Investigating the effects of increased salinity on leaf litter decomposition and mortality of an aquatic invertebrate detritivore (Caecidotea sp.)
Zachary B. Gordon A B * , Raymond P. Kidder
A B * , Raymond P. Kidder A
B
Abstract
Coastal freshwater wetlands and their associated communities are at an increased risk of salinity exposure because of a variety of contemporary and historical anthropogenic stressors. Salinization shifts the community structure of aquatic organisms, such as macroinvertebrates, leading to decreases in functional ecological integrity. These salinity-driven changes to communities have the potential to alter macroinvertebrate-mediated processes such as detrital decomposition.
Our study aimed to examine the relationship among salinity exposure, leaf decomposition and mortality of a common freshwater macroinvertebrate (isopod: genus Caecidotea).
Using an in-laboratory microcosm approach, we exposed tanks of isopods and detrital leaf material to varying salinity levels for 23 days, after which isopod mortality and decomposition of detrital material were measured.
We found that increases in salinity increased isopod mortality, but had no effect on leaf disc decomposition.
These findings demonstrated the negative effect of increased salinity on a common freshwater macroinvertebrate. Additional studies regarding the response of non-isopod decomposers, such as bacteria and fungi, are needed to provide a more complete understanding of the effects of salinity intrusion in at-risk freshwater habitats.
As we expect salinization to increase in the future, it is important to understand how organisms and the processes they contribute to and rely on will be affected. The detrital decomposition process and aquatic macroinvertebrates are key foundations of aquatic food webs, and significant bottom-up changes could have drastic implications for ecosystems.
Keywords: anthropogenic change, aquatic macroinvertebrates, Caecidotea, detrital decomposition, isopods, microcosms, salinity effects, salinity stress.
Introduction
A growing threat for coastal freshwater wetlands is the expected increase in saltwater intrusion events because of sea-level rise and an increase in the predicted number of large storm events. The rate of warming is predicted to accelerate, and sea-level rise is expected to increase 1–2 m by the year 2100 (Vermeer and Rahmstorf 2009). These impacts include the increase in severe storms and tidal amplitudes driving saltwater further inland (Quintino et al. 2009; Yin 2023). Saltwater intrusion into these systems can be accelerated further by anthropogenic coastal landscape modification, such as those performed on barrier islands. One example is the construction of artificial channels almost 200 years ago on Sapelo Island, Georgia, which has led to extensive surface-level saltwater movement to the interior of the island (Chalmers 1997; Kidder 2023). These at-risk habitats provide ecosystem services, including flood control and storm-surge protection for coastal areas (Costanza et al. 1997; Barbier 2013). The importance of detrital processing in these systems additionally provides services related to carbon sequestration, supplying organic matter to other ecosystems, and supporting other trophic guilds and higher-order taxa including birds, mammals, reptiles, etc. (Vannote et al. 1980; Polis et al. 1997).
Increases in salinity concentrations within otherwise non-saline aquatic habitats lead to the removal of salt-intolerant biota both directly through physiological stress (Peterson and Meador 1994; Tyree et al. 2016) and indirectly through competition with and predation by more salt-tolerant macroinvertebrate taxa (Peterson and Meador 1994; Hart et al. 2003). This can result in trophic cascades and community replacement coupled with diversity simplification by decreases in species richness and evenness (Ley et al. 1994; Nielsen and Brock 2009), loss of plant-influenced critical habitat (Hogue and Breon 2022) and alterations to the ionic chemistry and biogeochemical cycles (Herbert et al. 2015). These losses have potential positive feedback effects on climate-change impacts because of the loss in carbon sequestration by biota, such as in the case of ghost forests left behind by the salinization of freshwater systems (Chen et al. 2023).
Unlike large-order rivers or other exposed bodies of water, small, shaded streams and wetlands cannot rely on autochthonous inputs of carbon from primary producers such as algae and phytoplankton. Instead, these systems rely on allochthonous inputs of carbon, often mainly in the form of abscised leaf material from riparian areas (Vannote et al. 1980; Feio et al. 2021). Once entering the aquatic environment, organic and inorganic compounds start leaching into the water rapidly on the first day (Webster and Benfield 1986). A few days later, hyphomycete fungi and bacteria colonize and begin decomposing organic carbohydrates within the leaf material, softening it up; this process can also increase the nutritional content of leaf material (France 2011). Large detrital material is further broken down by aquatic macroinvertebrate ‘shredders’ that specialize in feeding by physical fragmentation and ingesting leaf material. This entire process creates a fine fraction of detrital material, which can then be further utilized by non-specialist macroinvertebrate consumers such as filterers or collectors (Cummins and Klug 1979). This multi-taxon decomposition pathway makes detrital processing a useful investigatory tool when considering the health of wetlands and small streams (Cummins and Klug 1979; Quintino et al. 2009).
An in-lab microcosm approach was used, following the methods of Connolly et al. (2014), to quantify increased salinity impacts on leaf detritus decomposition rates and the mortality of a common macroinvertebrate shredder (isopods, genus Caecidotea). Connolly et al. (2014) found that a species of this genus experience mortality at as low as 5 PSU, but many individuals can survive to at least 15 PSU. Kidder (2023) found that a species belonging to this genus predictably decreases in abundance along an increasing salinity gradient on Sapelo Island, Georgia. On the basis of their findings, it is expected that these isopods will not tolerate higher salinity and show a predictable decline in survival as a result of physiological stress.
Materials and methods
Experimental set up of microcosms
Microcosms were established using 24 aquaria (Lee’s Kritter Keeper mini rectangle 1 L, Lee’s Pet Products, San Marcos, CA, USA) and were randomly assigned to one of four salinity concentration exposure treatments (0, 5, 10 or 15 PSU). Practical salinity units (PSU) are equivalent to parts per thousand (ppt) (1 g salt L−1). These concentrations were selected to mirror the work by Connolly et al. (2014), in which they used these four treatments with an additional 20-PSU treatment, on the basis of the salinity gradient observed in the Hudson River Estuary. These concentrations also fit the gradient observed by Kidder (2023), which was occurring at sites along an artificial channel experiencing saltwater intrusion on Sapelo Island, Georgia. Aquaria were filled to ~500 mL with tap water, dechlorinated by sunlight exposure for 24–48 h, and mixed with Instant Ocean Sea Salt (Spectrum Brands, Blacksburg, VA, USA) to the determined salinity level. This salt was used as an alternative to regular sodium chloride salt, because this laboratory study was paired with a field study investigating impacts of saltwater intrusion (Kidder 2023). This artificial salt is designed to mimic the composition of seawater; seawater was not used to prevent the introduction of marine microbial communities. Aquaria were aerated throughout the duration of the experiment (37 days). Over the course of the experiment, water level and salinity were maintained by adjusting every 1–2 days. Salinity was measured with a refractometer. In situ water temperature was monitored to ensure consistency among aquaria.
Exposure trials
As a source of leaf litter material, red maple leaves (Acer rubrum) dried at 55°C in a drying oven for over 24 h were used to make leaf discs. Red maple was used because it is a common riparian species in the south-eastern USA and a species with a relatively fast breakdown rate among other leaf species, allowing for easier observations in mass loss (Benfield et al. 2017). Discs of 1 cm2 were created using a cork borer, with multiple discs taken from larger leaves; discs were made using the non-venated portions of the leaves to control for surface area, or total palatability of the material. The leaf discs were weighed in sets of 60, and each set was distributed to a random tank. The first 14 days of the experiment were set aside for leaching of the leaves and any colonization by microbes, specifically fungi already on the leaves before addition to water; while leaching rapidly occurs over the first day, it will then continue to progress slowly (Webster and Benfield 1986).
Isopods were obtained from Carolina Biological Supply Company (Burlington, NC, USA). Sets of 20 freshwater isopods (Caecidotea sp.) were randomly assigned to three tanks of each salinity treatment (60 per salinity). This left half of all tanks (12) as controls with no isopods. All 240 isopods were acclimated in 0-PSU water for 48 h. Isopods were additionally starved 24 h before tank distribution. To control detritivore length and mass, isopods were pre-measured using full body length to the nearest millimeter to ensure an even distribution of different sizes across tanks; the average length and standard deviation of all 240 individuals was 3.51 ± 0.86 mm.
An additional three sets of discs were weighed to control for handling loss that occurred between the initial disc weighing and their addition to the tanks. The average loss was removed from the final dry weights of the tank discs. Eight additional tanks, with two replicates of each salinity, were used to measure mass loss over the 14-day leaching period.
Data collection
The experiment ended after a total of 37 days. Leaves and isopods were removed 23 days after isopod addition on Day 14. The number of dead isopods at the end of the experiment was recorded to estimate percentage mortality; isopod time of death during the study was not measured. Leaf disk remnants were removed from the microcosms, dried in a 55°C drying oven for 24 h, and weighed to determine post-exposure mass remaining. Dry leaf material was subsequently burned in a muffle furnace at 500°C for 6 h to combust all organic matter, then weighed. The resulting leaf and ash mass values were used to calculate percentage remaining as defined by Benfield et al. (2017).
Data analysis
A one-way ANOVA with a Tukey post hoc test was used to compare rates of isopod mortality across treatments. A one-way ANOVA was used to compare the total decomposition among salinity treatments for tanks without isopods to determine variation in microbial decomposition. An analysis of covariance (ANCOVA) was used to compare decomposition between Day 14 and Day 37 with isopod tanks in the different salinities. Salinity was the treatment and time was the covariate; this covariate accounts for the natural progression of decomposition and helps isolate salinity treatment from temporal trends. Mean and standard deviation were collected for the salinity monitoring and water temperature over the course of the experiment, to ensure separate and distinct salinity regimes and consistent water temperature.
Results
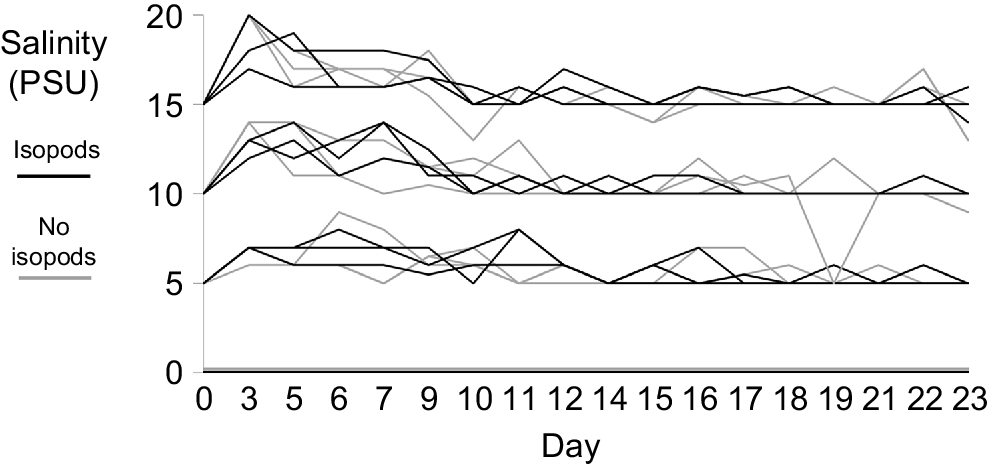
Distinct salinity regimes were maintained across treatments for the duration of the study (Fig. 1). The average salinity for the four treatments with standard deviations were as follows: 0 ± 0, 5.81 ± 0.94, 10.87 ± 1.38 and 15.87 ± 1.34 PSU. Tanks without isopods had averages of 0 ± 0, 5.86 ± 0.95, 10.89 ± 1.53 and 15.82 ± 1.50 PSU; tanks with isopods had averages of 0 ± 0, 5.86 ± 0.95, 10.89 ± 1.23 and 15.91 ± 1.18 PSU. There were no significant differences in salinity (P > 0.05) between tanks with and without isopods. Overall, the salinities remained in separate regimes, with more variation at the start of the experiment. There was only one instance of overlap that occurred in one of the 10-PSU no isopod replicates that dropped to 5 PSU on Day 33 of the study because too much freshwater was added. The mean water temperature throughout the study was 17.64°C ± 0.99.
Change in salinity since isopod addition (Day 0 = experiment Day 14, Day 23 = experiment Day 37) for each replicate. Salinity was measured as practical salinity units (PSU). Treatments included 0 (freshwater), 5, 10 and 15 PSU. Gray lines indicate treatments where no isopods were added to the tanks, and black lines indicate treatments where isopods were added to the tanks.
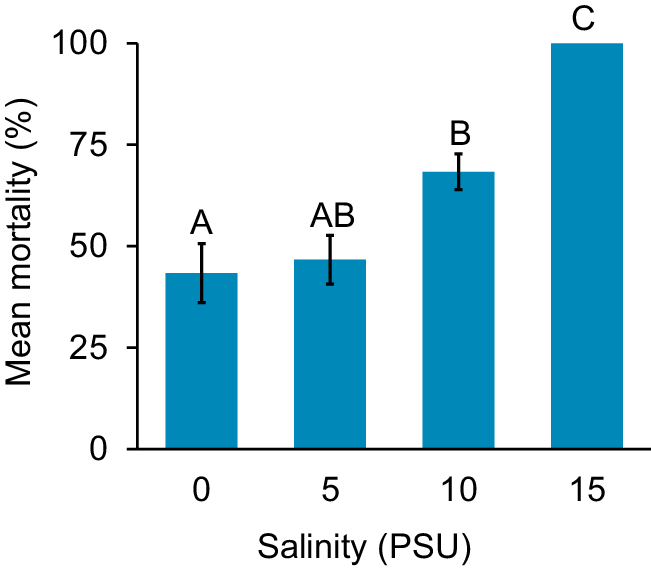
Isopod mortality increased with an increasing salinity (Fig. 2). The lowest average number of deceased isopods was from the 0-PSU (i.e. freshwater) treatments, with 43% mortality. At 5 PSU, average mortality slightly increased to 46.67%. At 10 PSU, the average mortality increased to 68.33%. However, no isopods survived the 15-PSU treatment, which resulted in an average mortality of 100%. Average isopod mortality differed among salinity treatments (one-way ANOVA, F3,11 = 25.1197, P = 0.0002), with the 0- and 10-PSU treatments differing significantly from each other (Tukey HSD, P = 0.0381), but not from the 5-PSU treatment (Tukey HSD, 0–5, P = 0.9672; 5–10 P = 0.0820). The highest salinity treatment (15 PSU) was significantly different from all other three salinity treatments (Tukey HSD, 0–15, P = 0.0003; 5–15, P = 0.0004; 10–15, P = 0.0112).
Mean percentage ± s.e. isopod mortality by salinity treatment (0, 5, 10, 15 PSU). Different letters denote a significant pairwise difference from Tukey post hoc.
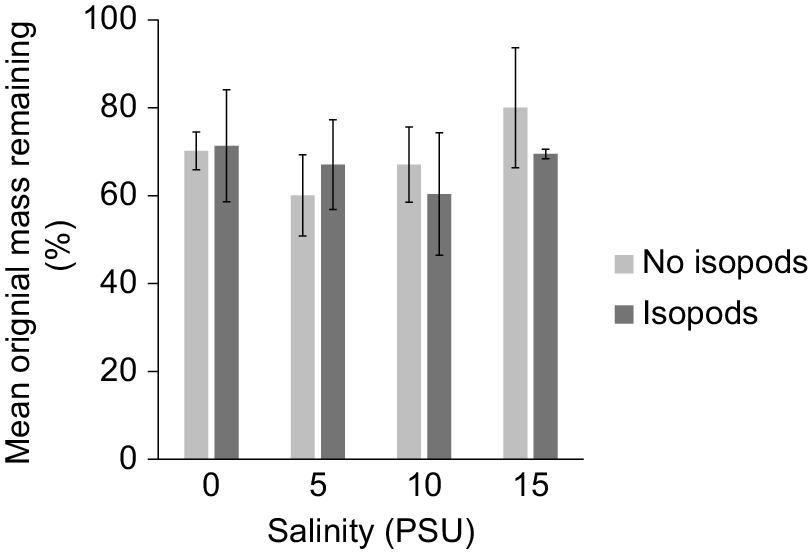
Decomposition of leaf material by isopods was not found to be significantly different among salinities between Day 14 and Day 37 (ANCOVA, F3,11 = 0.5451, P = 0.5946). Decomposition was also not significantly different across salinity in no isopod tanks (one-way ANOVA, F3,8 = 0.5963, P = 0.6350; Fig. 3).
Discussion
Our study demonstrated that increased salinity has a negative effect on freshwater macroinvertebrate shredders in a laboratory microcosm setting, but we did not find a measurable effect of salinity on decomposition. This has been corroborated by several studies that find increased mortality and decreased activity in freshwater shredders (e.g. Blasius and Merritt 2002; Connolly et al. 2014). Salinities of 15 PSU were lethal to isopod survival with 100% mortality. Similar responses were seen in Caecidotea sp. in a study by Connolly et al. (2014) where 15 PSU caused significantly higher mortality than did 10 PSU. Unfortunately, no tolerance studies aside from Connolly et al. (2014) have been performed on a Caecidotea species found on the eastern coast of the United States. A Caecidotea species found on Sapelo Island, Georgia, has been found to predictably decline across an artificial channel experiencing saltwater intrusion (Kidder 2023; Gordon 2025). This species dominates freshwater locations that do not receive salt input but decline as the frequency and intensity of salt intrusion increase. They are replaced by more tolerant taxa (i.e. Hemiptera, Odonata) in moderate salt conditions (5–10 PSU), extremely tolerant taxa (i.e. Chironomidae, Oligochaeta) in strong conditions (15–20 PSU), and estuarine taxa (i.e. Polychaeta, Sphaeromatidae) in tidal conditions (20–30 PSU). However, in this study isopods had a 43% mortality in 0 PSU, which seems high for the treatment closest to their natural freshwater environment. Higher than expected isopod mortality in freshwater may be associated with water quality decline. A build-up of ammonia for instance could have occurred because of extended days of trial without full water changes. However, this is speculative as nutrients were not measured in this study. Starvation because lack of food is unlikely because of similar leaf disc mass loss across salinity treatments (Fig. 3).
The possible absence of microbes in this experiment is a potential reason why decomposition was not significantly different in the no isopod tanks. Although the support for our hypothesis was absent in this experiment, it has been corroborated by several studies on the impacts of increased salinity on microbial decomposers. (e.g. Connolly et al. 2014; Tyree et al. 2016). Other limitations for this study include the small sample size of replicates; variation was very high in many of the results, and more replicates could have helped reduce this. Additionally, tanks were filled with 500 mL of water, aerated and lids had holes. All of these were potential problems for different reasons. The volume of water used was much greater than the amount needed for 60 1-cm2 leaf discs and 20 3.51 ± 0.86-mm isopods. Connolly et al. (2014) performed a similar study on isopod response with 20 mL of water with four isopods plus detritus. Therefore, only one-fifth of what we used would be needed to replicate their methods. The excess water makes accurate salinity measurement more difficult, because saltwater is denser than freshwater; water was not taken from the bottom to prevent any disturbances to decomposition and isopods. Although aeration was necessary for decomposition and isopods, it is also possible that any direct airflow onto leaf discs increased physical fragmentation of the leaves. This is also true in the addition of water to tanks, which was required because of evaporation through the holes in the lids. A closed system with aeration would have been preferable to prevent changes in salinity.
As salinity increases in freshwater wetlands because of sea-level rise, tidal intrusion and intensified storm events, the abundance and diversity of freshwater macroinvertebrates are expected to decline (Hart et al. 2003). Shredder macroinvertebrates and their microbial food sources play a central role in detrital-based food webs, supporting broader trophic dynamics (Cummins and Klug 1979; Marks 2019). Salt-tolerant taxa may replace freshwater communities (Hart et al. 2003; Kidder 2023), but whether key ecosystem functions persist remains uncertain. Over time, these wetlands may transition towards estuarine conditions (Feio et al. 2021; Chen et al. 2023), posing conservation risks for taxa without terrestrial life stages and for isolated habitats such as barrier islands (Ross et al. 2009; Woodward et al. 2010). Coastal development and climate change further exacerbate salinization and physiological stress (Peterson and Meador 1994; Hart et al. 2003).
Data availability
The data that support this study will be shared upon reasonable request to the corresponding author.
Declaration of funding
Financial support for this project was obtained from the National Science Foundation Research Experience for Undergraduates Site award to J. C. Colón-Gaud (DBI 1757536).
Acknowledgements
We thank Dmitry Apanaskevich for taxonomic identification help, Montana Carter for assistance with data collection, and John Carroll for assistance with methods and supplies for this project. We also thank the reviewers for suggestions that improved the paper.
References
Barbier E (2013) Valuing ecosystem services for coastal wetland protection and restoration: progress and challenges. Resources 2(3), 213-230.
| Crossref | Google Scholar |
Blasius BJ, Merritt RW (2002) Field and laboratory investigations on the effects of road salt (NaCl) on stream macroinvertebrate communities. Environmental Pollution 120(2), 219-231.
| Crossref | Google Scholar | PubMed |
Chalmers AG (Ed.) (1997) The ecology of the Sapelo Island National Estuarine research reserve. Prepared for the Sapelo Island National Estuarine Research Reserveand the Georgia Department of Natural Resources. (National Oceanic and Atmospheric Administration Office of Coastal Resource Management Sanctuaries and Reserves Division, Georgia Department of Natural Resources Parks and Historic Sites Division) Available at https://repository.library.noaa.gov/view/noaa/2734
Chen H, Rücker AM, Liu Y, Miller D, Dai J-N, Wang J-J, Suhre DO, Kuo L-J, Conner WH, Campbell BJ, Rhew RC, Chow AT (2023) Unique biogeochemical characteristics in coastal ghost forests – the transition from freshwater forested wetland to salt marsh under the influences of sea level rise. Soil & Environmental Health 1(1), 100005.
| Crossref | Google Scholar |
Connolly CT, Sobczak WV, Findlay SEG (2014) Salinity effects on Phragmites decomposition dynamics among the Hudson River’s freshwater tidal wetlands. Wetlands 34, 575-582.
| Crossref | Google Scholar |
Costanza R, d’Arge R, De Groot R, Farber S, Grasso M, Hannon B, Limburg K, Naeem S, O’Neill RV, Paruelo J, Raskin RG, Sutton P, Van Den Belt M (1997) The value of the world’s ecosystem services and natural capital. Nature 387, 253-260.
| Crossref | Google Scholar |
Cummins KW, Klug MJ (1979) Feeding ecology of stream invertebrates. Annual Review of Ecology and Systematics 10, 147-172.
| Crossref | Google Scholar |
Feio MJ, Serra SRQ, Neto JM (2021) From headwaters into the estuarine zone: changes in processes and invertebrate communities in response to abiotic conditions. Aquatic Ecology 55, 149-168.
| Crossref | Google Scholar |
France R (2011) Leaves as ‘crackers’, biofilm as ‘peanut butter’: exploratory use of stable isotopes as evidence for microbial pathways in detrital food webs. Oceanological and Hydrobiological Studies 40(4), 110-115.
| Crossref | Google Scholar |
Hart BT, Lake PS, Webb JA, Grace MR (2003) Ecological risk to aquatic systems from salinity increases. Australian Journal of Botany 51(6), 689-702.
| Crossref | Google Scholar |
Herbert ER, Boon P, Burgin AJ, Neubauer SC, Franklin RB, Ardón M, Hopfensperger KN, Lamers LPM, Gell P (2015) A global perspective on wetland salinization: ecological consequences of a growing threat to freshwater wetlands. Ecosphere 6(10), 1-43.
| Crossref | Google Scholar |
Hogue AS, Breon K (2022) The greatest threats to species. Conservation Science and Practice 4(3), e12670.
| Crossref | Google Scholar |
Ley JA, Montague CL, McIvor CC (1994) Food habits of mangrove fishes: a comparison along estuarine gradients in northeastern florida bay. Bulletin of Marine Science 54(3), 881-899.
| Google Scholar |
Marks JC (2019) Revisiting the fates of dead leaves that fall into streams. Annual Review of Ecology, Evolution, and Systematics 50, 547-568.
| Crossref | Google Scholar |
Nielsen DL, Brock MA (2009) Modified water regime and salinity as a consequence of climate change: prospects for wetlands of southern Australia. Climatic Change 95, 523-533.
| Crossref | Google Scholar |
Peterson MS, Meador MR (1994) Effects of salinity on freshwater fishes in coastal plain drainages in the southeastern US. Reviews in Fisheries Science 2(2), 95-121.
| Crossref | Google Scholar |
Polis GA, Anderson WB, Holt RD (1997) Toward an integration of landscape and food web ecology: the dynamics of spatially subsidized food webs. Annual Review of Ecology, Evolution, and Systematics 28, 289-316.
| Crossref | Google Scholar |
Quintino V, Sangiorgio F, Ricardo F, Mamede R, Pires A, Freitas R, Rodrigues AM, Basset A (2009) In situ experimental study of reed leaf decomposition along a full salinity gradient. Estuarine, Coastal and Shelf Science 85(3), 497-506.
| Crossref | Google Scholar |
Ross MS, O’Brien JJ, Ford RG, Zhang K, Morkill A (2009) Disturbance and the rising tide: the challenge of biodiversity management on low-island ecosystems. Frontiers in Ecology and the Environment 7(9), 471-478.
| Crossref | Google Scholar |
Tyree M, Clay N, Polaskey S, Entrekin S (2016) Salt in our streams: even small sodium additions can have negative effects on detritivores. Hydrobiologia 775, 109-122.
| Crossref | Google Scholar |
Vannote RL, Minshall GW, Cummins KW, Sedell JR, Cushing CE (1980) The river continuum concept. Canadian Journal of Fisheries and Aquatic Sciences 37(1), 130-137.
| Crossref | Google Scholar |
Vermeer M, Rahmstorf S (2009) Global sea level linked to global temperature. Proceedings of the National Academy of Sciences 106(51), 21527-21532.
| Crossref | Google Scholar |
Webster JR, Benfield EF (1986) Vascular plant breakdown in freshwater ecosystems. Annual Review of Ecology, Evolution, and Systematics 17, 567-594.
| Crossref | Google Scholar |
Woodward G, Perkins DM, Brown LE (2010) Climate change and freshwater ecosystems: impacts across multiple levels of organization. Philosophical Transactions of the Royal Society B: Biological Sciences 365, 2093-2106.
| Crossref | Google Scholar |
Yin J (2023) Rapid decadal acceleration of sea level rise along the US East and Gulf Coasts during 2010–22 and its impact on hurricane-induced storm surge. Journal of Climate 36(13), 4511-4529.
| Crossref | Google Scholar |