Skinks of Oceania, New Guinea, and Eastern Wallacea: an underexplored biodiversity hotspot
Alex Slavenko A B , Allen Allison C , Christopher C. Austin D , Aaron M. Bauer E , Rafe M. Brown F , Robert N. Fisher
A B , Allen Allison C , Christopher C. Austin D , Aaron M. Bauer E , Rafe M. Brown F , Robert N. Fisher  G , Ivan Ineich
G , Ivan Ineich  H , Bulisa Iova I , Benjamin R. Karin J , Fred Kraus K , Sven Mecke
H , Bulisa Iova I , Benjamin R. Karin J , Fred Kraus K , Sven Mecke  L , Shai Meiri
L , Shai Meiri  M , Clare Morrison N O , Paul M. Oliver N O , Mark O’Shea
M , Clare Morrison N O , Paul M. Oliver N O , Mark O’Shea  P , Jonathan Q. Richmond G , Glenn M. Shea
P , Jonathan Q. Richmond G , Glenn M. Shea  Q R , Oliver J. S. Tallowin S and David G. Chapple
Q R , Oliver J. S. Tallowin S and David G. Chapple  T *
T *
A Macroevolution and Macroecology Group, Fenner School of Environment & Society, The Australian National University, Acton, Canberra, ACT 0200, Australia.
B Cesar Australia, Brunswick, Vic. 3056, Australia.
C Bernice P. Bishop Museum, Honolulu, HI 96817, USA.
D Department of Biological Sciences, Louisiana State University, Baton Rouge, LA 70803, USA.
E Department of Biology and Center for Biodiversity and Ecosystem Stewardship, Villanova University, Villanova, PA 19085, USA.
F Department of Ecology and Evolutionary Biology & Biodiversity Institute, University of Kansas, Lawrence, KS 66044, USA.
G U.S. Geological Survey, Western Ecological Research Center, San Diego, CA 92101, USA.
H Institut de Systématique, Évolution, Biodiversité (ISYEB), Muséum national d’Histoire naturelle, Sorbonne Université, École Pratique des Hautes Études, Université des Antilles, CNRS - CP 30, Paris 75005, France.
I Papua New Guinea National Museum and Art Gallery, Port Moresby H5FQ+PX8, Papua New Guinea.
J Museum of Vertebrate Zoology and Department of Integrative Biology, University of California, Berkeley, CA 9420, USA.
K Department of Ecology and Evolutionary Biology, University of Michigan, Ann Arbor, MI 48109, USA.
L Universitätssammlungen, Philipps-Universität Marburg, 35032 Marburg, Germany.
M School of Zoology & The Steinahrdt Museum of Natural History, Tel Aviv University, Tel-Aviv 6997801, Israel.
N Centre for Planetary Health and Food Security, Griffith University, Brisbane, Qld 4121, Australia.
O Biodiversity and Geosciences Program, Queensland Museum, South Brisbane, Qld 4101, Australia.
P Faculty of Science and Engineering, University of Wolverhampton, Wolverhampton WV1 1LY, UK.
Q Australian Museum Research Institute, Australian Museum, Sydney, NSW 2010, Australia.
R Sydney School of Veterinary Science, B01, University of Sydney, NSW 2006, Australia.
S IUCN Biodiversity Assessment and Knowledge Team, Cambridge CB2 3QZ, UK.
T School of Biological Sciences, Monash University, Clayton, Vic. 3800, Australia.
Pacific Conservation Biology 29(6) 526-543 https://doi.org/10.1071/PC22034
Submitted: 13 September 2022 Accepted: 27 November 2022 Published: 6 January 2023
© 2023 The Author(s) (or their employer(s)). Published by CSIRO Publishing. This is an open access article distributed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND)
Abstract
Context: Skinks comprise the dominant component of the terrestrial vertebrate fauna in Oceania, New Guinea, and Eastern Wallacea (ONGEW). However, knowledge of their diversity is incomplete, and their conservation needs are poorly understood.
Aims: To explore the diversity and threat status of the skinks of ONGEW and identify knowledge gaps and conservation needs.
Methods: We compiled a list of all skink species occurring in the region and their threat categories designated by the International Union for Conservation of Nature. We used available genetic sequences deposited in the National Center for Biotechnology Information’s GenBank to generate a phylogeny of the region’s skinks. We then assessed their diversity within geographical sub-divisions and compared to other reptile taxa in the region.
Key results: Approximately 300 species of skinks occur in ONGEW, making it the second largest global hotspot of skink diversity following Australia. Many phylogenetic relationships remain unresolved, and many species and genera are in need of taxonomic revision. One in five species are threatened with extinction, a higher proportion than almost all reptile families in the region.
Conclusions: ONGEW contain a large proportion of global skink diversity on <1% of the Earth’s landmass. Many are endemic and face risks such as habitat loss and invasive predators. Yet, little is known about them, and many species require taxonomic revision and threat level re-assessment.
Implications: The skinks of ONGEW are a diverse yet underexplored group of terrestrial vertebrates, with many species likely facing extreme risks in the near future. Further research is needed to understand the threats they face and how to protect them.
Keywords: islands, knowledge gaps, Melanesia, molecular phylogenetics, Oceania, regional threat assessment, Scincidae, Wallacea.
Introduction
The global biodiversity crisis is among the chief challenges facing humanity in the 21st Century (Pereira et al. 2012). To ensure the long-term survival of the Earth’s biodiversity, we need a strong knowledge base to identify threatened taxa, evaluate biodiversity hotspots and inform conservation planning and action (Soulé 1985; Primack 2014). With this goal in mind, it has been a priority of conservation biologists to identify which species are most at risk, where, and why. The International Union for Conservation of Nature (IUCN) has been collecting and curating data on species distribution, demographics, population trends, and threats since 1964 to assess the extinction risk of the world’s biota (IUCN 2021). Global initiatives such as the IUCN Red List represent crucial datasets for use in identifying priority areas for conservation, including the recognition of global conservation hotspots – areas of exceptional biodiversity at high risk (Myers et al. 2000; Mittermeier et al. 2004; Brooks et al. 2006; IUCN 2016). However, it is becoming increasingly evident that conservation efforts should also include regional and local assessments to identify scale-dependent processes and threats requiring targeted management measures (Ferrier 2002; Knight et al. 2007; McDonald et al. 2022).
The region of Oceania and New Guinea encompasses Melanesia, Micronesia, and Polynesia (see exact definition below; Fig. 1). The region of Wallacea includes additional small and medium-sized Indonesian islands west of New Guinea, and the sovereign state of Timor-Leste. Despite containing less than 1% of the world’s landmass, this region is extremely biodiverse. Oceania and especially New Guinea are well recognised for their importance in global diversity for birds (Stattersfield et al. 1998), vertebrates in general (Myers et al. 2000), insects (Toussaint et al. 2014), vascular plants (Cámara-Leret et al. 2020), and human cultural diversity (Loh and Harmon 2005). However, Oceania also holds the infamous distinction of being one of the Earth’s extinction hotspots – with a particularly poor record of extinctions for terrestrial vertebrates, especially birds (Steadman 1995; Blackburn et al. 2004). It is perhaps no surprise that an area comprising many islands, with high levels of endemism, would be prone to high levels of extinction, because insular taxa are particularly vulnerable to human-induced threats (Fisher and Ineich 2012; Slavenko et al. 2016; Fromm and Meiri 2021). Indeed, available evidence suggests that colonisation of the area by humans has been associated with proportionally elevated (above ambient or background levels) rates of extinction, principally as a function of evolutionarily novel human predation and alien species introduction (Duncan et al. 2013).
|
|
One of the dominant features of Oceania, New Guinea, and Eastern Wallacea’s terrestrial vertebrate fauna are lizards and particularly those of the family Scincidae, generally referred to as ‘skinks’ (Squamata: Scincomorpha: Scincidae). Skinks make up almost one-quarter of the world’s lizard diversity and have a prominent richness hotspot in New Guinea (Chapple et al. 2021) which is second only to the global hotspot in Australia (Rabosky et al. 2007; Powney et al. 2010; Chapple et al. 2021). In addition to their high diversity in New Guinea, they are also the most diverse terrestrial vertebrates throughout the Pacific Ocean. New Caledonia is home to a particularly notable and large radiation of endemic skinks (Smith et al. 2007), many of which are endangered or vulnerable (Chapple et al. 2021). Some species and species complexes are extremely widespread, either due to variable microhabitat preferences (Richmond et al. 2021) or commensal habits, which facilitated human-mediated dispersal (Bruna et al. 1996; Austin 1999; Hamilton et al. 2010; Linkem et al. 2013; Klein et al. 2016; Tan 2016). However, the majority of species are more restricted: many species are habitat specialists (Greer and Simon 1982; Blom et al. 2019; Richmond et al. 2021; Slavenko et al. 2022), and their levels of endemism are high throughout the region (Smith et al. 2007; Meiri et al. 2018; Kraus 2021).
Despite the prominence of skinks among the terrestrial faunas of Oceania, New Guinea, and Eastern Wallacea, knowledge of their true diversity is incomplete. Little is known of their natural history, and relatively few species have so far been included in molecular phylogenetic analyses. Since the early years of herpetological research in Oceania and New Guinea (Lesson 1830; Duméril and Bibron 1839; Bavay 1869), many new species have been discovered and formally described. However, only relatively recently have studies begun to incorporate molecular markers to infer phylogenetic relationships and revise species boundaries – and many of these studies suggest that true diversity in the region is greatly underestimated, even as recognised species numbers are increasing at a rapid rate (Austin et al. 2010; Rodriguez et al. 2018; Slavenko et al. 2020, 2022; Richmond et al. 2021; Bernstein et al. 2022; Reilly et al. 2022). Thus, existing threat assessments (Cox et al. 2022) may be outdated or misleading if they apply to inaccurately understood species boundaries and limited species knowledge (Mace 2004; Pimm et al. 2014). A clear understanding of knowledge gaps and research priorities in the region is therefore crucial to provide the foundation for effective regional conservation planning.
We assess the composition, threat status, and state of knowledge for the skink biota of Oceania, New Guinea, and Eastern Wallacea as part of the larger framework of the IUCN Species Survival Commission (SSC) Skink Specialist Group: a global network of biologists and wildlife managers that study and conserve the world’s skinks (Chapple et al. 2021). We highlight the uniqueness of this region’s skink fauna in a global context, as well as some of the unique conservation challenges these species face, and we identify knowledge gaps that hinder the continued persistence of this unique fauna. We hope our work will inspire further research into this global biodiversity hotspot, particularly involving understudied, micro-endemic, less conspicuous, smaller, and difficult-to-study components of the terrestrial fauna.
Materials and methods
The focal study region is comprised of all Pacific island nations (excluding Japan, Taiwan, the Philippines, New Zealand, and Australia’s Overseas Territories, but including the UK and US Overseas Territories and Hawaii), all islands in the Indonesian provinces of Papua, Highland Papua, Central Papua, South Papua, West Papua, North Maluku, Maluku, and East Nusa Tenggara, and the sovereign state of Timor-Leste (Fig. 1). The Western boundary of the region roughly aligns with the boundary between the Oriental and Australasian biogeographic realms; however, it is somewhat arbitrary due to the complex geological history of the area and differs from other traditionally recognised turnover points, most notably Wallace’s Line (Wallace 1860; Mayr 1944; Simpson 1977; Ali and Heaney 2021). For our purposes we view our definition of the Western boundary as an appropriate, biologically defensible definition, because it corresponds with the western extent of multiple prominent skink clades (e.g. tribe Tiliquini, genus Carlia with the exception of Carlia nigrauris).
We further divided the region into five sub-regions based on their geological histories and skink assemblages. These include:
-
Eastern Wallacea: Timor-Leste and all Indonesian islands in the region, excluding New Guinea and its satellite islands that occur on the Australian continental plate (e.g. Aru Islands, Raja Ampat Islands).
-
New Guinea: the island of New Guinea and all of its satellite islands that occur on the Sahul shelf (e.g. Aru Islands, Raja Ampat Islands) and the islands of the Woodlark Basin (e.g. Trobriand Islands, D’Entrecasteaux Archipelago, Woodlark Island, and Louisiade Archipelago), but excluding Australian territories (Torres Strait Islands).
-
Solomon Islands: all islands on the Solomon Islands arc and the New Britain arc, comprising the islands of the Bismarck Archipelago (e.g. New Britain, New Ireland, Admiralty Islands), the entire nation of the Solomon Islands, and the islands of Bougainville and Buka in Papua New Guinea’s Autonomous Region of Bougainville.
-
New Caledonia: all islands on the French territory of New Caledonia, occurring on the mostly submerged continent of Zealandia.
-
Pacific: all remaining islands in the region, comprising most of the islands in the Western and Southern Pacific Ocean, including American Samoa, Caroline Islands, Clipperton Island (in the North-eastern Pacific Ocean), Cook Islands, Easter Island, Federated States of Micronesia, Fiji, French Polynesia, Guam, Hawaii, Kiribati, Marshall Islands, Nauru, Niue, Northern Mariana Islands, Palau, Pitcairn Islands, Samoa, Tokelau, Tonga, Tuvalu, US Overseas Territories in the Pacific, Vanuatu, and Wallis and Futuna.
We compiled a list of all species of skinks that occur in our focal region (Supplementary Table S1). The list was based on the most up-to-date list of global skink diversity (Chapple et al. 2021). We used global distribution maps of the world’s reptiles from an updated version of Roll et al. (2017; i.e. Caetano et al. 2022) and overlaid these maps with polygons defining our region of interest (Fig. 1). We included all species with ranges overlapping these polygons. We also included three skink species (Papuascincus buergersi, Papuascincus phaeodes, Prasinohaema parkeri) whose localities are not specific enough to be mapped but nonetheless occur in our region (namely, in New Guinea) based on verbal description of their ranges in their original description papers. We then assessed which sub-regions overlapped each species’ range, tallied species-richness values for each sub-region, and determined the number of species occurring over multiple sub-regions. We repeated this procedure for all mapped terrestrial reptile species to compare richness patterns of skinks in the region to patterns exhibited by other reptilian taxa.
To assess patterns of diversity and taxonomic knowledge, we recorded the description year for each skink species in the region. We then plotted cumulative species richness by year of description to compare rates of species description in the different sub-regions. Additionally, all authors (members of the IUCN SSC Skink Specialist Group specialising on the region) assessed for each species whether, to the best of their knowledge, it is in need of taxonomic revision either at the species (i.e. undescribed diversity likely exists in the species) or genus (i.e. genus likely in need of splitting or species likely needing reassignment to a different genus) level.
To explore patterns in threat status in the region, we compared IUCN threat categories. For each species we recorded its tribe, taxonomic authority, and year of description (Chapple et al. 2021; Shea 2021; Uetz et al. 2021). We then used the ‘iucn_summary’ and ‘iucn_status’ functions from the taxize package (Chamberlain and Szöcs 2013; Chamberlain et al. 2020) within the R data analysis platform v4.1.0 (R Core Team 2021) to query the IUCN Red List for the Red List status of all species in our list. We classified each species as being either Least Concern (LC), Near Threatened (NT), Threatened (VU, EN, or CR), or Unknown (DD or not evaluated [NE]). One species, Tachygia microlepis, is classified as Extinct (EX).
To assess knowledge gaps in molecular sequencing, we used the ‘entrez_search’ and ‘entrez_fetch’ functions from the rentrez package (Winter 2017) to query the National Center for Biotechnology Information (NCBI) GenBank nucleotide database for sequence data for all species of skinks in our list, and tallied for each species how many unique sequences, and how many loci, are available. We then selected the following genetic markers for downstream phylogenetic analyses to reconstruct a phylogeny of all sampled skinks in the region (although some sequences represent populations outside the region), and explore their phylogenetic relationships: the mitochondrial (mtDNA) markers ribosomal 12S rRNA (12S; 61 spp; 398 base pairs [bp]), ribosomal 16S rRNA (16S; 42 spp; 468 bp), ribosomal 18S rRNA (18S; 8 spp; 1770 bp), cytochrome c oxidase subunit I (COI; 103 spp; 645 bp), cytochrome b (CYTB; 42 spp; 270 bp), NADH dehydrogenase subunit 2 (ND2; 128 spp; 514 bp), NADH dehydrogenase subunit 4 (ND4; 66 spp; 706 bp), and the nuclear (nDNA) markers brain-derived neurotrophic factor (BDNF; 15 spp; 712 bp), breast cancer type 2 susceptibility protein (BRCA2; 19 spp; 1248 bp), proto-oncogene serine/threonine-protein kinase mos (c-mos; 69 spp; 547 bp), exophilin 5 (EXPH5; 21 spp; 697 bp), kinesin family member 24 (KIF24; 19 spp; 567 bp), nerve growth factor β polypeptide (NGFB; 34 spp; 582 bp), prolactin receptor (PRLR; 13 spp; 564 bp), protein tyrosine phosphatase non-receptor type 12 (PTPN12; 14 spp; 609 bp), RNA fingerprint protein 35 (R35; 33 spp; 636 bp), and recombination activating gene 1 (RAG1; 61 spp; 828 bp). These markers were selected because they were well represented (present for >5 species of multiple genera and tribes). We selected one sequence per-species per-marker to use in phylogenetic analyses, preferring when possible to use sequences from the same individual or geographic locality within species (Supplementary Table S2). In addition, we downloaded sequences of 16S, BDNF, c-mos, CYTB, EXPH5, ND4, NGFB, PRLR, PTPN12, and RAG1 for Acontias meleagris (of the skink subfamily Acontiinae, sister to all other skinks; Pyron et al. 2013) to use as an outgroup.
We aligned sequences using MUSCLE multiple-sequence alignment as implemented in MEGA 11 (Tamura et al. 2021). We partitioned the multiple-sequence alignment for phylogenetic analyses and selected substitution models by running ModelFinder (Kalyaanamoorthy et al. 2017) as implemented in IQ-TREE 2 (Minh et al. 2020) using a relaxed hierarchical clustering algorithm (Lanfear et al. 2014), and with initial partitions set to single partition for 12S, 16S, and 18S, and by codon for all protein-coding genes (all others). Partitions and models can be found in Supplementary Table S3.
We performed phylogenetic inference using three methods:
-
Maximum Likelihood (ML) analyses in IQ-TREE 2 (Minh et al. 2020), as implemented in the IQ-TREE web server (Trifinopoulos et al. 2016). We set the partitions to be edge-linked (same set of branch lengths among partitions, but with partition-specific rates). We assessed nodal support with 1000 ultrafast bootstrap (UFBoot) alignments with up to 1000 iterations as a stopping rule (Hoang et al. 2018). We considered nodes well-supported if they received UFBoot values ≥ 95%.
-
Maximum Likelihood (ML) analyses in RAxML v8 (Stamatakis 2014), as implemented in raxmlGUI v2.0.9 (Edler et al. 2021). We performed model selection using ModelTest-NG (Darriba et al. 2020) and used the selected GTR+G+I model of sequence evolution for all partitions. We assessed nodal support with 500 rapid bootstrap (BS) replicates, and considered nodes well-supported if they received BS values ≥ 80%.
-
Bayesian Inference (BI) analyses in MrBayes v3.2.7a (Ronquist et al. 2012) as implemented in CIPRES Science Gateway (Miller et al. 2010), using the BEAGLE library (Ayres et al. 2012). Since ModelFinder has more possible models than are accepted in MrBayes, we assigned the GTR model to all partitions where the best-fit model was K3P, TPM2, TPM, TIM, or TVM. In partitions where the best-fit models included the FreeRate model, we instead assigned the more restrictive (but still variable) gamma model for among-site rate variation. Nucleotide-substitution-model parameters were unlinked across partitions, and we allowed the different partitions to evolve at different rates. We performed two simultaneous parallel runs (Altekar et al. 2004) with four chains per run (three heated, one cold) for 107 generations, sampling every 1000 generations. We examined the standard deviation of the split frequencies between the two runs and the Potential Scale Reduction Factor (PSRF) diagnostic, and discarded the first 25% of trees as burn-in.
We then repeated the above analyses, from partitioning to phylogenetic inference, for concatenated mtDNA (seven markers; 173 spp; 4771 bp) and nDNA (10 markers; 112 spp; 6990 bp) alignments separately to assess agreement in topology between the different markers. Partitions and models for the mtDNA and nDNA datasets can be found in Supplementary Table S3.
Results
Skink diversity
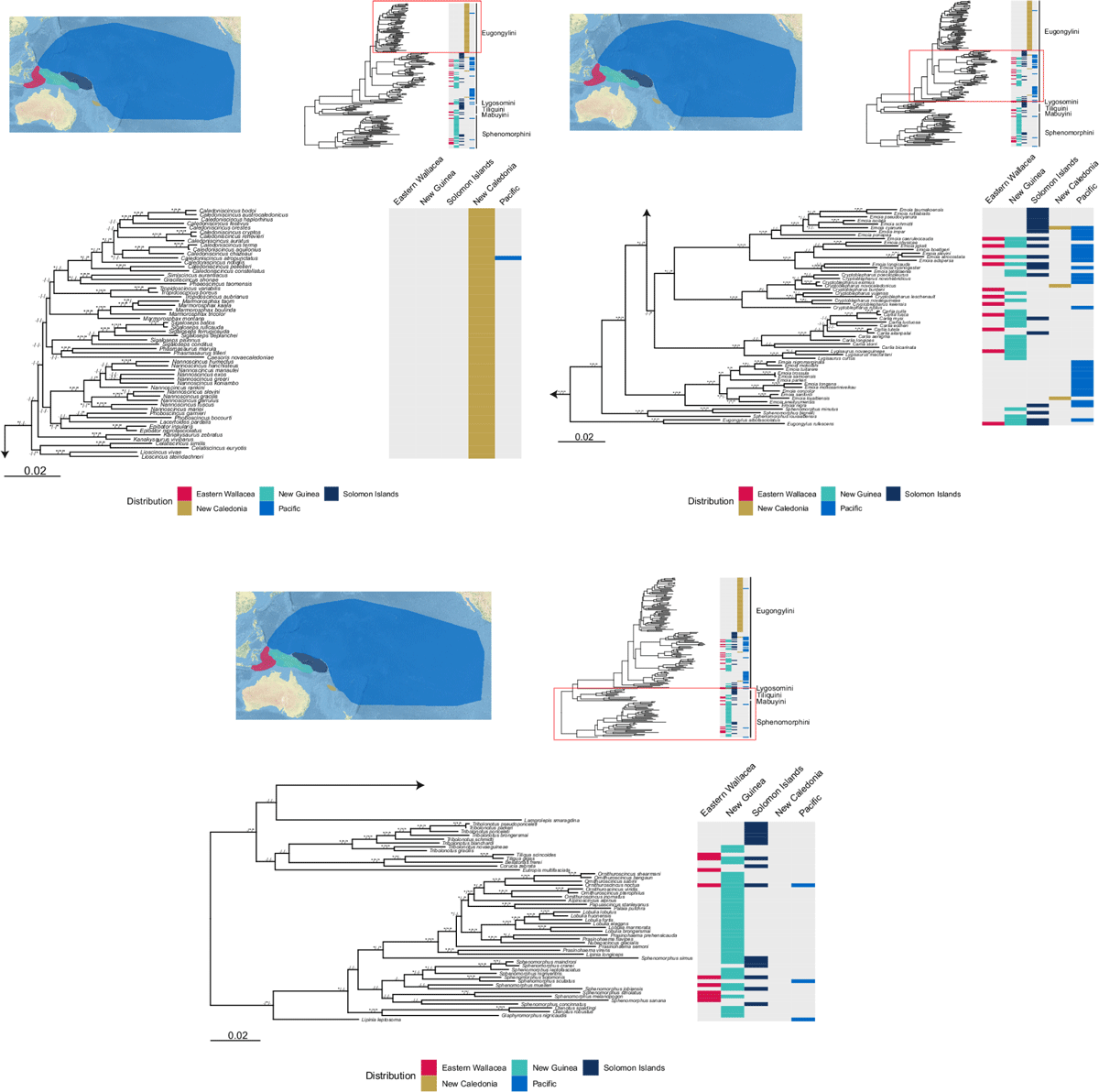
We identified 303 species of skinks that occur in our target region, one of which is introduced from Australia (Lampropholis delicata), and one of which is possibly native to New Guinea but its presence is unverified (Carlia quinquecarinata). Of the remaining 301 species, 18 are native but also occur elsewhere (mostly Australia), and 283 are endemic to the region. Together these 301 species constitute roughly 17% of the ~1740 species of skinks currently known worldwide. Almost half (146 species) occur in New Guinea alone (Fig. 1). Skink species vastly outnumber all other reptilian families in the region, particularly in New Guinea, but this pattern is also evident in all sub-regions (Fig. 2a).
All skinks in the region belong to the subfamily Lygosominae, and within this, most belong to the tribes Eugongylini and Sphenomorphini (Fig. 3), the two largest tribes in the subfamily (Chapple et al. 2021; Shea 2021). There are also several species from the tribe Tiliquini (most prominently crocodile skinks of the genus Tribolonotus, endemic to the region), two species of the tribe Mabuyini (one of which, Eutropis palauensis, is endemic), and a single species from the tribe Lygosomini (Lamprolepis smaragdina). Species from the tribe Eugongylini are widespread throughout the region and it is the dominant tribe in Eastern Wallacea, New Caledonia, the Solomon Islands, and the Pacific, with a prominent hotspot in New Caledonia where all skinks are eugongylins (Fig. 3). Conversely, the dominant tribe in New Guinea is Sphenomorphini – and indeed most sphenomorphin skinks in the region occur in New Guinea.
Extinction risk
Among the skinks of Oceania, New Guinea, and Eastern Wallacea, 56 species (18.7%) are evaluated by the IUCN as threatened with extinction, and one Tongan endemic (Tachygia microlepis) is extinct. Slightly over half (173 species) are listed as Least Concern, an additional eight are Near Threatened. Another 66 (22.0%) are either listed as Data Deficient or have not been assessed. When weighted by their species richness, the mean proportion of species in the Threatened categories across families in the region is 14.5%. Thus, skinks have a relatively high proportion of species threatened with extinction, higher than other speciose families such as Gekkonidae (121 spp., 9.9% Threatened), Colubridae (54 spp., 3.7% Threatened), Elapidae (42 spp., 7.1% Threatened), and Agamidae (36 spp., 2.8% Threatened).
Most New Caledonian skinks are classified as Threatened (63.1%; Fig. 3), followed by the Pacific (32.5%), and Eastern Wallacea (4.5%) sub-regions. New Guinea and the Solomon Islands both have no known Threatened species. New Guinea and Eastern Wallacea have the highest proportion of species with an unknown conservation status (26.0% and 18.2%, respectively).
Phylogenetic relationships
Despite the high absolute species richness, skinks are poorly sampled relative to other families: 174 species of skinks (57.1%) have sequences in NCBI genbank (Fig. 2c), lower than the mean proportion among reptile families in the region (66.3%). Our final dataset comprised 17 genetic markers (7 mtDNA, 10 nDNA) with a total length of 11 761 bp. Coverage averaged only 2.6 mtDNA markers and 1.7 nDNA markers per species (range 0–7, mean 36.8% and 15.6%, respectively). Coverage also varied between tribes and markers (Supplementary Table S4): eugongylin skinks had the lowest coverage for mitochondrial markers (range 1–7, mean 2.3 [33.3%] markers per species), followed by sphenomorphin skinks (range 0–5, mean 2.9 [41.2%] markers per species) and tiliquin skinks (range 2–6, mean 3.3 [46.4%] markers per species). Eugongylin skinks also had the lowest coverage for nDNA markers (range 0–7, mean 1.5 [14.0%] markers per species), followed by tiliquin skinks (range 0–6, mean 1.6 [14.4%] markers per species) and sphenomorphin skinks (range 0–6, mean 1.9 [17.5%] markers per species).
The higher-order relationships of skinks in our phylogeny were only moderately resolved (Fig. 4), with the monophyly of Tiliquini and Sphenomorphini receiving low support. In most regions, several different lineages of skinks are represented. However, most skink species on New Caledonia belong to a single, moderately supported eugongylin radiation (Fig. 4). Deeper relationships within the Sphenomorphini and Eugongylini are not well resolved (Fig. 4), particularly in the speciose genus Sphenomorphus and in the large radiation of New Caledonian eugongylin skinks. Most genera were supported as monophyletic, except Lipinia, Emoia (both with at least two distinct clades), Prasinohaema, and Sphenomorphus (both with at least three distinct clades).
With all three methods of phylogenetic inference, the full concatenated gene trees were mostly concordant with the concatenated mtDNA trees, and less so with the nDNA trees (Figs S1–S3). In general, concatenated nDNA trees had poorly supported topology, especially in the Eugongylini.
Among the full concatenated gene trees, the ML IQ-TREE phylogeny generally had better supported topology than the ML RAxML phylogeny (Fig. 4). However, the topology was similar between the two methods (Fig. S4), with major differences only in deeper nodes between genera within the Eugongylini (Carlia, Lygisaurus, Caesoris, Phasmasaurus, Kanakysaurus). Both the IQ-TREE and RAxML phylogenies showed much larger differences to the MrBayes phylogeny, although again most differences were in the relationships between genera within Eugongylini (Fig. S4).
Trends in species discoveries
The earliest described skink species from the region are widespread large species that would have been noticeable to early naturalists, some of which occur outside the region, including Tiliqua scincoides (Hunter 1790), Tiliqua gigas (Schneider 1801) and Eugongylus rufescens (Shaw 1802). Descriptions of species endemic to the region began accumulating throughout the 19th Century, with prominent spikes in descriptions of New Guinean and New Caledonian species towards the end of the century (Fig. 5). Throughout the 20th Century, descriptions of species from Eastern Wallacea, New Caledonia, the Pacific, and the Solomon Islands were published at a steady rate of ~0.19–0.33 species per year on average, whereas descriptions from New Guinea have been published at a much higher rate (~0.75 species per year on average). A pronounced upwards shift in these dynamics occurred at the start of the 21st Century. While rates of description in Eastern Wallacea, the Pacific, and the Solomon Islands remained static, average rates of description in New Caledonia and New Guinea increased dramatically to 1.4 and 1.7 species per year, respectively (Fig. 5).
|
|
Discussion
We identified Oceania, New Guinea, and Eastern Wallacea as a global hotspot of skink diversity, especially in the context of available land area – less than 1% of the world’s landmass. Despite its relatively small area, this region is home to almost a fifth of all skink species, making it second only to Australia in terms of absolute species richness (Rabosky et al. 2007; Powney et al. 2010; Chapple et al. 2021). The skink diversity of the region is impressive not only in its numbers, but also in terms of the range and magnitude of phenotypic and ecological variability including trophic spectra. Skinks in the region possess adaptations for extremely high elevations (Greer et al. 2005; Slavenko et al. 2021, 2022) and tolerance of highly saline conditions (Richmond et al. 2021). Some possess unique morphologies associated with aquatic (Greer and Simon 1982) or arboreal (Zippel et al. 1999) lifestyles. Others exhibit rare attributes among squamates in general, such as green blood pigmentation (Greer and Raizes 1969; Rodriguez et al. 2018), adhesive toe pads (Williams and Peterson 1982; Irschick et al. 1996), vocalisation (Hartdegen et al. 2001; Bauer et al. 2004), carcinophagy (Jowers et al. 2022), living inside ‘ant plants’ of the genus Hydnophytum (Brown and Gibbons 1986), herbivory, low reproductive rates, sociality, and colonial aggregations (Iverson 1982).
Knowledge gaps are still evident. Skinks are similar to other squamates (Tingley et al. 2016) in having a high proportion of species whose conservation status is unknown, with roughly a fifth of species in the region either not yet assessed by the IUCN, or assessed as Data Deficient (Tingley et al. 2016; Chapple et al. 2021; Cox et al. 2022). This assessment gap is particularly evident in New Guinea and Eastern Wallacea (Fig. 3). Data Deficient species are likely to be at least as threatened (Bland and Böhm 2016), or even more so (Gumbs et al. 2020; Caetano et al. 2022), than the general threat level for other reptiles. Species that have not yet been assessed are likely to be even more threatened due to their smaller geographic range sizes (Meiri 2016). Thus, the actual threat level of skinks presented here for Oceania and New Guinea is likely to be a conservative estimate (Chapple et al. 2021).
Another large knowledge gap involves species-level diversity. Although species richness of skinks in the region is high, this diversity is likely still underestimated. During the past decade alone, the rate of description for skinks in Oceania, New Guinea, and Eastern Wallacea has more than tripled to ~3 species per year (Fig. 5). This amounts to 15% of the ~20 species of skinks per year that have been described worldwide over the past decade (Chapple et al. 2021) in an area that takes up less than 1% of the Earth’s total landmass.
The foundations for our understanding of skink diversity of the region were set during the first half of the 19th Century mostly through the work of prominent French naturalists (Lescure 2002) such as René-Primevère Lesson (Lesson 1830), Auguste Duméril, and Gabriel Bibron (Duméril and Bibron 1839). However, the true process of exploration and description of the skink fauna of the region began in the latter half of the 19th Century and the beginning of the 20th Century (Fig. 5), starting with Arthur Bavay’s original description of the fauna of New Caledonia (Bavay 1869) and several monographs describing fauna collected in British, Italian, and German expeditions into the interior of New Guinea (Meyer 1874; Macleay 1877; Peters and Doria 1878; Boulenger 1887, 1897, 1903, 1914). The 20th Century saw a continuation of this trend, as species descriptions accumulated at a more or less steady rate (Fig. 5), with foundational treatises on the widespread eugongylin genera Cryptoblepharus (Mertens 1928, 1931, 1933, 1934, 1964) and Emoia (Brown 1953, 1954, 1983, 1991; Brown and Parker 1985; Brown and Allison 1986; Brown and Gibbons 1986), on New Guinea’s sphenomorphin skinks (Vogt 1932; Parker 1936; Loveridge 1945; Greer and Parker 1967a, 1967b, 1971, 1974; Greer 1973, 1974), on the eugongylin skinks of New Caledonia (Sadlier 1987), and on the tiliquin genus Tribolonotus (Zweifel 1966; Greer and Parker 1968; Cogger 1972). A revolution in taxonomic research in the region began towards the end of the 20th and into the 21st Century, with the advent of technological advancements that made molecular phylogenetic inference cheaper and easier than before. This revolution was most prominent in New Caledonia, which experienced a four-fold increase in species description rate in the 21st Century (Fig. 5), as its fauna was thoroughly researched and described (Sadlier et al. 1999; Bauer and Sadlier 2000; Sadlier et al. 2006, 2009a, 2009b, 2014a, 2014b, 2014c, 2019). New Guinea also experienced a more than two-fold increase in its skink species description rate, but many of these new descriptions were based on morphology alone (Günther 2000; Greer and Shea 2004; Zug 2004; Greer et al. 2005; Shea 2017; Kraus 2018, 2020; Shea and Allison 2021). Only relatively recently have the skinks of New Guinea and the Solomon Islands begun to be explored using molecular phylogenetic methods (Austin 1995; Austin et al. 2010; Rittmeyer and Austin 2017; Rodriguez et al. 2018; Slavenko et al. 2020, 2022), and the potential exists for New Guinea to experience a similar if not more spectacular taxonomic revolution as New Caledonia before it.
Despite these recent advances in taxonomic research in the region, some issues remain. Many phylogenetic relationships among the region’s skinks remain unresolved, particularly deeper nodes with short branches (Fig. 4). Parts of the topology in our phylogeny are likely erroneous, such as the low support for monophyly of Tiliquini and Sphenomorphini, two tribes whose monophyly is otherwise widely supported (Pyron et al. 2013). However, other nodes are strongly supported and represent cases in need of taxonomic revision – such as the non-monophyly of Emoia, Lipinia, Prasinohaema, and Sphenomorphus. We have identified 42 species (all occur in New Guinea and most are sphenomorphin) that likely contain undescribed diversity, and even more dramatically, 143 species in six genera that likely require either generic reassignment or a full revision of their genera and their contents. New Guinea and Eastern Wallacea remain especially poorly sampled genetically compared to the other sub-regions of the area (Fig. 3) – either due to lack of tissue samples, or due to lack of sequencing of available tissues. This is also evident in the weak resolution of deeper phylogenetic relationships among New Guinea’s sphenomorphin skinks (Fig. 4), likely a direct result of the patchy genetic coverage, particularly of nDNA markers, in these species (Supplementary Table S4 and Fig. S5).
New Guinea’s western half (Indonesian New Guinea) remains extremely poorly known especially compared to the better-sampled eastern half (Papua New Guinea) (Heads 2002; Slavenko et al. 2022), and the island of New Guinea in general has a huge diversity of habitats ranging from tropical rainforests and savannas to alpine vegetation at high elevation (Bryan and Shearman 2015). We thus suspect Eastern Wallacea and New Guinea, where much of our knowledge is based on fragmented, old collections, hold the most undescribed diversity in the entire region. Lack of accessibility to many localities remains a barrier that can only be overcome by political access and sufficient funding for fieldwork, which would be most efficient if done through local research, rather than ‘parachute science’. A strong emphasis should thus be placed on collections-based research tied to local capacity building through training of students and establishment of regional collections.
Although extinction risk is high throughout the region, with nearly a fifth of species in one of the IUCN’s threatened categories (Fig. 2), the skink fauna of New Caledonia especially emerges as a hotspot not only of biodiversity but also of extinction risk. Over 60% of New Caledonia’s skinks are listed as threatened (Fig. 3), with the largest threats to its terrestrial biodiversity being due to mining, particularly of nickel (Pascal et al. 2008; L’Huillier and Jaffré 2010). Other sub-regions, while seemingly in better shape in terms of their threat assessments (but note many assessments are lacking or outdated and may change pending taxonomic revisions; Chapple et al. 2021), face their own unique challenges. New Guinea is facing increasing development pressures, including from the mining, petroleum, plantation, and logging industries (Shearman et al. 2009; Laurance et al. 2011; Alamgir et al. 2019), threatening some of the largest remaining tracts of pristine primary rainforest on the planet. Many of New Guinea’s skinks are micro-endemics and microhabitat specialists (Slavenko et al. 2020, 2022; Kraus 2021). This places them at great risk if their habitats are not properly conserved. A clear example of the dangers that might threaten all of New Guinea is exemplified by Woodlark (Muyua) Island – an endemism hotspot that is under severe threat of habitat loss due to mining (Kraus 2021).
Habitat loss is also a major threat to skinks on other Pacific islands (Fisher and Ineich 2012), which on the whole face greater extinction risk than on New Guinea (Fig. 3). Logging, land clearance for palm oil, and nickel mining are key drivers of habitat loss in the Solomon Islands (Kabutaulaka 2000; Morrison et al. 2007; Wairiu 2007; Katovai et al. 2015; Lavery et al. 2021). Additionally, for especially charismatic species such as the Solomon Islands skink (Corucia zebrata) or crocodile skinks (Tribolonotus spp.), illegal harvesting for the pet trade can be a major threat (Leary 1991; Janssen and Shepherd 2018; Lavery et al. 2021). In addition to existing stressors, human-induced climate change is also likely to negatively affect species in Oceania, New Guinea, and Eastern Wallacea, especially lowland species that may be vulnerable to rising sea levels; or montane endemics, which are at risk of climatic conditions shifting beyond their thermal tolerances (Duffy 2011; Kingsford and Watson 2011).
Invasive species, especially predators, have proven to be one of the greatest threats to endemic insular skinks. Introduced wolf snakes (Lycodon capucinus) were a primary cause of the extinction of four or more skinks species on Christmas Island and Mauritius (Smith et al. 2012; Michaelides et al. 2015; Andrew et al. 2018; Oliver et al. 2018). In the Pacific, brown tree snakes (Boiga irregularis) have led to the local extinction of several lizard species on Guam (Rodda and Fritts 1992; Richmond et al. 2022), Indian brown mongooses (Urva fusca) prey on skinks in Fiji (Morley and Winder 2015; see also Clause et al. 2018), and little fire ants (Wasmannia auropunctata) are associated with declines of lizards in New Caledonia (Jourdan et al. 2001). Wolf snakes have been spreading eastward and establishing populations on additional islands in Wallacea and New Guinea, and may therefore create an additional threat in this region in particular (Kuch and McGuire 2004; Karin et al. 2018; O’Shea et al. 2018). Given how poorly known the fauna of these regions is, there is also a real risk that invasive species could (and may have already) eliminated species that have been overlooked before they were even scientifically described (McDonald et al. 2022).
A sobering cautionary tale can be found in one of the few documented reptile extinctions of the modern era: the Tonga ground skink (T. microlepis). This species has not been seen since the 19th Century when it was originally described based on two syntypes (Duméril and Bibron 1839). It was declared extinct by the IUCN in 1996 (Allison et al. 2022), its extinction likely caused by direct exploitation by the original Polynesian settlers, invasive species such as pigs, cats, and rats introduced by later European explorers, and extensive land-use change for pumpkin plantations all over the main island (Ineich and Zug 1996). Although its large body size likely made it especially vulnerable to such stresses (Slavenko et al. 2016), it should be seen not as an outlier but rather as a canary in the coal mine – a similar fate might await other skink species in Oceania, New Guinea, and Eastern Wallacea if their conservation needs are not properly met.
In conclusion, our analyses clearly establish the islands of Oceania, New Guinea, and Eastern Wallacea as hotspots of skink diversity, and potentially of large conservation concern not only for skinks but surely other taxa as well. As this fauna is relatively poorly known, it is also likely even richer and more threatened than we are currently aware. Habitat loss, mainly due to logging and agricultural land clearing, and invasive species emerge as the major threats to the skinks of the region (Rodda and Fritts 1992; Kabutaulaka 2000; Jourdan et al. 2001; Laurance et al. 2011; Fisher and Ineich 2012; Katovai et al. 2015; Morley and Winder 2015; Alamgir et al. 2019; McDonald et al. 2022). However, the region also faces unique conservation challenges: many skinks have not been assessed by the IUCN (Figs 2 and 3) and few are placed on national threatened species lists. Thus most species receive no official protection and do not attract species management plans. Local government capacity to manage threats or species conservation is often lacking, and greater involvement of local landowning communities is necessary to support conservation action (Keppel et al. 2012; Jupiter et al. 2017; Morrison et al. 2022). It is also likely that a large portion of the biota remains undescribed and unrecognised, suggesting a high risk of cryptic extinction (i.e. the loss of species before they are scientifically documented). Crucially – more data are needed, and local capacity to collect these data is lacking. However, local communities also offer a treasure trove of traditional knowledge approaches to conservation and data collection, which may fill many of the gaps in species ecology and distribution (McMillen et al. 2014; Pollard et al. 2014; Keppel et al. 2015). Oceania, New Guinea, and Eastern Wallacea are thus placed in a unique context even in the larger framework of global skink conservation (Chapple et al. 2021), and we encourage similar regional assessments in other regions and on other taxa. The knowledge gaps identified here may seem daunting, but they also present remarkable opportunities for further research in a fascinating, global biodiversity hotspot. However, the need to fill these gaps is urgent, and we must strive to do this before much of this unique diversity is lost.
Supplementary material
Supplementary material is available online.
Data availability
The species list compiled in this study is available in Table S1. All sequences used in phylogenetic analyses are publicly accessible on NCBI GenBank and accession numbers are available in Table S2.
Conflicts of interest
The authors declare no conflicts of interest.
Declaration of funding
DGC was supported by a grant from the Australian Research Council (FT200100108). Solomon Islands fieldwork by RMB and colleagues was supported by a grant from the US National Science Foundation (DEB-1557053). Research on New Guinea skinks by AS and colleagues, including fieldwork, was supported by Binational Science Foundation grants (2012143 to SM and AA and 002030900 to AS), a Naomi Foundation through the Tel Aviv University GRTF program grant (064181317 to AS), US National Science Foundation grants (DEB-0103794 and DEB-0743890 to FK and AA and DEB-1146033 and DEB-1926783 to CCA), and a National Geographic Explorer’s Grant (NGS-53506R-18) to CCA. RNF and JQR research in the Pacific Islands has been funded by many groups including Mohamed bin Zayed, Conservation International, Island Conservation, Wildlife Conservation Society, IUCN Oceania, SPREP, CEPF, San Diego Zoo Global, University of the South Pacific, and the U.S. Geological Survey. Any use of trade, firm, or product names is for descriptive purposes only and does not imply endorsement by the U.S. Government.
Acknowledgements
This study was performed under the larger framework of the IUCN SSC Skink Specialist Group (www.skinks.org).
References
Alamgir, M, Sloan, S, Campbell, MJ, Engert, J, Kiele, R, Porolak, G, Mutton, T, Brenier, A, Ibisch, PL, and Laurance, WF (2019). Infrastructure expansion challenges sustainable development in Papua New Guinea. PLoS ONE 14, .| Infrastructure expansion challenges sustainable development in Papua New Guinea.Crossref | GoogleScholarGoogle Scholar |
Ali, JR, and Heaney, LR (2021). Wallace’s line, Wallacea, and associated divides and areas: history of a tortuous tangle of ideas and labels. Biological Reviews 96, 922–942.
| Wallace’s line, Wallacea, and associated divides and areas: history of a tortuous tangle of ideas and labels.Crossref | GoogleScholarGoogle Scholar |
Allison A, Hamilton A, Tallowin O (2022) Tachygyia microlepis (amended version of 2012 assessment). The IUCN Red List of Threatened Species 2022: e.T21286A217760413. Available at https://dx.doi.org/10.2305/IUCN.UK.2022-1.RLTS.T21286A217760413.en
Altekar, G, Dwarkadas, S, Huelsenbeck, JP, and Ronquist, F (2004). Parallel Metropolis coupled Markov chain Monte Carlo for Bayesian phylogenetic inference. Bioinformatics 20, 407–415.
| Parallel Metropolis coupled Markov chain Monte Carlo for Bayesian phylogenetic inference.Crossref | GoogleScholarGoogle Scholar |
Andrew, P, Cogger, H, Driscoll, D, Flakus, S, Harlow, P, Maple, D, Misso, M, Pink, C, Retallick, K, Rose, K, Tiernan, B, West, J, and Woinarski, JCZ (2018). Somewhat saved: a captive breeding programme for two endemic Christmas Island lizard species, now extinct in the wild. Oryx 52, 171–174.
| Somewhat saved: a captive breeding programme for two endemic Christmas Island lizard species, now extinct in the wild.Crossref | GoogleScholarGoogle Scholar |
Austin, CC (1995). Molecular and morphological evolution in South Pacific scincid lizards: morphological conservatism and phylogenetic relationships of Papuan Lipinia (Scincidae). Herpetologica 51, 291–300.
Austin, CC (1999). Lizards took express train to Polynesia. Nature 397, 113–114.
| Lizards took express train to Polynesia.Crossref | GoogleScholarGoogle Scholar |
Austin, CC, Rittmeyer, EN, Richards, SJ, and Zug, GR (2010). Phylogeny, historical biogeography and body size evolution in Pacific Island Crocodile skinks Tribolonotus (Squamata; Scincidae). Molecular Phylogenetics and Evolution 57, 227–236.
| Phylogeny, historical biogeography and body size evolution in Pacific Island Crocodile skinks Tribolonotus (Squamata; Scincidae).Crossref | GoogleScholarGoogle Scholar |
Ayres, DL, Darling, A, Zwickl, DJ, Beerli, P, Holder, MT, Lewis, PO, Huelsenbeck, JP, Ronquist, F, Swofford, DL, Cummings, MP, Rambaut, A, and Suchard, MA (2012). BEAGLE: an application programming interface and high-performance computing library for statistical phylogenetics. Systematic Biology 61, 170–173.
| BEAGLE: an application programming interface and high-performance computing library for statistical phylogenetics.Crossref | GoogleScholarGoogle Scholar |
Bauer AM, Sadlier RA (2000) ‘The herpetofauna of New Caledonia.’ (Society for the Study of Amphibians and Reptiles: Ithaca, New York)
Bauer, AM, Jackman, T, Smith, SA, Sadlier, RA, and Austin, CC (2004). Note: Vocalization in Nannoscincus gracilis (New Caledonian Gracile Dwarf Skink). Herpetological Review 35, 268–269.
Bavay, A (1869). Catalogue des Reptiles de la Nouvelle-Calédonie et description d’espèces nouvelles. Mémoires de la Société Linnéenne de Normandie 15, 1–37.
Bernstein, JM, Jackman, TR, Sadlier, RA, Wang, Y-Y, and Bauer, AM (2022). A novel dataset to identify the endemic herpetofauna of the New Caledonia biodiversity hotspot with DNA barcodes. Pacific Conservation Biology 28, 36–47.
| A novel dataset to identify the endemic herpetofauna of the New Caledonia biodiversity hotspot with DNA barcodes.Crossref | GoogleScholarGoogle Scholar |
Blackburn, TM, Cassey, P, Duncan, RP, Evans, KL, and Gaston, KJ (2004). Avian extinction and mammalian introductions on Oceanic Islands. Science 305, 1955–1958.
| Avian extinction and mammalian introductions on Oceanic Islands.Crossref | GoogleScholarGoogle Scholar |
Bland, LM, and Böhm, M (2016). Overcoming data deficiency in reptiles. Biological Conservation 204, 16–22.
| Overcoming data deficiency in reptiles.Crossref | GoogleScholarGoogle Scholar |
Blom, MPK, Matzke, NJ, Bragg, JG, Arida, E, Austin, CC, Backlin, AR, Carretero, MA, Fisher, RN, Glaw, F, Hathaway, SA, Iskandar, DT, McGuire, JA, Karin, BR, Reilly, SB, Rittmeyer, EN, Rocha, S, Sanchez, M, Stubbs, AL, Vences, M, and Moritz, C (2019). Habitat preference modulares trans-oceanic dispersal in a terrestrial vertebrate. Proceedings of the Royal Society B: Biological Sciences 286, 20182575.
| Habitat preference modulares trans-oceanic dispersal in a terrestrial vertebrate.Crossref | GoogleScholarGoogle Scholar |
Boulenger GA (1887) ‘Catalogue of the lizards in the British Museum (Natural History) III. Lacertidae, Gerrhosauridae, Scincidae, Anelytropsidae, Dibamidae, Chamaeleontidae.’ (British Museum (Natural History): London)
Boulenger, GA (1897). II. – Descriptions of new lizards and frogs from Mount Victoria, Owen Stanley Range, New Guinea, collected by Mr. A. S. Anthony. Annals and Magazine of Natural History 19, 6–13.
| II. – Descriptions of new lizards and frogs from Mount Victoria, Owen Stanley Range, New Guinea, collected by Mr. A. S. Anthony.Crossref | GoogleScholarGoogle Scholar |
Boulenger, GA (1903). Descriptions of new reptiles from British New Guinea. Proceedings of the Zoological Society of London 1903, 125–129.
Boulenger, GA (1914). V. An annotated list of the batrachians and reptiles collected by the British Ornithologists’ Union Expedition and the Wollastolz Expedition in Dutch New Guinea. The Transactions of the Zoological Society of London 20, 247–274.
| V. An annotated list of the batrachians and reptiles collected by the British Ornithologists’ Union Expedition and the Wollastolz Expedition in Dutch New Guinea.Crossref | GoogleScholarGoogle Scholar |
Brooks, TM, Mittermeier, RA, da Fonseca, GAB, Gerlach, J, Hoffmann, M, Lamoreux, JF, Mittermeier, CG, Pilgrim, JD, and Rodrigues, ASL (2006). Global biodiversity conservation priorities. Science 313, 58–61.
| Global biodiversity conservation priorities.Crossref | GoogleScholarGoogle Scholar |
Brown, WC (1953). Results of the Archbold Expeditions. No. 69. A review of New Guinea lizards allied to Emoia baudini and Emoia physicae (Scincidae). American Museum Novitates 1627, 1–25.
Brown, WC (1954). Notes on several species of the genus Emoia, with descriptions of new species from the Solomon Islands. Fieldiana Zoology 34, 263–276.
Brown, WC (1983). A new species of Emoia Reptilia, Sauria, (Scincidae) from New Britain. Steenstrupia 8, 317–324.
Brown, WC (1991). Lizards of the genus Emoia (Scincidae) with observations on their evolution and biogeography. Memoirs of the California Academy of Sciences 15, 1–94.
Brown, WC, and Allison, A (1986). A new lizard of the genus Emoia (Scincidae) from Morobe province, Papua New Guinea. Occasional Papers Bernice P. Bishop Museum 26, 47–51.
Brown, WC, and Gibbons, JRH (1986). Species of the Emoia samoensis group of lizards (Scincidae) in the Fiji Islands, with descriptions of two new species. Proceedings of the California Academy of Sciences 44, 41–53.
Brown, WC, and Parker, F (1985). Three new lizards of the genus Emoia (Scincidae) from southern New Guinea. Breviora 480, 1–12.
Bruna, EM, Fisher, RN, and Case, TJ (1996). Morphological and genetic evolution appear decoupled in Pacific skinks (Squamata: Scincidae: Emoia). Proceedings of the Royal Society of London, Series B - Biological Sciences 263, 681–688.
Bryan JE, Shearman PL (2015) ‘The state of the forests of Papua New Guinea 2014: measuring change over the period 2002–2014.’ (University of Papua New Guinea: Port Moresby)
Caetano, GHdO, Chapple, DG, Grenyer, R, Raz, T, Rosenblatt, J, Tingley, R, Böhm, M, Meiri, S, and Roll, U (2022). Automated assessment reveals that the extinction risk of reptiles is widely underestimated across space and phylogeny. PLoS Biology 20, .
| Automated assessment reveals that the extinction risk of reptiles is widely underestimated across space and phylogeny.Crossref | GoogleScholarGoogle Scholar |
Cámara-Leret, R, Frodin, DG, Adema, F, Anderson, C, Appelhans, MS, Argent, G, Guerrero, SA, Ashton, P, Baker, WJ, Barfod, AS, Barrington, D, Borosova, R, Bramley, GLC, Briggs, M, Buerki, S, Cahen, D, Callmander, MW, Cheek, M, Chen, C-W, Conn, BJ, Coode, MJE, Darbyshire, I, Dawson, S, Dransfield, J, Drinkell, C, Duyfjes, B, Ebihara, A, Ezedin, Z, Fu, L-F, Gideon, O, Girmansyah, D, Govaerts, R, Fortune-Hopkins, H, Hassemer, G, Hay, A, Heatubun, CD, Hind, DJN, Hoch, P, Homot, P, Hovenkamp, P, Hughes, M, Jebb, M, Jennings, L, Jimbo, T, Kessler, M, Kiew, R, Knapp, S, Lamei, P, Lehnert, M, Lewis, GP, Linder, HP, Lindsay, S, Low, YW, Lucas, E, Mancera, JP, Monro, AK, Moore, A, Middleton, DJ, Nagamasu, H, Newman, MF, Lughadha, EN, Melo, PHA, Ohlsen, DJ, Pannell, CM, Parris, B, Pearce, L, Penneys, DS, Perrie, LR, Petoe, P, Poulsen, AD, Prance, GT, Quakenbush, JP, Raes, N, Rodda, M, Rogers, ZS, Schuiteman, A, Schwartsburd, P, Scotland, RW, Simmons, MP, Simpson, DA, Stevens, P, Sundue, M, Testo, W, Trias-Blasi, A, Turner, I, Utteridge, T, Walsingham, L, Webber, BL, Wei, R, Weiblen, GD, Weigend, M, Weston, P, de Wilde, W, Wilkie, P, Wilmot-Dear, CM, Wilson, HP, Wood, JRI, Zhang, L-B, and van Welzen, PC (2020). New Guinea has the world’s richest island flora. Nature 584, 579–583.
| New Guinea has the world’s richest island flora.Crossref | GoogleScholarGoogle Scholar |
Chamberlain, SA, and Szöcs, E (2013). taxize: taxonomic search and retrieval in R. F1000Research 2, .
| taxize: taxonomic search and retrieval in R.Crossref | GoogleScholarGoogle Scholar |
Chamberlain S, Szöcs E, Foster Z, Arendsee Z, Boettiger C, Ram K, Bartomeus I, Baumgartner J, O’Donnell J, Oksanen J, Tzovaras BG, Marchand P, Tran V, Salmon M, Li G, Grenié M (2020) taxize: taxonomic information from around the web. Available at https://github.com/ropensci/taxize
Chapple, DG, Roll, U, Böhm, M, Aguilar, R, Amey, AP, Austin, CC, Baling, M, Barley, AJ, Bates, MF, Bauer, AM, Blackburn, DG, Bowles, P, Brown, RM, Chandramouli, SR, Chirio, L, Cogger, H, Colli, GR, Conradie, W, Couper, PJ, Cowan, MA, Craig, MD, Das, I, Datta-Roy, A, Dickman, CR, Ellis, RJ, Fenner, AL, Ford, S, Ganesh, SR, Gardner, MG, Geissler, P, Gillespie, GR, Glaw, F, Greenlees, MJ, Griffith, OW, Grismer, LL, Haines, ML, Harris, DJ, Hedges, SB, Hitchmough, RA, Hoskin, CJ, Hutchinson, MN, Ineich, I, Janssen, J, Johnston, GR, Karin, BR, Keogh, JS, Kraus, F, LeBreton, M, Lymberakis, P, Masroor, R, McDonald, PJ, Mecke, S, Melville, J, Melzer, S, Michael, DR, Miralles, A, Mitchell, NJ, Nelson, NJ, Nguyen, TQ, de Campos Nogueira, C, Ota, H, Pafilis, P, Pauwels, OSG, Perera, A, Pincheira-Donoso, D, Reed, RN, Ribeiro-Júnior, MA, Riley, JL, Rocha, S, Rutherford, PL, Sadlier, RA, Shacham, B, Shea, GM, Shine, R, Slavenko, A, Stow, A, Sumner, J, Tallowin, OJS, Teale, R, Torres-Carvajal, O, Trape, J-F, Uetz, P, Ukuwela, KDB, Valentine, L, Van Dyke, JU, van Winkel, D, Vasconcelos, R, Vences, M, Wagner, P, Wapstra, E, While, GM, Whiting, MJ, Whittington, CM, Wilson, S, Ziegler, T, Tingley, R, and Meiri, S (2021). Conservation status of the world’s skinks (Scincidae): taxonomic and geographic patterns in extinction risk. Biological Conservation 257, .
| Conservation status of the world’s skinks (Scincidae): taxonomic and geographic patterns in extinction risk.Crossref | GoogleScholarGoogle Scholar |
Clause, AG, Thomas-Moko, N, Rasalato, S, and Fisher, RN (2018). All is not lost: herpetological “Extinctions” in the Fiji Islands. Pacific Science 72, 321–328.
| All is not lost: herpetological “Extinctions” in the Fiji Islands.Crossref | GoogleScholarGoogle Scholar |
Cogger, HG (1972). A new scincid lizard of the genus Tribolonotus from Manus Island, New Guinea. Zoologische Mededelingen 47, 202–210.
Cox, N, Young, BE, Bowles, P, Fernandez, M, Marin, J, Rapacciuolo, G, Böhm, M, Brooks, TM, Hedges, SB, Hilton-Taylor, C, Hoffmann, M, Jenkins, RKB, Tognelli, MF, Alexander, GJ, Allison, A, Ananjeva, NB, Auliya, M, Avila, LJ, Chapple, DG, Cisneros-Heredia, DF, Cogger, HG, Colli, GR, De Silva, A, Eisemberg, CC, Els, J, Fong, GA, Grant, TD, Hitchmough, RA, Iskandar, DT, Kidera, N, Martins, M, Meiri, S, Mitchell, NJ, Molur, S, Nogueira, CdeC, Ortiz, JC, Penner, J, Rhodin, AGJ, Rivas, GA, Rödel, M-O, Roll, U, Sanders, KL, Santos-Barrera, G, Shea, GM, Spawls, S, Stuart, BL, Tolley, KA, Trape, J-F, Vidal, MA, Wagner, P, Wallace, BP, and Xie, Y (2022). A global reptile assessment highlights shared conservation needs of tetrapods. Nature 605, 285–290.
| A global reptile assessment highlights shared conservation needs of tetrapods.Crossref | GoogleScholarGoogle Scholar |
Darriba, D, Posada, D, Kozlov, AM, Stamatakis, A, Morel, B, and Flouri, T (2020). ModelTest-NG: a new and scalable tool for the selection of DNA and protein evolutionary models. Molecular Biology and Evolution 37, 291–294.
| ModelTest-NG: a new and scalable tool for the selection of DNA and protein evolutionary models.Crossref | GoogleScholarGoogle Scholar |
Duffy, DC (2011). No room in the Ark? Climate change and biodiversity in the Pacific islands of Oceania. Pacific Conservation Biology 17, 192–200.
| No room in the Ark? Climate change and biodiversity in the Pacific islands of Oceania.Crossref | GoogleScholarGoogle Scholar |
Duméril AMC, Bibron G (1839) ‘Erpétologie générale ou histoire naturelle complète des reptiles. Tome Cinquième.’ (Roret: Paris)
Duncan, RP, Boyer, AG, and Blackburn, TM (2013). Magnitude and variation of prehistoric bird extinctions in the Pacific. Proceedings of the National Academy of Sciences 110, 6436–6441.
| Magnitude and variation of prehistoric bird extinctions in the Pacific.Crossref | GoogleScholarGoogle Scholar |
Edler, D, Klein, J, Antonelli, A, and Silvestro, D (2021). raxmlGUI 2.0: a graphical interface and toolkit for phylogenetic analyses using RAxML. Methods in Ecology and Evolution 12, 373–377.
| raxmlGUI 2.0: a graphical interface and toolkit for phylogenetic analyses using RAxML.Crossref | GoogleScholarGoogle Scholar |
Ferrier, S (2002). Mapping spatial pattern in biodiversity for regional conservation planning: where to from here? Systematic Biology 51, 331–363.
| Mapping spatial pattern in biodiversity for regional conservation planning: where to from here?Crossref | GoogleScholarGoogle Scholar |
Fisher, R, and Ineich, I (2012). Cryptic extinction of a common Pacific lizard Emoia impar (Squamata, Scincidae) from the Hawaiian Islands. Oryx 46, 187–195.
| Cryptic extinction of a common Pacific lizard Emoia impar (Squamata, Scincidae) from the Hawaiian Islands.Crossref | GoogleScholarGoogle Scholar |
Fromm, A, and Meiri, S (2021). Big, flightless, insular and dead: characterising the extinct birds of the Quaternary. Journal of Biogeography 48, 2350–2359.
| Big, flightless, insular and dead: characterising the extinct birds of the Quaternary.Crossref | GoogleScholarGoogle Scholar |
Greer, AE (1973). Two new lygosomine skinks from New Guinea with comments on the loss of the external ear in lygosomines and observations on previously described species. Breviora 406, 1–25.
Greer, AE (1974). The genetic relationships of the Scincid lizard genus Leiolopisma and its relatives. Australian Journal of Zoology Supplementary Series 22, 1–67.
| The genetic relationships of the Scincid lizard genus Leiolopisma and its relatives.Crossref | GoogleScholarGoogle Scholar |
Greer, AE, and Parker, F (1967a). A new scincid lizard from the northern Solomon Islands. Breviora 275, 1–20.
Greer, AE, and Parker, F (1967b). A second skink with fragmented head scales from Bougainville, Solomon Islands. Breviora 279, 1–12.
Greer, AE, and Parker, F (1968). A new species of Tribolonotus (Lacertilia: Scincidae) from Bougainville and Buka, Solomon Islands, with comments on the biology of the genus. Breviora 291, 1–23.
Greer, AE, and Parker, F (1971). A new scincid lizard from Bougainville, Solomon Islands. Breviora 364, 1–11.
Greer, AE, and Parker, F (1974). The fasciatus species group of Sphenomorphus (Lacertilia: Scincidae): notes on eight previously described species and descriptions of three new species. Papua New Guinea Scientific Society Proceedings 25, 31–61.
Greer, AE, and Raizes, G (1969). Green blood pigment in lizards. Science 166, 392–393.
| Green blood pigment in lizards.Crossref | GoogleScholarGoogle Scholar |
Greer, AE, and Shea, G (2004). A new character within the taxonomically difficult Sphenomorphus group of lygosomine skinks, with a description of a new species from New Guinea. Journal of Herpetology 38, 79–87.
| A new character within the taxonomically difficult Sphenomorphus group of lygosomine skinks, with a description of a new species from New Guinea.Crossref | GoogleScholarGoogle Scholar |
Greer, AE, and Simon, M (1982). Fojia bumui, an unusual new genus and species of scincid lizard from New Guinea. Journal of Herpetology 16, 131–139.
| Fojia bumui, an unusual new genus and species of scincid lizard from New Guinea.Crossref | GoogleScholarGoogle Scholar |
Greer, AE, Allison, A, and Cogger, HG (2005). Four new species of Lobulia (Lacertilia: Scincidae) from high altitude in New Guinea. Herpetological Monographs 19, 153–179.
| Four new species of Lobulia (Lacertilia: Scincidae) from high altitude in New Guinea.Crossref | GoogleScholarGoogle Scholar |
Gumbs, R, Gray, CL, Böhm, M, Hoffmann, M, Grenyer, R, Jetz, W, Meiri, S, Roll, U, Owen, NR, and Rosindell, J (2020). Global priorities for conservation of reptilian phylogenetic diversity in the face of human impacts. Nature Communications 11, .
| Global priorities for conservation of reptilian phylogenetic diversity in the face of human impacts.Crossref | GoogleScholarGoogle Scholar |
Günther, R (2000). In alten Sammlungen aus Neuguinea entdeckt: Zwei neue Arten der Gattung Lipinia (Squamata: Scincidae). Salamandra 36, 157–174.
Hamilton, AM, Zug, GR, and Austin, CC (2010). Biogeographic anomaly or human introduction: a cryptogenic population of tree skink (Reptilia: Squamata) from the Cook Islands, Oceania. Biological Journal of the Linnean Society 100, 318–328.
| Biogeographic anomaly or human introduction: a cryptogenic population of tree skink (Reptilia: Squamata) from the Cook Islands, Oceania.Crossref | GoogleScholarGoogle Scholar |
Hartdegen, RW, Russell, MJ, Young, B, and Reams, RD (2001). Vocalization of the crocodile skink, Tribolonotus gracilis (De Rooy, 1909), and evidence of parental care. Contemporary Herpetology 2001, 1–6.
| Vocalization of the crocodile skink, Tribolonotus gracilis (De Rooy, 1909), and evidence of parental care.Crossref | GoogleScholarGoogle Scholar |
Heads, M (2002). Regional patterns of biodiversity in New Guinea animals. Journal of Biogeography 29, 285–294.
| Regional patterns of biodiversity in New Guinea animals.Crossref | GoogleScholarGoogle Scholar |
Hoang, DT, Chernomor, O, von Haeseler, A, Minh, BQ, and Vinh, LS (2018). UFBoot2: improving the ultrafast bootstrap approximation. Molecular Biology and Evolution 35, 518–522.
| UFBoot2: improving the ultrafast bootstrap approximation.Crossref | GoogleScholarGoogle Scholar |
Ineich, I, and Zug, GR (1996). Tachygyia, the giant Tongan skink: extinct or extant? Cryptozoology 12, 30–35.
Irschick, DJ, Austin, CC, Petren, K, Fisher, RN, Losos, JB, and Ellers, O (1996). A comparative analysis of clinging ability among pad-bearing lizards. Biological Journal of the Linnean Society 59, 21–35.
| A comparative analysis of clinging ability among pad-bearing lizards.Crossref | GoogleScholarGoogle Scholar |
IUCN (2016) ‘A global standard for the identification of key biodiversity areas, Version 1.0.’ 1st edn. (IUCN: Gland)
IUCN (2021) IUCN red list of threatened species. Version 2021-3. Available at https://www.iucnredlist.org
Iverson JB (1982) Adaptations to herbivory in iguanine lizards. In ‘Iguanas of the world: their behavior, ecology, and conservation’. (Eds GM Burghardt, AS Rand) pp. 60–76. (Noyes Publications: Park Ridge, NJ)
Janssen, J, and Shepherd, CR (2018). Challenges in documenting trade in non CITES-listed species: a case study on crocodile skinks (Tribolonotus spp.). Journal of Asia-Pacific Biodiversity 11, 476–481.
| Challenges in documenting trade in non CITES-listed species: a case study on crocodile skinks (Tribolonotus spp.).Crossref | GoogleScholarGoogle Scholar |
Jourdan, H, Sadlier, RA, and Bauer, AM (2001). Little fire ant invasion (Wasmannia auropunctata) as a threat to New Caledonian lizards: evidences from a sclerophyll forest (Hymenoptera: Formicidae). Sociobiology 38, 283–302.
Jowers, MJ, Simone, Y, Herrel, A, Cabezas, MP, Xavier, R, Holden, M, Boistel, R, Murphy, JC, Santin, M, Caut, S, Auguste, RJ, van der Meijden, A, Andreone, F, and Ineich, I (2022). The Terrific Skink bite force suggests insularity as a likely driver to exceptional resource use. Scientific Reports 12, .
| The Terrific Skink bite force suggests insularity as a likely driver to exceptional resource use.Crossref | GoogleScholarGoogle Scholar |
Jupiter, SD, Wenger, A, Klein, CJ, Albert, S, Mangubhai, S, Nelson, J, Teneva, L, Tulloch, VJ, White, AT, and Watson, JEM (2017). Opportunities and constraints for implementing integrated land-sea management on islands. Environmental Conservation 44, 254–266.
| Opportunities and constraints for implementing integrated land-sea management on islands.Crossref | GoogleScholarGoogle Scholar |
Kabutaulaka TT (2000) Rumble in the jungle: land, culture and (un)sustainable logging in Solomon Islands. In ‘Culture and sustainable development in the Pacific’. (Ed. A Hooper) pp. 88–97. (Australian National University Press: Canberra)
Kalyaanamoorthy, S, Minh, BQ, Wong, TKF, von Haeseler, A, and Jermiin, LS (2017). ModelFinder: fast model selection for accurate phylogenetic estimates. Nature Methods 14, 587–589.
| ModelFinder: fast model selection for accurate phylogenetic estimates.Crossref | GoogleScholarGoogle Scholar |
Karin, BR, Stubbs, AL, Arifin, U, Bloch, LM, Ramadhan, G, Iskandar, DT, Arida, E, Reilly, SB, Kusnadi, A, and McGuire, JA (2018). The herpetofauna of the Kei Islands (Maluku, Indonesia): comprehensive report on new and historical collections, biogeographic patterns, conservation concerns, and an annotated checklist of species from Kei Kecil, Kei Besar, Tam, and Kur. Raffles Bulletin of Zoology 66, 704–738.
Katovai, E, Edwards, W, and Laurance, WF (2015). Dynamics of logging in Solomon Islands: the need for restoration and conservation alternatives. Tropical Conservation Science 8, 718–731.
| Dynamics of logging in Solomon Islands: the need for restoration and conservation alternatives.Crossref | GoogleScholarGoogle Scholar |
Keppel, G, Morrison, C, Watling, D, Tuiwawa, MV, and Rounds, IA (2012). Conservation in tropical Pacific Island countries: why most current approaches are failing. Conservation Letters 5, 256–265.
| Conservation in tropical Pacific Island countries: why most current approaches are failing.Crossref | GoogleScholarGoogle Scholar |
Keppel, G, Naikatini, A, Rounds, IA, Pressey, RL, and Thomas, NT (2015). Local and expert knowledge improve conservation assessment of rare and iconic Fijian tree species. Pacific Conservation Biology 21, 214–219.
| Local and expert knowledge improve conservation assessment of rare and iconic Fijian tree species.Crossref | GoogleScholarGoogle Scholar |
Kingsford, RT, and Watson, JEM (2011). Climate change in Oceania – a synthesis of biodiversity impacts and adaptations. Pacific Conservation Biology 17, 270–284.
| Climate change in Oceania – a synthesis of biodiversity impacts and adaptations.Crossref | GoogleScholarGoogle Scholar |
Klein, ER, Harris, RB, Fisher, RN, and Reeder, TW (2016). Biogeographical history and coalescent species delimitation of Pacific island skinks (Squamata: Scincidae: Emoia cyanura species group). Journal of Biogeography 43, 1917–1929.
| Biogeographical history and coalescent species delimitation of Pacific island skinks (Squamata: Scincidae: Emoia cyanura species group).Crossref | GoogleScholarGoogle Scholar |
Knight, AT, Smith, RJ, Cowling, RM, Desmet, PG, Faith, DP, Ferrier, S, Gelderblom, CM, Grantham, H, Lombard, AT, Maze, K, Nel, JL, Parrish, JD, Pence, GQK, Possingham, HP, Reyers, B, Rouget, M, Roux, D, and Wilson, KA (2007). Improving the key biodiversity areas approach for effective conservation planning. BioScience 57, 256–261.
| Improving the key biodiversity areas approach for effective conservation planning.Crossref | GoogleScholarGoogle Scholar |
Kraus, F (2018). A new species of Emoia (Squamata: Scincidae) from Papua New Guinea. Journal of Herpetology 52, 430–436.
| A new species of Emoia (Squamata: Scincidae) from Papua New Guinea.Crossref | GoogleScholarGoogle Scholar |
Kraus, F (2020). A new species of Lobulia (Squamata: Scincidae) from Papua New Guinea. Zootaxa 4779, 201–214.
| A new species of Lobulia (Squamata: Scincidae) from Papua New Guinea.Crossref | GoogleScholarGoogle Scholar |
Kraus, F (2021). A herpetofauna with dramatic endemism signals an overlooked biodiversity hotspot. Biodiversity and Conservation 30, 3167–3183.
| A herpetofauna with dramatic endemism signals an overlooked biodiversity hotspot.Crossref | GoogleScholarGoogle Scholar |
Kuch, U, and McGuire, JA (2004). Range extensions of Lycodon capucinus BOIE 1827 in eastern Indonesia. Herpetozoa 17, 191–193.
Lanfear, R, Calcott, B, Kainer, D, Mayer, C, and Stamatakis, A (2014). Selecting optimal partitioning schemes for phylogenomic datasets. BMC Evolutionary Biology 14, .
| Selecting optimal partitioning schemes for phylogenomic datasets.Crossref | GoogleScholarGoogle Scholar |
Laurance, WF, Kakul, T, Keenan, RJ, Sayer, J, Passingan, S, Clements, GR, Villegas, F, and Sodhi, NS (2011). Predatory corporations, failing governance, and the fate of forests in Papua New Guinea. Conservation Letters 4, 95–100.
| Predatory corporations, failing governance, and the fate of forests in Papua New Guinea.Crossref | GoogleScholarGoogle Scholar |
Lavery, TH, DeCicco, LH, Richmond, JQ, Tigulu, IG, Andersen, MJ, Boseto, D, and Moyle, RG (2021). New faunal records from a world heritage site in danger: Rennell Island, Solomon Islands. Pacific Science 75, 407–420.
| New faunal records from a world heritage site in danger: Rennell Island, Solomon Islands.Crossref | GoogleScholarGoogle Scholar |
Leary, T (1991). A review of terrestrial wildlife trade originating from Solomon Islands. Australian Zoologist 27, 20–27.
| A review of terrestrial wildlife trade originating from Solomon Islands.Crossref | GoogleScholarGoogle Scholar |
Lescure, J (2002). La naissance de l’herpétologie. Bulletin de la Société Herpétologique de France 101, 5–27.
Lesson R (1830) Description de quelques reptiles nouveaux ou peu connus. In ‘Voyage Autour du Monde, Exécuté par Ordre du Roi, Sur la Corvette de Sa Majesté, La Coquille, pendant les années 1822, 1823, 1824 et 1825. Zoologie Tome 2, Partie 1’. (Ed. LI Duperrey) pp. 34–65. (Arthus Bertrand: Paris)
L’Huillier L, Jaffré T (2010) L’exploitation des minerais de nickel en Nouvelle-Calédonie. In ‘Mines et Environnement en Nouvelle-Calédonie: les milieux sur substrats ultramafiques et leur restauration’. (Eds L L’Huillier, T Jaffré, A Wulff) pp. 21–31. (IAC editions: New Caledonia)
Linkem, CW, Brown, RM, Siler, CD, Evans, BJ, Austin, CC, Iskandar, DT, Diesmos, AC, Supriatna, J, Andayani, N, and McGuire, JA (2013). Stochastic faunal exchanges drive diversification in widespread Wallacean and Pacific island lizards (Squamata: Scincidae: Lamprolepis smaragdina). Journal of Biogeography 40, 507–520.
| Stochastic faunal exchanges drive diversification in widespread Wallacean and Pacific island lizards (Squamata: Scincidae: Lamprolepis smaragdina).Crossref | GoogleScholarGoogle Scholar |
Loh, J, and Harmon, D (2005). A global index of biocultural diversity. Ecological Indicators 5, 231–241.
| A global index of biocultural diversity.Crossref | GoogleScholarGoogle Scholar |
Loveridge, A (1945). New scincid lizards of the genera Tropidophorus and Lygosoma from New Guinea. Proceedings of the Biological Society of Washington 58, 47–52.
Mace, GM (2004). The role of taxonomy in species conservation. Philosophical Transactions of the Royal Society B: Biological Sciences 359, 711–719.
| The role of taxonomy in species conservation.Crossref | GoogleScholarGoogle Scholar |
Macleay, W (1877). The lizards of the Chevert Expedition. Proceedings of the Linnean Society of New South Wales 2, 97–104.
Mayr, E (1944). Wallace’s Line in the light of recent zoogeographic studies. The Quarterly Review of Biology 19, 1–14.
| Wallace’s Line in the light of recent zoogeographic studies.Crossref | GoogleScholarGoogle Scholar |
McDonald, PJ, Brown, RM, Kraus, F, Bowles, P, Arifin, U, Eliades, SJ, Fisher, RN, Gaulke, M, Grismer, LL, Ineich, I, Karin, BR, Meneses, CG, Richards, SJ, Sanguila, MB, Siler, CD, and Oliver, PM (2022). Cryptic extinction risk in a western Pacific lizard radiation. Biodiversity and Conservation 31, 2045–2062.
| Cryptic extinction risk in a western Pacific lizard radiation.Crossref | GoogleScholarGoogle Scholar |
McMillen, HL, Ticktin, T, Friedlander, A, Jupiter, SD, Thaman, R, Campbell, J, Veitayaki, J, Giambelluca, T, Nihmei, S, Rupeni, E, Apis-Overhoff, L, Aalbersberg, W, and Orcherton, DF (2014). Small islands, valuable insights: systems of customary resource use and resilience to climate change in the Pacific. Ecology and Society 19, .
| Small islands, valuable insights: systems of customary resource use and resilience to climate change in the Pacific.Crossref | GoogleScholarGoogle Scholar |
Meiri, S (2016). Small, rare and trendy: traits and biogeography of lizards described in the 21st century. Journal of Zoology 299, 251–261.
| Small, rare and trendy: traits and biogeography of lizards described in the 21st century.Crossref | GoogleScholarGoogle Scholar |
Meiri, S, Bauer, AM, Allison, A, Castro-Herrera, F, Chirio, L, Colli, G, Das, I, Doan, TM, Glaw, F, Grismer, LL, Hoogmoed, M, Kraus, F, LeBreton, M, Meirte, D, Nagy, ZT, Nogueira, CdeC, Oliver, P, Pauwels, OSG, Pincheira-Donoso, D, Shea, G, Sindaco, R, Tallowin, OJS, Torres-Carvajal, O, Trape, J-F, Uetz, P, Wagner, P, Wang, Y, Ziegler, T, and Roll, U (2018). Extinct, obscure or imaginary: the lizard species with the smallest ranges. Diversity and Distributions 24, 262–273.
| Extinct, obscure or imaginary: the lizard species with the smallest ranges.Crossref | GoogleScholarGoogle Scholar |
Mertens, R (1928). Neue Inselrassen von Cryptoblepharus boutonii (Desjardin). Zoologischer Anzeiger 78, 82–89.
Mertens, R (1931). Ablepharus boutonii (Desjardin) und seine geographische Variation. Zoologische Jahrbücher. Abteilung für Systematik, Geographie und Biologie der Tiere 61, 63–210.
Mertens, R (1933). Weitere Mitteilungen über die Rassen von Ablepharus boutonii (Desjardin). I. Zoologischer Anzeiger 105, 92–96.
Mertens, R (1934). Weitere Mitteilungen über die Rassen von Ablepharus boutonii (Desjardin). II. Zoologischer Anzeiger 108, 40–43.
Mertens, R (1964). Weitere Mitteilungen über die Rassen von Ablepharus boutonii (Desjardin). III. Zoologischer Anzeiger 173, 99–110.
Meyer, AB (1874). Eine Mittheilung von Hrn. Dr. Adolf Bernhard Meyer über die von ihm auf Neu-Guinea und den Inseln Jobi, Mysore und Mafoor im Jahre 1873 gesammelten Amphibien. Monatsberichte der Königlichen Preussische Akademie der Wissenschaften zu Berlin 1874, 128–140.
Michaelides, S, Cole, N, and Funk, SM (2015). Translocation retains genetic diversity of a threatened endemic reptile in Mauritius. Conservation Genetics 16, 661–672.
| Translocation retains genetic diversity of a threatened endemic reptile in Mauritius.Crossref | GoogleScholarGoogle Scholar |
Miller MA, Pfeiffer W, Schwartz T (2010) Creating the CIPRES Science Gateway for inference of large phylogenetic trees. In ‘Proceedings of the gateway computing environments workshop (GCE), 14 November’. pp. 1–8. (Institute of Electrical and Electronics Engineers [IEEE]: New Orleans, USA)
Minh, BQ, Schmidt, HA, Chernomor, O, Schrempf, D, Woodhams, MD, von Haeseler, A, and Lanfear, R (2020). IQ-TREE 2: new models and efficient methods for phylogenetic inference in the genomic era. Molecular Biology and Evolution 37, 1530–1534.
| IQ-TREE 2: new models and efficient methods for phylogenetic inference in the genomic era.Crossref | GoogleScholarGoogle Scholar |
Mittermeier RA, Gil PR, Hoffmann M, Pilgrim J, Brooks T, Mittermeier CG, Lamoreux J, Da Fonseca GAB (2004) ‘Hotspots revisited: earth’s biologically richest and most endangered terrestrial ecoregions.’ (Cemex-Agrupación Sierra Madre: Mexico City)
Morley, CG, and Winder, L (2015). Vulnerability of skinks to predation by introduced mongoose in the Fiji Islands. Pacific Science 69, 313–317.
| Vulnerability of skinks to predation by introduced mongoose in the Fiji Islands.Crossref | GoogleScholarGoogle Scholar |
Morrison, C, Pikacha, P, Pitakia, T, and Boseto, D (2007). Herpetofauna, community education and logging on Choiseul Island, Solomon Islands: implications for conservation. Pacific Conservation Biology 13, 250–258.
| Herpetofauna, community education and logging on Choiseul Island, Solomon Islands: implications for conservation.Crossref | GoogleScholarGoogle Scholar |
Morrison C, Keppel G, Moko N, Pikacha P, Brodie G (2022) Conservation in the Pacific. In ‘The Pacific Islands: environment and society’. (Ed. M Rapaport). (University of Hawaii Press: Honolulu, HI).
Myers, N, Mittermeier, RA, Mittermeier, CG, da Fonseca, GAB, and Kent, J (2000). Biodiversity hotspots for conservation priorities. Nature 403, 853–858.
| Biodiversity hotspots for conservation priorities.Crossref | GoogleScholarGoogle Scholar |
Oliver, PM, Blom, MPK, Cogger, HG, Fisher, RN, Richmond, JQ, and Woinarski, JCZ (2018). Insular biogeographic origins and high phylogenetic distinctiveness for a recently depleted lizard fauna from Christmas Island, Australia. Biology Letters 14, .
| Insular biogeographic origins and high phylogenetic distinctiveness for a recently depleted lizard fauna from Christmas Island, Australia.Crossref | GoogleScholarGoogle Scholar |
O’Shea, M, Kusuma, KI, and Kaiser, H (2018). First record of the Island Wolfsnake, Lycodon capucinus, from New Guinea, with comments on its widespread distribution and confused taxonomy, and a new record for the common sun skink, Eutropis multifasciata. IRCF Reptiles & Amphibians 25, 70–84.
Parker, HW (1936). V. – A Collection of reptiles and amphibians from the mountains of British New Guinea. Annals and Magazine of Natural History 17, 66–93.
| V. – A Collection of reptiles and amphibians from the mountains of British New Guinea.Crossref | GoogleScholarGoogle Scholar |
Pascal, M, de Forges, BR, Le Guyader, H, and Simberloff, D (2008). Mining and other threats to the New Caledonia biodiversity hotspot. Conservation Biology 22, 498–499.
| Mining and other threats to the New Caledonia biodiversity hotspot.Crossref | GoogleScholarGoogle Scholar |
Pereira, HM, Navarro, LM, and Martins, IS (2012). Global biodiversity change: the bad, the good, and the unknown. Annual Review of Environment and Resources 37, 25–50.
| Global biodiversity change: the bad, the good, and the unknown.Crossref | GoogleScholarGoogle Scholar |
Peters, WCH, and Doria, G (1878). Catalogo dei retilli e dei batraci raccolti da O. Beccari, L. M. D’Alberts e A. A. Bruijn. nella sotto-regione Austro-Malese. Annali del Museo Civico de Storia Naturale di Genova 13, 323–450.
Pimm, SL, Jenkins, CN, Abell, R, Brooks, TM, Gittleman, JL, Joppa, LN, Raven, PH, Roberts, CM, and Sexton, JO (2014). The biodiversity of species and their rates of extinction, distribution, and protection. Science 344, .
| The biodiversity of species and their rates of extinction, distribution, and protection.Crossref | GoogleScholarGoogle Scholar |
Pollard, E, Brodie, G, Thaman, R, and Morrison, C (2014). The use of herpetofauna and cultural values to identify priority conservation forests on Malaita, Solomon Islands. Pacific Conservation Biology 20, 354–352.
| The use of herpetofauna and cultural values to identify priority conservation forests on Malaita, Solomon Islands.Crossref | GoogleScholarGoogle Scholar |
Powney, GD, Grenyer, R, Orme, CDL, Owens, IPF, and Meiri, S (2010). Hot, dry and different: Australian lizard richness is unlike that of mammals, amphibians and birds. Global Ecology and Biogeography 19, 386–396.
| Hot, dry and different: Australian lizard richness is unlike that of mammals, amphibians and birds.Crossref | GoogleScholarGoogle Scholar |
Primack RB (2014) ‘Essentials of conservation biology.’ 6th edn. (Sinauer Associates: Sunderland, MA)
Pyron, RA, Burbrink, FT, and Wiens, JJ (2013). A phylogeny and revised classification of Squamata, including 4161 species of lizards and snakes. BMC Evolutionary Biology 13, .
| A phylogeny and revised classification of Squamata, including 4161 species of lizards and snakes.Crossref | GoogleScholarGoogle Scholar |
Rabosky, DL, Donnellan, SC, Talaba, AL, and Lovette, IJ (2007). Exceptional among-lineage variation in diversification rates during the radiation of Australia’s most diverse vertebrate clade. Proceedings of the Royal Society of London B: Biological Sciences 274, 2915–2923.
| Exceptional among-lineage variation in diversification rates during the radiation of Australia’s most diverse vertebrate clade.Crossref | GoogleScholarGoogle Scholar |
R Core Team (2021) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. Available at https://www.R-project.org/
Reilly, SB, Karin, BR, Stubbs, AL, Arida, E, Arifin, U, Kaiser, H, Bi, K, Hamidy, A, Iskandar, DT, and McGuire, JA (2022). Diverge and Conquer: phylogenomics of southern Wallacean forest skinks (Genus: Sphenomorphus) and their colonization of the Lesser Sunda Archipelago. Evolution; International Journal of Organic Evolution 76, 2281–2301.
| Diverge and Conquer: phylogenomics of southern Wallacean forest skinks (Genus: Sphenomorphus) and their colonization of the Lesser Sunda Archipelago.Crossref | GoogleScholarGoogle Scholar |
Richmond, JQ, Ota, H, Grismer, LL, and Fisher, RN (2021). Influence of niche breadth and position on the historical biogeography of seafaring scincid lizards. Biological Journal of the Linnean Society 132, 74–92.
| Influence of niche breadth and position on the historical biogeography of seafaring scincid lizards.Crossref | GoogleScholarGoogle Scholar |
Richmond, JQ, Wostl, E, Reed, RN, and Fisher, RN (2022). Range eclipse leads to tenuous survival of a rare lizard species on a barrier atoll. Oryx 56, 63–72.
| Range eclipse leads to tenuous survival of a rare lizard species on a barrier atoll.Crossref | GoogleScholarGoogle Scholar |
Rittmeyer, EN, and Austin, CC (2017). Two new species of Crocodile Skinks (Squamata: Scincidae: Tribolonotus) from the Solomon Archipelago. Zootaxa 4268, 71–87.
| Two new species of Crocodile Skinks (Squamata: Scincidae: Tribolonotus) from the Solomon Archipelago.Crossref | GoogleScholarGoogle Scholar |
Rodda, GH, and Fritts, TH (1992). The impact of the introduction of the colubrid snake Boiga irregularis on Guam’s lizards. Journal of Herpetology 26, 166–174.
| The impact of the introduction of the colubrid snake Boiga irregularis on Guam’s lizards.Crossref | GoogleScholarGoogle Scholar |
Rodriguez, ZB, Perkins, SL, and Austin, CC (2018). Multiple origins of green blood in New Guinea lizards. Science Advances 4, .
| Multiple origins of green blood in New Guinea lizards.Crossref | GoogleScholarGoogle Scholar |
Roll, U, Feldman, A, Novosolov, M, Allison, A, Bauer, AM, Bernard, R, Böhm, M, Castro-Herrera, F, Chirio, L, Collen, B, Colli, GR, Dabool, L, Das, I, Doan, TM, Grismer, LL, Hoogmoed, M, Itescu, Y, Kraus, F, LeBreton, M, Lewin, A, Martins, M, Maza, E, Meirte, D, Nagy, ZT, Nogueira, CdeC, Pauwels, OSG, Pincheira-Donoso, D, Powney, GD, Sindaco, R, Tallowin, OJS, Torres-Carvajal, O, Trape, J-F, Vidan, E, Uetz, P, Wagner, P, Wang, Y, Orme, CDL, Grenyer, R, and Meiri, S (2017). The global distribution of tetrapods reveals a need for targeted reptile conservation. Nature Ecology & Evolution 1, 1677–1682.
| The global distribution of tetrapods reveals a need for targeted reptile conservation.Crossref | GoogleScholarGoogle Scholar |
Ronquist, F, Teslenko, M, Van Der Mark, P, Ayres, DL, Darling, A, Höhna, S, Larget, B, Liu, L, Suchard, MA, and Huelsenbeck, JP (2012). MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Systematic Biology 61, 539–542.
| MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space.Crossref | GoogleScholarGoogle Scholar |
Sadlier, RA (1987). A review of the scincid lizards of New Caledonia. Records of the Australian Museum 39, 1–66.
| A review of the scincid lizards of New Caledonia.Crossref | GoogleScholarGoogle Scholar |
Sadlier, RA, Bauer, AM, and Colgan, DJ (1999). The scincid lizard genus Caledoniscincus (Reptilia: Scincidae) from New Caledonia in the Southwest Pacific: a review of Caledoniscincus austrocaledonicus (Bavay) and description of six new species from Province Nord. Records of the Australian Museum 51, 57–82.
| The scincid lizard genus Caledoniscincus (Reptilia: Scincidae) from New Caledonia in the Southwest Pacific: a review of Caledoniscincus austrocaledonicus (Bavay) and description of six new species from Province Nord.Crossref | GoogleScholarGoogle Scholar |
Sadlier, RA, Bauer, AM, and Smith, SA (2006). A new species of Nannoscincus Günther (Squamata: Scincidae) from high elevation forest in southern New Caledonia. Records of the Australian Museum 58, 29–36.
| A new species of Nannoscincus Günther (Squamata: Scincidae) from high elevation forest in southern New Caledonia.Crossref | GoogleScholarGoogle Scholar |
Sadlier RA, Smith SA, Bauer AM, Whitaker AH (2009a) Three new species of skink in the genus Marmorosphax Sadlier (Squamata: Scincidae) from New Caledonia. In ‘Zoologia Neocaledonica 7. Biodiversity studies in New Caledonia’. (Ed. P Grandcolas) pp. 373–390. (Muséum National d’Histoire Naturelle: Paris)
Sadlier, RA, Smith, SA, Whitaker, A, and Bauer, AM (2009b). A new live-bearing species of scincid lizard (Reptilia: Scincidae) from New Caledonia, southwest Pacific. Pacific Science 63, 123–136.
| A new live-bearing species of scincid lizard (Reptilia: Scincidae) from New Caledonia, southwest Pacific.Crossref | GoogleScholarGoogle Scholar |
Sadlier, RA, Bauer, AM, Smith, SA, Shea, GM, and Whitaker, AH (2014a). High elevation endemism on New Caledonia’s ultramafic peaks – a new genus and two new species of scincid lizard. Mémoires du Muséum National d’Histoire Naturelle 206, 115–125.
Sadlier, RA, Bauer, AM, Wood, PL, Smith, SA, Whitaker, AH, and Jackman, TR (2014b). Cryptic speciation in the New Caledonian lizard genus Nannoscincus (Reptilia: Scincidae) including the description of a new species and recognition of Nannoscincus fuscus Günther. Mémoires du Muséum National d’Histoire Naturelle 206, 45–68.
Sadlier, RA, Bauer, AM, Wood, PL, Smith, SA, Whitaker, AH, Jourdan, H, and Jackman, T (2014c). Localized endemism in the southern ultramafic bio-region of New Caledonia as evidenced by the lizards in the genus Sigaloseps (Reptilia: Scincidae), with descriptions of four new species. Mémoires du Muséum National d’Histoire Naturelle 206, 79–113.
Sadlier, RA, Debar, L, Chavis, M, Bauer, AM, Jourdan, H, and Jackman, TR (2019). Epibator insularis, a new species of scincid lizard from l’Ⓘle Walpole, New Caledonia. Pacific Science 73, 143–161.
| Epibator insularis, a new species of scincid lizard from l’Ⓘle Walpole, New Caledonia.Crossref | GoogleScholarGoogle Scholar |
Shea GM (2017) A new species of Sphenomorphus (Squamata: Scincidae) from the Doberai Peninsula of New Guinea, with a redescription of Sphenomorphus consobrinus (Peters et Doria, 1878). In ‘Biodiversity, biogeography and nature conservation in Wallacea and New Guinea’. (Eds D Telnov, MVL Barclay, OSG Pauwels) pp. 35–48. (The Entomological Society of Latvia: Rīga)
Shea, GM (2021). Nomenclature of supra-generic units within the Family Scincidae (Squamata). Zootaxa 5067, 301–351.
| Nomenclature of supra-generic units within the Family Scincidae (Squamata).Crossref | GoogleScholarGoogle Scholar |
Shea GM, Allison A (2021) A new species of Sphenomorphus (Squamata: Scincidae) from Mount Kaindi, Morobe Province, Papua New Guinea. In ‘Biodiversity, biogeography and nature conservation in Wallacea and New Guinea’. (Eds D Telnov, MVL Barclay, OSG Pauwels) pp. 49–60. (The Entomological Society of Latvia: Rīga)
Shearman, PL, Ash, J, Mackey, B, Bryan, JE, and Lokes, B (2009). Forest conversion and degradation in Papua New Guinea 1972–2002. Biotropica 41, 379–390.
| Forest conversion and degradation in Papua New Guinea 1972–2002.Crossref | GoogleScholarGoogle Scholar |
Simpson, GG (1977). Too many lines: the limits of the Oriental and Australian zoogeographic regions. Proceedings of the American Philosophical Society 121, 107–120.
Slavenko, A, Tallowin, OJS, Itescu, Y, Raia, P, and Meiri, S (2016). Late Quaternary reptile extinctions: size matters, insularity dominates. Global Ecology and Biogeography 25, 1308–1320.
| Late Quaternary reptile extinctions: size matters, insularity dominates.Crossref | GoogleScholarGoogle Scholar |
Slavenko, A, Tamar, K, Tallowin, OJS, Allison, A, Kraus, F, Carranza, S, and Meiri, S (2020). Cryptic diversity and non-adaptive radiation of montane New Guinea skinks (Papuascincus; Scincidae). Molecular Phylogenetics and Evolution 146, .
| Cryptic diversity and non-adaptive radiation of montane New Guinea skinks (Papuascincus; Scincidae).Crossref | GoogleScholarGoogle Scholar |
Slavenko, A, Allison, A, and Meiri, S (2021). Elevation is a stronger predictor of morphological trait divergence than competition in a radiation of tropical lizards. Journal of Animal Ecology 90, 917–930.
| Elevation is a stronger predictor of morphological trait divergence than competition in a radiation of tropical lizards.Crossref | GoogleScholarGoogle Scholar |
Slavenko, A, Tamar, K, Tallowin, OJS, Kraus, F, Allison, A, Carranza, S, and Meiri, S (2022). Revision of the montane New Guinean skink genus Lobulia (Squamata: Scincidae), with the description of four new genera and nine new species. Zoological Journal of the Linnean Society 195, 220–278.
| Revision of the montane New Guinean skink genus Lobulia (Squamata: Scincidae), with the description of four new genera and nine new species.Crossref | GoogleScholarGoogle Scholar |
Smith, SA, Sadlier, RA, Bauer, AM, Austin, CC, and Jackman, T (2007). Molecular phylogeny of the scincid lizards of New Caledonia and adjacent areas: evidence for a single origin of the endemic skinks of Tasmantis. Molecular Phylogenetics and Evolution 43, 1151–1166.
| Molecular phylogeny of the scincid lizards of New Caledonia and adjacent areas: evidence for a single origin of the endemic skinks of Tasmantis.Crossref | GoogleScholarGoogle Scholar |
Smith, MJ, Cogger, H, Tiernan, B, Maple, D, Boland, C, Napier, F, Detto, T, and Smith, P (2012). An oceanic island reptile community under threat: the decline of reptiles on Christmas Island, Indian Ocean. Herpetological Conservation and Biology 7, 206–218.
Soulé, ME (1985). What is conservation biology? BioScience 35, 727–734.
| What is conservation biology?Crossref | GoogleScholarGoogle Scholar |
Stamatakis, A (2014). RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 30, 1312–1313.
| RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies.Crossref | GoogleScholarGoogle Scholar |
Stattersfield AJ, Crosby MJ, Long AJ, Wege DC (1998) ‘Endemic bird areas of the World.’ (BirdLife International: Cambridge)
Steadman, DW (1995). Prehistoric extinctions of Pacific island birds: biodiversity meets zooarchaeology. Science 267, 1123–1131.
| Prehistoric extinctions of Pacific island birds: biodiversity meets zooarchaeology.Crossref | GoogleScholarGoogle Scholar |
Tamura, K, Stecher, G, and Kumar, S (2021). MEGA11: molecular evolutionary genetics analysis version 11. Molecular Biology and Evolution 38, 3022–3027.
| MEGA11: molecular evolutionary genetics analysis version 11.Crossref | GoogleScholarGoogle Scholar |
Tan, HH (2016). Commensal herpetofauna at Tufi Resort, Oro Province, Papua New Guinea. Southeast Asia Vertebrate Records 2016, 127–128.
Tingley, R, Meiri, S, and Chapple, DG (2016). Addressing knowledge gaps in reptile conservation. Biological Conservation 204, 1–5.
| Addressing knowledge gaps in reptile conservation.Crossref | GoogleScholarGoogle Scholar |
Toussaint, EFA, Hall, R, Monaghan, MT, Sagata, K, Ibalim, S, Shaverdo, HV, Vogler, AP, Pons, J, and Balke, M (2014). The towering orogeny of New Guinea as a trigger for arthropod megadiversity. Nature Communications 5, .
| The towering orogeny of New Guinea as a trigger for arthropod megadiversity.Crossref | GoogleScholarGoogle Scholar |
Trifinopoulos, J, Nguyen, L-T, von Haeseler, A, and Minh, BQ (2016). W-IQ-TREE: a fast online phylogenetic tool for maximum likelihood analysis. Nucleic Acids Research 44, W232–W235.
| W-IQ-TREE: a fast online phylogenetic tool for maximum likelihood analysis.Crossref | GoogleScholarGoogle Scholar |
Uetz P, Freed P, Aguilar R, Hošek J (2021) The reptile database. Available at http://www.reptile-database.org
Vogt, T (1932). Beitrag zur Reptilienfauna der ehemaligen Kolonie Deutsch-Neuguinea. Sitzungsberichte der Gesellschaft naturforschender Freunde zu Berlin 1932, 281–294.
Wairiu, M (2007). History of the forestry industry in Solomon Islands. The Journal of Pacific History 42, 233–246.
| History of the forestry industry in Solomon Islands.Crossref | GoogleScholarGoogle Scholar |
Wallace, AR (1860). On the zoological geography of the Malay archipelago. Journal of the Proceedings of the Linnean Society 4, 172–184.
| On the zoological geography of the Malay archipelago.Crossref | GoogleScholarGoogle Scholar |
Williams, EE, and Peterson, JA (1982). Convergent and alternative designs in the digital adhesive pads of scincid lizards. Science 215, 1509–1511.
| Convergent and alternative designs in the digital adhesive pads of scincid lizards.Crossref | GoogleScholarGoogle Scholar |
Winter, DJ (2017). rentrez: an R package for the NCBI eUtils API. The R Journal 9, 520–526.
| rentrez: an R package for the NCBI eUtils API.Crossref | GoogleScholarGoogle Scholar |
Zippel, KC, Glor, RE, and Bertram, JEA (1999). On caudal prehensility and phylogenetic constraint in lizards: the influence of ancestral anatomy on function in Corucia and Furcifer. Journal of Morphology 239, 143–155.
| On caudal prehensility and phylogenetic constraint in lizards: the influence of ancestral anatomy on function in Corucia and Furcifer.Crossref | GoogleScholarGoogle Scholar |
Zug, GR (2004). Systematics of the Carlia ‘fusca’ lizards (Squamata: Scincidae) of New Guinea and nearby islands. Bishop Museum Bulletin in Zoology 5, 1–83.
Zweifel, RG (1966). A new lizard of the genus Tribolonotus (Scincidae) from New Britain. American Museum Novitates 2264, 1–12.