Comparison of electronic cigarette chemical composition across Australia
Caitlin Jenkins A B , Jody Morgan
A B , Jody Morgan  A B # and Celine Kelso A B # *
A B # and Celine Kelso A B # *
A
B
# These authors contributed equally to this work.
Handling Editor: George Koutsantonis
Abstract
Electronic cigarette (e-cigarette) regulation in Australia varies between states and territories. E-cigarette products (N = 47) were purchased from six Australian states and territories across a range of different retailer types, and the chemical composition of their e-liquids was determined using gas chromatography–mass spectrometry. Nicotine was detected in e-cigarette products from all of the analysed states and territories, and all retailer types, with an average concentration of 42.7 mg mL–1 in 35 out of 47 samples. Samples generally contained no nicotine labelling or, in the case of two samples, inaccurate labelling. The cytotoxic flavouring chemical cinnamaldehyde was detected in two samples, which were purchased in two different states. Chemicals of unknown toxicity, WS-23 and acetals, were detected in the majority of samples.
Keywords: acetals, chemical composition, electronic cigarette, flavours, gas chromatography–mass spectrometry, nicotine, quantification, regulation, synthetic coolants.
Introduction
Within Australia, regulations regarding electronic cigarettes (e-cigarettes or vapes) vary by state or territory, although the legislation implemented by the federal government applies nationally.1–3 It is unclear how the different regulatory environments of different states influence the e-cigarette products available, both legal and illicit.
Australian federal legislation is currently undergoing reforms in response to the surge in popularity of these products and an extensive illegal market.4 The reforms, first announced in 2023, aim to address the illegal market and reduce the appeal of vaping products to adolescents and will be implemented throughout 2024 and 2025.2,4–6 The Therapeutic Goods (Standard for Therapeutic Vaping Goods) (TGO 110) Order 2021 regulates the composition of e-cigarettes sold in Australia and their packaging.2 This study was conducted when the 2021 version of TGO 1103 was in place. Under this version, all legal e-cigarettes were prohibited from including the following ingredients due to their associated health effects: 2,3-pentanedione, acetoin, benzaldehyde, cinnamaldehyde, diacetyl, ethylene glycol, diethylene glycol and vitamin E acetate.3 Updated product standards to be enforced in 2025 have modified the list of ingredients to include volatiles, nitrosamines and certain metals, and remove vitamin E acetate.2,5 Nicotine-containing products are also required to possess labelling indicating the presence of nicotine and its concentration.2,3 Currently, e-cigarette products in Australia that contain up to 20 mg mL–1 of nicotine can be legally sold to anyone over the age of 18 from a pharmacy, with higher concentrations requiring a prescription.7 At the time of this study, the legal purchase of e-cigarettes containing any concentration of nicotine in Australia required a prescription from a medical practitioner, the only country globally to have this requirement, with products able to be purchased from either an Australian pharmacy or imported into Australia through the personal importation scheme.1,8,9 Despite the legal pathways available to access these products, recent surveys have shown that Australians have accessed their e-cigarette products from a variety of retailer types without a prescription.10,11 This may have been facilitated by the presence of separate legislation for nicotine-containing and nicotine-free products at the time of this study, where nicotine-free products could be sold by any retailer to adults in most Australian regions.12 The removal of any mention of the presence of nicotine from the packaging of nicotine-containing e-cigarettes was observed in an attempt to continue the over-the-counter sale of these products through pathways intended for nicotine-free e-cigarette products.12
State legislation has also been implemented in some Australian states and territories to further restrict access to e-cigarettes. For instance, Western Australia has some of the toughest regulations, still requiring a prescription for purchase regardless of nicotine concentration.13 Access to e-cigarette products from pharmacists also varies between states, with people under the age of 18 unable to access these products in the Australian Capital Territory (ACT), South Australia (SA), Tasmania (Tas.) and Victoria (Vic.).14 At the time of this study, Western Australia (WA) also differed from other states and territories, with all e-cigarettes banned due to their resemblance to tobacco products.15
This paper is the first to analyse the labelling and chemical composition of e-cigarette samples from multiple Australian states or territories and retailer types for compliance with Australian legislation (April to May 2023, prior to the 2024 legislative reforms). This will facilitate a better understanding of e-cigarette product compliance in Australia on a national level and reveal the intricacies of the Australian market, particularly with regard to the sale of illicit nicotine-containing e-cigarettes. A greater understanding of e-cigarette content in Australia is essential to ensure legislation and recent reforms appropriately address current issues.
Experimental
The e-cigarette samples in the present study (N = 47) were a convenience sample set consisting of e-liquids (n = 5), disposable e-cigarettes (n = 41) and a pod (n = 1). All samples were purchased between April and May 2023, before the 2024 legislative reforms were announced and introduced and were thus subject to the 2021 version of the TGO 110 (Therapeutic Goods (Standard for Nicotine Vaping Products) Order 2021).3 Samples were purchased from corner stores (this includes petrol stations and convenience stores), tobacconists, vape stores or were ordered through social media (Facebook) and delivered. The purchased retailer type of five samples was not recorded and these samples have been labelled as unknown. Brands, models and flavours were chosen at random from the limited available products at each store. Samples were purchased from five Australian states and one territory: New South Wales (NSW), Queensland (Qld), SA, Vic., WA and ACT. For full details, see Supplementary Table S1.
Three separate aliquots of each sample were chemically analysed by gas chromatography–mass spectrometry (GC-MS) using the method detailed in Jenkins et al.12 Briefly, mass spectra were obtained using a Shimadzu QP2020 GC-MS single quadrupole system with electron ionisation (Japan) and a Shimadzu SH-Rxi-5Sil MS capillary column (30 m × 0.25 mm × 0.25 µm). The method had a total run time of 15.92 min with a solvent cut time of 1.63 min. Targeted compounds were selected from commonly reported ingredients in the literature, identified in previously analysed samples based on NIST17 matches and compounds prohibited under the TGO 110 legislation.3 Quantification of nicotine, flavouring chemicals and coolant molecules within samples was achieved through the creation of calibration curves using quinoline as the internal standard (25 µg mL–1). See Supplementary Table S2 for the list of chemicals used in this study and Table S3 for calibration curve details.
Results and discussion
At the time of this study, Australia was the only country with a prescription requirement for the purchase of nicotine-containing e-cigarette products.3 In addition to this, restrictions varied between the different states and territories of Australia, with WA having the most stringent rules and completely banning the sale of all e-cigarette products. Although varying restrictions between states is not unique to Australia, e.g. e-cigarette regulations in the US also vary greatly between states and include different restrictions on flavours or varied delivery sale laws,16,17 the differences across the regions may lead to a varied level of compliance countrywide when examining their sale, packaging and content.
Nicotine
Although at the time of this study, the purchase of all nicotine-containing vaping products required any individual to be of 18+ years of age with a valid prescription,3,10,11 all samples in this study were easily purchased across all states and territories from non-pharmacy retailers without a prescription. The five e-liquid refill samples were all labelled as nicotine-free and contained no detectable nicotine (Supplementary Table S4). Although all e-liquids in this study were appropriately labelled as nicotine-free, this dataset is small and previous analysis of Australian e-liquids labelled as nicotine-free by Chivers et al.18 found that similar products contained low nicotine concentrations.
Of the other two device styles analysed, the pod device contained no nicotine, with no labelling to indicate the presence or absence of nicotine, whereas nicotine was detected in 35 of the disposable samples (85.4%). In the disposables, nicotine was always present in the form of the nicotine benzoate salt, confirmed by the presence of benzoic acid (Supplementary Table S4; noting that the current method can only confirm the presence of benzoic acid and salicylic acid, not excluding the possibility that other organic acids (e.g. malic or tartaric acid) could have been used). The average nicotine concentration was 42.7 mg mL–1 and ranged between 30.8 ± 0.3 and 50.0 ± 0.5 mg mL–1. Only one device that contained nicotine was labelled with a nicotine warning and concentration, the HQD Cuvie Plus Melon Ice (DD-124). The labelled concentration listed both 5% and 50 mg mL–1 with a detected concentration of 44.7 ± 0.9 mg mL–1 (Supplementary Table S4), just outside the ±10% concentration accuracy labelling requirement according to the TGO 110. Other disposable products of the same brand and model were also analysed in this study and were found to have no nicotine labelling on the packaging. All but one of the HQD Cuvie Plus samples were labelled with a production date; the earliest of these (dated from 25 August 2021) was the sample which identified the presence and concentration of nicotine. The packaging of the disposable samples with no nicotine labelling were compared to products of the same brand and model from online sources which clearly showed a labelled nicotine concentration. This lack of consistency between the products sold for the Australian market and the same products sold online was consistent with our previous findings, which concluded that e-cigarette manufacturers appear to have deliberately removed nicotine labelling in an attempt to sell these products through the pathway intended for nicotine-free e-cigarette products in Australia.12
Concerningly, of the seven disposables that were labelled as nicotine-free, only six were accurate. The Dragbar F1000 Watermelon Ice (DD-110) sample was labelled as nicotine-free but contained 30.8 ± 0.3 mg mL–1 of nicotine (Supplementary Table S4). Therefore, the information provided to Australian consumers on these products cannot be used to appropriately determine their contents and may result in consumers unknowingly being exposed to nicotine. By combining the regulation of all vaping products under a single legislation and stricter quality control, the latest reforms (TGO 110 2024)5 aim to improve safety for consumers.
Of note, under the regulation in force at the time of this study, all nicotine-free e-cigarette products, including disposable devices, were legally allowed to be purchased (excluding WA) by anyone over the age of 18. The way products were obtained for this study was in agreement with previous studies showing that most people were accessing their products outside of the intended legal pathways.10,11 The illicit sale of nicotine-containing e‑cigarettes was also identified by the Australian Government as a priority and was addressed in the most current legislative reforms.4 These included the prohibition of disposable e-cigarettes, limitation of the sale of any e-cigarette product regardless of nicotine content to pharmacies and the removal of the prescription requirement for products with 20 mg mL–1 of nicotine or less (excluding WA).2,4
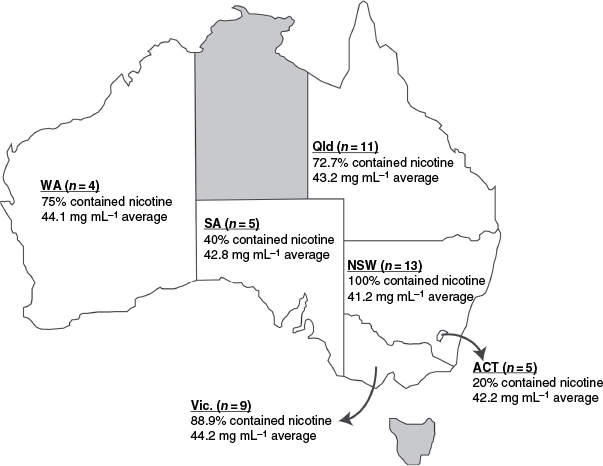
Nicotine-containing samples were purchased from all of the analysed states and territories and retailer types without a prescription or age verification (listed in Fig. 1 and Table 1 respectively). Although the number of samples analysed is limited and not equally distributed across the country, the findings from this study are reflective of the availability of e-cigarettes prior to the recent reforms. For example, the results from WA, where e-cigarettes were already prohibited, demonstrate the ease of access and extensive nature of Australia’s illegal market even with strict regulation. The stricter regulations in WA had no effect on the proportion of devices containing nicotine or the concentration of nicotine present. The percentage of samples that contained nicotine was lowest in the ACT, but this seems to reflect the retailer type they were purchased from, vape stores where they were labelled as nicotine-free, rather than a difference in local regulation. Therefore, the effectiveness of regulation relies on the closure of the illegal market; it remains to be seen whether the current reforms will be successful in preventing the illicit sale of e-cigarettes in Australia.
Summary of the percentage of samples (N = 47) containing nicotine and the average concentration of nicotine in these samples, by location. Sample origin: Australian Capital Territory (ACT), New South Wales (NSW), Queensland (Qld), South Australia (SA), Victoria (Vic.) and Western Australia (WA). The two regions not analysed in this study, Northern Territory and Tasmania, are shown in grey.
| Retailer type | Total number of samples | Samples containing nicotine (%) | Average nicotine concentration (mg mL–1) | |
|---|---|---|---|---|
| Corner store | 21 | 81.0 | 43.7 | |
| Social media | 3 | 100.0 | 43.0 | |
| Tobacconist | 5 | 100.0 | 41.0 | |
| Vape store | 13 | 38.5 | 41.3 | |
| Unknown | 5 | 100.0 | 42.3 |
The study samples were purchased from a variety of retailer types, including corner stores, tobacconists and vape stores across Australia. Vape stores were the most compliant of the analysed retailers, as only 38% of samples (n = 5) contained nicotine. In contrast, the majority of samples from the other analysed retailer types (corner stores, tobacconists and purchased through social media) contained nicotine. It should be noted that only a small number of samples were purchased through social media or from a tobacconist; therefore, a larger dataset would be required to obtain a more representative sample and make more definitive conclusions on trends in the nicotine content of products from these sources. The recent legislative changes, in force after this study was conducted, aimed to address the illegal market of nicotine-containing e-cigarettes through the regulation of all e-cigarettes under the same legislation, regardless of nicotine content.5 As a result of these new reforms, vape stores, which operated mainly to supply e-cigarette products, were the most affected by these changes and most have now closed. The other type of retailers examined in this study, particularly corner stores, appeared to already be supplying the majority of illicit products ‘under the counter’. The effectiveness of the most recent legislative changes in the disruption of supply from these retailer types will be reliant on successful enforcement measures at the state level. There is already anecdotal evidence of stores continuing to sell e-cigarette products despite the stricter regulations.19,20
Synthetic coolants
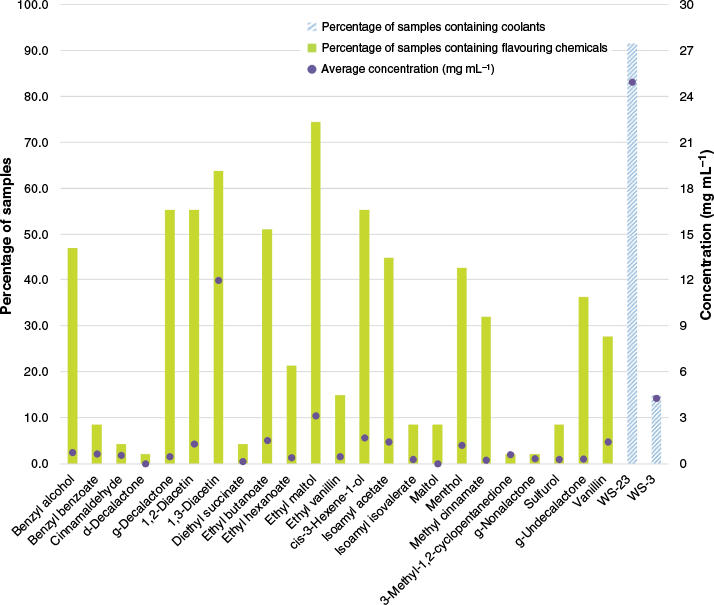
Synthetic coolants are compounds that produce a cooling sensation similar to menthol without the minty taste.21 Coolant data for all samples are provided in Fig. 2. WS-23 was detected in two refill e-liquids at low concentrations (<3 mg mL–1) and no WS-3 was detected. The pod device contained no WS-23 or WS-3. WS-3 was detected in seven disposable samples with an average concentration of 4.3 mg mL–1 and ranging between 0.752 ± 0.005 (DD-124) and 19.1 ± 0.4 mg mL–1 (DD-120) (Supplementary Table S5). All of the disposable samples (n = 41) contained WS-23 with an average concentration of 26.1 mg mL–1 and ranging between 14.0 ± 0.6 (DD-127) and 36 ± 4 mg mL–1 (DD-142) (Supplementary Table S5). This was, on average, the second-most concentrated ingredient in these samples (excluding the two carrier fluids). These results are similar to our previous studies,21 with the coolant WS-23 found in high concentrations in all disposable samples, whereas refill e-liquids contained low concentrations or no detectable WS-23. This trend was present regardless of brand, retailer type or purchase location.
Percentage of samples (N = 47) containing each chemical quantified in this study (flavouring molecules shown in green bars and coolants shown in blue, patterned bars) and the average concentration of each compound in milligrams per millilitre (purple dot).
Coolants were present in the disposable samples regardless of whether they contained a cooling component in their flavour name (e.g. ‘Ice’), indicating that their inclusion may be unrelated to the labelled flavour on the sample. WS-23 may be added to disposable e-cigarettes to reduce the throat irritation of high concentrations of nicotine and flavouring chemicals, potentially increasing uptake in nicotine-naive youth.21 Although there are little published data on the cytotoxicity of the coolant, there is evidence that the presence of WS-23 in concentrations below those observed in this study may produce adverse effects upon inhalation.22,23
Flavouring chemicals
Across all samples, 22 different flavouring chemicals were detected in one or more samples (Fig. 2). The most common flavouring molecules were ethyl maltol and 1,3-diacetin, which were detected in 74.5 and 63.8% of samples respectively. With the exception of 1,3-diacetin (average concentration 11.95 mg mL–1), flavour concentrations were, on average, present in all samples either in low concentrations (<2 mg mL–1) or moderate concentrations (2–5 mg mL–1) compared to other constituents such as nicotine and synthetic coolants. A list of tentative matches identified using the National Institute of Standards and Technology (NIST17) library is provided in Supplementary Table S6.
Acetals, new compounds that can form in the e-liquid solution under normal storage conditions by reactions between some flavouring chemicals and either of the carrier fluids, propylene glycol (PG) or vegetable glycerine (VG),24 were detected in five samples. An example of acetal formation depicting the reaction of benzaldehyde and PG to form the benzaldehyde PG acetal is shown in Fig. 3, noting that benzaldehyde PG acetal is also a known flavouring agent with floral notes.25 Vanillin VG acetals were detected in four samples, two of which also contained the vanillin PG acetals. One sample contained the benzaldehyde PG acetal but no detectable level of the parent flavour, benzaldehyde. The non-detection of the parent aldehyde in this case could be due to a combination of both the flavour addition as the acetal and the equilibrium favouring the product, benzaldehyde PG acetal, resulting in a benzaldehyde concentration below the method limit of detection (Fig. 3).24,26
Acetalisation reaction between benzaldehyde and propylene glycol (PG). The asterisk (*) indicates a chiral carbon.
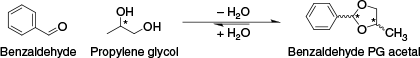
Previous research has found that acetals can be vapourised and would persist within physiological fluids long enough for airway exposure to occur, although little is known about their cytotoxicity.26 One study has established that the PG acetals identified here (vanillin and benzaldehyde PG acetals) were generally more cytotoxic than their parent flavour.27 Not all samples that contained vanillin or other potential acetal forming compounds24 (e.g. ethyl vanillin) contained identifiable levels of acetals. It is unclear whether additional components of the e-liquid may affect acetal formation and more research is required in this area in addition to further investigating the cytotoxicity of these acetals.
Prohibited ingredients
The analysis of these samples occurred prior to the most recent TGO 110 changes and therefore, the eight targeted prohibited compounds were those listed in the 2021 version of the TGO 110.3 Cinnamaldehyde, a potentially harmful chemical prohibited in e-cigarettes under the TGO 110,2,5 was identified in two of the samples (4.3%, Table 2) in low concentrations. The inclusion of cinnamaldehyde was disturbing as this flavouring chemical has been shown to be cytotoxic to monocytic cell types, causing an inflammatory response.28 The sample set contained another device of the same brand, model and flavour (IGET Legend – Cola Lemon Soda) purchased in a different state that did not contain any detectable cinnamaldehyde. As the samples analysed in this study were purchased outside of the intended prescription pathway, these products are illegal and their contents were not, strictly speaking, subject to compliance with the TGO 110. The samples containing cinnamaldehyde originated from two different states (Vic. and Qld) and retailer types (corner store and social media), but both were cola flavoured.
| Sample | Purchase state | Retailer type | Prohibited flavour | Concentration (mg mL–1) | |
|---|---|---|---|---|---|
| HQD Cuvie Plus – Ice Cola | Vic. | Corner Store | Cinnamaldehyde | 0.56 ± 0.01 | |
| IGET Legend – Cola Lemon Soda | Qld | Social Media | Cinnamaldehyde | <LOQ |
Cinnamaldehyde, and other prohibited ingredients, have been previously detected in Australian disposable e-cigarettes.12,29 Although the percentage of products containing these ingredients is small, it poses increased potential risks to anyone who intends to use these products. To improve the overall safety of these products, recent legislation reforms have increased the minimum quality control standards for these products by increasing restrictions on their content.4 This, in addition to the banning of disposables, may contribute to reducing, and ideally eliminating, the presence of these harmful chemicals in vaping products sold on the Australian market. It is important to note that despite tighter regulations, compliance of the chemical content of e-cigarette products will require detailed chemical analysis as the specific flavouring chemicals are not described, nor required to be detailed, on the product’s ingredient list.2,5
Limitations
The current study has several limitations. The absence of samples from Northern Territory and Tasmania prevented the provision of more complete observations. A greater number of samples from all Australian states and territories, especially from states with smaller sample sizes, would be necessary to provide a more comprehensive overview of the prevalence of illicit nicotine-containing e-cigarette products in Australia. However, due to the rapid and numerous legislative reforms,2,6,7,30 which have significantly changed the regulation of these products in Australia, further expansion of the presented sample set was not possible. As most samples were purchased from brick-and-mortar stores, the results presented here were not indicative of labelling and content trends for products available from online sources. Samples were also selected at random among the limited options available at each purchase location and therefore, were not representative of the range of brands and flavours available to Australian consumers. Although the majority of samples analysed in this study were disposable e-cigarettes and reflected the preference for these types of devices among young vapers,31 only a reasonably small number of pods and refill e-liquids were analysed. The results from this study represent the chemical composition of the e-liquid and not the chemical composition of the aerosol produced during the use of an e-cigarette. The amounts of each compound that users are exposed to may differ due to vapourisation efficiency and possible degradation that occurs during vapourisation.
Conclusion
This study analysed e-cigarette products from different retailer types across six Australian states and territories. Nicotine-containing products were sold without a prescription in all of the analysed regions from all of the analysed retailer types, despite the prescription requirement at the time of this study. These products generally did not possess any nicotine labelling despite containing high nicotine concentrations and even when adequate labelling was present, the labelled concentration was not accurate. The inhalational safety of these products was of concern as cinnamaldehyde, a chemical known to be cytotoxic, was found in two of the tested devices in addition to the identification of other chemicals of unknown inhalational toxicity, including coolants and acetals. These results provide insights into the national regulation compliance landscape of e-cigarette use in Australia, with the illicit sale of e-cigarette products, particularly disposables, widely present across Australia regardless of differences in local regulation. E-cigarette products present on the market are rapidly changing and ongoing studies are necessary to continue monitoring these changes and trends, especially in relation to future legislative amendments.
Data availability
All processed data are provided in the Supplementary material and raw data (GC-MS chromatograms) are available upon request by contacting the corresponding author.
Declaration of funding
This project was funded by the University of Wollongong, Faculty of Science, Medicine and Health Small Grant Scheme (2019/SPGHM-S/02 received by Celine Kelso and Jody Morgan) with samples provided by Channel 7 and The Australian news outlets.
Acknowledgements
The authors acknowledge Channel 7 and The Australian news outlets for the donation of samples included in this study, and the University of Wollongong Mass Spectrometry Facility, where all chemical analysis was performed.
References
1 Morgan J, Breitbarth AK, Jones AL. Risk versus regulation: an update on the state of e-cigarette control in Australia. Intern Med J 2019; 49: 110-113.
| Crossref | Google Scholar | PubMed |
2 Therapeutic Goods (Standard for Therapeutic Vaping Goods) (TGO 110) Order 2021. Compilation number 2. Commonwealth of Australia; 2024. Available at https://www.legislation.gov.au/F2021L00595/latest/text [Verified 11 February 2025]
3 Therapeutic Goods (Standard for Nicotine Vaping Products) (TGO 110) Order 2021. Commonwealth of Australia; 2021. Available at https://www.legislation.gov.au/F2021L00595/asmade/text [Verified 11 February 2025]
4 Butler M. Taking action on smoking and vaping. Department of Health and Aged Care; 2023. Available at https://www.health.gov.au/ministers/the-hon-mark-butler-mp/media/taking-action-on-smoking-and-vaping [Verified 11 February 2025]
5 Therapeutic Goods Legislation Amendment (Standard for Therapeutic Vaping Goods) (TGO 110) Instrument 2024. Commonwealth of Australia; 2024. Available at https://www.legislation.gov.au/F2024L01232/latest/text [Verified 11 February 2025]
6 Therapeutic Goods Legislation Amendment (Vaping) Regulations 2023. Commonwealth of Australia; 2023. Available at https://www.legislation.gov.au/F2023L01667/latest/text [Verified 11 February 2025]
7 Therapeutic Goods and Other Legislation Amendment (Vaping Reforms) Bill 2024. Commonwealth of Australia; 2024. Available at https://www.aph.gov.au/Parliamentary_Business/Bills_Legislation/Bills_Search_Results/Result?bId=r7169 [Verified 11 February 2025]
8 Department of Health and Aged Care. Reforms to the regulation of vapes. 2024. Available at https://www.tga.gov.au/products/unapproved-therapeutic-goods/vaping-hub/reforms-regulation-vapes [Verified 8 January 2024]
9 Therapeutic Goods Administration. Nicotine vaping products: Information for consumers. Department of Health and Aged Care; 2021. Available at https://www.tga.gov.au/products/medicines/prescription-medicines/nicotine-vaping-products-hub/nicotine-vaping-products-information-consumers [Verified 6 September 2023]
10 Cohen JE, Gartner C, Edwards R, Hammond D. Australia tightens its prescription-only regulation of e-cigarettes. BMJ 2023; 381: p1216.
| Crossref | Google Scholar | PubMed |
11 Pettigrew S, Miller M, Alvin Santos J, Raj TS, Brown K, Jones A. E-cigarette attitudes and use in a sample of Australians aged 15–30 years. Aust NZ J Public Health 2023; 47: 100035.
| Crossref | Google Scholar | PubMed |
12 Jenkins C, Powrie F, Morgan J, Kelso C. Labelling and composition of contraband electronic cigarettes: analysis of products from Australia. Int J Drug Policy 2024; 128: 104466.
| Crossref | Google Scholar | PubMed |
13 Tobacco Control Branch. Electronic cigarettes in Western Australia. Government of Western Australia; 2024. Available at https://www.health.wa.gov.au/Articles/A_E/Electronic-cigarettes-in-Western-Australia [Verified 10 February 2025]
14 Therapeutic Goods Administration. Prescribing and dispensing of prescription only therapeutic vaping goods to patients under 18 years of age. Department of Health and Aged Care; 2024. Available at https://www.tga.gov.au/products/unapproved-therapeutic-goods/vaping-hub/vapes-information-prescribers/prescribing-and-dispensing-therapeutic-vapes-patients-under-18-years [Verified 11 February 2025]
15 McCausland K, Maycock B, Leaver T, Wolf K, Freeman B, Jancey J. “Is it banned? Is it illegal?”: navigating Western Australia’s regulatory environment for e-cigarettes. Int J Drug Policy 2021; 94: 103177.
| Crossref | Google Scholar | PubMed |
16 Azagba S, Ebling T, Adekeye OT, Hall M, Jensen JK. Loopholes for underage access in e-cigarette delivery sales laws, United States, 2022. Am J Public Health 2023; 113: 568-576.
| Crossref | Google Scholar | PubMed |
17 Siegel M, Katchmar A. Effect of flavored e-cigarette bans in the United States: what does the evidence show? Prev Med 2022; 165: 107063.
| Crossref | Google Scholar | PubMed |
18 Chivers E, Janka M, Franklin P, Mullins B, Larcombe A. Nicotine and other potentially harmful compounds in “nicotine-free” e-cigarette liquids in Australia. Med J Aust 2019; 210: 127-128.
| Crossref | Google Scholar | PubMed |
19 Muroi M. Vape-flation: prices have surged, but retailers are still flouting the ban. In: The Sydney Morning Herald, 21 September 2024. Available at https://www.smh.com.au/politics/federal/vape-flation-prices-have-surged-but-retailers-are-still-flouting-the-ban-20240913-p5kad1.html [Verified 11 February 2025]
20 Cansdale D. Illegal retailers shirk Australia’s world-first anti-vaping laws as black market surges. In: ABC News, 22 November 2024. Available at https://www.abc.net.au/news/2024-11-22/vaping-black-market-surges-despite-laws-anti-vaping-laws/104618796 [Verified 11 February 2025]
21 Jenkins C, Morgan J, Kelso C. Synthetic cooling agents in Australian-marketed e-cigarette refill liquids and disposable e-cigarettes: trends follow the US market. Nicotine Tob Res 2024; 26: 380-384.
| Crossref | Google Scholar | PubMed |
22 Omaiye EE, Luo W, McWhirter KJ, Pankow JF, Talbot P. Disposable puff bar electronic cigarettes: chemical composition and toxicity of e-liquids and a synthetic coolant. Chem Res Toxicol 2022; 35: 1344-1358.
| Crossref | Google Scholar | PubMed |
23 Jabba SV, Erythropel HC, Torres DG, Delgado LA, Woodrow JG, Anastas PT, Zimmerman JB, Jordt S-E. Synthetic cooling agents in US-marketed e-cigarette refill liquids and popular disposable e-cigarettes: chemical analysis and risk assessment. Nicotine Tob Res 2022; 24: 1037-1046.
| Crossref | Google Scholar | PubMed |
24 Gschwend G, Jenkins C, Jones A, Kelso C, Morgan J. A wide range of flavoring–carrier fluid adducts form in e-cigarette liquids. Chem Res Toxicol 2023; 36: 14-22.
| Crossref | Google Scholar | PubMed |
25 2130: Benzaldehyde propylene glycol acetal. Flavor & Extract Manufacturers Association; 2025. Available at https://www.femaflavor.org/flavor-library/benzaldehyde-propylene-glycol-acetal [Verified 19 July 2025]
26 Erythropel HC, Jabba SV, DeWinter TM, Mendizabal M, Anastas PT, Jordt SE, Zimmerman JB. Formation of flavorant–propylene glycol adducts with novel toxicological properties in chemically unstable e-cigarette liquids. Nicotine Tob Res 2019; 21: 1248-1258.
| Crossref | Google Scholar | PubMed |
27 Jabba SV, Diaz AN, Erythropel HC, Zimmerman JB, Jordt S-E. Chemical adducts of reactive flavor aldehydes formed in e-cigarette liquids are cytotoxic and inhibit mitochondrial function in respiratory epithelial cells. Nicotine Tob Res 2020; 22: S25.
| Crossref | Google Scholar | PubMed |
28 Muthumalage T, Prinz M, Ansah KO, Gerloff J, Sundar IK, Rahman I. Inflammatory and oxidative responses induced by exposure to commonly used e-cigarette flavoring chemicals and flavored e-liquids without nicotine. Front Physiol 2018; 8: 1130.
| Crossref | Google Scholar | PubMed |
29 Larcombe A, Allard S, Pringle P, Mead-Hunter R, Anderson N, Mullins B. Chemical analysis of fresh and aged Australian e-cigarette liquids. Med J Aust 2022; 216: 27-32.
| Crossref | Google Scholar | PubMed |
30 Customs Legislation Amendment (Vaping Goods) Regulations 2023. Commonwealth of Australia; 2024. Available at https://www.legislation.gov.au/F2023L01666/latest/text [Verified 11 February 2025]
31 Jenkins C, Powrie F, Kelso C, Morgan J. Chemical analysis and flavor distribution of electronic cigarettes in Australian schools. Nicotine Tob Res 2024; 27: 997-1005.
| Crossref | Google Scholar | PubMed |


