Investigating the role of blow fly olfaction in flystrike in sheep
Guanjie Yan A B C , Anthony C. Schlink A , Shimin Liu
A B C , Anthony C. Schlink A , Shimin Liu  A C , Johan C. Greeff
A C , Johan C. Greeff  A D , Gavin R. Flematti
A D , Gavin R. Flematti  E and Graeme B. Martin
E and Graeme B. Martin  A C *
A C *
A
B
C
D
E
Abstract
Breech flystrike is a painful, debilitating and potentially lethal disease caused by the larvae of the blowfly, Lucilia cuprina, and, despite many years of research, it remains a serious financial and animal-welfare issue for the Merino sheep industry in Australia. The common methods of prevention, namely insecticides, crutching and ‘mulesing’, are problematical, so alternative approaches are needed. Breeding for resistance to breech strike is a fundamentally attractive proposition, but the trait itself is difficult and expensive to quantify in large numbers of sheep in extensive production systems. Several indirect traits are correlated with susceptibility to flystrike, but a large proportion of the variation in susceptibility remains unexplained. The common thread through those indirect traits is odour, so we turned to the biology of insect olfaction and its role in fly–sheep interactions. L. cuprina uses odours to detect and locate potential hosts over long distances, to guide orientation and landing behaviour, and to select egg-laying sites. Preliminary studies demonstrated the importance of confining our work to gravid female L. cuprina, and also validated the use of flies reared in the laboratory for experimentation. Using laboratory-reared flies and a combination of gas chromatography–mass spectrometry with electroantennographic detection, we identified odouriferous compounds from sheep that the antenna of L. cuprina can detect. To determine whether the identified compounds were attractive or repulsive, we needed to use a behaviour test. In preliminary studies, we compared four behaviour bioassays (Y-tube, landing time, visiting frequency, and trap) and found the trap to be the most effective. We observed that L. cuprina was attracted by several compounds in Merino wool, including octanal, nonanal and dimethyl trisulfide. We also found that the wool levels of octanal and nonanal are heritable in Merino sheep, suggesting that these compounds might be useful as traits in selection for flystrike resistance. Another possibility is that these olfactory-active compounds might guide efforts to modify the genome of sheep, or perhaps even L. cuprina. Success in these endeavours could save as much as A$200 m per year for the Australian Merino-based industries, while also improving the image of wool in world markets.
Keywords: antenna, behaviour, blow fly, breeding, electroantennography, flystrike, Lucilia cuprina, Merino.
Introduction
In warm, humid weather, adult blow flies lay eggs on the skin of livestock and, with ideal conditions such as open wounds or wool contaminated with faeces or urine, the emerging larvae feed on the skin and tissue. This disease, myiasis, known colloquially as ‘flystrike’, may prove fatal and is a major problem for sheep industries in many parts of the world (Lihou and Wall 2019). Several species of fly are responsible, including Lucilia cuprina, L. sericata, L. caesar, Wohlfahrtia magnifica, Calliphora stygia, Chrysomya rufifacies and Protophormia terraenovae. The prevalent species in Australia, L. cuprina (Wiedemann), a member of the family Calliphoridae, was probably introduced in the late 19th century, after which it found its way to New Zealand. It is now a major pest in both countries (Heath and Bishop 2006). In Australia, it is responsible for about 90% of flystrike (Anderson et al. 1988) and, in industries based on Merino sheep, it causes losses of about A$320 million per year due to lost production of wool and meat, animal deaths, and the costs of preventative measures (Shephard et al. 2022).
Sheep farmers use a variety of approaches for the control of blowfly-strike, ‘the rational basis for which is often not robust’ (Lihou and Wall 2019). Since the 1930s, the most efficient preventative measures have been (i) insecticides applied to the skin around the anus and vulva (the ‘breech’), (ii) ‘crutching’, the shearing of wool immediately around the ‘breech’, and (iii) ‘mulesing’ (Box 1).
| Box 1.Mulesing |
| The wool around the ‘breech’ (anus and vulva) of sheep can retain faeces and urine that attracts egg-laying blow flies, leading to ‘flystrike’ (technically, ‘myiasis’) with the blowfly larvae attacking the flesh of the host. The condition is clearly stressful for the sheep, and can lead to death if not treated, but it is also stressful for sheep managers who have to treat animals infested by larvae (‘maggots’) in a timely manner. The ‘mulesing’ operation is named after John Mules, a shearer who accidentally cut off some breech skin in the 1930s and noticed that when the skin healed it did not produce wool and thus avoided the soiling that attracted the flies (Beveridge 1984). Subsequently, a routine procedure was developed, particularly for Merino sheep, in which strips of skin are cut from around the breech of young lambs. |
| Despite its effectiveness, mulesing has long been controversial, with animal activists loudly voicing their concerns. In the early 2000s, campaigns were launched to target the international textile industry and thus inhibit the sale of wool sourced from mulesed animals (Sneddon and Rollin 2010). The sheep farming community countered this campaign by pointing out the effectiveness of the approach and by trying to develop more humane alternatives, none of which has been extensively adopted (Wells et al. 2011). Two significant advances have been the now widespread adoption of local analgesia during the procedure and the accreditation of mulesing practitioners (Colvin et al. 2022). Forward-thinking farmers, seeing a time when the practice will be banned, have themselves decided to abandon it for their own flocks and, instead, use other means of prevention, including insecticides, more intense surveillance for early strike detection, and breeding for resistance to flystrike (Greeff et al. 2018a) and for resistance to the helminths that cause diarrhoea in sheep (Karlsson and Greeff 2012). This change in practice is helped by a price advantage for producers of ‘non-mules’ wool, and by producer-demonstration events run by industry research and development corporations. |
In the 1940s, a combination of mulesing, insecticides, crutching and shearing, was widely adopted as a highly effective measure of flystrike prevention (Horton et al. 2020). For both crutching and mulesing, the aim is to prevent the accumulation of faecal matter, known colloquially as ‘dags’. However, crutching is an unwanted expense and, over the ensuing decades, three other issues arose, namely, (i) susceptible sheep were masked by the treatments, so their genes persisted in breeding flocks, (ii) the blow flies evolved resistance to widely used insecticides, and other insecticides were not biologically active because they bind to wool (Sandeman et al. 2014; Austin and Naidoo 2022; Benedetti Vallenari et al. 2023), (iii) mulesing became socially unacceptable, and is illegal in many countries. In any case, neither mulesing nor insecticides fit the drive towards a ‘clean, green and ethical’ image for the industry.
A better alternative is genetic selection for resistance to flystrike (Box 2).
| Box 2.Breeding for resistance to flystrike |
| It is generally acknowledged that the best long-term solution is to breed sheep that are resistant to flystrike (Karlsson and Greeff 2012; Kotze and James 2022). Flystrike is a heritable trait that is repeatable over an animal’s lifetime, with large differences among sire progeny groups (Greeff et al. 2013a). However, it is poorly suited as a direct selection trait in a breeding program because it depends on climate, it can be transient and unpredictable, and there are ethical issues in deliberately challenging sheep with flystrike. A more acceptable approach is to find indicator traits to allow indirect breeding for resistance. Proven indirect traits include the following: (i) skin wrinkles and wool cover around the anus and vulva (the ‘breech’) that provide sites readily contaminated with urine and faeces (Greeff et al. 2013a, 2018b); (ii) fleece rot: when the skin is wet for prolonged periods, bacterial populations develop rapidly and produce odours that attract flies (Ashworth and Wall 1994); (iii) ‘dags’, the colloquial term for faeces that accumulate on wool around the breech, and commonly caused by helminth burden, and one of the most significant factors predisposing sheep to breech strike (Heath and Bishop 2006; Greeff et al. 2013a, 2018b); (iv) urine stain is associated with the wetting of breech wool and causes bacterial growth (Belschner 1937); (v) skin odour and moisture levels in the fleece (Greeff et al. 2021, 2022). |
| Greeff et al. (2018b) showed that among these indirect selection traits, skin wrinkles and ‘dags’ are the most valuable for Merino sheep, although these two traits explain only about 40% of the variation among sheep in susceptibility to breech strike. The large proportion of unexplained variation drives the continuing search for more indirect traits. Notably, all of the indirect traits are related to odours produced by the sheep that attract gravid blowflies, and to the provision of a warm, humid environment for the development of the larvae. Indeed, it was found that dogs could be trained to identify wool from flystrike-resistant sheep prior to the sheep being struck (Greeff et al. 2013b). The common theme of odour led to investigation of the role of insect olfaction in susceptibility to breech strike. |
The evidence from quantitative genetics was clear, namely, susceptibility to breech strike is a moderately highly heritable trait, although the heritability is greater in a winter-rainfall environment (h2 = 0.5) than in a summer-rainfall environment (h2 = 0.3; Smith et al. 2009; Greeff et al. 2013a). Susceptibility to bre ech strike can respond to genetic selection, but breech strike itself is a discrete trait that is difficult and expensive to measure in large numbers of sheep in extensive production systems, and its expression can vary dramatically from year to year, depending on the prevailing environmental factors. Consequently, there has long been a focus on identifying indirect traits, namely factors associated with resistance that can be incorporated into breeding programs. Several have been documented, including the following:
Skin wrinkles: for Merino sheep in the early 1900s, wrinkled skin was seen as a way to increase wool production but, unfortunately, the wrinkles were a predisposing factor for flystrike (Seddon 1931; Sneddon and Rollin 2010). In particular, wrinkles and wool cover around the anus and vulva (the ‘breech’) are readily contaminated with urine and faeces that attract gravid blow flies (Greeff et al. 2013a, 2018b); the skin wrinkles, and therefore the wool, can be removed by ‘mulesing’ as a preventative measure.
Fleece rot: when the skin is wet for prolonged periods, populations of Pseudomonas spp. develop rapidly on the surface, provoking an acute inflammatory response (leakage of plasma proteins onto the skin surface; accumulation of inflammatory cells in the underlying dermis), and putrefaction-produced, sulfur-rich compounds that attract flies (Ashworth and Wall 1994); however, the diversity in bacterial populations on the skin around the breech in Merino sheep is more affected by environmental factors than by genetic factors (Greeff et al. 2021), perhaps explaining why fleece rot is more important for body strike than for breech strike (Colditz et al. 2021). Susceptibility to fleece rot is one possibility because it has a strong positive genetic correlation (>0.9) with body strike (rather than breech strike) and resistance to fleece rot is moderately heritable (h2 = 0.10 to 0.58; Atkins and McGuirk 1979; Raadsma 1991). For breech strike in a Mediterranean environment, dags and wrinkles are also major predisposing factors, although a large proportion of the variation in susceptibility remains unexplained in uncrutched and unmulesed sheep (Greeff et al. 2019).
‘Dags’, commonly caused by helminth burden, are one of the most significant factors predisposing sheep to breech strike in New Zealand and Australia (Heath and Bishop 2006; Greeff et al. 2013a, 2018a); soiled fleece must produce an odour that attracts gravid blow flies, and it then provides a warm, humid environment for larval development;
Urine stain is associated with the wetting of breech wool (Belschner 1937) and causes inflammation of the underlying skin, bacterial growth and, again, it must produce an odour that attracts gravid blow flies before offering an ideal environment for larval development.
In any case, variation in these breech characteristics explains only about 40% of the variation in breech flystrike (Greeff et al. 2018b), so other factors clearly play major roles in susceptibility. It is important to note that insects, including L. cuprina, use visual and gustatory cues as well as olfaction when foraging for egg-laying sites. However, odour is the common factor to the above characteristics that affect susceptibility to flystrike (Tellam and Bowles 1997). We therefore turned our attention to the processes of olfaction and behaviour in insects (Box 3), with the aim of testing whether an understanding of semio-chemical interactions between L. cuprina and Australian Merino sheep would lead to new indirect traits that could be used in selection for flystrike resistance. This review analyses recent progress.
| Box 3.Insect olfaction |
| The ‘odour-scape’ contains a complex mix of chemicals that provide information about the world. Insects have capitalised on this rich source of information and use their sense of smell (‘olfaction’) to locate and select food, find sites for laying eggs (‘oviposition’), find mates for reproduction, and to avoid predators (Wyatt 2014). Volatile organic compounds (odours) diffuse through pores in the exoskeleton into special olfactory sense organs that are called ‘sensilla’. The insect body is covered in a variety of sensilla, but the olfactory sensilla are concentrated on the antennae and some mouthparts (Ong and Stopfer 2012; Elgar et al. 2018). Once inside a sensillum, the odour molecule binds to a specific odorant-binding protein to form an odour–protein complex that then connects with an olfactory receptor embedded in the membrane of a sensillum nerve cell. This connection triggers an electrical signal that is transmitted to the central nervous system (Benton et al. 2007). The brain interprets and processes that signal on the basis of physiological state (hunger, mating status, egg load), age, experience, as well as other odour signals in the air, and even the time of day. The brain integrates all of this information and then changes behaviour, for example, by moving closer to or away from the odour source, depending on interest in feeding, finding a mate, or laying eggs (Leal 2013). The selectivity and sensitivity of insects to various odours is governed by complex molecular and cellular interactions, including crosstalk between sensilla. It is therefore not surprising that the olfactory system of insects is sophisticated and enables them to quickly adapt to changing conditions and to survive in diverse habitats. |
Experimentation with L. cuprina
Wild flies are not always readily available, so research programs often rely on flies reared under laboratory conditions, using the same simple diet for many sequential generations. This practice carries the risk that flies will evolve, perhaps to a point where their biology differs significantly from that of wild flies, thus invalidating conclusions from experimentation. We therefore tested the effect of long-term laboratory rearing on female L. cuprina of the F1, F6, and F11 generations. Fortunately, fecundity and responsiveness to attractive odours did not change with generation, and they were not affected by diet. In contrast, thorax length, wing length and wing aspect ratio did differ between the F1 and F11 generations, perhaps explaining a loss in flight performance (Yan et al. 2019a). Importantly, a consistent observation was that gravid flies were more attracted to wool and liver than were non-gravid flies. It thus became clear that we needed to confine our work to gravid flies, but we did not need to be overly concerned about the use of a single diet or long-term laboratory rearing with respect to fecundity or attraction to odour, two important variables in the context of flystrike biology.
Assessment of antennal responses and olfaction
In insects, semio-chemicals play important roles in foraging, host location, and selection of egg-laying sites (Myrick and Baker 2010). In adult females, odour molecules are typically detected by receptors located on the sensilla that are supported by the antennae (Elgar et al. 2018; Pang et al. 2020). Schneider (1957) discovered that there are small fluctuations in voltage between the tip and base of an insect antenna during stimulation with pheromones. These electrophysiological signals are the foundation of bioassays for volatile substances that can be perceived by the antennae, in which electroantennographic detection (EAD) is combined with gas chromatography–mass spectrometry (GC–MS; Box 4); so, molecules that elicit an antennal response can be immediately identified and their concentration measured. These GC–MS–EAD bioassays are now widely used for screening bioactive compounds, purification of extracts, and selection of active synthetic compounds.
| Box 4.Gas chromatography and mass spectrometry (GC–MS) |
| The combination of gas chromatography and mass spectrometry (GC–MS) allows the identification of tiny amounts of a substance within a sample. Gas chromatography is a powerful technique that separates volatile or semi-volatile compounds in complex mixtures. As the name suggests, it involves separating compounds in the gas phase, so it is essential that the compounds of interest are both volatile and thermally stable at high temperatures (up to 400°C). Those compounds are separated by injecting the sample, usually dissolved in a solvent, into a column housed in a temperature-controlled oven. The temperature of the column is then increased linearly over a time period (usually between 10 and 60 min), during which the individual compounds evaporate and are then carried through the column by an inert carrier gas (often helium). The individual compounds then either stay with the mobile gas phase passing through the column or are attracted to, and thus slowed down, by a stationary (liquid or solid) phase within the column. The compounds in the mix are separated by the interactions between the stationary phase and the constant flowing mobile carrier gas as they move through the column. The rate of movement of a compound through a column also depends on the temperature and the rate of flow of the carrier gas. The time taken for a compound to exit the column, i.e. its ‘retention time’, is usually unique for a given compound because it depends on its physical properties such as boiling points and polarity. As the compounds exit a column, various detectors are used to sense them. The most common detectors are flame ionisation detectors that sense changes in electrical conductivity as compounds pass through a flame, or mass spectrometers (MS) that give some indication of the molecular mass of a compound, as well as provide some structural information. The mass spectra for the separated compounds can be matched to libraries of known compounds. |
The success of EAD depends on the antenna preparation and the input to the subsequent amplifier (Syntech 2015). A silver wire, connected to a metal electrode, is inserted into a glass capillary filled with saline to ensure conduction (Fig. 1a), with which the trimmed tip of an antenna is brought into contact to form a closed loop (Staddon and Everton 1980). Antenna morphology is a major limiting factor, so access varies with species. There are three methods, as follows:
Access to antennae for detection of electrophysiological signals for electroantennographic detection (EAD). (a) An Ag–AgCl wire connected to a metal electrode is inserted into a glass capillary filled with saline. (b) Whole-insect access. (c) Whole-head access. (d) Antenna-only access to the EAD, as used in our studies of L. cuprina. Redrawn after Syntech (2015).
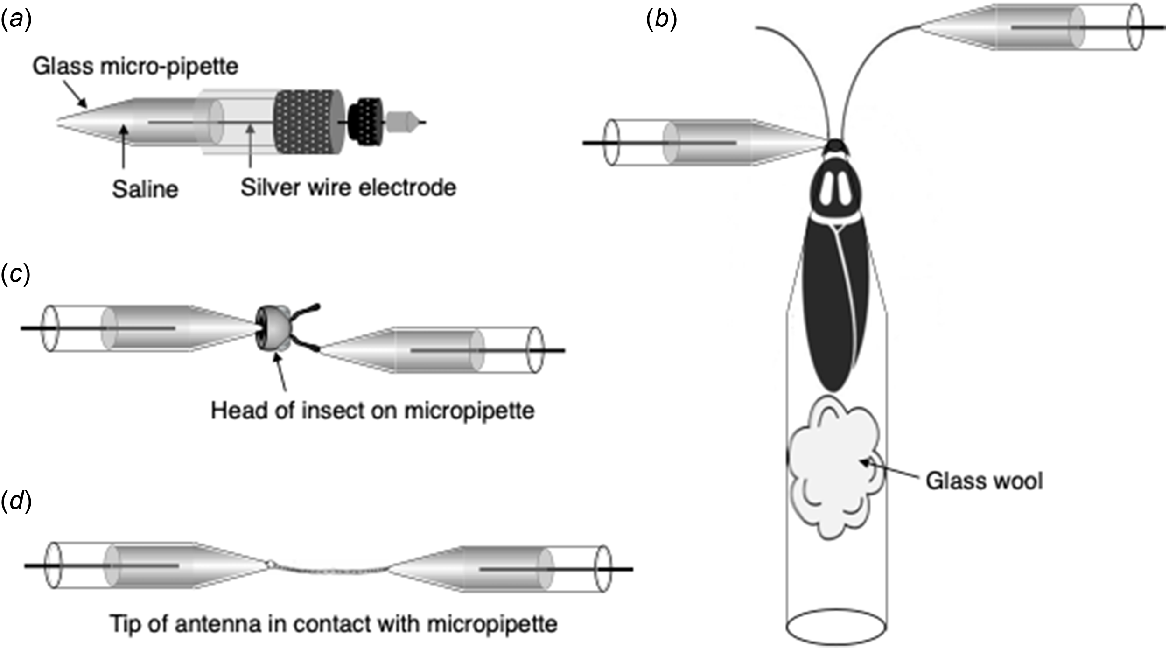
‘Whole-insect’ (Fig. 1b): after hypothermic anesthesia, the fly is fixed inside the tip of a disposable plastic pipette of diameter that restrains the body but allows the head to protrude; one electrode is placed in contact with the base of one antenna and the other is brought into contact the trimmed tip of the other antenna;
‘Whole head’ (Fig. 1c): after hypothermic anesthesia, the head is excised from the thorax and mounted between two electrodes, one placed in contact with the cut surface in the cavity in the underside of the head, while the other is brought into contact with the trimmed tip of an antenna;
‘Antenna-only’ (Fig. 1d): the antenna is carefully pulled out of the head and suspended between two electrodes; the antenna can also be mounted using an electrode gel or water-based saline.
The EAD method has achieved considerable success, including the detection of responses to (a) heptanal, octanal, nonanal and isobutyric acid in the sucking bug, Triatoma infestans (Guerenstein and Guerin 2001), (b) several compounds, particularly the 2-ketones and lactone in tsetse flies (Gikonyo et al. 2002), (c) heptanal, octanal, nonanal and decanal in mosquitoes (Ghaninia et al. 2008), (d) C7–C10 aldehydes and sulcatone in bed bugs (Harraca et al. 2012), advanced decay of rats in the fringed larder beetle, Dermestes frischii (Martin et al. 2020), and (e) 1-octen-3-ol, dimethyldisulfide (DMDS), 2-phenylethanol, dimethyl trisulfide (DMTS), butyric acid, and indole in L. cuprina (Park and Cork 1999; Yan et al. 2018).
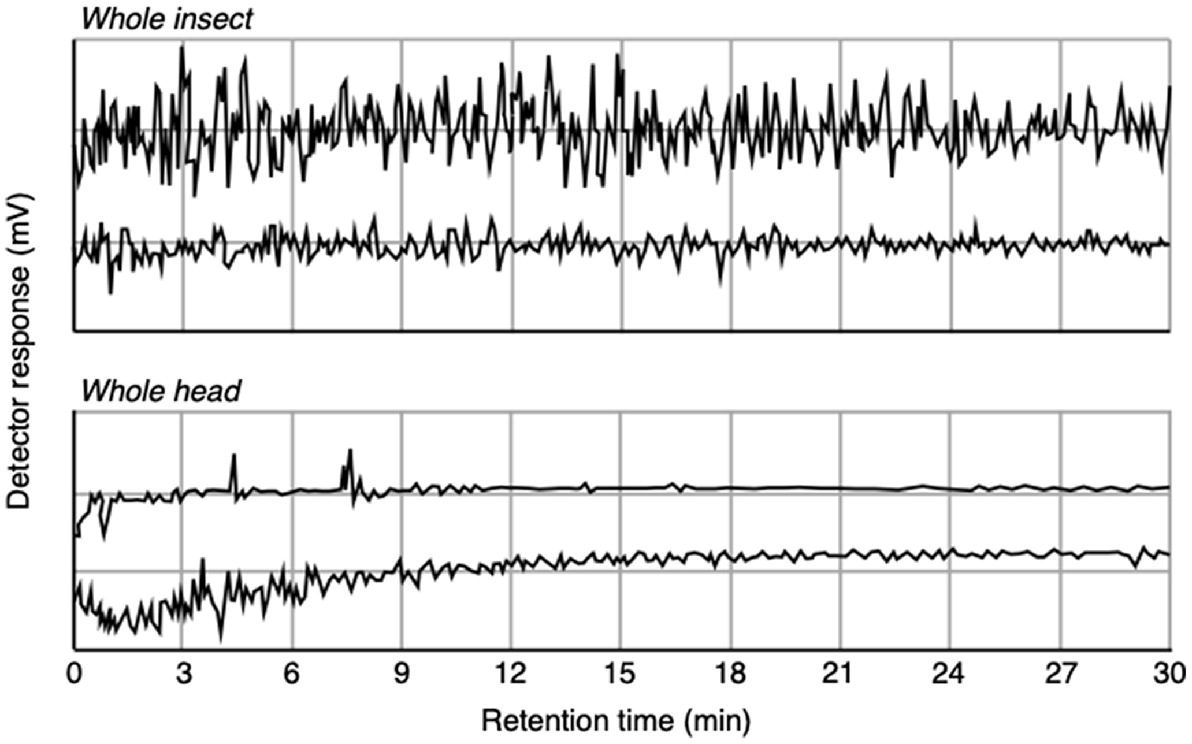
To determine the optimal access method for L. cuprina, we first tested the ‘whole-fly’ approach with the view that the antennae would retain their activity if they were not separated from the body (Yan et al. 2019b). However, the EAD signal fluctuated very widely and rapidly throughout the 30 min observation period, even without stimulation by putative odours (Fig. 2). We therefore moved to the ‘whole-head’ approach (Yan et al. 2019b), with which the signal fluctuated widely for the first 10 min in the absence of stimulation by a putative odour, but then stabilised for 20 min (Fig. 2).
An illustration of baseline electrophysiological recordings from the antennae of a gravid female L. cuprina in the absence of stimulation by putative odours, with the antenna accessed by the ‘whole insect’ and ‘whole head’ approaches (see Fig. 1). From Yan (2019).
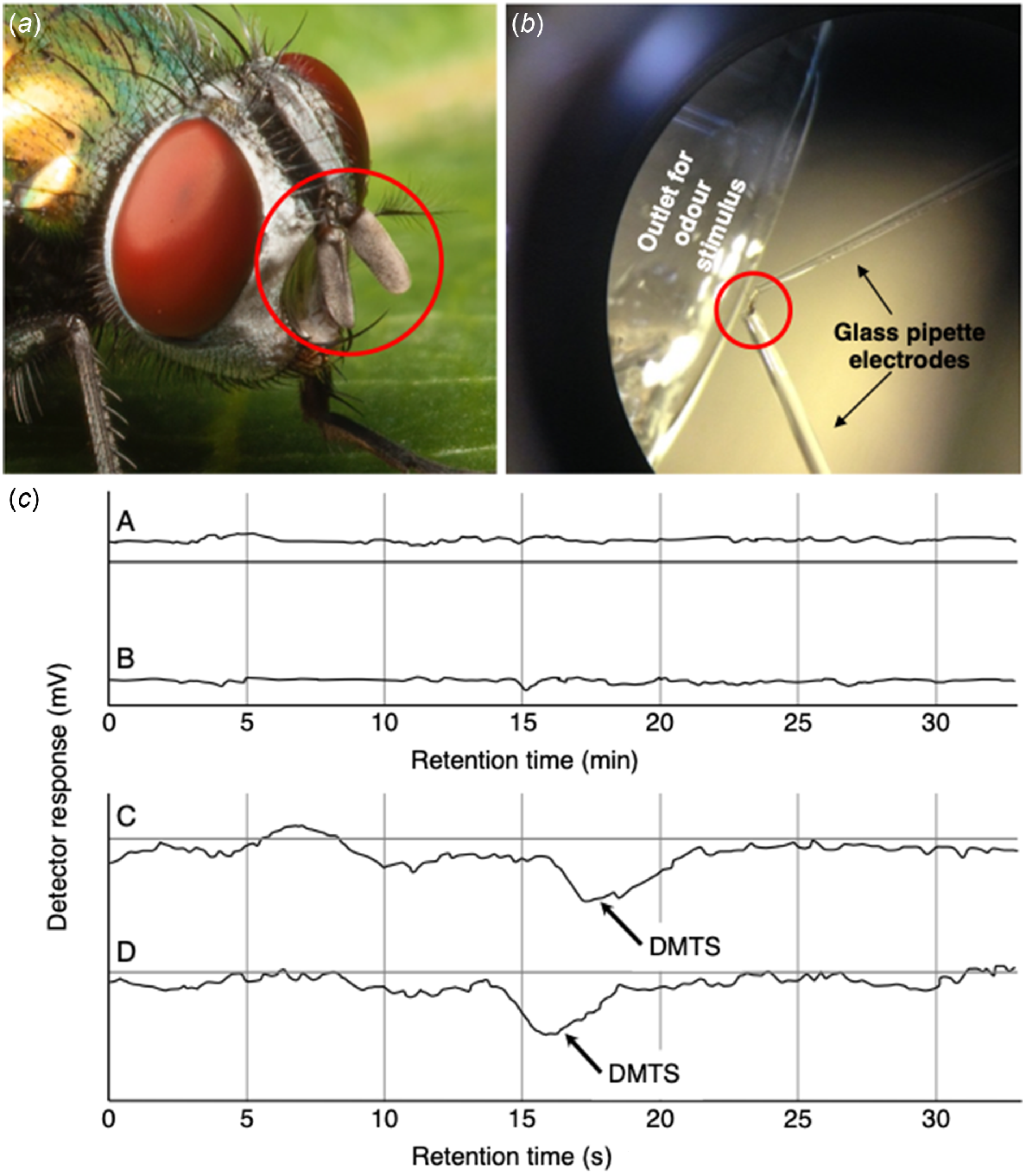
However, the risks of artifacts were too great for precise data acquisition, so we finally moved to antenna-only access (Yan et al. 2019b). The antennae of L. cuprina are very short and club-shaped (Fig. 3a) and we excised them from the head so we could access them directly using micropipette tips placed with the aid of a stereo microscope (Fig. 3b). With this preparation, the electrophysiological signal was stable without stimulation and showed clear responses to DMTS (Fig. 3c).
(a) Club-shaped antennae of L. cuprina (red circle). Image source: Alan Henderson, Minibeast Wildlife, Queensland, Australia. (b) The antenna-only method for access for electroantennography (see Fig. 1d). The antenna is detached from the head of the fly and each end is connected to an electrode. Test gases are passed over the gas chromatography column first, then via a Y-tube that divides it into two streams, one targeting the antenna and the other going to the mass spectrometer to identify the molecules that induce an antenna response. (c) An illustration of EAD recordings from the antennae of gravid female L. cuprina, accessed by the antenna-only method. Control recordings demonstrating signal stability over 30 min (Graphs A, B). Responses to stimulation (arrows) with dimethyl trisulfide (DMTS; Graphs C, D). Note the different time scales. From Yan (2019).
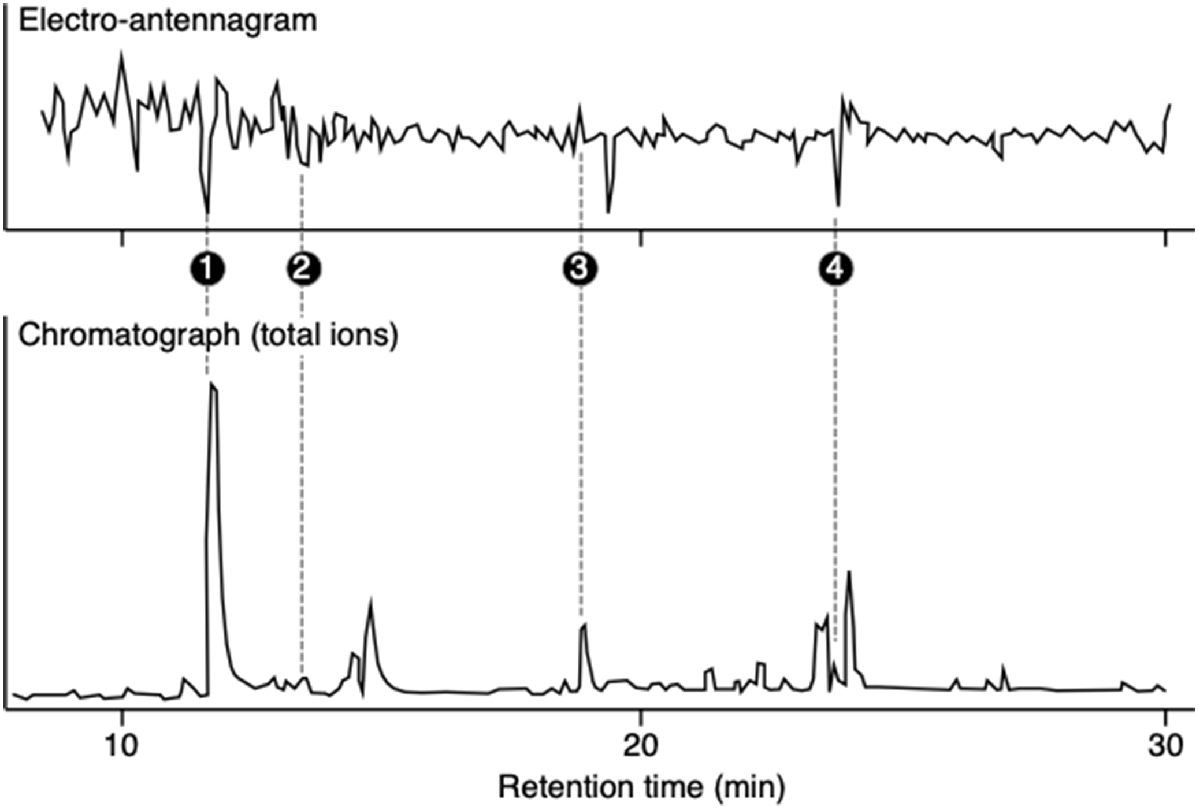
However, it is often difficult to distinguish between noise and the real antennal response, leading to random spikes in the recording, as illustrated in Fig. 4. Noise can be minimised by optimisation of the components the EAD system, including manipulating the mounts, electrode types and connection, using multiple electrodes, and varying the gel and Ringer solutions that are used to make contact. In addition, we make use of replication, an eluted compound was regarded as a candidate only when it elicited antennal responses in at least three of five valid antennae. Finally, as we describe below, the putative EAD response needs to be supported by relevant olfactory behavioural tests (Guerenstein and Guerin 2001; Yan et al. 2018; Martin et al. 2020).
An electrophysiological recording of the response of the antenna of a gravid female L. cuprina, aligned with the gas chromatography–mass spectrometer chromatogram after solid-phase microextraction of odours from wool that attracts flies. Four compounds elicited antennal responses in at least three of five valid antennae: dimethyl trisulfide (1); octanal (2); nonanal (3) and dimethyl tetrasulfide (4). These compounds were then subjected to olfactory behavioural tests (Yan 2019).
Behavioural bioassays
While the EAD can indicate detection of a volatile substance by the antenna, this information alone does not inform us about how the insects respond to that substance; for example, do they find the substance attractive or repellant? Therefore, EAD information must be supported by relevant olfactory behavioural tests, usually involving offering the insects a choice between treatment and control odours. Such tests include the following:
Y-tube test (Fig. 5a): the arms of Y-tube are connected to two odour sources by hoses, with one source being the Control (directly connected to the air pump) and the other being the treatment; a test fly is placed in the main arm of the Y-tube and its choice is recorded (Xu et al. 2015);
Landing time (Fig. 5b): a Petri dish (say, diameter 15 cm, depth 2.8 cm) is divided into two halves by using cardboard, and one side is filled with activated carbon (Control) while a test substance is placed in the other side; the whole dish is covered with gauze, to prevent the test flies gaining direct access, and a second Petri dish with a small hole for inserting a test fly is added; after adaptation, the number of seconds that the fly stays on the side with the test substance is recorded over a 10-min period; no duration is recorded if no exploration behaviour is observed (Cragg 1956);
Visiting frequency (Fig. 5c): flies are released into a wire mesh cage illuminated by fluorescent ceiling lights; they are allowed 1 h for acclimation after which two disposable paper cups (Control and treatment) covered with gauze are inserted; the number of flies on the gauze above each cup is recorded every 2 min during set times (Brodie et al. 2016);
Traps (Fig. 5d): test flies are released into a wire mesh cage illuminated by fluorescent ceiling lights; after acclimation, two traps (one Control and one treatment) are placed inside the cage and the number of flies caught in each trap is recorded after a set time (Brodie et al. 2015a).
The four methods for testing behavioural responses of flies to odours: (a) Y-tube; (b) landing time; (c) visit time; (d) trap. For L. cuprina, they were all compared by Yan (2019) and the Trap (d) was found to be the most reliable.
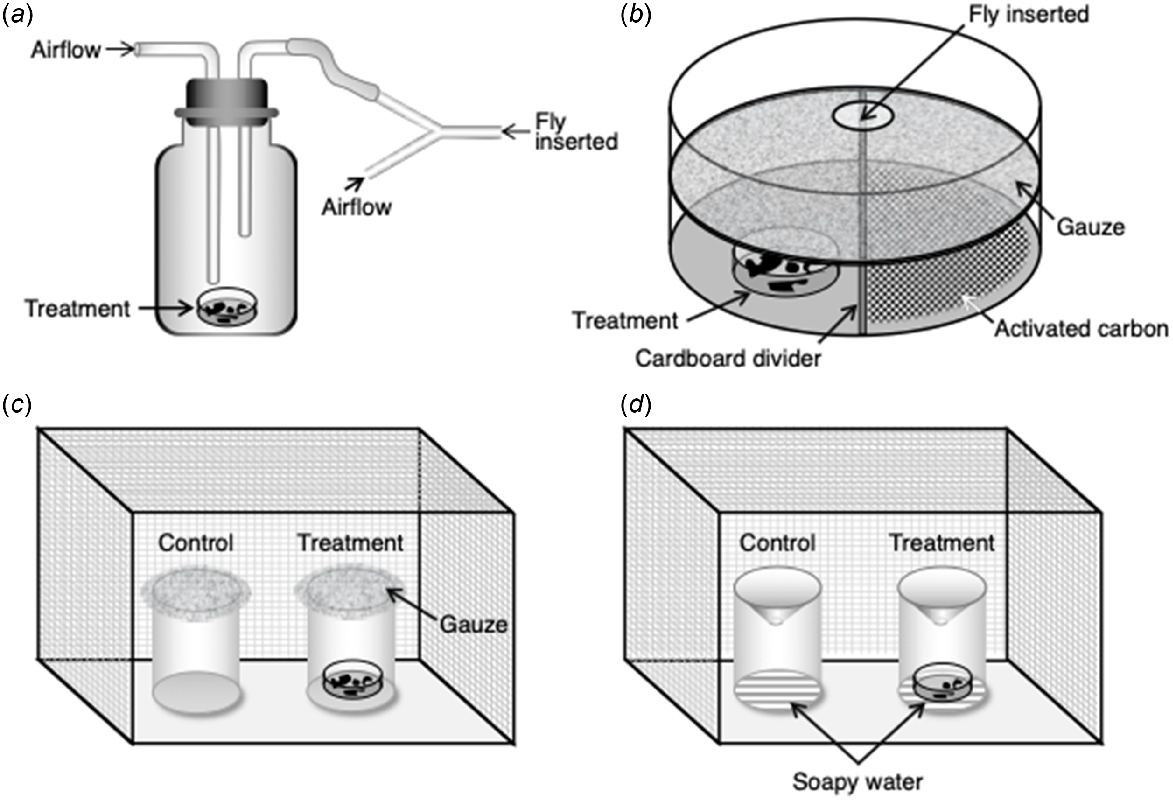
Unfortunately, some of these behavioural tests do not seem to work consistently for all insect species. For example, Cragg (1956) reported that L. cuprina showed a preference for wool samples in the Y-tube test, but we were unable to reproduce that outcome in our preliminary studies where we tested L. cuprina in all four of the above tests, as detailed by Yan (2019). We concluded that the Trap (Fig. 5d) was the most reliable for quantifying olfactory behavioural responses.
We tested four carrion-associated volatile organic compounds (VOCs), namely 1-octen-3-ol, indole, DMTS, and butyric acid, and found that DMTS and butyric acid evoked both EAD and behavioural responses with L. cuprina, whereas 1-octen-3-ol and indole evoked only EAD responses (Yan et al. 2018). Moreover, gravid flies were more sensitive to these compounds than were non-gravid flies, and produced both antennal and behavioural responses at lower doses. We then went on to study octanal and nonanal, two compounds identified in the odour of wool that attracts L. cuprina, and we found that they both elicit antennal responses and attract gravid flies in the behavioural test (Yan et al. 2019b).
Overall, therefore, we concluded that (1) behavioural testing is necessary to determine whether compounds that evoke antennal responses attract or repel the flies; (2) the trap was the most reliable behavioural test for L. cuprina; (3) the antennal and behavioural responses of L. cuprina are related to their reproductive status, with gravid flies being more sensitive than non-gravid flies (Yan et al. 2019b).
Finally, it is important to recognise that laboratory behavioural assays need to be supported by field testing to ensure that hypotheses developed with laboratory-reared insects in a controlled environment translate to the real world. It is well documented that semio-chemicals from livestock pelage can evoke antennal responses in a variety of insect pests (Guerenstein and Guerin 2001; Gikonyo et al. 2002; Ghaninia et al. 2008; Syed and Leal 2009; Harraca et al. 2012), yet, rarely seem to illicit positive behavioural responses in the field. Thus, in wild L. cuprina under field conditions, the antenna and behavioural responses could differ among individual semio-chemicals, or mixes of semio-chemicals.
Attraction of L. cuprina to its host
L. cuprina displays a sequence of behaviours, including initial activation, orientation and landing, that culminates in oviposition (Ashworth and Wall 1994). Olfactory and visual cues initiate the detection and location of a vertebrate host for egg-laying and for food-foraging (de Bruyne and Baker 2008). In addition to odours emitted directly from food resources, olfactory cues for aggregative oviposition seem to be produced by bacterial symbionts located in the fly digestive system (Brodie et al. 2015b; Uriel et al. 2020). The bacteria involved probably arose originally from environment, or from the host, and are associated with decay (the ‘necrobiome’) and produce volatile compounds that attract flies (von Hoermann et al. 2022). Indeed, in the field, L. cuprina repeatedly infests sheep and is repeatedly attracted to wool samples from flystruck sheep (Mackerras and Mackerras 1944).
In laboratory studies, L. cuprina was attracted to wool samples contaminated with serous exudate induced by L. cuprina myiasis (Eisemann 1995). More recently, we used antennagrams and behaviour responses to show that the attractiveness of wool samples was related to the presence of octanal and nonanal, whereas the attractiveness of carrion and faeces was related to butyric acid, dimethyl trisulfide (DMTS) and dimethyl tetrasulfide (Yan et al. 2018, 2019b). Similar outcomes have been reported for pests of cattle, horses and humans (Logan et al. 2009; Oyarzún et al. 2009; Baldacchino et al. 2014).
As flies near the host, visual cues come into play to help with orientation and landing behaviours (Wall and Fisher 2001). Many fly species, such as blowflies, hover flies, fruit flies and house flies, are attracted by yellow-coloured objects (Gomes et al. 2007; Diclaro et al. 2012). In L. sericata, for example, the attractiveness of yellow is enhanced by the floral odour of oxeye daisies (Brodie et al. 2014, 2015a).
Olfactory, visual and tactile cues are involved in the initiation of oviposition. Gravid L. sericata selects an oviposition site by using a combination of an odour, such as DMTS, and a dark colour (Brodie et al. 2014). In L. cuprina, 22 extra sensilla, located on each of the paired lateral leaflets of the ovipositor, are also thought to play a role in regulating egg-laying behaviour (Rice 1976). In this species, other cues, such as humidity, putrefaction and illuminance, also act as triggers for egg-laying on sheep fleece (Rice 1976).
After an oviposition site has been located, L. cuprina deposits batches of about 200 eggs in the wool (Wall 1993). At skin-surface temperatures, eggs hatch after 12–24 h (Wall et al. 1992) and the larvae feed on skin secretions, dermal tissues and blood as they proceed through three stages of development (Tellam and Bowles 1997); first-stage larvae feed on the skin surface tissue and digest it with proteolytic enzymes; the second and third stages develop mouth hooks that can abrade the skin surface, rapidly causing skin damage.
The development of L. cuprina larvae is rapid; a clean sheep can become heavily infested within only 1–2 days and, if larval feeding is not arrested, the resultant mechanical, chemical and enzymatic effects can become catastrophic and even lead to the death of the host (Morris 2000). After 3–4 days growth, the larvae stop feeding and enter a ‘wandering phase’, falling off the sheep and migrating into soil for pupation. Pupation lasts from one to several weeks, depending on the ambient temperature, after which the adult flies emerge to complete the life cycle.
Using semio-chemicals in flystrike control
In the early attempts to prevent flystrike, industry turned to chemicals such as dieldrin, benzene hexachoride and diazinon, all of which were very effective, but were subsequently banned because of their impacts on human health and the environment. Since then, a great deal of research has led to the development of several other insecticides, but it is difficult to avoid the flies developing resistance to any insecticides that are deployed extensively over long periods (Bisdorff and Wall 2008; Sandeman et al. 2014; Kotze and James 2022; Benedetti Vallenari et al. 2023).
In the quest for a more ‘clean, green and ethical’ solution to flystrike, there was initially some renewed interest in fly traps, a renewed emphasis on breeding flystrike-resistant sheep, and on genome modifications in both the blowfly and the sheep. All of these approaches could be aided by a greater understanding of olfaction in L. cuprina.
Fly-trap improvement
Traps that attract and capture flies to reduce the free-roaming population have been used for a century, during which, there has been a significant effort to improve trap efficacy and utility by improving the bait, design and materials (Hall 1995). However, the development of more attractive traps has commonly focused on baits made of mixtures, rather than individual compounds, so they often capture non-target insects. For example, when sheep carcase or offal treated with sodium sulfide was used, L. cuprina was trapped but so were large numbers of other, potentially beneficial, species (Heath and Leathwick 2001). Other attempts to enhance the efficacy, specificity and utility of baits have included a mixture of Proteus mirabilis and ovine gut mucus of sheep (Morris et al. 1998), cloth targets impregnated with sucrose and 10% triflumuron, a growth regulator insecticide (Smith and Wall 1998), a synthetic bait comprising sulfur-containing volatile compounds encapsulated in a slow-release casein matrix (Morris 2005), and freeze-dried liver (Broughan and Wall 2006). In addition to the problem of target specificity, these mixed-compound baits introduce complex interactions that depend on the types and proportions of compounds used; sometimes they are antagonistic and sometimes they are synergistic. For example, the most notable commercial trapping system in Australia, the Lucitrap™, is baited with mixtures including sodium sulfide, ‘technical flakes’, butanoic acid, 2-mercaptoethanol, and indole. These traps efficiently attract L. cuprina (Urech et al. 2004, 2009). Indole and 2-mercaptoethanol are added because it is known that they enhance the attractiveness of wet fleece (Eisemann 1995), but traps with only 2-mercaptoethanol or indole failed to attract L. cuprina (Yan et al. 2018). In addition, the need to purchase and maintain a large number of traps is not considered cost-effective. Thus, although there is little doubt that high-intensity trapping reduces the incidence of flystrike (Anderson et al. 1990), it seems unlikely that further investment in trapping efficiency will result in major improvements, even with our new knowledge of olfaction in L. cuprina.
Breeding flystrike-resistant sheep
The common thread of sheep odour in the indirect traits for flystrike led us to investigate the attractiveness to L. cuprina of wool from Merino sheep that had been bred to be resistant or susceptible to flystrike. As detailed above, we found that octanal and nonanal can be used by L. cuprina to distinguish between attractive and non-attractive wool (Yan et al. 2019b).
However, we need to consider the entire suite of odouriferous compounds from wool, and not only those that attract flies, for the same reason that single-compound baits have limited value in fly traps. To this end, we constructed a database of odours using wool samples from 1032 fully pedigreed Merino sheep and identified all of the odour compounds in each sample by using GC–MS. Dimethyl sulfone was present in greater concentrations in wool from flystrike-susceptible sheep than in wool from flystrike-resistant sheep. We also used our data to estimate heritabilities, which were 0.15 ± 0.07 for octanal, 0.19 ± 0.07 for nonanal, and 0.25 ± 0.09 for dimethyl sulfone (Yan 2019). These values are low but they do suggest that, after further work, the concentrations of octanal, nonanal and dimethyl sulfone in wool might be suitable indicator traits for breeding sheep that are less attractive to blow flies.
Clearly, further research is needed, including an evaluation of the genetic correlations of odour traits with incidence of flystrike (and other key production traits), and development of a method for measuring semio-chemicals in a commercial setting. Moreover, as mentioned above, we need to remember that semio-chemicals that come strongly to the fore under laboratory testing might not be important under field conditions. For example, octanal and nonanal, both of which have been identified previously in extracts of livestock pelage and are known to evoke antennal responses in a variety of insect pests (Guerenstein and Guerin 2001; Gikonyo et al. 2002; Ghaninia et al. 2008; Syed and Leal 2009; Harraca et al. 2012), rarely seem to illicit positive behavioural responses in the field.
Exploring genomic solutions
The sterile insect technique (SIT), in which sterilised males are released to reduce population numbers, has long been seen as having great potential for control of key insect pests because it is species-specific, non-polluting, and resistance-free. In Australia, from the 1960s to the 1980s, CSIRO focused on SIT programs for the control of L. cuprina, but the sheer size of the areas occupied by L. cuprina and the lack of climatic barriers greatly limited success. As an alternative, a ‘field female killing’ (FFK) strain was developed in which recessive eye-colour mutations rendered homozygous females functionally blind so they died in the field; in contrast, males are carriers but not blind and only semi-sterile, so they can mate with wild-type females and pass on the mutation (Foster et al. 1993; Black et al. 2011). In a trial on a small island in 1985–1986, FFK was so successful that L. cuprina became undetectable (Foster et al. 1993). However, a subsequent trial on larger islands in Bass Strait failed for several reasons, including practical difficulties with the mass rearing of flies, the unstable nature of the mutations, and the reduced fitness of the released flies compared with the wild flies.
Work on insect genomes continued, with exploration of odorant-binding proteins (OBPs) and odorant receptors, thus introducing semio-chemical options (Venthur and Zhou 2018). For example, (i) the oviposition preference of Drosophila melanogaster could be shifted by introducing OBP57d/e genes from D. sechellia (Matsuo et al. 2007), (ii) in Rhodnius prolixus, the vector of Chagas disease, oviposition and blood ingestion were reduced by knockdown of ORco, a major gene family involved in olfactory-evoked behaviours of insects and a required partner for all odorant receptors (Franco et al. 2016), and (iii) in Cochliomyia hominivorax, the response to floral-like and animal host-associated odours was impaired in ORco mutants that lost their odorant receptor function (Paulo et al. 2021).
Clearly, the identification of semio-chemicals offers considerable scope for manipulating host-searching behaviour or for interruption of fly sexual behaviour (Pickett 2014). For example, it is feasible that octanal and nonanal might offer opportunities for modification of the genome of L. cuprina to alter host attraction, or for modification of the genome of the sheep to decrease the production of attractive semio-chemicals, or perhaps promote the production of repellant semio-chemicals.
Conclusions
In the quest for new strategies for preventing breech flystrike in Australian Merino industries, breeding resistant sheep is an attractive option. To improve the efficiency of breeding programs, we need traits directly related to the cause of flystrike. To this end, we have focused on the olfactory biology of L. cuprina, particularly the semio-chemicals produced by sheep that underpin long-distance location of the host, orientation and landing behaviour, and selection of the oviposition site. With GCMS–EAD, we can determine which odour molecules the fly can detect and, with behaviour tests, we can determine whether the response is attraction or repulsion. Semio-chemicals that are specific for L. cuprina are not likely to revolutionise fly traps, but will probably assist by improving the selection of flystrike-resistant Merino sheep, and by offering guidance for modification of the L. cuprina genome. Success in these endeavours could save the Australian Merino-based industries as much as A$200 m per year, while also providing those industries with a more ‘clean, green and ethical’ image in world markets.
Data availability
Data sharing is not applicable because no new data were generated or analysed during this study. However, with a reasonable request, the authors can share previously published data.
Declaration of funding
We are grateful for the financial support for this project from Australian Wool Innovation (Contract ON-00169), and for the resources provided by the WA Department of Primary Industries and Regional Development, and The University of Western Australia. During his PhD studies in Australia, GJY was a recipient of a Postgraduate Scholarship (Award 201506300148) from the China Scholarship Council, People’s Republic of China.
References
Anderson PJ, Shipp E, Anderson JME, Dobbie W (1988) Population maintenance of Lucilia-cuprina (Wiedemann) in the arid zone. Australian Journal of Zoology 36, 241-249.
| Crossref | Google Scholar |
Anderson JME, Mcleod LJ, Shipp E, Swan A, Kennedy JP (1990) Trapping sheep blowflies using bait-bins. Australian Veterinary Journal 67, 93-97.
| Crossref | Google Scholar | PubMed |
Ashworth JR, Wall R (1994) Responses of the sheep blowflies Lucilia sericata and L. cuprina to odour and the development of semiochemical baits. Medical and Veterinary Entomology 8, 303-309.
| Crossref | Google Scholar | PubMed |
Atkins KD, McGuirk BJ (1979) Selection of Merino sheep for resistance to fleece-rot and body strike. Wool Technology and Sheep Breeding 27, 15-19.
| Google Scholar |
Austin CM, Naidoo V (2022) The efficacy of fluazuron in the management of blowfly strike in sheep. Experimental Parasitology 236–237, 108251.
| Crossref | Google Scholar | PubMed |
Baldacchino F, Manon S, Puech L, Buatois B, Dormont L, Jay-Robert P (2014) Olfactory and behavioural responses of tabanid horseflies to octenol, phenols and aged horse urine. Medical and Veterinary Entomology 28, 201-209.
| Crossref | Google Scholar | PubMed |
Benedetti Vallenari P, Bailey A, Horton BJ (2023) A model of flystrike pesticide resistance management on sheep: use of pesticide rotations. Animal Production Science 63, 802-815.
| Crossref | Google Scholar |
Benton R, Vannice KS, Vosshall LB (2007) An essential role for a CD36-relaeted receptor in pheromone detection in Drosophila. Nature 450, 289-293.
| Crossref | Google Scholar | PubMed |
Beveridge WIB (1984) The origin and early history of the Mules operation. Australian Veterinary Journal 61, 161-163.
| Crossref | Google Scholar | PubMed |
Bisdorff B, Wall R (2008) Sheep blowfly strike risk and management in Great Britain: a survey of current practice. Medical and Veterinary Entomology 22, 303-308.
| Crossref | Google Scholar | PubMed |
Black WC, IV, Alphey L, James AA (2011) Why RIDL is not SIT. Trends in Parasitology 27, 362-370.
| Crossref | Google Scholar | PubMed |
Brodie B, Gries R, Martins A, VanLaerhoven S, Gries G (2014) Bimodal cue complex signifies suitable oviposition sites to gravid females of the common green bottle fly. Entomologia Experimentalis et Applicata 153, 114-127.
| Crossref | Google Scholar |
Brodie BS, Smith MA, Lawrence J, Gries G (2015a) Effects of floral scent, color and pollen on foraging decisions and oocyte development of common green bottle flies. PLoS ONE 10, e0145055.
| Crossref | Google Scholar | PubMed |
Brodie BS, Wong WHL, VanLaerhoven S, Gries G (2015b) Is aggregated oviposition by the blow flies Lucilia sericata and Phormia regina (Diptera: Calliphoridae) really pheromone-mediated? Insect Science 22, 651-660.
| Crossref | Google Scholar | PubMed |
Brodie BS, Babcock T, Gries R, Benn A, Gries G (2016) Acquired smell? Mature females of the common green bottle fly shift semiochemical preferences from feces feeding sites to carrion oviposition sites. Journal of Chemical Ecology 42, 40-50.
| Crossref | Google Scholar | PubMed |
Broughan JM, Wall R (2006) Control of sheep blowfly strike using fly-traps. Veterinary Parasitology 135, 57-63.
| Crossref | Google Scholar | PubMed |
Colditz I, Vuocolo T, Denman S, Ingham A, Wijffels G, James P, Tellam R (2021) Fleece rot in sheep: a review of pathogenesis, aetiology, resistance and vaccines. Animal Production Science 62, 201-215.
| Crossref | Google Scholar |
Colvin AF, Reeve I, Kahn LP, Thompson LJ, Horton BJ, Walkden-Brown SW (2022) Australian surveys on incidence and control of blowfly strike in sheep between 2003 and 2019 reveal increased use of breeding for resistance, treatment with preventative chemicals and pain relief around mulesing. Veterinary Parasitology: Regional Studies and Reports 31, 100725.
| Crossref | Google Scholar | PubMed |
Cragg JB (1956) The olfactory behaviour of Lucilia species (diptera) under natural conditions. Annals of Applied Biology 44, 467-477.
| Crossref | Google Scholar |
de Bruyne M, Baker TC (2008) Odor detection in insects: volatile codes. Journal of Chemical Ecology 34, 882-897.
| Crossref | Google Scholar | PubMed |
Diclaro JW, II, Cohnstaedt LW, Pereira RM, Allan SA, Koehler PG (2012) Behavioral and physiological response of Musca domestica to colored visual targets. Journal of Medical Entomology 49, 94-100.
| Crossref | Google Scholar | PubMed |
Eisemann CH (1995) Orientation by gravid Australian sheep blowflies, Lucilia cuprina (Diptera: Calliphoridae), to fleece and synthetic chemical attractants in laboratory bioassays. Bulletin of Entomological Research 85, 473-477.
| Crossref | Google Scholar |
Elgar MA, Zhang D, Wang Q, Wittwer B, Pham HT, Johnson TL, Freelance CB, Coquilleau M (2018) Insect antennal morphology: the evolution of diverse solutions to odorant perception. The Yale Journal of Biology and Medicine 91, 457-469.
| Google Scholar | PubMed |
Franco TA, Oliveira DS, Moreira MF, Leal WS, Melo ACA (2016) Silencing the odorant receptor co-receptor RproOrco affects the physiology and behavior of the Chagas disease vector Rhodnius prolixus. Insect Biochemistry and Molecular Biology 69, 82-90.
| Crossref | Google Scholar | PubMed |
Ghaninia M, Larsson M, Hansson BS, Ignell R (2008) Natural odor ligands for olfactory receptor neurons of the female mosquito Aedes aegypti: use of gas chromatography-linked single sensillum recordings. Journal of Experimental Biology 211, 3020-3027.
| Crossref | Google Scholar | PubMed |
Gikonyo NK, Hassanali A, Njagi PGN, Gitu PM, Midiwo JO (2002) Odor composition of preferred (buffalo and ox) and nonpreferred (waterbuck) hosts of some savanna tsetse flies. Journal of Chemical Ecology 28, 969-981.
| Crossref | Google Scholar | PubMed |
Gomes L, Gomes G, Casarin FE, da Silva IM, Sanches MR, Von Zuben CJ, Fowler HG (2007) Interação entre fatores visuais e olfativo em localização de recursos pela mosca-varejeira, Chrysomya megacephala (Fabricius) (Diptera: Calliphoridae), em condições naturais. Neotropical Entomology 36, 633-639.
| Crossref | Google Scholar | PubMed |
Greeff JC, Karlsson LJE, Schlink AC (2013a) Identifying indicator traits for breech strike in Merino sheep in a Mediterranean environment. Animal Production Science 54, 125-140.
| Crossref | Google Scholar |
Greeff JC, Biggs A, Grewar W, Crumblin P, Karlsson LJE, Schlink AC, Smith J (2013b) Dogs can differentiate between odours from sheep that are resistant or susceptible to breech strike. Association for the Advancement of Animal Breeding and Genetics 20, 397-400.
| Google Scholar |
Greeff JC, Schlink AC, Karlsson LJE (2018a) Impact of sire on the lifetime susceptibility of their progeny to breech strike in a Mediterranean environment. Animal Production Science 58, 1522-1530.
| Crossref | Google Scholar |
Greeff JC, Karlsson LJE, Schlink AC, Gilmour AR, Greeff JC (2018b) Factors explaining the incidence of breech strike in a Mediterranean environment in unmulesed and uncrutched Merino sheep. Animal Production Science 58, 1279-1288.
| Crossref | Google Scholar |
Greeff JC, Karlsson LJE, Schlink AC (2019) Are breech strike, dags and breech wrinkle genetically the same trait in crutched, uncrutched and mulesed Merino sheep? Animal Production Science 59, 1777-1782.
| Crossref | Google Scholar |
Greeff JC, Paz EA, Munyard K, Schlink AC, Smith J, Karlsson LJE, Martin GB, Groth D (2021) Microbiome analysis of the skin of sheep that are resistant or susceptible to breech flystrike. Animal Production Science 61, 1774-1780.
| Crossref | Google Scholar |
Greeff JC, Schlink AC, Karlsson LJE, Vercoe PE, Gilmour AR (2022) Importance of humidity and temperature in breech strike of Merino sheep. Animal Production Science 63, 480-488.
| Crossref | Google Scholar |
Guerenstein PG, Guerin PM (2001) Olfactory and behavioural responses of the blood-sucking bug Triatoma infestans to odours of vertebrate hosts. Journal of Experimental Biology 204, 585-597.
| Crossref | Google Scholar | PubMed |
Hall MJR (1995) Trapping the flies that cause myiasis: their responses to host-stimuli. Annals of Tropical Medicine & Parasitology 89, 333-357.
| Crossref | Google Scholar | PubMed |
Harraca V, Ryne C, Birgersson G, Ignell R (2012) Smelling your way to food: can bed bugs use our odour? Journal of Experimental Biology 215, 623-629.
| Crossref | Google Scholar | PubMed |
Heath ACG, Bishop DM (2006) Flystrike in New Zealand: an overview based on a 16-year study, following the introduction and dispersal of the Australian sheep blowfly, Lucilia cuprina Wiedemann (Diptera: Calliphoridae). Veterinary Parasitology 137, 333-344.
| Crossref | Google Scholar | PubMed |
Horton BJ, Corkrey R, Smith J, Greeff J, Karlsson LJE (2020) Modelling of breech strike risk and protective efficacy of mulesing in adult Merino sheep. Animal Production Science 60, 1051-1060.
| Crossref | Google Scholar |
Karlsson LJE, Greeff JC (2012) Genetic aspects of sheep parasitic diseases. Veterinary Parasitology 189, 104-112.
| Crossref | Google Scholar | PubMed |
Kotze AC, James PJ (2022) Control of sheep flystrike: what’s been tried in the past and where to from here. Australian Veterinary Journal 100, 1-19.
| Crossref | Google Scholar | PubMed |
Leal WS (2013) Odorant reception in insects: roles of receptors, binding proteins, and degrading enzymes. Annual Review of Entomology 58, 373-391.
| Crossref | Google Scholar | PubMed |
Lihou K, Wall R (2019) Sheep blowfly strike: the cost of control in relation to risk. Animal 13, 2373-2378.
| Crossref | Google Scholar | PubMed |
Logan JG, Seal NJ, Cook JI, Stanczyk NM, Birkett MA, Clark SJ, Gezan SA, Wadhams LJ, Pickett JA, Mordue (Luntz) JM (2009) Identification of human-derived volatile chemicals that interfere with attraction of the Scottish biting midge and their potential use as repellents. Journal of Medical Entomology 46, 208-219.
| Crossref | Google Scholar | PubMed |
Martin C, Minchilli D, Francis F, Verheggen F (2020) Behavioral and electrophysiological responses of the fringed larder beetle Dermestes frischii to the smell of a cadaver at different decomposition stages. Insects 11, 238.
| Crossref | Google Scholar | PubMed |
Matsuo T, Sugaya S, Yasukawa J, Aigaki T, Fuyama Y (2007) Odorant-binding proteins OBP57d and OBP57e affect taste perception and host-plant preference in Drosophila sechellia. PLoS Biology 5, e118.
| Crossref | Google Scholar | PubMed |
Morris MC (2000) Ethical issues associated with sheep fly strike research, prevention, and control. Journal of Agricultural and Environmental Ethics 13, 205-217.
| Crossref | Google Scholar |
Morris MC (2005) Tests on a new bait for flies (Diptera: Calliphoridae) causing cutaneous myiasis (flystrike) in sheep. New Zealand Journal of Agricultural Research 48, 151-156.
| Crossref | Google Scholar |
Morris MC, Morrison L, Joyce MA, Rabel B (1998) Trapping sheep blowflies with lures based on bacterial cultures. Australian Journal of Experimental Agriculture 38, 125-130.
| Crossref | Google Scholar |
Myrick AJ, Baker TC (2010) Locating a compact odor source using a four-channel insect electroantennogram sensor. Bioinspiration & Biomimetics 6, 016002.
| Crossref | Google Scholar | PubMed |
Ong RC, Stopfer M (2012) Peripheral and central olfactory tuning in a moth. Chemical Senses 37, 455-461.
| Crossref | Google Scholar | PubMed |
Oyarzún MP, Palma R, Alberti E, Hormazabal E, Pardo F, Birkett MA, Quiroz A (2009) Olfactory response of Haematobia irritans (Diptera: Muscidae) to cattle-derived volatile compounds. Journal of Medical Entomology 46, 1320-1326.
| Crossref | Google Scholar | PubMed |
Pang X, Liu G, Wang Q, Elgar MA, Zhang D (2020) Oviposition preferences and antennal size in carrion flies. Entomologia Experimentalis et Applicata 168, 332-338.
| Crossref | Google Scholar |
Park KC, Cork A (1999) Electrophysiological responses of antennal receptor neurons in female Australian sheep blowflies, Lucilia cuprina, to host odours. Journal of Insect Physiology 45, 85-91.
| Crossref | Google Scholar | PubMed |
Paulo DF, Junqueira ACM, Arp AP, Vieira AS, Ceballos J, Skoda SR, Pérez-de-León AA, Sagel A, McMillan WO, Scott MJ, Concha C, Azeredo-Espin AML (2021) Disruption of the odorant coreceptor Orco impairs foraging and host finding behaviors in the New World screwworm fly. Scientific Reports 11, 11379.
| Crossref | Google Scholar | PubMed |
Pickett JA (2014) Chemical ecology in the post genomics era. Journal of Chemical Ecology 40, 319.
| Crossref | Google Scholar | PubMed |
Raadsma HW (1991) Fleece rot and body strike in Merino sheep. V. Heritability of liability to body strike in weaner sheep under flywave conditions. Australian Journal of Agricultural Research 42, 279-293.
| Crossref | Google Scholar |
Rice MJ (1976) Contact chemoreceptors on the ovipositor of Lucilia cuprina (Wied.), the Australian sheep blowfly. Australian Journal of Zoology 24, 353-360.
| Crossref | Google Scholar |
Sandeman RM, Levot GW, Heath ACG, James PJ, Greeff JC, Scott MJ, Batterham P, Bowles VM (2014) Control of the sheep blowfly in Australia and New Zealand – are we there yet? International Journal for Parasitology 44, 879-891.
| Crossref | Google Scholar | PubMed |
Schneider D (1957) Elektrophysiologische untersuchungen von chemound mechanoreceptoren de antenne des seidenspinners Bombyx mori L. Zeitschrift für vergleichende Physiologie 40, 8-41.
| Crossref | Google Scholar |
Seddon HR (1931) Conditions which predispose sheep to blowfly attack. Agricultural Gazette of New South Wales 42, 581-594.
| Google Scholar |
Smith KE, Wall R (1998) Suppression of the blowfly Lucilia sericata using odour-baited triflumuron-impregnated targets. Medical and Veterinary Entomology 12, 430-437.
| Crossref | Google Scholar | PubMed |
Smith JL, Brewer HG, Dyall T (2009) Heritability and phenotypic correlations for breech strike and breech strike resistance indicators in Merinos. Association for the Advancement of Animal Breeding and Genetics 18, 334-337.
| Google Scholar |
Sneddon J, Rollin B (2010) Mulesing and animal ethics. Journal of Agricultural and Environmental Ethics 23, 371-386.
| Crossref | Google Scholar |
Staddon BW, Everton IJ (1980) Haemolymph of the milkweed bug Oncopeltus fasciatus (Heteroptera; Lygaeidae): inorganic constituents and amino acids. Comparative Biochemistry and Physiology Part A: Physiology 65, 371-374.
| Crossref | Google Scholar |
Syed Z, Leal WS (2009) Acute olfactory response of Culex mosquitoes to a human- and bird-derived attractant. Proceedings of the National Academy of Sciences 106, 18803–18808. 10.1073/pnas.0906932106
Syntech (2015) Electroantennography—a practical introduction. Ockenfels Syntech GmbH, Kirchzarten, Germany. Available at www.syntech.nl.
Tellam RL, Bowles VM (1997) Control of blowfly strike in sheep: current strategies and future prospects. International Journal for Parasitology 27, 261-273.
| Crossref | Google Scholar | PubMed |
Urech R, Green PE, Rice MJ, Brown GW, Duncalfe F, Webb P (2004) Composition of chemical attractants affects trap catches of the Australian sheep blowfly, Lucilia cuprina, and other blowflies. Journal of Chemical Ecology 30, 851-866.
| Crossref | Google Scholar | PubMed |
Urech R, Green PE, Rice MJ, Brown GW, Webb P, Jordan D, Wingett M, Mayer DG, Butler L, Joshua E, Evans I, Toohey L, Dadour IR (2009) Suppression of populations of Australian sheep blowfly, Lucilia cuprina (Wiedemann) (Diptera: Calliphoridae), with a novel blowfly trap. Australian Journal of Entomology 48, 182-188.
| Crossref | Google Scholar |
Uriel Y, Gries R, Tu L, Carroll C, Zhai H, Moore M, Gries G (2020) The fly factor phenomenon is mediated by interkingdom signaling between bacterial symbionts and their blow fly hosts. Insect Science 27, 256-265.
| Crossref | Google Scholar | PubMed |
Venthur H, Zhou JJ (2018) Odorant receptors and odorant-binding proteins as insect pest control targets: a comparative analysis. Frontiers in Physiology 9, 1163.
| Crossref | Google Scholar | PubMed |
von Hoermann C, Weithmann S, Sikorski J, Nevo O, Szpila K, Grzywacz A, Grunwald J-E, Reckel F, Overmann J, Steiger S, Ayasse M (2022) Linking bacteria, volatiles and insects on carrion: the role of temporal and spatial factors regulating inter-kingdom communication via volatiles. Royal Society Open Science 9, 220555.
| Crossref | Google Scholar | PubMed |
Wall R (1993) The reproductive output of the blowfly Lucilia sericata. Journal of Insect Physiology 39, 743-750.
| Crossref | Google Scholar |
Wall R, Fisher P (2001) Visual and olfactory cue interaction in resource-location by the blowfly, Lucilia sericata. Physiological Entomology 26, 212-218.
| Crossref | Google Scholar |
Wall R, French N, Morgan KL (1992) Effects of temperature on the development and abundance of the sheep blowfly Lucilia sericata (Diptera: Calliphoridae). Bulletin of Entomological Research 82, 125-131.
| Crossref | Google Scholar |
Wells AED, Sneddon J, Lee JA, Blache D (2011) Farmer’s response to societal concerns about farm animal welfare: the case of mulesing. Journal of Agricultural and Environmental Ethics 24, 645-658.
| Crossref | Google Scholar |
Xu X, Cai X, Bian L, Luo Z, Xin Z, Chen Z (2015) Electrophysiological and behavioral responses of Chrysopa phyllochroma (Neuroptera: Chrysopidae) to plant volatiles. Environmental Entomology 44, 1425-1433.
| Crossref | Google Scholar | PubMed |
Yan G, Liu S, Schlink AC, Flematti GR, Brodie BS, Bohman B, Greeff JC, Vercoe PE, Hu J, Martin GB (2018) Behavior and electrophysiological response of gravid and non-gravid Lucilia cuprina (Diptera: Calliphoridae) to carrion-associated compounds. Journal of Economic Entomology 111, 1958-1965.
| Crossref | Google Scholar | PubMed |
Yan G, Schlink AC, Brodie BS, Hu J, Martin GB (2019a) The effects of diets and long-term laboratory rearing on reproduction, behavior and morphology of Lucilia cuprina (Wiedemann). Journal of Medical Entomology 56, 665-670.
| Crossref | Google Scholar | PubMed |
Yan G, Liu S, Schlink AC, Flematti GR, Brodie BS, Bohman B, Greeff JC, Vercoe PE, Hu J, Martin GB (2019b) Volatiles from Merino fleece evoke antennal and behavioural responses in the Australian sheep blow fly Lucilia cuprina. Medical and Veterinary Entomology 33, 491-497.
| Crossref | Google Scholar | PubMed |


