Benthic foraminifera as bioindicators for assessing reef condition in Kāne‘ohe Bay, O‘ahu, Hawai‘i
Gregor H. Mathes A B C * , Manuel J. Steinbauer A B D and Laura Cotton E F
A B C * , Manuel J. Steinbauer A B D and Laura Cotton E F
A Bayreuth Center of Ecology and Environmental Research, University of Bayreuth, Bayreuth, Germany.
B Department of Sport Science, University of Bayreuth, Bayreuth, Germany.
C GeoZentrum Nordbayern, Friedrich-Alexander-Universitat Erlangen-Nürnberg, Erlangen, Bayern, Germany.
D Department of Biological Sciences, University of Bergen, Bergen, Hordaland, Norway.
E Department of Geological Sciences, University of Florida, Gainesville, FL, USA.
F Natural History Museum of Denmark, University of Copenhagen, Copenhagen, Denmark.
Pacific Conservation Biology 29(3) 238-245 https://doi.org/10.1071/PC21027
Submitted: 17 April 2021 Accepted: 20 March 2022 Published: 12 April 2022
© 2023 The Author(s) (or their employer(s)). Published by CSIRO Publishing. This is an open access article distributed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND)
Abstract
Context: Tropical coral reef environments provide a wide variety of goods and ecosystem services but are experiencing growing pressure from coastal development and tourism. Assessing the status of reef communities along gradients of human pressure is therefore necessary to predict recovery and resilience capacity of reefs.
Aims: First, to determine the overall water quality in Kāne‘ohe Bay, O’ahu, Hawai‘i, by employing a low-cost monitoring approach for anthropogenic stress on coral reef areas. Second, to assess the suitability of the monitoring approach to complement existing monitoring programmes.
Methods: Sediment samples containing benthic foraminifera were used to determine water quality and stressor sources in Kāne‘ohe Bay, O’ahu, Hawai‘i, by applying the Foram Index (FI) and Bayesian regression analysis. The FI is based on relative abundance of functional groups of larger benthic foraminifera.
Key results: Overall water quality in Kāne‘ohe Bay may support active growth and recovery of coral reefs in the northern sector but deteriorates around Kāne‘ohe City.
Conclusions: Benthic foraminifera can be used as bio-indicators in Hawaiian reefs, providing an easy and fast-to-apply method for assessing short-term changes in water quality and stress sources. Implementing benthic foraminifera studies within existing long-term monitoring programs of Hawaiian reefs can be beneficial for conservation efforts.
Implications: Within a historic context, our findings illustrate the modest recovery of an ecosystem following pollution control measures but highlight the need of conservation efforts for reef environments adjacent to major human settlements.
Keywords: anthropogenic stress, assessment, coral reef, corals, foram index, marine, monitoring, pollution, reef crisis, reef health, water quality.
Introduction
Coral reef environments provide a wide variety of goods and services, including waste detoxification and vital food resources for millions of people (Holmlund and Hammer 1999; Adger et al. 2005; Woodhead et al. 2019). However, current climate warming, the increase of ocean pollution, acidification of the oceans, and the manifold forms of habitat destruction endanger modern coral reefs (Pandolfi et al. 2003; Barnosky et al. 2017). To evaluate and subsequently manage coral reef ecosystems, reefal, ecological, environmental, and anthropogenic characteristics must be considered (Sandin et al. 2008). Anthropogenic impacts in particular are a growing threat to coral environments, as the population of the Earth is projected to increase dramatically in the next 35 years (Dubois 2011). Coral reef environments on the Hawaiian Archipelago represent one of the most intensively studied reef systems worldwide, with an exceptional record of both natural and human-induced perturbations of the past. Coral reef ecosystems on Hawai‘i experienced major bleaching events (Burke et al. 2011) as well as rapid sea level rise (Leuliette 2012) and were subject of major anthropogenic impacts (Williams et al. 2008; Filous et al. 2017; Friedlander et al. 2018). Anthropogenic stressors on Hawai‘i likely have amplified in the past decades, as coastal development continues to increase with a growing human population. Current long-term monitoring programs focus mainly on the description of spatial and temporal dynamics of Hawaiian reef communities, and less on the potential anthropogenic drivers of these dynamics (Jokiel et al. 2004; Rodgers et al. 2015).
Here, we employ a low-cost approach to monitor anthropogenic stress on coral reef areas on Hawai‘i and assess its suitability to complement existing monitoring programs. The methodological approach was initially developed for western Atlantic-Caribbean reefs (Hallock et al. 2003) but has since been successfully extended to reefal areas all over the world (Hallock 2012). We first report the abundance and distribution of benthic foraminifera genera from 13 sediment samples in Kāne‘ohe Bay, Hawai‘i. As assemblages of benthic foraminiferal shells in sediment closely reflect water and sediment quality, they can be used to monitor high-resolution records of coastal pollution (Hallock et al. 2003; Frontalini and Coccioni 2008; Uthicke and Nobes 2008) and anthropogenic stress (Alve 1991; Frontalini and Coccioni 2008; Caruso et al. 2011). To do so, we transformed the raw abundance counts of foraminiferal shells into a well-established measure for water quality, the Foram Index (FI) (Hallock et al. 2003; Hallock 2012; Prazeres et al. 2020). The FI is based on the ratio of three functional groups of foraminifera: (1) taxa of larger foraminifera that host algal symbionts and reflect high water quality; (2) pollution-tolerant opportunistic foraminifera that dominate high-stress environments; and (3) small taxa that proliferate in response to nutrification. We then used the FI and distances to potential centres of anthropogenic stress (Kāne‘ohe City, Kahalu‘u City, and the Marine Corps Base Hawai‘i) to analyse whether spatial assemblage shifts are correlated with anthropogenic impacts in Kāne‘ohe Bay. Our results indicate that overall water quality is high in Kāne‘ohe Bay but deteriorates around Kāne‘ohe City. Given the potential applicability and a low expenditure of foraminiferal-based measures for water quality, we propose that implementing benthic foraminifera as bio-indicators for Hawaiian reefs can be beneficial for existing long-term monitoring programs.
Materials and methods
Regional setting
Kāne‘ohe Bay, situated on the windward coast of O‘ahu, Hawai‘i, is one of the most intensely studied estuarine and coral reef systems in the world (Bathen 1968; Banner 1974; Hunter and Evans 1995). It is located on the north-east coast of O‘ahu with a length of 13.5 km at its maximum and 4.5 km width from shore to the outer barrier reef (Fig. 1). The bay is bordered by the only barrier reef in the Hawaiian archipelago. The reef is cut by two natural channels and a dredged ship channel connecting the north and the south passages. Between the 1940s and 1970s, Kāne‘ohe Bay coral reefs suffered impacts to the reef community due to anthropogenic activities concomitant with land use changes, such as eutrophic conditions ensuing from sewage discharges into the bay, and channelisation of streams (Pastorok and Bilyard 1985; Ringuet and Mackenzie 2005). Additionally, extensive reef dredging amplified these impacts. Two large sewage outfalls were diverted from the bay in 1977–1978 (Smith et al. 1981; Laws and Redalje 1982), followed by a partial recovery of coral-reef dominated communities in Kāne‘ohe Bay (Hunter and Evans 1995). This trend, however, was slowing down since 1984 and subsequently even reversed, co-occurring with increasing size of the adjacent cities Kāne‘ohe and Kahalu‘u and the expansion of the marine corps-base (Hunter and Evans 1995). This urban growth concurred with non-point source pollution as well as increased runoff nutrient input into the bay linked to considerable impacts on the bay ecosystem (Ringuet and Mackenzie 2005; Hoover et al. 2006). Foraminiferal assemblages responded to these perturbations with a shift in composition and a severe decrease in abundance (P. Hallock, pers. comm.). Kāne‘ohe Bay is monitored since 1999 as part of the Hawai‘i Coral Reef Assessment and Monitoring Program. Between 1999 and 2002, coral reef coverage decreased in five out of six sampled stations in Kāne‘ohe Bay (Jokiel et al. 2004), whereas only one of the six stations showed a decrease over a 14-year period (Rodgers et al. 2015).
Sampling sites
Samples were collected during 2017 from Kāne‘ohe Bay by researchers from the Florida Museum of Natural History sampling surface sediment by scuba diving. Thirteen samples were taken across a variety of shallow water environments between 1 and 14 m water depth and a variety of distances from settlements on the island to examine the spatial variation in assemblage and any potential impact from anthropogenic sources (Supplementary Table S1). The locality in the bay, the longitude and latitude, the water depth, and the habitat were assigned to each individual sample. The distance to centres of anthropogenic stress (cities and military bases) were calculated by using the programme Google Earth (http://earth.google.com).
Sampling treatment
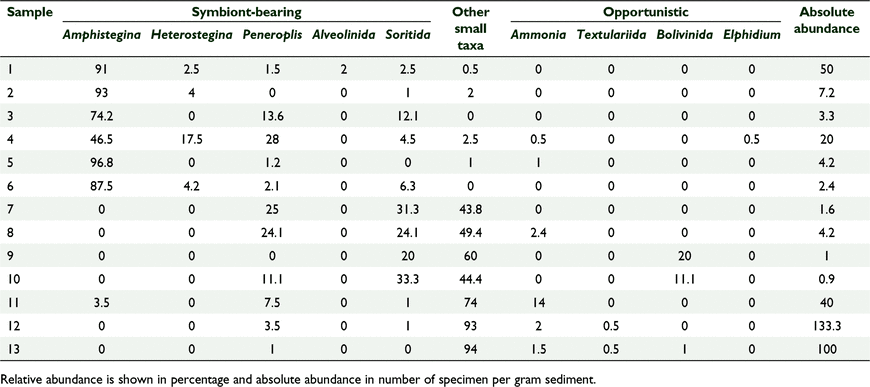
The foraminiferal assemblages were wet sieved through 63 μm and dried in a low temperature oven (~40°C). Following this, up to 200 foraminiferal specimens of each sample were picked under a stereo microscope following a standard protocol (Hallock et al. 2003). Each sample was split into smaller subsets of approximately 0.1 g and weighed. We then used the first weighed subset of the sample to pick out foraminiferal specimen until we reached a number of 200 specimen (Dix 2002). If less than 200 specimen were present in the subset, we repeated the picking procedure on a second 0.1 g subset from the sample. This procedure was repeated until 200 specimens were obtained or until the entire gram of sample was processed. Foraminiferal taxa were identified to generic level according to Loeblich and Tappan (2015). We used the FI (Hallock et al. 2003; Hallock 2012; Prazeres et al. 2020) to assess water quality and suitability for reef-building corals of the study area. The FI is defined by the ratio of large benthic foraminifera that host phototrophic endosymbionts to small heterotrophic foraminifera. Heterotrophic taxa proliferate under the input of nutrients into the sea water, while large symbiont-bearing taxa are constrained to water-quality conditions similar to those required by corals. Under extreme local nutrient input, with subsequent intermittent anoxia in the sediments, a few known taxa of heterotrophic, stress-tolerant foraminifera can become dominant (Alve and Bernhard 1995; Carnahan et al. 2009; Pisapia et al. 2017). Accordingly, we classified specimens into one of three functional groups: (1) symbiont-bearing; (2) opportunistic; or (3) other smaller taxa. For each sample, the FI was determined by the equation: FI = (10 × Ps) + (Po) + (2 × Ph), where ‘P’ is the proportion and where subscript ‘s’ represents symbiont-bearing foraminifera, subscript ‘o’ represents opportunistic foraminifera, and subscript ‘h’ represents other small, heterotrophic foraminifera. The FI scale ranges from 1 to 10, with FI >4 indicating environment conducive to reef growth, 2 < FI < 4 indicating environment marginal for reef growth and unsuitable for recovery, and FI <2 indicating stressed conditions unsuitable for reef growth. During specimen counting, the degree of bioclast preservation was also evaluated (Carnahan et al. 2009; Hallock 2012). Badly broken or possibly reworked specimen, which could not be identified to genus level, were omitted from the analysis (Hallock et al. 2003; Prazeres et al. 2020). Relative abundance (proportions of the subsample) and absolute abundance (numbers of specimens per gram of sediment) where calculated following standard procedures (Hallock et al. 2003).
Data analysis
All analysis were carried out using the R programming environment (R Core Team 2021). We used the ‘tidyverse’ package collection for data wrangling and visualisation (Wickham et al. 2019), the ‘vegan’ package (Oksanen et al. 2020) for non-metric multidimensional scaling (nMDS) ordination, and the ‘brms’ package for Bayesian regression analysis (Bürkner 2017). nMDS was conducted to analyse the community structure of all samples and was based on Bray-Curtis dissimilarity. Bayesian linear regression analysis was carried out to test if the water quality as indicated by the FI in the southern area of Kāne‘ohe Bay, which is mainly characterised by urban development, is lower compared to the northern sector, which is further away from cities and military bases. We first fitted three regression models with the FI as the outcome variable including an intercept only null model, a model with distances to all major human settlements in the bay (Kāne‘ohe City, Kahalu‘u City, and Marine Corps Base Hawai‘i (MCBH), and a model with a all settlements and additionally water depth as a predictor variable. This approach enabled us to compare the predictive effect of distance to human settlements to a null baseline as well as to water depth, which might be a possible confounding driver of the FI (Hallock 2012). Models were compared by means of leave-one-out cross-validation using Pareto-smoothed importance sampling (Vehtari et al. 2017). We transformed the outcome and all predictor variables to z-scores prior to model fitting to facilitate an easier calculation of the joint posterior probability distribution. All three models were fitted via the probabilistic programming language Stan using a Hamiltonian Monte Carlo Markov Chain (MCMC) and the No-U-Turn sampler (Gelman et al. 2015). We used weakly informative priors for all parameters that were easily exceeded by the actual data while reducing over-fitting compared to traditional frequentist approaches. The joint posterior probability distribution was estimated by four MCMC chains, a warm-up of 500 samples, and 2000 actual samples. We then used standard convergence and efficiency diagnostics to evaluate the sampling performance, based on Rhat values and the number of effective sample size (Vehtari et al. 2021).
Robustness testing
As a FI value of 10 is possible but unusual even in pristine regions (see Discussion), we further conducted a robustness test by removing all samples with values above 9.5 and repeating our analysis on this data subset. We then compared the results from the analysis based on the subset to the results based on all samples, to see whether potentially biassed samples with FI values above 9.5 might confound our findings.
Results
Community analysis
The assemblages show an average generic level-richness compared to other tropical warm water coral reefs (Hallock 2012). In total, 15 genera were identified and classified according to the three functional groups: (1) symbiont-bearing; (2) opportunistic; and (3) small heterotrophic foraminifera (Table 1). A clear spatial distribution of foraminiferal assemblages Kāne‘ohe Bay can be perceived: The northern sector is dominated by symbiont-bearing genera, in the middle sector all three functional groups are present, and the southern sector is characterised by heterotrophic genera (Fig. 1). Sample sites located on the barrier reef (1–6) are all dominated by symbiont-bearing foraminifera. In the middle sector of the bay, between the barrier reef and the coastline, the number of small heterotrophic genera increases. While the four samples that are located closest to the shore (9, 11–13) are dominated by small heterotrophic genera, the three samples in the middle sector (7, 8, 10) show an equal distribution between heterotrophic and symbiont-bearing taxa. Opportunistic genera are most abundant in the middle sector; however, they still remain the least abundant of the three functional groups even in the middle sector. Symbiont-bearing and opportunistic taxa are less abundant in the four near-shore samples. Overall, absolute abundance ranged from 0.9 to 133.3 individuals per gram of sediment, including three samples with less than two specimen per gram of sediment. The most abundant genera of the symbiont-bearing functional group were Amphistegina spp., Peneroplis spp., Sorites spp. and Heterostegina spp. (see Table S1 for relative and absolute abundance of all foraminiferal taxa). Opportunistic species were generally rare, and included Ammonia spp., Elphidium spp., and Bolivinida spp. The genus Amphistegina spp. from the symbiont bearing group had the greatest relative abundance. It dominated 46% of the assemblages, whereas the other 54% were dominated by small heterotrophic group genera. Amphistegina spp. also constituted 38% of the total foraminiferal population in Kāne‘ohe Bay and was present in 7 of the 13 sampling stations. However, Peneroplis spp. and Sorites spp. were found in 11 of the 13 sampling stations, making them the most widespread genera. Non-metric multidimensional scaling based on the foraminiferal assemblages show a clear clustering of the samples in three groups, closely corresponding to the three functional groups used to calculate the FI (Fig. S3).
|
Foram Index (FI)
The FI calculated for the sampled sites revealed values between 2.1 and 10, with a median of 6.8 (Fig. S1, Table S2). Four samples (9, 11–13, located close to the shore) are indicating environment marginal for reef growth and unsuitable for recovery, whereas the remaining nine samples are indicating environment conducive to reef growth. FI results mirror assemblage clusters attained by applying a nMDS scaling approach to the samples, indicating a strong biotic driver for foraminiferal distribution and emphasising the reliability of the FI.
Distance to human settlements
Model comparison showed that distance to human settlements (Kāne‘ohe City, Kahalu‘u City, and MCBH) is a robust predictor of the FI (Table S3). The Bayesian regression model revealed a substantial relationship between FI values and distance to Kāne‘ohe City, showing that samples scored lower FI values when they were located closer to Kāne‘ohe City (Figs 2 and 3). The model yielded no robust relationships between FI values and distance to Kahalu‘u City and MCBH, respectively. A regression model fitted on a subset of the data for robustness testing (see Materials and Methods and Fig. S2) yielded similar results, with a strong relationship between the FI and distance to Kāne‘ohe City while showing no consistent relationship for distance to Kahalu‘u City and to the MCBH. Our results hence indicate that a stress gradient is present in Kāne‘ohe Bay, with the highest stress close to Kāne‘ohe City and less further away from Kāne‘ohe City, while smaller settlements in the bay have less to no impact.
Discussion
Using a foraminiferal-based index for water quality, we found a clear spatial stress gradient in Kāne‘ohe Bay with good water quality in the outer bay and low water quality close to the shore. The distance of each sediment sample to Kāne‘ohe City turned out to be a strong predictor of this trend, while smaller settlements in the bay seemed to be less influential. This effect might result from non-point pollution by the adjacent city of Kāne‘ohe, or by organic matter input through the river mouths in this area. Our results are in line with other empirical studies showing periodical reef degradation in Kāne‘ohe Bay either through anthropogenic activities or natural processes such as freshwater flooding and erosional runoff (Hunter and Evans 1995; Laws and Allen 1996; Jokiel and Brown 2004; Neilson 2014). We further found the majority of the sampled area conducive to reef growth. One reason for these moderate to good conditions for coral reefs could be that the water body of Kāne‘ohe Bay is relatively well mixed vertically and horizontally under most conditions (Ringuet and Mackenzie 2005). Possible pollution sources around Kāne‘ohe are therefore quickly dispersed, as well as organic matter from riverine input. However, one-third of our samples indicated environment marginal for reef growth and unsuitable for recovery. This might be particularly warning as major coral bleaching events were observed in Kāne‘ohe Bay in the past (Jokiel and Brown 2004; Neilson 2014). Hence, reefs close to the shore and especially close to Kāne‘ohe City might not be able to recover after a period of perturbations, be it natural or anthropogenic stressors. We therefore agree with other current reef health assessments of Kāne‘ohe Bay that it is necessary to pay continuous attention to local pollution, impacts of climate change, sedimentation, and harvest issues (Jokiel et al. 2004; Bahr et al. 2015; Rodgers et al. 2015). Ongoing monitoring programs in the bay could benefit from the implementation of the FI as a fast and low expenditure method to assess conditions for reef growth. Although this index was not specifically developed for use in islands in the central Pacific Ocean (Hallock et al. 2003), our study shows that the application to Hawaiian reefs is feasible as our results are in line with other studies in Kāne‘ohe Bay using a variety of indicators for reef health and water quality (Maragos 1972; Hunter and Evans 1995; Fagan and Mackenzie 2007; Rodgers et al. 2015; Friedlander et al. 2018).
FI values obtained in this study appear similar to those from other regions with anthropogenic pollution (Barbosa et al. 2009; Carnahan et al. 2009; Caruso et al. 2011; Barbosa et al. 2012). However, FI values of 10 are seldom recorded in other studies even in pristine regions (Barbosa et al. 2009; Barbosa et al. 2012). In this study, five samples (1–3, 5, 6) recorded a FI value of approximately 10 in the outer bay of Kāne‘ohe, mainly consisting of lens-shaped Amphistegina spp. and Heterostegina spp. These genera tend to remain in the sediment for a prolonged time due to their test-shape and their robust nature. Hence, samples with a FI of 10 may have experienced reworking by currents for a longer time interval and could be therefore biased. However, these potentially biased samples do not confound our findings, as the robustness testing based on samples 6–13 showed equal results compared to the analysis of all samples. All other samples showed good preservation of delicate test-forms, indicating that the FI from these samples can be considered as reliable and represent accumulation over short time. North-easterly winds present in the northern area (Smith et al. 1981; Laws and Allen 1996) might have removed smaller foraminifera taxa from the sediment by grain size sorting, resulting in biased high FI values for this area. However, winter storm motion and trade wind influence is restricted to the northern area (Bathen 1968) and should not influence samples from the southern area. Although the FI can vary with other parameters such as sediment texture (Narayan and Pandolfi 2010), hydrodynamic regime, and light penetration (Barbosa et al. 2009), various studies have shown that the FI is primarily related to water quality (Uthicke and Nobes 2008; Koukousioura et al. 2011; Velásquez et al. 2011; Banner 1974; Oliver et al. 2014). The results from our Bayesian regression framework might support this as there was no apparent relationship between the FI and water depth (Table S3). Hence, high FI values of samples 1–5 could be biased by reworking and/or hydrodynamic sorting, but we expect remaining samples to be robust and reflect true water quality. Based on these, the coastal waters adjacent to Kāne‘ohe City in the southern sector seem to be impacted by anthropogenic stress and/or organic material input with eutrophic water conditions.
Based on our results, we emphasise that implementing benthic foraminifera studies within existing long-term monitoring programs of Hawaiian reefs can be beneficial for conservation efforts. We showed that benthic foraminifera can be used as bio-indicators in Hawaiian reefs, providing an easy and fast-to-apply method for assessing short-term changes in water quality and stress sources. Hence, abundance and distribution of benthic foraminiferal taxa reported in this study can be used as a baseline to compare changes in Kāne‘ohe Bay over both time and space. In conclusion, we found a clear and robust spatial pattern for reef suitability in Kāne‘ohe Bay, with areas closer to the shore and especially closer to Kāne‘ohe City being less suitable, while samples from the northern bay area indicated conditions more suitable for reef growth and recovery. Our findings highlight the need of an ongoing monitoring for reef areas in Kāne‘ohe Bay to protect the frail local ecosystem from both natural and anthropogenic impacts.
Supplementary material
Supplementary material is available online.
Data availability
All code and both raw and processed data are available on https://github.com/Ischi94/forams_on_hawaii.
Conflicts of interest
The authors declare no conflicts of interest.
Declaration of funding
G.H.M. and M.J.S. were supported by the Deutsche Forschungsgemeinschaft (DFG) grant STE 2360/2-1 as part of the Research Unit TERSANE (FO 2332). M.J.S. acknowledges support by ERC grant 741413 Humans on Planet Earth (HOPE).
Acknowledgements
We thank Pamela Hallock, Emilia Jarochowska, Nussaibah Raja and Wolfgang Kiessling for helpful scientific discussions and comments. We also thank Michal Kowalewski, Kristopher Kusnerik and Gustav Paulay of the Florida Museum of Natural History for the sample loans.
References
Adger, WN, Hughes, TP, Folke, C, Carpenter, SR, and Rockström, J (2005). Social-ecological resilience to coastal disasters. Science 309, 1036–1039.| Social-ecological resilience to coastal disasters.Crossref | GoogleScholarGoogle Scholar | 16099974PubMed |
Alve, E (1991). Benthic foraminifera in sediment cores reflecting heavy metal pollution in Sorfjord, western Norway. The Journal of Foraminiferal Research 21, 1–19.
| Benthic foraminifera in sediment cores reflecting heavy metal pollution in Sorfjord, western Norway.Crossref | GoogleScholarGoogle Scholar |
Alve, E, and Bernhard, JM (1995). Vertical migratory response of benthic foraminifera to controlled oxygen concentrations in an experimental mesocosm. Marine Ecology Progress Series 116, 137–151.
| Vertical migratory response of benthic foraminifera to controlled oxygen concentrations in an experimental mesocosm.Crossref | GoogleScholarGoogle Scholar |
Bahr, KD, Jokiel, PL, and Toonen, RJ (2015). The unnatural history of Kāne‘ohe Bay: coral reef resilience in the face of centuries of anthropogenic impacts. PeerJ 3, e950.
| The unnatural history of Kāne‘ohe Bay: coral reef resilience in the face of centuries of anthropogenic impacts.Crossref | GoogleScholarGoogle Scholar | 26020007PubMed |
Banner AH (1974) Kaneohe Bay, Hawai‘i: urban pollution and a coral reefs ecosystem. In ‘Proceedings of the 2nd International Coral Reef Symposium’. pp. 685–702. (The Great Barrier Reef Committee: Brisbane)
Barbosa, CF, de Freitas Prazeres, M, Ferreira, BP, and Seoane, JCS (2009). Foraminiferal assemblage and reef check census in coral reef health monitoring of East Brazilian margin. Marine Micropaleontology 73, 62–69.
| Foraminiferal assemblage and reef check census in coral reef health monitoring of East Brazilian margin.Crossref | GoogleScholarGoogle Scholar |
Barbosa, CF, Ferreira, BP, Seoane, JCS, Oliveira-Silva, P, Gaspar, ALB, Cordeiro, RC, and Soares-Gomes, A (2012). Foraminifer-based coral reef health assessment for southwestern Atlantic offshore archipelagos, Brazil. The Journal of Foraminiferal Research 42, 169–183.
| Foraminifer-based coral reef health assessment for southwestern Atlantic offshore archipelagos, Brazil.Crossref | GoogleScholarGoogle Scholar |
Barnosky, AD, Hadly, EA, Gonzalez, P, Head, J, Polly, PD, Lawing, AM, Eronen, JT, Ackerly, DD, Alex, K, Biber, E, Blois, J, Brashares, J, Ceballos, G, Davis, E, Dietl, GP, Dirzo, R, Doremus, H, Fortelius, M, Greene, HW, Hellmann, J, Hickler, T, Jackson, ST, Kemp, M, Koch, PL, Kremen, C, Lindsey, EL, Looy, C, Marshall, CR, Mendenhall, C, Mulch, A, Mychajliw, AM, Nowak, C, Ramakrishnan, U, Schnitzler, J, Das Shrestha, K, Solari, K, Stegner, L, Stegner, MA, Stenseth, NC, Wake, MH, and Zhang, Z (2017). Merging paleobiology with conservation biology to guide the future of terrestrial ecosystems. Science 355, eaah4787.
| Merging paleobiology with conservation biology to guide the future of terrestrial ecosystems.Crossref | GoogleScholarGoogle Scholar | 28183912PubMed |
Bathen KH (1968) ‘A descriptive study of the physical oceanography of Kaneohe Bay, Oahu, Hawai‘i.’ (Hawai‘i Institute of Marine Biology (formerly Hawai‘i Marine Laboratory))
Burke L, Reytar K, Spalding M, Perry A (2011) ‘Reefs at risk revisited.’ (World Resources Institute)
Bürkner, P-C (2017). brms: an R package for Bayesian multilevel models using Stan. Journal of Statistical Software 80, 1–28.
| brms: an R package for Bayesian multilevel models using Stan.Crossref | GoogleScholarGoogle Scholar |
Carnahan, EA, Hoare, AM, Hallock, P, Lidz, BH, and Reich, CD (2009). Foraminiferal assemblages in Biscayne Bay, Florida, USA: responses to urban and agricultural influence in a subtropical estuary. Marine Pollution Bulletin 59, 221–233.
| Foraminiferal assemblages in Biscayne Bay, Florida, USA: responses to urban and agricultural influence in a subtropical estuary.Crossref | GoogleScholarGoogle Scholar | 19744675PubMed |
Caruso, A, Cosentino, C, Tranchina, L, and Brai, M (2011). Response of benthic foraminifera to heavy metal contamination in marine sediments (Sicilian coasts, Mediterranean Sea). Chemistry and Ecology 27, 9–30.
| Response of benthic foraminifera to heavy metal contamination in marine sediments (Sicilian coasts, Mediterranean Sea).Crossref | GoogleScholarGoogle Scholar |
Dix TL (2002) The distribution and ecology of benthic foraminifera of Tampa Bay, Florida. PhD dissertation. Department of Marine Science, University of South Florida, Tampa.
Dubois O (2011) ‘The state of the world’s land and water resources for food and agriculture: managing systems at risk.’ (Earthscan)
Fagan, KE, and Mackenzie, FT (2007). Air–sea CO2 exchange in a subtropical estuarine-coral reef system, Kaneohe Bay, Oahu, Hawai‘i. Marine Chemistry 106, 174–191.
| Air–sea CO2 exchange in a subtropical estuarine-coral reef system, Kaneohe Bay, Oahu, Hawai‘i.Crossref | GoogleScholarGoogle Scholar |
Filous, A, Friedlander, AM, Koike, H, Lammers, M, Wong, A, Stone, K, and Sparks, RT (2017). Displacement effects of heavy human use on coral reef predators within the Molokini Marine Life Conservation District. Marine Pollution Bulletin 121, 274–281.
| Displacement effects of heavy human use on coral reef predators within the Molokini Marine Life Conservation District.Crossref | GoogleScholarGoogle Scholar | 28622990PubMed |
Friedlander, AM, Donovan, MK, Stamoulis, KA, Williams, ID, Brown, EK, Conklin, EJ, DeMartini, EE, Rodgers, KS, Sparks, RT, and Walsh, WJ (2018). Human-induced gradients of reef fish declines in the Hawaiian Archipelago viewed through the lens of traditional management boundaries. Aquatic Conservation: Marine and Freshwater Ecosystems 28, 146–157.
| Human-induced gradients of reef fish declines in the Hawaiian Archipelago viewed through the lens of traditional management boundaries.Crossref | GoogleScholarGoogle Scholar |
Frontalini, F, and Coccioni, R (2008). Benthic foraminifera for heavy metal pollution monitoring: a case study from the central Adriatic Sea coast of Italy. Estuarine, Coastal and Shelf Science 76, 404–417.
| Benthic foraminifera for heavy metal pollution monitoring: a case study from the central Adriatic Sea coast of Italy.Crossref | GoogleScholarGoogle Scholar |
Gelman, A, Lee, D, and Guo, J (2015). Stan: a probabilistic programming language for Bayesian inference and optimization. Journal of Educational and Behavioral Statistics 40, 530–543.
| Stan: a probabilistic programming language for Bayesian inference and optimization.Crossref | GoogleScholarGoogle Scholar |
Hallock P (2012) The FoRAM Index revisited: uses, challenges, and limitations. In ‘Proceedings of the 12th International Coral Reef Symposium, Cairns, Australia’. pp. 9–13. (James Cook University: Townsville, Qld, Australia)
Hallock, P, Lidz, BH, Cockey-Burkhard, EM, and Donnelly, KB (2003). Foraminifera as bioindicators in coral reef assessment and monitoring: the FORAM index. Environmental Monitoring and Assessment 81, 221–238.
| Foraminifera as bioindicators in coral reef assessment and monitoring: the FORAM index.Crossref | GoogleScholarGoogle Scholar | 12620018PubMed |
Holmlund, CM, and Hammer, M (1999). Ecosystem services generated by fish populations. Ecological Economics 29, 253–268.
| Ecosystem services generated by fish populations.Crossref | GoogleScholarGoogle Scholar |
Hoover, RS, Hoover, D, Miller, M, Landry, MR, DeCarlo, EH, and Mackenzie, FT (2006). Zooplankton response to storm runoff in a tropical estuary: bottom-up and top-down controls. Marine Ecology Progress Series 318, 187–201.
| Zooplankton response to storm runoff in a tropical estuary: bottom-up and top-down controls.Crossref | GoogleScholarGoogle Scholar |
Hunter, CL, and Evans, CW (1995). Coral reefs in Kaneohe Bay, Hawai‘i: two centuries of western influence and two decades of data. Bulletin of Marine Science 57, 501–515.
Jokiel, PL, and Brown, EK (2004). Global warming, regional trends and inshore environmental conditions influence coral bleaching in Hawai‘i. Global Change Biology 10, 1627–1641.
| Global warming, regional trends and inshore environmental conditions influence coral bleaching in Hawai‘i.Crossref | GoogleScholarGoogle Scholar |
Jokiel, PL, Brown, EK, Friedlander, A, Rodgers, SK, and Smith, WR (2004). Hawai‘i coral reef assessment and monitoring program: spatial patterns and temporal dynamics in reef coral communities. Pacific Science 58, 159–174.
| Hawai‘i coral reef assessment and monitoring program: spatial patterns and temporal dynamics in reef coral communities.Crossref | GoogleScholarGoogle Scholar |
Koukousioura, O, Dimiza, MD, Triantaphyllou, MV, and Hallock, P (2011). Living benthic foraminifera as an environmental proxy in coastal ecosystems: a case study from the Aegean Sea (Greece, NE Mediterranean). Journal of Marine Systems 88, 489–501.
| Living benthic foraminifera as an environmental proxy in coastal ecosystems: a case study from the Aegean Sea (Greece, NE Mediterranean).Crossref | GoogleScholarGoogle Scholar |
Laws, EA, and Allen, CB (1996). Water quality in a subtropical embayment more than a decade after diversion of sewage discharges. Pacific Science 50, 194–210.
Laws, EA, and Redalje, DG (1982). Sewage diversion effects on the water column of a subtropical estuary. Marine Environmental Research 6, 265–279.
| Sewage diversion effects on the water column of a subtropical estuary.Crossref | GoogleScholarGoogle Scholar |
Leuliette EW (2012) ‘Sea level trend map. National Oceanic and Atmospheric Administration, Laboratory for Satellite Altimetry sea-level rise products, online data’.
Loeblich AR Jr, Tappan H (2015) ‘Foraminiferal genera and their classification.’ (Springer)
Maragos JE (1972) ‘A study of the ecology of Hawaiian reef corals.’ (University of Hawai‘i)
Narayan, YR, and Pandolfi, JM (2010). Benthic foraminiferal assemblages from Moreton Bay, South-East Queensland, Australia: applications in monitoring water and substrate quality in subtropical estuarine environments. Marine Pollution Bulletin 60, 2062–2078.
| Benthic foraminiferal assemblages from Moreton Bay, South-East Queensland, Australia: applications in monitoring water and substrate quality in subtropical estuarine environments.Crossref | GoogleScholarGoogle Scholar | 20696442PubMed |
Neilson B (2014) Coral bleaching rapid response surveys September–October 2014. Available at http://dlnr.hawaii.gov/reefresponse/files/2014/10/DARCoralBleachingSrvyResults10.28.2014. pdf
Oksanen J, Guillaume Blanchet F, Friendly M, Kindt R, Legendre P, McGlinn D, Minchin PR, O’Hara RB, Simpson GL, Solymos P, Henry M, Stevens H, Szoecs E, Wagner H (2020) vegan: community ecology package. Available at https://CRAN.R-project.org/package=vegan
Oliver, LM, Fisher, WS, Dittmar, J, Hallock, P, Campbell, J, Quarles, RL, Harris, P, and LoBue, C (2014). Contrasting responses of coral reef fauna and foraminiferal assemblages to human influence in La Parguera, Puerto Rico. Marine Environmental Research 99, 95–105.
| Contrasting responses of coral reef fauna and foraminiferal assemblages to human influence in La Parguera, Puerto Rico.Crossref | GoogleScholarGoogle Scholar | 24840256PubMed |
Pandolfi, JM, Bradbury, RH, Sala, E, Hughes, TP, Bjorndal, KA, Cooke, RG, McArdle, D, McClenachan, L, Newman, MJH, Paredes, G, Warner, RR, and Jackson, JBC (2003). Global trajectories of the long-term decline of coral reef ecosystems. Science 301, 955–958.
| Global trajectories of the long-term decline of coral reef ecosystems.Crossref | GoogleScholarGoogle Scholar | 12920296PubMed |
Pastorok, RA, and Bilyard, GR (1985). Effects of sewage pollution on coral-reef communities. Marine Ecology Progress Series 21, 175–189.
| Effects of sewage pollution on coral-reef communities.Crossref | GoogleScholarGoogle Scholar |
Pisapia, C, El Kateb, A, Hallock, P, and Spezzaferri, S (2017). Assessing coral reef health in the North Ari Atoll (Maldives) using the FoRAM Index. Marine Micropaleontology 133, 50–57.
| Assessing coral reef health in the North Ari Atoll (Maldives) using the FoRAM Index.Crossref | GoogleScholarGoogle Scholar |
Prazeres, M, Martínez-Colón, M, and Hallock, P (2020). Foraminifera as bioindicators of water quality: the FoRAM Index revisited. Environmental Pollution 257, 113612.
| Foraminifera as bioindicators of water quality: the FoRAM Index revisited.Crossref | GoogleScholarGoogle Scholar | 31784269PubMed |
R Core Team (2021) R: a language and environment for statistical computing. (R Foundation for Statistical Computing: Vienna, Austria.) Available at https://www.R-project.org/
Reymond C, Uthicke S, Pandolfi JM (2012) Tropical foraminifera as indicators of water quality and temperature. In ‘Proceedings of the 12th International Coral Reef Symposium’. pp. 9–13. (James Cook University: Townsville, Qld, Australia)
Ringuet, S, and Mackenzie, FT (2005). Controls on nutrient and phytoplankton dynamics during normal flow and storm runoff conditions, southern Kaneohe Bay, Hawai‘i. Estuaries 28, 327–337.
| Controls on nutrient and phytoplankton dynamics during normal flow and storm runoff conditions, southern Kaneohe Bay, Hawai‘i.Crossref | GoogleScholarGoogle Scholar |
Rodgers, KS, Jokiel, PL, Brown, EK, Hau, S, and Sparks, R (2015). Over a decade of change in spatial and temporal dynamics of Hawaiian coral reef communities. Pacific Science 69, 1–13.
| Over a decade of change in spatial and temporal dynamics of Hawaiian coral reef communities.Crossref | GoogleScholarGoogle Scholar |
Sandin, SA, Smith, JE, DeMartini, EE, Dinsdale, EA, Donner, SD, Friedlander, AM, Konotchick, T, Malay, M, Maragos, JE, Obura, D, Pantos, O, Paulay, G, Richie, M, Rohwer, F, Schroeder, RE, Walsh, S, Jackson, JBC, Knowlton, N, and Sala, E (2008). Baselines and degradation of coral reefs in the Northern Line Islands. PLoS ONE 3, e1548.
| Baselines and degradation of coral reefs in the Northern Line Islands.Crossref | GoogleScholarGoogle Scholar | 18301734PubMed |
Smith, SV, Kimmerer, WJ, Laws, EA, Brock, RE, and Walsh, TW (1981). Kaneohe Bay sewage diversion experiment: perspectives on ecosystem responses to nutritional perturbation. Pacific Science 35, 279–402.
Uthicke, S, and Nobes, K (2008). Benthic foraminifera as ecological indicators for water quality on the Great Barrier Reef. Estuarine, Coastal and Shelf Science 78, 763–773.
| Benthic foraminifera as ecological indicators for water quality on the Great Barrier Reef.Crossref | GoogleScholarGoogle Scholar |
Vehtari, A, Gelman, A, and Gabry, J (2017). Practical Bayesian model evaluation using leave-one-out cross-validation and WAIC. Statistics and Computing 27, 1413–1432.
| Practical Bayesian model evaluation using leave-one-out cross-validation and WAIC.Crossref | GoogleScholarGoogle Scholar |
Vehtari, A, Gelman, A, Simpson, D, Carpenter, B, and Bürkner, P-C (2021). Rank-normalization, folding, and localization: an improved
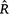 for assessing convergence of MCMC (with discussion). Bayesian Analysis 16, 667–718.
for assessing convergence of MCMC (with discussion). Bayesian Analysis 16, 667–718. | Rank-normalization, folding, and localization: an improved
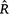 for assessing convergence of MCMC (with discussion).Crossref | for assessing convergence of MCMC (with discussion).&journal=Bayesian Analysis&volume=16&pages=667-718&publication_year=2021&author=A%20Vehtari&hl=en&doi=10.1214/20-BA1221" target="_blank" rel="nofollow noopener noreferrer" class="reftools">GoogleScholarGoogle Scholar |
for assessing convergence of MCMC (with discussion).Crossref | for assessing convergence of MCMC (with discussion).&journal=Bayesian Analysis&volume=16&pages=667-718&publication_year=2021&author=A%20Vehtari&hl=en&doi=10.1214/20-BA1221" target="_blank" rel="nofollow noopener noreferrer" class="reftools">GoogleScholarGoogle Scholar | Velásquez, J, López-Angarita, J, and Sánchez, JA (2011). Evaluation of the FORAM index in a case of conservation: Benthic foraminifera as indicators of ecosystem resilience in protected and non-protected coral reefs of the Southern Caribbean. Biodiversity and Conservation 20, 3591–3603.
| Evaluation of the FORAM index in a case of conservation: Benthic foraminifera as indicators of ecosystem resilience in protected and non-protected coral reefs of the Southern Caribbean.Crossref | GoogleScholarGoogle Scholar |
Wickham, H, Averick, M, Bryan, J, Chang, W, McGowan, LD, François, R, Grolemund, G, Hayes, A, Henry, L, and Hester, J (2019). Welcome to the Tidyverse. Journal of Open Source Software 4, 1686.
| Welcome to the Tidyverse.Crossref | GoogleScholarGoogle Scholar |
Williams, ID, Walsh, WJ, Schroeder, RE, Friedlander, AM, Richards, BL, and Stamoulis, KA (2008). Assessing the importance of fishing impacts on Hawaiian coral reef fish assemblages along regional-scale human population gradients. Environmental Conservation 35, 261–272.
| Assessing the importance of fishing impacts on Hawaiian coral reef fish assemblages along regional-scale human population gradients.Crossref | GoogleScholarGoogle Scholar |
Woodhead, AJ, Hicks, CC, Norström, AV, Williams, GJ, and Graham, NAJ (2019). Coral reef ecosystem services in the Anthropocene. Functional Ecology 33, 1023–1034.
| Coral reef ecosystem services in the Anthropocene.Crossref | GoogleScholarGoogle Scholar |


