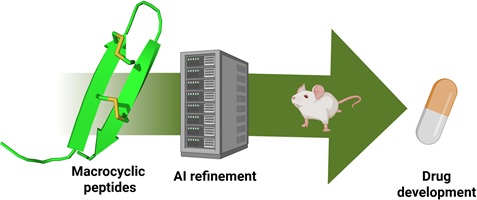
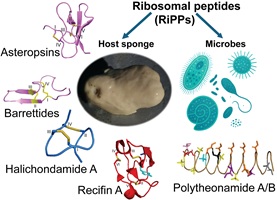
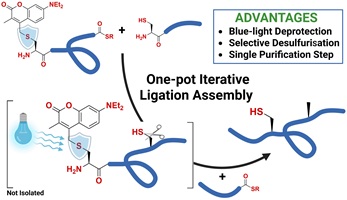
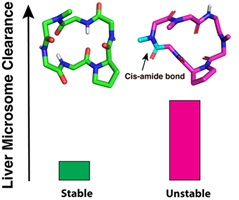
This collection honours the eminent peptide chemist, Professor David J. Craik, from the Institute for Molecular Bioscience, The University of Queensland, Australia, on the occasion of his 70th birthday. David’s many friends and colleagues have marked the splendid milestone of his reaching 70 years of age by contributing to this Special Issue of Australian Journal of Chemistry.
Prof. Craik discovered the cyclotide family of circular proteins and has characterised the structures of many animal toxins, including conotoxins from cone snail venoms. His research team focuses on applications of circular proteins, drugs in plants, toxins and NMR in drug design. He is author of over 800 scientific papers. He is a Fellow of the Royal Society and of the Australian Academy of Science, and an Officer (AO) of the Order of Australia. He has received numerous awards and is also Director of the ARC Centre of Excellence in Peptide and Protein Science (CIPPS).
This collection contains papers representing state-of-the-art investigations into peptide and protein science.
Guest Editors Prof. Ed Nice (Monash University), Prof. Mibel Aguilar (Monash University) & Prof. John Wade (The Florey)
Last Updated: 20 Oct 2025