Northern brown bandicoot (Isoodon macrourus) and common brushtail possum (Trichosurus vulpecula) density on the Tiwi Islands: insights and implications
Hugh F. Davies A D , Tiwi Land Rangers B , Emily Nicholson C and Brett P. Murphy A
A D , Tiwi Land Rangers B , Emily Nicholson C and Brett P. Murphy A
A NESP Threatened Species Recovery Hub, Research Institute for the Environment and Livelihoods, Charles Darwin University, Casuarina, NT 0810, Australia.
B Tiwi Land Rangers, Winnellie, NT 0810, Australia.
C Deakin University, Centre for Integrative Ecology, School of Life and Environmental Sciences, Burwood, Vic. 3125, Australia.
D Corresponding author. Email: hugh.davies@cdu.edu.au
Pacific Conservation Biology 28(3) 224-230 https://doi.org/10.1071/PC21020
Submitted: 29 March 2021 Accepted: 10 June 2021 Published: 6 July 2021
Journal Compilation © CSIRO 2022 Open Access CC BY-NC-ND
Abstract
Despite the ongoing collapse of native mammal populations across northern Australia, the paucity of robust estimates of population density limits our capacity to identify and understand population change. Here we aimed to provide the first estimates of native mammal density on the Tiwi Islands – one of Australia’s largest remaining refuge areas for native mammals. We conducted intensive live-trapping at four sites that represent varying combinations of fire frequency, feral cat density and feral herbivore presence. We used spatially-explicit capture-recapture models to investigate the density of common brushtail possum (Trichosurus vulpecula) and northern brown bandicoot (Isoodon macrourus). Compared with mainland northern Australia, populations of common brushtail possum and northern brown bandicoot have remained relatively healthy on the Tiwi Islands. Common brushtail possum density was significantly higher on Bathurst Island (1.06 possum ha−1) compared with Melville Island (0.32 possum ha−1), whereas northern brown bandicoot density varied across all four sites (ranging from 0.04 to 0.34 bandicoot ha−1). Unexpectedly, the very frequently burnt Ranku site (Bathurst Island) continues to support healthy populations of both species. These density estimates provide critical information for identifying and understanding future population change for two species that have suffered marked declines across the Australian monsoon tropics. Although the lack of replication limits our ability to draw conclusions regarding the ecological constraints of these mammal populations, our density observations align with a recent conceptual model postulating that the persistence of native mammal populations across northern Australian savannas reflects a complex, but spatially-variable interplay of ‘bottom-up’ and ‘top-down’ processes.
Keywords: Bathurst Island, capture-recapture, common brushtail possum, decline, habitat refuge, mammal extinction, Melville Island, monsoon tropics, native mammals, northern Australia, northern brown bandicoot, savannas, Tiwi Islands.
Introduction
Australia has experienced an extraordinarily high rate of mammal extinction in recent centuries. Ten percent of Australia’s native terrestrial mammal species have become extinct since European colonisation in 1788 (Woinarski et al. 2015), and many more have disappeared from most of their former ranges. The most dramatic declines occurred in arid central Australia prior to the mid-20th century. Until recent decades it was assumed that the vast, uncleared savannas of monsoonal northern Australia would retain a healthy assemblage of native mammals. However, research conducted since the 1990s has demonstrated that northern Australia’s native mammals are experiencing a rapid and severe decline (Woinarski et al. 2011), which, if continue unabated, will lead to more extinctions (Geyle et al. 2018). The drivers of the current decline of native mammals across northern Australia have proven difficult to identify. However, a conceptual model has emerged over the past decade, postulating that frequent fire and heavy grazing by feral herbivores have degraded habitat, disrupting both ‘bottom-up’ (e.g. resource availability) and ‘top-down’ (e.g. predation) processes, resulting in the widespread decline of native mammal populations (Stobo-Wilson et al. 2020a).
Robust estimates of population density increase our ability to understand population change and our capacity to mitigate population decline (Efford 2004), such as those currently occurring across northern Australia. Recently, advances in statistical modelling have improved our ability to accurately estimate population density. For example, spatially-explicit capture-recapture models take into account both the distribution and movement of individuals in relation to detectors (e.g. traps or motion-activated cameras) to provide estimates of density that are unbiased by edge-effects and imperfect detection (Efford 2004). Despite their utility, spatially-explicit estimates of population density are rare for northern Australian mammals. This likely reflects, in part, the diminished ability to obtain sufficient data required for these analytical methods due to the catastrophic declines suffered by native mammals across northern Australian savannas that occurred prior to the development of these methods. However, it appears that such data can still be obtained from areas of northern Australia, such as Groote Eylandt (Heiniger et al. 2018, 2020), the Tiwi Islands and Cobourg Peninsula (Stobo-Wilson et al. 2020a).
The Tiwi Islands, in the Australian monsoon tropics, are one of the last remaining areas in Australia to support an intact native mammal assemblage (Murphy and Davies 2014; Davies et al. 2018). However, due to the presence of threatening processes that have been implicated in the decline of native mammals across northern Australia including feral herbivores and feral cats (Davies et al. 2017, 2020), and worrying signs of initial decline (Davies et al. 2018), native mammal populations on these islands may be at risk. Here, we aimed to provide (1) estimates of native mammal density on the Tiwi Islands; and (2) insight into the ecological constraints of native mammal density in tropical savannas.
Materials and methods
Study site
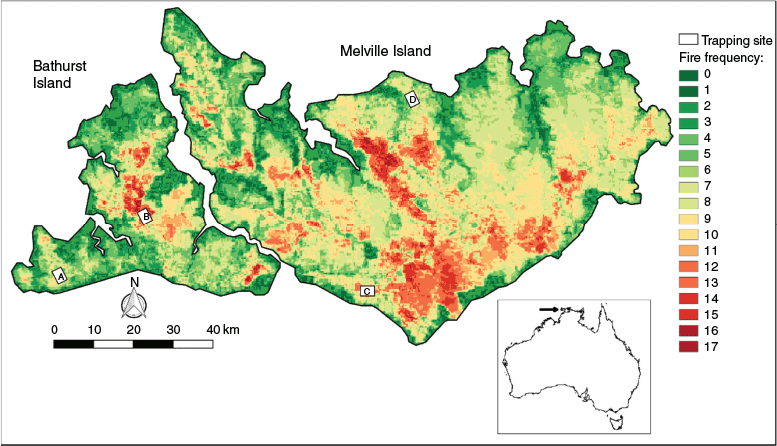
Our study was conducted on Melville (5786 km2) and Bathurst Island (2600 km2), Australia’s second and fifth largest islands, respectively. They are the two largest of the group of islands collectively known as the Tiwi Islands, located 20 km off the north coast of Australia’s Northern Territory (Fig. 1). The main vegetation type of the Tiwi Islands is lowland savanna dominated by Eucalyptus miniata, Eucalyptus tetrodonta and Corymbia nesophila with a predominantly grassy understorey. The islands experience a tropical monsoonal climate with a humid wet season (November–March) in which over 90% of the annual rainfall occurs, followed by a dry season (April–October). Fire frequency is very high but there is significant variation across the islands (Fig. 1). Feral cats (Felis catus) are present on both islands, but recent work suggests that the density of feral cats is lower on Bathurst Island than Melville Island (Davies et al. 2021). Feral herbivores (Asian water buffalo Bubalus bubalis and horse Equus caballus) are only present on Melville Island. Feral pigs (Sus scrofa) are widespread on Bathurst Island, but localised to the western half of Melville Island. Cane toads (Rhinella marina) are absent from both islands. Dingoes (Canis dingo) are widespread across both islands.
Data collection
We conducted intensive live-trapping at four sites across the Tiwi Islands: two on Bathurst Island (Cape Fourcroy and Ranku) and two on Melville Island (Pickertaramoor and Cache Point) (Fig. 1). At each site, we deployed a trapping grid consisting of 300 live-traps (30 rows of 10) spaced 20 m apart, covering an area of ~0.1 km2. Each grid was live-trapped for four consecutive nights at two separate times (June and September) in the dry season of 2019, a total of 9600 live-trap nights. On each grid, we used 75 cage traps (66 × 23 × 26 cm) and 225 box-type ‘Sherman’ traps (30 × 10 × 8 cm) baited with a standard mammal bait of oats, peanut butter and honey. Each captured animal was individually marked with a numbered ear tag (Style 1005-1; National Band and Tag Company, Newport, KY, USA) and a microchip (ID-100VB/1.4; Trovan Ltd, Cologne, Germany) implanted between the scapulae.
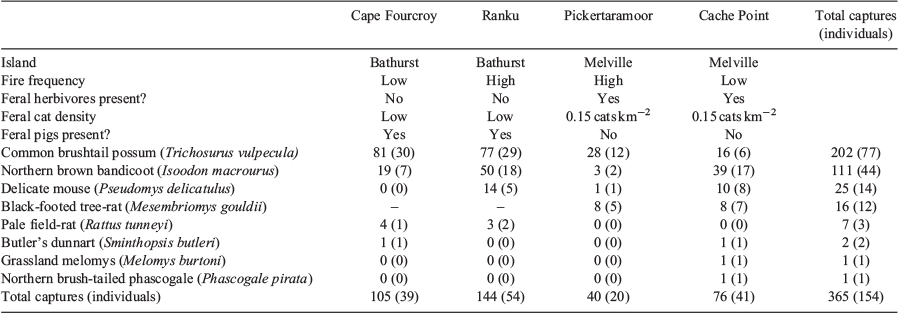
As we aimed to explore the environmental correlates of native mammal density across the Tiwi Islands, the locations of the four sites were chosen to represent varying combinations of fire frequency, feral cat density and feral herbivore presence (Table 1). The Pickertaramoor and Ranku sites were characterised by very high fire frequency, with the MODIS satellite-derived fire frequency recorded at each individual trap location, averaging 13.7 and 14.3 across each grid, respectively. The Cache Point and Cape Fourcroy sites were placed in areas with lower fire frequency, with the MODIS satellite-derived fire frequency recorded at each individual trap location, averaging 6.4 and 8.1 across each grid, respectively. Feral cat density was highest at Pickertaramoor and Cache Point, and exceptionally low at both the Ranku and Cape Fourcroy sites. Feral herbivores were present at both Pickertaramoor and Cache Point, and absent from Bathurst Island.
Data analysis
To investigate the variation in native mammal density across the four sites, we fitted spatially-explicit capture–recapture models using the ‘secr’ package (Efford 2020) in the statistical program R (R Development Core Team 2013). We collapsed the total number of trap nights conducted at each location (eight) into 24-h sampling occasions. We used a 500-m buffer around the outermost coordinates of the live-trapping grids to ensure that density was estimated over a large enough area to include all small native mammals exposed to our survey (Royle et al. 2013).
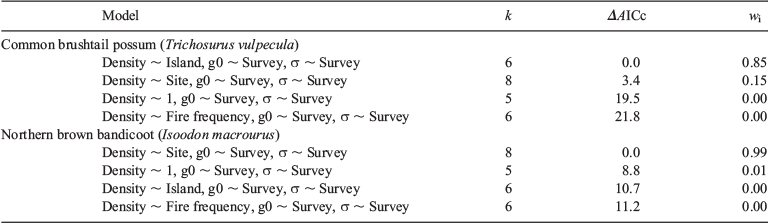
To investigate the variation of native mammal density across our four sites, we ran four multi-session models for each species: constant density across all sites (Density ~ 1), density predicted by site (Density ~ Site), density predicted by island (Density ~ Island) and density predicted by fire frequency (Density ~ Fire frequency). To account for seasonal variation in native mammal detectability, we included a categorical variable (Survey) as a predictor of the two detection parameters: g0 (the probability of detecting an animal (per occasion) if a trap was to be placed at its home-range centre) and σ (the reduction in detection probability with distance from a home-range centre). Model selection based on Akaike’s information criterion adjusted for small sample size (AICc) was used to identify the best fit model (Burnham and Anderson 2002), from which estimates of native mammal density were obtained (Table 2).
Results
From 9600 live-trap nights, we recorded a total of 365 captures of 154 individual native mammals, from eight species (Table 1). Common brushtail possum and northern brown bandicoot were by far the most commonly trapped species, with 202 captures of 77 individual possums and 111 captures of 44 individual bandicoots (Table 1). However, there was considerable variation in the number of captures of each of these species between the four sites. For example, we recorded 81 captures of 30 individual possums at Cape Fourcroy but only 16 captures of 6 individuals at Cache Point. Similarly, captures of northern brown bandicoot varied from 50 at Ranku to just 3 at Pickertaramoor.
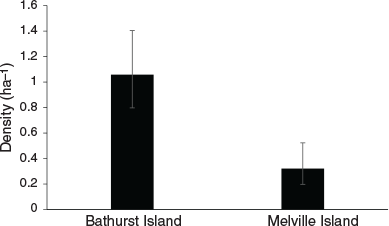
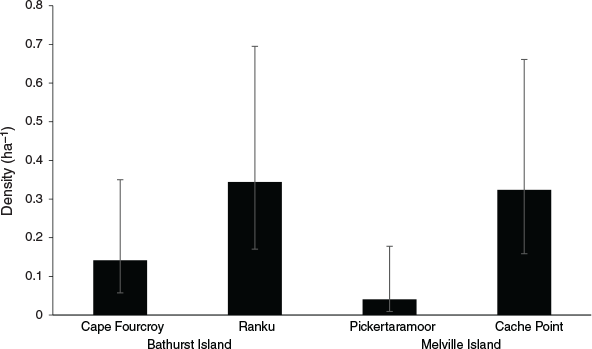
Capture rates were sufficient to investigate population density using spatially-explicit capture-recapture analyses for both the common brushtail possum and northern brown bandicoot. Variation in common brushtail possum density was best explained by island (Table 1), with the estimated possum density on Bathurst Island (1.06 ha−1) significantly higher than on Melville Island (0.32 ha−1) (Fig. 2). Variation in northern brown bandicoot density was best explained by site (Table 1), ranging from 0.04 ha−1 at Pickertaramoor to 0.34 ha−1 at Ranku (Fig. 3).
|
|
Discussion
We have provided the first robust population density estimates for common brushtail possum and northern brown bandicoot on the Tiwi Islands, one of the last remaining areas in Australia to retain a complete assemblage of native mammals (Burbidge et al. 2009). Our density estimates support the view that populations of these species on the Tiwi Islands have remained relatively healthy compared with adjacent parts of the mainland. Albeit from only four locations, we have provided critical information for identifying and understanding future population change for two species that have suffered marked declines across the Australian monsoon tropics. Although the lack of replication limits our ability to draw conclusions regarding the ecological constraints of these mammal populations, our observations provide valuable insight, and align with a recent conceptual model postulating that the persistence of native mammal populations across northern Australian savannas reflects a complex, but spatially-variable interplay of ‘bottom-up’ (e.g. resource availability) and ‘top-down’ (e.g. predation) processes.
Unfortunately, there are very few recent density estimates for the common brushtail possum or northern brown bandicoot that are directly comparable to our results. Kerle (1998) analysed survey data collected between 1979 and 1981 in an area of lowland woodland in Kakadu National Park and estimated the population density of common brushtail possum to be 3.1 ha−1, roughly three times higher than our estimate of possum density on Bathurst Island (1.06 ha−1). A decade later, using data collected as part of a large-scale fire experiment between 1990 and 1994 (Andersen et al. 1998), Griffiths (2013) estimated the density of possums and bandicoots across the Kapalga area of Kakadu National Park to be 0.78 and 0.79 ha−1, respectively. Pope (2001) estimated northern brown bandicoot density to range from 0.073 to 0.103 ha−1 in an area of dry eucalypt forest in the wet tropics of northern Queensland. The most directly comparable estimate of northern brown bandicoot population density comes from another island refuge off the northern Australian coast – Groote Eylandt (Heiniger et al. 2018). Using spatially-explicit capture-recapture models, Heiniger et al. (2018) estimated northern brown bandicoot population density to be 0.19 ha−1 (95% CI 0.13–0.36).
As common brushtail possum density on Bathurst Island (1.06 ha−1) remains higher than that recorded prior to widespread mammal decline in Kakadu (0.78 ha−1) (Griffiths 2013), our results concur with Firth et al. (2006), who concluded that possum populations on Bathurst Island have remained healthy compared with areas of mainland northern Australia. Although possum density on Melville Island (0.32 ha−1) is significantly lower than on Bathurst Island, a recent island-wide survey of historical sites on Melville Island demonstrated that possums are widespread, and found no significant decrease in possum trap-success since 2000, suggesting possum populations on Melville Island also remain healthy compared with areas on the mainland (Davies et al. 2018). Davies et al. (2018) found that while northern brown bandicoot trap-success on Melville Island decreased by 90% between 2000 and 2015, this species also remains widespread. Here, we have demonstrated that in some areas of the Tiwi Islands, northern brown bandicoot populations remain in relatively high densities, such as at Ranku (0.34 ha−1) and Cache Point (0.32 ha−1). However, only two individual bandicoots were recorded at the Pickertaramoor grid, with density estimated at a low 0.04 ha−1.
Given the paucity of robust and consistent estimates of population density, indices of relative abundance (i.e. trap-success) have more commonly been used to identify, and investigate the decline of native mammal species across northern Australian savannas (Woinarski et al. 2001, 2010). However, the validity of using indices of relative abundance as an accurate measure of population size has been questioned (Slade and Blair 2000), especially when imperfect detection is ignored (MacKenzie and Kendall 2002). Unfortunately, as the data required for spatially-explicit estimates of population density can be demanding to collect, there is often a trade-off with how many replicate sites can be surveyed. Our density estimates provide critical information for identifying and understanding future population change for two species that have suffered marked decline across the Australian monsoon tropics. Our density estimates also represent the robust information on population size that can ensure the accurate listing of species on the IUCN’s Red List of Threatened Species (IUCN 1996).
Patterns of native mammal persistence/decline across northern Australian savannas reflect a complex interplay of ‘bottom-up’ and ‘top-down’ processes (Stobo-Wilson et al. 2020a). As such, the variation in the density of common brushtail possum and northern brown bandicoot demonstrated here likely reflects multiple contributing factors. The high density of possums on Bathurst Island might reflect the greater availability of den sites (i.e. tree hollows) often associated with the dense, and well-developed eucalypt forests that occur on Bathurst Island (compared with Melville Island) (Woolley et al. 2018). The absence of the black-footed tree-rat (Mesembriomys gouldii) from Bathurst Island may also help explain the higher density of possums, due to less intraspecific competition for den sites compared with Melville Island (Pittman 2003; Firth et al. 2006). The higher density of possums on Bathurst Island may also reflect a higher availability of important food and shelter resources due to the absence of Asian water buffalo (Bubalus bubalis) and horse (Equus caballus), both of which are present on Melville Island (Stobo-Wilson et al. 2020a). The predation pressure on native mammal populations on Bathurst Island is also likely to be lower than on Melville Island due to the low density of feral cats (Davies et al. 2021).
Despite the very high frequency of fire at the Ranku site, populations of common brushtail possum and northern brown bandicoot in this area remain healthy. This was an unexpected result because frequent fire is often thought to make environmental conditions unfavourable for these species, due to the removal of critical food and shelter resources (Kerle 1998; Pardon et al. 2003; Griffiths and Brook 2015) and heightened exposure to predation (Stobo-Wilson et al. 2020b). It may be that in this area the negative impacts associated with very frequent fire are outweighed by the benefits associated with high productivity, the absence of feral herbivores and possibly lower predation pressure, thereby allowing populations to remain healthy. The Pickertaramoor site provides an interesting comparison to Ranku. Despite being similarly frequently burnt to the Ranku site, we recorded by far the lowest overall number of native mammal captures and individuals, and estimated a very low density of northern brown bandicoot (0.04 ha−1) at Pickertaramoor. Among our four sites, Pickertaramoor is characterised as the most degraded, with the highest activity of predators (feral cats and dingoes) and feral herbivores (Davies et al. 2021). In this area (contrary to the situation at Ranku), the negative impacts of very frequent fire are likely exacerbated by habitat degradation by feral herbivores and higher predation pressure, reducing the capacity of native mammal populations to persist.
Our results suggest that in productive northern Australian savannas, frequent fire may not necessarily drive species decline when other threats are either absent or relatively mild. However, it is important to note that we have only investigated fire frequency, not intensity. As northern brown bandicoot have been shown to prefer sites that have experienced fine-scale low intensity fires (compared with both unburnt and extensively burnt sites) (Pardon et al. 2003; Firth et al. 2006), the variation in bandicoot density across our sites could reflect variation in fire intensity. Given that large, high-intensity fires are likely to be particularly detrimental to small mammals (Firth et al. 2010; Lawes et al. 2015), we suggest that future research uses metrics that go beyond fire frequency and better gauge actual fire impact.
Another important caveat of our study is that it was conducted at only four locations. Hence, our capacity for robust inference of the ecological constraints on native mammal density is limited. Increasing the spatiotemporal replication of robust estimates of population density (for a greater number of species), will lead to a better understanding of population trajectories under varying combinations of threatening processes, as well as a better capacity to gauge the effectiveness of management interventions. However, our results clearly align with a recent conceptual model postulating that the state of native mammal populations across northern Australian savannas reflects a complex, and spatially-variable interplay of ‘bottom-up’ (e.g. resource availability) and ‘top-down’ processes (e.g. predation) (Stobo-Wilson et al. 2020a). This has important management implications. For example, as possum and bandicoot populations remain healthy at the frequently burnt Ranku site, targeted fire management aimed at reducing fire frequency in this area may be unnecessary and not offer the same benefits to these species as if it were applied in other areas, such as Pickertaramoor. In other words, the benefits of targeted management actions can vary across the landscape depending on how the occurrence and severity of disturbance processes are mitigated by landscape productivity. Given limited operational budgets for management agencies, a more spatially-tailored approach to management, based on a greater understanding of how the occurrence and severity of disturbance processes are mitigated by landscape productivity, may be more effective.
Data availability
The data used in this publication are owned by the Tiwi Land Council. Access to this data can be negotiated with the Tiwi Land Council.
Conflicts of interest
The authors declare no conflicts of interest.
Acknowledgements
We thank the traditional owners of the Tiwi Islands for their ongoing support of scientific research on their land. This study was supported by funding from the Australian Government’s National Environmental Science Program via the Threatened Species Recovery Hub, the Australian Research Council (LP170100305) and the Indigenous Land and Sea Corporation. Data were collected with ethics approval from Charles Darwin University (A19006) and a permit from the Northern Territory Government (permit no. 64 876). We thank Murray Efford for his helpful advice regarding the analyses, Graeme Gillespie for his input on field methods and study design, as well as the amazing field assistance of expert mammalogists Brien Roberts, Jaime Heiniger and Cara Penton.
References
Andersen, A. N., Braithwaite, R. W., Cook, G. D., Corbett, L. K., Williams, R. J., Douglas, M. M., Gill, A. M., Setterfield, S. A., and Muller, W. J. (1998). Fire research for conservation management in tropical savannas: introducing the Kapalga fire experiment. Australian Journal of Ecology 23, 95–110.| Fire research for conservation management in tropical savannas: introducing the Kapalga fire experiment.Crossref | GoogleScholarGoogle Scholar |
Burbidge, A. A., McKenzie, N. L., Brennan, K. E. C., Woinarski, J. C. Z., Dickman, C. R., Baynes, A., Gordon, G., Menkhorst, P. W., and Robinson, A. C. (2009). Conservation status and biogeography of Australia’s terrestrial mammals. Australian Journal of Zoology 56, 411–422.
| Conservation status and biogeography of Australia’s terrestrial mammals.Crossref | GoogleScholarGoogle Scholar |
Burnham, K. P. and Anderson, D. R. (2002). ‘Model selection and multimodel inference: a practical information-theoretic approach,’ (Springer-Verlag: New York.)
Davies, H. F., McCarthy, M. A., Firth, R. S. C., Woinarski, J., Gillespie, G. R., Andersen, A. N., Geyle, H., Nicholson, E., and Murphy, B. P. (2017). Top-down control of species distributions: feral cats driving the regional extinction of a threatened rodent in northern Australia. Diversity and Distributions 23, 272–283.
| Top-down control of species distributions: feral cats driving the regional extinction of a threatened rodent in northern Australia.Crossref | GoogleScholarGoogle Scholar |
Davies, H. F., McCarthy, M. A., Firth, R. S. C., Woinarski, J. C. Z., Gillespie, G. R., Andersen, A. N., Rioli, W., Puruntatameri, J., Roberts, W., Kerinaiua, C., Kerinauia, V., Womatakimi, K., and Murphy, B. P. (2018). Declining populations in one of the last refuges for threatened mammal species in northern Australia. Austral Ecology 43, 602–612.
| Declining populations in one of the last refuges for threatened mammal species in northern Australia.Crossref | GoogleScholarGoogle Scholar |
Davies, H. F., Maier, S. W., and Murphy, B. P. (2020). Feral cats are more abundant under severe disturbance regimes in an Australian tropical savanna. Wildlife Research 47, 624–632.
| Feral cats are more abundant under severe disturbance regimes in an Australian tropical savanna.Crossref | GoogleScholarGoogle Scholar |
Davies, H. F., Rangers, Tiwi Land, Rees, M. W., Stokeld, D., Miller, A. C., Gillespie, G. R., and Murphy, B. P. (2021). Variation in feral cat density between two large adjacent islands in Australia’s monsoon tropics. Pacific Conservation Biology , .
| Variation in feral cat density between two large adjacent islands in Australia’s monsoon tropics.Crossref | GoogleScholarGoogle Scholar |
Efford, M. (2004). Density estimation in live‐trapping studies. Oikos 106, 598–610.
| Density estimation in live‐trapping studies.Crossref | GoogleScholarGoogle Scholar |
Efford, M. (2020). secr: Spatially explicit capture-recapture models. R package version 4.2.2. Available at: https://CRAN.R-project.org/package=secr
Firth, R. S. C., Woinarski, J. C. Z., Brennan, K. G., and Hempel, C. (2006). Environmental relationships of the brush-tailed rabbit-rat, Conilurus penicillatus, and other small mammals on the Tiwi Islands, northern Australia. Journal of Biogeography 33, 1820–1837.
| Environmental relationships of the brush-tailed rabbit-rat, Conilurus penicillatus, and other small mammals on the Tiwi Islands, northern Australia.Crossref | GoogleScholarGoogle Scholar |
Firth, R. S. C., Brook, B. W., Woinarski, J. C. Z., and Fordham, D. A. (2010). Decline and likely extinction of a northern Australian native rodent, the brush-tailed rabbit-rat Conilurus penicillatus. Biological Conservation 143, 1193–1201.
| Decline and likely extinction of a northern Australian native rodent, the brush-tailed rabbit-rat Conilurus penicillatus.Crossref | GoogleScholarGoogle Scholar |
Geyle, H. M., Woinarski, J. C., Baker, G. B., Dickman, C. R., Dutson, G., Fisher, D. O., Ford, H., Holdsworth, M., Jones, M. E., and Kutt, A. (2018). Quantifying extinction risk and forecasting the number of impending Australian bird and mammal extinctions. Pacific Conservation Biology 24, 157–167.
| Quantifying extinction risk and forecasting the number of impending Australian bird and mammal extinctions.Crossref | GoogleScholarGoogle Scholar |
Griffiths, A. (2013). Beds are burning: small mammal responses to fire in tropical savannas of northern Australia. School of Environment, Charles Darwin University, Darwin, Northern Territory, Australia.
Griffiths, A. D., and Brook, B. W. (2015). Fire impacts recruitment more than survival of small-mammals in a tropical savanna. Ecosphere 6, 1–22.
| Fire impacts recruitment more than survival of small-mammals in a tropical savanna.Crossref | GoogleScholarGoogle Scholar |
Heiniger, J., Cameron, S. F., and Gillespie, G. (2018). Evaluation of risks for two native mammal species from feral cat baiting in monsoonal tropical northern Australia. Wildlife Research 45, 518–527.
| Evaluation of risks for two native mammal species from feral cat baiting in monsoonal tropical northern Australia.Crossref | GoogleScholarGoogle Scholar |
Heiniger, J., Davies, H. F., and Gillespie, G. R. (2020). Status of mammals on Groote Eylandt: safe haven or slow burn? Austral Ecology 45, 759–772.
| Status of mammals on Groote Eylandt: safe haven or slow burn?Crossref | GoogleScholarGoogle Scholar |
IUCN (1996). ‘1996 IUCN Red List of Threatened Animals.’ (IUCN: Gland, Switzerland.)
Kerle, J. (1998). The population dynamics of a tropical possum, Trichosurus vulpecula arnhemensis Collett. Wildlife Research 25, 171–181.
| The population dynamics of a tropical possum, Trichosurus vulpecula arnhemensis Collett.Crossref | GoogleScholarGoogle Scholar |
Lawes, M. J., Murphy, B. P., Fisher, A., Woinarski, J. C., Edwards, A., and Russell-Smith, J. (2015). Small mammals decline with increasing fire extent in northern Australia: evidence from long-term monitoring in Kakadu National Park. International Journal of Wildland Fire 24, 712–722.
| Small mammals decline with increasing fire extent in northern Australia: evidence from long-term monitoring in Kakadu National Park.Crossref | GoogleScholarGoogle Scholar |
MacKenzie, D. I., and Kendall, W. L. (2002). How should detection probability be incorporated into estimates of relative abundance? Ecology 83, 2387–2393.
| How should detection probability be incorporated into estimates of relative abundance?Crossref | GoogleScholarGoogle Scholar |
Murphy, B. P., and Davies, H. F. (2014). There is a critical weight range for Australia’s declining tropical mammals. Global Ecology and Biogeography 23, 1058–1061.
| There is a critical weight range for Australia’s declining tropical mammals.Crossref | GoogleScholarGoogle Scholar |
Pardon, L. G., Brook, B. W., Griffiths, A. D., and Braithwaite, R. W. (2003). Determinants of survival for the northern brown bandicoot under a landscape-scale fire experiment. Journal of Animal Ecology 72, 106–115.
| Determinants of survival for the northern brown bandicoot under a landscape-scale fire experiment.Crossref | GoogleScholarGoogle Scholar |
Pittman, G. W. (2003). Occurrence and use of tree hollows by mammals in fragmented and continuous savanna woodlands in northern Australia. B.Sc. (Hons.) thesis, Northern Territory University, Darwin, Northern Territory, Australia.
Pope, L. C. (2001). Population structure of the northern bettong, Bettongia tropica and the northern brown bandicoot, Isoodon macrourus. PhD thesis, School of Biological Sciences University of Queensland, St Lucia, Qld, Australia.
R Development Core Team (2013). ‘R: a language and environment for statistical computing.’ (R Foundation for Statistical Computing: Vienna, Austria.)
Royle, J. A., Chandler, R. B., Sollmann, R. and Gardner, B. (2013). ‘Spatial capture-recapture.’ (Academic Press: Cambridge, MA.)
Slade, N. A., and Blair, S. M. (2000). An empirical test of using counts of individuals captured as indices of population size. Journal of Mammalogy 81, 1035–1045.
| An empirical test of using counts of individuals captured as indices of population size.Crossref | GoogleScholarGoogle Scholar |
Stobo-Wilson, A., Stokeld, D., Einoder, L., Davies, H., Fisher, A., Hill, B., Murphy, B., Scroggie, M., Stevens, A., Woinarski, J., Rangers, Djelk, Rangers, Warddeken, and Gillespie, G. (2020a). Bottom-up and top-down processes influence contemporary patterns of mammal species richness in Australia’s monsoonal tropics. Biological Conservation 247, 108638.
| Bottom-up and top-down processes influence contemporary patterns of mammal species richness in Australia’s monsoonal tropics.Crossref | GoogleScholarGoogle Scholar |
Stobo-Wilson, A. M., Stokeld, D., Einoder, L. D., Davies, H. F., Fisher, A., Hill, B. M., Mahney, T., Murphy, B. P., Stevens, A., Woinarski, J. C. Z., Rangers, Djelk, Rangers, Warddeken, and Gillespie, G. R. (2020b). Habitat structural complexity explains patterns of feral cat and dingo occurrence in monsoonal Australia. Diversity and Distributions 26, 832–842.
| Habitat structural complexity explains patterns of feral cat and dingo occurrence in monsoonal Australia.Crossref | GoogleScholarGoogle Scholar |
Woinarski, J. C. Z., Milne, D. J., and Wanganeen, G. (2001). Changes in mammal populations in relatively intact landscapes of Kakadu National Park, Northern Territory, Australia. Austral Ecology 26, 360–370.
| Changes in mammal populations in relatively intact landscapes of Kakadu National Park, Northern Territory, Australia.Crossref | GoogleScholarGoogle Scholar |
Woinarski, J. C. Z., Armstrong, M., Brennan, K., Fisher, A., Griffiths, A. D., Hill, B., Milne, D. J., Palmer, C., Ward, S., Watson, M., Winderlich, S., and Young, S. (2010). Monitoring indicates rapid and severe decline of native small mammals in Kakadu National Park, northern Australia. Wildlife Research 37, 116–126.
| Monitoring indicates rapid and severe decline of native small mammals in Kakadu National Park, northern Australia.Crossref | GoogleScholarGoogle Scholar |
Woinarski, J. C. Z., Legge, S., Fitzsimons, J. A., Traill, B. J., Burbidge, A. A., Fisher, A., Firth, R. S. C., Gordon, I. J., Griffiths, A. D., Johnson, C. N., McKenzie, N. L., Palmer, C., Radford, I., Rankmore, B., Ritchie, E. G., Ward, S., and Ziembicki, M. (2011). The disappearing mammal fauna of northern Australia: context, cause, and response. Conservation Letters 4, 192–201.
| The disappearing mammal fauna of northern Australia: context, cause, and response.Crossref | GoogleScholarGoogle Scholar |
Woinarski, J. C. Z., Burbidge, A. A., and Harrison, P. L. (2015). Ongoing unraveling of a continental fauna: decline and extinction of Australian mammals since European settlement. Proceedings of the National Academy of Sciences 112, 4531–4540.
| Ongoing unraveling of a continental fauna: decline and extinction of Australian mammals since European settlement.Crossref | GoogleScholarGoogle Scholar |
Woolley, L.-A., Murphy, B. P., Radford, I. J., Westaway, J., and Woinarski, J. C. (2018). Cyclones, fire, and termites: the drivers of tree hollow abundance in northern Australia’s mesic tropical savanna. Forest Ecology and Management 419, 146–159.
| Cyclones, fire, and termites: the drivers of tree hollow abundance in northern Australia’s mesic tropical savanna.Crossref | GoogleScholarGoogle Scholar |