Continuous flow in situ shear stress induced encapsulation of curcumin within spheroidal bovine serum albumin-based nanoparticles
Abeer Yahia H. Alamry A B , Ahmed Hussein Mohammed Al-Antaki A C , Xuan Luo A and Colin L. Raston A *
A *
A Flinders Institute for Nanoscale Science and Technology, College of Science and Engineering, Flinders University, Adelaide, SA 5042, Australia.
B Department of Chemistry, Faculty of Science, University of Bisha, Bisha 67714, Saudi Arabia.
C Department of Chemistry, Faculty of Science, University of Kufa, Najaf 54001, Iraq.
Australian Journal of Chemistry 75(9) 772-779 https://doi.org/10.1071/CH21345
Submitted: 29 December 2021 Accepted: 11 March 2022 Published: 24 May 2022
© 2022 The Author(s) (or their employer(s)). Published by CSIRO Publishing. This is an open access article distributed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND)
Abstract
Nanospheres comprised of bovine serum albumin (BSA) crosslinked with glutaraldehyde possessing different pore sizes are accessible under continuous flow conditions using a vortex fluidic device (VFD) with a rapidly rotating tube tilt angle θ of −45° which overcomes an otherwise build-up of material occurring at θ +45°. The build-up can also be overcome at +45° under continuous flow using ethanol dehydrating conditions at 80°C without the need for crosslinking using glutaraldehyde. As-prepared BSA nanoparticles (BNPs) of ca. 531 nm in diameter were formed at 5k rpm in a single-step process. Similar rapid processing in the presence of curcumin affords composite BNPs@curcumin particles ca. 615 nm in diameter.
Keywords: bovine serum albumin, drug delivery, encapsulation, fluorescence, microfluidics, nanoparticle, vortex fluidic device.
Introduction
Protein-based nanoparticles such as bovine serum albumin nanoparticles (BNPs) have been widely used for drug delivery because of their biocompatibility, non-immunogenicity and non-toxicity, which can be advantageous over synthetic polymer-based nanoparticles.[1,2] BNPs have also gained interest as drug carriers which relates to their enriched functional groups allowing bioactive molecules to be attached covalently or adsorbed through non-covalent interactions.[3,4] Yu et al.[5] demonstrated the use of BNPs as a controlled release carrier to treat inner ear disorders. Morphologies of the BNPs determine the drug loading capacity and the encapsulation efficiency for such particles.[5,6] BNPs have been reported to exhibit a drug loading capacity typically around 15% with a maximum encapsulation efficiency for Rhodamine B (RhB) at 41%.[5] The encapsulation process is typically carried out as a separate process after the preparation of BNPs, and for RhB this involves incubating the BNPs in a solution containing varying amounts of RhB, as a time consuming process.[5]
Indeed, there are several methods to fabricate BNPs, including emulsification, thermal gelation, nanospray drying, self-assembly and desolvation.[7,8] BSA undergoes conformational changes and forms aggregates in the presence of desolvating agents such as ethanol. The size and shape of BNPs can be controlled by adjusting the nature and concentration of the desolvating agent along with the type of crosslinking reagents.[7] However, the fabrication of BNPs using batch stirring is inefficient requiring long crosslinking processing times. For example, Yang et al.[9] reported the preparation of fluorescent BNPs with a total processing time of ca. 18 h. A new era of possibilities regarding efficient nanoparticle synthesis has emerged using microfluidic technologies. The unique dimension of the device harnesses the fluid mechanics to allow efficient mass transport, improved heat transfer and reduced reaction time.[10,11] However, the limited diffusion and system clogging of conventional channel-based microfluidics remain to be solved, as is using colloidal materials and the build-up of products in the system.[12] Advances have been made in dramatically reducing the processing time, using high shear stress in a vortex fluidic device (VFD), but the processing is in the confined mode operation of the device for finite volume capacities (1–2 mL),[6] as a form of batch processing, thereby limiting scaling up of the process. The VFD is a versatile thin film microfluidic platform, having a number of applications, including preparing hydrogels,[13] protein folding,[14] protein purification[15] and accelerating enzymatic reactions.[16] In a more general sense, it is effective in controlling chemical transformations, materials processing and self-assembly.[13]
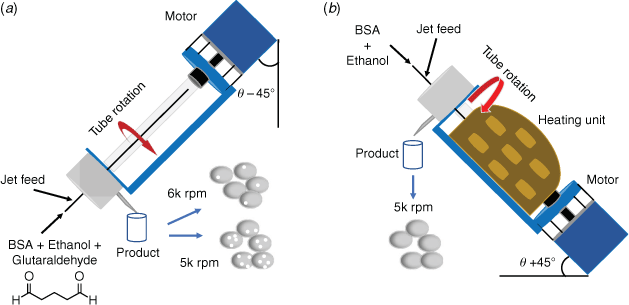
A VFD houses a rapidly rotating tube, typically as quartz of borosilicate glass, and inclined at +45° relative to the horizontal position. In addition to the aforementioned confined mode of operation of the VFD, there is the continuous flow mode where reagents are constantly fed into the tube and collected on exiting at the top, and this is where scalability is addressed up front for any downstream application. The rotational speed of the tube in the VFD can be varied, in systematically determining the optimum conditions for any process, and for all processing in the device the optimal tilt angle of the rotating tube θ is +45°, or −45° in a few cases (Fig. 1).[17] This processing harnesses the mechanical energy associated with different micron sized and submicron topological fluid flows in the device.[18]
|
Experimental
Materials and chemicals
Bovine serum albumin (BSA), phosphate buffered saline (PBS), glutaraldehyde, absolute ethanol and curcumin were obtained from Sigma–Aldrich as analytical grade.
Synthesis in confined mode
BNPs (from 1 mg mL–1 BSA solution) were prepared using the desolvation method at room temperature, as a modification of the method described by Yu et al.[5] Briefly, 15 mg of BSA was dissolved in 15 mL of PBS (pH = 7.45). Volume combinations for glutaraldehyde, BSA (1 mg mL–1) and ethanol used for the synthesis were 15, 300 and 900 μL, respectively. In this experiment, a standard borosilicate glass VFD tube (20 mm O.D., 17.5 mm I.D. and 19.4 mm length) was operated at different rotational speeds.
Synthesis in continuous flow
The high porosity BNPs (BNP-HP) were formed by loading 5 mL of BSA solution (5 mg mL–1), 15 mL of ethanol and 250 µL of glutaraldehyde into a syringe. The low porosity BNPs (BNP-LP) were formed by loading 5 mL of BSA solution (5 mg mL–1), 10 mL of ethanol and 170 µL of glutaraldehyde into a syringe. The mixture was delivered as close as possible to the base of the inverted tube titled at −45°,[19] Fig. 1a, by a syringe pump through a metal jet feed, at a flow rate of 0.5 mL min–1. The product was collected by centrifugation at 1200 × g for 2 min, and then washed three times with deionised water to remove excess reactants whereupon the samples were ready for analysis.
Entrapment of curcumin under flow
To gain access to glutaraldehyde-free BNPs, 10 mL of BSA solution (10 mg mL–1) and 15 mL of ethanol were subjected to continuous flow VFD processing, with the tube inclined at +45° (Fig. 1b). This mixture was then loaded into a syringe and delivered to the VFD tube through a jet feed at a flow rate of 0.5 mL min–1. For the synthesis of curcumin encapsulated BNPs or BNPs@curcumin, 10 mL of BSA solution (10 mg mL–1) was mixed with 15 mL of curcumin solution which was prepared by dissolving 7.5 mg of curcumin in 15 mL of ethanol. This mixture was then loaded into a syringe and delivered to the VFD tube through a jet feed at a flow rate of 0.5 mL min–1 with the tube rotating at 5k rpm at 80°C. After processing for preparing BNPs or BNPs@curcumin, heating was continued for another 5 min to ensure that the reactions were complete. Products were collected by centrifugation at 1200 × g for 2 min, followed by washing three times with deionised water.
Measurement
As-prepared BNPs were drop cast on a silicon wafer and air-dried. The samples were then coated with 5 nm of platinum prior to imaging. The morphology of the BNPs was determined using scanning electron microscopy (SEM-Inspect FEI F50 SEM). Dynamic light scattering (DLS) was carried out using a Malvern particle size analyser (Malvern Zetasizer). ATR-FTIR analysis was carried out in a Perkin Elmer instrument in the range of 550–4000 cm−1 (Perkin Elmer Frontier). X-Ray diffraction was carried out using a Bruker D8 ADVANCE ECO instrument (Co-Kα, λ = 1.7889 Å). UV-Vis absorbance and fluorescence of BNPs were investigated using a Varian Cary 50 EST 70772 spectrophotometer.
Results and discussion
In the present work, the fabrication of porous BNPs in the presence of glutaraldehyde has been established under continuous flow in the VFD thereby demonstrating it as a readily scalable process. The product is more uniform compared to the analogous product formed using the confined mode operation of the VFD.[6] In addition, by operating the tube at θ −45°, where the fluid dynamic response in the VFD appears to be similar to that at +45°, the issue of sample build-up in the tube as observed in our previous work was effectively circumvented.
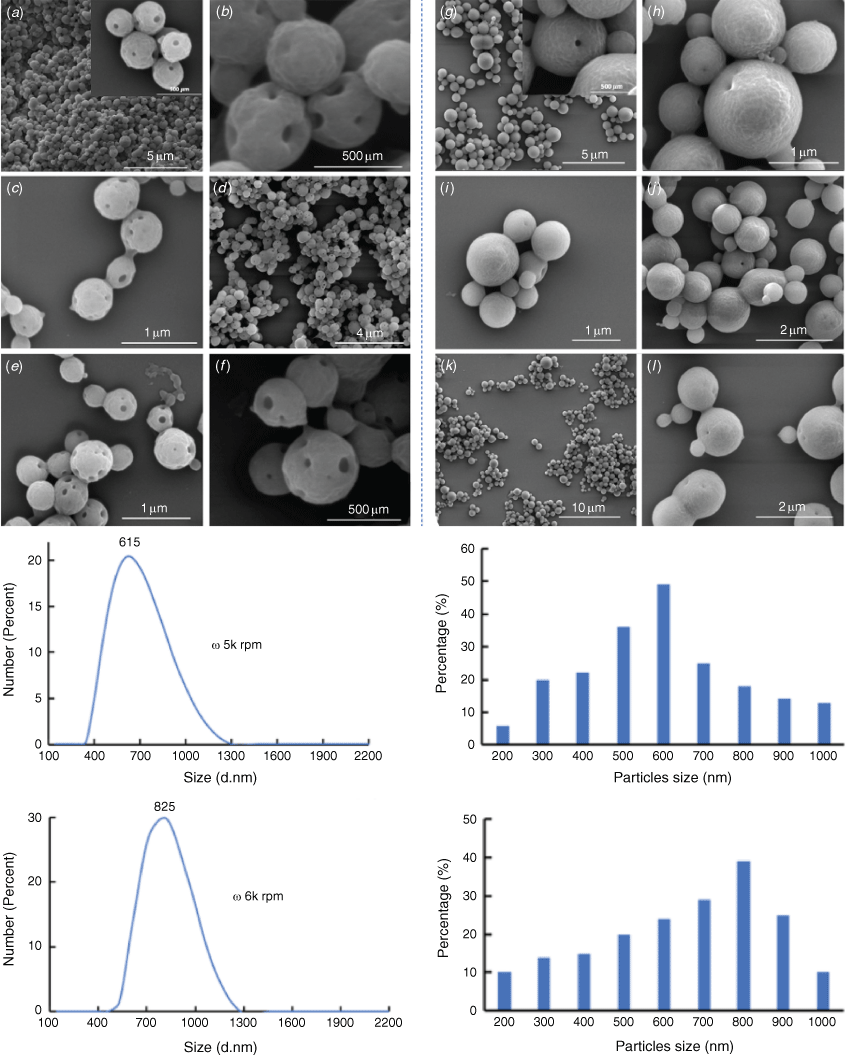
The optimisation of the fabrication process has been investigated at different processing temperatures, rotational speeds, flow rates and concentrations of starting reagents (Supplementary Figs S1–S4). The fabrication of BNPs in the VFD was efficient with well-developed and high yield spherioidal BNPs formed at 5k and 6k rpm at a flow rate of 0.5 mL min–1 (Fig. 2). Crosslinking with glutaraldehyde resulted in porous spheres for 5k rpm rotational speed (Fig. 2a–f). Increasing the rotational speed to 6k rpm resulted in the pore sizes in the spheroidal particles becoming smaller (Fig. 2g–l). These spheroidal particles are colloidally stable in solution for days and in the air for at least a week with the morphology and size of the particles unchanged, as established using SEM (Fig. 2c–f, i–l).
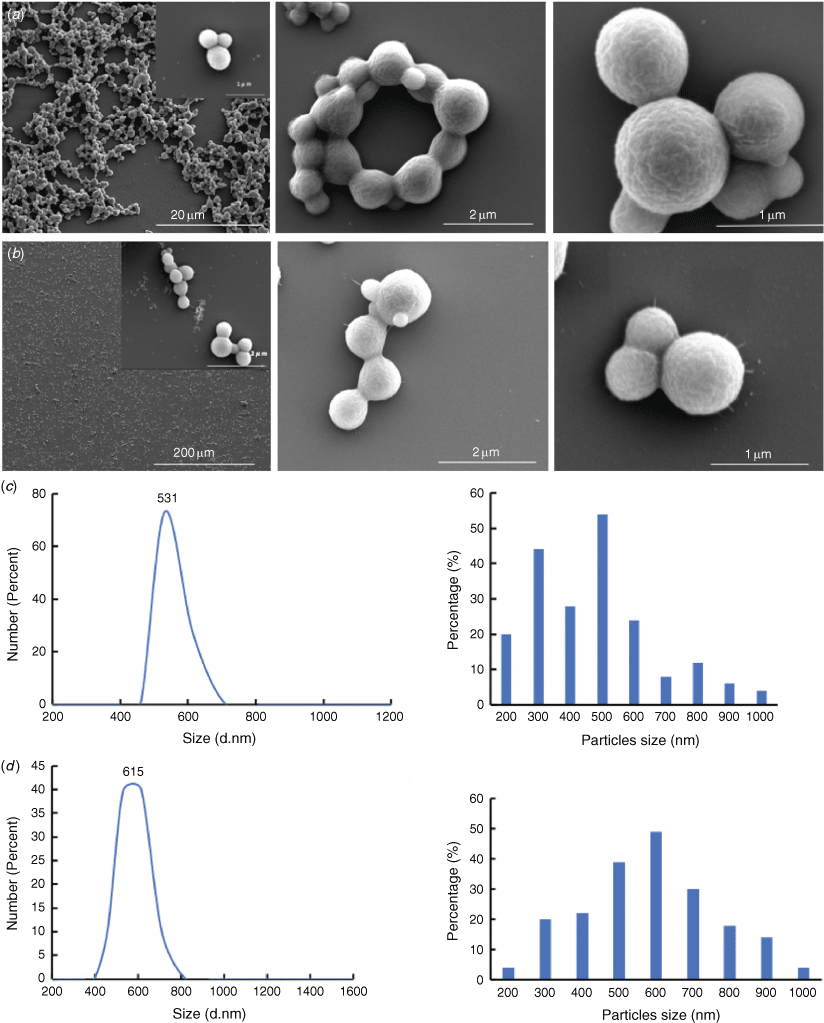
In addition, to translate the confined mode of preparing glutaraldehyde crosslinked BNPs, we also developed a continuous flow process using the VFD for preparing BNPs, which avoids the use of any crosslinking reagent, using ethanol as a dehydrating agent while the tube is heated using a modular heating unit attachment to the VFD itself (Fig. 1b). Avoiding the use of glutaraldehyde was based on its cytotoxicity when released into cells,[20] leading to the formation of unwanted products with toxic effects.[6,20] It was established that BNPs could also be efficiently formed without glutaraldehyde in the VFD (Fig. 3a, Supplementary Fig. S8). In the same way, the in situ encapsulation of curcumin into the BNPs was also established in the absence of the crosslinking reagent (Fig. 3b), which is of interest as a biocompatible drug delivery system.[21,22] For example, Jithan et al. have used BSA nanoparticles encapsulating curcumin as an anti-breast cancer drug.[21] In the present work, the encapsulation process is significantly simplified and efficient, involving processing and encapsulation in a simple one-step continuous flow process, thereby avoiding a separate processing step for the drug loading. Heating of the solution was necessary to stabilise the nanoparticle structure, presumably to unfold the protein molecules, which is facilitated by the intensive micromixing in the dynamic thin film in the VFD. The high stability of both BNPs and BNPs@curcumin formed in the absence of glutaraldehyde were similar (Supplementary Fig. S8). This establishes the ability of VFD processing to form stable BSA based nanoparticles without the need for chemical crosslinking. The resulting BNPs and BNPs@curcumin exhibited a similar morphology of essentially spheroidal particles (Fig. 3), with isolated yields of 17.4% and 21% for BNPs and BNPs@curcumin, which is consistent with the literature.[23–26]
In previous studies, the use of glutaraldehyde for crosslinking purposes resulted in the formation of porous spheroidal BNPs, whereas when they were formed herein in the absence of glutaraldehyde, and in the presence of curcumin, there was no evidence for large pores in the particles. Nevertheless, small pores down to nanometre dimensions are likely.
Further studies on particle sizes were conducted using DLS (Fig. 3c, d); this established that the BNPs obtained from BSA and ethanol under continuous flow VFD processing are ca. 531 nm in diameter. For BNPs@curcumin formed in situ, the particle size increases to ca. 615 nm (Fig. 3d). The BNPs@curcumin particles were more uniform with increased sizes compared to that of BNPs.
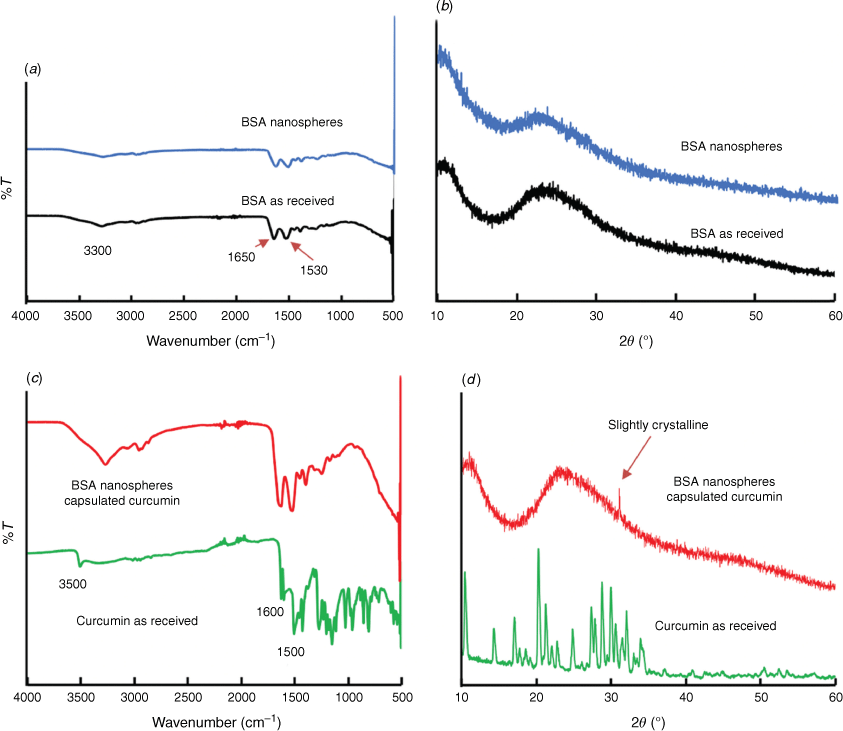
ATR-FTIR absorption peaks of BNPs at ~3300, 1650 and 1530 cm−1 correspond to O–H stretching, C═O (amide I) and N–H and C–N stretching vibration (amide II), respectively (Fig. 4a). This is consistent with the IR spectra of macroporous BNPs reported by Luo et al.[6] The characteristic vibration of curcumin at 1620 cm−1 (C═O) was shifted to 1600 cm−1 in the spectrum of BNPs@curcumin. The shifting of this peak was also observed on curcumin loaded poly(lactide)-tocopheryl polyethylene glycol succinate (PLA-TPGS) nanoparticles.[27] The in situ generated BNPs@curcumin also showed a curcumin characteristic phenolic O–H stretching vibration at 3500 cm−1 and C═C stretching vibration at 1500 cm−1.[27] XRD analysis of BSA, BNPs and BNPs@curcumin show that they were all amorphous (Fig. 4b, d). Thus the uptake of curcumin into the BNPs is not as small crystals of curcumin but rather as molecular curcumin incorporated into the overall structure of the particles. Thus processing under continuous flow in the VFD in the presence of curcumin does not appreciably change the nature of the spheroidal BNP particles themselves. The amorphous nature of these particles can be advantageous to improve the dissolution process, thereby enhancing both absorption and bioavailability during the drug delivery process.[28]
|
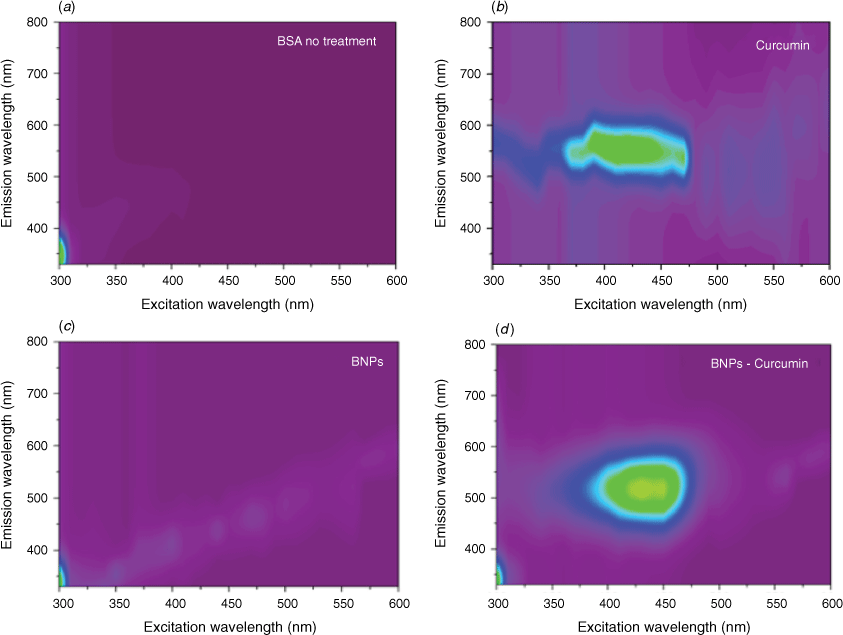
A UV-vis spectrum of the BNPs has a peak at 281 nm, with reduced intensity relative to BSA, reflecting a difference in folding in the protein structure from its native state (Supplementary Fig. S10a). This pattern was also observed in BNPs@curcumin (Supplementary Fig. S10b). From the 2D fluorescence, there is a significant shift of the emission for BNPs@curcumin, from 550 for the curcumin to ~500 nm (Fig. 5b, d). This emission was not observed for non-treated BSA and BNPs (Fig. 5a, c). According to the literature, when curcumin is loaded into the hydrophobic core of PLA-TPGS nanoparticles, the fluorescence spectra were also shifted, from 543 to 530 nm.[28] The shift might arise from the hydrophobic interactions between the nanoparticles and the curcumin molecule.[27] All of the above findings demonstrate the ability to load curcumin into BNPs in the absence of crosslinking reagent for fabrication carried out in the VFD.
Conclusions
We have established the ability to fabricate porous BNPs crosslinked with glutaraldehyde under continuous flow processing using a VFD thin film microfluidic platform, albeit requiring the VFD tube to be tilted at −45° to limit build-up of the product on the wall of the rotating tube. We have also established that BNPs can be formed in the absence of glutaraldehyde as a continuous flow VFD process, further adding to the utility of the thin film microfluidic platform. In addition, the ability to incorporate curcumin in the BNPs has been established, as an in situ process, also in the absence of other reagents and under continuous flow VFD processing. In the future, different drug molecules might be considered for encapsulation, in developing BSA-based drug delivery vehicles and meeting different applications.
Data availability
The data that support this study are available in the article and accompanying online supplementary material.
Conflicts of interest
There are no conflicts to declare.
Declaration of funding
This work was financially supported by the Australia Research Council (DP 200101106), the Government of South Australia, and the Australian Nanotechnology Network, through the Overseas Travel Fellowship program.
Supplementary material
Supplementary material is available online.
Acknowledgements
The authors appreciate Bisha University, Bisha, Saudi Arabia, for scholarship funding, and the authors also gratefully acknowledge financial support from the Australian Research Council and the Government of South Australia, the Australian Microscopy & Microanalysis Research Facility (AMMRF) and the Australian National Fabrication Facility (ANFF) for accessing microscopic facilities.
References
[1] IK Deshapriya, BS Stromer, A Pattammattel, CS Kim, R Iglesias-Bartolome, L Gonzalez-Fajardo, V Patel, JS Gutkind, X Lu, CV Kumar, Bio Chem 2015, 26, 396.[2] TH Kim, HH Jiang, YS Youn, CW Park, KK Tak, S Lee, H Kim, S Jon, X Chen, KC Lee, Inter Pharma 2011, 403, 285.
| Crossref | GoogleScholarGoogle Scholar |
[3] C Weber, J Kreuter, K Langer, Inter J Pharmac 2000, 196, 197.
| Crossref | GoogleScholarGoogle Scholar |
[4] M Skrt, E Benedik, Č Podlipnik, NP Ulrih, Food Chem 2012, 135, 2418.
| Crossref | GoogleScholarGoogle Scholar | 22980822PubMed |
[5] Z Yu, M Yu, Z Zhang, G Hong, Q Xiong, Nanoscale Res Lett 2014, 9, 343.
| Crossref | GoogleScholarGoogle Scholar | 25114637PubMed |
[6] X Luo, AHM Al-Antaki, DP Harvey, Y Ruan, S He, W Zhang, CL Raston, Acs Appl Mater Interf 2018, 10, 27224.
| Crossref | GoogleScholarGoogle Scholar |
[7] R Sadeghi, AA Moosavi-Movahedi, Z Emam-Jomeh, A Kalbasi, SH Razavi, M Karimi, J Kokini, J Nanoparticle Res 2014, 16, 2565.
| Crossref | GoogleScholarGoogle Scholar |
[8] AO Elzoghby, WM Samy, NA Elgindy, J Control Release 2012, 157, 168.
| Crossref | GoogleScholarGoogle Scholar | 21839127PubMed |
[9] Q Yang, Z Ye, M Zhong, B Chen, J Chen, R Zeng, L Wei, H-w Li, L Xiao, Acs Appl Mater Interf 2016, 8, 9629.
| Crossref | GoogleScholarGoogle Scholar |
[10] A-G Niculescu, C Chircov, AC Bîrcă, AM Grumezescu, Nanomaterials 2021, 11, 864.
| Crossref | GoogleScholarGoogle Scholar | 33800636PubMed |
[11] X Luo, P Su, W Zhang, CL Raston, Adv Mater Technol 2019, 4, 1900488.
| Crossref | GoogleScholarGoogle Scholar |
[12] X Luo, W Cai, K Vimalanathan, A Igder, Z Gardner, S Petticrew, S He, C Chuah, Y Tang, P Su, W Zhang, CL Raston, Acs Appl Nano Mater 2022, 5, 2875.
| Crossref | GoogleScholarGoogle Scholar |
[13] CL Tong, RA Boulos, C Yu, KS Iyer, CL Raston, RSC Adv 2013, 3, 18767.
| Crossref | GoogleScholarGoogle Scholar |
[14] TZ Yuan, CFG Ormonde, ST Kudlacek, S Kunche, JN Smith, WA Brown, KM Pugliese, TJ Olsen, M Iftikhar, CL Raston, GA Weiss, ChemBioChem 2015, 16, 393.
| Crossref | GoogleScholarGoogle Scholar | 25620679PubMed |
[15] X Luo, P Smith, CL Raston, W Zhang, ACS Sustainable Chem Eng 2016, 4, 3905.
| Crossref | GoogleScholarGoogle Scholar |
[16] J Britton, LM Meneghini, CL Raston, GA Weiss, Angew Chem Int Ed 2016, 55, 11387.
| Crossref | GoogleScholarGoogle Scholar |
[17] AHM Al-Antaki, X Luo, TMD Alharbi, DP Harvey, S Pye, J Zou, W Lawrance, CL Raston, RSC Adv 2019, 9, 22074.
| Crossref | GoogleScholarGoogle Scholar | 35518882PubMed |
[18] TMD Alharbi, M Jellicoe, X Luo, K Vimalanathan, IK Alsulami, BSAL Harbie, A Igder, FAJ Alrashaidi, X Chen, KA Stubbs, JM Chalker, W Zhang, RA Boulos, DB Jones, JS Quinton, CL Raston, Nanoscale Adv 2021, 3, 3064.
| Crossref | GoogleScholarGoogle Scholar |
[19] C Weber, C Coester, J Kreuter, K Langer, Inter J Pharmac 2000, 194, 91.
| Crossref | GoogleScholarGoogle Scholar |
[20] W Fürst, A Banerjee, Ann Thorac Surg 2005, 79, 1522.
| Crossref | GoogleScholarGoogle Scholar | 15854927PubMed |
[21] AV Jithan, K Madhavi, M Madhavi, K Prabhakar, Int J Pharm Investig 2011, 1, 119.
| Crossref | GoogleScholarGoogle Scholar | 23071931PubMed |
[22] BB Aggarwal, B Sung, Trends Pharmacol Sci 2009, 30, 85.
| Crossref | GoogleScholarGoogle Scholar | 19110321PubMed |
[23] L Yasmin, X Chen, KA Stubbs, CL Raston, Sci Rep 2013, 3, 2282.
| Crossref | GoogleScholarGoogle Scholar | 23884385PubMed |
[24] X Chen, NM Smith, KS Iyer, CL Raston, Chem Soc Rev 2014, 43, 1387.
| Crossref | GoogleScholarGoogle Scholar | 24346239PubMed |
[25] WH Lee, CY Loo, M Bebawy, F Luk, RS Mason, R Rohanizadeh, Curr Neuropharmacol 2013, 11, 338.
| Crossref | GoogleScholarGoogle Scholar | 24381528PubMed |
[26] KI Priyadarsini, Molecules 2014, 19, 20091.
| Crossref | GoogleScholarGoogle Scholar | 25470276PubMed |
[27] HN Nguyen, PT Ha, A Sao Nguyen, DT Nguyen, HD Do, QN Thi, MNH Thi, Adv Nat Sci: Nanosci Nanotech 2016, 7, 025001.
[28] JS Boateng, D Areago, Austin J Anal Pharm Chem 2014, 1, 1.