Phosphite for the prevention of disease caused by Phytophthora species in six rare or threatened species. Will it kill or cure?
K. L. McDougall A C * and E. C. Y. Liew B
A C * and E. C. Y. Liew B
A
B
C Present address:
Abstract
Phytophthora species are a significant threat to Australia’s biodiversity and many plant species are at risk of extinction as a result. For some plant species, the chemical phosphite (salts of phosphonic acid) can improve survival by activating defence mechanisms. However, phosphite at some concentrations can be phytotoxic. Uptake of and responses to phosphite vary greatly between species, so it is not possible to predict whether there will be positive or negative effects of its use. Glasshouse trials can be performed to test efficacy prior to field applications. The size of such trials can be constrained for rare or threatened species because of the difficulty of producing sufficient plants for adequate replication. In this study, we tested the effects of foliar application of phosphite on six threatened species in a glasshouse: three species were susceptible to infection by Phytophthora cinnamomi (Hibbertia circinata, Nematolepis rhytidophylla, and Phebalium squamulosum subsp. alpinum); and three species were susceptible to infection by Phytophthora gregata (Boronia deanei, Correa baeuerlenii, and Pimelea bracteata). We found that phosphite had severe phytotoxic effects on C. baeuerlenii but reduced disease and mortality of N. rhytidophylla. The effects on the other species were less equivocal. Alternative control options will be required for some species.
Keywords: chlorosis, Hibbertia, Nematolepis, phosphonic acid, Phytophthora cinnamomi, Phytophthora gregata, phytotoxicity, root pathogens.
Introduction
The oomycete pathogen Phytophthora cinnamomi is a significant threat to Australia’s flora (McDougall and Liew 2024) and is accordingly recognised as such in the listing of the disease it causes as a key threatening process under Commonwealth (Environment Protection and Biodiversity Conservation Act 1999) and New South Wales (NSW) legislation (Biodiversity Conservation Act 2016). Preventing the introduction of Phytophthora species through quarantine and hygiene is the most effective management response for protecting biodiversity assets because, once introduced, the likelihood of eradication is minimal and options for control are few (Cahill et al. 2008).
The only chemical widely used in Australia to control the spread of P. cinnamomi in native vegetation is phosphite (salts of phosphonic acid, H3PO3; Hardy et al. 2001). It works directly on the pathogen by inhibiting growth (Jackson et al. 2000) but may also activate a host defence response (Dalio et al. 2014), both of which can slow the spread of infection and increase the likelihood of plant survival. Phosphite can be applied as a foliar spray or injected into the stems of plants, its effectiveness varying with the technique and target plants (Crane and Shearer 2014), which can take up phosphite at greatly different rates (Pilbeam et al. 2000), and even with the isolate of P. cinnamomi (Wilkinson et al. 2001a). Phosphite has been used effectively both in the protection of individual plants in threatened species populations and aerially to slow pathogen spread in vulnerable habitat, especially habitat containing large numbers of species in the family Proteaceae (Barrett and Rathbone 2018; Boulle et al. 2023). However, not all plant species are protected from P. cinnamomi by phosphite and the chemical can be phytotoxic, causing chlorosis of leaves or even plant death (Aberton et al. 1999; Pilbeam et al. 2000).
As the taxonomy of the genus Phytophthora becomes more clear (Burgess et al. 2021), other species are increasingly being regarded as a threat to Australia’s flora (e.g. Phytophthora gregata, McDougall et al. 2018; and Phytophthora multivora, Migliorini et al. 2019). While phosphite can provide protection to some plant species harmed by P. cinnamomi, its effectiveness against other Phytophthora species is variable (Hunter et al. 2022) but largely unknown.
In the case of rare plant species, the potential for either positive or negative effects from phosphite makes its use in the wild problematic. In vitro experimental trials can be used to evaluate efficacy because of the advantage of controlling for plant age and growing conditions (Wilkinson et al. 2001b; Barrett et al. 2003); they are also prudent where the risk to native vegetation is great. In this trial, we tested the effects of P. cinnamomi and P. gregata on six rare or threatened plant species in a glasshouse trial. Three species are susceptible to infection by P. cinnamomi: (1) Hibbertia circinata (critically endangered); (2) Nematolepis rhytidophylla (critically endangered); and (3) Phebalium squamulosum subsp. alpinum (rare) (McDougall and Liew 2024). Three species are susceptible to infection by P. gregata: (1) Boronia deanei (vulnerable); (2) Correa baeuerlenii (vulnerable); and (3) Pimelea bracteata (critically endangered) (Wan et al. 2020). For most of these species, producing large numbers of plants for a well-replicated trial is not possible. The current trial also aims to evaluate whether small pot trials can be informative for assessing negative or positive effects of phosphite on threatened species.
Materials and methods
Plants
The three species that are susceptible to Phytophthora cinnamomi Rand are: (1) Hibbertia circinata K. L. McDougall and G. T. Wright (critically endangered); (2) Nematolepis rhytidophylla (Albr. and N. G. Walsh) Paul G. Wilson (critically endangered); and (3) Phebalium squamulosum subsp. alpinum (Benth.) Paul G.Wilson (rare). The three species that are susceptible to Phytophthora gregata T. Jung, M. J. C. Stukely and T. Burgess are: (1) Boronia deanei Maiden and Betche (vulnerable); (2) Correa baeuerlenii F. Muell. (vulnerable); and (3) Pimelea bracteata Threlfall (critically endangered). All plants were grown from cuttings at the Australian National Botanic Gardens Canberra and Booderee Botanic Gardens. Propagation stock-tubes (5 × 5 × 12 cm) were used with potting mix consisting of composted grade pine bark, coconut fibre, and propagation sand (Australian Standard AS3743; water holding capacity 45–55%, air-filled porosity of 16–20%, pH 5.6–6.3). The plants were delivered to the glasshouse in autumn 2022 and were acclimatised for approximately 4 months before the trial in winter/spring 2022. Nutrient solution (50 mL of half strength Seasol seaweed concentrate) was applied to all plants once a month before phosphite application and inoculation. The trial was conducted in a temperature-controlled greenhouse with temperature set at 20°C (actual temperatures ranging from 18°C to 26°C) throughout the trial period.
There were four treatments with each pathogen:
No pathogen inoculation + no phosphite application;
No pathogen inoculation + phosphite application;
Pathogen inoculation + no phosphite application;
Pathogen inoculation + phosphite application.
There were 6−10 plants in each treatment.
All plants designated for phosphite application were sprayed in mid-July 2022, 12 days before pathogen inoculation. The spray solution contained 5 g L−1 phosphite (in the form of mono- and di-potassium phosphonate; agrichem Agri-Fos 600) and 0.1% surfactant (Nufarm Pulse). This rate of phosphite application is considered to be suitable for field situations in native vegetation; rates above that can be more effective but often cause phytotoxicity (Tynan et al. 2001).
In preparation for the spray application, the plants were placed outside the greenhouse with direct morning sun and the soil surface of each pot covered with aluminium foil. The foliage of plants designated for phosphite application was sprayed with the phosphite/surfactant solution thoroughly until run-off, using a fine-mist manual-trigger spray bottle. All plants not designated for phosphite application were similarly sprayed with deionised water. The plants were returned to the greenhouse after approximately 5 h.
P. cinnamomi isolate RBG W1324 and P. gregata isolate RBG W1962 were used in the trial. These isolates were revitalised from cultures held at the Royal Botanic Gardens Sydney and propagated in the laboratory as described by Wan et al. (2019, 2020). Plants designated for pathogen inoculation were inoculated 12 days after the phosphite application, as described by Wan et al. (2019). Three days after inoculation, all pots (including controls) were flooded for 48 h with deionised water up to two-thirds of the pot height then drained; after 1 week, pots were flooded again for 48 h.
Throughout the trial, dead plants were removed, their entire root systems including the crown section were gently washed, surface-sterilised with 70% ethanol and plated onto Phytophthora selective media (V8 agar with Hymexazol 0.004% v/v, Rifampicin 0.004%, and Pimaricin 0.0004% v/v) agar plates. These plates were incubated at 24°C and assessed for pathogen re-isolation over 5 days. The duration of the P. cinnamomi component of the trial was 14 weeks from inoculation while that of P. gregata was 21 weeks. At the end of the trial, all root systems were removed, washed and plated onto Phytophthora selective media agar plates for pathogen re-isolation after incubation.
The following statistics were calculated:
Phytotoxicity: % of plants that died in Treatment 2 minus % of plants that died in Treatment 1.
Mortality associated with the pathogen: % of plants that died in Treatment 3 minus % of plants that died in Treatment 1.
Infection associated with the pathogen: % of plants from which the pathogen was recovered in Treatment 3 minus % of plants from which the pathogen was recovered in Treatment 1.
Mortality associated with phosphite: % of plants that died in Treatment 4 minus % of plants that died in Treatment 1.
Infection associated with phosphite: % of plants from which the pathogen was recovered in Treatment 4 minus % of plants from which the pathogen was recovered in Treatment 1.
Results
For the results of the experimental treatments, see Supplementary Table S1.
Phytotoxicity was greatest for C. baeuerlenii, where all sprayed, uninoculated plants died before the end of the trial, and least for N. rhytidophylla, where no plants died (Fig. 1a); for the other species, net mortality associated with phosphite ranged from 17% to 44% and damage to leaves was often visible soon after spraying. For C. baeuerlenii, there was at least some survival of inoculated plants to allow an assessment of phosphite effectiveness.
The effect and effectiveness of phosphite on six rare or threatened plant species. (a) Phytotoxicity, the net % of test plants that died after being sprayed with phosphite (equals % of plants that died after treatment with phosphite minus % of control plants that died). (b) Net % of test plants becoming infected (Infection) and that died (Mortality) after inoculation with Phytophthora cinnamomi or Phytophthora gregata (in comparison with control uninoculated plants). +p, plants were treated with phosphite.
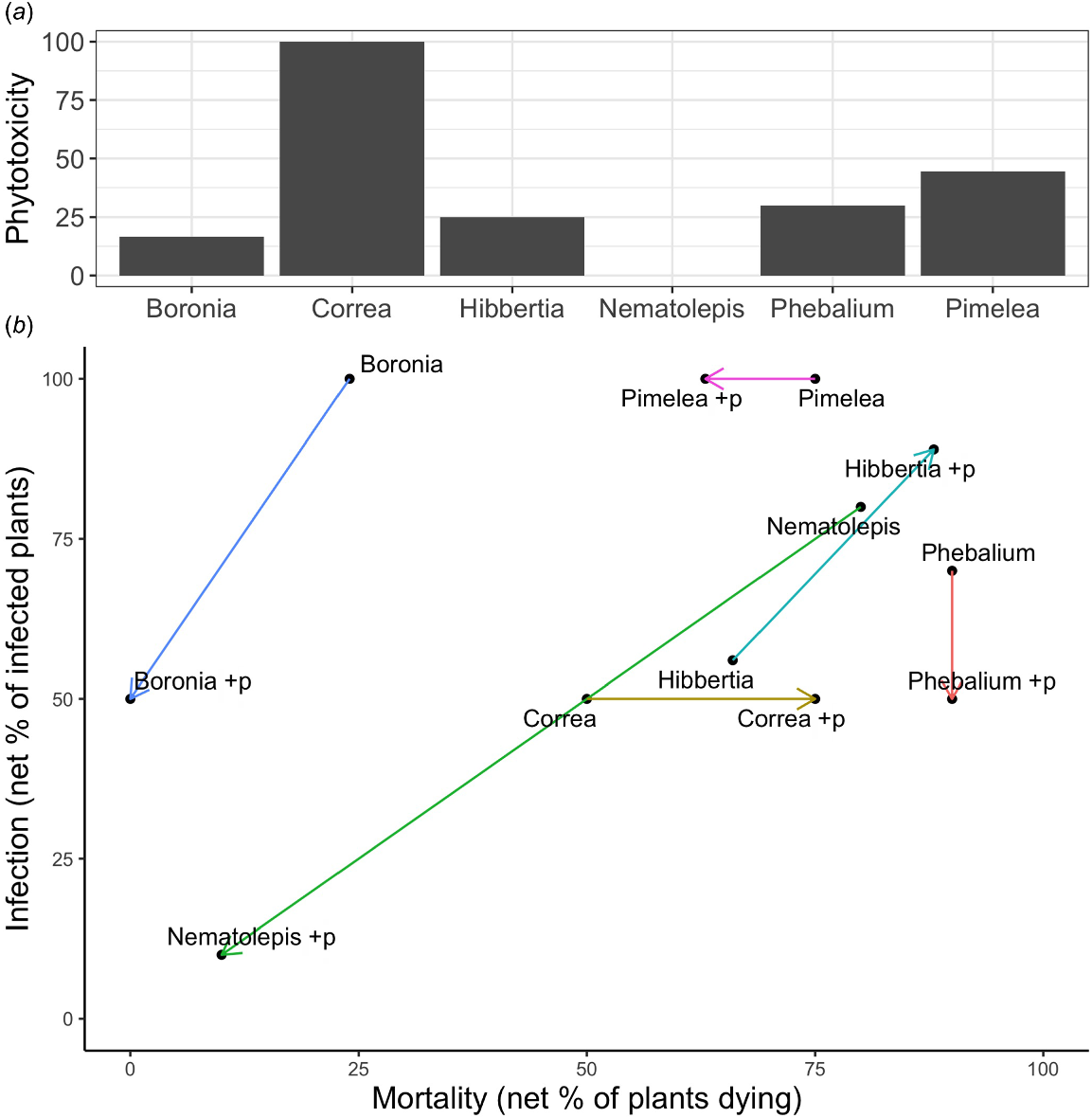
Without phosphite application, the pathogen was detected in at least half the plants of all species inoculated and for all but B. deanei, at least half of the inoculated plants died before the end of the trial in comparison with the control treatment (Fig. 1b). With phosphite application, this was also true for all species except N. rhytidophylla, where only one plant (10%) died and P. cinnamomi was recovered from its roots. The percentage of detections of P. gregata in B. deanei plants treated with phosphite was lower than that without phosphite. For species other than B. deanei and N. rhytidophylla, there was no improvement in survival or protection from infection. Indeed, for H. circinata and C. baeuerlenii, there were more deaths of phosphite-treated inoculated plants than control inoculated plants.
Discussion
The level of replication for this trial was unlikely to be great enough to produce unequivocal results unless the effects were profound. However, even if there was no ‘significant difference’ in phytotoxicity between treated and control plants, would land managers tolerate any deaths in small populations of critically endangered species? From our limited study, we can suggest that it should be safe to conduct a field trial using phosphite on N. rhytidophylla because the risk of phytotoxicity appears to be very low and the benefits appear to be potentially substantial. P. cinnamomi is apparently not present in the single population of this species, which occurs in a very remote area rarely visited by humans. However, it is instructive to know that there is a tool to help with control, should the pathogen find its way to the population. We identified a possible benefit from phosphite in reducing infection in B. deanei, and deaths from phytotoxicity in that species were few. Lowering the chance of infection may buy time for this species should P. gregata become a substantial threat in populations. Field trials will then be worthwhile; fortunately, some populations of B. deanei are large enough to permit this.
In field situations, phytotoxic effects may be reduced through low volume application of phosphite, for example using aerial spraying (Barrett 2001). While this could be trialled for plants of C. baeuerlenii, P. bracteata, and P. squamulosum subsp. alpinum because they are numerous enough to localise the risk, our small glasshouse trial suggests that phosphite will have little or no value in control of Phytophthora for these species. However, the risk from aerial spraying to the single small population of H. circinata is likely to be too great because it faces extinction in the coming decades without protection against P. cinnamomi (McDougall et al. 2023). Phosphite gave no apparent benefit and indeed may have weakened inoculated plants, increasing the likelihood of infection and death. For this species, alternative methods for phosphite delivery should be trialled. For instance, phosphite can be delivered effectively as a stem paint (Nyoni et al. 2021), possibly avoiding the scorching effects of a foliar application.
Ultimately, other control options will be required for H. circinata if it is to persist in the wild, and for other threatened species not aided by phosphite such as C. baeuerlenii. This is especially important because disease resistance and the selection of tolerant isolates have been identified with repeated phosphite use (Dobrowolski et al. 2008; Hunter 2018); multiple control options are desirable to avoid this risk. New oomycete-specific chemicals have become available in the past decade but to our knowledge, none has been trialled on native plants in Australia. One of the new chemicals, oxathiapiprolin, has proven effective at controlling Phytophthora agathidicida in kauri trees (Agathis australis) in New Zealand (Lacey et al. 2021). Oxathiapiprolin can be very effective at low concentration and it works in multiple ways to negatively affect the oomycete life cycle (for example, inhibition of zoospore release and sporangial germination, and the prevention of mycelial growth; Cohen 2015). While oxathiapiprolin and related chemicals have been applied to soils as drenches against soil-born oomycetes (Adaskaveg et al. 2024) and as foliar sprays against aerial pathogens (e.g. Phytophthora infestans; Cohen et al. 2018), there is also recent evidence that foliar spraying with oxathiapiprolin can be effective against a root pathogen (Wang et al. 2023). Trials of chemicals such as oxathiapiprolin are urgently needed to broaden control options, and especially for species on the brink of extinction such as Hibbertia circinata.
Our trial of the effectiveness of phosphite for the protection of six rare or threatened species against two Phytophthora species provided useful management information, despite its low replication; the use of phosphite on C. baeuerlenii seems inadvisable because of phytotoxicity and the chemical may be an important future tool for N. rytidophylla conservation. However, phosphite is unlikely to be a panacea for some threatened species; new control options are needed.
Declaration of funding
Funding for this project was provided by the NSW Department of Climate Change, Energy, the Environment and Water, as part of the Saving Our Species program.
Author contributions
KM prepared a first draft of the manuscript; ECYL maintained the plants in the glasshouse, inoculated pots and plated out roots for re-isolation; and KM and ECYL contributed to the preparation of the final manuscript.
Acknowledgements
We thank Maureen Phelan for the help with planting, general plant maintenance, inoculation and harvesting and for staff at the Australian National Botanic Gardens and National Botanic Garden for their expertise in growing the plants.
References
Aberton MJ, Wilson BA, Cahill DM (1999) The use of potassium phosphonate to control Phytophthora cinnamomi in native vegetation at Anglesea, Victoria. Australasian Plant Pathology 28(3), 225-234.
| Crossref | Google Scholar |
Adaskaveg JE, Förster H, O’Fallon C (2024) New fungicides for managing Phytophthora diseases of tree crops with foliar and soil applications. Journal of Plant Diseases and Protection 131, 1203-1209.
| Crossref | Google Scholar |
Barrett SR, Rathbone D (2018) Long-term phosphite application maintains species assemblages, richness and structure of plant communities invaded by Phytophthora cinnamomi. Austral Ecology 43(4), 360-374.
| Crossref | Google Scholar |
Barrett SR, Shearer BL, Hardy GESJ (2003) The efficacy of phosphite applied after inoculation on the colonisation of Banksia brownii stems by Phytophthora cinnamomi. Australasian Plant Pathology 32, 1-7.
| Crossref | Google Scholar |
Boulle M, Stewart BA, Barrett S (2023) To spray or not to spray: impact of phosphite spraying for Phytophthora cinnamomi control on Proteaceae species in southwestern Australia. Conservation Science and Practice 5(3), e12903.
| Crossref | Google Scholar |
Burgess TI, Edwards J, Drenth A, Massenbauer T, Cunnington J, Mostowfizadeh-Ghalamfarsa R, Dinh Q, Liew ECY, White D, Scott P, Barber PA, O’Gara E, Ciampini J, McDougall KL, Tan YP (2021) Current status of Phytophthora in Australia. Persoonia - Molecular Phylogeny and Evolution of Fungi 47, 151-177.
| Crossref | Google Scholar | PubMed |
Cahill DM, Rookes JE, Wilson BA, Gibson L, McDougall KL (2008) Phytophthora cinnamomi and Australia’s biodiversity: impacts, predictions and progress towards control. Australian Journal of Botany 56(4), 279-310.
| Crossref | Google Scholar |
Cohen Y (2015) The novel oomycide oxathiapiprolin inhibits all stages in the asexual life cycle of Pseudoperonospora cubensis – causal agent of cucurbit downy mildew. PLoS ONE 10(10), e0140015.
| Crossref | Google Scholar | PubMed |
Cohen Y, Rubin AE, Galperin M (2018) Oxathiapiprolin-based fungicides provide enhanced control of tomato late blight induced by mefenoxam-insensitive Phytophthora infestans. PLoS ONE 13(9), e0204523.
| Crossref | Google Scholar | PubMed |
Crane CE, Shearer BL (2014) Comparison of phosphite application methods for control of Phytophthora cinnamomi in threatened communities. Australasian Plant Pathology 43, 143-149.
| Crossref | Google Scholar |
Dalio RJD, Fleischmann F, Humez M, Osswald W (2014) Phosphite protects Fagus sylvatica seedlings towards Phytophthora plurivora via local toxicity, priming and facilitation of pathogen recognition. PLoS ONE 9(1), e87860.
| Crossref | Google Scholar | PubMed |
Dobrowolski MP, Shearer BL, Colquhoun IJ, O’Brien PA, Hardy GESJ (2008) Selection for decreased sensitivity to phosphite in Phytophthora cinnamomi with prolonged use of fungicide. Plant Pathology 57(5), 928-936.
| Crossref | Google Scholar |
Hardy GESJ, Barrett S, Shearer BL (2001) The future of phosphite as a fungicide to control the soilborne plant pathogen Phytophthora cinnamomi in natural ecosystems. Australasian Plant Pathology 30, 133-139.
| Crossref | Google Scholar |
Hunter SR (2018) Determining the risk of phosphite tolerance in Phytophthora species in New Zealand and the United States: a case study on the implications of long-term use of phosphite to control Phytophthora cinnamomi in avocado (Persea americana). MSc. thesis, The University of Waikato, New Zealand.
Hunter S, McDougal R, Williams N, Scott P (2022) Variability in phosphite sensitivity observed within and between seven Phytophthora species. Australasian Plant Pathology 51, 273-279.
| Crossref | Google Scholar |
Jackson TJ, Burgess T, Colquhoun I, Hardy GESJ (2000) Action of the fungicide phosphite on Eucalyptus marginata inoculated with Phytophthora cinnamomi. Plant Pathology 49(1), 147-154.
| Crossref | Google Scholar |
Lacey RF, Fairhurst MJ, Daley KJ, Ngata-Aerengamate TA, Patterson HR, Patrick WM, Gerth ML (2021) Assessing the effectiveness of oxathiapiprolin toward Phytophthora agathidicida, the causal agent of kauri dieback disease. FEMS Microbes 2, xtab016.
| Crossref | Google Scholar |
McDougall KL, Liew ECY (2024) The susceptibility of rare and threatened NSW species to the root-rot pathogen Phytophthora cinnamomi: 2. The identification of species requiring protection or further research. Australian Journal of Botany 72, BT23106.
| Crossref | Google Scholar |
McDougall KL, Wright GT, Burgess TI, Farrow R, Khaliq I, Laurence MH, Wallenius T, Liew ECY (2018) Plant, invertebrate and pathogen interactions in Kosciuszko National Park. Proceedings of the Linnean Society of New South Wales 140, 295-312.
| Google Scholar |
McDougall KL, Wright GT, Bredell PM, James EA, Simmons L (2023) Mount Imlay – an island of floristic significance on the brink. Cunninghamia 23, 1-9.
| Crossref | Google Scholar |
Migliorini D, Khdiar MY, Rodríguez Padrón C, Vivas M, Barber PA, Hardy GESJ, Burgess TI (2019) Extending the host range of Phytophthora multivora, a pathogen of woody plants in horticulture, nurseries, urban environments and natural ecosystems. Urban Forestry & Urban Greening 46, 126460.
| Crossref | Google Scholar |
Nyoni M, Mazzola M, Wessels JPB, McLeod A (2021) Phosphonate treatment effects on Phytophthora root rot control, phosphite residues and Phytophthora cactorum inoculum in young apple orchards. Plant Disease 105(12), 3835-3847.
| Crossref | Google Scholar | PubMed |
Pilbeam RA, Colquhoun IJ, Shearer B, Hardy GESJ (2000) Phosphite concentration: its effect on phytotoxicity symptoms and colonisation by Phytophthora cinnamomi in three understorey species of Eucalyptus marginata forest. Australasian Plant Pathology 29, 86-95.
| Crossref | Google Scholar |
Tynan KM, Wilkinson CJ, Holmes JM, Dell B, Colquhoun IJ, McComb JA, Hardy GESJ (2001) The long-term ability of phosphite to control Phytophthora cinnamomi in two native plant communities of Western Australia. Australian Journal of Botany 49(6), 761-770.
| Crossref | Google Scholar |
Wan JSH, McDougall KL, Liew ECY (2019) The susceptibility of rare and threatened NSW species to the root-rot pathogen Phytophthora cinnamomi: 1. Initial testing and identification of key research questions. Australian Journal of Botany 67(7), 510-516.
| Crossref | Google Scholar |
Wan JSH, McDougall KL, Liew ECY (2020) The susceptibility of seven threatened species to Phytophthora gregata and the aetiology of the disease caused by it. Australian Journal of Botany 68, 595-601.
| Crossref | Google Scholar |
Wang Z, Lv X, Wang R, He Z, Feng W, Liu W, Yang C, Wang Z, Ke Q, Tao K, Chen Q (2023) Use of oxathiapiprolin for controlling soybean root rot caused by Phytophthora sojae: efficacy and mechanism of action. Pest Management Science 79(1), 381-390.
| Crossref | Google Scholar | PubMed |
Wilkinson CJ, Shearer BL, Jackson TJ, Hardy GESJ (2001a) Variation in sensitivity of Western Australian isolates of Phytophthora cinnamomi to phosphite in vitro. Plant Pathology 50(1), 83-89.
| Crossref | Google Scholar |
Wilkinson CJ, Holmes JM, Tynan KM, Colquhoun IJ, McComb JA, Hardy GESJ, Dell B (2001b) Ability of phosphite applied in a glasshouse trial to control Phytophthora cinnamomi in five plant species native to Western Australia. Australasian Plant Pathology 30, 343-351.
| Crossref | Google Scholar |


