Ability of sulfur-oxidising bacteria to hasten degradation of ground rubber particles in soil for release of zinc as a fertiliser to correct deficiency in wheat
M. J. Asadollahzadeh A , A. H. Khoshgoftarmanesh A C and R. L. Chaney B
A C and R. L. Chaney B
A Department of Soil Sciences, College of Agriculture, Isfahan University of Technology, 84156-83111, Isfahan, Iran.
B Beltsville, MD 20705, USA.
C Corresponding author. Email: amirhkhosh@cc.iut.ac.ir
Crop and Pasture Science 70(1) 26-35 https://doi.org/10.1071/CP16316
Submitted: 26 August 2016 Accepted: 22 November 2018 Published: 15 January 2019
Abstract
Previous research has shown that ground rubber from tyres can be used to supply fertiliser zinc (Zn) for prevention of Zn-deficiency in crops, and that inoculation of the ground rubber with several bacterial species hastens the release of Zn to the soil. We evaluated the ability of several microbial combinations to speed the release of Zn from ground rubber and to decrease soil pH to favour phytoavailability of Zn to crops. In a batch experiment, treatment combinations of two rates of ground crumb rubber (nil or 300 mg kg–1, equal to 0 or 3.4 mg Zn kg–1) and 24 bacterial inoculants were incorporated into a Zn-deficient calcareous soil. In a pot experiment, two wheat cultivars were grown on the soil without or with ground rubber amendment or with equivalent Zn from ZnSO4 (15 mg kg–1) in combination with two selected microbial treatments. All microbial treatments significantly decreased soil pH at week 3, most notably the inoculant comprising Rhodococcus erythropolis and Acinetobacter calcoaceticus (RA) + Pseudomonas putida P41 (P1) + mixed Thiobacillus spp. (Mt). In the presence of tyre rubber, soil pH at week 10 was still significantly lower than the initial value, and soil DTPA-extractable Zn concentration increased until week 6 and then remained unchanged or slightly reduced at week 10. The greatest increase in DTPA-Zn concentration occurred with the RA inoculation. Microbial inoculation treatments were classified by cluster analysis into eight groups based on soil pH and concentrations of iron (Fe) and Zn. Group 8 produced the lowest pH and highest concentrations of DTPA-Fe ( average 6.92 mg kg–1) and DTPA-Zn (average 2.67 mg kg–1). Inoculations with RA and with RA + P1 + T. thioparus were the most effective in hastening an increase in DTPA-extractable Zn and significantly enhanced Zn uptake by wheat plants, whereas inoculations with P. putida P168 and with RA + P2 + Mt were most effective in decreasing soil pH and increasing plant Fe concentration.
Additional keywords: phytoavailable, rubber-biodegrading bacteria, tire rubber, Zn fertiliser.
Introduction
Accumulation of waste tyre rubber is a considerable environmental challenge in many countries (Adhikari et al. 2000; Gieré et al. 2004). These solid wastes contain sulfur, zinc oxide (ZnO), stearic acid, carbon black, proprietary additives and bead wires (Steudel and Steudel 2006). An emerging technology for recycling waste rubber is its use as an effective Zn fertilizer source with no risk of cadmium (Cd) contamination in agricultural lands (Chaney 2007; Taheri et al. 2011). Ground rubber contains 1–1.5% Zn, organic sulfur and the rubber matrix along with chemicals used to promote vulcanisation and protect the rubber against the environment.
Ground rubber added to Zn-deficient agricultural soils could provide an inexpensive and persistent source of Zn and sulfur as the particles are slowly biodegraded by soil microbes (Newman and Meneley 2006; Khoshgoftarmanesh et al. 2012). Taheri et al. (2011) observed that the addition of ground rubber increased DTPA-extractable soil Zn more than commercial fertiliser Zn and the control, but with a slow initial rate of Zn release to the soil because the Zn is within the cross-linked rubber particles (Taheri et al. 2011).
One reason for low efficiency of waste tyre rubber as a source of Zn for plants is the very slow release of Zn from the rubber matrix in alkaline soils. In acidic soils, Zn is released more rapidly and contributes to plant uptake of Zn (e.g. Groenevelt and Grunthal 1998). Rubber can be detoxified, devulcanised and degraded by various microorganisms. Rhodococcus erythropolis and Acinetobacter calcoaceticus DSMZ 590 are recognised as tyre-degrading bacteria. Inoculation with a combination of R. erythropolis + Escherichia coli + A. calcoaceticus was more effective at reducing soil pH and releasing Zn into the soil than inoculation with each of the bacteria alone (Khoshgoftarmanesh et al. 2012). Rhodococcus rhodochrous, Corynebacterium and Pseudomonas are able to break down 2-mercaptobenzothiazole (MBT) (Haroune et al. 2004), a toxic vulcanisation accelerator additive in rubber (Diepgen et al. 2006). Pseudomonas putida can degrade MBT and 2-methylthiobenzothiazole (MTBT) (El-Bassi et al. 2010) by using benzothiazole, MBT and MTBT as the sole source of carbon, nitrogen and sulfur when grown in minimum media (El-Bassi et al. 2010).
Sulfur-oxidising bacteria, specifically Thiobacillus spp., can oxidise disulfide linkages in rubber materials, and thereby devulcanise them (Panigrahi and Fung 2009; Jiang et al. 2010). Chritiansson et al. (1998) introduced T. ferrooxidans as the most efficient S-oxidising species in terms of released sulfate. Li et al. (2011) found that T. ferrooxidans was able to break sulfide bridges between polyisoprene chains and free up reactive sites where new crosslinks could form upon revulcanisation. Thiobacillus thioparus was more effective than T. ferrooxidans and T. thiooxidans at releasing sulfur from ground rubber, probably due to the fact that T. thioparus naturally grows at the neutral pH of rubber particles (Holst et al. 1998).
Although the ability of Thiobacillus spp. and P. putida as major rubber-degrading bacteria has been confirmed, no information is available on the effect of these bacteria on release of Zn and iron (Fe) into soil. Bacterial inoculation of ground rubber has been shown to be effective in hastening degradation of rubber and increasing DTPA-extractable Zn and Fe in calcareous soil (Khoshgoftarmanesh et al. 2012). Tyre rubber is not considered a source of Fe fertiliser but degradation of rubber in soil may decrease the soil pH and, consequently, result in higher solubility of soil Fe. In the present study, we extended our effort to identify microbial species that could hasten the release of rubber Zn for plant use. We evaluated the ability of P. putida and different Thiobacillus spp. alone or in combination with rubber-detoxifying bacteria (R. erythropolis and A. calcoaceticus DSMZ 590) to degrade ground rubber and release phytoavailable Zn into the soil.
Materials and methods
Experimental soil properties
Surface soil (0–30 cm) was collected for the experiment from the research field at the Agricultural Research Station of Rudasht, Isfahan province, Iran (32°29ʹN, 52°10ʹE; altitude 1507 m). Considering the critical deficiency level for DTPA-extractable soil Zn (1.0 mg kg–1) (Mortvedt 1985), soil was severely deficient in available Zn (see Table 1 for selected soil properties). Soil pH was measured in 1 : 2 soil–water suspension with a digital pH meter (Model 691; Metrohm AG, Herisau, Switzerland). Electrical conductivity (EC) was measured with an EC meter (Model 26 Ohm-644; Metrohm AG) in a soil saturation extract (Rhoades 1982). Organic matter content was determined by the Walkley–Black method (Nelson et al. 1996). The CaCO3 equivalent was determined by neutralising with HCl, and back-titration with NaOH (Black 1965). Available phosphorus content was determined by a colourimetric method, after extraction with 0.5 m NaHCO3 (Olsen and Sommers 1990). DTPA-extractable Zn, Fe, lead (Pb) and Cd were extracted by using the method of Lindsay and Norvell (1978) and then the elements were measured by flame atomic absorption spectrometry (AAS) with deuterium background correction.

|
Preparation and analysis of rubber particles
Samples of tyre rubber debris were obtained from the Yazd Tire, Yazd, Iran. After separating the metal wire, scrap tyres were reduced to shreds <10 cm by a slow-speed shredder. The shreds went through three, successively narrower blade shredders to reduce shreds further to <6 cm. The particles were processed to smaller sizes by grinding rolling mills and sieving them on a stainless-steel sieve (<0.3 mm screen). Samples of ground tyre rubber were obtained from Yazd Tire in Isfahan. For analysis, a subsample of sieved ground rubber was ashed in a furnace at 650°C for 6 h and the ash was extracted by using 5 mL 4 m HNO3. Concentrations of Zn, Fe, Pb and Cd in the solutions were determined by flame AAS (Table 2).
|
|
Preparation of bacterial inoculants
Thiobacillus thioparus PTCC 1668 and Acidithiobacillus ferrooxidans (basonym Thiobacillus ferrooxidans) PTCC 1647 were obtained as live cultures on agar slant, and Rhodococcus erythropolis PTCC 1767 and Acinetobacter calcoaceticus PTCC 1318 in the form of freeze-dried cultures, from the Persian Type Culture Collection (PTCC). Pseudomonas putida P41 and P168 were obtained in the form of freeze-dried cultures from Iranian Research Institute of Soil and Water.
Optimal pH and temperature for T. thioparus PTCC 1668 were reported as pH 9 and 30°C (Waksman 1922). Modified thiosulfate medium DSM 486 was used as the liquid medium. This medium contained nutrients (g L–1) KH2PO4 (2.0), K2HPO4 (2.0), NH4Cl (0.4), Na2CO3 (0.4), MgCl2·6H2O (0.2) and Na2S2O3·5H2O (5.0), plus vitamin and trace metals solutions (Abdehagh et al. 2011). Acidithiobacillus ferrooxidans PTCC 1647 was cultured at initial pH 1.6 in Fe(II)-based medium containing (g L–1) (NH4)2SO4 (3), KCl (0.1), MgSO4·7H2O (0.5), KH2PO4 (0.5) and FeSO4·7H2O (44.8) (Silverman and Lundgren 1959). FeSO4-free medium was autoclaved at 120°C for 20 min and the FeSO4 solution was separately sterilised by passage through a 0.2-μm filter and added aseptically to the Fe-free medium (Haghshenas et al. 2009). Rhodococcus erythropolis PTCC 1767, A. calcoaceticus PTCC 1318, and P. putida P41 and P168 were cultured in a liquid medium containing peptone and meat extract (Khadem Haghighat et al. 2003). A 0.4-mL volume of liquid medium (peptone 5 g L–1, meat extract 3 g L–1, deionised water 1 L) was added to each freeze-dried culture, and after homogenising, the suspensions were transferred to vials containing 20 mL liquid solution. The vials were incubated for 3–5 days at 25°C under dark conditions inside a shaker incubator. A 1-mL sample of each liquid medium was transferred to 200 mL liquid media and stored at the above mentioned conditions. Culture purity was checked by transferring a few drops of the suspensions to plates containing solid culture media (15 g L–1) and incubating them at the optimum temperature for growth of each bacterium.
Batch experiment
After preparing fresh bacterial inoculants, bacteria were collected by centrifugation at 5000g for 15 min at 10°C. The pelleted bacteria were then suspended in water and adjusted to ~5 × 107 cells mL–1 before mixing with the ground rubber. Inoculants (1 mL) were added to the ground rubber (300 mg kg–1, equal to 3.4 mg Zn kg–1) before mixing with the soil. In addition, a set of soil samples received inoculant treatments without ground rubber (0 mg Zn kg–1). The 24 bacterial inoculation treatments used are listed in Table 3. The soils were incubated at 25°C, with their moisture kept at 70% field capacity using deionised water. Subsamples of soil were collected from each treatment at 1, 3, 6 and 10 weeks after inoculation to investigate changes in soil pH and available concentrations of Fe and Zn over time. Soil pH and DTPA-extractable Zn, Fe, Pb and Cd were measured by flame AAS as described. The serial-dilution agar-plating technique was used at 3 weeks to enumerate the survival of bacterial cells (Cappuccino and Sherman 2001).
The experiment was set up in a quadruplicate, randomised, complete block design with a split-plot arrangement. The main plot was Zn treatment and the subplot was microbial inoculation treatment. Analysis of variance was conducted using the PROC GLM procedure of SAS version 8 (SAS Institute, Cary, NC, USA). Means separation was performed by Fisher’s protected least significant difference (l.s.d.) method at a significant level of P = 0.05.
Cluster analyses were performed for each soil parameter (soil pH, DTPA-extractable Zn and Fe) and for all three parameters combined at week 3 by using the Euclidean distances between treatments, as described by Johnson and Wichern (2002). The results of the analysis for each parameter were used to present average results over time for groups of treatments, and the results of the hierarchical clustering procedure for the three parameters combined were displayed graphically with a tree diagram.
Pot experiment with wheat
Based on the results of the batch experiment (inoculations with R. erythropolis and A. calcoaceticus (RA) and with RA + P. putida (P1) + T. thioparus (Tt) had the greatest effect on soil pH and available concentrations of Fe and Zn), these microbial treatments were chosen for the pot experiment. A bulk soil sample (the same soil used in the batch experiment) was air-dried, thoroughly mixed, sieved to <8 mm, and sterilised by autoclaving at 121°C for 1 h (Trevors 1996). Soil was mixed with either ground rubber (300 mg kg–1, applied as <0.3-mm-diameter particles) or ZnSO4·7H2O (15 mg kg–1), each equal to 3.4 mg Zn kg–1. The Zn amendments were inoculated with RA or RA + P1 + Tt, or were uninoculated. In addition, a set of soil samples received the inoculant treatments without Zn amendment. Treatments were thoroughly mixed with the soil and incubated moist (near water-holding capacity) at 25°C for about 1 week before seeding.
Homogenised soil samples weighing 800 g were placed in polyethylene pots. Fertiliser was applied based on recommended rates (Milani et al. 1998) and mixed thoroughly with soil before planting. Triple superphosphate was applied at 15 kg phosphorus ha–1 and urea at 200 kg nitrogen ha–1. Potassium (K) and Fe were applied as K2SO4 and Fe-EDTA at 100 kg K and 20 kg Fe ha–1. Pots were maintained near water-holding capacity with frequent watering to weight.
Seeds of a Zn-efficient bread wheat cultivar (Triticum aestivum cv. Roshan) and a Zn-inefficient durum wheat cultivar (Triticum turgidum subsp. durum cv. Arya) were surface-sterilised with 70% ethanol (v/v) for 30 s followed by 6% sodium hypochlorite for 15 min. They were then rinsed thoroughly with sterile water and germinated in quartz sand moistened with distilled water. These cultivars have widespread use in Iran. Wheat seedlings were then transferred to the pots of prepared soil and grown for 40 days. At harvest, shoots were cut at 2 cm above the soil surface and weighed; roots were removed and washed in tap water before weighing. Dry masses were recorded after drying root and shoot samples in an oven for 48 h at 70°C.
Dried plant samples (~0.50 g) digested in an APCU-40 75-mL TFM Teflon microwave vessel (START D microwave digestion system; Milestone Srl, Sorisole, Italy), using 5 mL HNO3 and 3 mL H2O2, and then filtered through Whatman No. 42 filter paper, transferred to 50-mL volumetric flasks, and diluted with deionised, distilled water. Elements were analysed by using flame AAS with deuterium background correction.
The experiment was set up as a quadruplicate, completely randomised design with a factorial arrangement. All data were subjected to analysis of variance and means were compared using Fisher’s protected l.s.d. (P < 0.05).
Results
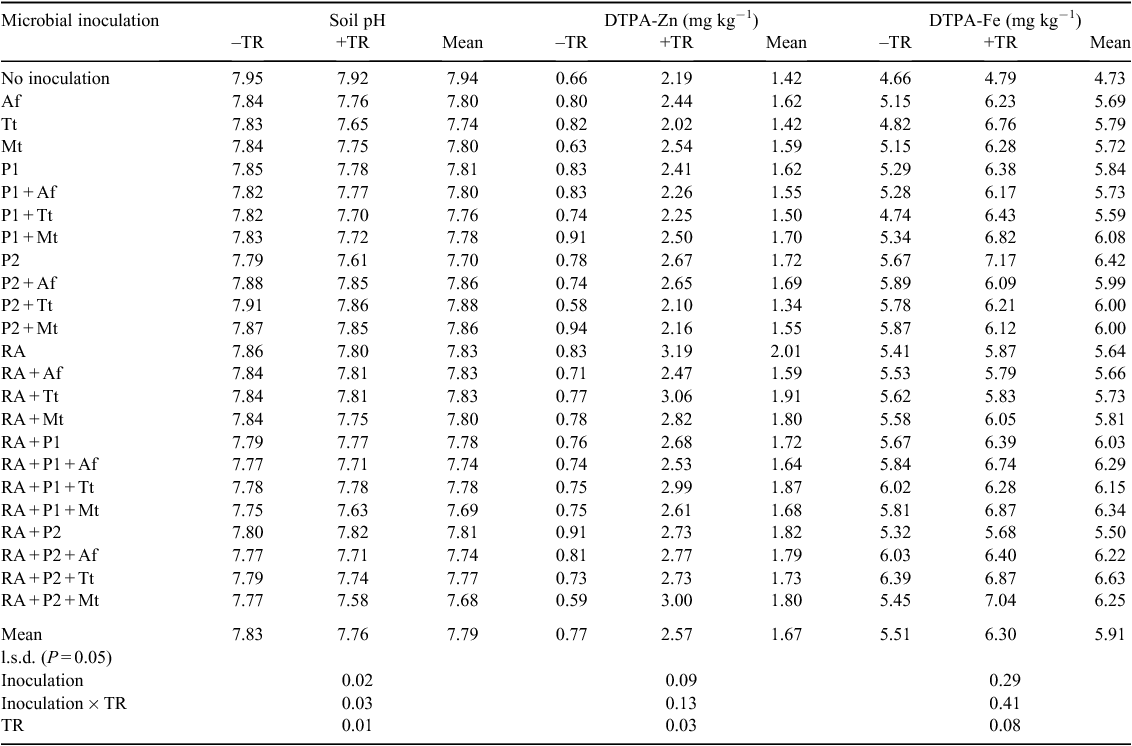
Changes in soil pH and DTPA-extractable Zn and Fe concentrations over time as affected by incorporated ground rubber with various microbial inoculants
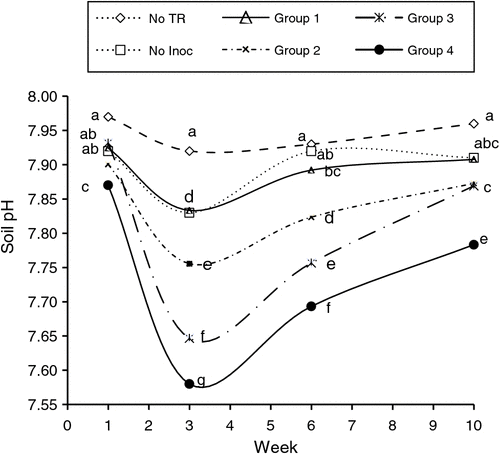
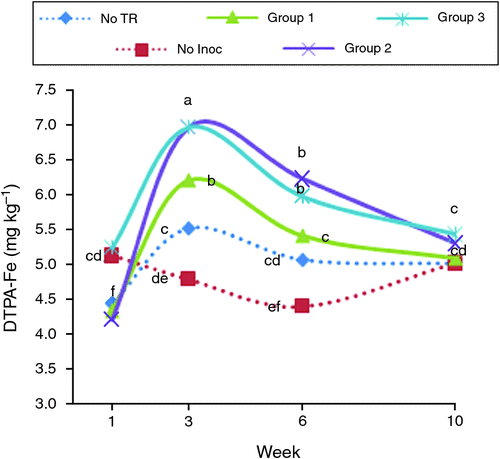
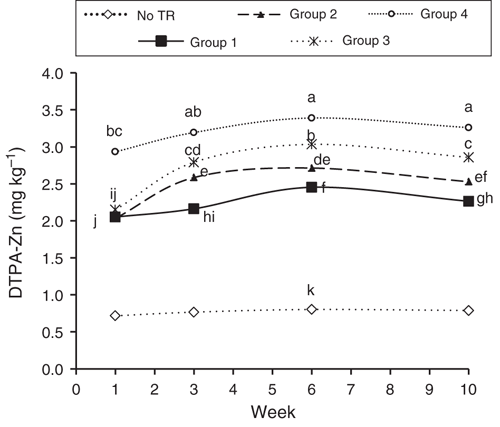
For each soil parameter, a cluster analysis was used to classify microbial treatments into different groups based on their effects; the average effects of individual microbial treatment groups on soil pH and DTPA-extractable concentrations of Fe and Zn over time are presented in Figs 1–3.
|
|
|
All microbial inoculation groups significantly reduced soil pH at week 3, although the amount varied among the inoculants (Fig. 1). The greatest reduction in soil pH was found for the RA + P1 + Mt treatment (Group 4, Fig. 1). Group 1 had the smallest effect on soil pH, followed by Groups 2 and 3. Application of tyre rubber resulted in a decrease in soil pH that varied depending on microbial treatment and exposure time; with application of ground rubber, soil pH at week 10 remained significantly lower than initial soil pH. In the inoculant-free tyre-rubber treatment, soil pH slightly decreased at week 3 and then remained unchanged until week 10.
All microbial inoculation treatments resulted in a significant increase in the DTPA-extractable Fe concentration at week 3 compared with no inoculation (Fig. 2). In all inoculated tyre-rubber treatments, DTPA-Fe concentrations increased up to week 3 and thereafter decreased, although they were still higher than their initial concentrations. RA + P2 + Mt and RA + P2 + Tt (from Groups 3 and 1 respectively,Fig. 2) were the most effective inoculation treatments for increasing the availability of Fe in rubber-treated soil.
In the presence of ground rubber, soil DTPA-extractable Zn concentration increased until week 6, then remained unchanged or slightly reduced at week 10 (Fig. 3). The greatest increase in DTPA-Zn concentration was found at the presence of RA microbial inoculation treatment (Group 4, Fig. 3).
Effect of ground tyre rubber and microbial inoculation on soil pH and DTPA-extractable Zn and Fe at week 3
From Figs 1–3, the maximum changes in pH and DTPA-extractable Zn and Fe resulting from the rubber and microbial inoculation treatments were observed at week 3. Therefore, data for that time-point are presented in detail; they show significant variation among the 24 microbial treatments in soil pH and concentrations of DTPA-extractable Zn and Fe (Table 3). For all microbial inoculation treatments, application of ground rubber resulted in greater reduction in pH and increase in DTPA-Zn and -Fe than without ground rubber. At week 3, the highest concentration of Fe and the lowest pH were found in soil treated with rubber inoculated with P2, RA + P2 + Mt and RA + P1 + Mt. Inoculation of ground rubber with all microbial inoculants except Tt increased DTPA-extractable Zn compared with the non-inoculated treatment, although this increase was smaller for the P2 + Tt and P2 +Mt treatments.
Cluster analysis
Hierarchical cluster analysis classified microbial inoculation treatments into eight groups based on soil pH and concentrations of DTPA-extractable Fe and Zn (Fig. 4). Significant differences were found among the groups (Table 4). Group 2 comprised the non-inoculated rubber treatment and had the highest pH (average 7.92) and the lowest DTPA-extractable Fe (on average 4.97 mg kg–1). Group 5 had the highest DTPA-Zn (average 3.13 mg kg–1) and Group 7 the lowest (average 2.02 mg kg–1), except for Group 1 (no tyre rubber, 0.77 mg kg–1). Microbial treatments classified into Group 8 produced the highest DTPA-Fe (on average 6.92 mg/kg) and high DTPA-Zn (on average 2.67 mg kg–1). Therefore, this group of microbial treatments was selected as the most effective for degradation of rubber and release of Zn and Fe into the soil.
Effect of ground rubber and microbial treatments on wheat yield
Incorporation of ground rubber into the Zn-deficient soil resulted in significant increase in shoot yield of bread wheat (on average 12%) and durum wheat (on average 22%) cultivars compared with the control treatment (Fig. 5). The effectiveness of rubber in increasing wheat shoot dry mass was similar to commercial ZnSO4 (except with RA microbial treatment for durum wheat, where rubber tended to be more effective). Microbial inoculation of ground rubber in general resulted in significant increases in shoot growth of both wheat cultivars; no significant difference was found between the RA and RA + P1 + Tt treatments.
Effects of ground rubber and microbial inoculation with on plant shoot element concentration
Without inoculation, shoot Zn of durum wheat was not significantly affected by ground tyre rubber or ZnSO4 fertiliser application treatments (Fig. 6). Without inoculation, shoot Zn of bread wheat tended to be increased by ground tyre rubber and was significantly increased ZnSO4. Application of inoculated ground tyre rubber caused a significant increase in shoot Zn concentration in both wheat cultivars. Wheat plants in the RA-inoculated ground rubber treatment accumulated higher shoot Zn concentration than those grown in the other treatments.
Cadmium and Pb concentrations in shoots of both wheat cultivars grown in all unamended and rubber-amended soils were below the detection limit of AAS (<0.02 mg kg–1 dry shoot).
Discussion
Application of 300 mg kg–1 of ground rubber without microbial inoculation had little effect in reducing soil pH. This agrees with the findings of Brown and Chaney (2000), who found that ground rubber in calcareous soil resulted in no or small changes in soil pH. By contrast, application of microbially inoculated ground rubber significantly reduced soil pH, the decrease depending on microbial treatment and inoculation time. High buffer capacity of the studied highly calcareous soil inhibited large and stable changes in soil pH.
Possible reasons for pH reduction in soil treated with microbially inoculated rubber are the release of sulfuric acid following oxidation of sulfur, and production of CO2 by microorganism respiration. Chritiansson et al. (1998) reported that T. ferrooxidans and T. thioparus are able to break S–S bonds at the surface of rubber particles. Li et al. (2011) found that T. ferrooxidans separated from the soil could break S–S bonds and thereby modify the chemical structure of rubber. Jiang et al. (2010) also found that T. thioparus oxidised C–S and S–S bonds and increased the O : S ratio of the rubber. In another study, inoculation with a combination of R. erythropolis + E. coli + A. calcoaceticus was reported to be more effective in reducing soil pH than inoculation with each bacterium alone or non-inoculation treatment (Khoshgoftarmanesh et al. 2012).
Application of non-inoculated tyre rubber had little effect in increasing soil DTPA-Fe. By contrast, Taheri et al. (2011) and Groenevelt and Grunthal (1998) reported significant increases in soil-available Fe by addition of ground tyre rubber. This variation in response might be due to different properties of the experimental soils and rubber studied. The amount of CaCO3 in the soil used in the present study was greater than in soils of the previous studies. In addition, the ground rubber applied in our experiment was of larger size and lower Fe content (75.6 mg kg–1) than the rubber applied in the previous experiments (108 mg kg–1). Increasing rubber size decreases the rate of degradation of rubber and its potential effect on soil pH (Taheri et al. 2011). Application of microbially inoculated ground rubber significantly increased soil available Fe. In general, an increase in the DTPA-Fe was associated with a reduction in soil pH. Each unit reduction in soil pH increases Fe solubility 1000-fold (Lucena 2000). Therefore, small decreases in pH of rubber-treated soils can effectively increase availability of Fe for plants (Lindsay and Norvell 1978). This should be confirmed in further experiments because we are unsure whether this is relevant to changes in DTPA-Fe after incubation with ground rubber. Any reduction of soil Fe(III) and re-precipitation would give higher DTPA extractability. Air drying a soil increases DTPA extractability of Fe and manganese (Mn) through chemical reaction of dissolved organic matter with Fe- and Mn-oxides during the drying process (Lucena 2000).
In the present study, inoculations with P. putida alone (P2) or in combination with R. erythropolis and A. calcoaceticus (RA + P2 + Mt and RA + P2 + Tt treatments) were more effective than the other microbial treatments in enhancing soil DTPA-Fe. One possible reason for the higher efficiency of P. putida with respect to increasing soil Fe solubility is its ability to release Fe-chelating siderophores (Alipour and Sobhanipour 2012). These microbial siderophores can form stable complexes with Fe and can increase solubility of this metal nutrient in soil (Alipour and Sobhanipour 2012).
As expected from previous results, addition of ground rubber, regardless of microbial-inoculation treatment, increased DTPA-Zn concentration. Ground rubber contains ~1% Zn in the form of ZnO. In soils treated with ground rubber, DTPA-Zn concentration was higher than the critical Zn level (0.5 mg kg–1) for wheat (Cakmak et al. 1996). The release of Zn from tyre rubber particles (<0.1 mm) into the soil is very slow, such that only 10–40% of rubber Zn was released into the soil after 1 year (Smolders and Degryse 2002). Microbial inoculation hastened the release of Zn from ground rubber into the soil plant-available pool. In the present study, at week 3, microbial inoculation of rubber increased soil DTPA-extractable Zn 4-fold compared with the non-inoculated rubber treatment. Inoculation of tyre rubber with RA (R. erythropolis and A. calcoaceticus) was more effective than with the other microbial treatments in increasing soil DTPA-Zn. Ability of A. calcoaceticus to degrade rubber has been reported by Bode et al. (2001) and Chengalroyen and Dabbs (2013). The Thiobacillus species were less effective than the other microbial inoculation treatments in degrading tyre rubber and increasing of DTPA-Zn in soil; however, these species in combination with rubber-degrading bacteria (R. erythropolis and A. calcoaceticus) significantly enhanced soil DTPA-Zn. It appears that Thiobacillus species are sensitive to toxic compounds released from tyre-rubber particles. Toxic chemicals in the tyre rubber, such as zinc oxides and some vulcanising chemicals, slow sulfur-oxidising and rubber-degrading bacteria from naturally breaking it down. Specific environmental conditions are necessary for effective microbial devulcanisation of tyre rubber. Therefore, tyre-rubber-detoxifying bacteria (i.e. R. erythropolis and A. calcoaceticus) can produce better conditions for Thiobacillus. Nowaczyk and Domka (1999) reported that toxic compounds released during degradation of tyre rubber by T. ferrooxidans have detrimental effects on growth of this bacterium. Rhodococcus erythropolis and A. calcoaceticus are able to break down or bio-transform MBT and thereby produce better conditions for activity of other microorganisms. MBT is a well-known toxic additive (Diepgen et al. 2006) used as a vulcanisation accelerator in rubber. Metabolites produced during MBT biodegradation are much less toxic (Haroune et al. 2004). El-Bassi et al. (2010) reported that P. putida strain HKT554 is able to break down toxic compounds in tyre rubber such as MBT and MTBT. Our results also showed that the presence of detoxifying bacteria enhanced the efficacy of sulfur-oxidising bacteria in the desulfurisation of tyre rubber.
Cluster analysis of microbial treatments based on their effects on soil pH and DTPA-extractable concentrations of Fe and Zn at week 3 showed that RA and RA + Tt were more effective than the other treatments in enhancing Zn release from the tyre rubber, whereas P2 and RA + P2 + Mt were most effective in reducing soil pH and enhancing Fe concentration in soil treated with tyre rubber. In the pot experiment, addition of 3 mg Zn kg–1 in the form of ground tyre rubber inoculated with RA or RA + P1 + Tt increased shoot dry mass and Zn concentration in wheat relative to the control and in some instances, relative to commercial ZnSO4. Inoculation of tyre rubber has been reported to be effective in increasing availability of Zn in soil and its uptake by maize (Zea mays) (Khoshgoftarmanesh et al. 2012). In the present study, the RA treatment was more effective than RA + P1 + Tt in enhancing shoot Zn concentration in wheat grown in rubber-treated soil, although no difference in shoot dry mass of wheat was found between these treatments.
Cadmium and Pb concentrations in shoots of both wheat cultivars were below the detection limit of AAS. This result that is in agreement with our previous findings (Khoshgoftarmanesh et al. 2012; Taheri et al. 2011), showing that Pb and Cd should not be an issue for using ground rubber as a Zn fertiliser, in strong contrast to several industrial by-products sold as Zn fertilisers that contained high levels of Cd (Mortvedt 1985).
Conclusions
Experiments showed that inoculation of ground rubber with detoxifying bacteria enhanced the efficacy of sulfur-oxidising bacteria in desulfurisation of tyre rubber and their effect on soil pH and plant-available Zn and Fe concentrations. Cluster analysis of microbial treatments based on their effects on soil pH and DTPA-Fe and -Zn at week 3 showed that RA (R. erythropolis PTCC 1767 and A. calcoaceticus PTCC 1318) and RA + P1 (P. putida P41) +Tt (T. thioparus PTCC 1668) were more effective than the other microbial treatments in enhancing Zn release from the tyre rubber, whereas P2 (P. putida P168) and RA + P2 + Mt (mix of Thiobacillus spp.) were the most effective treatments in reducing soil pH and enhancing Fe concentration in soil treated with ground rubber. In the pot experiment, addition of ground tyre rubber (300 mg kg–1) inoculated with RA and RA + P1 + Tt increased shoot dry mass and Zn concentration in wheat compared with the control (no added Zn) and, in some cases, commercial ZnSO4 treatment. The proper environmental conditions for detoxifying bacteria as well as for sulfur-oxidising bacteria could be better defined by further research works.
Conflicts of interest
No potential conflicts of interest is reported by the authors.
Acknowledgement
This research did not receive any specific funding.
References
Abdehagh N, Namini MT, Heydarian SM, Bonakdarpour B, Zare D (2011) Performance of a biotrickling filter employing Thiobacillus thioparus immobilized on polyurethane foam for hydrogen sulfide removal. Iranian Journal of Environnent and Heath Science Engineering 8, 245–254.Adhikari B, De D, Maiti S (2000) Reclamation and recycling of waste rubber. Progress in Polymer Science 25, 909–948.
| Reclamation and recycling of waste rubber.Crossref | GoogleScholarGoogle Scholar |
Alipour ZT, Sobhanipour A (2012) The effect of Thiobacillus and Pseudomonas fluorescent inoculation on maize growth and Fe uptake. Annals of Biological Research 3, 1661–1666.
Black CA (1965) ‘Methods of soil analysis.’ (American Society of Agronomy: Madison, WI, USA)
Bode HB, Kerkhoff K, Jendrossek D (2001) Bacterial degradation of natural and synthetic rubber. Biomacromolecules 2, 295–303.
| Bacterial degradation of natural and synthetic rubber.Crossref | GoogleScholarGoogle Scholar | 11749186PubMed |
Brown SL, Chaney RL (2000) Combining by-products to achieve specific soil amendment objectives. In ‘Land application of agricultural, industrial and municipal by-products’. SSSA Book Series No. 6. (Eds JF Power et al.) pp. 343–360. (Soil Science Society of America: Madison, WI, USA)
Cakmak I, Yilmaz A, Kalayci M, Ekiz H, Torun B, Erenoğlu B, Braun H (1996) Zinc deficiency as a critical problem in wheat production in Central Anatolia. Plant and Soil 180, 165–172.
| Zinc deficiency as a critical problem in wheat production in Central Anatolia.Crossref | GoogleScholarGoogle Scholar |
Cappuccino J, Sherman N (2001) Serial dilution-agar plate procedure to quantitate viable cells. In ‘Microbiology laboratory manual’. pp. 119–124. (Pearson Education: London)
Chaney RL (2007) Effect of ground rubber vs. ZnSO4 on spinach accumulation of Cd from Cd-mineralized California soil. In ‘Proceedings WEFTEC Residuals Conference’. Denver, CO. (Abstract). p. 993. (Water Environment Federation: Alexandria, VA, USA)
Chengalroyen MD, Dabbs E (2013) The biodegradation of latex rubber: a mini review. Journal of Polymers and the Environment 21, 874–880.
| The biodegradation of latex rubber: a mini review.Crossref | GoogleScholarGoogle Scholar |
Chritiansson M, Stenberg B, Wallenberg L, Holst O (1998) Reduction of surface sulphur upon microbial devulcanization of rubber materials. Biotechnology Letters 20, 637–642.
| Reduction of surface sulphur upon microbial devulcanization of rubber materials.Crossref | GoogleScholarGoogle Scholar |
Diepgen TL, Bruynzeel DP, Andersen KE, Brandao FM, Bruze M, Goncalo M, Goossens A, Lahti A, Mahler V, Menne T, White IR, Wilkinson D (2006) Mercaptobenzothiazole or the mercapto-mix: which should be in the standard series? Contact Dermatitis 55, 36–38.
| Mercaptobenzothiazole or the mercapto-mix: which should be in the standard series?Crossref | GoogleScholarGoogle Scholar | 16842552PubMed |
El-Bassi L, Iwasaki H, Oku H, Shinzato N, Matsui T (2010) Biotransformation of benzothiazole derivatives by the Pseudomonas putida strain HKT554. Chemosphere 81, 109–113.
| Biotransformation of benzothiazole derivatives by the Pseudomonas putida strain HKT554.Crossref | GoogleScholarGoogle Scholar | 20692014PubMed |
Gieré R, LaFree ST, Carleton LE, Tishmack JK (2004) Environmental impact of energy recovery from waste tyres. Geological Society of London, Special Publications 236, 475–498.
| Environmental impact of energy recovery from waste tyres.Crossref | GoogleScholarGoogle Scholar |
Groenevelt P, Grunthal P (1998) Utilisation of crumb rubber as a soil amendment for sports turf. Soil & Tillage Research 47, 169–172.
| Utilisation of crumb rubber as a soil amendment for sports turf.Crossref | GoogleScholarGoogle Scholar |
Haghshenas DF, Alamdari EK, Bonakdarpour B, Darvishi D, Nasernejad B (2009) Kinetics of sphalerite bioleaching by Acidithiobacillus ferrooxidans. Hydrometallurgy 99, 202–208.
| Kinetics of sphalerite bioleaching by Acidithiobacillus ferrooxidans.Crossref | GoogleScholarGoogle Scholar |
Haroune N, Combourieu B, Besse P, Sancelme M, Kloepfer A, Reemtsma T, De Wever H, Delort A-M (2004) Metabolism of 2-mercaptobenzothiazole by Rhodococcus rhodochrous. Applied and Environmental Microbiology 70, 6315–6319.
| Metabolism of 2-mercaptobenzothiazole by Rhodococcus rhodochrous.Crossref | GoogleScholarGoogle Scholar | 15466583PubMed |
Holst O, Stenberg B, Christiansson M (1998) Biotechnological possibilities for waste tyre-rubber treatment. Biodegradation 9, 301–310.
| Biotechnological possibilities for waste tyre-rubber treatment.Crossref | GoogleScholarGoogle Scholar | 10022073PubMed |
Jiang G, Zhao S, Luo J, Wang Y, Zhou Q (2010) Devulcanization effect of natural rubber crumb by Thiobacillus thioparus. China Synthetic Rubber Industry 33, 449–453.
Johnson R, Wichern D (2002) ‘Applied multivariate statistical analysis.’ 5th edn (Prentice Hall: New York)
Khadem Haghighat F, Mazaheri M, Eftekhar F (2003) Isolation of a dibenzothiophene desulfurizing bacterium from soil of Tabriz oil refinery. Iranian Journal of Biotechnology 1, 121–124.
Khoshgoftarmanesh AH, Behzadan HZ, SanaeiOstovar A, Chaney RL (2012) Bacterial inoculation speeds zinc release from ground tire rubber used as Zn fertilizer for corn and sunflower in a calcareous soil. Plant and Soil 361, 71–81.
| Bacterial inoculation speeds zinc release from ground tire rubber used as Zn fertilizer for corn and sunflower in a calcareous soil.Crossref | GoogleScholarGoogle Scholar |
Li Y, Zhao S, Wang Y (2011) Microbial desulfurization of ground tire rubber by Thiobacillus ferrooxidans. Polymer Degradation & Stability 96, 1662–1668.
| Microbial desulfurization of ground tire rubber by Thiobacillus ferrooxidans.Crossref | GoogleScholarGoogle Scholar |
Lindsay WL, Norvell WA (1978) Development of a DTPA soil test for zinc, iron, manganese, and copper. Soil Science Society of America Journal 42, 421–428.
| Development of a DTPA soil test for zinc, iron, manganese, and copper.Crossref | GoogleScholarGoogle Scholar |
Lucena JJ (2000) Effects of bicarbonate, nitrate and other environmental factors on iron deficiency chlorosis. A review. Journal of Plant Nutrition 23, 1591–1606.
| Effects of bicarbonate, nitrate and other environmental factors on iron deficiency chlorosis. A review.Crossref | GoogleScholarGoogle Scholar |
Milani P, Malakouti M, Khademi Z, Balali M, Mashayekhi M (1998) ‘A fertilizer recommendation model for the wheat field of Iran.’ (Soil Water Research Institute: Tehran)
Mortvedt JJ (1985) Plant uptake of heavy metals in zinc fertilizers made from industrial by-products. Journal of Environmental Quality 14, 424–427.
| Plant uptake of heavy metals in zinc fertilizers made from industrial by-products.Crossref | GoogleScholarGoogle Scholar |
Nelson DW, Sommers LE, Sparks D, Page A, Helmke P, Loeppert R, Soltanpour P, Tabatabai M, Johnston C, Sumner M (1996) Total carbon, organic carbon, and organic matter. Methods of Soil Analysis 961–1010.
Newman SE, Meneley JC (2006) Adaptation of waste tire rubber for green house media and zinc fertilizer. Final Report. Colorado School of Mines, Golden, CO, USA.
Nowaczyk K, Domka F (1999) Attempts at microbiological utilization of rubber wastes. Polish Journal of Environmental Studies 8, 101–106.
Olsen SR, Sommers LE (1990) Phosphorus. In ‘Methods of soil analysis. Part 2. Chemical and microbiological properties’. Agronomy Monograph No. 9. (Eds AL Page et al.) pp. 403–429. (Soil Science Society America: Madison, WI, USA)
Panigrahi S, Fung J (2009) Development of rubber and agricultural fiber based biocomposite for industrial application. International Journal of Vehicle Information and Communication Systems 1, 284–292.
Rhoades JD (1982) Soluble salts. In ‘Methods of soil analysis. Part 2. Chemical and microbiological properties’. Agronomy Monograph No. 9. 2nd edn (Eds AL Page et al.) pp. 167–179. (Soil Science Society of America: Madison, WI, USA)
Silverman MP, Lundgren DG (1959) Studies on the chemoautotrophic iron bacterium Ferrobacillus ferrooxidans: I. An improved medium and a harvesting procedure for securing high cell yields. Journal of Bacteriology 77, 642–647.
Smolders E, Degryse F (2002) Fate and effect of zinc from tire debris in soil. Environmental Science & Technology 36, 3706–3710.
| Fate and effect of zinc from tire debris in soil.Crossref | GoogleScholarGoogle Scholar |
Steudel R, Steudel Y (2006) Interaction of zinc oxide clusters with molecules related to the sulfur vulcanization of polyolefins (“rubber”). Chemistry 12, 8589–8602.
| Interaction of zinc oxide clusters with molecules related to the sulfur vulcanization of polyolefins (“rubber”).Crossref | GoogleScholarGoogle Scholar | 16953504PubMed |
Taheri S, Khoshgoftarmanesh AH, Shariatmadari H, Chaney RL (2011) Kinetics of zinc release from ground tire rubber and rubber ash in a calcareous soil as alternatives to Zn fertilizers. Plant and Soil 341, 89–97.
| Kinetics of zinc release from ground tire rubber and rubber ash in a calcareous soil as alternatives to Zn fertilizers.Crossref | GoogleScholarGoogle Scholar |
Trevors J (1996) Sterilization and inhibition of microbial activity in soil. Journal of Microbiological Methods 26, 53–59.
| Sterilization and inhibition of microbial activity in soil.Crossref | GoogleScholarGoogle Scholar |
Waksman SA (1922) Microorganisms concerned in the oxidation of sulfur in the soil: III. Media used for the isolation of sulfur bacteria from the soil. Soil Science 13, 329–336.
| Microorganisms concerned in the oxidation of sulfur in the soil: III. Media used for the isolation of sulfur bacteria from the soil.Crossref | GoogleScholarGoogle Scholar |