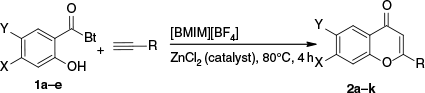A concise one-pot synthesis of flavones by cyclisation of o-(alkynon-1-yl)phenols
Khalid Widyan A *
A *
A
Abstract
A concise one-pot process for producing flavones and 2-alkyl-4H-benzopyran-4-one from a variety of salicylic acylbenzotriazoles is described. The in situ generation of o-(alkynon-1-yl)phenols in the ionic liquid 1-butyl-3-methylimidazoliumtetrafluoroborate ([BMIM][BF4]) is also described in this work. Three rounds of usage of the ionic liquid are possible without a noticeable decrease in efficiency.
Keywords: benzopyranone, flavones, green chemistry, ionic liquid, ionic liquid recycling, N-(o-hydroxyarylacyl)benzotriazoles, o-(alkynon-1-yl)phenols, one-pot synthesis.
Introduction
A major challenge of modern synthetic organic chemistry is the design of efficient chemical reaction sequences that afford molecules containing structural diversity with interesting applications in a minimum total number of synthetic steps. One-pot synthesis has attracted the attention of chemical research and has been developed as a useful synthetic route for the preparation of diverse heterocyclic compounds.1,2
Flavones, which are also known as 2-arylbenzopyrones, are present in a wide range of natural and synthetic products3–5 and have various bioactivities,6,7 including antiviral effects-such as inhibition of HIV-1, hepatitis C virus, influenza, dengue and enteroviruses8–10; antifungal activity demonstrated against Candida spp., Aspergillus spp., Trichophyton and dermatophytes11,12; anticancer potential targeting multiple pathways and kinase inhibition13,14; and antibacterial properties such as membrane disruption, inhibition of biofilm formation, ATP and nucleic acid synthesis suppression.15,16
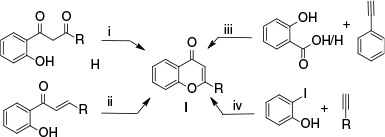
Flavones are frequently produced using a variety of protocols (Scheme 1): (i) cyclodehydration of 1-(2-hydroxyphenyl)-3-phenyl-1,3-propanediones. However, harsh conditions such as treatment with concentrated acid at high temperatures for a long time are required to promote cyclisation of the diketone,17–23 (ii) oxidative cyclisation of 2-hydroxychalcones. However, the synthetic challenges of chalcone derivatives have constrained their wider application,24–27 (iii) deoxygenation and cyclisation of salicylic acid derivatives or benzaldehyde with aryl acetylene; nevertheless, this strategy frequently depends on the use of expensive transition-metal catalysts28,29 and (iv) carbonylative cyclisation of 2-iodophenol with terminal alkynes. However, this protocol requires carbon monoxide under elevated temperatures and pressures, a costly palladium catalyst and an inert atmosphere.30–33
Survey of literature synthesis of flavones. i, Cyclodehydration of 1-(2-hydroxyphenyl)-1,3-diketones; ii, oxidative cyclisation of 2-hydroxychalcones; iii, deoxygenation/cyclisation of salicylic acid or benzaldehyde with aryl acetylene; iv, carbonylative cyclisation of 2-iodophenol with terminal alkynes.
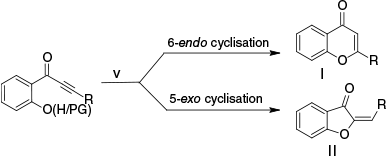
Alternatively, (v) intramolecular cyclisation of o-(alkynon-1-yl)phenols has gained attention as a practical method for flavone synthesis (Scheme 2). However, this approach requires a multi-step synthesis for achieving starting materials and produces both flavones through 6-endo-digonal cyclisation and aurones through 5-exo-digonal cyclisation with ratios influenced by reagents, substituents and solvents. Although 6-endo-digonal cyclisation is typically preferred,34 5-endo-digonal cyclisation is competitive, especially in protic solvents with basic conditions.35,36
To overcome this restriction, reaction conditions and reagents are modified37 to improve the regioselectivity of 6-endo cyclisation using potassium carbonate,38,39 triflic acid,40 4-(dimethylamino)pyridine,41 Tl(p-OTs)342 or sodium 2-pyridinethiolate.43 Others used the O-protected alkynones, using OTBS-protected alkynones requiring excess secondary amines in methanol under reflux,44 acetate-protected alkynones yield flavones with 2.5 equivalents of potassium methoxide.45
Even though these methods successfully produce flavones, a simpler procedure with fewer steps and using easily accessible starting materials under mild conditions is still needed.
N-Acyl benzotriazoles 1 have found a wide range of applications in organic synthesis, such as the preparation of esters, acyl azides and amides, as well as Weinreb amides. Most importantly, N-acylbenzotriazoles derived from salicylic acids are used in synthesis without prior protection of the phenolic hydroxy group.46–50 More recently, we have developed a transition metal-free protocol for the preparation of ynones51 and sequential synthesis of pyrazoles in 1-butyl-3-methylimidazolium tetrafluoroborate ([BMIM][BF4]) ionic liquid.52 Attracted by this strategy and continuing our interest in the synthesis of heterocycles using N-acylbenzotriazoles, we extend that mild and practical synthetic approach for obtaining flavones and 2-alkyl-4H-benzopyran-4-one by combining terminal alkynes with N-acylbenzotriazoles generated from salicylic acid derivatives.
Results and discussion
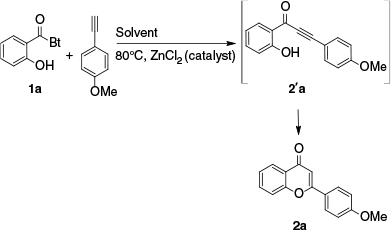
N-(o-Hydroxyaryl acyl)benzotriazoles 1a–e were readily obtained from the reaction of salicylic acid derivatives with 1,1′-sulfinylbis(1H-benzotriazole) [Bt2SO] produced in situ from a benzotriazole/thionyl chloride mixture in overall high yields according to an established procedure.46 Then, as a model reaction, benzotriazol-1-yl-(2-hydroxyphenyl)methanone (1a) was used as the substrate to investigate and optimise the coupling reaction conditions for preparation of 4′-methoxyflavone (2a) in the ionic liquid [BMIM][BF4] (Scheme 3, Table 1).
| Entry | Solvent | ZnCl2 equivalents | Temperature (°C) | Time (h) | Yield (%) | |
|---|---|---|---|---|---|---|
| 1 | [BMIM][BF₄] | None | 80 | 8 | 20 | |
| 2 | [BMIM][BF₄] | 0.05 | 80 | 4 | 60 | |
| 3 | [BMIM][BF₄] | 0.15 | 80 | 4 | 91 | |
| 4 | [BMIM][BF₄] | 0.2 | 80 | 4 | 93 | |
| 5 | CH3CN | 0.2 | 80 | 4 | 30 |
Reaction conditions: 2.0 mmol of 2a, catalytic amounts of ZnCl2. There was 5.0 mL of solvent. The yield was an isolated yield.
Harper et al. extensively studied reactions in ionic liquids and demonstrated that both rate constants and reaction outcomes are influenced by the proportion of ionic liquid used.53–56 Since this parameter was not investigated in the present study, the ionic liquid volume was fixed at 5.0 mL in all experiments, thereby ensuring that the outcomes of the reaction were not affected by the proportion of [BMIM][BF₄].
The reaction progress was followed using thin-layer chromatography, which shows the complete disappearance of 1a. After completion, the reaction mixture was extracted with diethyl ether and concentrated under reduced pressure to afford the desired product, 4′-methoxyflavone (2a).
Formation of 2a goes through formation of the o-(alkynon-1-yl)phenols intermediate 2-[3-(4-methoxyphenyl)propynoyl]phenol (2′a), which could be isolated in high yields using 0.1 equivalents of zinc chloride and heating the reaction mixture at a temperature of 60°C.
The best result was obtained when 1a was coupled with two equivalents of p-methoxyphenyl acetylene in [BMIM][BF4] for 4 h at a reaction temperature of 80°C with 0.15 equivalents of zinc chloride. This produced 2a in an excellent yield of 91% with complete conversion of 1a (Table 1, entry 3). A comparable yield was obtained in the same amount of time by raising the catalyst to 0.2 equivalents (Table 1, entry 4). Nevertheless, a lesser yield of 60% was obtained when 0.05 equivalents of the catalyst were used. A longer reaction time of 8 h was needed to perform the reaction, giving 2a in a low yield of 20%, in the absence of ZnCl2 (entry 1). The results of this optimisation demonstrate that the one-pot transformation proceeds under mild reaction conditions with high selectivity, a short reaction time and no side reactions.
The effect of solvent choice was also examined. When the reaction was carried out in acetonitrile under identical conditions, the desired flavone 2a was obtained only in a low yield of 30% (Table 1, entry 5). This result highlights the enhanced performance of the ionic liquid [BMIM][BF₄], which not only promotes higher yields but also ensures cleaner transformations compared to conventional molecular solvents.
Next, the method’s generality and scope were investigated. At a reaction mixture temperature of 80°C, various N-acyl benzotriazoles 1a–e with electron-donating and electron-withdrawing groups were converted into their respective flavones 2a–f and benzopyran-4-one 2g–k using various alkynes bearing alkyl and aryl groups in 4 h, with good to high yields of 80–92% (Scheme 4, Table 2). Alkynes with a bulky naphthyl group afforded benzopyran-4-one in slightly lower yields 2g,h presumably due to steric factors.
| 2 | X | Y | –R | Yield (%) | |
|---|---|---|---|---|---|
| a | H | H |  | 91 | |
| b | H | H |  | 91 | |
| c | H | H | 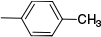 | 88 | |
| d | H | Br |  | 91 | |
| e | Cl | H |  | 88 | |
| f | CH3O | H |  | 90 | |
| g | Cl | H | 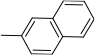 | 82 | |
| h | CH3O | H | 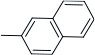 | 80 | |
| i | H | H |  | 92 | |
| j | H | H | –CH2CH2CH2CH3 | 91 | |
| k | H | H | –C(CH3)3 | 85 |
Reaction conditions: [BMIM][BF4] (5.0 mL), 0.15 equivalents of ZnCl2, 80°C, 4 h. The yield was an isolated yield.
The ionic liquid [BMIM][BF₄] was quantitatively recovered and reused for two consecutive cycles without a significant loss in yield (Table 3). A 2–3% variation in isolated yield falls within typical experimental variability and does not suggest any adverse effect from recycling. The recovery process was simple and efficient: after extracting the flavone with diethyl ether, the ionic liquid was dissolved in acetonitrile, filtered to remove impurities and vacuum dried following solvent evaporation. It was then reused without further purification.
Conclusion
In conclusion, the use of the ionic liquid [BMIM][BF₄] provided an effective reaction medium that enhanced selectivity and yielded a variety of flavones and benzopyran-4-one in good to high isolated yields. The protocol benefits from mild reaction conditions, such as low temperature and short reaction times, which minimise side reactions and improve overall efficiency. Additionally, the reduced formation of by-products simplified purification, while the optimised conditions ensured improved reproducibility across multiple runs. Notably, the process is a one-pot sequential reaction that eliminates the need to isolate the in situ generated o-(alkynon-1-yl)phenols.
Experimental
Melting points were determined and are uncorrected on a Reichert hot-stage microscope. NMR spectra were recorded for 1H at 300 MHz and 13C at 75 MHz on a Varian 300-MHz instrument. Spectra were obtained for the solution in CDCl3. Silica gel 200–245 mesh was used to perform column chromatography. N-Acylbenzotriazoles 1a–e were prepared according to previously published procedures.46
Typical procedure for preparation of 2-[3-(4-methoxyphenyl)propynoyl]phenol (2′a)
p-Methoxyphenyl acetylene (2.2 mmol) was added to a solution of 2.0 mmol of benzotriazol-1-yl-(2-hydroxyphenyl)methanone and zinc chloride (27.2 mg, 0.2 mmol) in 5.0 mL of [BMIM][BF4]. The mixture was stirred while keeping the temperature at 60°C for 2 h. The reaction mixture was extracted with diethyl ether (3 × 5 mL). The combined organic layer was then washed with diluted aqueous sodium hydrogen carbonate and water, then dried over anhydrous magnesium sulfate and filtered. The solvent was evaporated under a vacuum, and the residue was purified by flash column chromatography using hexane-ethyl acetate (1:2) to give the pure 2′a. Yellow solid, mp 80–81°C (lit. 79–80°C).40 IR (KBr) 3580, 2865, 2160, 1630, 1263 cm−1. 1H NMR (CDCl3): δ (ppm): 11.1 (s, 1H), 7.89–8.09 (m, 1H), 7.48–7.60 (m, 3H), 6.88–7.10 (m, 4H), 3.82 (s, 3H); 13C NMR (CDCl3): δ (ppm): 182.0, 162.4, 161.7, 136.6, 135.1, 132.5, 120.4, 118.9, 118.1, 114.3, 111.2, 97.6, 86.1, 55.4. Analytically calculated for C16H12O3: C 76.18, H 4.79; found: C 76.29, H 4.89.
Typical procedure for preparation of flavones (2a–k)
Terminal alkyne (4.1 mmol) was added to a solution of the appropriate N-acylbenzotriazole (2.0 mmol) and zinc chloride (40.8 mg, 0.3 mmol) in [BMIM][BF4] (5.0 mL). The mixture was stirred, keeping the temperature at 80°C for 4 h. The reaction mixture was extracted with diethyl ether (3 × 5 mL). The combined ether layer was then washed with dilute aqueous sodium hydrogen carbonate and water. The organic layer was dried over anhydrous magnesium sulfate and filtered. The solvent was evaporated under a vacuum, and the residue was purified by flash column chromatography using hexane-ethyl acetate (1:2) to give the pure flavone.
White solid, mp: 151–153°C (lit. 156°C).23 IR (KBr) 3582, 3425, 2930, 1643, 1608, 1270, 1070 cm−1. 1H NMR (CDCl3): δ (ppm): 8.23 (dd, J = 7.8, 1.6 Hz, 1H), 7.87 (d, J = 8.8 Hz, 2H), 7.61–7.72 (m, 1H), 7.55 (d, J = 8.3 Hz, 1H), 7.36–7.44 (m, 1H), 7.03 (d, J = 8.8 Hz, 2H), 6.75 (s, 1H), 3.87 (s, 3H). 13C NMR (CDCl3) δ (ppm): 178.4, 163.3, 162.5, 156.3, 133.6, 128.1, 125.8, 125.1, 124.3, 124.2, 118.1, 114.3, 106.3, 55.6. Analytically calculated for C16H12O3: C 76.18, H 4.79; found: C 76.38, H 4.61.
White solid, mp 95–96°C (lit. 97°C).23 IR (KBr) 3580, 3390, 1644, 1604, 1250, 1068 cm−1. 1H NMR (CDCl3): δ (ppm): 8.26 (d, J = 8.1 Hz, 1H), 7.90–8.11 (m, 2H), 7.61–7.74 (m, 1H), 7.51–7.59 (m, 4H), 7.45 (t, J = 7.6 Hz, 1H), 6.80 (s, 1H); 13C NMR (CDCl3): δ 178.6, 163.5, 156.4, 133.7, 131.6, 131.7, 129.2, 126.4, 125.6, 125.2, 124.1, 118.2, 107.7. Analytically calculated for C15H10O2: C 81.07, H 4.54; found: C 81.29, H 4.39.
Pale yellow solid, mp: 102–104°C (lit. 101–103°C).29 IR (KBr) 3080, 2962, 1640, 1580, 1440, 1050 cm−1. 1H NMR (CDCl3): δ (ppm): 8.21 (d, J = 7.6, 1H), 7.81 (d, J = 8.1 Hz, 2H), 7.64 (t, J = 8.6 Hz, 1H), 7.55 (d, J = 8.1 Hz, 1H), 7.43 (t, J = 7.6 Hz, 1H), 7.32 (d, J = 8.1 Hz, 2H), 6.76 (s, 1H), 2.41 (s, 3H); 13C NMR (CDCl3): δ (ppm): 178.3, 163.6, 156.2, 142.3, 133.4, 129.8, 128.6, 126.3, 125.1, 123.8, 118.2, 106.6, 21.4; analytically calculated for C16H12O2: C 81.34, H 5.12; found: C 81.49, H 4.98.
White solid, mp: 202–204°C (lit. 201–203°C).43 IR (KBr) 3085, 2950, 1639, 1586, 1290 cm−1. 1H NMR (CDCl3) δ (ppm): 8.34 (d, J = 2.4 Hz, 1H), 7.79 (d, J = 8.1 Hz, 2H), 7.76 (dd, J = 8.7, 2.4 Hz, 1H), 7.46 (d, J = 8.7 Hz, 1H), 7.33 (d, J = 8.1 Hz, 2H), 6.76 (s, 1H), 2.43 (s, 3H); 13C NMR (CDCl3) δ (ppm): 171.1, 163.8, 155.1, 142.7, 136.6, 129.7, 128.6, 128.5, 126.3, 125.3, 120.1, 118.6, 107.1, 21.4. Analytically calculated for C16H11BrO2: C 60.98, H 3.52; found: C 61.23, H 3.31.
White solid; mp: 155–157°C (lit. 156–157°C).19 IR (KBr) 3092, 1644, 1590, 1293 cm−1. 1H NMR (CDCl3): δ (ppm): 8.20 (d, J = 8.4 Hz, 1H), 7.85 (dd, J = 8.1, 2.1 Hz, 2H), 7.50–7.62 (m, 4H), 7.41 (dd, J = 8.4, 2.1 Hz, 1H), 6.78 (s, 1H); 13C NMR (CDCl3): δ (ppm): 177.4, 163.4, 156.4, 139.7, 131.7, 131.2, 129.0, 127.2, 126.4, 126.1, 122.4, 118.3, 107.9. Analytically calculated for C15H9ClO2: C 70.19, H 3.53; found: C 70.41, H 3.41.
White solid. mp: 108–110°C (lit. 111–112°C).19 IR (KBr) 3440, 3060, 1640, 1609, 1238, 1065 cm−1. 1H NMR (CDCl3): δ (ppm): 8.14 (d, J = 8.8 Hz, 1H), 7.86–7.94 (m, 2H), 7.42–7.59 (m, 3H), 6.90–7.02 (m, 2H), 6.73 (s, 1H), 3.91 (s, 3H). 13C NMR (CDCl3): δ (ppm): 177.6, 164.2, 163.1, 158.1, 131.7, 131.3, 129.1, 127.2, 126.3, 117.6, 114.3, 107.2, 100.4, 55.6. Analytically calculated for C16H12O3: C 76.18, H 4.79; found: C 76.37, H 4.61.
White solid. mp: 217–219°C (lit. 219–220°C).19 IR (KBr) 3090, 2928, 1640, 1530, 1265, 1031 cm−1. 1H NMR (CDCl3): δ (ppm): 8.46 (s, 1H), 8.21 (d, J = 8.6 Hz, 1H), 7.71–7.98 (m, 5H), 7.54–7.65 (m, 2H), 7.39 (dd, J = 8.6, 2.0 Hz, 1H), 6.89 (s, 1H). 13C NMR (CDCl3): δ (ppm): 177.3, 163.4, 156.6, 134.7, 133.1, 129.1, 129.0, 128.6, 128.3, 127.9, 127.3, 127.2, 127.1, 126.2, 122.8, 122.3, 118.1, 108.3. Analytically calculated for C19H11ClO2: C 74.40, H 3.61; found: C 74.59, H 3.48.
White solid; mp: 179–181°C (lit. 181–182°C).19 IR (KBr) 3092, 2935, 1641, 1590, 1483, 1021 cm−1; 1H NMR (CDCl3) δ (ppm): 8.41 (s, 1H), 8.11 (d, J = 8.9 Hz, 1H), 7.81–8.02 (m, 4H), 7.46–7.61 (m, 2H), 6.92–6.99 (m, 2H), 6.81 (s, 1H), 3.87 (s, 3H). 13C NMR (CDCl3): δ (ppm): 177.8, 164.2, 162.8, 158.2, 134.6, 128.8, 128.7, 128.6, 127.7, 127.5, 127.1, 126.8, 126.4, 122.4, 117.9, 114.3, 107.8, 100.3, 55.8. Analytically calculated for C20H14O3: C 79.46, H 4.67; found: C 79.61, H 4.59.
Colorless oil. IR film 3470, 2935, 1635, 1460, 1225, 1082 cm−1. 1H NMR (CDCl3): δ (ppm): 8.20 (dd, J = 8.1, 1.6 Hz, 1H), 7.61 (td, J = 8.8, 1.6 Hz, 1H), 7.40 (d, J = 8.4 Hz, 1H), 7.36 (td, J = 8.1, 1.2 Hz, 1H), 6.20 (s, 1H), 2.51 (tt, J = 11.4, 3.6 Hz, 1H), 2.03–2.16 (m, 2H), 1.82–1.96 (m, 2H), 1.71–1.80 (m, 1H), 1.20–1.56 (m, 5H). 13C NMR (CDCl3): δ (ppm): 178.8, 173.3, 156.4, 133.2, 125.8, 124.7, 123.6, 117.6, 107.8, 42.6, 30.2, 25.4. Analytically calculated for C15H16O2: C 78.92, H 7.06; found: C 79.13, H 7.19.
Colorless oil. IR film 3580, 1633, 1465, 1221, 1080 cm−1. 1H NMR (CDCl3): δ (ppm): 8.21 (dd, J = 8.1, 1.6 Hz, 1H), 7.62 (td, J = 8.8, 1.6 Hz, 1H), 7.38–7.48 (m, 2H), 6.21 (s, 1H), 2.61 (t, J = 7.6 Hz, 2H), 1.65–1.78 (m, 2H), 1.36–1.49 (m, 2H), 0.98 (t, J = 7.6 Hz, 3H). 13C NMR (CDCl3): δ (ppm): 178.3, 169.7, 156.6, 133.6, 125.5, 124.6, 123.8, 117.6, 109.9, 34.1, 28.6, 22.3, 13.6. Analytically calculated for C13H14O2: C 77.20, H 6.98; found: C 77.41, H 6.83.
Colorless oil. IR film 3382, 1638, 1460, 1335, 1075 cm−1. 1H NMR (CDCl3): δ (ppm): 8.20 (dd, J = 8.1, 1.6 Hz, 1H), 7.48–7.62 (m, 2H), 7.40 (td, J = 8.1, 1.2 Hz, 1H), 6.30 (s, 1H), 1.34 (s, 9H). 13C NMR (CDCl3): δ (ppm): 178.8, 176.1, 156.3, 133.2, 125.6, 124.6, 123.5, 117.6, 106.8, 36.2, 27.3. Analytically calculated for C13H14O2: C 77.20, H 6.98; found: C 77.43, H 7.18.
Dedication
Dedicated to the memory of the late Prof. Alan R. Katritzky for his excellent contributions to benzotriazole chemistry.
References
1 Nandi S, Jamatia R, Sarkar R, Sarkar FK, Alam S, Pal AK. One-pot multicomponent reaction: a highly versatile strategy for the construction of valuable nitrogen-containing heterocycles. ChemistrySelect 2022; 7: e202201901.
| Crossref | Google Scholar |
2 Ma X, Zhang W. Recent developments in one-pot stepwise synthesis (OPSS) of small molecules. iScience 2022; 25: 105005.
| Google Scholar |
3 Leonte D, Ungureanu D, Zaharia V. Flavones and related compounds: synthesis and biological activity. Molecules 2023; 28: 6528.
| Crossref | Google Scholar | PubMed |
4 Kshatriya R, Jejurkar VP, Saha S. In memory of Prof. Venkataraman: recent advances in the synthetic methodologies of flavones. Tetrahedron 2018; 74: 811-833.
| Crossref | Google Scholar |
5 Khadem S, Marles RJ. Chromone and flavonoid alkaloids: occurrence and bioactivity. Molecules 2012; 17: 191-206.
| Crossref | Google Scholar |
6 Lv XH, Liu H, Ren ZL, Wang W, Tang F, Cao HQ. Design, synthesis and biological evaluation of novel flavone Mannich base derivatives as potential antibacterial agents. Mol Divers 2019; 23: 299-306.
| Crossref | Google Scholar | PubMed |
7 Chagas MdSS, Behrens MD, Moragas-Tellis CJ, Penedo GXM, Silva AR, Gonçalves-de-Albuquerque CF. Flavonols and flavones as potential anti-inflammatory, antioxidant, and antibacterial compounds. Oxid Med Cell Longev 2022; 2022: 9966750.
| Crossref | Google Scholar |
8 Fujimoto KJ, Nema D, Ninomiya M, Koketsu M, Sadanari H, Takemoto M, Daikoku T, Murayama T. An in silico-designed flavone derivative, 6-fluoro-4'-hydroxy-3',5'-dimetoxyflavone, has a greater anti-human cytomegalovirus effect than ganciclovir in infected cells. Antivir Res 2018; 154: 10-16.
| Crossref | Google Scholar | PubMed |
9 Abou Baker DH. An ethnopharmacological review on the therapeutical properties of flavonoids and their mechanisms of actions: a comprehensive review based on up to date knowledge. Toxicol Rep 2022; 9: 445-469.
| Crossref | Google Scholar | PubMed |
10 Melrose J, Smith MM. Natural and semi-synthetic flavonoid anti-SARS-CoV-2 agents for the treatment of Long COVID-19 disease and neurodegenerative disorders of cognitive decline. Front Biosci (Elite Ed) 2022; 14(4): 27.
| Crossref | Google Scholar | PubMed |
11 Khdera HA, Saad SY, Moustapha A, Kandil F. Synthesis of new flavonoid derivatives based on 3-hydroxy-4′-dimethylamino flavone and study the activity of some of them as antifungal. Heliyon 2022; 8: e12062.
| Crossref | Google Scholar | PubMed |
12 Górniak I, Bartoszewski R, Króliczewski J. Comprehensive review of antimicrobial activities of plant flavonoids. Phytochem Rev 2019; 18: 241-272.
| Crossref | Google Scholar |
13 Bennardi DO, Ruiz DM, Romanelli GP, Baronetti GT, Thomas HJ, Autino JC. Efficient microwave solvent-free synthesis of flavones, chromones, coumarins and dihydrocoumarins. Lett Org Chem 2008; 5: 607-615.
| Crossref | Google Scholar |
14 Melrose J. The potential of flavonoids and flavonoid metabolites in the treatment of neurodegenerative pathology in disorders of cognitive decline. Antioxidants 2023; 12: 663.
| Crossref | Google Scholar | PubMed |
15 Reis J, Gaspar A, Milhazes N, Borges F. Chromone as a privileged scaffold in drug discovery: recent advances. J Med Chem 2017; 60: 7941-7957.
| Crossref | Google Scholar | PubMed |
16 Chen S, Wang X, Cheng Y, Gao H, Chen X. A review of classification, biosynthesis, biological activities and potential applications of flavonoids. Molecules 2023; 28: 4982.
| Crossref | Google Scholar | PubMed |
17 Stanek F, Stodulski M. Mild and efficient organocatalytic method for the synthesis of flavones. Tetrahedron Lett 2016; 57: 3841-3843.
| Crossref | Google Scholar |
18 Pérez M, Ruiz D, Autino J, Sathicq A, Romanelli G. A very simple solvent-free method for the synthesis of 2-arylchromones using KHSO4 as a recyclable catalyst. C R Chim 2016; 19: 551-555.
| Crossref | Google Scholar |
19 Pontes O, Costa M, Santos F, Sampaio-Marques B, Dias T, Ludovico P, Baltazar F, Proença F. Exploitation of new chalcones and 4H-chromenes as agents for cancer treatment. Eur J Med Chem 2018; 157: 101-114.
| Crossref | Google Scholar | PubMed |
20 Ares JJ, Outt PE, Kakodkar SV, Buss RC, Geiger JC. A convenient large-scale synthesis of 5-methoxyflavone and its application to analog preparation. J Org Chem 1993; 58: 7903-7905.
| Crossref | Google Scholar |
21 Tsukayama M, Kawamura Y, Ishizuka T, Hayas S, Torii F. Improved, rapid and efficient synthesis of polymethoxyflavones under microwave irradiation and their inhibitory effects on melanogenesis. Heterocycles 2003; 60: 2775-2784.
| Crossref | Google Scholar |
22 Kabalka GW, Mereddy AR. Microwave-assisted synthesis of functionalized flavones and chromones. Tetrahedron Lett 2005; 46: 6315-6317.
| Crossref | Google Scholar |
23 Zubaidha PK, Hashmi AM, Bhosale RS. FeCl3 catalyzed dehydrative cyclisation of 1,3-(diaryl diketones) to flavones. Heterocycl Commun 2005; 11: 97-100.
| Crossref | Google Scholar |
24 Hoshino Y, Oohinata T, Takeno N. The direct preparation of flavones from 2′-hydroxychalcones using disulfides. Bull Chem Soc Jpn 1986; 59: 2351-2352.
| Crossref | Google Scholar |
25 Selepe MA, van Heerden FR. Application of the Suzuki–Miyaura reaction in the synthesis of flavonoids. Molecules 2013; 18: 4739-4765.
| Crossref | Google Scholar | PubMed |
26 Du Z, Ng H, Zhang K, Zeng H, Wang J. Ionic liquid mediated Cu-catalyzed cascade oxa-Michael-oxidation: efficient synthesis of flavones under mild reaction conditions. J Org Biomol Chem 2011; 9: 6930-6933.
| Crossref | Google Scholar | PubMed |
27 Jung JC, Min JP, Park OS. A highly practical route to 2-methylchromones from 2-acetoxybenzoic acids. Synth Commun 2001; 31: 1837-1845.
| Crossref | Google Scholar |
28 Fan X, He C, Ji M, Sun X, Luo H, Li C, Tong H, Zhang W, Sun Z, Chu W. Visible light-induced deoxygenation/cyclization of salicylic acid derivatives and aryl acetylene for the synthesis of flavonoids. Chem Commun 2022; 58: 6348-6351.
| Crossref | Google Scholar | PubMed |
29 Baruah S, Kaishap PP, Gogoi S. Ru(II)-Catalyzed C-H activation and annulation of salicylaldehydes with monosubstituted and disubstituted alkynes. Chem Commun 2016; 52: 13004-13007.
| Crossref | Google Scholar | PubMed |
30 Miao H, Yang Z. Regiospecific carbonylative annulation of iodophenol acetates and acetylenes to construct the flavones by a new catalyst of palladium–thiourea–DPPP complex. Org Lett 2000; 2: 1765-1768.
| Crossref | Google Scholar | PubMed |
31 Xu S, Sun H, Zhuang M, Zheng S, Jian Y, Zhang W, Gao Z. Divergent synthesis of flavones and aurones via base-controlled regioselective palladium catalyzed carbonylative cyclization. Mol Catal 2018; 452: 264-270.
| Crossref | Google Scholar |
32 Zhu F, Wang Z, Li Y, Wu XF. Iridium-catalyzed and ligand-controlled carbonylative synthesis of flavones from simple phenols and internal alkynes. Chem Eur J 2017; 23: 3276-3279.
| Crossref | Google Scholar | PubMed |
33 Wu XF, Neumann H, Beller M. Palladium-catalyzed carbonylation reaction of aryl bromides with 2-hydroxyacetophenones to form flavones. Chem Eur J 2012; 18: 12595-12598.
| Crossref | Google Scholar | PubMed |
34 Baldwin JE, Thomas RC, Kruse LI, Silberman L. Rules for ring closure: ring formation by conjugate addition of oxygen nucleophiles. J Org Chem 1977; 42: 3846-3852.
| Crossref | Google Scholar |
35 García H, Iborra S, Primo J, Miranda MA. 6-Endo-dig vs. 5-exo-dig ring closure in o-hydroxyaryl phenylethynyl ketones. A new approach to the synthesis of flavones and aurones. J Org Chem 1986; 51: 4432-4436.
| Crossref | Google Scholar |
36 Brennan CM, Johnson CD, McDonnell PD. Ring closure to ynone systems: 5- and 6-endo- and -exo-dig modes. J Chem Soc Perkin Trans 1989; 2: 957-961.
| Crossref | Google Scholar |
37 Awuah E, Capretta A. Access to flavones via a microwave-assisted, one-pot Sonogashira-carbonylation-annulation reaction. Org Lett 2009; 11: 3210-3213.
| Crossref | Google Scholar | PubMed |
38 Garcia H, Iborra S, Primo J. 6-Endo-dig vs. 5-exo-dig ring closure in o-hydroxyaryl phenylethynyl ketones. A new approach to the synthesis of flavones and aurones. J Org Chem 1986; 51: 4432-4436.
| Crossref | Google Scholar |
39 Alabugin IV, Gilmore K, Manoharan M. Rules for anionic and radical ring closure of alkynes. J Am Chem Soc 2011; 133: 12608-12623.
| Crossref | Google Scholar | PubMed |
40 Yoshida M, Fujino Y, Doi T. Synthesis of γ-benzopyranone by TfOH-promoted regioselective cyclization of o-alkynoylphenols. Org Lett 2011; 13: 4526-4529.
| Crossref | Google Scholar | PubMed |
41 Yoshida M, Fujino Y, Saito K, Doi T. Regioselective synthesis of flavone derivatives via DMAP-catalyzed cyclization of o-alkynoylphenols. Tetrahedron 2011; 67: 9993-9997.
| Crossref | Google Scholar |
42 Lee JI, Kim HN. Synthesis of flavones by thallium(III) p-tosylate-catalyzed regioselective cyclization of o-(alkynon-1-yl)phenols. Bull Korean Chem Soc 2018; 39: 1113-1116.
| Crossref | Google Scholar |
43 Lee JI. Novel synthesis of flavones by regioselective cyclization of 1-(2-hydroxyphenyl)-3-phenyl-2-propyn-1-ones derived from 2-hydroxybenzoic acids. Bull Korean Chem Soc 2017; 38: 675-678.
| Crossref | Google Scholar |
44 Bhat AS, Whetstone JL, Brueggemeier RW. Novel synthetic routes suitable for constructing benzopyrone combinatorial libraries. Tetrahedron Lett 1999; 40: 2469-2472.
| Crossref | Google Scholar |
45 Chuang D-W, El-Shazly M, Balaji B, Chung Y-M, Chang F-R, Wu Y-C. Synthesis of flavones and γ-benzopyranones using mild sonogashira coupling and 18-crown-6 ether mediated 6-endo cyclization. Eur J Org Chem 2012; 2012(24): 4533-4540.
| Crossref | Google Scholar |
46 Katritzky AR, Singh SK, Cai C, Bobrov S. Direct synthesis of esters and amides from unprotected hydroxyaromatic and -aliphatic carboxylic acids. J Org Chem 2006; 71: 3364-3374.
| Crossref | Google Scholar | PubMed |
47 Katritzky AR, Singh SK, Akhmedova R, Cai C, Bobrov S. Efficient synthesis of 1,3-benzodioxin-4-one and benzoxazine-2,4-diones. Arkivoc 2007; 6: 6-13.
| Google Scholar |
48 Katritzky AR, Le KNB, Mohapatra PP. Synthesis of aliphatic hydroxyaryl ketones or (hetero)aryl hydroxyaryl ketones by acylation of organometallic reagents. Synthesis 2007; 2007(20): 3141-3146.
| Crossref | Google Scholar |
49 Katritzky AR, Widyan K, Kirichenko K. Preparation of polyfunctional acyl azides. J Org Chem 2007; 72: 5802-5804.
| Crossref | Google Scholar | PubMed |
50 Widyan K. An improved synthesis of polyfunctional acyl azides in PEG 400. Org Prep Proced Int 2021; 53: 120-126.
| Crossref | Google Scholar |
51 Widyan K. Acylation of terminal alkynes with N-acylbenzotriazole: synthesis of conjugated ynones in ionic liquids. Monatsh Chem 2023; 154: 645-649.
| Crossref | Google Scholar |
52 Widyan K. 3,5-Disubstituted-1H-pyrazoles: sequential syntheses from N-acylbenzotriazoles, alkynes, and hydrazine in ionic liquid. Chem Pap 2025; 79: 1649-1655.
| Crossref | Google Scholar |
53 Hsieh AY, Haines RS, Harper JB. Effects of ionic liquids on the nucleofugality of dimethyl sulfide. J Org Chem 2024; 89: 14929-14939.
| Crossref | Google Scholar | PubMed |
54 Hsieh AY, Haines RS, Harper JB. Effects of ionic liquids on the nucleofugality of bromide. J Org Chem 2024; 89: 6247-6256.
| Crossref | Google Scholar | PubMed |
55 Hsieh AY, Haines RS, Harper JB. The effects of ionic liquids on the ethanolysis of a chloroacenaphthene. Evaluation of the effectiveness of nucleofugality data to predict reaction outcome. RSC Adv 2023; 13: 21036-21043.
| Crossref | Google Scholar | PubMed |
56 Coney MD, Morris DC, Gilbert A, Prescott SW, Haines RS, Harper JB. Effects of ionic liquids on the nucleofugality of chloride. J Org Chem 2022; 87: 1767-1779.
| Crossref | Google Scholar | PubMed |