Ovulate cone and seed biology of Wollemia nobilis (Araucariaceae) and comparison with Agathis microstachya and Araucaria cunninghamii
Geoffrey E. Burrows A *
A *
A
Abstract
Most gymnosperms are dioecious (no self-pollination). It was not known if Wollemia nobilis (monoecious) could produce viable seeds via self-pollination.
In the nearly 30 years since its scientific discovery Wollemia nobilis (Araucariaceae) has been the focus of considerable scientific attention. While this includes several aspects of sexual reproduction, many features of ovulate cone morphology and seed biology have not been investigated.
Various aspects of ovulate cone morphology were quantified, as were seed morphology and germination (e.g. seed moisture content and rate of imbibition), in material from an isolated tree of Wollemia nobilis. Seeds of Agathis microstachya and Araucaria cunninghamii were also examined to allow comparisons across the Araucariaceae.
In Wollemia nobilis most seeds were empty (no formation of a megagametophyte and embryo (M + E)). Empty and filled seeds were morphologically very similar. On average, the fresh weights (FW) of empty and filled seeds were 6.2 and 33.1 mg, respectively. On average, the FW of the M + E was 25.8 mg (78% of seed mass, the other 22% was testa). The average moisture content (FW basis) of the testa and the M + E were 9.6% and 7.0%, respectively. The M + E imbibed water relatively slowly and imbibed, on average, to ~110% increase in FW after 48 h. In Agathis robusta and Wollemia nobilis the seeds separated freely from the cone scales. In Araucaria cunninghamii the M + E was an integral part of the bract/scale complex.
The seeds examined in this study came from an isolated, almost certainly self-fertilised tree. The seeds were of approximately the same dimensions as those from the wild populations of Wollemia nobilis, while average filled seed FW was greater and less variable. The filled seeds had a high viability and an excised M + E could germinate rapidly. This study shows that Wollemia nobilis can produce large, viable seeds via self-fertilisation. Successful self-fertilisation may be a factor in the low genetic diversity detected in the wild population.
Based on evidence from a single tree it would appear that Wollemia nobilis can produce large, viable, vigorous seeds via self-fertilisation. This has implications for the population structure of this species.
Keywords: germination, imbibition, megagametophyte, moisture content, monoecious, nucellus, self-fertilisation, Wollemi pine.
Introduction
Since it first came to scientific attention in 1994 the Wollemi pine (Wollemia nobilis, Araucariaceae) has been the focus of considerable scientific interest. Over 60 papers have been published in which Wollemia nobilis has been the research focus, with several of these studies focusing on aspects of female cone initiation, structure and development, along with subsequent seed production and germination. Various descriptions and images of whole female cones, cone scales and seeds have been published (e.g. Jones et al. 1995; Hill 1997, 2003; Chambers et al. 1998; Offord et al. 1999; Briggs 2000; McLoughlin and Vajda 2005; Pastoriza-Piñol 2007; Dettmann et al. 2012; Dörken and Rudall 2019; Ng et al. 2024) but several finer aspects of morphology, such as the nucellus, megagametophyte and embryo structure in filled and empty seeds have not been illustrated or described. Likewise, the quantification of various cone components (e.g. dimensions, and fresh and dry weights and thus moisture content) has been largely unexplored.
The Araucariaceae (order Araucariales) is divided into three genera: Araucaria, Agathis and Wollemia. Morphological and molecular-based studies indicate that Agathis and Wollemia are relatively closely related to each other and relatively distant from Araucaria (Escapa and Catalano 2013). Indeed, Page (2024) has recently split the Araucariales into two families – the existing Araucariaceae and the new Agathaceae – presumably to emphasise the similarities between Agathis and Wollemia, and their differences from Araucaria. Since the discovery of Wollemia nobilis various fossilised female cones of members of the Araucariaceae have been described (Cantrill and Raine 2006; Dettmann et al. 2012; Martínez et al. 2020; Stockey and Rothwell 2020). In some cases this material was found to be closer to Agathis and Wollemia than Araucaria (Cantrill and Raine 2006; Dettmann et al. 2012). Escapa and Catalano (2013) note that in fossils, conifer ovulate cones are the structures that preserve the highest number of major informative features. It has also been noted that well-described and illustrated material of extant species helps when interpreting the structure and relationships of fossilised cones (Stockey and Rothwell 2020).
Wollemia nobilis is monoecious (Dörken and Rudall (2019), thus self-fertilisation is possible. In the remainder of the Araucariaceae the other species are also monoecious, except for the two South American species from the section Araucaria (Araucaria araucana and Araucaria angustifolia) that are dioecious. Interestingly, ~65% of the c. 1000 species of gymnosperms are dioecious (Walas et al. 2018). Dioecy dominates in eight of the 12 gymnosperm families, while in the Araucariaceae (37 spp.), Pinaceae (224 spp.) and Sciadopityaceae (1 sp.) it occurs in 5, 0 and 0% of species, respectively. The main advantage of dioecy is usually stated to be complete exclusion of the risk of self-pollination.
Self-incompatibility is absent or, at best, poorly developed in gymnosperms (Sorensen 1982; Walas et al. 2018). Conifers are considered to be self-compatible, although with self-fertilisation relatively low levels of viable seed are usually formed (Runions and Owens 1998). Self-fertilisation in coniferous species can be associated with high levels of inbreeding depression, which can be expressed in the low viability of inbred embryos and in reduced growth of seedlings and saplings (e.g. Sorensen 1982; Kettle et al. 2007, 2008; Gernandt et al. 2011; Huang et al. 2022). Abate et al. (2025) notes that populations that have been selfing for long periods of time can be less prone to severe inbreeding depression. Thus, it will be informative to see if a self-pollinated Wollemi pine would produce any seeds and, if seeds are produced, how they compare to previously described material.
Wollemia nobilis female cones, cone scales and seeds were described, imaged and quantified to: (1) provide fine detail on the nucellus, megagametophyte and embryo that were previously undescribed, (2) provide more extensive comparison for characters such as variation in seed mass, (3) explore techniques to speed up the prolonged germination sequence, (4) illustrate features that will be useful when interpreting araucarian fossils and (5) determine if self-fertilisation can result in the formation of viable, vigorous seeds. Seeds of Agathis microstachya and Araucaria cunninghamii were also examined to provide a wider family perspective.
Materials and methods
In female cones of Wollemia and Agathis the bract and ovuliferous scales are almost completely fused, with the ovule/seed being almost entirely free from this structure. The seeds have integumentary wings. There has been ongoing discussion regarding these scale complexes about which parts represent the bract and ovuliferous scales (Dettmann et al. 2012). For this study this combined structure is termed a cone scale. In Araucaria the bract/ovulate scale complexes are incompletely fused, with unwinged seeds fully embedded in the bract/scale complex. For this study, this overall structure is termed a ‘seed’. For all three species a high proportion of empty seeds (no embryo) can be present in a cone or seed lot (e.g. Offord et al. 1999).
Wollemia nobilis
Wollemia nobilis female cones were collected in January 2025 from a 3 m high tree growing in a domestic garden (Figs 1–3). The tree was protected from afternoon sun by other plants, well mulched and regularly watered in summer. No other plants of this species were growing in the vicinity. Only a single plant was able to be sourced as, with long distance pollen dispersal, few plants were available that could be guaranteed to be entirely self-pollinated. There can also be variability in the number of female cones produced, between plants and between years (Zimmer et al. 2015), meaning that the possibility of finding another isolated tree with a good cone crop could be quite low. In addition, obtaining Wollemia seeds that will be destructively sampled is usually problematic.
At the time of collection the outer parts of the scales had started to turn brown and the cone scales usually separated easily from the cone axis. The fresh weights (FW) of eight cones were measured. The number of scales per cone was counted for five cones. The seeds were separated from the cone scales and the filled seeds (with megagametophyte and embryo (M + E)) were separated from the empty seeds (without M + E). This was easily accomplished by feeling the difference in thickness.
The FWs of 10 cone scales, 10 empty seeds and 10 filled seeds were measured for five cones. The filled seeds were dissected into testa and M + E, and FW was measured. Of the 10 M + E five were placed in aluminium trays and dried at 103°C for 24 h, then dry weight (DW) was measured and moisture content (MC) calculated on a FW basis. This is a slight modification of the International Seed Testing Association’s low constant-temperature drying method. The other five were placed on moistened paper towel in 9 cm diameter Petri dishes at 22°C. Their mass was measured at intervals over the next 24–72 h and percentage increase in mass was calculated. The cone scales, empty seeds and seed coats from the dissected seeds were also dried (as above) and weighed.
Thirty seeds from a single cone were used to further investigate imbibition and germination. Ten full seeds, 10 M + E (dissected from the testa) and 10 empty seeds (two replicates of five seeds for each treatment) were placed on moistened paper towel in 9 cm diameter Petri dishes at 22°C. Change in FW was measured over 7 d. At 7 d the M + E had either germinated or become bacterially infected, while the full seeds had only partially imbibed. The M + E were excised from the full seeds, placed on moist paper towel and subsequent changes in mass were recorded.
Seed viability was tested with triphenyl tetrazolium chloride (TTC). The liquid was applied to seeds cut in longitudinal section (LS) (two replicates of five seeds from two different cones).
The dimensions of cone scales, seeds and the detachment scar from the cone scale, along with the M + E and only E were measured using a Nikon SMZ25 dissecting microscope with NIS Elements software. Replicate numbers are listed in Table 1. In most of the abovementioned experiments replicate numbers were relatively low due to the limited number of seeds available.
| Measurement | Wollemia nobilis | Agathis microstachya | |
|---|---|---|---|
| No. cone scales/cone | 189 ± 15 (n = 5) | n.a. | |
| Cone FW (g) | 33 ± 4 (n = 8) | n.a. | |
| Cone scale FW (mg) | 104 ± 29 (n = 40) | n.a. | |
| Cone scale L × W, tip length (mm) | 15.5 ± 0.5, 18.1 ± 1.9, 6.0 ± 0.8 (n = 11) | n.a. | |
| Empty seed L × W × D (mm) | 11.7 ± 0.6 × 8.2 ± 0.7 × 0.5 ± 0.3 (n = 20) | 9.3 ± 0.3 × 6.5 ± 0.3 × 1.4 ± 0.3 (n = 10) | |
| Empty seed FW (mg) DW (mg) | 6.2 ± 0.9 (n = 30) 5.3 ± 0.8 (n = 30) | 16.6 ± 2.9 (n = 20) 15.3 ± 2.7 (n = 20) | |
| Empty seed MC (%) | 14 ± 3 (n = 30) | 8 ± 2 (n = 20) | |
| Filled seed L × W × D (mm) | 12.0 ± 0.7 × 8.1 ± 0.7 × 1.5 ± 0.2 (n = 20) | 10.1 ± 0.5 × 6.9 ± 0.5 × 2.8 ± 0.2 (n = 10) | |
| Filled seed FW (mg) | 33.1 ± 3.9 (n = 48) | 52.4 ± 8.0 (n = 43) | |
| T FW (mg) DW (mg) | 6.6 ± 0.7 (n = 40) 6.0 ± 0.7 (n = 40) | 13.1 ± 2.4 (n = 20) 12.4 ± 2.4 (n = 20) | |
| T (minus M + E) MC (%) | 9.6 ± 3.5 (n = 40) | 5.8 ± 3.0 (n = 20) | |
| % T of filled seeds, FW basis | 22% | 26% | |
| T thickness (μm) | 33 ± 7 (empty), 39 ± 10 (filled) (n = 10) | 306 ± 109 (empty), 134 ± 24 (filled) (n = 14) | |
| M + E L × W × D (mm) | 9.3 ± 0.3 × 5.7 ± 0.3 × 1.5 ± 0.2 (n = 10) | 8.8 ± 0.5 × 4.8 ± 0.4 × 2.3 ± 0.2 (n = 20) | |
| E dimensions, L × dia. (mm) | 5.4 ± 0.5, 0.7 ± 0.1 (n = 10) | 6.6 ± 0.4, 1.0 ± 0.1 (n = 10) | |
| M + E FW (mg) | 25.8 ± 3.7 (n = 48) | 38.5 ± 6.6 (n = 10) | |
| M + E DW (mg) | 24.0 ± 3.7 (n = 20) | 37.0 ± 6.4 (n = 20) | |
| M + E MC (%) | 7.0 ± 1.7 (n = 20) | 3.9 ± 0.9 (n = 20) | |
| % increase in FW when M + E were fully imbibed | c. 110 | 60–70 |
‘±’ indicates standard deviation (s.d.), all values with a s.d. are averages; moisture content (MC) was calculated on a fresh weight (FW) basis. DW, dry weight; E, embryo; M, megagametophyte; T, testa; L, length; W, width; D, depth.
Agathis microstachya
Seeds, but not cones or cone scales, of Agathis microstachya (bull kauri from far north Queensland) were obtained from a commercial supplier and most of the same measurements, as above, were taken (Table 1). As in Wollemia (see Fig. 4a), full and empty seeds could initially be quickly sorted by feeling the seed thickness. Seed weight was used to confirm this subjective assessment.
Araucaria cunninghamii
‘Seeds’ (scales fused with M + E) of Araucaria cunninghamii (hoop pine) were obtained from a commercial supplier. It was not possible to externally distinguish between empty and filled ‘seeds’ of this species (see Tompsett 1982). The only rapid way to determine if a hoop pine ‘seed’ was empty or filled was to cut the ‘seed’, which rendered it non-viable. The scale tissues surrounding the M + E were so tough that it was essentially impossible to excise the M + E from control ‘seeds’. In addition, there was a wide range of sizes and masses of viable ‘seeds’. These factors combined to limit the types of experiments that could be carried out.
Five main experiments were performed:
Thirty-eight of the larger ‘seeds’ were selected. They were cut in transverse section (TS) with a pair of heavy-duty secateurs. The cut surface was examined under a dissecting microscope and the ‘seeds’ were divided into those that were empty (14 seeds) and those that were filled (24 seeds). These ‘half pairs’ were weighed and placed in an oven at 103°C for 24–48 h, then reweighed and the MC of empty and filled ‘seeds’ was calculated.
‘Seeds’ were placed on moist paper towel for 2–3 days. With imbibition of the M + E (if present) the scale split apart along two lateral lines of weakness. The M + E could then be extracted and dried as above to find an approximate unimbibed DW starting mass.
Unimbibed seeds were cut in TS at either end and the remaining M + E was extracted, weighed, dried (as above), reweighed and MC (on a FW basis) was calculated.
Twenty ‘seeds’ were placed on moist paper towel at 24°C and allowed to imbibe and germinate. Seeds that did not split open were cut to assess the percentage that was empty and those that had a formed M + E.
Various dimensions associated with the ‘seeds’ were measured.
Please note that for Agathis microstachya and Araucaria cunninghamii the seeds were obtained from a commercial supplier. Thus, it is not known how many plants the seeds were collected from and whether the seeds were from natural stands, or parks or plantations. Thus material of the three species is not directly comparable, but illustration of the similarities in cone scale construction of Wollemia and Agathis, compared to the differences with Araucaria is valid, as are comparisons in seed moisture content and rate of imbibition between the three species.
Results and discussion
Wollemia nobilis
The tree from which the mature cones were collected is shown in Fig. 1. This image was taken in late March 2024, ~10 months before the 10–11 green cones that can be observed (and others on the far side of the tree) matured and provided the materials used in this study. Developing microsporangiate cones can also be observed. Although not apparent in the image, several of the upper branch tips have resting buds that were larger and more rounded than a typical vegetative resting bud. After 10 months of development they became the small green ovulate cones shown in Fig. 2.
Image, taken in late March 2024, of the top of the Wollemia nobilis tree examined in this study. Three female cones are indicated with long arrows that, with a further 10 months of development, matured to resemble the cone in Fig. 3a. Note, at this stage, they were uniformly green. Note the male cones (two indicated with short arrows). Note also the branch tip that is circled. This is a primordial female cone that would rapidly develop in late spring (October, November), possibly receiving pollen from the male cones. The stem at the bottom of the image is ~4 cm in diameter.

(a) Young female cone of Wollemia nobilis, ~12 months before cone scale and seed release. Scale in mm. (b) Cone from (a) cut in transverse section showing the adaxial surface of cone scales (cs), developing seeds (s) and cone axis (ca).
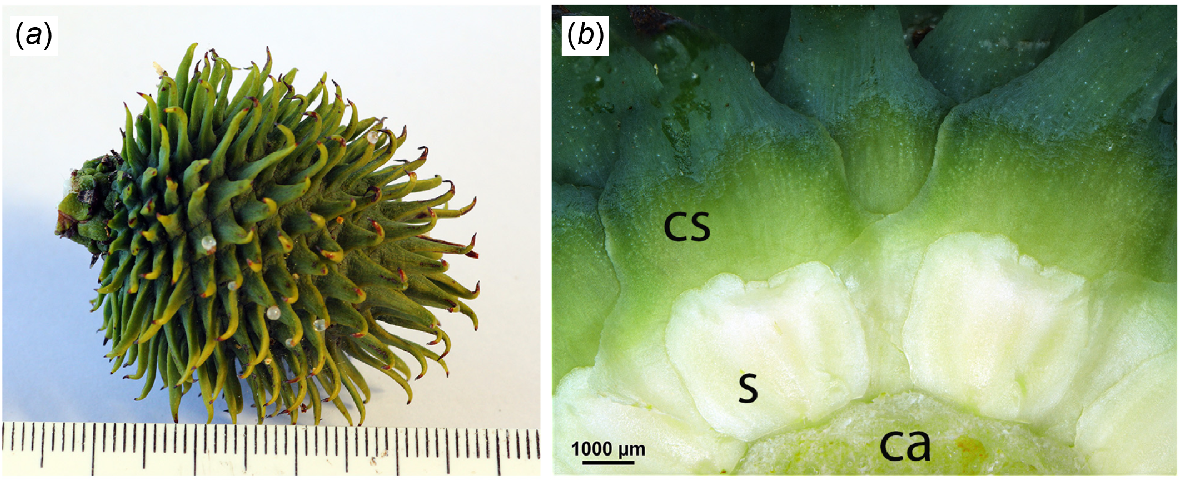
Immature cones (Fig. 2a), photographed in early February 2025, ~11 months before scale and seed release, showed the same basic construction described below. All cone components were smaller (except the scale tips), the cone scales were entirely green and the developing seeds were white (Fig. 2b).
Whole female cones had a FW of 28.1–38.2 g (average 33.2 g) (Table 1), with the variability related to size and likely also MC. Some cones were collected with the cone scales already separating from the axis (likely drier), while others were collected while still whole and needed several days in the laboratory before this separation occurred (likely higher MC). When cones were ready to release the scales the cone scale tips were still green, while the other outer parts of the scales had turned brown (Fig. 3a). In cones such as in Fig. 3a the cone scales and seeds were already brown inside the cone – indicating substantial moisture loss (see below). The cone scales were arranged with an 8:13 phyllotaxis, which is part of the Fibonacci sequence.
Images of a Wollemia nobilis female cone with associated cone scales and seeds. In images with a background grid the scale is in mm. (a) Mature cone. Note the green cone scale tips but the rest of the cone scales, both externally and internally, are brown. At this stage of maturity a gentle twist would release the cone scales from the cone axis. Scale at base in cm. (b) Adaxial surface of a cone scale with adpressed seed. (c) As per (b) but with seed detached and flipped over showing the area of attachment (arrows) of the seed to the cone scale. (d) Detail of the triangular attachment area showing three vascular bundles (arrows). (e) Seed with some of the testa (t) removed to show the megagametophyte and embryo covered in a thin brown nucellus. (f) Detail of (e) showing the convoluted nucellus at the micropylar end of the seed.
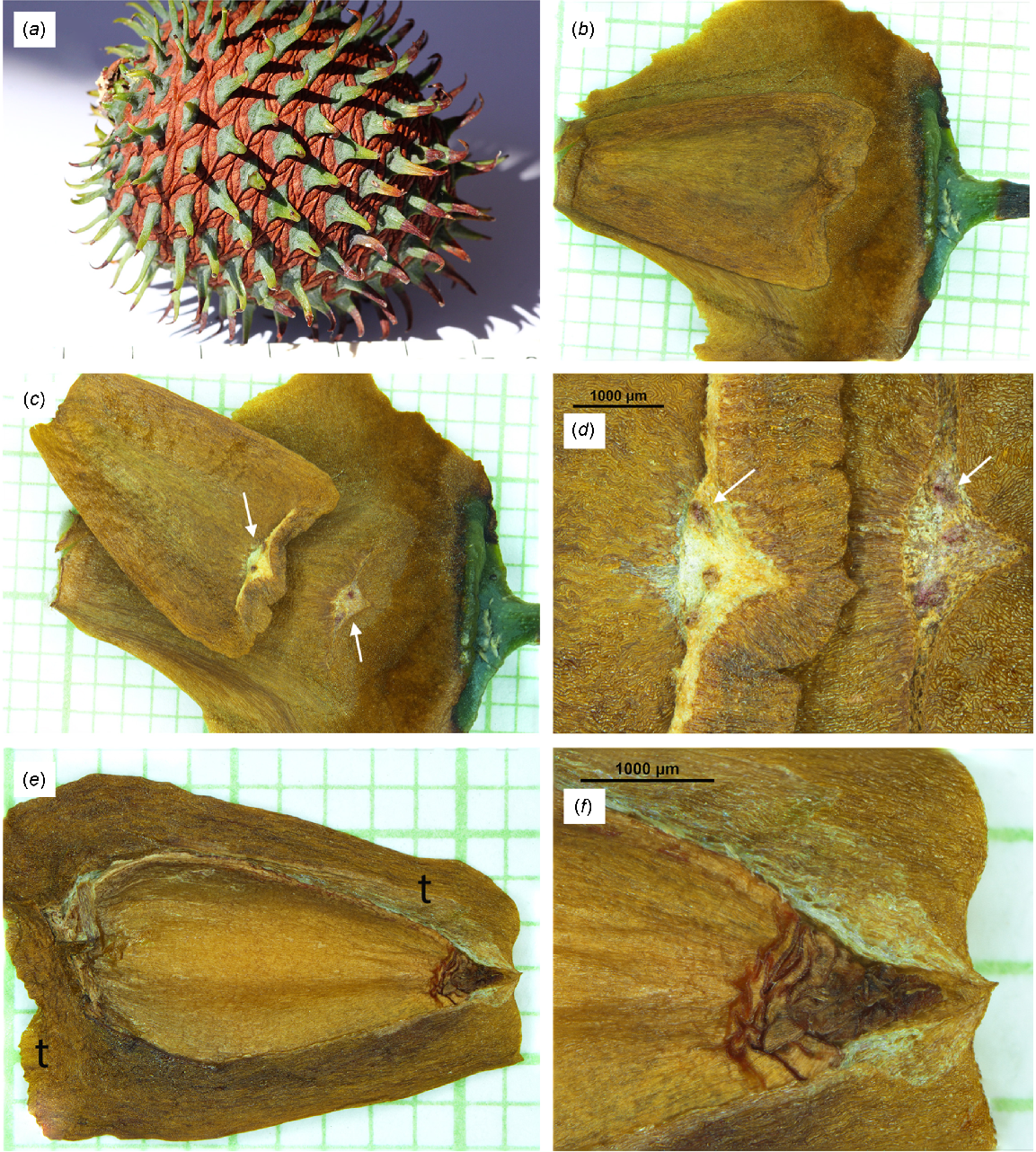
Excluding spines, the cones were ~60 mm long and 45 mm in diameter. After removing the cone scales, the cone axis length was 30–40 mm and the maximum diameter was 7–10 mm. Offord et al. (1999) recorded the cone axis as 27 mm long and 9 mm maximum width. While most of the central cone scales were perpendicular to the cone axis, the apical cone scales were almost parallel. This explains why the cone axis length was shorter than the overall cone length. Jones et al. (1995) recorded that female strobili could reach 125 mm long and 100 mm in diameter but in most studies, cone size appears to be ~60–70 mm in length and diameter (Jones et al. 1995; Offord et al. 1999; Hill 2003).
Jones et al. (1995) recorded >300 scales/cone, Offord et al. (1999) ~250 and Dörken and Rudall (2019) >100. In this study there were ~190 scales/cone (Table 1). Cone scales averaged 18 mm wide (i.e. tangential) and 16 mm long (i.e. radial), with a 6 mm long tip (Fig. 3b, c) (Table 1). This is within the ranges given by Jones et al. (1995). Dörken and Rudall (2019) recorded a green cone scale length of 30–40 mm, while in their fig. 1l the cone scale is indicated to be ~90 mm in length. These lengths would appear to be unusually long. Dettmann et al. (2012) illustrated young cone scales in their fig. 8, which were similar to those in Fig. 2 of this study.
Each seed was adpressed to the adaxial surface of a cone scale (Fig. 3b). The seed was attached to the scale at the seed’s abaxial outer edge (Fig. 3c, d). The main attachment was an approximately triangular area (sides averaged 1.2 ± 0.2 mm, with narrow tangential extensions that averaged 1.6 ± 0.2 mm long on either side), with 2–3 vascular bundles present within the central area (Fig. 3d). In most seeds a M + E had not formed. At most there were 20–30 filled seeds per cone (10–16% filled seeds/cone) and in several cones no filled seeds had formed. Offord et al. (1999) recorded that, in material from the wild populations, seed viability was ~10% (i.e. c. 25 filled seeds/cone).
The length and width of the filled and empty seeds were not statistically different (length 12.0 vs 11.7 mm, t(39) = 1.56, P = 0.13; width 8.1 vs 8.2 mm, t(39) = −0.19, P = 0.84) (Table 1), while filled seeds were, on average, approximately three times thicker than empty seeds (1.5 vs 0.5 mm) (Fig. 4a). While the cones studied by Jones et al. (1995) and Offord et al. (1999) had more cone scales, the seeds were similar in size as in this study. Compared to the data in Ng et al. (2024) (see below) the M + E in this study were, on average, heavier and more consistent in mass.
Cut seeds of Wollemia nobilis. (a) Seeds cut in TS. The upper seed was empty, while the lower seed had an embryo (e) surrounded by megagametophyte (m). (b) Embryo and megagametophyte excised from the testa, cut in LS. The seed had been imbibed for 2 days to make excising the embryo easier. Note the cotyledons (c), hypocotyl-stem axis (h), nucellus (n) and radicle (r).
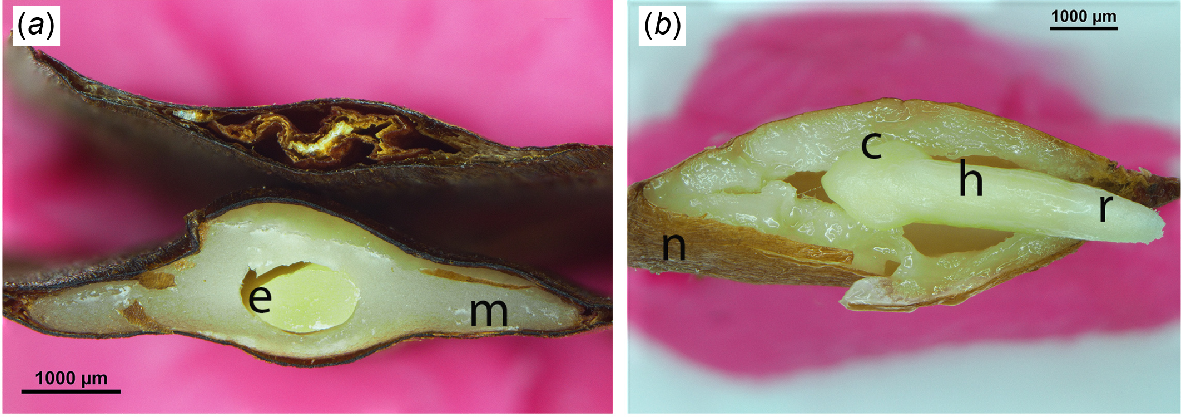
A thin brown nucellus covered the megagametophyte (Fig. 3e, f). At the micropylar end (i.e. closest to the cone axis) the nucellus was highly convoluted (Fig. 3e, f). This is a typically araucarian feature (Cantrill and Raine 2006; Stockey and Rothwell 2020) and is clearly shown in Late Cretaceous (100–66 Mya) permineralised cones of Araucaria famii (Stockey and Rothwell 2020, fig. 52). In Agathis and Araucaria the nucellus can extend beyond the ovule’s opening during fertilisation (Gelbart and Von Aderkas 2002). On average, the embryo was 5.4 mm long and 0.7 mm in diameter, with two cotyledons at the distal end and a radicle at the cone axis end (Fig. 4a, b).
The testa was, on average, 33 and 39 μm thick in empty and filled seeds, respectively (Fig. 4a). Timchenko et al. (2020), in an anatomical study of the Wollemia nobilis seed coat, recorded that the testa could be divided into an exotesta (1 layer thick – the epidermis), mesotesta (consisting of three parts – hypodermis (1–3 layers), peripheral (2–3 layers), internal (5–8 layers)) and the endotesta (1–2 layers) but provided no dimensions. In their figs 7, 9 the testa is indicated to be 150–200 μm thick. Their paper appears to make no mention of a nucellus. It appears that their ‘endotesta’ could be the nucellus (compare their fig. 7 with Fig. 4a, especially the upper empty seed, of this study).
The average FW of filled seeds was greater than that of the empty seeds (33.1 vs 6.2 mg, respectively) (Table 1). The average FW of empty seeds (essentially only the testa) and the testa of the filled seeds was similar (between 6 and 7 mg). The average FW of the M + E was 25.8 mg, thus the M + E was ~78% of the FW of the seed (Table 1). On average, the embryo was 11% of the FW of the M + E (n = 6).
Ng et al. (2024), in a supplementary table, provided FWs for 27 fresh seeds and 34 stored seeds, both as whole filled seeds and for what they termed the ‘kernel’ (i.e. the M + E). The average FWs for the fresh and stored seeds were 23.8 mg (s.d. 9.5) (range 6.7–44.4) and 26.6 mg (s.d. 11.6) (range 4.7–42.8), respectively. This is lighter and more variable than in this study (Table 1). Their fresh seeds included two that were so light (e.g. 6–8 mg) that they probably did not have a fully formed embryo. The stored seed had similar results, with six very light weight (5–10 mg) seeds. If the very light seeds of Ng et al.’s study were excluded, their averages increased to 25.2 and 30.8 mg, respectively, which is still less than the averages in this study. On average, in Ng et al.’s (2024) study, the kernel was ~80% of the seed FW, which is similar to the value from this study.
The average MC of cone scales was ~17%, for empty seeds it was 14%, the testa of filled seeds 10% and the M + E 7%. Using a different sample of seeds the MC of the megagametophyte was 6.9 ± 1.0% (89% of the M + E FW) and MC of the embryo was 9.5 ± 2.7% (n = 6). The MC of whole small green cones (as per Fig. 2) was 67%. While whole immature cones and the components of mature cones are not directly comparable, the MC values show considerable moisture loss by the time the cones are ready to shed scales and seeds.
Imbibition of the M + E was relatively slow. Average percentage increase in FW was 24, 91 and 112% after 4, 24 and 48 h, respectively.
TTC tests indicated that the filled seeds had 100% viability. Some of the M + E in the imbibition experiments germinated. In five of the cones 40% (2 of 5 M + E) germinated within 7 days and some germinated in less than 4 d. Offord and Meagher (2001) described Wollemia nobilis as having a prolonged germination pattern (see their graph of cumulative germination over time (~6 months)). Offord and Meagher (2001), using full seeds from the wild populations, recorded that, even in the most successful treatments, germination was less than 5% after 7 days. The rapid germination in this study might be related to using only the excised M + E, rather than full seeds. Excising the M + E was time consuming and it was easy to cause minor nicks to the nucellus and megagametophyte that often seemed to lead to microbial attack.
Whole seeds imbibed much more slowly than the excised M + E. Using a different batch of seeds from that described above whole seeds had an average initial FW of 35.2 mg, which increased to 55.8 mg (58% increase) after 7 d imbibition. The empty seeds (essentially only testa) had an average initial FW of 6.7 mg, which increased to 14.5 mg after 7 d imbibition. Thus, in the whole seeds, the average imbibed FW of the M + E after 7 d would have been ~41.3 mg. The average FW of the control M + E was ~28 mg (35.2 minus 6.7 mg). Thus, on average, there was a ~45% increase in weight of the M + E over 7 d. By comparison, the excised M + E had a 107% increase in weight after 2 d. It appears that some part of the testa and/or the nucellus slows but does not completely stop water uptake by the M + E.
The Wollemia nobilis seeds used in this study came from a single tree that was isolated from any other trees of the same species. The seeds produced were undoubtedly from self-fertilisation. Self-fertilisation can lead to inbreeding depression, which can lead to reduction in fitness of the subsequent population (Kettle et al. 2007). Nonetheless, compared to those from the wild populations, the filled seeds were as large as those recorded in Jones et al. (1995) and Offord et al. (1999) and, on average, heavier than those measured by Ng et al. (2024) and were of a relatively consistent mass. They were indicated to be 100% viable and some M + E could germinate rapidly. Molecular biology has indicated that the trees in the wild populations have very limited genetic diversity (Peakall et al. 2003; Greenfield et al. 2016). The limited seed set in the wild populations (Offord et al. 1999) indicates that wind pollination is not particularly efficient. Offord et al. (1999) recorded that viable seeds were mostly confined to the outer and most exposed part of the cone. Kettle et al. (2008) noted the low seed set in Wollemia nobilis. They also noted that Offord et al. (1999) had suggested pollen supply could be limiting. Kettle et al. (2008) also suggested that embryo abortion due to inbreeding depression might also be a factor. This study shows that an isolated tree can produce large viable and vigorous seeds. Given the arrangement of the seed cones and the mature tree crowns it is possible that many of the seeds produced in the wild are the result of self-fertilisation.
Agathis microstachya
In overall construction the seeds of Wollemia nobilis and Agathis microstachya were quite similar. Both species had: (1) a seed that was almost entirely free of the cone scale, (2) a dry brown testa that surrounded the M + E, (3) seeds with wings, and 4) the M + E was covered in a thin brown nucellus that was folded at the micropylar end.
Nonetheless, some distinct differences were present. The seeds of Wollemia nobilis were trapezium-shaped, with a wing that extended almost all the way around the edge of the seed (Fig. 3b, e). Seeds of Agathis microstachya were ‘Y’-shaped (Fig. 5a, b). The M + E was in the base of the ‘Y’, while at the top of the ‘Y’ were two wings (Fig. 5a, b), although one of the wings was usually less developed than the other. A major point of difference was the attachment of the seed to the cone scale. In Wollemia nobilis it was a small area, with only 2–3 vascular bundles, on the abaxial surface of the seed (Fig. 3c, d). In Agathis microstachya it formed an arc between the wings (Fig. 5c). It was much longer (8.1 ± 1.7 mm), wider (0.3 ± 0.1 mm) and had many more (5–11, average 8.1 ± 1.9) vascular bundles than in Wollemia. Average FWs of empty seeds (17 mg), filled seeds (52 mg) and M + E (39 mg) were all greater in Agathis than in Wollemia (Table 1). Agathis had a greater investment in the testa (25.5% of the whole seed mass) than in Wollemia. Wollemia wings were relatively membranous and flexible, while those in Agathis were rigid. The testa covering the M + E was ~40 μm thick in Wollemia and 130+ μm thick in Agathis (Table 1).
Seeds of Agathis microstachya. In images with a background grid the scale is in mm. (a) Filled seed on left (FW 56 mg), empty seed on right (FW 15 mg). Note the pair of wings on each seed. There was little morphological difference between the empty and filled seeds. (b) As per (a) but with some of the testa dissected away showing the megagametophyte and embryo on the left and a shrivelled nucellus on the right. (c) Looking between the wings, showing the area of attachment between the seed and cone scale. Note that it extends almost the full length of the wings and has numerous vascular bundles. (d) Seed cut in a transverse plane, showing the testa (arrowed), megagametophyte (m) and embryo (e). The darker spots in the embryo could be associated with resin ducts.
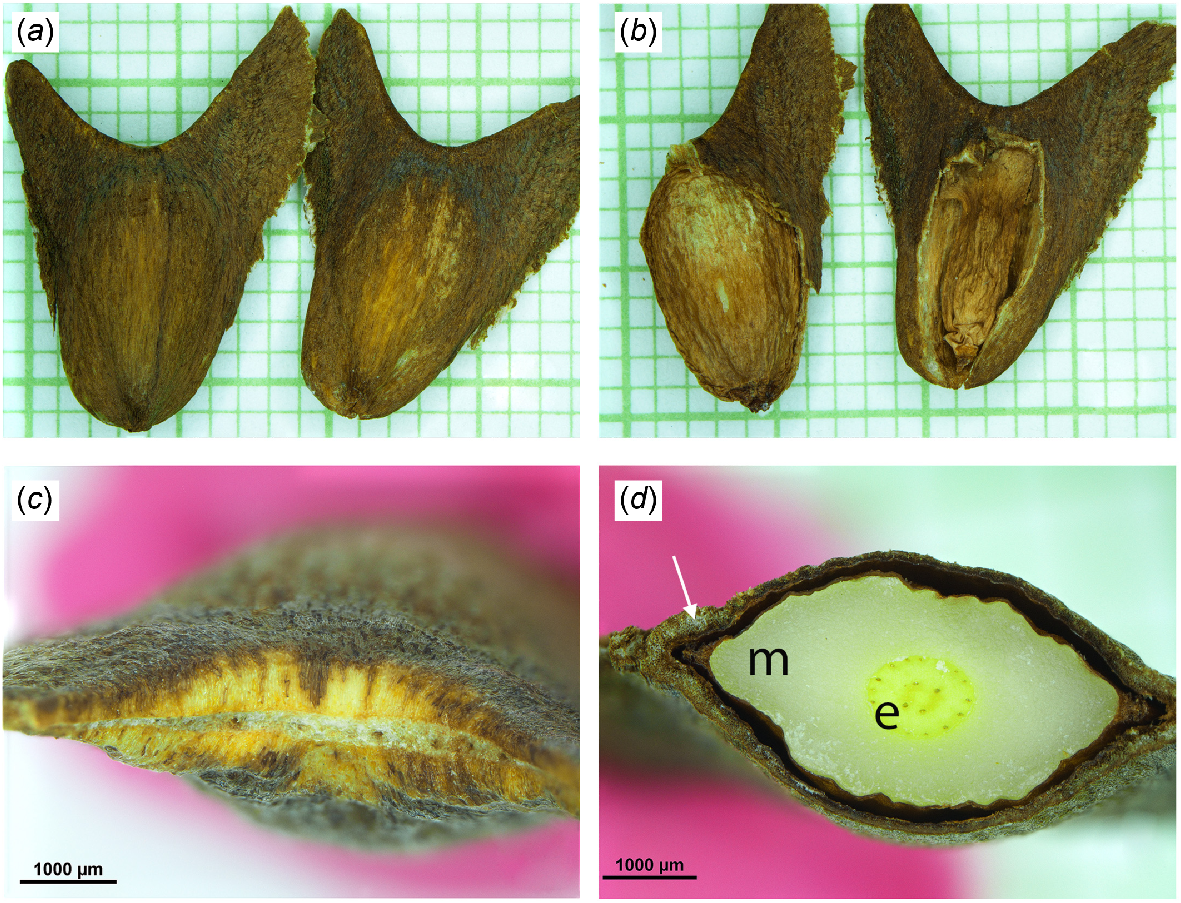
Little has been recorded about the seeds of Agathis microstachya. For example, no description was given in the Flora of Australia (Hill 1998) and in the Gymnosperm Database (https://www.conifers.org/ (accessed 28 March 2025)); the only description was ‘Seeds cordate, winged.’ Hyland et al. (1993) had no description or measurements of the seeds but included a line drawing showing a seed with two unequal wings. Farjon (2010) provided a relatively detailed description of the seed. The seed (i.e. the ovoid section with the M + E) was much the same size as in this study, but the wings were recorded as much larger – the bigger 20–25 mm long and the smaller 2–5 mm long. In this study the larger wing was much smaller – no longer than 10 mm.
In Agathis microstachya MC was 8%, 6% and 4% in empty seeds, testa and M + E, respectively (Table 1). The M + E MC is very low. The seeds imbibed relatively slowly and started germinating at only 60–70% increase in mass, with over 75% germination after 5 days (n = 38).
Araucaria cunninghamii
The central thicker part of the ‘seed’ was, on average, 23.4 ± 2.0 × 7.8 ± 0.7 × 4.6 ± 0.5 mm (n = 20) in length, width and thickness, respectively (Fig. 6a). Either side of this was a large (20.0 ± 1.4 × 9.0 ± 1.6 mm (n = 16), average length by width) wing that was very thin (Fig. 6a). A large range of ‘seed’ mass was present in the seed lot, both for full ‘seeds’ (252–428 mg), and empty ‘seeds’ (192–395 mg) (n = 78). Of these 78 seeds 50 (63%) were filled and 28 (37%) were empty (Fig. 6b); they had average FWs of 327 ± 41 and 276 ± 50 mg, respectively. The M + E was protected by, on average, ~1200 μm of tough fibrous scale tissue (Fig. 6c). The M + E was in a cavity, that in TS had three lobes, with megagametophyte in the two outer spaces and the embryo, surrounded by a thin layer of megagametophyte, in the middle space (Fig. 6c). In the fibrous tissue encircling the M + E a zone of relative weakness was present to the sides of the M + E (Fig. 6c). During imbibition and germination of full ‘seeds’ the ‘seed’ split into upper and lower halves through this zone (Fig. 6d), allowing the radicle to extend. Empty ‘seeds’ did not split during imbibition.
‘Seeds’ of Araucaria cunninghamii. In images with a background grid the scale is in mm. (a) Combined unit of bract and ovuliferous scales, and embedded megagametophyte and embryo. Note the extensive wings (w) are so thin that the grid scale can be seen through them. (b) Three empty seeds with the non-filled megagametophyte and embryo arrowed. These scales had lateral material excised to the point where the two halves of the ‘seeds’ could be split apart. (c) ‘Seed’ cut in TS, showing the thick, tough material that surrounds the megagametophyte (m) and embryo (e). The arrows point to the zones of relative weakness where the ‘seed’ splits when imbibition and subsequent germination occur. (d) After imbibing for 4–5 days the ‘seed’ had split and half of the ‘seed’ was easily removed, revealing the embryo (surrounded by megagametophyte) which was about to germinate.
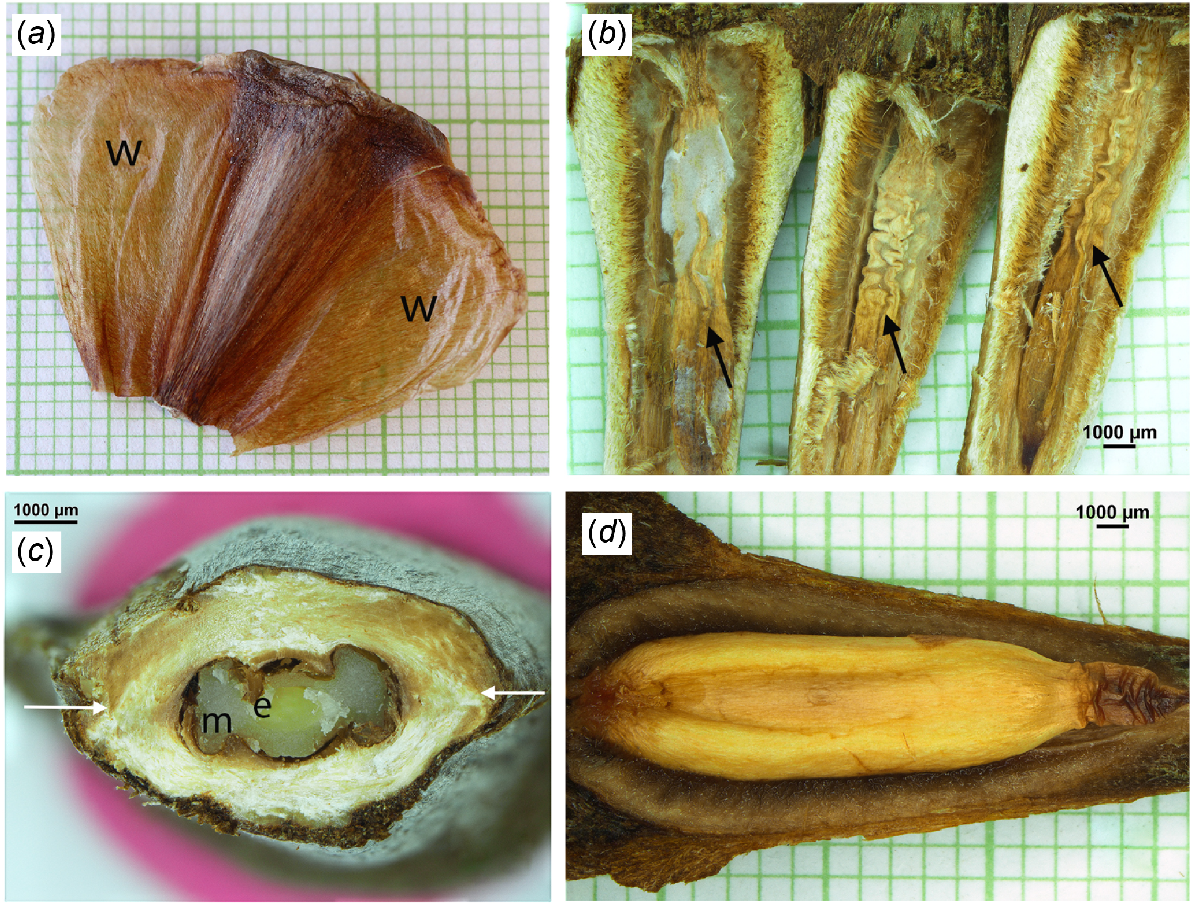
MC of the empty ‘seeds’ was 8.2 ± 0.4%, and filled ‘seeds’ 7.6 ± 0.5% (n = 38). M + E DW averaged 40.6 ± 10.9 mg (n = 7) which is ~14% of the whole ‘seed’ DW. The MC of the M + E was 4.4 ± 0.5% (n = 8).
The arrangement of the M + E and fused scales in Araucaria cunninghamii was very different from those of the other two species. Thus, there are few direct comparisons that can be made. The average DW of the M + E of the three species were similar (24–41 mg) and the M + E MC was less than 10% (4–7%) in the three species.
Conclusions
The seeds (including M + E) of Agathis microstachya and Wollemia nobilis separated freely from the cone scale, while in Araucaria cunninghamii the M + E was completely contained with the cone scale. Although this is a major difference, at the level of the nucellus, megagametophyte and embryo the three species were similar. The M + E DW and M + E MC were similar in the three species. All three species had orthodox seeds (see below) that were non-dormant. Once imbibed the ‘seeds’ of Araucaria cunninghamii and the seeds of Agathis microstachya germinated quickly. In Wollemia nobilis it appeared that the testa may slow imbibition. This finding should be further explored in terms of seed propagation. In all three species the tip of the nucellus was highly convoluted, a typically araucarian feature.
Seeds in the Araucariaceae can be orthodox (can be dried to 5–10% MC without losing viability) or recalcitrant (if dried below 25–40% MC viability is lost) in terms of their storage behaviour. Araucaria is divided into four sections – Bunya (1 sp.), Intermedia (1 sp.), Araucaria (2 spp.) and Eutacta (15 spp.) (Escapa and Catalano 2013). The species could be grouped into three MC categories. The first group includes the first three sections, where the seeds are large, starchy and recalcitrant (Tompsett 1984; Burrows et al. 2017). In Eutacta (smaller seeds with a high lipid content) there were two groups. The New Caledonian species could not be dried below 12% without damage (sub-orthodox – Bonner 1990), while Araucaria cunninghamii could be dried to 2% (orthodox). The seeds of Agathis also show a range of storage behaviour, from orthodox to recalcitrant (Dickie and Smith 1995; Sanderson 1998; Angkotta et al. 2022). The seeds of the three species studied all had an average MC of less than 8% without any specific drying treatment and are thus orthodox. Ashmore et al. (2011) noted that seeds of Wollemia nobilis were orthodox and could be dried to 5% MC, while still retaining viability. Ng et al. (2024) recorded that oils were 40–50% of the weight of the Wollemia nobilis kernel, which adds support to the orthodox classification.
Zimmer et al. (2015) studied year-to-year variation in female cone production in Wollemia nobilis. They posed four questions in relation to self-fertilisation in this species. The first was ‘How common is self-fertilisation in Wollemia nobilis?’ and the second, ‘Does selfing lead to fewer viable seeds?’ (p. 84). It is not known how common it is, but successful self-fertilisation is definitely possible. It is not known how selfing compares to outcrossing in terms of seed viability but the former can result in full-sized, viable and vigorous seeds. Although difficult to obtain it will be important to obtain cones from other isolated trees to provide replication.
Acknowledgements
I thank: (1) Joanne Ashnest for providing valuable comments on this manuscript, (2) Mark Filmer for editing assistance and (3) Integrifolia Native Seeds for assistance in obtaining the Agathis and Araucaria seeds.
References
Abate NB, Degu HD, Kalousová M, Abebe T (2025) Inbreeding depression manifested in progeny from fragmented populations of the wind-pollinated dioecious conifer Afrocarpus gracilior (Pilg.) C. N. Page. Ecology and Evolution 15(2), e70903.
| Crossref | Google Scholar |
Angkotta JC, Nugroho JD, Sinaga NI (2022) Characterization of cones and seeds of damar (Agathis labillardieri) from plantation in Klasaman, Sorong. Jurnal Sylva Lestari 10(1), 107-115.
| Crossref | Google Scholar |
Ashmore SE, Hamilton KN, Offord CA (2011) Conservation technologies for safeguarding and restoring threatened flora: case studies from Eastern Australia. In Vitro Cellular & Developmental Biology – Plant 47, 99-109.
| Crossref | Google Scholar |
Bonner FT (1990) Storage of seeds: potential and limitations for germplasm conservation. Forest Ecology and Management 35(1–2), 35-43.
| Crossref | Google Scholar |
Briggs BG (2000) What is significant – the Wollemi pine or the southern rushes? Annals of the Missouri Botanical Garden 87(1), 72-80.
| Crossref | Google Scholar |
Burrows GE, Heady RD, Smith JP (2017) Substantial resource reallocation during germination of Araucaria bidwillii (bunya pine), an Australian rainforest conifer with large seeds and cryptogeal germination. Trees 31(1), 115-124.
| Crossref | Google Scholar |
Cantrill DJ, Raine JI (2006) Wairarapaia mildenhallii gen. et sp. nov., a new Araucarian cone related to Wollemia from the Cretaceous (Albian-Cenomanian) of New Zealand. International Journal of Plant Sciences 167(6), 1259-1269.
| Crossref | Google Scholar |
Chambers TC, Drinnan AN, McLoughlin S (1998) Some morphological features of Wollemi pine (Wollemia nobilis: Araucariaceae) and their comparison to Cretaceous plant fossils. International Journal of Plant Sciences 159(1), 160-171.
| Crossref | Google Scholar |
Dettmann ME, Clifford HT, Peters M (2012) Emwadea microcarpa gen. et sp. nov. – anatomically preserved araucarian seed cones from the Winton Formation (late Albian), western Queensland, Australia. Alcheringa: An Australasian Journal of Palaeontology 36(2), 217-237.
| Crossref | Google Scholar |
Dickie JB, Smith RD (1995) Observations on the survival of seeds of Agathis spp. stored at low moisture contents and temperatures. Seed Science Research 5(1), 5-14.
| Crossref | Google Scholar |
Dörken VM, Rudall PJ (2019) Structure and abnormalities in cones of the Wollemi pine (Wollemia nobilis). Kew Bulletin 74, 3.
| Crossref | Google Scholar |
Escapa IH, Catalano SA (2013) Phylogenetic analysis of Araucariaceae: integrating molecules, morphology, and fossils. International Journal of Plant Sciences 174(8), 1153-1170.
| Crossref | Google Scholar |
Gelbart G, von Aderkas P (2002) Ovular secretions as part of pollination mechanisms in conifers. Annals of Forest Science 59(4), 345-357.
| Crossref | Google Scholar |
Greenfield A, McPherson H, Auld T, Delaney S, Offord CA, Van der Merwe M, Yap J-YS, Rossetto M (2016) Whole-chloroplast analysis as an approach for fine-tuning the preservation of a highly charismatic but critically endangered species, Wollemia nobilis (Araucariaceae). Australian Journal of Botany 64(8), 654-658.
| Crossref | Google Scholar |
Hill KD (1997) Architecture of the Wollemi pine (Wollemia nobilis, Araucariaceae), a unique combination of model and reiteration. Australian Journal of Botany 45(5), 817-826.
| Crossref | Google Scholar |
Hill KD (2003) The wollemi pine, another living fossil? Acta Horticulturae 615, 157-164.
| Crossref | Google Scholar |
Huang R, Zeng W, Deng H, Hu D, Wang R, Zheng H (2022) Inbreeding in Chinese fir: insight into the rare self-fertilizing event from a genetic view. Genes 13(11), 2105.
| Crossref | Google Scholar |
Jones WG, Hill KD, Allen JM (1995) Wollemia nobilis, a new living Australian genus and species in the Araucariaceae. Telopea 6(2–3), 173-176.
| Crossref | Google Scholar |
Kettle CJ, Hollingsworth PM, Jaffré T, Moran B, Ennos RA (2007) Identifying the early genetic consequences of habitat degradation in a highly threatened tropical conifer, Araucaria nemorosa Laubenfels. Molecular Ecology 16(17), 3581-3591.
| Crossref | Google Scholar | PubMed |
Kettle CJ, Ennos RA, Jaffré T, Gardner M, Hollingsworth PM (2008) Cryptic genetic bottlenecks during restoration of an endangered tropical conifer. Biological Conservation 141(8), 1953-1961.
| Crossref | Google Scholar |
Martínez LCA, Huacallo EP, Pujana RR, Padula H (2020) A new megaflora (leaves and reproductive structures) from the Huancane Formation (Lower Cretaceous), Peru. Cretaceous Research 110, 104426.
| Crossref | Google Scholar |
McLoughlin S, Vajda V (2005) Ancient wollemi pines resurgent. American Scientist 93(6), 540-547.
| Crossref | Google Scholar |
Ng MCH, Tran VH, Duke RK, Offord CA, Meagher PF, Cui PH, Duke CC (2024) Lipid profile of fresh and aged Wollemia nobilis seeds: omega-3 epoxylipid in older stored seeds. Lipidology 1(2), 92-104.
| Crossref | Google Scholar |
Offord CA, Meagher PF (2001) Effects of temperature, light and stratification on seed germination of Wollemi pine (Wollemia nobilis, Araucariaceae). Australian Journal of Botany 49(6), 699-704.
| Crossref | Google Scholar |
Offord CA, Porter CL, Meagher PF, Errington G (1999) Sexual reproduction and early plant growth of the Wollemi pine (Wollemia nobilis), a rare and threatened Australian conifer. Annals of Botany 84(1), 1-9.
| Crossref | Google Scholar |
Pastoriza-Piñol J (2007) 590. Wollemia nobilis. Curtis’s Botanical Magazine 24(3), 155-161.
| Crossref | Google Scholar |
Peakall R, Ebert D, Scott LJ, Meagher PF, Offord CA (2003) Comparative genetic study confirms exceptionally low genetic variation in the ancient and endangered relictual conifer, Wollemia nobilis (Araucariaceae). Molecular Ecology 12(9), 2331-2343.
| Crossref | Google Scholar | PubMed |
Sanderson KD (1998) Effect of storage conditions on viability of wind-dispersed seeds of some cabinet timber species from Australian tropical rainforests. Australian Forestry 61(2), 76-81.
| Crossref | Google Scholar |
Sorensen FC (1982) The roles of polyembryony and embryo viability in the genetic system of conifers. Evolution 36(4), 725-733.
| Crossref | Google Scholar | PubMed |
Stockey RA, Rothwell GW (2020) Diversification of crown group Araucaria: the role of Araucaria famii sp. nov. in the late Cretaceous (Campanian) radiation of Araucariaceae in the Northern Hemisphere. American Journal of Botany 107(7), 1072-1093.
| Crossref | Google Scholar |
Timchenko AS, Sorokin AN, Zdravchev NS, Bobrov AVFC, Romanov MS (2020) Comparative seed coat anatomy of the genus Wollemia (Araucariaceae). Problems of Botany of Southern Siberia and Mongolia 19(2), 134-139.
| Crossref | Google Scholar |
Tompsett PB (1982) The effect of desiccation on the longevity of seeds of Araucaria hunsteinii and A. cunninghamii. Annals of Botany 50(5), 693-704.
| Crossref | Google Scholar |
Tompsett PB (1984) Desiccation studies in relation to the storage of Araucaria seed. Annals of Applied Biology 105(3), 581-586.
| Crossref | Google Scholar |
Walas L, Mandryk W, Thomas PA, Tyrala-Wierucka Z, Iszkulo G (2018) Sexual systems in gymnosperms: a review. Basic and Applied Ecology 31, 1-9.
| Crossref | Google Scholar |
Zimmer HC, Meagher PF, Auld TD, Plaza J, Offord CA (2015) Year-to-year variation in cone production in Wollemia nobilis (Wollemi Pine). Cunninghamia 15, 79-85.
| Google Scholar |


