Australian Journal of Chemistry
Volume 76
Number 11 2023
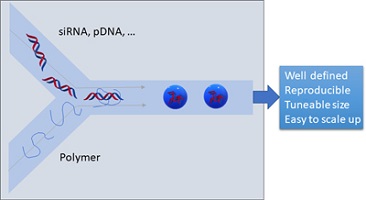
Treatment of diseases using nucleic acid-based approaches is of increasing importance. However, these drugs need to be delivered in nanoparticles to ensure high bioavailability. To ensure the formation of well-defined nanoparticles, flow assembly is increasingly employed. In this review, we map the pathways to nucleic acid-loaded polymer-based nanoparticles prepared by flow.
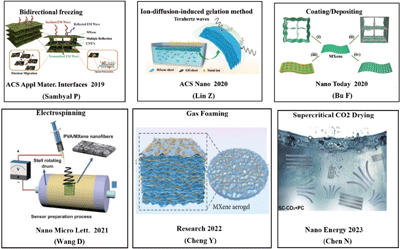
Constructing a 3D structure is an effective strategy for overcoming the electrochemical performance issues of MXenes. Generally, 3D MXene structures, being rich in funcitonal groups, can improve energy storage performance by an increased specific surface area and porosity, and decreasing ion transport distance. Therefore, 3D MXene structures have great potential in high-performance energy storage and conversion applications.
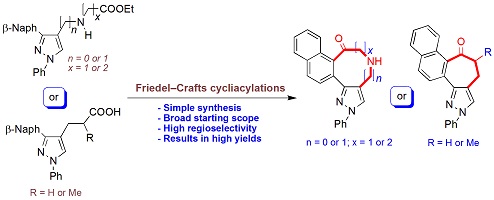
Condensed pyrazole scaffolds form the structural core of a large number of natural products and a wide range of pharmaceuticals. Herein, we explore an efficient construction of tetracyclic pyrazolo-fused carbo- and N-heterocyclic systems, in particular, naphtho-fused cycloheptapyrazolones, azepinopyrazolones, azocinopyrazolones and azoninopyrazolones, by intramolecular Friedel–Crafts cycliacylation reactions.
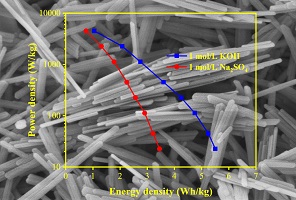
Rod-like porous α-Fe2O3 with large interplanar crystal spacing was prepared by static hydrothermal treatment at 160°C and used for a symmetric supercapacitor. The synthesized α-Fe2O3 exhibits good rate capability even at 20 A g–1 in 1 mol L–1 of KOH electrolyte. (Image credit: Fan-Ming Yang.)
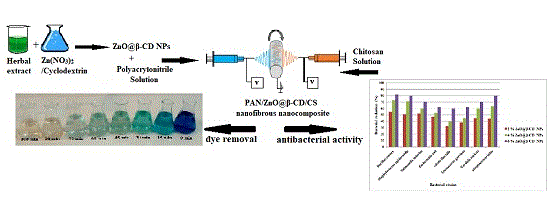
This work introduces a unique nano-biosorbent made of a polyacrylonitrile–ZnO@β-cyclodextrin–chitosan nanofibrous nanocomposite. The nanocomposite’s effectiveness as a novel antibacterial and photocatalyst agent for eliminating methylene blue was evaluated. Results verified the production of the nanocomposite, which exhibited high efficiency for methylene blue removal and acceptable antibacterial activity. (Image credit: Dadkhoda Ghazanfari.)
In the pursuit of new monomers for the sustainable production of polymers, the biomass-derived and platform molecule levoglucosenone (LGO, 1) as well as the pseudo-enantiomeric compound iso-levoglucosenone (iso-LGO, 3) were engaged in a series of Diels–Alder cycloaddition reactions with a range of cyclic dienes. Various manipulations of the resulting adducts provided new monomeric systems likely to be suitable for polymerisation.
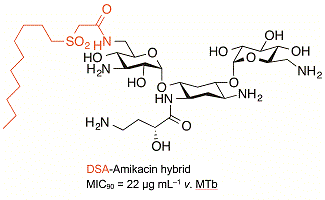
Two potent antitubercular compounds, one hydrophilic (amikacin) and one hydrophobic (DSA), have been combined in a single molecule and shown to retain activity against M. tuberculosis as well as selectivity relative to S. aureus activity. This has potential to improve bioavailability and circumvent resistance mechanisms. (Image credit: Todd A. Houston.)