Plant adaptation to climate change—opportunities and priorities in breeding
Scott C. Chapman A C D , Sukumar Chakraborty A , M. Fernanda Dreccer B C and S. Mark Howden CA CSIRO Plant Industry, Queensland Bioscience Precinct, 306 Carmody Road, St. Lucia, Qld 4067, Australia.
B CSIRO Plant Industry, Cooper Laboratory, The University of Queensland Gatton Campus, Warrego Highway, Gatton, Qld 4343, Australia.
C CSIRO Climate Adaptation Flagship, GPO Box 1700, Canberra, ACT 2601, Australia.
D Corresponding author. Email: scott.chapman@csiro.au
Crop and Pasture Science 63(3) 251-268 https://doi.org/10.1071/CP11303
Submitted: 4 November 2011 Accepted: 9 May 2012 Published: 28 May 2012
Journal Compilation © CSIRO Publishing 2012 Open Access CC BY-NC-ND
Abstract
Climate change in Australia is expected to influence crop growing conditions through direct increases in elevated carbon dioxide (CO2) and average temperature, and through increases in the variability of climate, with potential to increase the occurrence of abiotic stresses such as heat, drought, waterlogging, and salinity. Associated effects of climate change and higher CO2 concentrations include impacts on the water-use efficiency of dryland and irrigated crop production, and potential effects on biosecurity, production, and quality of product via impacts on endemic and introduced pests and diseases, and tolerance to these challenges. Direct adaptation to these changes can occur through changes in crop, farm, and value-chain management and via economically driven, geographic shifts where different production systems operate. Within specific crops, a longer term adaptation is the breeding of new varieties that have an improved performance in ‘future’ growing conditions compared with existing varieties.
In crops, breeding is an appropriate adaptation response where it complements management changes, or when the required management changes are too expensive or impractical. Breeding requires the assessment of genetic diversity for adaptation, and the selection and recombining of genetic resources into new varieties for production systems for projected future climate and atmospheric conditions. As in the past, an essential priority entering into a ‘climate-changed’ era will be breeding for resistance or tolerance to the effects of existing and new pests and diseases. Hence, research on the potential incidence and intensity of biotic stresses, and the opportunities for breeding solutions, is essential to prioritise investment, as the consequences could be catastrophic. The values of breeding activities to adapt to the five major abiotic effects of climate change (heat, drought, waterlogging, salinity, and elevated CO2) are more difficult to rank, and vary with species and production area, with impacts on both yield and quality of product. Although there is a high likelihood of future increases in atmospheric CO2 concentrations and temperatures across Australia, there is uncertainty about the direction and magnitude of rainfall change, particularly in the northern farming regions. Consequently, the clearest opportunities for ‘in-situ’ genetic gains for abiotic stresses are in developing better adaptation to higher temperatures (e.g. control of phenological stage durations, and tolerance to stress) and, for C3 species, in exploiting the (relatively small) fertilisation effects of elevated CO2. For most cultivated plant species, it remains to be demonstrated how much genetic variation exists for these traits and what value can be delivered via commercial varieties. Biotechnology-based breeding technologies (marker-assisted breeding and genetic modification) will be essential to accelerate genetic gain, but their application requires additional investment in the understanding, genetic characterisation, and phenotyping of complex adaptive traits for climate-change conditions.
Additional keywords: biosecurity, crop improvement, crop modelling, elevated CO2, stress, high temperature, water use efficiency.
Introduction
In addition to providing essential supplies of food and other plant products, cultivated plant species are significant contributors to the Australian economy, with a total production value greater than AU$20 billion per annum (p.a.). From 2004 to 2010, cereals, oilseeds, and legumes, grown on ~23 Mha of land, have had a gross value of production averaging $9 billion p.a., with 67% of that value realised through exports ($5.9 billion p.a.) (all production and value data from ABARE 2010). The value of sugarcane production across 390 000 ha ranges from $1.5 to 2 billion p.a., earning an average export value of ~$1.3 billion p.a. (ABARE 2010). Since 2004, viticulture exports have averaged $2.7 billion p.a. in value, while fruits and nuts (excluding grapes) had an average production value of $2.8 billion p.a., and vegetable production ~$3.1 billion p.a. In the non-food sector, cotton is a major contributor, with $651 million in production during 2009–10, following several periods of lower production during the droughts of the 1990s and 2000s. The other major non-food product is forestry, with production of $3.4 billion and exports of ~$2.3 billion p.a. in wood and paper products. Other indirect contributions of plant species include their substantial value via pasture production of beef, wool, lamb, and other animals ($18.4 billion p.a., with $13.4 billion exported). Plants also contribute a ‘difficult-to-measure’ value in providing ecosystem services, including through amenity species in facilities such as national and recreational parks, sports grounds, and golf courses, as well as in every residential garden.
Climate change and the associated elevation in atmospheric CO2 levels and temperature will provide novel challenges and potential opportunities for cultivated plant species. To cope with this and the additional issues of population growth/food security and competition for land and water resources, we are in need of a new ‘agricultural revolution’ to supply a global population that is projected to increase from 7 to 10 billion with a required increase in food production of 50–70% (Reynolds 2010). For >10 000 years, plant breeding has contributed to improvement in both the yield and quality of crops, first through selection by farmers, and, with great impact over the last 100–150 years, through selection in directed breeding programs. Selection and breeding has broadened the climatic adaptation of many species far beyond their geographical origin and indicates the ability of plants to adapt via recombination of their genetic makeup. In addition to adaptation to climatic and soil environments, plant species are bred for resistance to pests and diseases and for quality or market attributes associated with their end-use. Regarding the impacts of climate change on different crops, recent texts (Reynolds 2010; Yadav et al. 2011) have assembled comprehensive sets of reviews, several of which the reader will be directed to for detailed treatments of plant breeding as a potential adaptation response.
The papers in this symposium cover a diverse range of readership, and so it is instructive in this paper to mention the complexity and the power of the process of directed evolution of breeding. For example, a cross between two parental lines that differed for 25 genes could produce 325 unique genetic combinations (genotypes), which is in the range of the number of grains of sand on earth. Of course, many of these genotypes will produce a similar phenotype (the plant or product that is measured in a given environment). In reality, there are many more than 25 genes influencing plant growth, considering that crop species such as rice are estimated to have >30 000 genes. Hence, even in the largest of programs, breeders are always forced to work with extremely small samples of the potential genetic diversity to determine which genes are ‘most favourable’ for adaptation and end-use and how to identify and create the best combinations. Given the size of the combinatorial problem, and the complexity of interactions that occur among genetic effects and the environment, the main challenge in breeding has been increasingly recognised as the design of an efficient ‘search strategy’ to guide the accumulation of the desired, though rarely optimal, combinations of genes into cultivars (Messina et al. 2011).
The impacts of climate change on Australia’s plant-based production systems will vary with species, as will our opportunity to react through changes in the management of the farming system (Oliver et al. 2010; Stokes and Howden 2010; Howden and Crimp 2011; Hayman et al. 2012) or in the varieties that we grow. Due to existing climate variability, Australian farmers are already experienced with this kind of adaptation (White 2000). Across the diverse production zones of Australia, the effects of climate change could have both positive and negative impacts, only some of which are likely to be compensated for via market systems. The complete study of these types of impacts and opportunities for intervention requires application of ‘adaptation science’ (Howden et al. 2007; Meinke et al. 2009) and studies of the impact of agriculture itself on global warming (e.g. Biswas et al. 2010; Huth et al. 2010), but here we limit our interest to the more direct impacts of climate change on plant pests and plant production and quality in food, fibre, and forestry systems.
Implications of climate change and a role for plant breeding
Hatfield and Prueger (2011) concisely review the agro-ecological implications of climate change for plant responses, considering growth, yield, and quality, and they emphasise the importance of interactions among the factors of elevated CO2, temperature, rainfall patterns, and nitrogen (N) fertiliser. They summarise the implications as:
-
Elevated CO2 will lead to positive effects on plant growth (greater in C3 species), with less evident effects on grain yields;
-
Increasing temperatures will accelerate development with consistency across crop species;
-
Increasing temperatures will increase the rate of water use due to the effect of temperature on vapour pressure deficit;
-
Increasing CO2 will decrease stomatal conductance, leading to increased water-use efficiency (WUE), but this will vary with species and will depend on soil water status;
-
Interactions between CO2 and N dynamics in the canopy result in elevated CO2 having a generally positive effect on N response, often with reduced concentrations of N in organs; similar responses occur with other nutrients.
There is increasing evidence in the literature to suggest that, in the last 30–50 years, some of these effects have started to be realised as climate change impacts on agricultural production systems. For example, research in Europe (Brisson et al. 2010) and Australia (Asseng et al. 2011) has indicated negative impacts of temperature variability on wheat yields, whereas Conroy and Hocking (1993) suggested that some of the decrease in grain protein of Australian wheat from 1967 to 1990 could have been related to increased CO2. A modifying factor in this latter observation might be the increased use of parental lines from the ‘green revolution’ wheat, which at that time had lower quality grain than the Australian germplasm. Chapters in Yadav et al. (2011) discuss this impact of ‘recent climate change’ for a range of species and production environments.
In addition to the ‘average’ effects of climate are impacts related to increased risk of severe events associated with changes in temperature and/or rainfall. For example, heat waves—as in South Australia in the summers of 2008 and 2009 (Nitschke et al. 2011) and again in 2010—can impact on both production and quality of crops. In the last 3 years, two cyclones have successively destroyed almost 70% of the Australian production of bananas. In 2010 and 2011, in the more northern and eastern regions of Australia, high rainfall associated with La Niña conditions have contributed to high yields for wheat production, and also large accumulations of standing dry matter in the extensive rangelands. However, these conditions have also had a negative impact on wheat quality, as the N management for a ‘normal’ season has been insufficient, such that much of the wheat crop was downgraded from premium to feed-quality. This resulted in substantial reductions in the ratio of milling- to feed-grade wheat grain in storage from 89% milling grade in August 2010 to 52% a year later, with a consequent impact on price (ABS 2011). The wet seasonal climatic conditions in 2010 also led to severe disease epidemics further reducing wheat yield and grain quality (Neate and McIntyre 2011). In October 2011, the pasture areas of central-west Queensland suffered a final consequence of these extreme (‘favourable’) conditions for biomass production in 2010–11, when almost 4000 km2 of feed was burnt out by fires (Arthur and Phillips 2011). These ‘short-term’ impacts of climate variations on local production are expected to be more keenly experienced in 20–30 years or more, when the Australian population consumes a greater proportion of our current food exports, and when global food security becomes more strained by population pressure and ‘aspirational’ food consumption patterns, i.e. increased consumption of meat products (Howden et al. 2010). While these ‘extreme’ impacts do not have simple agronomic or genetic solutions, their existence will likely put pressure on policy decisions (i.e. influencing what to grow where) and on agronomic and genetic solutions to the less extreme impacts, i.e. moderate but not ‘lethal’ events such as increased temperature, drought, or waterlogging.
As a complement to changes in agronomic management, plant breeding is a convenient technological response to environmental challenges (e.g. Yadav et al. 2011), but considerable time and investment is required for this technology to deliver changes in the productivity or quality of varieties. The genetic basis of adaptation to environments is complex, and it is difficult to unravel what sets of genes are required for optimal performance and to explain the interactions of genes controlling any given trait. Investment in plant breeding requires assessment of when and where this is the most economical response for an industry in dealing with climate change. In some cases, such as the emergence of a new strain of a pathogen causing disease, or the need for a specific quality profile in a product, plant breeding is the only practical solution. The next sections will consider the projected climate changes affecting Australia, the relative risks of climate change for different cultivated plant species, and a summary of how breeding is currently utilised in these species. We then aim to provide some context to explain the process of modern plant breeding, particularly for Australia, and how the investments in understanding plant genetics can contribute to efforts to adapt to climate change and maintain food security.
Australian production environments and expected climate change effects on weather conditions
Human activities are increasing the atmospheric concentrations of key greenhouse gases such as CO2, methane (CH4), and nitrous oxide (N2O) (IPCC 2007). The increasing concentrations of these gases affect the radiation budget of the earth, keeping the atmosphere warmer than it would otherwise be. The concentration of CO2, the main human-emitted greenhouse gas, is now 392 µmol mol–1 (February 2012, www.co2now.org), 40% above the pre-industrial concentration of 280 µmol mol–1 (IPCC 2007). There is strong evidence that these anthropogenic changes in atmospheric composition are affecting the climate at global, continental, and even regional levels (IPCC 2007). In addition to the effect on climate processes, elevated CO2 has significant and relatively well-understood effects on crops, increasing water, solar radiation, and N-use efficiencies with—all else being equal—increasing yield, especially in C3 photosynthesis species (Tubiello et al. 2007b). Depending on which greenhouse emissions trajectory occurs, concentrations of CO2 may rise to up to 1000 µmol mol–1 by the end of this century.
The key climate variables for cropping systems are the maximum and minimum temperatures, rainfall, solar radiation, vapour pressure deficit, and wind speed. Changes in extreme temperatures, rainfall, and the duration and frequency of drought conditions will particularly affect agricultural industries. A range of indices may be computed from these climate variables, including chilling requirements and days above a temperature threshold that are critical for horticultural commodities. Here, we summarise briefly the projected changes over forthcoming decades, drawing on the IPCC Fourth Assessment Report (Hennessy et al. 2007).
Climate change projections using results from a range of global climate models and emissions scenarios suggest that average Australian temperatures in cropping regions will increase (relative to 1990), by 0.3–1.5°C in 2030, by 0.6–3.4°C in 2050, and by 1.2−5°C in 2070 (CSIRO and BoM 2007). Slightly greater increases are expected in the northern cropping regions (where summer crops are grown) than in the more southerly, winter cropping regions, and greater increases in inland sites compared with those nearer to the coast. These increases in average temperatures are consistent with the existing warming trends recorded over the past three decades. The projected increases in average temperatures are likely to be accompanied by marked increases in the frequency of hot days (temperatures >35°C), such that by 2030 the mean area of Australia experiencing what, historically, have been exceptionally hot years is likely to increase to 60–80% compared with the historical expectation of 5% (Hennessy et al. 2008). As well, there is a likelihood of decreases in cold nights and associated frost risk to crops, at least in the northern cropping region.
Climate model projections suggest annual decreases in rainfall over the cropping regions of south-east and south-west Australia, with a tendency for annual rainfall increases in much of Tasmania and summer rainfall increases in the north-east of the continent (CSIRO and BoM 2007). Substantial seasonal rainfall changes are projected, with decreases likely in winter and spring. However, it is important to note there is a large range and uncertainty around expected changes in future rainfall, including both seasonal declines and increases, requiring ongoing review of climate trends and their causes. Historical decreases in rainfall in south-east and south-west Australia over the last several decades, particularly in autumn and winter, respectively, have recently been attributed to climate change (Timbal et al. 2009).
For plant production, variation in rainfall will have impacts on the occurrence of drought events in rainfed production areas and on the supply of water for supplementary irrigation. Droughts are expected to increase in both frequency and spatial extent as a result of climate change, particularly in the south-west of Western Australia and Victorian regions. For example, under the high emissions climate change scenario used in Hennessy et al. (2008), using current definitions, droughts would occur almost twice as often in most regions and almost four times as often in the south-west of Western Australia. Rainfall changes will also affect the dynamics of surface and groundwater resources that supply the water used in irrigated cropping in Australia (Khan 2008). By 2030, annual water availability in the Murray–Darling Basin (MDB) could change significantly, with possible substantial decreases (26–45% reductions depending on catchment) to increases (up to 19% in one catchment), with the median scenario indicating consistent but varying reductions across all catchments. There is a tendency for more negative scenarios in the southern catchments than in the more northern catchments (CSIRO 2008). On the positive side, reductions in deep soil drainage arising from lower rainfall and higher evaporation rates may lessen the risk of dryland salinisation (van Ittersum et al. 2003).
The growing agreement between emergent climatic trends over the last 20–30 years and the projected changes in climate derived independently from global climate models provides additional confidence that the projected climate changes are not unrealistic and, hence, are a reasonable basis for assessing climate adaptation options.
Systems of cultivated plant species and relative production risks due to climate change
Plants are susceptible to both direct abiotic effects of climate (i.e. elevated CO2, heat, drought, salinity, waterlogging) and indirect biotic effects through climate effects on the population and virulence of various pests and pathogens. The effects of climate change on cultivated species will vary with the evolutionary history of the species as well as their agricultural history. In modern field production systems, the effects of climate variability and change can be mediated within-season by farming practices and by the choice of cultivar (genotype) that is grown (e.g. Stokes and Howden 2010). Many crop species are already grown in a diverse range of environments, such that adaptation to local climates has been a strong selection criterion. To adapt to climate change, the local farmers will need to have access to the right combination of genes for future climates—a ‘climate-change-ready’ cultivar. For any given location and crop, such cultivars may already exist but are currently grown in another geographic location in Australia or elsewhere; such locations could be considered proxies for ‘future’ climate at a given location. In the case of high-value, glasshouse-grown species, the impact of climate change is indirect and is mainly delivered via its effect on the energy costs of maintaining these artificial environments. Extreme catastrophic events (cyclones, flooding, long-term drought, heat-waves, fires, severe outbreaks of insect or disease) can particularly affect these high-value production systems, and breeding is rarely a viable solution, although it can sometimes contribute to the ability of plants to survive such events and recover. In general, these events are best handled via production-based risk-management at the farm, regional, and national levels as discussed elsewhere in this special issue.
In existing production systems, both management and breeding solutions contribute to the moderation of the effects of abiotic and biotic stress. Higher value species are frequently supplied with irrigation, protected from pests (by spraying protectants or breeding tolerant genotypes), or are grown in protected environments (glasshouses and greenhouses) to manage both abiotic and biotic stress. Beneficial insect and microbial species are also managed in such systems, such as pollinator insects or practices that promote beneficial soil microbes (Wakelin et al. 2010). Plant species are vulnerable to a range of bio-security threats. In addition to the potential effects of climate on the rate of incursion of exotic pests (Legreve and Duveiller 2010) or the evolution of endemic pathogen strains (Chakraborty and Datta 2003), climate change will influence interactions between pest and crop species, affecting the productivity and quality of the products. In plants, the mechanisms of resistance to pests and pathogens operate through structural (i.e. cell walls), biochemical, and physiological means. Climate change influences these mechanisms and can potentially make a resistant variety more susceptible (Chakraborty et al. 2008). We discuss the influence of climate change on pest and pathogens further in later sections.
The performance of different genotypes and the breeding methods used to realise performance are influenced by the end-use of the species; that is, plant products can be categorised into those based on biomass production (e.g. pastures, sugarcane, forestry, biofuels) and those based on the production of reproductive or specialised organs (grains, horticultural species). In some species, breeding focuses more on product yield, while in other species, quality or amenity is the major selection criterion. Where a pathogen produces toxins or other harmful compounds, breeding for disease resistance can improve quality and food safety in addition to increasing yield. Other than extreme events mentioned above, the impacts of climate change include those that affect patterns of heat and drought stress that may be experienced through the life cycle of the plant. There is also a potentially beneficial impact of climate change in plant species that use the C3-type photosynthesis pathway, in that elevated CO2 produces a ‘fertilisation’ effect by increasing the potential photosynthetic rate and increases the potential WUE of leaves (Tubiello et al. 2007a). However, these and other physiological changes can also modify resistance of crops to pest and diseases, a consideration in harnessing these benefits.
Biomass-based products are generally more tolerant of the impacts of climate change such as increased average temperatures, but genetic improvement to adapt through breeding can be quite slow due to long cycle times and longer ‘replacement’ times (i.e. time for a new variety to be introduced) in perennial and regenerative systems such as pastures, forests, and sugarcane. In southern regions of Australia, many pastures have been ‘improved’ over time through the inclusion of clovers or legumes into the species mixtures, together with the use of fertilisers, particularly phosphorus (P). Much of the rain-fed grain production region includes longer term rotations with pasture production, i.e. grain-and-graze systems. In the northern regions of Australia, pastures are potentially at greater risk of flooding, to the degree that climate change may be expected to increase the frequency of low pressure systems and cyclones, as is currently observed during La Niña events. These events have three major consequences for coastal weather: high rainfall, high wind speed, and low radiation levels. Regional sugarcane yields have already been shown to be vulnerable to La Niña high-rainfall seasons (Everingham and Reason 2011). While pastures and forests can be reasonably resilient to wet conditions including waterlogging, sugarcane productivity is severely compromised by low radiation, and by submergence and lodging. A local impact of intense low-pressure systems, including cyclones, is the combination of intense rainfall and high wind speeds, which causes lodging and reduction of sugar content in sugarcane (Singh et al. 2002) and, potentially, damage to plantation forests. Northern inland pasture systems are generally favoured by La Niña events (Park et al. 2003) and could respond positively to climate change in terms of productivity, albeit with a need to buffer production between years of high and low rainfall (O’Reagain et al. 2011). Any increase in growing season temperature or in high-temperature events will affect the dynamics of production, but adaptation can be implemented through pasture and animal management (Stokes and Howden 2010).
For pasture and forest systems across Australia, an additional impact of increased frequency of extreme seasons (cycles of wet and dry/hot seasons) is an increase in fire risk and fire intensity (Sullivan 2010; King et al. 2011). While moderate-heat fires are part of the cycle of regeneration of pastures (particularly in northern pasture areas), severe fires which can follow ‘good’ growing seasons can impact on both the future productivity and the species composition of pastures (Dyer and Stafford Smith 2003). Pasture and stocking management is generally the appropriate response to this type of impact, although breeding may have a potential role through improvements in fire tolerance, regeneration, and competitive ability of pasture species. In extensive pasture regions, the potential contribution from breeding is mostly limited to southern areas, as the majority of northern pastures are based on native species, partly augmented by the use of introduced legumes. Crimp et al. (2010) have reviewed the potential impacts of climate change on both livestock and pasture production for southern regions, and the implications for adaptive capacity.
The impacts of climate change on the quality of biomass products are not well documented. For their respective end-uses, the components of a product in terms of fibre, cellulose, protein, and energy content are essential contributors to value. During periods of accelerated growth (via elevated CO2 and/or temperature, and/or rainfall), pastures generally increase in fibre content (e.g. Tran et al. 2009), which decreases digestibility and efficiency of feed conversion. In sugarcane, the ratio of sugar to fibre is decreased at higher temperatures (Inman-Bamber et al. 2011), while for forests there are potential impacts on wood quality, as demonstrated in FACE (free-air CO2 enrichment) experiments with poplar species (Luo et al. 2005). In both tropical and temperate pasture systems, the composition of species could be influenced by climate resulting in a decrease in the overall nutritive value of the pasture (Schenk et al. 1997), but in other circumstances, increased legume competitiveness may increase nutritive value (e.g. Howden et al. 2008), with the expectation of flow-on impacts for animal weight gain in both cases. At present, little is known about the potential local and regional impacts of such climate influences on product quality, or the extent to which resilience to these effects on quality can be bred into the species.
Extreme temperature events (severe frost or heat waves) can be devastating for most cultivated plant species, especially if they do not have reserve capacity for growth (e.g. underground storage organs, ratooning capacity, etc.). Species that develop ‘organ-based’ products are also susceptible to moderate heat, frost, drought, or waterlogging stresses at specific stages of their life-cycle, especially when the plants are seedlings, or are in stages associated with the flowering period. For example, an increased incidence of high temperature ‘heat shock’ events impacts greatly on the reproductive potential of crops to maintain their yield and quality, whether they be field or horticultural crops (Stokes and Howden 2010). For products that are grown in controlled environments (use of irrigation, glasshouses, and greenhouses) and/or are relatively small in area and high in value, a practical response to potential climate change is to adjust the controlled environment (energy and water permitting) or to shift the industry to a more favourable production area given the changed climate. These adaptations are facilitated by management and policy responses, and are unlikely to be suited to a plant-breeding solution. The exceptions are when the product cannot be grown economically elsewhere or where the product must be grown in situ, i.e. Australia’s major grain crops (wheat, sorghum, barley), oil seeds (canola, chickpea), cotton, as well as amenity species such as turf which are needed in a specific location. In these species, the breeding solutions can build on existing global experience in the breeding of varieties for adaptation to a large range of environments (Braun et al. 2010).
Limited data are available about the genetic variation in response to elevated CO2. For several high-value species, such as tomato (Lindhout and Pet 1990), genetic variation for response to elevated CO2 is known to exist. Glasshouse production of major vegetables (tomato, pepper, cucumber) in temperate regions of Europe, USA, and Asia is frequently under artificially elevated CO2 conditions, such that vegetable breeding (largely within commercial companies) has been directed towards improvement for these conditions for up to 30 years. In Australia, relatively little horticultural production occurs in glasshouse environments. There are potentially opportunities to use known genetic resources that have been identified for elevated CO2 glasshouses in the development of new varieties for future elevated CO2 conditions in open fields or greenhouses.
Compared with biomass products, organ-based plant products in open-field conditions are frequently subject to management input of greater value, such as irrigation, fertiliser, protection against pests and diseases, and promotion of favourable insect and microbe species. These investments are all susceptible to local effects of climate, and therefore climate change. Poor management of these inputs results in decreased product quality and value and low efficiencies of resource use, with few options to moderate these via breeding, e.g. where the water use per unit of biomass produced (WUE) of plants is decreased by high temperature and low humidity conditions. For example, cotton quality has been shown to be decreased by heat tolerance, but useful genetic variation exists for this trait and could contribute to improved new varieties (Azhar et al. 2009). In warmer climates, the daily requirement for irrigation is greater than in cooler environments, although for annual crops, this may be moderated by an effect of temperature which shortens the crop cycle but in the process may reduce crop yield. Fertiliser requirements are moderated by both direct effects of climate (e.g. leaching and volatilisation by rainfall) and the indirect demands to match growth requirements. Dwyer et al. (2007) looked at changes in nutritional status, particularly N, as affected by climate change. They found a decreased N concentration in C3 species under elevated CO2. More recently, Erbs et al. (2010) demonstrated that the quality of flour would be lower when grown under elevated CO2 at low to moderate levels of N fertiliser. For both water and nutrients (especially N and P), there are active breeding programs with several field crops that aim to increase the efficiency with which these resources are used, i.e. more crop per unit of input.
The maintenance of yield and quality under pest and disease pressure has always been a core target of breeding programs, and will remain so with climate change. A major challenge is to anticipate the potentially devastating impacts of new diseases or pests in a given location. More recent considerations in adapting to climate effects have included management of the soil microbial communities to suppress disease development (Mazzola 2010); that is, plant breeding can contribute by increasing root exudation and rhizodeposition to selectively enrich species and communities of pathogen-suppressive microbes. To consider how plant breeding can contribute to adaptation to climate change, it is helpful to first consider the activities that are undertaken in a screening and selection program and the intervention points that can be used.
While much crop and pasture production in Australia is generally regarded as being quite marginal in terms of the impact of climate and climate extremes on profitability, it has been viable due to the broad scale of properties and operations. After its most rapid expansion in number of farmers after World War II, the last 30 years has seen a decline in farmer numbers and a substantial consolidation of properties in pastures, crops, and horticulture (George et al. 2005), as well as the privatisation of many of the previously state-owned forests. Even given a 2−3°C increase in temperature, much of Australia’s cropping and pasture regions will still be ‘within’ the existing physical climatic adaptation range when considering the climates of regions in other parts of the world where these crop and pasture species are produced. In most field crops, there would be a need for the Australian germplasm base to be ‘shifted’ from its current adaptation profile in terms of development rate and tolerance of higher temperature conditions (Zheng et al. 2012). At the lowest rainfall regions along the interior edges of the existing wheatbelt, the combination of existing or lower rainfall and higher temperatures could make wheat production unprofitable, depending on how much adaptation is available through breeding and management. Varieties of other major crops such as sorghum, sugarcane, and cotton should also be able to be developed for future temperature regimes, as these crops are already grown in locations hotter than in Australia. To the degree that breeding efforts elsewhere have developed varieties of fruits and vegetables with specific adaptation to high temperature conditions, it would be expected that breeding companies will bring that germplasm to Australia. The production levels and seed market for these species are not large enough to sustain ‘climate-change breeding programs’, although physiological research into potentially adaptive traits and their genetic control would likely be useful in informing international commercial breeding efforts.
Characteristics of breeding programs and opportunities to intervene to accelerate adaptation to climate change
Plant breeding is an incremental and cyclical process of selecting and crossing the most elite performing cultivars, and evaluating their progeny to identify those which are best adapted to a ‘target population of environments’ (TPE). Breeding is undertaken by both public and private breeding programs for a great range of environments. For example, private breeding companies are estimated to have contributed to >50% of the impressive improvements in maize yield in the USA, with genetic improvement predicted to have increased by 56 kg ha–1 year–1 between 1930 and 1990 in central Iowa (Duvick 1992). The remaining improvement is attributed to changes in crop management over time, although it is clear that the change was related to an interaction between genotype and management; the new maize hybrids only perform substantially better than the old ones when they are grown in modern high-density, high-input management conditions (Duvick 2005). For most of the major staple food crops, there are large public international breeding programs, for example those contributing to the Green Revolution of the 1960s and 70s in wheat and rice. As Braun et al. (2010) outlined, the structure of ‘global’ programs is designed to facilitate the identification and exchange of parental germplasm among smaller breeding programs operating in a diverse range of environments. Beyond the Green Revolution areas of irrigated production, Lantican et al. (2003) showed that the yield impact of such programs in drought- and heat-affected environments were also responsible for increases of 2–3% per annum through the 1980s and 1990s.
Breeding of any plant species takes time, especially in forest and plantation species. Even in annual crops, the breeding of specific adaptations may take 10–30 years in order to:
-
Assess the potential impact (intensity and incidence) of a new environmental challenge;
-
Identify traits for adaptation to the new environmental challenge;
-
Find genetic variation for the traits in existing or wild accessions of the species;
-
Introduce and select for these new genetic sources to develop cultivars through conventional, molecular, or engineering approaches;
-
Evaluate in large plots and release cultivars for adoption by growers.
Plant breeding programs comprise a parental pool of germplasm lines that is targeted to some geographic region and for which the environmental challenges to production, quality, and end-use can be defined (Fig. 1); see also Mullan and Barrett-Lennard (2010) for a detailed diagram explaining breeding processes for incorporating new genetic variation, and discussions by Dreccer et al. (2011), who outlined approaches to breeding wheat and rice for a changing climate. Crosses are made between existing parental lines, and also with introductions of new germplasm carrying potentially useful traits. The term ‘indirect selection’ is often used to describe the selection of genotypes in a breeding program based on traits other than those which are specifically of interest to growers of the crop. Indirect selection requires that the trait is heritable, can be efficiently measured, and has a genetically correlated relationship with the trait of interest, i.e. yield or quality. This process incrementally improves the ‘average’ adaptation of the parental pool, and with the generation of new genetic variability via crossing and introductions, this increases the opportunity to find lines that are better than the existing cultivars. The potential intervention points to influence a breeding program are to change the priorities for the traits being selected; to develop, introduce, and screen new sources of genetic variation for specific traits; to adjust the selection pressures and screening procedures for specific traits; and to utilise different breeding methods to accelerate the discovery of optimal genetic combinations. Across the many steps in the breeding program, there is an almost infinite number of possible manipulations of traits through various breeding methods and variations in population size and selection decisions. The variety of options is such that breeders in several commercial and public programs have developed and applied computer simulation tools to attempt to optimise these decisions for a whole range of breeding questions (Podlich and Cooper 1998; Chapman et al. 2002; Cooper et al. 2005).
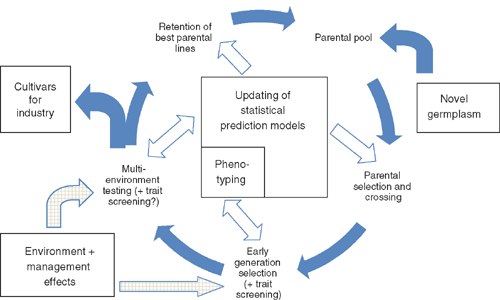
|
The following sections elaborate on the list of breeding tasks given above, and aim to provide an outline of how plant breeding decisions are made and the technological advantages and limitations of the methods currently available.
Assessing potential impact—defining target environments and adaptive traits
For public breeding programs, the geographic target region may be quite large and even trans-continental, while private breeding programs tend to focus on specific regions (hundreds to thousands of square kilometres) where profitable breeding outcomes can be delivered to farmers. Breeding programs have multiple targets for their end-products, and every new target that is included in the selection process has the potential to slow genetic gain towards other targets. Hence, breeding programs are not easily modified to include selection for new traits or environmental challenges, unless the challenges are essential for the breeding program to be viable within its mandate region. To remain viable, breeding programs need first to maintain the adaptation of a species to a region, and second to develop their cultivars to compete in the seed market for that region. If climate change becomes sufficiently severe to cause a species to no longer be grown in a region, then breeding would be no longer necessary for that area. The decisions that need to be made in breeding programs in the face of climate change are: (1) will the plant species remain viable in a given region, and (2) how can the plant species be adapted to continue to provide an economically desirable yield and quality.
Within a target breeding region for any species, there exists spatial variation in soil type and spatial and temporal variation in weather, disease spectrum, and management. This results in the possibility of many ‘niche’ adaptations, for which breeders cannot develop the optimal variety as the niches vary across space and time. The consequential genotype × environment (GE) interaction is perhaps the major challenge to developing new varieties with optimal adaptation across multiple seasons within a target breeding region. The phenomenon is described by quantitative genetic theory as when the same set of genotypes vary greatly in yield or quality between environments (heterogeneity of variance) or are re-ranked (lack of genetic correlation) (Cooper and Hammer 1996). To a large degree, plant breeders do not have ‘control’ over the sampling of environments, especially in non-irrigated locations, leading to poor sampling and prediction for the TPE. The TPE describes the possible set of ‘environmental types’ (ET) that could impact on a breeding program, i.e. pests, diseases, and seasonal patterns of environmental effects are all part of the TPE. Tolerance to pests or diseases tends to be an ‘all or nothing’ decision within a breeding program (i.e. the TPE includes ‘+ rust’ as an environment type), while interactions between the growing crop and its abiotic environment over a season generate a range of ETs within the TPE. Characterising the TPE for climate adaptation has been facilitated by the use of crop simulation models. For sorghum (Chapman et al. 2000), maize (Löffler et al. 2005), and wheat (Chenu et al. 2011) breeding programs, models have been run using historical weather records to characterise the frequency of different drought and heat ETs as affected by temperature, rainfall, and soil type, and these ETs have contributed to explaining the observed GE interaction in breeding trials. This approach is used to allow the analysis of breeding data with respect to the frequency of occurrence of different ETs and consequently to determine the need for the creation of a specific environmental screen, which ensures that genotypes are exposed to ETs that could affect their performance in the target region. In the context of climate change, this characterisation of the TPE can be undertaken using generated weather records for ‘future’ climate scenarios in order to determine which ETs (patterns of drought, heat stress, and waterlogging) will be experienced with what frequency, and hence to determine how that will impact on the efficiency of the breeding program and the design of relevant phenotypic screens, e.g. high temperature screens at different stages of growth.
Apart from adaptation to elevated CO2, the adaptive traits for climate change conditions (heat, drought, salinity, waterlogging) are already considered as part of most plant-species breeding programs. The challenge is to determine which traits will have greatest impact and therefore require more emphasis in the breeding program than is currently the case. This can be partially addressed by the same TPE concepts described above. However, such decisions are constrained by how well we can predict the degree and the potential spatial distribution of climate-change effects, i.e. the future TPE. The downscaling of predicted scenarios from global climate models is an uncertain science, and it is difficult to refine expected impacts much below scales of several 1000 km2 in area; however, this approximately coincides with the geographic target region of many breeding programs, and still possible to determine the potential range of climate change effects for a breeding region. Downscaling is typically needed to estimate the potential range of regional impacts based on different assumptions, as shown for wheat production in Victoria (O’Leary et al. 2011). As mentioned in the Introduction, the magnitude and the rate of projected change in climate also contain unavoidable uncertainty because of difficulty in accurately characterising human-induced emissions or the likelihood of scientific and technological discovery to transform society. Therefore, the use of the TPE approach may largely be limited to the determination of the frequency and intensity with which more extreme events are likely to occur, and whether that frequency and intensity is able to be tolerated by existing or potential germplasm lines.
Accessing and developing useful genetic resources for climate change
Plant species are subjected to a diverse range of selection and breeding activities. For most cultivated plant species used in Australian agricultural systems, there has been selective evaluation and/or breeding of the species since the arrival of European settlers. Many of the original crop introductions were not well adapted to Australian environments, which were typically warmer, drier, and at lower latitudes than Europe. For example, ‘winter’ wheats from the UK were poorly adapted to Australian environments as their vernalisation and photoperiod requirements for flowering were not met. William James Farrer developed and promoted the wheat variety ‘Federation’ in 1903 and is credited with having a substantial impact on the demonstration and continued contribution of wheat breeding in Australia (Fischer 2011). Wheat breeding programs were established by all of the wheat-growing states, supplying new varieties and exchanging parental lines between breeding programs. These programs also established long-term collaborations with international institutes such as CIMMYT in Mexico to access the high-yielding wheats developed during the Green Revolution (Reynolds and Borlaug 2006) and, more recently, ‘derived synthetic’ wheats, which were created at CIMMYT by new methods to cross wild wheat relatives into durum and bread wheat germplasm (Trethowan and Mujeeb-Kazi 2008). Together with changes in the rotation and management of wheat crops, these domestic and international breeding efforts in Australia have contributed to a consistent increase in mean wheat yield of 1–2% per year for >70 years, despite the rainfall constraints in our wheat-growing regions (Fischer 2011) and soil. Following a period of exotic introductions, similar long-term investments in breeding have occurred in the major crop species, i.e. sorghum, sugarcane, cotton, rice, and oilseeds. For most other cultivated species (i.e. pastures, forests, fruits, nuts, vegetables, grapes), changes in varieties occurred mainly through the evaluation of wild accessions, i.e. either from other regions of Australia or sourced internationally and brought through quarantine. In Australia, this introduction and consequent breeding of improved varieties happened mainly via public breeding programs, until the last 20 years, when public breeding efforts were largely privatised and commercial companies began to play a greater role in the supply of plant varieties in a whole range of species, both food and non-food.
The genetic resources of perennial species such as fruits, grapes, pastures, and forests are typically developed more slowly than for annual crops. While introduction via quarantine and multiplication of individuals can sometimes be accelerated by tissue culture and cloning, the testing of these species for adaptive traits is long term, due to their growth and production characteristics. As with annual species, the strong international links of both Australian research agencies and breeding companies are important in the identification and introduction of potentially useful varieties and parental lines. Even in Australian native species such as macadamia and eucalyptus, international selection and breeding activities have developed improved germplasm resources that should be monitored for their potential utility in addressing challenges of climate change.
For Australian farmers, continued access to germplasm banks and international breeding programs for major crops is particularly important to meet the potential challenges of climate change. These efforts will need to consider interactions with changing management due to climate change itself (climate adaptation and mitigation) and also anticipated alterations in technologies, social preferences (e.g. environmental regulation or subsidisation), and input and output prices. Even for annual crop species, the delivery of a new variety with a new combination of traits can easily take 10–15 years, and this does not really begin until the need for these traits has been identified. For the major crops (wheat, sorghum, sugarcane, cotton, grain legumes), Australia has research and development corporations (RDCs) that are jointly funded by producers and government. Part of the function of these RDCs is to fund germplasm collections and ‘pre-breeding’ activities that aim to anticipate the future needs of breeding companies for new traits and parental lines. However, it is only recently (last 2–3 years) that the RDCs and other research agencies have begun to consider whether and how to develop new genetic resources specifically for the challenges of climate change, mainly temperature.
Complementing breeding activities to develop useful germplasm resources, there have been rapid increases in the last 20 years in the characterisation and understanding of the genome sequences of major plant species and their use to investigate environmental stresses (e.g. Fleury et al. 2010). Initial approaches were to develop panels of genetic markers (tens to thousands) that could be used to characterise each germplasm line in a given collection. This method partitions germplasm collections into different groups that reflect their natural or artificial selection history, and allows breeders to choose lines and to define crossing and breeding strategies that best utilise the available genetic diversity. A more specific application of genetic characterisation is genome sequencing, which allows the determination of which versions (alleles) of any single gene exist in any given germplasm line, and where in the genome those genes are located, so that breeders can quickly select specific alleles and recombine these into new lines. Of the main crop species grown in Australia, only rice and sorghum have been fully genome-sequenced, although international research activities are under way in wheat, cotton, sugarcane, grape, eucalyptus, and various fruit, nut, vegetable, and pasture species. An example of the efficient combination of both germplasm and gene-sequence resources is for sorghum breeding in Australia. Researchers have catalogued the genetic ‘locations’ of major genes of known function (Mace and Jordan 2010), as well as quantitative trait loci (QTL), which are experiment-derived ‘estimates’ of other useful genes (Mace and Jordan 2011). This information is being used during the development of specific populations of lines that have been created by backcrossing a large number of exotic parents onto an adapted parent (Jordan et al. 2011). The lines in these populations are tested in extensive field experiments to estimate the value of exotic alleles, and to utilise these in developing new sorghum parents for delivery to commercial breeding programs.
‘Building’ and delivering new varieties
Once a new trait target is identified, together with a method of phenotypic screening for that trait, a breeding program typically screens its own germplasm, as well as germplasm from national and international collections, in order to locate genotypic lines with improved performance for the trait, e.g. reduced pollen sterility at high temperature. As discussed above, the effectiveness of the new trait typically needs to be validated in field tests before breeding for the trait. Once that is proven, the time in which the trait can be transferred to adapted lines will depend on the complexity of its genetic control (and thereby the heritability of the trait), and the degree to which the donor line is ‘exotic’. Existing commercial varieties of wheat, for example, have been tuned to Australian production environments over the last 50–100 years. So, the discovery of a useful trait in a wild relative of wheat will require that the trait be evaluated over several generations (and 5–15 years) as it is backcrossed several times to an otherwise adapted variety, with the progeny of each cross being screened and selected at each generation. In the case of high-value or novel traits, a breeding program may elect to apply biotechnology methods in a gene discovery activity in order to determine which versions of genes are responsible for the expression of the trait. Such methods are becoming more commonly used in wheat for the selection of genes of major effect (disease resistance, grain quality, and flowering time), with part of the challenge being to ‘optimise’ how to use such genes (e.g. Wang et al. 2009).
Two major advances in delivering varieties are the use of ‘indirect’ phenotyping (remote sensing) to measure plant growth (phenomics), and the use of biotechnology to accelerate selection and generation of new genotypes. The term ‘phenomics’ derives from ‘genomics’, which is the study of gene sequences, gene expression, and genetic diversity. The methods of phenomics include automated, robotic, and/or computer-aided technologies which aim to provide a high throughput of individuals (see Furbank 2009, and papers in the same issue of Functional Plant Biology). These measures can be applied in glasshouses (Sirault et al. 2009; Montes et al. 2011; White et al. 2012) or aerial vehicles (Merz and Chapman 2006; Lelong et al. 2008; Berni et al. 2009). Breeding companies have a strong demand for technologies that operate in realistic field-evaluation conditions. For example, a ‘wind machine’ has been developed that is able to generate the forces that cause corn stalks to break during late-season storms (Anon. 2009). This development is partially in response to climate change projections which indicate potential increases in the occurrence rate of more extreme events including storms and cyclones/hurricanes as well as drought periods.
Whitford et al. (2010) describe in detail how applications of biotechnology can contribute to breeding to improve adaptation to climate change, giving specific examples of previous experiences or opportunities. They summarise recent developments in biotechnology applications and how these can be used to address issues of greater temperature extremes, limited water supply, salination, disease challenges, and fertiliser use. The two major biotechnologies described are marker-assisted selection and genetic engineering. Marker-assisted selection leads to the identification of genetic markers that allow the tracking of pieces of DNA from the donor parent to ensure that these are transferred to progeny during the backcrossing. In the genetic engineering approach (also known as genetic modification, or GM), and where the control of the trait is ‘simple’, a single version of a gene may be identified from the same or different species and can be directly transferred into an existing variety. During the discovery phase, these biotechnological approaches inevitably take longer and are more expensive than conventional methods. However, once markers or a version of a gene have been identified, the process of delivering a useful trait into existing varieties can be typically achieved in <2 years, compared with 5–15 years for each transfer using conventional methods. Over time, as the trait becomes distributed throughout the parental pool, there is no longer any need to select for it directly, as all of the parental lines will carry the adaptive versions of the gene(s). The main advantage of GM approaches is a capability to introduce genetic variation from unrelated species into a target species. For example, in the case of heat tolerance, there are plant species that can tolerate extreme heat conditions, and which may carry genes for adaptations at biochemical level to increase the tolerance of plant tissues to such conditions.
In most crops, the final steps of conventional or biotechnology-assisted breeding programs are the broad-scale evaluation of genotypes for potential release into commercial testing, and increase for market. This stage usually requires at least 2 years of testing at multiple locations in the geographic target region of the breeding program. A range of other technologies are deployed at these and earlier steps in the breeding program. These include best-practice design and statistical analysis of breeding trials, and methodologies in crossing and propagation of genotypes that accelerate the generation times. These practices will continue to develop, but as with marker-assisted selection and genetic modification, none of these methods is specific to the breeding of species for climate change adaptation. The specific components of breeding for any type of adaptation trait are in determining the potential impact of increased trait expression on profitability or stability of product yield and quality, developing phenotypic or gene-marker based screens to monitor inheritance of the trait, and identifying and transferring useful genetic variation into relevant varieties.
In Australia, the breeding of the majority of food, fibre, and forestry species is now privatised or is via partnerships of public and private activities. The major crops of wheat, sorghum, sugarcane, and cotton have varying degrees of investment into research related to all five abiotic stresses, with the current priority on drought and salinity. In other species (horticultural and amenity species), these investments are modest, partly due to the greater environmental control producers can deploy (irrigation, greenhouses, etc.). For the food crops, public programs will continue to have a major role in identifying useful genetic resources and in developing these and suitable selection methods via ‘pre-breeding’ programs. With these adapted lines and selection methods, private breeders undertake the ‘fine-tuning’ of adaptation to local, geographically based production areas.
Priority adaptive ‘traits’ for potential impacts of climate change
Determination of the priority traits is conditional upon which environmental effects are likely to change most due to climate change, and the extent to which the traits are already being assessed and selected for in existing breeding programs. Adaptation to increased temperature is an obvious concern, but a major difficulty in the use of global climate model output is in trying to determine how and where climate change will affect the occurrence of heat waves (consecutive periods of temperature 5−10°C above average maximum or even short periods above physiological thresholds such as 35°C). For existing climatology, it is reasonable to say that heat waves are more common in locations (especially drier locations) that have the highest average temperatures, and Hennessy et al. (2008) suggest that it is reasonable to expect an increase both in average temperature, and in the incidence of extreme temperatures. The downscaling of global climate models for effects on rainfall are more challenging than for temperature, and consequently, the forecasting of local impacts on the occurrence of drought, waterlogging, and salinity are even more difficult to predict. In most food crops in Australia, these stresses are all currently the subject of research and breeding efforts and will need to remain within focus. Across multiple scenarios, the only regions that consistently have a prediction for change in rainfall are south-western and south-eastern Australia, where most models predict reductions due to climate change, and where recent climate changes have been in part attributed to human influence (CSIRO and BoM 2007). It could be argued that investment in drought research should be increased for crops in those regions in anticipation of this. For the food crops, the development of specific trait adaptations for these conditions have been reviewed regularly, and were discussed recently within the context of climate change adaptation (Mullan and Barrett-Lennard 2010; Reynolds et al. 2010b; also multiple chapters in Yadav et al. 2011). Given that increased temperature and elevated CO2 are the most predictable and ubiquitous effects of climate change, and that the genetic bases for adaptation to these effects are relatively less researched in Australia (cf. drought and salinity), this section focuses on the opportunities to breed for adaptation to these two environmental effects, and to changes in potentially associated biotic stresses.
Adaptation to high temperature and elevated CO2 conditions
Over the last 20 years, substantial effort has been invested in assessing the potential impacts of climate change on crop production (e.g. Lobell and Burke 2010, and references therein). While elevated CO2 has potentially had a positive influence on growth in conditions of favourable water supply, nutrition, and pest control, the size of this ‘fertilisation’ effect will be partially offset by accompanying higher temperature effects over the next 20–30 years, especially in dryland production areas (Howden et al. 2003; Hatfield and Prueger 2011). Higher temperatures influence plant growth by accelerating development (thereby causing a reduction in the number of ‘growing days’ and in the total radiation captured and biomass produced), and more directly by influencing growth processes to affect the photosynthetic capacity of leaves, the composition of biomass, and the establishment and filling of grains or fruits.
As shown by Lobell and Burke (2010) for several crops on a global scale and by Howden et al. (2003) and van Ittersum et al. (2003) for wheat in Australian environments, temperature increases of >~1−2°C are likely to have negative impacts if adaptation measures are not implemented. The primary impact will be by shortening of the season length of the crops, and secondary impacts occur through increased incidence of high temperature and drought stress. Howden and Crimp (2005) demonstrated that varietal change and alteration of planting windows could allow wheat to maintain productivity in Australian environments, while Craufurd and Wheeler (2009) describe how, for a range of crops and environments, flowering time can be manipulated to assist in avoidance of high temperature stress. Zheng et al. (2012) published a similar study for the Australian wheatbelt. The impact of higher temperature on development time is not limited to crops, and impacts from ‘recent’ climate changes have already been noted. Earlier maturity (onset of ripening) of grapes in south-eastern Australia has been observed in the last 15 years (Sadras and Petrie 2011; Webb et al. 2012), and higher temperatures have been associated elsewhere with reduced wine quality (Mira de Orduña 2010). Duchêne et al. (2010) found similar effects on phenology in French vines and commented on how much the phenology of grape varieties would need to change for future warmer climates. Due to the smaller number of growers involved in horticultural species (vegetables, fruits, and grapes), one rational industry response to climate change is to shift the industry geographically, rather than to breed new varieties. This shift has already begun in several industries, most notably in grape production, where several wine producers have bought properties in cooler regions to future-proof their production (Park et al. 2012).
For many crops, their current geographical distribution sees them adapted across a range of average seasonal temperatures that span about 3 to 10°C either side of their ‘optimum’. Hence, it is only in the most marginal areas of current production where the existing genetics could be completely unsuitable for an increase of 1−2°C. Wheat, for example has been adapted to environments from the equator to 60° latitude and to altitudes (and temperature regimes) ranging from sea level to 3000 m. However, the crop cannot be grown at low latitudes (<15–20°) near sea level where temperatures, even in winter, are too high. The challenge for breeding programs within a target region is to determine how to ‘shift’ the genetic adaptation profile of a crop. At the least, this will likely require transfer of genes from other germplasm resources to modify the length of development phases, and potentially to improve heat tolerance and/or tolerance to drought and waterlogging and, in the longer term, salinity tolerance if the soils are at risk. For crops where cultivars across a large area of the current production are already near the ‘edge’ of their physiological range, this breeding activity will be critical and could require a substantial investment to deliver new cultivars.
There has been much research into the adaptation of crops to higher temperature conditions; the reader is directed particularly to discussions and reviews contained in the recent publications edited by Reynolds (2010) and Yadav et al. (2011). The intention here is to place this work in the context of breeding programs and to provide only a brief summary of some of the major adaptation responses that can be exploited through genetics as related to changing phenology and tolerance to high temperatures (Table 1). Apart from phenological adaptation to avoid high temperature and/or to maintain season length (e.g. Zheng et al. 2012), the most critical traits are associated with the ability of tissues to ‘keep cool’ and tolerate either chronic heat stress (Wardlaw et al. 1989) or short-term heat-shock conditions (Hays et al. 2007). Molecular mechanisms such as heat-shock proteins associated with high-temperature tolerance in the model plant Arabidopsis have not been shown to have a clear role in cereal grain crops (Barnabas et al. 2008; see also references in Reynolds et al. 2010a). However, genetic variation for canopy temperature, under high temperature conditions, has been clearly demonstrated (Pinto et al. 2010). In both irrigated and dryland production, sufficient roots are required to supply the water needed for cooling aerial structures in exchange for CO2 uptake (Trethowan and Mahmood 2011), and canopy temperature is a potential indicator of this condition (Pinto et al. 2010). An alternative adaptation, leaf waxiness, has been demonstrated to reduce transpiration in wheat (Johnson et al. 1983) and rice (Wassmann et al. 2009). With respect to late-season conditions, Reynolds et al. (1994) noted that increased green leaf duration of different wheat cultivars was associated with better adaptation to high temperatures during grain filling. This trait is of potentially high value in both irrigated and dryland conditions when the plant canopy needs to continue to function during a potentially shortened grain-filling period. Many of the traits associated with tolerance to high temperature conditions are associated with reproductive stages, and include variation in the time of day of flowering (Jagadish et al. 2008), maintenance of pollen viability (Prasad et al. 2006), and variation in the ability of ovaries and grains to continue to grow under high temperature conditions. Reynolds et al. (2010a) reviewed in more detail these types of traits and their utility in irrigated v. dryland environments under high temperature conditions.

|
The consensus around the impact of the CO2 fertilisation effect is that there is expected to be an improvement in photosynthetic efficiency for C3 plants until the atmospheric concentration reaches ~750 µmol mol–1 (Seneweera and Norton 2011). Those authors provide summaries of proportional changes in growth and yield responses to elevated CO2 of different plant species. They further observe that there is a reasonable expectation that C3 plants will also have increases in efficiencies of use of radiation, water, and N resources, especially compared with C4 species. In comparing current with old (~1903) varieties of wheat, Ziska et al. (2004) claim that breeding strategies have not been selecting for response to elevated CO2, even given the ~20–30% increase in atmospheric [CO2] over this time period. Hence, they and others (Ainsworth 2008) have argued that there are appropriate traits related to increased photosynthetic capacity, and that decreased respiration that could improve response to elevated CO2. Engineering of RuBisCO activity has frequently been proposed as a target, particularly to allow the more efficient use of the N store that is associated with this major enzyme (Ainsworth 2008; Parry and Hawkesford 2010). Although there is extensive literature on the response of many species to elevated CO2, there is extremely limited information on the genetic variation in response within species.
In addition to temperature, a primary issue for adaptation to elevated CO2 environments concerns the two essential resources of water and N supply. While breeders are actively developing lines that have an increased efficiency of use of water and N, it is not known what impact elevated CO2 will have on such lines, especially in C3 species. Hatfield and Prueger (2011) outline many of the issues around these interactions as related to plant growth rate, energy balance and feedback, and the consequences for WUE. Increased WUE under elevated CO2 in C3 species occurs through an acceleration of plant growth, and its consequent effect on leaf area (via elevated CO2 effects on photosynthesis) generates a positive feedback on WUE over periods of days and weeks. An additional positive feedback on WUE is decreased stomatal conductance per unit leaf area, but this effect is partly countered by the small negative impact of increased foliage temperature (and evaporation rate) due to this decreased conductance.
Adaptation to new pests and pathogens
Despite the global effort in germplasm evaluation, selection, and plant breeding, an estimated US$200 billion is lost annually to plant diseases alone; soil-borne plant parasitic nematodes and other plant pathogens are responsible for more than half of these losses. Crop damage from pests and pathogens is frequently the consequence of complex biological interactions with weather at critical crop development stages. Plant protection strategies use short-term weather to forecast the likelihood of pest and disease outbreaks, in order to time the application of tactical control options such as chemical sprays. However, uncertainty under climate change adds another layer of complexity to pest and disease management and also to the utility of these tactical responses (Chakraborty and Newton 2011).
Plant breeding has been largely successful in controlling pathogens that have well-developed, highly specific genetic interactions with the host plant. These mostly biotrophic pathogens, such as rusts and smuts, which derive nutrients only from living host plants, have been controlled by manipulating single plant genes or a small number of plant genes with large effects. These genes often trigger a programmed cell death in plants when infected by a particular race and thus starve the pathogen of nutrients. The pathogen race is driven to near-extinction with widespread use of resistant varieties, but with a highly plastic genome, short generation time, and massive population size, new races evolve to overcome host resistance. The Ug99 strain of wheat stem rust is an example (Pretorius et al. 2000). Partial resistance that is effective against a large number of pathogen races can be often controlled by plant genes with intermediate and additive effects. Necrotrophic pathogens derive nutrition from dead host tissue, often producing toxins to first kill plant cells. Resistance, when available, is often controlled by a few genes and is only able to partially restrict the growth and development of the pathogen. Unlike their biotrophic cousins, necrotrophic pathogens can grow and maintain populations on crop residues and other organic matter, even if partially resistant varieties temporarily restrict their growth. Their impact on yield and quality can be magnified if crops are under abiotic stress such as drought and heat, particularly as some of their effects specifically damage root and internal transport systems of plants. Necrotrophic pathogens will likely become more damaging under climate change due to more frequent and severe abiotic stress episodes.
Climate change will influence pest and pathogens in three main ways, all with significant implications for plant breeding:
-
Change their geographical range and distribution;
-
Change their population genetics and biology, including virulence spectrum and rates of evolution;
-
Change the effectiveness of disease resistance genes and gene networks in plants.
With rising temperature, some cropping areas will shift pole-ward along altitudinal gradients, and pests and pathogens will migrate with their hosts. If crops suffer additional stress from unsuitable soil type, topography, etc., this will further modify the relative importance of pests and pathogens affecting a crop and the nature and extent of damage. There will be winners and losers, and the lifecycle of pests and pathogens will influence their survival and impact. Changes in other extreme events such as hurricanes will favour transcontinental invasion of fungal pathogens. Climate determines the distribution, development, and population dynamics of insect pests, and so warming climates with increased upper air movement will generally increase the frequency of outbreaks. Complex pest assemblages and weed species, more common in lower latitudes, will become common in temperate regions towards the poles. Hence, new pests and pathogens and new disease complexes will affect crops in new environments and breeding targets will have to be revised.
Changes in plant physiology at high CO2 concentrations influence the life cycle of insect pests, weeds, and plant pathogens. The fecundity of many biotrophic (Hibberd et al. 1996) and necrotrophic (Melloy et al. 2010) pathogens increases at elevated CO2. The enlarged crop canopy from a CO2 fertilisation effect increases the number of infection cycles to further boost pathogen population, potentially increasing the rate of evolution of new races (Chakraborty and Datta 2003). Although the probability of mutation from avirulence to virulence increases with increasing population size, mutation rate depends on the avirulence gene (Leach et al. 2001) and its fitness within a pathogen population, but predicting the evolution of new races has not been possible. Many of the common weeds present in Australian cropping systems have a C3 photosynthesis pathway and, therefore, would be expected to grow faster and become more competitive in elevated CO2 conditions. Pests and pathogens will quickly adapt to changing climate, and there are many examples in the literature for both insect pests (Zhou et al. 1995) and fungal pathogens (Milus et al. 2009). Changing crop phenology and an extended growing season in some regions will support more generations of insect pests each year, and infestations may start early and last long. Changing crop quality at elevated CO2 will influence palatability and concentrations of defensive chemicals, altering feeding behaviour and interactions with natural enemies and competitors.
Climate change can raise or lower resistance of plants to pests and diseases. Heat and drought stress will predispose plants to some pathogens but trigger defence mechanisms by raising the level of expression of some genes and gene networks, potentially increasing resistance to some pests and pathogens (Eastburn et al. 2011). However, the limited understanding of these complex interactions suggests that any breeding approach will have to target a specific pest or pathogen and that ‘one-size-fits-all’ approaches will not work. The effectiveness of many rust-resistance genes in wheat is determined by temperature and/or the stage of plant development. Temperature increases will make varieties with the stem rust resistance gene Sr15 susceptible, but the stripe rust resistance gene Yr18 will be more effective (Park et al. 1992).
Plant breeding in the future will need to deal with more complex traits controlled by series of interacting genes and gene networks such as those involved in defence reaction against pests and diseases. Abiotic stresses caused by drought and high temperature influence and interact with these networks. Here again, the distinction between pathogen lifestyles will be important, as different defence cascades work against biotrophic and necrotrophic pathogens. Current research in this area has been recently reviewed (Eastburn et al. 2011). A great deal of pre-breeding research will be necessary to understand the genetic architecture of these complex traits and how these networks are modified by climate change before they can be exploited through plant breeding. Early involvement of plant breeders will be necessary to dissect and to develop selection strategies for complex traits.
Historically, there has been limited success in breeding for resistance to soil-borne diseases, where the level of complexity rises further in the complex heterogeneous soil environment. Climate-change effects on soil biology and chemistry are difficult to predict. Several approaches, such as the use of pathogen-suppressive soils, organic amendments, and biological fumigation, have identified potentially useful mechanisms that can be exploited through plant breeding. Glucosinolate, which is produced by canola and decomposes in soil to produce compounds found in commercial soil fumigants effective against soil-borne pathogens, is an example (Gimsing and Kirkegaard 2009). Breeding can increase root exudation and rhizodeposition to selectively enrich species and communities of pathogen-suppressive microbes or better fortify roots to stop or reduce infection. While bacterial communities in the soil environment surrounding plant roots (rhizosphere) are known to be influenced by plant species (Marschner et al. 2004), so far, no genetic mechanism has been identified that would allow specific recognition by plant roots of bacteria implicated in the biological control of soil-borne pathogens.
Pre-emptive or anticipatory breeding (McIntosh and Brown 1997) against invasive species and predicted shifts/evolution in pest and pathogen strains/species can be mounted once the risk has been determined. As for direct environmental impacts on the TPE, models can be used to predict biosecurity risks for pest and pathogen incursion (Aurambout et al. 2009) to help align breeding objectives with best estimates of climate change scenarios.
Future priorities in phenotyping and breeding
Modern plant breeding utilises a large array of technologies to identify the genetic controls of simple and complex traits, with increasing capabilities to accelerate the incorporation of adaptive genetic variation into parental germplasm and cultivars of many species delivered to industry. In many crop species, there are established activities in the areas of breeding for increased adaptation to drought, waterlogging, and salinity, and ongoing maintenance breeding for existing pests and pathogens. There is a need for ongoing assessment of the potential for pests and pathogens to increase in population, to shift in their geographic distribution, and to evolve into new forms that could represent serious threats to production. In terms of direct environmental adaptation, elevated CO2 and potential increases in both average temperature and the occurrence of temperature extremes represent major challenges and opportunities for breeding. If increased temperatures force the major Australian production zones to ‘retreat’ southwards and/or to become shorter in duration, both of these outcomes have potentially serious consequences for local and national productivity of grain crops. Maintaining grain zones in their current locations will at the least require breeding activities to deliver changes in the phenology (flowering time) of the crops, and will likely require increased degrees of tolerance to a higher frequency of high temperature conditions. A practical response from breeding programs could be to identify current geographical locations that can serve as ‘proxies’ for future climate and to begin to undertake selection at those locations. Similar approaches have been used to screen for drought stress in the past. While changes in geographical distribution of seasonal temperature patterns will also affect perennial species (i.e. fruits and forests), it is more difficult to shift these usually higher value industries, and therefore management solutions are likely to be a more economical and effective solution compared with breeding in the shorter term (10–20 years).
For the major grain crops, there is a need to better document the opportunities for existing genetic variation in phenology, temperature tolerance, and response to elevated CO2 to contribute to improved yield, especially in regions where temperatures are predicted to increase; that is, what will the TPE look like for future climates? Especially at the rainfall-limited edges of the current grain-cropping regions, temperature increases may be such that novel genetic variation is required so that wheat and sorghum production, in particular, is still viable in most seasons. For C3 species such as wheat, rice, cotton, and grain oil crops, the prospect of slight increases in WUE due to elevated CO2 conditions is expected to partially offset some of the temperature impacts that result in a shortening of season length. However, this effect is not well quantified for any species across the Australian cropping region, and is not as beneficial for C4 species such as sorghum and sugarcane. Efficient methods to assess genetic variation in growth response to elevated CO2 are needed, and presuming that such methods will largely require glasshouse-type conditions (to assess large numbers of lines), it will be essential to demonstrate that genetic variation in trait responses to such conditions is predictive of performance in field conditions.
In all of these species, an improved understanding of the genetic control of development and flowering time will be essential to assist breeding programs to deliver new combinations of genes into existing varieties. Many of the genes that control flowering time through response to vernalisation and photoperiod have been identified in model species and several crop species, but the genetic control of response to temperature per se is not nearly as well understood. At present, it is also slow and difficult to screen genotypes for tolerance to high temperature in either vegetative or reproductive stages of growth. Breeding for these traits requires investment in the development of high-throughput phenotypic screens that allow the observation of canopy and ear temperature, and of the impacts of high temperature on leaf function, and processes associated with ovary development, pollen viability, grain set, and grain filling. Phenotyping methods based on imaging and monitoring of thermal condition will be essential here, but these need to be linked to methods that allow monitoring of the impact of the high temperature stress on plant function, i.e. leaf condition, pollen quality, and grain number.
Breeding for adaptation to new climatic environments is challenging. It could be argued that breeding programs will ‘naturally’ adapt to climate change, to the extent that climate change affects production environments at about the same rate as breeding programs deliver new varieties to industry. In the mainstream press, the major breeding programs already acknowledge that climate change is a potential risk to production, and even a marketing opportunity (e.g. ‘Biotech companies race for drought-tolerant crops’; www.enn.com/agriculture/article/29184). In plant breeding, the main danger, and the main opportunity, is if climate change leads to more variable production conditions such that industries are suddenly exposed to one or a sequence of seasons of extreme conditions, particularly if these seasons comprise high temperature events that could have catastrophic consequences.
Acknowledgments
This research has been undertaken with the support of the CSIRO Climate Adaptation Flagship and external research grants from the Australian Government Department of Agriculture, Fisheries and Forestry (DAFF) Australia’s Farming Future Climate Change Research Program (Project GMS-0335), and from the Australian Grains Research and Development Corporation (GRDC; Projects CSP00136 and CSP00125).
References
ABARE (2010) Australian Commodity Statistics. Australian Bureau of Agricultural and Resource Economics and Sciences, Canberra.ABS (2011) 7122.0.55.001 – Stocks of Grain Held by Bulk Handling Companies and Grain Traders, Australia. Australian Bureau of Statistics, Canberra.
Ainsworth EA (2008) Rice production in a changing climate: a meta-analysis of responses to elevated carbon dioxide and elevated ozone concentration. Global Change Biology 14, 1642–1650.
| Rice production in a changing climate: a meta-analysis of responses to elevated carbon dioxide and elevated ozone concentration.Crossref | GoogleScholarGoogle Scholar |
Anon. (2009) DuPont Unveils Innovative Mobile Wind Machines to Help Improve Corn Yield. DuPont Pioneer Hi-Bred, Des Moines, IA, USA.
Arthur C, Phillips A (2011) Disaster declaration mooted for fire-ravaged western Qld. Vol. 2011. News article (ABC). Available at: www.abc.net.au/news/2011-10-20/disaster-declaration-mooted-for-fire-ravaged-western-qld/3580708/?site=westqld§ion=news (accessed 31 October 2011).
Asseng S, Foster I, Turner NC (2011) The impact of temperature variability on wheat yields. Global Change Biology 17, 997–1012.
| The impact of temperature variability on wheat yields.Crossref | GoogleScholarGoogle Scholar |
Aurambout J-P, Finlay K, Luck J, Beattie G (2009) A concept model to estimate the potential of the Asiatic citrus psyllid (Diaphorina citri Kuwayama) in Australia under climate change—a means for assessing biosecurity risk. Ecological Modelling 220, 2512–2524.
| A concept model to estimate the potential of the Asiatic citrus psyllid (Diaphorina citri Kuwayama) in Australia under climate change—a means for assessing biosecurity risk.Crossref | GoogleScholarGoogle Scholar |
Azhar FM, Ali Z, Akhtar MM, Khan AA, Trethowan R (2009) Genetic variability of heat tolerance, and its effect on yield and fibre quality traits in upland cotton (Gossypium hirsutum L.). Plant Breeding 128, 356–362.
| Genetic variability of heat tolerance, and its effect on yield and fibre quality traits in upland cotton (Gossypium hirsutum L.).Crossref | GoogleScholarGoogle Scholar |
Barnabas B, Jager K, Feher A (2008) The effect of drought and heat stress on reproductive processes in cereals. Plant, Cell & Environment 31, 11–38.
Berni JAJ, Zarco-Tejada PJ, Suarez L, Fereres E (2009) Thermal and narrowband multispectral remote sensing for vegetation monitoring from an unmanned aerial vehicle. IEEE Transactions on Geoscience and Remote Sensing 47, 722–738.
| Thermal and narrowband multispectral remote sensing for vegetation monitoring from an unmanned aerial vehicle.Crossref | GoogleScholarGoogle Scholar |
Biswas WK, Graham J, Kelly K, John MB (2010) Global warming contributions from wheat, sheep meat and wool production in Victoria, Australia – a life cycle assessment. Journal of Cleaner Production 18, 1386–1392.
| Global warming contributions from wheat, sheep meat and wool production in Victoria, Australia – a life cycle assessment.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXhtVamtbvL&md5=e34a33526173bca9bc11643930ab5a11CAS |
Braun H-J, Atlin G, Payne T (2010) Multi-location testing as a tool to identify plant response to global climate change. In ‘Climate change and crop production. Vol. 1’. (Ed. MP Reynolds) pp. 115–138. (CABI: Wallingford, UK)
Brisson N, Gate P, Gouache D, Charmet G, Oury FX, Huard F (2010) Why are wheat yields stagnating in Europe? A comprehensive data analysis for France. Field Crops Research 119, 201–212.
| Why are wheat yields stagnating in Europe? A comprehensive data analysis for France.Crossref | GoogleScholarGoogle Scholar |
Chakraborty S, Datta S (2003) How will plant pathogens adapt to host plant resistance at elevated CO2 under a changing climate? New Phytologist 159, 733–742.
| How will plant pathogens adapt to host plant resistance at elevated CO2 under a changing climate?Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXns1Sgsr0%3D&md5=e224215d77b94405431728ffe661ba0eCAS |
Chakraborty S, Newton A (2011) Climate change, plant diseases and food security, an overview. Plant Pathology 60, 2–14.
| Climate change, plant diseases and food security, an overview.Crossref | GoogleScholarGoogle Scholar |
Chakraborty S, Luck J, et al (2008) Impacts of global change on diseases of agricultural crops and forest trees. CABI Reviews: Perspectives in Agriculture, Veterinary Science, Nutrition and Natural Resources 3, 1–15.
Chapman SC, Cooper M, Hammer GL, Butler DG (2000) Genotype by environment interactions affecting grain sorghum. II. Frequencies of different seasonal patterns of drought stress are related to location effects on hybrid yields. Australian Journal of Agricultural Research 51, 209–221.
| Genotype by environment interactions affecting grain sorghum. II. Frequencies of different seasonal patterns of drought stress are related to location effects on hybrid yields.Crossref | GoogleScholarGoogle Scholar |
Chapman SC, Hammer GL, Podlich DW, Cooper M (2002) Linking biophysical and genetic models to integrate physiology, molecular biology and plant breeding. In ‘Quantitative genetics, genomics, and plant breeding’. (Ed. MS Kang) pp. 167–187. (CABI: Wallingford, UK)
Chenu K, Cooper M, Hammer GL, Mathews KL, Dreccer MF, Chapman SC (2011) Environment characterization as an aid to wheat improvement: interpreting genotype-environment interactions by modelling water-deficit patterns in North-Eastern Australia. Journal of Experimental Botany 62, 1743–1755.
| Environment characterization as an aid to wheat improvement: interpreting genotype-environment interactions by modelling water-deficit patterns in North-Eastern Australia.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXjsFyis7w%3D&md5=a12d7c4440e7ad30bd625f133bed4bfdCAS |
Conroy J, Hocking P (1993) Nitrogen nutrition of C3 plants at elevated atmospheric CO2 concentrations. Physiologia Plantarum 89, 570–576.
| Nitrogen nutrition of C3 plants at elevated atmospheric CO2 concentrations.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2cXns1CrtA%3D%3D&md5=b97d9b893323f760f9ceeb313a2530a6CAS |
Cooper M, Hammer GL (Eds) (1996) ‘Plant adaptation and crop improvement.’ (CABI: Wallingford, UK)
Cooper M, Podlich DW, Smith OS (2005) Gene-to-phenotype models and complex trait genetics. Australian Journal of Agricultural Research 56, 895–918.
| Gene-to-phenotype models and complex trait genetics.Crossref | GoogleScholarGoogle Scholar |
Craufurd PQ, Wheeler TR (2009) Climate change and the flowering time of annual crops. Journal of Experimental Botany 60, 2529–2539.
| Climate change and the flowering time of annual crops.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXntlGisLY%3D&md5=f28a605a1f3a08a7339bb87170cd93d3CAS |
Crimp SJ, Stokes CJ, Howden SM, Moore AD, Jacobs B, Brown PR, Ash AJ, Kokic P, Leith P (2010) Managing Murray-Darling Basin livestock systems in a variable and changing climate: challenges and opportunities. The Rangeland Journal 32, 293–304.
CSIRO (2008) Water availability in the Murray-Darling Basin. A report to the Australian Government from the CSIRO Murray-Darling Sustainable Yields Project. CSIRO, Australia. Available at: www.csiro.au/Outcomes/Water/Water-for-the-environment/WaterAvailabilityInMurray-DarlingBasinMDBSY.aspx
CSIRO and BoM (2007) Climate Change in Australia. Technical Report 2007. CSIRO Marine and Atmospheric Research, Aspendale, Vic.
Dreccer MF, Bonnett DG, Lafarge T (2011) Breeding of new cultivarra des with a combination of approaches for a changing climate. In ‘Encyclopedia of sustainability science and technology’. (Ed. RA Meyers) (Springer Science+Business Media, LLC) (in press)
Duchêne E, Huard F, Dumas V, Schneider C, Merdinoglu D (2010) The challenge of adapting grapevine varieties to climate change. Climate Research 41, 193–204.
| The challenge of adapting grapevine varieties to climate change.Crossref | GoogleScholarGoogle Scholar |
Duvick DN (1992) Genetic contributions to advances in yield of United States maize. Maydica 37, 69–79.
Duvick DN (2005) The contribution of breeding to yield advances in maize (Zea mays L.). Advances in Agronomy 86, 83–145.
| The contribution of breeding to yield advances in maize (Zea mays L.).Crossref | GoogleScholarGoogle Scholar |
Dwyer SA, Ghannoum O, Nicotra A, Von Caemmerer S (2007) High temperature acclimation of C4 photosynthesis is linked to changes in photosynthetic biochemistry. Plant, Cell & Environment 30, 53–66.
| High temperature acclimation of C4 photosynthesis is linked to changes in photosynthetic biochemistry.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXitVCltb8%3D&md5=a18d203800323579d9eddc94a28b32a1CAS |
Dyer R, Stafford Smith DM (2003) Ecological and economic assessment of prescribed burning impacts in semi-arid pastoral lands of northern Australia. International Journal of Wildland Fire 12, 403–413.
| Ecological and economic assessment of prescribed burning impacts in semi-arid pastoral lands of northern Australia.Crossref | GoogleScholarGoogle Scholar |
Eastburn DM, McElrone AJ, Bilgin DD (2011) Influence of atmospheric and climatic change on plant–pathogen interactions. Plant Pathology 60, 54–69.
| Influence of atmospheric and climatic change on plant–pathogen interactions.Crossref | GoogleScholarGoogle Scholar |
Erbs M, Manderscheid R, Jansen G, Seddig S, Pacholski A, Weigel HJ (2010) Effects of free-air CO2 enrichment and nitrogen supply on grain quality parameters and elemental composition of wheat and barley grown in a crop rotation. Agriculture, Ecosystems & Environment 136, 59–68.
| Effects of free-air CO2 enrichment and nitrogen supply on grain quality parameters and elemental composition of wheat and barley grown in a crop rotation.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXhtVGrsr8%3D&md5=cbf3462c5ed850557234586737c1dac7CAS |
Everingham YL, Reason CJC (2011) Interannual variability in rainfall and wet spell frequency during the New South Wales sugarcane harvest season. International Journal of Climatology 31, 144–152.
| Interannual variability in rainfall and wet spell frequency during the New South Wales sugarcane harvest season.Crossref | GoogleScholarGoogle Scholar |
Fischer RA (2011) Wheat physiology: a review of recent developments. Crop & Pasture Science 62, 95–114.
| Wheat physiology: a review of recent developments.Crossref | GoogleScholarGoogle Scholar |
Fleury D, Jefferies S, Kuchel H, Langridge P (2010) Genetic and genomic tools to improve drought tolerance in wheat. Journal of Experimental Botany 61, 3211–3222.
| Genetic and genomic tools to improve drought tolerance in wheat.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXptVymsLc%3D&md5=7797f174d7dbd65457d1f6e4352c3d12CAS |
Furbank RT (2009) Plant phenomics: from gene to form and function. Functional Plant Biology 36, V–VI.
George AP, Broadley RH, Nissen RJ (2005) Can Australian horticulture survive and meet the global challenge? In ‘Proceedings of the International Symposium on Harnessing the Potential of Horticulture in the Asian-Pacific Region’. No. 694, pp. 289–294.
Gimsing A, Kirkegaard J (2009) Glucosinolates and biofumigation fate of glucosinolates and their hydrolysis in soil. Phytochemistry Reviews 8, 299–310.
| Glucosinolates and biofumigation fate of glucosinolates and their hydrolysis in soil.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXivFCntA%3D%3D&md5=4d812d15b2a8f09c3885d749435131acCAS |
Hatfield JL, Prueger JH (2011) Agroecology: implications for plant response to climate change. In ‘Crop adaptation to climate change’. (Eds SS Yadav, RJ Redden, JL Hatfield, H Lotze-Campen, AE Hall) pp. 27–43. (Wiley-Blackwell: Chichester, UK)
Hayman P, Rickards L, Eckard R, Lemerle D (2012) Climate change through the farming systems lens: challenges and opportunities for farming in Australia. Crop & Pasture Science 63, 203–214.
Hays DB, Do JH, Mason RE, Morgan G, Finlayson SA (2007) Heat stress induced ethylene production in developing wheat grains induces kernel abortion and increased maturation in a susceptible cultivar. Plant Science 172, 1113–1123.
| Heat stress induced ethylene production in developing wheat grains induces kernel abortion and increased maturation in a susceptible cultivar.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXkvFGitLw%3D&md5=902bd1c6193e2c1481ba06a348c1b29dCAS |
Hennessy KJ, Fitzharris B, Bates BC, et al. (2007) Australian and New Zealand. In ‘Climate Change 2007: Impacts, Adaptation and Vulnerability. Contribution of Working Group II to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change’. (Eds ML Parry, OF Canzani, JP Palutikof, et al.) pp. 507–540. (Cambridge University Press: Cambridge, UK)
Hennessy KJ, Fawcett R, Kirono D, et al. (2008) ‘An assessment of the impact of climate change on the nature and frequency of exceptional climatic events.’ (CSIRO and the Australian Bureau of Meteorology: Melbourne)
Hibberd JM, Whitbread R, Farrar JF (1996) Effect of elevated concentrations of CO2 on infection of barley by Erysiphe graminis. Physiological and Molecular Plant Pathology 48, 37–53.
| Effect of elevated concentrations of CO2 on infection of barley by Erysiphe graminis.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK28Xhs1ygs78%3D&md5=a927bd876f9cb281227a773a28b62372CAS |
Howden SM, Crimp S (2005) Assessing dangerous climate change impacts on Australia’s wheat industry. In ‘MODSIM 2005: International Congress on Modelling and Simulation: Advances and Applications for Management and Decision Making’. pp. 505–511.
Howden SM, Crimp S (2011) Regional impacts: Australia. In ‘Crop adaptation to climate change’. (Eds SS Yadav, RJ Redden, JL Hatfield, H Lotze-Campen, AE Hall) pp. 143–155. (Wiley-Blackwell: Chichester, UK)
Howden SM, Reyenga PJ, Meinke H (2003) Managing the quality of wheat grain under global change. In ‘MODSIM 2003: International Congress on Modelling and Simulation’. 1–4, pp. 35–40.
Howden SM, Soussana JF, Tubiello FN, Chhetri N, Dunlop M, Meinke H (2007) Adapting agriculture to climate change. Proceedings of the National Academy of Sciences of the United States of America 104, 19 691–19 696.
| Adapting agriculture to climate change.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXitFSltg%3D%3D&md5=00fb9f1d8e622a469270ec9de2bf6b90CAS |
Howden SM, Crimp SJ, Stokes CJ (2008) Climate change and Australian livestock systems: impacts, research and policy issues. Australian Journal of Experimental Agriculture 48, 780–788.
| Climate change and Australian livestock systems: impacts, research and policy issues.Crossref | GoogleScholarGoogle Scholar |
Howden SM, Crimp SJ, Nelson RN (2010) Australian agriculture in a climate of change. In ‘Managing climate change’. (Eds I Jubb, P Hopler, W Cai) pp. 101–111. (CSIRO: Melbourne)
Huth NI, Thorburn PJ, Radford BJ, Thornton CM (2010) Impacts of fertilisers and legumes on N2O and CO2 emissions from soils in subtropical agricultural systems: a simulation study. Agriculture, Ecosystems & Environment 136, 351–357.
| Impacts of fertilisers and legumes on N2O and CO2 emissions from soils in subtropical agricultural systems: a simulation study.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXisFenurk%3D&md5=88d98e92a989eccd2c45616eb5ce330aCAS |
Inman-Bamber G, Jackson PJ, Bourgault MB (2011) Genetic adjustment to changing climates: sugarcane. In ‘Crop adaptation to climate change’. (Eds SS Yadav, RJ Redden, JL Hatfield, H Lotze-Campen, AE Hall) pp. 439–447. (Wiley-Blackwell: Chichester, UK)
IPCC (2007) ‘Climate Change 2007: The Physical Science Basis. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change.’ (Cambridge University Press: Cambridge, UK)
Jagadish SVK, Craufurd PQ, Wheeler TR (2008) Phenotyping parents of mapping populations of rice for heat tolerance during anthesis. Crop Science 48, 1140–1146.
| Phenotyping parents of mapping populations of rice for heat tolerance during anthesis.Crossref | GoogleScholarGoogle Scholar |
Johnson DA, Richards RA, Turner NC (1983) Yield, water relations, gas-exchange, and surface reflectances of near-isogenic wheat lines differing in glaucousness. Crop Science 23, 318–325.
| Yield, water relations, gas-exchange, and surface reflectances of near-isogenic wheat lines differing in glaucousness.Crossref | GoogleScholarGoogle Scholar |
Jordan DR, Mace ES, Cruickshank AW, Hunt CH, Henzell RG (2011) Exploring and exploiting genetic variation from unadapted sorghum germplasm in a breeding program. Crop Science 51, 1444–1457.
| Exploring and exploiting genetic variation from unadapted sorghum germplasm in a breeding program.Crossref | GoogleScholarGoogle Scholar |
Khan S (2008) Managing climate risks in Australia: options for water policy and irrigation management. Australian Journal of Experimental Agriculture 48, 265–273.
| Managing climate risks in Australia: options for water policy and irrigation management.Crossref | GoogleScholarGoogle Scholar |
King KJ, de Ligt RM, Cary GJ (2011) Fire and carbon dynamics under climate change in south-eastern Australia: insights from FullCAM and FIRESCAPE modelling. International Journal of Wildland Fire 20, 563–577.
| Fire and carbon dynamics under climate change in south-eastern Australia: insights from FullCAM and FIRESCAPE modelling.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXnslOmtrY%3D&md5=1a9c8d18a6aa0a0b57b4f8e9f53bbf30CAS |
Lantican MA, Pingali PL, Rajaram S (2003) Is research on marginal lands catching up? The case of unfavourable wheat growing environments. Agricultural Economics 29, 353–361.
| Is research on marginal lands catching up? The case of unfavourable wheat growing environments.Crossref | GoogleScholarGoogle Scholar |
Leach JE, Vera Cruz CM, Bai J, Leung H (2001) Pathogen fitness penalty as a predictor of durability of disease resistance genes. Annual Review of Phytopathology 39, 187–224.
| Pathogen fitness penalty as a predictor of durability of disease resistance genes.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXmvVygs7s%3D&md5=92685a12572f070a541ac383f3da4ea1CAS |
Legreve A, Duveiller E (2010) Preventing potential disease and pest epidemics under a changing climate. In ‘Climate change and crop production. Vol. 1’. (Ed. MP Reynolds) pp. 50–70. (CABI: Wallingford, UK)
Lelong CCD, Burger P, Jubelin G, Roux B, Labbe S, Baret F (2008) Assessment of unmanned aerial vehicles imagery for quantitative monitoring of wheat crop in small plots. Sensors 8, 3557–3585.
| Assessment of unmanned aerial vehicles imagery for quantitative monitoring of wheat crop in small plots.Crossref | GoogleScholarGoogle Scholar |
Lindhout P, Pet G (1990) Effects of CO2 enrichment on young plant-growth of 96 genotypes of tomato (Lycopersicon esculentum). Euphytica 51, 191–196.
Lobell DB, Burke M (2010) Economic impacts of climate change on agriculture to 2030. In ‘Climate change and crop production. Vol. 1’. (Ed. MP Reynolds) pp. 38–49. (CABI: Wallingford, UK)
Löffler CM, Wei J, Fast T, Gogerty J, Langton S, Bergman M, Merrill B, Cooper M (2005) Classification of maize environments using crop simulation and geographic information systems. Crop Science 45, 1708–1716.
| Classification of maize environments using crop simulation and geographic information systems.Crossref | GoogleScholarGoogle Scholar |
Luo ZB, Langenfeld-Heyser R, Calfapietra C, Polle A (2005) Influence of free air CO2 enrichment (EUROFACE) and nitrogen fertilisation on the anatomy of juvenile wood of three poplar species after coppicing. Trees Structure and Function 19, 109–118.
| Influence of free air CO2 enrichment (EUROFACE) and nitrogen fertilisation on the anatomy of juvenile wood of three poplar species after coppicing.Crossref | GoogleScholarGoogle Scholar |
Mace ES, Jordan DR (2010) Location of major effect genes in sorghum (Sorghum bicolor (L.) Moench). Theoretical and Applied Genetics 121, 1339–1356.
| Location of major effect genes in sorghum (Sorghum bicolor (L.) Moench).Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXht12qu7rN&md5=06e28f1af3d002b20733bd429666d45dCAS |
Mace ES, Jordan DR (2011) Integrating sorghum whole genome sequence information with a compendium of sorghum QTL studies reveals uneven distribution of QTL and of gene-rich regions with significant implications for crop improvement. Theoretical and Applied Genetics 123, 169–191.
| Integrating sorghum whole genome sequence information with a compendium of sorghum QTL studies reveals uneven distribution of QTL and of gene-rich regions with significant implications for crop improvement.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC3MrhtVGksA%3D%3D&md5=4478c9ba3b9c6b916804441c6eeca46eCAS |
Marschner P, Crowley D, Yang C (2004) Development of specific rhizosphere bacterial communities in relation to plant species. Plant and Soil 261, 199–208.
| Development of specific rhizosphere bacterial communities in relation to plant species.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXlvVeht7Y%3D&md5=724bdf6e4fea588f66c17be0bc5f000fCAS |
Mazzola M (2010) Management of resident soil microbial community structure and function to suppress soilborne disease development. In ‘Climate change and crop production. Vol. 1’. (Ed. MP Reynolds) pp. 200–218. (CABI: Wallingford, UK)
McIntosh RA, Brown GN (1997) Anticipatory breeding for resistance to rust diseases in wheat. Annual Review of Phytopathology 35, 311–326.
| Anticipatory breeding for resistance to rust diseases in wheat.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2sXmtVGhsrc%3D&md5=115c21f0ca8a534c119a572b2641a14eCAS |
Meinke H, Howden SM, Struik PC, Nelson R, Rodriguez D, Chapman SC (2009) Adaptation science for agriculture and natural resource management – urgency and theoretical basis. Current Opinion in Environmental Sustainability 1, 69–76.
| Adaptation science for agriculture and natural resource management – urgency and theoretical basis.Crossref | GoogleScholarGoogle Scholar |
Melloy P, Hollaway G, Luck JO, Norton ROB, Aitken E, Chakraborty S (2010) Production and fitness of Fusarium pseudograminearum inoculum at elevated carbon dioxide in FACE. Global Change Biology 16, 3363–3373.
| Production and fitness of Fusarium pseudograminearum inoculum at elevated carbon dioxide in FACE.Crossref | GoogleScholarGoogle Scholar |
Merz T, Chapman SC (2006) Autonomous unmanned helicopter system for remote sensing missions in unknown environments. In ‘Conference on Unmanned Aerial Vehicle in Geomatics (UAV-g)’. 14–16 Sept. 2011, Zurich, Switzerland, Vol. XXXVIII-1/C22. (Eds H Eisenbeiss, M Kunz, H Ingensand) www.geometh.ethz.ch/uav_g/proceedings
Messina CD, Podlich D, Dong ZS, Samples M, Cooper M (2011) Yield-trait performance landscapes: from theory to application in breeding maize for drought tolerance. Journal of Experimental Botany 62, 855–868.
| Yield-trait performance landscapes: from theory to application in breeding maize for drought tolerance.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXhtVekurs%3D&md5=572935d16e3c9db3faecd1328fd881ccCAS |
Milus EA, Kristensen K, Hovmøller MS (2009) Evidence for increased aggressiveness in a Recent widespread strain of Puccini striiformis f. sp. tritici causing stripe rust of wheat. Phytopathology 99, 89–94.
| Evidence for increased aggressiveness in a Recent widespread strain of Puccini striiformis f. sp. tritici causing stripe rust of wheat.Crossref | GoogleScholarGoogle Scholar |
Mira de Orduña R (2010) Climate change associated effects on grape and wine quality and production. Food Research International 43, 1844–1855.
| Climate change associated effects on grape and wine quality and production.Crossref | GoogleScholarGoogle Scholar |
Montes JM, Technow F, Dhillon BS, Mauch F, Melchinger AE (2011) High-throughput non-destructive biomass determination during early plant development in maize under field conditions. Field Crops Research 121, 268–273.
| High-throughput non-destructive biomass determination during early plant development in maize under field conditions.Crossref | GoogleScholarGoogle Scholar |
Mullan DJ, Barrett-Lennard G (2010) Breeding crops for tolerance to salinity, waterlogging and inundation. In ‘Climate change and crop production. Vol. 1’. (Ed. MP Reynolds) pp. 92–114. (CABI: Wallingford, UK)
Neate S, McIntyre K (2011) Fusarium head blight (Head Scab). Queensland Department of Employment, Economic Development and Innovation, Toowoomba.
Nitschke M, Tucker GR, Hansen AL, Williams S, Zhang Y, Bi P (2011) Impact of two recent extreme heat episodes on morbidity and mortality in Adelaide, South Australia: a case-series analysis. Environmental Health 10, Art. No. 42
O’Leary GJ, Christy BP, Weeks A, Nuttall J, Riffkin PCB, Fitzgerald G (2011) Downscaling global climatic predictions to the regional level: a case study of regional effects of climate change on wheat crop production in Victoria, Australia. In ‘Crop adaptation to climate change’. (Eds SS Yadav, RJ Redden, JL Hatfield, H Lotze-Campen, AE Hall) pp. 12–26. (Wiley-Blackwell: Chichester, UK)
O’Reagain P, Bushell J, Holmes B (2011) Managing for rainfall variability: long-term profitability of different grazing strategies in a northern Australian tropical savanna. Animal Production Science 51, 210–224.
| Managing for rainfall variability: long-term profitability of different grazing strategies in a northern Australian tropical savanna.Crossref | GoogleScholarGoogle Scholar |
Oliver YM, Robertson MJ, Weeks C (2010) A new look at an old practice: benefits from soil water accumulation in long fallows under Mediterranean conditions. Agricultural Water Management 98, 291–300.
| A new look at an old practice: benefits from soil water accumulation in long fallows under Mediterranean conditions.Crossref | GoogleScholarGoogle Scholar |
Park R, Gavin J, Rees R (1992) Effects of temperature on the response of some Australian wheat cultivars to Puccinia striiformis f. sp. tritici. Mycological Research 96, 166–170.
| Effects of temperature on the response of some Australian wheat cultivars to Puccinia striiformis f. sp. tritici.Crossref | GoogleScholarGoogle Scholar |
Park JN, Cobon DH, Phelps DG (2003) Modelling pasture growth in the Mitchell grasslands. In ‘MODSIM 2003: International Congress on Modelling and Simulation’. 1–4, pp. 519–524.
Park SE, Marshall N, Jakku E, Dowd AM, Howden SM, Mendham E, Fleming A (2012) Informing adaptation responses to climate change through theories of transformation. Global Environmental Change 22, 115–126.
| Informing adaptation responses to climate change through theories of transformation.Crossref | GoogleScholarGoogle Scholar |
Parry MAJ, Hawkesford MJ (2010) Genetic approaches to reduce greenhouse gas emissions: increasing carbon capture decreasing environmental impact. In ‘Climate change and crop production. Vol. 1’. (Ed. MP Reynolds) pp. 139–150. (CABI: Wallingford, UK)
Pinto RS, Reynolds MP, Mathews KL, McIntyre CL, Olivares-Villegas JJ, Chapman SC (2010) Heat and drought adaptive QTL in a wheat population designed to minimize confounding agronomic effects. Theoretical and Applied Genetics 121, 1001–1021.
| Heat and drought adaptive QTL in a wheat population designed to minimize confounding agronomic effects.Crossref | GoogleScholarGoogle Scholar |
Podlich D, Cooper M (1998) QU-GENE: a simulation platform for quantitative analysis of genetic models. Bioinformatics 14, 632–653.
| QU-GENE: a simulation platform for quantitative analysis of genetic models.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1cXmvV2jtr4%3D&md5=fb41b898f14d3d49d0ffcb5edfefd17dCAS |
Prasad PVV, Boote KJ, Allen LH (2006) Adverse high temperature effects on pollen viability, seed-set, seed yield and harvest index of grain-sorghum [Sorghum bicolor (L.) Moench] are more severe at elevated carbon dioxide due to higher tissue temperatures. Agricultural and Forest Meteorology 139, 237–251.
| Adverse high temperature effects on pollen viability, seed-set, seed yield and harvest index of grain-sorghum [Sorghum bicolor (L.) Moench] are more severe at elevated carbon dioxide due to higher tissue temperatures.Crossref | GoogleScholarGoogle Scholar |
Pretorius ZA, Singh RP, Wagoire WW, Payne TS (2000) Detection of virulence to wheat stem rust resistance gene Sr31 in Puccinia graminis f. sp. tritici in Uganda. Plant Disease 84, 203
| Detection of virulence to wheat stem rust resistance gene Sr31 in Puccinia graminis f. sp. tritici in Uganda.Crossref | GoogleScholarGoogle Scholar |
Reynolds MP (2010) ‘Climate change and crop production.’ (CABI: Wallingford, UK)
Reynolds MP, Borlaug NE (2006) Impacts of breeding on international collaborative wheat improvement. The Journal of Agricultural Science 144, 3–17.
| Impacts of breeding on international collaborative wheat improvement.Crossref | GoogleScholarGoogle Scholar |
Reynolds MP, Balota M, Delgado MIB, Amani I, Fischer RA (1994) Physiological and morphological traits associated with spring wheat yield under hot, irrigated conditions. Australian Journal of Plant Physiology 21, 717–730.
| Physiological and morphological traits associated with spring wheat yield under hot, irrigated conditions.Crossref | GoogleScholarGoogle Scholar |
Reynolds MP, Hays D, Chapman SC (2010a) Breeding for adaptation to heat and drought stress. In ‘Climate change and crop production. Vol. 1’. (Ed. MP Reynolds) pp. 71–91. (CABI: Wallingford, UK)
Reynolds NP, Martin JM, Giroux MJ (2010b) Increased wheat grain hardness conferred by novel puroindoline haplotypes from Aegilops tauschii. Crop Science 50, 1718–1727.
| Increased wheat grain hardness conferred by novel puroindoline haplotypes from Aegilops tauschii.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXht1Omur7K&md5=d063dd8597f9f5de7c487fead1dbeb3bCAS |
Sadras VO, Petrie PR (2011) Climate shifts in south-eastern Australia: early maturity of Chardonnay, Shiraz and Cabernet Sauvignon is associated with early onset rather than faster ripening. Australian Journal of Grape and Wine Research 17, 199–205.
| Climate shifts in south-eastern Australia: early maturity of Chardonnay, Shiraz and Cabernet Sauvignon is associated with early onset rather than faster ripening.Crossref | GoogleScholarGoogle Scholar |
Schenk U, Jager HJ, Weigel HJ (1997) The response of perennial ryegrass white clover mini-swards to elevated atmospheric CO2 concentrations: effects on yield and fodder quality. Grass and Forage Science 52, 232–241.
| The response of perennial ryegrass white clover mini-swards to elevated atmospheric CO2 concentrations: effects on yield and fodder quality.Crossref | GoogleScholarGoogle Scholar |
Seneweera S, Norton RM (2011) Plant responses to increased carbon dioxide. In ‘Crop adaptation to climate change’. (Eds SS Yadav, RJ Redden, JL Hatfield, H Lotze-Campen, AE Hall) pp. 198–217. (Wiley-Blackwell: Chichester, UK)
Singh G, Chapman SC, Jackson PA, Lawn RJ (2002) Lodging reduces sucrose accumulation of sugarcane in the wet and dry tropics. Australian Journal of Agricultural Research 53, 1183–1195.
| Lodging reduces sucrose accumulation of sugarcane in the wet and dry tropics.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38Xps1Kgs7Y%3D&md5=02dde8a7c43b3d71781ee783c49f4f34CAS |
Sirault XRR, James RA, Furbank RT (2009) A new screening method for osmotic component of salinity tolerance in cereals using infrared thermography. Functional Plant Biology 36, 970–977.
| A new screening method for osmotic component of salinity tolerance in cereals using infrared thermography.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXhtlOgs7rI&md5=aeadf9dff9cda019237aaaa7a73134fcCAS |
Stokes C, Howden M (2010) ‘Adapting agriculture to climate change: preparing Australian agriculture, forestry and fisheries for the future.’ viii + 286. (CABI)
Sullivan AL (2010) Grassland fire management in future climate. Advances in Agronomy 106, 173–208.
| Grassland fire management in future climate.Crossref | GoogleScholarGoogle Scholar |
Timbal B, Fernandez E, Li Z (2009) Generalization of a statistical downscaling model to provide local climate change projections for Australia. Environmental Modelling & Software 24, 341–358.
| Generalization of a statistical downscaling model to provide local climate change projections for Australia.Crossref | GoogleScholarGoogle Scholar |
Tran H, Salgado P, Lecomte P (2009) Species, climate and fertilizer effects on grass fibre and protein in tropical environments. The Journal of Agricultural Science 147, 555–568.
| Species, climate and fertilizer effects on grass fibre and protein in tropical environments.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXhtVGrsrjO&md5=cf644781ea3cbfff7312705e5c640325CAS |
Trethowan RM, Mahmood T (2011) Genetics options for improving the productivity of wheat in water-limited and temperature-stressed environments. In ‘Crop adaptation to climate change’. (Eds SS Yadav, RJ Redden, JL Hatfield, H Lotze-Campen, AE Hall) pp. 218–237. (Wiley-Blackwell: Chichester, UK)
Trethowan RM, Mujeeb-Kazi A (2008) Novel germplasm resources for improving environmental stress tolerance of hexaploid wheat. Crop Science 48, 1255–1265.
| Novel germplasm resources for improving environmental stress tolerance of hexaploid wheat.Crossref | GoogleScholarGoogle Scholar |
Tubiello FN, Amthor JS, Boote KJ, Donatelli M, Easterling W, Fischer G, Gifford RM, Howden M, Reilly J, Rosenzweig C (2007a) Crop response to elevated CO2 and world food supply – a comment on “Food for Thought..” by Long et al., Science 312: 1918–1921, 2006. European Journal of Agronomy 26, 215–223.
| Crop response to elevated CO2 and world food supply – a comment on “Food for Thought..” by Long et al., Science 312: 1918–1921, 2006.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXitl2hsrw%3D&md5=ba61967cfe74f4c84dddf30f225cfb0eCAS |
Tubiello FN, Soussana JF, Howden SM (2007b) Crop and pasture response to climate change. Proceedings of the National Academy of Sciences of the United States of America 104, 19 686–19 690.
| Crop and pasture response to climate change.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXitFSlsQ%3D%3D&md5=c7c9665422e58fa6138cd275caf60e3bCAS |
van Ittersum MK, Howden SM, Asseng S (2003) Sensitivity of productivity and deep drainage of wheat cropping systems in a Mediterranean environment to changes in CO2, temperature and precipitation. Agriculture, Ecosystems & Environment 97, 255–273.
| Sensitivity of productivity and deep drainage of wheat cropping systems in a Mediterranean environment to changes in CO2, temperature and precipitation.Crossref | GoogleScholarGoogle Scholar |
Wakelin SA, Gupta VVSR, Forrester ST (2010) Regional and local factors affecting diversity, abundance and activity of free-living, N2-fixing bacteria in Australian agricultural soils. Pedobiologia 53, 391–399.
| Regional and local factors affecting diversity, abundance and activity of free-living, N2-fixing bacteria in Australian agricultural soils.Crossref | GoogleScholarGoogle Scholar |
Wang JK, Chapman SC, Bonnett DG, Rebetzke GJ (2009) Simultaneous selection of major and minor genes: use of QTL to increase selection efficiency of coleoptile length of wheat (Triticum aestivum L.). Theoretical and Applied Genetics 119, 65–74.
| Simultaneous selection of major and minor genes: use of QTL to increase selection efficiency of coleoptile length of wheat (Triticum aestivum L.).Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXmslOktrg%3D&md5=dcf820331a18a92828a99aa8fd4bcf16CAS |
Wardlaw IF, Dawson IA, Munibi P, Fewster R (1989) The tolerance of wheat to high-temperatures during reproductive growth. 1. Survey procedures and general response patterns. Australian Journal of Agricultural Research 40, 1–13.
| The tolerance of wheat to high-temperatures during reproductive growth. 1. Survey procedures and general response patterns.Crossref | GoogleScholarGoogle Scholar |
Wassmann R, Jagadish SVK, Heuer S, Ismail A, Redona E, Serraj R, Singh RK, Howell G, Pathak H, Sumfleth K (2009) Climate change affecting rice production: the physiological and agronomic basis for possible adaptation strategies. Advances in Agronomy 101, 59–122.
| Climate change affecting rice production: the physiological and agronomic basis for possible adaptation strategies.Crossref | GoogleScholarGoogle Scholar |
Webb LB, Whetton PH, Bhend J, Darbyshire R, Briggs PR, Barlow EWR (2012) Earlier wine-grape ripening driven by climatic warming and drying and management practices. Nature Climate Change 2, 259–264.
| Earlier wine-grape ripening driven by climatic warming and drying and management practices.Crossref | GoogleScholarGoogle Scholar |
White B (2000) The importance of climate variability and seasonal forecasting to the Australian economy. Applications of Seasonal Climate Forecasting in Agricultural and Natural Ecosystems 21, 1–22.
White JW, Andrade-Sanchez P, Gore MA, Bronson KF, Coffelt TA, Conley MM, Feldmann KA, French AN, Heun JT, Hunsaker DJ, Jenks MA, Kimball BA, Roth RL, Strand RJ, Thorp KR, Wall GW, Wang G (2012) Field-based phenomics for plant genetics research. Field Crops Research 133, 101–112.
| Field-based phenomics for plant genetics research.Crossref | GoogleScholarGoogle Scholar |
Whitford R, Gilbert M, Langridge P (2010) Biotechnology in agriculture. In ‘Climate change & crop production. Vol. 1’. (Ed. MP Reynolds) pp. 219–244. (CABI: Wallingford, UK)
Yadav SS, Redden RJ, Hatfield JL, Lotze-Campen H, Hall AE (Eds) (2011) ‘Crop adaptation to climate change.’ (Wiley-Blackwell: Chichester, UK)
Zheng B, Chenu K, Dreccer MF, Chapman SC (2012) Breeding for the future: what are the potential impacts of future frost and heat events on sowing and flowering time requirements for Australian bread wheat (Triticum aestivium) varieties? Global Change Biology
| Breeding for the future: what are the potential impacts of future frost and heat events on sowing and flowering time requirements for Australian bread wheat (Triticum aestivium) varieties?Crossref | GoogleScholarGoogle Scholar | in press.
Zhou X, Harrington R, Woiwod I, Perry J, Bale J, Clark S (1995) Effects of temperature on aphid phenology. Global Change Biology 1, 303–313.
| Effects of temperature on aphid phenology.Crossref | GoogleScholarGoogle Scholar |
Ziska LH, Morris CF, Goins EW (2004) Quantitative and qualitative evaluation of selected wheat varieties released since 1903 to increasing atmospheric carbon dioxide: can yield sensitivity to carbon dioxide be a factor in wheat performance? Global Change Biology 10, 1810–1819.
| Quantitative and qualitative evaluation of selected wheat varieties released since 1903 to increasing atmospheric carbon dioxide: can yield sensitivity to carbon dioxide be a factor in wheat performance?Crossref | GoogleScholarGoogle Scholar |


