Food and feed, mycotoxins and the perpetual pentagram in a changing animal production environment
Wayne L. BrydenThe University of Queensland, School of Agriculture and Food Sciences and Centre for Animal Science, Queensland Alliance for Agriculture and Food Innovation, Gatton, Qld 4343, Australia. Email: w.bryden@uq.edu.au
Animal Production Science 52(7) 383-397 https://doi.org/10.1071/AN12073
Submitted: 28 February 2012 Accepted: 30 March 2012 Published: 8 May 2012
Journal Compilation © CSIRO Publishing 2012 Open Access CC BY-NC-ND
Abstract
G. L. McClymont developed a unique paradigm in which to consider the challenges that confront agriculture and it is based on an understanding of the interrelationships of plants, animals, soils and water within an economic and social framework. The major changes in our environment are the consequence of rapid population growth and the need to increase world food supplies. Within this context, this paper provides an overview of the link between agriculture, especially animal production and population health and how mycotoxins, fungal secondary metabolites, can perturb this link. Examples from New Zealand and Australian animal agriculture are described. The underlying premise of this paper is that agriculture is a major determinant of human health through the supply of food derived from both plant and animal sources. In other words, nutrition is the conduit between agriculture and human health. Against this backdrop the potential role of mycotoxins in determining food and feed supplies is discussed. Globally, mycotoxins have significant human and animal health, economic and international trade implications.
Introduction
Professor G. L. (Bill) McClymont (1920–2000) was a veterinarian and nutritional scientist, who arrived at the University of New England (UNE) in 1955 to establish a new agricultural faculty that was to bring together aspects of traditional agricultural and veterinary science education with a focus on whole-farm production. The ecological degree that emerged, Rural Science, was unique in that it provided students with a holistic view of agriculture based on an understanding of plants, animals, soils and water within an economic and social framework; a paradigm in which to consider the challenges that confront agriculture. In many respects this approach to agricultural education and research is summed up by the title of McClymont’s 1955 inaugural lecture ‘All Flesh is Grass’ (McClymont 1996).
A biography of McClymont has not been published, but a former colleague, Professor J. S. Ryan has edited two books that provide an insightful overview of the man and his achievements. Rural Science: Philosophy and Application (Ryan 1996) contains many difficult to obtain papers by McClymont and contains his reflections and those of former colleagues and students. In this book Professor R. A. Leng described McClymont thus:
‘His vision was of a self-sustaining, soil and livestock agriculture based on ecological principles, where these principles were affected by social, political and international events. His vision was never static and it has evolved with changing world conditions.’ (Leng 1996)
McClymont’s vision is depicted in Fig. 1. The second book, McClymont’s Vision: The Challenge Remains (Ryan 2007) is mainly reflections by Rural Science graduates on the degree at UNE and celebrates the 50 years since its inception. At Easter 2011, my graduating class gathered in Armidale to reflect on the 40 years since our graduation. We agreed, that irrespective of our career paths (including animal or plant research and associated industries, farming, veterinary science, secondary and tertiary education, Government sector positions, finance, real estate and the Church) our undergraduate training in a holistic approach to problem solving had been of great assistance.
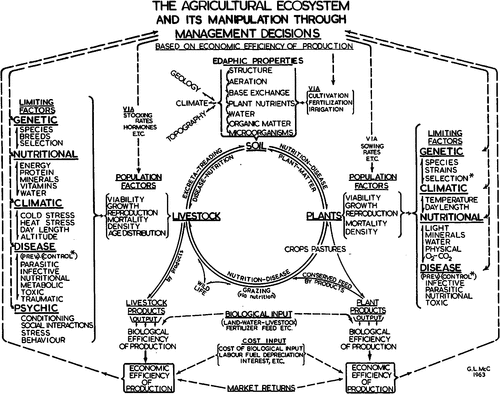
|
The title of this paper reflects my interests in nutrition and toxicology and in many respects mirrors McClymont’s interest in both these topics as title of his initial Chair at UNE was Chemical Pathology. Food and feed in the title refer to man and animals, respectively, and the growing competition between the two for world grain supplies. Fungi pose a major threat to world grain supply as plant pathogens and also due to their ability to contaminate grain with secondary metabolites or mycotoxins, some of which are carcinogens. Mycotoxins pose a threat to food/feed security and along with climate change, globalisation and an increasing world population are significant components of the changing environment in which we live. The final words in the title come from The perpetual pentagram: evolution, ecology, economics, ethics and education, a paper McClymont presented to the Ecology Society of Australia (McClymont 1970), which describes the breath of understanding required to define and to develop potential solutions to the major problems confronting the global community. Within this context, this paper provides an overview of the link between agriculture, especially animal production and population health and how mycotoxins can perturb this link. The underlying premise of this paper is that agriculture is a major determinant of human health through the supply of food derived from both plant and animal sources. In other words, nutrition is the conduit between agriculture and human health. Against this backdrop what potential role do mycotoxins play in determining global food and feed supplies?
Challenges of a changing global environment
The world population has reached 7 billion (October 2011) and is expected to climb to ~9 billion by the middle of this century and then stabilise and perhaps decline (Lutz and Samir 2010). It is the massive increase in the number of humans that has occurred over the last century, accompanied by increased affluence that is precipitating a cascade of environmental, economic, political and cultural changes including:
-
Globalisation and the global financial crisis;
-
Global warming and climate change;
-
Depletion of fossil fuel reserves;
-
Competition for resources and deforestation;
-
Scarcity of water;
-
Loss of biodiversity, both plant and animal;
-
Food security and safety;
-
Increased demand for meat;
-
Supermarket retailing and consumer preference;
-
Animal rights and liberation;
-
Zoonotic diseases.
These are all significant issues, most interacting, and many have far-reaching implications for life on earth and all have implications for animal production (Tribe 1994; Cheeke 2004; Cribb 2010; Farrell 2010; Smith 2011). The gravity of these issues can be appreciated by the number of books on these topics published by the popular press, some based on instinct and others on science. Awareness of the implications of an ever-increasing human population is not a recent phenomenon. It is a concern that has been voiced throughout human history as we have progressed from the agricultural, industrial and now the ‘i-phenomenon’ revolutions, most famously by Malthus (1798) over 200 years ago.
Population concerns in the past have been overcome by breakthroughs in science that have facilitated continued population growth, for example, the Green revolution and our ability to combat most infectious diseases of plants and animals. This has secured our food supply and when coupled with improvements in human disease prevention has allowed the human population to increase virtually unchecked, despite the many deaths that have occurred in wars (Lutz and Samir 2010). How many more people can be accommodated on the earth with increased rates of depletion of finite resources (fossil fuel, arable land, phosphates, water) is a legitimate concern on which McClymont also wrote (see Ryan 1996). Moreover, as all those involved in animal production appreciate, there is an optimum stocking density and beyond that production declines or in the case of the human animal, lifestyle diminishes or for those less fortunate, famine and pestilence consume them.
Globally the major concern for the majority of the world’s population is food security. Animal products play an important role in maintaining food supplies and contribute ~16% of energy and 38% of protein consumed globally (CAST 1999). There has been a significant increase in the demand for meat, milk and eggs over the last four decades (Speedy 2003; Thornton 2010). This reflects, not only population increase but also increasing affluence and what economists call Bennett’s Law: ‘as people become wealthier, they switch from simple starchy plant diets to a more varied food input that includes a range of vegetables, fruit, dairy products, and especially meat’ Godfray (2011).
The increased demand for animal products is accompanied by an increased utilisation of resources and as Thornton (2010) has intimated, future patterns of animal product demand will be modified by competition for resources, climate change, socio-cultural factors, ethical concerns and technological developments. Notwithstanding these drivers of change there is increasing concern about the competition between man and animals for the global supplies of grain which has been exacerbated by the use of cereal grains, especially maize, for biofuel production (Wu and Munkvold 2008). It is not possible within the present paper to discuss the complexities of this conflict between animals and man. It is the subject of another review in this issue (Hegarty 2012) and has been reviewed by others (see Cheeke 2004; Keyzer et al. 2005; Farrell 2010; Swick 2011). Likewise the global capacity to meet the increasing demand for cereal grains that will require both increased yields and cropping intensity has been the subject of numerous reviews (see Tester and Langridge 2010; Gregory and George 2011).
Plant disease is a significant factor that can adversely impact on the grain supply for both human and animal nutrition. Fungi are important plant pathogens globally and pose a threat to food security by reducing crop yields and in some instances contaminating grain with mycotoxins. The latter is also a significant food safety issue as some mycotoxins are carcinogens, mutagens and others teratogens (Haschek et al. 2002; Cawdell-Smith et al. 2007b). It has been estimated that some 25% of the world’s grain crops are affected annually by fungal invasion and mycotoxin contamination (Mannon and Johnson 1985), which indicates the enormity of the fungal threat. Most predictions indicate that with global warming, the threat from fungal invasion of crops will increase (Strange and Scott 2005; Garrett et al. 2006; Magan et al. 2011; Wu et al. 2011).
Mycotoxins and the link between agriculture and health
The link between human health and agriculture is through food; its sources, composition and distribution. Food sources include both plant and animal and the availability and composition of the latter is largely determined by the cost of plant-based feedstuffs. It is not surprising therefore, that any consideration of population demographics demonstrates the importance of agricultural production as a major determinant of public health (Scholthof 2003) as agriculture is the major source of our food. This would appear to be a straight forward proposition, embracing the adage ‘we are what we eat’, especially in tribal societies. However, as shown in Fig. 2, the relationship between agricultural production and human health is complex in a modern, developed society and measuring the impacts is difficult (Hawkesworth et al. 2010). For non-infectious human disease, the major cause is malnutrition whether it be a lack of food or excess consumption. Nutrient deficiencies are a major problem in many developing countries while excess intake leading to obesity and metabolic disease is an epidemic in developed countries. This double burden of nutrient deficiency and obesity is occurring simultaneously in some societies as the population becomes more affluent (Amuna and Zotor 2008).
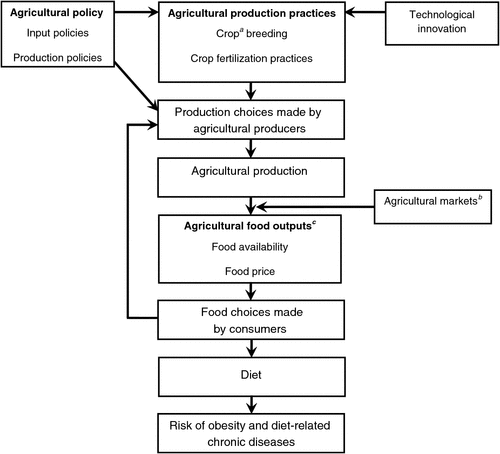
|
A feature of increasing affluence is that meat becomes a larger proportion of the diet. In many instances, the mechanism that allows impoverished families to improve their income and wellbeing is access to livestock or poultry (Delgado 2003). For many years there has been an ongoing debate about the benefit or otherwise of animal source foods, especially red meat consumption (see Givens 2010). In the past, claims of the detrimental effect of animal-sourced foods on human health have been made without rigorous scientific investigation (Blaxter 1991). There is no doubt, however, that animal source foods, including lean meat, fish, poultry, eggs and milk, are an excellent source of protein and micronutrients (Williams 2007; Givens 2010; Samman et al. 2012). It should not be forgotten that humans evolved as ‘meat eaters’ (see Cordain et al. 2004).
It is unlikely that we will curb our appetite for meat; as the demand grows, how will it be met? Following an in-depth, global and regional analysis, Keyzer et al. (2005) have made projections which show that the greatest demand will be for poultry meat, eggs, pork and dairy as Asia and Africa, the regions from where the largest demand is expected, have limited scope for expanded grazing. On the basis of their analysis, Keyzer et al. (2005) concluded that the world demand for cereal feed grain would be significantly higher over the next 30 years than currently estimated. Given this scenario, any factor that limits or reduces crop yields has the potential to significantly impact on the supply of human food of both plant and animal origin. Plant fungal diseases and associated mycotoxins have that capacity.
Fungi are ubiquitous and each agricultural commodity is susceptible to attack by fungi under both field and storage conditions (Smith and Henderson 1991). All food and feedstuffs can be contaminated with mycotoxins but the specific mycotoxin(s) found and level of contamination varies with location and the ecopathology of the infecting fungus. This reflects crops grown, agronomic practices and climatic conditions, which dictate the fungi that are present in a farming system (Magan 2006; Bryden 2009). Moreover, not all isolates of a toxigenic fungus produce toxins, toxin production is often unrelated to fungal biomass and most mycotoxin build-up occurs in crops before harvest (Magan 2006). Wheat, sorghum and maize can be infected by different fungal pathogens, some of which are mycotoxigenic but the degree of contamination is modified by many factors including plant variety, ambient temperature, rainfall and insect damage (Wicklow 1995). Mycotoxins may also be produced during storage and transport of food and feed. Importantly, mycotoxins are generally very stable chemicals and remain intact during food and feed manufacture (Humpf and Voss 2004; Meister and Springer 2004).
It is likely that mycotoxins have contaminated the food supply since the beginning of agriculture. The toxicity of mouldy grain has been appreciated for centuries with reports from China some 5000 years ago, the Bible, including the 10 plagues of Egypt and ergotism in the Middle Ages (Matossian 1989; Ramos et al. 2011). Moreover, the association of mouldy food with disease has been recognised in Japan, Russia and the USA for over a century (Richard 2008; Bryden 2009). However, the significance of mouldy foods and feeds as a possible cause of human and animal disease gained international attention in 1960 with outbreaks of ‘turkey X’ disease in England (Allcroft et al. 1961; Blount 1961). The causal agent, aflatoxin B1 was subsequently shown to be the most potent natural carcinogen known (Richard 2008; Wogan et al. 2012). This highlights the potential health hazard of these natural environmental contaminants. There are thousands of secondary fungal metabolites (Cole et al. 2003; Brase et al. 2009) of diverse chemical structures, some are beneficial (e.g. antibiotics) and ~400 classified as mycotoxins. Despite the potential toxicity of mycotoxins and their regulation in many countries (van Egmond and Jonker 2004) there is a public perception that these are innocuous chemical contaminants. However, as shown in Table 1 this view is not shared by scientists (see Pohland and Yess 1992; Wild 2007).
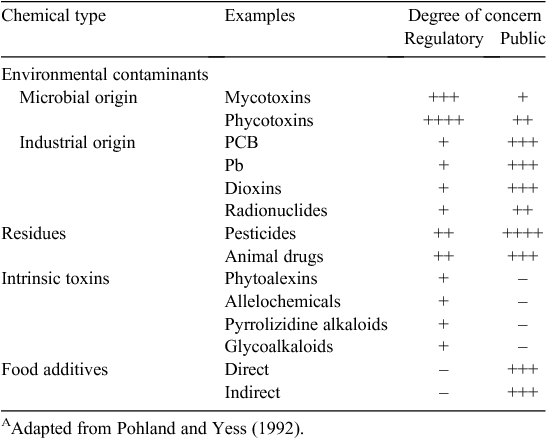
|
In view of the ranking of mycotoxins as very significant food contaminants it is interesting to read the work of the historian, Mary Matossian (1989) who contends that mycotoxins have probably been responsible for more loss and suffering in animals and man than any other class of food toxin. In particular, she makes the following observation:
‘It may seem irregular to use plant health and climatic indicators of plant health as an index of human health, but the two are intimately related. If plants that people eat are toxic or diseased, people will either be diseased or simply not as healthy and fertile as they would be if they were properly nourished. The population in the area identified more or less, in the heart of western civilisation, did not begin to thrive until the 16th century (and then only slowly until ~1750) and they grew at different rates in different regions. These regional differences in fact highlight the effects of plant health on human health because of the varied conditions of plant growth in different environments’.
She further explains that epidemics, outbursts of bizarre behaviour and low fertility and high death rates from the 14th to the 18th century may have been caused by food poisoning from mycotoxins in bread, the staple food in Europe and America during this period (Matossian 1989), especially ergot alkaloids.
Mycotoxin contamination can occur throughout all phases of the human food (Bryden 2009) and animal feed (Smith and Henderson 1991; Pettersson 2004; Bryden 2012) supply chains. Human populations are predominantly exposed to mycotoxins through plant-derived foods (Miller 2008) and the toxins most significant globally are aflatoxins, ochratoxin A, deoxynivalenol (DON), fumonisins, zearalenone and ergot alkaloids (Bryden 2007, 2009; Richard 2007). On occasion, people can be subjected to mycotoxins from the air where grain dust or airborne spores are the vehicle (Degen 2011). In contrast, animal products are an unlikely source of mycotoxins or their metabolites (Bryden 2009) with the possible exception of milk (Pettersson 2004; Fink-Gremmels 2008; Coffey et al. 2009). However, the impact of mycotoxins is different between developed and developing countries (Wild 2007; Shephard 2008). In developed countries, most mycotoxin contamination occurs pre-harvest, in contrast to developing countries where contamination occurs both pre- and post-harvest. The differences reflect agronomic practices in the different regions and also the extent and quality of storage facilities available in developed compared with developing countries. The major concern of mycotoxin contamination in developing countries is its impact on human health due to greater exposure to mycotoxins than their counterparts in developed countries (Miller 1998; Shier et al. 2005; Wild 2007). The human maladies that have been linked to mycotoxins are shown in Table 2. Although acute intoxication is rare in humans there is growing awareness in developing countries of the chronic effects of mycotoxins in human populations including reduced growth rate, immunosuppression and increased risk of cancer (Wild and Gong 2010). Mycotoxin contamination of the food and feed supply is tightly regulated to reduce human and animal exposure in developed countries The additional costs to the producer and/or the consumer to meet the economic burden of regulating the food and feed supply is the major mycotoxin concern in developed economies (Shier et al. 2005). The second major concern in both developed and developing countries is the impact of mycotoxins on animal health and production (Bryden 2012).
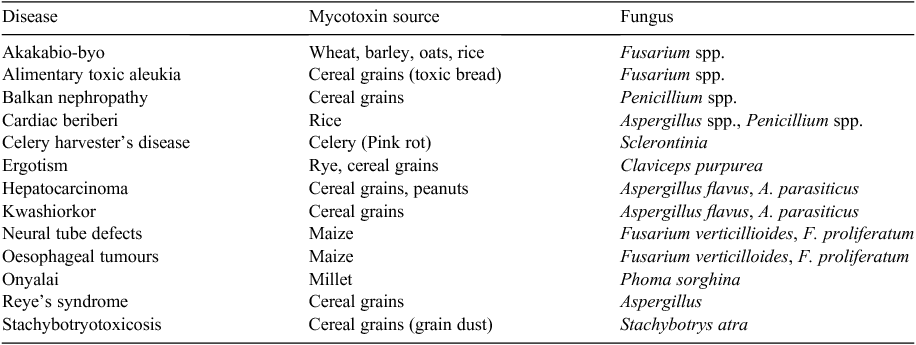
|
In Australia and New Zealand, there have been no cases of human mycotoxicoses reported. The latest annual Australian Total Diet Study conducted by Food Standards Australia and New Zealand did not find detectable levels of mycotoxins (aflatoxin B1, B2, G1, G2 and M1; DON; zearalenone; ochratoxin A; fumonisin B1 and B2; patulin) (FSANZ 2011). Nevertheless, peanuts and our major cereal crops (wheat, sorghum and maize) can be significantly contaminated with mycotoxins. The peanut industry has developed very sophisticated systems to essentially eliminate aflatoxin contamination, including novel biocontrol strategies (Dorner 2008). Contaminated grain is usually diverted to animal feed where dilution and the use of feed additives (see below) minimise animal effects. In contrast, the human consumer may experience increased costs due to reduced crop yield and the costs associated with monitoring. As discussed in the next section, all of the major mycotoxicoses, with the exception of ochratoxicosis, have been diagnosed in Australian and/or New Zealand livestock and poultry; providing unequivocal evidence of the presence of toxigenic fungi and mycotoxins in our food and feed chains.
Occurrence of mycotoxins on both sides of the Tasman
Australia and New Zealand are separated by the Tasman Sea and occupy different and latitudes. The geographical factors which determine rainfall, cropping practices and the fungi in agricultural systems vary between both countries and also between regions in each country. Therefore, mycotoxin occurrence and animal exposure will reflect these differences. The mycotoxin literature is dominated by studies that concentrate on the ramifications of mycotoxins in cereal grains, especially maize, and come largely from the northern hemisphere. In contrast, mycotoxins and associated mycotoxicoses in grasses and forages are a much greater source of economic loss in the Australian and New Zealand animal industries; reflecting our climate and animal production practices (Bryden 1998; Smith and Towers 2002).
Cereals, predominantly wheat, are grown and harvested under dryland conditions and then stored in very dry conditions. Maize, which is much more likely to be contaminated with mycotoxins than other cereal grains, (Miller 2008) is rarely used in Australian or New Zealand animal diets. This is in contrast to most other advanced animal production countries. In our environment, ruminants normally graze all year in contrast to the intensive feeding practices used to support these animals during northern hemisphere winters. This situation is changing with intensification of the dairy industry and continued expansion of beef feedlots and the greater reliance on compounded cereal-based feeds. With these changes in production systems, the range of mycotoxins to which animals are exposed will change. It should not be overlooked, but grazing animals may also consume mycotoxins when pastures are infected with fungi, especially endophytes (Bryden 1998; Cheeke 1998). The endophytic fungal genus Neotyphodium (formerly Acremonium) colonises pasture grasses (tall fescue and perennial ryegrass) and produces a series of ergot alkaloids and related compounds (Bryden 1994; Miles et al. 1998; Cawdell-Smith et al. 2007a). There is also increasing evidence that fungi, especially Fusarium species normally associated with cereal grain may also produce toxins in pasture (Smith and Towers 2002). For convenience, the following discussion is divided into intensively managed animals and grazing animals and largely reflects mycotoxins and mycotoxicoses associated with cereal grains and forages, respectively.
Intensive animal systems
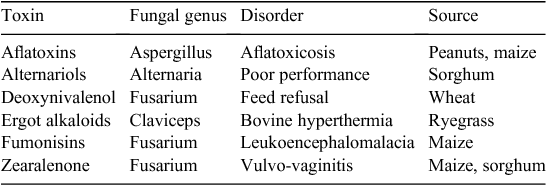
All of the major toxigenic fungi associated with cereal grains occur in Australia and New Zealand. There are documented cases of the associated mycotoxicoses in Australian livestock (Table 3) but there is not a similar list of documented cases for New Zealand. Nevertheless, grain surveys and toxicity screening of fungal isolates in New Zealand (Lauren et al. 1991, 1996; di Menna et al. 1997) demonstrate that the occurrence of grain contaminated with mycotoxins, especially those produced by Fusarium sp. is likely to be widespread. Moreover, there are New Zealand Government laboratory reports (Rammel 1991) of cases of aflatoxicosis and ergotism. The following discussion refers to major Australian cases that have been mainly associated with pigs and poultry.
|
Aflatoxin
Aflatoxin is produced by Aspergillus flavus and A. parasiticus, which are widespread in nature and local isolates are highly toxigenic (Bryden et al. 1975). However, due to climatic conditions and agronomic practices in this country this group of mycotoxins is not a major problem except for peanuts (Graham 1982; Wright et al. 2005). Maize and sorghum grown in northern Australia (Blaney 1985) can be contaminated occasionally. Aflatoxin has also been found in grains stored moist (Bryden et al. 1980). This toxin has many effects in animals at low dietary concentrations (see Bryden 2012) and is a major aetiological agent in the worldwide occurrence of human hepatocellular carcinoma (Henry et al. 1999; Wogan et al. 2012). Aflatoxicosis has occurred in poultry, sheep, pigs, cattle and dogs in Australia (Bryden 1982). Cyclopiazonic acid may also be a co-contaminant with aflatoxin. It is produced by many aflatoxigenic strains of A. flavus but it has not been reported in field outbreaks from Australia. Interestingly, there is evidence to suggest that the original outbreak of ‘turkey X’ disease may have been a combined toxicosis of aflatoxin and cyclopiazonic acid (Cole 1986b). This toxin, which perturbs calcium metabolism, is also produced by species of Penicillium, and is toxic to all farm animals (see Bryden et al. 2004).
Zearalenone
Zearalenone is a mycoestrogen and in biological systems mimics the effects of oestrogen (see Hagler et al. 2001; Fink-Gremmels and Malekinejad 2007). Hyperoestrogenism can be a problem in pigs consuming diets usually based on maize infected with Fusarium graminearium and containing 1–8 mg/kg zearalenone as demonstrated in a field outbreak in Queensland (Blaney and Williams 1991). Pigs are the most sensitive domestic species and disorders in pigs following exposure to zearalenone include infertility, characterised by constant oestrus, pseudopregnancy, reduced litter size, smaller and occasionally malformed offspring, and juvenile hyperoestrogenism (Hagler et al. 2001; Fink-Gremmels and Malekinejad 2007). Effects in cattle and avian species are much less pronounced than in pigs (Hagler et al. 2001).
Deoxynivalenol
DON (vomitoxin) is a trichothecene that is produced by Fusarium sp. and in Australia and New Zealand, predominantly F. graminiearum following infection of wheat, barley, sorghum and maize. It is interesting that the fungus has two chemotypes; one produces DON and its acetylate derivatives while the other chemotype produces nivalenol and its acetylate derivatives (Desjardins 2006). In Australia the DON chemotype dominates crops in northern New South Wales and southern Queensland while the nivalenol chemotype is found in far north Queensland on maize (Blaney and Dodman 2002). Both chemotypes are found together in New Zealand (Lauren et al. 1991) and both chemotypes produce zearalenone suggesting that animals may be exposed to several toxins simultaneously. Pigs are the most sensitive farm species to DON (Haschek et al. 2002). This toxin impairs feed intake and modifies the immune responsiveness of intoxicated animals (Pestka et al. 2004). Australian-grown wheat and triticale contaminated with DON have been associated with feed refusal and vomiting in pigs, the main clinical signs of intoxication (Moore et al. 1985; Bryden et al. 1987b). There is much disagreement in the literature as to the dietary concentration of DON needed to cause feed refusal in pigs. This may reflect the presence of the recently identified masked DON, which is not detected by normal analytical methods (Berthiller et al. 2009).
Fumonisins
Fumonisins are produced by Fusarium verticillioides (formerly F. moniliforme) and related species (Marasas et al. 2001). In human populations fumonisins have been associated with oesophageal cancer (Marasas et al. 2001) and neural tube defects (Gelineau-van Waes et al. 2005). The fungus has an endophytic relationship with maize and this crop has been found to be contaminated by fumonisins wherever it is grown with the possible exception of New Zealand (Shephard et al. 1996). The occurrence of F. verticillioides and fumonisins in Australian maize has been established (Bryden et al. 1995; Bricknell et al. 2008) and association with animal disease has been described (Bryden et al. 1998). Equine leucoencephalomalacia occurs in horses and donkeys following the consumption of maize contaminated with fumonisins. It is a neurological disease that results in liquefactive necrosis in the white matter of one or both cerebral hemispheres and this lesion is considered pathognomonic for equine leucoencephalomalacia (Haschek et al. 2002). Liver involvement is also seen in some horses. Pigs develop acute, massive pulmonary oedema, while sheep and cattle develop severe toxic nephrosis and hepatosis when dosed with fumonisins but poultry are relatively resistant (Voss et al. 2007). Equine leukoencephalomalacia has been diagnosed in Australia but there have been no reports of porcine pulmonary oedema (Bryden et al. 1998).
Ergot alkaloids
The term ‘ergot’ is the common name given to species of the Claviceps fungi. There are several fungi in this genera that cause classical or gangrenous ergotism (C. purpurea), paspalum staggers (C. paspali), and bovine hyperthermia (C. purpurea). Ergot also specifically refers to the sclerotium formed by C. purpurea when it parasitises the ovary of developing grass flowers (Bryden 1994). It is the diversity of ergot alkaloids produced by Claviceps species and found in the sclerotia that results in the toxicity of this genera. As discussed below, there are several closely related fungal endophytes that also produce a similar array of ergot alkaloids in grasses consumed by grazing animals (Bryden 1994; Cawdell-Smith et al. 2007a).
Ergots of C. purpurea can give rise to classical ergotism. In this disease, alkaloids contained within the sclerotium cause vasoconstriction of arterioles resulting in necrosis of extremities (nose, ears, tongue, tail and limbs) followed by dry gangrene (Bryden 1994). There has been one documented case of gangrenous ergotism in heifers reported from Western Australia (Fraser and Dorling 1983) and several cases of hyperthermia associated C. purpurea (Burgess et al. 1986; Jessup et al. 1987) in New South Wales in dairy cows and feedlot cattle. In the cases of bovine hyperthermia (41–42°C), increased respiration rate and excessive salivation occurred when daily temperatures exceeded 35°C and the syndrome has been reproduced by feeding beef and dairy cattle diets contaminated with the sclerotia of C. purpurea (Jang et al. 1987; Ross et al. 1989). There was no evidence of gangrene in any cattle, and the clinical expression of ergotism was mediated by ambient temperature (Bryden 1994). Death following hyperthermia of feedlot cattle has been reported in Western Australia after consumption of rations contaminated with C. purpurea (Peet et al. 1991).
C. purpurea infects cereal rye, ryegrass and some native grasses and its toxicity has been known for centuries (Barger 1931), but the toxicity of sorghum ergot (C. africana), was considered innocuous despite it being widespread in Africa and Asia. It was detected in Queensland in 1996 and infects only sorghum and related plants like Johnson grass (Ryley et al. 1996). However, C. africana-infected sorghum reduces performance in beef and dairy cattle, pigs and poultry (Blaney et al. 2000a, 2000b; Cawdell-Smith et al. 2007a). The alkaloids present in the contaminated sorghum are not as acutely toxic as those produced by C. purpurea (Blaney et al. 2003) but have a similar effect in depressing circulating levels of prolactin in animals as rye ergot alkaloids (Blaney et al. 2000a).
Alternaria mycotoxins
Alternaria is a common contaminant of cereal grains in Australia (Webley et al. 1997). Alternariol and its monomethyl ether, secondary metabolites of Alternaria, have been associated with poor production in broiler chickens but have such low toxicity in poultry that, their presence would not explain the production drops observed (Bryden et al. 1984). Nevertheless, many Australian isolates of Alternaria from wheat, barley and sorghum were toxic in a bioassay (Bryden et al. 1987a). However, field experience suggests that Alternaria mycotoxins pose little threat to animal production.
Grazing animal systems
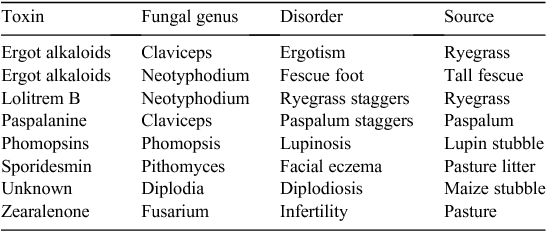
Ruminants and other grazing livestock (e.g. horses) are likely to encounter mycotoxins when grazing or consuming conserved fodder (Table 4). New Zealand has been at the forefront of research into the mycotoxicoses of grazing systems due to economic importance of cattle and sheep and the loss in productivity that a mycotoxicosis can cause. Facial eczema has been a major New Zealand problem for many years but is seen less frequently in Australia; it was described in detail by Clare (1952) before the discovery of aflatoxin. Staggers syndromes are important in both countries, lupinosis in Australia, and there is growing awareness in New Zealand of the potential of Fusarium sp. that are normally associated with cereal grains to produce mycotoxin on pasture. The significant mycotoxicoses of grazing animals are discussed below.
|
Facial eczema
di Menna et al. (2009) have reviewed in great detail the history of the occurrence and of the research that has delineated this mycotoxicosis. Spores produced by the fungus Pithomyces chartarum contain the mycotoxin sporidesmin, which causes facial eczema (pithomycotoxicosis), a hepatogenous photosensitisation in ruminants ingesting herbage containing the spores (Smith and Towers 2002). The toxin causes inflammation, damage and occlusion of bile ducts. Consequently, degradation products of chlorophyll (phylloerythrin) and bile are released into the general circulation, causing jaundice, photosensitisation, loss of production and in extreme cases death due to liver failure (Smith and Towers 2002). This is a major problem on the North Island of New Zealand, where it costs the dairy industry millions of dollars per year (Faull 1991), monitoring spore counts in pastures, administration of zinc salts at the time of exposure to reduce the amount of liver injury and breeding programs are all approaches used to alleviate this condition (Smith and Towers 2002). Facial eczema is seen in southern Australia, especially in the Gippsland region of Victoria and recently has been the subject of a major review by Dairy Australia (2011).
Lupinosis
Lupinosis is a mycotoxicosis primarily of sheep in Western Australia grazing dead lupin plants or stubble infected with Phomopsis leptostromiformis (Allen 1987). The teleomorph of this fungus has been classified as Diaporthe toxica (Williamson et al. 1994). The principal toxin, phomopsin A, is a linear, hexapeptide with a macrocylic ring (Edgar et al. 1986) and the liver is the primary organ affected. Natural outbreaks of the disease have also been reported in cattle, goats, donkeys and horses (Allen 1987).
Paspalum staggers
Paspalum staggers is caused by tremorgenic mycotoxins (paspalitrems) contained within the sclerotium or ergot of C. paspali. Clinical signs of paspalum staggers are typical of staggers syndromes in cattle and sheep and include tremors, incoordination, hyperexcitability and in severe cases, ataxia (Cole and Dorner 1986). An affected animal may appear normal until disturbed. This syndrome has been reported for over a century in New Zealand (Smith and Towers 2002) and on the east coast of Australia (Hurst 1942) in cattle, sheep, horses and deer grazing paspalum pastures. The clinical expression of the disease in horses has only recently been described (Cawdell-Smith et al. 2010). Due to changes in pasture composition and management practices the disease is now rarely encountered.
Fungal endophytes
The distinctive syndromes produced in animals by C. purpurea (gangrenous ergotism and hyperthermia) and C. paspali (paspalum staggers) have been described above and are identical to syndromes in animals grazing tall fescue (Festuca arundinacea Schreb) and perennial ryegrass (Lolium perenne), respectively (see Bryden 1994). In these instances the grasses are not infected by localised Claviceps sp. but by related systemic fungal endophytes. Endophytes are fungi that spend all or nearly all of their life cycles within the host plant and their presence has a positive effect on grass establishment and survival. The endophytes, Acremonium coenophialum and A. lolli, found in tall fescue and perennial ryegrass, respectively, have been reclassified from the genus Acremonium to the genus Neotyphodium (Smith and Towers 2002; Cawdell-Smith et al. 2007a).
Cattle grazing tall fescue may succumb to fescue foot or summer syndrome depending on whether it is winter or summer. These diseases are clinically identical to gangrenous ergotism and hyperthermia, respectively (Bryden 1994). With the isolation of ergovaline from endophyte-infected tall fescue it can be concluded that the major factors in the aetiology of the tall fescue-related disease and animal production problems are the concentrations of ergopeptine alkaloids in the grass and the prevailing climatic conditions (Reed et al. 2005; Cawdell-Smith et al. 2007a). Toxicoses associated with tall fescue occur occasionally in New Zealand (Smith and Towers 2002) but rarely in Australia (Culvenor 1974).
Ryegrass staggers (PRGS) can be a significant problem for animals grazing endophyte-infected perennial ryegrass in New Zealand (Smith and Towers 2002). It also costs graziers in southern Australia many millions dollars in lost production from sheep and cattle and has been the subject of a major review by Meat and Livestock Australia (Reed et al. 2005). Lolitrems are the toxins produced by the endophyte and are tremorgenic mycotoxins (Cole and Dorner 1986). Establishment of the association between the endophyte and PRGS was the result of New Zealand research (Fletcher and Harvey 1981). Once removed from contaminated pastures, animals usually recover. However, deaths from PRGS occur occasionally and are usually the result of accidents, e.g. drowning, or dying of dehydration or starvation (Cawdell-Smith et al. 2007a). It is now known that Neotyphodium lolli produces ergovaline in addition to lolitrem and the presence of ergovaline in perennial ryegrass may explain lamb losses when ewes graze these pastures. It is likely that the toxin reduces milk production by affected ewes. Reduced weight gains are often observed in sheep grazing endophyte-infected pastures, sometimes in the absence of overt staggers. These detrimental influences of the endophyte will be accompanied by a greater incidence of heat stress as the concentration of ergovaline in the grass increases (see Reed et al. 2005).
Fusarium related infertility and ill-thrift
New Zealand studies have demonstrated that zearalenone, is produced by Fusarium sp. on pastures (di Menna et al. 1987). Ewes ingesting these pastures have reduced reproductive performance without any other signs of intoxication and similar effects possibly occur in cattle (see Towers and Sprosen 1992; Smith and Morris 2006). A recent Australian survey suggests that zearalenone contamination of Australian pastures is widespread in some regions (Reed and Moore 2009). Interestingly, a derivative of zearalenone, α-zearalenol, has been used as a growth promoter in cattle (Hagler et al. 2001; Hunter 2010). Other New Zealand studies have demonstrated tricothecene production on pastures by Fusarium sp. (Lauren et al. 1988) and this is a possible cause of unexplained ill-thrift or poor growth in sheep and cattle (Litherland et al. 2004).
Implications for animal industries
The discussion above demonstrates that mycotoxins can cause overt disease in Australian and New Zealand animal enterprises. Fortunately, contamination levels in animal feedstuffs and forages are usually not high enough to cause an overt disease. Binder and her colleagues (Binder et al. 2007; Rodrigues and Griessler 2010; Rodrigues and Naehrer 2011) who have undertaken global surveys of mycotoxins (aflatoxin B1, DON, fumonisins ochratoxin and T-2 toxin) in animal feedstuffs annually since 2005 have demonstrated that mycotoxin contamination is widespread but at levels that would not cause overt disease. They found similar patterns of contamination each year but regional differences, especially between temperate and tropical areas. Significantly about half the samples analysed were contaminated with more than one toxin. However, low level toxin ingestion may cause an array of metabolic perturbations (Riley 1998; Haschek et al. 2002) resulting in poor animal productivity (Bryden 1982, 2012; Hamilton 1982; Smith and Henderson 1991; Dersjant-Li et al. 2003).
Diagnosis and impaired productivity
Bryden (2012) has reviewed the insidious consequences of mycotoxin ingestion, which impact negatively on all aspects of animal performance including:
-
Feed intake and growth rate;
-
Nutrient utilisation;
-
Reproduction;
-
Egg production and hatchability;
-
Immunomodulation;
-
Carcass quality and processing;
-
Tissue residues.
The severity of these disturbances in different animal production systems will depend on the level of mycotoxin present in the feed ingested, the duration of exposure, the physiological status of the animal and other environmental and disease factors (Haschek et al. 2002; Bryden 2012) that impact on the uptake, biotransformation, deposition and excretion of these toxins (Bryden 1982). The gut microflora may also modify mycotoxin toxicity (Annison and Bryden 1998; Eriksen et al. 2002). Of these effects, impairment of native and acquired resistance to disease is likely to have the most far-reaching consequences as these effects can occur at toxin intakes that do not impair feed intake, growth rate or egg production (Bryden 1982; Hamilton 1982). Under these circumstances, presence of a concurrent infection makes diagnosis of a mycotoxicosis difficult as the signs of the disease are associated with the infection rather than with the toxin that predisposed the animal to infection. Vaccine failures are also likely to occur in animals following low level mycotoxin exposure (Oswald et al. 2005).
Several authors (Feuell 1966; Hamilton 1978; Richard and Thurston 1986; Bryden 2012) have discussed the difficulties of diagnosing a mycotoxicosis when dietary concentrations of toxin are low. A confirmed mycotoxicosis largely depends upon the determination of the mycotoxin in the suspect feed at concentrations consistent with the clinical signs observed. It is not enough to isolate a toxigenic fungus (Richard and Thurston 1986). Changes in the composition of grain following fungal growth and alterations in nutrient intake and utilisation that accompany mycotoxin ingestion may further complicate diagnosis (Hamilton et al. 1988; Dersjant-Li et al. 2003; Bryden 2012). It is also likely that a mycotoxin-contaminated feed will contain low concentrations of more than one toxin. For example, aflatoxins, fumonisins, DON and zearalenone can occur together in the same grain (CAST 2003). Moreover, many fungi produce simultaneously several mycotoxins, especially Fusarium species (Desjardins 2006). Toxicity resulting from the interaction between mycotoxins is usually additive and not synergistic as often suggested (CAST 2003).
Costs associated with impaired productivity
Economic losses due to death are easy to determine but reduced animal productivity losses are difficult to quantify and may go unnoticed, especially when contamination is low (Charmley et al. 1994). Lost productivity may make it difficult to meet market contracts and have significant ramifications for producer viability, especially when profit margins are narrow. There are increased costs associated with feed loss, securing uncontaminated feed and the use of mycotoxin mitigating feed additives There is also additional cost to the industry as a whole, in terms of research, monitoring and extension; extra handling and distribution costs and possible legal suits (Bryden 2012). However, the major cost to the producer is determining the extent of contamination when a mycotoxin is suspected as the cause of lost production.
Sampling is the greatest source of error in quantifying mycotoxin contamination (Whitaker 2003) because of the difficulty of obtaining samples representative of the feed that may have caused a mycotoxicosis, the uneven distribution of toxins within a feed and the low concentrations, ranging from µg/g to mg/g, at which mycotoxins occur (CAST 1989, 2003). Quantification of these compounds requires sophisticated laboratory equipment and is a major analytical challenge due to the range of chemical compounds that mycotoxins represent and the vast array of feed matrices in which they occur (Cole 1986a; Shephard et al. 2011). Screening technology has been greatly assisted by the development of rapid, repeatable and sensitive enzyme-linked immunosorbent assays, which are commercially available for use in the field.
Reducing animal exposure
There are several approaches that can be taken to minimise mycotoxin contamination in the food and feed chains and these have been reviewed in detail by Bryden (2009). These involve prevention of fungal growth and therefore mycotoxin formation, strategies to reduce or eliminate mycotoxins from contaminated food commodities or diverting contaminated products to low risk uses and in many instances this means animal feed. The following general approaches, which apply to the mitigation of mycotoxin contamination of the human food chain (Bryden 2009) also apply to the animal feed chain:
-
Genetic modification of fungi and crops;
-
Agronomic and biological control measures;
-
Climate modelling to predict mycotoxin risk;
-
Storage management;
-
Food processing;
-
Detoxification;
-
Integrated mycotoxin management;
-
Human intervention.
Integrated mycotoxin management is an approach that attempts to reduce contamination throughout the chain by applying Hazard Analysis Critical Control Points (HACCP). The successful application of this approach should reduce mycotoxin contamination in all sectors of the chain and have the added advantage of increasing production efficiency (Lopez-Garcia 2001; Aldred and Magan 2004). Several HACCP programs have been developed for mycotoxins (Aldred and Magan 2004; Lopez-Garcia et al. 2008).
Where contamination occurs on-farm, simple measures such as storage of grain at a moisture level below 13% with rapid turnover of feed and/or the use of mould inhibitors significantly reduces the opportunity for fungal growth and mycotoxin production (Bryden 2012). Approaches to detoxification of mycotoxin-contaminated grain and feed have included physical, chemical and biological treatments (Cole 1989; Trenholm et al. 1989; CAST 2003; Kabak et al. 2006; Jouany 2007). All processes that seek to decontaminate feedstuffs, especially where rigorous chemical or heat treatments are involved, must be cost effective and must not reduce the nutritional content of the grain or feed if they are to be accepted generally. However, as indicated above, in many situations mycotoxin-contaminated cereal grains are diverted to livestock feed manufacture, thus attempts to mitigate the effects of mycotoxins in animals have largely centred around techniques that reduce exposure to the toxins, either by dilution with uncontaminated grain or feed application of binding or trapping agents.
The approach to mycotoxin mitigation that is most widely adopted by the animal feed industry is the use of feed additives that prevent toxin absorption. A diverse variety of substances have been investigated as potential mycotoxin-binding agents (Galvano et al. 2001; Huwig et al. 2001; Jouany 2007). Hydrated sodium calcium aluminosilicate is a high affinity adsorbent for aflatoxin, capable of forming a very stable complex with the toxin and hence reducing its bioavailability but is less effective with other mycotoxins (Phillips 1999). Jouany (2007) has reviewed the use of a yeast cell wall derived glucomanin prepared for Saccharomyces cerevisiae, which has been shown to adsorb aflatoxin, zearalenone and fumonisins with varying efficiency. Recently, considerable effort has concentrated on isolating microorganisms and/or enzymes from diverse sources that will metabolise a mycotoxin rendering it nontoxic (Molnar et al. 2004). Using this novel approach, success has been achieved with a stabilised bacterium that detoxifies trichothecenes (Binder et al. 2001; Fuchs et al. 2002) and enzymes from a soil bacterium (Sphingopyxis sp.) that degrade fumonisins (Heinl et al. 2010, 2011).
The perpetual pentagram revisited
In the 50 years since the discovery of aflatoxin, many other mycotoxins have been discovered and their toxic effects delineated. The ecopathology of many of the fungi involved is now much better understood, but as they are normal inhabitants of the natural environment, mycotoxins will continue to contaminate the food and feed chains. Reducing the occurrence and the impact of these toxins will require an integrated understanding of crop biology, agronomy, fungal ecology, harvesting methods, storage conditions, food or feed processing and detoxification strategies, animal production systems and human cultural practices (see Bryden 2007, 2009, 2012). These areas embody the basic and applied scientific aspects of mycotoxicology but the scope of the global problem generated by mycotoxin contamination of food and feed can only be gauged when the implications for human and animal health, economic sustainability and international trade (Wu 2004; Wild 2007; Bryden 2009; Wild and Gong 2010) are considered. Within this context, McClymont’s perpetual pentagram provides a framework to grapple with the enormity of the problem confronting those who seek to reduce the impact of mycotoxins. For as McClymont (1970) stated:
… ‘I believe that a great many of us have had to greatly expand our horizons as we became more conscious and confident of the importance of ecological concepts in facing up to our professional and personal involvement in the growing national and world problems; problems which have arisen from the piecemeal application of technology to management of resources and to man himself, with results ranging from local and world pollution to sickness of great cities and exploding world populations. For any grappling with these problems involves not only ecology, but culture and its evolutionary, ethical and economic bases, and education.’
All components of the perpetual pentagram (evolution, ecology, economics, ethics and education) can be applied to the study of fungi and mycotoxins. This would permit a more complete understanding of the ramifications of attempting to control the occurrence of these natural toxins in the food chain. For instance, the costs to animal production of mycotoxin-contaminated feed was mentioned above but this is only one aspect of the ‘cost’ of contamination. With the establishment of the high toxicity and carcinogenicity of some mycotoxins, international regulations that limit levels in food and feedstuffs for both man and animals have been imposed (van Egmond and Jonker 2004). These limits have implications for international trade in grain crops and in some instances can result in a trade barrier for the export or import of commodities from different parts of the world. Wu (2004) completed a comprehensive risk and economic analysis of lowering acceptable levels of fumonisins and aflatoxin in world trade. In that study she demonstrated that the United States would experience significant economic losses from tighter controls. The developing countries China and Argentina were more likely to experience greater economic losses than sub-Saharan Africa. The disturbing outcome of this detailed analysis was that tighter controls were, overall, unlikely to decrease health risks and may have the opposite effect (Wu 2004). Otsuki et al. (2001) estimated health risks would be reduced by 2.3 deaths per million people per year in the European Union if a lower aflatoxin standard was implemented. In other words, very stringent international trade regulations could lead to the situation where exporting countries, especially developing countries, would retain higher risk commodities, which would be available for their own populations, communities which are already exposed to higher levels of mycotoxins than consumers in developed countries (Wu 2008). These outcomes have both economic and ethical ramifications.
To do justice to the broader and very complicated aspects of mycotoxin production and contamination would require a book. In the relatively few words in this paper I hope I have conveyed to the reader the potential of fungi and their associated mycotoxins to disrupt both food and feed security. The impact of any disruption on society will be magnified if the prediction by Keyzer et al. (2005), that over the next three decades, increased demand for cereal feed supplies will greatly exceed the effect of genetically modified organisms and climate change on global food security, is correct. Moreover, as shown in Fig. 1, agroecology or the farming environment is very complex and coupled with poor public perception (see Table 1) there is an ongoing need for education (and research) at all levels as society becomes increasingly urbanised. Moreover, public education will be crucial if the mycotoxin burden is to be reduced in developing economies (Wild 2007; Wu and Khlangwiset 2010). The final word can be left to McClymont (1970), who made the case for education when he commented:
…. ‘to achieve these changes our education system must reassume a responsibility carried by the “education system” of our forebears, and discarded only in very recent evolutionary time, of transmitting the knowledge and ethics needed for man to live in balance with his environment and maintain quality of life; and this need is urgent: for time is running out.’
Acknowledgements
I am most grateful to the Australian Society of Animal Production for affording me the honour of presenting the 2012 McClymont lecture. It is of special personal interest as I had the privilege to be taught by Professor McClymont when completing a Rural Science degree at UNE. Being asked to present a lecture of this nature is not only a recognition of personal achievement but also reflects the contribution of former lecturers, colleagues, and past and current students. In this regard I owe a very special thanks to Professors Bill McClymont, Rob Cumming, Frank Annison and Dick Cole for their inspiring contributions.
References
Aldred D, Magan N (2004) The use of HACPP in the control of mycotoxins: the case of cereals. In ‘Mycotoxins in food’. (Eds N Magan, M Olsen) pp. 139–173. (Woodhead Publishing: Cambridge)Allcroft R, Carnaghan RBA, Sargeant K, O’Kelly J (1961) A toxic factor in Brazilian groundnut meal. The Veterinary Record 73, 428–429.
Allen JG (1987) Lupinosis. Veterinary Clinical Toxicology 103, 113–130.
Amuna P, Zotor FB (2008) Epidemiological and nutrition transition in developing countries: impact on human health and development. Proceedings of the Nutrition Society 67, 82–90.
Annison EF, Bryden WL (1998) Perspectives on ruminant nutrition and metabolism. I. Metabolism in the rumen. Nutrition Research Reviews 11, 173–198.
Barger G (1931) ‘Ergot and ergotism.’ (Guerney and Jackson: London)
Berthiller F, Schuhmacher R, Adam G, Krska R (2009) Formation, determination and significance of masked and other conjugated mycotoxins. Analytical and Bioanalytical Chemistry 395, 1243–1252.
Binder EM, Heidler D, Schatzmayrg G, Thimm N, Fuchs E, Schuh M, Krska R, Binder J (2001) Microbial detoxification of mycotoxins in animal feed. In ‘Mycotoxins and phytcotoxins in perspective at the turn of the millennium’. (Eds WJ de Koe, RA Samson, HP van Egmond, J Gilbert, M Sabino) pp. 271–277. (IUPAC: Amsterdam, The Netherlands)
Binder EM, Tan LM, Chin JL, Handl J, Richard J (2007) Worldwide occurrence of mycotoxins in commodities, feeds and feed ingredients. Animal Feed Science and Technology 137, 265–282.
Blaney BJ (1985) Mycotoxins in crops grown in different climatic regions of Queensland. In ‘Trichothecenes and other mycotoxins’. (Ed. J Lacey) pp. 97–108. (John Wiley and Sons Ltd: Chichester)
Blaney BJ, Dodman RL (2002) Production of zearalenone, deoxynivalenol, nivalenol, and acetylated derivatives by Australian isolates of Fusarium graminearum and F. pseudograminearum in relation to source and culturing conditions. Australian Journal of Agricultural Research 53, 1317–1326.
Blaney BJ, Williams KC (1991) Effective use in livestock feeds of mouldy and weather-damaged grain containing mycotoxins: case histories and economic assessments pertaining to pig and poultry industries of Queensland. Australian Journal of Agricultural Research 42, 993–1012.
Blaney BJ, Kopinski JS, Magee MH, McKenzie RA, Blight GW, Maryam R, Downing JA (2000a) Blood prolactin depression in growing pigs fed sorghum ergot (Claviceps africana). Australian Journal of Agricultural Research 51, 785–791.
Blaney BJ, McKenzie RA, Walters JR, Taylor LF, Bewg WS, Ryley MJ, Maryam R (2000b) Sorghum ergot associated with agalactia and feed refusal in pigs and dairy cattle. Australian Veterinary Journal 78, 102–107.
Blaney BJ, Maryam R, Murray S-A, Ryley MJ (2003) Alkaloids of the sorghum ergot pathogen (Claviceps africana): assay methods for grain and feed and variation between sclerotia/sphacelia. Australian Journal of Agricultural Research 54, 167–175.
Blaxter KL (1991) Animal production and food: real problems and paranoia. Animal Production 53, 261–269.
Blount WP (1961) Turkey ‘X’ disease. Turkeys 9, 52–77.
Brase S, Encinas A, Keck J, Nising CF (2009) Chemistry and biology of mycotoxins and related fungal metabolites. Chemical Reviews 109, 390–399.
Bricknell LK, Blaney BJ, Ng J (2008) Risk management for mycotoxin contamination of Australian maize. Australian Journal of Experimental Agriculture 48, 342–350.
Bryden WL (1982) Aflatoxin and animal production: an Australian perspective. Food Technology in Australia 34, 216–223.
Bryden WL (1994) The many guises of ergotism. In ‘Plant-associated toxins: agricultural, phytochemical and ecological aspects’. (Eds SM Colegate, PR Dorling) pp. 381–386. (CAB International: Wallingford, UK)
Bryden WL (1998) Mycotoxin contamination of Australian pastures and feedstuffs. In ‘Toxic plants and other natural toxicants’. (Eds T Garland, AC Barr) pp. 474–478. (CAB International: Wallingford, UK)
Bryden WL (2007) Mycotoxins in the food chain: human health implications. Asia Pacific Journal of Clinical Nutrition 16, 95–101.
Bryden WL (2009) Mycotoxins and mycotoxicoses: significance, occurrence and mitigation in the food chain. In ‘General and applied toxicology’. 3rd edn. (Eds B Ballantyne, T Marrs, T Syversen) pp. 3529–3553. (John Wiley & Sons Ltd: Chichester, UK)
Bryden WL (2012) Mycotoxin contamination of the feed supply chain: implications for animal productivity and feed security. Animal Feed Science and Technology 173, 134–158.
Bryden WL, Rajion MA, Lloyd AB, Cumming RB (1975) Surveys of Australian feedstuffs for toxigenic strains of Aspergillus flavus and for aflatoxin. Australian Veterinary Journal 51, 491–493.
Bryden WL, Lloyd AB, Cumming RB (1980) Aflatoxin contamination of Australian animal feeds and suspected cases of mycotoxicosis. Australian Veterinary Journal 56, 176–180.
Bryden WL, Suter DAI, Jackson CAW (1984) Response of chickens to sorghum contaminated with Alternaria. Proceedings of the Nutrition Society of Australia 9, 109.
Bryden WL, Bakau BJK, Burgess LW (1987a) Toxicity of Australian Alternaria isolates in a chick bioassay. Proceedings of the Nutrition Society of Australia 12, 171.
Bryden WL, Love RJ, Burgess LW (1987b) Feeding grain contaminated with Fusarium graminearum and Fusarium moniliforme to pigs and chickens. Australian Veterinary Journal 64, 225–226.
Bryden WL, Ravindran G, Salahifar H, Gill RJ, Burgess LW (1995) Fumonisin content of Australian maize. Proceedings of the Nutrition Society of Australia 19, 46
Bryden WL, Shanks GL, Ravindran G, Summerell BA, Burgess LW (1998) Occurrence of Fusarium moniliforme and fumonisins in Australian maize in relation to animal disease. In ‘Toxic plants and other natural toxicants’. (Eds T Garland, AC Barr) pp. 474–478. (CAB International: Wallingford, UK)
Bryden WL, Suksupath S, Taylor DJ, Prasongsidh BC (2004) Cyclopiazonic acid: food chain contamination? In ‘Plant toxins’. (Ed. T Acamovic) pp. 134–139. (CAB International: Wallingford, UK)
Burgess LW, Bryden WL, Jessup TM, Scrivener CJ, Barrow KD (1986) Role for ergot alkaloids in bovine hyperthermia. Proceedings of the Nutrition Society of Australia 11, 120
CAST (1989) Mycotoxins: economic and health risks. Report No. 116. Council for Agricultural Science and Technology, Ames, IA, USA.
CAST (1999) Animal agriculture and global food supply. Report No. 135. Council for Agricultural Science and Technology, Ames, IA, USA.
CAST (2003) Mycotoxins: risks in plant, animal and human systems. Report No. 139. Council for Agricultural Science and Technology, Ames, IA, USA.
Cawdell-Smith AJ, Bakau WJK, Scrivener CJ, Reed KFM, Blaney BJ, Bryden WL (2007a) Ergot toxins and animal disease in Australia. In ‘Poisonous plants: global research and solutions’. (Eds KE Panter, TL Wierenga, JA Pfister) pp. 519–524. (CABI Publishing: Wallingford, UK)
Cawdell-Smith AJ, Edwards MJ, Bryden WL (2007b) Mycotoxins and abnormal embryonic development. In ‘Poisonous plants: global research and solutions’. (Eds KE Panter, TL Wierenga, JA Pfister) pp. 525–532. (CABI Publishing: Wallingford, UK)
Cawdell-Smith AJ, Scrivener CJ, Bryden WL (2010) Staggers in horses grazing paspalum infected with Claviceps paspali. Australian Veterinarian Journal 88, 393–395.
Charmley LL, Rosenberg A, Trenholm HL (1994) Factors responsible for economic losses due to Fusarium mycotoxin contamination of grains, foods and feedstuffs. In ‘Mycotoxins in grains: compounds other than aflatoxin’. (Eds JD Miller, HL Trenholm) pp. 471–486. (Eagan Press: St Paul, MN)
Cheeke PR (1998) ‘Natural toxicants in feeds, forages and poisonous plants.’ (Interstate Publishers, Inc. Danville, IL)
Cheeke PR (2004) ‘Contemporary issues in animal agriculture.’ (Pearson Prentice Hall: New Jersey)
Clare NT (1952) ‘Photosensitization in diseases of domestic animals.’ (Commonwealth Agricultural Bureaux: Farnham Royal, England)
Coffey R, Cummins E, Ward S (2009) Exposure assessment of mycotoxins in dairy milk. Food Control 20, 239–249.
Cole RJ (Ed.) (1986a) ‘Modern methods in the analysis and structural elucidation of mycotoxins.’ (Academic Press: New York)
Cole RJ (1986b) Etiology of Turkey ‘X’ disease in retrospect: a case for the involvement of cyclopiazonic acid. Mycotoxin Research 2, 3–7.
Cole RJ (1989) Technology of aflatoxin decontamination. In ‘Mycotoxins and phycotoxins 88’. (Eds S Natori, K Hashimoto, Y Uueno) pp. 177–184. (Elsevier Scientific Publishing Co.: Amsterdam)
Cole RJ, Dorner JW (1986) Role of fungal tremorgens in animal disease. In ‘Mycotoxins and phycotoxins’. (Eds PS Steyn, R Vleggaar) pp. 501–511. (Elsevier Scientific Publishing Co.: Amsterdam, The Netherlands)
Cole RJ, Scheweikert MA, Javis BB (2003) ‘Handbook of secondary fungal metabolites.’ (Academic Press: San Diego, CA)
Cordain L, Eaton SB, Sebastian A, Mann N, Lindeberg S, Watkins BA, O’Keefe JH, Brand-Miller J (2004) Origins and evolution of the Western diet: health implications for the 21st century. The American Journal of Clinical Nutrition 81, 341–354.
Cribb J (2010) ‘The coming famine.’ (CSIRO Publishing: Melbourne)
Culvenor CCJ (1974) The hazard from toxic fungi in Australia. Australian Veterinary Journal 50, 69–78.
Dairy Australia (2011) ‘A review of facial eczema.’ Report of the Dairy Australia Facial Eczema Working Group. (Dairy Australia Ltd: Melbourne)
Degen GH (2011) Tools for investigating workplace-related risks for mycotoxin exposure. World Mycotoxin Journal 4, 315–327.
Delgado CL (2003) Rising consumption of meat and milk in developing countries has created a new food revolution. The Journal of Nutrition 133, 3902S–3910S.
Dersjant-Li Y, Verstegen MWA, Gerrits WJJ (2003) The impact of low concentrations of aflatoxin, deoxynivalenol or fumonisins in diets on growing pigs and poultry. Nutrition Research Reviews 16, 223–239.
Desjardins AE (2006) ‘Fusarium mycotoxins: chemistry genetics and biology.’ (APS Press: St Paul, MN)
di Menna ME, Lauren DR, Poole PR, Mortimer PH, Hill RA, Agnew MP (1987) Zearalenone in New Zealand pasture herbage and the mycotoxin-producing potential of Fusarium species from pasture. New Zealand Journal of Agricultural Research 30, 499–504.
di Menna ME, Lauren DR, Hardacre A (1997) Fusaria and Fusarium toxins in New Zealand maize plants. Mycopathologia 139, 165–173.
di Menna ME, Smith BL, Miles CO (2009) A history of facial eczema (pithomycotoxicosis) research. New Zealand Journal of Agricultural Research 52, 345–376.
Dorner JW (2008) Management and prevention of mycotoxins in peanuts. Food Additives and Contaminants 25, 203–208.
Edgar JA, Frahn JL, Cockrum PA, Culvenor CCJ (1986) Lupinosis: the chemistry and biochemistry of the fumonisins. In ‘Mycotoxins and phycotoxins’. (Eds PS Steyn, R Vleggaar) pp. 169–184. (Elsevier: Amsterdam)
Eriksen GS, Pettersson H, Johnsen K, Lindberg JE (2002) Transformation of trichothecenes in ileal digesta and faeces from pigs. Archives of Animal Nutrition 56, 263–274.
Farrell D (2010) ‘Great wealth, poor health: contemporary issues in eating and living.’ (Nottingham University Press: Nottingham)
Faull BW (1991) Prevalence and cost of facial eczema on dairy farms. Surveillance 18, 14–16.
Feuell AJ (1966) Toxic factors of mould origin. Tropical Science 8, 61–70.
Fink-Gremmels J (2008) Mycotoxins in cattle feeds and carry-over to dairy milk: a review. Food Additives and Contaminants 25, 172–180.
Fink-Gremmels J, Malekinejad H (2007) Clinical effects and biochemical mechanisms associated with exposure to the mycoestrogen zearalenone. Animal Feed Science and Technology 137, 326–341.
Fletcher LR, Harvey IC (1981) An association of a lolium endophyte with ryegrass staggers. New Zealand Veterinary Journal 29, 185–186.
Food Standards Australia New Zealand (2011) ‘The 23rd Australian total diet study.’ (FSANZ: Canberra)
Fraser DM, Dorling PR (1983) Suspected ergotism in two heifers. Australian Veterinary Journal 60, 303–305.
Fuchs E, Binder EM, Heidler D, Krska R (2002) Structural characterization of metabolites after microbial degradation of type A trichothecenes by the bacterial strain BBSH 797. Food Additives and Contaminants 19, 379–386.
Galvano F, Piva A, Ritieni A, Galvano G (2001) Dietary strategies to counteract the effects of mycotoxins: a review. Journal of Food Protection 64, 120–131.
Garrett KA, Dendy SP, Frank EE, Rouse MN, Travers SE (2006) Climate change effects on plant disease: genome to ecosystems. Annual Review of Phytopathology 44, 489–509.
Gelineau-van Waes J, Starr L, Maddox J, Alleman F, Voss KA, Wilberding J, Riley RT (2005) Maternal fumonisin exposure and risk for neural tube defects: mechanisms in an in vivo mouse model. Birth Defects Research. Part A, Clinical and Molecular Teratology 73, 487–497.
Givens DI (2010) Milk and meat in our diet; good or bad for health? Animal 4, 1941–1952.
Godfray CJ (2011) Food for thought. Proceedings of the National Academy of Sciences of the United States of America 108, 19845–19846.
Graham J (1982) Aflatoxin in peanuts: occurrence and control. Queensland Agricultural Journal 108, 119–122.
Gregory PJ, George TS (2011) Feeding nine billion: the challenge to sustainable crop production. Experimental Botany 62, 5233–5239.
Hagler WM Jr, Towers NR, Mirocha CJ, Eppley RM, Bryden WL (2001) Zearalenone: mycotoxin or mycoestrogen? In ‘Fusarium: Paul E. Nelson Memorial Symposium’. (Eds BA Summerell, JF Leslie, D Backhouse, WL Bryden, LW Burgess) pp. 321–331. (APS Press: St Paul, MN)
Hamilton PB (1978) Fallacies in our understanding of mycotoxins. Journal of Food Protection 41, 404–408.
Hamilton PB (1982) Mycotoxins and farm animals. Refu’ah Veterinarit 39, 17–45.
Hamilton RMG, Trenholm HL, Thompson BK (1988) Chemical, nutritive, deoxynivalenol and zearalenone content of corn relative to the site of inoculation with different isolates of Fusarium graminearum. Journal of the Science of Food and Agriculture 43, 37–47.
Haschek WM, Voss KA, Beasley VR (2002) Selected mycotoxins affecting animal and human health. In ‘Handbook of toxicological pathology, second edition. Volume 1’. (Eds WM Haschek, ECG Rousseaux, MA Wallig) pp. 645–699. (Academic Press: New York)
Hawkesworth S, Dangouri AD, Johnston D, Lock K, Poole P, Rushton J, Uauy R, Waage J (2010) Feeding the world healthily: the challenge of measuring the effects of agriculture on health. Philosophical Transactions of the Royal Society B 365, 3083–3097.
Hegarty RS (2012) Livestock nutrition: future needs in a resource challenged planet. Animal Production Science in press
Heinl S, Hartinger D, Thamhesl M, Vekiru E, Krska R, Schatzmayr G, Moll W-D, Grabherr R (2010) Degradation of fumonisin B1 by the consecutive action of two bacterial enzymes. Journal of Biotechnology 145, 120–129.
Heinl S, Hartinger D, Thamhesl M, Schatzmayr G, Moll W-D, Grabherr R (2011) An aminotransferase from bacterium ATCC 55552 deaminates hydrolysed fumonisin B1. Biodegradation 22, 25–30.
Henry SH, Bosch FX, Troxell TC, Bolger PM (1999) Reducing liver cancer – global control of aflatoxin. Science 286, 2453–2454.
Humpf HU, Voss KA (2004) Effects of thermal food processing on the chemical structure and toxicity of fumonisin mycotoxins. Molecular Nutrition & Food Research 48, 255–269.
Hunter RA (2010) Hormonal growth promotant use in the Australian beef industry. Animal Production Science 50, 637–659.
Hurst E (1942) ‘The poison plants of New South Wales.’ (New South Wales Poison Plants Committee: Sydney)
Huwig A, Freimund S, Käppeli O, Dutler H (2001) Mycotoxin detoxification of animal feed by different adsorbents. Toxicology Letters 122, 179–188.
Jang IH, Van der Tol J, Bryden WL, McDowell GH, Ross AD, Christie BM, Burgess LW (1987) Ergotism in dairy cattle – effects of ingestion of ergots of Claviceps purpurea. Proceedings of the Nutrition Society of Australia 12, 169
Jessup TW, Dent CHR, Kemp JB, Christie BM, Ahrens PJ, Burgess LW, Bryden WL (1987) Bovine idiopathic hyperthermia. Australian Veterinary Journal 64, 353–354.
Jouany JP (2007) Methods for preventing decontaminating and minimizing the toxicity of mycotoxins in feeds. Animal Feed Science and Technology 137, 342–362.
Kabak B, Dobson ADW, Var I (2006) Strategies to prevent mycotoxin contamination of food and animal feed: a review. Critical Reviews in Food Science and Nutrition 46, 593–619.
Keyzer MA, Merbis MD, Pavel IFPW, van Wesenbeeck CFA (2005) Diet shifts towards meat and the effects on cereal use: can we feed the animals in 2030? Ecological Economics 55, 187–202.
Lauren DR, diMenna ME, Greenhalgh R, Miller JD, Neish GA, Burgess LW (1988) Toxin-producing potential of some Fusarium species from a New Zealand pasture. New Zealand Journal of Agricultural Research 31, 219–225.
Lauren DR, Agnew MP, Smith WA, Sayer ST (1991) A survey of the natural occurrence of Fusarium mycotoxins in cereals grown in New Zealand in 1986–1989. Food Additives and Contaminants 8, 599–605.
Lauren DR, Jensen DJ, Smith WA, Dow BW, Sayer ST (1996) Mycotoxins in New Zealand maize: a study of some factors influencing contamination levels in grains. New Zealand Journal of Crop and Horticultural Science 24, 13–20.
Leng RA (1996) Bill McClymont: the man and his vision. In ‘Rural science: philosophy and application’. (Ed. JS Ryan) pp. 311–320. (School of Rural Science, University of New England: Armidale)
Litherland AJ, Layton DL, Boom CJ, Knight TL, Hyslop M, Lambert MG, Cook TL (2004) Ill-thrift in young growing cattle and sheep. Proceedings of the New Zealand Society of Animal Production 64, 197–202.
Lopez-Garcia R (2001) Mycotoxin management: an integrated approach. In ‘Mycotoxins and phycotoxins in perspective at the turn of the millennium’. (Eds WJ de Koe, RA Samson, HP van Egmond, J Gilbert, M Sabino) pp. 261–269. (IUPAC: Amsterdam, The Netherlands)
Lopez-Garcia R, Mallman CA, Pineiro M (2008) Design and management of an integrated system for ochratoxin A in the coffee production chain. Food Additives and Contaminants 25, 231–240.
Lutz W, Samir KC (2010) Dimensions of global population projections: what do we know about future population trends and structures. Philosophical Transactions of the Royal Society B 365, 2779–2791.
Magan N (2006) Mycotoxin contamination of food in Europe: early detection and prevention strategies. Mycopathologia 162, 245–253.
Magan N, Medina A, Aldred D (2011) Possible climate-change effects on mycotoxin contamination of food crops pre- and postharvest. Plant Pathology 60, 150–163.
Malthus T (1798) ‘An essay on the principle of population.’ (J. Johnson: London)
Mannon J, Johnson E (1985) Fungi down on the farm. New Scientist 105, 12–16.
Marasas WFO, Miller JD, Riley RT, Visconti A (2001) Fusmonisins – occurrence, toxicology, metabolism and risk assessment. In ‘Fusarium: Paul E. Nelson Memorial Symposium’. (Eds BA Summerell, JF Leslie, D Backhouse, WL Bryden, LW Burgess) pp. 332–359. (APS Press: St Paul, MN)
Matossian MK (1989) ‘Poisons of the past: molds, epidemics and history.’ (Yale University Press: Newhaven)
McClymont GL (1970) The perpetual pentagram: evolution, ecology, economics, ethics and education. Proceedings the Ecology Society of Australia 5, 169–182, reprinted 1996 in ‘Rural science: philosophy and application’. (Ed. JS Ryan) pp. 294–310. (School of Rural Science, University of New England: Armidale)
McClymont GL (1996) All flesh is grass. Inaugural lecture – 1955. In ‘Rural science: philosophy and application’. (Ed. JS Ryan) pp. 1–12. (School of Rural Science, University of New England: Armidale)
Meister U, Springer M (2004) Mycotoxins in cereals and cereal products – occurrence and changes during processing. Journal of Applied Botany and Food Quality 78, 168–173.
Miles CO, di Menna ME, Jacobs SWL, Garthwaite I, Lane GA, Prestidge RA, Marshall SL, Wilkinson HH, Schardl CL, Ball OJ-P, Latch GCM (1998) Endophytic fungi in indigenous Australasian grasses. Applied and Environmental Microbiology 64, 601–606.
Miller JD (1998) Global significance of mycotoxins. In ‘Mycotoxins and phycotoxins – developments in chemistry, toxicology and food safety’. (Eds M Miraglia, H van Egmond, C Brera, J Gilbert) pp. 3–15. (Alaken Inc.: Fort Collins, CO)
Miller JD (2008) Mycotoxins in small grains and maize: old problems, new challenges. Food Additives and Contaminants 25, 219–230.
Molnar O, Schatzmayr G, Fuchs E, Prillinger H (2004) Trichosporon mycotoxinivorans sp. nov., a new yeast species useful in biological detoxification of various mycotoxins. Systematic and Applied Microbiology 27, 661–671.
Moore CJ, Blaney BJ, Spencer RA, Dodman RL (1985) Rejection by pigs of mouldy grain containing deoxynivalenol. Australian Veterinary Journal 62, 60–62.
Oswald IP, Martin DE, Bouhet S, Pinton P, Taranu I, Accensi F (2005) Immunotoxiological risk of mycotoxins for domestic animals. Food Additives and Contaminants 22, 354–360.
Otsuki T, Wilson JS, Sewadeh M (2001) Saving two in a billion: quantifying the trade effect of European food safety standards on African exports. Food Policy 26, 493–514.
Peet RL, McCarthy MR, Barbetti MJ (1991) Hyperthermia and death in feedlot cattle associated with the ingestion of Claviceps purpurea. Australian Veterinary Journal 68, 121
Pestka JJ, Zhou H-R, Moon Y, Chung YJ (2004) Cellular and molecular mechanisms for immune modulation by deoxynivalenol and other trichothecenes: unravelling the paradox. Toxicology Letters 153, 61–73.
Pettersson H (2004) Controlling mycotoxins in animal feeds. In ‘Mycotoxins in food’. (Eds N Magan, M Olsen) pp. 262–304. (Woodhead Publishing: Cambridge)
Phillips TD (1999) Dietary clay in the chemoprevention of aflatoxin-induced disease. Toxicological Sciences 52, 118–126.
Pohland AE, Yess NJ (1992) Food contaminants; scientific and public health implications. Proceedings of the Nutrition Society of Australia 17, 1–12.
Rammel C (1991) Mycotoxins in feed. Surveillance 18, 7–13.
Ramos AJ, Sanchis V, Marin S (2011) The prehistory of mycotoxins: related cases from ancient times to the discovery of aflatoxins. World Mycotoxin Journal 4, 101–112.
Reed KFM, Moore DD (2009) A preliminary survey of zearalenone and other mycotoxins in Australian silage and pasture. Animal Production Science 49, 696–703.
Reed KFM, Page SW, Lean IJ (Eds) (2005) ‘Perennial ryegrass toxicosis in Australia.’ (Meat and Livestock Australia: Sydney)
Richard JL (2007) Some major mycotoxins and their mycotoxicoses: an overview. International Journal of Food Microbiology 119, 3–10.
Richard JL (2008) Discovery of aflatoxin and significant historical features. Toxin Reviews 27, 171–201.
Richard JL, Thurston JR (1986) ‘Diagnosis of mycotoxicoses.’ (Martinus Nijhoff: Boston)
Riley RT (1998) Mechanistic interactions of mycotoxins: theoretical considerations. In ‘Mycotoxins in agriculture and food safety’. (Eds KK Sinha, D Bhatnagar) pp. 227–253. (Marcel Dekker, Inc.: New York)
Rodrigues I, Griessler K (2010) Mycotoxin survey 2009: moulds remain a problem for the whole farm-to-fork. All About Feed 1, 12–14.
Rodrigues I, Naehrer K (2011) Biomin survey 2010: mycotoxins inseparable from animal commodities and feed. All About Feed 2, 17–20.
Ross AD, Bryden WL, Bakau W, Burgess LW (1989) Induction of heat stress in beef cattle by feeding the ergots of Claviceps puepurea. Australian Veterinary Journal 66, 247–249.
Ryan RS (Ed.) (1996) ‘Rural science: philosophy and application.’ (School of Rural Science, University of New England: Armidale)
Ryan RS (Ed.) (2007) ‘McClymont’s vision: the challenge remains.’ (School of Environment and Rural Science, University of New England: Armidale)
Ryley MJ, Alcorn JL, Kochman JK, Kong GA, Thompson SM (1996) Ergot on Sorghum spp. in Australia. Australasian Plant Pathology 25, 214
Samman S, Skeaf S, Thomson C, Truswell S (2012) Trace elements. In ‘Essentials of human nutrition’. 4th edn. (Eds JI Mann, AS Truswell) pp. 139–159. (Oxford University Press: Oxford)
Scholthof K-BG (2003) One foot in the farrow: linkages between agriculture, plant pathology, and public health. Annual Review of Public Health 24, 153–174.
Shephard GS (2008) Impact of mycotoxins on human health in developing countries. Food Additives and Contaminants 25, 146–151.
Shephard GS, Thiel PG, Stockenstrom S, Sydenham EW (1996) Worldwide survey of fumonisin contamination of corn and corn-based products. Journal of AOAC International 79, 671–687.
Shephard GS, Berthiller F, Burdaspal P, Crews C, Jonker MA, Krska R, MacDonald S, Malone B, Maragos C, Sabino M, Solfrizzo M, van Egmond HP, Whitaker T (2011) Developments in mycotoxin analysis: an update for 2009–2010. World Mycotoxin Journal 4, 3–28.
Shier WT, Abbas HK, Wearer MA, Horn BW (2005) The case for monitoring Aspergillus flavus aflatoxigenicity for food safety assessment in developing countries. In ‘Aflatoxin and food safety’. (Ed. HK Abbas) pp. 291–311. (CRC Press: Boca Raton, FL)
Smith BL, Towers NR (2002) Mycotoxicoses of grazing animals in New Zealand. New Zealand Veterinary Journal 50, 28–34.
Smith D (2011) ‘Dick’s Smith population crisis.’ (Allen & Unwin: Sydney)
Smith JE, Henderson RS (Eds) (1991) ‘Mycotoxins in animal foods.’ (CRC Press Inc.: Boca Raton, FL)
Smith JF, Morris CA (2006) Review of zearalenone studies with sheep in New Zealand. Proceedings of the New Zealand Society of Animal Production 66, 306–310.
Speedy AW (2003) Global production and consumption of animal source foods. The Journal of Nutrition 133, 4048S–4043S.
Strange RN, Scott PR (2005) Plant disease: a threat to global food security. Annual Review of Phytopathology 43, 83–116.
Swick RA (2011) Global feed supply and demand. Recent Advances in Animal Nutrition – Australia 18, 1–7.
Tester M, Langridge P (2010) Breeding technologies to increase crop production in a changing world. Science 327, 818–827.
Thornton PK (2010) Livestock production: recent trends, future prospects. Philosophical Transactions of the Royal Society B 365, 2853–2867.
Towers NR, Sprosen JM (1992) Fusarium mycotoxins in pastoral farming: zearalenone induced infertility in sheep. Recent Advances in Toxinology Research 3, 272–284.
Trenholm HL, Prelusky DB, Young JC, Miller JD (1989) A practical guide to prevention of Fusarium mycotoxins in grains and animal feedstuffs. Archives of Environmental Contamination and Toxicology 18, 443–451.
Tribe DE (1994) ‘Feeding and greening the world: The role of international agricultural research.’ (CAB International: Wallingford, UK)
van Egmond HP, Jonker MA (2004) Current regulations governing mycotoxin limits in food. In ‘Mycotoxins in food’. (Eds N Magan, M Olsen) pp. 49–68. (Woodhead Publishing: Cambridge)
Voss KA, Smith GW, Haschek WM (2007) Fumonisins: toxicokinetics, mechanism of action and toxicity. Animal Feed Science and Technology 137, 299–325.
Webley DJ, Jackson KL, Mullins JD, Hocking AD, Pitt JI (1997) Alternaria toxins in weather-damaged wheat and sorghum in the 1995–1996 harvest. Australian Journal of Agricultural Research 48, 1249–1255.
Whitaker TB (2003) Detecting mycotoxins in agricultural commodities. Molecular Biotechnology 23, 61–71.
Wicklow DT (1995) The mycology of stored grain: an ecological perspective. In ‘Stored-grain ecosystems’. (Eds DS Jayas, NDG White, WE Muir) pp. 197–249. (Marcel Dekker, Inc.: New York)
Wild CP (2007) Aflatoxin exposure in low and middle income countries: the critical interface of agriculture and health. Food and Nutrition Bulletin 28, S372–S380.
Wild CP, Gong YY (2010) Mycotoxins and human disease: a largely ignored global health issue. Carcinogenesis 31, 71–82.
Williams P (2007) Nutritional composition of red meat. Nutrition and Dietetics 64, S113–S119.
Williamson PM, Highet AS, Gams W, Siva-Sithamparam K, Cowling WA (1994) Diaporthe toxica sp. nov., the cause of lupinosis in sheep. Mycological Research 98, 1364–1368.
Wogan GN, Kensler TW, Groopman JD (2012) Present and future directions of translational research on aflatoxin and hepatocellular carcinoma: a review. Food Additives and Contaminants 29, 249–257.
Wright GC, Rachaputi NC, Chauhan YS, Robson A (2005) Increasing productivity and quality of peanuts using novel crop modeling and remote sensing technologies. In ‘Prospects and emerging opportunities for peanut quality and utilization technology. International peanut conference’. pp. 14–17. (Kasetsart University: Bangkok, Thailand)
Wu F (2004) Mycotoxin risk assessment for the purpose of setting international regulatory standards. Environmental Science & Technology 38, 4049–4055.
Wu F (2008) A tale of two commodities: how EU mycotoxin regulations have affected food industries. World Mycotoxin Journal 1, 71–78.
Wu F, Khlangwiset P (2010) Evaluating the technical feasibility of aflatoxin risk reduction strategies in Africa. Food Additives and Contaminants 27, 658–676.
Wu F, Munkvold GP (2008) Mycotoxin in ethanol co-products: modelling economic impacts on the livestock industry and management strategies. Journal of Agricultural and Food Chemistry 56, 3900–3911.
Wu F, Bhatnagar D, Bui-Klimke T, Cardone I, Hellmich R, Munkvold G, Paul P, Payne G, Takle E (2011) Climate change impacts on mycotoxin risks in US maize. World Mycotoxin Journal 4, 79–93.


