Tuning Packing, Structural Flexibility, and Porosity in 2D Metal–Organic Frameworks by Metal Node Choice
Witold M. Bloch A B , Christian J. Doonan A and Christopher J. Sumby A BA Department of Chemistry and Centre for Advanced Nanomaterials, School of Physical Sciences, The University of Adelaide, Adelaide, SA 5005, Australia.
B Corresponding authors. Email: witold.bloch@adelaide.edu.au; christopher.sumby@adelaide.edu.au
Australian Journal of Chemistry 72(10) 797-804 https://doi.org/10.1071/CH19215
Submitted: 10 May 2019 Accepted: 12 June 2019 Published: 15 July 2019
Abstract
Understanding the key features that determine structural flexibility in metal–organic frameworks (MOFs) is key to exploiting their dynamic physical and chemical properties. We have previously reported a 2D MOF material, CuL1, comprising five-coordinate metal nodes that displays exceptional CO2/N2 selectively (L1 = bis(4-(4-carboxyphenyl)-1H-pyrazolyl)methane). Here we examine the effect of utilising six-coordinate metal centres (CoII and NiII) in the synthesis of isostructural MOFs from L1, namely CoL1 and NiL1. The octahedral geometry of the metal centre within the MOF analogues precludes an ideal eclipse of the 2D layers, resulting in an offset stacking, and in certain cases, the formation of 2-fold interpenetrated analogues β-CoL1 and β-NiL1. We used a combination of thermogravimetric analysis (TGA), and powder and single crystal X-ray diffraction (PXRD and SCXRD) to show that desolvation is accompanied by a structural change for NiL1, and complete removal of the coordinated H2O ligands results in a reduction in long-range order. The offset nature of the 2D layers in combination with the structural changes impedes the adsorption of meaningful quantities of gases (N2, CO2), highlighting the importance of a five-coordinate metal centre in achieving optimal pore accessibility for this family of flexible materials.
Introduction
Elucidating the design principles that govern the synthesis of coordination polymers or metal–organic frameworks (MOFs) has been an area of intense interest since Robson’s early work in the area.[1–3] This class of materials is now well established and many of the early predictions[2] of utilising the chemically mutable porous networks have been realised.[4] MOFs have been shown to have possible applications in small molecule adsorption or separations,[5–7] in catalysis,[8,9] and recently even in the protection of biomacromolecules.[10,11]
Structurally flexible variants of MOFs, often referred to as third generation porous coordination polymers,[12] have been recognised as an important structural type. Since Kitagawa first highlighted this iteration, many contributions have developed the chemistry of flexible MOFs.[13–15] Two dimensional layered structures represent an important archetype of these materials with a range of flexible structures having been observed, either due to layer–layer sliding,[16,17] interlayer digitation,[18] expansion of the layer–layer contacts,[19] and trellis-like layer motion.[20] These intrinsically flexible materials have shown gated adsorption behaviour, guest-selective small molecule adsorption, and sensing behaviour.[15,21]
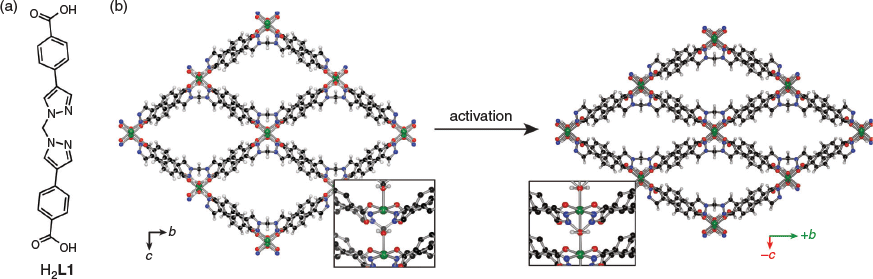
We have shown a variety of structural modifications for 2D MOFs that allow the engineering of trellis-like flexibility. Using a MOF design approach comprising a central six membered chelate ring core with appended bridging coordination groups, we have reported variants that show significant breathing behaviour and remarkable CO2/N2 selectivity.[20e,22,23] The originally reported 2D MOF, [Cu(L1)(H2O)]·xS (CuL1), packed in an eclipsed arrangement with notable close hydrogen bonding between the layers. Solvent guest exchange and then removal facilitated a simultaneous trellis-like and layer–layer contraction to a rigid 3D MOF (Fig. 1).[20e] In an effort to illuminate the design principles that govern this structural flexibility and these properties, we have explored the synthesis of variants with different structure metrics,[24,25] covalent linking,[26] or pillaring linkers,[27] and also varied the particle size.[28]
|
Herein, we report analogues of this 2D MOF structure type with octahedral rather than square pyramidal nodes. These materials, [M(L1)(H2O)2]n (ML1·xS) and [M(L1)(H2O)(C2H5OH)]n (β-ML1), where M = CoII or NiII, were synthesised from the dipyrazolylmethane-based ligand bis(4-(4-carboxyphenyl)-1H-pyrazolyl)methane (H2L1) and metal salt by a solvothermal synthesis method and characterised by single crystal (SCXRD) and powder X-ray diffraction (PXRD). The octahedral node necessitates introduction of a sixth donor to the coordination sphere, compared with CuL1, which either alters the layer–layer intermolecular contacts and packing (ML1·xS) or supports the formation of an interpenetrated structure (β-ML1). The additional aqua ligand in ML1·xS leads to a slightly offset layer–layer packing arrangement which reduces the pore dimensions of the as-synthesised material with respect to CuL1. SCXRD and PXRD reveal structural changes that occur upon solvent exchange and activation which help to rationalise the observation that NiL1-ac is essentially non-porous.
Experimental
Materials and General Experimental
Reagents were purchased from commercial sources and used without further purification. Bis(4-(4-carboxyphenyl)-1H-pyrazolyl)methane (H2L1) was prepared by a reported procedure.[20e] Elemental analyses were performed by the Campbell Microanalytical Laboratory at the University of Otago. Thermogravimetric analysis was performed on a Perkin-Elmer STA-6000 under a constant flow of N2 at a temperature ramp rate of 10°C min−1. IR spectra were recorded on a PerkinElmer Fourier-Transform Infrared (FT-IR) spectrometer on a zinc-selenide crystal. PXRD data were collected on a Rikagu Hiflux Homelab system using Cu Kα radiation (λ 1.5418 Å) with an RAxis IV++ image plate detector. Samples were mounted on plastic loops using paratone-N and data collected by scanning 90° in phi for 120–300 s exposures. The data were converted into xye format using the program Datasqueeze 2.2.2. Simulated PXRD patterns were generated from the single-crystal data using Mercury 3.0.
Synthesis of [Co(L1)(H2O)2]n (CoL1·xS)
In a screw cap vial, Co(NO3)2·6H2O (20.2 mg, 0.069 mmol) and H2L1 (23.0 mg, 0.059 mmol) were combined and dissolved in a mixture of DMF (3 mL), water (1 mL), ethanol (1 mL), and 4 drops of glacial acetic acid. The mixture was heated at 65°C for 3 days resulting in maroon block-shaped crystals. The crystals were washed in DMF (× 3) and methanol (× 5) and heated to 260°C for 5 min (14.7 mg, 52 %). νmax (neat)/cm−1 2896–3350 (br, O–H), 1612 (w, C=C), 1579 (m, C=C), 1526 (m, C=C), 1371 (s, C-O). Found C 52.7, H 3.7, N 11.8, C21H18N4O6Co requires: C 52.4, H 3.8, H 11.6 %. The interpenetrated form [Co(L1)(H2O)(C2H5OH)]n (β-CoL1) could also be prepared by a subtle modification to the procedure. In a screw cap vial, Co(NO3)2·6H2O (14.1 mg, 0.048 mmol) and H2L1 (16.0 mg, 0.041 mmol) were combined in a mixture of DMF (1.5 mL), ethanol (0.5 mL), and 2 drops of HNO3 (1 M). Heating the mixture at 85°C for 1 day yielded small maroon block-shaped crystals and the isolated material was shown to be a distinct phase by PXRD.
Synthesis of [Ni(L1)(H2O)2]}n (NiL1·xS)
In a screw cap vial, Ni(NO3)2·6H2O (20.1 mg, 0.069 mmol) and H2L1 (22.0 mg, 0.057 mmol) were combined and dissolved in a mixture of DMF (3 mL), water (1 mL), ethanol (1 mL), and 4 drops of glacial acetic acid. The mixture was heated at 65°C for 3 days resulting in teal block-shaped crystals. The crystals were washed in DMF (× 3) and methanol (× 3) and heated to 260°C for 5 min. (16.5 mg, 59 %). νmax (neat)/cm−1 2891–3301 (br, O–H), 3104 (w, C–H), 1610 (m, C=O), 1582 (m, C=C), 1535, (m, C=C), 1380 (s, C–O). Found C 51.0, H 3.9, N 11.4, C21H18N4O6Ni·0.75H2O requires: C 50.1, H 4.0, N 11.3 %. Again, the interpenetrated form [Ni(L1)(H2O)(C2H5OH)]n (β-NiL1) could also be prepared by a subtle modification to the procedure. In a screw cap vial, Ni(NO3)2·6H2O (15.0 mg, 0.050 mmol) and H2L1 (16.5 mg, 0.043 mmol) were combined in a mixture of DMF (1.5 mL), ethanol (0.5 mL), and 2 drops of HNO3 (1 M). Heating the mixture at 85°C for 1 day yielded small teal coloured rod-like crystals and the isolated material was shown to be a distinct phase by PXRD.
Gas Adsorption Analysis
Gas sorption isotherm measurements were performed on an ASAP 2020 Surface Area and Pore Size Analyzer. UHP grade (99.999 %) N2 and CO2 were used for all measurements. As required, the temperatures were maintained at 77 K (liquid nitrogen bath or cryo-cooler) or 293 K (room temperature water bath), respectively.
X-Ray Crystallography
Single crystals were mounted in paratone-N oil on a plastic loop. X-Ray diffraction data were collected at 150(2) K on an Oxford X-Calibur single crystal diffractometer or at 100(2) K on the MX1 beamline at the Australian Synchrotron for NiL1·Tol and β-NiL1[29] (λ 0.71073 Å). Datasets were corrected for absorption using a multi-scan method, and structures were solved by direct methods using SHELXS-97[30] and refined by full-matrix least-squares on F2 by SHELXL-2014[31] interfaced through the program X-Seed.[32] In general, all non-hydrogen atoms were refined anisotropically and hydrogen atoms were included as invariants at geometrically estimated positions, unless specified otherwise in additional details below. X-Ray experimental data is given in Table 1. Figures were produced using the program CrystalMaker or Mercury 3.1. CIF data have been deposited with the Cambridge Crystallographic Data Centre, CCDC reference numbers CCDC 1910941–1910944 and 1914827 (CoL1, 1910944; NiL1, 1910943; NiL1·Tol, 1914827; β-CoL1, 1910942; β-NiL1, 1910941).
|
Variata for CoL1·xS and NiL1·xS: The hydrogen atoms of the water ligands could be located in the difference map but despite using DFIX and DANG restraints did not refine in a chemically sensible manner; these were placed in calculated positions. The structures have large solvent accessible voids. These contained several diffuse electron density peaks that could not be adequately identified and refined as solvent. The SQUEEZE routine of PLATON was applied to the collected data for both structures, which resulted in significant reductions in R1 and wR2 and an improvement in the goodness of fit (GOF). CoL1·xS: R1, wR2, and GOF before SQUEEZE routine: 12.16 %, 43.31 %, and 1.973; after SQUEEZE routine: 4.13 %, 13.67 %, and 1.168. The contents of the solvent region calculated from the result of the SQUEEZE routine equate to one DMF molecule and one water molecule per asymmetric unit and were included in the formula reported. NiL1·xS: R1, wR2, and GOF before SQUEEZE routine: 11.20 %, 42.26 %, and 1.989; after SQUEEZE routine: 3.82 %, 12.73 %, and 1.167. The contents of the solvent region calculated from the result of the SQUEEZE routine equates to one DMF molecule and one water molecule per asymmetric unit and were included in the formula reported. For NiL1·Tol: The hydrogen atoms of the water ligands were located in the difference map and refined with suitable DFIX restraints. The structures have large solvent accessible voids containing disordered solvent (toluene). The SQUEEZE routine of PLATON was applied to the collected data, which resulted in significant reductions in R1 and wR2 and an improvement in the GOF (R1, wR2, and GOF before SQUEEZE routine: 10.75 %, 38.87 %, and 1.876; after SQUEEZE routine: 4.77 %, 14.08 %, and 1.077). The contents of the solvent region calculated from the result of the SQUEEZE routine equates to 0.8 of a toluene per asymmetric unit and were included in the formula reported. For β-CoL1 and β-NiL1: A coordinated ethanol solvate molecule was disordered over a mirror plane in the structures. Hydrogen atoms were added to the carbon atoms of the molecule but not to the oxygen atom; due to the disorder, the maximum electron density around the oxygen atom was not in a chemically sensible position. Similarly, the hydrogen atoms of the coordinated water molecules could not be refined in a chemically sensible manner. These hydrogen atoms were added to the formula unit of the structure.
Results and Discussion
Synthesis
In our previous work, the synthesis of CuL1 was achieved by reacting a slight excess of metal salt to ligand in a mixture of DMF, alcohol (usually ethanol), and water, with a small addition of acid to slow down the rate of crystallisation.[20e] Using this approach, we sought to prepare topologically identical materials containing octahedral rather than square pyramidal metal centres. Thus, heating slightly more than one equivalent of Co(NO3)2·6H2O and H2L1 in a mixture of DMF, EtOH, water, and 4 drops of glacial acetic acid at 65°C for three days gave large maroon coloured crystals of [Co(L1)(H2O)2]n (CoL1·xS) in 52 % yield. Similarly, combining Ni(NO3)2·6H2O with H2L1 under the same conditions gave teal coloured crystals of [Ni(L1)(H2O)2]n (NiL1·xS) in 59 % yield. Single-crystal X-ray crystallography revealed that CoL1·xS and NiL1·xS are isostructural, and are comprised of pseudo-eclipsed 2D layers. The synthesis of both of these materials was readily reproducible and elemental analysis for each material was consistent with the respective crystallographic formula shown above. Interestingly, CuL1 was typically prepared in the presence of nitric acid rather than acetic acid (DMF, water, ethanol, and 1 drop of 70 % HNO3, 85°C). These conditions however did not yield either the Co or Ni-based MOFs, but repeating the reaction with a lower dilution of HNO3 (1 M) yielded maroon coloured crystals of a different, 2-fold interpenetrated 2D MOF [Co(L1)(H2O)(C2H5OH)]n (β-CoL1) after 1 day. The nickel variant [Ni(L1)(H2O)(C2H5OH)]n (β-NiL1) could also be isolated as light blue/teal coloured crystals. Single crystal X-ray crystallography confirmed the interpenetrated structure for both materials which, as a result, are close-packed and lack solvent accessible porous regions.
Structures of CoL1 and NiL1
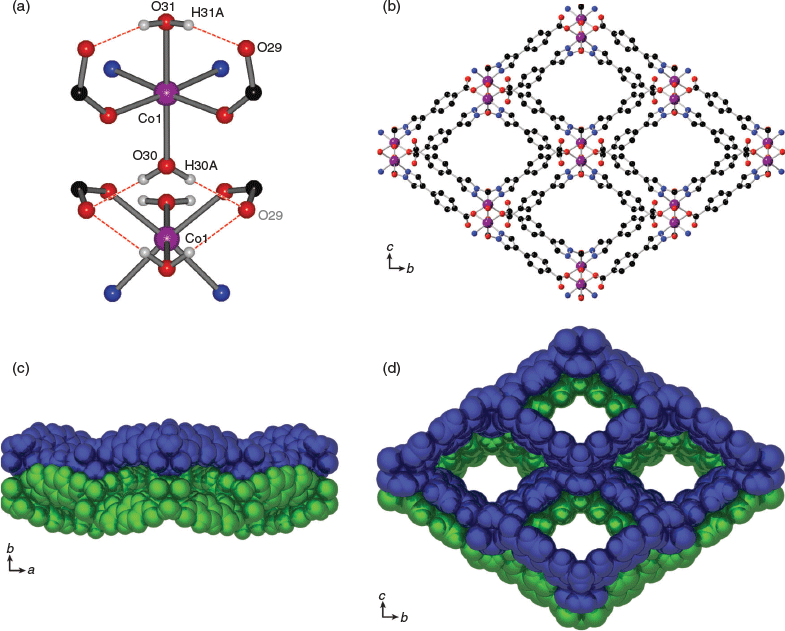
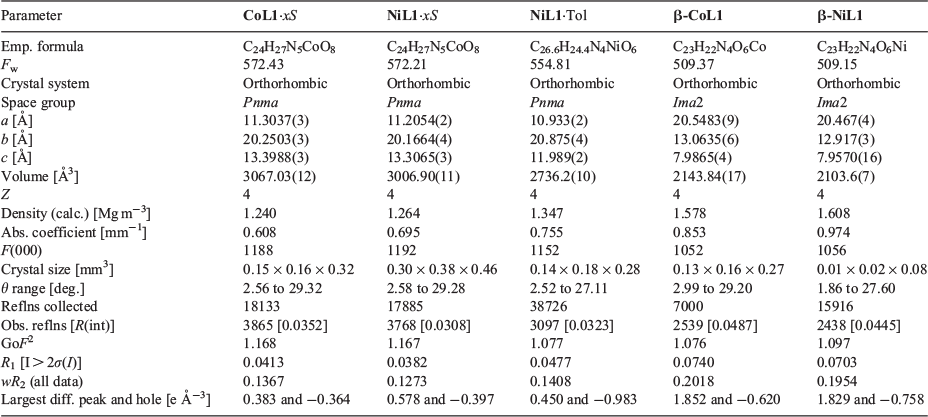
The CoII and NiII 2D MOFs, CoL1·xS and NiL1·xS, are isostructural and as a consequence are considered together. These materials are 2D frameworks with 4,4 nets identical to that of CuL1. In contrast, however, the 2D layers are not eclipsed as seen in CuL1, but stack with a subtle offset to each other. Both materials crystallise in the orthorhombic space group Pnma with essentially the same asymmetric unit as CuL1; half of a molecule of L1 and half a metal centre coordinated by donors from L1 and two, rather than one, water ligands. The metal node possesses an octahedral geometry with a coordination environment comprising two pyrazole donors (chelating), two monodentate carboxylate donors (bridging), and two water ligands located in the axial positions. The hydrogen atoms of both water ligands were successfully located in the difference map which revealed their participation in hydrogen bonding within and between the 2D layers. Within the layers, the hydrogen atoms of one water ligand (H31A) point towards an adjacent carbonyl oxygen (O29) atom of a coordinated carboxylate donor (Fig. 2a). The hydrogen bonding interaction between H31A and O29 measures 1.97 Å (O⋯O distance 2.74 Å, O–H⋯O angle 152.0°) for CoL1·xS and 1.95 Å (O⋯O distance 2.72 Å, O–H⋯O angle 151.4°) for NiL1·xS. The other water ligand (O30) hydrogen bonds to a pair of carbonyl oxygen atoms from a separate layer (Fig. 2a), with a distance of 1.97 Å (O⋯O distance 2.77 Å, O–H⋯O angle 157.6°) for CoL1·xS and 1.99 Å (O⋯O distance 2.78 Å, O–H⋯O angle 157.4°) for NiL1·xS. Similar to the CuII analogue CuL1, the 2D sheets of Co/NiL1·xS are puckered (Fig. 2c) and are stabilised by an array of hydrogen bonds. The 2D sheets, however, are not perfectly eclipsed owing to the octahedral metal centres which are arranged along the a-axis in a zig-zag conformation (Fig. S2b, Supplementary Material). While the b and c-axes are close-packed, 1D microporous channels can be seen along the a-axis (Fig. 2b, d). When taking into consideration the van der Waals radii of surrounding atoms, these channels measure ~3.9 × 6.0 Å.
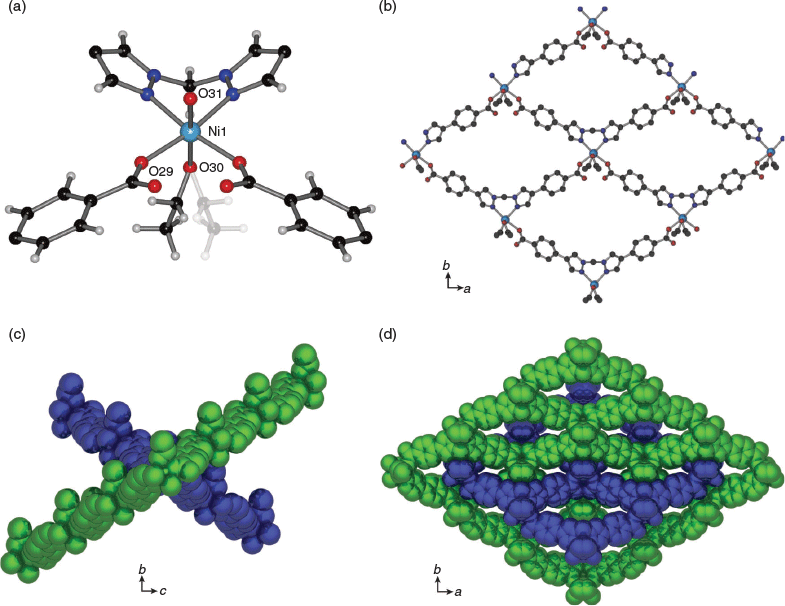
Structures of β-CoL1 and β-NiL1
Under certain conditions (see experimental) it was possible to also obtain phase pure samples of the interpenetrated forms of CoL1 and NiL1, but each of these materials, β-CoL1 and β-NiL1, were more commonly isolated as a mixture, often dominated by the non-interpenetrated variants. β-CoL1 and β-NiL1 are 2-fold interpenetrated 2D layered materials with 4,4 nets (Fig. 3b–d). The inclined interpenetration of the 2D sheets results in a structure that is essentially close-packed. The isostructural MOFs both crystallise in the orthorhombic space group Ima2, and contain half of the formula [M(L1)(H2O)(C2H5OH)] (M = Co or Ni) in the asymmetric unit. The octahedral metal centres are composed of two N-donors from a chelating bis-pyrazolyl moiety, two monodentate carboxylate donors, a water ligand, and an ethanol ligand. In the case of β-CoL1, the water ligand, metal ion, and methylene of the ethanol ligand lie on a centre of inversion with the methyl of the ethanol ligand thus half-occupied over two symmetry related positions. β-NiL1 differs from β-CoL1 in that the entire alkyl chain of the ethanol ligand is half-occupied over two symmetry related positions (Fig. 3a and Fig. S1, Supplementary Material). Although the connectivity of the 4,4 net is analogous to that previously described for CuL1, CoL1, and NiL1, the octahedral geometry of the metal atom and flattened conformation of the 2D layers disfavour an eclipsed packing. Due to the 2-fold interpenetration, the structures of β-CoL1 and β-NiL1 are close-packed and do not contain any solvent accessible channels (Fig. 3c, d).
The structures of β-CoL1 and β-NiL1 are stabilised by weak π–π stacking between two phenyl rings of ligand L1 from two separate layers, with centroid–centroid distances of 3.99 and 3.98 Å respectively. Although the hydrogen atoms of the water ligands could not be located in the difference map, the water ligand and adjacent carbonyl oxygen atoms from the carboxylate donors are in close proximity (O⋯O distance = 3.36 and 3.31 Å for β-CoL1 and β-NiL1 respectively), indicative of hydrogen bonding.
Interestingly, CoL1·xS and NiL1·xS have the same 4,4 net connectivity as the interpenetrated analogues β-CoL1 and β-NiL1. Control over interpenetration of these structures is achieved through modification of the reaction conditions. Although the concentration, acidic modulator, and co-solvents are important parameters,[33] in this case, a higher temperature (85°C) leads to an interpenetrated framework, while a lower temperature (65°C) gives a non-interpenetrated structure. This observation is consistent with work reported by Zaworotko and co-workers, who found that lower temperatures favour the formation of a non-interpenetrated MOF material.[34] An additional point of difference is that the metal nodes of β-CoL1 and β-NiL1 are coordinated by a water and an ethanol ligand, whereas the non-interpenetrated structures CoL1·xS and NiL1·xS contain two water ligands in the axial coordination sites. Accordingly, there is a greater extent of (and notably stronger) hydrogen bonding in CoL1·xS and NiL1·xS which favours a layered packing instead of an interpenetrated structure. Thus, the reaction conditions also influence the composition of the metal node which in turn favours interpenetration of the 2D layers.
Characterisation
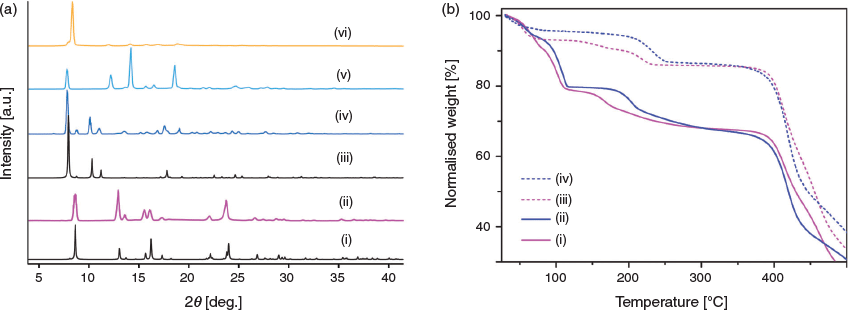
The crystalline phase purity of CoL1·xS and NiL1·xS were confirmed by PXRD experiments, which revealed a good correspondence between the crystalline samples and the respective simulated patterns (Fig. 4a and Fig. S4, Supplementary Material). Furthermore, analysis of the PXRD patterns revealed that the interpenetrated phase (β-CoL1 or β-NiL1) was absent, indicating that the synthetic conditions successfully provided a phase-pure sample. Similarly, for β-CoL1 and β-NiL1 the purity of both materials was confirmed by PXRD, which showed an excellent correlation between the experimental patterns and those simulated from the respective crystal structure (Fig. 4a and Fig. S3, Supplementary Material). Due to the close-packed nature of these materials, no further analysis was undertaken.
TGA was performed on the as-synthesised samples CoL1·xS and NiL1·xS to investigate their thermal stability and desolvation behaviour. It was also of interest to examine whether the coordinated water ligands are integral for the stability of the MOF structure. Both materials behaved similarly, showing an initial weight loss up to 110°C followed by a plateau and a further weight loss step (total of ~32 %) before decomposition at 360°C (Fig. 4b). TGA was also performed on methanol-exchanged samples of the respective materials. For CoL1·xCH3OH, a two-step weight loss of 7.0 % over a temperature range of 110–220°C was observed. NiL1·xCH3OH showed a one-step weight loss of 8.2 % over a temperature range 165–240°C (Fig. 4b). The loss of two water molecules from the structure of both these materials amounts to 7.5 %, which corresponds well to the percentage weight losses observed. This suggests that the coordinated water molecules can be thermally removed without decomposition of the material. The observed colour changes of the respective samples after the stepped weight loss support this assertion. After 220°C, the colour of CoL1·xCH3OH changed to deep blue, whereas at a similar temperature NiL1·xCH3OH changed to a light brown/yellow colour, consistent with a six-coordinate to four-coordinate change in geometry occurring around the respective metal centres.[35] Elemental analysis was performed on the methanol-exchanged samples pre-heated to 260°C. Both samples analysed with at least two water molecules as part of the formula, suggesting that while the water ligands can be removed at high temperatures, the structure thereafter adsorbs moisture upon exposure to air.
We performed PXRD experiments to garner insight into structural changes for NiL1·xCH3OH before and after the loss of the water ligands (due to the isomorphous structures of these materials, further studies focussed only on NiL1). PXRD of a sample heated to 100°C revealed that a structural change indeed occurs with preservation of crystallinity (Fig. 4a). The structural similarities of this material with CuL1 suggests that an analogous trellis-like contraction (Fig. 1) may take place upon initial desolvation. When the same sample was heated to 260°C however, PXRD revealed a notable reduction in crystallinity and an absence of high angle diffraction (Fig. 4a). As indicated by the X-ray structure, the intra- and inter-layer hydrogen bonding interactions of the water ligands contribute significantly to packing of the structure. Thus, removal of these ligands is consistent with the observed loss of long-range order for the material.
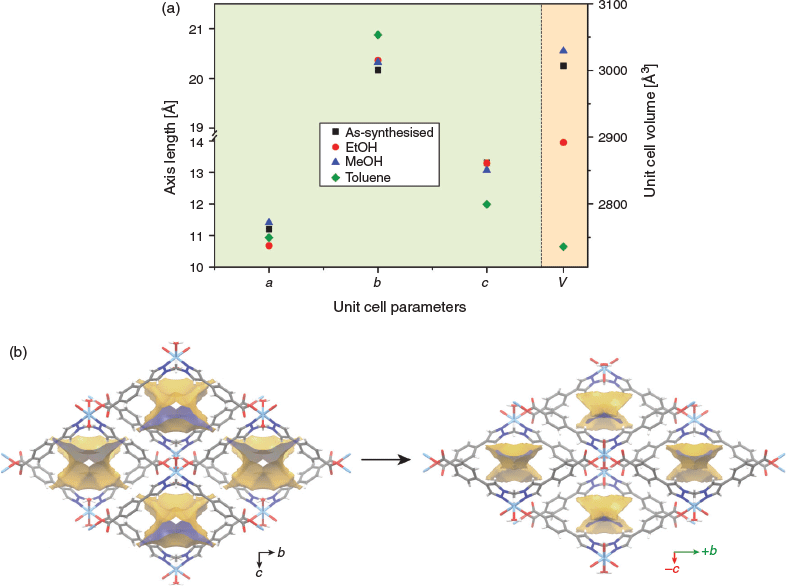
Previously, we had observed that solvent-exchange can lead to noticeable changes to the unit cell dimensions for CuL1.[28] Therefore, in order to confirm the trellis-like flexibility of NiL1 and to understand changes upon activation, we prepared solvent-exchanged samples and analysed the unit cell parameters by SCXRD (Fig. 5a and Table S1, Supplementary Material). In all cases, the samples underwent structural changes in a single-crystal to single-crystal fashion, retaining the orthorhombic space group Pnma. Interestingly, the ethanol exchanged sample underwent the greatest changes along the a axis (interlayer separation) and a small increase along the b axis, resulting in an overall unit cell contraction of 3.8 %. The methanol exchanged sample showed very subtle changes with an overall 1 % expansion of the unit cell. The most dramatic changes were observed for the toluene exchanged sample, NiL1·Tol, which showed a 9 % decrease of the cell volume (relative to the as-synthesised sample), corresponding to an increase in the b axis (+ 0.7 Å) and decrease in the c axis (−1.3 Å). This indicates that the MOF is indeed flexible and responds structurally to solvent exchange along all three axes. The X-ray structure of NiL1·Tol (Fig. 5b) confirmed the expected structural changes and also revealed that the intra and inter-layer hydrogen bonding interactions had shortened (1.90 and 1.94 Å respectively for NiL1·Tol vs 1.97 Å and 1.97 Å for NiL1·xS). A calculation of the void volume with a 3.30 Å diameter probe revealed that the solvent accessible volume of NiL1·Tol is 62.1 Å3. This represents a 72 % decrease relative to NiL1·xS (~218.8 Å3) and indicates that NiL1·Tol is essentially non-porous.
In order to confirm our hypothesis for a contracted, non-porous structure for the activated form of NiL1 (NiL1-ac),[36] we measured the N2 isotherms at 77 and 293 K. These experiments revealed the material was essentially non-porous to N2 (Fig. S6, Supplementary Material, and Fig. 7). However, at 293 K, a maximum uptake of CO2 of 0.63 mmol g−1 (14.3 cm3 g−1) was recorded (Fig. S7, Supplementary Material). These data indicate that the pore diameter is of a size accessible only to the higher boiling point probe molecule CO2. Due to the already narrow pore diameter of NiL1·xS (3.9 × 6.0 Å), and the structural contraction that occurs upon solvent exchange (as indicated by SCXRD, Fig. 5), activation of NiL1 may yield a material with limiting pore dimensions approaching that of the kinetic diameter of CO2 (3.30 Å). This hypothesis is supported by PXRD experiments (Fig. 4a) and the negligible uptake of N2 as well as the evident hysteresis in the 293 K CO2 isotherm.
Discussion
Our previously reported Cu-based 2D MOF, CuL1, is composed of chelating bis-pyrazolyl moieties that bridge to adjacent square pyramidal copper(ii) centres via the phenyl-carboxylate groups.[20e] Due to the layers being eclipsed, the single water molecule is positioned in the pocket below the vacant coordinating site on the copper(ii) of the next layer and primed for linking the layered 2D structure into a 3D material upon activation. Similar observations were observed for the isoreticulated variants of this material.[24] Here however, the octahedral coordination environment of the node in ML1·xS and the lack of a vacant coordination site precludes the layers from packing in an eclipsed manner and furthermore from easily undergoing the structural transformation to a 3D material.
For ML1·xS the layers were slightly offset relative to one another due to the perpendicular relationship between adjacent metal centres (Fig. S2, Supplementary Material), giving narrower pore diameters of 3.9 × 6.0 Å in comparison to CuL1 (~6.9 Å). Confirmation that ML1·xS is structurally flexible comes from SCXRD and PXRD data that show different solvates and a partially desolvated sample to have different crystalline phases. Upon heating however, the formation of a new bond between the coordinated water and the metal in the adjacent layer appears disfavoured and instead the coordinated water molecules around the metal centre are displaced by heating the material up to ~260°C (this was accompanied with a partial loss of crystallinity). Gas adsorption studies of the NiII analogue, NiL1-ac, revealed that the material was non-porous to N2 and sparingly porous to CO2, supporting the occurrence of a trellis-like structural contraction upon desolvation that is similar to CuL1.
Modification of the synthetic conditions also yielded materials composed of interpenetrated 4,4 nets for both CoII and NiII salts. These MOFs were also composed of a singular octahedral metal node containing both N-donors and O-donors from ligand L1, a water ligand, but with an ethanol ligand replacing the second water molecule. This combination of donors supports an interpenetrated packing of the layers and the formation of a close-packed material. Combined, the two sets of results further the understanding of the coordination chemistry of L1 and demonstrate the sensitivity of these four-connected networks towards interpenetration.
Conclusion
Here we have reported the synthesis of a two isostructural CoII and NiII MOF materials and their interpenetrated analogues. SCXRD revealed that all the 2D MOFs are based upon a 4,4 net structure, with the donor composition of the octahedral metal centres dictating framework interpenetration. The presence of H2O and EtOH ligands on the axial sites of the mononuclear metal nodes supported 2-fold interpenetrated structures that were essentially closed-packed. Modifying the synthetic conditions yielded non-interpenetrated forms with two water molecules coordinated to the metal node, resulting in a perpendicular relationship and an array of hydrogen bonds between adjacent CoII or NiII metal centres. This structural feature induced a staggered arrangement of the 2D layers in CoL1 and NiL1, giving smaller pore dimensions compared with our previously reported CuL1 MOF. For NiL1, we showed by PXRD, SCXRD, and TGA analysis that solvent exchange and partial desolvation leads to a structural contraction, while complete removal of the solvent (including coordinated H2O molecules) leads to a loss of long-range order. Due to the already narrow pore-size, the structural contraction observed upon activation of NiL1 led to a phase with negligible uptake of N2 at 77 and 293 K and a low uptake of CO2 at 293 K. This work highlights the importance of metal centre geometry in the trellis-like contraction and pore diameter tuning for this class of materials.
Supplementary Material
Additional structural information for β-ML1 and ML1 (M = CoII or NiII), unit cell parameters for the solvent-exchanged forms of NiL1, and PXRD and gas adsorption data are available on the Journal’s website.
Conflicts of Interest
The authors declare no conflicts of interest.
Acknowledgements
W.M.B., C.J.S., and C.J.D. gratefully acknowledge the Australian Research Council for funding (DE190100327, FT0991910, and FT100100400). This research was supported by the Science and Industry Endowment Fund. Aspects of this research were undertaken on the MX1 beamline at the Australian Synchrotron, Victoria, Australia.
References
[1] B. F. Hoskins, R. Robson, J. Am. Chem. Soc. 1989, 111, 5962.| Crossref | GoogleScholarGoogle Scholar |
[2] B. F. Hoskins, R. Robson, J. Am. Chem. Soc. 1990, 112, 1546.
| Crossref | GoogleScholarGoogle Scholar |
[3] R. Robson, J. Chem. Soc., Dalton Trans. 2000, 3735.
| Crossref | GoogleScholarGoogle Scholar |
[4] Y. Cui, B. Li, H. He, W. Zhou, B. Chen, G. Qian, Acc. Chem. Res. 2016, 49, 483.
| Crossref | GoogleScholarGoogle Scholar | 26878085PubMed |
[5] H. Furukawa, K. E. Cordova, M. O’Keeffe, O. M. Yaghi, Science 2013, 341,
| Crossref | GoogleScholarGoogle Scholar | 23990564PubMed |
[6] B. Chen, S. Xiang, G. Qian, Acc. Chem. Res. 2010, 43, 1115.
| Crossref | GoogleScholarGoogle Scholar | 20450174PubMed |
[7] K. Sumida, D. L. Rogow, J. A. Mason, T. M. McDonald, E. D. Bloch, Z. R. Herm, T.-H. Bae, J. R. Long, Chem. Rev. 2012, 112, 724.
| Crossref | GoogleScholarGoogle Scholar | 22204561PubMed |
[8] J. Lee, O. K. Farha, J. Roberts, K. A. Scheidt, S. T. Nguyen, J. T. Hupp, Chem. Soc. Rev. 2009, 38, 1450.
| Crossref | GoogleScholarGoogle Scholar | 19384447PubMed |
[9] J. Liu, L. Chen, H. Cui, J. Zhang, L. Zhang, C.-Y. Su, Chem. Soc. Rev. 2014, 43, 6011.
| Crossref | GoogleScholarGoogle Scholar | 24871268PubMed |
[10] R. Ricco, W. Liang, S. Li, J. J. Gassensmith, F. Caruso, C. J. Doonan, P. Falcaro, ACS Nano 2018, 12, 13.
| Crossref | GoogleScholarGoogle Scholar | 29309146PubMed |
[11] C. Doonan, R. Riccò, K. Liang, D. Bradshaw, P. Falcaro, Acc. Chem. Res. 2017, 50, 1423.
| Crossref | GoogleScholarGoogle Scholar | 28489346PubMed |
[12] K. Uemura, R. Matsuda, S. Kitagawa, J. Solid State Chem. 2005, 178, 2420.
| Crossref | GoogleScholarGoogle Scholar |
[13] S. Kitagawa, R. Matsuda, Coord. Chem. Rev. 2007, 251, 2490.
| Crossref | GoogleScholarGoogle Scholar |
[14] G. Ferey, C. Serre, Chem. Soc. Rev. 2009, 38, 1380.
| Crossref | GoogleScholarGoogle Scholar | 19384443PubMed |
[15] A. Schneemann, V. Bon, I. Schwedler, I. Senkovska, S. Kaskel, R. A. Fischer, Chem. Soc. Rev. 2014, 43, 6062.
| Crossref | GoogleScholarGoogle Scholar | 24875583PubMed |
[16] M.-H. Zeng, X.-L. Feng, X.-M. Chen, Dalton Trans. 2004, 2217.
| Crossref | GoogleScholarGoogle Scholar | 15278110PubMed |
[17] K. Biradha, Y. Hongo, M. Fujita, Angew. Chem. Int. Ed. 2002, 41, 3395.
| Crossref | GoogleScholarGoogle Scholar |
[18] R. Kitaura, K. Seki, G. Akiyama, S. Kitagawa, Angew. Chem. Int. Ed. 2003, 42, 428.
| Crossref | GoogleScholarGoogle Scholar |
[19] D. Tanaka, K. Nakagawa, M. Higuchi, S. Horike, Y. Kubota, T. C. Kobayashi, M. Takata, S. Kitagawa, Angew. Chem. 2008, 120, 3978.
| Crossref | GoogleScholarGoogle Scholar |
[20] (a) Most trellis-like structural transformations occur for defined layers within 3D MOFs where the layers are connected by metal ligand interactions through a bridging ligand. For reviews, see: W. Li, S. Henke, A. K. Cheetham, APL Mater. 2014, 2,
| Crossref | GoogleScholarGoogle Scholar |
(b) Z.-J. Lin, J. Lü, M. Hong, R. Cao, Chem. Soc. Rev. 2014, 43, 5867.
| Crossref | GoogleScholarGoogle Scholar |
(c) F.-X. Coudert, Chem. Mater. 2015, 27, 1905.
| Crossref | GoogleScholarGoogle Scholar |
(d) In certain instances, where non-covalent secondary interactions stabilise the layer-layer contacts, trellis-like transformations can be observed: H.-L. Zhou, J.-P. Zhang, X.-M. Chen, Front. Chem. 2018, 6, 306.
| Crossref | GoogleScholarGoogle Scholar |
(e) W. M. Bloch, R. Babarao, M. R. Hill, C. J. Doonan, C. J. Sumby, J. Am. Chem. Soc. 2013, 135, 10441.
| Crossref | GoogleScholarGoogle Scholar |
[21] S. Horike, S. Shimomura, S. Kitagawa, Nat. Chem. 2009, 1, 695.
| Crossref | GoogleScholarGoogle Scholar | 21124356PubMed |
[22] W. M. Bloch, C. J. Sumby, Chem. Commun. 2012, 48, 2534.
| Crossref | GoogleScholarGoogle Scholar |
[23] W. M. Bloch, C. J. Sumby, Eur. J. Inorg. Chem. 2015, 3723.
| Crossref | GoogleScholarGoogle Scholar |
[24] A. Burgun, W. M. Bloch, C. J. Doonan, C. J. Sumby, Aust. J. Chem. 2017, 70, 566.
| Crossref | GoogleScholarGoogle Scholar |
[25] W. M. Bloch, C. J. Doonan, C. J. Sumby, CrystEngComm 2013, 15, 9663.
| Crossref | GoogleScholarGoogle Scholar |
[26] C. J. Coghlan, C. J. Sumby, C. J. Doonan, CrystEngComm 2014, 16, 6364.
| Crossref | GoogleScholarGoogle Scholar |
[27] O. M. Linder-Patton, C. J. Doonan, C. J. Sumby, J. Coord. Chem. 2016, 69, 1802.
| Crossref | GoogleScholarGoogle Scholar |
[28] O. M. Linder-Patton, W. M. Bloch, C. J. Coghlan, K. Sumida, S. Kitagawa, S. Furukawa, C. J. Doonan, C. J. Sumby, CrystEngComm 2016, 18, 4172.
| Crossref | GoogleScholarGoogle Scholar |
[29] N. P. Cowieson, D. Aragao, M. Clift, D. J. Ericsson, C. Gee, S. J. Harrop, N. Mudie, S. Panjikar, J. R. Price, A. Riboldi-Tunnicliffe, R. Williamson, T. Caradoc-Davies, J. Synchrotron Radiat. 2015, 22, 187.
| Crossref | GoogleScholarGoogle Scholar | 25537608PubMed |
[30] G. M. Sheldrick, Acta Crystallogr. Sect. A 1990, 46, 467.
| Crossref | GoogleScholarGoogle Scholar |
[31] G. M. Sheldrick, Acta Crystallogr. Sect. C 2015, 71, 3.
| Crossref | GoogleScholarGoogle Scholar |
[32] L. J. Barbour, J. Supramol. Chem. 2001, 1, 189.
| Crossref | GoogleScholarGoogle Scholar |
[33] O. K. Farha, J. T. Hupp, Acc. Chem. Res. 2010, 43, 1166.
| Crossref | GoogleScholarGoogle Scholar | 20608672PubMed |
[34] J. Zhang, L. Wojtas, R. W. Larsen, M. Eddaoudi, M. J. Zaworotko, J. Am. Chem. Soc. 2009, 131, 17040.
| Crossref | GoogleScholarGoogle Scholar | 19891485PubMed |
[35] R. H. Lee, E. Griswold, J. Kleinberg, Inorg. Chem. 1964, 3, 1278.
| Crossref | GoogleScholarGoogle Scholar |
[36] The material used for gas sorption studies (NiL1-ac) was prepared by heating a methanol exchanged sample at 260 °C for 1 h under vacuum.