Tuning the photoreactivity of photocycloaddition by halochromism†
Vinh X. Truong A B * and Christopher Barner-Kowollik A B C *
A B C *
A Centre for Materials Science, Queensland University of Technology (QUT), 2 George Street, Brisbane, Qld 4000, Australia.
B School of Chemistry and Physics, Queensland University of Technology (QUT), 2 George Street, Brisbane, Qld 4000, Australia.
C Institute of Nanotechnology, Karlsruhe Institute of Technology (KIT), Hermann-von-Helmholtz-Platz 1, 76344 Eggenstein-Leopoldshafen, Germany.
Handling Editor: Curt Wentrup
Australian Journal of Chemistry 75(11) 899-905 https://doi.org/10.1071/CH22103
Submitted: 12 May 2022 Accepted: 16 June 2022 Published: 25 July 2022
© 2022 The Author(s) (or their employer(s)). Published by CSIRO Publishing. This is an open access article distributed under the Creative Commons Attribution 4.0 International License (CC BY)
Abstract
Harnessing the power of light for chemical transformation is a long-standing goal in organic synthesis, materials fabrication and engineering. Amongst all photochemical reactions, [2 + 2] photocycloadditions are inarguably the most important and most frequently used. These photoreactions have green characteristics by enabling new bond formation in a single step procedure under light irradiation, without the need for heat or chemical catalysis. More recently, substantial progress has been made in red-shifting the activation wavelength of photocycloadditions in response to research trends moving towards green and sustainable processes, and advanced applications in biological environments. In the past 5 years, our team has further expanded the toolbox of photocycloaddition reactions that can be triggered by visible light. In our exploration of photochemical reactivity, we found that reactivity is often red-shifted compared to the substrate’s absorption spectrum. Our efforts have resulted in red-shifted photochemical reactions, providing some of the lowest energy – and catalyst-free – photo-activated [2 + 2] cycloadditions (up to 550 nm). More recently, we introduced an additional level of control over such finely wavelength gated reactions by altering the pH of the reaction environment, thus exploiting halochromic effects to enhance or impede the photoreactivity of red-shifted [2 + 2] photocycloaddition reactions. In this account, we discuss the current state of halochromically regulated photochemical reactions and their potential in soft matter materials on selected examples.
Keywords: halochromism, hydrogels, photocrosslinking, photocycloaddition, regioselectivity, single chain nanoparticle, visible light, wavelength-orthogonal.
Introduction
In June 1908, Giacomo Ciamician presented a comprehensive account of his work on photochemistry, mostly in collaboration with his associate Paul Silber, at the Société Française de Chimie (French Chemical Society) meeting,[1] illustrating his vision of employing light as green energy to fuel organic synthesis.[2] Among the series of photochemical reactions presented, an important group of bond forming reactions, including the dimerization of cinnamic acid, stilbene, and coumarin, were described as the initial reports of [2 + 2] photocycloaddition.[3] These new bond forming reactions have critical value in organic synthesis – they provide excellent atom economy and occur in a single step transformation under mild conditions that allow control of the follow-up processes. Ciamician’s pioneering work has thus opened a vibrant research area of photocycloaddition, and extensive information collected in the 1960s and 1970s further revealed the cleavage of the cycloadducts upon irradiation with shorter UV wavelengths, along with mechanistic work that provides an understanding of the characteristics of stilbene photocycloaddition chemistry.[4]
Towards the 1980s, research on photocycloaddition reactions has extended significantly from organic synthesis into soft matter materials. The characteristic of wavelength-controlled covalent bond formation and dissociation of reversible [2 + 2] photodimerization has enabled universal applications, e.g. the synthesis and modification of polymer architectures,[5,6] tuning properties of plastics,[7–9] hydrogels[10–12] and metal-organic frameworks,[13–15] as well as controlling biological processes in situ or in vitro.[16,17] More recently, the increasing interest in advanced applications under environmentally and biologically benign conditions has strongly enhanced the development of red-shifted reversible photochemical systems. In contribution to such endeavours, our team and others have introduced new [2 + 2] photocycloaddition reactions that can be triggered by long wavelength of visible light, up to green (λ < 550 nm) wavelength.[18,19] The longer activation wavelength further allows for their use in conjunction with other photochemical reactions, activated by shorter wavelength of light, in sequence-independent λ-orthogonal systems.[20–23]
While red-shifted absorption of the chromophores can be developed by engineering the chemical structure to expand the π-system via conjugation with aromatic systems, e.g. napthalene,[24,25] anthracene,[26] pyrene,[19] or pyranocarbazole,[27] their photoreactivity can be further controlled by the environment. In particular, the λ-dependent reactivity and kinetics of the cycloaddition can be altered significantly when the chromophores are in crystal form[28], in confined environments,[29,30] or attached to a surface.[31,32] Furthermore, chromophores with halochromic properties, i.e. compounds that can change their absorbance reversibly at different pH levels, can have different photoreactivities depending on their protonation state. Such substances have important utility in λ-orthogonal systems where the pH switch can assist in the orthogonal initiation of two photochemical reactions, one of which is not pH-dependent, under irradiation of a single wavelength regime. In the current account, we discuss recent developments in the halochromic regulation of the photoreactivity of [2 + 2] cycloaddition reactions. Specifically, we focus on intermolecular photodimerization, rather than intramolecular photocycloaddition systems that have practical significance mostly in natural product synthesis.[4,33–35] We further highlight the potential applications of such photochemical systems in soft matter material design.
Halochromic enhancement of photodimerization in confined environment
The remarkable effect of acid on the regioselectivity of [2 + 2] photodimerization of trans-styrylpyridine, specifically the majority formation of the syn head-to-tail dimer, (Fig. 1a) was reported in the early 2000s.[36,37] Such a high regioselectivity is due to the preorientation of the pyridinium salt during the photocycloaddition, particularly the cation-π interactions between the pyridinium and aromatic rings that enable the molecules to arrange themselves in a head-to-tail pattern.[38,39] Further studies on these systems indicate that the strength of the cation π-interaction can be enhanced by increasing the size of the conjugated π-structures.[40] In 2017, Yamada and Nojiri noticed the acidic conditions provide the extended π-systems, such as nathylvinylpyridines, not only excellent regio- and stereoselectivity but also a red-shift (10–30 nm) in the absorbance, with a shoulder extending into the 400 nm region, thus enabling the photocycloaddition of the protonated species by visible light (Fig. 1a).[25]
Recently, we combined the highly conjugated π-system of pyrene with pyridine, establishing a new halochromic chromophore (pyren)vinylpyridine (PyPy) for red-shifted photocycloaddition.[41] Meticulous analysis of the PyPy reaction pathway under irradiation of various wavelengths revealed interesting characteristics that differ from the previously reported pyridine-based systems. In neutral conditions, trans-PyPy undergoes reversible photocycloaddition under blue light (λmax = 450 nm) and UV light (λmax = 360 nm) irradiation, respectively (Fig. 1b). The cycloaddition adducts [PyPy]2 entail three main regioisomers (anti- head-to-tail: 10%, syn- head-to-tail: 37%, and syn- head-to-head: 53%) formed in 94% yield, together with a small amount (2%) of cis-PyPy. The protonated state PyPyH+, induced by addition of trifluoroacetic acid, displays a red-shifted absorbance yet does not undergo photocycloaddition. Instead, we observed photoisomerization of PyPyH+ under irradiation at wavelengths up to 620 nm (Fig. 1c). The photoisomerization (trans- to cis-) was found to take place in common polar organic solvents, with the conversion in the range of 70–80%. The cis-form is retained after neutralization, displaying good thermal stability with no cis- to trans-isomerization after being left in the dark for 24 h. Subsequent photocycloaddition (λmax = 450 nm) of the cis-form results in a higher regioselectivity, specifically the formation of syn- head-to-head (60%) and anti- head-to-tail (25%) as the major regioisomers.
When attaching the chromophores to a polymer chain to increase the proximity of the photoreactive moieties, we observed the photocycloaddition of the protonated PyPyH+ by green (λmax = 525 nm) or orange (λmax = 590 nm) light irradiation (Fig. 1d) and folding of the polymer chains, as evident by both size exclusion chromatography and NMR analysis of the polymers post-irradiation. In the neutral state, the polymer folding via photocycloaddition only occurred under irradiation at wavelength up to 450 nm. Clearly, the confined environment of a single polymer chain can overcome the restrictions blocking the photocycloaddition of the protonated PyPyH+ moiety. Our work thus opens up new opportunities in controlling the reaction pathways of the styryl moiety, and our preliminary demonstration in polymer folding indicates the applicability of the halochromic effect in tuning polymer architectures under irradiation of long wavelength visible light.
Halochromic inhibition of photodimerization
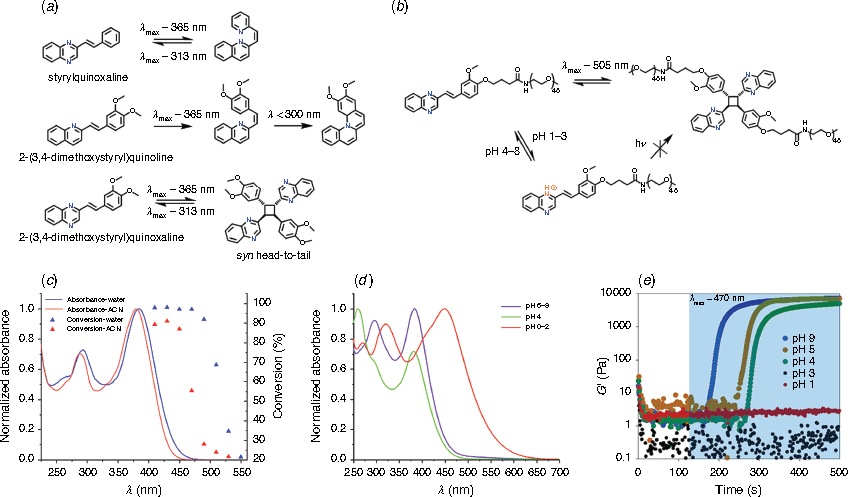
The photochemical behaviors of heterocyclic nitrogen-containing stilbene derivatives can differ significantly depending on their structural arrangement, such as the number of nitrogens and their placement, and substituents on the aromatic rings. For example, styrylquinoxaline only undergoes reversible trans-/cis-photoisomerization (Fig. 2a);[42,43] while quinoline compounds could potentially participate in photocyclization in the cis-form, followed by oxidation.[44,45] However, these compounds do not show any photodimerization under full light irradiation in solution. Interestingly, the team of Fedorov reported that addition of two methoxy groups at the para- and meta-positions on the styryl component enables reversible [2 + 2] photocycloaddition, with exclusive formation of the syn head-to-head isomer out of 11 possible regioisomers (Fig. 2a).[46] The authors proposed that the head-to-tail dimer organization of dimethoxystyrylquinoxaline in solution is due to the dipole interaction between the donor dimethoxyphenyl moiety of one molecule and the acceptor quinoxaline residue of the other one, enabling the formation of the cycloadduct under irradiation of UV light (λmax = 365 nm).
In the absence of methoxy substituents, [2 + 2] photodimerization of styrylquinoxaline can take place in Langmuir-Blodgett (LB) films due to the two-dimensional confined environment.[47,48] In this case, molecular preorientation is promoted by side-to-side packing of the amphiphilic (hydrophilic styrylquinoxaline head and hydrophobic alkyl chain) molecules, forming exclusively syn head-to-head dimer adducts.[49] While the halochromic properties of styrylquinoxaline LB films have been widely investigated for applications in sensors, only recently Lu and co-workers discovered that the dimerization rate was significantly reduced when the LB films were exposed to HCl gas.[50] Therefore, it is likely that the protonated forms disrupt the head-to-head aggregated structures of the monolayer, preventing the photodimerization processes.
In light of the recent reports on photocycloaddition of the styrylquinoxaline (SQ) derivatives, we sought to employ such chromophores in polymer ligation and crosslinking. Our main interest lies in biological applications, and we thus developed a synthetic protocol to attach the SQ chromophore to a water soluble poly(ethylene glycol) (PEG), while retaining the (2,3-dimethoxy)styryl motif (Fig. 2b).[51] By mapping the photodimerization as the function of wavelength using a monochromatic tuneable laser system, we found green light (λ < 520 nm) can be used to trigger photocycloaddition in an aqueous environment (Fig. 2c). Furthermore, acidic conditions (pH < 4) result in a red-shift in the absorption (~100 nm) of the compound in solution (Fig. 2d), and inhibition of the photocycloaddition. By attaching the styrylquinoxaline to an 8-armedPEG, we were able to form hydrogels by irradiation of the polymer solution with blue or green light, and the photo-crosslinking process was further controlled by adjusting the pH of the solution (Fig. 2e).
To further red-shift the activation wavelength for photodimerization, we developed a new heterocyclic styryl derivative with three nitrogen centres, namely styrylpyrido[2,3-b]pyrazine (SPP) (Fig. 3a).[52] In a neutral aqueous environment, the SPP can participate in [2 + 2] photocycloaddition under irradiation of wavelength up to 550 nm, which is the longest wavelength employed in photocycloaddition in solution and without the assistance of confined environment conditions. Critically, replacement of the phenyl with a pyrido subunit results in the increase in the pKa of the resultant compound (SQ pKa = 0.6; SPP pKa = 1.15), and consequently enhancement of the halochromic response. When mapping the photodimerization ratio as a function of pH, we observed a significant decrease in the monomer conversion when pH was switched from 7 to 6 (~50 to ~20%) under identical irradiation condition at λmax = 480 nm. This enhanced halochromic response enables the use of an internal pH regulator, which was a water soluble spiropyran photoacid generator (PAG),[53] to restrict the activation wavelength of photodimerization (Fig. 3a). Under excitation at λ = 400–510 nm, the PAG undergoes photoisomerization and protonates the SPP function, limiting its photodimerization to only under green light (λ = 520–550 nm) irradiation.
Taking advantage of the restricted activation wavelength of SPP in the presence of the PAG, we established a λ-orthogonal photoactivation of two photodimerization processes, one of which is the photocycloaddition of non-halochromic acrylamidylpyrene (AP, Fig. 3b) under irradiation at λ = 410–490 nm. We integrated this photochemical system into a polymer crosslinking concept, and demonstrated the λ-orthogonal stiffening of hydrogel materials by either blue or green light (Fig. 3c). We expect that all chromophores, including the PAG, SPP, and AP can be covalently integrated into a single system for modulation of mechanical properties. The exclusive use of visible light to finely tune the moduli of hydrogel materials is highly attractive for applications in cell mechanotransduction studies.
Concluding remarks
It is evident that the herein described progress in halochromic tuning of photochemical transformations of the heterocylic N-containing stilbene represents a valuable tool for soft matter materials modification, with applications in biological environments. Critically, the halochromic control over the photochemical transformation can provide additional orthogonal control over two or more spatiotemporal elements, which is highly beneficial for regulation of multiple biological events, such as enzymatic signalling pathways,[54] microRNA interactions in cellular processes,[55] or microorganism growth.[56] Currently, we are investigating the potential of photoinduced [2 + 2] cycloaddition for DNA modification and exploiting precision photochemistry for tracing processes by live-tagging cells. Given that chromaticity is highly pH dependent, we submit that the prospects for establishing sensitive and biorthogonal halochromic systems are realistic. Therefore, it will be interesting to establish if the pH gated control of photochemical reactivity can be exploited in living systems, where pH levels tend to site- and morbidity dependently vary (e.g. in cancerous tissue, where cancer cells have a lower extracellular pH of ~6.7 compared to pH of 7.4 in normal cells).[57] For this to occur, even finer control of photochemical reactivity in response to even slight pH changes needs to be established.
In addition to the opportunities available for halochromic control in biological systems, halochromic control over photo-induced coupling can be exploited in sensor applications, where system status can be potentially read-out by photochemical reactivity responses.[48] Thus, increasing the sensitivity of the halochromic response will also be critical for such applications outside the biological arena. Finally, it might be possible to dual gate photochemical reactivity not only with halochromaticity, but with an additional gate such as supramolecular templating[58] or organocatalysis activation,[59] opening avenues to safe-guarded photochemical responses for the forward reaction but possibly also for the reverse. Such dual gated photochemically induced debonding reactions could find use in fail-safe adhesives,[60] where two triggers have to come to together before release occurs.
Data availability
The data that support this study will be shared upon reasonable request to the corresponding authors.
Conflicts of interest
The authors declare no conflicts of interest.
Declaration of funding
This research is funded by an ARC Laureate Fellowship, grant number: FL170100014.
Acknowledgements
C. B.-K. acknowledges funding in the context of an ARC Laureate Fellowship underpinning his photochemical research program. C. B.-K. and V. X. T. are grateful for the PhD students and post-doctoral research fellows that have contributed to the development of these studies over time, as well as their academic colleagues who were a critical part of the described research studies.
References
[1] A Albini, M Fagnoni, ChemSusChem 2008, 1, 63.| Crossref | GoogleScholarGoogle Scholar |
[2] G Ciamician, Science 1912, 36, 385.
| Crossref | GoogleScholarGoogle Scholar |
[3] A Albini, V Dichiarante, Photochem Photobiol Sci 2009, 8, 248.
| Crossref | GoogleScholarGoogle Scholar |
[4] S Poplata, A Tröster, Y-Q Zou, T Bach, Chem Rev 2016, 116, 9748.
| Crossref | GoogleScholarGoogle Scholar |
[5] T Junkers, Eur Polym J 2015, 62, 273.
| Crossref | GoogleScholarGoogle Scholar |
[6] BT Tuten, S Wiedbrauk, C Barner-Kowollik, Prog Polym Sci 2020, 100, 101183.
| Crossref | GoogleScholarGoogle Scholar |
[7] M Abdallh, C Yoshikawa, MTW Hearn, GP Simon, K Saito, Macromolecules 2019, 52, 2446.
| Crossref | GoogleScholarGoogle Scholar |
[8] T Hughes, GP Simon, K Saito, Polym Chem 2019, 10, 2134.
| Crossref | GoogleScholarGoogle Scholar |
[9] C-M Chung, Y-S Roh, S-Y Cho, J-G Kim, Chem Mater 2004, 16, 3982.
| Crossref | GoogleScholarGoogle Scholar |
[10] VX Truong, F Li, F Ercole, JS Forsythe, ACS Macro Lett 2018, 7, 464.
| Crossref | GoogleScholarGoogle Scholar |
[11] S Ludwanowski, D Hoenders, K Kalayci, H Frisch, C Barner-Kowollik, A Walther, Chem Commun 2021, 57, 805.
| Crossref | GoogleScholarGoogle Scholar |
[12] TM Almutairi, HH Al-Rasheed, M Monier, FS Alatawi, NH Elsayed, Int J Biol Macromol 2022, 210, 208.
| Crossref | GoogleScholarGoogle Scholar |
[13] R Ou, H Zhang, C Zhao, HM Hegab, L Jiang, VX Truong, H Wang, Chem Mater 2020, 32, 10621.
| Crossref | GoogleScholarGoogle Scholar |
[14] Y-J Zhang, C Chen, L-X Cai, B Tan, X-D Yang, J Zhang, M Ji, Dalton Trans 2017, 46, 7092.
| Crossref | GoogleScholarGoogle Scholar |
[15] R Medishetty, I-H Park, SS Lee, JJ Vittal, Chem Commun 2016, 52, 3989.
| Crossref | GoogleScholarGoogle Scholar |
[16] K Kalayci, H Frisch, C Barner-Kowollik, VX Truong, Adv Funct Mater 2020, 30, 1908171.
| Crossref | GoogleScholarGoogle Scholar |
[17] Y Zhang, PPY Chan, AE Herr, Angew Chem Int Ed 2018, 57, 2357.
| Crossref | GoogleScholarGoogle Scholar |
[18] M Sicignano, RI Rodríguez, J Alemán, Eur J Org Chem 2021, 2021, 3303.
| Crossref | GoogleScholarGoogle Scholar |
[19] VX Truong, C Barner-Kowollik, Trends Chem 2022, 4, 291.
| Crossref | GoogleScholarGoogle Scholar |
[20] K Hiltebrandt, M Kaupp, E Molle, JP Menzel, JP Blinco, C Barner-Kowollik, Chem Commun 2016, 52, 9426.
| Crossref | GoogleScholarGoogle Scholar |
[21] A Shahrokhinia, P Biswas, JF Reuther, J Polym Sci 2021, 59, 1748.
| Crossref | GoogleScholarGoogle Scholar |
[22] VX Truong, K Ehrmann, M Seifermann, PA Levkin, C Barner-Kowollik, Eur J Chem 2022, 28, e202104466.
| Crossref | GoogleScholarGoogle Scholar |
[23] H Frisch, FR Bloesser, C Barner-Kowollik, Angew Chem Int Ed 2019, 58, 3604.
| Crossref | GoogleScholarGoogle Scholar |
[24] J Liu, K Ye, Y Shen, J Peng, J Sun, R Lu, J Mater Chem C 2020, 8, 3165.
| Crossref | GoogleScholarGoogle Scholar |
[25] S Yamada, Y Nojiri, Molecules 2017, 22, 491.
| Crossref | GoogleScholarGoogle Scholar |
[26] J Bai, Z Shi, X Ma, J Yin, X Jiang, Macromol Rapid Commun 2022, 43, 2200055.
| Crossref | GoogleScholarGoogle Scholar |
[27] K Fujimoto, S Sasago, J Mihara, S Nakamura, Org Lett 2018, 20, 2802.
| Crossref | GoogleScholarGoogle Scholar |
[28] FH Quina, DG Whitten, J Am Chem Soc 1977, 99, 877.
| Crossref | GoogleScholarGoogle Scholar |
[29] J-S Wang, K Wu, C Yin, K Li, Y Huang, J Ruan, X Feng, P Hu, C-Y Su, Nat Commun 2020, 11, 4675.
| Crossref | GoogleScholarGoogle Scholar |
[30] H Frisch, JP Menzel, FR Bloesser, DE Marschner, K Mundsinger, C Barner-Kowollik, J Am Chem Soc 2018, 140, 9551.
| Crossref | GoogleScholarGoogle Scholar |
[31] S Wilsey, L González, MA Robb, KN Houk, J Am Chem Soc 2000, 122, 5866.
| Crossref | GoogleScholarGoogle Scholar |
[32] R Farwaha, P de Mayo, YC Toong, J Chem Soc, Chem Commun 1983, 739.
| Crossref | GoogleScholarGoogle Scholar |
[33] J Iriondo-Alberdi, MF Greaney, Eur J Org Chem 2007, 2007, 4801.
| Crossref | GoogleScholarGoogle Scholar |
[34] T Bach, JP Hehn, Angew Chem Int Ed 2011, 50, 1000.
| Crossref | GoogleScholarGoogle Scholar |
[35] N Hoffmann, Chem Rev 2008, 108, 1052.
| Crossref | GoogleScholarGoogle Scholar |
[36] S Yamada, M Kusafuka, M Sugawara, Tetrahedron Lett 2013, 54, 3997.
| Crossref | GoogleScholarGoogle Scholar |
[37] X-H Li, L-Z Wu, L-P Zhang, C-H Tung, Org Lett 2002, 4, 1175.
| Crossref | GoogleScholarGoogle Scholar |
[38] S Yamada, Y Nojiri, M Sugawara, Tetrahedron Lett 2010, 51, 2533.
| Crossref | GoogleScholarGoogle Scholar |
[39] S Yamada, N Uematsu, K Yamashita, J Am Chem Soc 2007, 129, 12100.
| Crossref | GoogleScholarGoogle Scholar |
[40] R Amunugama, MT Rodgers, Int J Mass Spectrom 2003, 227, 1.
| Crossref | GoogleScholarGoogle Scholar |
[41] D Kodura, LL Rodrigues, SL Walden, AS Goldmann, H Frisch, C Barner-Kowollik, J Am Chem Soc 2022, 144, 6343.
| Crossref | GoogleScholarGoogle Scholar |
[42] SC Shim, KT Lee, P-H Bong, J Photochem Photobiol A 1987, 40, 381.
| Crossref | GoogleScholarGoogle Scholar |
[43] MS Kim, KT Lee, BM Jeong, BH Lee, SC Shim, Photochem Photobiol 1991, 54, 517.
| Crossref | GoogleScholarGoogle Scholar |
[44] EN Gulakova, DV Berdnikova, TM Aliyeu, YV Fedorov, IA Godovikov, OA Fedorova, J Org Chem 2014, 79, 5533.
| Crossref | GoogleScholarGoogle Scholar |
[45] TM Aliyeu, DV Berdnikova, OA Fedorova, EN Gulakova, C Stremmel, H Ihmels, J Org Chem 2016, 81, 9075.
| Crossref | GoogleScholarGoogle Scholar |
[46] OA Fedorova, AE Saifutiarova, EN Gulakova, EO Guskova, TM Aliyeu, NE Shepel, YV Fedorov, Photochem Photobiol Sci 2019, 18, 2208.
| Crossref | GoogleScholarGoogle Scholar |
[47] L Liu, L Zhang, T Wang, M Liu, Phys Chem Chem Phys 2013, 15, 6243.
| Crossref | GoogleScholarGoogle Scholar |
[48] M Yin, H Gong, B Zhang, M Liu, Langmuir 2004, 20, 8042.
| Crossref | GoogleScholarGoogle Scholar |
[49] Y Ma, Y Xie, L Lin, L Zhang, M Liu, Y Guo, Z Lan, Z Lu, J Phys Chem C 2017, 121, 23541.
| Crossref | GoogleScholarGoogle Scholar |
[50] Y Ma, L Lin, L Zhang, M Liu, Y Guo, Z Lu, Chin Chem Lett 2017, 28, 1285.
| Crossref | GoogleScholarGoogle Scholar |
[51] K Kalayci, H Frisch, VX Truong, C Barner-Kowollik, Nat Commun 2020, 11, 4193.
| Crossref | GoogleScholarGoogle Scholar |
[52] VX Truong, J Bachmann, A-N Unterreiner, JP Blinco, C Barner-Kowollik, Angew Chem Int Ed 2022, 61, e202113076.
| Crossref | GoogleScholarGoogle Scholar |
[53] Z Shi, P Peng, D Strohecker, Y Liao, J Am Chem Soc 2011, 133, 14699.
| Crossref | GoogleScholarGoogle Scholar |
[54] MA Priestman, L Sun, DS Lawrence, ACS Chem Biol 2011, 6, 377.
| Crossref | GoogleScholarGoogle Scholar |
[55] Y Yamano, K Murayama, H Asanuma, Eur J Chem 2021, 27, 4599.
| Crossref | GoogleScholarGoogle Scholar |
[56] WA Velema, JP van der Berg, W Szymanski, AJM Driessen, BL Feringa, ACS Chem Biol 2014, 9, 1969.
| Crossref | GoogleScholarGoogle Scholar |
[57] X Zhang, Y Lin, RJ Gillies, J Nucl Med 2010, 51, 1167.
| Crossref | GoogleScholarGoogle Scholar |
[58] KB Sutyak, W Lee, PV Zavalij, O Gutierrez, JT Davis, Angew Chem Int Ed 2018, 57, 17146.
| Crossref | GoogleScholarGoogle Scholar |
[59] C Müller, A Bauer, T Bach, Angew Chem Int Ed 2009, 48, 6640.
| Crossref | GoogleScholarGoogle Scholar |
[60] LC Chambers, C Barner-Kowollik, L Barner, L Michalek, H Frisch, ACS Macro Lett 2022, 11, 532.
| Crossref | GoogleScholarGoogle Scholar |
† Prof. Barner-Kowollik is the recipient of the 2022 David Craig Medal of the Australian Academy of Science.