Validation of age determination using otoliths of the European anchovy (Engraulis encrasicolus L.) in the Bay of Biscay
A. Uriarte A D , I. Rico A , B. Villamor B , E. Duhamel C , C. Dueñas B , N. Aldanondo A and U. Cotano AA AZTI Tecnalia, Marine Research Division, Herrera Kaia Portualdea z/g, E-20110 Pasaia, Spain.
B Instituto Español de Oceanografía (IEO), Promontorio de San Martín s/n, E-39080 Santander, Cantabria, Spain.
C Institut Français de Recherche et d’Exploitation de la Mer (IFREMER), Lorient station, 8 rue François Toullec F-56100, Lorient, France.
D Corresponding author. Email: auriarte@azti.es
Marine and Freshwater Research 67(7) 951-966 https://doi.org/10.1071/MF15092
Submitted: 3 March 2015 Accepted: 30 January 2016 Published: 28 April 2016
Journal Compilation © CSIRO Publishing 2016 Open Access CC BY-NC-ND
Abstract
Validation of the age determination procedure using otoliths of European anchovy in the Bay of Biscay was achieved by monitoring very strong year-classes in successive spring catches and surveys, as well as the seasonal occurrence of edge types. Historical corroboration of the ageing method was obtained by cross-correlation between successive age groups by year-classes in catches and surveys (1987–2013). Summary annual growth in length is also presented. Yearly annuli consist of a hyaline zone (either single or composite) and a wide opaque zone, disrupted occasionally by some typical checks (mainly at age-0 and age-1 at peak spawning time). Age determination, given a date of capture, requires knowledge of the typical annual growth pattern of otoliths, their seasonal edge formation by ages and the most typical checks. Most opaque growth occurs in summer and is minimal (translucent) in winter. Opaque zone formation begins earlier in younger fish (in spring), and this helps distinguish age-1 from age-2+.
Additional keywords: age estimation, checks, Engraulidae, otolith formation.
Introduction
Age determination for short-lived fish species by examination of otoliths can be difficult because of the predominance of young fish (0, 1 or 2 year olds), which often show false rings or checks (Melo 1984; Waldron 1994; Waldron and Kerstan 2001; Panfili et al. 2002). The assessment of growth and demography of short-lived species in temperate and tropical latitudes often uses length-based methods (Palomares et al. 1987; Bellido et al. 2000; Cubillos et al. 2001). However, insights into the growth dynamics and actual demography gained from the use of otoliths for age determination have led many groups working with short-lived pelagic species to adopt such procedures (Aguayo 1976; Thomas 1983, 1985; Melo 1984; Morales-Nin and Pertierra 1990; Hoedt 1992).
Age determination of sardines and anchovies in several areas of the Mediterranean and Atlantic waters is problematic (ICES 2009, 2011; Arneri et al. 2011). The European anchovy (Engraulis encrasicolus L.) supports an important fishery in the Bay of Biscay (latitude 42–48°N). Anchovy spawning takes place primarily from April to July during the spring warming up of sea surface temperatures (SST) between 13 and 19°C (Motos et al. 1996; Petitgas et al. 2010). There is a traditional Spanish fishery that takes place in spring based on purse seining, and a French fishery that occurs primarily during the second half of the year and based mostly on pelagic trawling (Uriarte et al. 1996; ICES 2014). Stock assessment relies on direct monitoring by surveys, namely an acoustic survey (Pelgas series; Massé 1996; Massé et al. 2016) and a daily egg production method (DEPM) survey (Bioman series; Motos et al. 2005; Santos et al. 2016). Both surveys supply biomass and population at age estimates in May for the integrated assessment carried out by the ICES (2014), which applies a Bayesian biomass-based model (Ibaibarriaga et al. 2011) structured on two age groups: 1 and ≥2 years older age classes (referred to as age-1 and age-2+).
Early studies on the growth of anchovy in the Bay of Biscay had been performed by direct examination of catch by length distributions (Navaz and Lozano 1966; Guerault and Avrilla 1973). However, otolith examination soon became the preferred method (Guerault and Avrilla 1974; Cendrero et al. 1981; Astudillo 1986; Junquera 1986). In recent decades, age determination from otolith examinations has followed procedures first applied by Uriarte and Astudillo (1987) and described and agreed through several workshops on anchovy age reading that have occurred since 1990 (reports are available in the repository of ICES Planning Group on Commercial. Catches, Discards and Biological Sampling (PGCCDBS), see http://www.ices.dk/community/Pages/PGCCDBS-doc-repository.aspx, accessed 2 January 2016; complete references available in ICES 2009). These reports have assessed the consistency and precision of age determinations. However, the original validation studies supporting this method were not published.
The purpose of this article is to present results of a study of the annual and seasonal growth of otoliths that served to validate age determination from otoliths for the anchovy in the Bay of Biscay, completed by historical corroboration of the age determination method up to the present day and a summary of the annual growth pattern (in length) of this anchovy resulting from it. Supplementary material is available online containing a seasonal description of otoliths by ages and of their marginal edge formation (with pictures; see first section of the Supplementary material), a quantitative analysis of the size of the annual otolith increments by ages (see second section of the Supplementary material) and a summary of the incidence of checks between 1984 and 1991 (see the third section of the Supplementary material).
The method has served to deduce the demography of the catches and of the population estimates from surveys since 1987. It provided the foundations for several studies on spatial distribution of adults at spawning time (Motos et al. 1996; Vaz et al. 2002; Ibaibarriaga et al. 2013) and on the modelling of otolith shape (Gonzalez-Salas and Lenfant 2007) and growth (Hernández et al. 2009, Pecquerie et al. 2009, 2012). Beyond this, recent studies on otolith micro-increment formation of this anchovy have enabled the daily rhythm of deposition to be established in larvae (Aldanondo et al. 2008), as well as in juveniles and adults (Cermeño et al. 2003), and the method for otolith daily increment examination (Cermeño et al. 2008). A validation of the formation of the first annulus for this anchovy is also presented in this special issue by daily increment examination of reared juveniles (Aldanondo et al. 2016).
Materials and methods
Sampling
Age validation was based on otoliths from monthly collections from the Spanish (national and regional) catch sampling programs between 1984 and 1992, taken at random up to 1986 and stratified to size categories from 1987 onwards (Table 1). Some additional samples came from acoustic surveys (SARACUS 1985 (Instituto Español de Oceanografía (IEO), Spain) and EIGAS 1985 and 1986 (Institut Français de Recherche et d’Exploitation de la Mer (IFREMER), France)). Length distribution and age composition of the Spanish spring fishery between 1983 and 1987 has been reported in Astudillo (1986) and Uriarte and Astudillo (1987), whereas for 1988–92 the data have been published in ICES reports (Anon. 1989, 1991, 1992, 1993). Although no otoliths were available for 1983, for the purposes of the present study we used the rough inference from lengths made by Uriarte and Astudillo (1987). Finally, for 1984, a new age–length key (ALK) was prepared based on the current age determination criteria and applied to the length distribution in Astudillo (1986).
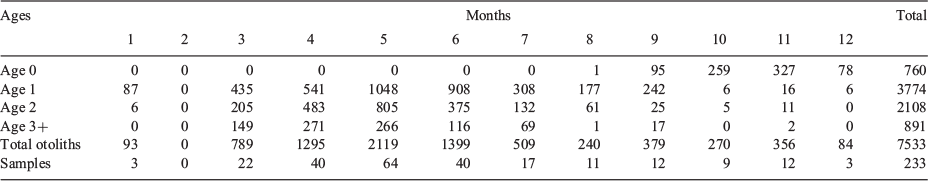
|
Biological samples consisted of a minimum of 40 fish, taken randomly from the landings, for which total length (TL; mm), weight (g) and sexual maturity was recorded and sagittal otoliths extracted. Otoliths were washed with water and dried for 24 h before setting the entire otolith in transparent resin inside holes made over black plastic slide containers. Both otoliths were laid in parallel with the sulcus facing down.
Age determination procedures
The otoliths were observed under a binocular microscope at a magnification of 20×, applying incident double oblique light (from both sides) as recommended in ICES (2009). The basic information required for age determination is the date of capture. Knowledge of individual fish length is not required. For this anchovy, conventional birthdate is set at 1 January. Very doubtful age determinations were discarded from posterior processing if not agreed after a second examination by two readers.
Age determination is based on knowledge of the annual growth pattern of anchovy otoliths, seasonal growth of the edge (by ages) and of the most typical checks. The ageing criteria agreed in the 2002 workshop (Uriarte et al. 2002), following the original validation, are listed below.
-
Number of complete annual growth zones (annuli): the assigned age equals the number of complete opaque growth zones corresponding to the expected annual growth pattern of the otoliths, excluding the marginal edge development of the year. The translucent zones are usually formed in winter, but are not necessarily present from the beginning of the year. Typically, successive annual opaque growth zones are expected to be of decreasing width (Morales-Nin and Panfili 2002). Where the number of opaque zones does not correspond to the typical expected annual growth pattern for the presumed age, the existence of false increments (checks) can be suspected.
-
Conformity of marginal edge development with the expected type of edge at the month of capture for the presumed age: if the edge does not match the expectation, then alternative interpretations could be considered, including the potential occurrence of checks.
This anchovy can lay down several typical checks (mainly during age-0 and age-1) and it is important to recognise these for correct age determination. These checks are easily recognised in old fish, for which annual growth patterns are well established and age determination is easy. The checks are usually faint translucent zones, clearly visible but of less intensity than true winter translucent zones placed within the typical annual opaque growth of otoliths. The checks do not always form a complete zone around the otolith.
Checks have been typified and are named with a ‘C’ plus two digits according to the age of the fish when formed (first digit) and to the approximate relative position over the expected annual growth of the otolith at that age (second digit). For example, the most typical checks formed during the first year of life (age-0) are termed ‘C05’ or ‘C08’ because they are formed at ~50 or ~80% respectively of the expected annual growth of the otolith at age-0. Other typical checks are ‘C12’, ‘C15’ or ‘C18’, which correspond to checks formed at age-1 at ~20, 50 or 80% respectively of the expected annual growth of the otolith for that age. The formation of checks C12/15 was studied along with evaluation of edge formation for age-1, noting the time of year when those checks were already recognisable.
The age compositions of catches were obtained by applying the respective ALK to the length distribution, usually on a half-yearly basis (but occasionally quarterly). Usually, more than 1000 age determinations support every ALK, along with considerable sampling for length distribution (ICES 2014), well above the minimum standard sampling requirement established in the European Union Community programme for the collection of data in the Fisheries sector (see https://datacollection.jrc.ec.europa.eu/c/document_library/get_file?uuid=296dffd3-9c81- 4759-b691-9b1654ea66b9&groupId=10213, accessed 2 January 2016). For the surveys, spatial explicit ALKs were applied to length distribution of the population estimates of the respective spatial strata (Boyra et al. 2013; Massé et al. 2016; Santos et al. 2016).
Age validations
The age determination method and otolith growth pattern were established through three complementary validation studies.
First, the annual growth pattern of otoliths (or annuli identification) was achieved by establishing the correspondence between the types of otoliths and ages in spring through the following two indirect methods (sensu Panfili et al. 2002):
-
Monitoring of the progression of strong year-classes in the catches over several years (correspondence of successive modal lengths with modal otolith types), which is a well-suited indirect method for short-lived species and for the first age groups of other species (Holden and Raitt 1974; Campana 2001). This was applied to the progression of the 1982 year class in the Spanish spring purse seine fishery between 1983 and 1986 (up to age-4, which was also seen as age-5 in 1987) and for the 1989 year class in the Spanish fishery throughout the year (between 1989 and 1991 up to age-2).
-
Verifying the consistency between sharp spawning biomass fluctuations recorded in the spring DEPM surveys 1987–92 and fluctuations in the biomass of the (presumed) 1-year-old recruits, which validates absolute correspondence between Type I otoliths and the age-1 group. This relies on the fact that for a fish fully mature at age-1 (like this anchovy; Motos 1996), if the survey’s observations are rather precise, any sharp increase in the spawning biomass has to be due to a major increase in the 1-year-old recruits.
Second, the seasonal growth pattern of otoliths was established by following the seasonal formation of the otolith margin edge throughout the year using the following semidirect qualitative validation method (Panfili et al. 2002):
-
Monitoring of the occurrence of edge types throughout the year by age classes validates the yearly rhythm of annuli formation, improves the understanding of the growth pattern of otoliths throughout the year and completes the age determination criteria in terms of the expected seasonal otolith edge type by ages. We basically followed the nomenclature for the edge types in otoliths adopted by the International Commission for the Southeast Atlantic fisheries (ICSEAF) for hake (ICSEAF 1983; which originated from Jensen 1965) by naming the narrow (N) and wide (W) hyaline-translucent (H) or opaque (O) edges as HN, HW, ON and OW respectively.
Historical corroboration of the age determination method
Finally, a set of corroborative indirect validation methods was applied to all available historical age composition of catches and population estimates from surveys (between 1987 and 2013), as reported to ICES (2014) by the Spanish and French Fishery Institutes (applying the current age determination procedures):
-
Checking correlation between the abundance of successive age groups of the same year classes either in catches or in population estimates from surveys. Significant correlations are demonstrative of coherent correlative age estimations (Panfili et al. 2002)
-
Checking the consistency between the yearly biomass fluctuations in surveys and changes in percentages of 1-year-old recruits in the respective population estimates, which is a generalisation of the second validation method detailed above. This was tested by applying the following model, which relates the ratio of biomasses in two consecutive years of a survey series to the ratio of age-1 proportions over older fish in the second year (the odds ratio for age-1; Uriarte 2015):
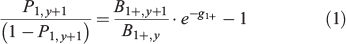
where B1+,y and B1+, y+1 refer to the total biomass (all ages) in years y and y+1, P1+,y+1 refer to the percentage in mass of the age-1 group over the total biomass in the second year (y + 1), and g1+ is the instantaneous rate of biomass decay/increase of all ages pooled together (g1+ = G1+ – M1+ – F1+, with G1+, F1+ and M1+ corresponding to the rates (equal for all ages) of individual growth in mass, natural mortality and fishing mortality respectively). As such, this is a linear model with an intercept of –1 (offset) and a slope (parameter) equal to the inverse of the average survival in biomass of a population from year to year (exp(–g1+)). Because G, M and F usually vary across ages and may change along the time series, the slope cannot properly be considered a constant, but is subject to structural and process error. Although if such variability turns out to be of little magnitude, finding a significant fitting to such relationship should be indicative of an overall satisfactory performance of the age determination, as well as of the biomass estimation procedures of the surveys. This is a suitable model for anchovy because the population mostly consists of two age groups; the 1- and 2+-year-old fish (Ibaibarriaga et al. 2008). -
Checking the correlation between the juvenile (age-0) estimates in an autumn acoustic survey series started in 2003 (JUVENA; Boyra et al. 2013), with the age-1 recruit estimates in the spring surveys (both DEPM and acoustic) of the following year. This served to verify that juveniles (0 group in autumn) and recruits at age-1 (1 year old in spring) are correctly identified from otoliths.
Annual growth pattern in length and growth parameters
Annual growth in length has been studied using the mean length at ages in the Spanish spring fishery 1985–2013, and from the mean lengths at ages in the population obtained from the spring DEPM survey (1990–2013). The mean length at age class was integrated into the von Bertalanffy growth equation:
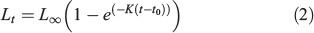
where Lt is the mean fish length at age t and L∞, K and t0 are parameters that determine the shape of the growth curve: L∞ is defined as the asymptotic mean length, K the rate at which the curve approaches the asymptote and t0 the age at which mean length is zero (Ricker 1975). The von Bertalanffy growth function (VBGF) was fitted by direct minimisation of squared residuals to the former mean lengths in an Excel spreadsheet (Microsoft Office 2010, Microsoft Corporation) between ages 1 and 5 (the latter from a single year). Comparison with the growth of other Engraulidae was based on the mean length at age-1 and age-2, on the von Bertalanffy equation parameters and their combination in Pauly and Munro’s (1984) growth performance index (φ′):
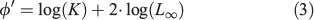
where K and L∞ are VBGF parameters.
Results
Validations
Annual growth pattern (annuli)
Fig. 1 shows the series of typical otoliths in spring, ordered by an increasing number of opaque growth zones, labelled as otolith Types I, II, III, IV and V (although the latter was only seen in 1987). The length distribution of the spring Spanish catches between 1983 and 1986 (Fig. 2) showed that there was an increasing modal length during those years, whereas catches peaked in 1984 and later decreased. This was indicative of a very strong year-class (1982) passing through the fishery being followed by weak year-classes so that the progression in length of the cohort could be tracked year after year. Although in 1983 no collection of otoliths was available, the modal incidence of otolith Types II and III in 1984 and 1985 catches and the maximum incidence of otolith Type IV in the series occurring in 1986 led us to conclude that those otoliths labelled as Type I, II, III and IV corresponded to age-1, -2, -3 and -4 respectively. Finally, in 1987, a new group of otoliths showing an additional opaque growth band (compared with age-4) appeared for the first time in a rather remarkable frequency, which corresponded to age-5 of the 1982 cohort. The progression of modal lengths in the Spanish catches between the autumn of 1989 and the spring of 1991 (Fig. 3) also revealed the passing of a big year-class (1989) and corroborated the former correspondence of otoliths Type I and II with age-1 and age-2 respectively.
The close correspondence between the ups and down of the population between 1987 and 1992, as estimated by the DEPM surveys, and the incidence of otolith Type I (age-1) in those surveys (Fig. 4) provided evidence that Type I otoliths actually corresponded to age-1 and completed the former validation procedure.
Seasonal growth pattern of otoliths by ages
The timing of formation of the opaque edges by age is summarised in Fig. 5. For age-1, growth resumes usually during March, and by April most show marginal opaque growth. However, for the age-2 group, only a few individuals start laying down the marginal opaque growth by May, many do it during June and most have already resumed opaque growth by July. For age-3, it is only in June when some individuals start showing marginal opaque growth, whereas by July most of them will have resumed otolith opaque growth and by August all individuals show opaque edges. So, the older the fish, the later the formation of marginal opaque growth resumes. As such, for age-2 and age-3 (and older), spring is a period when the hyaline zone, laid down in winter, intensifies and becomes more pronounced and wide (HW), whereas for age-1 it is a period of intense growth and formation of an opaque edge.
In addition, we noticed that by September otoliths of all ages show such a wide marginal opaque growth that they seem to have culminated the expected total annual opaque growth.
Further detailed seasonal description of the otoliths by ages (with pictures) and of their monthly margin edge formation is available in the first section of the Supplementary material. Overall, the extent of otolith growth during the first year of life (as age-0, until the first winter translucent zone) is vast and usually accounts for the major part of the otoliths (even for the oldest fish). Otolith growth at age-1 and age-2 diminishes to approximately one-half and one-third of that at the previous age respectively, whereas at older ages the reduction of the annual increments slows down, leading to a gradual (less intense) narrowing of subsequent increments (Fig. 1). The variability around this pattern is large (see a quantitative analysis of otolith growth in the second section of the Supplementary material).
Checks
The most typical checks are C05, C08, C12, C15 and C18 (Fig. 1). Checks at age-2 (e.g. checks C22 or C25) are very rare. Not all otoliths have checks and their incidence can change considerably between years. The most frequent checks occur at age-1 (C12–C15), with an incidence ranging between 15 and 60% depending on year-class (see the varying incidence of most typical checks in the period 1984–1991 in the third section of the Supplementary material). We noticed that the presence of C12/15 in the otoliths of the 1-year-old fish increases from mid-year to autumn (from ~10% in June to ~30–40% in July–September). The semihyaline edges that are rather common for the age-1 fish in June–July (Fig. 5) should correspond to the formation of this check.
Historical corroboration of the age determination method
Using the current ageing method to infer the age composition of catches in the period 1987–2013 resulted in a significant correlation of successive catches at age by year-class in the international fishery (Fig. 6). This is indirect validation of the otolith age determination method because ages have to be determined rather precisely for it to be true. Parallel analysis of the national fisheries reveals that the French catches at age show significant relationships for all ages throughout the entire time series (with r = 0.829 (P = 1 × 10–5) and r = 0.555 (P = 0.011) for ages-1–2 and ages-2–3 respectively). For the Spanish spring fishery, the correlations were also significant up to the closure in 2005 (with r = 0.489 (P = 0.046) and r = 0.657 (P = 0.004) for ages-1–2 and ages-2–3 respectively), but the addition of the years after the reopening of the fishery (2010–13) rendered the relationship between age-1 and age-2 not significant (P = 0.160) because of the unusually large quantity of catches of age-2 over age-1 compared with previous years; however, the relationship between age-2 and age-3 remained significant (P = 0.013).
The same analysis for surveys shows significant correlation of successive age groups by year-class of the populations at age estimates (at an α of 10%, not always at 5%). For brevity, no graphs are shown, but correlations between successive population estimates at age-1 and age-2, as well as age-2 and age-3 for the DEPM survey (n = 20) were 0.435 (P = 0.055) and 0.677 (P = 0.001) respectively, whereas for the acoustics survey (n = 14) these correlations were 0.819 (P = 0.0003) and 0.509 (P = 0.063) respectively.
Two additional indirect historical validations of the age determination were achieved with surveys. First, a significant linear relationship was found between the ratio of biomasses in two consecutive years of the spring surveys and the odds ratio for age-1 in the second year (r = 0.722, P < 0.0000, n = 37; Fig. 7a). This implies that globally the surveys were properly tracking the biomass oscillations of the anchovy population and that the age readings of age-1 and age-2+ were globally accurate. Second, a significant linear relationship was found between the abundance of juvenile anchovies (age-0) estimated by the JUVENA acoustic survey series in autumn and the estimates of recruits at age-1 in either of the two spring surveys of the following year (for the DEPM survey, r = 0.955 and P < 1.6 × 10–5; for the acoustic survey, r = 0.931, P < 9.1 × 10–5; 10 observations in each; Fig. 7b). This implied both that surveys were consistently estimating the biomass of recruits and that age-0 and age-1 groups were well identified from otoliths.
Annual growth pattern in length and growth parameters
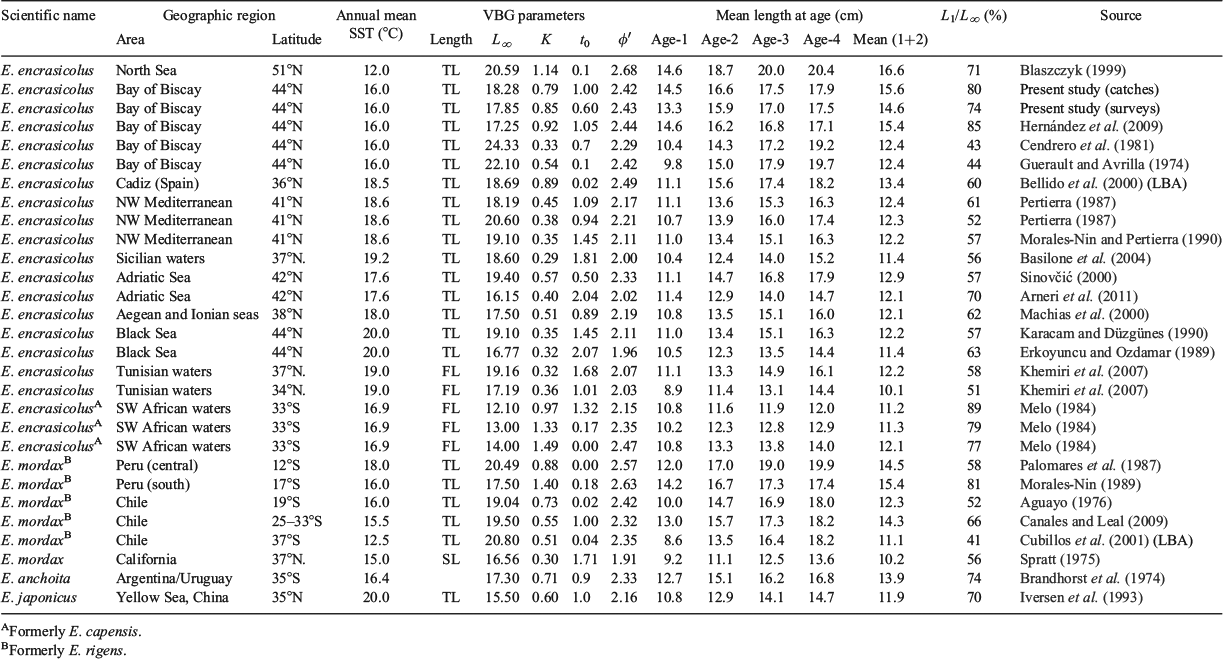
There were no major trends in the series of mean length at age in catches of the Spanish spring fishery (see the series and arithmetic mean values in Fig. 8a). However, mean lengths at age in catches were larger than those measured by the direct surveys of the population in May as part of the DEPM survey (Fig. 8c). In recent years, there has been a slight declining trend in the population mean length at age estimated by the DEPM survey (significant for age-1 and age-2; Fig. 8c). The VBGF curves are shown in Fig. 8b, d, and the parameters are given in Table 2.
|
Discussion
Validation methods
Our validation was successful in applying the method of tracking strong year-classes, particularly for that of 1982 in the spring Spanish fishery (Fig. 2). Certainly, the moderate fishing effort at the beginning of the study and the occurrence of a strong (1982) cohort, followed by several medium or weak year-class, allowed us to track the 1982 year class up to the age of 5. Previously, Uriarte and Astudillo (1987) reported similar progressions of modal lengths in catches in this fishery, also indicative of the passing of spasmodic strong year classes. However, in 1987 the fishing effort increased with the addition of the French pelagic fishery and subsequently individuals of age-3+ became scarcer. Monitoring of the progression of strong year-classes in the catches afterwards would have been more difficult. For example, the 1989 strong cohort was only followed-up to the age of 3. Since then, many cohorts have disappeared after age-3: of the 19 cohorts from 1986 to 2004, only eight cohorts were seen at age-4).
The early detection of the 1989 year class in the catches of the second half of 1989 (at age-0) and the monitoring during 1990 and 1991 up to age-2 (Fig. 3) validated that Type I otoliths correspond to age-1. In addition, the combination of the study of the 1984 and 1989 year classes in catches with direct monitoring of several year classes between 1987 and 1992 in surveys concluded the validation procedure, because the strong increases in the population between years ought to be due to sharp increases in populations at age-1 (with Type I otoliths), as was, in fact, found by the surveys. The use of microstructure analysis by Aldanondo et al. (2016) has added a new validation of the first annulus deposition for this anchovy. Finally, our former validation of yearly growth patterns was confirmed and complemented with the monitoring of the occurrence of edge types throughout the year (Fig. 5).
Using surveys to support the validation of the ageing method is particularly suitable for short-lived species because the typical strong fluctuations in recruitment (which constitute the major part of the population) should result in rather parallel biomass fluctuations, provided that no major catchability problems affect the surveys. Therefore, age composition should support the interpretation of the biomass fluctuations being caused by recruitment fluctuations. For short-lived species for which there are ageing difficulties, but for which routine surveys of the population are conducted, the validation method described herein could be used to compare different ageing procedures. We used the method based on surveys to validate our initial ageing procedure and, subsequently, to corroborate the historical performance of the application of such an ageing method. The corroboration was expanded to the cross-correlation between the abundance of juvenile (age-0) and subsequent age-1 adults in surveys performed in autumn and spring respectively. This emphasises the considerable potential support of surveys when dealing with age validation problems for short-lived species.
The historical corroboration presented herein, making use of the whole series of age structures of catches and population estimates, was dependent, in part, on the precision and accuracy of the age determinations, as well as on the variability of the yearly catchability of the fishery and surveys (plus natural mortality). The overall level of precision in workshops on age determination is high, particularly for age-0 and age-1 (with agreements >93%, CVs <10% and negligible bias) but a bit poorer for older ages (ICES 2009). However, for correlations between successive age groups of the same year class in catches to be significant, a rather constant fishing mortality at age (and hence fishing effort, as well as catchability and selectivity at age) between successive years and throughout the series is required so that the proportionality between catches of successive age groups remains relatively constant throughout the time series. However, the fishing mortality as a result of the fishery is known to have changed considerably since 1987 (ICES 2014), and is probably subject to yearly variability. In support of the latter, it is worth noting that the CV of the historical series of catches of the Spanish purse seine fishery is far lower than the CV of survey biomass estimates since 1987. For this reason we suspect that a major part of the wide variability found in Fig. 6, between pairs of catches of successive age groups of the same year classes, is probably due to yearly changes in effective fishing mortality beyond any inherent errors in the age determination. In addition, the fact that this relationship was no longer significant for the Spanish purse seine fishery when the last 4 years were added likely indicates an intense change in fishing mortality in recent years as a result of the strong drop in fishing effort and a reduction in the fisheries during the first quarter and second half of the year as a result of the implementation of a management plan (ICES 2014). This may result in a higher survival at age-2 than before the closure of the fishery, leading to the abnormally higher catch rates of age-2 over age-1 individuals in recent years.
In parallel, the proportionality (linearity) of the abundance of successive age groups of the same year classes relies on the assumption of constant catchabilities at age at the time of the surveys. Although this constancy is presumed, the existence of occasional strong yearly catchability phenomena is also acknowledged in all surveys series, as revealed by the occasional strong divergence in the series of the two spring surveys (ICES 2014). Therefore, a considerable part of the wide variability between the abundance of juveniles in autumn and age-1 individuals the following spring, or in the relationship between relative changes in successive spring biomass estimates and the odds of the proportion of individuals of age-1 in the second year (Fig. 7), must be due to random realisation errors in yearly catchability.
Finally, in addition to the former considerations, variability in natural mortality may introduce process errors in the correlation between catches or in the abundance of successive age groups of the same year-class.
Therefore, the corroboration methods used in the present study are rough approaches that are subject to quite a few observational and process errors. The fact that all the relationships turned out to be significant proved both that the age readings were globally accurate and that observational and process errors affecting those relationships were not strong enough to mask the expected relationships.
Growth pattern of otoliths and age determination criteria
The validations demonstrated that annuli consist of a translucent zone (either a single or composite zone) plus a wider opaque zone, disrupted occasionally by some typical checks. The otolith growth is intense until the end of the second year of life (i.e. for the first 18–20 months), whereby opaque growth at age-1 is still substantial: ~50% that at age-0. As such, most otoliths are the result of growth periods at age-0 and age-1, with the growth at age-0 alone usually accounting for the major part of the otolith. Otolith increments at age-2 and older become gradually and steadily narrower. In this way, the general principle by which year growth increments gradually decrease (Morales-Nin and Panfili 2002) is obeyed, but the particular way in which otoliths grow in this anchovy is the first general scheme age readers should be aware of for the examination of these otoliths.
In addition to this general otolith growth pattern, there is large individual variability in the size of annual opaque growth increments by age. Much of this variability is due to the inverse relationship between the growth achieved during the first year of life (at age-0 until the first hyaline annulus) and the second and third years of life (at age-1 and age-2), as noted by Petitgas and Grellier (2003) and in the quantitative analysis presented in the second section in the Supplementary material. This is indicative of the compensatory growth (the smaller fish tend to catch up with larger ones), which is observed in other marine species, also as a result of following the von Bertalanffy model or similar growth patterns (Taylor 1962; Ricker 1975; Xiao 1994).
Seasonal otolith growth pattern
Generally, the maximum occurrence of the translucent zone for all ages corresponded to the period of coldest sea temperature in late winter, whereas the maximum occurrence of opaque growth edges occurred with increasing temperatures during late spring and summer. This is typical for fish species at mid latitudes (Beckman and Wilson 1995; Cappo et al. 2000), including several small pelagic species, such as the Tunisian anchovy (Khemiri et al. 2007), Californian anchovy (Mallicote and Parrish 1981) and tapertail anchovy (He et al. 2008). However, the seasonal development of the marginal growth of otoliths differs between ages, with young anchovy (1 year old) resuming opaque growth earlier (from March) than older anchovy, which resume growth in late spring or early summer. This tendency for the younger fish to resume growth earlier than older fish was first noted for this anchovy by Guerault and Avrilla (1974), and it seems to be a widespread feature (Williams and Bedford 1974; Holden and Raitt 1974), also affecting clupeids such as the Baltic sprat, herring and Atlantic sardine (ICES 2008a, 2008b, 2011). Because most of the expected annual opaque increment of the otoliths is completed for all ages by the end of September, otolith growth for the 1-year-old fish occurs from March to September (in ~7 months), whereas otolith growth for fish aged 2 and 3 years old occurs from June and July to September (i.e. in ~4 and 3 months respectively). As such, there is some parallelism between the decreasing gain in length by age and the decreasing time of opaque growth formation in otoliths by ages.
Understanding the seasonal development of the marginal edge by ages was relevant to improving the accuracy of age determination, particularly discriminating age-1 from older fish during spring, when most of the 1-year-old fish will show an opaque narrow edge type whereas older fish are still laying down hyaline edges. For this reason, it could be worth considering this issue further to improve age determination procedures in small short-lived pelagic fishes of other areas too.
Checks
Many fish species lay down checks in their otoliths associated with non-periodic stress, such as environmental conditions (storms, cooling, starvation), life history transitions, endogenous processes (hatching, maturation, spawning) or cyclic environmental issues (Pannella 1971; Campana and Neilson 1985; Casselman 1987). The incidence of checks is particularly common in young fish (from age-0 to age-2) of many species (Swan and Gordon 2001; Waldron and Kerstan 2001; Santiago and Arrizabalaga 2005), including pelagics (Thomas 1983, 1984), and hence probably affects the predominant age group of short-lived species. Therefore, our observations of several checks in this anchovy were not surprising. Fortunately, they are recurrent and become typically identifiable, so that readers can become familiar with them.
The earliest checks are formed in autumn (October–December) when anchovy juveniles move from the surface waters over wide regions of the Bay of Biscay to deeper and more coastal waters where they overwinter (Uriarte et al. 2001; Irigoien et al. 2007). This change may induce a first check, associated with either a change in water temperature or temporary poor feeding conditions. This possibility has been supported by the bioenergetics modelling of otolith biomineralisation for this anchovy by Pecquerie et al. (2012), who were able to simulate check formation in the age-0 group of anchovies by simulating poor feeding conditions before winter. These types of checks before the first annulus have also been reported in south-west African pilchard and anchovy (Thomas 1983; Melo 1984) and in the Chilean anchovy (Aguayo 1976).
Later, during winter, split rings can occur, especially in fast-growing age-0 fish, where the translucent zone is interrupted by one or two short periods with opaque growth if food and temperature conditions are good. These split rings are commonly associated with the first translucent zone of many fish species (Panfili et al. 2002; Santiago and Arrizabalaga 2005) and have also been reported for pilchard off south-west Africa (Thomas 1983, 1984).
The most common check is observed in age-1 fish from July onwards, but is formed in the middle of the second opaque zone in June during the peak spawning time of the 1-year-old anchovies (Motos 1996). In older fish, maturation and peak spawning is earlier, and the opaque growth of the otolith does not resume before spawning, but rather mainly in June–July. This check could be a spawning check laid down by the fast-growing 1-year-old anchovy after its initial opaque growth during spring, whereas older anchovies may be merging the hyaline winter annulus with the spawning check (resulting in the widening of the hyaline zone during spring). This probably reflects the different relative investment of energy in growth and reproduction of the younger v. older anchovies, with the former investing relatively more in growth whereas the latter invest more in reproduction (as also suggested by the dynamic energy budget modelling of this anchovy; Pecquerie et al. 2009). Spawning checks are reported to occur in many fish (Casselman 1987), including South African anchovy (Melo 1984; Waldron 1994) and Chilean anchovy (Aguayo 1976). In addition, a similar check within the opaque growth of the second year of life (age-1) has been reported for Pacific anchovy (Collins and Spratt 1969) and Mediterranean anchovy (Pertierra 1987). In some cases, if spawning is close to winter, a composite hyaline zone may be formed of the spawning check plus the winter annulus, or it can be merged in older fish, as seems to happen for the Bay of Biscay anchovy and for the south-west African pilchard (Thomas 1983, 1984) and other fish (Casselman 1987; Hsu and Tzeng 2009).
The present study corroborated that false checks are not equally laid down in all fish or in all year classes, as reported for other species too (Thomas 1983, 1984; Campana and Neilson 1985; Casselman 1987). This irregular incidence impeded the formulation of a simple rule based purely on the counting of every hyaline zone.
Annual growth patterns in length and growth parameters
Since the current methodology was applied in the late 1980s, the mean lengths at age have remained almost constant throughout the time series in the catches of the spring Spanish fishery, with von Bertalanffy growth parameters similar to those calculated by Hernández et al. (2009), also for the Spanish fishery, and Vaz et al. (2002) for the acoustic surveys in the Bay of Biscay. However, these results are in contrast with those published previously by Cendrero et al. (1981) for the Spanish fishery and by Guerault and Avrilla (1974) in the French area, which reported length at age-1 of ~10 cm and length at age-2 of ~14.5 cm in catches (i.e. the latter corresponds to values obtained using the method described herein for mean length at age-1; Table 2). Furthermore, using the current procedure of age determination using otoliths resulted in a shift in the proportion of major age groups supporting the Spanish spring catches, by which age-1, which was formerly almost absent, became very abundant (Anon. 1993). The differences with the results reported by Cendrero et al. (1981) are probably due to the initial use by those authors of probabilistic techniques for the analysis of polymodal length–frequency distributions for several years (Cort et al. 1976) before the use of otoliths. In addition, there could also be some differences in ageing criteria. Conversely, the mean lengths at age calculated by Guerault and Avrilla (1974) were back-calculated at the time of translucent annulus formation; this, coupled with the fact that they were working on the French shelf close to shore (including the Gironde area, where anchovies are usually smaller), may explain the differences in results.
The present study evidenced lower mean lengths at age (and weights) in the population (as estimates from the DEPM surveys) than in the catches of the concurrent spring Spanish fishery, so that they resulted in a slightly higher growth coefficient (K) and lower theoretical maximum length (L∞) (Table 2). The reason for these differences is related to the spatial pattern of the Spanish fishery because it operates in regions of deep water rather close to the Spanish coast where the bigger anchovies are found (Uriarte et al. 1996; Ibaibarriaga et al. 2013). In contrast, the surveys systematically cover all areas occupied by the population, with the major part of the population often placed around the northern coastal shelf close to the mouth of the Gironde River where age-1 predominates and the mean lengths at ages are smaller than in the remaining areas (Motos et al. 1996; Vaz et al. 2002; Ibaibarriaga et al. 2013).
This anchovy population has an intense growth rate compared with other populations of the same species or with other Engraulidae (Table 2). The rather high L∞ and K values result in it having among the highest φ′ parameter of this genus, only equalled or surpassed by anchovies in the North Sea and by anchovies in Cadiz. However, looking at the mean of lengths at age-1 and age-2, only E. encrasicolus in the North Sea seem to be bigger (Blaszczyk 1999); however, a more recent comparison with otoliths from the International Bottom Trawl Surveys in the North Sea along the Dutch coast shows rather comparable mean lengths at age (Petitgas et al. 2012). Despite several populations having similar or higher L∞ in the Mediterranean, the low K means they have smaller mean lengths at ages 1 and 2 and lower φ′. So, the average size and growth of these populations are smaller. E. encrasicolus of South Africa has a higher K but smaller L∞ so that despite its rather similar φ′, mean sizes at age-1 and age-2 are also smaller. The Peruvian and northern Chilean anchovies are those that have rather similar growth rates and mean lengths at age-1 and age-2 (Aguayo 1976; Palomares et al. 1987; Morales-Nin 1989). Most other Engraulidae have smaller sizes at age-1 and age-2 regardless of their actual growth rates or φ′ (Hoedt 1990; Tiroba et al. 1990), except for the large tropical anchovy Thryssa hamiltoni (Hoedt 1992). Anchovies inhabiting estuarine areas also tend to be smaller (Iversen et al. 1993; Newberger and Houde 1995; He et al. 2008). Aldanondo et al. (2011), analysing micro-increments in the otoliths of juveniles, has shown that this anchovy in the Bay of Biscay can reach a size of 6–8 cm in ~70–90 days. All these observations reflect the life strategy of this short-lived anchovy, namely very intense growth during its first year of life so that it maximizes its size at the age of first maturity (i.e. at age-1 and, thus, L1/L∞) and hence the reproductive output of its first spawning season. This, coupled with a still substantial growth during its second year of life, should maximize the overall reproductive output across its expected life, because survivors at older ages become negligible.
Acknowledgements
The authors thank Patrick Prouzet and Patrick Grellier for their discussions, which helped refine the ageing method described herein. In addition, the authors thank S. Junquera (Oviedo University, Oviedo, Spain), A. Astudillo (IEO) and J. Massé (IFREMER) for providing some otoliths for the original validation study. Many of the measurements presented in the second section of the Supplementary material were provided by Aitor Astoreka (AZTI), within a study funded by European Union (EU) project number 044132 AFISA (Automated Fish Ageing). The surveys and catch at age composition and mean lengths at age were produced by the National and Regional Institutes of Spain (IEO and AZTI) and of France (IFREMER) with the support of the their respective fishery departments (including the Fishery Department of the Basque Country in Spain). Since 2000, the catch sampling program and surveying monitoring system have been supported, in part, by the EU Community framework for the collection and management of data needed to conduct common fisheries policy (and legislation) updates. The authors thank Prof. Audrey Geffen and three anonymous referees for helpful suggestions to improve the paper. This paper is contribution number 754 from AZTI (Marine Research).
References
Aguayo, H. M. (1976). Edad y crecimiento de la anchoveta (Engraulis ringens) del norte de Chile (Arica-Iquique). Investigaciones Pesqueras 23, 1–25.Aldanondo, N., Cotano, U., Etxebeste, E., Irigoien, X., Álvarez, P., Martínez de Murguía, A., and Herrero, D. L. (2008). Validation of daily increments deposition in the otoliths of European anchovy larvae (Engraulis encrasicolus L.) reared under different temperature conditions. Fisheries Research 93, 257–264.
| Validation of daily increments deposition in the otoliths of European anchovy larvae (Engraulis encrasicolus L.) reared under different temperature conditions.Crossref | GoogleScholarGoogle Scholar |
Aldanondo, N., Cotano, U., and Etxebeste, E. (2011). Growth of young-of-the-year European anchovy (Engraulis encrasicolus L.) in the Bay of Biscay. Scientia Marina 75, 227–235.
Aldanondo, N., Cotano, U., Álvarez, P., and Uriarte, A. (2016). Validation of the first annual increment deposition in the otoliths of European anchovy in the Bay of Biscay based on otolith microstructure analysis. Marine and Freshwater Research 67, 943–950.
| Validation of the first annual increment deposition in the otoliths of European anchovy in the Bay of Biscay based on otolith microstructure analysis.Crossref | GoogleScholarGoogle Scholar |
Anon. (1989). Report of the Working Group on the Assessment of Pelagic Stocks in Divisions VIIIc and IXa and Horse Mackerel. ICES CM 1989. International Council for the Exploration of the Sea (ICES), Copenhagen.
Anon. (1991). Report of the Working Group on the Assessment of the Stocks of Sardine, Horse Mackerel and Anchovy. ICES CM 1991. International Council for the Exploration of the Sea (ICES), Copenhagen.
Anon. (1992). Report of the Working Group on the Assessment of the Stocks of Mackerel Horse Mackerel, Sardine and Anchovy. ICES CM 1992. International Council for the Exploration of the Sea (ICES), Copenhagen.
Anon. (1993). Report of the Working Group on the Assessment of the Stocks of Mackerel Horse Mackerel, Sardine and Anchovy. ICES Document CM 1993. International Council for the Exploration of the Sea (ICES), Copenhagen.
Arneri, E., Carpi, P., Donato, F., and Santojanni, A. (2011). Growth in small pelagic fishes and its implications in their population dynamics. Biologia Marina Mediterranea 18, 106–113.
Astudillo, A. (1986). The Anchovy in the Bay of Biscay: Recent Data on the Fishery. ICES CM 1986/H:45. International Council for the Exploration of the Sea (ICES), Copenhagen.
Basilone, G., Guisande, C., Patti, B., Mazzola, S., Cuttitta, A., Bonanno, A., and Kallianiotis, A. (2004). Linking habitat conditions and growth in the European anchovy (Engraulis encrasicolus). Fisheries Research 68, 9–19.
| Linking habitat conditions and growth in the European anchovy (Engraulis encrasicolus).Crossref | GoogleScholarGoogle Scholar |
Beckman, D. W., and Wilson, C. A. (1995). Seasonal timing of opaque zone formation in fish otoliths. In ‘Recent Developments in Fish Otolith Research’. (Eds D. H. Secor, J. M. Dean and S. E. Campana.) pp. 27–43. (University of South Carolina Press: Columbia, SC.)
Bellido, J. M., Pierce, G. J., Romero, J. L., and Millan, M. (2000). Use of frequency analysis methods to estimate growth of anchovy (Engraulis encrasicolus L. 1758) in the Gulf of Cádiz (SW, Spain). Fisheries Research 48, 107–115.
| Use of frequency analysis methods to estimate growth of anchovy (Engraulis encrasicolus L. 1758) in the Gulf of Cádiz (SW, Spain).Crossref | GoogleScholarGoogle Scholar |
Blaszczyk, P. (1999). Length and age composition and growth of anchovy Engraulis encrasicolus (L. 1758) caught in the East Schelde, The Netherlands, in 1987. Folia Universitatis Agriculturae Stetinensis 192, 5–11.
Boyra, G., Martinez, U., Cotano, U., Santos, M., Irigoien, X., and Uriarte, A. (2013). Acoustic surveys for juvenile anchovy in the Bay of Biscay: abundance estimate as an indicator of the next year’s recruitment and spatial distribution patterns. ICES Journal of Marine Science 70, 1354–1368.
| Acoustic surveys for juvenile anchovy in the Bay of Biscay: abundance estimate as an indicator of the next year’s recruitment and spatial distribution patterns.Crossref | GoogleScholarGoogle Scholar |
Brandhorst, W., Castello, J. P., Cousseau, M. B., and Capezzani, D. A. (1974). Evaluation of anchovy resources (Engraulis anchoita) offshore Argentina and Uruguay. VIII. Spawning, growth and population structure. Physis (Rio de Janeiro, Brazil) 33, 37–58.
Campana, S. E. (2001). Accuracy, precision and quality control in age determination, including a review of the use and abuse of age validation methods. Journal of Fish Biology 59, 197–242.
| Accuracy, precision and quality control in age determination, including a review of the use and abuse of age validation methods.Crossref | GoogleScholarGoogle Scholar |
Campana, S. E., and Neilson, J. D. (1985). Microstructure of fish otoliths. Canadian Journal of Fisheries and Aquatic Sciences 42, 1014–1032.
| Microstructure of fish otoliths.Crossref | GoogleScholarGoogle Scholar |
Canales, T. M., and Leal, E. (2009). Parámetros de historia de vida de la anchoveta Engraulis ringens Jenyns, 1842, en la zona centro norte de Chile. Revista de Biología Marina y Oceanografía 44, 173–179.
Cappo, M., Eden, P., Newman, S. J., and Robertson, S. (2000). A new approach to validation of periodicity and timing of opaque zone formation in the otoliths of eleven species of Lutjanus from the central Great Barrier Reef. Fishery Bulletin 98, 474–488.
Casselman, J. M. (1987). Determination of age and growth. In ‘The Biology of Fish Growth’. (Eds A. H. Weatherley and H. S. Gill.) pp. 209–242. (Academic Press: London.)
Cendrero, O., Cort, J. L., and Cárdenas, E. (1981). Revisión de algunos datos sobre la biología de la anchoa, Engraulis encrasicholus (L.) del Mar Cantábrico. Boletín del Instituto Español de Oceanografía 311, 118–123.
Cermeño, P., Uriarte, A., De Munguía, A. M., and Morales-Nin, B. (2003). Validation of daily increment formation in otoliths of juvenile and adult European anchovy. Journal of Fish Biology 62, 679–691.
| Validation of daily increment formation in otoliths of juvenile and adult European anchovy.Crossref | GoogleScholarGoogle Scholar |
Cermeño, P., Uriarte, A., Morales-Nin, B., Cotano, U., and Álvarez, P. (2008). Setting up interpretation criteria for ageing juvenile European anchovy otoliths. Scientia Marina 72, 733–742.
Collins, R. A., and Spratt, J. D. (1969). Age determination of northern anchovies, Engraulis mordax, from otoliths. In ‘The Northern Anchovy (Engraulis mordax) and its Fishery 1965–1968’. (Ed. J. D. Messersmith.) Department of Fish and Game, Fish Bulletin 147, pp. 39–55. (State of California, The Resources Agency.)
Cort, J. L., Cendrero, O., and Iribar, X. (1976). La anchoa, Engraulis encrasicholus (L.), del Cantábrico. Resultados de las campañas de 1974, 1975 y 1976. Boletín del Instituto Español de Oceanografía 220, 1–34.
Cubillos, L. A., Arcos, D. F., Bucarey, D. A., and Canales, M. T. (2001). Seasonal growth of small pelagic fish off Talcahuano, Chile (37°S, 73°W): a consequence of their reproductive strategy to seasonal upwelling? Aquatic Resources 14, 115–124.
| Seasonal growth of small pelagic fish off Talcahuano, Chile (37°S, 73°W): a consequence of their reproductive strategy to seasonal upwelling?Crossref | GoogleScholarGoogle Scholar |
Erkoyuncu, I., and Ozdamar, E. (1989). Estimation of the age, size and sex composition and growth parameters of anchovy (Engraulis encrasicholus L.) in the Black sea. Fisheries Research 7, 241–247.
| Estimation of the age, size and sex composition and growth parameters of anchovy (Engraulis encrasicholus L.) in the Black sea.Crossref | GoogleScholarGoogle Scholar |
Gonzalez-Salas, C., and Lenfant, P. (2007). Interannual variability and intraannual stability of the otolith shape in European anchovy Engraulis encrasicolus (L.) in the Bay of Biscay. Journal of Fish Biology 70, 35–49.
| Interannual variability and intraannual stability of the otolith shape in European anchovy Engraulis encrasicolus (L.) in the Bay of Biscay.Crossref | GoogleScholarGoogle Scholar |
Guerault, D., and Avrilla, J. (1973). L’anchois du golfe de Gascogne, captures de 1972, données biologiques et biometriques. ICES CM 1973/J:11. International Council for the Exploration of the Sea (ICES), Copenhagen.
Guerault, D., and Avrilla, J. (1974). L’anchois du golfe de Gascogne, taille, âge et croissance. ICES CM 1974/J:17. International Council for the Exploration of the Sea (ICES), Copenhagen.
He, W., Li, Z., Liu, J., Li, Y., Murphy, B. R., and Xie, S. (2008). Validation of a method of estimating age, modelling growth, and describing the age composition of Coilia mystus from the Yangtze Estuary, China. ICES Journal of Marine Science 65, 1655–1661.
| Validation of a method of estimating age, modelling growth, and describing the age composition of Coilia mystus from the Yangtze Estuary, China.Crossref | GoogleScholarGoogle Scholar |
Hernández, C., Villamor, B., Abaunza, P., and Landa, J. (2009). Age and growth of European anchovy (Engraulis encrasicolus) in the Bay of Biscay (NE Atlantic), 1994–2008. In ‘Report of the Workshop on Age Reading of European Anchovy (WKARA)’, 9–13 November 2009, Sicily, Italy. ICES CM 2009/ACOM:43. (International Council for the Exploration of the Sea (ICES): Copenhagen.) Available at http://www.ices.dk/sites/pub/Publication%20Reports/Expert%20Group%20Report/acom/2009/WKARA/WKARA%20Report%202009.pdf [Verified 2 January 2016].
Hoedt, F. E. (1990). Growth of the tropical anchovy, Stolephorus nelsoni, in Northern Australia. In ‘Tuna Baitfish in the Indo-Pacific Region: Proceedings of a Workshop’, 11–13 December 1989, Honiara, Solomon Islands. (Eds S. J. M. Blaber and J. W. Copland.) pp. 147–149. (Australian Centre for International Agricultural Research: Canberra.)
Hoedt, F. E. (1992). Age and growth of a large tropical anchovy, Thryssa hamiltoni (Gray): a comparison of ageing techniques. Australian Journal of Marine and Freshwater Research 43, 953–971.
| Age and growth of a large tropical anchovy, Thryssa hamiltoni (Gray): a comparison of ageing techniques.Crossref | GoogleScholarGoogle Scholar |
Holden, M. J., and Raitt, D. F. S. (1974). Manual of Fisheries Science. Part 2. Methods of Resource Investigations and Their Application. FAO Fisheries Technical Paper number 115. FAO, Rome.
Hsu, C. C., and Tzeng, W. N. (2009). Validation of annular deposition in scales and otoliths of flathead mullet Mugil cephalus. Zoological Studies 48, 640–648.
Ibaibarriaga, L., Fernandez, C., Uriarte, A., and Roel, B. A. (2008). A two-stage biomass dynamic model for the Bay of Biscay anchovy: a Bayesian approach. ICES Journal of Marine Science 65, 191–205.
| A two-stage biomass dynamic model for the Bay of Biscay anchovy: a Bayesian approach.Crossref | GoogleScholarGoogle Scholar |
Ibaibarriaga, L., Fernandez, C., and Uriarte, A. (2011). Gaining information from commercial catch for a Bayesian two-stage biomass dynamic model: application to Bay of Biscay anchovy. ICES Journal of Marine Science 68, 1435–1446.
| Gaining information from commercial catch for a Bayesian two-stage biomass dynamic model: application to Bay of Biscay anchovy.Crossref | GoogleScholarGoogle Scholar |
Ibaibarriaga, L., Uriarte, A., Laconcha, U., Bernal, M., Santos, M., Chiflet, M., and Irigoien, X. (2013). Modelling the spatio-temporal distribution of age-1 Bay of Biscay anchovy (Engraulis encrasicolus) at spawning time. Scientia Marina 77, 461–472.
| Modelling the spatio-temporal distribution of age-1 Bay of Biscay anchovy (Engraulis encrasicolus) at spawning time.Crossref | GoogleScholarGoogle Scholar |
ICES (2008a). Report of the Workshop on Age Reading of Baltic Herring (WKARBH), 9–13 June 2008, Riga, Latvia. ICES CM 2008/ACOM:36. (International Council for the Exploration of the Sea (ICES): Copenhagen.)
ICES (2008b). Report of the Workshop on Age Reading on Baltic Sprat (WKARBS), 17–20 March 2008, Klaipeda, Lithuania. ICES CM 2008/ACOM:37. (International Council for the Exploration of the Sea (ICES): Copenhagen.)
ICES (2009). Report of the Workshop on Age Reading of European Anchovy (WKARA), 9–13 November 2009, Sicily, Italy. ICES CM 2009/ACOM:43. (International Council for the Exploration of the Sea (ICES): Copenhagen.)
ICES (2011). Report of the Workshop on Age Reading of European Atlantic Sardine (WKARAS), 14–18 February 2011, Lisbon, Portugal. ICES CM 2011/ACOM:42. (International Council for the Exploration of the Sea (ICES): Copenhagen.)
ICES (2014). Report of the Working Group on Southern Horse Mackerel, Anchovy and Sardine (WGHANSA), 20–25 June 2014, Copenhagen, Denmark. ICES CM 2014/ACOM:16. (International Council for the Exploration of the Sea (ICES): Copenhagen.)
ICSEAF (1983). Otolith Interpretation Guide. Number 1 Hake. International Commission for the Southeast Atlantic fisheries, Madrid.
Irigoien, X., Fiksen, Ø., Cotano, U., Uriarte, A., Alvarez, P., Arrizabalaga, H., Boyra, G., Santos, M., Sagarminaga, Y., Otheguy, P., Etxebeste, E., Zarauz, L., Artetxe, I., and Motos, L. (2007). Could Biscay Bay anchovy recruit through a spatial loophole? Progress in Oceanography 74, 132–148.
| Could Biscay Bay anchovy recruit through a spatial loophole?Crossref | GoogleScholarGoogle Scholar |
Iversen, S. A., Zhu, D., Johannessen, A., and Toresen, R. (1993). Stock size, distribution and biology of anchovy in the Yellow Sea and East China Sea. Fisheries Research 16, 147–163.
| Stock size, distribution and biology of anchovy in the Yellow Sea and East China Sea.Crossref | GoogleScholarGoogle Scholar |
Jensen, A. C. (1965). A standard terminology and notation for otolith readers. ICNAF Research Bulletin 2, 5–7.
Junquera, S. (1986). Pêche de l’anchois (Engraulis encrasicholus) dans le golfe de Gascogne et sur le littoral atlantique de Galice depuis 1920. Variations quantitatives. Revue des Travaux de l’Institut des Pêches Maritimes 48, 133–142.
Karacam, H., and Düzgünes, E. (1990). Age, growth and meat yield of the European anchovy (Engraulis encrasicolus, L. 1758) in the Black Sea. Fisheries Research 9, 181–186.
| Age, growth and meat yield of the European anchovy (Engraulis encrasicolus, L. 1758) in the Black Sea.Crossref | GoogleScholarGoogle Scholar |
Khemiri, S., Gaamour, A., Meunier, F. J., and Zylberger, L. (2007). Age and growth of Engraulis encrasicolus (Clupeiforme: Engraulidae) in the Tunisian waters. Cahiers de Biologie Marine 48, 259–269.
Machias, A., Somarakis, S., Drakopoulos, P., Magoulas, A., and Koutsikopoulos, C. (2000). Evaluation of the Southern Greek Anchovy Stocks. Project 97–0048, Final Report. Institute of Marine Biology, Crete.
Mallicote, D. L., and Parrish, R. H. (1981). Seasonal growth patterns of California stocks of northern anchovy, Engraulis mordax, pacific mackerel, Scomber japonicus, and jack mackerel, Trachurus symmetrlcus. California Cooperative Oceanic Fisheries Investigations Report 22, 69–81.
Massé, J. (1996). Acoustic observations in the Bay of Biscay: schooling, vertical distribution, species assemblages and behaviour. Scientia Marina 60, 227–234.
Massé, J., Duhamel, E., Petitgas, P., Doray, M., and Huret, M. (2016). Spring acoustic surveys: Pelgas survey. In ‘Pelagic Surveys Series for Sardine and Anchovy in ICES Areas VIII and IX (WGACEGG): Towards an Ecosystem Approach’. (Eds J. Masse, A. Uriarte, M. M. Angelico and P. Carrera.) ICES Cooperative Research Report 332. (International Council for the Exploration of the Sea (ICES): Copenhagen.)
Melo, Y. C. (1984). Ages studies on anchovy Engraulis capensis Gichrist off south west Africa. South African Journal of Marine Science 2, 19–31.
| Ages studies on anchovy Engraulis capensis Gichrist off south west Africa.Crossref | GoogleScholarGoogle Scholar |
Morales-Nin, B. (1989). Age and growth of the southern stock of Peruvian anchoveta based on otolith microstructures and length frequency analysis. In ‘ICLARM Conference Proceedings 18. The Peruvian Upwelling System: Dynamics and Interactions’. (Eds D. Pauly, P. Muck, J. Mendo, and I. Tsukayama.) pp. 179–188. (Instituto del Mar del Perú (IMARPE): Callao, Peru; Deutsche Gesellschaft für Technische Zusammenarbeit (GTZ), GmbH; Eschborn, Germany; and International Center for Living Aquatic Resources Management: Manila, Philippines.)
Morales-Nin, B., and Panfili, J. (2002). Age estimation. In ‘Manual of Fish Sclerochronology’. (Eds J. Panfili, H. Troadec, H. de Pontual and P. J. Wright.) pp. 91–98. (IFREMER and IRD: Brest, France).
Morales-Nin, B., and Pertierra, J. P. (1990). Growth rates of the anchovy Engraulis encrasicolus and the sardine Sardina pilchardus in the northwestern Mediterranean Sea. Marine Biology 107, 349–356.
| Growth rates of the anchovy Engraulis encrasicolus and the sardine Sardina pilchardus in the northwestern Mediterranean Sea.Crossref | GoogleScholarGoogle Scholar |
Motos, L. (1996). Reproductive biology and fecundity of the Bay of Biscay anchovy population (Engraulis encrasicolus L.). Scientia Marina 60, 195–207.
Motos, L., Uriarte, A., and Valencia, V. (1996). The spawning environment of the Bay of Biscay anchovy (Engraulis encrasicolus L.). Scientia Marina 60, 117–140.
Motos, L., Uriarte, A., Prouzet, P., Santos, M., Alvarez, P., and Sagarminaga, Y. (2005). Assessing the Bay of Biscay anchovy population by DEPM: a review 1989–2001. In ‘Report of the SPACC Meeting on Small Pelagic Fish Spawning Habitat Dynamics and the Daily Egg Production Method (DEPM)’. (Eds L. R. Castro, P. Freón, C. D. van der Lingen and A. Uriarte.) GLOBEC Report 22. pp. 88–90. (GLOBEC International Project Office: Plymouth, UK.)
Navaz J.M. and Lozano F. (1966). Donnes sur la taille, l’âge et la croissance de l’anchois (Engraulis encrasicholus L.) de la côte Basque Espagnole. ICES CM 1966/J:6. International Council for the Exploration of the Sea (ICES), Copenhagen.
Newberger, T. A., and Houde, E. D. (1995). Population biology of bay anchovy Anchoa mitchilli in the mid Cheasapeake Bay. Marine Ecology Progress Series 116, 25–37.
| Population biology of bay anchovy Anchoa mitchilli in the mid Cheasapeake Bay.Crossref | GoogleScholarGoogle Scholar |
Palomares, M. L., Muck, P., Mendo, J., Chuman, E., Gomez, O., and Pauly, D. (1987). Growth of the Peruvian anchoveta (Engraulis ringens), 1953 to 1982. In ‘The Peruvian Anchoveta and its Upwelling Ecosystem: Three Decades of Change’. (Eds D. Pauly and I. Tsukayama.) ICLARM Studies and Reviews. 15, pp. 117–141. (Instituto del Mar del Peru (IMARPE): Callao, Peru; Deutsche Gesellschaft für Technische Zusammenarbeit (GTZ), GmbH: Eschborn, Germany; and International Center for Living Aquatic Resources Management: Manila, Philippines.).
Panfili, J., Troadec, H., de Pontual, H., and Wright, P. J. (Eds) (2002). ‘Manual of Fish Sclerochronology.’ (IFREMER and IRD: Brest, France.)
Pannella, G. (1971). Fish otoliths: daily growth layers and periodical patterns. Science 173, 1124–1127.
| Fish otoliths: daily growth layers and periodical patterns.Crossref | GoogleScholarGoogle Scholar |
Pauly, D., and Munro, J. L. (1984). Once more on the comparison of growth in fish and invertebrates. Fishbyte 2, 21.
Pecquerie, L., Petitgas, P., and Kooijman, S. A. L. M. (2009). Modeling fish growth and reproduction in the context of the dynamic energy budget theory to predict environmental impact on anchovy spawning duration. Journal of Sea Research 62, 93–105.
| Modeling fish growth and reproduction in the context of the dynamic energy budget theory to predict environmental impact on anchovy spawning duration.Crossref | GoogleScholarGoogle Scholar |
Pecquerie, L., Fablet, T., de Pontual, H., Bonhommeau, S., Alunno-Bruscia, M., Petitgas, P., and Kooijman, S. A. L. M. (2012). Reconstructing individual food and growth histories from biogenic carbonates. Marine Ecology Progress Series 447, 151–164.
| Reconstructing individual food and growth histories from biogenic carbonates.Crossref | GoogleScholarGoogle Scholar |
Pertierra, J. P. (1987). Crecimiento del boquerón (Engraulis encrasicolus, L. 1758) (Pisces, Engraulidae) de la costa catalana (Mediterráneo noroccidental). Investigaciones Pesqueras 51, 263–275.
Petitgas, P., and Grellier, P. (2003). Size selective processes for anchovy in Biscay, 2000–2002: recruitment, adult survival and spawning. ICES CM, N:07. International Council for the Exploration of the Sea (ICES), Copenhagen.
Petitgas, P., Uriarte, A., Nogueira, E., Massé, J., and Cotano, U. (2010). Bay of Biscay anchovy. In ‘Life Cycle Spatial Patterns of Small Pelagic Fish in the Northeast Atlantic’. (Ed. P. Petitgas.) ICES Cooperative Research Report number 306, pp. 40–44. (International Council for the Exploration of the Sea (ICES): Copenhagen.)
Petitgas, P., Alheit, J., Peck, M. A., Raab, K., Irigoien, X., Huret, M., van der Kooij, J., Pohlmann, T., Wagner, C., Zarraonaindia, I., and Dickey-Collas, M. (2012). Anchovy population expansion in the North Sea. Marine Ecology Progress Series 444, 1–13.
| Anchovy population expansion in the North Sea.Crossref | GoogleScholarGoogle Scholar |
Ricker, W. E. (1975). Computation and Interpretation of Biological Statistics of Fish Populations. Bulletin 191, Fisheries Research Board of Canada, Ottawa, ON.
Santiago, J., and Arrizabalaga, H. (2005). An integrated growth study for North Atlantic albacore (Thunnus alalunga Bonn. 1788). ICES Journal of Marine Science 62, 740–749.
| An integrated growth study for North Atlantic albacore (Thunnus alalunga Bonn. 1788).Crossref | GoogleScholarGoogle Scholar |
Santos, M., Uriarte, A., Boyra, G., and Ibaibarriaga, L. (2016). Anchovy DEPM surveys 2003–2012 in the Bay of Biscay (subarea VIII) BIOMAN. In ‘Pelagic Surveys Series for Sardine and Anchovy in ICES Areas VIII and IX (WGACEGG): Towards an Ecosystem Approach’. (Eds J. Massé, A. Uriarte, M. M. Angelico and P. Carrera.) ICES Cooperative Research Report 332. (International Council for the Exploration of the Sea (ICES): Copenhagen.)
Sinovčić, G. (2000). Anchovy, Engraulis encrasicolus (Linnaeus, 1758): biology, population dynamics and fisheries case study. Acta Adriatica 41, 3–53.
Spratt, J. D. (1975). Growth rate of northern anchovy Engraulis Mordax, in Southern California waters calculated from otoliths. California Fish and Game 61, 116–126.
Swan, S. C., and Gordon, J. D. M. (2001). A review of age estimation in macrourid fishes with new data on age validation of juveniles. Fisheries Research 51, 177–195.
| A review of age estimation in macrourid fishes with new data on age validation of juveniles.Crossref | GoogleScholarGoogle Scholar |
Taylor, C. C. (1962). Growth equations with metabolic parameters. Journal du Conseil International pour l’Exploration de la Mer 27, 270–286.
| Growth equations with metabolic parameters.Crossref | GoogleScholarGoogle Scholar |
Thomas, R. M. (1983). Back-calculation of time of hyaline ring formation in the otoliths of the pilchard off South West Africa. South African Journal of Marine Science 1, 3–18.
| Back-calculation of time of hyaline ring formation in the otoliths of the pilchard off South West Africa.Crossref | GoogleScholarGoogle Scholar |
Thomas, R. M. (1984). A method of age determination for the South West African pilchard Sardinops ocellata. South African Journal of Marine Science 2, 63–70.
| A method of age determination for the South West African pilchard Sardinops ocellata.Crossref | GoogleScholarGoogle Scholar |
Thomas, R. M. (1985). Growth rate of the pilchard off south west Africa, 1971–1983. Investigational report 128, Sea Fisheries Research Institute, South Africa.
Tiroba, G., Rawlinson, N. J. F., Nichols, P. V., and Leqata, J. L. (1990). Length–frequency Analysis of Major Baitfish Species in Solomon Islands. In ‘Tuna Baitfish in the Indo-Pacific Region: Proceedings of a Workshop’, 11–13 December 1989, Honiara, Solomon Islands. (Eds S. J. M. Blaber and J. W. Copland.) pp. 114–133. (Australian Centre for International Agricultural Research: Canberra.)
Uriarte, A. (2015). Consistency of relative changes in biomass and proportions at age 1 in anchovy surveys. Preliminary results. In ‘ICES 2015. First Interim Report of the Working Group on Acoustic and Egg Surveys for Sardine and Anchovy in ICES Areas VII, VIII and IX (WGACEGG)’, 17–21 November 2014, Vigo, Spain. ICES CM 2014/SSGESST:21, pp. 88–91. (International Council for the Exploration of the Sea (ICES). Copenhagen.)
Uriarte, A., and Astudillo, A. (1987). The anchovy in the Bay of Biscay: new data and analysis of the fishery, 1974–1987. ICES CM 1987/H:20. International Council for the Exploration of the Sea (ICES), Copenhagen.
Uriarte, A., Prouzet, P., and Villamor, B. (1996). Bay of Biscay and Ibero Atlantic anchovy populations and their fisheries. Scientia Marina 60, 237–255.
Uriarte, A., Sagarminaga, Y., Scalabrin, C., Valencia, V., Cermeño, P., De Miguel, E, Gómez Sánchez, J.A., Jiménez, M. (2001). Ecology of Anchovy Juveniles in the Bay of Biscay 4 Months after Peak Spawning: Do They Form Part of the Plankton? ICES CM/W:20. International Council for the Exploration of the Sea (ICES), Copenhagen.
Uriarte, A., Blanco, M., Cendrero, O., Grellier, P., Millán, M., Morais, A., and Rico, I. (2002). Report of the Workshop on Anchovy Otoliths from Subarea VIII and Division IXa. Working Document to the ICES Working Group on the Assessment of Mackerel, Horse Mackerel, Sardine and Anchovy. Available at http://www.ices.dk/community/Documents/PGCCDBS/ANCHOVY%20OTOLITH%20WORKSHOP_2002.pdf [Verified 28 February 2015].
Vaz, S., Petitgas, P., Beillois, P., and Masse, J. (2002). Time and Spatial Variations of Anchovy Biometric Parameters in the Bay of Biscay from 1983 to 2002. ICES Document CM 2002/O:27. International Council for the Exploration of the Sea (ICES), Copenhagen.
Waldron, M. E. (1994). Validation of annuli of South African anchovy Engraulis capensis, using daily otoliths increments. ICES Journal of Marine Science 51, 233–234.
| Validation of annuli of South African anchovy Engraulis capensis, using daily otoliths increments.Crossref | GoogleScholarGoogle Scholar |
Waldron, M. E., and Kerstan, M. (2001). Age validation in horse mackerel (Trachurus trachurus) otoliths. ICES Journal of Marine Science 58, 806–813.
| Age validation in horse mackerel (Trachurus trachurus) otoliths.Crossref | GoogleScholarGoogle Scholar |
Williams, T., and Bedford, B. C. (1974). The use of otoliths for age determination. In ‘The Ageing of Fish’. (Ed. T. B. Bagenal.) pp 114–123. (Unwin Brothers: London, UK.)
Xiao, Y. (1994). Von Bertalanffy growth models with variability in, and correlation between, K and L∞. Canadian Journal of Fisheries and Aquatic Sciences 51, 1585–1590.
| Von Bertalanffy growth models with variability in, and correlation between, K and L∞.Crossref | GoogleScholarGoogle Scholar |


