Trajectories of ecology past and future
Mark Westoby A § *
A § *
A
§ This contribution by Professor Mark Westoby is the first in a new journal category - Reflections. These are invited reviews authored by a leader in a research field on a topic of their choosing that relates to the development of Botanical Science. The reviews will reflect historically on our science and participating scientists, providing context for the current state of a discipline.
Handling Editor: John Morgan
Abstract
Ecosystems have many different processes going on. Researchers need to select and simplify, and so development of ecology as a discipline has involved finding different possible ways to select and simplify. The history is summarised via six strands: zonation of vegetation physiognomy, single-species population dynamics, population interactions, ecosystems as machines through which energy cascades and nutrients cycle, episodic events and landscape mosaics, and generalisation across species. Australia has been influential in several of these strands. So then, where might ecology head in future? Future ecosystems are likely to be different from the past, partly owing to climate change, but also because of technologies such as cell culture milk and meat, new synbio organisms, and agricultural and land-management robotics. The most important framing for the future will be first-principle rules for how ecosystems are constructed. First-principle questions include the following: (1) what are the resources; where do energy and mineral nutrients come from; and linked to that (2) what is the disturbance regime and how does succession between disturbances work; (3) how does competition for resources get worked out; (4) what physical structure is contributed by habitat-forming species; and how is this influenced by competition, disturbance regime and predation on habitat-forming species? The six historical strands discussed continue to be important for addressing these questions. Ecology is seen by many students and citizens as aligned with conservation, as a Cassandra-science mainly concerned with what might go wrong in ecosystems. By framing ecology curriculum as a science of ecosystem construction, we can look more to the future and to new possibilities.
Keywords: competition and competitive exclusion, ecology curriculum, ecosystem structure, foundation species, history of ecology, population dynamics, succession, trait-based ecology, vegetation physiognomy.
Ecology as a discipline
For researchers, ecology is about population dynamics, relationships of species to habitat and each other, and transactions of energy and nutrients in assemblages of more than one species. It deals with situations such as agriculture and aquaculture, zoonotic diseases, sewage plants, biofilms and fermentations, as well as with forests and grasslands and waters.
The words ‘ecology’ and ‘ecosystem’ have meanwhile also become widely used in general conversation. One hears of the financial ecosystem and the wellness ecosystem, for example. Sometimes ‘ecological’ is used with overtones of ethics or almost religion (Westoby 1997). But for this paper, ecology is treated as a discipline within biological sciences.
This present paper has two components. The first is outline history of how ecology as a discipline has developed up to the present. The second is opinion about the future: what would be the best way for ecology to develop, going forward?
How has ecology developed?
In ecosystems there are a lot of things happening. Energy is being moved and processed. Water is being transported from soil to atmosphere. Mineral nutrients are being cycled. Populations of species are increasing and decreasing, and influencing other populations as those in turn increase and decrease. Resources are being traded in mutualisms between organisms with different specialisations and comparative advantage, such as plants and mycorrhizal fungi. Local populations are going extinct, and being recolonised from elsewhere. Some species are substantially modifying the physical environment (these are called habitat-forming or foundation species). Temperatures and humidities and windspeeds are being modified by the canopy and by the litter of leaves and twigs that have fallen to the ground. Fire regimes depend on that litter.
Over the past 100 years, data acquisition and mechanistic understanding have been greatly strengthened for the different processes occurring in ecosystems. Also, and because so many different things are happening, advances in ecology have often come about through fresh ideas about what questions or framings or simplifications will be most productive.
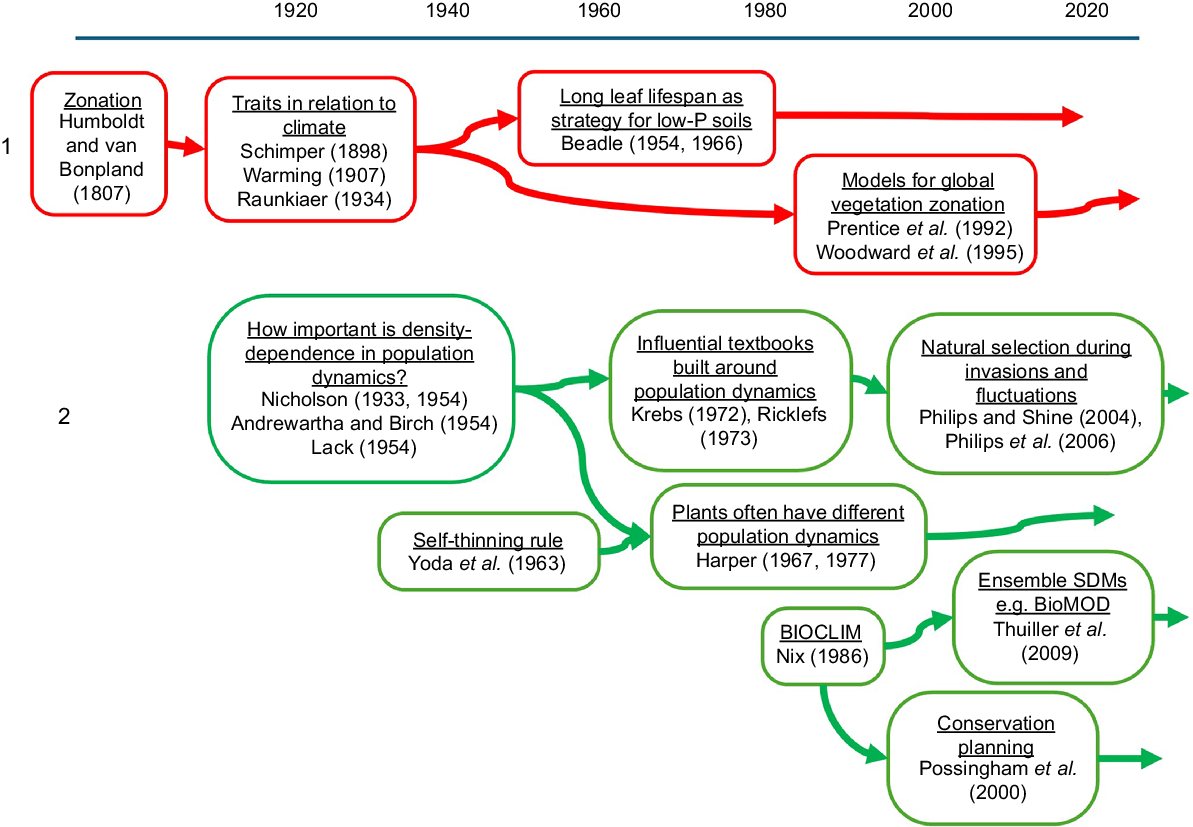
The first part of this paper offers a brief history of ecology expressed as six strands (Figs 1–3). The strands are (1) zonation of vegetation physiognomy, (2) single-species population dynamics, (3) population interactions, (4) ecosystems as energy cascades, (5) episodic events and landscape mosaics, and (6) generalisation across species. The strands are not neatly separated; braiding of ideas and questions has been commonplace. The material selected is somewhat weighted towards Australia-related advances and terrestrial plant ecology. As readers might expect, the strands reflect my own opinions and enthusiasms.
Strand 1: zonation of vegetation physiognomy
Large-scale zonation of plant physiognomy with latitude and with altitude is arguably the most important pattern in ecology. It is conspicuous; students and the public rightly expect the world map of biome-types to be something explained by ecology. Also the shifts in plant growth-form and phenology and tissue properties with latitude powerfully influence habitat and resources for all the other residents. Zonation was extensively described already by von Humboldt and Bonpland (1807) (Strand 1 in Fig. 1). At the opening of the 20th century, founders of ecology as a discipline (Schimper 1898; Warming 1909; Raunkiaer 1934 English translation) were focused on why plant traits varied among different climates.
A distinct Australian contribution came from Beadle (1954, 1966), who showed that sclerophylly could be a response to low soil phosphorus as well as to seasonal drought. The sclerophylly confers long leaf lifespans that economise on nutrient costs. The point was subsequently taken up in the northern hemisphere (Loveless 1961).
From the 1990s on, the most comprehensive account of global vegetation patterns has been expressed through dynamic global vegetation models (DGVMs) and earth system models. Models such as BIOME (Prentice et al. 1992; Haxeltine and Prentice 1996) and Sheffield (Woodward et al. 1995) have led on to a wide variety of others, reviewed by Zaehle (2019). Fire regimes are an important input for modelling global pattern successfully (Bond et al. 2005; Hantson et al. 2016). The LPJ-GUESS model, for example (Smith et al. 2014), embodies patch succession and fire processes, as well as modulation of seasonal productivity and leaf cover by climate and soil water.
Strand 2: single-species populations
Much ecological research focuses around individual species, how much their populations fluctuate and why, and how to manage them for harvest or suppression or conservation (Strand 2 in Fig. 1). One of the main definitions for ecology has been that it is about the distribution and abundance of organisms (Andrewartha and Birch 1954). Density dependence regulates abundance, and a debate about whether density dependence was important continued from the 1930s into the 1980s. Key contributors were Australian, both emphasising density dependence (Nicholson 1933, 1954) and downplaying its importance (Andrewartha and Birch 1954). The population regulation debate became the backbone of key textbooks (e.g. Krebs 1972).
Up to the 1960s, population dynamics research in the anglosphere was driven mainly by interest in insect pests and small mammals, especially in some species with strong cyclic fluctuations. In parallel from the 1950s onward, there was very creative research on density effects in plant populations in Japan. This gave rise notably to the ‘self-thinning rule’ or Yoda rule (Yoda et al. 1963). The rule was brought to western consciousness through Harper’s (1967) presidential address to British Ecological Society, and subsequently in his magisterial book on plant population dynamics (Harper 1977). The self-thinning rule points to two important differences compared with animal population dynamics: (1) slower growth conditions actually decrease rather than increasing mortality, and (2) carrying capacities are not a function of numbers only, but a joint function of biomass and numbers.
For plants, it was clear from the outset that density was important. Effects of planting density and of shading are obvious to any farmer or gardener as well as to researchers. Key ideas for animal populations included the stable age distribution. Plant populations in some situations, such as forests driven by treefall gaps, and meadows and tundras, can indeed be approximated as having continuing turnover and stable distributions of age and size. But in many other situations, such as the vegetation types with regimes of recurrent fire that occupy most of Australia, individuals develop as stands that are even-aged from seedlings, or if resprouting from the base, then starting from even-sized so far as canopy deployment is concerned.
Birth and mortality rates are forces of natural selection as well as of population change. It was noticed quite early that this meant that there could be evolutionary change during strong population fluctuations or invasions (e.g. Pimentel 1968). However, the importance of evolutionary change during invasions was not fully appreciated until the studies from Shine laboratory of cane toads and their predators at the invasion front across northern Australia (Phillips and Shine 2004; Phillips et al. 2006).
Research on species distributions, in other words on their geographic boundaries, has gone along largely separate from research on species abundance. The seminal species distribution model (SDM) was the Australian BIOCLIM, first available in 1984 (Nix 1986; Booth et al. 2014). SDMs map known distributions onto climate and other environmental surfaces. Many different SDMs now exist, to the point that software packages provide for ensembles of models to be applied (Thuiller et al. 2009). They have become very widely used in applied ecology, including forecasting future distributions of invasive species (e.g. Elith 2017), future distributions under climate change (e.g. Elith et al. 2010), and conservation planning (Possingham et al. 2000). These models are correlative, and are not able to identify by what mechanism a species has zero abundance beyond the edge of its distribution. Ecology still has disconcertingly little hard evidence about how distribution boundaries are determined (Westoby 2022) (see Substrand 3d below).
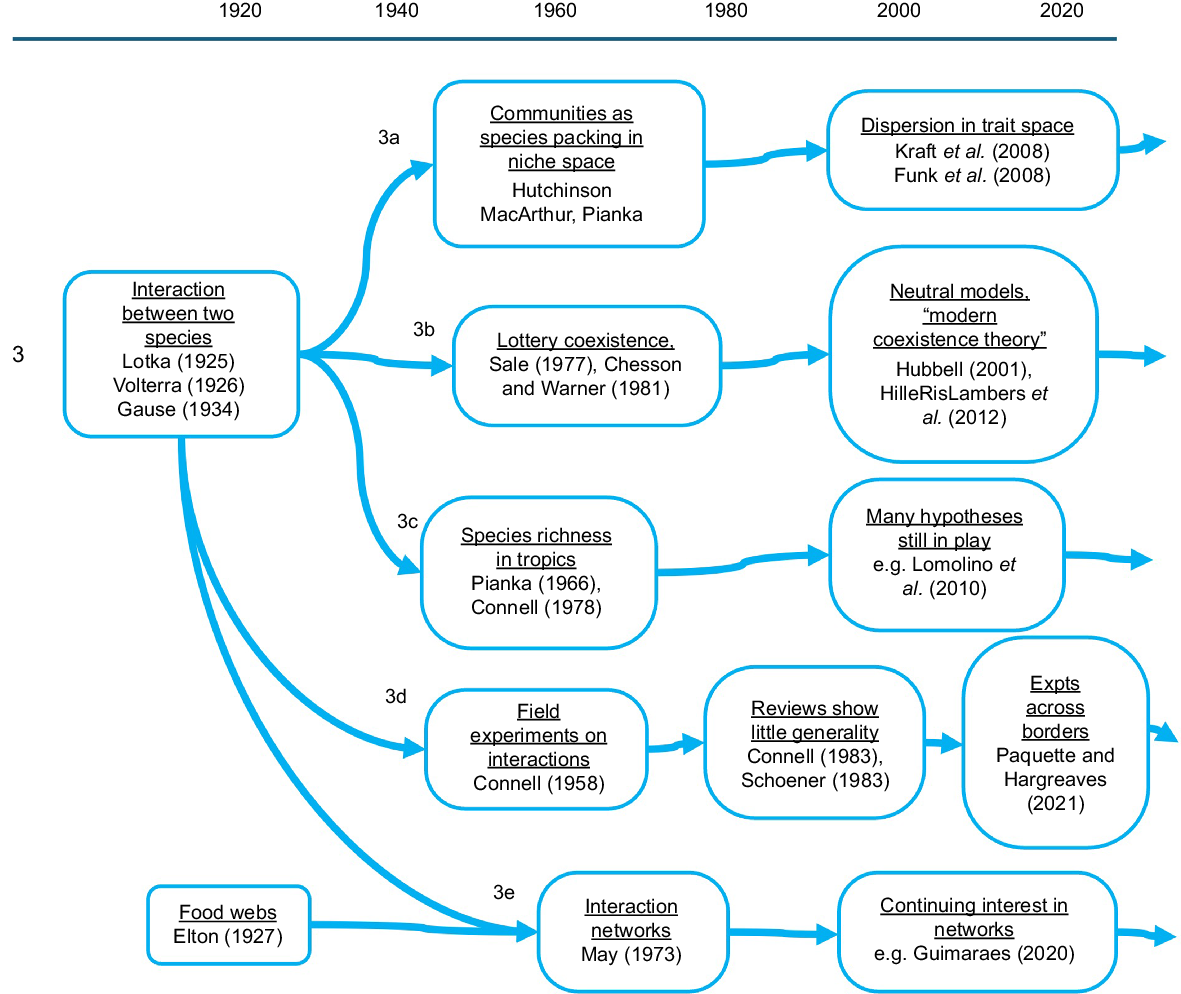
Strand 3: population interactions
Mathematics for competitive and predator–prey interactions between pairs of species populations was worked out by the 1930s (Lotka 1925; Volterra 1926; Elton 1927; Gause 1934). This work provided foundation for five different substrands continuing into the present (Fig. 2, Strands 3a–e).
Substrand 3a, the Hutchinson–MacArthur revolution, was the study of communities as a set of competing and coexisting species (Hutchinson 1959; MacArthur 1965; Macarthur and Levins 1967). Hutchinson shifted the concept of niche of a species from verbal description of habitat and way of life, to visualising a volume in n-dimensional hyperspace with resources and biotic tolerances as dimensions. In key textbooks of the 1970s (Krebs 1972; Ricklefs 1973; Pianka 1978), communities were thought of as a total available niche volume in multidimensional space, occupied by a set of balloon-shaped volumes pressed together and representing realised niches of species. The agenda was the species-packing problem, i.e. to understand the total volume of niche space available, then the niche locations and volumes of each species, and then the limits to similarity; how closely adjacent niches could be packed in together without competitive exclusion. Pursuit of this agenda really included pursuit of all the relevant biology: which niche dimensions were important, how much niche space was available in a given physical and resource setting, the niche use by each species, and the rules for competition. Among the predictions was the number of coexisting species. The idea that species richness is the test of whether we understand communities is still with us.
The idea of overdispersion or limiting similarity in niche space has been translated into an expectation of overdispersion in trait space, both for natural communities (e.g. Kraft et al. 2008) and in search of rules for what kinds of species can become invasive (Funk et al. 2008; Divíšek et al. 2018).
However, by the 1970s it had also become evident that using distinct depletable resources (niche separation) could not be the only mechanism for coexistence. An alternative ‘lottery coexistence’ mechanism emerged from Peter Sale’s (Sale 1974, 1978) experiments on coexisting territorial fish on the Great Barrier Reef (Substrand 3b). The mechanism is partly that established fish are able to hold their site for a long time, and partly that when sites do become vacant, it is near enough a lottery for which species colonises it. These mechanisms can slow down competitive exclusion so much that minor fluctuations in relative advantage can maintain coexistence. They became known as storage effects (Chesson 1994), and were eventually elaborated into ‘modern coexistence theory’ (HilleRisLambers et al. 2012).
These properties, of persistent established adults together with a large dose of chance in who establishes in vacant living sites, were obviously applicable to perennial vegetation as well as to territorial reef fish. ‘Neutral theory’ (Hubbell 2001) extended to geographical and evolutionary timescales this idea that which species took over vacant sites was stochastically determined. Broadly, there is a distinction in ecology between sessile and mobile communities, more so than between plants and animals. Sessile species compete for space occupancy more so than for distinct resources. Their population structures and size distributions are shaped by disturbance regimes, more so than by reaching equilibrium under a given survivorship curve.
Given that species richness is not necessarily a product of differentiated niches and species interaction rules, differences among communities in species richness will not reliably tell us anything about interaction processes or about which niche dimensions are most important. Nevertheless, species richness continues to be treated as a cardinal property of communities. This seems to me unfortunate; surely, it is more interesting to know what sorts of species are present, what traits they have (see Strand 6), than it is to know how many there are?
Substrand 3c (Fig. 2), the question why species diversity is greater in the tropics than in the temperate zone (for most taxa), has been a natural partner to Substrand 3a about how coexistence versus competitive exclusion works. The latitudinal diversity gradient has impressed everyone (Brown 2014), from von Humboldt, Wallace and Darwin through Dobzhansky, Elton and Hutchinson up to the present day. Lists of hypotheses have expanded from 6 (Pianka 1966) to 32 (Lomolino et al. 2010). These hypotheses are not mutually exclusive, and several or many may turn out to have explanatory power in parallel. To explain the latitudinal diversity gradient is not quite the same question as explaining why diversity seems impressively high in the tropics. Studies that show a diversity-sustaining process operating in the tropics do not actually resolve the question about the latitudinal diversity gradient. Rather, studies are needed that show a particular diversity-sustaining process operating more powerfully in the tropics than at higher latitudes.
Substrand 3d (Fig. 2) consists of actual manipulative experiments about competition. This strand began from Connell’s barnacle experiments (Connell 1961). In the 1960s, it was exciting and transformative to realise that the pervasive cross-correlations in ecology could be successfully dispelled by manipulative field experiments. Textbooks ever since have illustrated the barnacle experiments. Krebs’s (1972) influential text was pointedly titled ‘Ecology: the experimental analysis of distribution and abundance’. Through the 1970s and 1980s, field experiments were the most respected research style in ecology, the gold coin of evidence.
By the 1980s, it was already possible to review hundreds of competition experiments (Connell 1983; Schoener 1983). However, generality was hard to find. Also these experiments were much more often directed at measuring the intensity of competition within communities, than at understanding the boundaries of distributions along environmental and geographical gradients. A recent review (Paquette and Hargreaves 2021) emphasised that we still have rather little reliable experimental evidence about the control of boundaries (Westoby 2022). With temperature zones moving poleward, it makes an important difference whether a warm-edge boundary of a species range is decided by climate directly or rather by competition with other species, or perhaps by the impact of pathogens.
Substrand 3e of population interactions deals with food webs, with the idea that ecosystems can be thought of as networks of interactions among species, going back to Elton (1927). May’s (1973) book modelled interaction webs through large sets of pairwise population equations mediated by Lotka–Volterra interaction coefficients drawn from random distributions. The idea that network configuration will tell us something interesting persists through to the present day (e.g. Guimarães 2020). My own view is that the details of how the most important interactions work, for example, whether herbivores can or cannot suppress the accumulation of aboveground vegetation structure, are more important than is the network structure. This point was actually made by May (1973). That book is best remembered for showing that there was no automatic tendency for networks with more nodes (species) to be more dynamically stable. But May (1973) also made the point that this network model did not capture any of the more complex biological features of real interactions. Models that did, would likely show different behaviour.
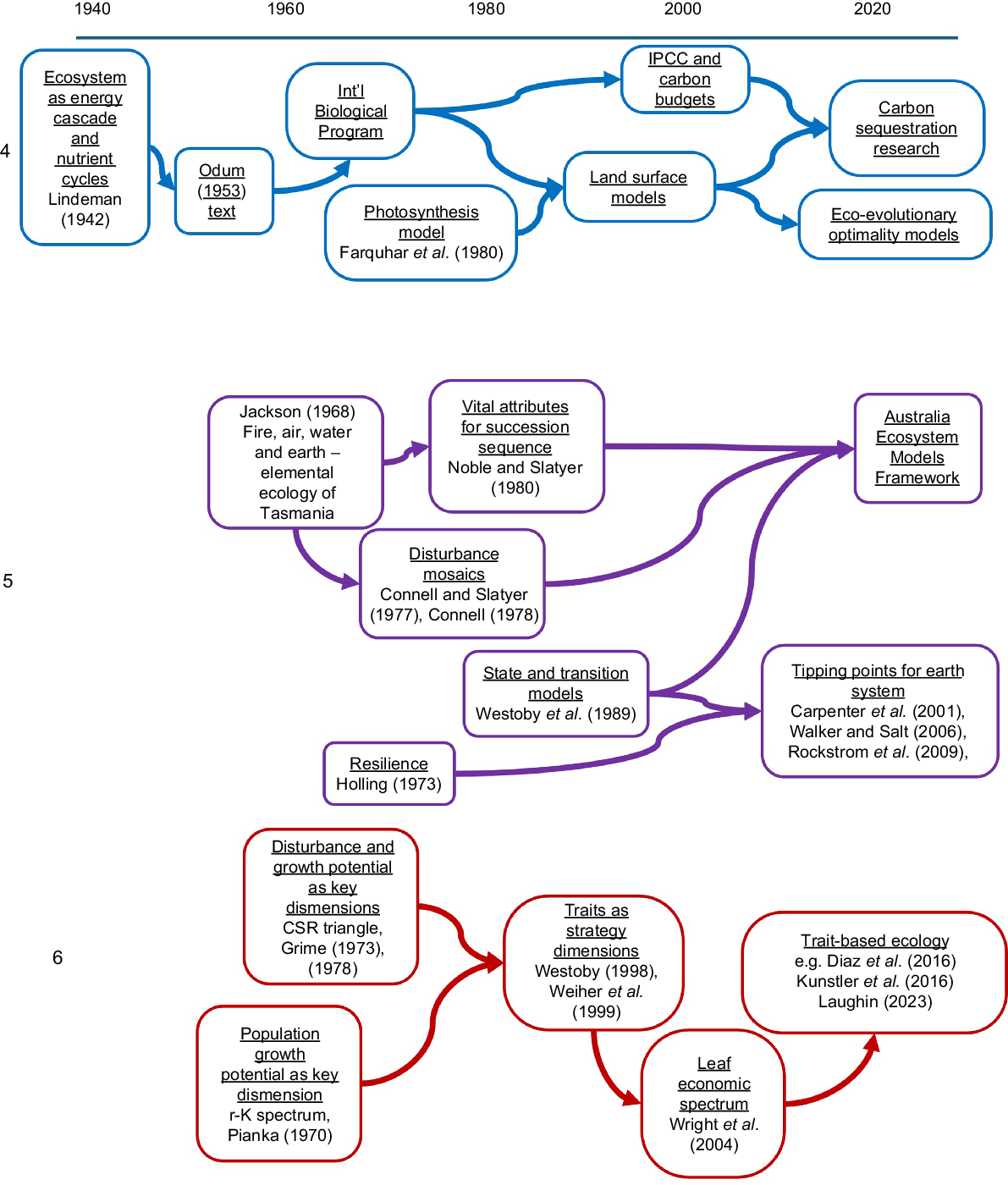
Strand 4: ecosystems as energy cascades and nutrient cycles
Visualising ecosystems as machines driven by a quantifiable flow of energy traces to Lindeman (1942) (Fig. 3, Strand 4). E. P. Odum’s textbook (Odum 1953) made this into a standard part of ecology curriculum by the 1960s. During the 1960s, estimating energy flow was the focus of the International Biological Program. This formulation was not much pursued in Australia through the 1960s to 2000s. Over the past 20 years, it has risen to importance in the specific form of estimating and forecasting carbon sequestration (Roxburgh et al. 2006; Haverd et al. 2018). For this purpose, seminal Australian contributions to plant physiology (Farquhar et al. 1980; Cowan 1982) have been important; the Farquhar–von Caemmerer photosynthesis scheme remains the basis for modelling photosynthesis in vegetation models worldwide. Next-generation models are expected to deduce trait values from eco-evolutionary optimality (Franklin et al. 2020; Harrison et al. 2021), rather than empirically estimating them for plant functional types. For example, least-cost theory for balancing the use of water in transpiration against the use of nitrogen in CO2 fixation capacity (Wright et al. 2003; Prentice et al. 2014) successfully predicts CO2 drawdown within leaves.
Strand 5: episodic events and landscape mosaics
Australia was early in recognising that recurrent fires were to be expected, and that many vegetation landscapes could be understood as successional stages (Jackson 1968) (Fig. 3, Strand 5). During the 1970s it became internationally established (Connell 1978) to visualise landscapes and reefscapes as disturbance mosaics. There was strong Australian connection, with Connell’s reef and rainforest sites both being in northern Queensland. In parallel, understanding about alternative mechanisms of succession was consolidated (Connell and Slatyer 1977), and a ‘vital attributes’ system of ecological strategies was developed to express differences in species response to succession (Noble and Slatyer 1980). State land management authorities have subsequently developed databases listing between-fire intervals that are acceptable for different species (e.g. Ferrer-Paris and Keith 2022).
Similarly in arid zone ecology and range management, the equilibrium range succession formulation began to be replaced by an alternative state-and-transition language (Westoby et al. 1989; Smith and Morton 1990). The observation that some transitions are not easily reversible was connected to the ideas of tipping points and resilience. Subsequently, Brian Walker, working through the Stockholm-based Resilience Alliance, developed these ideas on the larger stage of earth tipping points (Carpenter et al. 2001; Walker and Salt 2006; Rockström et al. 2009; Walker and Westoby 2011). Currently in Australia, the Australian Ecosystem Models Framework project (https://research.csiro.au/biodiversity-knowledge/projects/models-framework/) aims to develop state-and-transition narratives for all terrestrial ecosystems continent-wide, and the intention is to embed these within a National Ecological Assessment System for Australia (https://www.tern.org.au/neasa/).
Strand 6: generalisation across species
The desire to put plants into ecologically meaningful categories goes back to the Lyceum of Athens and Theophrastos’s Enquiry into Plants (~320 BC) (Weiher et al. 1999). Depressingly for plant ecologists, plants were a second thought even then, following on from the Enquiry into Animals by Theophrastos’s mentor Aristotle. A sharper focus came with Raunkiaer (1934), whose life-form system expressed regeneration after the most difficult season of the year. From the 1970s, Grime’s (1974, 1979) competitive, stress tolerant, ruderal CSR triangle expressed variation as continuous spectra (Fig. 3, Strand 6).
Grime’s Unit of Comparative Plant Ecology at Sheffield was also a groundbreaker in developing systematic measurement of traits across a range of species (Grime and Hunt 1975; Grime et al. 1988). Nevertheless, the two spectra within the CSR triangle remained conceptual, rather than it being possible to calculate a CSR position from traits. For this reason, during the 1990s it was suggested that selected trait measurements should instead be used as axes of strategy schemes (Westoby 1998). This frame-shift made international comparisons possible, initially for seed mass (Lord et al. 1995; Moles et al. 2005) and for a bundle of leaf traits that came to be known as leaf economics (Wright et al. 2004). In the mid-2000s, the Australia–New Zealand Network for Vegetation Function was one of the organisers for the international trait database TRY (Kattge et al. 2011). Subsequently a wide variety of global-scale trait analyses have appeared (Moles et al. 2007, 2009; Cornwell et al. 2008; Choat et al. 2012; Díaz et al. 2016; Kunstler et al. 2016), many led from Australia. Trait-based ecology has been a big trend over the past 25 years (Laughlin 2023; Westoby 2025).
Summation
Taking together these six strands of development in ecology as a discipline, a phase of rapid transformation can be seen in the 1960–1970s. Up to the 1950s, ecology was seen as a specialisation within either zoology or botany. During the 1950–1960s, the population regulation debate was crystallised, field experimental ecology emerged, coexistence equations developed into a theory of community coexistence and species richness, and ecosystem energy and nutrient transactions began to be measured. These were key components of new textbooks (Krebs 1972; Ricklefs 1973; and later Begon et al. 1986) that dominated the curriculum for the next 30 years, and thence to a substantial extent the research agenda also.
Since the 1990s, progress in ecology has taken the form of more extensive data and more complex analyses, more so than of new ways to think about ecosystems. A major trend has been a shift to global scales. An increasing proportion of research has been directed at consequences of climate change. Rich digital datasets have developed, including products from satellite imagery, point location records for species, and species lists and abundances from plots (Sabatini et al. 2021). These are survey-type data and are pervaded with cross-correlations. Models to interpret them have become more complex (e.g. Bruelheide et al. 2018; Soley-Guardia et al. 2024; Cai et al. 2025a), which helps to quantify the cross-correlations but does not resolve causation. A sense of dissatisfaction with inconsistent results from local studies has motivated disseminated projects by collaborative networks (e.g. Borer et al. 2014; Henry et al. 2022; Zanne et al. 2022), projects simple at each location, but repeated across many sites worldwide. Dissatisfaction with inconsistent results has also been a motivation for trait-based ecology at global scale (Strand 6, Fig. 3), seeking to understand different ecosystem processes through the predictive power of measurable species traits.
I think (as do others, e.g. Dormann and Mello 2023) that there is currently no consensus curriculum, no agreed priorities on what ecology should look like as a discipline. Different people’s priorities range all the way from field knowledge of botany or zoology, through pollutant dispersal and ecotoxicology, to population theory and community structure, to global budgets for carbon, water and nutrients, and to integration of ecology with human social science. With the emergence of online resources, printed textbooks are no longer the consensus force they once were. And ecology has not managed to develop a unifying online curriculum structure in the way that economics (https://www.core-econ.org/) and information technology (Alrumaih et al. 2018) have.
Future of ecology
Tendencies
The future of ecology will be shaped by two main tendencies. They are connected to each other. One tendency is that 50–100 years from now, many ecosystems will be substantially different. Global change is one driver. Warming by a global average of at least 3°C seems now more likely than not (IPCC 2023), with associated changes in atmospheric CO2 and in incidence of fires, extreme temperatures, and droughts. Human population and land clearing continue to increase. But other drivers will also be important (Table 1), such as large-scale replacement of livestock by cell culture products, genetic technologies for suppression of selected species, novel synthetic biology organisms, and robotics for agriculture and for fire management.
| Anthropogenic climate change | • Warming 3–5°C, faster water cycle, higher atmospheric CO2; broadly favourable for plant life, although usually for different plants from those currently at a given location | |
| • Disproportionate increase in heatwave events | ||
| • More extremes of drought and rainfall | ||
| • More frequent extreme fire weather, tending to produce shorter intervals between hot fires | ||
| Cell culture or other food synthesis superseding traditional agriculture | • Withdrawal of livestock from rangelands and dairy lands | |
| • Oil crops such as oil palm plantations replaced by lipid-producing yeasts grown on lignocellulosic residue (Parsons et al. 2020) | ||
| • Replacement of fish farming by cell culture | ||
| Synbio organisms for use in mixed cultures | Modified fermentations, e.g. for wine Biomining and bioremediation | |
| Novel habitats, either deliberately constructed or by-products | • ‘Living seawalls’ – engineered structures designed to support rocky-shore life | |
| • Bat caves in cities | ||
| • Offshore kelp farms attached to rafts | ||
| • Space station food production | ||
| • Plastisphere | ||
| • Solar arrays (e.g. discussion in Reinert 2024), wind farms | ||
| Altered physical geography | • Sea-level rise; consequences for coastal areas depend very much on whether sediment accretion can keep up (Saintilan et al. 2023) | |
| • Redistribution of riparian and floodplain sediments by more extreme rainfalls | ||
| Field robotics | • Ecological management over wide areas has always been limited by expense or by number of people. Management methods need to be self-powering. Traditional forms of self-powering are fire and grazing animals. Field robotics (Lipson and Sukkarieh 2023) powered by renewable energy sources creates a new situation potentially. Individual decision makers can be supported by many robotic operators in the field. | |
| • For example, drones can be designed to initiate fires and to emulate the information gathering and the decision process used by skilled practitioners of cultural fire and of hazard-reduction burning | ||
| • In agricultural ecosystems, field robotics relaxes the need for crops to ripen all at same time and be mass-harvested. Fruits and seed-heads can potentially be individually visualised and harvested. This opens the scope for wide-area agriculture that is more perennial and more mixed-species than most present-day cropping. | ||
| Targeted suppression of selected species populations by new genetic technologies | • For example buffel grass (Cenchrus ciliaris), an exotic introduction that in Australia is likely to shorten fire intervals with substantial consequences | |
| • Selected mosquito species: suppression to reduce disease transmission, but with consequences for freshwater food webs | ||
| • Suppression of feral cats for protection of small native mammals |
The second tendency will be a return from framing questions as risks to nature, i.e. from ecology as conservation and restoration, to first principles for ecosystem construction. Given a future world with changed physical environment and new opportunities via technology, ecology should be explaining from first principles what ecosystem structures can potentially emerge in a given situation.
Ecosystem structure from first principles
Ecosystem structure means two things mainly. First, it means foundation species or habitat-forming species, and the physical structure and habitat modification they provide. Ecosystems often are named after foundation species, as in grasslands, kelp forests and oyster beds. Second, where do energy and nutrients come from, and what is the destination of the organic matter produced, to herbivore–carnivore food chains, to decomposition food chains, to ecosystems elsewhere, or to fire?
At almost any site above freezing temperature, some sort of ecosystem will be found. In context of climate change, there is much talk of ecosystem collapse (Bergstrom et al. 2021; Canadell and Jackson 2021). But some form of productive life will develop in almost all habitats, and will continue to grow and multiply until something stops it. So ecologists ought to ask not only about persistence of the present ecosystem, but about what may come next. Basic principles (Box 1) begin from the idea that for any particular ecosystem we need to know where the resources come from. Does the energy to produce new organic matter come from photosynthesis (as it does for most terrestrial and surface-water ecosystems), or from other organic matter (as for headwater streams, benthos, sewage plants and biotech manufacturing), or from chemosynthesis (as in hot springs, hydrothermal vents and methane seeps)? Similarly, where do mineral nutrients come from?
| Box 1.Principles of ecosystem construction. These principles have much in common with the function-based ecosystem typology in Keithet al.(2022). They are expressed also in Fig. 4 via the boxes in red running down the centre of the schematic. |
|
| These principles can be contrasted with Box 2, which shows a typical list of principles of ecology from an introductory ecology curriculum. The principles given there are not incorrect, but they do serve to illustrate how the present curriculum does not add up to a system for predicting what kinds of ecosystem can be found in what kind of situation. |
Closely linked to the question where the resources come from is the nature of the disturbance regime and the succession in between disturbance events (Box 1). Some successions begin from an injection of organic matter (faecal deposit, dead whale, wine fermentation) and proceed by depletion of the resources. In water, some successions begin from an upwelling of nutrient-rich water that allows phytoplankton growth. On land, many successions begin with vegetation being cleared or cut or burned to ground level, and proceed by stems reaching for the light.
Third (Box 1), what ecological strategies are successful over time in the competition for those resources? In particular (and fourth in Box 1), do habitat-forming or foundation species develop a structure that modifies the microenvironment and provides habitat for other residents? And how do disturbance regimes or herbivory shape the physical framework that can accumulate?
The principles in Box 1 are illustrated via three examples. The examples illustrate (respectively) alternative states and new physical structures, new possibilities arising from synthetic biology, and new environments arising from climate change.
Illustration 1: kelp forests
Kelp forests are a classic case of alternative ecosystem states, and also an illustration of ecosystem possibilities arising from new physical structures. Rock substrate in the subtidal can potentially support a forest of kelp, brown algae that attach to rock, but can extend tens of metres towards the light (Principles 2 and 3 in Box 1, P2, P3). The habitat provided is an important refuge for smaller fish and invertebrates (P4). The carbon fixation or organic-matter output from the kelp is substantial. An alternative state for these rocky reefs is turf-forming algae, maintained by grazing sea urchins or herbivorous fish. Some of the fish or urchins are able to attack the holdfasts of the kelp, and so when these herbivores reach higher densities, the kelp forest can be cleared and converted to the turf-forming condition, sometimes also called an urchin barren. These alternative states can be mediated by predators on the sea urchins. In the northern hemisphere, human suppression of sea otters and of lobsters has allowed sea urchin populations to increase and kelp forests to be converted to urchin barrens (Filbee-Dexter and Scheibling 2014). On this basis, sea otters and lobsters have been called keystone species, although more logically it is the kelp that is the keystone of the ecosystem.
Rapid kelp growth demands mineral nutrient in the water (P1), and kelp species favour colder waters to varying degrees. Kelp can be killed by marine heatwaves. A 2011 heatwave contracted the Western Australian boundary of Ecklonia radiata 100 km poleward (Wernberg 2021), and kelp forests are contracting globally (Filbee-Dexter and Wernberg 2018). Following the Western Australian heatwave, herbivorous fish also established further south, preventing kelp re-establishment (Wernberg 2021).
Methods for wide-area re-establishment of kelp have been developed (‘green gravel’, Wood et al. 2024). However, these are not going to develop into kelp forests at locations where marine heatwaves and pressures from herbivores continue.
New kelp ecosystems are being constructed, potentially at scale, in the form of ‘ocean macroalgal afforestation’ (OMA), with kelp being grown from floating rafts or lines in offshore waters. Unless they are in upwelling situations in cool waters, this growth is likely to be restricted by supply of iron and other nutrients (Paine et al. 2023). It will be interesting to see what sort of fish and invertebrate communities they support, and under what circumstances herbivorous fish become a serious risk to them. It can be expected that their equatorward boundary will continue to be limited by temperature, backed by the tendency of warmer waters to hold fewer nutrients and the greater effectiveness of herbivorous fish in warmer water.
Illustration 2: wine fermentations
Wine fermentations are ecosystems that stand to be modified in future via application of biological technologies, especially new organisms created by synthetic biology.
Fermentations are depletion successions that begin from organic matter that includes sugars (P1). Other depletion successions operate in faecal deposits, cadavers and fallen tree trunks. Ripe fleshy fruits undergo fermentations naturally (Janzen 1977). Human-managed examples include cheese, wine, and yoghurt. The organic matter is processed anaerobically. The fermentation starting from crushed grapes and leading to wine is driven by a microbial assemblage made up of a mixture of yeasts plus bacteria. As well as using up sugars and accumulating alcohol (P2), this ecosystem produces various flavour and aroma compounds. The mixture of aromas decides how attractive the wine is to humans.
One suggested target for synthetic biology is to gather all the relevant genes together into a single yeast strain (Belda et al. 2021). If such a portmanteau yeast could be developed, would it be effective? Could such an ecosystem be constructed? This would depend on whether it maintained substantial abundance through the whole duration of the succession, in the face of competition from all the other yeasts entering on the surfaces of the harvested fruit (P3). Three distinct types of competitiveness are relevant. One is rapid multiplication to convert resources into DNA copies or evolutionary success. A second is the ability to sustain a population on much-depleted resource concentrations. The third is tolerance of toxins produced by competitors, with the most important toxin in this case being alcohol.
Among bacteria and among plants, rapid growth on plentiful resources demands different traits from those needed for persistence on depleted resources (Díaz et al. 2016; Westoby et al. 2021). Similarly in yeasts, it is very unlikely that a single organism can dominate throughout the fermentation succession. Desired aroma elements would need to be distributed into at least three different yeasts, perhaps more.
A range of other possibilities have been floated for improving wine fermentations via synthetic biology (Walker and Pretorius 2022). These include biosensor yeasts designed to signal faults such as volatile phenols, precursors of ethyl carbamate, or incomplete fermentations.
Illustration 3: vegetation on land
How do the principles of Box 1 work themselves out for natural vegetation on land? The key resources are light and water (P1). Some of the resulting photosynthate is used to acquire mineral nutrients (P1). The transpiration stream made possible by water input (rainfall and/or run-on), together with evaporative power of the atmosphere, limits the total amount of leaf area (leaf area index, LAI) that can be supported (Cai et al. 2025b; Zhou et al. 2025). Beyond some LAI, leaves at the bottom of the canopy do not receive enough light to return a profit to their plant (P2, P3). In most forests, this LAI is ~6, but vegetation with very steep leaf orientations, such as sugar cane crops, can go higher.
On land, herbivores rarely suppress LAI strongly below what transpiration and light can support. Hairston et al. (1960) observed not only that ‘the world is green’, but also that it was not self-evident why this should be so. Why should there not instead be terrestrial ecosystems with a few scraps of green pursued by a substantial standing crop of herbivores? This is, after all, the typical ecosystem structure in pelagic waters and on some rocky reefs. Hairston et al.’s (1960) answer was that in general on land, predators suppressed herbivore populations. More complex answers were proposed subsequently (Lawton and McNeill 1979).
Unless LAI is too low for significant shading, there is an arms race for light, with plants investing in vertical stems at the expense of foliage. Plants are the habitat-forming or foundation species in terrestrial ecosystems. Their stems and the vertical foliage profile develop a physical structure that influences light, diurnal temperature cycle, humidity, windspeed and lines of visibility. In many cases, they also literally provide habitat, via tree hollows, rolled leaves and litter on the ground (P4).
The habitat-forming or foundational plants are also strongly affected by how frequently there is substantial reduction of biomass, by fire, landslip, cyclone, human clearing or concentrated herbivory (P2). This disturbance regime is shaped partly by the physical environment, partly by human intervention and partly by the vegetation itself.
Currently dynamic global vegetation models (DGVMs) provide our best first-principles understanding for vegetation structure around the world (Strand 1 in Fig. 1). The LPJ-GUESS model (Smith et al. 2001, 2014; Hantson et al. 2020; Martín Belda et al. 2022), for example, simulates processes including plant growth, establishment, and mortality. Plant functional types (PFTs) compete for light and for water at different depths. This competition is mediated by mortality of established plants and by wildfires. It is arguable that some of the success of DGVMs is achieved through omission. For example, although there is no species diversity within each PFT, this does not lead to outbreaks of herbivores or pathogens in the model, but that is because animals and pathogens do not appear in the model at all, rather than because species diversity or any other mechanism limits their impact. In summary, what DGVMs include, and also what they do not include, expresses the state of the art for first-principles understanding of vegetation structure.
Conclusion
The argument here has been that the central research aim for ecology should be capacity to predict what ecosystem structures are possible, in any given physical situation and with known sources for energy, water and mineral nutrients (Fig. 4). Similarly, curriculum for introductory ecology units should be organised around those principles for what ecosystem structures are achievable. This is a rearrangement, it does not mean discarding existing knowledge. In Fig. 4, the principles for ecosystem construction from Box 1, run down the centre. Fig. 4 also shows the six strands of ecological research described in the first half of this paper. Each of them relates in one way or another to the principles for ecosystem structure.
Schematic for the proposition that future ecology research and curriculum should focus on principles for how ecosystems are constructed, that is, what sort of ecosystem structure is possible in any particular setting. Those principles are in the red boxes running down the centre of the schematic. The six strands of ecology that were discussed earlier in this paper are in blue. They continue to be important as inputs to, or outputs from, the principles for ecosystem construction.
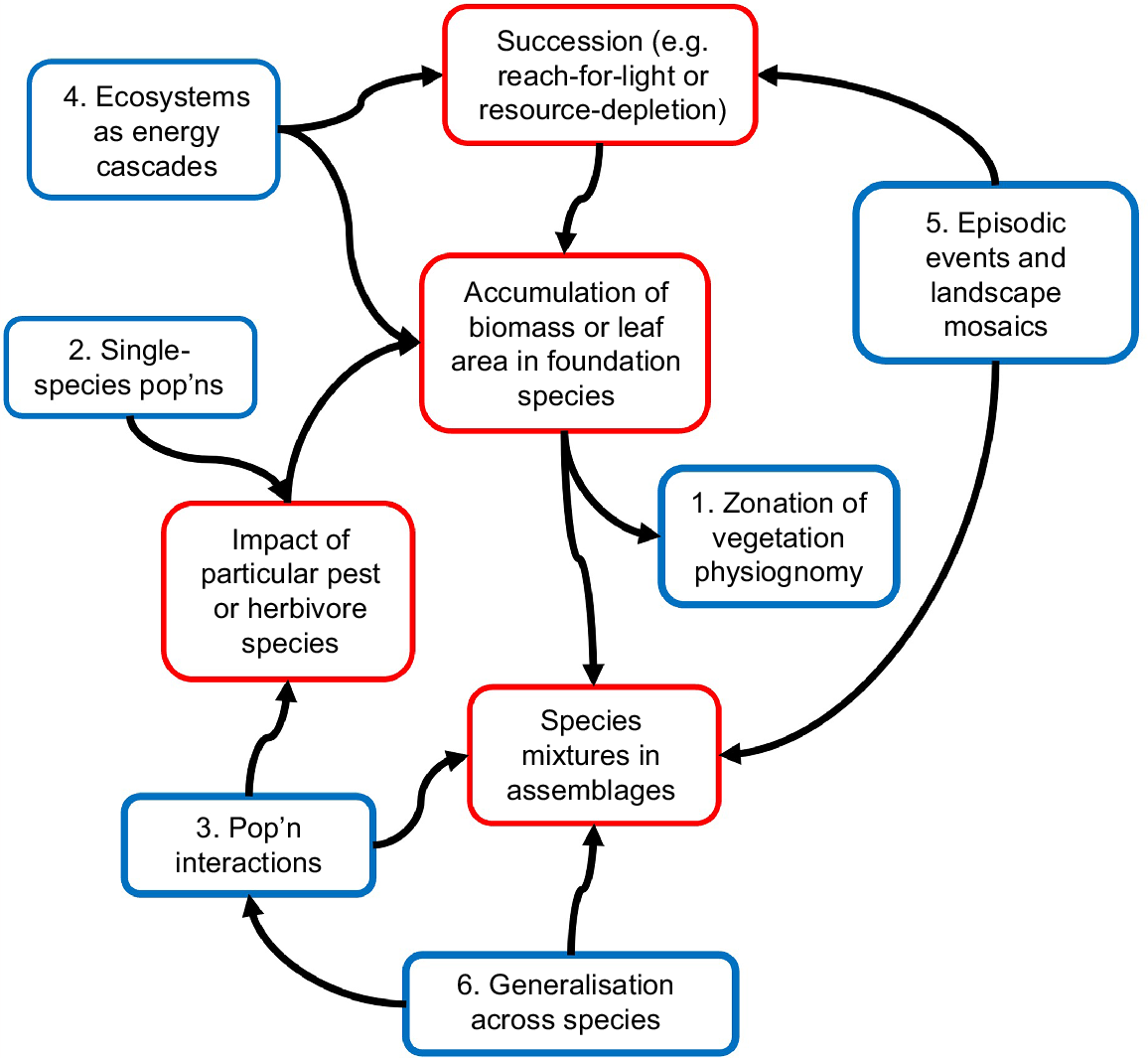
Future physical situations that do not yet exist are valuable challenges. They prevent us working simply from observation of the present-day. Together with a focus on principles for ecosystem construction, they give students a sense that ecology can be a powerful world-building discipline, not just a Cassandra-science.
Acknowledgements
Warm thanks to John Morgan for suggesting this Reflection. David Coleman, Martyna Kotowska, Mark Ooi, Francesco Santi, Julian Schrader, Reuben Stone, and an anonymous reviewer provided very helpful comments on a draft.
References
Beadle NCW (1954) Soil phosphate and the delimitation of plant communities in eastern Australia. Ecology 35, 370-375.
| Crossref | Google Scholar |
Beadle NCW (1966) Soil phosphate and its role in molding segments of the Australian flora and vegetation, with special reference to xeromorphy and sclerophylly. Ecology 47, 992-1007.
| Crossref | Google Scholar |
Belda I, Williams TC, de Celis M, Paulsen IT, Pretorius IS (2021) Seeding the idea of encapsulating a representative synthetic metagenome in a single yeast cell. Nature Communications 12(1), 1599.
| Crossref | Google Scholar |
Bergstrom DM, Wienecke BC, van den Hoff J, Hughes L, Lindenmayer DB, Ainsworth TD, Baker CM, Bland L, Bowman DMJS, Brooks ST, Canadell JG, Constable AJ, Dafforn KA, Depledge MH, Dickson CR, Duke NC, Helmstedt KJ, Holz A, Johnson CR, McGeoch MA, Melbourne-Thomas J, Morgain R, Nicholson E, Prober SM, Raymond B, Ritchie EG, Robinson SA, Ruthrof KX, Setterfield SA, Sgrò CM, Stark JS, Travers T, Trebilco R, Ward DFL, Wardle GM, Williams KJ, Zylstra PJ, Shaw JD (2021) Combating ecosystem collapse from the tropics to the Antarctic. Global Change Biology 27(9), 1692-1703.
| Crossref | Google Scholar | PubMed |
Bond WJ, Woodward FI, Midgley GF (2005) The global distribution of ecosystems in a world without fire. New Phytologist 165(2), 525-538.
| Crossref | Google Scholar | PubMed |
Booth TH, Nix HA, Busby JR, Hutchinson MF (2014) BIOCLIM: the first species distribution modelling package, its early applications and relevance to most current MaxEnt studies. Diversity and Distributions 20(1), 1-9.
| Crossref | Google Scholar |
Borer ET, Harpole WS, Adler PB, Lind EM, Orrock JL, Seabloom EW, Smith MD (2014) Finding generality in ecology: a model for globally distributed experiments. Methods in Ecology and Evolution 5(1), 65-73.
| Crossref | Google Scholar |
Brown JH (2014) Why are there so many species in the tropics? Journal of Biogeography 41(1), 8-22.
| Crossref | Google Scholar | PubMed |
Bruelheide H, Dengler J, Purschke O, Lenoir J, Jiménez-Alfaro B, Hennekens SM, Botta-Dukát Z, Chytrý M, Field R, Jansen F, Kattge J, Pillar VD, Schrodt F, Mahecha MD, Peet RK, Sandel B, van Bodegom P, Altman J, Alvarez-Dávila E, Arfin Khan MAS, Attorre F, Aubin I, Baraloto C, Barroso JG, Bauters M, Bergmeier E, Biurrun I, Bjorkman AD, Blonder B, Čarni A, Cayuela L, Černý T, Cornelissen JHC, Craven D, Dainese M, Derroire G, Sanctis MD, Díaz S, Doležal J, Farfan-Rios W, Feldpausch TR, Fenton NJ, Garnier E, Guerin GR, Gutiérrez AG, Haider S, Hattab T, Henry G, Hérault B, Higuchi P, Hölzel N, Homeier J, Jentsch A, Jürgens N, Kącki Z, Karger DN, Kessler M, Kleyer M, Knollová I, Korolyuk AY, Kühn I, Laughlin DC, Lens F, Loos J, Louault F, Lyubenova MI, Malhi Y, Marcenò C, Mencuccini M, Müller JV, Munzinger J, Myers-Smith IH, Neill DA, Niinemets Ü, Orwin KH, Ozinga WA, Penuelas J, Pérez-Haase A, Petřík P, Phillips OL, Pärtel M, Reich PB, Römermann C, Rodrigues AV, Sabatini FM, Sardans J, Schmidt M, Seidler G, Silva Espejo JE, Silveira M, Smyth A, Sporbert M, Svenning J-C, Tang Z, Thomas R, Tsiripidis I, Vassilev K, Violle C, Virtanen R, Weiher E, Welk E, Wesche K, Winter M, Wirth C, Jandt U (2018) Global trait–environment relationships of plant communities. Nature Ecology & Evolution 2, 1906-1917.
| Crossref | Google Scholar | PubMed |
Cai L, Kreft H, Denelle P, Taylor A, Craven D, Dawson W, Essl F, van Kleunen M, Pergl J, Pyšek P, Winter M, Cabezas FJ, Wagner V, Pelser PB, Wieringa JJ, Weigelt P (2025a) Environmental filtering, not dispersal history, explains global patterns of phylogenetic turnover in seed plants at deep evolutionary timescales. Nature Ecology & Evolution 9(2), 314-324.
| Crossref | Google Scholar |
Cai W, Zhu Z, Harrison SP, Ryu Y, Wang H, Zhou B, Prentice IC (2025b) A unifying principle for global greenness patterns and trends. Communications Earth & Environment 6(1), 1-11.
| Crossref | Google Scholar |
Carpenter S, Walker B, Anderies JM, Abel N (2001) From metaphor to measurement: resilience of what to what? Ecosystems 4(8), 765-781.
| Crossref | Google Scholar |
Chesson P (1994) Multispecies competition in variable environments. Theoretical Population Biology 45(3), 227-276.
| Crossref | Google Scholar |
Choat B, Jansen S, Brodribb TJ, Cochard H, Delzon S, Bhaskar R, Bucci SJ, Feild TS, Gleason SM, Hacke UG, Jacobsen AL, Lens F, Maherali H, Martínez-Vilalta J, Mayr S, Mencuccini M, Mitchell PJ, Nardini A, Pittermann J, Pratt RB, Sperry JS, Westoby M, Wright IJ, Zanne AE (2012) Global convergence in the vulnerability of forests to drought. Nature 491(7426), 752-755.
| Crossref | Google Scholar | PubMed |
Connell JH (1961) The influence of interspecific competition and other factors on the distribution of the barnacle Chthamalus Stellatus. Ecology 42(4), 710-723.
| Crossref | Google Scholar |
Connell JH (1978) Diversity in tropical rain forests and coral reefs. Science 199(4335), 1302-1310.
| Crossref | Google Scholar | PubMed |
Connell JH (1983) On the prevalence and relative importance of interspecific competition: evidence from field experiments. The American Naturalist 122, 661-696.
| Crossref | Google Scholar |
Connell JH, Slatyer RO (1977) Mechanisms of succession in natural communities and their role in community stability and organization. The American Naturalist 111, 1119-1144.
| Crossref | Google Scholar |
Cornwell WK, Cornelissen JHC, Amatangelo K, Dorrepaal E, Eviner VT, Godoy O, Hobbie SE, Hoorens B, Kurokawa H, Pérez-Harguindeguy N, Quested HM, Santiago LS, Wardle DA, Wright IJ, Aerts R, Allison SD, van Bodegom P, Brovkin V, Chatain A, Callaghan TV, Diaz S, Garnier E, Gurvich DE, Kazakou E, Klein JA, Read J, Reich PB, Soudzilovskaia NA, Vaieretti MV, Westoby M (2008) Plant species traits are the predominant control on litter decomposition rates within biomes worldwide. Ecology Letters 11(10), 1065-1071.
| Crossref | Google Scholar | PubMed |
Díaz S, Kattge J, Cornelissen JHC, Wright IJ, Lavorel S, Dray S, Reu B, Kleyer M, Wirth C, Colin Prentice I, Garnier E, Bönisch G, Westoby M, Poorter H, Reich PB, Moles AT, Dickie J, Gillison AN, Zanne AE, Chave J, Joseph Wright S, Sheremet’ev SN, Jactel H, Baraloto C, Cerabolini B, Pierce S, Shipley B, Kirkup D, Casanoves F, Joswig JS, Günther A, Falczuk V, Rüger N, Mahecha MD, Gorné LD (2016) The global spectrum of plant form and function. Nature 529(7585), 167-171.
| Crossref | Google Scholar | PubMed |
Divíšek J, Chytrý M, Beckage B, Gotelli NJ, Lososová Z, Pyšek P, Richardson DM, Molofsky J (2018) Similarity of introduced plant species to native ones facilitates naturalization, but differences enhance invasion success. Nature Communications 9(1), 4631.
| Crossref | Google Scholar |
Dormann CF, Mello MAR (2023) Why we need a canonical ecology curriculum. Basic and Applied Ecology 71, 98-109.
| Crossref | Google Scholar |
Elith J, Kearney M, Phillips S (2010) The art of modelling range-shifting species. Methods in Ecology and Evolution 1(4), 330-342.
| Crossref | Google Scholar |
Farquhar GD, von Caemmerer S, Berry JA (1980) A biochemical model of photosynthetic CO2 assimilation in leaves of C3 species. Planta 149(1), 78-90.
| Crossref | Google Scholar | PubMed |
Ferrer-Paris JR, Keith DA (2022) Fire ecology traits for plants: a database for fire research and management. Available at http://13.54.3.205/index
Filbee-Dexter K, Scheibling RE (2014) Sea urchin barrens as alternative stable states of collapsed kelp ecosystems. Marine Ecology Progress Series 495, 1-25.
| Crossref | Google Scholar |
Filbee-Dexter K, Wernberg T (2018) Rise of turfs: a new battlefront for globally declining kelp forests. BioScience 68(2), 64-76.
| Crossref | Google Scholar |
Franklin O, Harrison SP, Dewar R, Farrior CE, Brännström Å, Dieckmann U, Pietsch S, Falster D, Cramer W, Loreau M, Wang H, Mäkelä A, Rebel KT, Meron E, Schymanski SJ, Rovenskaya E, Stocker BD, Zaehle S, Manzoni S, van Oijen M, Wright IJ, Ciais P, van Bodegom PM, Peñuelas J, Hofhansl F, Terrer C, Soudzilovskaia NA, Midgley G, Prentice IC (2020) Organizing principles for vegetation dynamics. Nature Plants 6(5), 444-453.
| Crossref | Google Scholar | PubMed |
Funk JL, Cleland EE, Suding KN, Zavaleta ES (2008) Restoration through reassembly: plant traits and invasion resistance. Trends in Ecology & Evolution 23(12), 695-703.
| Crossref | Google Scholar | PubMed |
Grime JP (1974) Vegetation classification by reference to strategies. Nature 250, 26-31.
| Crossref | Google Scholar |
Grime JP, Hunt R (1975) Relative growth-rate: its range and adaptive significance in a local flora. The Journal of Ecology 63, 393-422.
| Crossref | Google Scholar |
Guimarães PR (2020) The structure of ecological networks across levels of organization. Annual Review of Ecology, Evolution, and Systematics 51, 433-460.
| Crossref | Google Scholar |
Hairston NG, Smith FE, Slobodkin LB (1960) Community structure, population control, and competition. The American Naturalist 94(879), 421-425.
| Crossref | Google Scholar |
Hantson S, Arneth A, Harrison SP, Kelley DI, Prentice IC, Rabin SS, Archibald S, Mouillot F, Arnold SR, Artaxo P, Bachelet D, Ciais P, Forrest M, Friedlingstein P, Hickler T, Kaplan JO, Kloster S, Knorr W, Lasslop G, Li F, Mangeon S, Melton JR, Meyn A, Sitch S, Spessa A, van der Werf GR, Voulgarakis A, Yue C (2016) The status and challenge of global fire modelling. Biogeosciences 13(11), 3359-3375.
| Crossref | Google Scholar |
Hantson S, Kelley DI, Arneth A, Harrison SP, Archibald S, Bachelet D, Forrest M, Hickler T, Lasslop G, Li F, Mangeon S, Melton JR, Nieradzik L, Rabin SS, Prentice IC, Sheehan T, Sitch S, Teckentrup L, Voulgarakis A, Yue C (2020) Quantitative assessment of fire and vegetation properties in simulations with fire-enabled vegetation models from the Fire Model Intercomparison Project. Geoscientific Model Development 13(7), 3299-3318.
| Crossref | Google Scholar |
Harper JL (1967) A Darwinian approach to plant ecology. Journal of Ecology 55(2), 247-270.
| Crossref | Google Scholar |
Harrison SP, Cramer W, Franklin O, Prentice IC, Wang H, Brännström Å, de Boer H, Dieckmann U, Joshi J, Keenan TF, Lavergne A, Manzoni S, Mengoli G, Morfopoulos C, Peñuelas J, Pietsch S, Rebel KT, Ryu Y, Smith NG, Stocker BD, Wright IJ (2021) Eco-evolutionary optimality as a means to improve vegetation and land-surface models. New Phytologist 231(6), 2125-2141.
| Crossref | Google Scholar | PubMed |
Haverd V, Smith B, Nieradzik L, Briggs PR, Woodgate W, Trudinger CM, Canadell JG, Cuntz M (2018) A new version of the CABLE land surface model (Subversion revision r4601) incorporating land use and land cover change, woody vegetation demography, and a novel optimisation-based approach to plant coordination of photosynthesis. Geoscientific Model Development 11(7), 2995-3026.
| Crossref | Google Scholar |
Haxeltine A, Prentice IC (1996) BIOME3: an equilibrium terrestrial biosphere model based on ecophysiological constraints, resource availability, and competition among plant functional types. Global Biogeochemical Cycles 10, 693-709.
| Crossref | Google Scholar |
Henry GHR, Hollister RD, Klanderud K, Björk RG, Bjorkman AD, Elphinstone C, Jónsdóttir IS, Molau U, Petraglia A, Oberbauer SF, Rixen C, Wookey PA (2022) The International Tundra Experiment (ITEX): 30 years of research on tundra ecosystems. Arctic Science 8(3), 550-571.
| Crossref | Google Scholar |
HilleRisLambers J, Adler PB, Harpole WS, Levine JM, Mayfield MM (2012) Rethinking community assembly through the lens of coexistence theory. Annual Review of Ecology, Evolution, and Systematics 43(1), 227-248.
| Crossref | Google Scholar |
Hutchinson GE (1959) Homage to Santa Rosalia or why are there so many kinds of animals? The American Naturalist 93(870), 145-159.
| Crossref | Google Scholar |
Jackson WD (1968) Fire, air, water and earth – an elemental ecology of Tasmania. Proceedings of the Ecological Society of Australia 3, 9-16.
| Google Scholar |
Janzen DH (1977) Why fruits rot, seeds mold, and meat spoils. The American Naturalist 111, 691-713.
| Crossref | Google Scholar |
Kattge J, Díaz S, Lavorel S, Prentice IC, Leadley P, Bönisch G, Garnier E, Westoby M, Reich PB, Wright IJ, Cornelissen JHC, Violle C, Harrison SP, Van Bodegom PM, Reichstein M, Enquist BJ, Soudzilovskaia NA, Ackerly DD, Anand M, Atkin O, Bahn M, Baker TR, Baldocchi D, Bekker R, Blanco CC, Blonder B, Bond WJ, Bradstock R, Bunker DE, Casanoves F, Cavender-Bares J, Chambers JQ, Chapin FS, III, Chave J, Coomes D, Cornwell WK, Craine JM, Dobrin BH, Duarte L, Durka W, Elser J, Esser G, Estiarte M, Fagan WF, Fang J, Fernández-Méndez F, Fidelis A, Finegan B, Flores O, Ford H, Frank D, Freschet GT, Fyllas NM, Gallagher RV, Green WA, Gutierrez AG, Hickler T, Higgins SI, Hodgson JG, Jalili A, Jansen S, Joly CA, Kerkhoff AJ, Kirkup D, Kitajima K, Kleyer M, Klotz S, Knops JMH, Kramer K, Kühn I, Kurokawa H, Laughlin D, Lee TD, Leishman M, Lens F, Lenz T, Lewis SL, Lloyd J, Llusià J, Louault F, Ma S, Mahecha MD, Manning P, Massad T, Medlyn BE, Messier J, Moles AT, Müller SC, Nadrowski K, Naeem S, Niinemets Ü, Nöllert S, Nüske A, Ogaya R, Oleksyn J, Onipchenko VG, Onoda Y, Ordoñez J, Overbeck G, Ozinga WA, Patiño S, Paula S, Pausas JG, Peñuelas J, Phillips OL, Pillar V, Poorter H, Poorter L, POSCHLOD P, Prinzing A, Proulx R, Rammig A, Reinsch S, Reu B, Sack L, Salgado-Negret B, Sardans J, Shiodera S, Shipley B, Siefert A, Sosinski E, Soussana J-F, Swaine E, Swenson N, Thompson K, Thornton P, Waldram M, Weiher E, White M, White S, Wright SJ, Yguel B, Zaehle S, Zanne AE, Wirth C (2011) TRY – a global database of plant traits. Global Change Biology 17(9), 2905-2935.
| Crossref | Google Scholar |
Keith DA, Ferrer-Paris JR, Nicholson E, Bishop MJ, Polidoro BA, Ramirez-Llodra E, Tozer MG, Nel JL, Mac Nally R, Gregr EJ, Watermeyer KE, Essl F, Faber-Langendoen D, Franklin J, Lehmann CER, Etter A, Roux DJ, Stark JS, Rowland JA, Brummitt NA, Fernandez-Arcaya UC, Suthers IM, Wiser SK, Donohue I, Jackson LJ, Pennington RT, Iliffe TM, Gerovasileiou V, Giller P, Robson BJ, Pettorelli N, Andrade A, Lindgaard A, Tahvanainen T, Terauds A, Chadwick MA, Murray NJ, Moat J, Pliscoff P, Zager I, Kingsford RT (2022) A function-based typology for Earth’s ecosystems. Nature 610(7932), 513-518.
| Crossref | Google Scholar | PubMed |
Kraft NJB, Valencia R, Ackerly DD (2008) Functional traits and niche-based tree community assembly in an amazonian forest. Science 322(5901), 580-582.
| Crossref | Google Scholar | PubMed |
Kunstler G, Falster D, Coomes DA, Hui F, Kooyman RM, Laughlin DC, Poorter L, Vanderwel M, Vieilledent G, Wright SJ, Aiba M, Baraloto C, Caspersen J, Cornelissen JHC, Gourlet-Fleury S, Hanewinkel M, Herault B, Kattge J, Kurokawa H, Onoda Y, Peñuelas J, Poorter H, Uriarte M, Richardson S, Ruiz-Benito P, Sun I-F, Ståhl G, Swenson NG, Thompson J, Westerlund B, Wirth C, Zavala MA, Zeng H, Zimmerman JK, Zimmermann NE, Westoby M (2016) Plant functional traits have globally consistent effects on competition. Nature 529(7585), 204-207.
| Crossref | Google Scholar | PubMed |
Lawton JH, McNeill S (1979) Between the devil and the deep blue sea: on the problem of being an herbivore. Symposium of the British Ecological Society 20, 223-244.
| Google Scholar |
Lindeman RL (1942) The trophic – dynamic aspect of ecology. Ecology 23(4), 399-417.
| Crossref | Google Scholar |
Lipson H, Sukkarieh S (2023) Robots may transform the way we produce and prepare food. Nature Reviews Bioengineering 1(11), 795-798.
| Crossref | Google Scholar |
Lord J, Westoby M, Leishman M (1995) Seed size and phylogeny in six temperate floras: constraints, niche conservatism, and adaptation. The American Naturalist 146(3), 349-364.
| Crossref | Google Scholar |
Loveless AR (1961) A nutritional interpretation of sclerophylly based on differences in the chemical composition of sclerophyllous and mesophytic leaves. Annals of Botany 25, 168-184.
| Crossref | Google Scholar |
MacArthur RH (1965) Patterns of species diversity. Biological Reviews 40, 510-533.
| Crossref | Google Scholar |
Macarthur R, Levins R (1967) The limiting similarity, convergence, and divergence of coexisting species. The American Naturalist 101(921), 377-385.
| Crossref | Google Scholar |
Martín Belda D, Anthoni P, Wårlind D, Olin S, Schurgers G, Tang J, Smith B, Arneth A (2022) LPJ-GUESS/LSMv1.0: a next-generation land surface model with high ecological realism. Geoscientific Model Development 15(17), 6709-6745.
| Crossref | Google Scholar |
Moles AT, Ackerly DD, Webb CO, Tweddle JC, Dickie JB, Westoby M (2005) A brief history of seed size. Science 307(5709), 576-580.
| Crossref | Google Scholar | PubMed |
Moles AT, Ackerly DD, Tweddle JC, Dickie JB, Smith R, Leishman MR, Mayfield MM, Pitman A, Wood JT, Westoby M (2007) Global patterns in seed size. Global Ecology and Biogeography 16(1), 109-116.
| Crossref | Google Scholar |
Moles AT, Warton DI, Warman L, Swenson NG, Laffan SW, Zanne AE, Pitman A, Hemmings FA, Leishman MR (2009) Global patterns in plant height. Journal of Ecology 97(5), 923-932.
| Crossref | Google Scholar |
Nicholson AJ (1933) Supplement: the balance of animal populations. Journal of Animal Ecology 2(1), 131-178.
| Crossref | Google Scholar |
Nicholson AJ (1954) An outline of the dynamics of animal populations. Australian Journal of Zoology 2(1), 9-65.
| Crossref | Google Scholar |
Noble IR, Slatyer RO (1980) The use of vital attributes to predict successional changes in plant communities subject to recurrent disturbances. Vegetatio 43, 5-21.
| Crossref | Google Scholar |
Paine ER, Boyd PW, Strzepek RF, Ellwood M, Brewer EA, Diaz-Pulido G, Schmid M, Hurd CL (2023) Iron limitation of kelp growth may prevent ocean afforestation. Communications Biology 6(1), 607.
| Crossref | Google Scholar |
Paquette A, Hargreaves AL (2021) Biotic interactions are more often important at species’ warm versus cool range edges. Ecology Letters 24(11), 2427-2438.
| Crossref | Google Scholar | PubMed |
Parsons S, Raikova S, Chuck CJ (2020) The viability and desirability of replacing palm oil. Nature Sustainability 3(6), 412-418.
| Crossref | Google Scholar |
Phillips BL, Shine R (2004) Adapting to an invasive species: toxic cane toads induce morphological change in Australian snakes. Proceedings of the National Academy of Sciences 101(49), 17150-17155.
| Crossref | Google Scholar |
Phillips BL, Brown GP, Webb JK, Shine R (2006) Invasion and the evolution of speed in toads. Nature 439(7078), 803.
| Crossref | Google Scholar | PubMed |
Pianka ER (1966) Latitudinal gradients in species diversity: a review of concepts. The American Naturalist 100, 33-46.
| Crossref | Google Scholar |
Pimentel D (1968) Population regulation and genetic feedback. Science 159(3822), 1432-1437.
| Crossref | Google Scholar | PubMed |
Prentice IC, Cramer W, Harrison SP, Leemans R, Monserud RA, Solomon AM (1992) Special Paper: a global biome model based on plant physiology and dominance, soil properties and climate. Journal of Biogeography 19, 117-134.
| Crossref | Google Scholar |
Prentice IC, Dong N, Gleason SM, Maire V, Wright IJ (2014) Balancing the costs of carbon gain and water transport: testing a new theoretical framework for plant functional ecology. Ecology Letters 17(1), 82-91.
| Crossref | Google Scholar | PubMed |
Reinert M (2024) How science is helping farmers to find a balance between agriculture and solar farms. Nature Spotlight France.
| Crossref | Google Scholar |
Rockström J, Steffen W, Noone K, Persson A, Chapin FS, Lambin EF, Lenton TM, Scheffer M, Folke C, Schellnhuber HJ, Nykvist B, de Wit CA, Hughes T, van der Leeuw S, Rodhe H, Sörlin S, Snyder PK, Costanza R, Svedin U, Falkenmark M, Karlberg L, Corell RW, Fabry VJ, Hansen J, Walker B, Liverman D, Richardson K, Crutzen P, Foley JA (2009) A safe operating space for humanity. Nature 461, 472-475.
| Crossref | Google Scholar | PubMed |
Roxburgh SH, Wood SW, Mackey BG, Woldendorp G, Gibbons P (2006) Assessing the carbon sequestration potential of managed forests: a case study from temperate Australia. Journal of Applied Ecology 43(6), 1149-1159.
| Crossref | Google Scholar |
Sabatini FM, Lenoir J, Hattab T, Arnst EA, Chytrý M, Dengler J, De Ruffray P, Hennekens SM, Jandt U, Jansen F, Jiménez-Alfaro B, Kattge J, Levesley A, Pillar VD, Purschke O, Sandel B, Sultana F, Aavik T, Aćić S, Acosta ATR, Agrillo E, Alvarez M, Apostolova I, Arfin Khan MAS, Arroyo L, Attorre F, Aubin I, Banerjee A, Bauters M, Bergeron Y, Bergmeier E, Biurrun I, Bjorkman AD, Bonari G, Bondareva V, Brunet J, Čarni A, Casella L, Cayuela L, Černý T, Chepinoga V, Csiky J, Ćušterevska R, De Bie E, de Gasper AL, De Sanctis M, Dimopoulos P, Dolezal J, Dziuba T, El-Sheikh MAE-RM, Enquist B, Ewald J, Fazayeli F, Field R, Finckh M, Gachet S, Galán-de-Mera A, Garbolino E, Gholizadeh H, Giorgis M, Golub V, Alsos IG, Grytnes J-A, Guerin GR, Gutiérrez AG, Haider S, Hatim MZ, Hérault B, Hinojos Mendoza G, Hölzel N, Homeier J, Hubau W, Indreica A, Janssen JAM, Jedrzejek B, Jentsch A, Jürgens N, Kącki Z, Kapfer J, Karger DN, Kavgacı A, Kearsley E, Kessler M, Khanina L, Killeen T, Korolyuk A, Kreft H, Kühl HS, Kuzemko A, Landucci F, Lengyel A, Lens F, Lingner DV, Liu H, Lysenko T, Mahecha MD, Marcenò C, Martynenko V, Moeslund JE, Monteagudo Mendoza A, Mucina L, Müller JV, Munzinger J, Naqinezhad A, Noroozi J, Nowak A, Onyshchenko V, Overbeck GE, Pärtel M, Pauchard A, Peet RK, Peñuelas J, Pérez-Haase A, Peterka T, Petřík P, Peyre G, Phillips OL, Prokhorov V, Rašomavičius V, Revermann R, Rivas-Torres G, Rodwell JS, Ruprecht E, Rūsiņa S, Samimi C, Schmidt M, Schrodt F, Shan H, Shirokikh P, Šibík J, Šilc U, Sklenář P, Škvorc Ž, Sparrow B, Sperandii MG, Stančić Z, Svenning J-C, Tang Z, Tang CQ, Tsiripidis I, Vanselow KA, Vásquez Martínez R, Vassilev K, Vélez-Martin E, Venanzoni R, Vibrans AC, Violle C, Virtanen R, von Wehrden H, Wagner V, Walker DA, Waller DM, Wang H-F, Wesche K, Whitfeld TJS, Willner W, Wiser SK, Wohlgemuth T, Yamalov S, Zobel M, Bruelheide H (2021) sPlotOpen – An environmentally balanced, open-access, global dataset of vegetation plots. Global Ecology and Biogeography 30(9), 1740-1764.
| Crossref | Google Scholar |
Saintilan N, Horton B, Törnqvist TE, Ashe EL, Khan NS, Schuerch M, Perry C, Kopp RE, Garner GG, Murray N, Rogers K, Albert S, Kelleway J, Shaw TA, Woodroffe CD, Lovelock CE, Goddard MM, Hutley LB, Kovalenko K, Feher L, Guntenspergen G (2023) Widespread retreat of coastal habitat is likely at warming levels above 1.5°C. Nature 621(7977), 112-119.
| Crossref | Google Scholar | PubMed |
Sale PF (1974) Overlap in resource use, and interspecific competition. Oecologia 17(3), 245-256.
| Crossref | Google Scholar | PubMed |
Sale PF (1978) Coexistence of coral reef fishes – a lottery for living space. Environmental Biology of Fishes 3(1), 85-102.
| Crossref | Google Scholar |
Schoener TW (1983) Field experiments on interspecific competition. The American Naturalist 122, 240-285.
| Crossref | Google Scholar |
Smith DMS, Morton SR (1990) A framework for the ecology of arid Australia. Journal of Arid Environments 18, 255-278.
| Crossref | Google Scholar |
Smith B, Prentice IC, Sykes MT (2001) Representation of vegetation dynamics in the modelling of terrestrial ecosystems: comparing two contrasting approaches within European climate space. Global Ecology and Biogeography 10(6), 621-637.
| Crossref | Google Scholar |
Smith B, Wårlind D, Arneth A, Hickler T, Leadley P, Siltberg J, Zaehle S (2014) Implications of incorporating N cycling and N limitations on primary production in an individual-based dynamic vegetation model. Biogeosciences 11(7), 2027-2054.
| Crossref | Google Scholar |
Soley-Guardia M, Alvarado-Serrano DF, Anderson RP (2024) Top ten hazards to avoid when modeling species distributions: a didactic guide of assumptions, problems, and recommendations. Ecography 2024(4), e06852.
| Crossref | Google Scholar |
Thuiller W, Lafourcade B, Engler R, Araújo MB (2009) BIOMOD – a platform for ensemble forecasting of species distributions. Ecography 32(3), 369-373.
| Crossref | Google Scholar |
Volterra V (1926) Variazioni e fluttuazioni del numero d’individui in specie animali conviventi. Memoria della Reale Accademia Nazionale dei Lincei 2, 31-113 [In Italian].
| Google Scholar |
Walker RSK, Pretorius IS (2022) Synthetic biology for the engineering of complex wine yeast communities. Nature Food 3(4), 249-254.
| Crossref | Google Scholar | PubMed |
Walker B, Westoby M (2011) States and transitions: the trajectory of an idea, 1970–2010. Israel Journal of Ecology and Evolution 57, 17-22.
| Crossref | Google Scholar |
Weiher E, van der Werf A, Thompson K, Roderick M, Garnier E, Eriksson O (1999) Challenging Theophrastus: a common core list of plant traits for functional ecology. Journal of Vegetation Science 10(5), 609-620.
| Crossref | Google Scholar |
Westoby M (1997) What does ‘ecology’ mean? Trends in Ecology & Evolution 12(4), 166.
| Crossref | Google Scholar |
Westoby M (1998) A leaf-height-seed (LHS) plant ecology strategy scheme. Plant and Soil 199, 213-227.
| Crossref | Google Scholar |
Westoby M (2022) Field experiments on mechanisms influencing species boundary movement under climate change. Plant and Soil 476(1), 527-534.
| Crossref | Google Scholar |
Westoby M (2025) Trait-based ecology, trait-free ecology, and in between. New Phytologist 245(1), 33-39.
| Crossref | Google Scholar | PubMed |
Westoby M, Walker B, Noy-Meir I (1989) Opportunistic management for rangelands not at equilibrium. Journal of Range Management 42, 266-274.
| Crossref | Google Scholar |
Westoby M, Gillings MR, Madin JS, Nielsen DA, Paulsen IT, Tetu SG (2021) Trait dimensions in bacteria and archaea compared to vascular plants. Ecology Letters 24, 1487-1504.
| Crossref | Google Scholar | PubMed |
Wood GV, Filbee-Dexter K, Coleman MA, Valckenaere J, Aguirre JD, Bentley PM, Carnell P, Dawkins PD, Dykman LN, Earp HS, Ennis LB, Francis P, Franco JN, Hayford H, Lamb JB, Ling SD, Layton C, Lis E, Masters B, Miller N, Moore PJ, Neufeld C, Pocklington JB, Smale D, Stahl F, Starko S, Steel SC, Verbeek J, Vergés A, Wilding CM, Wernberg T (2024) Upscaling marine forest restoration: challenges, solutions and recommendations from the Green Gravel Action Group. Frontiers in Marine Science 11, 1364263.
| Crossref | Google Scholar |
Woodward FI, Smith TM, Emanuel WR (1995) A global land primary productivity and phytogeography model. Global Biogeochemical Cycles 9(4), 471-490.
| Crossref | Google Scholar |
Wright IJ, Reich PB, Westoby M (2003) Least-cost input mixtures of water and nitrogen for photosynthesis. The American Naturalist 161(1), 98-111.
| Crossref | Google Scholar | PubMed |
Wright IJ, Reich PB, Westoby M, Ackerly DD, Baruch Z, Bongers F, Cavender-Bares J, Chapin T, Cornelissen JHC, Diemer M, Flexas J, Garnier E, Groom PK, Gulias J, Hikosaka K, Lamont BB, Lee T, Lee W, Lusk C, Midgley JJ, Navas M-L, Niinemets U, Oleksyn J, Osada N, Poorter H, Poot P, Prior L, Pyankov VI, Roumet C, Thomas SC, Tjoelker MG, Veneklaas EJ, Villar R (2004) The worldwide leaf economics spectrum. Nature 428(6985), 821-827.
| Crossref | Google Scholar | PubMed |
Yoda K, Kira T, Ogawa H, Hozumi H (1963) Self-thinning in overcrowded pure stands under cultivated and natural conditions. Journal of the Institute of Polytechnics, Osaka City University, Series D 14, 107-129.
| Google Scholar |
Zanne AE, Flores-Moreno H, Powell JR, Cornwell WK, Dalling JW, Austin AT, Classen AT, Eggleton P, Okada K-i, Parr CL, Adair EC, Adu-Bredu S, Alam MA, Alvarez-Garzón C, Apgaua D, Aragón R, Ardon M, Arndt SK, Ashton LA, Barber NA, Beauchêne J, Berg MP, Beringer J, Boer MM, Bonet JA, Bunney K, Burkhardt TJ, Carvalho D, Castillo-Figueroa D, Cernusak LA, Cheesman AW, Cirne-Silva TM, Cleverly JR, Cornelissen JHC, Curran TJ, D’Angioli AM, Dallstream C, Eisenhauer N, Evouna Ondo F, Fajardo A, Fernandez RD, Ferrer A, Fontes MAL, Galatowitsch ML, González G, Gottschall F, Grace PR, Granda E, Griffiths HM, Guerra Lara M, Hasegawa M, Hefting MM, Hinko-Najera N, Hutley LB, Jones J, Kahl A, Karan M, Keuskamp JA, Lardner T, Liddell M, Macfarlane C, Macinnis-Ng C, Mariano RF, Méndez MS, Meyer WS, Mori AS, Moura AS, Northwood M, Ogaya R, Oliveira RS, Orgiazzi A, Pardo J, Peguero G, Penuelas J, Perez LI, Posada JM, Prada CM, Přívětivý T, Prober SM, Prunier J, Quansah GW, Resco de Dios V, Richter R, Robertson MP, Rocha LF, Rúa MA, Sarmiento C, Silberstein RP, Silva MC, Siqueira FF, Stillwagon MG, Stol J, Taylor MK, Teste FP, Tng DYP, Tucker D, Türke M, Ulyshen MD, Valverde-Barrantes OJ, van den Berg E, van Logtestijn RSP, Veen GF(Ciska), Vogel JG, Wardlaw TJ, Wiehl G, Wirth C, Woods MJ, Zalamea P-C (2022) Termite sensitivity to temperature affects global wood decay rates. Science 377(6613), 1440-1444.
| Crossref | Google Scholar | PubMed |
Zhou B, Cai W, Zhu Z, Wang H, Harrison SP, Prentice IC (2025) A general model for the seasonal to decadal dynamics of leaf area. Global Change Biology 31(3), e70125.
| Crossref | Google Scholar |