicated that the preparation method could affect the quality of the results, and that interpretations were being made differently between the laboratories. Ages ranged from 10–70 years using unburnt image enhancement to 11–62 years after burning by PBS to 13–50 years after burning by BIO. There was obvious bias between both the methods and the interpretation of the laboratories such that more increments were visible in older fish (>40 years) after burning than before burning (Fig. 3). However, the method alone did not explain all the difference in interpretation between laboratories. Older S. fasciatus could be interpreted as having either many fine narrow annuli or fewer broad ‘split’ annuli (Fig. 4), and both laboratories reported having difficulty deciding which interpretation to make. In the end, one laboratory elected to interpret the otoliths as old, whereas the other laboratory elected to interpret them as younger. However, both laboratories were capable of ageing them either way, and there were no significant differences in ages if both laboratories used similar ageing criteria. The younger ages were later entered into the statistical analyses.
Bomb radiocarbon assays and age validation
The date of formation of the redfish otolith cores was estimated in two ways: (1) through age determination of the fish based on otolith growth increment counts; and (2) through comparison of otolith core Δ14C values with the values known to be present in surface marine waters of the NWA at the time (the NWA reference chronology). Agreement between the increment- and Δ14C-based dates confirms that the annuli were interpreted correctly for age estimation, at least on average.
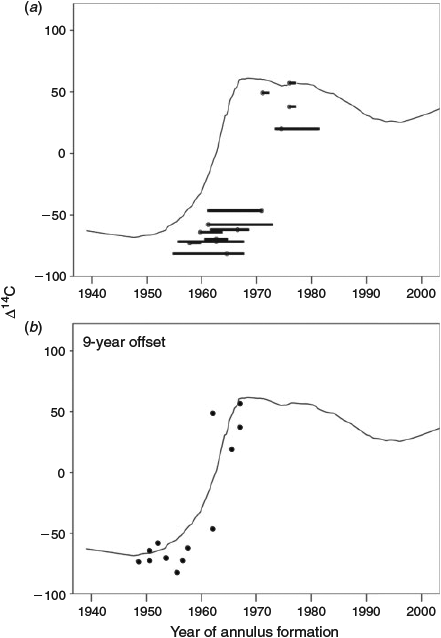
In all, 13 otolith cores were assayed for radiocarbon, and the resulting range of Δ14C values (–82 to +57) was that expected of pre- to post-bomb radiocarbon in the NWA (Table 1). However, there was no overlap between the NWA reference chronology and the chronology derived from the redfish otolith cores, even when uncertainty in the growth increment count was considered by using the entire plausible age range (Fig. 5a). Somewhat surprisingly, there was a close correspondence between the reference and redfish core chronologies if it is assumed that one or the other of the chronologies was offset by 9 years (Fig. 5b). This correspondence indicates either that all the redfish ages were underaged by 9 years, regardless of whether the fish was young or old, or that the NWA reference chronology representative of surface marine waters was inappropriate for use on deep-water redfish. As discussed later, if it was determined that a reference chronology phase lagged by 9 years was appropriate for use with redfish, this would indicate that the age determinations based on growth increment counts were accurate up to an age of at least 46 years.
|
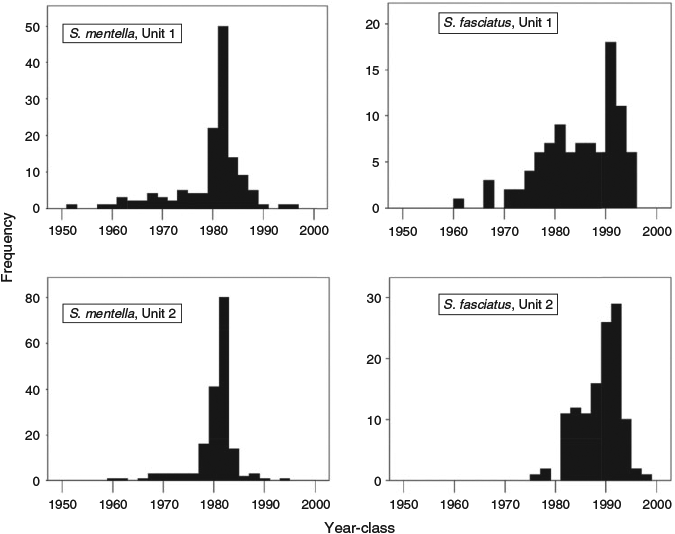
Deep-water redfish recruitment in the Gulf of St Lawrence is known to be strongly episodic and various stock assessments have commented on the record size of the 1980–81 year-classes since 1983 (DFO 2010). To determine whether the age assignments based on growth increment counts were consistent with the presence of these strong year-classes, the year of birth was calculated for all Unit 1 and 2 redfish (Fig. 6). The calculated 1980 and 1981 year-classes based on age reading dominated all other year-classes of S. mentella in both Units 1 and 2, indicating that the age reading must have been accurate up to at least an age of 22 years. In contrast, relative year-class strength was much more dispersed in S. fasciatus, a species not known for the same level of episodic recruitment in Units 1 and 2.
|
Growth analyses
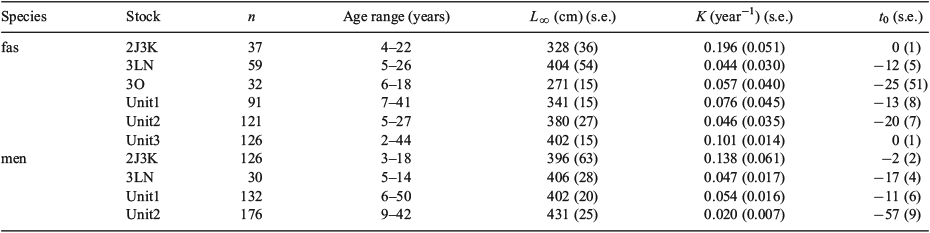
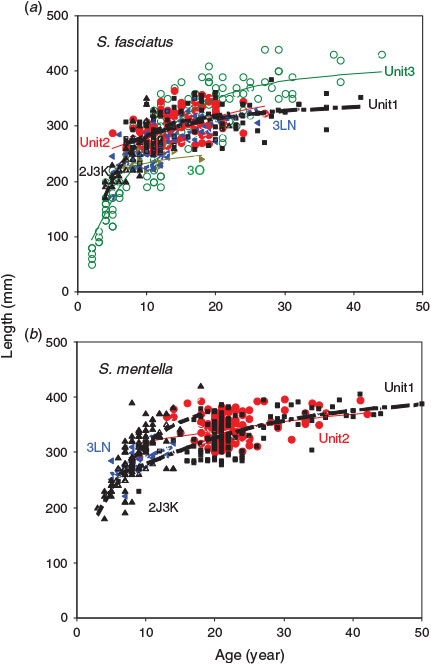
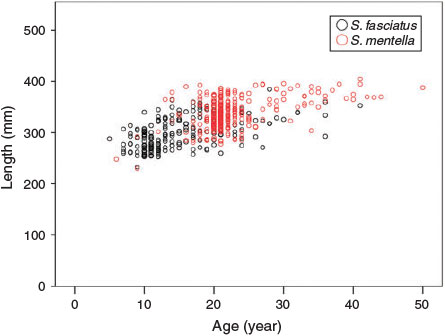
In all, 470 S. fasciatus and 470 S. mentella from six management units were aged to develop growth curves, with maximum observed ages of 44 and 50 years respectively for the two species. Both species showed similar growth patterns, with relatively rapid growth to an age of 10–15 years, after which growth slowed markedly (Fig. 7). The growth of S. fasciatus appeared to be fairly similar across stocks, with the exception of the Scotian Shelf stock (Unit 3), which appeared to grow somewhat more quickly. Sebastes mentella also appeared to grow similarly in all stocks, with the exception of the NAFO 2J3KL stock, where it may have grown more quickly; however, the scarcity of NAFO 2J3KL individuals with ages >15 years made it difficult to determine whether their absolute growth rate was actually higher than that of other stocks. Within the best-sampled management unit, the growth of the two species appeared to follow similar trajectories, with S. mentella growing to slightly larger asymptotic lengths and ages (Fig. 8). The von Bertalanffy growth models fit to each species and stock resulted in estimates of asymptotic length that tended to be <400 mm for S. fasciatus and >400 mm for S. mentella (Table 2; Fig. 7). With the exception of redfish from NAFO 2J3KL, all estimates of the growth coefficient K were <0.1 year–1, indicating slow growth. Likelihood ratio tests indicated that the growth coefficients of S. fasciatus in Unit 3 and NAFO 2J3KL were significantly greater than those in other stocks (P < 0.05). Small but significant differences were evident in some other stocks as well, but those results were associated with small sample sizes and may not be biologically important. For all stocks and both species, there were very few samples with lengths <180 mm, implying that modelled lengths below this size were solely a function of the K and t0 growth parameters. Indeed, some of the t0 values reported in Table 2 differ considerably from zero, highlighting the growth uncertainty of very small redfish. For S. mentella, samples from NAFO 2J3KL grew significantly faster and those in Unit 2 significantly slower than those in other stocks (P < 0.05). The growth rate differed significantly between sexes for both species in almost all stocks, whereby females grew more rapidly and to larger sizes than males, at least after ages 7–10 years (likelihood ratio test, P < 0.01).
|
|
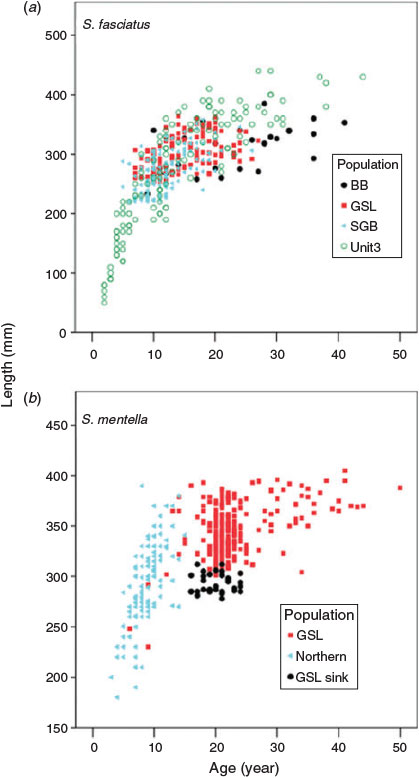
The growth curves of 430 S. fasciatus and 467 S. mentella from the genetically identified populations of Valentin et al. (2014) suggested that some or all of the observed differences in length at age could be attributed to environmental rather than genetic differences (Fig. 9). Environmental effects were most evident in S. mentella, where there were large and significant differences (P < 0.01) in growth rate between the Gulf of St Lawrence population and the genetically identical sink population in the Saguenay fjord. Slower growth was also observed in the Bonne Bay population of S. fasciatus, but because this population is both environmentally and genetically distinct, it was not possible to disentangle the effects. For all other populations of both species, it appeared that there was little evidence of differences in length at age; significant differences were evident in some cases, but those differences were probably due to very different length or age differences among samples.
|
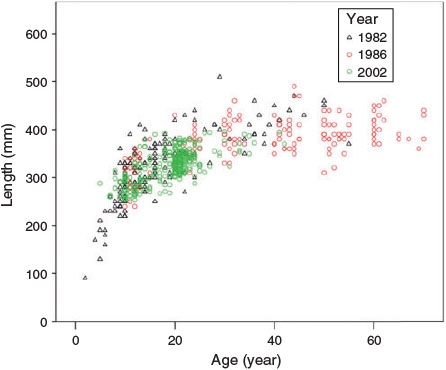
A comparison of the recent length at age of S. mentella in Unit 2 with that evident in 1982 and 1986 suggested that there had been no visible change in growth trajectory over a 20-year interval (Fig. 10). Large changes in age composition were evident, with maximum ages of ~70 years observed in 1986 compared with 42 years in 2002. However, it is not clear whether that difference represents a true change in age composition or merely reflects non-routine sampling from deep-water stations in the 1980s.
|
Discussion
Otolith transverse thin sections have become the standard method for age determination of fishes around the world, particularly for long-lived species (Beamish and McFarlane 1987). Thus, it is somewhat curious that C&B otolith preparations have persisted as the preparation of choice for Sebastes spp. and a small number of other species in both the Pacific and Atlantic oceans (Stransky et al. 2005b; CARE 2006). To some extent, this may reflect convention, because Beamish’s (1979) first report on the longevity of Pacific Sebastes was based on C&B otoliths. However, subsequent comparative research on various Sebastes species has demonstrated that C&B and otolith thin sections provided comparable age estimates up to an age of over 100 years (Andrews et al. 2002; Stransky et al. 2005b). Early ageing studies recognised the advantages of working with cross-sectional views of otoliths, even if their methods were sometimes cruder (otoliths broken by hand; Kelly and Wolf 1959; Sandeman 1969; Mayo et al. 1981), presumably because the methods avoid the age underestimation bias associated with either scales or whole otoliths at ages >30 years (Campana et al. 1990; Nedreaas 1990). The results reported herein extend the findings of Stransky et al. (2005b), demonstrating comparability between C&B and image-enhanced sections to an age of ~40 years. However, annual increments in old Sebastes spp. are known to be both narrow and difficult to interpret (Nedreaas 1990; Stransky et al. 2005b), which probably explains why image-enhanced C&B preparations using cut surfaces provided superior visibility to even image-enhanced sections at ages >40 years. Given the relative ease of preparing a cut transverse surface without embedding, the ability to capture and archive a high-quality digital image before fading of the burnt surface and the significant benefits of rapid image enhancement, a slight reduction in productivity would appear to be an acceptable limitation of the method. Thus image-enhanced C&B otoliths using cut surfaces are highly recommended for routine age determination of Sebastes spp. at least in older fish.
Bomb radiocarbon is well established as an effective method of age validation for long-lived species, often with a precision of as little as 1–2 years (Kalish 1993; Campana 2001). Given that two previous radiochemical studies have confirmed the overall accuracy of C&B preparations and sections for determining the age of S. mentella and S. marinus in the Atlantic (albeit with low precision; Campana et al. 1990; Stransky et al. 2005a), and in light of another two studies that have confirmed the accuracy of these methods for ageing S. mentella to an age of 10 years (Mayo et al. 1981; Saborido-Rey et al. 2004), the bomb radiocarbon results in the present study were initially puzzling. The consistent offset of 9 years between the NWA reference chronology and the redfish core chronology indicates that either our growth increment counts were underaged by 9 years in every fish that we aged or the NWA surface marine chronology was inappropriate for use on Sebastes spp. off eastern Canada. There is no Δ14C reference chronology available for the Gulf of St Lawrence at depths of ~400 m, although the surface water chronology shows a 3-year delay relative to the NWA (Morin et al. 2013). A 3-year delay is insufficient to explain the assay results in the present study. However, several lines of evidence suggest it was the delayed penetration of the bomb radiocarbon signal to the 390–450 m living depth of the redfish that produced the offset and thus made the NWA surface chronology inappropriate for use. It is well known that the bomb signal in dissolved inorganic carbon (DIC) was initially confined to the surface marine layers, but has penetrated to increasingly deeper waters over time. Through 14C assays of both the core and edge of the otoliths of deep-dwelling fish species, Horn et al. (2010) were able to demonstrate that there was a delay of 10–15 years in the penetration of the 14C signal to depths of 300–600 m in the south-west Pacific. Independent and more detailed otolith radiocarbon assays by Grammer et al. (2015) documented a 5–10-year delay in radiocarbon penetration to a depth of 400–500 m in the Tasman Sea. In the north-east Atlantic, the Δ14C penetration depth increased by 1000 m over a period of 18 years (Nydal 1993); assuming a linear increase in depth through time, starting from the base of the 200-m mixed layer, this would imply that it would take 4.5 years for the signal to penetrate to the mean 450-m depth of the redfish in the present study. Similarly, the bomb signal took 14 years to penetrate to a depth of 800 m in the Indian Ocean (Rubin and Key 2002); given the same calculations described above, this would imply a 6-year penetration time to a depth of 500 m. Friess and Sedberry (2011) also observed a 5-year lag between surface and otolith Δ14C values at depths of 450–600 m, although they attributed this to otolith ageing error. Therefore, it not only appears inevitable that the bomb signal would be delayed in reaching the depth of redfish in the present study, but the length of the delay is consistent with the 9-year delay we observed, suggesting that our redfish ages were, indeed, accurate.
Although the bomb radiocarbon results were consistent with our ages based on growth increment counts, uncertainty about the exact magnitude of the delay in the bomb signal meant that we could not validate our ages with the 1–2-year precision normally expected of the method. However, the dominance of the 1980–81 year-classes of S. mentella in Units 1 and 2 confirmed the accuracy of our ages for redfish up to an age of 22 years, because inaccurate ages would not have identified the correct birth years in fish collected in 2003. Age determination of dominant year-classes is a valid means of age validation (Campana 2001) but is only useful when recruitment is periodic and highly variable in strength. Fortunately, this is the case with many of the Sebastes populations in the Atlantic, and several previous studies have used it to validate their age interpretations of younger redfish (Mayo et al. 1981; Nedreaas 1990; Saborido-Rey et al. 2004). In the present study, the youngest fish assayed for bomb radiocarbon was ~20 years old. Given that the dominant year-class method validated our age interpretations to an age of 22 years, this suggests that our bomb radiocarbon results (with a 9-year delay) were also accurate, extending our age validation to an age of 46 years.
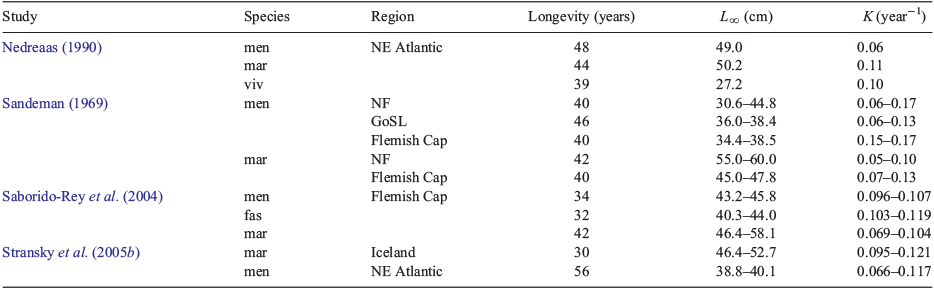
The results of the present study indicate that S. mentella grows to a larger size and with a lower growth coefficient than S. fasciatus, but that the growth trajectories and longevities of the two species can be quite similar. Few other studies have attempted to compare the growth of these two Sebastes species, in part because of their problematic species identification. In comparing the growth and longevity of S. fasciatus, S. mentella and S. marinus on the Flemish Cap, Saborido-Rey et al. (2004) commented on the difficulty of distinguishing between the first two species, but then neglected to state how they identified the species. They concluded that S. marinus grew fastest, S. mentella grew slowest and that longevities ranged from 32 to 34 years for S. mentella and S. fasciatus (Table 3). None of the other published studies considered S. fasciatus. However, most of these studies reported that S. mentella grew slower and to smaller size than S. marinus, with longevities on the order of 40–56 years (Sandeman 1969; Nedreaas 1990; Stransky et al. 2005b). The von Bertalanffy parameters in the present study are relatively similar to those that have been published (Table 3), with the possible exception of those published by Saborido-Rey et al. (2004). To our knowledge, the present study is the only age determination study that has unequivocally differentiated between Atlantic redfish species on the basis of genetic markers.
The genus Sebastes is extremely speciose in the north-east Pacific, where there at least 72 reported species (Love et al. 2002). Across these species, longevities ranging from 18 to 205 years (mean 62 years) have been reported, with mean concomitant K and L∞ values of 0.15 (0.03–0.62 year–1) and 42 cm (15–86 cm) respectively (Love et al. 2002). These mean values of longevity and asymptotic length are reasonably similar to those we report here for the well-sampled Gulf of St Lawrence–Scotian Shelf (Unit 1–3) stocks (mean longevity 41 years; L∞ = 39 cm), although the mean Pacific K value is somewhat higher than the Units 1–3 mean K value of 0.06 year–1. Sebastes spp. in Alaskan waters appear to grow more slowly (Malecha et al. 2007) and are thus more similar to those in the NWA, presumably reflecting the colder ambient waters.
Although there were clear differences in growth and longevity between redfish species, it was difficult to determine whether there were biologically meaningful differences among stocks within a species. Certainly, there were significant differences among many of the stocks, but low sample sizes and limited size ranges of the S. fasciatus samples from NAFO 3LN, 3O and 2J3KL may have affected accurate estimation of growth parameters in those regions. However, for the better-sampled Unit 1–3 stocks, the results suggest that there is relatively little difference within species for Units 1 and 2, but that S. fasciatus grew faster in the warmer slope waters of Unit 3. Examination of growth rates by genetic population, rather than management unit, clearly highlighted some of the growth heterogeneity that was present. In particular, the genetically identical sink population (Saguenay fjord) of the Gulf of St Lawrence population indicated that environmental effects could affect growth rate, even in a deep-water species such as Sebastes. Nevertheless, the overall perspective was one of a consistent growth trajectory for each species in the area of southern Newfoundland and the Gulf of St Lawrence, with growth differences evident only for isolated populations (Bonne Bay) or sink populations (Saguenay fjord).
Given the relative constancy of water temperature at the ambient depth of the redfish, it is perhaps not surprising that there was little evidence of long-term (20-year) changes in growth rate within Unit 2 redfish, despite marked changes in age composition. Whether the reduced apparent longevity in Unit 2 in recent years represents a real change, perhaps because of increased fishing mortality, is unclear. However, it likely represents more intensive sampling of deep-water stations in the 1980s, at locations that have not been sampled since. Similar longevities and von Bertalanffy growth parameters were reported for S. mentella in the only other study that has looked at redfish growth in and around the Gulf of St Lawrence (Sandeman 1969).
Examples of the successful management of deep-water fisheries are few and far between (Roberts 2002). In part, the failures are because of the slow growth, delayed age at maturation and infrequent recruitment characteristic of many deep-sea species (Musick 1999). High initial biomass often leads to unsustainably high catch rates, which then plummet as the virgin stock is exploited. This pattern has been repeated in many deep-sea fisheries around the world (Devine et al. 2006) and it has the potential to develop with Sebastes in the Atlantic Ocean as well. Musick (1999) noted that von Bertalanffy growth coefficient (K) values <0.10 year–1 are often associated with stocks that are particularly vulnerable to rapid and unsustainable overexploitation, and most of the Sebastes stocks in the NWA fall into this category. Both S. mentella and S. fasciatus in and around the Gulf of St Lawrence are characterised by growth curves that show a virtual cessation of somatic growth after an age of 10–15 years, corresponding to the approximate age of sexual maturation. Thus, it is of concern to see that recent stock assessments have placed the biomass of Unit 1 S. mentella at <10% of its value in 1990 (DFO 2010). Given the intrinsically lower growth and productivity of S. mentella compared with S. fasciatus, and in light of the apparent inevitability of mixed species catches in Units 1 and 2, it appears that at least some of the continued decline in S. mentella abundance and biomass can be attributed to the impact of fishing mortality on a multispecies mixture containing stocks of differing productivities (Kell et al. 2004).
Acknowledgements
The authors are grateful to Jean-Marie Sévigny of the Institut Maurice Lamontagne (Mont Joli, Québec, Canada) for directing the genetic species identifications through funding by a Genomics R&D Initiative grant. The authors also thank Don Power (Northwest Atlantic Fisheries Science Centre in St John’s) and Peter Comeau (Bedford Institute of Oceanography) for providing some of the redfish otoliths. Anna MacDonnell, Mark Fowler, Dan Houlihan and Warren Joyce provided expert technical assistance. Allen H. Andrews provided a helpful review of the manuscript. Funding was provided by the Groundfish Enterprise Allocation Council (GEAC) and Fisheries and Oceans Canada.
References
Andrews, A. H., Cailliet, G. M., Coale, K. H., Munk, K. M., Mahoney, M. A., and O’Connell, V. M. (2002). Radiometric age validation of the yelloweye rockfish (Sebastes ruberrimus) from southeastern Alaska. Marine and Freshwater Research 53, 139–146.| Radiometric age validation of the yelloweye rockfish (Sebastes ruberrimus) from southeastern Alaska.Crossref | GoogleScholarGoogle Scholar |
Andrews, A. H., Tracey, D. M., and Dunn, M. R. (2009). Lead–radium dating of orange roughy (Hoplostethus atlanticus): validation of a centenarian life span. Canadian Journal of Fisheries and Aquatic Sciences 66, 1130–1140.
| Lead–radium dating of orange roughy (Hoplostethus atlanticus): validation of a centenarian life span.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXotlegtr8%3D&md5=6640921523bebac419f0016602623e48CAS |
Beamish, R. J. (1979). New information on the longevity of Pacific ocean perch (Sebastes alutus). Journal of the Fisheries Research Board of Canada 36, 1395–1400.
| New information on the longevity of Pacific ocean perch (Sebastes alutus).Crossref | GoogleScholarGoogle Scholar |
Beamish, R. J., and McFarlane, G. A. (1987). Current trends in age determination methodology. In ‘Age and Growth of Fish’. (Eds R. C. Summerfelt and G. E. Hall.) pp. 15–42. (Iowa State University Press: Ames, IA.)
Cailliet, G. M., Andrews, A. H., Burton, E. H., Watters, D. L., Kline, D. E., and Ferry-Graham, L. A. (2001). Age determination and validation studies of marine fishes: do deep-dwellers live longer? Experimental Gerontology 36, 739–764.
| Age determination and validation studies of marine fishes: do deep-dwellers live longer?Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD3Mris1eqtQ%3D%3D&md5=01ec9e4a76efd30a41ab3af8da2d1a38CAS | 11295512PubMed |
Campana, S. E. (2001). Accuracy, precision and quality control in age determination, including a review of the use and abuse of age validation methods. Journal of Fish Biology 59, 197–242.
| Accuracy, precision and quality control in age determination, including a review of the use and abuse of age validation methods.Crossref | GoogleScholarGoogle Scholar |
Campana, S. E., Zwanenburg, K. C. T., and Smith, J. N. (1990). 210Pb/226Ra determination of longevity in redfish. Canadian Journal of Fisheries and Aquatic Sciences 47, 163–165.
| 210Pb/226Ra determination of longevity in redfish.Crossref | GoogleScholarGoogle Scholar |
Campana, S. E., Casselman, J. M., and Jones, C. M. (2008). Bomb radiocarbon chronologies in the Arctic, with implications for the age validation of lake trout (Salvelinus namaycush) and other Arctic species. Canadian Journal of Fisheries and Aquatic Sciences 65, 733–743.
| Bomb radiocarbon chronologies in the Arctic, with implications for the age validation of lake trout (Salvelinus namaycush) and other Arctic species.Crossref | GoogleScholarGoogle Scholar |
CARE (2006). Manual on generalized age determination procedures for groundfish. Committee of Age Reading Experts. Technical Subcommittee of the Canada–US Groundfish Committee. Available at http://care.psmfc.org/docs/CareManual2006.pdf [Verified January 2015].
Charles, K. D., MacLellan, S. E., and Little, D. (2013). ‘A Guide to Sectioning Otoliths for Age Determination. Canadian Technical Report of Fisheries and Aquatic Science.’ (Fisheries and Oceans Canada: Ottawa, ON.).
Clarke, M. W., Kelly, C. J., Connolly, P. L., and Molloy, J. P. (2003). A life history approach to the assessment and management of deepwater fisheries in the Northeast Atlantic. Journal of Northwest Atlantic Fishery Science 31, 401–411.
Devine, J. A., Baker, K. D., and Haedrich, R. L. (2006). Deep-sea fishes qualify as endangered. Nature 439, 29.
| Deep-sea fishes qualify as endangered.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28Xht1aluw%3D%3D&md5=162727d45b81bd9595c11cefe1808a06CAS | 16397489PubMed |
DFO (2010). Assessment of redfish stocks (Sebastes fasciatus and S. mentella) in Units 1 and 2 in 2009. Canadian Science Advisory Secretariat Science Advisory Report 2010/037, Department of Fisheries and Oceans Canada, Ottawa, ON.
DFO (2012). Reference Points for Redfish (Sebastes mentella and Sebastes fasciatus) in the Northwest Atlantic. Canadian Science Advisory Secretariat Science Advisory Report 2012/004, Department of Fisheries and Oceans Canada, Ottawa, ON.
Francis, R. I. C. C., Campana, S. E., and Neil, H. L. (2010). Validation of fish ageing methods should involve bias estimation rather than hypothesis testing: a proposed approach for bomb radiocarbon validations. Canadian Journal of Fisheries and Aquatic Sciences 67, 1398–1408.
| Validation of fish ageing methods should involve bias estimation rather than hypothesis testing: a proposed approach for bomb radiocarbon validations.Crossref | GoogleScholarGoogle Scholar |
Friess, C., and Sedberry, G. R. (2011). Age, growth, and spawning season of red bream (Beryx decadactylus) off the southeastern United States. US Fishery Bulletin 109, 20–33.
Grammer, G. L., Fallon, S. J., Izzo, C., Wood, R., and Gillanders, B. M. (2015). Investigating bomb radiocarbon transport in the southern Pacific Ocean with otolith radiocarbon. Earth and Planetary Science Letters 424, 59–68.
| Investigating bomb radiocarbon transport in the southern Pacific Ocean with otolith radiocarbon.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2MXotlGkt7c%3D&md5=aa8b45ae278d1210437828d396d2ed0aCAS |
Haddon, M. (2001). ‘Modelling and Quantitative Methods in Fisheries’. (Chapman and Hall/CRC: New York.)
Horn, P. L., Neil, H. L., Paul, L. J., and Marriott, P. (2010). Age validation and growth of bluenose Hyperoglyphe antarctica using the bomb chronometer method of radiocarbon ageing. Journal of Fish Biology 77, 1552–1563.
| Age validation and growth of bluenose Hyperoglyphe antarctica using the bomb chronometer method of radiocarbon ageing.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC3cbnt1SjsQ%3D%3D&md5=6c2e8fbda3ff66151fb2e9deb4abd402CAS | 21078018PubMed |
Kalish, J. M. (1993). Pre- and post-bomb radiocarbon in fish otoliths. Earth and Planetary Science Letters 114, 549–554.
| Pre- and post-bomb radiocarbon in fish otoliths.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK3sXitFKqs7k%3D&md5=6388f8e4a23774019baf9da1c1445145CAS |
Kell, L. T., Crozier, W. W., and Legault, C. M. (2004). Mixed and multi-stock fisheries. ICES Journal of Marine Science 61, 1330.
| Mixed and multi-stock fisheries.Crossref | GoogleScholarGoogle Scholar |
Kelly, G. F., and Wolf, R. S. (1959). Age and growth of the redfish (Sebastes marinus) in the Gulf of Maine. Fishery Bulletin 60, 1–31.
Love, M. S., Yoklavich, M., and Thorsteinson, L. (2002). ‘The Rockfishes of the Northeast Pacific’. (University of California Press: Berkeley, CA.)
MacLellan, S. E. (1997). How to age Rockfish (Sebastes) using S. alutus as an example. The otolith burnt section technique. Canadian Technical Report of Fisheries and Aquatic Sciences 2146, 1–39.
Malecha, P. W., Hanselman, D. H., and Heitetz, J. (2007). Growth and Mortality of Rockfishes (Scorpaenidae) from Alaska Waters. NOAA Technical Memorandum NMFS-AFSC-172,.US Department of Commerce, Seattle, WA.
Mayo, R. K., Gifford, V. M., and Jerald, A. (1981). Age validation of redfish, Sebastes marinus, from the Gulf of Maine–Georges Bank region. Journal of Northwest Atlantic Fishery Science 2, 13–19.
| Age validation of redfish, Sebastes marinus, from the Gulf of Maine–Georges Bank region.Crossref | GoogleScholarGoogle Scholar |
Morin, R., LeBlanc, S. G., and Campana, S. E. (2013). Bomb radiocarbon validates age and long-term growth declines in American plaice in the southern Gulf of St Lawrence. Transactions of the American Fisheries Society 142, 458–470.
| Bomb radiocarbon validates age and long-term growth declines in American plaice in the southern Gulf of St Lawrence.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXjslegt7o%3D&md5=45c7a78ad87847f404eeefc0d08b2e13CAS |
Munk, K. (2001). Maximum ages of groundfishes in waters off Alaska and British Columbia and considerations of age determination. Alaska Fishery Research Bulletin 8, 12–21.
Musick, J. A. (1999). Ecology and conservation of long-lived marine animals. American Fisheries Society Symposium 23, 1–10.
Nedreaas, K. (1990). Age determination of northeast Atlantic Sebastes species. ICES Journal of Marine Science 47, 208–230.
| Age determination of northeast Atlantic Sebastes species.Crossref | GoogleScholarGoogle Scholar |
Nydal, R. (1993). Application of bomb C-14 as a tracer in the global carbon cycle. Trends in Geophysical Research 2, 355–364.
Perlmutter, A., and Clarke, G. M. (1949). Age and growth of immature rosefish (Sebastes marinus) in the Gulf of Maine and off western Nova Scotia. Fishery Bulletin 51, 207–228.
Roberts, C. M. (2002). Deep impact: the rising toll of fishing in the deep sea. Trends in Ecology & Evolution 17, 242–245.
| Deep impact: the rising toll of fishing in the deep sea.Crossref | GoogleScholarGoogle Scholar |
Roques, S., Sévigny, J.-M., and Bernatchez, L. (2001). Evidence for broadscale introgressive hybridization between two redfish (genus Sebastes) in the northwest Atlantic redfish: a rare example. Molecular Ecology 10, 149–165.
| Evidence for broadscale introgressive hybridization between two redfish (genus Sebastes) in the northwest Atlantic redfish: a rare example.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXjs1ejtLc%3D&md5=805620258b7d9ff229c421c796f65ca2CAS | 11251794PubMed |
Rubin, S. I., and Key, R. M. (2002). Separating natural and bomb-produced radiocarbon in the ocean: the potential alkalinity method. Global Biogeochemical Cycles 16, 52-1–52-19.
| Separating natural and bomb-produced radiocarbon in the ocean: the potential alkalinity method.Crossref | GoogleScholarGoogle Scholar |
Saborido-Rey, F., Garabana, D., and Cervino, S. (2004). Age and growth of redfish (Sebastes marinus, S. mentella and S. fasciatus) on the Flemish Cap (northwest Atlantic). ICES Journal of Marine Science 61, 231–242.
| Age and growth of redfish (Sebastes marinus, S. mentella and S. fasciatus) on the Flemish Cap (northwest Atlantic).Crossref | GoogleScholarGoogle Scholar |
Sandeman, E. J. (1969). Age determination and growth rate of redfish, Sebastes sp., from selected areas around Newfoundland. ICNAF Research Bulletin 6, 79–106.
Smith, D. C., Fenton, G. E., Robertson, S. G., and Short, S. A. (1995). Age determination and growth of orange roughy (Hoplostethus atlanticus): a comparison of annulus counts with radiometric ageing. Canadian Journal of Fisheries and Aquatic Sciences 52, 391–401.
| Age determination and growth of orange roughy (Hoplostethus atlanticus): a comparison of annulus counts with radiometric ageing.Crossref | GoogleScholarGoogle Scholar |
Stransky, C., Kanisch, G., Kruger, A., and Purkl, S. (2005a). Radiometric age validation of golden redfish (Sebastes marinus) and deep-sea redfish (S. mentella) in the northeast Atlantic. Fisheries Research 74, 186–197.
| Radiometric age validation of golden redfish (Sebastes marinus) and deep-sea redfish (S. mentella) in the northeast Atlantic.Crossref | GoogleScholarGoogle Scholar |
Stransky, C., Gudmundsdottir, S., Sigurdsson, T., Lemvig, S., Nedreaas, K., and Saborido-Rey, F. (2005b). Age determination and growth of Atlantic redfish (Sebastes marinus and S. mentella): bias and precision of age readers and otolith preparation methods. ICES Journal of Marine Science 62, 655–670.
| Age determination and growth of Atlantic redfish (Sebastes marinus and S. mentella): bias and precision of age readers and otolith preparation methods.Crossref | GoogleScholarGoogle Scholar |
Stuiver, M., and Polach, H. A. (1977). Reporting of 14C data. Radiocarbon 19, 355–363.
Tracey, D. M., and Horn, P. L. (1999). Background and review of ageing orange roughy (Hoplostethus atlanticus, Trachichthyidae) from New Zealand and elsewhere. New Zealand Journal of Marine and Freshwater Research 33, 67–86.
| Background and review of ageing orange roughy (Hoplostethus atlanticus, Trachichthyidae) from New Zealand and elsewhere.Crossref | GoogleScholarGoogle Scholar |
Valentin, A., Sévigny, J.-M., Power, D., Branton, R. M., and Morin, B. (2006). Extensive sampling and concomitant use of meristic characteristics and variation at the MDH-A* locus reveal new information on redfish species distribution and spatial pattern of introgressive hybridization in the northwest Atlantic. Journal of Northwest Atlantic Fishery Science 36, 65–80.
| Extensive sampling and concomitant use of meristic characteristics and variation at the MDH-A* locus reveal new information on redfish species distribution and spatial pattern of introgressive hybridization in the northwest Atlantic.Crossref | GoogleScholarGoogle Scholar |
Valentin, A., Penin, X., Power, D., Chanut, J.-P., and Sévigny, J.-M. (2014). Combining microsatellites and geometric morphometrics for the study of redfish (Sebastes spp.) population structure in the northwest Atlantic. Fisheries Research 154, 102–119.
| Combining microsatellites and geometric morphometrics for the study of redfish (Sebastes spp.) population structure in the northwest Atlantic.Crossref | GoogleScholarGoogle Scholar |