Industry environmental offset funding facilitates a large multi-species fauna translocation program
Judy Dunlop A B C D F , Andrew Smith D E , Allan H. Burbidge D , Neil Thomas D , Neil A. Hamilton D and Keith Morris D
A B C D F , Andrew Smith D E , Allan H. Burbidge D , Neil Thomas D , Neil A. Hamilton D and Keith Morris D
A Western Australian Feral Cat Working Group, 58 Sutton St, Mandurah, WA 6210, Australia.
B School of Biological Sciences, University of Western Australia, Crawley, WA 6009, Australia.
C Institute for Land, Water and Society, School of Environmental Science, Charles Sturt University, Albury, NSW 2640, Australia.
D Biodiversity and Conservation Science, Department of Biodiversity, Conservation and Attractions, Locked Bag 104, Bentley Delivery Centre, WA 6983, Australia.
E Chevron Australia Pty Ltd, 250 St Georges Terrace, Perth, WA 6000, Australia.
F Corresponding author. Email: judy.dunlop@wafcwg.org.au
Pacific Conservation Biology 28(3) 231-246 https://doi.org/10.1071/PC20036
Submitted: 20 April 2020 Accepted: 21 June 2021 Published: 16 July 2021
Journal Compilation © CSIRO 2022 Open Access CC BY
Abstract
Worldwide deterioration in natural communities has led to an increased use of fauna translocations to improve conservation status and restore ecological function. However, few translocation programs have sufficient resources to involve multiple species and destination locations with appropriate threat management and monitoring before and after release. As part of conservation actions to mitigate impacts of the Chevron Australia Gorgon liquefied natural gas project on Barrow Island Nature Reserve, biodiversity offset funding was provided to benefit species impacted by the development. Animals were translocated from three islands to two mainland locations in Western Australia. We aimed to: (1) improve conservation status and security of several threatened species; and (2) contribute to reconstruction of pre-European fauna assemblages. Nine hundred and seventy five individuals of six mammal and two bird species were translocated. These included 421 golden bandicoots (Isoodon auratus barrowensis), 111 spectacled hare-wallabies (Lagorchestes conspicillatus conspicillatus), 105 Barrow Island boodies (Bettongia lesueur ssp. Barrow Island), 104 brushtail possums (Trichosurus vulpecula hypoleucus), 62 mala (Lagorchestes hirsutus ssp. Tanami), 88 djoongari (Pseudomys fieldi), 37 black and white fairy-wrens (Malurus leucopterus edouardi) and 47 spinifexbirds (Eremiornis carteri). Of 11 new populations, only two failed to establish; attributed to native and feral predators. Additional populations of four species of threatened mammal (one of which has now been reduced in conservation listing) and one species of threatened bird were established. To our knowledge, this is the largest translocation effort ever undertaken in Australia and is a rare example of an offset that has provided tangible threatened species benefit.
Keywords: Australia, conservation biology, fauna reconstruction, marsupials, reintroduction.
Introduction
Ecosystems worldwide are experiencing unprecedented levels of biodiversity decline and extinction due to human impacts (Dirzo et al. 2014; Otto 2018). Islands have suffered disproportionate levels of loss, often because of introduction of invasive species including rodents (Rattus spp.), red foxes (Vulpes vulpes) and feral cats (Felis catus) (Jones et al. 2008; Medina et al. 2011; Pimm et al. 2014; Doherty et al. 2016). Of these global extinctions, Australia has the world’s worst record with at least 53 vertebrate species becoming extinct since European settlement in 1788, including three in the past 10 years (Waller et al. 2017; Woinarski et al. 2017). Australia has the dubious honour as the site of approximately 30% of the world’s modern mammal extinctions (Burbidge and Manly 2002; Woinarski et al. 2015b), and seven Australia mammal species are forecast to be extinct by 2038 unless conservation management improves (Geyle et al. 2018). In addition to extinctions, many other species have suffered significant range contractions and local extinctions due to population decline (Woinarski et al. 2015a, 2015b; Moore et al. 2019) in Western Australia alone, 15 mammal species have become locally extinct from 10 islands (Burbidge and Abbott 2017). Such losses have negative flow-on impacts to ecosystem health and function (Fleming et al. 2014).
Despite these losses, the opportunity exists to reconstruct the fauna assemblages of suitably managed areas, thereby restoring ecological processes and the conservation status of their extirpated occupants (Burbidge 1999; Ostendorf et al. 2016). Translocation of animals from stable populations to previous parts of their range to boost, reinstate or insure the species is an increasingly common strategy being employed by conservationists (Armstrong and Seddon 2007). Indeed, reintroduction is a management option recommended for many threatened Australian vertebrates to combat severe range contractions, population declines and local extinctions (Pedler et al. 2018; Ringma et al. 2018). However, many reintroduction programs fail due to the difficulty of removing the threatening processes that initially caused the local extinction (Moseby et al. 2011; Clayton et al. 2014; Andrew et al. 2018), poor habitat quality or suitability at release sites (Bennett et al. 2013; Short et al. 2019), or other environmental factors such as stress of translocation and establishing in a new location (Cabezas et al. 2007; White and Pyke 2008). Islands with predator free enclosures or areas where threatening process have been managed are therefore appealing choices for successful translocation.
Critical to successfully undertaking such reconstruction programs is access to adequate resources, funding and personnel to manage the threats and habitat quality, undertake the translocation and determine its success afterwards (Sheean et al. 2011; Pérez et al. 2012). In this case, financial resources for the translocation program were provided by biodiversity offsets for the clearing of 332 ha for the development of a liquid natural gas (LNG) processing facility on Barrow Island A-Class Reserve (Government of Western Australia 2003; Lagdon and Moro 2013). Biodiversity offsetting is a conservation tool that aims to generate biodiversity benefits in one area to compensate for losses in another (Maron et al. 2016). Generally, offsetting is used to permit some habitat loss due to industry or development whilst restoring or revegetating another location in an attempt to achieve no overall net loss in habitat extent or condition (Gibbons and Lindenmayer 2007). However, concerns have been raised about the actual conservation benefits from enacting compensatory offsets (Lindenmayer et al. 2017; May et al. 2017). In particular, projects demonstrating a net positive impact are rare because of the uncertainty in defining the counterfactual scenario if the offset was not created and the development did not go ahead (Maron et al. 2018). Other issues with effective offsetting include lack of ‘like for like’ habitat, a flawed ecological basis to secure a net benefit, or allocating an already ‘safe’ parcel as an offset resulting in no additionality (May et al. 2017).
The significant conservation values of Barrow Island prompted an extensive environmental, social and economic review and impact assessment prior to the construction of the LNG facility. A terrestrial fauna translocation program was developed and implemented by Western Australia’s Department of Biodiversity, Conservation and Attractions (DBCA) to offset this habitat loss by establishing new populations of threatened and priority species of fauna from Barrow Island to other suitable habitats. We also used the opportunity to undertake two additional species reintroductions from other Pilbara islands while the facility to translocate relevant animals was available. The goal of all these translocations was primarily to improve the conservation status of these species and facilitate fauna reconstruction at recipient sites and included follow-up monitoring and management of populations and habitats. The total value of this project was AUD11 million, indexed to 2007 dollars, over 14 years (Government of Western Australia 2003, 2009). It is an example of an unusually large fauna translocation involving multiple species and destination sites as well as the resources for monitoring of effectiveness and management actions.
This paper describes the criteria used for the selection of species to be translocated, and the translocation sites, details of the translocations and their outcomes using short-, medium- and longer-term success criteria. We discuss this translocation as an offset strategy and whether there was a net benefit to biodiversity.
Methods
Source of animals
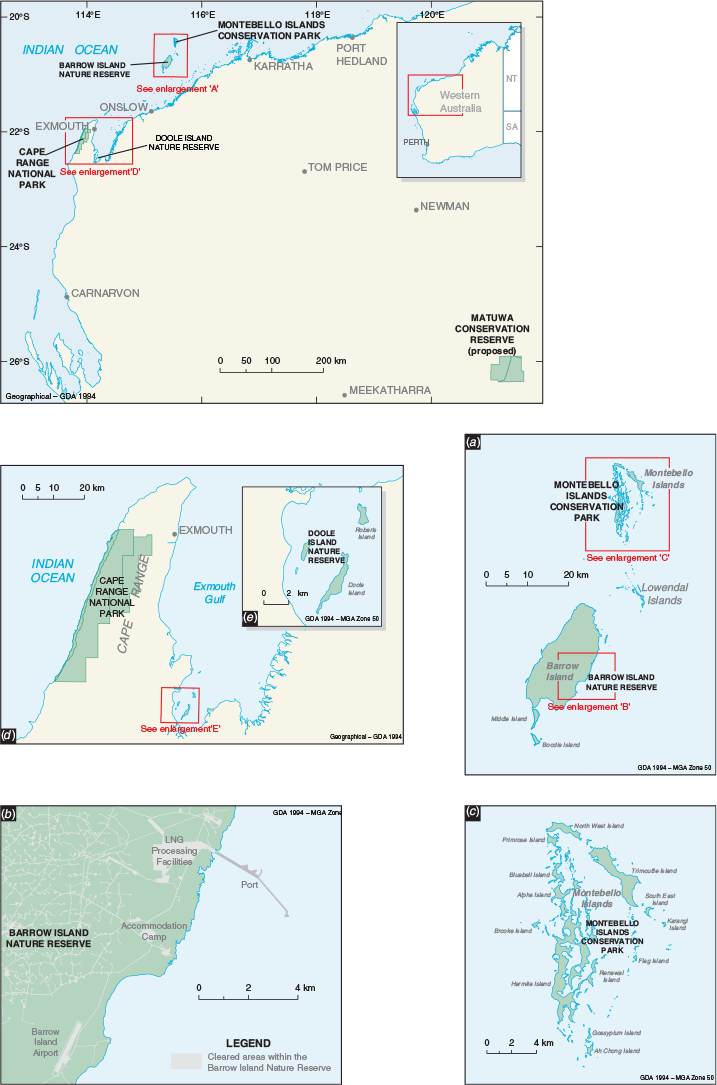
Animals were sourced from Barrow Island Nature Reserve and the Montebello Islands Conservation Park. Barrow Island (23 567 ha; –20°795′S, 115°390′E) is Western Australia’s second largest island, approximately 25 km long and 10 km wide, lying about 55 km north-west of the Pilbara coast (Fig. 1). The Montebello Islands (–20°437′S, 115°535′S) are a group of 218 islands, islets and rocks 20 km north of Barrow Island and 80 km from the Pilbara coast (Fig. 1) (Lohr et al. 2014). All these islands were separated from the mainland during sea level rise approximately 8000 years ago (Dortch and Morse 1984). The islands experience a maritime, semi-arid climate with highly variable cyclone-driven rainfall (average rainfall on Barrow Island of about 320 mm per year). The primary habitat types on the islands are mostly spinifex (Triodia spp.) hummock grasslands, Spinifex longifolius tussock-grassland, Acacia dominated shrublands and mangrove thickets. Barrow Island has been a Nature Reserve since 1910, and since 1964 has been used for oil extraction and shipping activities. In 2009 construction of the Gorgon gas processing plant commenced on the island and gas exports commenced in 2016.
Barrow Island is one of Australia’s most important A-Class Nature Reserves and supports a significant vertebrate assemblage of 15 mammal, 119 bird, 44 reptile, one frog and one subterranean fish species (Moro and Lagdon 2013), including 22 threatened taxa (Government of Western Australia 2018). Because of its geographic isolation, large size (~23 000 ha), and no introduced predators, the assemblage of flora and fauna species has remained essentially intact since European colonisation of Australia. Black rats (Rattus rattus) and house mice (Mus musculus) have been inadvertently introduced to the island several times since the 1960s but were successfully eradicated by baiting and trapping (Morris 2002). Several taxa present on the island are virtually extinct on the nearby mainland, such as the golden bandicoot (Isoodon auratus), and spectacled hare-wallaby (Lagorchestes conspicillatus), and others are recognised as unique from the mainland form, such as Barrow Island euro (Osphranter robustus isabellinus), Barrow Island boodie (Bettongia lesueur ssp. Barrow and Boodie Island), and Barrow Island black and white fairy-wren (Malurus leucopterus edouardi) (Government of Western Australia 2018). Barrow Island therefore represents a critically important natural ark for a number of Australia’s endemic species.
The nearby source populations of mala (Lagorchestes hirutus spp. Tanami) on Trimouille Island and djoongari (Pseudomys fieldi) on North-West Island in the Montebello group were both conservation introductions. Thirty mala were released on Trimouille Island from the Tanami Desert in 1998 when the last remaining wild population was being predated by feral cats (Langford and Burbidge 2001). Djoongari were introduced to North West Island from Bernier Island (via breeding facilities at Perth Zoo) in 1999. Both conservation introductions were successful with abundant populations of both species within three years of release.
Selection of species to be translocated
The following criteria were used to select species of highest importance for translocation (Morris and Yates 2016):
Species that were of conservation significance, endemic or threatened, and conservation status would be improved by establishing other populations.
Species that had been lost from areas of former habitat, and where repatriation would be of benefit to the ecosystem.
Sufficient knowledge was available on the distribution and abundance of the source population, and that it was sufficiently large to tolerate harvest of animals for translocation.
Species that were likely to be impacted by the clearing activities at the LNG site on Barrow Island.
Species for which there was good knowledge about translocation and monitoring techniques, and that had a good chance of translocation success.
There were suitable translocation sites with adequate areas of quality habitat for the selected species with threatening processes mitigated.
The fauna species selected for translocation were Barrow Island golden bandicoot (I. a. barrowensis), Barrow Island spectacled hare-wallaby (Lagorchestes conspicillatus conspicillatus), Barrow Island boodie (Bettongia lesueur ssp. Barrow and Boodie Islands), brushtail possum (Trichosurus vulpecula hypoleucus), rakali (Hydromys chrysogaster), Barrow Island black and white fairy-wren (Malurus leucopterus edouardi), and spinifexbird (Eremiornis carteri) (Table 1). These species largely met all the above criteria for selection. Other threatened taxa were considered but not selected were the endemic Barrow Island euro (Osphranter robustus isabellinus) and Barrow Island leopard skink (Ctenotus pantherinus acripes), both of which had never been translocated before, or were known from many other locations. Two additional species were also translocated; although not at risk from the Barrow Island development; populations of mala (Lagorchestes hirsutus ssp. Tanami Desert) and djoongari (Pseudomys fieldi) were present on islands nearby to Barrow and were value-added to the translocation as part of a fauna reconstruction project being undertaken at Matuwa Indigenous Protected Area (Matuwa) (Algar et al. 2013), while the facilities to translocate were present.
|
The majority of these translocations were reintroductions; that is, there was good evidence of recent occurrence of those species previously occurring at the destination sites (Table 1). On surveys of the Montebello Islands in 1914, anthropologist and naturalist P. D. Montague noted the presence of spectacled hare-wallabies and ‘there was formerly a Bandicoot, Isoodon barrowensis, Thom., which until very recently inhabited Hermite Island, but has now been exterminated. Of introduced species, cats and black rats (M. rattus rattus) are numerous, and… doing great damage to the endemic fauna.’ (Montague 1914). Evidence for other species included prior distribution, skeletal remains from caves or currently unoccupied boodie warrens (Abbott and Burbidge 1995; Abbott 2006; Burbidge and Abbott 2017). The species were all locally extinct at the destination sites at the time of release (Appendix 1). As per IUCN translocation guidelines, every effort was made to ensure the likelihood of establishment of new populations and ensuring adequate genetic diversity by releasing as many individuals as possible, whilst not endangering the source population by overharvesting (IUCN SSC 2013). Table 1 details the estimated source populations and the number translocated; in most cases fewer than 5% of the source population were harvested as founders. For this reason, translocations of rakali were not undertaken in 2010–2011 due to a lack of information on the distribution and abundance of the Barrow Island rakali that would be the translocation ‘source’ population.
Selection of destination sites
Release sites for translocated fauna were selected using the following criteria (Morris and Yates 2016):
There was evidence of the former occurrence of the species to be translocated at the site, or it was within its former range.
The threatening process(es) responsible for the decline or extinction of the target species was/were adequately managed or eliminated.
The site contained adequate area(s) of habitat to support a viable translocated population.
There was secure tenure and adequate management presence at the translocation site.
It was logistically feasible to access the translocation site for monitoring and ongoing management.
Using the site selection criteria above, three island and two mainland sites were selected for translocation releases: Hermite and Alpha Islands in the Montebello Island Conservation Park, Doole Island Nature Reserve in Exmouth Gulf, Cape Range National Park, and a predator proof enclosure at Matuwa in the rangelands (Fig. 1). All these sites, except Matuwa, are conservation reserves managed by the WA State Government. Matuwa is managed by the Wiluna Martu traditional owners, Tarlka Matuwa Piarku Aboriginal Corporation and the WA State Government. Further details for each site can be found in Appendix 1.
Description of offset
This project was funded largely by an environmental offset provided by Chevron Australia as part of a ministerial agreement with the WA Government and approval to clear 332 ha of natural vegetation for the construction of the Gorgon LNG gas processing plant on the Barrow Island Nature Reserve. Additional funding as in-kind support for the translocation program was provided by Chevron Australia and the then Department of Environment and Conservation (DEC), who undertook the translocations. The Gorgon offset funding commenced in November 2009 and will continue to 2023. This offset did not follow traditional format as it has Parliamentary Acts associated with it, and required funds to be delivered to DEC, with DEC required to undertake the work and manage the funds in a way seen fit for the species. There were no specific completion criteria outlined in the Acts, so under May et al. (2017), it would fall into the category of ‘research, rehabilitation and restoration’. DEC set goals of securing threatened and priority species populations and enhancing fauna restoration programs. Under the Gorgon Gas Processing and Infrastructure Project Agreement (Government of Western Australia 2003) and Variation Agreement (Government of Western Australia 2009), the initial funding agreement was for a total of AUD $10 million to be provided over 12 years; $1.3 million per year for 5 years, then $0.5 million per year for 7 years. An additional $0.5 million per year for 2 years was provided to the fauna translocation program in 2012 as an offset for the clearing of an additional 32 ha of land to support construction activities. The offset funding was indexed to 2007-dollar values and increased by Consumer Price Index for successive years.
Methods of capture and release
Mammals were captured using a variety of traps, including Elliott (Type A, Elliott Scientific Company, Upwey, Vic., Australia), medium cage traps (Sheffield Wire Products, Welshpool, WA, Australia), soft-walled Thomas traps (Sheffield Wire Products) or hand-netting. Traps were baited with peanut butter, oats and sardines, and were cleared twice per night to maximise captures of targeted species. On Barrow Island, we primarily targeted the LNG development envelope but to achieve adequate founder numbers, some mammals were trapped from a 2 km radius around the development site. Trap effort to collect founders totalled over 4200 trap nights through 2010–2011, the majority (85%) in January and February 2010. All captured mammals were assessed at the site for retention or release.
Mist-netting to collect black and white fairy-wrens and spinifexbirds for translocation was undertaken for a total of 26 days in May 2010 and June 2011 at 39 sites located adjacent to the LNG site, and near the west and south coasts of Barrow Island. Nets of a mesh size appropriate for small passerines (maximum stretched diagonal mesh size of 25 mm) were deployed, with netting attempts restricted to early morning and late afternoon. Call broadcasting was often used to attract individuals from the immediate location. Mist-netting operations were cancelled during hot or windy weather. One to three operators accredited by the Australian Bird and Bat Banding Scheme (ABBBS) and experienced in catching and extracting small passerines from mist-nets were present on the team during the entire field program. Fairy-wrens and spinifexbirds to be translocated were placed in calico bags (one bird per bag), given Spark Liquid electrolyte supplement (Vetafarm, Wagga Wagga, NSW, Australia) and then placed in specially designed shower-proof cardboard transport boxes for transport.
Non-target species and animals not suitable for translocation were released immediately. Reasons for being deemed unsuitable included females that were lactating or had large pouch young, animals that appeared in poor condition or were non-independent juveniles. Biological samples (tissue, swabs, blood or scats) were taken from a sub-set of each species per destination to provide a pre-translocation health and genetic reference. All mammals were implanted with a Passive Integrated Transponder (PIT) Allflex 12 mm FD-X transponder (Allflex, Australia) providing each with an individual identity number, and a full set of biometric data including measurements of weight, head and pes length, age, gender, reproductive status, presence of ectoparasites and intended final destination were recorded for each. Birds were leg-banded with uniquely numbered metal bands provided by the ABBBS, followed by weighing and taking morphometric measurements. Macropods are susceptible to capture myopathy if unduly stressed (Jackson 2007; Vogelnest and Portas 2008), so veterinary administration of sedatives (Azaperone) and Vitamin E/selenium was used to reduce anxiety of spectacled hare-wallabies and mala during the translocation process.
All founder mammals were released at their destination site within 24 h of their initial capture. Some birds were held up to 48 h before release and provided with food (limited to termites) and water while held captive. All animals were held in a darkened and quiet air-conditioned room (21–26°C) for holding prior to early morning transport. Mammals were transported individually in black cotton drill bags, inside airline-approved pet carriers (K9 Pet Carrier 53 × 37 × 37 cm PP20). A quarantine-compliant helicopter was used to transport animals between islands (44 km) or to Karratha airport (143 km). Animals destined for Matuwa (781 km) or Exmouth (325 km) were transported from Karratha via a Beechcraft Bonanza fixed-wing aircraft. At the destination sites, birds were released on the morning of arrival, and mammals were held in a quiet, cool and darkened location and released at dusk (Parker et al. 2012).
Translocation monitoring
The translocated mammal populations were initially monitored via very high frequency radiotracking to provide preliminary survivorship and movement information. A combination of monitoring methods was used to monitor the populations in the following 8 years, and included radiotracking, trapping, resighting during transect searches, and camera trapping. Newly recruited individual mammals captured during trapping surveys were implanted with unique PITs. These were subsequently used in population estimates, calculated using RMARK openCR population models (Dunlop 2015), or Known To Be Alive (Department of Parks and Wildlife 2013). Area of occupancy was calculated from camera trap or sighting occupancy and analysed using program Presence (MacKenzie et al. 2017). Translocated birds on Hermite Island were monitored by aural and visual detection, during walked area or transect searches, using call-broadcast procedures to lure birds at sites where none were detected by passive survey. Population estimates were based on distance analysis calculations from leg band sightings. Frequency of monitoring decreased with time after release. Populations were approximated to our best knowledge at time of writing, and publications for each species or site will follow with more specific detail.
Definitions of success
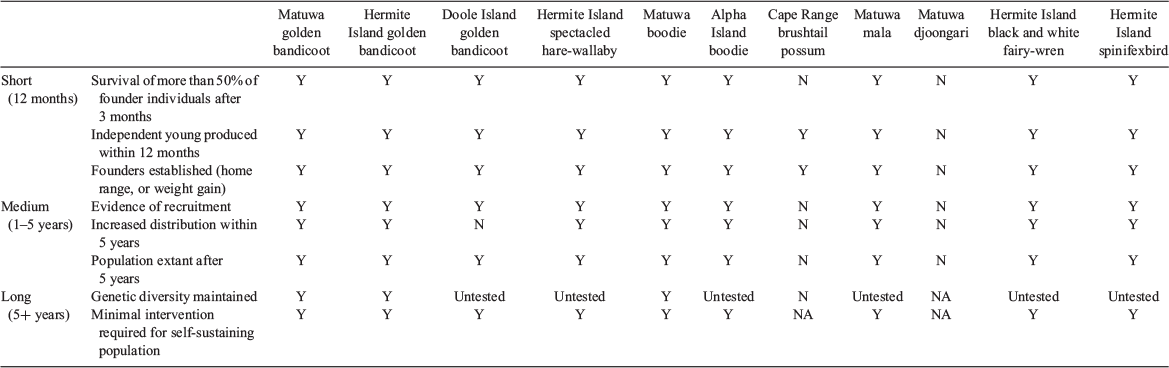
Assessment of success or failure of each translocation was based on guidelines within IUCN SSC (2013) (Table 2), and further divided to short term (release to 12 months), medium term (1–5 years) and longer term (5+ years) in order to better identify success or failure. These time frames related to generation time; short-term criteria relate to the survival and establishment of founders, medium-term criteria relate to ongoing persistence, evidence of successful recruitment (evidence of breeding, and recapture/resight of newly marked animals surviving to adulthood), and expansion beyond the release sites to occupation of suitable habitat (based on camera trap arrays, or evidence of occupation of a new area) (IUCN SSC 2013). Longer-term criteria relate to the self-sustaining survival of the population without intervention (population increase from founder number), including gene pool health (IUCN SSC 2013). Assessing at which point an establishing population failed, and causes of failure, are critical to learning from the experience and improving translocation outcomes (Moseby et al. 2011; Lloyd et al. 2019). We also assessed the success of the offset, according to the framework set out in May et al. (2017). We assessed four aspects of the translocations that we felt were relevant here for each species; net individual loss/gain, new population locations created, whether the reintroductions were culturally significant to Traditional Owners, and whether the species was part of a faunal reconstruction providing ecosystem services to the landscape.
|
Results
Summary of translocations
In total, 975 individuals of nine species were translocated to five different destination sites as part of this program (Table 1). This included 11 separate repatriated populations as there were several populations of some species. Two of these were considered conservation introductions under IUCN SSC (2013) guidelines, as there was not good evidence to suggest that boodies previously inhabited Alpha Island in the Montebellos group, or golden bandicoots occurred on Doole Island (Baynes and Jones 1993; Burbidge et al. 2000). The release of fairy-wrens is considered a reintroduction, as they existed on Hermite Island in 1912, and were detected on Trimouille Island (also within Montebellos group) in 1950 (Burbidge et al. 2000). The remaining eight translocations, as well as the rakali translocation planned for 2022, are reintroductions of species to areas they previously inhabited but have become extirpated. A total of 825 individuals of six species were sourced from Barrow Island, in particular targeting those that would be directly impacted from the clearing of the LNG site. The remaining 150 individual mammals came from the Montebello Islands (Trimouille and North West Islands). As a result of these translocations, five threatened species have established new populations.
Successes and failures
Of the 11 translocated populations, nine have persisted and are currently self-sustaining (populations have remained the same or increased following release) with minimal intervention in the long-term (Tables 1, 3). The population of djoongari released within the fenced enclosure at Matuwa did not establish, nor did the population of brushtail possums released at Cape Range National Park. Trapping revealed that the djoongari at Matuwa survived for up to 1 year, but long-term survival was unlikely, with evidence of predation by mulgara (Dasycercus blythi) and other native predators (barn owls, varanids, and snakes) resident within the enclosure (Lohr 2018). Seventy-four percent (20/27) of the radio-collared possums died within 6 months of release at Cape Range National Park and all radio-collared animals had died by 14 months post release. Despite 1080 fox baiting as part of the regional Western Shield program, fox predation was confirmed via DNA swabs for at least 85% of the collared animal deaths. While possums persisted within the gorge systems for up to 4 years post-release, and there was evidence of breeding, there was no evidence of recruitment and possums have not been recorded at Cape Range National Park since 2014.

|
Black and white fairy-wren and spinifexbird
By September 2012, 15 months after the last birds were translocated, a minimum of 60 fairy-wrens and an estimated 100 spinifexbirds were recorded on Hermite Island, an approximate doubling of the founder populations of 37 and 47, respectively. Both species had persisted at their release sites and dispersed well beyond. At least 80% of the fairy-wrens and 96% of the spinifexbirds were unbanded individuals; since all translocated birds had been fitted with metal leg bands, this indicates that successful breeding and recruitment had occurred. By 2015, both species were widespread on Hermite Island and the spinifexbird had dispersed to another six islands adjacent to Hermite Island (Table 3). At that time, the population estimate for fairy-wrens on Hermite Island was ~70 birds, and there were more than 300 spinifexbirds. Both translocations are considered to be successful in the short and medium-term, and self-sustaining in the long term, but genetic analysis is required to assess diversity (Table 2). Further detail on the bird translocations will be presented in Burbidge A, Blythman M, Danks A, Hamilton N (unpubl. data).
Golden bandicoot
All three golden bandicoot translocations were successful in the short, medium and likely long-term (Table 2). Genetic assessment of the translocated populations compared with the source population indicated that the founder population was suitable to retain genetic diversity, but that occasional supplementation is required in a 10-year timeframe (Ottewell et al. 2014). Current population estimates are in the range of 580–2800 additional animals established at three locations, with a combined occupation area of more than 2367 ha (Table 3).
All three founder populations of golden bandicoots showed a dramatic increase in mass and skeletal body size following release (Dunlop and Morris 2018). Female bandicoots on Hermite Island and at Matuwa exhibited higher reproductive output in the number, frequency and average mass of pouch young when compared with the founder population on Barrow Island (Dunlop and Morris 2018). More than 70% of female bandicoots at Matuwa were in a reproductive state during trapping efforts for the first 5 years, but this rate has declined as the population stabilised (Dunlop and Morris 2018; Lohr 2018). By May 2012, it was estimated there were 132–200 golden bandicoots inside the Matuwa enclosure, and current estimates are 130–680 (Dunlop 2015; Lohr 2018). In 2015, 92 bandicoots were released outside the enclosure at Matuwa, and small juvenile animals move through the fence netting and establish outside the enclosure. Currently, the population outside the enclosure is persisting, albeit at low densities. By September 2012, 31 months after release, it was estimated that there were at least 254 bandicoots on Hermite Island (Dunlop 2015). Bandicoots from Hermite Island also spread to nearby Buttercup Island (5.5 ha), which connects to Hermite Island at low tides (Department of Parks and Wildlife 2013).
At Doole Island, new animals were detected in the population within 10 months of release, and there was a 25% increase in average bodyweight of animals trapped. The estimated population size has remained between 80 and 100 individuals since the translocation in 2011; however, up to 80% of these animals are new to the population suggesting a relatively high turnover of individuals on the island. Up to 10% of the females are breeding during the monitoring periods.
Spectacled hare-wallaby
The spectacled hare-wallaby translocation to Hermite Island was successful in the short and medium term and is considered self-sustaining in the long term (Table 2). Eighty seven percent of the of the initial radio-collared founders survived the first 4 months, and by September 2012, hare-wallabies had occupied most of Hermite Island and in 2015 they were estimated to occupy 94% of available habitat. In 2016, the population density was estimated at 0.6–0.7 hare-wallabies per hectare of occupied area, equating to approximately 500–600 hare-wallabies on Hermite Island. Hare-wallabies increased bodyweight by 15–25% within 12 months of release on Hermite Island. Trapping up to 2016 indicated between 75% and 100% of females trapped were either carrying a pouch young or were lactating. Current population estimates are between 400 and 600 individuals. Genetic variability of this population in the long term is currently unknown, but tissue samples are retained by DBCA.
Boodie
Both boodie translocations were considered successful in the short and medium term, with population estimates of approximately 250–700 individuals at Matuwa and 200–500 individuals on Alpha Island from an initial founder population of 65 and 40, respectively. The total area occupied by these two translocated populations is 1203 ha. The Matuwa boodie population was genetically supplemented with Dorre Island sourced animals that had been in the Dryandra captive breeding facility, increasing their genetic variation with no apparent negative impacts (Rick et al. 2019; Thavornkanlapachai et al. 2019). Short-term survival of released boodies was determined by mark–recapture methods and radiotracking of 15 individuals to be 82.5% (Dunlop 2015). By May 2012, 90% of trapped females carried pouch young or were lactating (Dunlop 2015). The boodie population at Matuwa is highly seasonal, with estimates varying between 250 and 700 individuals depending on rainfall (Lohr 2018). Boodies were active across all Alpha Island by April 2012, and 70% of the females were carrying pouch young. In April 2013, at least 67.5% of the founders were still alive, and new recruits to the population were recorded. Boodies are now occupying all available warren habitat on Alpha Island.
Mala
Radiotelemetry provided some information on early survival and movements. Targeted trapping and spotlight monitoring of radio-tagged animals have indicated long-term survival of individuals in the enclosure, with good body condition and breeding activity (Sims et al. 2017). Mala are occasionally still trapped and captured on camera trap, and there is evidence of many tracks and scats throughout the spinifex areas of the enclosure. Attempts at population estimates within the enclosure via trapping and spotlight transects were unsuccessful due to low number of sightings (Sims et al. 2017; Lohr 2018).
Discussion
Translocation success
To our knowledge, this is Australia’s largest multi-species fauna translocation project, involving 975 individuals of eight species released at five different locations. Of the 11 translocated populations, nine were a success against our longer term (5+ year) criteria. These translocations have also progressed the fauna reconstruction of the Montebello Islands, and the Matuwa Rangelands Restoration project. This program was built on the experience of over 230 fauna translocations for conservation in WA over the past 40 years (Morris et al. 2015).
Critical reviews of other translocations (Sheean et al. 2011; Pérez et al. 2012) have highlighted the failure of translocations and introductions due to lack of adequate resourcing and long-term commitment of the proponents to undertake suitable and adequate monitoring. The requirement by Chevron Australia to offset the impact of the Gorgon LNG development site on the Barrow Island biodiversity values has allowed a translocation program to be undertaken that has resulted in the establishment of five populations of three threatened species and contributed to the down-listing of a fourth threatened species (boodie). The funding over a 12-year period has ensured that adequate monitoring and continued management of all the translocated populations occurred, enabling a quantitative assessment of the success/failure of each population.
Offset success
For an offset to be a success, there should be measurable and enduring benefits that counterbalance the impacts of the development. A frequent issue with determining the effectiveness of offsets is the uncertainty around the counterfactual scenario (i.e. what would have happened to the species if there was no development and no offset). In this case, although the translocations were listed as high priority within species recovery plans or habitat reconstruction projects, it is unlikely that they would have ever occurred without significant coordinated funding. Therefore, the counterfactual scenario for these species is that those populations remained locally extinct and the 332 ha on Barrow Island would have remained intact. We used the three-criteria framework produced by May et al. (2017) to evaluate the success of this offset.
1. The offset was successful in producing a desired or intended results
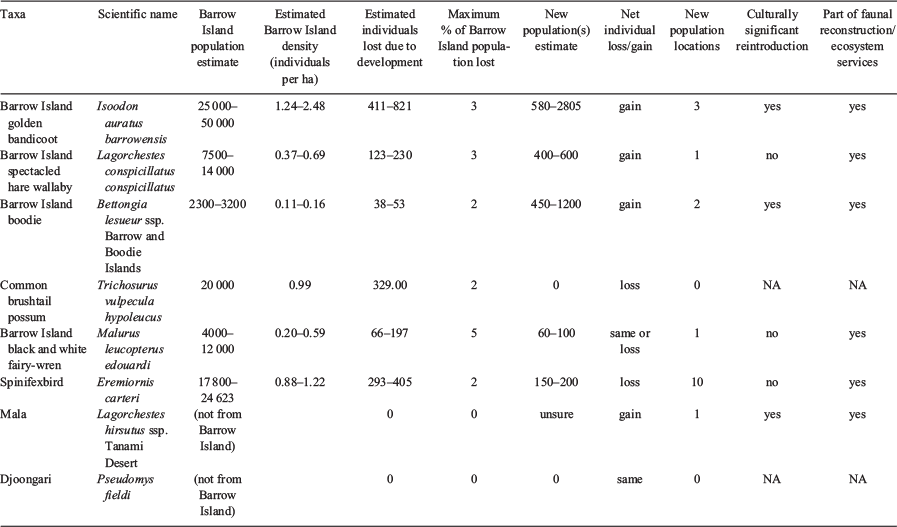
The goal of the offset was to improve the conservation status of species impacted by the gas works and to contribute to faunal reconstructions elsewhere in the state. For an improved conservation status, the combined source and translocated populations must have a lower risk of extinction than the counterfactual scenario – a scenario where no gas development took place. For the purposes of gauging offset success, extinction risk can be measured as combination of the number of target species populations and total population size. Six different species gained one or more secure new populations, and at least five of the eight species translocated have the same or greater total population size than was predicted to occur prior to the development, within the first 10 years.
2. The offset brought measurable, successful, long-term benefits
The creation of new populations of threatened species that are severely limited in range provides a significant insurance against extinction by catastrophic impacts (Waller et al. 2017). At the time of this program, the golden bandicoot, spectacled hare-wallaby, boodie and black and white fairy-wren were regarded as Barrow Island endemic subspecies listed as threatened (Vulnerable) species (Table 1). Islands can function as both a refuge for threatened species as well as a death trap in catastrophic circumstances such as wildfire, disease, predator invasion or climate change, and establishment of additional populations of unique island taxa reduces their risk of extinction via catastrophe (Woinarski et al. 2011; Waller et al. 2017). For example, threatening processes on Christmas Island have proved overwhelming for several species recently, resulting in the extinction of the Christmas Island pipistrelle (Pipistrellus murrayi) and Christmas Island forest skink (Emoia nativitatis) and the extinction in the wild of the blue-tailed skink (Cryptoblepharus egeriae) and Lister’s gecko (Lepidodactylus listeri) (Woinarski et al. 2017; Andrew et al. 2018). Establishing additional populations of unique Barrow Island taxa, and extremely limited taxa such as Tanami Desert mala reduces this risk, as well as enhances their overall conservation status.
There is also a significant ecosystem benefit to returning species to areas they previously inhabited, especially when considering species that provide ecosystem services (James and Eldridge 2007). The Montebello Islands presumably suffered considerable loss in ecosystem function in the local extinction of five species of medium sized mammals and small passerine birds exterminated by feral cats, black rats, and nuclear weapons testing (Burbidge et al. 2000; Burbidge 2004). Analysis of vegetation cover on the islands before, during, and after feral cat and black rat eradications revealed the negative effect black rats had on vegetation density despite occurrence of good growing conditions, whereas vegetation density continued to increase in response to rainfall after the release of native mammals (Lohr et al. 2014).
Returning iconic mammal species to Country has significant cultural importance to Indigenous custodians beyond pure population numbers. Species such as golden bandicoots, boodies and mala reintroduced to Matuwa are culturally significant for lore, food resources, and for the benefits they provide to Country. Traditional Owners were involved in the Matuwa translocations from the first release, and as owners of exclusive native title rights dictate the land management activities occurring at this site.
New populations of threatened species have the potential to enhance species genetic diversity. For example, the combination of boodies from two different genotypes has produced a hybrid variety considered to be more diverse and fitter than the substantially bottlenecked founder populations (Rick et al. 2019; Thavornkanlapachai et al. 2019).
3. The benefit of the offset counterbalanced significant residual impacts or risk of a project
In this case, we can demonstrate that several species made a net gain in habitat area and population that compensates for the 332 ha habitat loss on Barrow Island. While we do not have a population estimate due to their cryptic nature and difficulty of trapping in the presence of boodie and bandicoots, mala gained a new persistent population location without being impacted by the development, as they were not present on Barrow Island.
Benefits of these translocations extend beyond pure numbers of animals lost/gained, and that returning iconic species to Country is culturally important (Table 4). Species successfully translocated from Barrow Island gained additional population numbers (four of eight species; Table 4), distribution locations (six of eight species) and contributed to a reconstruction of the pre-European fauna. For example, golden bandicoots were successfully reintroduced to mainland and island habitats where they persisted, successfully bred and occupied the available habitat (Dunlop and Morris 2018). Estimates of new populations exceed predicted loss from Barrow Island (Table 4).
|
Biodiversity offsets are a popular tool due to their potential ability to meet the objectives of biodiversity conservation and economic development in tandem (Bull et al. 2013). Offsets may be used to counterbalance impacts on biodiversity values, ecosystem function or ecosystem services (May et al. 2017), and schemes are underway in nearly 40 countries (Maron et al. 2015). However, biodiversity offsets are rarely effective for their predetermined purpose, and lack rigorous standards for compliance, definitions of success or accurate tallies on the environmental losses that triggered them (Gibbons and Lindenmayer 2007; Maron et al. 2012). For example, an offset in NSW included the replacement of hollow bearing trees by nest boxes that were subsequently found to be poorly used by the intended species and therefore failed to be effective in counteracting the loss of hollow-bearing trees, a long term environmental impact (Lindenmayer et al. 2017). In Western Australia, a wealthy first-nation state with strict environmental regulation, at most 39% of offsets delivered an outcome and could be considered effective (May et al. 2017).
Since the translocations, the conservation status for boodies as a species has been revised downwards to be Conservation Dependent under the Western Australian Biodiversity Conservation Act 2016 but remains ‘Vulnerable’ under the EPBC Act 1999. This is in part due to the establishment of two additional populations within this project. While we can consider this offset to be an example of a net positive outcome for several threatened species and ecosystems, issues around defining nature as a tradable commodity, or eroding the value of reserves by allowing industry values to be prioritised still remain and must be treated with caution (McCauley 2006; Ives and Bekessy 2015).
The overall increased likelihood of population persistence for four of the eight Barrow Island species would suggest residual impacts have been counterbalanced. However, the predicted lifespan of the project is 40 years, and ongoing impacts are possible. Predicting long-term consequences for Barrow Island species viability is difficult based on short-term data, so ongoing monitoring at the source and translocation sites will be critical for detecting long-term population trends.
The key reasons for the successes of this offset in the first 10 years included:
recognition of the conservation values of Barrow Island, it was guided by legislated agreements and government statements that outlined the nature and extent of the translocation program.
it was a well-resourced program and had an ecologically sensible timeframe that allowed for adequate site management and monitoring.
it was planned and implemented by an independent body (WA State Government), with oversight on funding, and in the context of a legislated agreement and government statements (Government of Western Australia 2018).
the translocation sites had been subject to appropriate management such as eradication/control of introduced species well before this program commenced (Algar et al. 2002; Burbidge 2004; Jones et al. 2016).
the value and timeframe of the offset included follow-up management and monitoring.
it was a large resource, allowing for high quality translocations to be undertaken.
the source locations supported large numbers of the targeted species allowing large numbers of founders to be translocated.
the value of the offset was unusually high for the area of disturbance.
While the mammal transfers proceeded without incident, there were several unexpected deaths of birds between capture at source site and release at their destinations. In 2010, of the 31 fairy-wrens captured, 3 died and 1 was euthanised, and of the 38 spinifexbirds captured, three died prior to release. In 2011, of the 15 fairy-wrens captured, 5 died and of the 16 spinifexbirds, 4 died in the process of being translocated. Although appropriate husbandry protocols for malurids (Holland 2007) were followed, these guidelines seemed inadequate for the situation encountered on Barrow Island. Keeping very small wild-caught insectivores in a captive situation can be challenging, often with species-specific requirements (Shephard 1994; Holland 2007). The increased holding time on Barrow Island over two nights increased the risk of death for both species (Burbidge 2011), probably because of stress response through capture, transport and holding (Dickens et al. 2009). Age of the birds and stage of the breeding cycle may also have been relevant (birds might be more stressed at the beginning of a breeding season (Goutte et al. 2010)) but we did not have the opportunity to determine the stage of breeding season on Barrow Island where breeding is less predictable than on the mainland (Ambrose and Murphy 1994). For any future translocations of these species, we recommend birds be transported to the translocation site within 24 h of capture to minimise stress or disruption of social networks.
The failure of the brushtail possum translocation can be attributed to predation by the red fox. Prior to the translocation, the baiting regime was via aerially deployed dried meat baits. Although baiting was intended to be undertaken four times annually, several logistical issues meant that the baiting regime leading up to this translocation was interrupted. Genetic testing of swabbed brushtail possum collars indicated that at least six individual foxes were responsible for the possum deaths, indicating that the baiting had not been sufficiently effective. There may also have been issues of predator naivety for an island population not exposed to mammalian predators for ~8000 years (Whitwell et al. 2012; Jolly et al. 2018), and perhaps future attempts at reintroduction to this site should not involve animals sourced from predator naive populations. In 2015, an integrated fox and feral cat control program was commenced at Cape Range National Park. Using Eradicat baits has been successful at reducing fox activity to almost zero, and feral cat activity to 40–70% of pre-baiting activity.
Conclusion
This series of translocations have improved our knowledge of reintroduction biology through the planning and implementation of large-scale translocations to improve the conservation outlook for threatened species. Several publications have resulted including from studies in reintroduction genetics (Ottewell et al. 2014; Rick et al. 2019; Thavornkanlapachai et al. 2019), predator–prey interactions (Wysong et al. 2019), parasite transmission ecology (Dunlop and Watson, in press), cost–benefit analyses of exclosure fence building (Bode et al. 2012) the demography of translocated populations (Dunlop and Morris 2018) and their impact on the environment (Lohr et al. 2014; Chapman 2015a, 2015b). Other large-scale fauna translocations in WA, such as the reconstruction of the Dirk Hartog Island fauna (Morris et al. 2017), are building on the information resulting from this translocation program.
The program was successful in making major contributions to fauna reconstruction programs at the Montebello Islands, Doole Island and at the Matuwa Indigenous Protected Area. The conservation status of one species, the boodie, was improved with a change in IUCN listing from ‘Vulnerable’ to ‘Near Threatened’, largely because of these translocations. Three other species of threatened mammal and one bird have additional secure populations as a result of these efforts, and one species of mammal, rakali is scheduled to be reintroduced to the Montebello Islands in 2021–2023. To our knowledge, this is the largest translocation effort ever undertaken in Australia and is an example of a biodiversity offset that has provided a tangible benefit to threatened species and semiarid ecosystems.
Conflict of interest
The authors declare no conflicts of interest.
Acknowledgements
This project received funding as part of the additional environmental undertakings of the Barrow Island Act 2003 to ameliorate any potential impacts of the Gorgon Gas Development on the Barrow Island fauna. We thank Chevron Australia for additional in-kind support (airfares, accommodation) and their Barrow Island based staff, particularly Lee Mould and Todd Baldock who assisted with the program; their efforts in assisting in the trapping program, coordinating laboratory facilities, vehicles and field equipment was greatly appreciated. Bristow Helicopters (Barrow Island) assisted with logistics of animal movements at Barrow Island airport, Jayrow Helicopters (Karratha) transported animals from Barrow Island to the Montebello Islands and Karratha airport. We thank Peter McGinty for his efforts in flying animals from Karratha to Exmouth, and to Lorna Glen. Andy Edwards and the crew of Keshi Mer were invaluable for their assistance accessing islands. We are grateful to the assistance provided by Barrow Island based DEC staff Peter Kendrick, Paul Connelly, Misty Shipway, Brad Daw, Kim Onton and Wes Manson. Numerous other DEC staff assisted with initial planning, logistics and field work, including John Angus, Sophie Arnall, Ann Biasol, Mark Blythman, Wes Caton, Sarah Comer, Alan Danks, Ryan Ellis, Sean Garretson, Sandy Grose, Brent Johnson, Marion Massam, Marika Maxwell, Bill Muir, Jon Pridham, Kelly Rayner, Karen Rusten, Chris Schroder, Colleen Sims, Adrian Wayne and many volunteers who assisted with the translocation and monitoring. The City of Karratha provided a suitable animal holding facility at Karratha airport. Cheryl Lohr provided helpful advice for the manuscript. This project was approved by the DEC Animal Ethics Committee (approvals 2010/01, 2010/02, 2010/13, 2010/14 and 2013/32).
References
Abbott, I. (2006). Mammalian faunal collapse in Western Australia, 1875–1925: The hypothesised role of epizootic disease and a conceptual model of its origin, introduction, transmission, and spread. Australian Zoologist 33, 530–561.| Mammalian faunal collapse in Western Australia, 1875–1925: The hypothesised role of epizootic disease and a conceptual model of its origin, introduction, transmission, and spread.Crossref | GoogleScholarGoogle Scholar |
Abbott, I. (2008). Historical perspectives of the ecology of some conspicuous vertebrate species in south-west Western Australia. Conservation Science Western Australia 6, 1–214.
Abbott, I., and Burbidge, A. (1995). The occurrence of mammal species on the islands of Australia: a summary of existing knowledge. CALMScience 1, 259–324.
Abbott, I. (2012). Original distribution of Trichosurus vulpecula (Marsupialia: Phalangeridae) in Western Australia, with particular reference to occurrence outside the southwest. Journal of the Royal Society of Western Australia 95, 83–93.
Algar, D., Burbidge, A. A., and Angus, G. J. (2002). Cat eradication on Hermite Island, Montebello Islands, Western Australia. In ‘Turning the Tide: the Eradication of Invasive Species’, (Eds C. R. Veitch and M. N. Clout), pp. 465–473. (IUCN SSC Invasive Species Specialist Group: Gland, Switzerland and Cambridge, UK.)
Algar, D., Onus, M., and Hamilton, N. (2013). Feral cat control as part of rangelands restoration at Lorna Glen (Matuwa), Western Australia: the first seven years. Conservation Science Western Australia 8, 367–381.
Ambrose, S. J., and Murphy, D. P. (1994). Synchronous breeding of land birds on Barrow Island, Western Australia, after cyclonic summer rains. Emu-Austral Ornithology 94, 55–58.
| Synchronous breeding of land birds on Barrow Island, Western Australia, after cyclonic summer rains.Crossref | GoogleScholarGoogle Scholar |
Andrew, P., Cogger, H., Driscoll, D., Flakus, S., Harlow, P., Maple, D., Misso, M., Pink, C., Retallick, K., Rose, K., Tiernan, B., West, J., and Woinarski, J. C. Z. (2018). Somewhat saved: a captive breeding programme for two endemic Christmas Island lizard species, now extinct in the wild. Oryx 52, 171–174.
| Somewhat saved: a captive breeding programme for two endemic Christmas Island lizard species, now extinct in the wild.Crossref | GoogleScholarGoogle Scholar |
Armstrong, D. P., and Seddon, P. J. (2007). Directions in reintroduction biology. Trends in Ecology and Evolution 23, 20–25.
| Directions in reintroduction biology.Crossref | GoogleScholarGoogle Scholar | 18160175PubMed |
Baynes, A. (2006). Preliminary assessment of the original mammal fauna of Lorna Glen station. Unpublished Report to the Department of Environment and Conservation, Western Australia: Department of Environment and Conservation, Perth, Western Australia.
Baynes, A., and Jones, B. (1993). The mammals of Cape Range peninsula, north-western Australia. Records of the Western Australian Museum 45, 207–225.
Bennett, V. A., Doerr, V. A. J., Doerr, E. D., Manning, A. D., Lindenmayer, D. B., and Yoon, H. (2013). Causes of reintroduction failure of the brown treecreeper: Implications for ecosystem restoration. Austral Ecology 38, 700–712.
| Causes of reintroduction failure of the brown treecreeper: Implications for ecosystem restoration.Crossref | GoogleScholarGoogle Scholar |
Bettink, K. A. (2016). Shedding light on rakali: Genetic and morphological differentiation in the Australo-Papuan golden-bellied water rat (Hydromys chrysogaster), with notes on the Barrow Island population. PhD thesis, University of Western Australia, Perth.
Bode, M., Brennan, K., Morris, K., Burrows, N., and Hague, N. (2012). Choosing cost-effective locations for conservation fences in the local landscape. Wildlife Research 39, 192–201.
| Choosing cost-effective locations for conservation fences in the local landscape.Crossref | GoogleScholarGoogle Scholar |
Bull, J. W., Suttle, K. B., Gordon, A., Singh, N. J., and Milner-Gulland, E. J. (2013). Biodiversity offsets in theory and practice. Oryx 47, 369–380.
| Biodiversity offsets in theory and practice.Crossref | GoogleScholarGoogle Scholar |
Burbidge, A. (1999). Conservation values and management of Australian islands for non-volant mammal conservation. Australian Mammalogy 21, 67–74.
| Conservation values and management of Australian islands for non-volant mammal conservation.Crossref | GoogleScholarGoogle Scholar |
Burbidge, A. A. (2004). Montebello Renewal: Western Shield review-February 2003. Conservation Science Western Australia 5, 194.
Burbidge, A. (2011). Unexpected deaths of spinifexbirds and black-and-white fairy-wrens. Report to the DEC Animal Ethics Committee: Department of Environment and Conservation, Perth.
Burbidge, A. A., and Manly, B. F. J. (2002). Mammal extinctions on Australian islands: causes and conservation implications. Journal of Biogeography 29, 465–473.
| Mammal extinctions on Australian islands: causes and conservation implications.Crossref | GoogleScholarGoogle Scholar |
Burbidge, A. A., and Abbott, I. (2017). Mammals on Western Australian islands: occurrence and preliminary analysis. Australian Journal of Zoology 65, 183–195.
| Mammals on Western Australian islands: occurrence and preliminary analysis.Crossref | GoogleScholarGoogle Scholar |
Burbidge, A., Blythman, M., Danks, A., and Hamilton, N. (2019). Bringing back the birds of the Montebellos. Landscope 34, 28–33.
Burbidge, A. A., Blyth, J. D., Fuller, P. J., Kendrick, P. G., Stanley, F. J., and Smith, L. E. (2000). The terrestrial vertebrate fauna of the Montebello Islands, Western Australia. CALM Science 3, 95–107.
Cabezas, S., Blas, J., Marchant, T. A., and Moreno, S. (2007). Physiological stress levels predict survival probabilities in wild rabbits. Hormones and Behavior 51, 313–320.
| Physiological stress levels predict survival probabilities in wild rabbits.Crossref | GoogleScholarGoogle Scholar | 17258747PubMed |
Chapman, T. F. (2015a). Reintroduced burrowing bettongs (Bettongia lesueur) scatter hoard sandalwood (Santalum spicatum) seed. Australian Journal of Zoology 63, 76–79.
| Reintroduced burrowing bettongs (Bettongia lesueur) scatter hoard sandalwood (Santalum spicatum) seed.Crossref | GoogleScholarGoogle Scholar |
Chapman, T. F. (2015b). Comparison of soils and plants on the active and relic parts of a recolonised burrowing bettong (Bettongia lesueur) warren. Pacific Conservation Biology 21, 298–306.
| Comparison of soils and plants on the active and relic parts of a recolonised burrowing bettong (Bettongia lesueur) warren.Crossref | GoogleScholarGoogle Scholar |
Chapman, T., and Burrows, N. (2015). Lorna Glen (Matuwa) small vertebrate fauna monitoring program 2002–2010 – preliminary analysis and review. Department of Parks and Wildlife, Perth.
Chevron Australia. (2015). Gorgon gas development and Jansz feed gas pipeline: Five year environmental performance report. Chevron Australia, Perth.
Clayton, J. A., Pavey, C. R., Vernes, K., and Tighe, M. (2014). Review and analysis of Australian macropod translocations 1969–2006. Mammal Review 44, 109–123.
| Review and analysis of Australian macropod translocations 1969–2006.Crossref | GoogleScholarGoogle Scholar |
Department of Parks and Wildlife. (2013). Gorgon Gas Development: Threatened and priority species translocation and reintroduction program. Annual Report 2012-2013. Department of Parks and Wildlife, Perth.
Dickens, M. J., Delehanty, D. J., and Romero, L. M. (2009). Stress and translocation: alterations in the stress physiology of translocated birds. Proceedings of the Royal Society B: Biological Sciences 276, 2051–2056.
| Stress and translocation: alterations in the stress physiology of translocated birds.Crossref | GoogleScholarGoogle Scholar | 19324794PubMed |
Dirzo, R., Young, H. S., Galetti, M., Ceballos, G., Isaac, N. J. B., and Collen, B. (2014). Defaunation in the Anthropocene. Science 345, 401–406.
| Defaunation in the Anthropocene.Crossref | GoogleScholarGoogle Scholar | 25061202PubMed |
Doherty, T. S., Dickman, C. R., Johnson, C. N., Legge, S. M., Ritchie, E. G., and Woinarski, J. C. Z. (2016). Impacts and management of feral cats in Australia. Mammal Review 47, 87–97.
| Impacts and management of feral cats in Australia.Crossref | GoogleScholarGoogle Scholar |
Dortch, C. E., and Morse, K. (1984). Prehistoric stone artefacts on some offshore islands in Western Australia. Australian Archaeology 19, 31–47.
| Prehistoric stone artefacts on some offshore islands in Western Australia.Crossref | GoogleScholarGoogle Scholar |
Dunlop, J. A. (2015). The ecology and host parasite dynamics of a fauna translocation in Australia. PhD thesis, Murdoch University, Perth.
Dunlop, J., and Morris, K. (2018). Environmental determination of body size in mammals: Rethinking ‘island dwarfism’ in the golden bandicoot. Austral Ecology 43, 817–827.
| Environmental determination of body size in mammals: Rethinking ‘island dwarfism’ in the golden bandicoot.Crossref | GoogleScholarGoogle Scholar |
Dunlop, J. A., and Watson, M. J. (in press). The hitchhiker’s guide to Australia: a parasitological perspective on conservation translocations. Austral Ecology.
Dunlop, J. A., Sims, C., and Morris, K. (2011). Translocation Proposal: Reintroduction of Mala from Trimouille Island, Peron Captive Breeding Centre and Return to Dryandra, to Lorna Glen Conservation Park. Department of Environment and Conservation.
Fleming, P. A., Anderson, H., Prendergast, A. S., Bretz, M. R., Valentine, L. E., and Hardy, G. E. S. (2014). Is the loss of Australian digging mammals contributing to a deterioration in ecosystem function. Mammal Review 44, 94–108.
| Is the loss of Australian digging mammals contributing to a deterioration in ecosystem function.Crossref | GoogleScholarGoogle Scholar |
Geyle, H. M., Woinarski, J. C. Z., Baker, G. B., Dickman, C. R., Dutson, G., Fisher, D. O., Ford, H., Holdsworth, M., Jones, M. E., Kutt, A., Legge, S., Leiper, I., Loyn, R., Murphy, B. P., Menkhorst, P., Reside, A. E., Ritchie, E. G., Roberts, F. E., Tingley, R., and Garnett, S. T. (2018). Quantifying extinction risk and forecasting the number of impending Australian bird and mammal extinctions. Pacific Conservation Biology 24, 157–167.
| Quantifying extinction risk and forecasting the number of impending Australian bird and mammal extinctions.Crossref | GoogleScholarGoogle Scholar |
Gibbons, P., and Lindenmayer, D. B. (2007). Offsets for land clearing: No net loss or the tail wagging the dog. Ecological Management & Restoration 8, 26–31.
| Offsets for land clearing: No net loss or the tail wagging the dog.Crossref | GoogleScholarGoogle Scholar |
Goutte, A., Antoine, É., Weimerskirch, H., and Chastel, O. (2010). Age and the timing of breeding in a long-lived bird: a role for stress hormones. Functional Ecology 24, 1007–1016.
| Age and the timing of breeding in a long-lived bird: a role for stress hormones.Crossref | GoogleScholarGoogle Scholar |
Government of Western Australia. (2003). Barrow Island Act 2003. Government of Western Australia, Perth.
Government of Western Australia (2009). Variation Agreement, 1–15.
Government of Western Australia. (2018). Wildlife conservation (Specially protected fauna) notice. Government of Western Australia, Perth.
Holland, G. (2007). Encyclopedia of aviculture. Hancock House, Surrey, B.C. Canada.
IUCN SSC. (2013). Guidelines for reintroductions and other conservation translocations. Reintroduction and Invasive Species Specialist Groups’ Task Force, Gland, Switzerland.
Ives, C. D., and Bekessy, S. A. (2015). The ethics of offsetting nature. Frontiers in Ecology and the Environment 13, 568–573.
| The ethics of offsetting nature.Crossref | GoogleScholarGoogle Scholar |
Jackson, S. (2007). Australian mammals: biology and captive management. CSIRO Publishing, Melbourne.
James, A. I., and Eldridge, D. J. (2007). Reintroduction of fossorial native mammals and potential impacts on ecosystem processes in an Australian desert landscape. Biological Conservation 138, 351–359.
| Reintroduction of fossorial native mammals and potential impacts on ecosystem processes in an Australian desert landscape.Crossref | GoogleScholarGoogle Scholar |
Jolly, C. J., Webb, J. K., and Phillips, B. L. (2018). The perils of paradise: an endangered species conserved on an island loses antipredator behaviours within 13 generations. Biology Letters 14, 20180222.
| The perils of paradise: an endangered species conserved on an island loses antipredator behaviours within 13 generations.Crossref | GoogleScholarGoogle Scholar | 29875211PubMed |
Jones, H. P., Tershy, B. R., Zavaleta, E. S., Croll, D. A., Keitt, B. S., Finkelstein, M. E., and Howald, G. R. (2008). Severity of the effects of invasive rats on seabirds: a global review. Conservation Biology 22, 16–26.
| Severity of the effects of invasive rats on seabirds: a global review.Crossref | GoogleScholarGoogle Scholar | 18254849PubMed |
Jones, H. P., Holmes, N. D., Butchart, S. H. M., Tershy, B. R., Kappes, P. J., Corkery, I., Aguirre-Muñoz, A., Armstrong, D. P., Bonnaud, E., Burbidge, A. A., Campbell, K., Courchamp, F., Cowan, P. E., Cuthbert, R. J., Ebbert, S., Genovesi, P., Howald, G. R., Keitt, B. S., Kress, S. W., Miskelly, C. M., Oppel, S., Poncet, S., Rauzon, M. J., Rocamora, G., Russell, J. C., Samaniego-Herrera, A., Seddon, P. J., Spatz, D. R., Towns, D. R., and Croll, D. A. (2016). Invasive mammal eradication on islands results in substantial conservation gains. Proceedings of the National Academy of Sciences 113, 4033–4038.
| Invasive mammal eradication on islands results in substantial conservation gains.Crossref | GoogleScholarGoogle Scholar |
Lagdon, R., and Moro, D. (2013). The Gorgon gas development and its environmental commitments. Records of the Western Australian Museum 9, 011.
| The Gorgon gas development and its environmental commitments.Crossref | GoogleScholarGoogle Scholar |
Langford, D., and Burbidge, A. A. (2001). Translocation of mala (Lagorchestes hirsutus) from the Tanami Desert, Northern Territory to Trimouille Island, Western Australia. Australian Mammalogy 23, 37–46.
| Translocation of mala (Lagorchestes hirsutus) from the Tanami Desert, Northern Territory to Trimouille Island, Western Australia.Crossref | GoogleScholarGoogle Scholar |
Lindenmayer, D. B., Crane, M., Evans, M. C., Maron, M., Gibbons, P., Bekessy, S., and Blanchard, W. (2017). The anatomy of a failed offset. Biological Conservation 210, 286–292.
| The anatomy of a failed offset.Crossref | GoogleScholarGoogle Scholar |
Lloyd, N. A., Hostetter, N. J., Jackson, C. L., Converse, S. J., and Moehrenschlager, A. (2019). Future directions to escalate benefits of the stepping‐stone approach for conservation translocations. Animal Conservation 22, 122–123.
| Future directions to escalate benefits of the stepping‐stone approach for conservation translocations.Crossref | GoogleScholarGoogle Scholar |
Lohr, C. (2018). Closure report for SPP 2012-0024: Rangelands restoration – reintroduction of native mammals to Lorna Glen (Matuwa). Department of Biodiversity, Conservation and Attractions, Perth.
Lohr, C., Van Dongen, R., Huntley, B., Gibson, L., and Morris, K. (2014). Remotely monitoring change in vegetation cover on the Montebello Islands, Western Australia, in response to introduced rodent eradication. PloS One 9, e114095.
| Remotely monitoring change in vegetation cover on the Montebello Islands, Western Australia, in response to introduced rodent eradication.Crossref | GoogleScholarGoogle Scholar | 25436454PubMed |
MacKenzie, D., Nichols, J. D., Royle, J. A., Pollock, K., Bailey, L. L., and Hines, J. (2017). Occupancy Estimation and Modeling: Inferring Patterns and Dynamics of Species Occurrence: Second Edition , .
| Occupancy Estimation and Modeling: Inferring Patterns and Dynamics of Species Occurrence: Second EditionCrossref | GoogleScholarGoogle Scholar | 29119578PubMed |
Maron, M., Hobbs, R. J., Moilanen, A., Matthews, J. W., Christie, K., Gardner, T. A., Keith, D. A., Lindenmayer, D., and McAlpine, C. A. (2012). Faustian bargains? Restoration realities in the context of biodiversity offset policies. Biological Conservation 155, 141–148.
| Faustian bargains? Restoration realities in the context of biodiversity offset policies.Crossref | GoogleScholarGoogle Scholar |
Maron, M., Gordon, A., Mackey, B. G., Possingham, H. P., and Watson, J. E. M. (2015). Conservation: stop misuse of biodiversity offsets. Nature News 523, 401.
| Conservation: stop misuse of biodiversity offsets.Crossref | GoogleScholarGoogle Scholar |
Maron, M., Ives, C. D., Kujala, H., Bull, J. W., Maseyk, F. J. F., Bekessy, S., Gordon, A., Watson, J. E. M., Lentini, P. E., Gibbons, P., Possingham, H. P., Hobbs, R. J., Keith, D. A., Wintle, B. A., and Evans, M. C. (2016). Taming a Wicked Problem: Resolving Controversies in Biodiversity Offsetting. Bioscience 66, 489–498.
| Taming a Wicked Problem: Resolving Controversies in Biodiversity Offsetting.Crossref | GoogleScholarGoogle Scholar |
Maron, M., Brownlie, S., Bull, J. W., Evans, M. C., von Hase, A., Quétier, F., Watson, J. E. M., and Gordon, A. (2018). The many meanings of no net loss in environmental policy. Nature Sustainability 1, 19–27.
| The many meanings of no net loss in environmental policy.Crossref | GoogleScholarGoogle Scholar |
May, J., Hobbs, R. J., and Valentine, L. E. (2017). Are offsets effective? An evaluation of recent environmental offsets in Western Australia. Biological Conservation 206, 249–257.
| Are offsets effective? An evaluation of recent environmental offsets in Western Australia.Crossref | GoogleScholarGoogle Scholar |
McCauley, D. J. (2006). Selling out on nature. Nature 443, 27–28.
| Selling out on nature.Crossref | GoogleScholarGoogle Scholar | 16957711PubMed |
McKenzie, N. L., Morris, K. D., and Dickman, C. R. (2008). Golden Bandicoot, Isoodon auratus. In ‘The mammals of Australia.’ Third edn. (Eds S. Van Dyck and R. Strahan) pp. 176–178. (Reed New Holland: Sydney.)
McKenzie, N. L., Morris, K. D., and Dickman, C. R. (2013). Golden Bandicoot, Isoodon auratus. In ‘Field companion to the mammals of Australia’ (Eds S. Van Dyck and I. Gynther). (New Holland Publishers: Sydney.)
Medina, F. M., Bonnaud, E., Vidal, E., Tershy, B. R., Zavaleta, E. S., Josh Donlan, C., Keitt, B. S., Le Corre, M., Horwath, S. V., and Nogales, M. (2011). A global review of the impacts of invasive cats on island endangered vertebrates. Global Change Biology 17, 3503–3510.
| A global review of the impacts of invasive cats on island endangered vertebrates.Crossref | GoogleScholarGoogle Scholar |
Montague, P. D. (1914). 35. A Report on the Fauna of the Monte Bello Islands. Proceedings of the Zoological Society of London 84, 625–652.
| 35. A Report on the Fauna of the Monte Bello Islands.Crossref | GoogleScholarGoogle Scholar |
Moore, H. A., Dunlop, J. A., Valentine, L. E., Woinarski, J. C. Z., Ritchie, E. G., Watson, D. M., and Nimmo, D. G. (2019). Topographic ruggedness and rainfall mediate geographic range contraction of a threatened marsupial predator. Diversity and Distributions 25, 1818–1831.
| Topographic ruggedness and rainfall mediate geographic range contraction of a threatened marsupial predator.Crossref | GoogleScholarGoogle Scholar |
Moro, D., and Lagdon, R. (2013). History and environment of Barrow Island. Records of the Western Australian Museum Supplement 83, 1–8.
| History and environment of Barrow Island.Crossref | GoogleScholarGoogle Scholar |
Morris, K. D. (2002). The eradication of the black rat (Rattus rattus) on Barrow and adjacent islands off the north-west coast of Western Australia. In ‘Turning the tide: the eradication of invasive species’, (Eds C. R. Veitch and M. N. Clout), pp. 219–225. (IUCN SSC Invasive Species Specialist Group: Gland, Switzerland and Cambridge, UK.)
Morris, K. D., and Yates, C. (2016). Barrow Island threatened and priority species translocation and reintroduction program. Strategic Plan 2010–2023: Department of Parks and Wildlife, Perth.
Morris, K., Speldewinde, P., Orell, P., and Unit, C. (2000). Djoongari (Shark Bay mouse) recovery plan. Wildlife Management Program 17: Department of Conservation and Land Management, Perth.
Morris, K., Page, M., Kay, R., Renwick, J., Desmond, A., Comer, S., Burbidge, A., Kuchling, G., and Sims, C. (2015). Forty years of fauna translocations in Western Australia: lessons learned. In ‘Advances in Reintroduction Biology of Australian and New Zealand Fauna’, (Eds D. P. Armstrong, M. W. Hayward, D. Moro and P. J. Seddon), pp. 217–235. (CSIRO Publishing: Clayton South, Victoria.)
Morris, K. D., Page, M., Thomas, N., and Ottewell, K. (2017). A strategic framework for the reconstruction and conservation of the vertebrate fauna of Dirk Hartog Island, 2017–2030. Department of Parks and Wildlife, Perth.
Moseby, K. E., Read, J. L., Paton, D. C., Copley, P., Hill, B. M., and Crisp, H. A. (2011). Predation determines the outcome of 10 reintroduction attempts in arid South Australia. Biological Conservation 144, 2863–2872.
| Predation determines the outcome of 10 reintroduction attempts in arid South Australia.Crossref | GoogleScholarGoogle Scholar |
Ostendorf, B., Boardman, W. S. J., and Taggart, D. A. (2016). Islands as refuges for threatened species: multispecies translocation and evidence of species interactions four decades on. Australian Mammalogy 38, 204–212.
| Islands as refuges for threatened species: multispecies translocation and evidence of species interactions four decades on.Crossref | GoogleScholarGoogle Scholar |
Ottewell, K., Dunlop, J., Thomas, N., and Morris, K. (2014). Evaluating success of translocations in maintaining genetic diversity in a threatened mammal. Biological Conservation 171, 209–219.
| Evaluating success of translocations in maintaining genetic diversity in a threatened mammal.Crossref | GoogleScholarGoogle Scholar |
Otto, S. P. (2018). Adaptation, speciation and extinction in the Anthropocene. Proceedings of the Royal Society B 285, 20182047.
| Adaptation, speciation and extinction in the Anthropocene.Crossref | GoogleScholarGoogle Scholar | 30429309PubMed |
Parker, K. A., Dickens, M. J., Clarke, R. H., and Lovegrove, T. G. (2012). The theory and practice of catching, holding, moving and releasing animals. In ‘Reintroduction Biology: Integrating Science and Management’, (Eds J. G. Ewen, D. P. Armstrong, K. A. Parker and P. J. Seddon), pp. 105–137. (Wiley-Blackwell: West Sussex.)
Pedler, R. D., West, R. S., Read, J. L., Moseby, K. E., Letnic, M., Keith, D. A., Leggett, K. D., Ryall, S. R., and Kingsford, R. T. (2018). Conservation challenges and benefits of multispecies reintroductions to a national park – a case study from New South Wales, Australia. Pacific Conservation Biology 24, 397.
| Conservation challenges and benefits of multispecies reintroductions to a national park – a case study from New South Wales, Australia.Crossref | GoogleScholarGoogle Scholar |
Pérez, I., Anadón, J. D., Díaz, M., Nicola, G. G., Tella, J. L., and Giménez, A. (2012). What is wrong with current translocations? A review and a decision‐making proposal. Frontiers in Ecology and the Environment 10, 494–501.
| What is wrong with current translocations? A review and a decision‐making proposal.Crossref | GoogleScholarGoogle Scholar |
Pimm, S. L., Jenkins, C. N., Abell, R., Brooks, T. M., Gittleman, J. L., Joppa, L. N., Raven, P. H., Roberts, C. M., and Sexton, J. O. (2014). The biodiversity of species and their rates of extinction, distribution, and protection. Science 344, 1246752.
| The biodiversity of species and their rates of extinction, distribution, and protection.Crossref | GoogleScholarGoogle Scholar | 24876501PubMed |
Pruett-Jones, S., and O’Donnell, E. (2004). Land birds on Barrow Island: status, population estimates, and responses to an oil-field development. Journal of the Royal Society of Western Australia 87, 101.
Richardson, J., Watson, G., and Kregor, G. (2006). The distribution of terrestrial vertebrate fauna in the Montebello Islands. Conservation Science Western Australia 5, 269–271.
Rick, K., Ottewell, K., Lohr, C., Thavornkanlapachai, R., Byrne, M., and Kennington, W. J. (2019). Population Genomics of Bettongia lesueur: Admixing Increases Genetic Diversity with no Evidence of Outbreeding Depression. Genes 10, 851.
| Population Genomics of Bettongia lesueur: Admixing Increases Genetic Diversity with no Evidence of Outbreeding Depression.Crossref | GoogleScholarGoogle Scholar |
Ringma, J., Legge, S., Woinarski, J., Radford, J., Wintle, B., and Bode, M. (2018). Australia’s mammal fauna requires a strategic and enhanced network of predator-free havens. Nature Ecology and Evolution 2, 410–411.
| Australia’s mammal fauna requires a strategic and enhanced network of predator-free havens.Crossref | GoogleScholarGoogle Scholar | 29335573PubMed |
Sedgwick, E. H. (1978). A population study of the Barrow Island avifauna. Western Australian Naturalist 14, 85–108.
Sheean, V. A., Manning, A. D., and Lindenmayer, D. B. (2011). An assessment of scientific approaches towards species relocations in Australia. Austral Ecology 37, 204–215.
| An assessment of scientific approaches towards species relocations in Australia.Crossref | GoogleScholarGoogle Scholar |
Shephard, M. (1994). Aviculture in Australia. Keeping and Breeding Aviary Birds. Reed, Chatswood.
Short, J., and Turner, B. (1991). Distribution and abundance of spectacled hare-wallabies and euros on Barrow Island, western Australia. Wildlife Research 18, 421–429.
| Distribution and abundance of spectacled hare-wallabies and euros on Barrow Island, western Australia.Crossref | GoogleScholarGoogle Scholar |
Short, J., and Turner, B. (1994). A test of the vegetation mosaic hypothesis: a hypothesis to explain the decline and extinction of Australian mammals. Conservation Biology 8, 439–449.
| A test of the vegetation mosaic hypothesis: a hypothesis to explain the decline and extinction of Australian mammals.Crossref | GoogleScholarGoogle Scholar |
Short, J., Copley, P., Ruykys, L., Morris, K., Read, J., and Moseby, K. (2019). Review of translocations of the greater stick-nest rat (Leporillus conditor): lessons learnt to facilitate ongoing recovery. Wildlife Research 46, 455–475.
| Review of translocations of the greater stick-nest rat (Leporillus conditor): lessons learnt to facilitate ongoing recovery.Crossref | GoogleScholarGoogle Scholar |
Sims, C., Morris, K., and Blythman, M. (2017). Rangelands Restoration: Fauna recovery at Matuwa (Lorna Glen) Western Australia. Annual Report. Department of Parks and Wildlife, Perth.
Thackway, R., and Cresswell, I. D. (1995). An Interim Biogeographic Regionalisation for Australia: a framework for establishing the national system of reserves, Version 4.0. Australian Nature Conservation Agency, Canberra.
Thavornkanlapachai, R., Mills, H. R., Ottewell, K., Dunlop, J., Sims, C., Morris, K., Donaldson, F., and Kennington, W. J. (2019). Mixing Genetically and Morphologically Distinct Populations in Translocations: Asymmetrical Introgression in A Newly Established Population of the Boodie (Bettongia lesueur). Genes 10, 729.
| Mixing Genetically and Morphologically Distinct Populations in Translocations: Asymmetrical Introgression in A Newly Established Population of the Boodie (Bettongia lesueur).Crossref | GoogleScholarGoogle Scholar |
Vogelnest, L., and Portas, T. (2008). Macropods. Medicine of Australian Mammals , 133–225.
| Macropods.Crossref | GoogleScholarGoogle Scholar |
Waller, N. L., Gynther, I. C., Freeman, A. B., Lavery, T. H., and Leung, L. K.-P. (2017). The Bramble Cay melomys rubicola (Rodentia: Muridae): a first mammalian extinction caused by human-induced climate change. Wildlife Research 44, 9–21.
| The Bramble Cay melomys rubicola (Rodentia: Muridae): a first mammalian extinction caused by human-induced climate change.Crossref | GoogleScholarGoogle Scholar |
White, A. W., and Pyke, G. H. (2008). Frogs on the hop: translocations of Green and Golden Bell Frogs Litoria aurea in Greater Sydney. Australian Zoologist 34, 249–260.
| Frogs on the hop: translocations of Green and Golden Bell Frogs Litoria aurea in Greater Sydney.Crossref | GoogleScholarGoogle Scholar |
Whitwell, S. M., Amiot, C., Mclean, I. G., Lovegrove, T. G., Armstrong, D., Brunton, D. H., and Weihong, J. I. (2012). Losing anti‐predatory behaviour: A cost of translocation. Austral Ecology 37, 413–418.
| Losing anti‐predatory behaviour: A cost of translocation.Crossref | GoogleScholarGoogle Scholar |
Woinarski, J. C. Z., Ward, S., Mahney, T., Bradley, J., Brennan, K., Ziembicki, M., and Fisher, A. (2011). The mammal fauna of the Sir Edward Pellew island group, Northern Territory, Australia: refuge and death-trap. Wildlife Research 38, 307–322.
| The mammal fauna of the Sir Edward Pellew island group, Northern Territory, Australia: refuge and death-trap.Crossref | GoogleScholarGoogle Scholar |
Woinarski, J. C. Z., Burbidge, A. A., and Harrison, P. L. (2015a). A review of the conservation status of Australian mammals. Therya 6, 155–166.
| A review of the conservation status of Australian mammals.Crossref | GoogleScholarGoogle Scholar |
Woinarski, J. C. Z., Burbidge, A., and Harrison, P. L. (2015b). Ongoing unraveling of a continental fauna: Decline and extinction of Australian mammals since European settlement. Proceedings of the National Academy of Sciences 112, 4531–4540.
| Ongoing unraveling of a continental fauna: Decline and extinction of Australian mammals since European settlement.Crossref | GoogleScholarGoogle Scholar |
Woinarski, J. C. Z., Garnett, S. T., Legge, S. M., and Lindenmayer, D. B. (2017). The contribution of policy, law, management, research, and advocacy failings to the recent extinctions of three Australian vertebrate species. Conservation Biology 31, 13–23.
| The contribution of policy, law, management, research, and advocacy failings to the recent extinctions of three Australian vertebrate species.Crossref | GoogleScholarGoogle Scholar |
Wysong, M. L., Tulloch, A. I. T., Valentine, L. E., Hobbs, R. J., Morris, K., and Ritchie, E. G. (2019). The truth about cats and dogs: assessment of apex- and mesopredator diets improves with reduced observer uncertainty. Journal of Mammalogy 100, 410–422.
| The truth about cats and dogs: assessment of apex- and mesopredator diets improves with reduced observer uncertainty.Crossref | GoogleScholarGoogle Scholar |
Appendix 1. Translocation site and species selection
Three island and two mainland sites were selected for the translocations (Table 1; Fig. 1).
Hermite Island (836 ha; 20°28.04′S, 115°31.40′E) is the largest island in the Montebello Island Conservation Park and is located approximately 30 km north of Barrow Island Nature Reserve (Fig. 1). Until the early 20th century, Hermite Island supported golden bandicoots, spectacled hare-wallabies, spinifexbirds, and possibly black-and-white fairy-wrens (Montague 1914; Burbidge et al. 2000, 2019). Water rats persisted until the 1980s, but have not been detected on any subsequent survey (Burbidge 2004; Richardson et al. 2006) despite intensive management presence on the islands (Algar et al. 2002; Lohr et al. 2014). The proposal for the re-establishment of these species on Hermite Island was part of the Montebello Renewal program following the successful eradication of feral cats and black rats from the archipelago (Algar et al. 2002; Burbidge 2004).
Another island in the Montebello group, Alpha Island (86 ha; 20° 24.67′S, 115° 31.65′E) was selected as a conservation introduction site for the boodie (Barrow Island subsp.) as this taxon was only known from two other sites, Barrow and Boodie Islands. This island has been disturbed considerably as it was the site of an atomic weapons test by the British in 1956. No other mammal species were recorded on Alpha Island.
Doole Island Nature Reserve (210 ha; 22°27.92′ S, 114°9.89′E) lies at the southern end of Exmouth Gulf, approximately 230 km south-west of Barrow Island. No native mammal species or introduced animal species have been recorded on the island; however, djoongari were introduced for conservation purposes in 1993 but did not persist (Morris et al. 2000). Doole Island is within the former range of the golden bandicoot (McKenzie et al. 2008, 2013), and has vegetation assemblages (Acacia shrubs, tussock grasses on sandy soils) considered suitable for golden bandicoots.
Matuwa Indigenous Protected Area (256 000 ha; 26°13.75′S, 121°19.41′E) is an ex-pastoral lease (formerly Lorna Glen) purchased by the State Government for conservation purposes in 2000. It is located 160 km north-east of Wiluna in the northern Goldfields region, and ~820 km south-east of Barrow Island. A successful Native Title claim resulted in an Indigenous Protected Area being declared in 2015. The property extends across the boundary of the Gascoyne and Murchison Interim Biogeographic Regionalisation for Australia regions (Thackway and Cresswell 1995), and consequently supports a diversity of landscape systems. The area once also supported a range of small and medium-sized mammals, many of which are now extinct in the rangelands (Chapman and Burrows 2015). Pre-European skeletal remains of golden bandicoots were found at two locations on Lorna Glen and neighbouring Earaheedy stations (Baynes 2006), and the multitude of abandoned boodie warrens throughout the area indicates their former presence at the site, potentially until the 1950s (Abbott 2008). Matuwa is the site of an integrated rangelands restoration project, including feral cat control, implementation of a small mosaic fire regime and control of feral herbivores, followed by the reintroduction of 11 species of medium-sized mammals (Algar et al. 2013; Lohr 2018). Boodies, golden bandicoots and mala were among those species selected for reintroduction. The reintroduction strategy involved the construction of an 1100 ha introduced predator proof enclosure into which some of the more predator naïve reintroduced mammals would be initially located (Bode et al. 2012). Following acclimatisation to the environment and native predators, and increasing abundances, the intention was to translocate the mammals outside the fenced enclosure, but within the area subject to ongoing feral cat control.
Cape Range National Park (CRNP, 50 600 ha; 22°10.80′S, 113°54.16′E) is located adjacent to the town of Exmouth on the Cape Range Peninsula, ~200 km south-west of Barrow Island. Thirty-eight species of ground-dwelling native mammals have been recorded from the Cape Range peninsula, but 27 of these have become locally extinct and only 11 species persist now. Species that once occurred in this area include the golden bandicoot, boodie and brushtail possum (Baynes and Jones 1993). Fox control to protect nesting marine turtles and rock-wallaby (Petrogale lateralis) populations has been underway at CRNP since the 1980s. In 2015, an integrated fox and feral cat control program was commenced, with the goal of protecting critical weight range species.