HuGAI1: a key transcription factor upregulated by trypsin, regulating phenylpropanoid biosynthesis, and enhancing fruit shelf life in Hylocereus undatus
Xinyue Pang A * , Xinxin Chen B , Hemin Wang B , Jiaju Sun B , Enyan Chen B , Fuxin Li B , Jingyu Jia B , Bairu Li B and Xin Li B C D *
B C D *
A
B
C
D
Abstract
DELLA proteins can participate in the biosynthesis pathway of flavonoids. It has been shown that trypsin can induce flavonoid synthesis, thereby enhancing the storage quality of Hylocereus undatus (H. undatus) fruit. However, whether trypsin induces flavonoid biosynthesis and improves fruit quality during storage by regulating the phenylpropanoid synthesis pathway through DELLA remains to be further elucidated. To investigate the molecular mechanism of trypsin-induced flavonoid synthesis in H. undatus, we conducted transcriptomic analysis and verified it through virus-induced gene silencing (VIGS). Analysis of transcription factors showed that the top five genes with the largest expression differences regulated by trypsin all belonged to the GRAS family. Further protein network interaction analysis identified HuGAI1 as a hub protein in the GRAS family. Trypsin treatment was able to extend the shelf life of fruit. However, after the expression of HuGAI1 was silenced, the storage quality of the fruit declined. GO and KEGG analysis after HuGAI1 silencing revealed that differentially expressed genes (DEGs) were mainly concentrated in metabolic pathways such as phenylpropanoid, flavonoid, and flavonol biosynthesis. Trypsin can upregulate the expression of HuGAI1. And HuGAI1, by participating in the phenylpropanoid biosynthesis pathway, regulates the biosynthesis of flavonoids and flavonols, leading to an increase in antioxidant flavonoid content and, consequently, enhancing fruit storage.
Keywords: flavonoids, HuGAI1, phenylpropanoid biosynthesis, preservation, transcription factor, transcriptomics, trypsin, virus-induced gene silencing (VIGS).
Introduction
Post-harvest senescence refers to the gradual loss of freshness and quality in fruits after harvesting. During this process, fruits are influenced not only by external environmental factors such as light, temperature, and humidity but also by various internal factors (Tian et al. 2013). The GRAS family is a group of transcription factors widely present in plants, named after the first three identified family members: Gibberellic acid insensitive (GAI), Repressor of GAI (RGA), and Scarecrow (SCR) (Tian et al. 2004). Research has shown that the GRAS family in plants interacts with other transcription factors, participating in various metabolic pathways, thereby influencing plant physiological processes and development (Aoyanagi et al. 2020; Jaiswal et al. 2022; Khan et al. 2022). However, their functions are still being continually studied and explored, and they hold significant importance in revealing the regulatory mechanisms in plants.
DELLA genes are among the first-discovered members of the GRAS family (Peng et al. 1997; Gómez et al. 2019). Currently, in Arabidopsis, including GAI, five DELLA protein family-like genes have been identified (Pysh et al. 1999). DELLA proteins can interact with other transcription factors, thereby regulating the expression of genes involved in flavonoid synthesis (Yoshida et al. 2014; Wang et al. 2020). Research on the functions of DELLA proteins is more extensive in Arabidopsis and some other model plants (Tyler et al. 2004; Blanco-Touriñán et al. 2020; Phokas et al. 2023). For example, in Arabidopsis, DELLA proteins interact and cooperate with the MYB transcription factor TT8 to promote the expression of key genes in the flavonoid synthesis pathway in petals (Xie et al. 2016). Tan et al. (2019) discovered that DELLA proteins (RGA and GAI) in Arabidopsis interact with SG7MYB (MYB12 and MYB111), reducing flavonol biosynthesis to promote root growth. DELLA proteins directly bind to MYBL2 and JAZ repressor factors, forming active complexes of R2R3-MYB, basic helix-loop-helix (bHLH), and WD40-repeat proteins, and then activate the anthocyanin biosynthesis pathway (Xie et al. 2016).
The preliminary results of this study have shown that trypsin can significantly eliminate superoxide anions and induce the biosynthesis of related substances in the phenylpropanoid pathway, such as flavonoids, demonstrating excellent protective effects on plant cells (Li et al. 2019). Flavonoids, as natural antioxidants and protective compounds, possess strong antioxidant capabilities that can help inhibit oxidative reactions in fruits and vegetables, thus extending their shelf life (Xie et al. 2015). Numerous studies have indicated that DELLA proteins can participate in the biosynthesis pathway of flavonoid compounds, influencing the production of flavonoids. However, whether trypsin regulates the phenylpropanoid synthesis pathway through DELLA, affecting the biosynthesis of flavonoids, and thereby enhancing fruit quality during storage, still requires further clarification.
Hylocereus undatus (H. undatus) is rich in nutrients and has a unique flavor, but it has a short shelf life (Huang et al. 2021). Therefore, extending storage time and improving storage quality are important tasks in H. undatus research. In this study, based on H. undatus transcriptome data, hub genes in the GRAS family of H. undatus regulated by trypsin were selected. Through virus-induced gene silencing (VIGS) technology and physicochemical index experiments, it was verified that silencing HuGAI1 reduced the fruit’s storage quality and weakened the preservative effect of trypsin. Finally, using transcriptomic analysis of silenced plants, it was preliminarily demonstrated that HuGAI1 is a key transcription factor induced by trypsin to regulate the phenylpropanoid synthesis pathway and promote the synthesis of flavonoid compounds, thereby preserving the fruit. This research provides a reference for trypsin’s ability to enhance the preservation of H. undatus fruit by influencing GRAS family genes.
Materials and methods
Experimental materials and reagents
H. undatus (Vietnamese variety 1) was harvested from Ruyang County, Luoyang City, China. Fruit selected for the experiments were free from surface mechanical damage, uniform in size, and similar in color.
Trypsin (bovine source, 5 × 105 units/g) was purchased from Amersco, USA.
Treatment procedure
H. undatus fruits were divided into two groups: the control group (CK group) and the trypsin treatment group (Try group). The fruits in the trypsin treatment group were immersed in a trypsin solution of 2.41 × 10−6 mol/L for 80 s. The optimal concentration of trypsin was determined in a previous study (Pang et al. 2020; Li et al. 2021; Zhang et al. 2022) and was used in this research. The control group was immersed in distilled water. For each group, six fruits were used in parallel for the experiment. The fruits were placed in a constant-temperature incubator at 25°C, and observations, photographs, records, and dehydration rate measurements were made every 24 h (Wang et al. 2023a). After 1 week of storage, the treated H. undatus peel was excised using a scalpel, mixed thoroughly, rapidly placed in liquid nitrogen for 3–5 min, and stored at −80°C for subsequent transcriptomic analysis.
Transcriptomic analysis
The eukaryote mRNA sequencing technology primarily utilizes high-throughput sequencing to perform sequence analysis of mRNA expressed in specific tissues and cells at a particular time point. The sequencing process was completed by Shanghai Meiji Biomedical Technology Co., Ltd. In this study, sequencing experiments were conducted using the Illumina TruSeqTM RNA Sample Prep Kit to construct libraries. Total RNA was extracted from tissue samples, and its quantity and purity were measured using a Nanodrop 2000, while integrity was assessed by agarose gel electrophoresis and the RNA Integrity Number (RIN) was determined using an Agilent 2100. Illumina HiSeq sequencing involved the following steps: (1) PCR amplification for 15 cycles to enrich the library; (2) recovery of the target band from a 2% agarose gel; (3) pooling the samples in proportion to the data and quantifying using TBS380 (Picogreen); (4) bridge PCR amplification using cBot to generate clusters; and (5) Illumina HiSeq sequencing (PE library, read length 2 × 150 bp). Differential expression analysis of the transcriptome was conducted using DESeq2 software, which identifies differentially expressed genes (DEGs) between the two samples using strict algorithms with default parameters: p-adjust ≤0.05 and |log2FC| ≥ 1. The obtained sequencing data were then subjected to differential gene expression analysis, Gene Ontology (GO) enrichment analysis, and Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analysis (Zhang et al. 2022).
Transcription factor family analysis
The identification of plant transcription factors was carried out using the Plant Transcription Factor Database PlantTFDB 5.0 (http://planttfdb.gao-lab.org/). The analysis involved examining the domain information present in the gene transcript products for transcription factor prediction and family classification. Clean reads obtained from Illumina sequencing were assembled and then subjected to BlastX alignment against the NR database (E ≤ 1e−5). Based on the annotated gene’s DNA-binding domains and related conserved motifs, transcription factors were annotated and classified (Sarwar et al. 2019).
VIGS vector construction
Total RNA from H. undatus peel was extracted using the RNAprep Pure Micro Kit (DP432, TIANGEN), and cDNA was synthesized using the TransScript® II One-Step gDNA Removal and cDNA Synthesis SuperMix (AH311-02, TransGen). The HuGAI1-specific primers were used for amplification. The primers used for VIGS were 5′CCGGAATTCGCTTTCTGAGTTTAACCCTAACC-3′ (sense) and 5′CGCGGATCCATCTGATGCCGTTTTCTTGTG-3′ (antisense), which were synthesized by Genscript Biotech Corporation. The PCR products were subjected to agarose gel electrophoresis, the target bands were recovered, and then ligated with the pEASY®-Blunt Cloning vector (CB101-01, TransGen Biotech Co., Ltd.). The ligation products were heat-shocked into E. coli TOP10 competent cells, and colonies that tested positive by PCR were sent to Sangon Biotech (Shanghai) Co., Ltd. for sequencing. After confirming the correctness of the sequencing results, the plasmids were digested with EcoR I and BamH I restriction enzymes, and the fragments were ligated with the pTRV2 vector using T4 DNA Ligase to construct the HuGAI1-pTRV2 recombinant plasmid (Zhang et al. 2021). After PCR verification, the recombinant plasmid was transformed into Agrobacterium GV3101 for subsequent experiments.
Preparation and infection of bacterial solution
One percent of the bacterial cultures containing pTRV1, pTRV2, and HuGAI1-pTRV2 were separately inoculated into 20 mL of Luria-Bertani (LB) liquid medium. The cultures were grown at 28°C with shaking at 200 r/min until the bacterial suspension became turbid (24–36 h). Then, 2 mL of bacterial suspension was transferred to 200 mL of LB medium and cultured at 28°C with shaking at 200 r/min until the OD600 of the bacterial suspension reached approximately 0.6. The suspension was then centrifuged at 4000g for 10 min, the supernatant was discarded, and the bacterial pellet was collected. The bacterial pellet was resuspended in freshly prepared infection buffer (containing 200 μmol/L acetosyringone solution (AS), 10 mmol/L 2-(N-morpholino) ethanesulfonic acid (MES), and 10 mmol/L MgCl2) (Zhang et al. 2023). The suspension containing pTRV1 was mixed in equal volumes with the suspension containing HuGAI1-pTRV2, and the mixture was used to infect H. undatus peel by injection (Li et al. 2024). For the control experiment, the suspension containing pTRV1 was mixed in equal volumes with the suspension containing pTRV2 empty vector and injected into peel of H. undatus. The injected fruits were kept in the dark for 48 h. After infection, the fruits were soaked in trypsin solution and then stored under normal light conditions.
RT-qPCR validation and physicochemical index determination
The experiment used the perfectStart® Green qPCR Supermix kit for RT-qPCR. The experiments were performed in 20 μL of reaction volume and SYBR Premix Ex TaqII (TakaRa) and an iCycleriQ multi-color real-time PCR Detection System (Bio-Rad, California, CA, USA) was used (Yang et al. 2017). H. undatus Actin gene was used as the reference gene (Nie et al. 2015), with primers listed in Table 1. The relative expression levels of the gene in the two sample groups were calculated using the 2−ΔΔCt method (Li et al. 2022). The weight loss rate was periodically measured using Wang’s method (Wang et al. 2023a). Flavonoid content was determined using the method of Ghasemzadeh et al. (2016).
| Gene name | Gene ID | Primer sequence | |
|---|---|---|---|
| HuGAI1 | TRINITY_DN40817_c0_g1 | 5′-CCTTCTCCATCGTCAATCTCACC-3′ | |
| 3′-CTTGTGAGTCTACCAGCACCACC-5′ | |||
| Actin | Nie et al. (2015) | 5′-TCTGCTGAGCGAGAAAT-3′ | |
| 3′-AGCCACCACTAAGAACAAT-5′ | |||
| HuSCL1 | TRINITY_DN50311_c0_g1 | 5′-AGCTCATCCACCCATCAGATGC-3′ | |
| 3′-CACTCTTCATCAAGCTCCATGC-5′ | |||
| HuSCL4 | TRINITY_DN42012_c0_g1 | 5′-TCTGCTACAAGCCCTGGCAAC-3′ | |
| 3′-GCGACCAGGGTTTCGTCTAAG-5′ | |||
| HuSCL14 | TRINITY_DN51826_c3_g2 | 5′-CAAATGAAAAGGAGGCGGCCC-3′ | |
| 3′-CAGGGATGTTCCCTTCTCGGA-5′ | |||
| HuSCL3 | TRINITY_DN47379_c5_g1 | 5′-TTACTAAGGGCTTGGCAGCCC-3′ | |
| 3′-CAGGTCGAACACTCAATGCCT-5′ |
Statistical analysis
Significance tests and correlation analyses were conducted using SPSS (11.0.1) software. Principal component analysis (PCA) was employed to analyze intergroup differences. All experiments were set up with three replicates, and paired sample t-tests were used to analyze inter-sample variances. Significance or high significance was evaluated with P-values of <0.05 or <0.01 respectively (Liu et al. 2021).
Results
Gene expression analysis in control H. undatus and H. undatus treated with trypsin
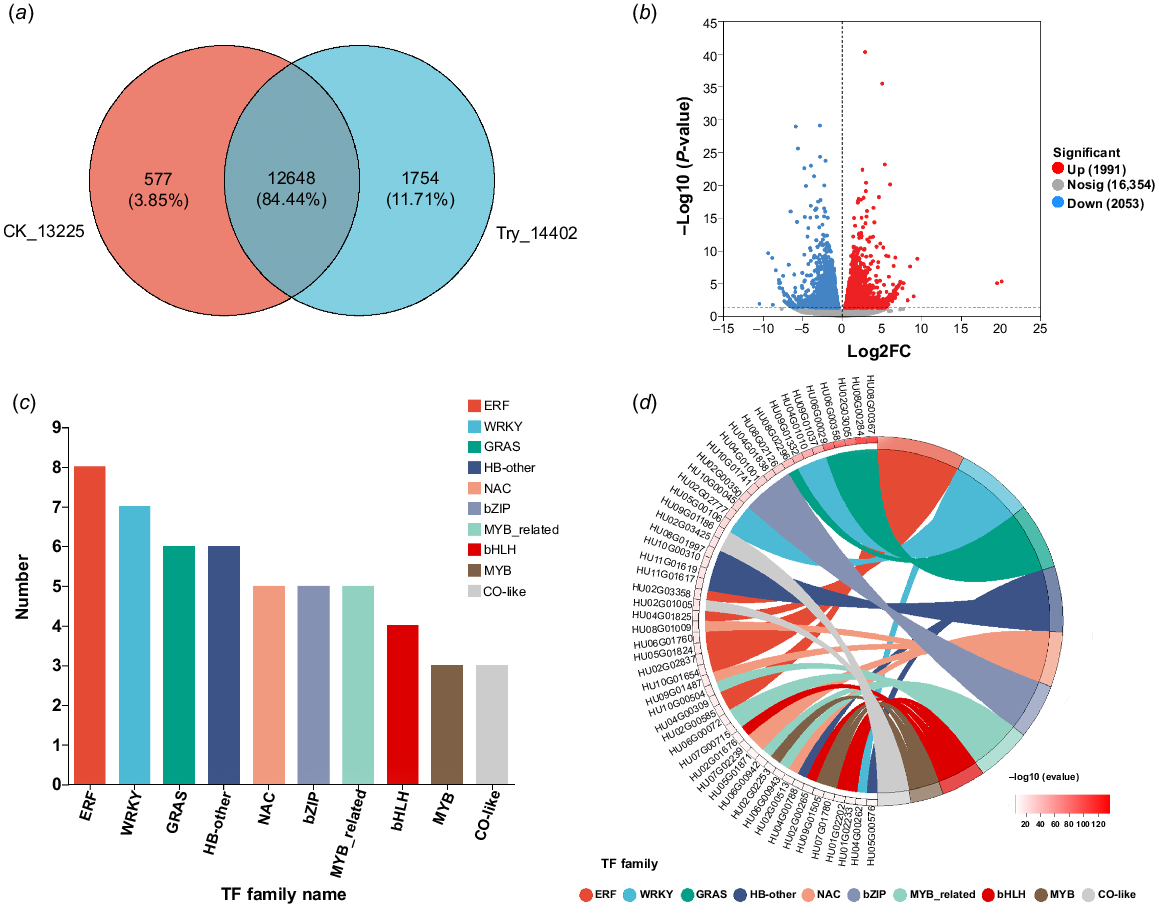
Comparative analysis was conducted between the CK group and the Try group during the transcriptome process using the public databases including Swiss-Prot, NR, COG, Pfam, GO, and KEGG. A Venn plot showing the unique and common genes between the two samples revealed that the CK group had 577 unique genes, the Try group had 1754 unique genes, and both groups shared 12,648 genes (Fig. 1a). A total of 20,398 genes were identified from the differential expression analysis, with 1991 genes upregulated and 2053 genes downregulated (Fig. 1b). The DEGs were used to construct gene sets, and transcription factor prediction and family analysis of these genes were performed using the PlantTF database. Based on the transcription factor prediction results, the transcription factor families were statistically analyzed (Fig. 1c). It was found that they mainly concentrated in 10 families, including ERF, WRKY, GRAS, NAC, among others, with the GRAS family containing six genes. From the chord plot of transcription factor family analysis (Fig. 1d). It was found that the top five genes with the most significant expression belong to the GRAS family. This suggests that trypsin may exert its preservation effect on H. undatus by influencing the GRAS family.
Transcriptomic analysis of DEGs regulated by trypsin. (a) Venn plot between samples. (b) Volcano plot of expression difference analysis between samples. (c) Bar graph of transcription factor family analysis of DEGs. (d) Chordal graph of transcription factor family analysis of expressed DEGs. On the left side are the genes, with the −log10 of the evalue shown in descending order from top to bottom. The genes are arranged in order of significance (evalue), with a larger evalue indicating more significant gene expression. On the right side are the transcription factor families to which the genes belong.
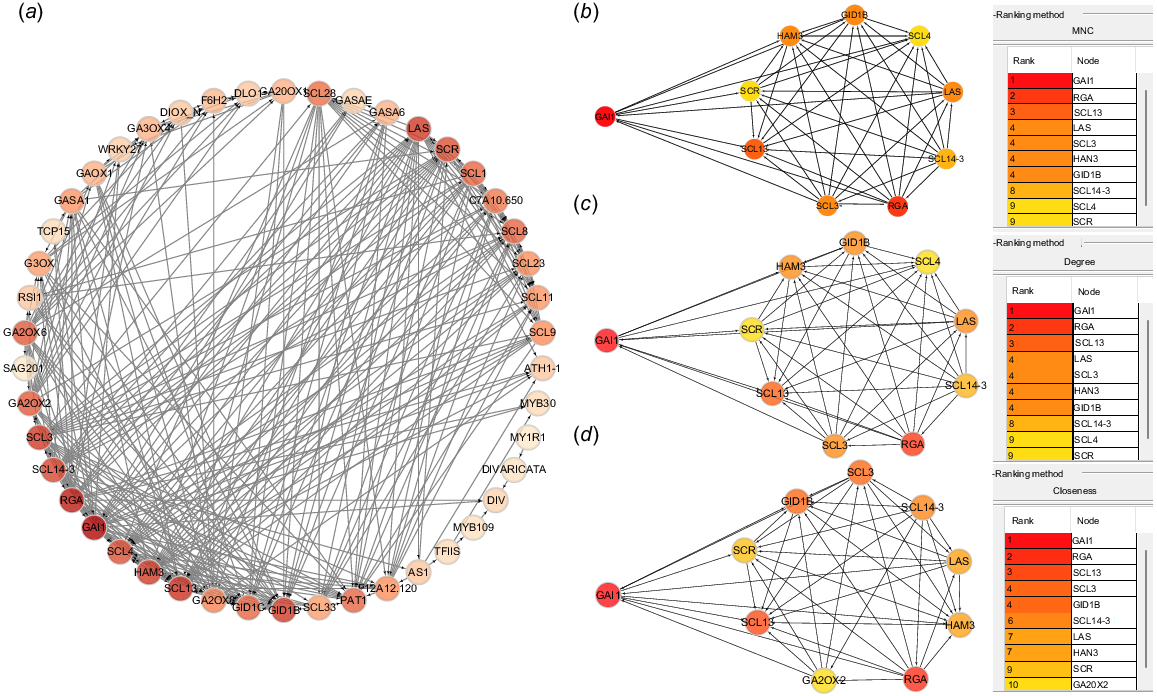
A total of 68 GRAS family-related genes were selected through transcriptomic analysis, and a PPI network was constructed using Cytoscape for correlation analysis (Fig. 2a). The co-expression network constructed using Cytoscape consisted of 46 nodes and 288 edges. The Top 10 proteins were analyzed among the 68 genes. Hub proteins in the GRAS family were identified as HuGAI1 based on maximum neighborhood component (MNC), degree, and closeness centrality (Fig. 2b–d).
Fig. 3a shows the specific primers and sequencing sequence alignment of HuGAI1. By observing the phenotypic changes of the fruit, it was found that at the early stage of storage, all H. undatus fruits had bright colors and good quality. After 12 days of storage, the scales of the CK group fruits were almost completely dried, and the overall color of the fruit became dull. The scales of the Try group were dried, but the appearance remained relatively good. The T-pTRV2 (empty vector) group fruits had slight yellow spots, with no significant difference from the Try group. However, in the TV-GAI1 (silenced HuGAI1) group, the fruit scales were completely wilted, and the decay began from the scales. Obvious lesions appeared on the surface of the fruit (Fig. 3b).
Detection of HuGAI1 gene silencing sequence and physicochemical indicators of H. undatus fruit. (a) Sequence comparison of HuGAI1 gene fragment sequencing results. (b) Phenotypic changes of H. undatus after different treatments. (c) H. undatus weight loss rate. (d) The total flavonoid content in H. undatus peel. Data are presented as mean values ± s.d. The style of connecting is spline. The area under the curve is filled. A paired two-tailed t-test was used for all statistical analyses. No adjustments were made for multiple comparisons. *Represents P < 0.05, and **represents P < 0.01. Note: CK represents blank control group, Try represents trypsin treated group, T-pTRV2 represents trypsin-treated empty vector group; TV-GAI1 represents the VIGS group. 0 d or 12 d represent fruit storage for 0 days or 12 days.
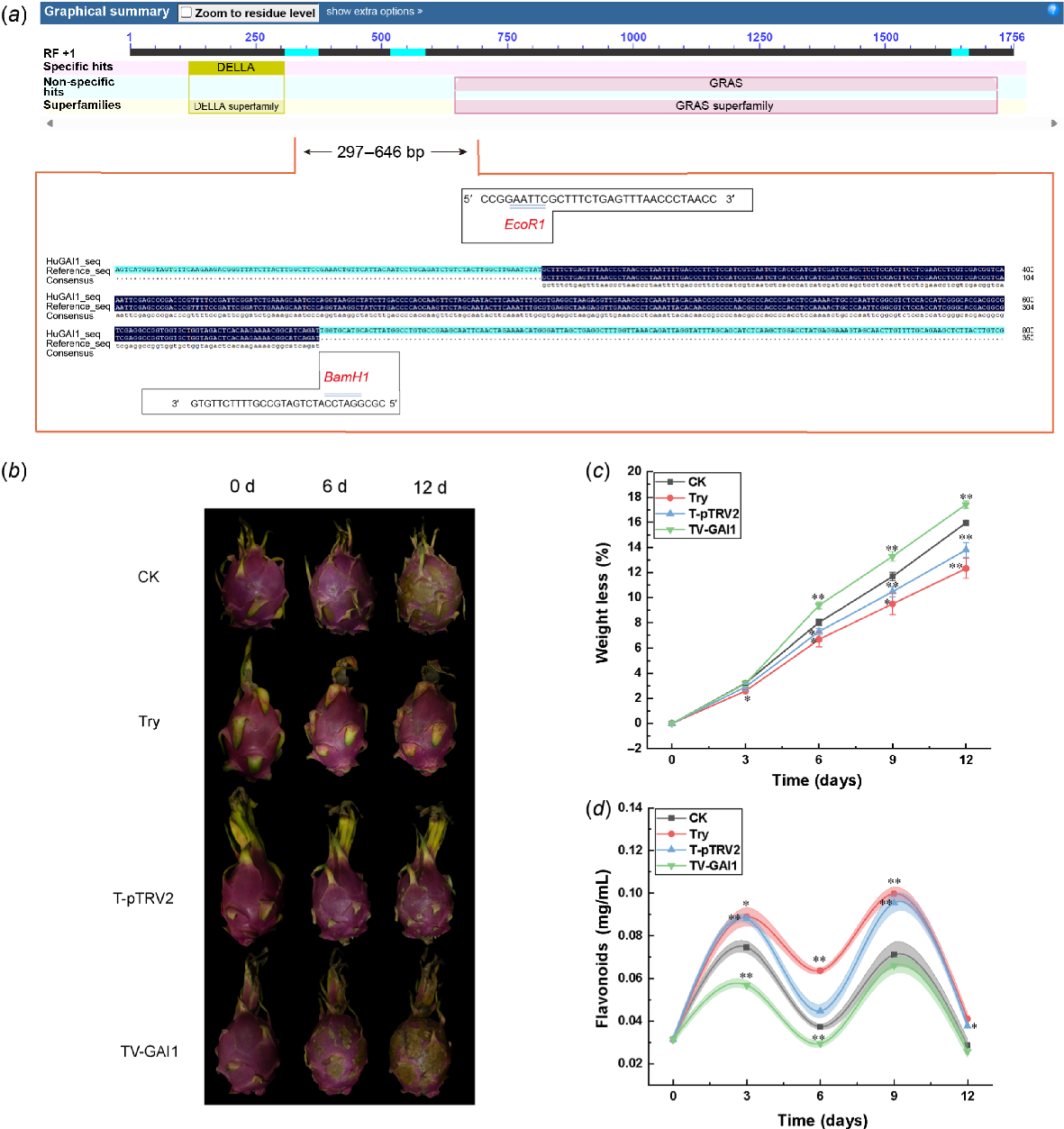
With the increase in storage days, the weight loss rate of H. undatus in all groups increased, with the TV-GAI1 group showing the fastest rate of weight loss. After 12 days of storage, the TV-GAI1 group had the highest weight loss rate (17.41%), followed by the CK group (15.95%), the T-pTRV2 group (13.79%), and the Try group, which had the lowest weight loss rate (12.32%). This suggests that silencing the HuGAI1 gene exacerbated the water loss of the fruit, leading to accelerated decay of H. undatus (Fig. 3c).
During the storage period, the flavonoid content in H. undatus peel was higher on the 3rd and 9th day (Fig. 3d), showing a ‘bimodal’ pattern. Throughout the storage process, the flavonoid content in the TV-GAI1 group remained at a lower level, lower than the other three groups. On the 9th day, the TV-GAI1 group had a lower flavonoid content (0.065 mg/mL), while the Try group (0.099 mg/mL) and the T-pTRV2 group (0.095 mg/mL) had higher flavonoid content, which was higher than that of the CK group (0.077 mg/mL). On the 12th day, the flavonoid content in the protease-treated H. undatus peel remained higher than in the TV-GAI1 group (0.024 mg/mL). Silencing of the HuGAI1 gene resulted in a decrease in the flavonoid content of the fruit peel, while the flavonoid content of H. undatus peel treated with trypsin remained at a high level, indicating that trypsin can improve the preservation effect by increasing the flavonoid content in H. undatus peel.
Transcriptomic analysis of HuGAI1 silencing lines
A comparison of the transcriptomic data between the trypsin-treated group (Try) and the HuGAI1 silencing group (TV-GAI1) was conducted to analyze DEGs. The results of PCA analysis (Fig. 4a) indicated that biological replicates of each group, as well as the three samples, clustered together. This suggests that the gene data from the different H. undatus groups had good stability, with no significant deviations during the measurement process. The contribution rate of Principal Component 1 (PC1) in PCA analysis was 41.65%, while that of Principal Component 2 (PC2) was 13.33%. Different samples showed clear separation trends in the two dimensions, indicating certain differences in the transcriptomes of H. undatus from different groups.
Differentially expressed gene (DEG) analysis between HuGAI1 silencing and trypsin-treated groups. (a) PCA plot between samples. (b) Up/down plot of DEGs. (c) Venn diagram of DEGs. (d) Bubble plot of GO analysis of flavonoid related DEGs. (e) KEGG pathway map of significantly enriched flavonoid related genes. The vertical axis indicates the GO term and KEGG pathway to which multiple genes were enriched in the set, the horizontal axis indicates the ratio of the number of DEGs annotated to the GO term and KEGG pathway to the total number of DEGs, the size of the dots represents the number of genes annotated to the GO term and KEGG pathway, and the color of the dots corresponds to the different p-adjust ranges.
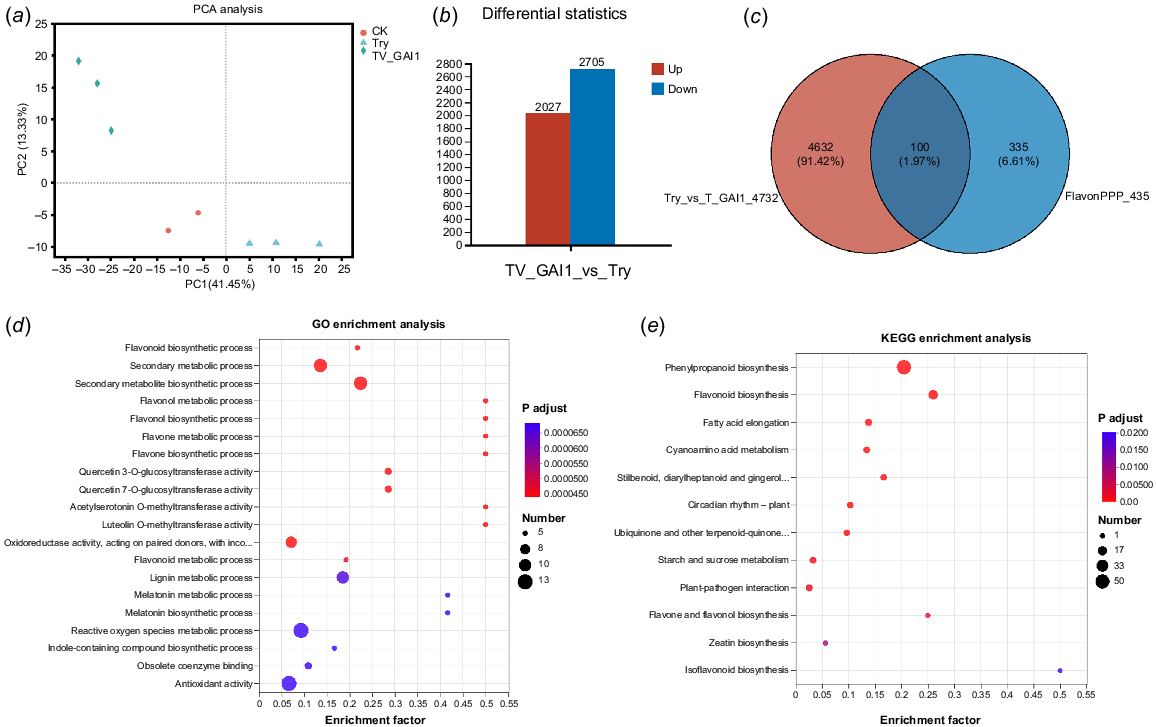
Based on the differential screening criteria (p-adjust <0.05, |log2FC| >= 1), a total of 4732 DEGs were identified between the Try group and the TV-GAI1 group (Fig. 4b). Among these, 2027 genes were upregulated, and 2705 genes were downregulated. The detailed information of these 4732 DEGs is shown in Supplementary Table S1. Venn analysis was performed on 435 genes related to the synthesis of flavonoids and other phenylpropanoid biosynthesis from transcriptome data, as well as 4732 DEGs silenced by HuGAI1. A total of 100 genes were annotated to coexist in both gene sets (Fig. 4c).
In order to further elucidate the functions of differentially expressed HuGAI1 genes and their metabolic pathways under trypsin regulation, we performed GO and KEGG enrichment analysis on the annotated 100 genes.
The GO functional enrichment analysis was performed to validate the enrichment of DEGs in ‘Cellular Component’ (CC), ‘Molecular Function’ (MF), and ‘Biological Process’ (BP) categories (Table S2). Top five enriched terms for the 100 DEGs were mainly in the BP category, including flavonoid biosynthetic process, secondary metabolic process, secondary metabolite biosynthetic process, flavonol metabolic process, and flavonol biosynthetic process (Fig. 4d). The results of GO enrichment analysis suggest that in the fruit preservation process of H. undatus, trypsin achieves preservation by inducing the biosynthesis and metabolism of flavonoid compounds.
In KEGG enrichment analysis, 100 DEGs were annotated, and the KEGG enrichment annotation results are displayed in a bubble chart, indicating annotations to 12 KEGG pathways (Fig. 4e). Among these, DEGs were mainly enriched in the biosynthesis of phenylpropanoids and flavonoids, with 50 genes and 19 genes, respectively. The pathway with the most annotated genes was the biosynthesis of phenylpropanoids. The results of KEGG pathway enrichment analysis suggest that under the regulation of trypsin, HuGAI1 affects genes in the phenylpropanoid biosynthesis pathway, which in turn affects the synthesis of flavonoid compounds. This plays a crucial role in promoting H. undatus preservation.
RT-qPCR validation
Through RT-qPCR experiments, it was validated that compared to the CK group, the expression of HuGAI1 was upregulated in the Try group, the expression of HuGAI1 in the T-pTRV2 group was similar to that in the Try group, and the expression of HuGAI1 in the TV-GAI1 group was downregulated, indicating that the silencing vector was successfully constructed (Fig. 5a).
Schematic diagram of the expression of some genes in H. undatus after trypsin treatment by RNA-seq and RT-qPCR. (a) Expression of HuGAI1 in different samples. (b) RNA-seq analysis and RT-qPCR confirmation of the expression of 5 GRAS family genes in H. undatus peel of CK group and Try group. The FC (Try/CK) indicates the Fold Change of expression of genes in Try and CK group. **P < 0.01.
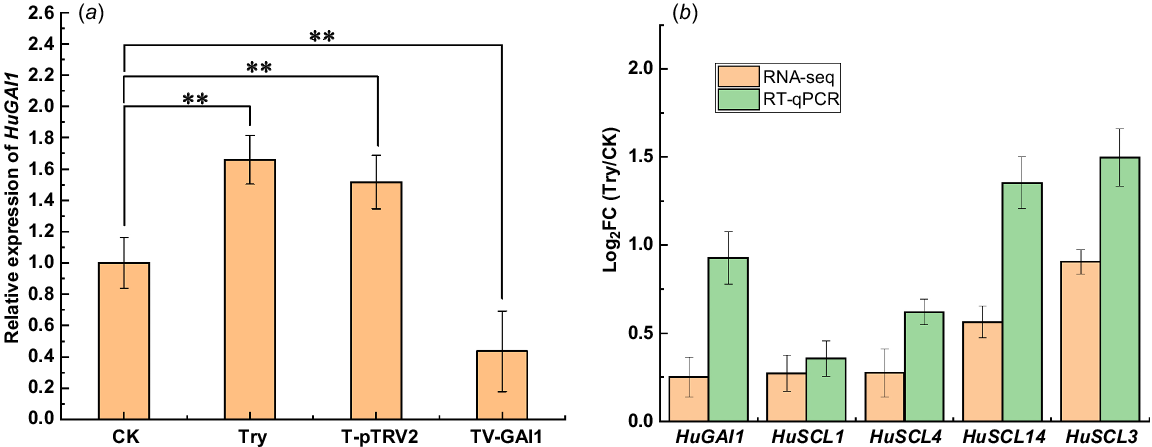
Additionally, RT-qPCR was used to assess the expression changes of four GRAS family genes in the CK group and Try group. The gene information is provided in Table 1. The gene expression results from RT-qPCR were consistent with the RNA-seq data, confirming the reliability of the transcriptome data (Fig. 5b).
Discussion
DELLA family genes play a role in plant growth and development, and their functions have been extensively studied in crops such as Arabidopsis, rice, and wheat (Tian et al. 2004; Locascio et al. 2013). It has been reported that GAI, a member of the DELLA family, is involved in the response to drought stress in Arabidopsis by regulating plant antioxidant capacity and osmotic regulation (Zhang et al. 2011). In this study, PPI network interaction analysis of DEGs were performed in H. undatus, and HuGAI1 was selected as the hub protein from the GRAS transcription factor family.
The application of trypsin treatment to post-harvest fruits and vegetables is an effective method to slow down fruit senescence and decay (Li et al. 2017). Transcriptomic results showed that trypsin upregulated the expression of HuGAI1. However, whether the low expression of HuGAI1 can weaken fruit storage quality, thereby promoting fruit senescence and decay, needs further validation. Therefore, we used VIGS technology to silence HuGAI1, and the experimental results showed that after 12 days of storage, the silencing group had the fastest increase in fruit weight loss rate, while the trypsin-treated group had the lowest weight loss rate. Furthermore, the results of this study show a bimodal trend of flavonoid compounds during the storage of H. undatus fruit (Li et al. 2024). Treatment with trypsin maintained a higher flavonoid content in the fruit peel, while silencing the HuGAI1 gene significantly reduced the flavonoid content. This suggests that trypsin can preserve the moisture content of the fruit and the high flavonoid levels in the peel, enhancing storage quality of fruit and thereby delaying the decay of H. undatus fruit.
The changes induced by trypsin treatment are directly related to the signal mechanisms involved in phenylpropanoid biosynthesis, flavonoid biosynthesis, and flavonol biosynthesis pathways. Pang et al.’s research (Pang et al. 2021) indicated that trypsin controlled the fruit storage quality by promoting the biosynthesis of endogenous flavonoids, particularly the biosynthesis of catechin gallate. Trypsin also activated CsMYC2, which promoted the biosynthesis of endogenous phenylpropanoids in cucumber fruits (Wang et al. 2023a). These pathways play a role in cell growth, fruit maturation, senescence, and related mechanisms (Farag et al. 2008; Chen et al. 2019). Currently, research on the metabolism pathways of flavonoid compounds is mainly focused on Arabidopsis, and their synthesis begins with the phenylpropanoid metabolic pathway (Wang et al. 2019; Dong and Lin 2021). Combining the transcriptomic analysis after VIGS silencing of HuGAI1, GO enrichment analysis of DEGs revealed that their biological functions were mainly related to flavonoid biosynthetic processes. Furthermore, through KEGG pathway enrichment, it was found that the most highly enriched metabolic pathway was phenylpropanoid biosynthesis, followed by flavonoid and flavonol biosynthesis. The results of GO and KEGG enrichment analysis suggest that trypsin, as a novel superoxide scavenger (Li et al. 2021; Pang et al. 2021; Wang et al. 2023b, 2023a), has a regulatory effect on the phenylpropanoid biosynthesis system.
To summarise, HuGAI1 was a key transcription factor involved in the regulation of phenylpropanoid compound synthesis by trypsin. Under the action of trypsin, HuGAI1 participated in the biosynthesis pathways of phenylpropanoids, flavonoids, and flavonols, leading to an increase in the content of antioxidant flavonoid compounds. This, in turn, enhanced the fruit’s resistance to senescence, ultimately achieving the goal of delaying the senescence and decay of H. undatus. The research presented in this study provided new theoretical support for understanding the mechanism by which trypsin enhanced the preservation of fruits and vegetables.
Conclusion
Trypsin, as a novel biological preservative, significantly delays the dehydration process of fruits and improves their quality. Previously, our laboratory had discovered that Trypsin possesses the capability to eliminate superoxide anions and exerts a significant regulatory effect on the antioxidant system of H. undatus peel. In this study, through transcriptomic data analysis, we identified the HuGAI1 transcription factor. Subsequently, we confirmed through VIGS that after silencing HuGAI1, the fruit’s water loss rate increased, the total flavonoid content in the peel decreased, and the storage quality of H. undatus fruit significantly deteriorated. Combining transcriptomic analysis, GO enrichment results indicated a significant enrichment in the flavonoid biosynthesis process. Moreover, KEGG enrichment analysis revealed that the phenylpropanoid biosynthesis pathway had the highest enrichment rate. These results further validate the ability of HuGAI1 to regulate the phenylpropanoid biosynthesis pathway, enhance the content of flavonoid compounds, and thereby achieve the goal of preserving fruits and vegetables.
Data availability
The NCBI BioProject accession number is PRJNA509494. The link with the data is https://trace.ncbi.nlm.nih.gov/Traces/sra_sub/sub.cgi?acc=SRP173572.
Declaration of funding
This work was supported by the National Key Research and Development Program of China (2017YFC1600802).
Author contributions
HM Wang: writing, original draft and editing; EY Chen, FX Li, JY Jia, XX Chen, BR Li, JJ Sun: formal analysis, investigation, preparation; X Li and XY Pang: editing.
Acknowledgements
The authors thank Prof. Jianye Chen of South China Agricultural University for providing pTRV2 VIGS vectors and Agrobacterium strain (GV3101) for this work. We are grateful for the free online platform of Majorbio I-Sanger Cloud Platform (www.i-sanger.com).
References
Aoyanagi T, Ikeya S, Kobayashi A, Kozaki A (2020) Gene regulation via the combination of transcription factors in the INDETERMINATE DOMAIN and GRAS families. Genes 11(6), 613.
| Crossref | Google Scholar | PubMed |
Blanco-Touriñán N, Serrano-Mislata A, Alabadí D (2020) Regulation of DELLA proteins by post-translational modifications. Plant and Cell Physiology 61(11), 1891-1901.
| Crossref | Google Scholar | PubMed |
Chen X, Wang H, Li X, Ma K, Zhan Y, Zeng F (2019) Molecular cloning and functional analysis of 4-Coumarate: CoA ligase 4 (4CL-like 1) from Fraxinus mandshurica and its role in abiotic stress tolerance and cell wall synthesis. BMC Plant Biology 19(1), 231.
| Crossref | Google Scholar | PubMed |
Dong N-Q, Lin H-X (2021) Contribution of phenylpropanoid metabolism to plant development and plant–environment interactions. Journal of Integrative Plant Biology 63(1), 180-209.
| Crossref | Google Scholar | PubMed |
Farag MA, Huhman DV, Dixon RA, Sumner LW (2008) Metabolomics reveals novel pathways and differential mechanistic and elicitor-specific responses in phenylpropanoid and isoflavonoid biosynthesis in Medicago truncatula cell cultures. Plant Physiology 146(2), 323-324.
| Crossref | Google Scholar |
Ghasemzadeh A, Jaafar HZE, Rahmat A (2016) Variation of the phytochemical constituents and antioxidant activities of Zingiber officinale var. rubrum theilade associated with different drying methods and polyphenol oxidase activity. Molecules 21(6), 780.
| Crossref | Google Scholar |
Gómez MD, Fuster-Almunia C, Ocaña-Cuesta J, Alonso JM, Pérez-Amador MA (2019) RGL2 controls flower development, ovule number and fertility in Arabidopsis. Plant Science 281, 82-92.
| Crossref | Google Scholar | PubMed |
Huang Y, Brennan MA, Kasapis S, Richardson SJ, Brennan CS (2021) Maturation process, nutritional profile, bioactivities and utilisation in food products of red pitaya fruits: a review. Foods 10(11), 2862.
| Crossref | Google Scholar | PubMed |
Jaiswal V, Kakkar M, Kumari P, Zinta G, Gahlaut V, Kumar S (2022) Multifaceted roles of GRAS transcription factors in growth and stress responses in plants. iScience 25(9), 105026.
| Crossref | Google Scholar | PubMed |
Khan Y, Xiong Z, Zhang H, Liu S, Yaseen T, Hui T (2022) Expression and roles of GRAS gene family in plant growth, signal transduction, biotic and abiotic stress resistance and symbiosis formation – a review. Plant Biology 24(3), 404-416.
| Crossref | Google Scholar | PubMed |
Li X, Tang Z, Pang X, Zhao C, Li X, Liu Y (2017) Trypsin slows the aging of mice due to its novel superoxide scavenging activity. Applied Biochemistry and Biotechnology 181(4), 1549-1560.
| Crossref | Google Scholar | PubMed |
Li X, Liu X, Yin Y, Yu H, Zhang M, Jing H, Ma Y, Xiong X, Pang X (2019) Transcriptomic analysis reveals key genes related to antioxidant mechanisms of Hylocereus undatus quality improvement by trypsin during storage. Food & Function 10(12), 8116-8128.
| Crossref | Google Scholar | PubMed |
Li X, Zhang Y, Zhao S, Li B, Cai L, Pang X (2021) Omics analyses indicate the routes of lignin related metabolites regulated by trypsin during storage of pitaya (Hylocereus undatus). Genomics 113(6), 3681-3695.
| Crossref | Google Scholar | PubMed |
Li X, Zhang Y, Wu Y, Li B, Sun J, Gu S, Pang X (2022) Lipid metabolism regulated by superoxide scavenger trypsin in Hylocereus undatus through multi-omics analyses. Journal of Food Biochemistry 46(7), e14144.
| Crossref | Google Scholar | PubMed |
Li X, Li B, Gu S, Pang X, Mason P, Yuan J, Jia J, Sun J, Zhao C, Henry R (2024) Single-cell and spatial RNA sequencing reveal the spatiotemporal trajectories of fruit senescence. Nature Communications 15(1), 3108.
| Crossref | Google Scholar | PubMed |
Liu J, Gao Y, Gong F, Hou F, Zhang Z, Cheng X, Du W, Zhang L, Wang J, Xu J, Xing G, Kang X, Li S (2021) The transcriptome and metabolome reveal stress responses in sulfur-fumigated Cucumber (Cucumis sativus L.). Frontiers in Plant Science 12, 778956.
| Crossref | Google Scholar | PubMed |
Locascio A, Blázquez MA, Alabadí D (2013) Genomic analysis of DELLA protein activity. Plant & Cell Physiology 54(8), 1229-1237.
| Crossref | Google Scholar | PubMed |
Nie Q, Gao G-L, Fan Q-J, Qiao G, Wen X-P, Liu T, Peng Z-J, Cai Y-Q (2015) Isolation and characterization of a catalase gene “HuCAT3” from pitaya (Hylocereus undatus) and its expression under abiotic stress. Gene 563(1), 63-71.
| Crossref | Google Scholar | PubMed |
Pang X, Li X, Liu X, Cai L, Li B, Li X (2020) Transcriptomic analysis reveals Cu/Zn SODs acting as Hub genes of SODs in Hylocereus undatus induced by trypsin during storage. Antioxidants 9(2), 162.
| Crossref | Google Scholar | PubMed |
Pang X, Zhao S, Zhang M, Cai L, Zhang Y, Li X (2021) Catechin gallate acts as a key metabolite induced by trypsin in Hylocereus undatus during storage indicated by omics. Plant Physiology and Biochemistry 158, 497-507.
| Crossref | Google Scholar | PubMed |
Peng J, Carol P, Richards DE, King KE, Cowling RJ, Murphy GP, Harberd NP (1997) The Arabidopsis GAI gene defines a signaling pathway that negatively regulates gibberellin responses. Genes & Development 11(23), 3194-3205.
| Crossref | Google Scholar | PubMed |
Phokas A, Meyberg R, Briones-Moreno A, Hernandez-Garcia J, Wadsworth PT, Vesty EF, Blazquez MA, Rensing SA, Coates JC (2023) DELLA proteins regulate spore germination and reproductive development in Physcomitrium patens. The New Phytologist 238(2), 654-672.
| Crossref | Google Scholar | PubMed |
Pysh LD, Wysocka-Diller JW, Camilleri C, Bouchez D, Benfey PN (1999) The GRAS gene family in Arabidopsis: sequence characterization and basic expression analysis of the SCARECROW-LIKE genes. The Plant Journal 18(1), 111-119.
| Crossref | Google Scholar | PubMed |
Sarwar MB, Ahmad Z, Rashid B, Hassan S, Gregersen PL, Leyva MdlO, Nagy I, Asp T, Husnain T (2019) De novo assembly of Agave sisalana transcriptome in response to drought stress provides insight into the tolerance mechanisms. Scientific Reports 9(1), 396.
| Crossref | Google Scholar | PubMed |
Tan H, Man C, Xie Y, Yan J, Chu J, Huang J (2019) A crucial role of GA-regulated flavonol biosynthesis in root growth of Arabidopsis. Molecular Plant 12(4), 521-537.
| Crossref | Google Scholar | PubMed |
Tian C, Wan P, Sun S, Li J, Chen M (2004) Genome-wide analysis of the GRAS gene family in rice and Arabidopsis. Plant Molecular Biology 54(4), 519-532.
| Crossref | Google Scholar | PubMed |
Tian S, Qin G, Li B (2013) Reactive oxygen species involved in regulating fruit senescence and fungal pathogenicity. Plant Molecular Biology 82(6), 593-602.
| Crossref | Google Scholar | PubMed |
Tyler L, Thomas SG, Hu J, Dill A, Alonso JM, Ecker JR, Sun T-P (2004) Della proteins and gibberellin-regulated seed germination and floral development in Arabidopsis. Plant Physiology 135(2), 1008-1019.
| Crossref | Google Scholar | PubMed |
Wang R, Wang G-L, Ning Y (2019) PALs: emerging key players in broad-spectrum disease resistance. Trends in Plant Science 24(9), 785-787.
| Crossref | Google Scholar | PubMed |
Wang P, Zhang Q, Chen Y, Zhao Y, Ren F, Shi H, Wu X (2020) Comprehensive identification and analysis of DELLA genes throughout the plant kingdom. BMC Plant Biology 20(1), 372.
| Crossref | Google Scholar | PubMed |
Wang J, Tian P, Sun J, Li B, Jia J, Yuan J, Li X, Gu S, Pang X (2023a) CsMYC2 is involved in the regulation of phenylpropanoid biosynthesis induced by trypsin in cucumber (Cucumis sativus) during storage. Plant Physiology and Biochemistry 196, 65-74.
| Crossref | Google Scholar |
Wang J, Jia J, Sun J, Pang X, Li B, Yuan J, Chen E, Li X (2023b) Trypsin preservation: CsUGT91C1 regulates Trilobatin Biosynthesis in Cucumis sativus during Storage. Plant Growth Regulation 100(3), 633-646.
| Crossref | Google Scholar |
Xie Y, Yang W, Tang F, Chen X, Ren L (2015) Antibacterial activities of flavonoids: structure-activity relationship and mechanism. Current Medicinal Chemistry 22(1), 132-149.
| Crossref | Google Scholar | PubMed |
Xie Y, Tan H, Ma Z, Huang J (2016) DELLA proteins promote anthocyanin biosynthesis via sequestering MYBL2 and JAZ suppressors of the MYB/bHLH/WD40 complex in Arabidopsis thaliana. Molecular Plant 9(5), 711-721.
| Crossref | Google Scholar | PubMed |
Yang A, Yu L, Chen Z, Zhang S, Shi J, Zhao X, Yang Y, Hu D, Song B (2017) Label-free quantitative proteomic analysis of chitosan oligosaccharide-treated rice infected with Southern rice black-streaked dwarf virus. Viruses 9(5), 115.
| Crossref | Google Scholar | PubMed |
Yoshida H, Hirano K, Sato T, Mitsuda N, Nomoto M, Maeo K, Koketsu E, Mitani R, Kawamura M, Ishiguro S, Tada Y, Ohme-Takagi M, Matsuoka M, Ueguchi-Tanaka M (2014) DELLA protein functions as a transcriptional activator through the DNA binding of the indeterminate domain family proteins. Proceedings of the National Academy of Sciences 111(21), 7861-7866.
| Crossref | Google Scholar |
Zhang Z-L, Ogawa M, Fleet CM, Zentella R, Hu J, Heo J-O, Lim J, Kamiya Y, Yamaguchi S, Sun T-P (2011) Scarecrow-like 3 promotes gibberellin signaling by antagonizing master growth repressor DELLA in Arabidopsis. Proceedings of the National Academy of Sciences 108(5), 2160-2165.
| Crossref | Google Scholar |
Zhang L, Chen C, Xie F, Hua Q, Zhang Z, Zhang R, Chen J, Zhao J, Hu G, Qin Y (2021) A Novel WRKY Transcription Factor HmoWRKY40 Associated with Betalain Biosynthesis in Pitaya (Hylocereus monacanthus) through Regulating HmoCYP76AD1. International Journal of Molecular Sciences 22(4), 2171.
| Crossref | Google Scholar | PubMed |
Zhang Y, Li B, Zhang M, Jia J, Sun S, Chen X, Yuan J, Bi X, Pang X, Li X (2022) Transcriptome analyses and virus-induced gene silencing identify HuWRKY40 acting as a hub transcription factor in the preservation of Hylocereus undatus by trypsin. Journal of Food Biochemistry 46(12), e14437.
| Crossref | Google Scholar | PubMed |
Zhang Y, Niu N, Li S, Liu Y, Xue C, Wang H, Liu M, Zhao J (2023) Virus-Induced Gene Silencing (VIGS) in Chinese Jujube. Plants 12(11), 2115.
| Crossref | Google Scholar | PubMed |