A comparison of reproductive parameters of the Bering skate, Bathyraja interrupta, from two Alaskan large marine ecosystems
Shaara M. Ainsley A B , David A. Ebert A and Gregor M. Cailliet AA Pacific Shark Research Center, Moss Landing Marine Laboratories, 8272 Moss Landing Road, Moss Landing, CA 95039, USA.
B Corresponding author. Email: shaaraainsley@fishbio.com
Marine and Freshwater Research 62(6) 557-566 https://doi.org/10.1071/MF10140
Submitted: 16 June 2010 Accepted: 25 February 2011 Published: 24 June 2011
Journal Compilation © CSIRO Publishing 2011 Open Access CC BY-NC-ND
Abstract
Estimates of size at maturity are crucial to fisheries stock assessments and may change spatially and temporally. This study directly compares life-history characteristics of a skate species in two large marine ecosystems in a region where there is both a directed fishery and considerable skate by-catch in other fisheries. The Bering skate, Bathyraja interrupta, is one of the most common skate species in Alaskan waters, occurring in two large marine ecosystems, the eastern Bering Sea (EBS) and the Gulf of Alaska (GOA), but little is known about its life history. Skates were sampled from both regions between 2004 and 2007. In the GOA, the size at maturity was estimated to be 69 cm total length (TL) for males and 71 cm TL for females, while in the EBS size at maturity was estimated as 70 cm and 72 cm TL for males and females, respectively. Median size at maturity differed by sex but not by region. Our results indicate that B. interrupta shows late maturity, typical of most skate species, suggesting that more detailed monitoring of skate populations and precautionary management is warranted as skate fisheries expand.
Additional keywords: by-catch, elasmobranch, skate fishery.
Introduction
Elasmobranchs are considered to be especially vulnerable to overfishing owing to their slow growth, late age at maturation and low fecundity (Dulvy et al. 2000; Stevens et al. 2000; Dulvy and Reynolds 2002). Despite these characteristics, skates have received comparatively little research relative to sharks, although some species are the target of major fisheries worldwide (Food and Agriculture Organization 2009). Owing to their prevalence as by-catch in groundfish fisheries and in directed fisheries, skates have become a taxon of concern. They are especially vulnerable to groundfish fisheries because of their generally large size and demersal life style (Walker and Hislop 1998; Ebert et al. 2008a, 2008b). The decline of some skate populations in recent decades, especially in the North Atlantic, has been attributed to fishing pressures (Walker and Hislop 1998; Frisk et al. 2005).
Skate landings in Alaskan waters have been increasing and greatly exceed those of all other North American states combined, with an annual skate catch in the Gulf of Alaska estimated to range from ~5500 to 9000 metric tonnes (Ormseth and Matta 2007a). Large numbers of skates are taken as by-catch in longline and bottom trawl fisheries (Matta et al. 2006). In addition, a directed fishery for skates has recently re-emerged in Alaskan waters. In the past, skates have been a relatively unprofitable target species; however, the intent of the fishery is to provide a cheap, easy means for entry-level fishers to join the industry and supplement the local economy when the salmon fishery is closed. The Alaskan fishery opened despite concerns of insufficient life-history data available for proper management, demonstrating an expansion or shift into inferior, less profitable fisheries. Furthermore, the emerging directed fishery in the Gulf of Alaska (GOA) has indicated potential for expanded development of a skate fishery in the eastern Bering Sea (EBS), where skates are only taken as by-catch currently.
While estimates of median size at maturity are crucial to fisheries stock assessments, these may change both spatially and temporally owing to environmental factors, population density or patchy fishing pressures (Rochet 2000; Sosebee 2005; Walker 2007). Regional differences in size at maturity have been noted for several species of elasmobranchs (Templeman 1987; Yamaguchi et al. 2000; Lombardi-Carlson et al. 2003). Often in cases where regional comparisons have been described for elasmobranchs, the same authors did not conduct the studies being compared; therefore, differences may be attributed to methodology rather than biology. In addition, studies of life-history parameters have shown considerable variation among individual skate species (Ebert 2005; McFarlane and King 2006), yet they are generally managed as aggregate groups. The re-emergence of the Alaskan skate fishery has seen a shift towards species-specific management initiatives, but a deficiency in life-history data for this species-complex has inhibited better management practices (Matta et al. 2006).
Bathyraja interrupta, the Bering skate, is the second most common species on the EBS shelf, and is one of the five most commonly caught skates in the GOA (Ormseth and Matta 2007a, 2007b). It is generally found at depths of 55 to 1372 m in the EBS and the GOA (Mecklenburg et al. 2002). To date, there have been few studies on the ecology and reproductive biology of B. interrupta (Ebert 2005).
Given that size at maturity may change spatially and considering the differences in environmental conditions and fishing pressures between the two large marine ecosystems (GOA and EBS), we tested two hypotheses: (1) median size at maturity differs by sex; and (2) median size at maturity differs by region. Significant differences in these parameters may affect the way the populations respond to fishing pressures and should be taken into account in the management of elasmobranch species with broad geographic ranges.
Materials and methods
Sources of samples
Specimens of B. interrupta from the EBS were collected from Unalaska and Akutan islands (54°N, 166°W) to the Navarin Canyon (61°N, 180°W) on the USA–Russia border during the 2004 National Marine Fisheries Service Alaska Fisheries Science Center (NMFS-AFSC) Eastern Bering Sea Continental Slope survey (Hoff and Britt 2005). In the GOA, specimens were obtained from surveys conducted by the Alaska Department of Fish and Game (ADFG) and the NMFS-AFSC along Alaskan Peninsula (54°N, 164°W) to Kamishak Bay and Kodiak Island (59°N, 152°W) between 2005 and 2007. Port sampling of direct and indirect fishery landings in Kodiak provided additional samples in 2005 and 2006.
Biological measurements
External and internal morphometrics were taken upon capture at sea or dockside, including total length (TL) and disc width (DW), measured to the nearest millimetre (mm). The TL measurements are presented here rounded to the nearest centimetre (cm). Inner clasper length was measured from the insertion of the clasper near the pelvic fin to the tip, to the nearest mm for each male. The widths of the oviducal gland and uterus were measured to the nearest mm at the widest point for each female. When the yellow vitellogenic ova were intact, the number of ova was counted in each ovary and the maximum ovum diameter (MOD) for each female was measured to the nearest mm. The total number of mature ova for each individual was calculated as the sum of the left and right ova counts. Weight (W) was recorded when possible and was reported to the nearest 0.01 kg. Complete metrics were not obtained from every individual, therefore not all sample sizes add up to the overall totals. Following recommendations by Francis (2006), TL was used as the main body measurement, although equations for the relationship between TL and DW are presented to facilitate conversion.
Linear regressions were fitted to the TL and DW data, and an analysis of covariance (ANCOVA) was used to examine differences in the linear relationship between sexes and regions. Residual plots were examined to confirm that the error terms met the assumptions of normality and homogeneity of variance. Power regressions were used to describe the weight–length data for each sex and region following the equation from Ricker (1973), W = aTLb, in which a and b are fitted constants (SigmaPlot 10.0, SPSS Inc., Chicago, IL). To facilitate comparison and meet assumptions, linear regressions were fitted to the log-transformed TL and weight data and an ANCOVA was used to compare differences in this relationship between sexes and regions.
Each individual was assigned a maturity status, determined by examination of the gonads and, in males, the rigidity of the claspers. The criteria were modified from the reproductive status guidelines used by Ebert (2005): 1 = embryos; 2 =juveniles; 3 = adolescents; 4 = adults; and 5 = gravid females.
Juvenile males had short and flexible claspers and thin, threadlike testes without obvious coiling in the epididymis. In adolescent males, the claspers extended beyond the posterior margin of the pelvic fins. However, the terminal cartilage elements were not yet fully calcified and the epididymis was loosely coiled. Adult males had elongated, rigid calcified claspers and a tightly coiled epididymis. A subsample of whole reproductive tracts from male individuals was preserved in 10% formaldehyde for histology.
Juvenile females lacked ovarian or oviduct development and the oviducal gland appeared as a slight bulge in the thin, transparent uterus. Adolescent females showed small, slightly differentiated ovaries that lacked vascularisation, and the oviducal gland was a noticeable bulge rather than the kidney-bean shape of a developed gland. Adult females had vascularised, usually yellowish, ovarian eggs, and the oviducal gland was distinctly differentiated from the oviducts, forming a kidney-bean shape. Gravid females had egg cases in utero.
A Chi-square goodness-of-fit test was used to test for a 1 : 1 sex ratio for each region and maturity status. The overall sex ratios include all individuals that were sexed during the study, while the analysis of sex ratios by maturity status was limited to the individuals that were assigned maturity during the study period. Except where stated otherwise, all statistical analyses were performed using SPSS 11.0 (SPSS Inc.).
Maturity
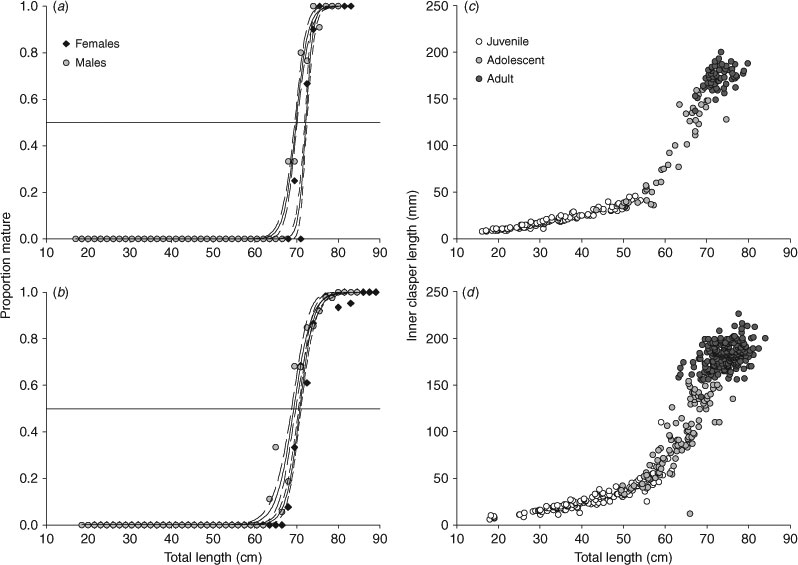
First maturity was considered to be the size of the smallest mature individual examined for each sex and location. To calculate the median size at maturity, the initial maturity condition was modified to a binomial dataset (immature = 0 and mature = 1). Individuals were pooled into size bins (1.5 cm TL) and the proportion of mature individuals was calculated for each bin. Using the statistical program SigmaPlot 10.0, a logistic regression was fitted by sex and region to the length data (Roa et al. 1999; Ebert 2005), and 95% confidence intervals were calculated:
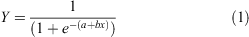
where x = TL and Y = maturity status.
Median TL at maturity (TL50) was calculated as ‘–a/b’. Two hypotheses were tested using logistic regression model Wald χ2 likelihood ratio tests: (1) median size at maturity differs by sex; and (2) median size at maturity differs by region. Since each factor had only two categories (region = EBS or GOA and sex = male or female), there were not enough degrees of freedom to test for an interaction between the two factors.
Histology
Histological techniques were employed to investigate seasonal changes in the testes and to corroborate the macroscopic maturity assessments. A tissue sample (3–4 mm thick) was removed from preserved testes, placed in cassettes for sectioning and stored in 70% ethanol for transportation. Fixed tissues were further dehydrated in a graded series of ethanol and subsequently embedded in a series of paraffin baths, sectioned to a thickness of 8–10 μm and stained using standard haematoxylin and eosin staining following Maruska et al. (1996). Once prepared, the sections were examined under a compound microscope. The stages of spermatogenesis were classified as: stage I, germinal zone; stage II, early spermatocysts; stage III, spermatocytes; stage IV, spermatids; stage V, immature spermatozoa; stage VI, mature spermatocysts; and stage VII, empty spermatocysts (Maruska et al. 1996; Ebert et al. 2008a). Since the developmental stages of spermatogenesis have been well described for skates, and hormonal analysis has confirmed that spermatocyst and spermatid stages are associated with reproductive readiness (Sulikowski et al. 2005, 2006), we concentrated on stages III–VI (Maruska et al. 1996). For ease of interpretation, stage VII, consisting of empty spermatocysts and free spermatogonia, was combined with stage VI. Spermatocysts were counted and assigned stages along a transect traversing a full and representative section of a testis lobe. The numbers of spermatocysts per stage were summed across individuals for each month, and graphed as a percentage of the total for each stage by month.
Reproduction
The maternity status of a mature female was defined by the presence of egg capsules in the uteri (present = gravid; absent = non-gravid). A paired t-test was used to test for a difference between the mean number of mature ova in the left and right ovaries. Data were tested for the assumption of normality of differences using the one-sample Kolmogorov–Smirnov test, and in cases of significant deviations, a non-parametric test (Wilcoxon Signed Ranks) was conducted. For each region, ANCOVAs were used to test for the effects of month (factor) or maternity status (factor) on the relationship between MOD (dependent) and female TL (fixed covariate). The same approach was used to test the effects of month (factor) or maternity status (factor) on the relationship between total number of mature ova (dependent) and female TL (fixed covariate). In all of the above analyses, residual plots were examined to confirm that the error terms met the assumptions of normality and homogeneity of variance. To test whether the proportions of mature females with developing egg cases in utero were equal among months, a Chi-square goodness of fit test was performed. The expected values were calculated by multiplying the total sample size for each month by the total mean percentage gravid for all months combined. Following Cohen (1988), a power analysis was conducted to assess the Chi-square test.
Results
Biological measurements
Eastern Bering Sea
In total, 720 B. interrupta individuals were collected in the EBS between 2004 and 2007, although reproductive data and maturity stages were only recorded in 2004. The TLs ranged from 18 to 87 cm (mean = 63 cm, s.d. = 18 cm, n = 362) for females and 16 to 89 cm for males (mean = 58 cm, s.d. = 18 cm, n = 352). The weights ranged from 0.36 to 4.16 kg for females (mean = 2.33 kg, s.d. = 0.88 kg, n = 49) and 0.25 to 3.00 kg for males (mean = 1.96 kg, s.d. = 0.55 kg, n = 44); no individuals less than 33 cm TL were weighed. The overall sex ratio in the EBS did not significantly differ from the expected 1 : 1 (χ0.05,12 = 0.200, P = 0.655). However, when broken down by maturity stage, significantly more juvenile males were caught than females (juveniles: χ0.05,12 = 6.821, P = 0.009), but no significant difference was observed for adolescents (χ0.05,12 =0.505, P = 0.477) or adults (χ0.05,12 = 3.706, P = 0.054).
Gulf of Alaska
In total, 1325 skates were collected in the GOA between 2005 and 2007. The TLs ranged from 17 to 88 cm for females (mean = 66 cm, s.d. = 15 cm, n = 700) and 18 to 84 cm for males (mean = 62 cm, s.d. = 16 cm, n = 616). The weights ranged from 0.03 to 4.00 kg for females (mean = 2.00, s.d. = 0.89 kg, n = 149) and 0.05 to 2.70 kg for males (mean = 1.56 kg, s.d. = 0.77 kg, n = 94); a few individuals of both sexes between 13 and 33 cm TL were weighed, providing a slightly broader range than the EBS. There was a significant departure from the expected 1 : 1 sex ratio, with more females than males (χ0.05,12 = 5.079, P = 0.024) caught overall. However, when the data were broken down by individual maturity stages, there was no significant difference in sex ratios (juveniles: χ0.05,12 =0.294, P = 0.588; adolescents: χ0.05,12 = 0.486, P = 0.486; adults: χ0.05,12 = 0.252, P = 0.616).
Comparisons
A significant difference was found in the TL-DW relationship between regions (ANCOVA: F1,1653 = 116.156, P < 0.001). However, no significant difference was found between sexes (F1,1653 = 0.021, P = 0.884), and there was no significant interaction found between region and sex (F1,1653 = 0.826, P =0.363). Therefore, sexes were pooled and a strong linear TL-DW relationship was identified for both the GOA (n = 1259, DW =0.650(TL) – 1.011, R2 = 0.980) and for the EBS (n = 399, DW =0.668(TL) – 1.111, R2 = 0.993).
There was a significant difference in the TL-W relationship between males and females (ANCOVA: F1,329 = 20.392, P <0.001) and between individuals from the EBS and GOA (F1,329 = 9.707, P = 0.002), with no significant interaction between the factors of sex or region (F1,329 = 0.636, P = 0.426). The TL-W relationships are described by the following:
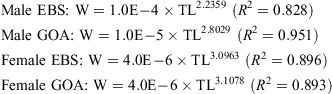
Maturity
Eastern Bering Sea
Maturity stages were assigned for 226 males and 177 females. The largest immature male was 85 cm TL, while the smallest mature male was 65 cm (Fig. 1). The largest immature female was 84 cm TL, while the smallest mature female was 69 cm. The TL at 50% maturity (TL50) was estimated as 70 cm TL for males and 72 cm TL for females (males: R2 = 0.988, P < 0.0001, n = 43; females: R2 = 0.985, P < 0.0001, n = 43) (Fig. 1).
Gulf of Alaska
Maturity stages were assigned for 572 males and 636 females. The largest immature male was 78 cm TL, while the smallest mature male was 63 cm TL (Fig. 1). The largest immature female was 86 cm TL, while the smallest mature female was 67 cm. The TL50 was estimated as 69 cm TL for males and 71 cm TL for females (males: R2 = 0.981, P < 0.0001, n = 45; females: R2 = 0.993, P < 0.0001, n = 46) (Fig. 1).
Maturity hypotheses
Sex and TL were statistically significant predictors of maturity (hypothesis 1 – sex: χ0.05,12 = 13.617, P < 0.001; TL: χ0.05,12 =1546.423, P < 0.001), while region (GOA versus EBS) was not significant (hypothesis 2 – χ0.05,12 = 0.005, P = 0.945).
Reproduction
Eastern Bering Sea
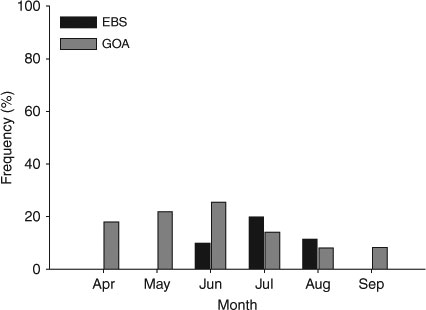
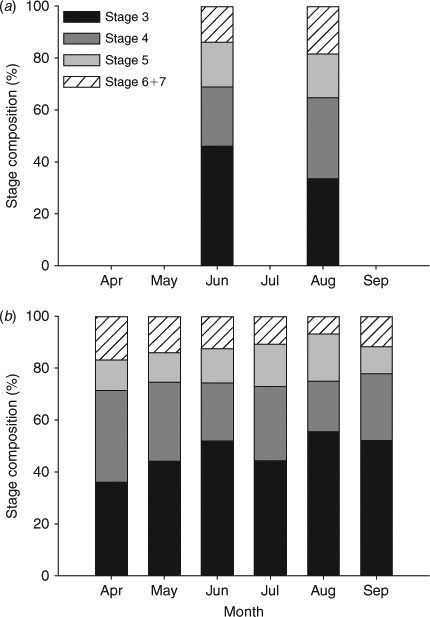
The mean number of mature ova, when mature ova were present, was 11.6 ova (±6.2 s.d., n = 45), and the mean MOD was 30.5 mm (±5.4 mm s.d., n = 45). No difference was found in the mean number of mature ova in the left and right ovaries using a paired t-test (t0.05(2),41 = –0.556, P = 0.581). No significant relationship was found between the MOD and female TL when accounting for the month of sampling (ANCOVA: F1,39 = 0.606, P = 0.441) or maternity status (F1,41 = 0.835, P = 0.366). Additionally, no significant relationship was found between the number of mature ova and the female TL for the EBS, regardless of the month of sampling (ANCOVA: F1,39 = 1.690, P = 0.201) or the maternity status of the female (F1,41 = 2.250, P = 0.141). Owing to sample size limitations, a statistical comparison of the proportion of mature females with developing egg cases in utero was not possible; nevertheless, proportions from June, July and August appear approximately equal, with a slight peak in July (Fig. 2). Only six histology samples out of nine were available from the EBS; males with mature spermatocysts were found in both months examined (Fig. 3).
|
|
Gulf of Alaska
The mean number of mature ova was 10.3 (±4.3 s.d., n = 257), and the MOD was 32.1 mm (±6.7 mm s.d., n = 298). As differences in left and right ova counts were not normally distributed (Kolmogorov–Smirnov test: Z = 1.416, P = 0.036, n = 256), a Wilcoxon Signed Ranks test was conducted and found no significant difference between left and right ova counts in the GOA (Z0.05(2) = –0.329, P = 0.742, n = 256).
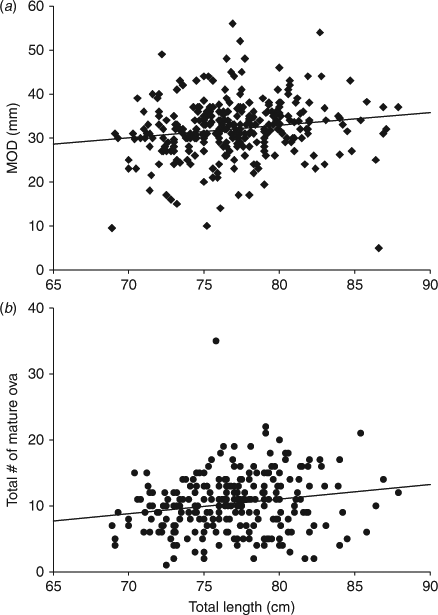
While the female TL-MOD relationship was significant (ANCOVA: F1,286 = 12.787, P < 0.001), it did not differ by month of sampling (F5,286 = 0.851, P = 0.514) and no significant interaction was detected between month and TL (F5,286 = 0.933, P = 0.460). Additionally, when all months were pooled and the relationship was tested for differences between gravid and non-gravid mature females (maternity status), no significant relationship with female TL was detected (ANCOVA: F1,294 = 0.887, P = 0.347). The MOD-female TL relationship can be described as a significant, but highly variable linear regression (R2 = 0.025, F1.296 = 7.631, P = 0.006; Fig. 4).
|
A positive significant relationship was found between female TL and the total number of mature ova (ANCOVA: F1,245 =17.280, P < 0.001); the relationship did not differ among months of sampling (F5,245 = 1.554, P = 0.174) and there was no significant interaction between maternity status and female TL (F5,245 = 1.748, P = 0.124). When the relationship was tested for differences between gravid and non-gravid mature females with all months pooled, a positive significant relationship was detected for the GOA (ANCOVA: F1,253 = 10.372, P = 0.001). The relationship did not differ between maternity statuses (F1,253 = 2.470, P = 0.117), and there was no significant interaction between month and TL (F1,253 = 2.579, P = 0.110). Therefore, the relationship between female TL and total number of mature ova can be described by a significant, but highly variable linear regression (R2 = 0.033, F1,255 = 8.830, P = 0.003; Fig. 4).
A Chi-square test comparing the proportion of mature females with developing egg cases in utero failed to reject the null hypothesis of equal proportions among months (χ0.05,52 = 8.27, P > 0.10, n = 343; Fig. 2). However, a post hoc power analysis determined that there was only a 54% probability of rejecting the null if the null was false (α = 0.05, d.f. = 5, n = 51, w = 0.41, power = 54).
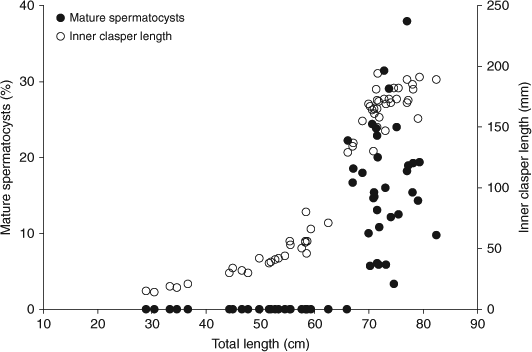
Results of the histological analysis demonstrate that spermatocysts began to mature in males of 62.0 cm TL or greater and were present in all males over 66 cm TL (Fig. 5). Of the 70 testes from the GOA prepared for histology, 67 were useable for analysis. Males with mature spermatocysts were found in each month studied (Fig. 3). However, statistical analyses were not conducted owing to the highly variable sample sizes.
|
Discussion
Sexual dimorphism
The largest B. interrupta reported in this study (a male from the EBS with TL = 89.0 cm) exceeded the maximum size presently reported in literature (Mecklenburg et al. 2002; Ebert 2005). The largest female was 87.9 cm (GOA), supporting the findings of Ebert (2005) that there is little to no sexual dimorphism in maximum size of this species. In general, contrary to the commonly held axiom that elasmobranchs exhibit sexual dimorphism in size, this is not a uniform characteristic in elasmobranchs, especially in small- and medium-sized skates (<150 cm) (Ebert et al. 2008a, 2008b). Regional differences can occur in maximum size, for example both sexes of three skate species from southern Africa attained a larger maximum size on the west coast (= Benguela Current Large Marine Ecosystem) than they do on the south coast (= Agulhas Current Large Marine Ecosystem) (Ebert et al. 2008b). There was no difference in maximum size observed between regions for B. interrupta, although there were differences in weight at length. Males from the EBS were heavier at length than GOA males until they reached maturity. Conversely, EBS and GOA females had a very similar TL-W relationship until they reached 60 cm TL.
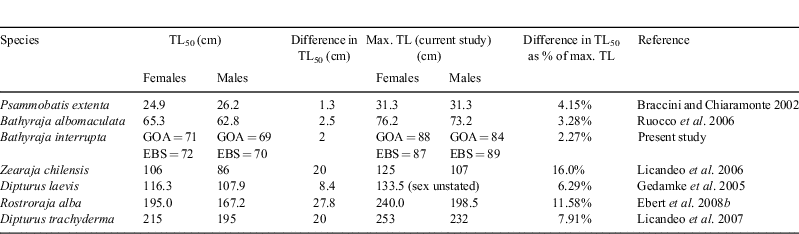
Although there was no sexual dimorphism found in maximum size, B. interrupta females matured at a larger size than males in both regions. This is true for many elasmobranchs (Cortés 2000), yet not always true in skates (Ebert 2005; Ruocco et al. 2006). Skates vary considerably in sexual dimorphism in size at maturity (Table 1), with those species exhibiting strong sexual dimorphism generally being larger skate species (>1.2 m TL). However, many small- and medium-sized skate species show distinct, but relatively small differences in size at sexual maturity, such as B. interrupta.
|
Similarly, there may be a difference between sexes in age at maturity, which is an essential maturity parameter for fisheries management. Determining whether sexual dimorphism exists in age at maturity becomes important in the application of demographic models, which typically project the rate of population change for a single sex. If sexual dimorphism in age at maturity does not exist, as found by Ainsley (2009) for B. interrupta, then pooling the sexes can reduce variability in this parameter.
Regional variation in size at maturity
Regional differences in size at maturity have been noted for several species of elasmobranchs (Templeman 1987; Lombardi-Carlson et al. 2003), and this variability is important to recognise when managing widely distributed species. While this study did not detect a significant difference in B. interrupta maturity curves (hypothesis 2), Ainsley (2009) found a significant difference in age at maturity for B. interrupta between the EBS and GOA, indicating that differences in growth can lead to differences in age at maturity among regions, even where there is no difference in size at maturity. The results obtained in the present study may have been confounded by temporal variance since no reproductive samples were obtained from the EBS after 2004 and all GOA samples were collected between 2005 and 2007. However, the TL50 values for the EBS estimated from this study were very similar to those estimated by Ebert (2005) for samples collected in 2002, so it is unlikely that the relatively short mismatch in sampling periods confounded the regional comparison.
Studies have found evidence of significant differences in size at maturity along a latitudinal gradient for viviparous and oviparous chondrichthyans (Lombardi-Carlson et al. 2003; Colonello et al. 2007; Barnett et al. 2009), and in general the later-to-mature populations are found in the higher latitudes (Cope 2006). The sampling locations in the two regions compared in the present study occupy the same latitudinal range (54° to 60°N), but there may be a latitudinal rather than regional difference in size at maturity in B. interrupta. Bizzarro and Vaughn (2009), using the same maturity criteria, found the median size at maturity of B. interrupta in insular waters of south-eastern Alaska (54°N) to be several cm smaller than the estimates from this study. Given this, maturity studies of B. interrupta from further south, in British Columbia, Canada and Washington State, USA, should be conducted using similar criteria for reproductive classifications to elucidate whether this trend in size at maturity is real or an effect of sampling.
Late maturation
The tendency of elasmobranchs to mature relatively later and larger than teleosts is often used to explain why they may be more vulnerable to overfishing (Walker and Hislop 1998; Dulvy et al. 2000; Dulvy and Reynolds 2002). While most teleosts begin to mature between 40 and 80% of their maximum size (Beverton and Holt 1959), elasmobranchs mature towards the higher end of this spectrum (Holden 1974), with skates maturing between 75 and 90% of their maximum TL (Ebert 2005; Gedamke et al. 2005). B. interrupta matures at a larger size (78–82% of the maximum observed TL) and later in life (~57% of oldest estimated age) relative to many other species managed in commercial fisheries (Ainsley 2009). The delay in size and age at maturity indicates that immature individuals may make up a larger proportion of direct or indirect catch than in typical teleost fisheries, and traditional teleost management strategies may not be appropriate for skates.
Determination of the maturity status
Past studies have used an abrupt change in clasper length as an indication of male sexual maturity. Eleven of the sixty-seven males (16%) examined for spermatocycst counts had been given a maturity level of 3 in the field based on clasper rigidity, but were later determined to have mature spermatozoa. In at least two of these 11 cases, some coiling was visible but the claspers were still flexible, indicating that despite the presence of mature spermatozoa, these males could not have successfully copulated. A study of the thorny skate, Amblyraja radiata, found that size at 50% maturity calculated using clasper length was smaller than size calculated using histological analysis (Sulikowski et al. 2006). This contrasts with results of the present study, which indicate the claspers developed more gradually than spermatozoa, in some cases fully maturing after the spermatozoa. To ensure the most accurate determination of the maturity status of a skate in a species that reproduces year round, at least two of these parameters (rigidity of the claspers and the presence of mature spermatozoa) should be considered when practical.
Female size and fecundity
An increase in the number of embryos with increasing female TL is not uncommon in viviparous elasmobranchs (Lenanton et al. 1990; Yamaguchi et al. 2000; McAuley et al. 2007; Walker 2007). However, this relationship is more difficult to examine in elasmobranchs that lay egg cases over an extended period of time. The present study showed a significant but highly variable relationship between TL and the total number of mature ova. A similar relationship was also detected for B. parmifera (Matta 2006), implying that, like many teleosts and sharks, larger and older female skates potentially have higher annual fecundity. Additionally, a relationship was found between female TL and the MOD, regardless of month of sampling, which is in agreement with previous work on B. interrupta and several other Alaskan Bathyraja species (Ebert 2005; Matta 2006). Hoff (2007) found a significant but weak relationship between the embryo size and egg case width and a significant but weak relationship between egg case width and female size in B. parmifera, indicating that there may be a positive correlation between female size and embryo size. Since the largest ovum in a female may be days or weeks away from being deposited, it would be more informative to use the size of ova in a fully enclosed egg case ready to be deposited.
Reproductive resting stage
Females of some species may go through periods of ‘resting’ or senescence during which they are reproductively inactive (Richards et al. 1963; Holden et al. 1971), which seemed to be the case for a few adult females in both regions that were assigned a maturity condition of ‘3+/4–’ (EBS = 6/178 and GOA = 7/638). An adult female in a resting or spent stage has greatly reduced ovaries that appear similar to those of a juvenile or adolescent, but the uterus walls appear considerably thicker and more stretched out than those of an adolescent (Ebert 2005). In study of five captive female Japanese common skates, Okamejei kenojei (Ishihara et al. 2002), one skate that was kept for over 7 years laid fewer egg cases in the last 2 years of the study, indicating possible decreased fecundity and impending senescence. The ‘3+/4–’ individuals found in this study were not necessarily the largest or the oldest and may represent maturing females entering their first reproductive cycle. Additionally, there does not appear to be any seasonal pattern to the proportion of resting B. interrupta females, as found for Alantoraja cyclophora (Oddone and Vooren 2005). Koob et al. (1986) suggested that Raja erinacea, which reproduces year round, undergoes cyclic (though not necessarily synchronised) periods of reproductive inactivity and ovarian recrudescence between spawning periods. If skates have inconsistent periods of reproductive resting, this would be important to account for when quantifying lifetime fecundity for demography and fisheries management purposes.
Reproductive seasonality
Although 51 gravid females were obtained from the GOA and 7 from the EBS, sample collection was limited to only 6 and 3 consecutive months, respectively. As a consequence, results do not conclusively support year-round egg case deposition. However, previous studies of skate reproduction have revealed this to be the case in several Bathyraja species from the eastern North Pacific (Davis 2006; Matta 2006). As such, year-round egg production is likely in B. interrupta, but samples from the winter months are necessary to confirm this.
Maximum total length and susceptibility
From results obtained for B. interrupta , it can be inferred that skates exhibit variable life-history traits. Previous studies have suggested that the maximum TL of fish species may be an acceptable proxy for estimating susceptibility to overfishing (Jennings et al. 1998; Frisk et al. 2001), and that species may be prioritised for management by size. This is based on the notion that skates of similar sizes share similar life-history parameters, and specifically that larger species of elasmobranchs have a higher longevity and lower growth rates (growth coefficient, k). Since there does not appear to be a connection between maximum TL and life-history traits for skates in the eastern North Pacific (Ebert et al. 2007, 2009), this is not a useful proxy and is not recommended for use by managers of skate fisheries as an approximation of susceptibility to overfishing.
Conclusions
The present study provides critical life-history information on an individual skate species such that management agencies can begin to address concerns regarding lack of species-specific data for the Alaskan skate complex. The results of the study indicate that B. interrupta shows late maturity, typical of most skate species. Additionally, a statistically significant difference in size at maturity was found between sexes. While this difference may not be biologically significant, the distinct parameters can be incorporated into the demographic models for the regional management plans. High levels of fishing mortality have been implicated in dramatic changes in the abundance, distribution and size composition of several skate species in other marine regions and can be perceptible in less than a decade of fishing (Walker and Hislop 1998; Agnew et al. 2000). Although B. interrupta is currently not a targeted skate species, it is caught as bycatch and occasionally retained (Stevenson and Lewis 2010). As fisheries expand secondary elasmobranch species can increasingly become commercially important and it is possible that smaller species may be targeted in the future. Given the relatively large size at maturity for B. interrupta and other species, the magnitude of fishing effort within the region and elsewhere, and the susceptibility of skates to fishing mortality before maturity, more detailed monitoring of skate populations and precautionary management is warranted. The findings of this study highlight the broader importance of estimating critical life-history information on skate species-complexes not only on a species-specific basis, but also within geographically extensive stocks.
Acknowledgements
We thank Eric Brown, Sarah Gaichas, Christopher Gburski, Dan Kimura, Ned Laman, Bob Lauth, Frank Shaw, and Jim Stark from National Marine Fisheries Service Alaska Fisheries Science Center in Seattle; Rob Swanson (NMFS-AFSC), Lynne Mattes and Kally Spalinger of the Alaska Department of Fish and Game (ADFG) in Kodiak; Ken Goldman, and Mike Byerly (ADFG) in Homer; Tory O’Connell (ADFG) in Sitka; James Sulikowski (University of New England); and Louisiana State University veterinary laboratories for their invaluable assistance on various portions of this study. We especially thank the following individuals at the Pacific Shark Research Center at Moss Landing Marine Laboratories: Lewis Barnett, Joe Bizzarro, Mariah Boyle, Simon Brown, Jasmine Maurer, Diane Haas, Ashley Neway, and Wade Smith. Animal care approval was obtained from the Institutional Animal Care and Use Committee (IACUC #801) at San José State University and specimens were collected under an ADFG fish resource permit (CF-06–052). We thank the Guest Editors, the anonymous referees, and James Lindholm for their insightful comments that improved the manuscript. Funding for this project was provided by the North Pacific Research Board under project 510 and by NOAA/NMFS to the National Shark Research Consortium and Pacific Shark Research Center. San Jose State University Lottery Funds, Earl and Ethel Myers Foundation Grant, and the David and Lucille Packard Foundation provided additional funding support. This is North Pacific Research Board publication #290.
References
Agnew, D. J., Nolan, C. P., Beddington, J. R., and Baranowski, R. (2000). Approaches to the assessment and management of multispecies skate and ray fisheries using the Falkland Islands fishery as an example. Canadian Journal of Fisheries and Aquatic Sciences 57, 429–440.| Approaches to the assessment and management of multispecies skate and ray fisheries using the Falkland Islands fishery as an example.Crossref | GoogleScholarGoogle Scholar |
Ainsley, S. M. (2009). Age, growth and reproduction of the Bering skate, Bathyraja interrupta (Gill and Townsend, 1897), from the eastern Bering Sea and Gulf of Alaska. M.S. Thesis, California State University, Monterey Bay, CA.
Barnett, L. A. K., Earley, R. L., Ebert, D. A., and Cailliet, G. M. (2009). Maturity, fecundity, and reproductive cycle of the spotted ratfish, Hydrolagus colliei. Marine Biology 156, 301–316.
| Maturity, fecundity, and reproductive cycle of the spotted ratfish, Hydrolagus colliei.Crossref | GoogleScholarGoogle Scholar |
Beverton, R. J. H., and Holt, S. J. (1959). A review of the lifespans and mortality rates of fish in nature and the relation to growth and other physiological characteristics In ‘Ciba Foundation, Colloquia in Ageing V. The Life Span of Animals’. (Eds G. E. W. Wolstenholme and M. O’Conner.) pp. 142–177. (Churchill: London.)
Bizzarro, J. J., and Vaughn, M. T. (2009). Aspects of the biology and species composition of skates (Rajiformes) from insular waters of southeastern Alaska. Northwestern Naturalist (Olympia, Wash.) 90, 247–256.
| Aspects of the biology and species composition of skates (Rajiformes) from insular waters of southeastern Alaska.Crossref | GoogleScholarGoogle Scholar |
Braccini, J. M., and Chiaramonte, G. E. (2002). Reproductive biology of Psammobatis extenta. Journal of Fish Biology 61, 272–288.
| Reproductive biology of Psammobatis extenta.Crossref | GoogleScholarGoogle Scholar |
Cohen, J. (1988). ‘Statistical Power Analysis for the Behavioral Sciences.’ (Erlbaum Associates: Hillsdale, NJ.)
Colonello, J. H., Lucifora, L. O., and Massa, A. M. (2007). Reproduction of the angular angel shark (Squatina guggenheim): geographic differences, reproductive cycle, and sexual dimorphism. ICES Journal of Marine Science 64, 131–140.
Cope, J. M. (2006). Exploring intraspecific life history patterns in sharks. Fishery Bulletin 104, 311–320.
Cortés, E. (2000). Life history patterns and correlations in sharks. Reviews in Fisheries Science 8, 299–344.
Davis, C. (2006). Age, growth and reproduction of the roughtail skate, Bathyraja trachura (Gilbert, 1892), from the eastern North Pacific. M.S. Thesis, California State University, Monterey Bay, CA.
Dulvy, N. K., and Reynolds, J. D. (2002). Predicting extinction vulnerability in skates. Conservation Biology 16, 440–450.
| Predicting extinction vulnerability in skates.Crossref | GoogleScholarGoogle Scholar |
Dulvy, N. K., Metcalfe, J. D., Glanville, J., Pawson, M. G., and Reynolds, J. D. (2000). Fishery stability, local extinctions, and shifts in community structure in skates. Conservation Biology 14, 283–293.
| Fishery stability, local extinctions, and shifts in community structure in skates.Crossref | GoogleScholarGoogle Scholar |
Ebert, D. A. (2005). Reproductive biology of skates, Bathyraja (Ishiyama) along the eastern Bering Sea continental slope. Journal of Fish Biology 66, 618–649.
| Reproductive biology of skates, Bathyraja (Ishiyama) along the eastern Bering Sea continental slope.Crossref | GoogleScholarGoogle Scholar |
Ebert, D. A., Smith, W. D., Haas, D. L., Ainsley, S. M., and Cailliet, G. M. (2007). Life history and population dynamics of Alaskan skates: providing essential biological information for effective management of bycatch and target species. Final Report 510. North Pacific Research Board, Anchorage, AK.
Ebert, D. A., Smith, W. D., and Cailliet, G. M. (2008). Reproductive biology of two commercially exploited skates, Raja binoculata and R. rhina, in the western Gulf of Alaska. Fisheries Research 94, 48–57.
| Reproductive biology of two commercially exploited skates, Raja binoculata and R. rhina, in the western Gulf of Alaska.Crossref | GoogleScholarGoogle Scholar |
Ebert, D. A., Compagno, L. J. V., and Cowley, P. D. (2008). Aspects of the reproductive biology of skates (Chondrichthyes: Rajiformes: Rajoidei) from southern Africa. ICES Journal of Marine Science 65, 81–102.
| Aspects of the reproductive biology of skates (Chondrichthyes: Rajiformes: Rajoidei) from southern Africa.Crossref | GoogleScholarGoogle Scholar |
Ebert, D. A., Maurer, J. R., Ainsley, S. M., Barnett, L. A. K., and Cailliet, G. M. (2009). Life history and population dynamics of four endemic Alaskan skates: determining essential biological information for effective management of bycatch and target species. Final Report 715. North Pacific Research Board, Anchorage, AK.
Food and Agriculture Organization (2009). The state of world marine fishery resources. Food and Agriculture Organization, Rome.
Francis, M. P. (2006). Morphometric minefields – towards a measurement standard for chondrichthyan fishes. Environmental Biology of Fishes 77, 407–421.
| Morphometric minefields – towards a measurement standard for chondrichthyan fishes.Crossref | GoogleScholarGoogle Scholar |
Frisk, M. G., Miller, T. J., and Fogarty, M. J. (2001). Estimation and analysis of biological parameters in elasmobranch fishes: a comparative life history study. Canadian Journal of Fisheries and Aquatic Sciences 58, 969–981.
| Estimation and analysis of biological parameters in elasmobranch fishes: a comparative life history study.Crossref | GoogleScholarGoogle Scholar |
Frisk, M. G., Miller, T. J., and Dulvy, N. K. (2005). Life histories and vulnerability to exploitation of elasmobranchs: inferences from elasticity, perturbation and phylogenetic analyses. Journal of Northwest Atlantic Fishery Science 35, 27–45.
| Life histories and vulnerability to exploitation of elasmobranchs: inferences from elasticity, perturbation and phylogenetic analyses.Crossref | GoogleScholarGoogle Scholar |
Gedamke, T., DuPaul, W. D., and Musick, J. A. (2005). Observations on the life history of the barndoor skate, Dipturus laevis, on Georges Bank (western north Atlantic). Journal of Northwest Atlantic Fishery Science 35, 67–78.
| Observations on the life history of the barndoor skate, Dipturus laevis, on Georges Bank (western north Atlantic).Crossref | GoogleScholarGoogle Scholar |
Hoff, G. R. (2007). Reproductive biology of the Alaska skate, Bathyraja parmifera, with regard to nursery sites, embryo development and predation. Ph.D. Thesis, University of Washington, Seattle, WA.
Hoff, G. R., and Britt, L. L. (2005). Results of the 2004 Eastern Bering Sea upper continental slope survey of groundfish and invertebrate resources. Technical Memorandum NMFS-AFSC-156. National Oceanic and Atmospheric Administration, Seattle, WA.
Holden, M. J. (1974). Problems in the rational exploitation of elasmobranch populations and some suggested solutions. In ‘Sea Fisheries Research’. (Ed. F. R. Harden-Jones.) pp. 117–137. (John Wiley and Sons: New York.)
Holden, M. J., Rout, D. W., and Humphreys, C. N. (1971). The rate of egg laying by three species of ray. Journal du Conseil International pour l’Exploration de la Mer 33, 335–339.
Ishihara, H., Mochizuki, T., Homma, K., and Taniuchi, T. (2002). Reproductive strategy of the Japanese common skate (spiny rasp skate) Okameji kenojei. In ‘Elasmobranch Biodiversity, Conservation and Management: Proceedings of the International Seminar and Workshop, Sabah, Malaysia, July, 1997’. (Eds S. L. Fowler, T. M. Reed and F. A. Dipper.) pp. 236–240. (IUCN SSC Shark Specialist Group: Gland.)
Jennings, S., Reynolds, J. D., and Mills, S. C. (1998). Life history correlates of response to fisheries exploitation. Proceedings of the Royal Society of London. Series B. Biological Sciences 265, 333–339.
| Life history correlates of response to fisheries exploitation.Crossref | GoogleScholarGoogle Scholar |
Koob, T. J., Tsang, P., and Callard, I. P. (1986). Plasma estradiol, testosterone, and progesterone levels during the ovulatory cycle of the skate (Raja erinacea). Biology of Reproduction 35, 267–275.
| Plasma estradiol, testosterone, and progesterone levels during the ovulatory cycle of the skate (Raja erinacea).Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL28Xls12gurs%3D&md5=c46e76666cf36c3a36dcdeb4f72064f0CAS | 3768454PubMed |
Lenanton, R. C. J., Heald, D. I., Platell, M., Cliff, M., and Shaw, J. (1990). Aspects of the reproductive biology of the gummy shark, Mustelus antarcticus Günther, from waters off the south coast of Western Australia. Australian Journal of Marine and Freshwater Research 41, 807–822.
| Aspects of the reproductive biology of the gummy shark, Mustelus antarcticus Günther, from waters off the south coast of Western Australia.Crossref | GoogleScholarGoogle Scholar |
Licandeo, R. R., Lamilla, J. G., Rubilar, P. G., and Vega, R. M. (2006). Age, growth, and sexual maturity of the yellownose skate Dipturus chilensis in the south-eastern Pacific. Journal of Fish Biology 68, 488–506.
| Age, growth, and sexual maturity of the yellownose skate Dipturus chilensis in the south-eastern Pacific.Crossref | GoogleScholarGoogle Scholar |
Licandeo, R., Cerna, F., and Céspedes, R. (2007). Age, growth, and reproduction of the roughskin skate, Dipturus trachyderma, from the southeastern Pacific. ICES Journal of Marine Science 64, 141–148.
Lombardi-Carlson, L. A., Cortés, E., Parsons, G. R., and Manire, C. A. (2003). Latitudinal variation in life-history traits of bonnethead sharks, Sphyran tiburo, (Carcharhiniformes: Sphyrnidae) from the eastern Gulf of Mexico. Marine and Freshwater Research 54, 875–883.
| Latitudinal variation in life-history traits of bonnethead sharks, Sphyran tiburo, (Carcharhiniformes: Sphyrnidae) from the eastern Gulf of Mexico.Crossref | GoogleScholarGoogle Scholar |
Maruska, K. P., Cowie, E. G., and Tricas, T. C. (1996). Periodic gonadal activity and protracted mating in elasmobranch fishes. The Journal of Experimental Zoology 276, 219–232.
| Periodic gonadal activity and protracted mating in elasmobranch fishes.Crossref | GoogleScholarGoogle Scholar |
Matta, M. E. (2006). Aspects of the life history of the Alaska skate, Bathyraja parmifera, in the eastern Bering Sea. M.S. Thesis, School of Aquatic and Fisheries Sciences, University of Washington, Seattle, WA.
Matta, M. E., Gaichas, S., Lowe, S., Stevenson, D., Hoff, J., et al. (2006). Bering Sea and Aleutian Islands Skates. In ‘Stock Assessment and Fishery Evaluation Report for the Groundfish Resources of the Bering Sea and Aleutian Islands for 2007’. (Ed. The Plan Team for the Groundfish Fisheries of the Bering Sea and Aleutian Islands.) pp. 1017–1062. (North Pacific Fishery Management Council: Anchorage, AK.)
McAuley, R. B., Simpfendorfer, C. A., Hyndes, G. A., and Lenanton, R. C. J. (2007). Distribution and reproductive biology of the sandbar shark, Carcharinus plumbeus (Nardo), in Western Australian waters. Marine and Freshwater Research 58, 116–126.
| Distribution and reproductive biology of the sandbar shark, Carcharinus plumbeus (Nardo), in Western Australian waters.Crossref | GoogleScholarGoogle Scholar |
McFarlane, G. A., and King, J. R. (2006). Age and growth of big skate (Raja binoculata) and longnose skate (Raja rhina) in British Columbia waters. Fisheries Research 78, 169–178.
| Age and growth of big skate (Raja binoculata) and longnose skate (Raja rhina) in British Columbia waters.Crossref | GoogleScholarGoogle Scholar |
Mecklenburg, C. W., Mecklenburg, T. A., and Thorsteinson, L. K. (2002). ‘Fishes of Alaska.’ (American Fisheries Society: Bethesda, MD.)
Oddone, M. C., and Vooren, C. M. (2005). Reproductive biology of Atlantoraja cyclophora (Regan 1903) (Elasmobranchii: Rajidae) off southern Brazil. ICES Journal of Marine Science 62, 1095–1103.
| Reproductive biology of Atlantoraja cyclophora (Regan 1903) (Elasmobranchii: Rajidae) off southern Brazil.Crossref | GoogleScholarGoogle Scholar |
Ormseth, O., and Matta, B. (2007a). Gulf of Alaska Skates. In ‘Stock Assessment and Fishery Evaluation Report for the Groundfish Resources of the Gulf of Alaska’. (Ed. The Plan Team for the Groundfish Fisheries of the Gulf of Alaska.) pp. 957–1008. (North Pacific Fishery Management Council: Anchorage, AK.)
Ormseth, O., and Matta, B. (2007b). Bering Sea and Aleutian Islands Skates. In ‘Stock Assessment and Fishery Evaluation Report for the Groundfish Resources of the Bering Sea and Aleutian Islands’. (Ed. The Plan Team for the Groundfish Fisheries of the Bering Sea and Aleutian Islands). pp. 909–1001. (North Pacific Fishery Management Council: Anchorage.)
Richards, S. W., Merriman, D., and Calhoun, L. H. (1963). Studies on the marine resources of southern New England. IX. The biology of the little skate, Raja erinacea (Mitchell). Bulletin of the Bingham Oceanographic Collection 18, 1–67.
Ricker, W. E. (1973). Linear regressions in fishery research. Journal of the Fisheries Research Board of Canada 30, 409–434.
Roa, R., Ernst, B., and Tapia, F. (1999). Estimation of size at sexual maturity: an evaluation of analytical and resampling procedures. Fishery Bulletin 97, 570–580.
Rochet, M. J. (2000). May life history traits be used as indices of population viability? Journal of Sea Research 44, 145–157.
| May life history traits be used as indices of population viability?Crossref | GoogleScholarGoogle Scholar |
Ruocco, N. L., Lucifora, L. O., Diaz de Astarloa, J. M., and Wöhler, O. (2006). Reproductive biology and abundance of the white-dotted skate, Bathyraja albomaculata, in the Southwest Atlantic. ICSE Journal of Marine Science 63, 105–116.
| Reproductive biology and abundance of the white-dotted skate, Bathyraja albomaculata, in the Southwest Atlantic.Crossref | GoogleScholarGoogle Scholar |
Sosebee, K. A. (2005). Are density-dependent effects on elasmobranch maturity possible? Journal of Northwest Atlantic Fishery Science 35, 115–124.
| Are density-dependent effects on elasmobranch maturity possible?Crossref | GoogleScholarGoogle Scholar |
Stevens, J. D., Bonfil, R., Dulvy, N. K., and Walker, P. A. (2000). The effects of fishing on sharks, rays, and chimaeras (chondrichthyans), and the implications for marine ecosystems. ICES Journal of Marine Science 57, 476–494.
| The effects of fishing on sharks, rays, and chimaeras (chondrichthyans), and the implications for marine ecosystems.Crossref | GoogleScholarGoogle Scholar |
Stevenson, D. E., and Lewis, K. A. (2010). Observer-reported skate bycatch in the commercial groundfish fisheries of Alaska. Fishery Bulletin 108, 208–217.
Sulikowski, J. A., Tsang, P. C. W., and Howell, W. H. (2005). Age and size at sexual maturity for the winter skate, Leucoraja ocellata, in the western Gulf of Maine based on morphological, histological and steroid hormone analyses. Environmental Biology of Fishes 72, 429–441.
| Age and size at sexual maturity for the winter skate, Leucoraja ocellata, in the western Gulf of Maine based on morphological, histological and steroid hormone analyses.Crossref | GoogleScholarGoogle Scholar |
Sulikowski, J. A., Kneebone, J., Elzey, S., Jurek, J., Danley, P. D., et al. (2006). Using the composite variables of reproductive morphology, histology and steroid hormones to determine age and size at sexual maturity for the thorny skate Amblyraja radiata in the western Gulf of Maine. Journal of Fish Biology 69, 1449–1465.
| Using the composite variables of reproductive morphology, histology and steroid hormones to determine age and size at sexual maturity for the thorny skate Amblyraja radiata in the western Gulf of Maine.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XhtlWhtbbF&md5=073c4676f68887cbb4606382ac2564a5CAS |
Templeman, W. (1987). Differences in sexual maturity and related characteristics between populations of thorny skate (Raja radiata) in the Northwest Atlantic. Journal of Northwest Atlantic Fishery Science 7, 155–167.
| Differences in sexual maturity and related characteristics between populations of thorny skate (Raja radiata) in the Northwest Atlantic.Crossref | GoogleScholarGoogle Scholar |
Walker, T. I. (2007). Spatial and temporal variation in the reproductive biology of gummy shark Mustelus antarcticus (Chondrichthyes: Triakidae) harvested off southern Australia. Marine and Freshwater Research 58, 67–97.
| Spatial and temporal variation in the reproductive biology of gummy shark Mustelus antarcticus (Chondrichthyes: Triakidae) harvested off southern Australia.Crossref | GoogleScholarGoogle Scholar |
Walker, P. A., and Hislop, J. R. G. (1998). Sensitive skates or resilient rays? Spatial and temporal shifts in ray species composition in the central and north-western North Sea between 1930 and the present day. ICES Journal of Marine Science 55, 392–402.
| Sensitive skates or resilient rays? Spatial and temporal shifts in ray species composition in the central and north-western North Sea between 1930 and the present day.Crossref | GoogleScholarGoogle Scholar |
Yamaguchi, A., Taniuchi, T., and Shimizu, M. (2000). Geographic variations in reproductive parameters of the starspotted dogfish, Mustelus manazo, from five localities in Japan and in Taiwan. Environmental Biology of Fishes 57, 221–233.
| Geographic variations in reproductive parameters of the starspotted dogfish, Mustelus manazo, from five localities in Japan and in Taiwan.Crossref | GoogleScholarGoogle Scholar |