Element composition of shark vertebrae shows promise as a natural tag
J. C. A. Pistevos A B E , P. Reis-Santos
A B E , P. Reis-Santos  A C , C. Izzo
A C , C. Izzo  A D and B. M. Gillanders
A D and B. M. Gillanders  A
A
A Southern Seas Ecology Laboratories, School of Biological Sciences, The University of Adelaide, SA 5005, Australia.
B Paris Sciences et Lettres (PSL) Research University, EPHE-UPVD-CNRS, USR 3278 CRIOBE and Laboratoire d’Excellence CORAIL, BP 1013, 98729 Papetoai, Moorea, French Polynesia.
C MARE – Marine and Environmental Sciences Centre, Faculdade de Ciências, Universidade de Lisboa, Campo Grande, PT-1749-016, Lisboa, Portugal.
D Fisheries Research and Development Corporation, PO Box 2733, Kent Town, SA 5071, Australia.
E Corresponding author. Email: jennifer.pistevos@ephe.sorbonne.fr
Marine and Freshwater Research 70(12) 1722-1733 https://doi.org/10.1071/MF18423
Submitted: 2 November 2018 Accepted: 1 May 2019 Published: 13 August 2019
Journal Compilation © CSIRO 2019 Open Access CC BY-NC-ND
Abstract
Reconstructing movements and environmental histories of sharks may be possible by using the element composition of vertebrae, but unlocking such possibilities requires an understanding of the effects of extrinsic and intrinsic factors on element composition. We assessed water temperature and pH effects (independently and in combination) on vertebral chemistry of Port Jackson sharks while accounting for intrinsic factors (condition and sex) using indoor aquaria and outdoor mesocosm environments, where the latter may better reflect natural field conditions. We analysed eight element : Ca ratios (7Li, 8B, 24Mg, 55Mn, 65Cu, 88Sr, 138Ba and 238U) by laser ablation inductively coupled plasma mass spectrometry and found positive temperature-dependant responses for multiple elements, including B : Ca, Mn : Ca, Sr : Ca and Ba : Ca (r2 = 0.43, 0.22, 0.60 and 0.35 respectively), whereas pH had a minor effect on vertebral Mg : Ca and Li : Ca (r2 = 0.10 and 0.31 respectively). As shown for teleost otoliths, condition affected element composition (Mn : Ca), suggesting potential physiological influences on element uptake. The suitability of vertebral chemistry as a natural tag appears to be element specific, and likely governed by a suite of potentially codependent extrinsic and intrinsic factors. Overall, variations in vertebrae chemistry show promise to reconstruct movements and habitat use of cartilaginous fishes. Yet, further research is required to understand the ubiquitous nature of the findings presented here.
Additional keywords: acidification, condition, elasmobranchs, Heterodontus portusjacksoni, hydroxyapatite, vertebrae chemistry.
Introduction
Calcified structures of organisms contain chemical information that can unlock patterns of movement and habitat use by molluscs and fishes, including the chemical history of their habitats. In fishes, otoliths (ear bones) are inert aragonitic structures of choice because they are not prone to resorption, biomineralise with daily or annual growth increments and incorporate chemicals from the environment that can be calibrated against time (Radtke and Shafer 1992; Campana 1999; Elsdon et al. 2008). Increasingly, other structures of fish are also being investigated, especially for threatened or endangered species, to provide additional information to that obtained from otoliths (Gillanders et al. 2011; Izzo et al. 2016a; Tzadik et al. 2017). In elasmobranchs, which do not possess otoliths, alternative cartilaginous structures, such as jaw cartilage, vertebrae or fin spines, have increasingly been used for reconstructing environmental histories, population structure, ontogenetic movement and habitat use (McMillan et al. 2017a).
Vertebrae of elasmobranchs are widely used for age and growth studies because, like otoliths, they show growth increments that, for several species, have been validated as forming annually (Cailliet et al. 2004, 2006). Because vertebrae form a birth band and grow through accretion at the marginal edge, information within vertebrae can be correlated with the age of the elasmobranch and, if time of collection is known, to a year of formation. Elasmobranch vertebrae are comprised of calcium phosphate (Ca3(PO4)2), with no evidence of post-deposition resorption or remodelling of the chemical structure of the mineral or protein matrix of the vertebrae during growth (Clement 1992; Dean and Summers 2006; Dean et al. 2015). Therefore, these structures are metabolically inert and provide stable chronological records of shark life histories and their surrounding environment (Tillett et al. 2011; Smith et al. 2013; McMillan et al. 2017a; Mohan et al. 2018).
Several studies have examined sources of intra-individual natural variation in element composition of shark vertebrae and demonstrated no variation among regions of the vertebral column (e.g. cervical, thoracic, precaudal; Lewis et al. 2017), among vertebrae within similar regions of the vertebral column (e.g. Lewis et al. 2017; McMillan et al. 2017a), with the exception of Zn (Raoult et al. 2018), and little to no variation within similar regions of a vertebra (e.g. Tillett et al. 2011; Smith et al. 2016; Lewis et al. 2017). Other studies have also examined the effects of storage and sample cleaning methods on shark vertebral chemistry, finding that storage in ethanol or by freezing had little effect, although bleaching should be minimised because prolonged exposure can result in changes to Na, Mg and Mn concentrations (Tillett et al. 2011; Lewis et al. 2017; McMillan et al. 2017a; Mohan et al. 2017).
Element uptake and incorporation in calcified structures is a complex process, involving passage and fractionation of dissolved ions across multiple membranes or pathways (e.g. gills, gut, blood, endolymph; Campana 1999; McMillan et al. 2017a). Unlike otoliths, for which there are many experimental studies examining how extrinsic (environmental) and intrinsic (biological) factors affect otolith chemistry (e.g. Kalish 1989; Fowler et al. 1995; Bath et al. 2000; Elsdon and Gillanders 2003; Reis-Santos et al. 2013; Sturrock et al. 2015; Martino et al. 2017; Izzo et al. 2018), few studies have examined these effects on shark vertebrae. In fact, we are aware of only a single study that directly examines the effect of extrinsic and intrinsic factors on elasmobranch vertebral chemistry (Smith et al. 2013). In that study, no effects or consistent relationships between somatic growth or vertebrae precipitation on element concentrations of vertebrae were found, but Ba in the water was positively correlated with Ba in the vertebrae of round stingray Urobatis halleri. Temperature also negatively affected incorporation of Mg and Ba, and positively affected incorporation of Mn, but no effect was found for Sr (Smith et al. 2013).
Although knowledge of factors affecting vertebrae chemistry is not required for applications such as determining stock structure, connectivity, and assessing natal and juvenile habitats (e.g. Tillett et al. 2011; Werry et al. 2011; Izzo et al. 2016b; McMillan et al. 2017a, 2018), it helps in the interpretation of such results and is required for applications such as use as an environmental tracer. In addition, experimental studies, although difficult because of logistical issues with holding elasmobranchs in aquaria or mesocosms, would improve the generality of results to date. Moreover, increases of sea surface temperature allied to ocean acidification due to rising atmospheric carbon dioxide (CO2) and consequent decreases in ocean pH (e.g. Doney et al. 2009; McNeil and Sasse 2016) generate a myriad of potential effects on marine biota, including on elasmobranchs’ physiology, acid–base balance, metabolism, growth or behaviour (e.g. Green and Jutfelt 2014; Rosa et al. 2014; Pistevos et al. 2015, 2016). Considering that increased temperatures can exacerbate effects of increased CO2 (Harvey et al. 2013; Pistevos et al. 2015; Lefevre 2016) and that the combination of these effects may also hinder the applicability of the chemical composition of calcified structures as natural tags in changing ocean conditions, it is important to further evaluate their combined effects on the chemical composition of vertebrae.
The aim of the present study was to investigate the effects of water temperature and pH (independently and in combination) on vertebral element composition of an elasmobranch while simultaneously: (1) considering a set of biological or intrinsic factors (individual shark condition and specimen sex); and (2) evaluating patterns of element uptake in experimental indoor aquaria v. outdoor mesocosms in relation to temperature and pH, where the latter better replicates natural field conditions. Experimental studies on otolith element incorporation in fish have identified differential responses between controlled aquaria conditions and field observations (e.g. Elsdon and Gillanders 2005; Miller 2009; Reis-Santos et al. 2013, 2018). Therefore, unravelling the causes for these discrepancies, including the potential effects of intrinsic factors under different experimental conditions on element composition is essential to help interpret these data in complex and dynamic environments.
We used the Port Jackson shark Heterodontus portusjacksoni, a medium-sized (with a maximum total length (TL) of 1.5 m) oviparous benthic shark endemic to southern Australia, as our test species (Last and Stevens 2009). Every 9–12 months, individuals lay batches of eggs that each contain a single embryo. Development occurs over a 10 to 11-month period, with initial development occurring in an egg capsule sealed by a mucous plug. After 4 months, the mucous plug breaks down, surrounding the embryo and yolk with seawater (Rodda and Seymour 2008). After hatching, juveniles are benthic. Overall, H. portusjacksoni provides an excellent model elasmobranch species for laboratory and mesocosm trials because of its restricted size, reduced habitat requirements and diet based on benthic invertebrates (e.g. echinoderms, molluscs, crustaceans).
Materials and methods
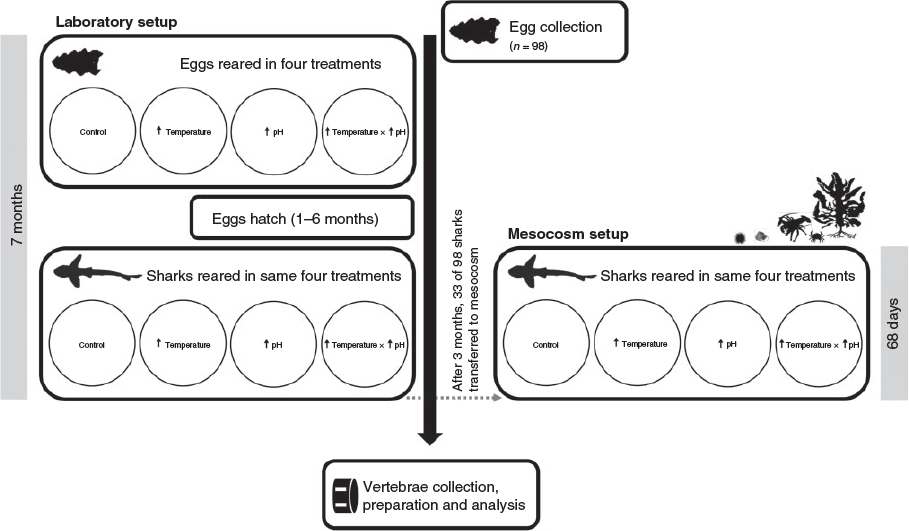
Egg collection and rearing of H. portusjacksoni
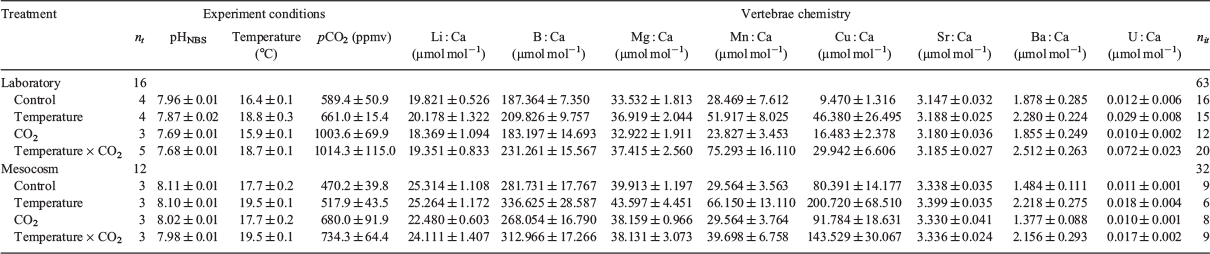
H. portusjacksoni eggs were collected from Edithburg, Gulf St Vincent (South Australia) by snorkelling (7 and 28 June 2013; n = 98). Eggs were immediately transported to a temperature-controlled laboratory and placed in 40-L treatment tanks (maximum 8 eggs per tank) with an internal biological filter and filled with natural sand-filtered seawater that was partially exchanged every 2–3 days (minimum 40% water change). The embryos within the eggs were in full contact with the treatment water because they lacked the mucus plug (eggs were >4 months old). Treatment acclimation took place over 7 days, whereby temperature and CO2 were gradually increased by 1°C and ~0.1 pH units for the elevated temperature and CO2 treatments. In total, there were four replicate tanks per treatment, with eggs kept in either control (16°C) or elevated (19°C) temperatures crossed with control (~400 parts per million per volume, ppmv) or elevated (~1000 ppmv) CO2 conditions (Fig. 1). Temperatures were maintained using heater–chiller units (TR15 Aquarium Chillers; TECO Refrigeration Technologies, Ravenna, Italy) and 300-W glass heaters. Pumps connected to the chiller units enabled even temperature distribution throughout the water baths. Salinity was not manipulated and was constant at 40 for the duration of the study, including the acclimation period and the laboratory and mesocosm experimental duration. Exposure before hatching ranged from a minimum of 65 days to a maximum of 179 days (for data on hatching, see Pistevos et al. 2015).
Thermal mass flow meters and controllers (PEGAS 4000 MF Gas Mixer; Columbus Instruments, Columbus, OH, USA) were used to regulate CO2 concentration in the seawater by bubbling enriched air directly into the experimental tanks. Temperature and the pHNBS (National Bureau of Standards, NBS, scale) of each tank were measured daily using a temperature and pH meter (SevenGo SG2; Mettler Toledo, Columbus, OH, USA) calibrated with fresh buffers each day. In addition, salinity and oxygen (HandyPolaris, OxyGuard, Farum, Denmark) were measured daily. Total alkalinity of seawater was estimated by Gran titration (888 Titrando; Metrohm, Herisau, Switzerland) weekly. Variability in pCO2 measures are higher than for pH because pCO2 was calculated using weekly measurements of total alkalinity, whereas pH was measured on a daily basis.
Research activities described herein were undertaken with approval from the University of Adelaide Animal Ethics Committee (Permit S2013-095).
Experimental setup
Laboratory conditions
As the juvenile sharks hatched they were relocated to new large tanks (volume 100 or 150 L) with the exact same treatments and manipulation methods as described above, and again with four tanks per treatment. The number of sharks in each tank ranged from one to eight for the 150-L tanks and from one to four for the 100-L tanks (because of differences in hatching time and because sharks were also transferred for the mesocosm experiment; see below and Fig. 1). Sharks were fed ad libitum with thawed frozen prawns daily and kept in their respective tank and treatment for a total of 68 days. Water parameters were measured daily (Table 1) and conditions significantly differed among treatments (see Table S1, available as Supplementary material to this paper), with water changes made every other day (minimum 40% total volume). Water parameter measurement methods are provided above.
Mesocosm conditions
Once an equal number of sharks hatched from all treatments, randomly selected individuals per treatment (total n = 36) were relocated to a treatment matched mesocosm setup (Fig. 1). Three sharks were introduced in each of the 12 mesocosm tanks (volume 2000 L). Sand-filtered natural seawater in the mesocosms was from the same source as the water used in the laboratory experiments, and temperature and CO2 were manipulated using TC60 Aquarium heater and chillers (TECO Refrigeration Technologies) and identical thermal mass flow meters and controllers as in the laboratory experiments. As with the laboratory experiments, temperature, salinity and pH were measured daily (Table 1) and conditions differed significantly among treatments (see to Table S1). The mesocosms were set up to mimic the local shallow temperate reef habitat and included 5 kelp plants (Ecklonia radiata; mean weight 250 g per plant), 1 spiny rock lobster (Jasus edwardsii; ~2 kg in weight), 1 crab (Ozius truncatus), 15 snails (Turbo undulatus), 6 urchins (Heliocidaris erythrogramma) and >1000 herbivorous amphipods (Cymadusa pemptos). The substratum was covered by naturally growing turf algae. Kelp, snails and urchins were replenished (three times) throughout the experiment. Like fish in the laboratory, sharks were fed ad libitum with thawed frozen prawns daily, and the mesocosm trial lasted the same 68 days as the laboratory trial. A schematic summarising the experiment is shown in Fig. 1.
Vertebral sampling and preparation
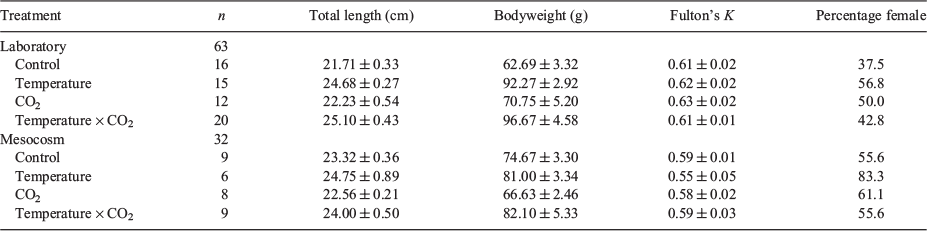
At the end of the laboratory and mesocosm experimental periods, all sharks were killed with clove oil and TL (cm), bodyweight (g) and specimen sex were recorded (Table 2). Fulton’s K condition index was calculated using the following formula:

|
Sections of vertebrae (n = 6–12 per individual) were excised posterior to the cranium, separated and cleaned of excess tissue before being oven dried at 50°C for 24 h. Vertebrae were then embedded in a clear-setting epoxy resin spiked with indium to discriminate the vertebrae from the epoxy during elemental analysis.
Transverse sections (~500 µm) through the vertebral focus were made using a low-speed diamond saw (Isomet; Buehler, Lake Bluff, IL, USA), wet polished on progressively finer grades of lapping film (30, 9, 3 μm) and cleaned in ultrapure water. Sections were then mounted onto microscope slides with indium-spiked Crystalbond (509) glue (Crystalbond CPI, West Chester, PA, USA). Finally, to remove any surface contamination, slides were sonicated in ultrapure water for 3 min, triple rinsed in ultrapure water, and dried with lint-free tissues before being individually stored in sealed plastic bags.
Element analysis
The multielement composition of vertebrae was analysed using a New Wave NWR213-ESI 213 nm ultraviolet (UV) laser (Elemental Scientific Lasers, Omaha, NE, USA) connected to an Agilent 7900cx (Agilent Technologies INC, Santa Clara CA, USA) inductively coupled plasma mass spectrometer (ICP-MS; housed at Adelaide Microscopy, The University of Adelaide). Element concentrations quantified included 7Li, 8B, 24Mg, 55Mn, 65Cu, 88Sr, 138Ba and 238U; 43Ca was used as an internal standard and 115In was measured as a marker to distinguish between spiked resin or CrystalBond and the sample. Laser ablations were performed in a sealed chamber with resulting analyte transported to the ICP-MS by a smoothing manifold in an Ar and He stream.
In all, 95 vertebrae (laboratory, n = 63; mesocosm, n = 32) were analysed at the marginal edge of the corpus calcareum, therefore mitigating any potentially confounding effects of maternal contributions to element composition (Smith 2013; Lewis et al. 2016). Vertebral growth was estimated to range from 98 to 130 µm among treatments based on sharks’ growth rates from Pistevos et al. (2015) fit to a linear relationship between TL and centrum diameter (C. Izzo, unpubl. data). Given that all sharks were maintained since their time of collection as eggs and, most importantly, from the time of hatching to the end of the experiment in the exact same treatment conditions, we are confident that ablated material represents recent element incorporation during the period of experimental growth.
Triplicate spot ablations per vertebrae were made using a laser spot size of 40 µm, with a repetition rate of 5 Hz and an approximate fluence of 10 J cm–2. Background concentrations of elements within the sample chamber were measured for 30 s before each ablation. For all ablations, we excluded the first 3–4 s of elemental signal (characterised by a sharp peak in the raw counts data) during the signal selection process, which cleans or removes the effect of any possible surface contaminants on the sample.
The National Institute of Standards glass standard (NIST-612) and the USGS (United States Geological Survey) micro-analytical carbonate standard (MACS-3) were analysed at the start, end and periodically throughout each session (after every 12 ablations) to correct for instrument drift and measure external precision respectively (the CV for repeated measures for all elements was <5%). Data reductions and raw count data conversions to element concentrations (ppm) were performed using Iolite software (ver. 3, Iolite, The University of Melbourne, Vic., Australia) and the Trace Element IS data reduction scheme (Paton et al. 2011), with individual elements then expressed as ratios to Ca, based on a 43Ca percentage weight value for H. portusjacksoni vertebrae of 44.8 (McMillan et al. 2017a). Any outlying values (identified as deviating ±2 s.d. of the mean of the three replicate ablations made per vertebrae) were excluded from the dataset (in all, 24 ablations of a total 285 were removed). For each vertebrae, a minimum of two ablations was averaged to obtain a single value per specimen. Limits of detection are given in Table S2.
Data analysis
All analyses were undertaken in R (ver. 3.5.3, R Foundation for Statistical Computing, Vienna, Austria). To confirm the success of experimental manipulations, variations in temperature, pH and pCO2 were compared by univariate analysis of variance (ANOVA; Table S1).
The effects of treatment tank-level extrinsic (temperature, pH) and intrinsic (individual Fulton’s K condition, specimen sex) covariates on vertebral element : Ca composition were explored with general linear models using the lme4 package (ver. 1.1-19, D. Bates, M. Maechler, B. Bolker and S. Walker, see https://github.com/lme4/lme4/). Prior to the modelling approach, multi-collinearity of the data was evaluated among the suite of covariates using pairwise Pearson correlations. There was no evidence of collinearity among covariates, except among TL, bodyweight and temperature (Fig. S1). TL and bodyweight were excluded from subsequent analysis, but Fulton’s K condition index was kept as a predictor because it is an adequate proxy for individual growth and condition.
All vertebral element data were transformed with a log(x + 1) function to meet the model assumptions of normality and homoscedasticity, with the quality of model fit visually confirmed by observation of model residuals (Fig. S2). A suite of models comprising all combinations of the fixed extrinsic and intrinsic covariates including experiment type (laboratory v. mesocosm) was fit to individual element data using the ‘dredge’ function in the MuMIn package (ver. 1.15.1, K. Bartoń, see http://CRAN.R-project.org/package=MuMIn/; Bartoń 2019). The Akaike information criterion corrected for small sample sizes (AICc) was used to identify the best model.
The best-ranked model (based on AICc) was then rerun and the covariate effects extracted from the model. Model parsimony and frequency of inclusion (and coherence of direction of effect) were taken into consideration if there were no differences in AICc among the top-ranked models. In those instances where experiment type was identified as one of the factors affecting vertebrae element composition, the data were partitioned into laboratory and mesocosm datasets and the suite of models was rerun to better understand how element incorporation differed between experiment types.
Results
Temperature and pH manipulations were successful, reflected the desired treatment levels and showed significant differences in all stages of the experimental procedure, even if temperature exhibited higher within-treatment variability (Table 1; for ANOVA, see Table S1).
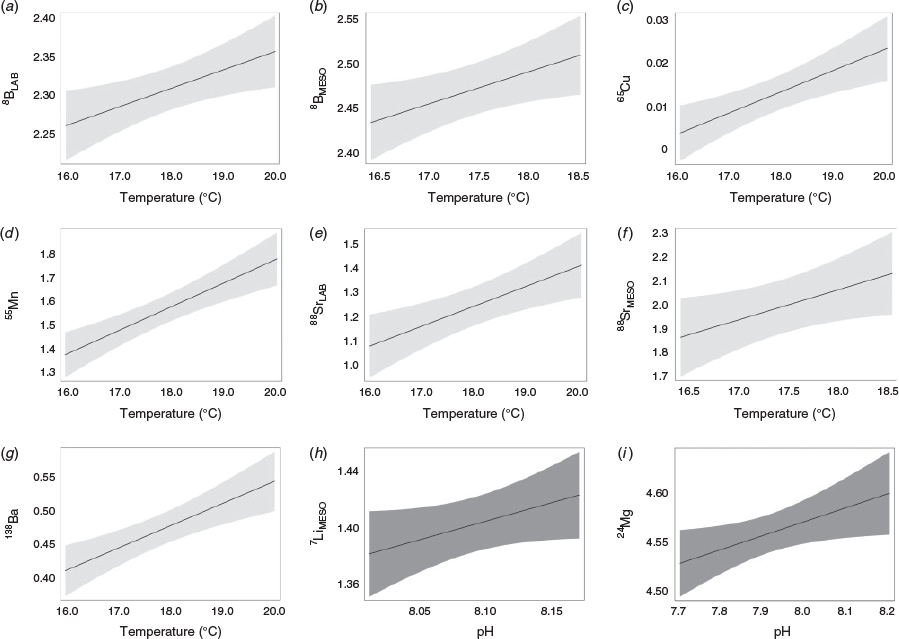
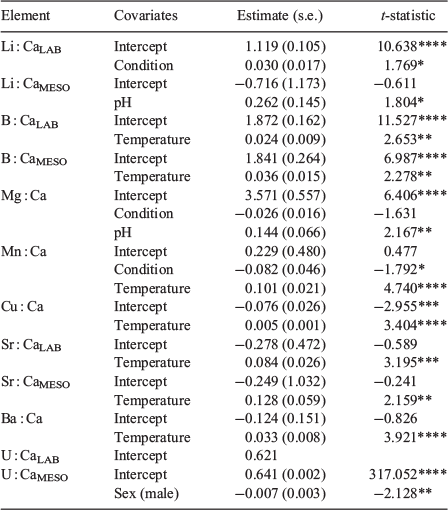
General linear models identified and ranked the influence of each of the extrinsic and intrinsic covariates on element vertebral composition and enabled us to relate vertebral element composition at the individual level to the conditions of the individual temperature and pH treatments. The covariates most often included in the top five models for all elements were temperature and experiment type, with specimen sex largely excluded (Table 3; Fig. 2; see also Table S3). When temperature was present in the best-ranked model, this covariate was included in all the top five best-ranked models (Table S3). Overall, consistent positive temperature effects on vertebral element composition were observed for five of the eight elements investigated (see B : Ca, Mn : Ca, Cu : Ca, Sr : Ca, Ba : Ca in Table 3; Fig. 2a–g), even if in some of the cases the overall variance explained by the models was reduced.
|
Analysing each element individually, the highest ranked model for Li : Ca (based on the dredge AICc model ranking; see to Table S3) included the covariates experiment type, condition (Fulton’s K) and pH (r2 = 0.30). The top ranked laboratory model contained condition, whereas the best-ranked mesocosm model contained the term pH (Fig. 2h). Under the different experimental setups, both condition and pH had a positive effect on Li : Ca, but with marginal significance (P < 0.05; Table 3).
Model ranking for vertebral B : Ca indicated that the highest-ranked model of the suite of 40 contained the covariates experiment type and water temperature (r2 = 0.43). When the laboratory and mesocosm models were reranked independently (Table S4), temperature was identified as the common influential covariate for both experiment types, with a positive and significant effect on vertebral B : Ca composition (Table 3; Fig. 2a, b).
For Mg : Ca, the model that contained the covariates condition and pH was the highest ranked of the model suite (Table S3). Overall, this model provided a relatively poor fit to the data (r2 = 0.10; lowest of all the elements analysed), with covariates revealing opposing effects on vertebral Mg : Ca chemistry. pH had a positive effect (Fig. 2i), whereas increasing condition (non-significantly) decreased Mg : Ca (Table 3).
The best-ranked Mn : Ca model included condition and temperature as influential covariates (Table S3), and provided a moderate fit to the data (r2 = 0.22). Temperature had a significant positive effect on vertebral Mn : Ca (Fig. 2d) and condition had a significant negative effect (Table 3). The second highest-ranked model for Mn : Ca only included the temperature covariate, although model fit was reduced based on r2 (albeit a minor reduction; Table S3).
The model containing temperature was the highest ranked for Cu : Ca (Table S3), although with poor fit to the data (r2 = 0.11), with temperature having a significant positive effect on vertebral Cu : Ca (Table 3; Fig. 2c). The second highest-ranked model that included pH in addition to temperature provided minor improvement to the model fit (based on r2; Table S3).
The top-ranked Ba : Ca model included temperature, with this predictor present in all top five models (Table S3); however, the fit of the highest-ranked model was relatively poor (r2 = 0.14). Vertebral Ba : Ca was significantly and positively affected by temperature (Table 3; Fig. 2g).
Temperature had a positive and significant effect on Sr : Ca vertebral composition (Table 3). For Sr : Ca, the top-ranked model incorporated the covariates temperature and experiment type (Table S3), and presented overall the highest model fit of all the elements analysed (r2 = 0.60). Reranking the laboratory and mesocosm datasets independently revealed that the best-ranked models for both experimental setups contained temperature (Fig. 2e, f; Table S4).
Experiment type was the only covariate in the best-ranked model for U : Ca (Table S3). Overall, the goodness of fit for the best U : Ca model (r2 = 0.35) was the third best overall, following only that of Sr : Ca and B : Ca. When the data were partitioned by experiment type, the model reranking indicated that the null model (containing no covariates) was the best-ranked model for the laboratory-reared sharks. In contrast, for the mesocosm sharks, the model containing specimen sex (male v. female) was the best-ranked model, with females having significantly higher U : Ca ratios relative to their male counterparts (Table 3).
Discussion
Although we observed that intrinsic factors (condition and individual’s sex) can affect elasmobranch vertebral chemistry, extrinsic factors (temperature and pH) also affected elasmobranch vertebral chemistry and may help with the reconstruction of historical movements and habitats of sharks. To date, only one other study has tested the effects of temperature and biomineralisation on vertebral element incorporation in elasmobranchs (Smith et al. 2013); the present study is the first to also assess the effect of pH, as well as to simultaneously evaluate how intrinsic individual-based factors may affect shark vertebral chemistry. Overall, we found positive temperature-dependent responses for most elements, whereas pH had a comparatively minor (positive) effect. Akin to observations in teleost otoliths, individual condition played a role in the incorporation of specific elements (e.g. Mg or Mn; Chang and Geffen 2013; Izzo et al. 2015; Sturrock et al. 2015; Reis-Santos et al. 2018). Yet, the lack of analogous elasmobranch studies against which to compare the present results, in combination with the relative importance of ‘experiment type’ and the reduced overall variance of some models, limit the explanatory power of some inferences, even if our results provide a valuable framework for both future laboratory and field measurements.
Consistent positive temperature effects on vertebral element chemistry were observed for five of the eight elements investigated (B : Ca, Mn : Ca, Cu : Ca, Sr : Ca and Ba : Ca). Although these findings are consistent with trends observed by Smith et al. (2013) in U. halleri, there are also some clear contrasting results between studies. Overall, Smith et al. (2013) identified positive temperature effects on vertebral Mn : Ca and Zn : Ca (not tested here), but showed negative or no effects for Mg : Ca, Ba : Ca and Sr : Ca. In both studies, Li : Ca composition in vertebrae was not sensitive to temperature. Interspecific differences in the effects of extrinsic factors, such as temperature, on the element chemistry of calcified structures are common and widely reported in the teleost otolith element literature, including among species in a location or between laboratory and wild-caught fish of the same species (e.g. Elsdon and Gillanders 2005; Reis‐Santos et al. 2008; Chang and Geffen 2013; Izzo et al. 2018). These differences have been attributed to a range of physiological, metabolic or kinetic factors, including modes of incorporation (e.g. via the gut v. the gill), an element’s role in physiological processes (e.g. essential v. non-essential) and the binding fate in the carbonate structure (e.g. substituting for calcium or forming metal–protein complexes; Izzo et al. 2015; Stanley et al. 2015; Sturrock et al. 2015; Grønkjær 2016; Thomas et al. 2017). In fact, because temperature poses osmotic and physiological constraints on ion regulation and uptake kinetics, it can drive element uptake and affect the chemical composition of calcified tissues both directly and indirectly (Loewen et al. 2016; Reis-Santos et al. 2018). Smith et al. (2013) also described temperature effects on rates of vertebral element incorporation (by partition factors). However, we cannot make inferences on changes to incorporation rates in H. portusjacksoni (even though we saw changes in vertebral chemistry) because of a lack of water chemistry data. Nevertheless, the results of this study reinforce the apparent importance of the chemical composition of biogenic tissues, namely of Sr : Ca and Ba : Ca, as natural tags for environmental reconstructions (Elsdon et al. 2008; Tillett et al. 2011; Smith et al. 2013; Izzo et al. 2016a). Here, approximately 3°C of temperature induced changes in chemical composition (albeit with varying ranges per element); nevertheless, a key issue to consider is what magnitude of variation in the factor of interest (i.e. temperature) promotes a meaningful change in element chemistry (Sturrock et al. 2012; Walther 2019).
The pervasive increase in pCO2 in coastal systems potentially hinders the accretion of biogenic calcified structures in aquatic systems, even though mixed responses have been observed (Wood et al. 2008; Ries et al. 2009; Cattano et al. 2018). Those few studies exploring the effects of pH (or acidification by increased pCO2) in teleost otoliths from natural (i.e. volcanic vents) and laboratory conditions have not identified effects on otolith chemistry (e.g. Martino et al. 2017; Mirasole et al. 2017). The results of the present study indicated that pH can affect vertebral Mg : Ca and Li : Ca (albeit a weak effect for Li : Ca, and thus some caution must be taken). Considering the present study is the first to explore ocean acidification on shark element chemistry, we are yet to gauge how ubiquitous these results may be. However, such attempts will be key to confirming the suitability of using these structures as natural tags and tracers of environmental acidification in changing oceans. Uranium concentrations have been shown to increase with the acidity of the environment in marine molluscan shells (Doubleday et al. 2017) and cephalopod statoliths (Frieder et al. 2014), and we hypothesised a similar trend for U : Ca in shark vertebrae. However, that was not the case. Overall, apatite is poorly crystallised relative to other biogenic calcified structures, and its chemical properties (i.e. element incorporation) are expected to differ from those of otoliths and other structures (see McMillan et al. 2017a, 2017b), with both biogenic (from Serratia sp. bacteria) and non-biogenic hydroxyapatite showing stable strontium and cobalt sorption under a range of pH conditions (Handley-Sidhu et al. 2016). Further evaluation of the sensitivity of δ13C and δ44Ca to acidification in elasmobranch vertebrae may provide further insights into the effects of pH on these calcified structures and advance the use of vertebrae as natural tags (Hinojosa et al. 2012; Mirasole et al. 2017).
Despite positive indications, our ability to interpret variations in vertebrae chemistry as tracers of elasmobranch environmental histories is still limited, and further evaluations of the effects of extrinsic factors, including temperature, salinity, water chemistry and pH, are paramount to better understand common trends across species and environments, as well as to gain insights into the scale and magnitude at which we are able to reconstruct elasmobranchs’ past environmental histories or patterns of habitat use. Moreover, the extent to which intrinsic factors can buffer or magnify environmentally mediated element incorporation in calcified structures and how that potentially introduces interpretation error in environmental and life history reconstructions remain uncertain (Kalish 1989; Sturrock et al. 2014, 2015; Loewen et al. 2016; Grammer et al. 2017; Reis-Santos et al. 2018). Here, we found that shark condition (Fulton’s K) increased Mn : Ca and Li : Ca (for the laboratory sharks only) and assume that this is evidence of an intrinsic control or physiological influence on element uptake and vertebrae chemical composition (as per Izzo et al. 2015), even if further investigation is required. Moreover, it is unclear, for example, what role diet has in affecting both the condition of an individual and vertebral element composition, where both are likely to be affected by extrinsic factors such as temperature (Webb et al. 2012), although diet did not vary among experimental treatments in the present study. This is consistent with hypothesised among-element differences in how vertebral elements are affected by intrinsic as opposed to extrinsic factors (McMillan et al. 2017b), with Mn and Li linked to metabolism (Watanabe et al. 1997) and osmoregulation (Fleishman et al. 1986) respectively. In teleost otoliths, variations in composition and element incorporation of Mn : Ca and Li : Ca have also been related to intrinsic factors directly (e.g. ontogeny, growth, genetics, metabolism) or indirectly (e.g. environmental conditions influence metabolic or physiological processes, including osmoregulation or biomineralisation, and thus element uptake kinetics) (Campana 1999; Clarke et al. 2011; Sturrock et al. 2015; Loewen et al. 2016; Grammer et al. 2017; Reis-Santos et al. 2018). However, it is worth noting that Mn : Ca was also significantly affected by temperature, and Li : Ca in the mesocosm sharks was affected by pH, suggesting that physiological controls on vertebral elements are complex and likely influenced by environmentally mediated processes. For example, increased temperatures have been shown to significantly decrease H. portusjacksoni growth when food is limiting (Pistevos et al. 2015), and elicit declines in (Fulton’s K) condition (data not shown), which is coincident with increases in vertebral Mn : Ca (Smith et al. 2013; present study). The complexity of potentially codependent extrinsic and intrinsic processes and their effects on element uptake require measures of changes in plasma ion–protein concentrations, because these are the shared mechanism by which physiological processes modify uptake, incorporation and chemical composition (Grønkjær 2016).
Here, we evaluated the consistency in patterns of vertebrae element composition in controlled experimental indoor aquaria v. outdoor mesocosms, where the latter may better reflect natural field conditions. Mesocosms have also become paramount for acidification trials, which cannot be performed in the natural environment, with the exception of natural sea vents (Doubleday et al. 2017; Mirasole et al. 2017). Despite the controlled conditions being similar between the laboratory and mesocosm, such as water source, salinity and diet (albeit sharks sporadically predated on some organisms in the mesocosms), the inclusion of experiment type in some top-ranked models for individual elements likely reflects biological (intrinsic) and chemical interactions (that may affect element availability) taking place in the more complex natural ecosystem of the mesocosm, which would be similar to what occurs in the field, where most applications of vertebrae chemistry are aimed. The absence of water chemistry impeded the ability to test for potential element variations or availability in the experimental setup (e.g. due to microbial activity), but given our focus was on temperature and pH variation, this was not required. Still, it is recommended that future studies comparing trends between wild-caught and experimentally reared sharks (and fish) analyse the element composition of the surrounding water to help interpretation of the findings. Such water chemistry may also provide a means of standardising the two datasets to improve comparisons (i.e. using partition coefficients; Elsdon and Gillanders 2005). Overall, together with the influence of unquantified (and hard to control for) individual-level intrinsic factors, there is uncertainty over whether trends observed in controlled laboratory conditions would reflect field conditions and changes in natural populations (Elsdon and Gillanders 2005). The continued challenge for the application and interpretation of chemical signals to resolve environmental reconstructions, or other key questions, is to account for the suite of influential extrinsic and intrinsic factors (Sturrock et al. 2014, 2015). In fact, Walther (2019) suggests a signal-to-noise approach to differentiate target data from the background of other dynamic factors (natural variability, or intrinsic and extrinsic factors). Key to this is understanding the magnitude of non-target data, because this will allow us to resolve the magnitude of change required in the chemical gradient that enables detectable or relevant environmental or movement reconstructions. The magnitude and range of chemical variations observed were small for some elements, which, together with natural variability and lower model fits, emphasises the need to further address these issues under both laboratory and mesocosm or field conditions.
Several other assumptions also need to be addressed to confirm the suitability of vertebral elements as an environmental tracer, including the need to understand potential ontogenetic and diagenetic effects (see McMillan et al. 2017a). In previous studies, vertebral element incorporation was not related to somatic growth or vertebral biomineralisation rates (Smith et al. 2013), with the exception of Zn : Ca (Raoult et al. 2018). However, ontogeny and biomineralisation have been shown to influence rates of element uptake in otoliths of teleost species in some (but not all) manipulative experiments (Morales-Nin 2000; DiMaria et al. 2010; Fablet et al. 2011; Loewen et al. 2016). Therefore, analogous experiments designed to evaluate the effects of somatic and vertebral growth are suggested to compare to Smith et al. (2013), and to evaluate trends and the generality of results in elasmobranchs. Furthermore, information is lacking on the relative effects of water chemistry and diet on element composition in elasmobranch vertebrae (but see Walther and Thorrold 2006; Doubleday et al. 2013) and how this is mediated by environmental conditions (as observed in teleosts; e.g. Webb et al. 2012).
In conclusion, we support further research into the use of shark vertebrate as natural tags. Although our work helps understand trends in elasmobranch chemistry, the suitability of vertebral chemistry as a natural tag appears to be element specific, as it is likely governed by a suite of potentially codependent extrinsic and intrinsic factors. Hence, advances to using the element composition of vertebrae as a tracer to reconstruct movements and environmental histories will be reliant on unravelling how both extrinsic and intrinsic factors contribute to the element chemistry, and further understanding the mechanisms that directly and indirectly influence uptake and incorporation pathways. Further research will also help in our understanding of the ubiquitous nature of the findings presented here and in other studies that explore the suitability of vertebral element composition as a natural tag for cartilaginous fishes.
Conflicts of interest
Bronwyn Gillanders is an Associate Editor for Marine and Freshwater Research. Despite this relationship, she did not at any stage have Associate Editor-level access to this manuscript while in peer review, as is the standard practice when handling manuscripts submitted by an editor to this journal. Marine and Freshwater Research encourages its editors to publish in the journal and they are kept totally separate from the decision-making process for their manuscripts. The authors have no further conflicts of interest to declare.
Declaration of funding
The authors thank I. Nagelkerken and S. D. Connell for assistance and providing funding (Australian Research Council FT120100183 and FT0991953); additional funding was provided from Bronwyn M. Gillanders (FT100100767), the Environment Institute and the School of Biological Sciences (The University of Adelaide). This study also had the support of the Fundação para a Ciência e Tecnologia (UID/MAR/04292/2019) and a postdoctoral grant (SFRH/BPD/95784/2013) to P. Reis-Santos.
Acknowledgements
The authors thank I. Nagelkerken, S. D. Connell and the three anonymous reviewers for their insightful comments that improved the manuscript. The authors also thank T. Rossi, G. Ghedini and B. Florance for their assistance with the collection of shark eggs and additional staff who helped with the daily maintenance of eggs and sharks in the laboratory and mesocosms (M. Olmos, N. Mertens, K. Heldt, K. Anderson, L. Falkenberg, P. Munguia, B. Russell and M. Rutte). The authors thank Adelaide Microscopy, The University of Adelaide, and the Australian Microscopy and Microanalysis Research Facility for the use of equipment, and in particular S. Gilbert for assistance with the laser ablation inductively coupled plasma mass spectrometry.
References
Bartoń, K. (2019). Package ‘MuMIn’, ver. 1.43.6. Available at https://cran.r-project.org/web/packages/MuMIn/MuMIn.pdf [Verified 8 August 2019].Bath, G. H., Thorrold, S. T., Jones, C. M., Campana, S. E., McLaren, J. W., and Lam, J. W. (2000). Strontium and barium uptake in aragonitic otoliths of marine fish. Geochimica et Cosmochimica Acta 64, 1705–1714.
| Strontium and barium uptake in aragonitic otoliths of marine fish.Crossref | GoogleScholarGoogle Scholar |
Cailliet, G. M., Goldman, K. J., Carrier, J. C., Musick, J. A., and Heithaus, M. R. (2004). Age determination and validation in chondrichthyans fishes. In ‘Biology of Sharks and Their Relatives’. (Eds J. Carrier, J. A. Musick, and M. Heithaus.) pp. 399–447. (CRC Press: San Diego, CA, USA.)
Cailliet, G. M., Smith, W. D., Mollet, H. F., and Goldman, K. J. (2006). Age and growth studies of chondrichthyan fishes: the need for consistency in terminology, verification, validation, and growth function fitting. Environmental Biology of Fishes 77, 211–228.
| Age and growth studies of chondrichthyan fishes: the need for consistency in terminology, verification, validation, and growth function fitting.Crossref | GoogleScholarGoogle Scholar |
Campana, S. E. (1999). Chemistry and composition of fish otoliths: pathways, mechanisms and applications. Marine Ecology Progress Series 188, 263–297.
| Chemistry and composition of fish otoliths: pathways, mechanisms and applications.Crossref | GoogleScholarGoogle Scholar |
Cattano, C., Claudet, J., Domenici, P., and Milazzo, M. (2018). Living in a high CO2 world: a global meta‐analysis shows multiple trait‐mediated fish responses to ocean acidification. Ecological Monographs 88, 320–335.
| Living in a high CO2 world: a global meta‐analysis shows multiple trait‐mediated fish responses to ocean acidification.Crossref | GoogleScholarGoogle Scholar |
Chang, M., and Geffen, A. J. (2013). Taxonomic and geographic influences on fish otolith microchemistry. Fish and Fisheries 14, 458–492.
| Taxonomic and geographic influences on fish otolith microchemistry.Crossref | GoogleScholarGoogle Scholar |
Clarke, L. M., Thorrold, S. R., and Conover, D. O. (2011). Population differences in otolith chemistry have a genetic basis in Menidia menidia. Canadian Journal of Fisheries and Aquatic Sciences 68, 105–114.
| Population differences in otolith chemistry have a genetic basis in Menidia menidia.Crossref | GoogleScholarGoogle Scholar |
Clement, J. (1992). Re-examination of the fine structure of endoskeletal mineralization in Chondrichthyans: implications for growth, ageing and calcium homeostasis. Marine and Freshwater Research 43, 157–181.
| Re-examination of the fine structure of endoskeletal mineralization in Chondrichthyans: implications for growth, ageing and calcium homeostasis.Crossref | GoogleScholarGoogle Scholar |
Dean, M. N., and Summers, A. P. (2006). Mineralized cartilage in the skeleton of chondrichthyan fishes. Zoology 109, 164–168.
| Mineralized cartilage in the skeleton of chondrichthyan fishes.Crossref | GoogleScholarGoogle Scholar | 16584875PubMed |
Dean, M. N., Ekstrom, L., Monsonego-Ornan, E., Ballantyne, J., Witten, E. P., Riley, C., Habraken, W., and Omelon, S. (2015). Mineral homeostasis and regulation of mineralization processes in the skeletons of sharks, rays and relatives (Elasmobranchii). Seminars in Cell & Developmental Biology 46, 51–67.
| Mineral homeostasis and regulation of mineralization processes in the skeletons of sharks, rays and relatives (Elasmobranchii).Crossref | GoogleScholarGoogle Scholar |
DiMaria, R., Miller, J., and Hurst, T. (2010). Temperature and growth effects on otolith elemental chemistry of larval Pacific cod, Gadus macrocephalus. Environmental Biology of Fishes 89, 453–462.
| Temperature and growth effects on otolith elemental chemistry of larval Pacific cod, Gadus macrocephalus.Crossref | GoogleScholarGoogle Scholar |
Doney, S. C., Fabry, V. J., Feely, R. A., and Kleypas, J. A. (2009). Ocean acidification: the other CO2 problem. Marketing Science 1, 169–192.
| Ocean acidification: the other CO2 problem.Crossref | GoogleScholarGoogle Scholar |
Doubleday, Z., Izzo, C., Woodcock, S., and Gillanders, B. (2013). Relative contribution of water and diet to otolith chemistry in freshwater fish. Aquatic Biology 18, 271–280.
| Relative contribution of water and diet to otolith chemistry in freshwater fish.Crossref | GoogleScholarGoogle Scholar |
Doubleday, Z. A., Nagelkerken, I., and Connell, S. D. (2017). Ocean life breaking rules by building shells in acidic extremes. Current Biology 27, R1104–R1106.
| Ocean life breaking rules by building shells in acidic extremes.Crossref | GoogleScholarGoogle Scholar | 29065288PubMed |
Elsdon, T., and Gillanders, B. (2003). Relationship between water and otolith elemental concentrations in juvenile black bream Acanthopagrus butcheri. Marine Ecology Progress Series 260, 263–272.
| Relationship between water and otolith elemental concentrations in juvenile black bream Acanthopagrus butcheri.Crossref | GoogleScholarGoogle Scholar |
Elsdon, T., and Gillanders, B. (2005). Consistency of patterns between laboratory experiments and field collected fish in otolith chemistry: an example and applications for salinity reconstructions. Marine and Freshwater Research 56, 609–617.
| Consistency of patterns between laboratory experiments and field collected fish in otolith chemistry: an example and applications for salinity reconstructions.Crossref | GoogleScholarGoogle Scholar |
Elsdon, T. S., Wells, B. K., Campana, S. E., Gillanders, B. M., Jones, C. M., Limburg, K. E., Secor, D. H., Thorrold, S. R., and Walther, B. D. (2008). Otolith chemistry to describe movements and life-history parameters of fishes: hypotheses, assumptions, limitations and inferences. Oceanography and Marine Biology – an Annual Review 46, 297–330.
| Otolith chemistry to describe movements and life-history parameters of fishes: hypotheses, assumptions, limitations and inferences.Crossref | GoogleScholarGoogle Scholar |
Fablet, R., Pecquerie, L., de Pontual, H., Høie, H., Millner, R., Mosegaard, H., and Kooijman, S. A. (2011). Shedding light on fish otolith biomineralization using a bioenergetic approach. PLoS One 6, e27055.
| Shedding light on fish otolith biomineralization using a bioenergetic approach.Crossref | GoogleScholarGoogle Scholar | 22110601PubMed |
Fleishman, D. G., Saulus, A. A., and Vasilieva, V. F. (1986). Lithium in marine elasmobranchs as a natural marker of rectal gland contribution in sodium balance. Comparative Biochemistry and Physiology – A. Physiology 84, 643–648.
| Lithium in marine elasmobranchs as a natural marker of rectal gland contribution in sodium balance.Crossref | GoogleScholarGoogle Scholar |
Fowler, A. J., Campana, S. E., Thorrold, S. R., and Jones, C. M. (1995). Experimental assessment of the effect of temperature and salinity on elemental composition of otoliths using laser ablation ICPMS. Canadian Journal of Fisheries and Aquatic Sciences 52, 1431–1441.
| Experimental assessment of the effect of temperature and salinity on elemental composition of otoliths using laser ablation ICPMS.Crossref | GoogleScholarGoogle Scholar |
Frieder, C. A., Gonzalez, J. P., and Levin, L. A. (2014). Uranium in larval shells as a barometer of molluscan ocean acidification exposure. Environmental Science & Technology 48, 6401–6408.
| Uranium in larval shells as a barometer of molluscan ocean acidification exposure.Crossref | GoogleScholarGoogle Scholar |
Gillanders, B. M., Elsdon, T. S., Halliday, I. A., Jenkins, G. P., Robins, J. B., and Valesini, F. J. (2011). Potential effects of climate change on Australian estuaries and fish utilising estuaries: a review. Marine and Freshwater Research 62, 1115–1131.
| Potential effects of climate change on Australian estuaries and fish utilising estuaries: a review.Crossref | GoogleScholarGoogle Scholar |
Grammer, G. L., Morrongiello, J. R., Izzo, C., Hawthorne, P. J., Middleton, J. F., and Gillanders, B. M. (2017). Coupling biogeochemical tracers with fish growth reveals physiological and environmental controls on otolith chemistry. Ecological Monographs 87, 487–507.
| Coupling biogeochemical tracers with fish growth reveals physiological and environmental controls on otolith chemistry.Crossref | GoogleScholarGoogle Scholar |
Green, L., and Jutfelt, F. (2014). Elevated carbon dioxide alters the plasma composition and behaviour of a shark. Biology Letters 10, 20140538.
| Elevated carbon dioxide alters the plasma composition and behaviour of a shark.Crossref | GoogleScholarGoogle Scholar | 25232027PubMed |
Grønkjær, P. (2016). Otoliths as individual indicators: a reappraisal of the link between fish physiology and otolith characteristics. Marine and Freshwater Research 67, 881–888.
| Otoliths as individual indicators: a reappraisal of the link between fish physiology and otolith characteristics.Crossref | GoogleScholarGoogle Scholar |
Handley-Sidhu, S., Mullan, T. K., Grail, Q., Albadarneh, M., Ohnuki, T., and Macaskie, L. E. (2016). Influence of pH, competing ions, and salinity on the sorption of strontium and cobalt onto biogenic hydroxyapatite. Scientific Reports 6, 23361.
| Influence of pH, competing ions, and salinity on the sorption of strontium and cobalt onto biogenic hydroxyapatite.Crossref | GoogleScholarGoogle Scholar | 26988070PubMed |
Harvey, B. P., Gwynn‐Jones, D., and Moore, P. J. (2013). Meta‐analysis reveals complex marine biological responses to the interactive effects of ocean acidification and warming. Ecology and Evolution 3, 1016–1030.
| Meta‐analysis reveals complex marine biological responses to the interactive effects of ocean acidification and warming.Crossref | GoogleScholarGoogle Scholar | 23610641PubMed |
Hinojosa, J. L., Brown, S. T., Chen, J., DePaolo, D. J., Paytan, A., Shen, S., and Payne, J. L. (2012). Evidence for end-Permian ocean acidification from calcium isotopes in biogenic apatite. Geology 40, 743–746.
| Evidence for end-Permian ocean acidification from calcium isotopes in biogenic apatite.Crossref | GoogleScholarGoogle Scholar |
Izzo, C., Doubleday, Z., Schultz, A., Woodcock, S., and Gillanders, B. (2015). Contribution of water chemistry and fish condition to otolith chemistry: comparisons across salinity environments. Journal of Fish Biology 86, 1680–1698.
| Contribution of water chemistry and fish condition to otolith chemistry: comparisons across salinity environments.Crossref | GoogleScholarGoogle Scholar | 26033292PubMed |
Izzo, C., Doubleday, Z. A., Grammer, G. L., Gilmore, K. L., Alleway, H. K., Barnes, T. C., Disspain, M. C., Giraldo, A., Mazloumi, N., and Gillanders, B. M. (2016a). Fish as proxies of ecological and environmental change. Reviews in Fish Biology and Fisheries 26, 265–286.
| Fish as proxies of ecological and environmental change.Crossref | GoogleScholarGoogle Scholar |
Izzo, C., Huveneers, C., Drew, M., Bradshaw, C. J. A., Donnellan, S. C., and Gillanders, B. M. (2016b). Vertebral chemistry demonstrates movement and population structure of bronze whaler. Marine Ecology Progress Series 556, 195–207.
| Vertebral chemistry demonstrates movement and population structure of bronze whaler.Crossref | GoogleScholarGoogle Scholar |
Izzo, C., Reis‐Santos, P., and Gillanders, B. M. (2018). Otolith chemistry does not just reflect environmental conditions: a meta‐analytic evaluation. Fish and Fisheries 19, 441–454.
| Otolith chemistry does not just reflect environmental conditions: a meta‐analytic evaluation.Crossref | GoogleScholarGoogle Scholar |
Kalish, J. M. (1989). Otolith microchemistry: validation of the effects of physiology, age and environment on otolith composition. Journal of Experimental Marine Biology and Ecology 132, 151–178.
| Otolith microchemistry: validation of the effects of physiology, age and environment on otolith composition.Crossref | GoogleScholarGoogle Scholar |
Last, P., and Stevens, J. (2009). ‘Sharks and Rays of Australia’, 2nd edn. (CSIRO Publishing: Melbourne, Vic., Australia.)
Lefevre, S. (2016). Are global warming and ocean acidification conspiring against marine ectotherms? A meta-analysis of the respiratory effects of elevated temperature, high CO2 and their interaction. Conservation Physiology 4, cow009.
| Are global warming and ocean acidification conspiring against marine ectotherms? A meta-analysis of the respiratory effects of elevated temperature, high CO2 and their interaction.Crossref | GoogleScholarGoogle Scholar | 27766156PubMed |
Lewis, J. P., Patterson, W. F., Carlson, J. K., and McLachlin, K. (2016). Do vertebral chemical signatures distinguish juvenile blacktip shark (Carcharhinus limbatus) nursery regions in the northern Gulf of Mexico? Marine and Freshwater Research 67, 1014–1022.
| Do vertebral chemical signatures distinguish juvenile blacktip shark (Carcharhinus limbatus) nursery regions in the northern Gulf of Mexico?Crossref | GoogleScholarGoogle Scholar |
Lewis, J., Patterson, W., and Carlson, J. (2017). Natural variability and effects of cleaning and storage procedures on vertebral chemistry of the blacktip shark Carcharhinus limbatus. Journal of Fish Biology 91, 1284–1300.
| Natural variability and effects of cleaning and storage procedures on vertebral chemistry of the blacktip shark Carcharhinus limbatus.Crossref | GoogleScholarGoogle Scholar | 29023719PubMed |
Loewen, T. N., Carriere, B., Reist, J. D., Halden, N. M., and Anderson, W. G. (2016). Linking physiology and biomineralization processes to ecological inferences on the life history of fishes. Comparative Biochemistry and Physiology – A. Molecular & Integrative Physiology 202, 123–140.
| Linking physiology and biomineralization processes to ecological inferences on the life history of fishes.Crossref | GoogleScholarGoogle Scholar |
Martino, J., Doubleday, Z. A., Woodcock, S. H., and Gillanders, B. M. (2017). Elevated carbon dioxide and temperature affects otolith development, but not chemistry, in a diadromous fish. Journal of Experimental Marine Biology and Ecology 495, 57–64.
| Elevated carbon dioxide and temperature affects otolith development, but not chemistry, in a diadromous fish.Crossref | GoogleScholarGoogle Scholar |
McMillan, M. N., Izzo, C., Wade, B., and Gillanders, B. (2017a). Elements and elasmobranchs: hypotheses, assumptions and limitations of elemental analysis. Journal of Fish Biology 90, 559–594.
| Elements and elasmobranchs: hypotheses, assumptions and limitations of elemental analysis.Crossref | GoogleScholarGoogle Scholar | 27859234PubMed |
McMillan, M. N., Izzo, C., Junge, C., Albert, O., Jung, A., and Gillanders, B. M. (2017b). Analysis of vertebral chemistry to assess stock structure in a deep-sea shark, Etmopterus spinax. ICES Journal of Marine Science 74, 793–803.
| Analysis of vertebral chemistry to assess stock structure in a deep-sea shark, Etmopterus spinax.Crossref | GoogleScholarGoogle Scholar |
McMillan, M. N., Huveneers, C., Semmens, J. M., and Gillanders, B. M. (2018). Natural tags reveal populations of conservation dependent school shark use different pupping areas. Marine Ecology Progress Series 599, 147–156.
| Natural tags reveal populations of conservation dependent school shark use different pupping areas.Crossref | GoogleScholarGoogle Scholar |
McNeil, B. I., and Sasse, T. P. (2016). Future ocean hypercapnia driven by anthropogenic amplification of the natural CO2 cycle. Nature 529, 383.
| Future ocean hypercapnia driven by anthropogenic amplification of the natural CO2 cycle.Crossref | GoogleScholarGoogle Scholar | 26791726PubMed |
Miller, J. A. (2009). The effects of temperature and water concentration on the otolith incorporation of barium and manganese in black rockfish Sebastes melanops. Journal of Fish Biology 75, 39–60.
| The effects of temperature and water concentration on the otolith incorporation of barium and manganese in black rockfish Sebastes melanops.Crossref | GoogleScholarGoogle Scholar | 20738481PubMed |
Mirasole, A., Gillanders, B., Reis-Santos, P., Grassa, F., Capasso, G., Scopelliti, G., Mazzola, A., and Vizzini, S. (2017). The influence of high pCO2 on otolith shape, chemical and carbon isotope composition of six coastal fish species in a Mediterranean shallow CO2 vent. Marine Biology 164, 191.
| The influence of high pCO2 on otolith shape, chemical and carbon isotope composition of six coastal fish species in a Mediterranean shallow CO2 vent.Crossref | GoogleScholarGoogle Scholar |
Mohan, J. A., TinHan, T. C., Miller, N. R., and Wells, R. J. (2017). Effects of sample cleaning and storage on the elemental composition of shark vertebrae. Rapid Communications in Mass Spectrometry 31, 2073–2080.
| Effects of sample cleaning and storage on the elemental composition of shark vertebrae.Crossref | GoogleScholarGoogle Scholar | 28940897PubMed |
Mohan, J. A., Miller, N. R., Herzka, S. Z., Sosa-Nishizaki, O., Kohin, S., Dewar, H., Kinney, M., Snodgrass, O., and Wells, D. R. (2018). Elements of time and place: manganese and barium in shark vertebrae reflect age and upwelling histories. Proceedings of the Royal Society of London – B. Biological Sciences 285, 20181760.
| Elements of time and place: manganese and barium in shark vertebrae reflect age and upwelling histories.Crossref | GoogleScholarGoogle Scholar |
Morales-Nin, B. (2000). Review of the growth regulation processes of otolith daily increment formation. Fisheries Research 46, 53–67.
| Review of the growth regulation processes of otolith daily increment formation.Crossref | GoogleScholarGoogle Scholar |
Paton, C., Hellstrom, J., Paul, B., Woodhead, J., and Hergt, J. (2011). Iolite: freeware for the visualisation and processing of mass spectrometric data. Journal of Analytical Atomic Spectrometry 26, 2508–2518.
| Iolite: freeware for the visualisation and processing of mass spectrometric data.Crossref | GoogleScholarGoogle Scholar |
Pistevos, J. C., Nagelkerken, I., Rossi, T., Olmos, M., and Connell, S. D. (2015). Ocean acidification and global warming impair shark hunting behaviour and growth. Scientific Reports 5, 16293.
| Ocean acidification and global warming impair shark hunting behaviour and growth.Crossref | GoogleScholarGoogle Scholar | 26559327PubMed |
Pistevos, J. C., Nagelkerken, I., Rossi, T., and Connell, S. D. (2016). Antagonistic effects of ocean acidification and warming on hunting sharks. Oikos 126, 241–247.
| Antagonistic effects of ocean acidification and warming on hunting sharks.Crossref | GoogleScholarGoogle Scholar |
Radtke, R., and Shafer, D. (1992). Environmental sensitivity of fish otolith microchemistry. Marine and Freshwater Research 43, 935–951.
| Environmental sensitivity of fish otolith microchemistry.Crossref | GoogleScholarGoogle Scholar |
Raoult, V., Howell, N., Zahra, D., Peddemors, V. M., Howard, D. L., de Jonge, M. D., Buchan, B. L., and Williamson, J. E. (2018). Localized zinc distribution in shark vertebrae suggests differential deposition during ontogeny and across vertebral structures. PLoS One 13, e0190927.
| Localized zinc distribution in shark vertebrae suggests differential deposition during ontogeny and across vertebral structures.Crossref | GoogleScholarGoogle Scholar | 29324879PubMed |
Reis‐Santos, P., Vasconcelos, R., Ruano, M., Latkoczy, C., Günther, D., Costa, M., and Cabral, H. (2008). Interspecific variations of otolith chemistry in estuarine fish nurseries. Journal of Fish Biology 72, 2595–2614.
| Interspecific variations of otolith chemistry in estuarine fish nurseries.Crossref | GoogleScholarGoogle Scholar |
Reis-Santos, P., Tanner, S. E., Elsdon, T. S., Cabral, H. N., and Gillanders, B. M. (2013). Effects of temperature, salinity and water composition on otolith elemental incorporation of Dicentrarchus labrax. Journal of Experimental Marine Biology and Ecology 446, 245–252.
| Effects of temperature, salinity and water composition on otolith elemental incorporation of Dicentrarchus labrax.Crossref | GoogleScholarGoogle Scholar |
Reis-Santos, P., Vasconcelos, R. P., Tanner, S. E., Fonseca, V. F., Cabral, H. N., and Gillanders, B. M. (2018). Extrinsic and intrinsic factors shape the ability of using otolith chemistry to characterise estuarine environmental histories. Marine Environmental Research 140, 332–341.
| Extrinsic and intrinsic factors shape the ability of using otolith chemistry to characterise estuarine environmental histories.Crossref | GoogleScholarGoogle Scholar | 30251648PubMed |
Ries, J. B., Cohen, A. L., and McCorkle, D. C. (2009). Marine calcifiers exhibit mixed responses to CO2-induced ocean acidification. Geology 37, 1131–1134.
| Marine calcifiers exhibit mixed responses to CO2-induced ocean acidification.Crossref | GoogleScholarGoogle Scholar |
Rodda, K., and Seymour, R. (2008). Functional morphology of embryonic development in the Port Jackson shark Heterodontus portusjacksoni (Meyer). Fish Biology 72, 961–984.
| Functional morphology of embryonic development in the Port Jackson shark Heterodontus portusjacksoni (Meyer).Crossref | GoogleScholarGoogle Scholar |
Rosa, R., Baptista, M., Lopes, V. M., Pegado, M., Paula, J., Trübenbach, K., Leal, M., Calado, R., and Repolho, T. (2014). Early-life exposure to climate change impairs tropical shark survival. Proceedings of the Royal Society of London – B. Biological Sciences 281, 20141738.
| Early-life exposure to climate change impairs tropical shark survival.Crossref | GoogleScholarGoogle Scholar |
Smith, W. D. (2013). Vertebral elemental markers in elasmobranchs: potential for reconstructing environmental history and population structure. Ph.D. Thesis, Oregon State University, Corvallis, OR, USA.
Smith, W. D., Miller, J. A., and Heppell, S. S. (2013). Elemental markers in elasmobranchs: effects of environmental history and growth on vertebral chemistry. PLoS One 8, e62423.
| Elemental markers in elasmobranchs: effects of environmental history and growth on vertebral chemistry.Crossref | GoogleScholarGoogle Scholar | 24098320PubMed |
Smith, W. D., Miller, J. A., Márquez-Farías, J. F., and Heppell, S. S. (2016). Elemental signatures reveal the geographic origins of a highly migratory shark: prospects for measuring population connectivity. Marine Ecology Progress Series 556, 173–193.
| Elemental signatures reveal the geographic origins of a highly migratory shark: prospects for measuring population connectivity.Crossref | GoogleScholarGoogle Scholar |
Stanley, R. R., Bradbury, I. R., DiBacco, C., Snelgrove, P. V., Thorrold, S. R., and Killen, S. S. (2015). Environmentally mediated trends in otolith composition of juvenile Atlantic cod (Gadus morhua). ICES Journal of Marine Science 72, 2350–2363.
| Environmentally mediated trends in otolith composition of juvenile Atlantic cod (Gadus morhua).Crossref | GoogleScholarGoogle Scholar |
Sturrock, A., Trueman, C., Darnaude, A., and Hunter, E. (2012). Can otolith elemental chemistry retrospectively track migrations in fully marine fishes? Journal of Fish Biology 81, 766–795.
| Can otolith elemental chemistry retrospectively track migrations in fully marine fishes?Crossref | GoogleScholarGoogle Scholar | 22803735PubMed |
Sturrock, A. M., Trueman, C. N., Milton, J. A., Waring, C. P., Cooper, M. J., and Hunter, E. (2014). Physiological influences can outweigh environmental signals in otolith microchemistry research. Marine Ecology Progress Series 500, 245–264.
| Physiological influences can outweigh environmental signals in otolith microchemistry research.Crossref | GoogleScholarGoogle Scholar |
Sturrock, A., Hunter, E., Milton, A. J., EIMF Johnson, R. C., Waring, C. P., and Trueman, C. N. (2015). Quantifying physiological influences on otolith microchemistry. Methods in Ecology and Evolution 6, 806–816.
| Quantifying physiological influences on otolith microchemistry.Crossref | GoogleScholarGoogle Scholar |
Thomas, O. R., Ganio, K., Roberts, B. R., and Swearer, S. E. (2017). Trace element–protein interactions in endolymph from the inner ear of fish: implications for environmental reconstructions using fish otolith chemistry. Metallomics 9, 239–249.
| Trace element–protein interactions in endolymph from the inner ear of fish: implications for environmental reconstructions using fish otolith chemistry.Crossref | GoogleScholarGoogle Scholar | 28091665PubMed |
Tillett, B., Meekan, M., Parry, D., Munksgaard, N., Field, I., Thorburn, D., and Bradshaw, C. (2011). Decoding fingerprints: elemental composition of vertebrae correlates to age-related habitat use in two morphologically similar sharks. Marine Ecology Progress Series 434, 133–142.
| Decoding fingerprints: elemental composition of vertebrae correlates to age-related habitat use in two morphologically similar sharks.Crossref | GoogleScholarGoogle Scholar |
Tzadik, O. E., Curtis, J. S., Granneman, J. E., Kurth, B. N., Pusack, T. J., Wallace, A. A., Hollander, D. J., Peebles, E. B., and Stallings, C. D. (2017). Chemical archives in fishes beyond otoliths: a review on the use of other body parts as chronological recorders of microchemical constituents for expanding interpretations of environmental, ecological, and life-history changes. Limnology and Oceanography, Methods 15, 238–263.
| Chemical archives in fishes beyond otoliths: a review on the use of other body parts as chronological recorders of microchemical constituents for expanding interpretations of environmental, ecological, and life-history changes.Crossref | GoogleScholarGoogle Scholar |
Walther, B. D. (2019). The art of otolith chemistry: interpreting patterns by integrating perspectives. Marine and Freshwater Research , .
| The art of otolith chemistry: interpreting patterns by integrating perspectives.Crossref | GoogleScholarGoogle Scholar |
Walther, B., and Thorrold, S. (2006). Water, not food, contributes the majority of strontium and barium deposited in the otoliths of a marine fish. Marine Ecology Progress Series 311, 125–130.
| Water, not food, contributes the majority of strontium and barium deposited in the otoliths of a marine fish.Crossref | GoogleScholarGoogle Scholar |
Watanabe, T., Kiron, V., and Satoh, S. (1997). Trace minerals in fish nutrition. Aquaculture 151, 185–207.
| Trace minerals in fish nutrition.Crossref | GoogleScholarGoogle Scholar |
Webb, S., Woodcock, S., and Gillanders, B. (2012). Sources of otolith barium and strontium in estuarine fish and the influence of salinity and temperature. Marine Ecology Progress Series 453, 189–199.
| Sources of otolith barium and strontium in estuarine fish and the influence of salinity and temperature.Crossref | GoogleScholarGoogle Scholar |
Werry, J., Lee, S., Otway, N., Hu, Y., and Sumpton, W. (2011). A multi-faceted approach for quantifying the estuarine–nearshore transition in the life cycle of the bull shark, Carcharhinus leucas. Marine and Freshwater Research 62, 1421–1431.
| A multi-faceted approach for quantifying the estuarine–nearshore transition in the life cycle of the bull shark, Carcharhinus leucas.Crossref | GoogleScholarGoogle Scholar |
Wood, H. L., Spicer, J. I., and Widdicombe, S. (2008). Ocean acidification may increase calcification rates, but at a cost. Proceedings of the Royal Society of London – B. Biological Sciences 275, 1767–1773.
| Ocean acidification may increase calcification rates, but at a cost.Crossref | GoogleScholarGoogle Scholar |