The evidence for and against competition between the European honeybee and Australian native bees
Kit S. Prendergast A * , Kinglsey W. Dixon A and Philip W. Bateman
A * , Kinglsey W. Dixon A and Philip W. Bateman  A
A
A School of Molecular and Life Sciences, Curtin University, Kent Street, Bentley, WA 6102, Australia.
Pacific Conservation Biology 29(2) 89-109 https://doi.org/10.1071/PC21064
Submitted: 12 October 2021 Accepted: 27 January 2022 Published: 3 March 2022
© 2023 The Author(s) (or their employer(s)). Published by CSIRO Publishing. This is an open access article distributed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND)
Abstract
In Australia, the European honeybee (Apis mellifera) is an exotic, abundant, super-generalist species. Introduced two centuries ago, it thrives in the absence of many diseases adversely impacting honeybees elsewhere. Australia’s native bees may be vulnerable to competition with honeybees, leading to reduced abundances, reproductive output or even loss of bee species. We review the literature concerning competition between honeybees and Australian native bees in order to: (1) identify the valuence and strength of honeybee associations with native bees, and how this varies according to the response variable measured; (2) assess potential research biases; (3) use ecological theory to explain variation in results; and (4) identify key knowledge gaps. We found honeybees typically comprised the majority of individuals in surveys of Australian bee communities. Data on whether honeybees outcompete native bees is equivocal: there were no associations with native bee abundance, species richness, or reproductive output in most cases. However, there were more negative than positive associations. Data indicate effects of honeybees are species-specific, and more detailed investigations regarding how different species and life-history traits affect interactions with honeybees is needed. We propose the following investigations to address deficiencies in the current literature: greater geographic and landscape representation; trait-based investigations; quantifying resource availability and overlap; disease and predator interactions; experimental feral colony removals; and studies spanning multiple seasons and years. Identifying conditions under which honeybees have negative, neutral or positive effects on native bees, and how the ecological traits of native bees are affected by honeybee competition can guide conservation and management.
Keywords: Australian pollinators, conservation, exotic pollinators, interspecific competition, introduced species, pollinators, resource overlap, wild bees.
Introduction
Invasive species are one of the leading causes of species endangerment (Clavero and García-Berthou 2005; Aizen et al. 2008; González-Varo et al. 2013; Bellard et al. 2016). The European or western honeybee (Apis mellifera) is one such invasive species that is posited to cause native bee declines in its non-native range (Geslin et al. 2017). The European honeybee has a broad native range covering Africa, western Asia and south-east Europe, and now occurs on all inhabited continents, having been introduced for honey production and pollination services, especially for agricultural crops (Moritz et al. 2005; Geslin et al. 2017). The vigourous swarming behaviour of honeybees means they now occur as feral colonies in their introduced range (Manning et al. 2006).
Honeybees have been particularly successful in Australia following their introduction in the 1820s (Paton 1996; Goulson 2003) where they are important generalist pollinators, especially of introduced crops (New 1997; Rural Industries Research & Development Corporation (RIRDC) 2010). Honeybees occur in every state and territory of Australia, with high densities in the south-west, east coast and Tasmania (ALA (Atlas of Living Australia) 2021). Domesticated honeybees occur in managed hives, with beekeepers operating at scales from small local hobby beekeeping ventures, through to commercial ventures involving hundreds of hives at single locations during ‘honey flow’ periods when mass flowering occurs or during crop flowering periods. Honeybees also occur in ‘un-managed’ feral colonies in regions of moderate climate with hollow-bearing trees, where they represent an invasive introduced species (Moritz et al. 2005). Both managed and feral colonies are thriving in Australia due to the lack of pathogens and diseases such as Varroa destructor mite and Deformed Wing Virus that cause colony losses on other continents, and appear to be less exposed or susceptible to lethal levels of pesticides (Goulson 2003; De la Rúa et al. 2009; vanEngelsdorp and Meixner 2010; Manning 2018).
The Australian honey bee industry consists of approximately 12 400 registered beekeepers, with about 520 000 hives, producing 25 000–30 000 tonnes of honey annually (Karasinski 2018a). In 2019, Australia was 44th in the world out of 340 countries in honey yield (FAO Food & Agriculture Organization of the United Nations, 2021). This industry has an overall estimated gross value of production of AUD1.75 billion/year in direct hive products, with an additional AUD14.2 billion/year through pollination services (BeeAware 2014; Karasinski 2018b). In Australia, honeybees are not only transported at high densities into areas to provide crop pollination services (where native bees tend to be in relatively low numbers due to unfavourable forage conditions, especially for oligolectic bees (Batley and Hogendoorn 2009; Prendergast et al. 2021b), but major influxes of honeybees also occur in natural areas such as parks and reserves in order for beekeepers to capitalise on flowering of major honey-source species; key species of high value in terms of production and honey quality include Jarrah (Eucalyptus marginata), Yellow Box (Eucalyptus melliodora), Leatherwood (Eucalyptus lucida), Marri (Corymbia calophylla), and tea tree (Leptospermum spp.).
There are concerns that this massively introduced managed species (sensu. Geslin et al. 2017) exerts negative impacts on flower-visiting native fauna. Given that native bees rely on nectar and pollen as both adults and larvae, they can be expected to be in competition for these resources, through interspecific interference (contest) and/or resource (scramble) competition. Heterospecific interference competition, where one species physically interferes with another species’ ability to use a resource, tends to be strongly asymmetric, with aggressive, larger or group-foraging bees displacing less-aggressive, smaller, solitary bees, respectively (Johnson and Hubbell 1975; Lichtenberg et al. 2010) (but see Ali et al. 2015). Interference competition may exclude inferior competitors from accessing preferred, high-quality resources (Thomson 1989), and even the presence of honeybees has been observed to disrupt visitation to shared resources and inhibit co-existence (Chen 1993). The primary means by which honeybees appear to compete with native bees is via resource competition, monopolising limiting resources (nectar and/or pollen) (Roubik 1978; Torné-Noguera et al. 2016). Negative effects on biological fitness (an organism’s success at surviving and reproducing, thereby passing on its genes) through resource competition will only occur if there is high niche overlap and if resources are limiting (Tilman 2004).
Honeybees are potentially able to monopolise and consume a large fraction of floral resources at a site (Geslin et al. 2017), especially under conditions when resources may be limiting, and may therefore exert a negative effect on the fitness of native bees; however, whether honeybees outcompete native bees for floral resources (nectar and/or pollen) such that they negatively affect populations of native bees, or under what conditions this might occur, remains unresolved (Eickwort and Ginsberg 1980). Global studies have reported both negative (Goulson 2004; Thomson 2004; Shavit et al. 2009; Torné-Noguera et al. 2016), as well as no effects of honeybees on native bee diversity and abundance (Forup and Memmott 2005; Steffan-Dewenter and Tscharntke 2000; Roubik and Wolda 2001; Mallinger et al. 2017). A recent global review of literature concerning the impact of managed bees (both exotic and native) on wild bees (which included only nine studies from Australia) also found mixed effects, with 53% reporting negative effects on wild bees, while 28% reported no effects and 19% reported mixed effects (varying with the bee species or variables examined) (Mallinger et al. 2017). Our study goes further than previous analyses in a number of ways, through:
-
Importantly, distinguishing between abundance vs richness effects, as well as effects on reproduction.
-
Considering the ‘strength’ of the evidence.
-
Considering management status (managed vs feral), which may be especially pertinent.
-
Including studies that allow honeybee–native bee relationships to be assessed yet were not explicitly focused on competition, thereby discerning if there may be biases; and
-
Identifying major knowledge gaps, with recommendations on how to address these.
With an Australian focus, we may expect competition to be particularly severe in an Australian context due to contrasting evolutionary histories of Australian native bees and A. mellifera (Mallinger et al. 2017), and with other Australian taxonomic groups appearing to be highly vulnerable to the effect of introduced species (Jackson et al. 2016). In Europe, where honeybees have co-evolved with the flora and fauna, even high densities of managed honeybees that overlap in resource use with other bees by almost 50% have no adverse impact on bee species richness or abundance, or reproductive success (Steffan-Dewenter and Tscharntke 2000). In contrast, honeybees have been present in Australia for some 200 years. These different histories over which wild bee assemblages have interacted with honeybees suggest we are unlikely to be able to extrapolate findings from Europe to Australia (Strauss et al. 2006).
Within Australia, as is the case in other regions of the world, there is no scientific consensus on whether honeybees are negatively impacting native bees (Manning 1997). Australia has a high and distinctive diversity of mostly endemic native bees, with an estimated >2000 species, many of which are undescribed (Houston 2018). The Australian native bee fauna also has a unique taxonomic composition (Batley and Hogendoorn 2009). Large-bodied social Bombus, a major component in Eurasian and American ecosystems, and members of the family Melittidae and Andrenidae are absent. The family Stenotritidae is endemic to Australia, and unlike in most regions in the world, Colletidae is the most species-rich family, of which the most species-rich subfamily in Australia, the Euryglossinae, are endemic (Michener 2007). Only 11 species in two genera (Austroplebia and Tetragonula) are highly eusocial (Heard 2016). Australia’s bee fauna is diverse compared with other continents, and the absence of honeybees as competitors may be a contributing factor (Michener 1965; Michener 1979). Given the economic importance of the Australian honeybee industry, if Australia’s unique diversity of native bees is to be preserved, it is crucial to understand whether honeybees are exerting a negative impact. In this review, we focus on negative impacts that may arise through resource competition, which can lead to reduced fitness, translating to population declines and extirpation of a native bee species. In the literature assessed, proxies for such negative impacts are negative associations between honeybees and native bees in terms of abundance of a species or assemblage, number of species, or reproductive output. Here, we review the current known information on the impact of honeybees on native bee communities and outline key knowledge gaps and future research priorities. The aims of this review are to: (1) summarise the state of the literature to date; (2) identify the strength and direction of honeybee influences on native bee abundance, species richness and reproductive output, and how this varies according to the response variable measured; (3) discern potential biases; (4) provide explanations for variation in results, including how these may relate to ecological theory; and (5) identify key areas requiring investigation and make recommendations for future studies.
Materials and methods
Studies reviewed
To review evidence for competitive effects of honeybees on native bees in Australia, publications were sourced (Google Scholar and ProQuest) using combinations of the search terms ‘honey bees, competition, Apis mellifera, honeybees, interactions, introduced bee, exotic bee, feral bee, invasive species, pollination, Australia*’ (search fields are connected by the Boolean search term ‘and’) published up to 29 June 2021. Other references were found in citation lists and relevant papers on Australian bees. Therefore, publications included in this review are not biased towards those explicitly looking for competition.
Fifty four cases evaluating the impact of honeybees on Australian native bees in 46 publications were found, published between 1977 and 2021. Eleven were reviews or opinion papers, leaving 43 empirical cases that form our review of the evidence for and against honeybee competition (Table 1; see Supplementary Material).

|
Twenty three of these cases were explicitly aimed at addressing competition between honeybees and native bees. It should be noted that of these, most failed to define or indicate how competition was measured.
Assessing the relationship between honeybees and native bees
In our systematic review on the relationship between honeybees and Australian native bees, we used a vote counting analysis to quantify the direction and ‘strength’ of evidence for the relationship between the introduced honeybee and Australian native bees. For this analysis, we only used original data papers, thus excluded reviews and opinion papers. Some publications evaluated more than one response variable, or contained results from more than one study or experiment; hence, there were a greater number of cases than publications. Publications included were those where there was a quantitative measure of both honeybees (presence/absence of colonies in space or time; abundance or density of colonies; visitation rates; distance from hives or apiaries) which could be related to that of native bees (abundance; visitation rates; species richness; reproductive rates). Publications were excluded that did not measure these metrics (e.g. if a study measured only pathogen transmission it would not be included), or if they were not in English. The limited number of publications overall, and the limited number of publications that provided quantifiable outcomes on the effect of honeybees on native bees (such as publications that did not explicitly analyse the effect of honeybees on native bees, or publications in which no statistical analyses were performed) precluded us from being able to perform a meta-analysis. Therefore, we indexed the effect of honeybees on native bee parameters according to a three-point scale, similar to that of Hoffmann and Andersen (2003): 1, weak apparent response, possibly representing background noise (P < 0.1), or no statistical tests performed; 2, apparently clear response, statistically tested (P < 0.05); and 3, very strong response, statistically tested (P < 0.01). The valence was recorded as negative (−) or positive (+). We also included a category for n.s. (no significant difference, P > 0.05), or unknown (? – not mentioned/addressed). We looked at three main parameters: native bee abundance, species richness, and reproductive output. Some studies measured more than one parameter (e.g. both abundance and species richness), and there were also studies where, when conducted over more than 1 year, or looking at different native bee guilds, the response metric varied (e.g. the strength or valence differed from 1 year to the next), and thus some studies had more than one response for a given parameter (see Supplementary Material).
Results
Honeybees are a dominant component of Australian ecosystems, with 93.3% of studies (out of n = 30) reporting that honeybees numerically dominated the assemblage. When abundance data was available (n = 20), honeybees represented on average 65 ± 6% of total bees recorded. None of the previous honeybee reviews unequivocally supported or refuted the hypothesis that honeybees had a negative effect on native bees, all concluding that the evidence was ambiguous or insufficient, emphasising the need for the current review to evaluate the strength of the evidence across all studies to date, and what factors may cause this variation in the relationship between honeybees and native bees in Australian ecosystems. We found only one early opinion piece that asserted that honeybees caused the extirpation of native bees, but was based on anecdotal evidence (Douglas 1977) (see summary in Supplementary Material).
Quantitative studies
Abundance was the most commonly measured metric of native bees (42 responses), followed by species richness (22 responses); only three studies measured reproductive output (Table 1). Of studies with data for assessing the relationship between honeybees and native bee response variables, 19 were conducted in New South Wales, 10 in Western Australia, six in Tasmania, four in Victoria, only one each in South Australia and the Northern Territory, with none in the Australian Capital Territory (Fig. 1). Regarding landscape type, 75% were from natural landscapes, 15.9% in agricultural landscapes, and 9.1% in urbanised landscapes (Table 1). The majority of studies measured the response of the native bee community, with only eight studies focusing on measuring the response of a particular species. These species-focused studies were further taxonomically restricted (Table 1). Despite Meliponini comprising a minority of Australian species (AFD (Australian Fauna Directory) 2021), one-quarter of species-level studies were with Tetragonula carbonaria. In contrast, no studies on the impact of honeybees have involved species in the subfamilies Euryglossinae and Neopasiphaeinae, Callomelittinae and Diphaglossinae (previously grouped together as Colletinae in Michener (2007; Almeida et al. 2012, 2019), which represent together over half of the native bee fauna and include many oligolectic species (Houston 2018; AFD (Australian Fauna Directory) 2021). Only a single study involved an oligolege (Hylaeus alcyoneus) (Paini et al. 2005).
|
|
Across all studies presenting data reviewed here, we found scant conclusive evidence that resource competition occurs between honeybees and native bees. Only eight cases (17.4%) concluded or provided evidence for resource competition between the taxa; however, resource competition was inferred, but not demonstrated in 14 cases (30.4%). Ten cases (21.7%) concluded or provided evidence that did not support asserting that resource competition was occurring (Table 1), and in 14 cases (30.4%) it was ambiguous (Table 1). Only two studies reported interference competition (Williams and Adam 1997; Gross and Mackay 1998) (see Supplementary Material). These results describing resource competition will; however, be influenced by the particular resources under study (some studies looked at a single plant species, others a plant community; see Supplementary Material). Studies were also dominated by investigating native flora (87.8% of studies), with little research into managed horticultural or crop species, or exotic plant species.
Mensurative and manipulative studies
Mensurative studies (also known as observational studies) involve taking advantage of natural variation and making measurements of uncontrolled events, with space and/or time being the only experimental variable, whereas manipulative studies involve two or more treatments, where experimental units in each treatment receive controlled manipulations (Hulbert 1984). Of the 43 cases involving data on relationships between honeybees and native bees, one quarter (12) involved manipulating honeybees, and the remainder (31) were mensurative (Table 1). Of manipulative studies, there were seven cases where no association was found, five negative associations, and only two positive associations. Moreover, two of the negative associations were highly statistically significant (Fig. 2). Of mensurative studies, 27 cases of no associations were found, and similar to manipulative studies, more negative than positive associations were found (16 vs 9). However, there were fewer highly statistically significant associations (Fig. 2). Proportions of cases finding negative, positive and no associations were similar between mensurative and manipulative studies, but mensurative studies appeared to have a slightly higher proportion of findings of no association.
Studies investigating honeybee competition compared with those that do not
Of all studies, 24 explicitly looked at the effect of honeybees on native bees; the remaining 19 provided information on honeybees and native bees but were not conducted with the aim of investigating honeybee impact on native bees (Table 1). All highly significant instances of negative impacts on native bee abundance (three out of 24) were from studies that explicitly investigated the impact of honeybees (Fig. 3). In contrast, all negative associations reported by studies not specifically investigating honeybee competition were non-significant or were not statistically analysed (Fig. 3). However, whilst this may suggest a bias towards detecting negative impacts of honeybees on native bees, the majority of positive associations (nine out 12) also came from studies investigating honeybee impacts on native bees. A similar proportion of cases for no association between honeybees and native bees being reported occurred for both types of studies (50% and 53.6%, respectively). Contrary to the potential for researchers who were investigating competition to report negative associations, there was a greater proportion of positive associations from these studies than those not focused on looking at the impact of honeybees.
Response of native bee abundance
Non-significant associations between honeybees and native bee abundance dominated, making up just over 50% of responses (Figs 2a, 3a). There were more negative than positive associations (12 vs 7) (Figs 2a, 3a), although when excluding those cases where no tests were performed or there was a trend (P = 0.05–0.1), there was an equal number of statistically significant positive and negative associations (Figs 2a, 3a).
Response of native bee reproductive output
Only four studies measured reproductive output, with two showing no association with honeybees, one showing a statistically negative association, and one a highly significant positive association. With such a low sample size, it is difficult to draw any conclusions. All of these were studies explicitly investigating competition (Fig. 2b).
Response of native bee species richness
Responses in terms of species richness appeared to show the greatest support that honeybees may be exerting negative impacts on some species: eight studies had negative associations, with half of this number showing positive associations (Figs 2c, 3b). An equal number of studies had responses that were statistically significant (two each), and ten studies exhibited no association of honeybees with native bee species richness (Figs 2c, 3b).
Overall impact of honeybees on native bees
Combining all response parameters, non-significant effects dominated (Figs 2, 3). Overall, from studies where an outcome could be evaluated, non-significant responses comprised over 50%. However, almost twice as many negative associations were reported than positive (21 vs 11).
Discussion
Based on the surveyed literature there is limited strong evidence to support or to reject the hypothesis that honeybees have a deleterious impact on Australian native bees (Table 1; see Supplementary Material).
Abundant resources and unsaturated niches
Why might there be such variation in reported results? The impact of an introduced competitive species on native communities through resource competition will depend upon resource overlap between the introduced and resident species, resource availability and whether communities are ‘saturated’ (Stachowicz and Tilman 2005). The ease with which honeybees have spread and established throughout Australia implies that communities were not saturated, and there existed ‘open niches’ for honeybees.
The diversity and abundance of floral resources in most Australian ecosystems (ABRS (Australian Biological Resources Study) 2015) may explain both the ease of honeybee invasion, and why this introduced species has had minimal conclusive competitive effects on endemic bees (Simberloff 1981). In addition, honeybee foragers exhibit short-term flower constancy (Leonhardt and Blüthgen 2012), but undergo shifts throughout the day or season as the cost–benefit value of remaining in a patch changes. This behaviour is likely to leave resources incompletely diminished, such that nectar remains for native bees. Moreover, many native bees are small and solitary, with consequently lower nectar requirements (Eickwort and Ginsberg 1980; Goulson 1994; Manning 1997). This is supported by findings of substantial quantities of nectar remaining unexploited by honeybees, even near apiaries (Corbet and Delfosse 1984; Paton 1996; Horskins and Turner 1999). In Western Australian forests, short-term agistments of commercial apiarists on registered apiary sites results in only 40% of the available nectar crop being harvested (Manning 1997). Many Australian flowering plant species that are highly visited by both honeybees and native pollinators are mass flowering, with easily accessed open-faced flowers, and produce a seasonal superabundance of nectar; e.g. Myrtaceae (Williams and Adam 1994). This suggests that nectar is seldom fully depleted and nectar resources are therefore not limiting. This reduces the potential for honeybees to adversely affect native bees through resource competition, even those species with overlapping niches (Manning 1989; Schwarz and Hurst 1997).
However, resources are not always at surplus levels (Zimmerman and Pleasants 1982; Dupont et al. 2004), and honeybees are documented to remove up to 97.2% of the nectar and 99.0% of pollen produced by some native Australian flora (Paton 1990). A study on Tasmanian leatherwood (Eucryphia lucida), a nectar resource highly sought after by commercial honey producers, found that honeybees rapidly removed pollen, and standing crops of nectar sugar were significantly depressed at apiary sites compared with control sites (situated 2 km away from apiaries), where pollen remained in flowers until the female phase (Mallick and Driessen 2009). However, despite this thorough resource use by honeybees, no competitive effects on native insect visitors, in terms of numbers of visits to leatherwood trees, were observed and visiting rates by native insects did not differ between control and apiary sites. It should be noted that the control sites still had a high abundance of feral honeybees (73% of that recorded at commercial apiary sites), which may explain the lack of difference in native bees since feral honeybees may have usurped resources to an extent that larger native bee populations could not be supported (Mallick and Driessen 2009). This study also found that across all sites honeybees were the dominant visitors, and carried significantly more pollen than did native bees.
Differences in life-history traits
Divergences in ecological and life-history traits leading to differences in foraging behaviour, floral preferences, or habitat preferences may also result in negative correlations between honeybees and native bees (Williams et al. 2010). Honeybees tend to be more abundant in agricultural areas or regions with high abundances of introduced plants, whereas native bees are more abundant and species-rich in areas with larger amounts of native vegetation (Martins et al. 2018). In Australia, differences in habitat use and flower species have been found in studies comparing native bee and honeybee abundance between habitat types (Heard et al. 1990; Threlfall et al. 2015; Prendergast et al. 2022) and flower species (Hingston 2002; Prendergast and Ollerton 2022). Unlike most native bees, honeybees can achieve their highest densities in communities with extensive human-induced ecosystem disturbance (Cairns et al. 2005). Stored resources in the hive may make honeybees better-equipped to persist in such landscapes, whereas native bees must constantly forage over a brief active season such that costs of foraging in impoverished landscapes are prohibitively high (Tomlinson et al. 2017). The large flight range of honeybees also allows them to be more resilient to landscape fragmentation, allowing them to more easily cross the matrix to reach forage resources. Honeybees are also highly polylectic, and will forage on a wide diversity of flora, including exotic crops, whereas many of Australia’s native bees have co-evolved with the flora and have preferences for, or are specialised on, a more narrow range of native flora (Houston 2000).
Interactions with shared predators and flora
Honeybees may have a beneficial impact on native bees because as an abundant, energy-rich food source, bee-eating birds and spiders may reduce their per capita predation rate upon native bees (a ‘dilution’ or ‘satiation’ effect) (Schwarz et al. 1991; Schwarz et al. 1992; Manning 1997; Schwarz and Hurst 1997; DEC 2012). Honeybees may also indirectly have a beneficial effect on native bees by enhancing reproductive success of plants on which native bees depend (Heard et al. 1990). If native pollinators decline owing to anthropogenic disturbances, they may be at insufficient densities to effect maximal pollination, leading to pollination deficits. Because managed honeybees can be at least partly buffered against erratic periods of low bloom when beekeepers provide supplemental feeding, their ongoing pollination services in such conditions may halt otherwise cascading extinctions and breakdown of mutualist networks through occupying key nodes in invaded communities (Harrison and Winfree 2015). Nevertheless, it cannot be assumed that honeybees have positive influences on pollination networks, and indeed recent evidence has demonstrated honeybees not only are not ecologically equivalent to native bees in their roles in pollination networks, but they also have disruptive influences on pollination networks, dominate interactions, and are associated with lower network stability and high indices of competition (Prendergast and Ollerton 2022).
Honeybees may also reduce plant reproductive success, as has been found both in Australia (Gross 1993; Gross and Mackay 1998; Delnevo et al. 2020; Prendergast and Ollerton 2022), and elsewhere on the globe (Hargreaves et al. 2010; Magrach et al. 2017; Valido et al. 2019; Agüero et al. 2020). They may also indirectly harm native bees by favouring spread of weeds (Goulson and Derwent 2004; Simpson et al. 2005).
Caveats to the literature and potential biases
The lack of consistent agreement between studies may be influenced by the nature of the studies themselves. The limited number of sites (median of 6) and duration of studies also has implications for interpreting results: it is likely that many of the studies reporting non-significant associations lacked the power to detect significant negative associations (James-Pirri et al. 2007). All studies have been of limited durations, some spanning only a few days, with a median of 3 months; most were conducted in just 1 year (median number of years: 1, mean 2.3 ± 0.2), over one, rarely two, seasons. The few long-term studies on honeybee interactions with native bee assemblages in other countries have found that negative effects of honeybees on other bees are transitory (Roubik 1983; Schaffer et al. 1983). Limitations in sample size and variability means that the scoring system used here also has caveats: a large mean difference yet with much variation may not have the power to be statistically significant, whereas a statistically significant difference may represent a small ‘biologically meaningful’ difference.
Many of the studies examined here (n = 9) came from a single set of experiments that were published as part of a report (Pyke and Balzer 1985). As such, a large proportion of the literature has not undergone the scrutiny of peer-review. Many studies also fail to consider confounding factors, spatial or temporal scale and levels of shared or alternative resources. Competition is often inferred by looking at resource overlap or changes in foraging patterns (e.g. visiting rates) which are indirect measures of competition, rather than assessing whether honeybees affect fitness, as measured by changes in native bee survival, fecundity, and population density. In addition, negative associations between honeybees and native bees can only be found if there is variation in these two parameters (see Prendergast et al. 2021a); and indeed, consistent high numbers of honeybees in relation to native bees was a common feature of studies in this review.
Another factor to consider is biases in expectations for finding competition. A novel aspect of this review was including data from studies where associations between honeybees and native bees were presented, but the researchers did not set out to examine competition. The limited sample size means no strong conclusions can be drawn; however, we can see that negative effects are represented to a greater extent in studies looking at competition, with non-significant effects being represented to a greater extent in studies not looking for competition. Although this difference in the representation and strength of negative associations may be due to the experimental design in studies investigating honeybee competition, it may also be due to bias in looking for evidence of honeybees competing with native bees, and a bias against publishing non-significant results.
Our review has assessed the relationship between honeybees and Australian native bees reported in the literature, but we acknowledge that it remains contentious for what determines a competitive effect: a negative correlation or association is a necessary but not sufficient attribute but does not necessarily mean competition.
Response metrics
Our review emphasises the importance of the response metric being used to measure the impact of competition, and they may not necessarily be congruent, even within a study. Consider honeybee competition exerting negative impacts on the bee assemblages through resource competition (Fig. 4), a number of scenarios are possible:
|
|
-
both abundance and species richness may decline;
-
abundance of each species may decline, but not to a level where any species becomes locally extinct such that the number of species remains the same;
-
the abundance of vulnerable species may decline to such an extent that they become locally extinct; these species however may have been in competition with other native bee species that are not in competition with honeybees, leading to a release from competition. Consequently populations of these remaining species would increase, leading to no net decrease in abundance at the community level (Dale 2017).
With a greater proportion of studies finding honeybees were negatively associated with native bee species richness rather than abundance, this third, complex diffuse competition scenario may be operating. This has yet to be investigated in the context of honeybee competition, but has been investigated in the context of plant communities which may serve as a model for such studies in the context of direct and indirect interactions between pollinator assemblages (Brooker et al. 2008; Xiao and Michalet 2013; Aschehoug and Callaway 2015; Godoy et al. 2017).
Determining whether competition with honeybees exerts negative impacts on native bee populations requires measuring their survival and reproduction (Begon et al. 2005). Yet only four studies have measured this response metric, with no consistent result. Furthermore, these studies all involved cavity-nesting bee species. Given that over 50% of native bee species are ground-nesting (Houston 2018), and some of these ground-nesting bees are pollen specialists (e.g. the ground-nesting Euryglossinae (Michener 2007), Trichocolletes (Batley and Houston 2012), some species of Leioproctus (e.g. Houston 1989)), this is an under-researched topic. It is challenging to measure reproductive output of ground-nesting bees; however, there are studies that have successfully estimated reproductive success of this nesting guild, e.g. Willis Chan and Raine (2021).
Key knowledge gaps
We identified seven key knowledge gaps (or biases), and areas of further research to help elucidate relationships between honeybees and native Australian bees.
Geographic biases
There is a geographic bias in studies, with a third of studies reviewed conducted in just one state (NSW) (Fig. 1). Australia is a large continent, possessing a diversity of environments. As such, studies on honeybees in a bioregion may not be valid for other regions. For example, in Australia’s arid regions (Smith 2008), native bees are likely to have a competitive edge over honeybees through greater drought tolerance. Organisms adapted to Australia’s arid environments characterised by environmental unpredictability often have evolved broad niches and high plasticity, which should reduce competitive exclusion (Levins 1968; Halcroft 2012). No studies on honeybee competition have been conducted in such regions.
Landscape type biases
A major knowledge gap is honeybee impacts on native bees in environments other than natural habitats. Three-quarters of studies were conducted in natural landscapes, 15.9% in agricultural, and only 9% were conducted in more urbanised landscapes (see Supplementary Material). The potential for competition may differ greatly in disturbed habitats compared with natural habitats. Firstly, because native bees tend to be at reduced population sizes in landscapes with fragmented native vegetation (Kremen et al. 2002a, 2002b; Steffan-Dewenter et al. 2005), whereas honeybees tend to do better in more disturbed landscapes, especially those with mass flowering crops (Steffan-Dewenter et al. 2002; Carré et al. 2009; Threlfall et al. 2015). Secondly, with the loss of native flowering plants, resources will be more limiting, exacerbating the potential for resource competition. Thirdly, given that islands are particularly vulnerable to invasive species (Kato et al. 1999; Traveset and Richardson 2011), habitat fragments, within inhospitable matrices, may be more conducive to native bees being displaced by honeybees. Conversely, it is possible that when presented with mass flowering crops in agricultural landscapes (Martins et al. 2018), or a diversity of exotics in urban landscapes (McKinney 2008), honeybees will preferentially forage on these non-native flowers, reducing niche overlap and competition with native bees. However, recent research has indicated that this is not the case, and instead honeybee competition is more intense in more urbanised habitats because there is a reduced amount of native flora available for native bees resulting in resource limitation, and a greater diversity of primarily exotic flora, favouring honeybees (Prendergast et al. 2021a; Prendergast and Ollerton 2022).
In Australia, honeybees attain high densities in urban areas (Prendergast and Ollerton 2022), which contrasts with most urban bee fauna surveys in the northern hemisphere where overall abundance is dominated by native bees (Cane et al. 2005). This disparity may stem from a higher density of urban beekeepers in Australia. It may also be because V. destructor mites, and associated viruses and hive disorders, that have caused colony losses of honeybees in Europe and the USA, are absent in Australia, allowing healthier colonies and a greater densities of feral colonies (vanEngelsdorp and Meixner 2010). There have been proposals to intensively use urban lands for communal apiaries in order to reduce commercial honeybee impacts in supposedly superior natural habitats (Sugden et al. 1996). Yet, urban habitats may be a refuge for native bees, that may be eliminated if honeybee densities are allowed to rise (McFrederick and LeBuhn 2006; Ropars et al. 2019). With urban beekeeping and urban crop cultivation increasing (Moore and Kosut 2013; Saggin 2013; Lorenz and Stark 2015), it is important to identify opportunities for supporting both wild native bees as well as managed honeybees in urban contexts (MacIvor and Packer 2016).
Evaluating how species’ traits influence competitive interactions
Predicting situations where honeybees will have adverse effects on native bees will be improved by information about the autoecology of native bees and factors limiting their populations, yet such data is poor for most of Australia’s native bees (Batley and Hogendoorn 2009), and many studies suffer from poor taxonomic practices (Prendergast and Hogendoorn 2021). Morphological, ecological and behavioural similarities between native and alien pollinators are important in predicting competitive interaction intensities (Dohzono and Yokoyama 2010) and therefore, the traits of a bee species – body size (and associated energetic requirements and flight range), tongue length, floral preferences and lecty (extent of specialisation on pollen hosts), phenology – will influence the intensity and outcome of competition (Lancaster et al. 2017). However, the majority of studies have analysed changes in native bee abundance at the aggregate community level, and moreover have not distinguished between taxonomic groups. Testing to see if native bee taxonomic or trait-based guilds show variation in their vulnerability to honeybee competition should be a focus of further investigations. Importantly, recent research has revealed the importance of species’ traits in their susceptibility to honeybee competition and how aggregate responses can mask vulnerability to competitive impacts (Prendergast et al. 2021a). Importance of a species’ autoecology is underscored in two studies conducted in the same location investigating honeybee competition on two divergent species: a summer-active long-tongued megachilid, and a winter-active short-tongued Hylaeus, which arrived at opposing conclusions (Paini and Roberts 2005; Paini et al. 2005). Research into honeybee impacts across a far greater diversity of native bee species is needed given only seven species have been investigated. For example, there have been no studies on Euryglossinae species, which is the most species-rich subfamily of Australian bees, the majority of which are oligolectic (Houston 2018).
Even if honeybees do not affect native bee abundance or species richness, they nevertheless may cause shifts in species composition, which are masked by these aggregate measures. This may occur where sensitive species are replaced by species that are unaffected by honeybee competition.
The greater the niche similarity between species, the greater the harm invaders are predicted to cause for native communities (Traveset and Richardson 2011). This may explain the susceptibility of Bombus to honeybee competition, as this genus comprises ‘long-tongued’, mainly large, social species. Australia lacks native taxa with a similar niche, but we may expect that the large-bodied, long-tongued Amegilla and Xylocopa may be most susceptible based on their niche similarity with honeybees in these life-history traits. Phylogeny has been proposed as a predictor for competition (Moritz et al. 2005; Violle et al. 2011; Rohr and Bascompte 2014), based on the premise that species that are phylogenetically closely-related have similar fundamental niches (Cahill et al. 2008). Indeed, studies have demonstrated negative competitive effects of honeybees on Bombus (Thomson 2004, 2006, 2016; Goulson et al. 2008; Elbgami et al. 2014; Torné-Noguera et al. 2016): a well-studied genus, phylogenetically close to Apis – both Apinae, Corbiculata (Bossert et al. 2019). Under this assumption, it can be predicted that competition will be relatively weak for most Australian species, but will be strongest between native apid genera (e.g. Xylocopa, Tetragonula, Austroplebeia, Amegilla) (Bossert et al. 2019).
Quantifying resource overlap and resource levels
To understand and predict competition, a measurement of resource overlap in terms of nectar and pollen plants, taking into account resource levels, should be quantified. In conjunction resource requirements of honeybees and native bees needs to be determined. This is crucial because even under low resource levels, resources may not be limiting for solitary native bees due to their relatively low per capita resource requirements, whereas the health of a honeybee colony relies on a flow of comparatively high amounts of nectar and pollen. However, the resources required to sustain solitary or social bee populations, and what represents a sustainable population size, are not known for many bee species (but see Franzén and Nilsson (2010) for the non-Australian bee, Andrena hattorfiana).
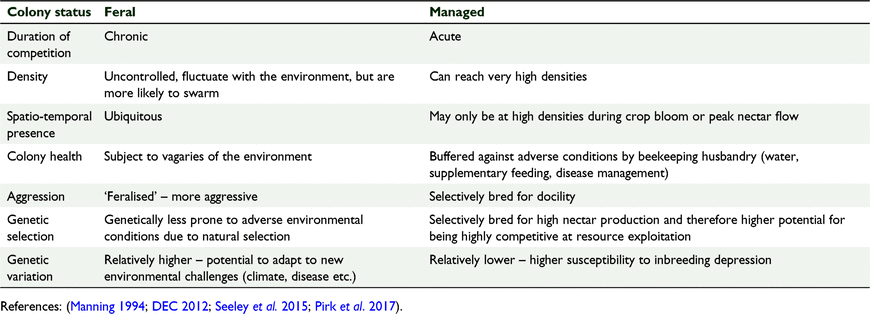
Feral honeybees
Feral honeybees have been present in Australia since the introduction of honeybees in the 1820s (Gibbs and Muirhead 1998). Feral and managed honeybees generate different predictions on the level of competitive threat they represent to native bees (Table 2). For example, feral honeybees represent chronic levels of competition and would be present when resources are limiting, whereas beekeepers tend to only place hives in areas where there is adequate, or at least greater amounts, of forage. Managed hives can however, be more vigourous due to beekeeping husbandry, and occur at higher densities. No feral hive removal experiments have been conducted in Australia, yet this may represent a more harmonious solution to honeybee management, being favourable to both commercial beekeepers and conservationists particularly as density or reproduction of inferior competitor species increases following removal of the superior competitor (Palmer et al. 2003).
|
Feral and managed honeybees appear to compete more strongly against each other than against other species (Mallick and Driessen 2009) and introductions of commercial honeybees in Europe exert the strongest effects on feral honeybees, reducing feral honeybee densities and causing significant shifts temporally, spatially, and with respect to flora visited, yet did not affect native bumblebee foraging, or densities, which increased under addition of managed honeybees (Walther-Hellwig et al. 2006).
The number of feral colonies in Australia is unknown, yet feral honeybees appear to be most dense along waterways owing to high water requirements for cooling hives in hot Australian summers (DEC 2012). In a riparian woodland of north-west Victoria, feral honeybees occurred at densities of 50–150/km2, fluctuating over the 3 years study (Oldroyd et al. 1997). Based on these estimates, it appears that feral honeybee colonies represent a greater population than do managed bees in Australia (Pirk et al. 2017). However, feral colony estimates may have been overestimated by an order of magnitude, and are also reduced in disturbed habitats (Arundel 2015). As techniques exist to remove feral hives (DEC 2012), feral honeybee removal experiments in Australia are warranted.
Finally, potential threatening processes on native bees can act in a combinatory or compensatory fashion, leading to potentially complex and often counterintuitive responses regarding the relationship of native bees to one or more potential explanatory variables (González-Varo et al. 2013; Goulson et al. 2015). It is therefore important to evaluate honeybee competition within the context of other threatening processes, and how environmental conditions may exacerbate or ameliorate competition.
Disease transmission
Honeybees suffer from parasites, viruses and other pathogens (Goulson et al. 2015). Despite being free of the major diseases that affect honeybee populations across the globe, Australian honeybees still are subject to many parasites and diseases (Frost 2019). There is therefore potential for honeybees to adversely affect native bees by transmitting diseases (Fürst et al. 2014; Goulson and Hughes 2015; Graystock et al. 2016). Indeed, of all potential negative impacts by honeybees on native bees, disease spill-over has received empirical support with no contradicting conclusions (Fürst et al. 2014; Russo 2016). However, clear declines due to disease transmission from honeybees appears to be restricted to closely-related bees (other Apis species, and some Bombus species) (Goulson et al. 2015; McMahon et al. 2015), whereas the phylogenetically-divergent native bee fauna of Australia may be relatively immune to this potential threat. Nevertheless, research has revealed pathogen sharing between honeybees and native Australian bees (Brettell et al. 2020).
The influence of predators in mediating competition
An Australian study on predation by spiders found honeybees were more vulnerable to predation than native bees (Heiling and Herberstein 2004). A potential explanation of increased numbers of native bees in the presence of higher honeybee abundances may therefore be due to predator saturation effects (Schwarz et al. 1991; Schwarz et al. 1992; Schwarz and Hurst 1997). However, greater abundances of honeybees can increase predator numbers, and consequently consumption of native bees could also increase (Wilson and Holway 2010). Solitary bees would be more negatively impacted by predation than would the colonial honeybee (Moller 1996). Future studies should consider identifying predators, and conducting manipulative studies measuring predator population responses and predation rates on honeybees and native bees under different honeybee abundances.
Considerations for future honeybee competition studies in Australia
Given that almost one quarter of publications on the effect of honeybees on native bees in Australia are reviews/opinion pieces, there is an over-representation of opinion on the topic with a comparatively small set of data from which to draw conclusions. We provide recommendations to advance research on this topic below.
Ecological theory predicts that under competition, either the superior competitor will eventually cause the extinction of the inferior competitor, or natural selection will cause species to evolve mechanisms such as niche shifts (e.g. character displacement, partitioning of resources among multiple dimensions) to reduce competition (Sommer and Worm 2002). Alternatively, non-equilibrium conditions, trade-offs, environmental heterogeneity, patch dynamics, recruitment and aggregation, as well as tritrophic interactions (such as disease or predators, discussed above), may enable co-existence (Sommer and Worm 2002). At what time scale these eco-evolutionary dynamics take place is unknown but would be important, because plant–pollinator dynamics following the invasion of honeybees can result in evolutionary changes to accommodate such new interactions within mere decades (Briggs 2014; Herrera and Pellmyr 2009). Conducting studies on whether native bees are affected by honeybees may be critical if time lags between invasion and competitive elimination occurs and may be vital to reverse the situation for species on the brink of extinction (Kuussaari et al. 2009).
Identifying under what conditions honeybees do or do not have an impact on native bees, and identifying vulnerable native bee species, can allow more targeted, evidence-based management. Studies reviewed above that found positive correlations between honeybee and native bee abundance suggests that opportunities exist to promote both categories of pollinators. Further studies need to be undertaken to identify what factors determine conditions where both native bees and honeybees can thrive.
Ambiguity about whether honeybees outcompete native bees may stem in part from the difficulties of rigorously testing the competition hypothesis in the field, where there are many interacting and potentially confounding variables. Conducting competition experiments in controlled conditions in enclosures can reduce the effects of environmental vagaries. For example, such an approach provided clear evidence that honeybees could significantly depress fitness of the native European megachilid (Osmia bicornis) (Hudewenz and Klein 2015).
Overall, greater recognition, research, investment, and monitoring of Australia’s native bee fauna is urgently required. Even though honeybees are not endangered, bee conservation is presented as being synonymous with saving honeybees and their pollination services (Batra 1995; Ollerton et al. 2012). Actions to help honeybees are unlikely to preserve native bees because of divergent foraging preferences and nesting habits; moreover, honeybee declines (where present) are largely driven not by loss of natural habitat, but rather due to their unique diseases and poor husbandry (essentially a domestic animal management issue) (Ollerton et al. 2012; Packer et al. 2016; Geldmann and González-Varo 2018).
Native bees can be key pollinators of both native and managed flora, and their co-evolutionary history with native flora means some oligolectic species perform superior pollination for particular native plants compared with honeybees (Houston 2000, 2014; Phillips et al. 2010). Even if honeybees compensate for native bees in terms of pollination services for some flora (e.g. exotic crops), ensuring native pollinators are preserved or restored to landscapes is still a fundamental endeavour, as native pollinators represent a unique evolutionary history that, unlike their pollination services, are irreplaceable (Senapathi et al. 2015; Prendergast 2020).
A precautionary approach to impacts of Apis mellifera in Australia
Well-resourced, long-term and, in some instances, manipulative experiments, are required to help determine under what circumstances there is a risk for honeybees to exert negative impacts on native bees. The published literature is insufficient to conclude with confidence if, when and where honeybees are competing with native bees. When resource competition is occurring, the published literature also is inconclusive regarding if the severity of competition results in negative consequences to biological fitness, translating to declines at the population level. Investigating all parameters to evaluate competition is challenging (Minckley et al. 2003), but a thorough assessment would require measuring honeybee density, resource levels, and resource overlap, and how this relates to both native bee population density and reproductive output over time, and across scales ranging from the patch, to the habitat, to the landscape. For example, in over 50% of studies, it was assumed that indirect measures of resource competition were associated with fitness costs. Furthermore, indirect measures of competition included overlap in resource use, which is a necessary requirement for competition, but also, conversely, divergences in resource use as can occur under competitive exclusion, Whether indirect measures of resource competition result in reductions in fitness will depend upon resource availability and resource requirements of native bee populations (e.g. Minckley et al. 2003; Cane and Tepedino 2017), which have yet to be measured in Australian environments and for Australian bee species. Wild bees face fluctuating resource levels, and especially in urban and natural landscapes, they forage in heterogeneous landscapes. Therefore, when and where competition occurs, and if this translates to negative population outcomes, likely varies over time, as well as space (e.g. Roubik 2001; Prendergast et al. 2021a). For example, with adequate resource levels, honeybees and native bees could partition resources between patches, enabling co-existence, with no negative population impacts at larger scales. There has been far greater solid, ongoing experimental research of resource competition between introduced honey bees and native bees in the Neotropics (Roubik 2001; Roubik and Wolda 2001). Such studies may be considered a template to build a research agenda in Australia. However, it would be challenging to extrapolate these results to an Australian context due to a different climate, floristically rich habitats of different species, and a different honey bee subspecies (the Africanised honey bee) (Villanueva-Gutiérrez and Roubik 2004). Moreover, unlike the Neotropics, which has 33 genera (391 species) of Meliponini (Freitas et al. 2009), polylectic eusocial species are only a very minor component of Australian bee fauna (Houston 2018), and therefore, much of the Australian endemic fauna have not co-evolved with bees of this niche as a major selection pressure.
It is evident that studies to date are insufficient to draw solid conclusions about the net impact of the introduced European honeybee. However, a lack of evidence should not be interpreted to mean a lack of impacts. Rather, there is a solid theoretical foundation for expecting that under certain conditions this introduced abundant species may exert competitive pressures on native bees with consequences to biological fitness, which can be predicted to be exacerbated due to conditions causing limitations in resources and favouring honeybees over native bees (climate change, land-clearing, increased proportions of exotic flora, increased backyard beekeepers). Therefore, a precautionary principle is warranted (Pyke 1999).
Conclusion
Our review shows that it is premature to conclude that honeybees have either a benign influence or a net detrimental impact on Australian native bee abundance, species richness, or reproductive output. Because the effect of invasive species is likely to be strongly context-dependent and will vary upon the environmental and biotic conditions in space and time (Pyšek et al. 2012), further robust experimental studies on the impact of honeybees on native plant–pollinator networks in Australia are essential. Indeed, significant negative impacts appear to be more likely to be revealed through controlled manipulative studies. A major conclusion from our review was that part of the variation in results stems from variation in species ecology and therefore, more detailed investigations into how particular native bee species or guilds are impacted is needed rather than coarse approaches which may obscure the impacts of this introduced bee on vulnerable species. Future studies should focus on the areas we identified above with the aim of managing honeybees in such a way that does not jeopardise the conservation of native bees.
Supplementary material
Supplementary material is available online.
Data availability
All data involved in this review is available in the Supplementary Material.
Conflicts of interest
The authors declare that they have no conflicts of interest.
Declaration of funding
The lead author began writing this review whilst undertaking a Ph.D. funded by a Forrest Research Foundation Scholarship.
Acknowledgements
The authors thank previous reviewers for their comments on earlier versions of this manuscript. This research was supported by a Forrest Research Foundation scholarship awarded to K.S.P. We acknowledge Australia’s Traditional Land Owners, for whom this country has always been a place of learning. K. Prendergast undertook this review during a PhD with a scholarship from the Forrest Research Foundation.
References
ABRS (Australian Biological Resources Study) (2015) Flora of Australia. (Australian Biological Resources Study: Canberra). Available at http://www.ausflora.org.au. [Accessed 11 March 2021]AFD (Australian Fauna Directory) (2021) Apiformes. Australian Faunal Directory. Australian Biological Resources Study, Canberra. Available at https://biodiversity.org.au/afd/taxa/APIFORMES. [Accessed 10 March 2021]
Agüero, JI, Pérez-Méndez, N, Torretta, J, et al. (2020). Impact of invasive bees on plant-pollinator interactions and reproductive success of plant species in mixed Nothofagus Antarctica forests. Neotropical Entomology 49, 557–567.
| Impact of invasive bees on plant-pollinator interactions and reproductive success of plant species in mixed Nothofagus Antarctica forests.Crossref | GoogleScholarGoogle Scholar | 32734552PubMed |
Aizen, MA, Morales, CL, and Morales, JM (2008). Invasive mutualists Erode native pollination webs. PLoS Biology 6, e31.
| Invasive mutualists Erode native pollination webs.Crossref | GoogleScholarGoogle Scholar | 18271628PubMed |
ALA (Atlas of Living Australia) (2021) Apis (Apis) mellifera Linnaeus, 1758. Available at https://bie.ala.org.au/species/urn:lsid:biodiversity.org.au:afd.taxon:1a490f00-368f-427c-8d4c-fa3f3271d75f#overview. [Accesssed 10 March 2021]
Ali, M, Saeed, S, Sajjad, A, and Akbar, A (2015). Linking pollination effectiveness and interspecific displacement success in bees. Neotropical Entomology 44, 101–108.
| Linking pollination effectiveness and interspecific displacement success in bees.Crossref | GoogleScholarGoogle Scholar | 26013126PubMed |
Almeida, EAB, Pie, MR, Brady, SG, and Danforth, BN (2012). Biogeography and diversification of colletid bees (Hymenoptera: Colletidae): emerging patterns from the southern end of the world. Journal of Biogeography 39, 526–544.
| Biogeography and diversification of colletid bees (Hymenoptera: Colletidae): emerging patterns from the southern end of the world.Crossref | GoogleScholarGoogle Scholar |
Almeida, EAB, Packer, L, Melo, GAR, Danforth, BN, Cardinal, SC, Quinteiro, FB, and Pie, MR (2019). The diversification of neopasiphaeine bees during the Cenozoic (Hymenoptera: Colletidae). Zoologica Scripta 48, 226–242.
| The diversification of neopasiphaeine bees during the Cenozoic (Hymenoptera: Colletidae).Crossref | GoogleScholarGoogle Scholar |
Arthur, AD, Li, J, Henry, S, and Cunningham, SA (2010). Influence of woody vegetation on pollinator densities in oilseed Brassica fields in an Australian temperate landscape. Basic and Applied Ecology 11, 406–414.
| Influence of woody vegetation on pollinator densities in oilseed Brassica fields in an Australian temperate landscape.Crossref | GoogleScholarGoogle Scholar |
Arundel JP (2015) The spatio-temporal distribution of honey bees and floral resources in Australia. PhD thesis, University of Melbourne, Melbourne.
Aschehoug, ET, and Callaway, RM (2015). Diversity increases indirect interactions, attenuates the intensity of competition, and promotes coexistence. The American Naturalist 186, 452–459.
| Diversity increases indirect interactions, attenuates the intensity of competition, and promotes coexistence.Crossref | GoogleScholarGoogle Scholar | 26655569PubMed |
Bailey W (1994) Feral bees: their potential effect on the native insect fauna. In ‘Impact and control of feral animals in Southwestern Australia’. (Eds R Siewert, N Robinson, P Horwitz) pp. 19–28. (Conservation Council of Western Australia: Perth, WA)
Batley, M, and Hogendoorn, K (2009). Diversity and conservation status of native Australian bees. Apidologie 40, 347–354.
| Diversity and conservation status of native Australian bees.Crossref | GoogleScholarGoogle Scholar |
Batley, M, and Houston, TF (2012). Revision of the Australian bee genus Trichocolletes Cockerell (Hymenoptera: Colletidae: Paracolletini). Records of the Australian Museum 64, 1–50.
| Revision of the Australian bee genus Trichocolletes Cockerell (Hymenoptera: Colletidae: Paracolletini).Crossref | GoogleScholarGoogle Scholar |
Batra, SWT (1995). Bees and pollination in our changing environment. Apidologie 26, 361–370.
| Bees and pollination in our changing environment.Crossref | GoogleScholarGoogle Scholar |
BeeAware (2014) BeeAware - industry. BeeAware. Available at https://beeaware.org.au/industry/. [Accessed 10 March 2021]
Begon M, Harper JL, Townsend CR (2005) ‘Ecology.’ (John Wiley & Sons, Incorporated)
Bellard, C, Cassey, P, and Blackburn, TM (2016). Alien species as a driver of recent extinctions. Biology Letters 12, 20150623.
| Alien species as a driver of recent extinctions.Crossref | GoogleScholarGoogle Scholar | 26888913PubMed |
Bernhardt, P (1987). A comparison of the diversity, density, and foraging behavior of bees and wasps on Australian Acacia. Annals of the Missouri Botanical Garden 74, 42–50.
| A comparison of the diversity, density, and foraging behavior of bees and wasps on Australian Acacia.Crossref | GoogleScholarGoogle Scholar |
Bossert, S, Murray, EA, Almeida, EAB, et al. (2019). Combining transcriptomes and ultraconserved elements to illuminate the phylogeny of Apidae. Molecular Phylogenetics and Evolution 130, 121–131.
| Combining transcriptomes and ultraconserved elements to illuminate the phylogeny of Apidae.Crossref | GoogleScholarGoogle Scholar | 30326287PubMed |
Brettell, LE, Riegler, M, O’Brien, C, and Cook, JM (2020). Occurrence of honey bee-associated pathogens in Varroa-free pollinator communities. Journal of Invertebrate Pathology 171, 107344.
| Occurrence of honey bee-associated pathogens in Varroa-free pollinator communities.Crossref | GoogleScholarGoogle Scholar | 32081716PubMed |
Briggs, JC (2014). Invasions, adaptive radiations, and the generation of biodiversity. Environmental Skeptics and Critics 3, 8–16.
Brooker, RW, Maestre, FT, Callaway, RM, Lortie, CL, Cavieres, LA, Kunstler, G, Liancourt, P, Tielbörger, K, Travis, JMJ, Anthelme, F, Armas, C, Coll, L, Corcket, E, Delzon, S, Forey, E, Kikvidze, Z, Olofsson, J, Pugnaire, F, Quiroz, CL, Saccone, P, Schiffers, K, Seifan, M, Touzard, B, and Michalet, R (2008). Facilitation in plant communities: the past, the present, and the future. Journal of Ecology 96, 18–34.
| Facilitation in plant communities: the past, the present, and the future.Crossref | GoogleScholarGoogle Scholar |
Cahill, JF, Kembel, SW, Lamb, EG, and Keddy, PA (2008). Does phylogenetic relatedness influence the strength of competition among vascular plants? Perspectives in Plant Ecology, Evolution and Systematics 10, 41–50.
| Does phylogenetic relatedness influence the strength of competition among vascular plants?Crossref | GoogleScholarGoogle Scholar |
Cairns, CE, Villanueva-Gutiérrez, R, Koptur, S, and Bray, DB (2005). Bee populations, forest disturbance, and Africanization in Mexico. Biotropica 37, 686–692.
| Bee populations, forest disturbance, and Africanization in Mexico.Crossref | GoogleScholarGoogle Scholar |
Cane, JH, and Tepedino, VJ (2017). Gauging the effect of honey bee pollen collection on native bee communities. Conservation Letters 10, 205–210.
| Gauging the effect of honey bee pollen collection on native bee communities.Crossref | GoogleScholarGoogle Scholar |
Cane JH, Johnson E, Klemens M (2005) Bees, pollination, and the challenges of sprawl. In ‘Nature in fragments: the legacy of sprawl’. (Eds EA Johnson, MW Klemens) pp. 109–124. (Columbia University Press: New York)
Carré, G, Roche, P, Chifflet, R, et al. (2009). Landscape context and habitat type as drivers of bee diversity in European annual crops. Agriculture, Ecosystems & Environment 133, 40–47.
| Landscape context and habitat type as drivers of bee diversity in European annual crops.Crossref | GoogleScholarGoogle Scholar |
Chapman NC, Oldroyd BP (2020) A report to the Queensland Parks and Wildlife Service on the effects of commercial honey bees on native flora and fauna. QPaW Service, Sydney, Australia
Chen Y (1993) ‘Apiculture in China.’ (Agricultural Sci-tech Publishing House of China: China)
Clavero, M, and García-Berthou, E (2005). Invasive species are a leading cause of animal extinctions. Trends in Ecology & Evolution 20, 110.
| Invasive species are a leading cause of animal extinctions.Crossref | GoogleScholarGoogle Scholar |
Corbet, SA, and Delfosse, ES (1984). Honeybees and the nectar of Echium plantagineum L. in southeastern Australia. Australian Journal of Ecology 9, 125–139.
| Honeybees and the nectar of Echium plantagineum L. in southeastern Australia.Crossref | GoogleScholarGoogle Scholar |
Dale MRT (2017) Competition: hierarchies and reversals. In ‘Applying graph theory in ecological research’. (Ed. MRT Dale) pp. 128–146. (Cambridge University Press: Cambridge)
DEC (2012) Notes from the Feral Bee Forum. In ‘Feral bees in Western Australia: Proceedings of a forum - green skills and the land for wildlife scheme, Albany, 7 Dec 2012’. (WA Department of Environment and Conservation: Albany, WA, Australia)
De la Rúa, P, Jaffé, R, Dall’Olio, R, Muñoz, I, and Serrano, J (2009). Biodiversity, conservation and current threats to European honeybees. Apidologie 40, 263–284.
| Biodiversity, conservation and current threats to European honeybees.Crossref | GoogleScholarGoogle Scholar |
Delnevo, N, van Etten, EJ, Byrne, M, Petraglia, A, Carbognani, M, and Stock, WD (2020). Habitat fragmentation restricts insect pollinators and pollen quality in a threatened Proteaceae species. Biological Conservation 252, 108824.
| Habitat fragmentation restricts insect pollinators and pollen quality in a threatened Proteaceae species.Crossref | GoogleScholarGoogle Scholar |
Dohzono, I, and Yokoyama, J (2010). Impacts of alien bees on native plant-pollinator relationships: A review with special emphasis on plant reproduction. Applied Entomology and Zoology 45, 37–47.
| Impacts of alien bees on native plant-pollinator relationships: A review with special emphasis on plant reproduction.Crossref | GoogleScholarGoogle Scholar |
Douglas, A (1977). Some inimical effects of the domestic bee on the native fauna and flora. The Western Australian Naturalist 14, 1–2.
Dupont, YL, Hansen, DM, Valido, A, and Olesen, JM (2004). Impact of introduced honey bees on native pollination interactions of the endemic Echium wildpretii (Boraginaceae) on Tenerife, Canary Islands. Biological Conservation 118, 301–311.
| Impact of introduced honey bees on native pollination interactions of the endemic Echium wildpretii (Boraginaceae) on Tenerife, Canary Islands.Crossref | GoogleScholarGoogle Scholar |
Eickwort, GC, and Ginsberg, HS (1980). Foraging and mating behavior in Apoidea. Annual Review of Entomology 25, 421–446.
| Foraging and mating behavior in Apoidea.Crossref | GoogleScholarGoogle Scholar |
Elbgami, T, Kunin, WE, Hughes, WOH, and Biesmeijer, JC (2014). The effect of proximity to a honeybee apiary on bumblebee colony fitness, development, and performance. Apidologie 45, 504–513.
| The effect of proximity to a honeybee apiary on bumblebee colony fitness, development, and performance.Crossref | GoogleScholarGoogle Scholar |
Elliott, B, Wilson, R, Shapcott, A, Keller, A, Newis, R, Cannizzaro, C, Burwell, C, Smith, T, Leonhardt, SD, Kämper, W, and Wallace, HM (2021). Pollen diets and niche overlap of honey bees and native bees in protected areas. Basic and Applied Ecology 50, 169–180.
| Pollen diets and niche overlap of honey bees and native bees in protected areas.Crossref | GoogleScholarGoogle Scholar |
Ettershank G, Ettershank JA (1990) Insects associated with flowers of Tasmanian leatherwoods (Eucryphia spp.). In ‘Proceedings of the Tasmanian National Rainforest Conservation Program’. pp. 65–71.
Ettershank G, Ettershank J (1993) ‘Tasmanian leatherwoods (Eucryphia spp.)-floral phenology and the insects associated with flowers.’ (Forestry Commission: Tasmania)
Evans, LJ, Jesson, L, Read, SFJ, Jochym, M, Cutting, BT, Gayrard, T, Jammes, MAS, Roumier, R, and Howlett, BG (2021). Key factors influencing forager distribution across macadamia orchards differ among species of managed bees. Basic and Applied Ecology 53, 74–85.
| Key factors influencing forager distribution across macadamia orchards differ among species of managed bees.Crossref | GoogleScholarGoogle Scholar |
FAO (Food & Agriculture Organization of the United Nations) (2021) FAOSTAT: honey, natural, 2019. Available at http://www.fao.org/faostat/en/#data/QL. [Accessed 8 March 2021]
Forup, ML, and Memmott, J (2005). The relationship between the abundances of bumblebees and honeybees in a native habitat. Ecological Entomology 30, 47–57.
| The relationship between the abundances of bumblebees and honeybees in a native habitat.Crossref | GoogleScholarGoogle Scholar |
Franzén, M, and Nilsson, SG (2010). Both population size and patch quality affect local extinctions and colonizations. Proceedings of the Royal Society B: Biological Sciences 277, 79–85.
| Both population size and patch quality affect local extinctions and colonizations.Crossref | GoogleScholarGoogle Scholar | 19793747PubMed |
Freitas BM, Imperatriz-Fonseca VL, Medina LM, Kleinert AdMP, Galetto L, Nates-Parra G, Quezada-Euán JJG (2009) Diversity, threats and conservation of native bees in the Neotropics. Apidologie 40(3), 332–346.
Frost E (2019) primefact: pests and diseases of honey bees. (State of New South Wales Department of Primary Industries: NSW). Available at https://www.dpi.nsw.gov.au/__data/assets/pdf_file/0007/1148992/pest-and-diseases-of-honey-bees.pdf. [Accessed 19 March 2020]
Fürst, MA, McMahon, DP, Osborne, JL, Paxton, RJ, and Brown, MJF (2014). Disease associations between honeybees and bumblebees as a threat to wild pollinators. Nature 506, 364–366.
| Disease associations between honeybees and bumblebees as a threat to wild pollinators.Crossref | GoogleScholarGoogle Scholar | 24553241PubMed |
Geldmann, J, and González-Varo, JP (2018). Conserving honey bees does not help wildlife. Science 359, 392–393.
| Conserving honey bees does not help wildlife.Crossref | GoogleScholarGoogle Scholar | 29371456PubMed |
Geslin B, Gauzens B, Baude M, Dajoz I, Fontaine C, Henry M, Ropars L, Rollin O, Thébault E, Vereecken NJ (2017) Chaptet Four: Massively introduced managed species and their consequences for plant–pollinator interactions. In ‘Advances in ecological research’. (Eds DA Bohan, AJ Dumbrell, F Massol) pp. 147–99. (Academic Press) 10.1016/bs.aecr.2016.10.007
Gibbs DM, Muirhead IF (1998) The economic value and environmental impact of the Australian beekeeping industry: a report prepared for the Australian beekeeping industry. (Australian beekeeping industry: Canberra). Available at https://honeybee.org.au/doc/Muirhead.doc. [Accessed 3 March 2020]
Gilpin, A-M, Denham, AJ, and Ayre, DJ (2019a). Are there magnet plants in Australian ecosystems: pollinator visits to neighbouring plants are not affected by proximity to mass flowering plants. Basic and Applied Ecology 35, 34–44.
| Are there magnet plants in Australian ecosystems: pollinator visits to neighbouring plants are not affected by proximity to mass flowering plants.Crossref | GoogleScholarGoogle Scholar |
Gilpin, A-M, Denham, AJ, and Ayre, DJ (2019b). Do mass flowering agricultural species affect the pollination of Australian native plants through localised depletion of pollinators or pollinator spillover effects? Agriculture, Ecosystems & Environment 277, 83–94.
| Do mass flowering agricultural species affect the pollination of Australian native plants through localised depletion of pollinators or pollinator spillover effects?Crossref | GoogleScholarGoogle Scholar |
Godoy, O, Stouffer, DB, Kraft, NJB, and Levine, JM (2017). Intransitivity is infrequent and fails to promote annual plant coexistence without pairwise niche differences. Ecology 98, 1193–1200.
| Intransitivity is infrequent and fails to promote annual plant coexistence without pairwise niche differences.Crossref | GoogleScholarGoogle Scholar | 28241383PubMed |
González-Varo, JP, Biesmeijer, JC, Bommarco, R, Potts, SG, Schweiger, O, Smith, HG, Steffan-Dewenter, I, Szentgyörgyi, H, Woyciechowski, M, and Vilà, M (2013). Combined effects of global change pressures on animal-mediated pollination. Trends in Ecology & Evolution 28, 524–530.
| Combined effects of global change pressures on animal-mediated pollination.Crossref | GoogleScholarGoogle Scholar |
Goulson, D (1994). A model to predict the influence of insect flower constancy on interspecific competition between insect pollinated plants. Journal of Theoretical Biology 168, 309–314.
| A model to predict the influence of insect flower constancy on interspecific competition between insect pollinated plants.Crossref | GoogleScholarGoogle Scholar |
Goulson, D (2003). Effects of introduced bees on native ecosystems. Annual Review of Ecology, Evolution, and Systematics 34, 1–26.
| Effects of introduced bees on native ecosystems.Crossref | GoogleScholarGoogle Scholar |
Goulson, D (2004). Keeping bees in their place: impacts of bees outside their native range. Bee World 85, 45–46.
| Keeping bees in their place: impacts of bees outside their native range.Crossref | GoogleScholarGoogle Scholar |
Goulson, D, and Derwent, LC (2004). Synergistic interactions between an exotic honeybee and an exotic weed: pollination of Lantana camara in Australia. Weed Research 44, 195–202.
| Synergistic interactions between an exotic honeybee and an exotic weed: pollination of Lantana camara in Australia.Crossref | GoogleScholarGoogle Scholar |
Goulson, D, and Hughes, WOH (2015). Mitigating the anthropogenic spread of bee parasites to protect wild pollinators. Biological Conservation 191, 10–19.
| Mitigating the anthropogenic spread of bee parasites to protect wild pollinators.Crossref | GoogleScholarGoogle Scholar |
Goulson, D, Lye, GC, and Darvill, B (2008). Decline and conservation of bumble bees. Annual Review of Entomology 53, 191–208.
| Decline and conservation of bumble bees.Crossref | GoogleScholarGoogle Scholar | 17803456PubMed |
Goulson, D, Nicholls, E, Botías, C, and Rotheray, EL (2015). Bee declines driven by combined stress from parasites, pesticides, and lack of flowers. Science 347, 1255957.
| Bee declines driven by combined stress from parasites, pesticides, and lack of flowers.Crossref | GoogleScholarGoogle Scholar | 25721506PubMed |
Graystock, P, Blane, EJ, McFrederick, QS, Goulson, D, and Hughes, WO (2016). Do managed bees drive parasite spread and emergence in wild bees? International Journal for Parasitology: Parasites and Wildlife 5, 64–75.
| Do managed bees drive parasite spread and emergence in wild bees?Crossref | GoogleScholarGoogle Scholar | 28560161PubMed |
Gross, CL (1993). The breeding system and pollinators of Melastoma affine (Melastomataceae); a pioneer shrub in tropical Australia. Biotropica , 468–474.
| The breeding system and pollinators of Melastoma affine (Melastomataceae); a pioneer shrub in tropical Australia.Crossref | GoogleScholarGoogle Scholar |
Gross, CL, and Mackay, D (1998). Honeybees reduce fitness in the pioneer shrub Melastoma affine (Melastomataceae). Biological Conservation 86, 169–178.
| Honeybees reduce fitness in the pioneer shrub Melastoma affine (Melastomataceae).Crossref | GoogleScholarGoogle Scholar |
Gross, C (2001). The effect of introduced honeybees on native bee visitation and fruit-set in Dillwynia juniperina (Fabaceae) in a fragmented ecosystem. Biological Conservation 102, 89––95.
Halcroft MT (2012) Investigations into the biology, behaviour and phylogeny of a potential crop pollinator: the Australian stingless bee, Austroplebeia australis. Doctor of Philosophy thesis, University of Western Sydney.
Hargreaves, AL, Harder, LD, and Johnson, SD (2010). Native pollen thieves reduce the reproductive success of a hermaphroditic plant, Aloe maculata. Ecology 91, 1693–1703.
| Native pollen thieves reduce the reproductive success of a hermaphroditic plant, Aloe maculata.Crossref | GoogleScholarGoogle Scholar | 20583711PubMed |
Harrison, T, and Winfree, R (2015). Urban drivers of plant-pollinator interactions. Functional Ecology 29, 879–888.
| Urban drivers of plant-pollinator interactions.Crossref | GoogleScholarGoogle Scholar |
Heard, TA (1994). Behaviour and pollinator efficiency of stingless bees and honey bees on macadamia flowers. Journal of Apicultural Research 33, 191––198.
Heard T (2016) ‘The Australian native bee book: keeping stingless bee hives for pets, pollination and sugarbag honey.’ (Sugarbag Bees: Brisbane)
Heard, TA, Vithanage, V, and Chacko, EK (1990). Pollination biology of cashew in the Northern Territory of Australia. Australian Journal of Agricultural Research 41, 1101–1114.
| Pollination biology of cashew in the Northern Territory of Australia.Crossref | GoogleScholarGoogle Scholar |
Heiling, AM, and Herberstein, ME (2004). Predator–prey coevolution: Australian native bees avoid their spider predators. Proceedings of the Royal Society of London B: Biological Sciences 271, S196–S198.
| Predator–prey coevolution: Australian native bees avoid their spider predators.Crossref | GoogleScholarGoogle Scholar |
Hermansen, TD, Britton, DR, Ayre, DJ, and Minchinton, TE (2014). Identifying the real pollinators? Exotic honeybees are the dominant flower visitors and only effective pollinators of Avicennia marina in Australian temperate mangroves. Estuaries and Coasts 37, 621–635.
| Identifying the real pollinators? Exotic honeybees are the dominant flower visitors and only effective pollinators of Avicennia marina in Australian temperate mangroves.Crossref | GoogleScholarGoogle Scholar |
Herrera CM, Pellmyr O (2009) ‘Plant animal interactions: an evolutionary approach.’ (John Wiley & Sons)
Hingston AB (2002) ‘Pollination ecology of Eucalyptus globulus subsp. globulus and Eucalyptus nitens (Myrtaceae).’ (University of Tasmania: Tasmania)
Hingston, AB, Potts, BM, and McQuillan, PB (2004a). Pollination services provided by various size classes of flower visitors to Eucalyptus globulus ssp. globulus (Myrtaceae). Australian Journal of Botany 52, 353–369.
| Pollination services provided by various size classes of flower visitors to Eucalyptus globulus ssp. globulus (Myrtaceae).Crossref | GoogleScholarGoogle Scholar |
Hingston, AB, Potts, BM, and McQuillan, PB (2004b). The swift parrot Lathamus discolor (Psittacidae), social bees (Apidae), and native insects as pollinators of Eucalyptus globulus ssp. globulus (Myrtaceae). Australian Journal of Botany 52, 371–379.
| The swift parrot Lathamus discolor (Psittacidae), social bees (Apidae), and native insects as pollinators of Eucalyptus globulus ssp. globulus (Myrtaceae).Crossref | GoogleScholarGoogle Scholar |
Hoffmann, BD, and Andersen, AN (2003). Responses of ants to disturbance in Australia, with particular reference to functional groups. Austral Ecology 28, 444–464.
| Responses of ants to disturbance in Australia, with particular reference to functional groups.Crossref | GoogleScholarGoogle Scholar |
Holm E (1988) The native pollinating insects with a particular note on the honeybee. In ‘On pollination and pollinators in Western Australia’. (Ed. E Holm) pp. 125–126. (Gelved: Denmark)
Hopper S (1985) Impact of honeybees on Western Australia’s nectarivorous fauna. In ‘Beekeeping and land management: proceedings of a workshop’. (Ed. J Blyth) pp. 59–72. (Department of Conservation and Land Management: Perth, WA)
Horskins, K, and Turner, VB (1999). Resource use and foraging patterns of honeybees, Apis mellifera, and native insects on flowers of Eucalyptus costata. Australian Journal of Ecology 24, 221–227.
| Resource use and foraging patterns of honeybees, Apis mellifera, and native insects on flowers of Eucalyptus costata.Crossref | GoogleScholarGoogle Scholar |
Houston, TF (1989). Leioproctus bees associated with western Australian smoke bushes (Conospermum spp.) and their adaptations for foraging and concealment (Hymenoptera: Colletidae: Paracolletini). Records of the Western Australian Museum 14, 275–292.
Houston TF (2000) ‘Native bees on wildflowers in Western Australia.’ (Western Australian Insect Study Society: WA)
Houston TF (2014) Pollination vectors: invertebrates. In ‘Plant life on the sand plains in Southwest Australia: a global biodiversity hotspot’. (Ed. H Lambers). (UWA Press: Crawley, WA)
Houston TF (2018) ‘A guide to the native bees of Australia.’ (CSIRO Publishing: Melbourne)
Hudewenz, A, and Klein, A-M (2015). Red mason bees cannot compete with honey bees for floral resources in a cage experiment. Ecology and Evolution 5, 5049–5056.
| Red mason bees cannot compete with honey bees for floral resources in a cage experiment.Crossref | GoogleScholarGoogle Scholar | 26640681PubMed |
Hulbert, J (1984). Pseudoreplication and the design of field experiments in ecology. Ecological Monographs 54, 187––211.
Jackson WJ, Argent RM, Bax NJ, Bui E, Clark GF, Coleman S, Cresswell ID, Emmerson KM, Evans K, Hibberd MF, Johnston EL, Keywood MD, Klekociuk A, Mackay R, Metcalfe D, Murphy H, Rankin A, Smith DC, Wienecke B (2016) Overview: Invasive species are a potent, persistent and widespread threat to Australia’s environment. In ‘Australia state of the environment 2016.’ (Australian Government Department of the Environment and Energy: Canberra). Available at https://soe.environment.gov.au/theme/overview/topic/invasive-species-are-potent-persistent-and-widespread-threat-australias. [Accessed 9 October 2021]
James-Pirri, M-J, Roman, CT, and Heltshe, JF (2007). Power analysis to determine sample size for monitoring vegetation change in salt marsh habitats. Wetlands Ecology and Management 15, 335–345.
| Power analysis to determine sample size for monitoring vegetation change in salt marsh habitats.Crossref | GoogleScholarGoogle Scholar |
Johanson, LG, Hoffmann, AA, Walker, KL, and Nash, MA (2019). Bees of the Victorian Alps: network structure and interactions of introduced species. Austral Ecology 44, 245–254.
| Bees of the Victorian Alps: network structure and interactions of introduced species.Crossref | GoogleScholarGoogle Scholar |
Johnson, LK, and Hubbell, SP (1975). Contrasting foraging strategies and coexistence of two bee species on a single resource. Ecology , 1398–1406.
| Contrasting foraging strategies and coexistence of two bee species on a single resource.Crossref | GoogleScholarGoogle Scholar |
Karasinski J (2018a) The economic valuation of Australian managed and wild honey bee pollinators.’ (Curtin University: WA)
Karasinski J (2018b) ‘The economic value of Australia’s insect crop pollinators in 2014–2015.’ (Curtin University: WA)
Kato, M, Shibata, A, Yasui, T, and Nagamasu, H (1999). Impact of introduced honeybees, Apis mellifera, upon native bee communities in the Bonin (Ogasawara) Islands. Population Ecology 41, 217–228.
| Impact of introduced honeybees, Apis mellifera, upon native bee communities in the Bonin (Ogasawara) Islands.Crossref | GoogleScholarGoogle Scholar |
Kremen, C, Bugg, RL, Nicola, N, Smith, SA, Thorp, RW, and Williams, NM (2002a). Native bees, native plants and crop pollination in California. Fremontia 30, 41–49.
Kremen, C, Williams, NM, and Thorp, RW (2002b). Crop pollination from native bees at risk from agricultural intensification. Proceedings of the National Academy of Sciences of the United States of America 99, 16812–16816.
| Crop pollination from native bees at risk from agricultural intensification.Crossref | GoogleScholarGoogle Scholar | 12486221PubMed |
Kuussaari, M, Bommarco, R, Heikkinen, RK, et al. (2009). Extinction debt: a challenge for biodiversity conservation. Trends in Ecology & Evolution 24, 564–571.
| Extinction debt: a challenge for biodiversity conservation.Crossref | GoogleScholarGoogle Scholar |
Ladd, PG, Yates, CJ, Dillon, R, and Palmer, R (2019). Pollination ecology of Tetratheca species from isolated, arid habitats (banded iron formations) in Western Australia. Australian Journal of Botany 67, 248–255.
| Pollination ecology of Tetratheca species from isolated, arid habitats (banded iron formations) in Western Australia.Crossref | GoogleScholarGoogle Scholar |
Lancaster, LT, Morrison, G, and Fitt, RN (2017). Life history trade-offs, the intensity of competition, and coexistence in novel and evolving communities under climate change. Philosophical Transactions of the Royal Society B: Biological Sciences 372, 20160046.
| Life history trade-offs, the intensity of competition, and coexistence in novel and evolving communities under climate change.Crossref | GoogleScholarGoogle Scholar |
Leonhardt, SD, and Blüthgen, N (2012). The same, but different: pollen foraging in honeybee and bumblebee colonies. Apidologie 43, 449–464.
| The same, but different: pollen foraging in honeybee and bumblebee colonies.Crossref | GoogleScholarGoogle Scholar |
Levins R (1968) ‘Evolution in changing environments: some theoretical explorations.’ (Princeton University Press: Princeton, NJ)
Lichtenberg, EM, Imperatriz-Fonseca, VL, and Nieh, JC (2010). Behavioral suites mediate group-level foraging dynamics in communities of tropical stingless bees. Insectes Sociaux 57, 105–113.
| Behavioral suites mediate group-level foraging dynamics in communities of tropical stingless bees.Crossref | GoogleScholarGoogle Scholar | 20098501PubMed |
Lomov, B, Keith, DA, and Hochuli, DF (2010). Pollination and plant reproductive success in restored urban landscapes dominated by a pervasive exotic pollinator. Landscape and Urban Planning 96, 232–239.
| Pollination and plant reproductive success in restored urban landscapes dominated by a pervasive exotic pollinator.Crossref | GoogleScholarGoogle Scholar |
Lorenz, S, and Stark, K (2015). Saving the honeybees in Berlin? A case study of the urban beekeeping boom. Environmental Sociology 1, 116–126.
| Saving the honeybees in Berlin? A case study of the urban beekeeping boom.Crossref | GoogleScholarGoogle Scholar |
MacIvor, JS, and Packer, L (2016). The bees among us: modelling occupancy of solitary bees. PLoS ONE 11, e0164764.
| The bees among us: modelling occupancy of solitary bees.Crossref | GoogleScholarGoogle Scholar | 27911954PubMed |
Magrach, A, González-Varo, JP, Boiffier, M, et al. (2017). Honeybee spillover reshuffles pollinator diets and affects plant reproductive success. Nature Ecology & Evolution 1, 1299–1307.
| Honeybee spillover reshuffles pollinator diets and affects plant reproductive success.Crossref | GoogleScholarGoogle Scholar |
Mallick, SA, and Driessen, MM (2009). Impacts of hive honeybees on Tasmanian leatherwood Eucryphia lucida Labill.(Eucryphiaceae). Austral Ecology 34, 185–195.
| Impacts of hive honeybees on Tasmanian leatherwood Eucryphia lucida Labill.(Eucryphiaceae).Crossref | GoogleScholarGoogle Scholar |
Mallinger, RE, Gaines-Day, HR, and Gratton, C (2017). Do managed bees have negative effects on wild bees?: a systematic review of the literature. PLoS ONE 12, e0189268.
| Do managed bees have negative effects on wild bees?: a systematic review of the literature.Crossref | GoogleScholarGoogle Scholar | 29220412PubMed |
Manning R (1989) ‘Literature review of environmental issues concerning the honeybee Apis mellifera in Western Australia.’ (Western Australian Department of Agriculture: Perth)
Manning, R (1994). Honey production from managed feral bee colonies. Australasian Beekeeper 96, 110–112.
Manning, R (1997). The honey bee debate: a critique of scientific studies of honey bees Apis mellifera and their alleged impact on Australian wildlife. Victorian Naturalist 114, 13–22.
Manning, R (2018). Chemical residues in beebread, honey, pollen and wax samples collected from bee hives placed on canola crops in Western Australia. Journal of Apicultural Research 57, 696–708.
| Chemical residues in beebread, honey, pollen and wax samples collected from bee hives placed on canola crops in Western Australia.Crossref | GoogleScholarGoogle Scholar |
Manning R, Cadman R, Beard J, Hawkins C (2006) Surveillance of swarms and feral honey bees (Apis mellifera) for the presence of American foulbrood (Paenibacillus larvae sub. sp. larvae) spores and their habitat preferences in Western Australia. (Department of Agriculture and Food: Perth, WA). Bulletin 4680. Available at https://researchlibrary.agric.wa.gov.au/cgi/viewcontent.cgi?article=1234&context=bulletins. [Accessed 10 March 2021]
Martins, KT, Albert, CH, Lechowicz, MJ, and Gonzalez, A (2018). Complementary crops and landscape features sustain wild bee communities. Ecological Applications 28, 1093–1105.
| Complementary crops and landscape features sustain wild bee communities.Crossref | GoogleScholarGoogle Scholar | 29495110PubMed |
McFrederick, QS, and LeBuhn, G (2006). Are urban parks refuges for bumble bees Bombus spp. (Hymenoptera: Apidae)? Biological Conservation 129, 372–382.
| Are urban parks refuges for bumble bees Bombus spp. (Hymenoptera: Apidae)?Crossref | GoogleScholarGoogle Scholar |
McKinney, ML (2008). Effects of urbanization on species richness: a review of plants and animals. Urban Ecosystems 11, 161–176.
| Effects of urbanization on species richness: a review of plants and animals.Crossref | GoogleScholarGoogle Scholar |
McMahon, DP, Fürst, MA, Caspar, J, Theodorou, P, Brown, MJF, and Paxton, RJ (2015). A sting in the spit: widespread cross-infection of multiple RNA viruses across wild and managed bees. Journal of Animal Ecology 84, 615–624.
| A sting in the spit: widespread cross-infection of multiple RNA viruses across wild and managed bees.Crossref | GoogleScholarGoogle Scholar |
Michener, CD (1965). A classification of the bees of the Australian and South Pacific regions. Bulletin of the American Museum of Natural History 130, 1–362.
Michener, CD (1979). Biogeography of the bees. Annals of the Missouri Botanical Garden 66, 277–347.
| Biogeography of the bees.Crossref | GoogleScholarGoogle Scholar |
Michener CD (2007) ‘The Bees of the World.’ 2nd edn. (Johns Hopkins: Baltimore)
Minckley, RL, Cane, JH, Kervin, L, and Yanega, D (2003). Biological impediments to measures of competition among introduced honey bees and desert bees (Hymenoptera: Apiformes). Journal of the Kansas Entomological Society 76, 306–319.
Moller, H (1996). Lessons for invasion theory from social insects. Biological Conservation 78, 125–142.
| Lessons for invasion theory from social insects.Crossref | GoogleScholarGoogle Scholar |
Moore LJ, Kosut M (2013) ‘Buzz: urban beekeeping and the power of the bee.’ (NYU Press: New York)
Moritz, RFA, Härtel, S, and Neumann, P (2005). Global invasions of the western honeybee (Apis mellifera) and the consequences for biodiversity. Écoscience 12, 289–301.
| Global invasions of the western honeybee (Apis mellifera) and the consequences for biodiversity.Crossref | GoogleScholarGoogle Scholar |
New, T (1997). Significance of honey bees in the Australian environment: setting the scene. Victorian Naturalist 114, 4–6.
Oldroyd, BP, Thexton, EG, Lawler, SH, and Crozier, RH (1997). Population demography of Australian feral bees (Apis mellifera). Oecologia 111, 381–387.
| Population demography of Australian feral bees (Apis mellifera).Crossref | GoogleScholarGoogle Scholar | 28308133PubMed |
Ollerton, J, Price, V, Armbruster, WS, Memmott, J, Watts, S, Waser, NM, Totland, Ø, Goulson, D, Alarcón, R, Stout, JC, and Tarrant, S (2012). Overplaying the role of honey bees as pollinators: a comment on Aebi and Neumann (2011). Trends in Ecology & Evolution 27, 141–142.
| Overplaying the role of honey bees as pollinators: a comment on Aebi and Neumann (2011).Crossref | GoogleScholarGoogle Scholar |
Packer L, MacIvor J, Dumesh S, Colla S, Couto O, Harpur B, Sheffield C, Zayed A (2016) ‘Bees of Toronto: a guide to their remarkable diversity.’ (City of Toronto: Toronto, Canada)
Paini DR (2004) The impact of the European honey bee (Apis mellifera) on Australian native bees. PhD thesis, University of Western Australia Perth, Australia.
Paini, DR, and Roberts, JD (2005). Commercial honey bees (Apis mellifera) reduce the fecundity of an Australian native bee (Hylaeus alcyoneus). Biological Conservation 123, 103–112.
| Commercial honey bees (Apis mellifera) reduce the fecundity of an Australian native bee (Hylaeus alcyoneus).Crossref | GoogleScholarGoogle Scholar |
Paini, DR, Williams, MR, and Roberts, JD (2005). No short-term impact of honey bees on the reproductive success of an Australian native bee. Apidologie 36, 613–621.
| No short-term impact of honey bees on the reproductive success of an Australian native bee.Crossref | GoogleScholarGoogle Scholar |
Palmer, TM, Stanton, ML, and Young, TP (2003). Competition and coexistence: exploring mechanisms that restrict and maintain diversity within mutualist guilds. The American Naturalist 162, S63–S79.
| Competition and coexistence: exploring mechanisms that restrict and maintain diversity within mutualist guilds.Crossref | GoogleScholarGoogle Scholar | 14583858PubMed |
Paton AJ (1990) Budgets for the use of floral resources in mallee heath. In ‘The mallee lands: a conservation perspective’. (Eds J Noble, P Joss, C Jones) pp. 189–193. (CSIRO: Melbourne, Australia)
Paton, DC (1993). Honeybees in the Australian environment. BioScience , 95–103.
| Honeybees in the Australian environment.Crossref | GoogleScholarGoogle Scholar |
Paton DC (1996) ‘Overview of feral and managed honeybees in Australia: distribution, abundance, extent of interactions with native biota, evidence of impacts and future research.’ (Australian Nature Conservation Agency)
Paton, DC (1997). Honey bees Apis mellifera and the disruption of plant-pollinator systems in Australia. Victorian Naturalist 114, 23–29.
Peel, MC, Finlayson, BL, and McMahon, TA (2007). Updated world map of the Köppen-Geiger climate classification. Hydrology and Earth System Sciences 11, 1633–1644.
| Updated world map of the Köppen-Geiger climate classification.Crossref | GoogleScholarGoogle Scholar |
Phillips, RD, Hopper, SD, and Dixon, KW (2010). Pollination ecology and the possible impacts of environmental change in the Southwest Australian Biodiversity Hotspot. Philosophical Transactions of the Royal Society of London B: Biological Sciences 365, 517–528.
| Pollination ecology and the possible impacts of environmental change in the Southwest Australian Biodiversity Hotspot.Crossref | GoogleScholarGoogle Scholar | 20047877PubMed |
Pirk, CWW, Crewe, RM, and Moritz, RFA (2017). Risks and benefits of the biological interface between managed and wild bee pollinators. Functional Ecology 31, 47–55.
| Risks and benefits of the biological interface between managed and wild bee pollinators.Crossref | GoogleScholarGoogle Scholar |
Prendergast, KS (2020). Beyond ecosystem services as justification for biodiversity conservation. Austral Ecology 45, 141–143.
| Beyond ecosystem services as justification for biodiversity conservation.Crossref | GoogleScholarGoogle Scholar |
Prendergast, KS, and Hogendoorn, K (2021). FORUM: methodological shortcomings and lack of taxonomic effort beleaguer Australian bee studies. Austral Ecology 46, 880–884.
| FORUM: methodological shortcomings and lack of taxonomic effort beleaguer Australian bee studies.Crossref | GoogleScholarGoogle Scholar |
Prendergast, K, and Ollerton, J (2022). Impacts of the introduced European honeybee on Australian bee-flower network properties in urban bushland remnants and residential gardens. Austral Ecology 47, 35–53.
| Impacts of the introduced European honeybee on Australian bee-flower network properties in urban bushland remnants and residential gardens.Crossref | GoogleScholarGoogle Scholar |
Prendergast, KS, Dixon, KW, and Bateman, PW (2021a). Interactions between the introduced European honey bee and native bees in urban areas varies by year, habitat type and native bee guild. Biological Journal of the Linnean Society 133, 725–743.
| Interactions between the introduced European honey bee and native bees in urban areas varies by year, habitat type and native bee guild.Crossref | GoogleScholarGoogle Scholar |
Prendergast, KS, Leclercq, N, and Vereecken, NJ (2021b). Honey bees (Hymenoptera: Apidae) outnumber native bees in Tasmanian apple orchards: perspectives for balancing crop production and native bee conservation. Austral Entomology 60, 422–435.
| Honey bees (Hymenoptera: Apidae) outnumber native bees in Tasmanian apple orchards: perspectives for balancing crop production and native bee conservation.Crossref | GoogleScholarGoogle Scholar |
Prendergast, KS, Tomlinson, S, Dixon, KW, Bateman, PW, and Menz, MHM (2022). Urban native vegetation remnants support more diverse native bee communities than residential gardens in Australia’s southwest biodiversity hotspot. Biological Conservation 265, 109408.
Pyke, G (1999). The introduced honeybee Apis mellifera and the precautionary principle: reducing the conflict. Australian Zoologist 31, 181–186.
| The introduced honeybee Apis mellifera and the precautionary principle: reducing the conflict.Crossref | GoogleScholarGoogle Scholar |
Pyke G, Balzer L (1985) The effects of the introduced honeybee (Apis Mellifera) on Australian native bees: a report prepared for NSW National Parks & Wildlife Service. National Parks and Wildlife Service, NSW.
Pyšek, P, Jarošík, V, Hulme, PE, Pergl, J, Hejda, M, Schaffner, U, and Vilà, M (2012). A global assessment of invasive plant impacts on resident species, communities and ecosystems: the interaction of impact measures, invading species’ traits and environment. Global Change Biology 18, 1725–1737.
| A global assessment of invasive plant impacts on resident species, communities and ecosystems: the interaction of impact measures, invading species’ traits and environment.Crossref | GoogleScholarGoogle Scholar |
Rohr, RP, and Bascompte, J (2014). Components of phylogenetic signal in antagonistic and mutualistic networks. The American Naturalist 184, 556–564.
| Components of phylogenetic signal in antagonistic and mutualistic networks.Crossref | GoogleScholarGoogle Scholar | 25325741PubMed |
Ropars, L, Dajoz, I, Fontaine, C, Muratet, A, and Geslin, B (2019). Wild pollinator activity negatively related to honey bee colony densities in urban context. PLoS ONE 14, e0222316.
| Wild pollinator activity negatively related to honey bee colony densities in urban context.Crossref | GoogleScholarGoogle Scholar | 31513663PubMed |
Roubik, DW (1978). Competitive interactions between neotropical pollinators and Africanized honey bees. Science 201, 1030–1032.
| Competitive interactions between neotropical pollinators and Africanized honey bees.Crossref | GoogleScholarGoogle Scholar | 17743636PubMed |
Roubik, DW (1983). Experimental community studies: time-series tests of competition between African and neotropical bees. Ecology 64, 971–978.
| Experimental community studies: time-series tests of competition between African and neotropical bees.Crossref | GoogleScholarGoogle Scholar |
Roubik, DW (2001). Ups and downs in pollinator populations: when is there a decline? Conservation Ecology 5, 2.
Roubik, DW, and Villanueva-Gutierrez, R (2009). Invasive Africanized honey bee impact on native solitary bees: a pollen resource and trap nest analysis. Biological Journal of the Linnean Society 98, 152––160.
Roubik, DW, and Wolda, H (2001). Do competing honey bees matter? Dynamics and abundance of native bees before and after honey bee invasion. Population Ecology 43, 53–62.
| Do competing honey bees matter? Dynamics and abundance of native bees before and after honey bee invasion.Crossref | GoogleScholarGoogle Scholar |
Rural Industries Research & Development Corporation (RIRDC) (2010) Pollination aware: the real value of pollination in Australia (RIRDC Pub. No. 10-081, August 2010). (AgriFutures Australia: Wagga Wagga NSW). Available at https://www.agrifutures.com.au/product/pollination-aware-the-real-value-of-pollination-in-australia-fact-sheet/. [Accessed 7 March 2021]
Russo, L (2016). Positive and negative impacts of non-native bee species around the world. Insects 7, 69.
| Positive and negative impacts of non-native bee species around the world.Crossref | GoogleScholarGoogle Scholar |
Rymer, PD, Whelan, RJ, Ayre, DJ, Weston, PH, and Russell, KG (2005). Reproductive success and pollinator effectiveness differ in common and rare Persoonia species (Proteaceae). Biological Conservation 123, 521–532.
| Reproductive success and pollinator effectiveness differ in common and rare Persoonia species (Proteaceae).Crossref | GoogleScholarGoogle Scholar |
Saggin G (2013) Urban beehives on the increase as global bee numbers decline. ABC. Available at https://www.abc.net.au/news/2013-11-15/urban-beehive-movement-in-australia-and-around-the-world/5093764. [Accessed 2 November 2021]
Schaffer, WM, Zeh, DW, Buchmann, SL, Kleinhans, S, Schaffer, MV, and Antrim, J (1983). Competition for nectar between introduced honey bees and native North American bees and ants. Ecology 64, 564–577.
| Competition for nectar between introduced honey bees and native North American bees and ants.Crossref | GoogleScholarGoogle Scholar |
Schwarz, M, and Hurst, P (1997). Effects of introduced honey bees on Australia’s native bee fauna. Victorian Naturalist 114, 7–12.
Schwarz M, Gross C, Kukuk P (1991) Assessment of competition between honeybees and native bees. July 1991 Progress Report to the World Wildlife Fund. Project P158. Australia.
Schwarz, M, Kukuk, P, and Gross, C (1992). Assessment of competition between honeybees and native bees. World Wildlife Fund Australia Project P 158, 1–9.
Seeley, TD, Tarpy, DR, Griffin, SR, Carcione, A, and Delaney, DA (2015). A survivor population of wild colonies of European honeybees in the northeastern United States: investigating its genetic structure. Apidologie 46, 654––666.
Senapathi, D, Biesmeijer, JC, Breeze, TD, Kleijn, D, Potts, SG, and Carvalheiro, LG (2015). Pollinator conservation—the difference between managing for pollination services and preserving pollinator diversity. Current Opinion in Insect Science 12, 93–101.
| Pollinator conservation—the difference between managing for pollination services and preserving pollinator diversity.Crossref | GoogleScholarGoogle Scholar |
Shavit, O, Dafni, A, and Ne’eman, G (2009). Competition between honeybees (Apis mellifera) and native solitary bees in the Mediterranean region of Israel—implications for conservation. Israel Journal of Plant Sciences 57, 171–183.
| Competition between honeybees (Apis mellifera) and native solitary bees in the Mediterranean region of Israel—implications for conservation.Crossref | GoogleScholarGoogle Scholar |
Simberloff D (1981) Community effects of introduced species. In ‘Biotic crises in ecological and evolutionary time’. (Ed. MH Nitecki) pp. 53–81. (Academic Press: New York)
Simpson, SR, Gross, CL, and Silberbauer, LX (2005). Broom and honeybees in Australia: an Alien Liaison. Plant Biology 7, 541–548.
| Broom and honeybees in Australia: an Alien Liaison.Crossref | GoogleScholarGoogle Scholar | 16163620PubMed |
Smith, MS (2008). The ‘desert syndrome’ - causally-linked factors that characterise outback Australia. The Rangeland Journal 30, 3–14.
| The ‘desert syndrome’ - causally-linked factors that characterise outback Australia.Crossref | GoogleScholarGoogle Scholar |
Sommer U, Worm B (2002) ‘Competition and coexistence.’ (Springer Science & Business Media)
Spessa A (1999) The behavioural and population ecology of an Australian native bee, Amphylaeus morosus Smith (Colletidae: Hylaeinae). Doctor of Philosophy thesis, Australian National University, Canberra.
Stachowicz JJ, Tilman D (2005) Species invasions and the relationships between species diversity, community saturation, and ecosystem functioning. In ‘Species invasions: insights into ecology, evolution, and biogeography’. (Eds DF Sax, JJ Stachowicz, SD Gaines) pp. 41–64. (Sinauer Associates Incorporated: Sunderland, Massachusetts)
Steffan-Dewenter, I, and Tscharntke, T (2000). Resource overlap and possible competition between honey bees and wild bees in central Europe. Oecologia 122, 288–296.
| Resource overlap and possible competition between honey bees and wild bees in central Europe.Crossref | GoogleScholarGoogle Scholar | 28308384PubMed |
Steffan-Dewenter, I, Münzenberg, U, Bürger, C, Thies, C, and Tscharntke, T (2002). Scale-dependent effects of landscape context on three pollinator guilds. Ecology 83, 1421–1432.
| Scale-dependent effects of landscape context on three pollinator guilds.Crossref | GoogleScholarGoogle Scholar |
Steffan-Dewenter, I, Potts, SG, and Packer, L (2005). Pollinator diversity and crop pollination services are at risk. Trends in Ecology & Evolution 20, 651–652.
| Pollinator diversity and crop pollination services are at risk.Crossref | GoogleScholarGoogle Scholar |
Strauss, SY, Webb, CO, and Salamin, N (2006). Exotic taxa less related to native species are more invasive. Proceedings of the National Academy of Sciences of the United States of America 103, 5841–5845.
| Exotic taxa less related to native species are more invasive.Crossref | GoogleScholarGoogle Scholar | 16581902PubMed |
Sugden, EA, and Pyke, GH (1991). Effects of honey bees on colonies of Exoneura asimillima, an Australian native bee. Australian Journal of Ecology 16, 171–181.
| Effects of honey bees on colonies of Exoneura asimillima, an Australian native bee.Crossref | GoogleScholarGoogle Scholar |
Sugden, EA, Thorp, RW, and Buchmann, SL (1996). Honey bee-native bee competition: focal point for environmental change and apicultural response in Australia. Bee World 77, 26–44.
| Honey bee-native bee competition: focal point for environmental change and apicultural response in Australia.Crossref | GoogleScholarGoogle Scholar |
Thomson, JD (1989). Reversal of apparent feeding preferences of bumble bees by aggression from Vespula wasps. Canadian Journal of Zoology 67, 2588–2591.
| Reversal of apparent feeding preferences of bumble bees by aggression from Vespula wasps.Crossref | GoogleScholarGoogle Scholar |
Thomson, D (2004). Competitive interactions between the invasive European honey bee and native bumble bees. Ecology 85, 458–470.
| Competitive interactions between the invasive European honey bee and native bumble bees.Crossref | GoogleScholarGoogle Scholar |
Thomson, DM (2006). Detecting the effects of introduced species: a case study of competition between Apis and Bombus. Oikos 114, 407–418.
| Detecting the effects of introduced species: a case study of competition between Apis and Bombus.Crossref | GoogleScholarGoogle Scholar |
Thomson, DM (2016). Local bumble bee decline linked to recovery of honey bees, drought effects on floral resources. Ecology Letters 19, 1247–1255.
| Local bumble bee decline linked to recovery of honey bees, drought effects on floral resources.Crossref | GoogleScholarGoogle Scholar | 27539950PubMed |
Threlfall, CG, Walker, K, Williams, NSG, Hahs, AK, Mata, L, Stork, N, and Livesley, SJ (2015). The conservation value of urban green space habitats for Australian native bee communities. Biological Conservation 187, 240–248.
| The conservation value of urban green space habitats for Australian native bee communities.Crossref | GoogleScholarGoogle Scholar |
Tilman, D (2004). Niche tradeoffs, neutrality, and community structure: a stochastic theory of resource competition, invasion, and community assembly. Proceedings of the National Academy of Sciences of the United States of America 101, 10854–10861.
| Niche tradeoffs, neutrality, and community structure: a stochastic theory of resource competition, invasion, and community assembly.Crossref | GoogleScholarGoogle Scholar | 15243158PubMed |
Tomlinson, S, Dixon, KW, Didham, RK, and Bradshaw, SD (2017). Landscape context alters cost of living in honeybee metabolism and feeding. Proceedings of the Royal Society B: Biological Sciences 284, 20162676.
| Landscape context alters cost of living in honeybee metabolism and feeding.Crossref | GoogleScholarGoogle Scholar | 28179522PubMed |
Torné-Noguera, A, Rodrigo, A, Osorio, S, and Bosch, J (2016). Collateral effects of beekeeping: impacts on pollen-nectar resources and wild bee communities. Basic and Applied Ecology 17, 199–209.
| Collateral effects of beekeeping: impacts on pollen-nectar resources and wild bee communities.Crossref | GoogleScholarGoogle Scholar |
Traveset A, Richardson DM (2011) Mutualisms: key drivers of invasion.ey casualties of invasions. In ‘Fifty years of invasion ecology: the legacy of Charles Elton’. (Ed. DM Richardson) pp. 143–160. (Wiley-Blackwell: Oxford)
Valido, A, Rodríguez-Rodríguez, MC, and Jordano, P (2019). Honeybees disrupt the structure and functionality of plant-pollinator networks. Scientific Reports 9, 4711.
| 30886227PubMed |
vanEngelsdorp, D, and Meixner, MD (2010). A historical review of managed honey bee populations in Europe and the United States and the factors that may affect them. Journal of Invertebrate Pathology 103, S80–S95.
| A historical review of managed honey bee populations in Europe and the United States and the factors that may affect them.Crossref | GoogleScholarGoogle Scholar | 19909973PubMed |
Villanueva-Gutiérrez, R, and Roubik, DW (2004). Why are African honey bees and not European bees invasive? Pollen diet diversity in community experiments. Apidologie 35, 481––491.
Violle, C, Nemergut, DR, Pu, Z, and Jiang, L (2011). Phylogenetic limiting similarity and competitive exclusion. Ecology Letters 14, 782–787.
| Phylogenetic limiting similarity and competitive exclusion.Crossref | GoogleScholarGoogle Scholar | 21672121PubMed |
Walther-Hellwig, K, Fokul, G, Frankl, R, Büchler, R, Ekschmitt, K, and Wolters, V (2006). Increased density of honeybee colonies affects foraging bumblebees. Apidologie 37, 517–532.
| Increased density of honeybee colonies affects foraging bumblebees.Crossref | GoogleScholarGoogle Scholar |
Williams, G, and Adam, P (1994). A review of rainforest pollination and plant-pollinator interactions with particular reference to Australian subtropical rainforests. Australian Zoologist 29, 177–212.
| A review of rainforest pollination and plant-pollinator interactions with particular reference to Australian subtropical rainforests.Crossref | GoogleScholarGoogle Scholar |
Williams, G, and Adam, P (1997). The composition of the bee (Apoidea: Hymenoptera) fauna visiting flowering trees in New South Wales lowland subtropical rainforest remnants. Proceedings-Linnean Society of New South Wales 118, 69–96.
Williams, NM, Crone, EE, Roulston, TH, Minckley, RL, Packer, L, and Potts, SG (2010). Ecological and life-history traits predict bee species responses to environmental disturbances. Biological Conservation 143, 2280–2291.
| Ecological and life-history traits predict bee species responses to environmental disturbances.Crossref | GoogleScholarGoogle Scholar |
Willis Chan, DS, and Raine, NE (2021). Population decline in a ground-nesting solitary squash bee (Eucera pruinosa) following exposure to a neonicotinoid insecticide treated crop (Cucurbita pepo). Scientific Reports 11, 4241.
| Population decline in a ground-nesting solitary squash bee (Eucera pruinosa) following exposure to a neonicotinoid insecticide treated crop (Cucurbita pepo).Crossref | GoogleScholarGoogle Scholar | 33608633PubMed |
Wills, RT, Lyons, MN, and Bell, DT (1990). The European honey bee in western Australian kwongan: foraging preferences and some implications for management. Proceedings of the Ecological Society of Australia 16, 167–176.
Wilson, EE, and Holway, DA (2010). Multiple mechanisms underlie displacement of solitary Hawaiian Hymenoptera by an invasive social wasp. Ecology 91, 3294–3302.
| Multiple mechanisms underlie displacement of solitary Hawaiian Hymenoptera by an invasive social wasp.Crossref | GoogleScholarGoogle Scholar | 21141190PubMed |
Xiao, S, and Michalet, R (2013). Do indirect interactions always contribute to net indirect facilitation? Ecological Modelling 268, 1–8.
| Do indirect interactions always contribute to net indirect facilitation?Crossref | GoogleScholarGoogle Scholar |
Yates, C, Hopper, S, and Taplin, R (2005). Native insect flower visitor diversity and feral honeybees on Jarrah (Eucalyptus marginata) in Kings Park, an urban bushland remnant. Journal of the Royal Society of Western Australia 88, 147–153.
Zimmerman, M, and Pleasants, JM (1982). Competition among pollinators: quantification of available resources. Oikos 38, 381–383.
| Competition among pollinators: quantification of available resources.Crossref | GoogleScholarGoogle Scholar |


