Ribosomally synthesised and post-translationally modified peptides (RiPPs) from marine demosponges and their microsymbionts
Lakmini Kosgahakumbura A B # , Jayani Gamage A B # , Chamari M. Hettiarachchi B , Paco Cárdenas A C and Sunithi Gunasekera
A B # , Chamari M. Hettiarachchi B , Paco Cárdenas A C and Sunithi Gunasekera  A *
A *
A
B
C

Lakmini Kosgahakumbura completed her PhD at the University of Colombo, Sri Lanka, in collaboration with Uppsala University, Sweden. Her doctoral research focused on integrative taxonomy and isolation of bioactive natural products from marine sponges. Her particular interests are the isolation, characterisation, and evaluation of the biological significance of bromopyrrole alkaloids and Ribosomally synthesised and post-translationally modified peptides (RiPPs). The potential bioactivities of these compounds serve as valuable leads for future drug discovery. |

Jayani Gamage completed her PhD at the University of Colombo, Sri Lanka, in collaboration with Uppsala University, Sweden. Her doctoral research focused on the integrative taxonomy and antimicrobial potential of shallow-water marine sponges, along with the phylogenetic relationships of selected species. This research contributed to documenting understudied marine biodiversity in the region and highlighted the bioactive potential of sponges as a source of natural products. She has a strong interest in combining conventional taxonomy with modern molecular and chemical approaches to better understand sponge diversity and bioactive potential. |

Chamari Hettiarachchi is a professor in molecular biology and biochemistry at the Department of Chemistry, University of Colombo, Sri Lanka. Her research interests span molecular biology, biochemistry and microbiology, with a strong focus on cellular and molecular mechanisms and their applications in biotechnology. She has published over 50 peer-reviewed articles and has actively engaged in collaborative research with institutes in Sweden and India. Her ongoing work emphasises integrating fundamental molecular insights with applied research to address contemporary challenges in medicine, industry and the environment. |

Paco Cárdenas is a sponge biologist and head curator of zoology at the Museum of Evolution, Uppsala University, Sweden. He obtained his PhD at the University of Bergen, Norway, on the systematics of tetractinellid sponges. His expertise today centres on the evolution, taxonomy and classification of demosponges, using different sets of characters including genetics and chemistry. He has described more than 30 new species of sponges worldwide. Paco also contributes to sponge pharmacognosy and metabolomics research in the Pharmacognosy group at Uppsala University. |

Sunithi Gunasekera is a adjunct lecturer and associate professor in the Pharmacognosy group, Department of Pharmaceutical Biosciences, Uppsala University, Sweden. She obtained her PhD in Structural Biology in 2009 from The University of Queensland, Australia. During her doctoral research, Sunithi worked on early cyclotide bioactivity grafting applications, which sparked her interest in peptide therapeutic development. Sunithi’s research expertise spans peptide synthesis and NMR-based structural analysis. Currently, Sunithi is engaged in ribosomal peptide discovery from marine sponges from underexplored marine environments across the tropics and the North Atlantic. |
# These authors equally contributed to this work.
Handling Editor: Mibel Aguilar
Abstract
Marine sponges are among the oldest animals to have emerged on Earth. They are metazoan holobionts that host diverse microbial symbionts, which constitute more than 40% of their biomass. Despite their morphological simplicity, sponges exhibit complex genetic architecture, unquestionably encoding ribosomally synthesised and post-translationally modified peptides (RiPPs) and proteins, essential for their biological functions. In addition to host-derived compounds, the associated microbiota also produce RiPPs, introducing further complexity in distinguishing the origin of these molecules. To date, marine sponge RiPPs research is confined to species within the class Demospongiae, with peptidomic, transcriptomic and genomic approaches employed for their discovery. This review provides a comprehensive account of current research on ribosomal peptides in marine sponges and associated microsymbionts, emphasising the need for expanded discovery efforts. Unravelling the genetic basis and biosynthetic pathways of these peptides will deepen our understanding of sponge biology and open new opportunities for peptide-based drug discovery.
Keywords: demosponges, marine, microsymbionts, metagenomics, peptides, peptidomics, post-translational modifications, RiPPs, sponges, transcriptomics.
Introduction
Sponges (phylum Porifera) are among the first animals to appear on Earth1–3 and are still present today, with more than 9736 species distributed worldwide.4 Sponges are sessile, benthic animals, occupying a wide range of freshwater and marine habitats, from shallow to the deep sea, down to 8840-m depth.5 Sponges’ biodiversity is especially remarkable when taking into account their close association with highly rich and diverse microbial communities, which can occupy up to 40% of their volume.6 This close relationship makes sponges complex ‘holobionts’-small ecosystems including the host and its microbiota.7 The multiple molecular interactions within sponge-associated microsymbiont communities, and with their hosts, may be one of the major reasons for the prodigious chemical diversity observed in sponges.8,9
Compounds from all kinds of natural product classes are found in sponges: alkaloids, terpenoids, quinones, steroids and peptides, among many others.10,11 The huge structural and stereochemical complexity of these chemical compounds plays a major ecological role, notably enabling sponges to survive, defend themselves and adapt to different environments. This huge structural diversity in compounds is accompanied by many attractive pharmacological properties, such as antibacterial, antifungal, antiviral and anticancer activities.12–15 Numerous other compounds have the ability to inhibit enzymes,16 prevent marine fouling17 or cause toxicity to cells.18 The antimicrobial and antifouling compounds are likely important in maintaining host-microbial balance, microbial homeostasis and overall sponge health. The cytotoxic compounds act as chemical defences to deter predators from feeding. In addition, they may exhibit allelopathy to obtain a competitive advantage for survival in marine environments.19 Many enzyme inhibitors produced by sponges function as defensive mechanisms against microbes, helping to regulate microbial growth on their surface or within their microbial communities. An example includes the production of the enzyme laccase by the demosponge Suberites domuncula (order: Suberitida, family: Suberitidae) that kills bacteria, including Escherichia coli, using a free radical mechanism.20 Therefore, many of these chemical compounds are of medicinal, agricultural or biotechnological importance, with most in preclinical investigations and some further progressing into clinical studies.21
Ribosomally synthesised and post-translationally modified peptides (RiPPs) in sponges form a natural product class on its own with remarkable structural features and a wide range of potential pharmaceutical and biotechnological applications. To date, only a limited number of RiPPs have been identified from sponges and for the majority of them, their precise biosynthetic origin remains largely unclear: the host sponge or one of its numerous microsymbionts (bacteria, archaea, fungi or other unicellular eukaryotes). Sponge RiPPs have all been isolated from the class Demospongiae; none have been found in the three other classes of sponges (Calcarea, Homoscleromorpha, Hexactinellida). Several previous reviews have extensively covered bioactive peptides from marine sponges, with a predominant focus on nonribosomal peptides (NRPs).22–24 Other reviews have compiled sponge-derived peptides, primarily emphasising their anticancer potential, but offering limited discussion on the distinction between ribosomal and nonribosomal origins, or the identification of their true producer.25,26
When we began reviewing this field, it became evident that NRPs have historically dominated marine natural product discovery: their striking structural diversity and potent bioactivities made them appealing targets for isolation. The underrepresentation of marine sponge RiPPs can be attributed to the scarcity of high-quality sponge genomic data, as well as technical challenges in distinguishing host-encoded RiPPs from those produced by associate microbial symbionts. However, despite the availability of dozens of sponge genomes,27,28 the identification and characterisation of RiPPs encoded by the sponges themselves remain challenging.29 This review presents the first dedicated and comprehensive account of RiPPs from marine demosponges. Furthermore, it seeks to reinvigorate research interest in sponge RiPPs by highlighting their significant potential for future drug discovery.
Marine sponges as a source of bioactive peptides
Marine sponges are renowned for their exceptional chemical diversity, producing a wide range of bioactive compounds that play a crucial role in their ecology. Living in an exceptionally wide range of habitats, from intertidal shallow waters to the deep-sea (up to 8800-m depth), from tropical to polar waters, and with a wide range of spatial competitors and predators (fish, turtles, gastropods, sea urchins, sea stars, etc.) and no possibility to move, sponges rely heavily on chemicals to survive.5,19 The ecological pressure that drives the evolution of these chemical adaptations and defences also results in compounds with potent pharmacological properties, making sponges a valuable source of novel drug candidates.
Among the various classes of compounds produced by sponges, peptides are unique due to their structural complexity, specificity and potent bioactivities. To date, peptides isolated from sponges include linear peptides,30 cyclic peptides,31 lipopeptides,32 depsipeptides,33 modified peptides having unusual amino acids,34 RiPPs28 and NRPs.35 These peptides bear diverse bioactivities that have important ecological roles for sponge survival and growth. The most common bioactivities include antimicrobial activity,15 antifouling activity,36 anticancer activity,37 enzyme inhibition activity,38 anti-inflammatory39 and neuromodulatory effects.40 In other examples, collagen-derived peptides extracted from Chondrosia reniformis exhibit antioxidant and photoprotective properties, significantly enhancing fibroblast and keratinocyte migration and proliferation, promoting in vitro wound closure.41 Thus, sponge-derived peptides not only support sponge survival in complex marine ecosystems but also hold significant promise for human therapeutic applications.
Symbiotic microbiota and the complexity of peptide origin
Marine sponges harbour dense and diverse communities of symbiotic microorganisms, including bacteria, archaea, and fungi, which can constitute up to 40% of the sponge’s biomass.42 These symbionts play essential ecological roles in nutrient cycling, chemical defence and the production of secondary metabolites.7 One remarkable feature of these microbial communities is their ability to biosynthesise a wide array of structurally complex and biologically active peptides. These peptides exhibit diverse chemical scaffolds, including cyclic, linear and hybrid structures, often featuring non-proteinogenic amino acids and post-translational modifications (PTMs).43 The complexity arises from both the host sponge and its microbial associates, with many peptides encoded by microbial biosynthetic gene clusters (e.g. nonribosomal peptide synthetase, NRPS, and polyketide synthase, PKS, systems).44,45 Nevertheless, these microbes also produce ribosomal peptides using microbial ribosomes that subsequently undergo extensive PTMs.43
For instance, some marine ribosomal peptides are produced by marine microbes such as Actinobacteria (e.g. Streptomyces spp.),46,47 Proteobacteria (e.g. Pseudomonas spp.),48 Firmicutes (e.g. Bacillus)49,50 and Cyanobacteria (e.g. Prochloron spp.).51–53 Notably, cyanobacterial metabolites, including peptides of both ribosomal and nonribosomal origin, are also commonly found in sponges.54–56
One of the well-characterised RiPPs directly associated with marine sponges is polytheonamides, produced by candidatus Entotheonella factor, a bacterial symbiont of Theonella swinhoei chemotype Y (order: Tetractinellida, family: Theonellidae).43,53 Polytheonamides will be discussed in detail later in this review in a dedicated section. The biosynthetic origin of other peptides from Theonella species, including orbiculamide57 and koshikamides,58 remains unresolved. In similar cases, the biosynthetic origin of cytotoxic peptides phakellistatins from Stylissa carteri (order: Scopalinida, family: Scopalinidae) and Phakellia fusca (order: Bubarida, family: Bubaridae), as well as discodermins from Discodermia species, has remained elusive for a long time.59 Recent findings support the hypothesis that phakellistatins are likely RiPPs produced by sponges.60 However, the origin of discodermins remains unresolved and could potentially involve production by the sponge-associated microbiota.
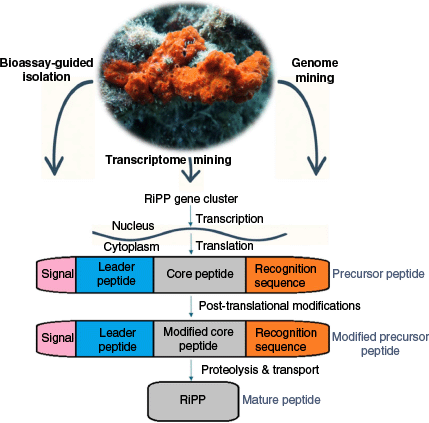
Defining RiPPs
RiPPs are found in a variety of organisms, in all three domains of the tree of life: bacteria, archaea and eukarya.61–63 These molecules are derived from long precursor peptides encoded by structural genes, as illustrated in Fig. 1. The precursor peptide consists of two major parts, a ‘core peptide’ and a ‘leader peptide’. The leader peptide, acting as a recognition site for the PTM enzymes, is usually attached to the N-terminus of the core peptide and frequently maintains the precursor in an inactive state. PTM enzymes include primary enzymes that introduce class-defining PTMs, as well as secondary enzymes that carry out compound-specific modifications, referred to as ‘tailoring reactions’. In RiPPs with multiple PTMs, distinct regions of the leader or follower peptides are recognised by specific biosynthetic enzymes by defined recognition sequences. More rarely, the leader peptide is found at the C terminus of the core peptide, in which case it is referred to as a ‘follower peptide’. Some precursor peptides, such as those in cyanobactins, dikaritins and cyclotides, contain multiple core peptides and recognition sequences, enabling the production of multiple RiPPs from a single precursor.52 Binding of biosynthetic enzymes to the leader peptide facilitates the modifications of the core peptide and subsequent removal of the leader peptide by proteolysis, resulting in the modified core peptide (Fig. 1).61,64 These PTMs, in turn, affect the three-dimensional structure of the molecule, fundamentally altering its shape, an aspect that can be critical for receptor binding, enzyme resistance and the intrinsic biological activity of the core peptide. In 2013, a community effort by scientists working on the research area of RiPPs led to a consensus on the defining commonalities of RiPPs, resulting in a comprehensive review. An overview of divergent classes of RiPPs from all kingdoms of life was presented: lanthipeptides, linear azoline-containing peptides, bottromycins, microcins, proteusins, lasso peptides, amatoxins and phallotoxins, cyanobactins and microviridins, thiopeptides, cyclotides and conopeptides.61 In a later review in 2019,65 the newly identified families of RiPPs since the 2013 review, along with their biosynthetic logic and enzymatic machinery, have been extensively further described. A recurring question in peptide classification is whether certain amino acids or topological features (e.g. disulfide bonds) can be used to reliably distinguish RiPPs from NRPs.
Typically, cysteines found outside of disulfide-bonded contexts have been associated more with NRPs.64 But there are some examples where disulfide bonds occur in NRPs and, conversely, where RiPPs entirely lack disulfide linkages. For instance, amatoxins and phallotoxins, prominent fungal RiPP peptide subclasses, contain cysteine residues that do not participate in disulfide bonding.66 The depsipeptides, FK22867 and spiruchostatin,68 which are naturally discovered in the fermentation broth of Chromobacterium violaceum number 968 and Pseudomonas extract respectively, consist of a disulfide linkage between L and D configured cysteine residues. These depsipeptides are synthesised by either NRPS or PKS systems.67,68 Such cases underscore that, although amino acid composition (notably cysteine abundance and disulfide topology) can provide useful hints, they are not definitive criteria for distinguishing RiPPs from NRPs. Altogether, the most reliable and consistent determinant remains the genetic basis of biosynthesis.
Structural diversity and biosynthetic mechanisms of sponge RiPPs
Asteropine A and Asteropsins
Asteropine A (APA) was serendipitously discovered by researchers at the University of Tokyo during a screening effort of specialised metabolites for anti-sialidase activity.69 It was isolated from an extract of the marine sponge Asteropus sp.1 (order: Tetractinellida, family: Ancorinidae), collected from shallow waters of Shikine-jima Island, Japan. A subsequent re-examination of the voucher specimen ZMAPOR 16718 (Naturalis, Leiden, Netherlands) by P. Cárdenas clarified that the sponge was an Asteropus sp.1 but not Asteropus simplex, as originally reported. APA possessed a molecular weight of 3817.5 Da and contains an Inhibitory Cysteine Knot (ICK) framework comprising six cysteine residues arranged to form three disulfide bridges: CysI–CysIV, CysII–CysV and CysIII–CysVI. The latter disulfide bond penetrates a macrocycle formed by the other two, along with the inter-connecting backbone regions, producing the characteristic knotted topology of knottins. The RiPP identity of APA is unknown at this point without genetic or biosynthetic analyses. However, the structural features of APA, particularly the presence of multiple cysteine residues forming disulfide-stabilised topology, are reminiscent of several well-characterised RiPP families, including cyclotides, conotoxins and θ-Defensins.
APA attracted significant interest due to its rigid conformation maintained by an ICK disulfide framework. This inspired a group of scientists at Pusan National University to search for asteropine-like candidates in a shallow-water species from Geoje Island, South Korea. Their efforts led to the successful discovery of seven additional ICK peptides, named asteropsin A (ASPA) to asteropsin G (ASPG) from Asteropus sp.2 (Fig. 2).40,70–72 A re-examination of the voucher specimen J05B-3 (courtesy of Jee H. Jung) by one of us (P. Cárdenas) confirmed that this species is taxonomically distinct from Asteropus sp.1 and Asteropus simplex. However, this re-examination also showed that the voucher is a mix of two sponge species: the main species, Asteropus sp.2 (grey colour), is entirely covered with a second unidentified Hymeniacidon sponge (orange colour) (order: Suberitida; family: Halichondriidae); a deck picture of the voucher shows this clearly (fig. S9 in Li et al.71). Therefore, asteropsins A–G were actually isolated from a two-sponge association. Although peptides from Asteropus sp.1 and sp.2 belong to the knottin class, the asteropsins A–G share significantly greater sequence similarity with each other than with APA, which is unique in sequence.
Exemplified structures of Asteropus peptides and their sequence alignment. The top panel shows the structures of three selected peptides: Asteropsin A (PDB: 2LQA), Asteropsin E (PDB: 2M3J) and Asteropsin G (PDB: 2N3P). The N-terminal pGlu is depicted by X (blue) where applicable. Observed masses are indicated for either (M + H)+ or (M + Na)+ (highlighted by asterisks, *) ions.

Knottin peptides are found across a variety of organisms61–63,73 but relatively few have been reported from marine organisms outside the cone snail family. Therefore, the discovery of eight knottins from the genus Asteropus to date, establishes a promising platform for Porifera, or at least the genus Asteropus, as a source for unusual knottins.
All Asteropus peptides exhibit several conserved structural features. The tertiary structural framework in Asteropus peptides features a triple-stranded antiparallel β sheet stabilised by an extensive hydrogen bond network. This overall stable fold is conserved across all Asteropus peptides. An exception is Asteropsin G (ASPG), which contains only two β strands. Additionally, an N-terminal pyroglutamic acid (pGlu) occurs as a PTM in five of the asteropsins except APA, ASPE and ASPF. Li et al. also discovered two N-terminal L-pyroglutamyl dipeptides probably from the same Hymeniacidon and Asteropus sp.2 association, that harboured the seven asteropsins, suggesting that this modification may serve a unique ecological or functional role.74
All Asteropus peptides exhibit remarkable stability in human plasma and resistance to degradation by gastrointestinal enzymes, primarily attributed to a dense hydrogen bonding network stabilising their structures.70–72 Although some asteropsins contain an N-terminal pGlu, this feature is not universally conserved, indicating that it is not a key determinant of their proteolytic resistance. Yet, the presence of the pGlu may confer additional thermal stability. ASPA and ASPC, which contain this modification, exhibit greater thermal stability than ASPE and ASPF, which are devoid of it. Asteropsins also exhibit a uniquely high prevalence of cis-proline residues, uncommon among knottins, which likely contribute to their structural rigidity and protease resistance.
The exceptional stability of the peptide scaffolds found in Asteropus sp. may have significant implications for oral drug development and merits further analysis. Kalata B1, an archetypal cyclotide and representative of a cyclic cystine-knot peptide family, exhibits remarkable resistance to gastrointestinal proteolysis and is thus considered a promising scaffold for oral drug delivery.75 Notably, epitope-grafted analogues of kalata B1 in their linear form have demonstrated substantially reduced oral bioavailability, despite the preservation of the knottin fold.76 Therefore, it will be important to assess the plasticity of the asteropsins to accommodate foreign epitopes within their inter-cysteine loops, while preserving the native disulfide connectivity and maintaining pharmacokinetic properties, particularly oral bioavailability.
Asteropsins offer several advantageous properties, including a linear backbone that bypasses the need for head-to-tail cyclisation and stability in human plasma for extended durations. These attributes collectively highlight their potential as lead compounds for therapeutic development. Importantly, none of the tested asteropsins exhibited hemolytic activity at concentrations up to 50 µM, and their lack of cytotoxicity further supports their suitability as inert pharmacophore carriers.72 This stands in contrast to kalata B1, which, despite being proposed as a promising scaffold, showed 74% hemolysis under identical conditions, a potential limitation for its development as a drug carrier.72
Most knottins exhibit a net positive surface charge, consistent with their function as cation channel blockers. By contrast, the eight Asteropus knottins contain net negatively charged surfaces due to an abundance of acidic amino acid residues. Of the two peptides ASPA and ASPC, ASPA uniquely induced Ca2+ influx of murine cerebrocortical neurons, but only in the presence of Na+ channel activator, with an EC50 of 14 nM. When administered alone, even at micromolar concentrations, ASPA did not affect voltage-gated ion channels.40 Comparative surface analysis of ASPA and ASPC revealed a distinct hydrophilic patch in ASPA, formed by Glu6, Glu8, Ser9, Asp31 and Thr33, flanked by hydrophobic residues such as Phe5, Phe14, Tyr15, Leu21, Ile26, Tyr35 and Leu37.70 By contrast, ASPC exhibits a markedly different surface composition. Hydrophobic residues Tyr29 and Ile31 in ASPC replace the acidic Asp31 and polar Thr33 found in ASPA, preventing the formation of a hydrophilic region. Furthermore, ASPC lacks key residues such as Phe5, Tyr15 and Leu21 present in ASPA, which are necessary to establish a hydrophobic region in the same area. These variations in surface profile may account for ASPC’s inability to induce neuronal Ca2+ influx.70
Among the eight knottin peptides, APA stands out with notable structural and functional distinctions. The acidic residues implicated for bioactivity in APA are exposed to the solvent, in contrast to ASPA, where such residues are buried within the core.40 Aside from the cystine bridges, APA lacks other PTMs and is the only member shown to selectively inhibit bacterial sialidase/neuraminidase.40 To date, none of the other asteropins except ASPA have been evaluated for anti-neuraminidase activity against bacterial or viral strains.
Taken together, Asteropus knottins represent a structurally unique and functionally versatile class of peptides. Although plasticity of the scaffolds or epitope grafting applications are yet to be explored, their exceptional enzymatic stability, non-hemolytic nature and stable core architecture with inter-cysteine loops make them attractive scaffolds for potential bioengineering and therapeutic development.
Barrettides
Barrettide A and B were isolated from the Swedish deep-sea sponge Geodia barretti (order: Tetractinellida, family: Geodiidae) at Uppsala University.77 Sequences consist of 31 amino acids with one amino acid difference – the position 19 (Valine v. Leucine). The structure of barrettides is characterised by a unique folding pattern of the long antiparallel β sheets, cross-braced by two disulfide bonds in a ladder like-arrangement. Of the three proline residues in each barrettide, P20 is in cis configuration. An overall net charge of −4 makes the surface of barrettides highly negative, and thus acidic in nature, similar to asteropsins. In 2021, our research group at Uppsala University discovered five more barrettides, barrettide C to G, with molecular masses between 3 and 4 kDa using a combined genomic, transcriptomic and in silico discovery route (Fig. 3).36 Synthesised barrettide C exhibited similar structural features to barrettide A with the same disulfide arrangement. A short α-helix is formed on the C-terminal side of the β-hairpin of barrettide C, which is stapled to the N-terminal tail by the CysI–CysIV disulfide bond.36
Identification of barrettides across different Geodia barretti samples. The top panel shows the three-dimensional structures of barrettide A (PDB ID: 6CFB, left) and barrettide C (PDB ID: 7SAG, right). The bottom panel displays a sequence alignment of all barrettide peptides identified to date. P indicates detection in peptide extracts, T in transcriptomes, whereas M and G indicate identification in a single metatranscriptome and genome respectively.

Our research group has conducted several experiments to determine the biosynthetic origin of barrettides. One of the experiments searched for analogous features to modular precursor peptides of RiPPs in the transcriptome, which can contain a signal domain, leader and core peptide, and recognition sequences. In addition, the presence of a poly-A tail (A refers to adenine) at the end of the nucleotide sequence suggests the mature transcript to be eukaryotic and therefore most probably of sponge origin. However, this is not a foolproof argument since the poly-A tails can occasionally be found in bacteria and archaea.78 Therefore, the length and sequence similarity of the introns (noncoding regions in transcripts that are removed prior to translation) were used to confirm the eukaryotic origin of transcripts.36 Generally, prokaryotes only produce two characteristic types of introns (group I and group II),79,80 different from the ones that were found in the barrettide transcripts. Finally, the barrettide genes were also found in the assembled G. barretti genome.28,36 Altogether, by identifying the genes of barrettides in the G. barretti genome and transcriptomes, the authors are confident that the sponge host is the producer of these peptides and that they are coded by genes; barrettides can therefore be considered RiPPs. Interestingly, a subsequent metabolomic study consistently detected barrettides A, B, and C from a series of G. barretti specimens from the Davis Strait (between Canada and Greenland), irrespective of the depth of sample collection from 400 to 1400 m deep, and despite important changes in the microbiome community and metabolome.81
Biofouling, the settlement of micro or macro organisms on surfaces, causes significant problems in the marine industry. Antifouling agents are used to deter the fouling on marine equipment, but restrictions imposed on chemical biocides in industrial formulations have triggered the need for alternative, environmentally friendly compounds.82 Marine sources such as sponges, ascidians, algae and seagrasses are being heavily explored for their ability to harbour anti-biofouling compounds. Barrettides A, B and C inhibit the settlement of barnacle larvae Amphibalanus improvisus at a low micromolar concentration without apparent cytotoxicity.36,77 These data suggest barrettides may be a family of antifouling peptides that might have a unique functional or ecological role important for the sponge.
Typically, the cationic nature of peptides facilitates interaction with negatively charged bacterial cell membranes, imparting antibacterial activity. No bacterial growth inhibition was observed for barrettides against the Gram-positive Staphylococcus aureus or the Gram-negative E. coli, up to a concentration of 80 µM. Owing to their overall negatively charged surface, barrettides do not appear to interact with the membranes, leading to poor antibacterial properties.77
Aculeines
Aculeine A, B and C are polypeptides from the shallow water Japanese marine sponge Axinyssa aculeata (order: Suberitida, family: Halichondriidae) with a molecular size between 2.5 and 7 kDa.83 Matsunaga et al. also provide genetic evidence confirming that aculeines are ribosomally synthesised peptides.83 Using 3′ and 5′ Rapid Amplification of cDNA Ends (RACE) sequencing, it was identified that aculeines are gene-encoded precursor peptides with a 3′ poly-A terminal, which undergo PTMs, including the addition of long-chain polyamines. The poly-A terminal suggests that aculeines are produced by the sponge, but again, this is not definitive proof. Identification of introns or simply whole genome sequencing may confirm this hypothesis.
Only the primary sequences of aculeine A and B have been determined. Both peptides have a polypeptide chain consisting of 44 amino acid residues. Aculeine A and B differ from each other by the length of the polyamine tail attached to their N-terminal. This unique N-terminal modification most likely affects their structure and biological functions. The structure of the polyamine tail of aculeine B was later confirmed to be equal to protoaculeine B, a polyamine attached to an unusual tricyclic tryptophan derivative isolated from the same sponge (Fig. 4).84,85 Some important structural features of the polyamine tail of aculeine A were described in the same study with incomplete structure elucidation.85 Although aculeines are assumed to be knottin peptides, their native disulfide connectivity has not been confirmed by either chemical disulfide mapping or NMR structural analysis.85
Proposed structures of Aculeine A and B.84 Both aculeine A and B consist of a common polypeptide chain of 44 amino acid residues. In addition, aculeine B has an N-terminally attached protoaculeine B unit, a long chain polyamine (12–15 mer) connected to the polypeptide chain by a tryptophan-derived amino acid residue.

Aculeines are neuroactive compounds due to convulsant behaviour in mice observed after intracerebroventricular injection of the aqueous extract (20 µg).83 In addition, hemolytic activity against erythrocytes of several different animal species83 and moderate cytotoxic activities were observed against cultured human cancer cell-lines MDA-MB-231, A 549 and HT-29 (GI50 values of ~0.5 µM).83 Furthermore, experiments suggest that aculeine A acts either by disrupting membranes or by penetrating cells, depending on the cell type.86
Proline-rich macrocyclic peptides
Cyclic peptides or N to C macrocyclic peptides are widespread in nature and are associated with potent bioactivities.15,59 Among them, proline-rich macrocyclic peptides (PRMPs) are well known in marine sponges87 and 93 PRMPs have been recorded to date in the MarinLit database (Royal Society of Chemistry, see https://marinlit.rsc.org/). These peptides consist of fewer than 15 amino acid residues and have multiple proline residues. The proline residue plays an important structural role by reducing the structural flexibility, thereby enhancing the stability.88,89 The exact biosynthetic origin of all these peptides is unknown. Some have been shown to be produced by the microorganisms associated with sponges. An example is the isolation of two PRMPs from the cell extract of a bacterium, Ruegeria, a strain associated with Suberites domuncula (order: Suberitida, family: Suberitidae).90 Another two PRMPs produced by marine cyanobacteria include pitiprolamide and croissamide.91,92
Multiple experimental approaches were conducted by Lin et al. to determine the exact origin of PRMPs produced by the two sponge genera Stylissa and Axinella.60 In the absence of RiPP or NRP sequences that might encode the desired products in the microbial genomes, the precursor peptide sequences were searched in the sponge transcriptome. The cyclic peptide sequences were linearised and used as queries with Stylissa carteri translated transcriptomic assemblies using BlastP (ver. 2025_03, see https://www.uniprot.org/). Forty of the query sequences exactly matched with 79 predicted protein sequences from S. carteri, providing them as possible precursor peptides.60 To validate the accuracy of the possible precursor peptides, the highly conserved recognition regions that direct proteolysis and cyclisation of PRMPs were searched. Sixteen PRMP precursor candidates were found that share at least two conserved motifs. In addition, multiple copies of the same motif were observed within a single precursor, which is a key characteristic of cyclic RiPP precursors across kingdoms of life.52 Between these motifs, putative core sequences were identified, some of which were identical to sequences known to encode chemically characterised PRMPs from sponges. In addition, a few more facts were confirmed that support the hypothesis of the biosynthesis of PRMPs by sponges. These include the presence of signal peptide sequences in the N-terminal of the precursor peptide that carry information for peptide secretion. To validate the results further, transcriptomic data of Cymbaxinella corrugata (order: Agelasida, family: Hymerhabdiidae) were searched for similar PRMP precursor peptides found in S. carteri, leading to the identification of 35 precursor peptides, including 31 unique core peptide sequences.93 A chemical extract of C. corrugata was also obtained and analysed using mass spectrometry to search for the unique core peptide sequences. Eleven core peptides were found, of which four were known PRMPs, stylissamides A–D from Stylissa caribica (order: Scopalinida, family: Scopalinidae).60,94 The remaining seven were not reported previously, highlighting the structural diversity of the genomic predicted peptides. In conclusion, S. carteri and C. corrugata sponges were confirmed to be the true producers of the PRMPs. PRMPs have also been reported elsewhere in the genus Hymeniacidon and Phakellia.95,96
Stylissamides A–D (Fig. 5) are heptapeptide PRMPs isolated from the Caribbean sponge S. caribica.94 These are shorter peptides with molecular masses of 845.4555, 812.4311, 862.4490 and 828.4657 Da corresponding to stylissamide A, B, C and D respectively. Stylissamides A, C and D contain three proline residues, whereas stylissamide B has four. The proline residues of stylissamide A and B are in both cis and trans configurations, whereas it is only cis in the latter two compounds. All the amino acids of the four peptides are in the L-configuration.94 Of these four peptides, stylissamide B has been successfully synthesised using Fmoc solid-phase peptide synthesis followed by cyclisation in the solution phase.97 So far, no bioactivities have been reported for these peptides.
Exemplified structures of Stylissamide A, B, C and D with their calculated and experimental masses (a–d).94

Recifin A
During screening for potential inhibitors of Tyrosyl-DNA phosphodiesterase 1 (TDP1), a molecular target for the sensitisation of cancer cells to FDA-approved topoisomerase inhibitors, recifin A (Fig. 6), was isolated from the marine sponge Axinella sp.98 The sponge sample was collected from Thunderbolt Reef, located south-south-west of Cape Recife Nature Reserve, South Africa. Recifin A consists of 42 amino acids and has a molecular mass of 4912.9661 Da. With a disulfide connectivity between CysI–CysIII, CysII–CysV and CysIV–CysVI, recifin A does not belong to the ICK peptide family. In fact, when recifin A was first structurally characterised, it showed an unprecedented three-dimensional structure, with a central four-stranded β-sheet motif, sandwiched by two helical turns. It is not a cyclic peptide as it lacks either a head-to-tail cyclic backbone or a side-chain-to-backbone cross-link. The most unique feature is the embedded ring that is formed by the three disulfides and β strands I and IV which is then threaded by the third β strand. A tyrosine residue further stabilises the resultant structure by locking it in three-dimensional space. Consequently, recifin A has been classified as the founding member of a new peptide family, called the Tyr-lock peptide family. Notably, it contains an N-terminal pGlu moiety, which renders Edman degradation ineffective unless selective cleavage is performed by Pfu pyroglutamate aminopeptidase. Although genetic data are currently unavailable, recifin A displays typical structural traits found in RiPPs from sponges, given its molecular mass, complex structural features and particularly high cysteine content arranged to form a unique disulfide connectivity.
Three-dimensional structure of Recifin A (PDB ID: 6XN9). The N-terminal pGlu residue is highlighted in dark blue, and Tyr6, which is involved in the Tyr-lock motif, is highlighted in cyan.

Recifin A inhibited the cleavage of the phosphodiester bond by TDP1 with a 50% inhibition of the full-length TDP1 enzymaticactivity byrecifin A (IC50) of 190 nM. The enzyme kinetic studies revealed allosteric binding of recifin A to a regulatory domain of TDP1, instead of affecting the activity by binding to the catalytic domain. Clinically, TDP1 can counteract the effects of Topoisomerase I (TOP I)-inhibiting chemotherapeutic agents, such as camptothecin derivatives topotecan and irinotecan, by reducing DNA damage. As a compound that inhibits TDP1, recifin A is of significant potential therapeutic value. Recently, the synthesis of recifin A was accomplished by ligating two assembled peptide fragments by native chemical ligation.99 Notably, the linear form of recifin A spontaneously folded into its native conformation without the need for selective disulfide protection, indicating that this fold is energetically favourable. This synthetic advancement has paved the way for obtaining first insights into the structure–activity relationship (SAR) studies towards its cancer target TDP1, as analogues of recifin A have now been successfully synthesised.99
Neopetrosiamides
Neopetrosiamides were discovered during a screening for novel inhibitors of amoeboid invasion of the extracellular matrix (ECM) by metastatic cancer cells.100 Amoeboid migration is characterised by rapid shape changes that allow cells to traverse pre-existing spaces within the ECM. Typically, cancer cells can transition from enzyme-dependent mesenchymal migration to amoeboid migration, a process known as the mesenchymal–amoeboid transition (MAT), which plays a critical role in metastasis.
A crude methanolic extract of Neopetrosia sp. (order: Haplosclerida, family: Petrosiidae), collected from Papua New Guinea, exhibited promising inhibitory activity in the bioassay to detect MAT. Bioassay-guided isolation subsequently led to the identification of two tricyclic diastereomers, neopetrosiamide A and B, as the active constituents. Neopetrosiamide A and B are 28-residue peptides, each containing six cysteine residues. NMR analysis revealed a disulfide connectivity of CysI–CysV, CysII–CysIII and CysIV–CysVI, forming a constrained tricyclic structure. 101 In a follow up study on the chemical synthesis of neopetrosiamides, it was discovered that the originally proposed disulfide connectivity was incorrect.101 Notably, the synthetic peptides displayed different retention times compared to the native product after oxidative folding, indicating a discrepancy in the originally proposed disulfide connectivity. Disulfide mapping of neopetrosiamides was then performed using a combination of partial reduction, proteolytic cleavage and selective labelling that enabled the disulfide connectivity to be revised as CysI–CysV, CysII–CysIV and CysIII–CysVI. However, a revised 3-D structure reflecting these changes is not currently available. All proline residues in neopetrosiamides adopt trans proline configuration.100 Both peptides are linear and composed exclusively of L-amino acids. A key PTM is the oxidation of a methionine residue to methionine sulfoxide, which is the only structural difference between neopetrosiamide A and B, giving rise to distinct configurations of the sulfoxide moiety. Thus, neopetrosiamide exists as a mixture of diastereomers, differing only in the stereochemistry at the methionine sulfoxide residue. This epimeric difference is not detected in amino acid or Marfey’s analyses, as methionine sulfoxide is reduced to methionine during these degradation-based assays.
A number of Matrix Metalloproteinase (MMP) inhibitors targeting ECM-degrading enzymes have been developed, but their limited clinical efficacy is believed to stem from the ability of cancer cells to undergo amoeboid invasion. Thus, the discovery of clinically relevant compounds capable of blocking amoeboid invasion holds significant therapeutic potential. Accordingly, neopetrosiamides have emerged as promising lead molecules for anticancer applications.
Halichondamide A
Halichondamide A, a unique undecapeptide featuring two fused bicyclic motifs stabilised by two disulfide bridges (Fig. 7), was identified in the shallow-water Halichondria (Halichondria) bowerbanki (order: Suberitida, family: Halichondriidae). Specimens were collected at Santa Barbara, CA, USA, where it is likely an introduced species102 since it is originally described from the North Atlanto-Mediterranean region. Halichondamide A exhibits mild cytotoxic activity against hepatic and breast cancer cell lines, with IC50 values exceeding 100 µM. The presence of two disulfide bonds, CysI–CysIII and CysII–CysIV, imparts significant conformational rigidity to the central peptide backbone, despite the molecule being linear. Notably, only a single native disulfide isomer is observed in sponge-derived extracts, suggesting that a specific conformation is selectively stabilised in the natural environment.
Halichondamide A (PDB ID: 9bhn), a disulfide-rich undecapeptide isolated from Halichondria bowerbanki. The structure contains two disulfide bonds (CysI–CysIII and CysII–CysIV), contributing to a constrained, yet, acyclic topology.

To investigate its biosynthetic assembly, the authors employed molecular dynamics simulations on halichondamide A.102 Their findings suggest that pre-installation of the CysII–CysIV disulfide bond leads to a conformational ensemble characterised by a significantly reduced average interatomic distance between CysI and CysIII, thereby facilitating subsequent disulfide bond formation. By contrast, reduction of the CysII–CysIV linkage results in a broader, less-ordered range of conformations, unfavourable for CysI–CysIII coupling. Based on these insights, the proposed biosynthetic model is: (i) enzymatic formation of the CysII–CysIV disulfide bond leading to (ii) the second disulfide bond.
A summary of the RiPPs from sponges described above is available in Table 1.
| Name of the compound | True producer | Voucher in museum (Identifier) | Order/family | Species | Biological activity | Class of the peptide | [M + H]+ | References | |
|---|---|---|---|---|---|---|---|---|---|
| Asteropine A | ? | ZMAPOR 16718 (R.W.M. van Soest) | Tetractinellida/Ancorinidae | Asteropus sp.1 | Bacterial sialidase inhibition | ICK | 3817.5 | 69 | |
| Asteropsin A (ASPA) | ? | J06B-2, J05B-3 (C. J. Sim) | Tetractinellida/Ancorinidae | Asteropus sp.2/Hymeniacidon sp. | Enhancement of the Ca2+ influx with the administration of veratridine | ICK | 4244.7 | 40 | |
| ASPB | ? | Suberitida/Halichondriidae | Extreme stability in human plasma, proteolytic stability against major gastrointestinal enzymes | ICK | 3897.5 | 70– 72 | |||
| ASPC | 3765.5 | ||||||||
| ASPD | 3426.2 | ||||||||
| ASPE | 3539.5 | ||||||||
| ASPF | 3540.2 | ||||||||
| ASPG | 3517.6 | ||||||||
| Barrettide A | marine sponge | UPSZMC 184975-77 (P. Cárdenas) | Tetractinellida/Geodiidae | Geodia barretti | Antifouling activity | – | 3213.3862 | 77 | |
| Barrettide B | 3227.4175 | ||||||||
| Aculeine A | ? | QM G321104 (J.N.A. Hopper) | Suberitida/Halichondriidae | Axinyssa aculeata | Cytotoxic activity | – | 6574 | 83 | |
| Aculeine B | 5706 | ||||||||
| Recifin A | ? | 0CDN7410 (–) | Axinellida/Axinellidae | Axinella sp. | Inhibits the TDP1 | Tyr-lock | 4912.9661 | 98 | |
| Stylissamide A | marine sponge | – (–) | Scopalinida/Scopalinidae | Stylissa caribica | – | PRMP | 845.4555 | 60, 94 | |
| Stylissamide B | 812.4311 | ||||||||
| Stylissamide C | 862.4490 | ||||||||
| Stylissamide D | 828.4657 | ||||||||
| Neopetrosiamide A | ? | ZMAPOR 18339 (R.W.M. van Soest) | Haplosclerida/Petrosiidae | Neopetrosia sp. | Inhibition of amoeboid invasion | – | 3071 | 100 | |
| Neopetrosiamide B | 3071 | ||||||||
| Halichondamide A | ? | UCSB-IZC00069004 (T. L. Turner) | Suberitida/Halichondriidae | Halichondria (Halichondria) bowerbanki | Mild cytotoxic activity | – | 1322.5175 | 102 |
RiPPs produced by sponge-associated microbial communities
A large majority of the peptides of microbial origin are NRPs, produced by highly complex nonribosomal machinery, often using unusual proteinogenic amino acid residues.103 Microorganisms, including bacteria, archaea and fungi are also known producers of RiPPs, in the case of the latter, these are known as fungal-RIPP (F-RiPP).104 Given that several comprehensive reviews53,63,105,106 have already addressed the diversity and biosynthetic pathways of RiPPs from these sources, we will not elaborate on each microbial RiPP class in detail. Similarly, cyanobactins produced by cyanobacteria have been extensively discussed in the literature107 and are therefore not addressed here. Notably, some of these microorganisms may exist as part of sponge-associated holobionts, contributing to the microbial biomass within marine sponges. Among the RiPPs identified from sponge-associated microbiota, representatives of the lanthipeptide and proteusin classes have been reported. Additionally, a few thiopeptides have also been found in sponge-associated bacteria that are assumed to be RiPPs (Table 2). In the following sections, we highlight selected examples from these classes and briefly discuss their defining structural and biosynthetic features.
| Name of the compound | Producer | Sponge host | Biological activity | Class of the peptide | [M + H]+ | Reference | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Phylum | Species | Order | Family | Species | ||||||
| Polytheonamide A | Tectomicrobia | Candidatus ‘Entotheonella factor’ | Tetractinellida | Theonellidae | Theonella swinhoei | Cytotoxic activity | Thiopeptide | 5033 | 43 | |
| Polytheonamide B | Tectomicrobia | Candidatus ‘Entotheonella factor’ | Tetractinellida | Theonellidae | Theonella swinhoei | Cytotoxic activity | Thiopeptide | 5033 | 43 | |
| Subtilomycin | Bacillota | Bacillus subtilis strain MMA7 | Haplosclerida | Chalinidae | Haliclona (Haliclona) simulans | Antimicrobial activity | Lanthipeptide | 3235.6 | 108 | |
| YM 266183 | Bacillota | Bacillus subtilis | Suberitida | Halichondriidae | Halichondria (Halichondria) japonica | Antibiotic activity | Thiopeptide | 1158.1791 | 109 | |
| YM 266184 | Bacillota | Bacillus subtilis | Suberitida | Halichondriidae | Halichondria (Halichondria) japonica | Antibiotic activity | Thiopeptide | 1172.1948 | 109 | |
Proteusins
Proteusins are a class of RiPPs produced by certain bacteria, notably those associated with marine sponges.61 These peptides are characterised by their complex structures resulting from extensive PTMs. The name ‘proteusin’ is derived from the shapeshifting Greek sea god Proteus, reflecting the structural diversity conferred by these extensive PTMs.110 Like other RiPPs, proteusins are synthesised as precursor peptides consisting of an N-terminal leader sequence and a C-terminal core peptide. Although other RiPP families are often classified based on the type of PTM or a structural motif present in the mature product, proteusins are defined by their conserved and unusually long leader sequences, which include a rigid N-terminal domain and a flexible, disordered C-terminal region.110,111 The disordered region is particularly important for interacting with modifying enzymes. The prototypical proteusins, polytheonamides, were the first to be isolated from a natural source, the marine sponge T. swinhoei chemotype Y, and are biosynthesised by a sponge-associated bacterium.110 Genomic and metagenomic studies have revealed polytheonamide-type proteusins (such as aeronamide A and polygeonamides) and non-polytheonamide proteusins (such as landornamides) from non-sponge sources, have cytotoxic and antiviral activities.112,113 Recent metagenomic surveys of mesophotic coral ecosystem sponges revealed a vast diversity of proteusin biosynthetic gene clusters (BGCs), identifying 107 proteusin BGCs out of 293 RiPP BGCs associated with sponge symbionts like candidatus Entotheonella and Acidobacteriota.114 These findings underscore the potential of marine sponges as rich reservoirs of novel proteusin-type natural products and emphasise proteusins as a rapidly emerging subclass of RiPPs with significant promise for drug discovery, particularly from marine sponge-associated microbiomes. Of these, polytheonamides will be discussed below.
Polytheonamides A and B from the Japanese marine sponge T. swinhoei chemotype Y are extensively post-translationally modified ribosomal peptides (Fig. 8).43,73 These modifications create a highly tailored β helical structure with low pico-molar cytotoxicity to mammalian cell lines.43,115 Owing to the presence of 48 PTMs, including 18 epimerisations and 17 methylations, it was initially believed to be synthesised by a nonribosomal pathway. However, its biosynthesis by a RiPP pathway came to light with the identification of the BCG of polytheonamide (poy) in the sponge metagenome.73 With single-cell and metagenomic analyses, Wilson et al. identified the true producer of polytheonamide as the uncultivated bacterial symbiont candidatus Entotheonella factor.116 Subsequently, expression and mutational studies characterised the involvement of seven enzymes involved in generating the mature polytheonamides.115
Structure of polytheonamide B (PDB ID: 2RQO). The peptide backbone is shown in sand colour, with selected post-translationally modified amino acids highlighted: N-terminal glycine blocked with a 5,5-dimethyl-2-oxo-hexanoyl group (red), β-methylisoleucine (pink), β-hydroxyvaline (cyan), β-methylglutamine (black), γ-N-methyl-threo-β-hydroxyasparagine (purple), tert-leucines (yellow), γ-N-methylasparagines (orange) and a β,β-dimethylmethionine sulfoxide residue (grey). Polytheonamide A is an epimer of polytheonamide B, differing only in the stereochemistry of the sulfoxide of the 44th residue.
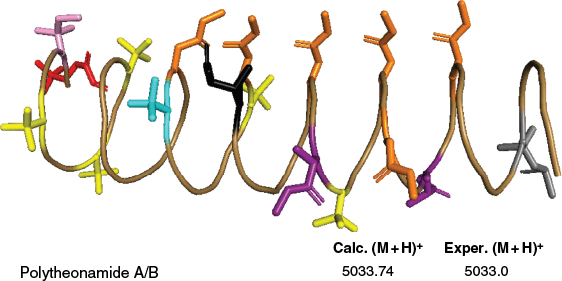
Polytheonamides A and B displayed potent cytotoxic activity against P388 murine leukemia cells with IC50 values of 78, 68 pg mL–1 respectively.43 The cytotoxicity of polytheonamide B is likely due to alternating D- and L-amino acid residues throughout the 48 amino acids, suggesting the formation of a β-helix similar to gramicidin channels. This facilitates polytheonamide B to act as a monovalent cation-selective channel.117 When polytheonamide B penetrates vectorially into the membrane, it forms a channel using a single molecule and remains in the membrane. This unique functional property may account for its specific cytotoxic activity.117
Lanthipeptides and lantibiotics
Lanthipeptides are a class of RiPPs characterised by thioether and methylene bridges, primarily lanthionine (Lan) and methyllanthionine (MeLan), which confer high thermal, chemical and proteolytic stability.61,118,119 These Lan and MeLan residues occur through a two-step PTM process that begins with the dehydration of serine and threonine residues, converting them into 2,3-didehydroalanine (Dha) and 2,3-didehydrobutyrine (Dhb) respectively. Subsequently, the thiol group of a cysteine residue performs a Michael-type addition to the Dha or Dhb, resulting in the formation of Lan or MeLan.61 Based on variations in the enzymes responsible for these modifications, lanthipeptides are currently classified into five distinct classes. In Class I lanthipeptides, the dehydration and cyclisation steps are catalysed by two separate enzymes: the dehydratase LanB and the zinc-dependent cyclase LanC. By contrast, Classes II, III and IV rely on multifunctional enzymes, LanM, LanKC and LanL respectively. More recently, a newly discovered lanthipeptide, lexapeptide, was shown to be synthesised by a three-enzyme system comprising LxmK, LxmX and LxmY, and has been designated as the prototype of the newly established Class V lanthipeptides.119 Lanthipeptides with antimicrobial activity are known as lantibiotics.61 Even though there are well-known lantibiotics such as nisin and subtilin, the only known lantibiotic from sponges is subtilomycin produced by Bacillus subtilis strain MMA7, originally isolated from the marine sponge Haliclona (Haliclona) simulans (order: Haplosclerida, family: Chalinidae).108 Moreover, metagenomic analysis of Red Sea sponge Hyrtios erectus (order: Dictyoceratida, family: Thorectidae) revealed numerous RiPP BCGs, including putative novel lanthipeptides.120 Along similar lines, 30 BGCs for lanthipeptide biosynthesis were identified in the genomes of 10 Streptomyces strains isolated from Antho (Antho) dichotoma (order: Poecilosclerida, family: Microcionidae).46 Of these, subtilomycin will be discussed below.
Subtilomycin is a class I lantibiotic produced by Bacillus subtilis strain MMA7, which was originally isolated from the marine sponge H. (H.) simulans. Subtilomycin features multiple thioether-bridge rings (lanthionine and methyllanthionine), similar to typical lantibiotics, which enhance its structural stability and function.108,121 The subtilomycin BCG, identified as the sub (or apn) cluster, encodes a set of enzymes required for lantibiotic maturation, export and immunity. These include the precursor peptide (apnA), modification enzymes (apnB and apnC), a dedicated transporter (apnT), a protease (apnP), a putative immunity gene (apnI) and others.122 The subtilomycin has been found in Bacillus strains associated with both marine sponges and plants, but is notably absent from many terrestrial B. subtilis strains.108,123
Although lantibiotics are typically produced by specific strains and show antibacterial properties primarily towards Gram-positive bacteria, there is growing evidence of variants with an extended spectrum of activity including Gram-negative bacteria. This is further supported by subtilomycin, which exhibits activity against both Gram-positive and Gram-negative pathogens, as well as several pathogenic Candida species. The activity of subtilomycin remains stable across a wide range of temperatures, including 100°C for 30 min and it also retains full activity over a broad pH range (2–8.5). Moreover, subtilomycin is not susceptible to proteolytic degradation, as demonstrated by resistance to proteinase K and α-chymotrypsin digestion.108
It is worth noting that subtilomycin isolated from B. subtilis strain BSn5, an endophyte of the plants Arabidopsis thaliana and Amorphophallus konjac, was shown to evade host plant immune responses by binding to its own flagellin. This interaction masks the flagellin epitope flg22, thereby preventing detection by the plant’s FLS2 receptor and downstream immune activation, facilitating endophytic colonisation.123 This discovery positions subtilomycin not only as an antimicrobial agent, but also as an immunomodulatory molecule, expanding the known functional landscape of lantibiotics.
Thiopeptides (YM 266183 and YM 266184)
Thiopeptides are structurally complex peptides defined by a central pyridine ring and a core macrocyclic ring, both of which are formed by an unusual enzymatic [4 + 2] cycloaddition on two Dha-containing fragments. A tail is linked to the macrocyclic ring by the central pyridine. The thiopeptide macrocycle is further characterised by the presence of multiple thiazole, oxazole and dehydroamino acid residues.119 Thiopeptides are also referred to as thiazolyl peptides or pyridinyl polythiazolyl peptides. Structurally, thiopeptides can be classified into five major series (a–e) based on the oxidation state and substitution pattern of their central nitrogen-containing heterocycle. Series ‘a’ features a fully saturated piperidine ring as the central core; series ‘b’ contains a dehydropiperidine ring; series ‘c’ includes a rare imidazopiperidine core; the most common class, series ‘d’, includes a trisubstituted pyridine ring as the central scaffold; and series ‘e’ features a pyridine ring distinguished by a hydroxyl group at the fifth position.61
Thiopeptides are well known for their antibacterial activity, particularly against Gram-positive bacteria, while typically exerting little to no effect on Gram-negative species.61 Notable thiopeptides originating from sponges include YM-266183 and YM-266184, produced by Bacillus cereus strain QN03323, which inhabits the marine sponge Halichondria (Halichondria) japonica (order: Suberitida, family: Halichondriidae) (Fig. 9). These compounds were identified through targeted screening for antibiotics active against drug-resistant bacteria, and their production was confirmed under controlled fermentation conditions. Antimicrobial activity testing revealed that both compounds are highly active against Gram-positive bacteria, including drug-resistant strains such as Methicillin-resistant Staphylococcus aureus, methicillin-resistant Streptococcus epidermidis and vancomycin-resistant enterococci, but showed no activity against Gram-negative bacteria, fungi or yeasts.109
Exemplified structures of YM 266183 and YM 266184 with their calculated and experimental masses.109

Despite their structural complexity, advances in bioinformatics and genome mining are uncovering new thiopeptide BCGs, including from underexplored sources such as marine sponges, highlighting their potential as scaffolds for next-generation antibiotics. One notable example is a thiopeptide BGC identified in the genomes of 10 Streptomyces strains isolated from the sponge A. (A.) dichotoma.46
Current tools and techniques for RiPP identification
The discovery of RiPPs has evolved significantly over the past few decades. Once limited to bioassay-guided compound isolation, RiPP discovery is now driven by genome mining, metagenomics and integrative -omics approaches.119,124 These advances have enabled access to the vast biosynthetic potential of both cultivable and uncultivable microbes, particularly those residing in marine sponges. Genome and metagenome mining offer alternatives for accessing uncultivated microbes, revealing diverse and novel microbial RiPP BGCs from otherwise inaccessible sources.114
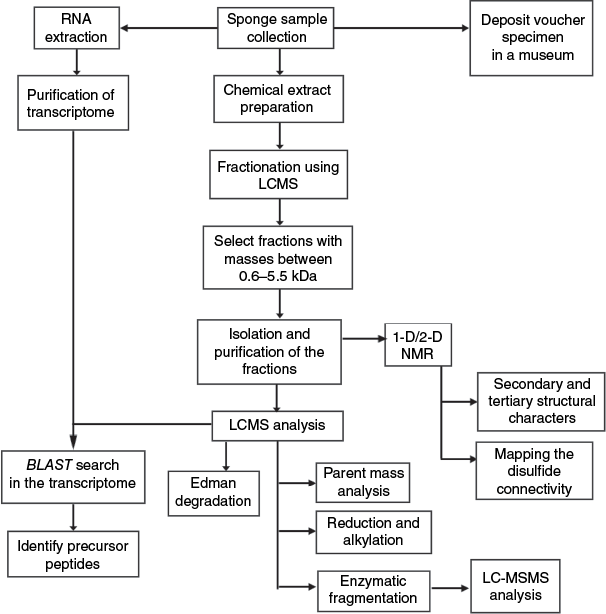
Recent research on the deep-sea sponge G. barretti has identified BGCs within the sponge genome itself, although such instances remain rare.28 The majority of BGCs associated with sponges are typically derived from their symbiotic microbial communities, indicating that these microbes are often the true producers of the diverse secondary metabolites traditionally attributed to sponges. Unlike bacteria, sponge genomes are large (58–492 Mb),28 highly repetitive and often poorly annotated. Therefore, for the discovery of RiPPs potentially synthesised by sponges themselves, transcriptomic and proteomic approaches may be more appropriate. The following sections will discuss peptidomic analysis, transcriptome mining, phylogenetic analysis and genomic analysis in the context of RiPP discovery. The general approach for the isolation and characterisation of RiPPs from marine sponges is depicted in Fig. 10.
Peptidomic and structural analysis
In this section, we highlight the general approaches used for RiPPs characterisation. Notably, a multidisciplinary approach, integrating advanced analytical platforms such as NMR spectroscopy, high-resolution mass spectrometry and comprehensive -omics data sets (as outlined in the following section), are required to unravel peptide sequence information, their structural complexity and PTMs. Typically, the freshly collected sponge specimen is preserved immediately either in ethanol or frozen (−20°C) until the chemical extraction is initiated. In our experience, a moderately hydrophilic solvent system, typically 60% methanol, is suitable for preparing an extract containing potential peptides or a ‘peptide extract’. In the literature, a plethora of techniques are used for peptide extraction: stepwise extraction using methanol, ethanol and acetone,69 methanol alone,71 dichloromethane:methanol (1:1 v/v),102 water,98,125 stepwise extraction using 60, 30 and 0% acetonitrile in water,77 have all been used for RiPP isolation. When methanol is used as the extraction medium, the mixture is typically partitioned between water and dichloromethane. The aqueous layer is then further extracted with n-BuOH and water, where the peptides preferentially partition into the organic layer.40,69 This peptide-enriched extract is then fractionated on C18 medium, typically using flash chromatography or MPLC, with acetonitrile–water or methanol–water as the elution medium, to generate fractions based on peptide polarity.40,69 Coupled MS analysis is essential for identifying and targeting fractions that contain peptide-like compounds during downstream isolation. Molecular masses exceeding 600 Da, with distinct ionisation and isotopic patterns corresponding to multiple charge states, may suggest that the compounds are likely of peptide origin. The presence of amide proton signals between 7 and 9 ppm in a 1-D 1H NMR spectrum further supports their identification as peptides. Once confirmed at the semi-fractionated stage, further isolation and purification of the peptides can be achieved using semi-preparative and analytical RP-HPLC.
Mass spectroscopic analysis followed by de novo sequencing of the purified compounds is essential to further validate their peptidic nature. Edman degradation, a stepwise chemical method to identify amino acids from the N-terminus of the peptide, has been frequently employed to sequence sponge RiPPs.69 In the case of asteropsins, the N-terminal pGlu residue impedes sequence analysis by this method, requiring pretreatment steps such as digestion with pyroglutamate aminopeptidase following reduction and alkylation.40 To confirm the presence of disulfides and facilitate sequencing, purified peptides are commonly reduced using dithiothreitol to open the disulfide bonds and then alkylated using iodoacetamide to protect the reactive cysteines.77 An increment of 57.02 Da per cysteine is indicative of intramolecular disulfide bond formation and can aid in determining the number of such bonds. The reduced and alkylated peptides are typically cleaved using enzymes, including trypsin, chymotrypsin or Glu-C endopeptidase, followed by MS/MS fragmentation to determine their amino acid sequence. In parallel, NMR analysis is employed for complete structural validation and to determine the three-dimensional structure of the purified peptides. Although the total number of cysteine residues can be readily determined as described above, elucidating their specific disulfide pairing presents an additional challenge. NMR can aid this process by identifying inter-cysteine proximities through Nuclear Overhauser Effect between cysteine beta protons (Hβ-NOEs).69 Furthermore, a strategy involving partial reduction of disulfide bonds followed by alkylation and subsequent secondary reduction with an orthogonal alkylating agent, has been employed to resolve the native disulfide connectivity.69
Chemical analysis, particularly solid-phase peptide synthesis, offers an alternative strategy to produce sufficient peptide quantities for bioactivity assays and functional studies, especially when natural yields are not sufficient.36,97,99 Nevertheless, the chemical synthesis and correct folding of complex peptides, particularly those containing disulfide bonds or unusual modifications, add additional layers of complexity. In particular, if an enzyme is responsible for site-specific modifications, replicating such modifications synthetically may require multiple orthogonal chemical reactions, which can be technically challenging. Additionally, determining the optimal oxidative folding conditions to achieve the correct native disulfide connectivity presents another significant hurdle. A notable example of a synthetic approach to circumvent low natural abundance was demonstrated by Steffen et al., who employed Fmoc-based solid-phase peptide synthesis to generate barrettides identified from transcriptomes, as the native peptides were not expressed in sufficient quantities for functional studies.36 The presence of correct disulfide connectivity in the oxidatively folded synthetic peptides was validated through co-elution studies with the native peptide. In other examples, to ensure proper folding into the native disulfide connectivity, regioselective disulfide bond formation is employed using selectively protected cysteine residues. In the case of neopetrosiamides,100 a stepwise disulfide bond formation protocol was designed to replicate the native fold, revealing inconsistencies with the initially proposed NMR structure. This synthesis-guided approach prompted a revision of the disulfide connectivity and ultimately unravelled an error in the original structural assignment.
Transcriptome mining
Despite peptidomic analyses suggesting that the isolated peptides are likely RiPPs, transcriptomic mining is essential to identify their true producer and to determine whether their biosynthesis is ribosomal or nonribosomal. Transcriptome mining has been employed as a tool to confirm the ribosomal origin of two categories of RiPPs: barrettides and PRMPs discussed in the present review.36,60 The following section outlines a widely adopted approach for transcriptome analysis.36 To achieve this, a freshly collected sample of the sponge’s choanosome (inner part) is immediately preserved in RNAlater stabilisation solution to maintain RNA integrity. High-quality total RNA is extracted, purified and sequenced. The transcriptome is then assembled and sometimes annotated. A local BLAST database is constructed using the assembled transcriptome sequences. Short amino acid fragments (~10–15 residues) derived from the MS/MS fragmentation of the purified peptides are employed as queries in tblastn searches, conducted, for example, using the Unipro UGENE software package (ver. 52.1, see https://ugene.net/).126 Candidate hits exhibiting high sequence identity (typically more than 80%) are translated into protein sequences. Sequences that match the experimentally observed molecular weights or conserved cysteine framework are retained for further analysis. Additionally, signal peptides are predicted using SignalP (ver. 6.0, see https://github.com/fteufel/signalp-6.0)127 to assess secretion potential. Taxonomic classification tools such as Kraken2 (ver. 2.1.6, see https://github.com/DerrickWood/kraken2)128 or BLAST (ver. 1.4.0, see https://blast.ncbi.nlm.nih.gov/)129 are employed to determine whether the transcripts originate from the sponge or the microbial symbionts. Where possible, surrounding transcripts or annotations could also be inspected for genes encoding biosynthetic enzymes or RiPP pathways, and predicted structures may be analysed using tools like AlphaFold (ver. 2.3.2, see https://deepmind.google/science/alphafold/)130 to further support candidate identification.
In other examples, transcriptomic analysis has investigated the innate immune responses of sponges, including Aplysina aerophoba (order: Verongiida, family: Aplysinidae) and Dysidea avara (order: Dictyoceratida, family: Dysideidae), following exposure to microbial-associated molecular patterns such as lipopolysaccharides and peptidoglycans.131 Although the primary focus was on immune gene expression, particularly identifying pattern recognition receptors such as nucleotide-binding domain and leucine-rich repeat-containing receptors (NLRs) and scavenger receptor cysteine-rich domain-containing proteins (SRCRs), the use of transcriptomic profiling enabled a broader survey of gene regulation, including genes potentially involved in defence-related biosynthetic pathways. This study highlights the potential for discovering genes linked to RiPPs that may play a host defence role in the sponges. Immune-related transcripts have also been discovered in a number of other sponge species.132 Given the structural similarity of these receptors to those in other metazoans known to bind microbial peptides, it is plausible that sponge receptors may also bind peptide-based molecules, including bacterial toxins, quorum-sensing peptides or host-derived signalling peptides. High-resolution transcriptomic datasets can thus be mined for hallmark RiPP biosynthetic elements, including precursor peptide-encoding genes and associated tailoring enzymes. These datasets provide a foundational resource for future efforts to uncover and functionally characterise RiPP biosynthetic potential in marine sponges, particularly in the context of host–microbe interactions.
Phylogenetic analysis
In the field of natural products, phylogenetic analysis enables the investigation of the evolutionary history and classification of BGCs, facilitating the discovery of novel secondary metabolites and their functional annotation across diverse lineages. Another approach is to construct phylogenetic trees of different species (using ribosomal genes or other common gene markers), including species already known for producing secondary metabolites of interest, to predict the presence of unknown RiPPs in phylogenetically close species. This strategy enables identification of prolific metabolite-producing lineages, which can then be prioritised for targeted isolation, sampling and genome mining.124 For instance, the distribution of RiPPs among mesophotic sponge-associated bacterial Metagenome-Assembled Genomes (MAGs) revealed a higher prevalence of RiPPs in Nitrospirota, Acidobacteriota, Proteobacteria, Poribacteria and Gemmatimonadota.114 This uneven taxonomic distribution implies a role for RiPPs in enhancing host microbial fitness and provides direction for antibiotic discovery efforts. Furthermore, distinct microbial compositions were observed between sponge species; for example, Chloroflexota was predominantly associated with Melophlus (order: Tetractinellida, family: Geodiidae), Terpios (order: Suberitida, family: Suberitidae) and Xestospongia (order: Haplosclerida, family: Petrosiidae), whereas Actinobacteriota were common in Axinella and Rhabderemia (order: Biemnida, family: Rhabderemiidae) and Terpios.114 As all samples were collected from the same geographical region, these patterns are likely driven by host specificity and ecological interactions rather than environmental variables. Nevertheless, interpretations must be approached cautiously due to the influence of horizontal gene transfer (HGT), which plays a critical role in shaping the evolution of secondary metabolite biosynthesis. As an example, a proteusin-like RiPP GCF, FAM_01143, was identified across different MAGs within single sponge samples, indicating potential horizontal gene transfer or lineage-specific diversification.114 Another aspect is comparative studies of BGCs from conspecific organisms sampled across different geographical locations. These comparisons are useful to uncover strain-level or location-dependent variations in BGC architecture.124
A second phylogenetic strategy involves constructing gene trees for secondary metabolite biosynthetic genes directly. These gene trees trace the evolutionary history of individual biosynthetic genes and gene clusters, often yielding more precise functional predictions than sequence similarity-based approaches alone.124 As an example, gene cluster families (GCFs) FAM_01149 and FAM_01143 found in mesophotic sponge-associated bacteria formed a distinct proteusin family cluster. Proteusin BGCs are characterised by conserved core genes, two transporter genes, a YcaO cyclodehydratase and a precursor gene. Despite these conserved features, the structural diversity of the final products and the presence of flanking genes with uncharacterised functions suggest a rich reservoir of unexplored chemical diversity.114
The general workflow for phylogenetic analysis begins with the selection of homologous sequences sharing common ancestry to ensure meaningful resolution, often incorporating outgroup sequences to root the tree or using midpoint rooting when necessary.133 These sequences are then aligned using tools such as MAFFT (ver. 7.526, see https://mafft.cbrc.jp/alignment/software/),134 ClustalW (see https://www.genome.jp/tools-bin/clustalw)135 or MUSCLE (ver. 5, see http://www.drive5.com/muscle/),136 followed by manual editing to remove artefacts and standardise length using software like BioEdit (ver. 7.7, see https://bioedit.software.informer.com/)137 or Mesquite (ver. 4.01, see http://www.mesquiteproject.org). Then an appropriate evolutionary model, such as GTR or JTT, is selected.138 Phylogenetic trees are subsequently constructed using methods like Maximum Likelihood (ML), Bayesian Inference, or Neighbour-Joining (NJ), employing software such as RAxML (ver. 8.2.12, see https://github.com/stamatak/standard-RAxML),139 MrBayes (ver. 3.2.7, see https://github.com/NBISweden/MrBayes/)140,141 or PhyML (ver. 3.3, see http://www.atgc-montpellier.fr/phyml/).142 Finally, tree robustness is evaluated through bootstrap analysis or Bayesian posterior probabilities.138
Genomic and metagenomic analysis
Genome mining involves analysing the complete genome of a single organism to identify BGCs of natural products, and their possible functional and chemical interactions.143 By contrast, metagenomic analysis examines the collective DNA from an organism and its associated organisms, also called the holobiont, including uncultured microbes, to discover BGCs directly from environmental samples.144
Genomic and metagenomic analyses of RiPPs begin with collecting the specimen, followed by high-quality DNA extraction and purification. The DNA is then quantified and assessed for integrity before preparing shotgun or long-read sequencing libraries, using platforms like Illumina, PacBio, or Oxford Nanopore.145 After sequencing, metagenomic reads are assembled into contigs or MAGs using tools like MEGAHIT (ver. 1.2.9, see https://github.com/voutcn/megahit)146 or metaSPAdes (ver. 4.2.0, see https://github.com/ablab/spades).147 BGCs encoding RiPPs are identified based on conserved motifs and domain structures using specialised tools such as antiSMASH (ver. 8.0.4, see https://github.com/antismash/antismash),148 BAGEL (see https://github.com/ByteDance-Seed/Bagel),149 DeepRiPP (see http://deepripp.magarveylab.ca/),150 PRISM (ver. 9.0.537, see https://github.com/PrismLibrary/Prism)151 and RiPPER (see https://github.com/streptomyces/ripper).152,153 These techniques provide insights into the metabolic logic of novel RiPP families by predicting precursor peptides, transporters and modifying enzymes (such as cyclodehydratases, oxidases and methyltransferases).148,151,153 Importantly, even in extremely fragmented or low-coverage datasets, the accuracy of RiPP precursor prediction has been greatly increased by using deep learning-based algorithms, including NeuRiPP (see https://github.com/emzodls/neuripp) and DeepRiPP.150,154 Structure prediction algorithms built into these platforms enable the inference of potential chemical scaffolds in addition to gene cluster identification, which helps prioritise high-value candidates for total chemical synthesis or downstream synthetic biology.151 Moreover, assembly and binning techniques such as MAGs help assign RiPP BGCs to specific microbial taxa within the sponge microbiome, offering clues on the ecological roles and evolutionary relationships of RiPP producers.155,156 One such study investigated BGCs in 10 phylogenetically diverse Streptomyces strains isolated from the marine sponge A. (A.) dichotoma, collected at the bottom of the Trondheim Fjord (Norway). An important observation was the evidence for horizontal gene transfer of BGCs encoding RiPP classes, especially lantibiotics and thiopeptides, among the isolates. This highlights the importance of comparative genomic analysis in accurately identifying the true producer of secondary metabolites.46
Another study revealed the widespread conservation of a BGC encoding proteusins, across phylogenetically and geographically dispersed sponge species from both the Pacific and Atlantic oceans.157 This BGC was identified in the microbiome of three sponge species from different orders and families collected from a single location (Florida Keys, FL, USA): Verongula sp. (order: Verongiida, family: Aplysinidae), Aiolochroia sp. (order: Verongiida, family: Aplysinidae), and Aplysinella sp. (order: Verongiida, family: Aplysinellidae). Furthermore, the same BGC was found in the microbiomes of Aiolochroia sp. and Pseudoceratina sp. (order: Verongiida, family: Pseudoceratinidae) but not Verongula sp. when collected from a different location (Puerto Rico). The gene cluster was also detected in Ircinia sp. (order: Dictyoceratida, family: Irciniidae) from the Indo-Pacific region. These results demonstrate the broad conservation of cryptic BGCs across diverse sponge microbiomes, which are often missed by traditional natural product screening approaches. Additionally, metagenomic analysis of three sponge species with high microbial abundance, A. aerophoba (order: Verongiida, family: Aplysinidae), G. barretti and Petrosia (Petrosia) ficiformis (order: Haplosclerida, family: Petrosiidae) revealed numerous uncharacterised BGC families potentially involved in RiPP biosynthesis.158 Some of these gene clusters were conserved among all three species, suggesting common biosynthetic capabilities across phylogenetically distinct hosts.158 This taxonomic resolution is critical for designing targeted isolation strategies or for expressing RiPP pathways heterologously in suitable host organisms.
Although genome mining provides a powerful means to predict novel RiPP BGCs, experimental validation is essential to confirm the production, structure and bioactivity of the encoded compounds. In many cases, particularly with sponge-associated microbiomes where the native hosts are unculturable or slow-growing, heterologous expression has emerged as a key strategy for functional verification.
Heterologous expression involves cloning the predicted BGC into a model microbial host (e.g. E. coli or Streptomyces coelicolor M145) capable of supporting the necessary PTM machinery.159 Advanced synthetic biology tools, including codon optimisation and inducible promoter systems, are often used to ensure proper transcription, translation and modification of the RiPP precursor peptide.160
In parallel, metabolomics approaches, including comparative metabolite profiling and molecular networking using Global Natural Products Social (GNPS) molecular networking, can be applied to sponge tissue or microbial extracts to detect RiPP products that match genome-predicted structures.161 This non-targeted analysis helps to confirm natural production in the native environment and guides isolation for full structural elucidation.
Overall, metagenomic sequencing, when paired with sophisticated annotation pipelines and integrative analysis, holds tremendous promise for expanding the chemical diversity of RiPPs in marine sponges and understanding their functional roles in host-microbe interactions.
Future perspectives
To date, RiPPs have been identified exclusively within the Demospongiae class of marine sponges. No such reports exist for the other three sponge classes (Hexactinellida, Calcarea and Homoscleromorpha), highlighting a significant knowledge gap that warrants further investigation. Targeting these underexplored classes in future studies could potentially unveil novel RiPPs with unique properties. Another promising strategy involves examining phylogenetically related sponge families, especially those closely aligned with known peptide-producing species. For instance, considering the eight promising RiPPs isolated from Asteropus, other Ancorinidae genera such as Stelletta, Ancorina and especially Stryphnus, all of which are phylogenetically close to Asteropus,162 should be explored for similar ICK RiPPs. Altogether, expanding the search across broader taxonomic groups and ecological niches holds great promise for uncovering novel bioactive RiPPs with diverse structures and functions.
The revision of asteropine and asteropsins vouchers illustrates once more the critical importance of properly identifying the sponge material163 and depositing vouchers in sponge natural product studies.164 Accessible vouchers ensure that species identifications can be revised by an expert, thus increasing the reproducibility and the validity of the study. We have listed in Table 1 the known vouchers of the sponge peptide articles listed in this review. Species misidentification remains a major issue in sponge-derived natural product research. In one of our previous studies on isomalabaricane triterpenes, nearly 50% of voucher specimens were misidentified, and ~66% of studies used incorrect species names.164 These errors hinder reproducibility and the interpretation of bioactivity data. Accurate taxonomic identification and proper voucher deposition are essential in the search for new marine sponge RiPPs.
Another key future direction is the systematic mining of existing sponge transcriptomes for RiPP-encoding genes, which may contribute to fundamental aspects of metazoan biology. Sponges across all four classes, Demospongiae, Calcarea, Homoscleromorpha and Hexactinellida, exhibit remarkably diverse and complex gene repertoires involved in processes including development, signalling, immunity, apoptosis, cytoskeletal and structural organisation and cell adhesion,165 providing a rich genomic context in which RiPP biosynthetic potential may reside. Taking a step further, candidate RiPPs identified through such transcriptomic surveys can be chemically synthesised and subjected to functional validation. These efforts are expected to uncover lineage-specific RiPPs unique to marine sponges, providing new insights into their chemical ecology and the molecular basis of their interactions with symbiotic microbes and environmental cues.
In parallel, deepening our understanding of the biosynthetic logic behind sponge-derived RiPPs remains an essential goal. Structural modification observed in RiPPs could potentially suggest the presence of novel and uncharacterised PTM enzymes. BGCs associated with sponge RiPPs remain largely unexplored, in part due to the genomic complexity of the holobionts and challenges in assigning gene products to either the host or microsymbionts.45,166 Although the integration of long-read metagenomics, single-cell sequencing and gene cluster reconstruction may significantly enhance our ability to resolve RiPP biosynthetic pathways and assign them to specific holobiont members, unambiguous producer identification may still require complementary approaches.
Data availability
Data sharing is not applicable as no new data were generated or analysed during this study.
Declaration of funding
The research was funded by the Swedish Research Council under an international collaborative linkage grant awarded to S. Gunasekera as the principal investigator, C. Hettiarachchi as the international partner and P. Cárdenas as the collaborating scientist (award number: 2017-05416).
Declaration of use of AI
OpenAI was used to help create Fig. 1 and the image used for the online table of contents.
References
1 Feuda R, Dohrmann M, Pett W, Philippe H, Rota-Stabelli O, Lartillot N, et al. Improved modeling of compositional heterogeneity supports sponges as sister to all other animals. Curr Biol 2017; 27: 3864-3870.
| Crossref | Google Scholar | PubMed |
2 Nielsen C. Early animal evolution: a morphologist’s view. R Soc Open Sci 2019; 6: 190638.
| Crossref | Google Scholar | PubMed |
3 Simion P, Philippe H, Baurain D, Jager M, Richter DJ, Di Franco A, et al. A large and consistent phylogenomic dataset supports sponges as the sister group to all other animals. Curr Biol 2017; 27: 958-967.
| Crossref | Google Scholar | PubMed |
4 de Voogd N, Alvarez B, Boury-Esnault N, Cárdenas P, Díaz M, Dohrmann M, et al. The World Porifera database. 2025. Available at https://www.marinespecies.org/porifera/index.php
5 Van Soest RW, Boury-Esnault N, Vacelet J, Dohrmann M, Erpenbeck D, De Voogd NJ, et al. Global diversity of sponges (Porifera). PLoS ONE 2012; 7: e35105.
| Crossref | Google Scholar | PubMed |
6 Webster NS, Taylor MW. Marine sponges and their microbial symbionts: love and other relationships. Environ Microbiol 2012; 14: 335-346.
| Crossref | Google Scholar | PubMed |
7 Pita L, Rix L, Slaby BM, Franke A, Hentschel U. The sponge holobiont in a changing ocean: from microbes to ecosystems. Microbiome 2018; 6: 46.
| Crossref | Google Scholar | PubMed |
9 Cheng M-M, Tang X-L, Sun Y-T, Song D-Y, Cheng Y-J, Liu H, et al. Biological and chemical diversity of marine sponge-derived microorganisms over the last two decades from 1998 to 2017. Molecules 2020; 25: 853.
| Crossref | Google Scholar | PubMed |
11 Hong L-L, Ding Y-F, Zhang W, Lin H-W. Chemical and biological diversity of new natural products from marine sponges: a review (2009–2018). Mar Life Sci Tech 2022; 4: 356-372.
| Crossref | Google Scholar | PubMed |
12 Nadar VM, Manivannan S, Chinnaiyan R, Govarthanan M, Ponnuchamy K. Review on marine sponge alkaloid, aaptamine: a potential antibacterial and anticancer drug. Chem Biol Drug Des 2022; 99: 103-110.
| Crossref | Google Scholar | PubMed |
13 Pokharkar O, Lakshmanan H, Zyryanov G, Tsurkan M. In silico evaluation of antifungal compounds from marine sponges against COVID-19-associated mucormycosis. Mar Drugs 2022; 20: 215.
| Crossref | Google Scholar | PubMed |
14 Varijakzhan D, Loh J-Y, Yap W-S, Yusoff K, Seboussi R, Lim S-HE, et al. Bioactive compounds from marine sponges: Fundamentals and applications. Mar Drugs 2021; 19: 246.
| Crossref | Google Scholar | PubMed |
15 Vitali A. Antimicrobial peptides derived from marine sponges. Am J Clin Microbiol Antimicrob 2018; 1: 1006.
| Google Scholar |
16 Ruocco N, Costantini S, Palumbo F, Costantini M. Marine sponges and bacteria as challenging sources of enzyme inhibitors for pharmacological applications. Mar Drugs 2017; 15: 173.
| Crossref | Google Scholar | PubMed |
17 Sánchez-Lozano I, Hernández-Guerrero CJ, Muñoz-Ochoa M, Hellio C. Biomimetic approaches for the development of new antifouling solutions: study of incorporation of macroalgae and sponge extracts for the development of new environmentally friendly coatings. Int J Mol Sci 2019; 20: 4863.
| Crossref | Google Scholar | PubMed |
18 Tian T, Takada K, Ise Y, Ohtsuka S, Okada S, Matsunaga S. Microsclerodermins N and O, cytotoxic cyclic peptides containing a p-ethoxyphenyl moiety from a deep-sea marine sponge Pachastrella sp. Tetrahedron 2020; 76: 130997.
| Crossref | Google Scholar |
19 Singh A, Thakur NL. Significance of investigating allelopathic interactions of marine organisms in the discovery and development of cytotoxic compounds. Chem Biol Interact 2016; 243: 135-147.
| Crossref | Google Scholar | PubMed |
20 Li Q, Wang X, Korzhev M, Schröder HC, Link T, Tahir MN, et al. Potential biological role of laccase from the sponge Suberites domuncula as an antibacterial defense component. Biochim Biophys Acta 2015; 1850: 118-128.
| Crossref | Google Scholar | PubMed |
21 Mayer AM, Glaser KB, Cuevas C, Jacobs RS, Kem W, Little RD, et al. The odyssey of marine pharmaceuticals: a current pipeline perspective. Trends Pharm Sci 2010; 31: 255-265.
| Crossref | Google Scholar | PubMed |
22 Agrawal S, Acharya D, Adholeya A, Barrow CJ, Deshmukh SK. Nonribosomal peptides from marine microbes and their antimicrobial and anticancer potential. Front Pharmacol 2017; 8: 828.
| Crossref | Google Scholar | PubMed |
23 Bharathi D, Lee J. Recent advances in marine-derived compounds as potent antibacterial and antifungal agents: a comprehensive review. Mar Drugs 2024; 22: 348.
| Crossref | Google Scholar | PubMed |
24 Ribeiro R, Pinto E, Fernandes C, Sousa E. Marine cyclic peptides: antimicrobial activity and synthetic strategies. Mar Drugs 2022; 20: 397.
| Crossref | Google Scholar | PubMed |
25 Kang HK, Choi M-C, Seo CH, Park Y. Therapeutic properties and biological benefits of marine-derived anticancer peptides. Int J Mol Sci 2018; 19: 919.
| Crossref | Google Scholar | PubMed |
26 Nazarian M, Hosseini SJ, Nabipour I, Mohebbi G. Marine bioactive peptides with anti-cancer potential. Iran South Med J 2015; 18: 607-629.
| Google Scholar |
27 McKenna V, Archibald JM, Beinart R, Dawson MN, Hentschel U, Keeling PJ, et al. The aquatic symbiosis genomics project: probing the evolution of symbiosis across the tree of life. Wellcome Open Res 2021; 6: 254.
| Crossref | Google Scholar | PubMed |
28 Steffen K, Proux-Wéra E, Soler L, Churcher A, Sundh J, Cárdenas P. Whole genome sequence of the deep-sea sponge Geodia barretti (Metazoa, Porifera, Demospongiae). G3 2023; 13: jkad192.
| Crossref | Google Scholar | PubMed |
29 Macedo MWFS, Cunha NB, Carneiro JA, Costa RA, Alencar SA, Cardoso MH, et al. Marine organisms as a rich source of biologically active peptides. Front Mar Sci 2021; 8: 667764.
| Crossref | Google Scholar |
30 Urda C, Perez M, Rodriguez J, Jimenez C, Cuevas C, Fernandez R. Pembamide, a N-methylated linear peptide from a sponge Cribrochalina sp. Tetrahedron Lett 2016; 57: 3239-3242.
| Crossref | Google Scholar |
31 Afifi AH, El-Desoky AH, Kato H, Mangindaan RE, de Voogd NJ, Ammar NM, et al. Carteritins A and B, cyclic heptapeptides from the marine sponge Stylissa carteri. Tetrahedron Lett 2016; 57: 1285-1288.
| Crossref | Google Scholar |
32 Tan KC, Wakimoto T, Abe I. Sulfoureido lipopeptides from the marine sponge Discodermia kiiensis. J Nat Prod 2016; 79: 2418-2422.
| Crossref | Google Scholar | PubMed |
33 Coello L, Reyes F, Martín MJ, Cuevas C, Fernández R. Isolation and structures of pipecolidepsins A and B, cytotoxic cyclic depsipeptides from the madagascan sponge Homophymia lamellosa. J Nat Prod 2014; 77: 298-303.
| Crossref | Google Scholar | PubMed |
34 Fernández R, Bayu A, Aryono Hadi T, Bueno S, Pérez M, Cuevas C, et al. Unique polyhalogenated peptides from the marine sponge Ircinia sp. Mar Drugs 2020; 18: 396.
| Crossref | Google Scholar | PubMed |
35 Molinski TF. Cyclic azole-homologated peptides from marine sponges. Org Biomol Chem 2018; 16: 21-29.
| Crossref | Google Scholar |
36 Steffen K, Laborde Q, Gunasekera S, Payne CD, Rosengren KJ, Riesgo A, et al. Barrettides: a peptide family specifically produced by the deep-sea sponge Geodia barretti. J Nat Prod 2021; 84: 3138-3146.
| Crossref | Google Scholar | PubMed |
37 Mokhlesi A, Stuhldreier F, Wex KW, Berscheid A, Hartmann R, Rehberg N, et al. Cyclic cystine-bridged peptides from the marine sponge Clathria basilana induce apoptosis in tumor cells and depolarize the bacterial cytoplasmic membrane. J Nat Prod 2017; 80: 2941-2952.
| Crossref | Google Scholar | PubMed |
38 Ko S-C, Jang J, Ye B-R, Kim M-S, Choi I-W, Park W-S, et al. Purification and molecular docking study of angiotensin I-converting enzyme (ACE) inhibitory peptides from hydrolysates of marine sponge Stylotella aurantium. Process Biochem 2017; 54: 180-187.
| Crossref | Google Scholar |
39 Kita M, Gise B, Kawamura A, Kigoshi H. Stylissatin A, a cyclic peptide that inhibits nitric oxide production from the marine sponge Stylissa massa. Tetrahedron Lett 2013; 54: 6826-6828.
| Crossref | Google Scholar |
40 Li H, Bowling JJ, Fronczek FR, Hong J, Jabba SV, Murray TF, et al. Asteropsin A: an unusual cystine-crosslinked peptide from porifera enhances neuronal Ca2+ influx. Biochim Biophys Acta 2013; 1830: 2591-2599.
| Crossref | Google Scholar | PubMed |
41 Pozzolini M, Millo E, Oliveri C, Mirata S, Salis A, Damonte G, et al. Elicited ROS scavenging activity, photoprotective, and wound-healing properties of collagen-derived peptides from the marine sponge Chondrosia reniformis. Mar Drugs 2018; 16: 465.
| Crossref | Google Scholar | PubMed |
42 Taylor MW, Radax R, Steger D, Wagner M. Sponge-associated microorganisms: evolution, ecology, and biotechnological potential. Microbiol Mol Biol Rev 2007; 71: 295-347.
| Crossref | Google Scholar | PubMed |
43 Hamada T, Matsunaga S, Yano G, Fusetani N. Polytheonamides A and B, highly cytotoxic, linear polypeptides with unprecedented structural features, from the marine sponge, Theonella swinhoei. J Am Chem Soc 2004; 127: 110-118.
| Crossref | Google Scholar |
44 Siegl A, Hentschel U. PKS and NRPS gene clusters from microbial symbiont cells of marine sponges by whole genome amplification. Environ Microbiol Rep 2010; 2: 507-513.
| Crossref | Google Scholar | PubMed |
45 Storey MA, Andreassend SK, Bracegirdle J, Brown A, Keyzers RA, Ackerley DF, et al. Metagenomic exploration of the marine sponge Mycale hentscheli uncovers multiple polyketide-producing bacterial symbionts. MBio 2020; 11: 10.1128/mbio.02997-19.
| Crossref | Google Scholar | PubMed |
46 Guerrero-Garzón JF, Zehl M, Schneider O, Rückert C, Busche T, Kalinowski J, et al. Streptomyces spp. from the marine sponge Antho dichotoma: analyses of secondary metabolite biosynthesis gene clusters and some of their products. Front Microbiol 2020; 11: 437.
| Crossref | Google Scholar | PubMed |
47 Oves-Costales D, Sánchez-Hidalgo M, Martín J, Genilloud O. Identification, cloning and heterologous expression of the gene cluster directing RES-701-3,-4 lasso peptides biosynthesis from a marine Streptomyces strain. Mar Drugs 2020; 18: 238.
| Crossref | Google Scholar | PubMed |
48 Miranda KJ, Jaber S, Atoum D, Arjunan S, Ebel R, Jaspars M, et al. Pseudomonassin, a new bioactive ribosomally synthesised and post-translationally modified peptide from Pseudomonas sp. SST3. Microorganisms 2023; 11: 2563.
| Crossref | Google Scholar | PubMed |
49 Scholz R, Molohon KJ, Nachtigall J, Vater J, Markley AL, Süssmuth RD, et al. Plantazolicin, a novel microcin B17/streptolysin S-like natural product from Bacillus amyloliquefaciens FZB42. J Bacteriol 2011; 193: 215-224.
| Crossref | Google Scholar | PubMed |
50 Yi Y, Liang L, de Jong A, Kuipers OP. A systematic comparison of natural product potential, with an emphasis on RiPPs, by mining of bacteria of three large ecosystems. Genomics 2024; 116: 110880.
| Crossref | Google Scholar | PubMed |
51 Bibi F, Faheem M, Azhar EI, Yasir M, Alvi SA, Kamal M, et al. Bacteria from marine sponges: a source of new drugs. Curr Drug Metab 2017; 18: 11-15.
| Crossref | Google Scholar | PubMed |
52 Rubin GM, Ding Y. Recent advances in the biosynthesis of RiPPs from multicore-containing precursor peptides. J Ind Microbiol Biotechnol 2020; 47: 659-674.
| Crossref | Google Scholar | PubMed |
53 Sukmarini L. Marine bacterial ribosomal peptides: recent genomics- and synthetic biology-based discoveries and biosynthetic studies. Mar Drugs 2022; 20: 544.
| Crossref | Google Scholar | PubMed |
54 Welker M, von Döhren H. Cyanobacterial peptides – nature’s own combinatorial biosynthesis. FEMS Microbiol Rev 2006; 30: 530-563.
| Crossref | Google Scholar | PubMed |
55 Fidor A, Konkel R, Mazur-Marzec H. Bioactive peptides produced by cyanobacteria of the genus Nostoc: a review. Mar Drugs 2019; 17: 561.
| Crossref | Google Scholar | PubMed |
56 Sivonen K, Leikoski N, Fewer DP, Jokela J. Cyanobactins – ribosomal cyclic peptides produced by cyanobacteria. Appl Microbiol Biotechnol 2010; 86: 1213-1225.
| Crossref | Google Scholar | PubMed |
57 Fusetani N, Sugawara T, Matsunaga S, Hirota H. Orbiculamide A: a novel cytotoxic cyclic peptide from a marine sponge Theonella sp. J Am Chem Soc 1991; 113: 7811-7812.
| Crossref | Google Scholar |
58 Araki T, Matsunaga S, Nakao Y, Furihata K, West L, Faulkner DJ, et al. Koshikamide B, a cytotoxic peptide lactone from a marine sponge Theonella sp. J Org Chem 2008; 73: 7889-7894.
| Crossref | Google Scholar | PubMed |
60 Lin Z, Agarwal V, Cong Y, Pomponi SA, Schmidt EW. Short macrocyclic peptides in sponge genomes. Proc Natl Acad Sci 2024; 121: e2314383121.
| Crossref | Google Scholar | PubMed |
61 Arnison PG, Bibb MJ, Bierbaum G, Bowers AA, Bugni TS, Bulaj G, et al. Ribosomally synthesized and post-translationally modified peptide natural products: overview and recommendations for a universal nomenclature. Nat Prod Rep 2013; 30: 108-160.
| Crossref | Google Scholar | PubMed |
62 Dong S-H, Liu A, Mahanta N, Mitchell DA, Nair SK. Mechanistic basis for ribosomal peptide backbone modifications. ACS Cent Sci 2019; 5: 842-851.
| Crossref | Google Scholar | PubMed |
63 Kessler SC, Chooi Y-H. Out for a RiPP: challenges and advances in genome mining of ribosomal peptides from fungi. Nat Prod Rep 2022; 39: 222-230.
| Crossref | Google Scholar | PubMed |
64 McIntosh JA, Donia MS, Schmidt EW. Ribosomal peptide natural products: bridging the ribosomal and nonribosomal worlds. Nat Prod Rep 2009; 26: 537-559.
| Crossref | Google Scholar | PubMed |
65 Montalbán-López M, Scott TA, Ramesh S, Rahman IR, van Heel AJ, Viel JH, et al. New developments in RiPP discovery, enzymology and engineering. Nat Prod Rep 2021; 38: 130-239.
| Crossref | Google Scholar | PubMed |
66 Walton JD, Hallen-Adams HE, Luo H. Ribosomal biosynthesis of the cyclic peptide toxins of Amanita mushrooms. Biopolymers 2010; 94: 659-664.
| Crossref | Google Scholar | PubMed |
67 Cheng Y-Q, Yang M, Matter AM. Characterization of a gene cluster responsible for the biosynthesis of anticancer agent FK228 in Chromobacterium violaceum No. 968. Appl Environ Microbiol 2007; 73: 3460-3469.
| Crossref | Google Scholar | PubMed |
68 Doi T, Iijima Y, Shin-Ya K, Ganesan A, Takahashi T. A total synthesis of spiruchostatin A. Tetrahedron Lett 2006; 47: 1177-1180.
| Crossref | Google Scholar |
69 Takada K, Hamada T, Hirota H, Nakao Y, Matsunaga S, van Soest RW, et al. Asteropine A, a sialidase-inhibiting conotoxin-like peptide from the marine sponge Asteropus simplex. Chem Biol 2006; 13: 569-574.
| Crossref | Google Scholar | PubMed |
70 Li H, Bowling JJ, Su M, Hong J, Lee B-J, Hamann MT, et al. Asteropsins B–D, sponge-derived knottins with potential utility as a novel scaffold for oral peptide drugs. Biochim Biophys Acta 2014; 1840: 977-984.
| Crossref | Google Scholar | PubMed |
71 Li H, Su M, Hamann MT, Bowling JJ, Kim HS, Jung JH. Solution structure of a sponge-derived cystine knot peptide and its notable stability. J Nat Prod 2014; 77: 304-310.
| Crossref | Google Scholar | PubMed |
72 Su M, Li H, Wang H, Kim EL, Kim HS, Kim E-H, et al. Stable and biocompatible cystine knot peptides from the marine sponge Asteropus sp. Bioorg Med Chem 2016; 24: 2979-2987.
| Crossref | Google Scholar | PubMed |
73 Freeman MF, Gurgui C, Helf MJ, Morinaka BI, Uria AR, Oldham NJ, et al. Metagenome mining reveals polytheonamides as posttranslationally modified ribosomal peptides. Science 2012; 338: 387-390.
| Crossref | Google Scholar | PubMed |
74 Li H, Dang HT, Li J, Sim CJ, Hong J, Kim DK, et al. Pyroglutamyl dipeptides and tetrahydro-β-carboline alkaloids from a marine sponge Asteropus sp. Biochem Syst Ecol 2010; 38: 1049-1051.
| Crossref | Google Scholar |
75 Getz JA, Rice JJ, Daugherty PS. Protease-resistant peptide ligands from a knottin scaffold library. ACS Chem Biol 2011; 6: 837-844.
| Crossref | Google Scholar | PubMed |
76 Wong CT, Rowlands DK, Wong CH, Lo TW, Nguyen GK, Li HY, et al. Orally active peptidic bradykinin B1 receptor antagonists engineered from a cyclotide scaffold for inflammatory pain treatment. Angew Chem Int Ed 2012; 51: 5620-5624.
| Crossref | Google Scholar | PubMed |
77 Carstens BB, Rosengren KJ, Gunasekera S, Schempp S, Bohlin L, Dahlström M, et al. Isolation, characterization, and synthesis of the barrettides: disulfide-containing peptides from the marine sponge Geodia barretti. J Nat Prod 2015; 78: 1886-1893.
| Crossref | Google Scholar | PubMed |
78 Sarkar N. Polyadenylation of mRNA in prokaryotes. Annu Rev Biochem 1997; 66: 173-197.
| Crossref | Google Scholar | PubMed |
79 Hausner G, Hafez M, Edgell DR. Bacterial group I introns: mobile RNA catalysts. Mob DNA 2014; 5: 8.
| Crossref | Google Scholar | PubMed |
81 Steffen K, Indraningrat AAG, Erngren I, Haglöf J, Becking LE, Smidt H, et al. Oceanographic setting influences the prokaryotic community and metabolome in deep-sea sponges. Sci Rep 2022; 12: 3356.
| Crossref | Google Scholar | PubMed |
82 Champ MA. Economic and environmental impacts on ports and harbors from the convention to ban harmful marine anti-fouling systems. Mar Pollut Bull 2003; 46: 935-940.
| Crossref | Google Scholar | PubMed |
83 Matsunaga S, Jimbo M, Gill MB, Wyhe LL, Murata M, Nonomura K, et al. Isolation, amino acid sequence and biological activities of novel long‐chain polyamine‐associated peptide toxins from the sponge Axinyssa aculeata. ChemBioChem 2011; 12: 2191-2200.
| Crossref | Google Scholar | PubMed |
84 Irie R, Miyako K, Matsunaga S, Sakai R, Oikawa M. Structure revision of protoaculeine B, a post-translationally modified N-terminal residue in the peptide toxin aculeine B. J Nat Prod 2021; 84: 1203-1209.
| Crossref | Google Scholar | PubMed |
85 Matsunaga S, Kishi R, Otsuka K, Fujita MJ, Oikawa M, Sakai R. Protoaculeine B, a putative N-terminal residue for the novel peptide toxin aculeines. Org Lett 2014; 16: 3090-3093.
| Crossref | Google Scholar | PubMed |
86 Watari H, Kishi R, Matsunaga S, Nishikawa T, Sawada Y, Honda A, et al. Interaction of polyamine-modified marine peptide aculeine A with cell membranes: disruption or entry. Chem Lett 2023; 52: 185-189.
| Crossref | Google Scholar |
87 Fang W-Y, Dahiya R, Qin H-L, Mourya R, Maharaj S. Natural proline-rich cyclopolypeptides from marine organisms: chemistry, synthetic methodologies and biological status. Mar Drugs 2016; 14: 194.
| Crossref | Google Scholar | PubMed |
88 Mechnich O, Messier G, Kessler H, Bernd M, Kutscher B. Cyclic heptapeptides axinastatin 2, 3, and 4: conformational analysis and evaluation of the biological potential. Helv Chim Acta 1997; 80: 1338-1354.
| Crossref | Google Scholar |
89 Malešević M, Schumann M, Jahreis G, Fischer G, Lücke C. Design of cyclic peptides featuring proline predominantly in the cis conformation under physiological conditions. ChemBioChem 2012; 13: 2122-2127.
| Crossref | Google Scholar | PubMed |
90 Mitova M, Popov S, De Rosa S. Cyclic peptides from a Ruegeria strain of bacteria associated with the sponge Suberites domuncula. J Nat Prod 2004; 67: 1178-1181.
| Crossref | Google Scholar | PubMed |
91 Iwasaki K, Iwasaki A, Sumimoto S, Sano T, Hitomi Y, Ohno O, et al. Croissamide, a proline-rich cyclic peptide with an N-prenylated tryptophan from a marine cyanobacterium Symploca sp. Tetrahedron Lett 2018; 59: 3806-3809.
| Crossref | Google Scholar |
92 Montaser R, Abboud KA, Paul VJ, Luesch H. Pitiprolamide, a proline-rich dolastatin 16 analogue from the marine cyanobacterium Lyngbya majuscula from Guam. J Nat Prod 2011; 74: 109-112.
| Crossref | Google Scholar | PubMed |
93 Mistry J, Finn RD, Eddy SR, Bateman A, Punta M. Challenges in homology search: HMMER3 and convergent evolution of coiled-coil regions. Nucleic Acids Res 2013; 41: e121.
| Crossref | Google Scholar | PubMed |
94 Schmidt G, Grube A, Köck M. Stylissamides A–D – new proline‐containing cyclic heptapeptides from the marine sponge Stylissa caribica. Eur J Org Chem 2007; 24: 4103-4110.
| Crossref | Google Scholar |
95 Kobayashi Ji, Nakamura T, Tsuda M. Hymenamide F, new cyclic heptapeptide from marine sponge Hymeniacidon sp. Tetrahedron 1996; 52: 6355-6360.
| Crossref | Google Scholar |
96 Zhang H-J, Yi Y-H, Yang G-J, Hu M-Y, Cao G-D, Yang F, et al. Proline-containing cyclopeptides from the marine sponge Phakellia fusca. J Nat Prod 2010; 73: 650-655.
| Crossref | Google Scholar | PubMed |
97 Li Y, Ling H, Teruya K, Konno H. Synthesis of Stylissamide B, a pro‐rich cyclic heptapeptide isolated from the marine sponge Stylissa caribica. J Pept Sci 2025; 31: e70028.
| Crossref | Google Scholar | PubMed |
98 Krumpe LRH, Wilson BAP, Marchand C, Sunassee SN, Bermingham A, Wang W, et al. Recifin A, initial example of the tyr-lock peptide structural family, is a selective allosteric inhibitor of tyrosyl-DNA phosphodiesterase I. J Am Chem Soc 2020; 142: 21178-21188.
| Crossref | Google Scholar | PubMed |
99 Smallwood TB, Krumpe LR, Payne CD, Klein VG, O’Keefe BR, Clark RJ, et al. Picking the tyrosine-lock: chemical synthesis of the tyrosyl-DNA phosphodiesterase I inhibitor recifin A and analogues. Chem Sci 2024; 15: 13227-13233.
| Crossref | Google Scholar | PubMed |
100 Williams DE, Austin P, Diaz-Marrero AR, Soest RV, Matainaho T, Roskelley CD, et al. Neopetrosiamides, peptides from the marine sponge Neopetrosia sp. that inhibit amoeboid invasion by human tumor cells. Org Lett 2005; 7: 4173-4176.
| Crossref | Google Scholar | PubMed |
101 Liu H, Boudreau MA, Zheng J, Whittal RM, Austin P, Roskelley CD, et al. Chemical synthesis and biological activity of the Neopetrosiamides and their analogues: revision of disulfide bond connectivity. J Am Chem Soc 2010; 132: 1486-1487.
| Crossref | Google Scholar | PubMed |
102 Zhong W, Olugbami JO, Rathakrishnan P, Mohanty I, Moore SG, Garg N, et al. Discovery and folding dynamics of a fused bicyclic cysteine knot undecapeptide from the marine sponge Halichondria bowerbanki. J Org Chem 2024; 89: 12748-12752.
| Crossref | Google Scholar | PubMed |
103 Finking R, Marahiel MA. Biosynthesis of nonribosomal peptides. Annu Rev Microbiol 2004; 58: 453-488.
| Crossref | Google Scholar | PubMed |
104 Ozaki T, Minami A, Oikawa H. Recent advances in the biosynthesis of ribosomally synthesized and posttranslationally modified peptides of fungal origin. J Antibiot 2023; 76: 3-13.
| Crossref | Google Scholar | PubMed |
105 Nie Q, Sun C, Liu S, Gao X. Exploring bioactive fungal RiPPs: advances, challenges, and future prospects. Biochemistry 2024; 63: 2948-2957.
| Crossref | Google Scholar | PubMed |
106 Liang H, Song ZM, Zhong Z, Zhang D, Yang W, Zhou L, et al. Genomic and metabolic analyses reveal antagonistic lanthipeptides in Archaea. Microbiome 2023; 11: 74.
| Crossref | Google Scholar | PubMed |
107 Czekster CM, Ge Y, Naismith JH. Mechanisms of cyanobactin biosynthesis. Curr Opin Chem Biol 2016; 35: 80-88.
| Crossref | Google Scholar | PubMed |
108 Phelan RW, Barret M, Cotter PD, O’Connor PM, Chen R, Morrissey JP, et al. Subtilomycin: a new lantibiotic from Bacillus subtilis strain MMA7 isolated from the marine sponge Haliclona simulans. Mar Drugs 2013; 11: 1878-1898.
| Crossref | Google Scholar | PubMed |
109 Nagai K, Kamigiri K, Arao N, Suzumura K, Kawano Y, Yamaoka M, et al. YM-266183 and ym-266184, novel thiopeptide antibiotics produced by Bacillus cereus isolated from a marine sponge I. taxonomy, fermentation, isolation, physico-chemical properties and biological properties. J Antibiot 2003; 56: 123-128.
| Crossref | Google Scholar | PubMed |
111 Nguyen NA, Vidya F, Yennawar NH, Wu H, McShan AC, Agarwal V. Disordered regions in proteusin peptides guide post-translational modification by a flavin-dependent RiPP brominase. Nat Commun 2024; 15: 1265.
| Crossref | Google Scholar | PubMed |
112 Bhushan A, Egli PJ, Peters EE, Freeman MF, Piel J. Genome mining- and synthetic biology-enabled production of hypermodified peptides. Nat Chem 2019; 11: 931-939.
| Crossref | Google Scholar | PubMed |
113 Bösch NM, Borsa M, Greczmiel U, Morinaka BI, Gugger M, Oxenius A, et al. Landornamides: antiviral ornithine-containing ribosomal peptides discovered through genome mining. Angew Chem Int Ed 2020; 59: 11763-11768.
| Crossref | Google Scholar | PubMed |
114 Chen N, Liu L, Wang J, Mao D, Lu H, Shishido TK, et al. Novel gene clusters for secondary metabolite synthesis in mesophotic sponge-associated bacteria. Microb Biotechnol 2025; 18: e70107.
| Crossref | Google Scholar | PubMed |
115 Freeman MF, Helf MJ, Bhushan A, Morinaka BI, Piel J. Seven enzymes create extraordinary molecular complexity in an uncultivated bacterium. Nat Chem 2017; 9: 387-395.
| Crossref | Google Scholar | PubMed |
116 Wilson MC, Mori T, Rückert C, Uria AR, Helf MJ, Takada K, et al. An environmental bacterial taxon with a large and distinct metabolic repertoire. Nature 2014; 506: 58-62.
| Crossref | Google Scholar | PubMed |
117 Iwamoto M, Shimizu H, Muramatsu I, Oiki S. A cytotoxic peptide from a marine sponge exhibits ion channel activity through vectorial-insertion into the membrane. FEBS Lett 2010; 584: 3995-3999.
| Crossref | Google Scholar | PubMed |
118 Maheshwari N, Jermiin LS, Cotroneo C, Gordon SV, Shields DC. Insights into the production and evolution of lantibiotics from a computational analysis of peptides associated with the lanthipeptide cyclase domain. R Soc Open Sci 2024; 11: 240491.
| Crossref | Google Scholar | PubMed |
119 Zhong G, Wang Z-J, Yan F, Zhang Y, Huo L. Recent advances in discovery, bioengineering, and bioactivity-evaluation of ribosomally synthesized and post-translationally modified peptides. ACS Bio Med Chem Au 2023; 3: 1-31.
| Crossref | Google Scholar | PubMed |
120 Samak ME, Solyman SM, Hanora A, Zakeer S. Metagenomic mining of two Egyptian Red Sea sponges associated microbial community. BMC Microbiol 2024; 24: 315.
| Crossref | Google Scholar | PubMed |
121 Janssen K, Krasenbrink J, Strangfeld S, Kroheck S, Josten M, Engeser M, et al. Elucidation of the bridging pattern of the lantibiotic pseudomycoicidin. ChemBioChem 2023; 24: e202200540.
| Crossref | Google Scholar | PubMed |
122 Deng Y, Li C-Z, Zhu Y-G, Wang P-X, Qi Q-D, Fu J-J, et al. ApnI, a transmembrane protein responsible for subtilomycin immunity, unveils a novel model for lantibiotic immunity. Appl Environ Microbiol 2014; 80: 6303-6315.
| Crossref | Google Scholar | PubMed |
123 Deng Y, Chen H, Li C, Xu J, Qi Q, Xu Y, et al. Endophyte Bacillus subtilis evade plant defense by producing lantibiotic subtilomycin to mask self-produced flagellin. Commun Biol 2019; 2: 368.
| Crossref | Google Scholar | PubMed |
124 Ziemert N, Alanjary M, Weber T. The evolution of genome mining in microbes – a review. Nat Prod Rep 2016; 33: 988-1005.
| Crossref | Google Scholar | PubMed |
125 McCloud TG. High throughput extraction of plant, marine and fungal specimens for preservation of biologically active molecules. Molecules 2010; 15: 4526-4563.
| Crossref | Google Scholar | PubMed |
126 Okonechnikov K, Golosova O, Fursov M, The UGENE Team. Unipro UGENE: a unified bioinformatics toolkit. Bioinformatics 2012; 28: 1166-1167.
| Crossref | Google Scholar | PubMed |
127 Teufel F, Almagro Armenteros JJ, Johansen AR, Gíslason MH, Pihl SI, Tsirigos KD, et al. SignalP 6.0 predicts all five types of signal peptides using protein language models. Nat Biotechnol 2022; 40: 1023-1025.
| Crossref | Google Scholar | PubMed |
128 Lu J, Rincon N, Wood DE, Breitwieser FP, Pockrandt C, Langmead B, et al. Metagenome analysis using the Kraken software suite. Nat Protoc 2022; 17: 2815-2839.
| Crossref | Google Scholar | PubMed |
129 Camacho C, Coulouris G, Avagyan V, Ma N, Papadopoulos J, Bealer K, et al. BLAST+: architecture and applications. BMC Bioinform 2009; 10: 421.
| Crossref | Google Scholar |
130 Jumper J, Evans R, Pritzel A, Green T, Figurnov M, Ronneberger O, et al. Highly accurate protein structure prediction with AlphaFold. Nature 2021; 596: 583-589.
| Crossref | Google Scholar | PubMed |
131 Pita L, Hoeppner MP, Ribes M, Hentschel U. Differential expression of immune receptors in two marine sponges upon exposure to microbial-associated molecular patterns. Sci Rep 2018; 8: 16081.
| Crossref | Google Scholar | PubMed |
132 Rathinam RB, Acharya A, Robina AJ, Banu H, Tripathi G. The immune system of marine invertebrates: Earliest adaptation of animals. Comp Immunol Rep 2024; 7: 200163.
| Crossref | Google Scholar |
134 Katoh K, Standley DM. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol Biol Evol 2013; 30: 772-780.
| Crossref | Google Scholar | PubMed |
135 Thompson JD, Higgins DG, Gibson TJ. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res 1994; 22: 4673-4680.
| Crossref | Google Scholar | PubMed |
136 Edgar RC. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res 2004; 32: 1792-1797.
| Crossref | Google Scholar | PubMed |
137 Hall T, Biosciences I, Carlsbad C. BioEdit: an important software for molecular biology. GERF Bull Biosci 2011; 2: 60-61.
| Google Scholar |
139 Stamatakis A. RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 2014; 30: 1312-1313.
| Crossref | Google Scholar | PubMed |
140 Huelsenbeck JP, Ronquist F. MRBAYES: Bayesian inference of phylogenetic trees. Bioinformatics 2001; 17: 754-755.
| Crossref | Google Scholar | PubMed |
141 Ronquist F, Teslenko M, van der Mark P, Ayres DL, Darling A, Höhna S, Larget B, Liu L, Suchard MA, Huelsenbeck JP. MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Systematic Biology 2012; 61(3): 539-542.
| Crossref | Google Scholar |
142 Guindon S, Dufayard J-F, Lefort V, Anisimova M, Hordijk W, Gascuel O. New algorithms and methods to estimate maximum-likelihood phylogenies: assessing the performance of PhyML 3.0. Syst Biol 2010; 59: 307-321.
| Crossref | Google Scholar | PubMed |
143 Albarano L, Esposito R, Ruocco N, Costantini M. Genome Mining as new challenge in natural products discovery. Mar Drugs 2020; 18: 199.
| Crossref | Google Scholar | PubMed |
144 Valenzuela L, Chi A, Beard S, Orell A, Guiliani N, Shabanowitz J, et al. Genomics, metagenomics and proteomics in biomining microorganisms. Biotechnol Adv 2006; 24: 197-211.
| Crossref | Google Scholar | PubMed |
145 Strehlow BW, Schuster A, Francis WR, Canfield DE. Metagenomic data for Halichondria panicea from Illumina and nanopore sequencing and preliminary genome assemblies for the sponge and two microbial symbionts. BMC Res Notes 2022; 15: 135.
| Crossref | Google Scholar | PubMed |
146 Li D, Liu C-M, Luo R, Sadakane K, Lam T-W. MEGAHIT: an ultra-fast single-node solution for large and complex metagenomics assembly via succinct de Bruijn graph. Bioinform 2015; 31: 1674-1676.
| Crossref | Google Scholar |
147 Nurk S, Meleshko D, Korobeynikov A, Pevzner PA. metaSPAdes: a new versatile metagenomic assembler. Genome Res 2017; 27: 824-834.
| Crossref | Google Scholar | PubMed |
148 Blin K, Shaw S, Kloosterman AM, Charlop-Powers Z, van Wezel GP, Medema H, et al. antiSMASH 6.0: improving cluster detection and comparison capabilities. Nucleic Acids Res 2021; 49: W29-W35.
| Crossref | Google Scholar | PubMed |
149 de Jong A, van Hijum SA, Bijlsma JJ, Kok J, Kuipers OP. BAGEL: a web-based bacteriocin genome mining tool. Nucleic Acids Res 2006; 34: W273-W279.
| Crossref | Google Scholar | PubMed |
150 Merwin NJ, Mousa WK, Dejong CA, Skinnider MA, Cannon MJ, Li H, et al. DeepRiPP integrates multiomics data to automate discovery of novel ribosomally synthesized natural products. Proc Natl Acad Sci 2020; 117: 371-380.
| Crossref | Google Scholar | PubMed |
151 Skinnider MA, Merwin NJ, Johnston CW, Magarvey NA. PRISM 3: expanded prediction of natural product chemical structures from microbial genomes. Nucleic Acids Res 2017; 45: W49-W54.
| Crossref | Google Scholar | PubMed |
152 Kloosterman AM, Medema MH, van Wezel GP. Omics-based strategies to discover novel classes of RiPP natural products. Curr Opin Biotechnol 2021; 69: 60-67.
| Crossref | Google Scholar | PubMed |
153 Santos-Aberturas J, Chandra G, Frattaruolo L, Lacret R, Pham TH, Vior NM, et al. Uncovering the unexplored diversity of thioamidated ribosomal peptides in Actinobacteria using the RiPPER genome mining tool. Nucleic Acids Res 2019; 47: 4624-4637.
| Crossref | Google Scholar | PubMed |
154 de los Santos ELC. NeuRiPP: neural network identification of RiPP precursor peptides. Sci Rep 2019; 9: 13406.
| Crossref | Google Scholar | PubMed |
155 Oliveira RS, Pinto OH, Quirino BF, de Freitas MA, Thompson FL, Thompson C, et al. Genome-resolved metagenomic analysis of Great Amazon Reef System sponge-associated Latescibacterota bacteria and their potential contributions to the host sponge and reef. Front Microbiomes 2023; 2: 1206961.
| Crossref | Google Scholar |
156 Parks DH, Rinke C, Chuvochina M, Chaumeil P-A, Woodcroft BJ, Evans PN, et al. Recovery of nearly 8,000 metagenome-assembled genomes substantially expands the tree of life. Nat Microbiol 2017; 2: 1533-1542.
| Crossref | Google Scholar | PubMed |
157 Nguyen NA, Lin Z, Mohanty I, Garg N, Schmidt EW, Agarwal V. An obligate peptidyl brominase underlies the discovery of highly distributed biosynthetic gene clusters in marine sponge microbiomes. J Am Chem Soc 2021; 143: 10221-10231.
| Crossref | Google Scholar | PubMed |
158 Loureiro C, Galani A, Gavriilidou A, Chaib de Mares M, van der Oost J, Medema H, et al. Comparative metagenomic analysis of biosynthetic diversity across sponge microbiomes highlights metabolic novelty, conservation, and diversification. mSystems 2022; 7: e0035722.
| Crossref | Google Scholar | PubMed |
159 Gomez-Escribano JP, Bibb MJ. Engineering Streptomyces coelicolor for heterologous expression of secondary metabolite gene clusters. Microb Biotechnol 2011; 4: 207-215.
| Crossref | Google Scholar | PubMed |
160 Zhang Y, Chen M, Bruner SD, Ding Y. Heterologous production of microbial ribosomally synthesized and post-translationally modified peptides. Front Microbiol 2018; 9: 1801.
| Crossref | Google Scholar | PubMed |
161 Wang M, Carver JJ, Phelan VV, Sanchez LM, Garg N, Peng Y, et al. Sharing and community curation of mass spectrometry data with Global Natural Products Social molecular networking. Nat Biotechnol 2016; 34: 828-837.
| Crossref | Google Scholar | PubMed |
162 Cárdenas P, Xavier JR, Reveillaud J, Schander C, Rapp HT. Molecular phylogeny of the Astrophorida (Porifera, Demospongiaep) reveals an unexpected high level of spicule homoplasy. PLoS ONE 2011; 6: e18318.
| Crossref | Google Scholar | PubMed |
163 Cárdenas P. Who produces ianthelline? The arctic sponge Stryphnus fortis or its sponge epibiont Hexadella dedritifera: a probable case of sponge–sponge contamination. J Chem Ecol 2016; 42: 339-347.
| Crossref | Google Scholar | PubMed |
164 Cárdenas P, Gamage J, Hettiarachchi CM, Gunasekera S. Good practices in sponge natural product studies: revising vouchers with isomalabaricane triterpenes. Mar Drugs 2022; 20: 190.
| Crossref | Google Scholar | PubMed |
165 Riesgo A, Farrar N, Windsor PJ, Giribet G, Leys SP. The analysis of eight transcriptomes from all poriferan classes reveals surprising genetic complexity in sponges. Mol Biol Evol 2014; 31: 1102-1120.
| Crossref | Google Scholar | PubMed |
166 Nowak VV, Hou P, Owen JG. Microbial communities associated with marine sponges from diverse geographic locations harbor biosynthetic novelty. Appl Environ Microbiol 2024; 90(12): e0072624.
| Crossref | Google Scholar | PubMed |
 Lakmini Kosgahakumbura completed her PhD at the University of Colombo, Sri Lanka, in collaboration with Uppsala University, Sweden. Her doctoral research focused on integrative taxonomy and isolation of bioactive natural products from marine sponges. Her particular interests are the isolation, characterisation, and evaluation of the biological significance of bromopyrrole alkaloids and Ribosomally synthesised and post-translationally modified peptides (RiPPs). The potential bioactivities of these compounds serve as valuable leads for future drug discovery. |
 Jayani Gamage completed her PhD at the University of Colombo, Sri Lanka, in collaboration with Uppsala University, Sweden. Her doctoral research focused on the integrative taxonomy and antimicrobial potential of shallow-water marine sponges, along with the phylogenetic relationships of selected species. This research contributed to documenting understudied marine biodiversity in the region and highlighted the bioactive potential of sponges as a source of natural products. She has a strong interest in combining conventional taxonomy with modern molecular and chemical approaches to better understand sponge diversity and bioactive potential. |
 Chamari Hettiarachchi is a professor in molecular biology and biochemistry at the Department of Chemistry, University of Colombo, Sri Lanka. Her research interests span molecular biology, biochemistry and microbiology, with a strong focus on cellular and molecular mechanisms and their applications in biotechnology. She has published over 50 peer-reviewed articles and has actively engaged in collaborative research with institutes in Sweden and India. Her ongoing work emphasises integrating fundamental molecular insights with applied research to address contemporary challenges in medicine, industry and the environment. |
 Paco Cárdenas is a sponge biologist and head curator of zoology at the Museum of Evolution, Uppsala University, Sweden. He obtained his PhD at the University of Bergen, Norway, on the systematics of tetractinellid sponges. His expertise today centres on the evolution, taxonomy and classification of demosponges, using different sets of characters including genetics and chemistry. He has described more than 30 new species of sponges worldwide. Paco also contributes to sponge pharmacognosy and metabolomics research in the Pharmacognosy group at Uppsala University. |
 Sunithi Gunasekera is a adjunct lecturer and associate professor in the Pharmacognosy group, Department of Pharmaceutical Biosciences, Uppsala University, Sweden. She obtained her PhD in Structural Biology in 2009 from The University of Queensland, Australia. During her doctoral research, Sunithi worked on early cyclotide bioactivity grafting applications, which sparked her interest in peptide therapeutic development. Sunithi’s research expertise spans peptide synthesis and NMR-based structural analysis. Currently, Sunithi is engaged in ribosomal peptide discovery from marine sponges from underexplored marine environments across the tropics and the North Atlantic. |