Meiobenthos in intermittently open/closed coastal lakes in New South Wales: spatial and temporal patterns in densities of major taxa
A. H. DyeCentre for Research on Ecological Impacts of Coastal Cities, University of Sydney, NSW 2006, Australia. Email: adye@bio.usyd.edu.au
Marine and Freshwater Research 56(8) 1055-1067 https://doi.org/10.1071/MF05050
Submitted: 24 March 2005 Accepted: 6 October 2005 Published: 3 November 2005
Abstract
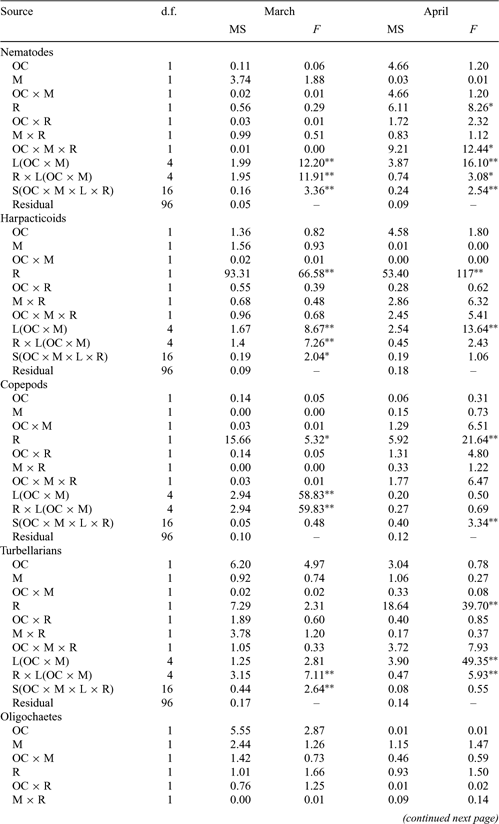
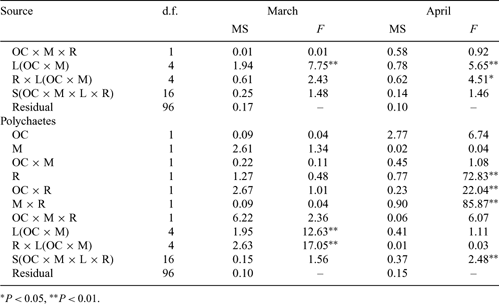
Intermittently open/closed coastal lakes and lagoons (ICOLLs) are common in Australia. Isolation from the sea makes them susceptible to nutrient enrichment and pollution and many are considered degraded. Understanding of their ecology and the effects of anthropogenic activity is limited. Many lakes are kept open artificially to improve water quality and mitigate the effects of floods. The present study examined the relationship between multivariate and univariate patterns in higher taxa of meiobenthos and compared their densities and distributions in naturally open and closed lakes with those in managed lakes. The degree of correspondence between multivariate and univariate patterns was taxon and locality dependent. Differences in densities between types of lakes was not related to physical factors. Within lakes, meiobenthos generally correlated negatively with salinity and organic content, but positively with silt. Densities reflected the degree of isolation from the sea, but the influence of this factor varied among lakes within categories and between taxa. Most taxa were less abundant in isolated localities, such as the inner reaches of lakes and in closed lakes. Meiobenthos were more spatially variable in closed and in managed lakes. The influence of frequency and duration of closure on the ecology of coastal lakes is discussed.
Extra keywords: coastal lagoons, invertebrates, isolation, meiofauna.
Acknowledgments
The author acknowledges the support of the Centre for Research on Ecological Impacts of Coastal Cities, University of Sydney, and funding from the Australian Research Council (through the Centre). In particular, I thank Professor A. J. Underwood and Dr T. Lasiak for comments on the manuscript and, with Dr F. Barros, for valuable discussions during the planning and implementation of this project. Dr F. Barros, Mr W. Green and Mr T. Probst are thanked for their assistance in the field.
Alongi, D. M. (1989). Community dynamics of free-living nematodes in some tropical mangrove and sandflat habitats. Bulletin of Marine Science 46, 358–373.
Commito, J. A. , and Tita, G. (2002). Differential dispersal rates in an intertidal meiofauna assemblage. Journal of Experimental Marine Biology and Ecology 268, 237–256.
| Crossref | GoogleScholarGoogle Scholar |
Dye, A. H. , and Barros, F. (2005a). Spatial patterns in meiobenthic assemblages in intermittently open/closed coastal lakes in New South Wales, Australia. Estuarine, Coastal and Shelf Science 62, 575–593.
| Crossref | GoogleScholarGoogle Scholar |
Gerlach, S. A. (1978). Food chain relationships in subtidal silty marine sediments and the role of meiofauna in stimulating bacterial productivity. Oecologia 33, 55–69.
| Crossref | GoogleScholarGoogle Scholar |
Roy, P. S. , Williams, R. J. , Jones, A. R. , Yassini, I. , Gibbs, P. J. , Coates, B. , West, R. J. , Scanes, P. R. , Hudson, J. P. , and Nichol, S. (2001). Structure and function of south-east Australian estuaries. Estuarine, Coastal and Shelf Science 53, 351–384.
| Crossref | GoogleScholarGoogle Scholar |

Sandulli, R. , and de Nicola-Giudici, M. (1990). Pollution effects on the structure of meiobenthos communities in the Bay of Naples. Marine Pollution Bulletin 21, 144–153.
| Crossref | GoogleScholarGoogle Scholar |

Schratzberger, M. , and Warwick, R. M. (1998). Effects of physical disturbance on nematode communities in sand and mud: a microcosm experiment. Marine Biology 130, 643–650.
| Crossref | GoogleScholarGoogle Scholar |

Schratzberger, M. , and Warwick, R. M. (1999). Differential effects of various types of disturbances on the structure of nematode assemblages: an experimental approach. Marine Ecology Progress Series 181, 227–236.

Schratzberger, M. , Gee, J. M. , Rees, H. L. , Boyd, S. E. , and Wall, C. M. (2000). The structure and taxonomic composition of sublittoral meiobenthos assemblages as an indicator of the status of marine environments. Journal of the Marine Biological Association of the UK 80, 969–980.
| Crossref | GoogleScholarGoogle Scholar |

Somerfield, P. J. , Gee, G. M. , and Warwick, R. M. (1994). Soft-sediment meiofaunal community structure in relation to a long-term heavy metal gradient in the Fal estuary system. Marine Ecology Progress Series 105, 79–88.

Stark, J. S. (1998). Heavy metal pollution and macrobenthic assemblages in soft sediments in two Sydney estuaries. Marine and Freshwater Research 49, 533–540.
| Crossref | GoogleScholarGoogle Scholar |

Tenore, K. R. , Tietjen, J. H. , and Lee, J. J. (1977). Effect of meiobenthos on incorporation of aged eelgrass, Zostera marina, detritus by the polychaete Nepthys incisa. Journal of the Fisheries Research Board of Canada 34, 563–567.

Tietjen, J. H. (1980). Microbial–meiobenthos interrelationships: a review. Microbiology 1980, 335–338.

Tita, G. , Desrosiers, G. , Vincx, M. , and Nozais, C. (2000). Predation and sediment disturbance effects of the intertidal polychate Nereis virens (Sars) on associated meiofaunal assemblages. Journal of Experimental Marine Biology and Ecology 243, 261–282.
| Crossref | GoogleScholarGoogle Scholar |

Udy, J. W. , and Dennison, W. C. (1997). Physiological responses of seagrasses used to identify anthropogenic nutrient inputs. Marine and Freshwater Research 48, 605–614.
| Crossref | GoogleScholarGoogle Scholar |

Underwood, A. J. , and Chapman, M. G. (1998). Spatial analyses of intertidal assemblages on sheltered rocky shores. Australian Journal of Ecology 23, 138–157.

Walters, K. , and Moriarty, D. J. W. (1993). The effects of complex trophic interactions on a marine microbenthic community. Ecology 74, 1475–1489.

Warwick, R. M. (1988). The level of taxonomic discrimination required to detect pollution effects on marine benthic communities. Marine Pollution Bulletin 19, 259–268.
| Crossref | GoogleScholarGoogle Scholar |

Warwick, R. M. (1993). Environmental impact studies on marine communities: pragmatical considerations. Australian Journal of Ecology 18, 63–80.

Warwick, R. M. , and Clarke, K. R. (1993). Increased variability as a symptom of stress in marine communities. Journal of Experimental Marine Biology and Ecology 172, 215–226.
| Crossref | GoogleScholarGoogle Scholar |

Warwick, R. M. , and Gee, J. M. (1984). Community structure of estuarine benthos. Marine Ecology Progress Series 18, 97–111.

|
|


