Effects of extracellular matrices and lectin Dolichos biflorus agglutinin on cell adhesion and self-renewal of bovine gonocytes cultured in vitro
Sung-Min Kim A C , Mayako Fujihara A B , Mahesh Sahare A , Naojiro Minami A , Masayasu Yamada A and Hiroshi Imai A DA Laboratory of Reproductive Biology, Graduate School of Agriculture, Kyoto University, Kyoto 606-8502, Japan.
B Smithsonian Conservation Biology Institute, Center for Species Survival, National Zoological Park, VA 22630, USA.
C Present address: Institute for Biogenesis Research, John A. Burns School of Medicine, University of Hawaii, Honolulu, Hawaii 96813, USA.
D Corresponding author. Email: imai@kais.kyoto-u.ac.jp
Reproduction, Fertility and Development 26(2) 268-281 https://doi.org/10.1071/RD12214
Submitted: 5 July 2012 Accepted: 13 December 2012 Published: 21 February 2013
Abstract
Surface molecules of primitive male germ cells, gonocytes, are essential components for regulating cell adhesion and maintaining self-renewal in mammalian species. In domestic animals, the stage-specific glycan epitope α-N-acetylgalactosamine (GalNAc) is recognised by the lectin Dolichos biflorus agglutinin (DBA) and is found on the surface of gonocytes and spermatogonia. Gonocytes from bovine testis formed mouse embryonic stem-like cell colonies on plates that had been coated with DBA or extracellular matrix (ECM) components, such as gelatin (GN), laminin (LN) and poly-L-lysine (PLL). The number of colonies on the DBA-coated plate was significantly higher than that on the GN-, LN- and PLL-coated plates. Pretreating gonocytes with DBA to neutralise the terminal GalNAc residues strongly suppressed colony formation. Furthermore, expression of a germ cell-specific gene and pluripotency-related transcription factors was increased considerably on the DBA-coated plates. These results suggest that the GalNAc residues on gonocytes can recognise precoated DBA on plates and the resulting GalNAc–DBA complexes support germ cell and stem cell potentials of gonocytes in vitro. These glycan complexes, through the GalNAc epitope, may provide a suitable microenvironment for the adhesion and cell proliferation of gonocytes in culture.
Additional keywords: cattle, DBA, ECM, glycan epitope, male germ cells, N-acetylgalactosamine, spermatogonia, testis.
Introduction
A population of germ cells has the unique ability to transmit genetic information to the next generation. Gonocytes are primitive germ cells that are present in the early stage of the neonatal testis and give rise to spermatogonia. Spermatogonia have the potential for self-renewal and differentiation to spermatozoa, thereby initiating spermatogenesis. In rodents, gonocytes growing in culture acquire the characteristics of spermatogonia, exhibit stem cell potential, as indicated by their self-renewal (Kanatsu-Shinohara et al. 2003, 2005), and can contribute to spermatogenesis after transplantation into immune-deficient nude mouse testes (Orwig et al. 2002a, 2002b). Similarly, gonocytes present in the testis of 3-month-old bulls are only germ cells and are replaced with spermatogonia at around 4 months of age, with the initiation of spermatogenesis observed between 16 and 24 weeks (Curtis and Amann 1981). However, in domestic animals, little is known about whether gonocytes have stem cell activity during germ cell development. Culture conditions for maintaining germ cells have been established for various species, including the mouse (Nagano et al. 1998, 2003; Kubota et al. 2004; Kanatsu-Shinohara et al. 2005), rat (Hamra et al. 2005), hamster (Kanatsu-Shinohara et al. 2008a) and rabbit (Kubota et al. 2011). However, culture systems are not available for domestic animal species and cell lines, such as embryonic germ (EG) cells in the mouse, have not been established.
In the testis, the dynamic events during spermatogenesis occur through the basement membrane of the seminiferous tubule and interactions with Sertoli cells. In fact, the basement membrane of the seminiferous tubule is composed of extracellular matrix (ECM), the major components of which are collagen and laminin (Siu and Cheng 2004). Recent studies have revealed that adhesion molecules on the surface of spermatogonial stem cells (SSCs) specifically recognise ECM components, which have been used to identify and purify the population of germ cells in mixed testicular cells (Shinohara et al. 1999; Orwig et al. 2002c; Hamra et al. 2005). Furthermore, adhesion molecules, such as β1- and α6-integrin are known to be receptors for laminin. These molecules, which are present on the surface of mouse SSCs, support the long-term proliferation of SSCs in culture (Shinohara et al. 1999; Kanatsu-Shinohara et al. 2005) and play critical roles in the reconstruction of the stem cell niche after transplantation into immunodeficient mouse testis (Kanatsu-Shinohara et al. 2008b). Therefore, the adhesion of cells to ECM molecules seems to be associated with their survival and proliferation, both in vitro and in vivo. However, in the case of cattle, little is known about the mechanism by which germ cells adhere to the ECM.
One approach to distinguishing and characterising germ cells in a mixed testicular cell population is to identify a stage-specific glycosylation event. The lectin Dolichos biflorus agglutinin (DBA), which recognises a terminal N-acetylgalactosamine (GalNAc) residue (Piller et al. 1990), is a specific marker for germ cells such as gonocytes and Type A spermatogonia in both the pig (Goel et al. 2007) and cattle (Ertl and Wrobel 1992; Izadyar et al. 2002). In addition, DBA can be used to enrich germ cells using magnetic-activated cell sorting (MACS; Herrid et al. 2009). Therefore, germ cells isolated by DBA can be a useful model for understanding the roles of cell surface glycans in the adhesion and proliferation of germ cells both in vivo and in vitro.
In domestic animals, a procedure for the long-term culture of germ cells has not been established. To achieve this, the expression of vital pluripotency-related genes, such as NANOG and POU5F1, is essential, but their expression gradually decreases as the passage number increases (Goel et al. 2009). The pluripotent state in cultured germ cells can be supported by using ECM components that interact with adhesion molecules on the cell surface (Chai and Leong 2007), which suggests that some cell surface molecules can regulate the expression of genes associated with a pluripotent state in cultured germ cells. However, the effects of biomaterials, such as ECM molecules and DBA, on the adhesion, proliferation and stem cell potential of germ cells remain unknown in domestic animals.
In the present study, we tested the hypothesis that adhesion molecules, including carbohydrate chains, on the surface of germ cells affect cell survival and proliferation in culture. Our results suggest that the terminal glycan residues of cell surface carbohydrates are involved in the proliferation and stem cell potential of bovine gonocytes in culture.
Materials and methods
Collection of the testes and isolation of gonocytes
Testes were collected from 3-month-old Holstein bulls (Bos taurus) kept on a local farm in Japan and were placed immediately in Dulbecco’s modified Eagle’s medium (DMEM)/F12 medium (GIBCO BRL Invitrogen, Carlsbad, CA, USA) supplemented with 100 IU mL−1 penicillin (Sigma-Aldrich, St Louis, MO, USA), 50 mg mL−1 streptomycin (Sigma-Aldrich), 40 mg mL−1 gentamicin sulfate (Sigma-Aldrich) and 15 mM HEPES (Wako, Osaka, Japan). The collected testes were transported to the laboratory at 4°C within 24 h. Part of the testis was fixed with Bouin’s fixative or 4% (w/v) paraformaldehyde (PFA) solution for immunohistochemical analysis.
To collect testicular cells, the testes were treated with three-step enzymatic digestions and isolated cells were subjected to discontinuous density gradient Percoll centrifugation, as described previously (Fujihara et al. 2011) with some modifications. Briefly, to obtain a testicular cell suspension, the decapsulated testicular tissue was minced into small pieces and treated with a first enzymatic solution that was supplemented with 2 mg mL−1 collagenase (Type IV; Sigma-Aldrich) and 1 mg mL−1 DNase I (Sigma-Aldrich) in DMEM/F12 for 30 min at 37°C. Testicular cells were washed three times in DMEM/F12 and digested sequentially with a second enzymatic solution containing 2 mg mL−1 collagenase (Type IV; Sigma-Aldrich), 2 mg mL−1 hyaluronidase (Sigma-Aldrich) and 1 mg mL−1 DNase I for 30 min at 37°C and then washed with DMEM/F12. The collected cells were incubated with a third enzymatic solution (0.25% trypsin and 0.53 mM EDTA in phosphate-buffered saline (PBS)) containing 5 mg mL−1 DNase I for 10 min at 37°C, washed with DMEM/F12 and filtered through a 50-µm nylon mesh (Kyoshin Rikoh, Tokyo, Japan). The isolated cells were then subjected to discontinuous density gradient Percoll centrifugation. Gonocytes were fractionated between 40% and 50% and identified by DBA staining and morphological definition (large diameter cells). Gonocytes were collected at a density of 1 × 106 cells g−1 testis tissue and were enriched ≤40% by density gradient purification. The viability of purified cells was ≥95%, as determined by Trypan blue exclusion.
In vitro culture of gonocytes
Freshly collected gonocytes were seeded at a density of 2 × 105 cells cm−2 onto culture dishes (Iwaki, Tokyo, Japan). The culture medium used was DMEM/F12 supplemented with 10 µg mL−1 insulin (Sigma-Aldrich), 10 µg mL−1 apotransferrin (Sigma-Aldrich), 100 IU mL−1 penicillin (Sigma-Aldrich), 50 µg mL−1 streptomycin (Sigma-Aldrich), 40 µg mL−1, gentamicin sulfate (Sigma-Aldrich), single strength non-essential amino acid solution (GIBCO BRL Invitrogen), 1 mM pyruvate (Sigma-Aldrich), 1.5 µL mL−1 60% (w/v) sodium lactate (Sigma-Aldrich), 0.01 mM β-mercaptoethanol (Wako), 20 ng mL−1 basic fibroblast growth factor (bFGF; Upstate, Temecula, CA, USA), 20 ng mL−1 glial cell line-derived neurotrophic factor (GDNF; R&D System, Minneapolis, MN, USA), 50 ng mL−1 epidermal growth factor (EGF), 1% (v/v) fetal bovine serum (FBS; JRH Biosciences, Lenexa, KS, USA) and 15% (v/v) knockout serum replacement (KSR). The culture medium was changed every other day and cells were passaged every 7–10 days using 0.25% (w/v) Trypsin and 0.53 mM EDTA solution or mechanical dissociation methods using a fire-polished Pasteur pipette. Cells were cultured in a CO2 incubator at 37°C in a water-saturated atmosphere with 95% air and 5% CO2.
Preparation of ECM plates and assessment of germ cell binding affinity
Culture dishes were precoated with ECM molecules (0.2% (w/v) gelatin (GN; Sigma-Aldrich), 20 µg mL−1 laminin (LN; Sigma-Aldrich) and 10 µg mL−1 poly-L-lysine (PLL; Sigma-Aldrich)) or 30 µg mL−1 DBA (Vector Laboratories, Burlingame, CA, USA) overnight at 37°C, before being washed with PBS and blocked with 5% bovine serum albumin (BSA) in PBS for 1 h at 37°C to prevent non-specific binding.
To analyse the binding affinity of gonocytes to culture dishes, freshly collected gonocytes were plated in 4- or 24-well culture dishes (Iwaki) precoated with different ECM molecules. Cells were incubated for 4 h at 37°C in an adherent medium, namely DMEM/F12 supplemented 10% FBS without KSR and growth factors to enhance the attachment of germ cells on the ECM. After 4 h culture, floating cells were discarded, and adherent cells were gently washed and collected in the culture medium. Adherent cells were characterised by immunocytochemical staining to distinguish germ and somatic cell populations. Antibodies were used for germ cell markers, ubiquitin carboxy-terminal hydrolase L1 (UCHL1) and DBA, and a Sertoli cell (vimentin). The average number of cells positive for germ cell-specific markers was determined in six randomly selected microscopic field per sample at ×200 magnification. Each experiment was performed using a single animal and the experiment was repeated at least four times using four to five animals.
Assessment of colony formation on the different ECM
Freshly isolated gonocytes were seeded at a density of 2 × 105 cells cm−2 onto 4- or 24-well plates or 35-mm dishes. Gonocytes were incubated in the adherent medium on dishes that had been precoated with different ECM components or DBA for 12 h at 37°C. Gonocytes were then preincubated with DBA (30 µg mL−1) for 30 min at 37°C to neutralise GalNAc residues on the surface of the gonocytes. After preincubation, gonocytes were seeded at a density of 2 × 105 cells cm−2 onto 4- or 24-well plates or 35-mm dishes. The DBA pretreated cells were incubated on GN plates (D30_GN) and DBA plates (D30_DBA) for 12 h at 37°C. After 12 h culture, floating cells were decanted and the adherent cells were washed with culture medium before being cultured with adherent medium for another 4–7 days on dishes coated with different ECM components or DBA. To examine the effects of glycan epitopes on colony formation, gonocytes were preincubated with DBA (30 µg mL−1) for 30 min at 37°C to neutralise GalNAc residues on the surface of the gonocytes. After preincubation, gonocytes were seeded at a density of 2 × 105 cells cm−2 onto 4-or 24-well plates or 35-mm dishes and were incubated on the GN plates (D30_GN) or DBA plates (D30_DBA) for 12 h at 37°C. The culture medium was changed every 2 days. On Day 5, the total number of colonies was counted in each well of the 4- or 24-well plates to obtain the average number of colonies. These procedures were replicated four times for the each group.
Immunochemistry of testicular tissues and cultured gonocytes
Gonocytes were identified in the testicular tissues and in cultured testicular cells using fluorescein isothiocyanate (FITC)-conjugated DBA (1 : 50; Vector Laboratories, Burlingame, CA, USA) and anti-UCHL1 (PGP9.5; 1 : 100; Biomol, Exeter, UK). The presence of Sertoli cells in cultured testicular cells was confirmed by using anti-vimentin (clon v9; 1 : 100; Sigma-Aldrich). The expression of pluripotency-specific markers on gonocytes in bovine testis and cultured testicular cells was examined using anti-NANOG (1 : 200; Chemicon International, Temecula, CA, USA) and anti-POU5F1 (1 : 50; C-10; Santa Cruz Biotechnology, Santa Cruz, CA, USA), as described previously (Goel et al. 2008; Fujihara et al. 2011). Briefly, testis sections were fixed with Bouin’s fixative or 4% PFA, washed several times with 0.2% (v/v) Tween 20 in Tris-buffered saline (TBST), incubated in 5% (w/v) BSA in Tris-buffered saline (TBS) for 90 min to block non-specific binding, incubated with DBA-FITC and primary antibodies overnight at 4°C, washed with TBST three times, incubated with the corresponding secondary antibody (Alexa 546-conjugated anti-rabbit IgG antibody (1 : 500; Molecular Probes, Eugene, OR, USA) or Alexa 546-conjugated anti-mouse IgG antibody (1 : 500; Molecular Probes)) for 1 h at 37°C, rinsed three times with TBST, stained with Hoechst 33342 (Sigma-Aldrich) for 10 min, mounted with 50% glycerol in phosphate-buffered saline (PBS) and observed under an immune fluorescence microscope (BX 50; Olympus, Tokyo, Japan).
Cultured cells were examined for the presence of gonocytes using germ cell-specific markers, DBA and anti-DEAD (Asp-Glu-Ala-Asp) box polypeptide 4 (DDX4), and for stem cell potential using pluripotent-specific markers (anti-NANOG and anti-POU5F1). Samples were fixed with Bouin’s fixative or 4% PFA, washed several times with TBST, incubated with 0.3% (v/v) H2O2 in PBS for 15 min to block endogenous peroxidase activity, washed with PBS several times, incubated in 5% (w/v) BSA in PBS for 30 min to block non-specific binding and incubated with DBA and primary antibodies overnight at 4°C. The primary antibodies used were anti-NANOG (1 : 200 dilution), anti-POU5F1 (1 : 50 dilution), and anti-DDX4 (1 : 300; Chemicon). After incubation with the primary antibodies, samples were washed with TBST three times, incubated with substrate–chromogen mix for DBA or the corresponding horseradish peroxidase-conjugated secondary antibodies (i.e. sheep anti-rabbit IgG (1 : 100; GE Healthcare, Buckinghamshire, UK) or sheep anti-mouse IgG (1 : 100; Amersham Biosciences, Buckinghamshire, UK)) for 1 h at room temperature, rinsed several times with TBST, mixed with substrate–chromogen mix for 3–5 min to colorimetrically measure peroxidase activity, washed with TBS several times, counterstained with haematoxylin, mounted on slides and observed under a microscope (BX 50; Olympus).
To examine the stem cell potential of gonocytes in cattle, purified gonocytes were double stained with DBA-FITC, anti-UCHL1, anti-NANOG and anti-POU5F1 antibodies using immune fluorescence labelling as described above.
Reverse transcription–polymerase chain reaction
Testicular cells were cultured for 4 days on the different ECM. Total RNAs were prepared from these cells using a ToTally RNA kit (Ambion, Austin, TX, USA) according to the manufacturer’s instructions. In addition, RNA was isolated from 3-month-old testes as a positive control (T). Oligo(dT) primers and RNase OUT (both from Invitrogen) were added to the RNA solution, incubated for 5 min at 65°C and set on ice. For reverse transcription, ReverTra Ace (MMLV reverse transcriptase RNaseH-; Toyobo, Tokyo, Japan) was added to the RNA solution and incubated for 10 min at 30°C, for 60 min at 42°C and for 5 min at 99°C (RT+). At the same time, the reaction without the addition of ReverTra Ace was performed to check genomic DNA contamination (RT–). The PCR amplification was performed on 2 μL cDNA per 20 μL PCR reaction mixture containing 2 mM MgCl2, 0.25 mM dNTPs, 1× PCR buffer, 10 pmol of each primer and 1 U Taq DNA polymerase (ExTaq; TaKaRa, Ohtsu, Japan). The primer sequences used for the amplification of specific genes are given in Table 1. The PCR products were separated and visualised on 2% (w/v) agarose gels containing 0.5 μg mL−1 ethidium bromide. All PCR products were sequenced to confirm their identity.

|
Statistical analysis
All data are presented as the mean ± s.e.m. (n = 4–5 in each group). To determine the significance of differences among experimental groups, one- or two-way ANOVA was performed using GraphPad Prism 4.0 (GraphPad Software, San Diego CA, USA). All data were subjected to Tukey’s multiple-comparison test to determine the significance of differences between groups. Differences were considered significant at P < 0.05.
Results
Characterisation of the stem cell potential of developing germ cells
To examine DBA binding affinity and the expression pattern of pluripotent-specific markers in bovine testis, 3-month-old testes sections were stained immunohistochemically. The binding of DBA and the expression of UCHL1 were observed in populations of gonocytes. These cells were easily distinguished from other somatic cell populations by two morphological features, namely a large nucleus and a basal location in the seminiferous tubule (Fig. 1a–d). DBA was found on the cell surface or in the cytoplasmic part of gonocytes (Fig. 1a–b), whereas UCHL1 expression was observed in the germ cells (Fig. 1c–d). Double immunostaining for UCHL1 (a germ cell-specific marker) and DBA revealed that UCHL1 is expressed in most DBA-positive cells (Fig. 1e–g), whereas a small number of UCHL1-positive cells (see Fig. 1e) were negative for DBA (Fig. 1f, g). To examine the stem cell potential of DBA-positive germ cells, sections were double stained with DBA and anti-POU5F1 (Fig. 1h–j) or anti-NANOG (Fig. 1k–m). Most cells expressing POU5F1 (Fig. 1h) were DBA positive (Fig. 1h–j). Expression of POU5F1 was detected in most DBA-positive cells, but some POU5F1-positive cells did not show the DBA signal (Fig. 1h–j). NANOG expression was also detected in the seminiferous tubules (Fig. 1k). Some NANOG-positive cells were DBA positive and some were not (Fig. 1j–m). Some NANOG-negative cells were also DBA positive (Fig. 1k–m), indicating that DBA and NANOG expression is not coincident in germ cells of the prepubertal bovine testis.
Cultivation and characterisation of bovine gonocytes
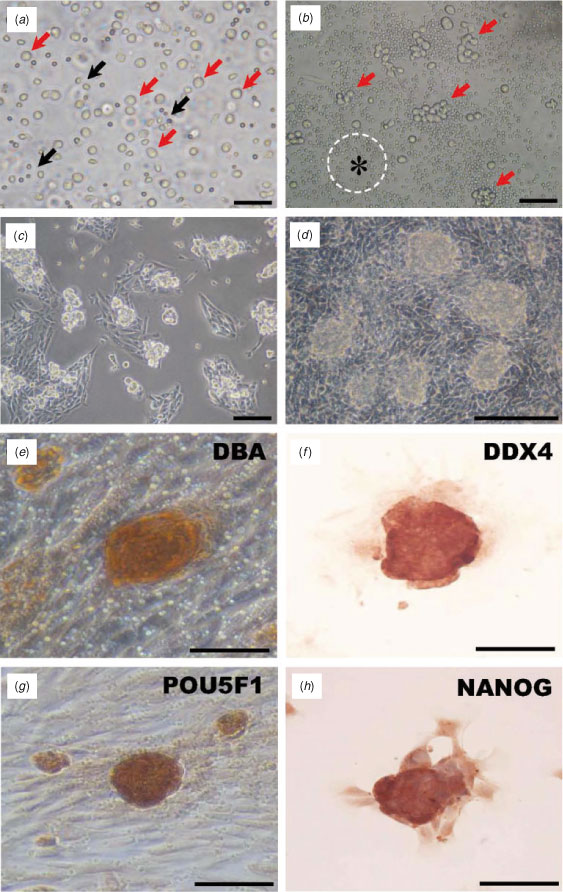
Bovine gonocytes were isolated and enriched by Percoll centrifugation (Fig. 2a). When the isolated cells were cultured on a GN-coated dish, they formed cell clumps on Day 1 of culture (Fig. 2b) and formed mouse embryonic stem (ES)-like colonies by Days 3–4 (Fig. 2c), which became compacted at around Days 6–7 (Fig. 2d) and enlarged gradually during the culture period. Most of these colonies were stained with germ cell-specific markers, namely DBA (Fig. 2e) and DDX4 (Fig. 2f) and stem cell-specific markers, namely POU5F1 (Fig. 2g) and NANOG (Fig. 2h), suggesting that gonocyte colonies in culture still have stem cell potential.
|
Binding of gonocytes to DBA and different ECM
The binding of gonocytes to different ECM and DBA was examined 4 h after cell plating (Fig. 3). The average number of testicular cells was significantly higher on the PLL-precoated plates (192.0 ± 14.7 cells; P < 0.01) and lower on the LN-precoated plates (79.0 ± 9.6 cells; Fig. 3a) compared with numbers on the GN- and DBA-precoated plates. The average number of testicular cells on the GN- and DBA-precoated plates was similar (98.3 ± 22.6 and 104.6 ± 9.1 cells, respectively; Fig. 3a). In the case of Sertoli cells, which are identified by staining for vimentin, approximately equal numbers of cells bound to each of the different ECM components, and the number of negative cells increased significantly on PLL-precoated plates compared with numbers on the other palates (Fig. 3a). Although the number of attached testicular cells was highest on the PLL-precoated plates, it is interesting that the number of gonocytes was significantly higher on the DBA-precoated plates (4.21 ± 0.49%) than on the GN- and LN-precoated plates (2.03 ± 0.59% and 0.75 ± 0.43%, respectively; P < 0.05 and P < 0.01), but not significantly different from the number of cells on the PLL-precoated plates (2.08 ± 0.52%; Fig. 3b). Cells that adhered to the DBA- and ECM-precoated plate were detected using the germ cell marker DBA and the Sertoli cell marker vimentin (Fig. 3c). Gonocytes stained only with DBA and Hoechst 33342. Cells that adhered to the DBA-precoated plate also expressed UCHL1 and had a large nucleus that stained with Hoechst 33342. However, vimentin-positive cells did not stain with DBA and had a small nucleus.
Colony formation on the DBA- and ECM component-precoated plates
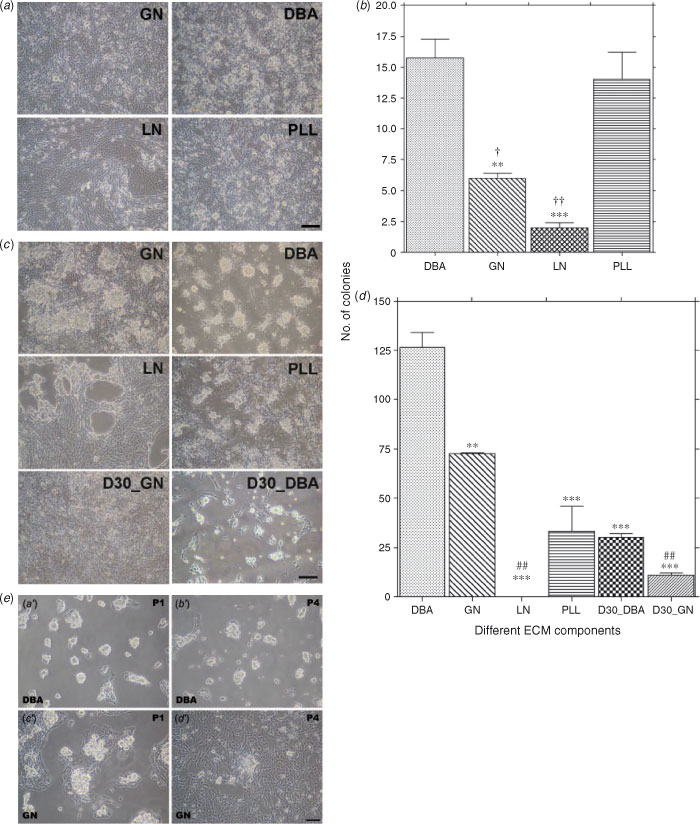
Freshly collected cells were cultured on plates coated with DBA and different ECM components for 5 days (Fig. 4a, b) and the numbers of colonies estimated. The number of colonies on the DBA- and PPL-precoated plates was significantly greater than the number of colonies on the GN- and LN-precoated plates (15.8 ± 1.5 and 14.0 ± 4.4 vs 6.0 ± 0.4 and 2.0 ± 0.4, respectively; Fig. 4b). However, these colonies gradually disappeared on most plates around Day 7 of culture.
After 12 h of positive selection of attached testicular cells followed by 5 days culture, colonies were observed on the DBA-, GN- and PLL-precoated plates, but not on the LN-precoated plates (Fig. 4c). Interestingly, more colonies formed on the DBA-precoated plate than on the ECM component-precoated plates (Fig. 4c, d). The average number of colonies on the DBA-precoated plates was significantly higher than the number of colonies on the GN-, LN- and PLL-precoated plates (126.5 ± 7.5 vs 72.5 ± 0.5 (P < 0.05), 0 (P < 0.001) and 33 ± 13.0 (P < 0.01), respectively; Fig. 4d). Conversely, the proliferation of somatic cells was effectively suppressed on the DBA-precoated plate, but not on the ECM-precoated plates (Fig. 4c). When isolated gonocytes were pretreated with 30 µg mL−1 DBA and then cultured on the DBA-precoated plates (D30_DBA) and GN-precoated plates (D30_GN), the average number of colonies was significantly decreased on both the GN (11.0 ± 1.0; P < 0.001) and DBA (30.0 ± 2.0; P < 0.001) plates (Fig. 4c, d). In addition, the growth of somatic cells was strongly suppressed on the DBA-precoated plates (Fig. 4e, a′), but not on the GN-precoated plates (Fig. 4e, c′). After 1.5 months (passage (P) 4), colonies of gonocytes had formed successfully on the DBA-precoated plates, but were not able to be maintained on the GN-precoated plates (Fig. 4 e, c′, d′). These results indicate that GalNAc residues on the surface of gonocytes are associated with the cell adhesion and colony formation of gonocytes on the DBA-precoated plates.
Characterisation of gonocytes on the DBA-precoated plates
Three-dimensional colonies on the ECM were double stained with anti-UCHL1 and anti-vimentin. Most of the colonies on the DBA-, GN- and PLL-precoated plates were positive for UCHL1, and some of the cells in the colonies were partially positive for anti-vimentin (Fig. 5a). On the LN-precoated plate, few of the colonies were UCHL1 positive, whereas most were vimentin-positive (Fig. 5a).
To estimate the stem cell characteristics of gonocytes on the DBA-precoated plate, colonies that formed after 5 days of culture were double stained with germ cell markers (DBA and UCHL1) and stem markers (NANOG and POU5F1). Most colonies were strongly positive for DBA staining and were colocalised with UCHL1, and were also positive for NANOG and POU5F1 with DBA staining (Fig. 5b).
Results of RT-PCR analysis
Testes tissues and cultured cells were subjected to semiquantitative RT-PCR analysis to identify stem cell-specific transcripts such as NANOG, POU5F1, SOX2, C-MYC and REX1 (Fig. 6a, b). Most transcripts, with the exception of C-MYC, were detected in testis sections, with the expression of NANOG greater than that of the other transcripts. Transcripts of these genes were also detected in cultured cells, but the expression patterns of transcripts for 4-day cultures were markedly different for cells grown on plates precoated with the different ECM components and DBA (Fig. 6). For example, C-MYC transcripts were more abundant in most cultured cells than in freshly collected testicular cells, whereas NANOG transcripts were less abundant in the cultured cells. Furthermore, the expression of POU5F1 and UCHL1 was markedly increased on DBA-precoated plates, and that of SOX, C-MYC and REX was considerably upregulated on the DBA-precoated plate compared with the other plates, but the expression of NANOG was relatively low. Conversely, on LN-precoated plates, the expression of NANOG and C-MYC transcripts was weak, whereas POU5F1 and SOX2 transcript expression was not detected.
Discussion
One of the unique biological features of differentiating germ cells is their adhesion to the basement membrane of the seminiferous tubule for the abilities as a stem cell potential and the differentiation. The present study investigated the effects of ECM components and DBA on the adhesion and growth of gonocytes, as well as on their stem cell characteristics in culture.
Germ cells usually require feeder cells for their survival, proliferation and maintenance in culture (Nagano et al. 2003). However, it has been revealed that feeder cells are not essential because they can be replaced with ECM molecules such as laminin (Kanatsu-Shinohara et al. 2005). The results of the present study indicate that ECM molecules are not effective in enriching or purifying gonocytes from the prepubertal testis (Fig. 3). In addition, ES-like colony formation from gonocytes was not stimulated by the ECM molecules, but was stimulated by the presence of DBA after selection of DBA-positive cells (Fig. 4). ECM molecules have been used as components of culture media for various types of cells. The requirement of the ECM for cell survival and growth varies depending on cell type; for example, LN is suitable for the culture of post-migratory primordial germ cells (PGCs; Garcia-Castro et al. 1997), GN is suitable for muscle cells and endothelial cells (Richler and Yaffe 1970; Folkman et al. 1979) and PLL is suitable for neuronal cells (Yavin and Yavin 1980). In the present study, testicular cell cultures (after positive cell selection) resulted in different cell populations on each of the ECM molecule-precoated plates (Fig. 4c). For example, cells grown on the DBA-precoated plates consisted mainly of gonocytes with ES-cell like morphology, whereas cells grown on the LN-precoated plates were mainly vimentin-positive and epithelial-type cells, indicating that they are Sertoli cells (Herrid et al. 2007). Therefore, the cell type-specific growth pattern of testicular cells, including gonocytes, may be affected by ECM molecules or DBA, which are closely associated with cell surface molecules, suggesting that ligands for the cell surface molecules are essential components for cell adhesion and regulate the physiological features of gonocytes in culture.
The use of DBA, which recognises α- and β-linked GalNAc residues (Kamada et al. 1991; Klisch et al. 2008), has been reported for the detection of gonocytes and SSCs in domestic species such as the pig (Goel et al. 2007) and cattle (Ertl and Wrobel 1992; Izadyar et al. 2002; Herrid et al. 2007). In addition, DBA has been used to enrich germ cells by MACS (Herrid et al. 2009), indicating that it can be a ligand for the surface glycan epitopes of germ cells. The specific affinity of the terminal GalNAc residues for their ligands may be associated with the cell surface interaction of gonocytes. Similarly, a terminal carbohydrate, such as mannose (Huang and Stanley 2010) and N-acetylglucosamine (GlcNAc; Akama et al. 2002), may be involved in the interaction between germ cells and Sertoli cells, indicating that the binding of germ cells to Sertoli cells depends on the terminal carbohydrate. Although these reports suggest that terminal carbohydrates on the surface of germ cells are associated with cell adhesion, there is no evidence that terminal GalNAc residues are involved in the adhesion activity in the testis. At the beginning of the present study, we hypothesised that GalNAc residues on the surface of gonocytes in the bovine testis that are specifically recognised by DBA affect cell survival and expansion in vitro. Our finding that the number of adherent gonocytes was significantly higher on DBA-coated plates than on the ECM component-coated plates (Fig. 3b) indicates that DBA can support the cell adhesion associated with the cell survival and cell growth of cultured gonocytes.
The results shown in Fig. 4 indicate that the DBA-coated plates support the binding of gonocytes to the plates and result in an increased number of colonies. The GalNAc residues on the surface of gonocytes are a part of the Sda-glycotopes on glycoproteins, which are associated with cell surface interactions (Klisch et al. 2011). The surface interaction of terminal glycan epitopes, such as GlcNAc-terminated N-linked glycans, which are combined with proteins or lipids, was found to affect the adhesion and differentiation of gonocytes on Sertoli cells in the mouse (Akama et al. 2002). Similarly, O-linked glycoproteins on mouse ES cells, which also have GalNAc residues and are recognised by DBA, are associated with the transition of the cells to a pluripotent state (Nash et al. 2007). The finding that masking of the terminal GalNAc residues of gonocytes by DBA pretreatment suppressed colony formation on both GN- and DBA-precoated plates (Fig. 4c, d) indicates that the proliferation and adhesion of gonocytes can be stimulated by terminal GalNAc residues. Because structural changes to the glycoproteins on a cell surface can affect cell–cell interactions and signal transduction (Dennis et al. 2009; Varki and Lowe 2009), the formation of a GalNAc–DBA complex on gonocytes may affect cell growth, cell survival and colony formation in culture.
The ability to maintain germ cells in culture depends on the presence of supporting cells that are associated with reconstruction of the niche microenvironment (Wu et al. 2010). Sertoli cells are key somatic cells that secrete growth factors, such as GDNF and bFGF, which are critical factors for the self-renewal and colony formation of germ cells in mice (Meng et al. 2000; Kubota et al. 2004). The presence of Sertoli cells in culture is known to improve the growth of germ cells (Koruji et al. 2009; Mohamadi et al. 2012). However, the flat cells surrounding the colonies of gonocytes in the present study were mainly Sertoli cells on the LN-precoated plate that did not support colony formation (Fig. 4), whereas the DBA-precoated plates on which the growth of somatic cells was suppressed supported colony formation (Fig. 4). The absence of colonies on the LN-precoated plates was considered to be due to the extensive growth of testicular somatic cells that inhibited the proliferation of germ cells (Kanatsu-Shinohara et al. 2005). The higher number of colonies on the DBA-precoated plates than on the plates precoated with the ECM components (Fig. 3) suggests that proper stimulation of somatic cells, including Leydig cells, Sertoli cells and endothelial cells (which are necessary for the survival and proliferation of germ cells; Aponte et al. 2008), supports the colony formation of gonocytes on the DBA-precoated plates. In fact, the colony formation of bovine gonocytes can be supported on GN-coated plates for the first week of culture (Fujihara et al. 2011). However, adhesion affinity and colony formation of gonocytes were lower on GN- compared with DBA-precoated plates (Fig. 3). On the DBA-precoated plates, the ability to form colonies was maintained up to 1.5 months by increasing the number of passages (Fig. 4e). In addition, the growth of testicular somatic cells was suppressed on the DBA-precoated plates, but not on the GN-precoated plates (Fig. 4c, e), suggesting that DBA-coated plates may provide suitable conditions for efficient colony formation compared with GN-precoated plates. Conversely, interactions between gonocytes and Sertoli cells were not observed during the initial phase of culture (Fig. 3c). However, Sertoli cells were associated with germ cell colonies after 7 days of culture (Fig. 5), indicating the characteristic change in gonocytes during culture.
Colonies of bovine gonocytes have stem cell potential, as identified by the expression of stem cell-specific genes (NANOG and POU5F1; Fujihara et al. 2011). The colony formation of testicular cells in culture depends on the presence of germ cell populations (Aponte et al. 2008), and these cell populations were strongly associated with the expression of NANOG and POU5F1 (Fig. 5b). Transcripts of other pluripotency-related genes, such as SOX2 and REX1, were expressed in 3-month-old bovine testis (Fig. 6a). The expression patterns of these genes depended on the culture plates, indicating that adhesion molecules on the plates are associated with the characteristics, including stem cell potential, of germ cells in culture. The expression of most of the pluripotency-related genes (POU5F1, SOX2, REX1 and C-MYC; but not NANOG) was considerably increased on the DBA-precoated plates (Fig. 6). The expression of these genes may be required for the survival and proliferation of gonocytes. In the pig, upregulation of pluripotency-related genes in gonocytes in primary culture was shown to stimulate the proliferation and stem cell potential of the gonocytes (Goel et al. 2009), suggesting that DBA-coated plates can support the proliferation and survival of cultured bovine germ cells. Meanwhile, the expression of NANOG on the DBA-precoated plates was decreased, whereas UCHL1 expression increased. In the 3-month-old bovine testis, heterogeneous distribution of NANOG expression was observed, but expression was no longer evident in gonocytes when they reach the basement membrane of seminiferous tubules (Fig. 1k–m), indicating that gonocytes may have differentiated into advanced stages of spermatogenesis (i.e. SSCs and/or spermatogonia). Similarly, decreasing NANOG expression is observed in differentiating gonocytes (Mitchell et al. 2008), with detection of UCHL1 expression in SSCs during asymmetrical division of the cells (Luo et al. 2009). Therefore, our results suggest the possibility that DBA-precoated plates may have resulted in transit characteristics of gonocytes towards SSCs and/or spermatogonia. This hypothesis could be supported by the evidence that characteristic changes of gonocytes have been observed when they reach the basement membrane of the seminiferous tubules (Culty 2009), and the proliferative activity of cultured gonocytes seems to be induced by the transition towards SSCs and/or spermatogonia in mice (Kanatsu-Shinohara et al. 2005). The finding that the characteristic changes in colony formation and the expression of pluripotency-related genes support both the growth of colonies and the differentiation of some gonocytes indicates that the glycan epitope of gonocytes may be associated with further progression for the differentiation towards SSCs and/or spermatogonia during postnatal developmental stages. Although the ligand for GalNAc residues was not determined in the present study, the cell surface glycan epitope seems to contribute to supporting proliferation and survival during the progressive differentiation of gonocytes in culture. This may be determined by analysis of proteoglycans carrying GalNAc residues and their natural receptors on Sertoli cells and/or testicular cells.
Acknowledgements
The authors thank Drs K. Konishi and Y. Hashiyada (National Livestock Breeding Center, Fukushima, Japan), and Dr Y. Hoshino (Gifu Prefectural Livestock Research Institute, Gifu, Japan) for providing the bovine testes. This research was supported by a grant from the Research Fellowship Program of the Japan Society for the Promotion of Science (JSPS Research Fellow) to SMK and a grant from the Ministry of Education, Culture, Sports, Science and Technology of Japan (no. 22380150 to HI).
References
Akama, T. O., Nakagawa, H., Sugihara, K., Narisawa, S., Ohyama, C., Nishimura, S., O’Brien, D. A., Moremen, K. W., Millan, J. L., and Fukuda, M. N. (2002). Germ cell survival through carbohydrate-mediated interaction with Sertoli cells. Science 295, 124–127.| Germ cell survival through carbohydrate-mediated interaction with Sertoli cells.Crossref | GoogleScholarGoogle Scholar | 11778047PubMed |
Aponte, P. M., Soda, T., Teerds, K. J., Mizrak, S. C., van de Kant, H. J., and de Rooij, D. G. (2008). Propagation of bovine spermatogonial stem cells in vitro. Reproduction 136, 543–557.
| Propagation of bovine spermatogonial stem cells in vitro.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXhsVCjtrnL&md5=e6f6f8bfd0efb715e584f162b141e94eCAS | 18663014PubMed |
Chai, C., and Leong, K. W. (2007). Biomaterials approach to expand and direct differentiation of stem cells. Mol. Ther. 15, 467–480.
| Biomaterials approach to expand and direct differentiation of stem cells.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXhtVKhsr%2FN&md5=bfe9de4b104a0955b1ca046e7c04a9c7CAS | 17264853PubMed |
Culty, M. (2009). Gonocytes, the forgotten cells of the germ cell lineage. Birth Defects Res. C Embryo Today 87, 1–26.
| Gonocytes, the forgotten cells of the germ cell lineage.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXksVKrsrY%3D&md5=076f94d5a219337bc768d2c70e449ad0CAS | 19306346PubMed |
Curtis, S. K., and Amann, R. P. (1981). Testicular development and establishment of spermatogenesis in Holstein bulls. J. Anim. Sci. 53, 1645–1657.
| 1:STN:280:DyaL387ovFOhuw%3D%3D&md5=c3bdcbdde4589a3a1ecf48b1fb5ced8dCAS | 7341622PubMed |
Dennis, J. W., Nabi, I. R., and Demetriou, M. (2009). Metabolism, cell surface organization, and disease. Cell 139, 1229–1241.
| Metabolism, cell surface organization, and disease.Crossref | GoogleScholarGoogle Scholar | 20064370PubMed |
Ertl, C., and Wrobel, K. H. (1992). Distribution of sugar residues in the bovine testis during postnatal ontogenesis demonstrated with lectin–horseradish peroxidase conjugates. Histochemistry 97, 161–171.
| Distribution of sugar residues in the bovine testis during postnatal ontogenesis demonstrated with lectin–horseradish peroxidase conjugates.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK38XhsVyqtro%3D&md5=e75b15410756fa2ed4562065937611f4CAS | 1559848PubMed |
Folkman, J., Haudenschild, C. C., and Zetter, B. R. (1979). Long-term culture of capillary endothelial cells. Proc. Natl Acad. Sci. USA 76, 5217–5221.
| Long-term culture of capillary endothelial cells.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaL3c%2Fls1Kltw%3D%3D&md5=ea246bb14c300776b08234ba79ed9cc2CAS | 291937PubMed |
Fujihara, M., Kim, S. M., Minami, N., Yamada, M., and Imai, H. (2011). Characterization and in vitro culture of male germ cells from developing bovine testis. J. Reprod. Dev. 57, 355–364.
| Characterization and in vitro culture of male germ cells from developing bovine testis.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXpslOiur0%3D&md5=965d5a51eca3e82e275fcbc44d70ad70CAS | 21289464PubMed |
Garcia-Castro, M. I., Anderson, R., Heasman, J., and Wylie, C. (1997). Interactions between germ cells and extracellular matrix glycoproteins during migration and gonad assembly in the mouse embryo. J. Cell Biol. 138, 471–480.
| Interactions between germ cells and extracellular matrix glycoproteins during migration and gonad assembly in the mouse embryo.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2sXkvFCjsrY%3D&md5=9ba0718b82d68290dccae560b20872d8CAS | 9230086PubMed |
Goel, S., Sugimoto, M., Minami, N., Yamada, M., Kume, S., and Imai, H. (2007). Identification, isolation, and in vitro culture of porcine gonocytes. Biol. Reprod. 77, 127–137.
| Identification, isolation, and in vitro culture of porcine gonocytes.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXntV2gtrk%3D&md5=080a814e02085fee82ee922377679f1bCAS | 17377141PubMed |
Goel, S., Fujihara, M., Minami, N., Yamada, M., and Imai, H. (2008). Expression of NANOG, but not POU5F1, points to the stem cell potential of primitive germ cells in neonatal pig testis. Reproduction 135, 785–795.
| Expression of NANOG, but not POU5F1, points to the stem cell potential of primitive germ cells in neonatal pig testis.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXnsVOksrs%3D&md5=9c69798dcee117a5c21d6bd1f5936816CAS | 18367503PubMed |
Goel, S., Fujihara, M., Tsuchiya, K., Takagi, Y., Minami, N., Yamada, M., and Imai, H. (2009). Multipotential ability of primitive germ cells from neonatal pig testis cultured in vitro. Reprod. Fertil. Dev. 21, 696–708.
| Multipotential ability of primitive germ cells from neonatal pig testis cultured in vitro.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXmsVCrurY%3D&md5=7bcedbd306dcedfebe221a8d8850885dCAS | 19486607PubMed |
Hamra, F. K., Chapman, K. M., Nguyen, D. M., Williams-Stephens, A. A., Hammer, R. E., and Garbers, D. L. (2005). Self renewal, expansion, and transfection of rat spermatogonial stem cells in culture. Proc. Natl Acad. Sci. USA 102, 17 430–17 435.
| Self renewal, expansion, and transfection of rat spermatogonial stem cells in culture.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXhtlSqtb%2FF&md5=61dcfecdc16ff4c3ce185a51ea9ffbbdCAS |
Herrid, M., Davey, R. J., and Hill, J. R. (2007). Characterization of germ cells from pre-pubertal bull calves in preparation for germ cell transplantation. Cell Tissue Res. 330, 321–329.
| Characterization of germ cells from pre-pubertal bull calves in preparation for germ cell transplantation.Crossref | GoogleScholarGoogle Scholar | 17593396PubMed |
Herrid, M., Davey, R. J., Hutton, K., Colditz, I. G., and Hill, J. R. (2009). A comparison of methods for preparing enriched populations of bovine spermatogonia. Reprod. Fertil. Dev. 21, 393–399.
| A comparison of methods for preparing enriched populations of bovine spermatogonia.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXisFemtr8%3D&md5=8f7f4201e970f113faafc394537ee3ddCAS | 19261216PubMed |
Huang, H. H., and Stanley, P. (2010). A testis-specific regulator of complex and hybrid N-glycan synthesis. J. Cell Biol. 190, 893–910.
| A testis-specific regulator of complex and hybrid N-glycan synthesis.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXhtFygsr7O&md5=7868ce6277c49c1bf0bf5e0bffaf7df8CAS | 20805325PubMed |
Izadyar, F., Spierenberg, G. T., Creemers, L. B., den Ouden, K., and de Rooij, D. G. (2002). Isolation and purification of type A spermatogonia from the bovine testis. Reproduction 124, 85–94.
| Isolation and purification of type A spermatogonia from the bovine testis.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38XmtFaksL8%3D&md5=9970823eefe8a9dc3e9a45524bbfc122CAS | 12090922PubMed |
Kamada, Y., Muramatsu, H., Arita, Y., Yamada, T., and Muramatsu, T. (1991). Structural studies on a binding site for Dolichos biflorus agglutinin in the small intestine of the mouse. J. Biochem. 109, 178–183.
| 1:CAS:528:DyaK3MXhsVShu74%3D&md5=5851e8b20e87e61f259e6a24ed28716cCAS | 2016266PubMed |
Kanatsu-Shinohara, M., Ogonuki, N., Inoue, K., Miki, H., Ogura, A., Toyokuni, S., and Shinohara, T. (2003). Long-term proliferation in culture and germline transmission of mouse male germline stem cells. Biol. Reprod. 69, 612–616.
| Long-term proliferation in culture and germline transmission of mouse male germline stem cells.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXlvVerurc%3D&md5=bed0c7eff283021cc98d8fd5c5a88969CAS | 12700182PubMed |
Kanatsu-Shinohara, M., Miki, H., Inoue, K., Ogonuki, N., Toyokuni, S., Ogura, A., and Shinohara, T. (2005). Long-term culture of mouse male germline stem cells under serum- or feeder-free conditions. Biol. Reprod. 72, 985–991.
| Long-term culture of mouse male germline stem cells under serum- or feeder-free conditions.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXis12hsLY%3D&md5=27daa2ad31c034b5d749e6cae92777e2CAS | 15601913PubMed |
Kanatsu-Shinohara, M., Muneto, T., Lee, J., Takenaka, M., Chuma, S., Nakatsuji, N., Horiuchi, T., and Shinohara, T. (2008a). Long-term culture of male germline stem cells from hamster testes. Biol. Reprod. 78, 611–617.
| Long-term culture of male germline stem cells from hamster testes.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXjvVaitbg%3D&md5=6acab49c3c511b001d18240fe337af1aCAS | 18094355PubMed |
Kanatsu-Shinohara, M., Takehashi, M., Takashima, S., Lee, J., Morimoto, H., Chuma, S., Raducanu, A., Nakatsuji, N., Fassler, R., and Shinohara, T. (2008b). Homing of mouse spermatogonial stem cells to germline niche depends on beta1-integrin. Cell Stem Cell 3, 533–542.
| Homing of mouse spermatogonial stem cells to germline niche depends on beta1-integrin.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXhsVaqs7%2FO&md5=27828157885cc47a179c5acc24839e78CAS | 18983968PubMed |
Klisch, K., Jeanrond, E., Pang, P. C., Pich, A., Schuler, G., Dantzer, V., Kowalewski, M. P., and Dell, A. (2008). A tetraantennary glycan with bisecting N-acetylglucosamine and the Sda antigen is the predominant N-glycan on bovine pregnancy-associated glycoproteins. Glycobiology 18, 42–52.
| A tetraantennary glycan with bisecting N-acetylglucosamine and the Sda antigen is the predominant N-glycan on bovine pregnancy-associated glycoproteins.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXhtFeisw%3D%3D&md5=2cfd267150234d79cdbec5b041bfa9f9CAS | 17951374PubMed |
Klisch, K., Contreras, D. A., Sun, X., Brehm, R., Bergmann, M., and Alberio, R. (2011). The Sda/GM2-glycan is a carbohydrate marker of porcine primordial germ cells and of a subpopulation of spermatogonia in cattle, pigs, horses and llama. Reproduction 142, 667–674.
| The Sda/GM2-glycan is a carbohydrate marker of porcine primordial germ cells and of a subpopulation of spermatogonia in cattle, pigs, horses and llama.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXhsFOhsbrO&md5=fdb44192a524fc6c77c5348e0f90e572CAS | 21896636PubMed |
Koruji, M., Movahedin, M., Mowla, S. J., Gourabi, H., and Arfaee, A. J. (2009). Efficiency of adult mouse spermatogonial stem cell colony formation under several culture conditions. In Vitro Cell. Dev. Biol. Anim. 45, 281–289.
| Efficiency of adult mouse spermatogonial stem cell colony formation under several culture conditions.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXmtF2rtrs%3D&md5=bd3a8bac762af28d7e2e406457a0b98aCAS | 19221844PubMed |
Kubota, H., Avarbock, M. R., and Brinster, R. L. (2004). Growth factors essential for self-renewal and expansion of mouse spermatogonial stem cells. Proc. Natl Acad. Sci. USA 101, 16 489–16 494.
| Growth factors essential for self-renewal and expansion of mouse spermatogonial stem cells.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXhtVOisbvP&md5=8eb8a97b6e2751c72f7f7b6f80e409b2CAS |
Kubota, H., Wu, X., Goodyear, S. M., Avarbock, M. R., and Brinster, R. L. (2011). Glial cell line-derived neurotrophic factor and endothelial cells promote self-renewal of rabbit germ cells with spermatogonial stem cell properties. FASEB J. 25, 2604–2614.
| Glial cell line-derived neurotrophic factor and endothelial cells promote self-renewal of rabbit germ cells with spermatogonial stem cell properties.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXpvFers74%3D&md5=4064d23e9875343392d8a76e7f7a9faaCAS | 21525489PubMed |
Luo, J., Megee, S., and Dobrinski, I. (2009). Asymmetric distribution of UCH-L1 in spermatogonia is associated with maintenance and differentiation of spermatogonial stem cells. J. Cell. Physiol. 220, 460–468.
| Asymmetric distribution of UCH-L1 in spermatogonia is associated with maintenance and differentiation of spermatogonial stem cells.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXotFags7c%3D&md5=982da295520d4efa784d405828772210CAS | 19388011PubMed |
Meng, X., Lindahl, M., Hyvonen, M. E., Parvinen, M., de Rooij, D. G., Hess, M. W., Raatikainen-Ahokas, A., Sainio, K., Rauvala, H., Lakso, M., Pichel, J. G., Westphal, H., Saarma, M., and Sariola, H. (2000). Regulation of cell fate decision of undifferentiated spermatogonia by GDNF. Science 287, 1489–1493.
| Regulation of cell fate decision of undifferentiated spermatogonia by GDNF.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3cXhsV2qtL8%3D&md5=9fef5f444bc2f28ca398345054c353b2CAS | 10688798PubMed |
Mitchell, R. T., Cowan, G., Morris, K. D., Anderson, R. A., Fraser, H. M., McKenzie, K. J., Wallace, W. H., Kelnar, C. J., Saunders, P. T., and Sharpe, R. M. (2008). Germ cell differentiation in the marmoset (Callithrix jacchus) during fetal and neonatal life closely parallels that in the human. Hum. Reprod. 23, 2755–2765.
| Germ cell differentiation in the marmoset (Callithrix jacchus) during fetal and neonatal life closely parallels that in the human.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXhsVWmt77F&md5=cb0a05ad6fe0db931ae223a80103c71dCAS | 18694875PubMed |
Mohamadi, S. M., Movahedin, M., Koruji, S. M., Jafarabadi, M. A., and Makoolati, Z. (2012). Comparison of colony formation in adult mouse spermatogonial stem cells developed in Sertoli and STO coculture systems. Andrologia 44, 431–437.
| Comparison of colony formation in adult mouse spermatogonial stem cells developed in Sertoli and STO coculture systems.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38Xhs1WkurjI&md5=68036605d99cfd5ef7f9873d6ec0535bCAS | 21762195PubMed |
Nagano, M., Avarbock, M. R., Leonida, E. B., Brinster, C. J., and Brinster, R. L. (1998). Culture of mouse spermatogonial stem cells. Tissue Cell 30, 389–397.
| Culture of mouse spermatogonial stem cells.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK1M%2FgtlOnsg%3D%3D&md5=74c0a1c61db4c5815831b2366c73a5d6CAS | 9787472PubMed |
Nagano, M., Ryu, B. Y., Brinster, C. J., Avarbock, M. R., and Brinster, R. L. (2003). Maintenance of mouse male germ line stem cells in vitro. Biol. Reprod. 68, 2207–2214.
| Maintenance of mouse male germ line stem cells in vitro.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXks1Ggsbk%3D&md5=623ab7cb129a40b468ecae458f470c32CAS | 12606373PubMed |
Nash, R., Neves, L., Faast, R., Pierce, M., and Dalton, S. (2007). The lectin Dolichos biflorus agglutinin recognizes glycan epitopes on the surface of murine embryonic stem cells: a new tool for characterizing pluripotent cells and early differentiation. Stem Cells 25, 974–982.
| The lectin Dolichos biflorus agglutinin recognizes glycan epitopes on the surface of murine embryonic stem cells: a new tool for characterizing pluripotent cells and early differentiation.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXls1Kmtrc%3D&md5=173b32a5862d5a4efabe49294c63026bCAS | 17170066PubMed |
Orwig, K. E., Avarbock, M. R., and Brinster, R. L. (2002a). Retrovirus-mediated modification of male germline stem cells in rats. Biol. Reprod. 67, 874–879.
| Retrovirus-mediated modification of male germline stem cells in rats.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38XmsV2jsbw%3D&md5=f0db7db7a8dbc24f7ab5b746ed6bab9aCAS | 12193397PubMed |
Orwig, K. E., Ryu, B. Y., Avarbock, M. R., and Brinster, R. L. (2002b). Male germ-line stem cell potential is predicted by morphology of cells in neonatal rat testes. Proc. Natl Acad. Sci. USA 99, 11 706–11 711.
| Male germ-line stem cell potential is predicted by morphology of cells in neonatal rat testes.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38XntFWqsbc%3D&md5=add9c0157d4db16db5ff5b2401f09402CAS |
Orwig, K. E., Shinohara, T., Avarbock, M. R., and Brinster, R. L. (2002c). Functional analysis of stem cells in the adult rat testis. Biol. Reprod. 66, 944–949.
| Functional analysis of stem cells in the adult rat testis.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38XitlCltb4%3D&md5=12303e4921d97151f66ce18a79c71aa8CAS | 11906912PubMed |
Piller, V., Piller, F., and Cartron, J. P. (1990). Comparison of the carbohydrate-binding specificities of seven N-acetyl-d-galactosamine-recognizing lectins. Eur. J. Biochem. 191, 461–466.
| Comparison of the carbohydrate-binding specificities of seven N-acetyl-d-galactosamine-recognizing lectins.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK3cXkvFGqtrk%3D&md5=6d7bc8c876bbdb073a163aa6647c2cebCAS | 2384093PubMed |
Richler, C., and Yaffe, D. (1970). The in vitro cultivation and differentiation capacities of myogenic cell lines. Dev. Biol. 23, 1–22.
| The in vitro cultivation and differentiation capacities of myogenic cell lines.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaE3M%2Fjslymtg%3D%3D&md5=90099d6a66d1821f55f9955b217d59e9CAS | 5481965PubMed |
Shinohara, T., Avarbock, M. R., and Brinster, R. L. (1999). Beta1- and alpha6-integrin are surface markers on mouse spermatogonial stem cells. Proc. Natl Acad. Sci. USA 96, 5504–5509.
| Beta1- and alpha6-integrin are surface markers on mouse spermatogonial stem cells.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1MXjtFCnsbg%3D&md5=ab2f2648b68bcf3fd809c6278c843617CAS | 10318913PubMed |
Siu, M. K., and Cheng, C. Y. (2004). Dynamic cross-talk between cells and the extracellular matrix in the testis. Bioessays 26, 978–992.
| Dynamic cross-talk between cells and the extracellular matrix in the testis.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXnvFWlt7c%3D&md5=e34e3ca126f8834ccf4578983af9e422CAS | 15351968PubMed |
Varki, A., and Lowe, J. (2009), Biological roles of glycans. In ‘Essentials of Glycobiology’. (Eds A. Varki, R. Cummings, J. Esko, H. Freeze, P. Stanley, C. Bertozzi, G. Hart and M. Etlzler.) pp. 75–88. (Cold Spring Harbor Laboratory Press: New York.)
Wu, X., Oatley, J. M., Oatley, M. J., Kaucher, A. V., Avarbock, M. R., and Brinster, R. L. (2010). The POU domain transcription factor POU3F1 is an important intrinsic regulator of GDNF-induced survival and self-renewal of mouse spermatogonial stem cells. Biol. Reprod. 82, 1103–1111.
| The POU domain transcription factor POU3F1 is an important intrinsic regulator of GDNF-induced survival and self-renewal of mouse spermatogonial stem cells.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXmvVCgt70%3D&md5=4cc4cfd7c941c551d3dbdcdf9cc5ac1cCAS | 20181621PubMed |
Yavin, Z., and Yavin, E. (1980). Survival and maturation of cerebral neurons on poly(L-lysine) surfaces in the absence of serum. Dev. Biol. 75, 454–459.
| Survival and maturation of cerebral neurons on poly(L-lysine) surfaces in the absence of serum.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL3cXhslyhu7g%3D&md5=403f03c12f46e4493b34868dc83a7befCAS | 6989691PubMed |