Variation in boldness behaviour across individuals, sexes and strains of the guppy (Poecilia reticulata)
Darrell J. Kemp A * , K. E. Lynch A and St. Jean Samantha A
A * , K. E. Lynch A and St. Jean Samantha A
A Department of Biological Sciences, Macquarie University, North Ryde, NSW 2109, Australia.
Marine and Freshwater Research 73(4) 441-453 https://doi.org/10.1071/MF21129
Submitted: 6 May 2021 Accepted: 17 November 2021 Published: 4 February 2022
© 2022 The Author(s) (or their employer(s)). Published by CSIRO Publishing. This is an open access article distributed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND)
Abstract
The concept of animal personality is based on consistent individual differences in behaviour, yet little is known about the factors responsible for such variation. Theory based on sex-specific selection predicts sexual dimorphism in personality-related traits and, in some cases, differences in trait variances between the sexes. In this study, we examined the sources of individual variation for boldness behaviour in guppies (Poecilia reticulata). We first demonstrated heightened boldness expression in males relative to females across feral wild types, artificially selected domestic ‘designer’ guppies, and putative hybrids of the two. Boldness and body size covaried at the strain level but were not associated among individuals within strains. We also found high and repeatable behavioural differences among individuals (0.40 > intraclass r > 0.60) in all sex/strain groups except hybrid strain females. However, there was no evidence for the heightened inter-individual male variance anticipated for personality traits subject to certain forms of directional sex-specific selection. Domestic fish were boldest overall, and indicated the largest sex difference, which is consistent with genetic linkage between boldness and male ornamental colouration. Consistently high intrinsic variation in boldness behaviour, which extends to inbred domesticated fish, may in part underpin the invasive potential of this species.
Keywords: behavioural syndrome, domestication, invasiveness, mate choice, personality, Poecilid, repeatability, sexual dimorphism.
Introduction
Considerable research effort over the past several decades has been given to understanding the causes and consequences of intra-population level variation in behaviour. Theory and empiricism in this area have dealt particularly with the magnitude and consistency of behavioural differentiation among population members. Here, evidence from disparate taxa supports the widespread occurrence of correlated suites of behaviours, which (a) vary more among than within individuals, and (b) are expressed consistently over time and/or across different ecological contexts (Sih et al. 2004; Réale et al. 2007; Wolf and Weissing 2012; Jäger et al. 2019). These intrinsic behavioural suites are conceptualised as a type of multidimensional reaction norm known as a ‘behavioural syndrome’ and referred to in shorthand as ‘personality’ (Dingemanse et al. 2004; Sih et al. 2004; Réale et al. 2007). Discovery of this phenomenon has informed our basic understanding of how behaviour is regulated at multiple levels of biological organisation (Réale et al. 2010). Moreover, recent treatments have elucidated the importance of individual temperament for understanding broader-scale ecological and evolutionary processes (Réale et al. 2007; Schuett et al. 2010; Wolf and Weissing 2012).
Aside from its theoretical value, the concept of animal personality is also anticipated to aid and inform practice in applied realms spanning captive breeding, conservation biology and ecosystem management (McDougall et al. 2006; Réale et al. 2007; Chapple et al. 2012; Carere and Gherardi 2013). Expectations in this regard have been well spelt-out for aquatic systems and for fishes in particular (e.g. Huntingford 2004; Conrad et al. 2011; Cote et al. 2011; Mittelbach et al. 2014). Perhaps the most fundamental issue here concerns behavioural diversity; that is, the extent to which populations, cohorts, or other focal groups comprise individuals of varied personality type. Greater diversity is considered to favour ecological resilience and to enhance establishment potential, and therefore presents as a key objective for the management of threatened, restricted and/or reintroduced fish populations (McDougall et al. 2006; Conrad et al. 2011). By the same token, the presence of diverse behavioural types is likely to influence the outcome of species invasions (Chapple et al. 2012; Carere and Gherardi 2013) and artificial stocking programs (Huntingford 2004). Considerations such as these place precedence on a better understanding of the processes that engender and maintain inter-individual diversity in animal personality and its constituent traits.
One source of variation that has until recently received little attention concerns the potential for personality differences among the sexes (Schuett et al. 2010; Rangel-Patino et al. 2018; Kralj-Fiser et al. 2019). As with sexual dimorphism more generally, adaptive variation in personality may arise between the sexes either because natural selection favours divergent phenotypic optima, because of sexual selection, or because of some measure of both. Theoretically, sex-specific selection has the potential to generate and consolidate relationships either among individual personality traits per se, or between such traits and the broader phenotype. Two areas of theory are potentially relevant. The first is pace-of-life syndrome (POLS), a framework that considers underlying relationships among fundamental behavioural, physiological and life history-based components of animal phenotypes (Réale et al. 2010; Hämäläinen et al. 2018; Immonen et al. 2018; Tarka et al. 2018). POLS theory predicts that alternative complimentary combinations of traits exist along a fast−slow pace-of-life continuum and are favoured in a manner akin to the concept of r- versus K-selection (sensu MacArthur and Wilson 1967; Reznick et al. 2002). Precisely because of their divergent reproductive roles, the sexes are expected to occupy different positions along this continuum (or different trait variance/covariance structures altogether), with males being expected to exhibit higher levels of key behaviours such as aggressiveness, boldness and exploration (Hämäläinen et al. 2018; Immonen et al. 2018; Tarka et al. 2018; Moschilla et al. 2019).
The second candidate area of theory is sexual selection. Aside from indirectly shaping behaviour by driving sex-specific pace-of-life evolution (as above), sexual selection has also been argued to directly target personality or its constituent traits (Scherer et al. 2020, and references therein). Schuett et al. (2010) presented evidence and argument for the role of sexual selection in this regard, formulating predictions for how it should influence trait expression levels and variances. The following two complimentary components of behavioural variation were considered: (a) inter-individual variation, which describes the extent to which different individuals vary in their average level of behaviour, and (b) intra-individual consistency, which describes the extent to which individuals are consistent in their level of behavioural expression over time or contexts (these are equivalent to the slopes and intercepts obtained from intra-population reaction norms; see below). Schuett et al. (2010) hypothesised that sexual selection could directly target behavioural consistency if this is costly to achieve and therefore revealing of individual quality (e.g. Hunt 2004). Costliness may in this case arise from maintaining consistent behaviour irrespective of context, such as, for example, in prey species across conditions of varied predation risk. Selection may act further to maintain individual variation if population members mate assortatively vis-à-vis personality (e.g. Montiglio et al. 2016; Johnson et al. 2017; Collins et al. 2019).
In this study, we investigated sex-specific variation in a key personality trait among guppies (Poecilia reticulata). We focus on the shyness−boldness continuum (hereafter: ‘boldness’), a dimension of temperament describing the propensity to engage in risky behaviour (Wilson et al. 1994; Fraser et al. 2001; Réale et al. 2007). Aside from the potential for sexual differences outlined above, variation in this trait is thought to stem from the different ways in which risk aversion influences individual fitness across environments and/or ecological contexts ( Réale et al. 2007). Although bolder phenotypes may benefit from greater access to mates and food resources, for example, the magnitude of such benefits is ultimately modulated by features such as the intensity of social and interspecific competition (e.g. Dingemanse et al. 2004). Likewise, the lifetime fitness value of bolder behaviour will depend on the strength of predation, which may frequently vary through space and time. These considerations have been used to infer a prominent role for divergent selection in shaping boldness phenotypes. As for animal personality more broadly, this, in turn, supports the notion that individual-level variation is maintained according to gene–environment interactions for lifetime fitness (Réale et al. 2007).
Consistent individual variation in boldness has been established across many different animal groups and contexts, and is expressed via behaviours spanning the exploration of novel environments/objects (Lucon-Xiccato and Dadda 2016), the time spent in exposed locations (Sneddon 2003; King et al. 2013), and startle responses, freezing and similar aversive reactions to perceived predation risk (Godin and Dugatkin 1996; Bleakley et al. 2006; Piyapong et al. 2010). It generally covaries with measures of overall activity (Sneddon 2003; Bell 2005; Dingemanse et al. 2007) and integrates with additional personality features such as aggression (Archard and Braithwaite 2011) and sociality (Irving and Brown 2013). The study of boldness has proceeded with great strides in guppies and their relatives, and arguably to such an extent that poecilids pose a model group for such work (Lucon-Xiccato and Dadda 2016). Boldness has a known heritable basis in guppies (White and Wilson 2019) and differs among individuals according to brain size (Kotrschal et al. 2014), learning ability (Sneddon 2003) and cognitive lateralisation (Reddon and Hurd 2009). In males, this trait is related to ornamentation (Godin and Dugatkin 1996) and sperm number (Gasparini et al. 2019), constituents of pre- and post-copulatory mating success respectively. Further, bolder individuals have been shown to detect predators earlier (Godin and Dugatkin 1996) and survive better under predation risk (Smith and Blumstein 2010). All of this is consistent with boldness as a broadly integrated feature of individual quality (sensu Rowe and Houle 1996; Hunt et al. 2004).
Sex differences in boldness-related behaviour have previously been reported for guppies (Harris et al. 2010; Piyapong et al. 2010; White et al. 2019). However, there is some discordance as to the strength of the difference, and not all cases present evidence for sexual dimorphism according to the strict metric of Schuett et al. (2010). Interest in sexual differences comes from knowledge that female guppies prefer bolder as well as more highly ornamented males (Godin and Dugatkin 1996). By imposing directional selection on boldness in males, such a mating preference should favour the sex-specific elaboration of trait level, and generate characteristic patterns of variance across the sexes (Schuett et al. 2010; see below). However, knowledge of mate choice for bold male guppies is limited to Godin and Dugatkin’s (1996) study population, which originated from the Quare drainage in Trinidad. It is not presently known whether this preference extends to different populations, but the finding at least implicates sexual selection as a potential influence on male guppy boldness. Accordingly, we address whether and how observable variation within and among the sexes for this trait aligns with Schuett’s et al. (2010) predictions for animal personality traits under sexual selection.
Study design, aims and predictions
We sought to investigate the mean levels and magnitude of variation in boldness behaviour among/within individuals and sexes for guppies sourced from the following three distinct populations:
a pure feral wild-type Australian strain;
inbred domestic ‘designer’ or ‘fancy’ guppies, and;
a naturally occurring population of hybridising feral and domestic fish.
Incorporation of these strains was motivated, first, by the opportunity to contrast trait values between fish from natural versus artificial selective environments (i.e. the wild and domestic strains), and, second, to explore the outcome of a putative genetic admixture between the two. Our use of domestic guppies also followed a prior study (Bleakley et al. 2006) that showed variation in personality-type measures among different colour-defined varieties but, unfortunately, neither reported data separately for each sex nor accounted for individual variation. These fish come from lines that are artificially selected for male ornamental colour (and body size) over many generations in captivity. They are, consequentially, highly inbred and possess reduced neutral genetic variation (Bleakley et al. 2008; see below). Naturally occurring vectors of sexual selection are largely, if not entirely, absent in these lines, which therefore excludes any female preference favouring bold males (sensu Godin and Dugatkin 1996). Both sexes are also freed from the pressures of predation and environmental stochasticity, which might otherwise select against boldness. However, it is possible that domestic males may experience indirect selection for increased boldness because of linkage between colour and boldness genes (Godin and Dugatkin 1996; Bleakley et al. 2006). Fish for the hybridising strain originated from a semi-isolated watercourse colonised primarily by the feral population, yet with occasional influxes of domestic escapees from nearby residential ponds (see below). This population should receive periodic inputs of genetic material from domesticated guppies and is therefore anticipated to represent a genetic admixture between feral and domestic populations.
Our primary aim was to assess the mean level of boldness expression across males and females of each study strain. Secondarily, and within the constraints posed by sample size, we aimed to contrast the extent of inter-individual variation for this behaviour between the sexes, and among the different strains. This sought partly to address theoretical predictions for personality traits under sexual selection (Schuett et al. 2010), chiefly the prediction for higher and more consistent levels of boldness in males. We considered that this prediction should be most clearly testable in the studied feral wild-type strain, which represents a large, outbred population in which mate choice proceeds without human interference. Aside from this, we expect domestic fish to be bolder than their feral counterparts, as known generally for captive-bred fish populations (Huntingford 2004), and to exhibit lower inter-individual variation owing to reduced population genetic diversity. If present, pleiotropy or genetic linkage between colour and boldness (as noted above) should exacerbate both of these differences because domesticated male boldness would then be targeted indirectly by artificial selection for colour exaggeration. We expect that this would drive higher phenotypic boldness levels in domestic males (and possibly females), coupled with reduced variance as a consequence of high-value alleles being indirectly selected to fixation.
For the hybrid strain, the putative admixture of feral/domestic genes should engender a level of boldness that is intermediate between the two populations, coupled with increased inter-individual variation. All above predictions are contingent on the extent to which boldness differences among strains trace from additive genetic variation (which appears likely to be large; see, e.g. White and Wilson 2019). In addition to boldness, we also assess mature body size among sex/strain groups. This trait is markedly greater in domestic fish because of artificial selection, and is otherwise known to co-vary with boldness behaviour in poecilids (Brown and Braithwaite 2004); hence, it offers a basis for comparison with levels of boldness expression within and among study strains.
Materials and methods
Ethical note
All aspects of this research were conceived and weighed in relation to the guiding principles of animal welfare (‘the 3Rs’; Russell and Burch 1959). To this end, we co-opted individuals that were already held in similar captive aquarium environments where possible (reduction). Procedures consisted of observation, minor handling and light anaesthesia, all of which drew on extensive precedents in aligned studies of this species over many decades (refinement). We conducted all work in accordance with the ‘Australian code for the care and use of animals for scientific purposes’ (8th edition, 2013). The approach was formally approved by the Macquarie University animal ethics committee (Animal Research Authority 2013/024), as overseen by the New South Wales Department of Primary Industries under their Animal Research Regulation, and in accordance with the Australian Animal Research Act 1985.
Provenance and description of study strains
Guppies for the feral wild-type population originated from Alligator Creek, an undisturbed rainforest stream located in the Bowling Green Bay National Park, ∼30 km from Townsville in northern Queensland, Australia. Guppies have existed at this location for over 60 years. They exhibit colour and behavioural phenotypes similar to those of fish from low- to medium-predation Trinidadian streams (Brooks and Endler 2001). We sourced fish from an Alligator Creek stock population held at the University of New South Wales (UNSW), Sydney. This population was founded by several hundred individuals captured upstream of the main Alligator Creek swimming hole in 1999, which has been actively managed for the retention of genetic variation (VG) over ∽30 generations since (at the time of this study). In evolutionary genetic terms, the sustained rearing of laboratory populations with finite VG will often engender adaptation to captivity and the loss of rare alleles, hence the erosion of VG, because of drift (Falconer 1981). However, a comparison between our UNSW stock and the F1 descendants of dams sampled from precisely the same Alligator Creek location in 2017 has indicated little divergence in either mature body size or male colour phenotype (D. J. Kemp, unpubl. data). This argues against significant departure from the wild-type state because these traits are otherwise known to evolve quickly under altered selection in guppies (i.e. in as little as several years; Endler 1980; Reznick et al. 1997).
Adult domestic guppies were obtained direct from Bay Fish, Brisbane. This company is the principal Australian importer and supplies the commercial pet trade, with fish sourced directly from breeders in Singapore and Thailand. We obtained a mixture of three popular and widely propagated varieties known as ‘gold neon’ (n = 32), ‘red sunset’ (n = 12) and ‘green snakeskin’ (n = 12; fewer of the two latter varietier were available from suppliers at the time of this study). These varieties resemble purebred guppy phenotypes documented in prior studies of population genetics and behaviour (Lindholm et al. 2005; Bleakley et al. 2006, 2008). The latter two varieties are superficially identical to the ‘snakeskin’ and ‘½ yellow’ fish used by Bleakley et al. (2006) to investigate predator response behaviours (see Discussion). Genetic studies of domesticated guppy varieties have indicated high inbreeding coefficients (F = 0.26–0.45) and neutral allelic diversity at around half the wild-type level (Bleakley et al. 2008). Phenotypically, both sexes mature at much larger size than do wild fish, and the males display greatly exaggerated colouration and fin morphology (refer to exemplar images for each study strain in Supplementary Fig. S1). We elected to source a mix of three domestic varieties here to match the likely varied constitution of fish thought to hybridise with wild guppies, which may be important if behaviour differs among varieties (sensu Bleakley et al. 2006). Subsequently, however, we found no evidence of a difference in mean boldness, and likewise for body size (Supplementary Tables S1, S2). In terms of effect size, the mean difference in boldness level among domestic varieties (females = 0.41; males = 0.22) was less than half that between the overall domestic strain and the other two strains (see Results and Supplementary Fig. S2). We therefore treat the three varieties as representatives of a single domestic strain, although recognising that pooling them may slightly increase group variance.
Hybrid fish derived from a freshwater drainage channel located in Darwin, Northern Territory, Australia. This channel is connected to a greater network of suburban aqueducts that are ultimately contiguous with the Howard and Elizabeth rivers. The entire system carries an extensive resident wild-type guppy population. The channel itself is upstream of an estuarine watercourse and is occasionally visited by predatory fish (Trompf and Brown 2014). Importantly, it runs for several kilometres directly adjacent to residential back yards containing ponds stocked with domestic guppies. The aspect of surrounding terrain is such that pond overflow drains directly into the channel, and the ponds overflow during periodic excessive rainfall events each tropical wet season. We therefore treat fish in this strain as putative hybrids, although the precise degree of wild/domestic genetic admixture is unknown. We also do not assume that feral fish local to the Darwin region are phenotypically identical to the studied wild-type strain, only that they each fall within the bounds of expectation for naturalised wild-type guppy populations (and, hence, are categorically more similar to each other than to domesticated phenotypes). Several hundred hybrid strain individuals were initially collected in May 2011 (Irving and Brown 2013) and have been maintained since in a large mixed-sex population at Macquarie University.
General fish handling procedures
Thirty reproductively mature males and females of the wild and hybrid strains were haphazardly selected for this study in early March 2014. These fish were housed for several weeks prior to testing in single-sex shoals (N = 30 fish) in 200 L tanks outfitted with filtration, aeration, a gravel substrate and aquatic vegetation. They were fed once daily with crushed Tetramin tropical flakes. Domestic fish were handled similarly, albeit with slightly lower densities of N = 27 males and N = 29 females housed per 200 L tank. Four days prior to testing, we placed subjects into individual holding tanks (225 × 155 × 151 mm) containing a gravel substrate only. We continued daily feeding and conducted one-third water changes over this period as a substitute for continuous filtration. All tanks were housed in the same laboratory under 25.0 ± 1.0°C and a 12 h light−12 h dark cycle, with overhead fluorescent lighting at ∼500 lumens m−2 intensity during daylight hours. Two females and one male from the wild population were excluded from the study prior to the first round of testing because of signs of ill-health.
After being tested for behaviour, each subject was lightly anesthetised with a pH-buffered solution of ethyl 3-aminobenzoate methane sulfonic acid salt and photographed against matte black cardstock (methods as per Kemp et al. 2018). Body size (standard length) was measured from these photographs as the distance from snout tip to caudal peduncle, to the nearest 0.05 mm.
Boldness assays
Boldness was quantified using a standard procedure known as the open-field or emergence test (Brown et al. 2005, 2007; Harris et al. 2010; Irving and Brown 2013). This test has been applied across fishes, mammals and birds (see Burns 2008). We chose this assay specifically to enable comparison with the existing literature on behavioural repeatability in guppies (e.g. Harris et al. 2010). Importantly, this assay has been found to exhibit high internal validity in guppies (reliability across alternate forms of the test) as well as high divergent validity (specificity to the measurement of putative boldness–shyness; Burns 2008). Note that potential problems associated with ‘darting’ reported by Burns (2008) were not observed in our trials. The assay is also known to generate reliable scores that integrate highly with other measures of boldness–shyness in zebrafish (Toms and Echevarria 2014). Although not validated against wild behaviour for guppies, this test has been shown to predict the dispersal/exploration of Rivulus hartii following their release into the wild, and that this correlates with individual growth rate under predation risk in wild environments where R. hartii exists in fragmented distributions (Fraser et al. 2001). In laboratory populations of guppies, boldness measured in ways known to correlate with emergence test scores has been linked to survival under the risk of predation (Smith and Blumstein 2010).
Strains were assayed in discrete blocks throughout March and April 2014, with sexes being tested in a random order within testing days. Subjects participated in two sequential trials, 1 day apart, and were housed in their individual tanks between trials. Testing began between 9:00 am and 11:00 am each day and took ∼5 h to complete. There was no indication that time of day (or intra-day testing order) systematically influenced emergence time. The test arena (illustrated in Supplementary Fig. S3) comprised a 33 L tank containing a black plastic start box with a remotely operable vertical sliding door. Plastic objects and rocks were distributed throughout the arena to present a standard novel environment. Test subjects were gently transferred from their holding tanks into the start box and allowed to settle for 300 s prior to raising the sliding door. We used this acclimation time because it is known to furnish high test reliability (Burns 2008). Investigators were not visible to fish at this time. For consistency with prior studies (Harris et al. 2010), we judged boldness as latency until the fish’s snout first emerged from the start box. Lower values for emergence time therefore equate to greater boldness. We defined a ceiling time for emergence of 10 min on the basis of work showing 96% successful emergence given this duration (Toms and Echevarria 2014). All subjects emerged prior to this ceiling time, except for two wild population fish (one of each sex) in the first round of trials. These individuals subsequently exhibited signs of ill-health and were removed from the experiment.
Variance estimation
Behavioural variances can be calculated in several ways and these have different utility for the study of animal personality (Schuett et al. 2010; Wilson 2018; Dochtermann and Royauté 2019). The basic components are among-subject variance 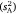 and within-subject variance
and within-subject variance 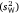 , which together sum for total observed variance. The former component
, which together sum for total observed variance. The former component 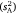 gives the raw or unstandardised variance among individuals and is most relevant to assessing the absolute magnitude of inter-individual variation in the trait (Wilson 2018; Dochtermann and Royauté 2019). This component can then be expressed as a proportion of total variance via the intraclass correlation coefficient (Lessells and Boag 1987), as follows:
gives the raw or unstandardised variance among individuals and is most relevant to assessing the absolute magnitude of inter-individual variation in the trait (Wilson 2018; Dochtermann and Royauté 2019). This component can then be expressed as a proportion of total variance via the intraclass correlation coefficient (Lessells and Boag 1987), as follows:
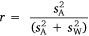
This gives a variance-standardised ratio that is widely used and equated with the concept of behavioural repeatability or population-level consistency (e.g. Boake 1989; Bell et al. 2009). When calculated across a series of different groups, this parameter will vary from 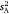 to the extent that
to the extent that 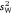 varies among them (i.e. r and
varies among them (i.e. r and 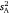 would co-vary perfectly were
would co-vary perfectly were 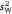 is equal across groups). Along with r, Dochtermann and Royauté (2019) advocated strongly for a repeatability index that is mean-standardised because this may represent the magnitude of effect size on the most biologically relevant scale. In group contrasts, the argument is that repeatability estimates are most appropriately standardised by the mean level of behavioural expression within each respective group (X). Mean standardised variance is calculated as a dimensionless ratio according to the following formula:
is equal across groups). Along with r, Dochtermann and Royauté (2019) advocated strongly for a repeatability index that is mean-standardised because this may represent the magnitude of effect size on the most biologically relevant scale. In group contrasts, the argument is that repeatability estimates are most appropriately standardised by the mean level of behavioural expression within each respective group (X). Mean standardised variance is calculated as a dimensionless ratio according to the following formula:
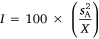
Both indices (r and I) are derivatives of 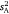 and all of them may therefore co-vary across sex/strain groups to some extent. We analysed all parameters but report the indices primarily as descriptors of the effect size (Nakagawa and Cuthill 2007). Guidelines derived from several relevant meta-analyses (refer to Baker et al. 2018) describe a large effect as intraclass r ≥ 0.40, a medium effect as 0.2 < r < 0.4, and a small effect as r ≤ 0.2. We refer to these in the interpretation of intraclass r as well as the difference in r between contrast groups.
and all of them may therefore co-vary across sex/strain groups to some extent. We analysed all parameters but report the indices primarily as descriptors of the effect size (Nakagawa and Cuthill 2007). Guidelines derived from several relevant meta-analyses (refer to Baker et al. 2018) describe a large effect as intraclass r ≥ 0.40, a medium effect as 0.2 < r < 0.4, and a small effect as r ≤ 0.2. We refer to these in the interpretation of intraclass r as well as the difference in r between contrast groups.
Statistical analysis
We used a generalised linear mixed modelling (GLMM) approach to partition the variance in boldness within and among individuals and strains. The full model included sex, strain, sex × strain, trial order and body size as fixed effects, and individual (nested within sex and strain) as a random effect. Fixed effects were tested for significance using conditional Wald F-tests and the Kenward and Roger adjustment (Kenward and Roger 1997) to respect the marginality relationships among fixed GLMM factors (for further detail, see Gilmour et al. 2015).
The random (sparse) part of the model generated estimates for among-subject variance 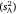 versus within-subject variance
versus within-subject variance 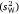 . These values informed intra-individual variation (which was simply
. These values informed intra-individual variation (which was simply 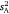 ) and enabled calculation of both repeatability indices (r and I). We applied the same analytical approach for each parameter. The first step involved modelling the data using an unconstrained diagonal variance structure to estimate the observational values of
) and enabled calculation of both repeatability indices (r and I). We applied the same analytical approach for each parameter. The first step involved modelling the data using an unconstrained diagonal variance structure to estimate the observational values of 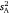 ,
, 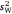 , r and I separately for each sex/strain group. At this point we tested whether each parameter was significant in each group by constraining it to zero and assessing the change in overall fit from a model in which it was free to vary.
, r and I separately for each sex/strain group. At this point we tested whether each parameter was significant in each group by constraining it to zero and assessing the change in overall fit from a model in which it was free to vary.
We next used an iterative model-fitting procedure to formally test for differences in variances 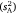 and variance ratios (r and I) between sexes and among strains. Starting from a model in which all sex/strain groups were estimated separately, we progressively fit models whereby the values of each parameter were constrained to equality, first across the males and females of each strain, and second across each pair of strains. Overall goodness of fit (G) was tested at each step according to twice the log-likelihood difference between the current and prior model, which was evaluated using the chi-squared distribution with n degrees of freedom (equal to a change in n estimated parameters). This presents a formal test for homogeneity among the sex/strain groupings constrained to equality in each step (Gilmour et al. 2015). A non-significant loss of model fit indicated that the variances were statistically homogeneous. Such changes were successively incorporated, and the final (most parsimonious) model was used in the estimation and testing of fixed effects. We used a simplified version of this model-fitting approach to estimate and contrast phenotypic variance in body size among sexes and strains.
and variance ratios (r and I) between sexes and among strains. Starting from a model in which all sex/strain groups were estimated separately, we progressively fit models whereby the values of each parameter were constrained to equality, first across the males and females of each strain, and second across each pair of strains. Overall goodness of fit (G) was tested at each step according to twice the log-likelihood difference between the current and prior model, which was evaluated using the chi-squared distribution with n degrees of freedom (equal to a change in n estimated parameters). This presents a formal test for homogeneity among the sex/strain groupings constrained to equality in each step (Gilmour et al. 2015). A non-significant loss of model fit indicated that the variances were statistically homogeneous. Such changes were successively incorporated, and the final (most parsimonious) model was used in the estimation and testing of fixed effects. We used a simplified version of this model-fitting approach to estimate and contrast phenotypic variance in body size among sexes and strains.
Means are quoted with standard errors throughout, unless otherwise indicated. Random variance components are accompanied by symmetric standard errors (equivalent to what may elsewhere be termed ‘uncertainty’) that were derived as the square root of the diagonal elements of the inverse information submatrix (Gilmour et al. 2015). Boldness values were natural log-transformed to comply with the assumptions of a normal distribution. Cohen’s standardised effect size (d; Cohen 1988) was used to describe the magnitude of mean group differences in body size and boldness. We conducted all analyses using ASReml (Gilmour et al. 2015) and Statistica Ver. 7, Statsoft, OH, USA.
Results
Body size
Body size differed between sexes (Wald F1,165 = 228.6, P < 0.001) and among strains (Wald F2,165 = 267.0, P < 0.001), and there was a significant sex × strain interaction (Wald F2,165 = 47.8, P < 0.001). Feral wild-type fish were, on average, smaller than hybrids, which were in-turn smaller than domestics (Fig. 1a). Females were larger than males in both the wild and hybrid strains (Tukey’s honest significant difference: P < 0.001), but there was no evidence for sexual dimorphism in domestic fish (Tukey’s honest significant difference: P = 0.64). In terms of phenotypic variance, female size varied more than male size in the feral wild strain (G1 = 11.16, P < 0.001) and the hybrid strain (G1 = 8.27, P < 0.005) but not in the domestic strain (G1 = 0.45, P = 0.50). The overall pattern was therefore for larger and more variable females relative to males in all cases except the domestic strain (Fig. 1a).
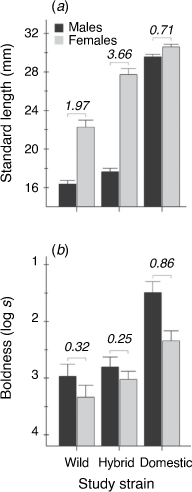
|
Level of boldness expression
Boldness differed between sexes (Wald F1,335 = 9.63, P < 0.005), among strains (Wald F2,335 = 23.5, P < 0.001) and according to trial order (Wald F2,335 = 13.8, P < 0.001). As expected, males were bolder than females, and boldness overall scaled from lowest in feral wild-type fish to highest in domestic fish (Fig. 1b). The order effect was such that fish behaved less boldly in their second trial (estimate = 0.35 ± 0.094). In contrast to body size, the sex difference in boldness was statistically equivalent across strains (sex × strain interaction: Wald F2,335 = 1.56, P = 0.21). The standardised effect sizes associated with these sex differences were also lower than those seen for body size, except in the domestic strain (Fig. 1b). Body size was not a significant predictor of boldness either overall (Wald F1,93.6 = 0.01, P = 0.913), or in any sex/strain group (Pearson’s correlation: −0.20> r > 0.15, P > 0.31).
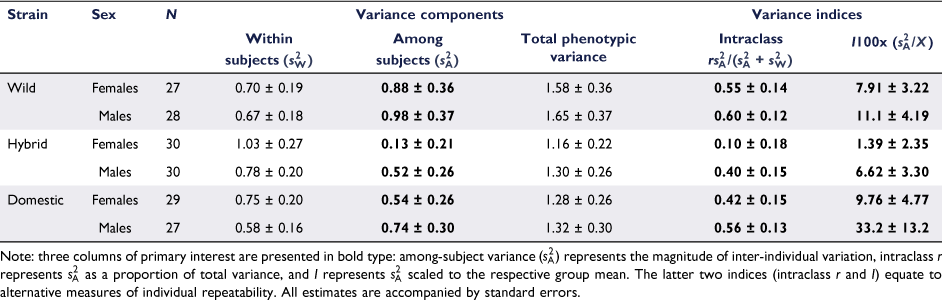
Behavioural variances
Components of inter-individual variation 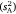 ranged across the sex/strain groups from 0.12 to 0.98, which corresponded to intraclass r estimates ranging from 0.10 to 0.60 and mean-standardised variance estimates (I) from 1.4 to 33.2 (Table 1). Variances were marginally higher for males across the board, with highest values seen for the wild strain. Tests for whether each of the three variance parameters (
ranged across the sex/strain groups from 0.12 to 0.98, which corresponded to intraclass r estimates ranging from 0.10 to 0.60 and mean-standardised variance estimates (I) from 1.4 to 33.2 (Table 1). Variances were marginally higher for males across the board, with highest values seen for the wild strain. Tests for whether each of the three variance parameters (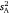 , intraclass r, I) differed from zero across the sex/strain groups all simply converged on the solution for
, intraclass r, I) differed from zero across the sex/strain groups all simply converged on the solution for 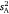 (because all parameters are derivatives of
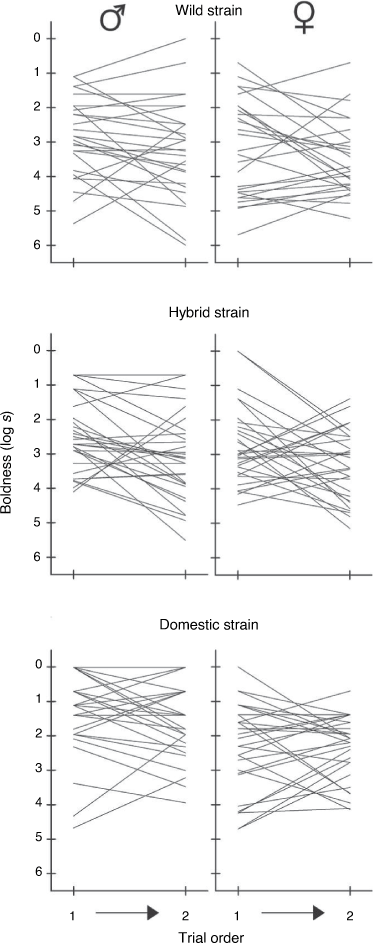
(because all parameters are derivatives of 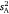 ). These tests indicated a significant departure from zero for all sex/strain groups (G1 > 5.13, P < 0.05), except hybrid strain females (G1 = 0.35, P = 0.55), thereby supporting the presence of consistent individual differences in behaviour (Fig. 2). Considering intraclass r as an estimate of effect size likewise indicated that consistent individual differences existed in all groups aside from hybrid females (Table 1).
). These tests indicated a significant departure from zero for all sex/strain groups (G1 > 5.13, P < 0.05), except hybrid strain females (G1 = 0.35, P = 0.55), thereby supporting the presence of consistent individual differences in behaviour (Fig. 2). Considering intraclass r as an estimate of effect size likewise indicated that consistent individual differences existed in all groups aside from hybrid females (Table 1).
|
|
Differences among the sexes
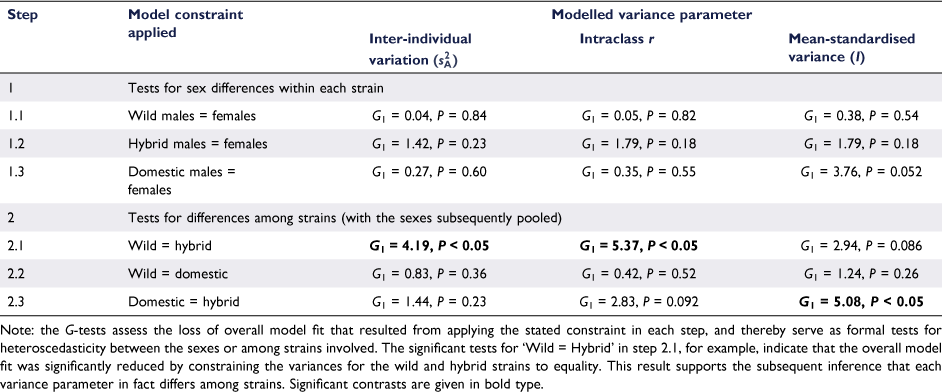
Although modelled estimates of behavioural variance were numerically higher for males in all three strains (Table 1), formal model fitting showed that sex differences were non-significant across the board (Table 2). However, the sex difference in mean-standardised variance for domestic fish was nearing the margin of significance (i.e. P = 0.052), suggesting a trend toward greater male variance in this strain.
|
Differences among the strains
Progressive modelling with the sexes subsequently pooled within each strain showed evidence for differences between wild and hybrid fish in both intra-individual variation and intraclass r (accompanied by a marginally non-significant difference in mean-standardised variance). Here, values for wild fish (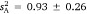 and r = 0.594 ± 0.088) exceeded those for hybrid fish (
and r = 0.594 ± 0.088) exceeded those for hybrid fish (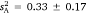 and r = 0.275 ± 0.121). There was also evidence for greater mean-standardised variance in domestic as opposed to hybrid fish (I = 16.5 ± 5.65 versus 3.84 ± 2.00 respectively), with the corresponding intraclass r contrast also just above the margin of significance. On the balance, these findings support lower behavioural variance among hybrid fish, and particularly females, as opposed to the two other studied strains.
and r = 0.275 ± 0.121). There was also evidence for greater mean-standardised variance in domestic as opposed to hybrid fish (I = 16.5 ± 5.65 versus 3.84 ± 2.00 respectively), with the corresponding intraclass r contrast also just above the margin of significance. On the balance, these findings support lower behavioural variance among hybrid fish, and particularly females, as opposed to the two other studied strains.
Discussion
Recent years have seen burgeoning interest in the causes and consequences of personality variation among individuals as well as among the sexes (e.g. Schuett et al. 2010; Hämäläinen et al. 2018; Tarka et al. 2018). Here, we sought to define and contrast each source of variance in guppy strains derived from highly divergent ecological and evolutionary genetic backgrounds. Guppies pose particular interest for such contrasts because of the potential for female choice favouring bolder males (Godin and Dugatkin 1996), which would levy sex-specific selection on this key personality feature. Our data showed clear differences in the level of boldness expression among strains and between sexes within each strain, with the former contrast indicating heightened boldness in domesticated relative to wild-type guppies coupled with intermediate trait expression in their putative hybrids. We also demonstrated significant inter-individual variation (equating to population-level repeatability) in all sex−strain combinations, except for females of the hybrid strain. Evidence for differences in trait variance among strains, and in particular between sexes, proved difficult to establish statistically, but the direction of differences was, nevertheless, inconsistent with prediction. We discuss the key findings in relation to sex-specific selection, domestication, and the maintenance of behavioural variation across individuals and sexes.
Sexual selection is thought to drive the sex-specific expression of indicator traits to costly levels while at the same time favouring integration with the genetic basis of viability (Rowe and Houle 1996). This leads to condition-dependence and heightened trait variation in the selected sex. For personality traits, it is also predicted to reinforce, if not amplify, individual differences in this sex (Schuett et al. 2010). Whereas our data indicated marginally higher observational variances for male boldness across the board, they provided no statistical evidence for sex differences in variance parameters. Although it stands that our power is low for detecting all but large effects (equivalent to a between-groups difference of r ≈ 0.45), the observed wild male–female effect size of r = 0.05 implied negligible sex differentiation in the population where we would otherwise expect it to the greatest. One explanation, based on the potentially context-dependent nature of behaviour, is that meaningful variation may be revealed only in the presence of an explicit threat. In demonstrating female preference for bolder males, for example, Godin and Dugatkin (1996) assessed boldness via the willingness to inspect a dangerous predator, the pike cichlid. Others have sought to incorporate threat using startle stimuli in conjunction with emergence or open-field assays (e.g. Bell 2005; Bleakley et al. 2006; Piyapong et al. 2010; Biro 2012; Edenbrow and Croft 2013; Swaney et al. 2015). The clear perception of risk, if suitably standardised across trials, may be ultimately necessary to subdue the expression of boldness in all fish, except in males of increasing quality. An alternative but non-exclusive explanation is that a meaningful assay of variation may require a female audience. This goes to the possibility that high-quality males do not express their full potential for boldness unless they perceive an immediate benefit in doing so. Godin and Dugatkin’s (1996) experiments also provided support for this in the form of interactive relationships among colouration, boldness and female presence, although more recent work has implied that such interactions may not be universal (Piyapong et al. 2010). Aside from test efficacy, it is also possible that the bold–shy axis of behaviour has simply not been subject to sexual selection in the studied strains. If so, we would, however, need to look elsewhere to explain greater male boldness, which is evidently ubiquitous across populations of guppies and related poecilids (see below).
We appraised individual variation in behaviour using both raw and standardised estimates of among-individual variance. The variance-standardised index, known as intraclass r, is widely considered as a population-level index of individual consistency or repeatability (Schuett et al. 2010). There is a robust comparative literature for this parameter because efforts to establish repeatability are routine in personality research. Our intraclass r estimates generally exceed the average reported for repeatability of non-courtship behaviours in vertebrates (r = 0.33; Bell et al. 2009). Further, our wild strain estimates occur towards the upper end of values reported for fishes across such behaviours as activity, aggression, boldness, courtship, exploration, inspection and sociality (Smith and Blumstein 2010; Edenbrow and Croft 2013; Rezucha and Reichard 2016; Baker et al. 2018; Barbosa et al. 2018; Roy and Bhat 2018). The most salient comparison comes from two existing studies that near-precisely replicated the open-field test protocol used here. These reported mean emergence time values for wild-caught Trinidadian guppies (♂ 33 s; ♀ 61 s; Harris et al. 2010) and wild-caught Gambusia affinis (♂ 39 s; ♀ 50 s; Chen et al. 2018) that are notably similar to our wild strain (♂ 32 s; ♀ 50 s). Each study also found significant repeatability, as judged from the intraclass correlation, with estimates for guppies proving lower than ours (♂ 0.34; ♀ 0.29; cf. Table 1), yet those for Gambusia almost identical (♂ 0.59; ♀ 0.56). This degree of concordance is surprising, particularly given the uncertainty attached to estimating variances. It is also notable considering that all studies used only two sequential trials, which is otherwise expected to heighten the importance of extrinsic factors (e.g. handling conditions, inter-trial duration) and intrinsic factors (e.g. acclimatisation and learning) for within-subject variation (Biro 2012). At the very least, this comparison supports the persistence of considerable levels of trait variance in our wild-type strain despite its captive-bred tenure. It is also interesting to note that Harris et al. (2010) studied guppies from several locations on the Quare River, which was also the source of fish for Godin and Dugatkin’s (1996) mate-choice study. Although our intraclass r estimates exceeded those of Harris et al. (2010), both datasets agree on the absence of heightened male values that may otherwise be engendered by sexual selection (Schuett et al. 2010).
Our finding of universally greater male boldness is consistent with results established across multiple wild Trinidadian guppy populations (e.g. Harris et al. 2010; White et al. 2019). This sex difference has, likewise, been reported for several poecilids in the genus Brachyrhaphis (Brown et al. 2007; Ingley et al. 2014), and in other model species such as zebrafish (Roy and Bhat 2018) and sticklebacks (King et al. 2013). The balance of evidence among small freshwater fishes in fact points to males as being bolder, more aggressive, more exploratory and/or generally more willing to engage in risky behaviours. This is expected if male personality is subject to sexual selection (Schuett et al. 2010), but is also explainable by sex-specific pace-of-life evolution (Hämäläinen et al. 2018; Immonen et al. 2018; Tarka et al. 2018; Moschilla et al. 2019). The optimisation of risk-taking behaviour under life-history trade-offs should, for example, generally favour this syndrome in males (Kemp 2002; Wolf and Weissing 2012). Interestingly, our data showed that the heightened male boldness effect is actually largest in the inbred domesticated strain (Fig. 1b). Captive breeding is expected to select inadvertently for increased boldness because of the absence of predation, overcrowding and competition for access to food (Huntingford 2004). Bolder genotypes may also be favoured because they are easier to catch (King et al. 2013) and, consequently, more likely to participate in designed crosses. However, there is little reason to expect that selective biases of this nature should apply more to males than to females. Assuming that sex differences reflect genetic differences rather than plasticity, the greater elaboration of male boldness implies a sex-specific consequence of domestication. Our data are in this sense consistent with the hypothesis of genetic linkage between boldness and male colouration (Godin and Dugatkin 1996; Bleakley et al. 2006), if not pleiotropy. Given that guppy colouration has a broad genetic basis, encompassing both autosomal and sex-linked loci (Haskins et al. 1970), defining this source of this relationship will ultimately require a quantitative genetic approach.
The hybrid strain was included in this study as an additional naturally occurring population but with the potential feature of domestic interbreeding. We found intermediate trait values for both body size and behaviour in this strain (Fig. 1), which is consistent with a hypothesis of admixture involving additive genes. However, there are various caveats. Foremost of which is that we must assume that the phenotypes of putatively hybridising wild and domestic guppies at the Darwin sampling site are reasonably well characterised by those of the studied wild-type and domestic strains. Some difference in each case is inevitable (sensu Lindholm et al. 2014), and particularly so for domestics because the candidates for hybridisation (i.e. commercially purchased garden pond fish) will be represented by varieties additional to those studied here (e.g. see Bleakley et al. 2006). We consider such variation likely to be greatly exceeded by the magnitude of the overall divergence between wild-type and domestic phenotypes, but it, nevertheless, remains unknown. Second, periodic influxes of highly inbred domestic genomes may influence the population phenotype via non-additive mechanisms involving changes in dominant and epistatic interactions and/or disruption of linkage complexes (Neiman and Linksvayer 2006). Functionally additive trait variance in the domestic strain arising from ‘converted’ epistatic or dominance variance (Goodnight 1988), for example, is less likely to express additive effects in an outbred gene pool. Such considerations warn against ascribing the hybrid phenotype to a simple additive outcome, and could in fact explain why behavioural trait variances were observationally lowest in this strain (and, indeed, not different from zero in females).
We know of only two other investigations into the boldness-type behaviours of domesticated ornamental guppies; one which reported on males only (Swaney et al. 2015), and another which pooled male and female data (Bleakley et al. 2006). The latter study is most directly relevant here. This study reports differences among specific ‘designer’ colour varieties for behaviours such as agitated swimming and freezing in response to a predator, as well as predator inspection behaviour. These varieties are genetically differentiated (pairwise FST = 0.23–0.49; Bleakley et al. 2008), which implies a genetic basis to their behavioural differences. Our study was not designed to examine differences among varieties, and nor did we detect any, even with sample sizes exceeding the n = 10 per variety used by Bleakley et al. (2006). Importantly, however, two of our varieties, namely ‘snakeskin’ and ‘gold neon’, closely match phenotypes contrasted by Bleakley et al. (2006) and for which they found no differences across five studied behaviours. These are also the least genetically differentiated pair of studied domestic varieties (FST = 0.23; Bleakley et al. 2008). Given that no similar basis exists for comparing the ‘red sunset’ variety, we consider the available information to support agreement between our findings and those of Bleakley et al. (2006). However, the main problem with interpreting variation among domestic colour varieties is the lack of information on the original provenance of genetic stock and then precisely on how phenotypes were derived via selection.
On a final note, whereas the high absolute boldness of domesticated fish agrees with expectation (e.g. Huntingford 2004), we were surprised by the evidence for significant individual variation in this strain. However, this does complement existing knowledge concerning the behavioural differentiation among domestic colour varieties (Bleakley et al. 2006), as well as indications that domesticated guppies retain ecologically important traits such as predator avoidance behaviour (Swaney et al. 2015). Putting aside the question of precisely how behavioural variation is maintained in captivity (and indeed despite sustained artificial selection on colour), its presence is likely to have important ecological implications. Perhaps the most salient consideration concerns the invasive potential of this species. Invasiveness has recently been linked to animal personality (Cote et al. 2011; Wolf and Weissing 2012; Carere and Gherardi 2013), and in particular to the presence of individual-level variation (such as we report here for boldness). A mix of differentially bold individuals, for example, may facilitate successful invasion owing to reasons spanning enhanced habitat-use flexibility and dispersal to greater metapopulation stability and the capacity to co-exist with novel biological communities (Wolf and Weissing 2012). Furthermore, individual variation may reflect additive genetic differentiation (Réale et al. 2007) and the subsequent potential for behaviour to adaptively evolve (Falconer 1981). Guppies have an ongoing legacy of human transport around the globe as ornamental aquarium pets and for mosquito control (El-Sabaawi et al. 2016), but have also readily established and invaded throughout their new wild environments. This has proceeded such a degree that naturalised guppy populations are now near ubiquitous in (sub)tropical freshwater systems around the world. Our data, coupled with those of Bleakley et al. (2006) and Swaney et al. (2015), point to a working hypothesis that such success relates not only to the existence of different guppy personality types, but also to the persistence of such variation even despite long-term breeding in captivity.
Data availability
The data corresponding to the paper are to be lodged in the dryad repository.
Conflicts of interest
The authors declare that they have no conflicts of interest.
Declaration of funding
The Australian Research Council supported this research via the award of Discovery-Projects Grant DP160103668 to D. J. Kemp.
Supplementary material
Supplementary material is available online.
Acknowledgements
We thank David Reznick and Pierre-Olivier Montiglio for insights into guppy biology and the study of animal personality, Rob Brooks and Culum Brown for access to laboratory stock, and Amanda Baxter for her unwavering care and maintenance of fish throughout this study.
References
Archard, GA, and Braithwaite, VA (2011). Variation in aggressive behaviour in the poeciliid fish Brachyrhaphis episcopi: population and sex differences. Behavioural Processes 86, 52–57.| Variation in aggressive behaviour in the poeciliid fish Brachyrhaphis episcopi: population and sex differences.Crossref | GoogleScholarGoogle Scholar | 20850509PubMed |
Baker, MR, Goodman, AC, Santo, JB, and Wong, RY (2018). Repeatability and reliability of exploratory behavior in proactive and reactive zebrafish, Danio rerio. Scientific Reports 8, 12114.
| Repeatability and reliability of exploratory behavior in proactive and reactive zebrafish, Danio rerio.Crossref | GoogleScholarGoogle Scholar | 30108258PubMed |
Barbosa, M, Deacon, AE, Janeiro, MJ, Ramnarine, I, Morrissey, MB, and Magurran, AE (2018). Individual variation in reproductive behaviour is linked to temporal heterogeneity in predation risk. Proceedings of the Royal Society B. Biological Sciences 285, 20171499.
| Individual variation in reproductive behaviour is linked to temporal heterogeneity in predation risk.Crossref | GoogleScholarGoogle Scholar |
Bell, AM (2005). Behavioural differences between individuals and two populations of stickleback (Gasterosteus aculeatus). Journal of Evolutionary Biology 18, 464–473.
| Behavioural differences between individuals and two populations of stickleback (Gasterosteus aculeatus).Crossref | GoogleScholarGoogle Scholar | 15715852PubMed |
Bell, AM, Hankison, SJ, and Laskowski, KL (2009). The repeatability of behaviour: a meta-analysis. Animal Behaviour 77, 771–783.
| The repeatability of behaviour: a meta-analysis.Crossref | GoogleScholarGoogle Scholar | 24707058PubMed |
Biro, PA (2012). Do rapid assays predict repeatability in labile (behavioural) traits? Animal Behaviour 83, 1295–1300.
| Do rapid assays predict repeatability in labile (behavioural) traits?Crossref | GoogleScholarGoogle Scholar |
Bleakley, BH, Martell, CM, and Brodie, ED (2006). Variation in anti-predator behavior among five strains of inbred guppies, Poecilia reticulata. Behavior Genetics 36, 783–791.
| Variation in anti-predator behavior among five strains of inbred guppies, Poecilia reticulata.Crossref | GoogleScholarGoogle Scholar | 16502137PubMed |
Bleakley, BH, Eklund, AC, and Brodie, ED (2008). Are designer guppies inbred? Microsatellite variation in five strains of ornamental guppies, Poecilia reticulata, used for behavioral research. Zebrafish 5, 39–48.
| Are designer guppies inbred? Microsatellite variation in five strains of ornamental guppies, Poecilia reticulata, used for behavioral research.Crossref | GoogleScholarGoogle Scholar | 18361681PubMed |
Boake, CRB (1989). Repeatability: its role in evolutionary studies of mating behavior. Evolutionary Ecology 3, 173–182.
| Repeatability: its role in evolutionary studies of mating behavior.Crossref | GoogleScholarGoogle Scholar |
Brooks, R, and Endler, JA (2001). Direct and indirect sexual selection and quantitative genetics of male traits in guppies (Poecilia reticulata). Evolution 55, 1002–1015.
| Direct and indirect sexual selection and quantitative genetics of male traits in guppies (Poecilia reticulata).Crossref | GoogleScholarGoogle Scholar | 11430637PubMed |
Brown, C, and Braithwaite, VA (2004). Size matters: a test of boldness in eight populations of the poeciliid Brachyraphis episcopi. Animal Behaviour 68, 1325–1329.
| Size matters: a test of boldness in eight populations of the poeciliid Brachyraphis episcopi.Crossref | GoogleScholarGoogle Scholar |
Brown, C, Jones, F, and Braithwaite, V (2005). In situ examination of boldness-shyness traits in the tropical poeciliid, Brachyraphis episcopi. Animal Behaviour 70, 1003–1009.
| In situ examination of boldness-shyness traits in the tropical poeciliid, Brachyraphis episcopi.Crossref | GoogleScholarGoogle Scholar |
Brown, C, Burgess, F, and Braithwaite, VA (2007). Heritable and experiential effects on boldness in a tropical poeciliid. Behavioral Ecology and Sociobiology 62, 237–243.
| Heritable and experiential effects on boldness in a tropical poeciliid.Crossref | GoogleScholarGoogle Scholar |
Burns, JG (2008). The validity of three tests of temperament in guppies (Poecilia reticulata). Journal of Comparative Psychology 122, 344–356.
| The validity of three tests of temperament in guppies (Poecilia reticulata).Crossref | GoogleScholarGoogle Scholar | 19014258PubMed |
Carere, C, and Gherardi, F (2013). Animal personalities matter for biological invasions. Trends in Ecology and Evolution 28, 5–6.
| Animal personalities matter for biological invasions.Crossref | GoogleScholarGoogle Scholar | 23131537PubMed |
Chapple, DG, Simmonds, SM, and Wong, BBM (2012). Can behavioral and personality traits influence the success of unintentional species introductions? Trends in Ecology and Evolution 27, 57–64.
| Can behavioral and personality traits influence the success of unintentional species introductions?Crossref | GoogleScholarGoogle Scholar | 22001529PubMed |
Chen, BJ, Liu, K, Zhou, LJ, Gomes-Silva, G, Sommer-Trembo, C, and Plath, M (2018). Personality differentially affects individual mate choice decisions in female and male Western mosquitofish (Gambusia affinis). PLoS One 13, 23.
| Personality differentially affects individual mate choice decisions in female and male Western mosquitofish (Gambusia affinis).Crossref | GoogleScholarGoogle Scholar |
Cohen J (1988) ‘Statistical power analysis for the behavioral sciences’, 2nd edn. (Lawrence Earlbaum and Associates: Hillsdale, NJ, USA)
Collins, SM, Hatch, SA, Elliott, KH, and Jacobs, SR (2019). Boldness, mate choice and reproductive success in Rissa tridactyla. Animal Behaviour 154, 67–74.
| Boldness, mate choice and reproductive success in Rissa tridactyla.Crossref | GoogleScholarGoogle Scholar |
Conrad, JL, Weinersmith, KL, Brodin, T, Saltz, JB, and Sih, A (2011). Behavioural syndromes in fishes: a review with implications for ecology and fisheries management. Journal of Fish Biology 78, 395–435.
| Behavioural syndromes in fishes: a review with implications for ecology and fisheries management.Crossref | GoogleScholarGoogle Scholar | 21284626PubMed |
Cote, J, Fogarty, S, Brodin, T, Weinersmith, K, and Sih, A (2011). Personality-dependent dispersal in the invasive mosquitofish: group composition matters. Proceedings of the Royal Society B. Biological Sciences 278, 1670–1678.
| Personality-dependent dispersal in the invasive mosquitofish: group composition matters.Crossref | GoogleScholarGoogle Scholar |
Dingemanse, NJ, Both, C, Drent, PJ, and Tinbergen, JM (2004). Fitness consequences of avian personalities in a fluctuating environment. Proceedings of the Royal Society B. Biological Sciences 271, 847–852.
| Fitness consequences of avian personalities in a fluctuating environment.Crossref | GoogleScholarGoogle Scholar |
Dingemanse, NJ, Wright, J, Kazem, AJN, Thomas, DK, Hickling, R, and Dawnay, N (2007). Behavioural syndromes differ predictably between 12 populations of three-spined stickleback. Journal of Animal Ecology 76, 1128–1138.
| Behavioural syndromes differ predictably between 12 populations of three-spined stickleback.Crossref | GoogleScholarGoogle Scholar |
Dochtermann, NA, and Royaute, R (2019). The mean matters: going beyond repeatability to interpret behavioural variation. Animal Behaviour 153, 147–150.
| The mean matters: going beyond repeatability to interpret behavioural variation.Crossref | GoogleScholarGoogle Scholar |
El-Sabaawi, RW, Frauendorf, TC, Marques, PS, Mackenzie, RA, Manna, LR, Mazzoni, R, Phillip, DAT, Warbanski, ML, and Zandonà, E (2016). Biodiversity and ecosystem risks arising from using guppies to control mosquitoes. Biology Letters 12, 20160590.
| Biodiversity and ecosystem risks arising from using guppies to control mosquitoes.Crossref | GoogleScholarGoogle Scholar | 28120806PubMed |
Edenbrow, M, and Croft, DP (2013). Environmental and genetic effects shape the development of personality traits in the mangrove killifish Kryptolebias marmoratus. Oikos 122, 667–681.
| Environmental and genetic effects shape the development of personality traits in the mangrove killifish Kryptolebias marmoratus.Crossref | GoogleScholarGoogle Scholar |
Endler, JA (1980). Natural selection on color patterns in Poecilia reticulata. Evolution 34, 76–91.
| Natural selection on color patterns in Poecilia reticulata.Crossref | GoogleScholarGoogle Scholar | 28563214PubMed |
Falconer DS (1981) ‘Introduction to quantitative genetics’, (Longman: London, UK)
Fraser, DF, Gilliam, JF, Daley, MJ, Le, AN, and Skalski, GT (2001). Explaining leptokurtic movement distributions: intrapopulation variation in boldness and exploration. American Naturalist 158, 124–135.
| Explaining leptokurtic movement distributions: intrapopulation variation in boldness and exploration.Crossref | GoogleScholarGoogle Scholar |
Gasparini, C, Speechley, EM, and Polverino, G (2019). The bold and the sperm: positive association between boldness and sperm number in the guppy. Royal Society Open Science 6, 6.
| The bold and the sperm: positive association between boldness and sperm number in the guppy.Crossref | GoogleScholarGoogle Scholar |
Gilmour AR, Gogel BJ, Cullis BR, Welham SJ, Thompson R (2015) ‘ASReml user guide release 4.1 functional specification’, (VSN International: Hemel Hempstead, UK)
Godin, JGJ, and Dugatkin, LA (1996). Female mating preference for bold males in the guppy, Poecilia reticulata. Proceedings of the National Academy of Sciences of the United States of America 93, 10262–10267.
| Female mating preference for bold males in the guppy, Poecilia reticulata.Crossref | GoogleScholarGoogle Scholar |
Goodnight, CJ (1988). Epistasis and the effect of founder events on the additive genetic variance. Evolution 42, 441–454.
| Epistasis and the effect of founder events on the additive genetic variance.Crossref | GoogleScholarGoogle Scholar | 28564006PubMed |
Hämäläinen, A, Immonen, E, Tarka, M, and Schuett, W (2018). Evolution of sex-specific pace-of-life syndromes: causes and consequences. Behavioral Ecology and Sociobiology 72, 1–15.
| Evolution of sex-specific pace-of-life syndromes: causes and consequences.Crossref | GoogleScholarGoogle Scholar |
Harris, S, Ramnarine, IW, Smith, HG, and Pettersson, LB (2010). Picking personalities apart: estimating the influence of predation, sex and body size on boldness in the guppy Poecilia reticulata. Oikos 119, 1711–1718.
| Picking personalities apart: estimating the influence of predation, sex and body size on boldness in the guppy Poecilia reticulata.Crossref | GoogleScholarGoogle Scholar |
Haskins, CP, Young, P, Hewitt, RE, and Haskins, EF (1970). Stabilized heterozygosis of supergenes mediating Y-linked color patterns in Lebistes reticulatus. Heredity 25, 575–589.
Hunt, J, Bussiere, LF, Jennions, MD, and Brooks, R (2004). What is genetic quality? Trends in Ecology and Evolution 19, 329–333.
| What is genetic quality?Crossref | GoogleScholarGoogle Scholar | 16701279PubMed |
Huntingford, FA (2004). Implications of domestication and rearing conditions for the behaviour of cultivated fishes. Journal of Fish Biology 65, 122–142.
| Implications of domestication and rearing conditions for the behaviour of cultivated fishes.Crossref | GoogleScholarGoogle Scholar |
Immonen, E, Hämäläinen, A, Schuett, W, and Tarka, M (2018). Evolution of sex-specific pace-of-life syndromes: genetic architecture and physiological mechanisms. Behavioral Ecology and Sociobiology 72, 23.
| Evolution of sex-specific pace-of-life syndromes: genetic architecture and physiological mechanisms.Crossref | GoogleScholarGoogle Scholar |
Ingley, SJ, Rehm, J, and Johnson, JB (2014). Size doesn’t matter, sex does: a test for boldness in sister species of Brachyrhaphis fishes. Ecology and Evolution 4, 4361–4369.
| Size doesn’t matter, sex does: a test for boldness in sister species of Brachyrhaphis fishes.Crossref | GoogleScholarGoogle Scholar | 25540696PubMed |
Irving, E, and Brown, C (2013). Examining the link between personality and laterality in a feral guppy Poecilia reticulata population. Journal of Fish Biology 83, 311–325.
| Examining the link between personality and laterality in a feral guppy Poecilia reticulata population.Crossref | GoogleScholarGoogle Scholar | 23902308PubMed |
Jäger, HY, Han, CS, and Dingemanse, NJ (2019). Social experiences shape behavioral individuality and within-individual stability. Behavioral Ecology 30, 1012–1019.
| Social experiences shape behavioral individuality and within-individual stability.Crossref | GoogleScholarGoogle Scholar |
Johnson, KVA, Aplin, LM, Cole, EF, Farine, DR, Firth, JA, Patrick, SC, and Sheldon, BC (2017). Male great tits assort by personality during the breeding season. Animal Behaviour 128, 21–32.
| Male great tits assort by personality during the breeding season.Crossref | GoogleScholarGoogle Scholar |
Kemp, DJ (2002). Sexual selection constrained by life history in a butterfly. Proceedings of the Royal Society B. Biological Sciences 269, 1341–1345.
| Sexual selection constrained by life history in a butterfly.Crossref | GoogleScholarGoogle Scholar |
Kemp, DJ, Batistic, FK, and Reznick, DN (2018). Predictable adaptive trajectories of sexual coloration in the wild: Evidence from replicate experimental guppy populations. Evolution 72, 2462–2477.
| Predictable adaptive trajectories of sexual coloration in the wild: Evidence from replicate experimental guppy populations.Crossref | GoogleScholarGoogle Scholar | 30055021PubMed |
Kenward, MG, and Roger, IH (1997). The precision of mixed effects estimates from restricted maximum likelihood. Biometrics 53, 983–997.
| 9333350PubMed |
King, AJ, Furtbauer, I, Mamuneas, D, James, C, and Manica, A (2013). Sex-differences and temporal consistency in stickleback fish boldness. PLoS One 8, 6.
| Sex-differences and temporal consistency in stickleback fish boldness.Crossref | GoogleScholarGoogle Scholar |
Kotrschal, A, Lievens, EJP, Dahlbom, J, Bundsen, A, Semenova, S, Sundvik, M, Maklakov, AA, Winberg, S, Panula, P, and Kolm, N (2014). Artificial selection on relative brain size reveals a positive genetic correlation between brain size and proactive personality in the guppy. Evolution 68, 1139–1149.
| Artificial selection on relative brain size reveals a positive genetic correlation between brain size and proactive personality in the guppy.Crossref | GoogleScholarGoogle Scholar | 24359469PubMed |
Kralj-Fiser, S, Laskowski, KL, and Garcia-Gonzalez, F (2019). Sex differences in the genetic architecture of aggressiveness in a sexually dimorphic spider. Ecology and Evolution 9, 10758–10766.
| Sex differences in the genetic architecture of aggressiveness in a sexually dimorphic spider.Crossref | GoogleScholarGoogle Scholar | 31624579PubMed |
Lessells, CM, and Boag, PT (1987). Unrepeatable repeatabilities: a common mistake. Auk 104, 116–121.
| Unrepeatable repeatabilities: a common mistake.Crossref | GoogleScholarGoogle Scholar |
Lindholm, AK, Breden, F, Alexander, HJ, Chan, WK, Thakurta, SG, and Brooks, R (2005). Invasion success and genetic diversity of introduced populations of guppies Poecilia reticulata in Australia. Molecular Ecology 14, 3671–3682.
| Invasion success and genetic diversity of introduced populations of guppies Poecilia reticulata in Australia.Crossref | GoogleScholarGoogle Scholar | 16202088PubMed |
Lindholm, AK, Head, ML, Brooks, RC, Rollins, LA, Ingleby, FC, and Zajitschek, SRK (2014). Causes of male sexual trait divergence in introduced populations of guppies. Journal of Evolutionary Biology 27, 437–448.
| Causes of male sexual trait divergence in introduced populations of guppies.Crossref | GoogleScholarGoogle Scholar | 24456226PubMed |
Lucon-Xiccato, T, and Dadda, M (2016). Guppies show behavioural but not cognitive sex differences in a novel object recognition test. PLoS One 11, 12.
| Guppies show behavioural but not cognitive sex differences in a novel object recognition test.Crossref | GoogleScholarGoogle Scholar |
MacArthur RH, Wilson EO (1967) ‘The theory of Island biogeography’, (Princeton University Press: Princeton, NJ, USA)
McDougall, PT, Réale, D, Sol, D, and Reader, SM (2006). Wildlife conservation and animal temperament: causes and consequences of evolutionary change for captive, reintroduced, and wild populations. Animal Conservation 9, 39–48.
| Wildlife conservation and animal temperament: causes and consequences of evolutionary change for captive, reintroduced, and wild populations.Crossref | GoogleScholarGoogle Scholar |
Mittelbach, GG, Ballew, NG, and Kjelvik, MK (2014). Fish behavioral types and their ecological consequences. Canadian Journal of Fisheries and Aquatic Sciences 71, 927–944.
| Fish behavioral types and their ecological consequences.Crossref | GoogleScholarGoogle Scholar |
Montiglio, PO, Wey, TW, Chang, AT, Fogarty, S, and Sih, A (2016). Multiple mating reveals complex patterns of assortative mating by personality and body size. Journal of Animal Ecology 85, 125–135.
| Multiple mating reveals complex patterns of assortative mating by personality and body size.Crossref | GoogleScholarGoogle Scholar |
Moschilla, JA, Tomkins, JL, and Simmons, LW (2019). Sex-specific pace-of-life syndromes. Behavioral Ecology 30, 1096–1105.
| Sex-specific pace-of-life syndromes.Crossref | GoogleScholarGoogle Scholar |
Nakagawa, S, and Cuthill, IC (2007). Effect size, confidence interval and statistical significance: a practical guide for biologists. Biological Reviews 82, 591–605.
| Effect size, confidence interval and statistical significance: a practical guide for biologists.Crossref | GoogleScholarGoogle Scholar | 17944619PubMed |
Neiman, M, and Linksvayer, TA (2006). The conversion of variance and the evolutionary potential of restricted recombination. Heredity 96, 111–121.
| The conversion of variance and the evolutionary potential of restricted recombination.Crossref | GoogleScholarGoogle Scholar | 16333302PubMed |
Piyapong, C, Krause, J, Chapman, BB, Ramnarine, IW, Louca, V, and Croft, DP (2010). Sex matters: a social context to boldness in guppies (Poecilia reticulata). Behavioral Ecology 21, 3–8.
| Sex matters: a social context to boldness in guppies (Poecilia reticulata).Crossref | GoogleScholarGoogle Scholar |
Rangel-Patino, C, Garcia-Morales, C, Mastachi-Loza, C, Carmen-Cristobal, JM, and Ruiz-Gomez, MD (2018). Personality and its variation between sexes in the black-bellied bunchgrass lizard Sceloporus aeneus during the breeding season. Ethology 124, 796–803.
| Personality and its variation between sexes in the black-bellied bunchgrass lizard Sceloporus aeneus during the breeding season.Crossref | GoogleScholarGoogle Scholar |
Réale, D, Reader, SM, Sol, D, McDougall, PT, and Dingemanse, NJ (2007). Integrating animal temperament within ecology and evolution. Biological Reviews 82, 291–318.
| Integrating animal temperament within ecology and evolution.Crossref | GoogleScholarGoogle Scholar | 17437562PubMed |
Réale, D, Garant, D, Humphries, MM, Bergeron, P, Careau, V, and Montiglio, PO (2010). Personality and the emergence of the pace-of-life syndrome concept at the population level. Philosophical Transactions of the Royal Society B. Biological Sciences 365, 4051–4063.
| Personality and the emergence of the pace-of-life syndrome concept at the population level.Crossref | GoogleScholarGoogle Scholar |
Reddon, AR, and Hurd, PL (2009). Individual differences in cerebral lateralization are associated with shy-bold variation in the convict cichlid. Animal Behaviour 77, 189–193.
| Individual differences in cerebral lateralization are associated with shy-bold variation in the convict cichlid.Crossref | GoogleScholarGoogle Scholar |
Reznick, DN, Shaw, FH, Rodd, FH, and Shaw, RG (1997). Evaluation of the rate of evolution in natural populations of guppies (Poecilia reticulata). Science 275, 1934–1937.
| Evaluation of the rate of evolution in natural populations of guppies (Poecilia reticulata).Crossref | GoogleScholarGoogle Scholar | 9072971PubMed |
Reznick, DN, Bryant, MJ, and Bashey, F (2002). r- and K-selection revisited: the role of population regulation in life-history evolution. Ecology 83, 1509–1520.
| r- and K-selection revisited: the role of population regulation in life-history evolution.Crossref | GoogleScholarGoogle Scholar |
Rezucha, R, and Reichard, M (2016). The association between personality traits, morphological traits and alternative mating behaviour in male Endler’s guppies, Poecilia wingei. Ethology 122, 456–467.
| The association between personality traits, morphological traits and alternative mating behaviour in male Endler’s guppies, Poecilia wingei.Crossref | GoogleScholarGoogle Scholar |
Rowe, L, and Houle, D (1996). The lek paradox and the capture of genetic variance by condition dependent traits. Proceedings of the Royal Society B. Biological Sciences 263, 1415–1421.
| The lek paradox and the capture of genetic variance by condition dependent traits.Crossref | GoogleScholarGoogle Scholar |
Roy, T, and Bhat, A (2018). Repeatability in boldness and aggression among wild zebrafish (Danio rerio) from two differing predation and flow regimes. Journal of Comparative Psychology 132, 349–360.
| Repeatability in boldness and aggression among wild zebrafish (Danio rerio) from two differing predation and flow regimes.Crossref | GoogleScholarGoogle Scholar | 30451523PubMed |
Russell WMS, Burch RL (1959) ‘The principles of humane experimental technique’, (Methuen: London, UK)
Scherer, U, Godin, JGJ, and Schuett, W (2020). Do female rainbow kribs choose males on the basis of their apparent aggression and boldness? A non-correlational mate choice study. Behavioral Ecology and Sociobiology 74, 15.
| Do female rainbow kribs choose males on the basis of their apparent aggression and boldness? A non-correlational mate choice study.Crossref | GoogleScholarGoogle Scholar |
Schuett, W, Tregenza, T, and Dall, SRX (2010). Sexual selection and animal personality. Biological Reviews 85, 217–246.
| Sexual selection and animal personality.Crossref | GoogleScholarGoogle Scholar | 19922534PubMed |
Sih, A, Bell, A, and Johnson, JC (2004). Behavioral syndromes: an ecological and evolutionary overview. Trends in Ecology and Evolution 19, 372–378.
| Behavioral syndromes: an ecological and evolutionary overview.Crossref | GoogleScholarGoogle Scholar | 16701288PubMed |
Smith, BR, and Blumstein, DT (2010). Behavioral types as predictors of survival in Trinidadian guppies (Poecilia reticulata). Behavioral Ecology 21, 919–926.
| Behavioral types as predictors of survival in Trinidadian guppies (Poecilia reticulata).Crossref | GoogleScholarGoogle Scholar |
Sneddon, LU (2003). The bold and the shy: individual differences in rainbow trout. Journal of Fish Biology 62, 971–975.
| The bold and the shy: individual differences in rainbow trout.Crossref | GoogleScholarGoogle Scholar |
Swaney, WT, Cabrera-Alvarez, MJ, and Reader, SM (2015). Behavioural responses of feral and domestic guppies (Poecilia reticulata) to predators and their cues. Behavioural Processes 118, 42–46.
| Behavioural responses of feral and domestic guppies (Poecilia reticulata) to predators and their cues.Crossref | GoogleScholarGoogle Scholar | 26003138PubMed |
Tarka, M, Guenther, A, Niemela, PT, Nakagawa, S, and Noble, DWA (2018). Sex differences in life history, behavior, and physiology along a slow-fast continuum: a meta-analysis. Behavioral Ecology and Sociobiology 72, 13.
| Sex differences in life history, behavior, and physiology along a slow-fast continuum: a meta-analysis.Crossref | GoogleScholarGoogle Scholar |
Toms, CN, and Echevarria, DJ (2014). Back to basics: searching for a comprehensive framework for exploring individual differences in zebrafish (Danio rerio) behavior. Zebrafish 11, 325–340.
| Back to basics: searching for a comprehensive framework for exploring individual differences in zebrafish (Danio rerio) behavior.Crossref | GoogleScholarGoogle Scholar | 24921670PubMed |
Trompf, L, and Brown, C (2014). Personality affects learning and trade-offs between private and social information in guppies, Poecilia reticulata. Animal Behaviour 88, 99–106.
| Personality affects learning and trade-offs between private and social information in guppies, Poecilia reticulata.Crossref | GoogleScholarGoogle Scholar |
White, SJ, and Wilson, AJ (2019). Evolutionary genetics of personality in the Trinidadian guppy I: maternal and additive genetic effects across ontogeny. Heredity 122, 1–14.
| Evolutionary genetics of personality in the Trinidadian guppy I: maternal and additive genetic effects across ontogeny.Crossref | GoogleScholarGoogle Scholar | 29773896PubMed |
White, SJ, Houslay, TM, and Wilson, AJ (2019). Evolutionary genetics of personality in the Trinidadian guppy II: sexual dimorphism and genotype-by-sex interactions. Heredity 122, 15–28.
| Evolutionary genetics of personality in the Trinidadian guppy II: sexual dimorphism and genotype-by-sex interactions.Crossref | GoogleScholarGoogle Scholar | 29795179PubMed |
Wilson, AJ (2018). How should we interpret estimates of individual repeatability? Evolution Letters 2, 4–8.
| How should we interpret estimates of individual repeatability?Crossref | GoogleScholarGoogle Scholar | 30283660PubMed |
Wilson, DS, Clark, AB, Coleman, K, and Dearstyne, T (1994). Shyness and boldness in humans and other animals. Trends in Ecology and Evolution 9, 442–446.
| Shyness and boldness in humans and other animals.Crossref | GoogleScholarGoogle Scholar |
Wolf, M, and Weissing, FJ (2012). Animal personalities: consequences for ecology and evolution. Trends in Ecology and Evolution 27, 452–461.
| Animal personalities: consequences for ecology and evolution.Crossref | GoogleScholarGoogle Scholar | 22727728PubMed |


 . As in
. As in 