Alleviation of field water stress in wheat cultivars by using silicon and salicylic acid applied separately or in combination
Kobra Maghsoudi A E , Yahya Emam B , Muhammad Ashraf C and Mohammad Javad Arvin D
A E , Yahya Emam B , Muhammad Ashraf C and Mohammad Javad Arvin D
A University of Advanced Technology, Kerman, Iran.
B Department of Crop Production and Plant Breeding, College of Agriculture, Shiraz University, Shiraz, Iran.
C Institute of Molecular Biology and Biotechnology, The University of Lahore, Lahore, Pakistan.
D Department of Horticulture, College of Agriculture, Shahid Bahonar University, Kerman, Iran.
E Corresponding author. Email: k_maghsoudi1982@yahoo.com
Crop and Pasture Science 70(1) 36-43 https://doi.org/10.1071/CP18213
Submitted: 15 July 2018 Accepted: 9 December 2018 Published: 15 January 2019
Abstract
The role of exogenous individual or combined application of silicon (Si) and salicylic acid (SA) (control, 6 mm Si, 1 mm SA, and 6 mm Si + 1 mm SA) on grain yield and some key physiological characteristics of wheat (Triticum aestivum L.) cvv. Shiraz (drought-sensitive) and Sirvan (drought-tolerant) was investigated under field water-stress conditions (100% and 40% field capacity). Drought stress caused a considerable reduction in biological yield, yield and yield components, relative water content and leaf water potential of both cultivars. Application of Si and SA effectively improved these parameters in water-deficit treatments. Moreover, water-limited conditions markedly promoted the activities of key antioxidant enzymes including peroxidase, ascorbate peroxidase, catalase and superoxide dismutase as well as the levels of malondialdehyde (MDA) and hydrogen peroxide (H2O2), while enhancing the accumulation of soluble sugars, potassium, magnesium and calcium in leaf tissues. Application of Si and SA further enhanced the activities of the key antioxidant enzymes and accumulation of osmolytes, and decreased the levels of H2O2 and MDA in drought-stressed plants; the positive effects of Si were greatest when it was applied with SA. Synergistic effects of Si + SA application on yield and physiological parameters were apparent compared with Si or SA applied separately. Water-stress alleviation and yield improvement in the wheat cultivars by Si and SA application was attributable to partly improved osmotic adjustment and antioxidant activity as well as to more favourable water status under stress conditions. Overall, Si and SA application proved to have great potential in promoting grain yield of wheat in drought-prone areas.
Additional keywords: membrane stability, osmoregulation, osmotic stress, plant growth regulator.
Introduction
Wheat (Triticum aestivum L.) is a vital food crop around the world, but often its yield potential cannot be achieved because of the presence of environmental stresses such as heat, salinity and drought (Emam 2011). Although all abiotic stresses adversely affect the wheat growth and production, water scarcity imposes the most severe effects on this crop (González et al. 2010). Water scarcity adversely affects all phases of growth, most strikingly noted at the reproductive phase and grain filling, leading to fewer grains and smaller grain size in cereal crops including wheat. Impairment of assimilate partitioning and of activities of vital enzymes taking part in the synthetic processes of key carbohydrates including starch and sucrose reduces grain filling (Guttieri et al. 2001; Erocli et al. 2007). Drought stress is also believed to affect the uptake, transport and accumulation of key inorganic nutrients in plants (Sinclair 2011).
Silicon (Si) occurs abundantly in soils, but it is not as important as other known inorganic elements such as nitrogen (N), potassium (K), phosphorus (P), calcium (Ca) and magnesium (Mg) for plant growth (Ashraf et al. 2009; Zhang et al. 2017). However, Si is believed to play a beneficial role in plants subjected to stressful cues (Li et al. 2009; Maghsoudi et al. 2015; Maghsoudi et al. 2016). For example, Si has been reported to be effective in mitigating the harmful effects of salinity, drought, high temperature and metal stress on plants (Ma and Takahashi 2002; Parveen and Ashraf 2010; Maghsoudi et al. 2016). Furthermore, beneficial effects of Si have been reported in plants subjected to water-deficit treatments, with respect to drought-induced regulation of metabolic processes and water relations (Liang et al. 2007; Ashraf 2009; Zhang et al. 2017). However, the mechanism by which Si can effectively alleviate drought-induced harmful effects remains unknown.
Various plant growth regulators (PGRs) are currently used to achieve enhanced growth and production of different crops worldwide (Hayat et al. 2000; Tuna et al. 2007; Arora et al. 2008). Of several PGRs, salicylic acid (SA) is believed to be very effective in masking the adverse effects of different abiotic and biotic stresses on crops as well as being an essential component of the signal-transduction pathways operating in plants exposed to environmental cues including drought stress (Hayat et al. 2010). Ashraf and Foolad (2007) reported that SA also has a crucial function in the mechanism of plant water-stress tolerance. Exogenously applied SA has been reported to influence uptake and transport of nutrients (Kaydan et al. 2007), stomatal regulation (Morris et al. 2000), growth and photosynthetic rate (Khan et al. 2003), chlorophyll synthesis (Misra and Saxena 2009) and transpiration (Morris et al. 2000).
Furthermore, both Si (Gong et al. 2008; Ashraf 2009) and SA (Senaratna et al. 2000; Khodary 2004) can increase the antioxidative defence systems, both enzymatic and non-enzymatic, and thereby alleviate damage from reactive oxygen species (ROS) induced by stresses. Application of Si (Isa et al. 2010) and SA (Misra and Saxena 2009) also increases synthesis of osmolytes, improving plant tolerance against stresses. The positive role of osmolytes in osmoregulation has been reported (Sonobe et al. 2010; Murmu et al. 2017). Szabados and Savoure (2010) suggested that the accumulation of osmolytes in leaves might be involved in one or more of the above processes and contribute to drought tolerance.
Although, it has been shown that exogenous supplementation of Si or SA can effectively promote the endurance of plants against a variety of stresses (Senaratna et al. 2000; Ashraf et al. 2009; Hayat et al. 2010; Zhang et al. 2017), the literature has little information on the role of Si and SA applied in combination in alleviating drought-induced injurious effects on plants. Therefore, in the present study, we appraised the effects of exogenous Si and SA applied individually or in combination on wheat growth and grain yield under water-deficit conditions.
Materials and methods
Plant materials and growth conditions
Two wheat cultivars, Shiraz (relatively drought-sensitive) and Sirvan (drought-tolerant), were selected. Seeds of uniform size of both cultivars were sown in a field at the Research Farm of the College of Agriculture (altitude 1810 m a.m.s.l.), Shiraz University, Iran, during the 2013–14 growing season. The crop was irrigated with good-quality irrigation water. The soil texture is loam, pH(H2O) 7.7 and electrical conductivity (EC) 2.55 dS m–1.
Experimental design and treatments
The experiment was set up in a split-split-plot complete randomised block design with three replicates. Watering treatments (100% and 40% field capacity (FC)) were considered as main plots; foliar application of Si and SA (control (nil), 6 mm Si, 1 mm SA, and 6 mm Si + 1 mm SA) as subplots; and the two wheat cultivars as sub-subplots. The seeds were hand-sown (150 kg ha−1) during the first week of November in 2013. Each plot was 3 m wide and 2 m long. The soil was fertilised with 150 kg ha−1 of urea before sowing, and at mid-tillering and anthesis stages. Until the anthesis stage, all plots were irrigated to maintain 100% FC. From anthesis to ripening, water-stress treatment was initiated to maintain 40% FC, while the control plots were maintained at 100% FC. Silicon (as Na2Si3O7) and SA were sprayed onto the leaves of the appropriate plants at tillering and anthesis. These chemicals were sprayed for three consecutive days to ensure their uptake by the plants.
Measurements
All measurements based on fresh plant samples were done before the grain-filling stage. The fully expanded flag leaves were used for all biochemical analysis. Measurements included relative water content (RWC) (Castillo 1996), soluble sugars (Zhang et al. 2006) and soluble proteins (Bradford 1976); activities of peroxidase (POD) (Cakmak et al. 1993), ascorbate peroxidase (APX) (Nakano and Asada 1981), catalase (CAT) (Aebi 1984) and superoxide dismutase (SOD) (Dhindsa and Matow 1981); levels of hydrogen peroxide (H2O2) (Veljovic-Jovanovic et al. 2002) and malondialdehyde (MDA) (Hodges et al. 1999); concentrations of Ca, K and Mg by flame photometer (model 410; Corning Inc., Corning, NY, USA); and leaf water potential (Ψw) (PMS Instrument Company, Albany, OR, USA).
At maturity, grain yield, number of grains per spike, 1000-grain weight and harvest index were measured.
Statistical analyses
Analysis of variance was performed on data for each parameter by using SAS version 9.1 software (SAS Institute, Cary, NC, USA). Significant differences among mean values were compared using Duncan’s multiple range test (at P ≤ 0.05).
Results
Yield and yield components
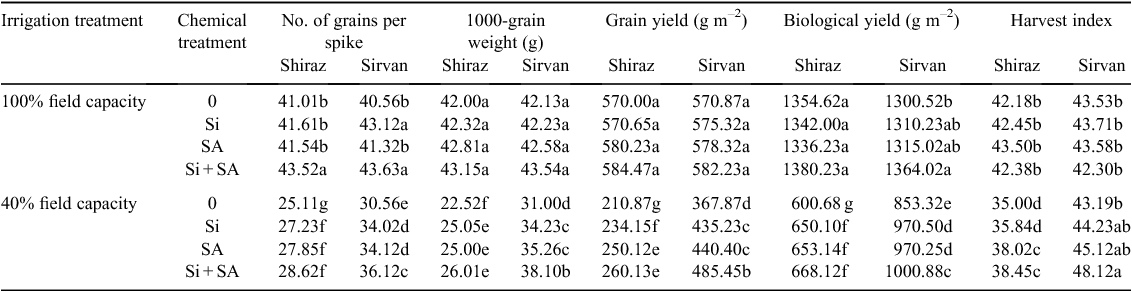
Water stress (40% FC) significantly reduced grain number per spike by 24.65% in cv. Sirvan and 38.77% in cv. Shiraz. The negative impact of water stress on number of grains per spike was alleviated by application of Si and SA. Under water stress, foliar application of Si, SA and Si + SA caused an increase of 11.32%, 11.64% and 18.19%, respectively, in grain number per spike in cv. Sirvan, and 8.44%, 10.91% and 13.97% in cv. Shiraz (Table 1). Furthermore, in both wheat cultivars, 1000-grain weight decreased significantly under water stress. The drought-tolerant cultivar Sirvan had higher 1000-grain weight than drought-sensitive Shiraz under water stress (Table 1). The decline in 1000-grain weight was considerably less in plants supplied with Si, SA or Si + SA than that when these treatments were not applied. Therefore, foliar application of these treatments can significantly improve 1000-grain weight under field water-deficit conditions; maximum benefit was recorded with Si + SA when applied under water-stress conditions to cv. Sirvan, increasing 1000-grain weight by 22.90% (Table 1).
Grain yield decreased significantly under water-stress conditions, by 35.55% in drought-tolerant cv. Sirvan and 63.00% in drought-sensitive cv. Shiraz. However, foliar application of SA, Si and Si + SA caused a significant increase in grain yield under water-limited conditions. The effect of Si + SA was greater than of Si or SA applied separately (Table 1). With applications of Si, SA and Si + SA, grain yield was 18.31%, 19.71% and 31.96% higher, respectively, for cv. Sirvan, and 11.03%, 18.61% and 23.36% higher for cv. Shiraz than with no foliar application under water stress (Table 1). In both cultivars, the biological yield decreased significantly under water-stress conditions; however, Si- and SA-treated plants had higher biological yield than untreated plants under water stress alone. The effect of Si + SA application on biological yield was greater than of Si or SA applied separately (Table 1). Water stress decreased harvest index of drought-sensitive Shiraz only. Foliar application of Si + SA significantly promoted harvest index of both wheat varieties under water-limited conditions (Table 1).
Organic substances (soluble sugars and soluble proteins) and inorganic ions
Soluble sugar concentration in the flag leaf increased significantly under water-stress conditions, by 19.09% in cv. Shiraz and 43.83% in cv. Sirvan (Table 2). Plants treated with Si and SA had significantly higher soluble sugar content than untreated plants under water stress alone. The influence of Si + SA on soluble sugars in plants under water stress tended to be greater than of Si or SA applied separately. The response of cultivars to Si and SA varied significantly, with cv. Sirvan more responsive; in Si, SA and Si + SA treatments and under water stress, soluble sugar content was 21.75%, 15.20% and 29.57% higher, respectively, in cv. Sirvan, and 13.70%, 15.71% and 21.10% higher in cv. Shiraz than with no foliar application (Table 2).
In both cultivars the levels of soluble proteins decreased markedly under water-limited conditions. Application of Si and SA improved the soluble protein levels of water-stressed plants of both cultivars compared with plants exposed to drought stress without Si and SA application, and the effect of Si + SA on soluble protein content was greater than of Si or SA applied separately. Foliar application of Si + SA also significantly increased soluble protein content by 6.96% and 17.61%, respectively in cv. Shiraz and cv. Sirvan under non-stress conditions (Table 2).
Concentrations of K, Mg and Ca increased significantly under water stress, by 38.06%, 76.19% and 62.20%, respectively, in cv. Sirvan, and 15.62%, 25.21% and 12.36% in cv. Shiraz (Table 2). Drought-stressed plants fed with Si and SA accumulated a greater concentration of K than control plants. Supplementation with SA and Si + SA caused a marked increase in Mg concentration in water-stressed plants compared with those receiving no foliar treatment (Table 2). Calcium concentration increased significantly in both cultivars under water stress; foliar application of Si, SA and Si + SA caused a further increase in this nutrient only in cv. Sirvan. The concentrations of the three mineral nutrients K, Mg and Ca were greater in cv. Sirvan than in cv. Shiraz under water-stress conditions (Table 2).
Antioxidant enzyme activities, H2O2 and MDA
The activity of POD was significantly increased due to water stress, by 75.06% in cv. Sirvan and 5.49% in cv. Shiraz. In both cultivars, application of Si, SA and Si + SA significantly increased POD activity of water-stressed plants; the influence of Si + SA was greater than of Si or SA applied separately. POD was much higher in cv. Sirvan than in cv. Shiraz under water-stress conditions, especially with foliar-applied Si + SA (Table 3).
Activity of SOD rose significantly under water-stress conditions, by 34.61% in cv. Shiraz and 62.50% in cv. Sirvan. Plants treated with Si or SA had greater SOD activity than those grown solely under water limitation. The effect of Si + SA was greater than of Si or SA applied separately. Varietal response to Si and SA varied significantly for SOD activity; cv. Sirvan was more responsive. In addition, under normal water conditions, combined application of Si + SA significantly promoted SOD activity relative to no foliar application in both wheat varieties (Table 3).
Activity of APX also increased in both wheat varieties under water stress, and this increase was more pronounced in cv. Sirvan. Application of Si and/or SA had no significant effect on APX activity in cv. Shiraz under either water regime, whereas in cv. Sirvan, APX significantly increased with application of SA and Si + SA under normal water conditions and with application of Si, SA and Si + SA under water stress (Table 3).
In both cultivars, water stress increased the CAT activity. Application of Si or SA supplementation had no significant effect on CAT activity in cv. Shiraz under either water-limited and normal watering conditions, whereas in cv. Sirvan, CAT activity increased with application of SA and Si + SA under drought-stress conditions (Table 3).
Levels of H2O2 increased markedly under water-limited conditions. Plants treated with Si and/or SA had lower H2O2 levels than plants under water stress alone. Furthermore, the influence of Si + SA application on H2O2 content was greater than with either Si or SA applied separately. With application of Si, SA and Si + SA and under water stress, H2O2 content was lower than with no foliar application, in both cultivars (Table 3).
In addition, drought stress caused a significant increase in the levels of MDA in both wheat cultivars. Although the cultivars did not differ significantly from each other under normal watering, cv. Shiraz (drought-sensitive) had considerably higher levels of MDA than cv. Sirvan (drought-tolerant) under water-limited conditions. Treatment with Si, SA and Si + SA decreased MDA levels under both non-stress and water-limited regimes in both cultivars, but the influence was more evident under water deficit (Table 3).
Relative water content and leaf water potential (Ψw)
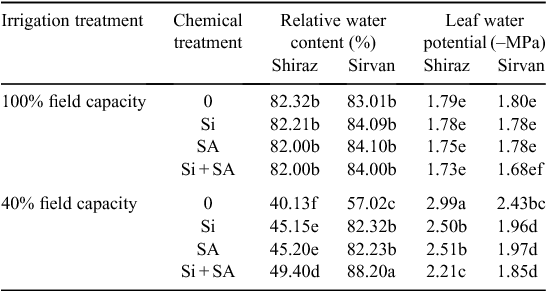
Water-deficit treatments caused a marked suppression in RWC and Ψw in both wheat varieties. However, cv. Sirvan had higher RWC and Ψw than cv. Shiraz under drought stress. Application of Si, SA and Si + SA significantly improved the RWC and Ψw of water-stressed plants in both cultivars (Table 4).
|
Discussion
Water stress (40% FC) imposed from anthesis to grain ripening strongly reduced the yield of two wheat cultivars of differing drought tolerance in the present study. Indeed, the importance of water availability during grain filling for yield formation of bread wheat was demonstrated here. Generally, drought imposed during anthesis and grain filling leads to small-sized and fewer grains (Guttieri et al. 2001; Erocli et al. 2007; Sinclair 2011). Impaired grain filling was reported to be attributable to reduced partitioning of assimilates and reduced activities of key enzymes involved in sucrose and starch synthesis (Sinclair 2011). However, reduction of grain yield, yield components and biological yield in both wheat cultivars was lower in the presence of externally applied Si, SA, and especially combined Si + SA. Therefore, application of Si and SA could improve grain yield under water-stress conditions. Similarly, Tahir et al. (2006) observed that exogenous application of Si promoted grain yield in a wheat crop (by 50%) under stressful environmental conditions. A marked improvement was observed in biomass under drought stress, showing a promising effect of exogenous application of Si in counteracting the injurious effects of drought (Parveen and Ashraf 2010; Maghsoudi et al. 2016; Zhang et al. 2017). These results are similar to reports for sorghum (Sorghum bicolor) (Sonobe et al. 2010; Ahmed et al. 2011). Furthermore, foliar application of SA was previously reported to result in a significant increase in yield of wheat under water-stress conditions (Ahmad et al. 2014; Zamaninejad et al. 2013).
Water-stress conditions are believed to affect physiological responses and growth of several cereal crops (Maghsoudi and Maghsoudi Moud 2008; Yao et al. 2009), and several studies suggest that Si could improve endurance of plants under stressful conditions (Hattori et al. 2007; Liang et al. 2008; Maghsoudi et al. 2015; Mauad et al. 2016). Si-induced growth promotion under water-starved regimes has been reported in different crops, e.g. wheat (Gong et al. 2005; Gong and Chen 2012; Maghsoudi et al. 2016), rice (Oryza sativa) (Chen et al. 2011; Mauad et al. 2016) and soybean (Glycine max) (Shen et al. 2010).
Silicon is indispensable for promoting growth of several crops including cereals (Broadley et al. 2012), and SA, like several other known plant growth regulators, plays a key role in promoting plant resistance against drought stress (Ashraf and Foolad 2007). Some reports show the vital role of exogenous supply of SA in counteracting injurious effects of stressful environments in different plants (Hayat et al. 2010). Both SA, as a plant growth regulator, and Si, as a mineral, are believed to regulate different physio-biochemical processes in plants including photosynthesis, stomatal regulation and ion uptake. Thus, Si and SA have potential roles in activating plant growth and productivity (Ashraf et al. 2009; Hayat et al. 2010; Mauad et al. 2016; Ali and Hassan 2017).
Application of Si can effectively mitigate drought-induced injury in different plants (Gong et al. 2005; Hattori et al. 2005; Liang et al. 2007; Parveen and Ashraf 2010; Ahmed et al. 2011; Cooke and Leishman 2011; Broadley et al. 2012; Mauad et al. 2016; Ali and Hassan 2017). The benefits of soil-applied Si in counteracting both abiotic and biotic stresses have been reported by several researchers (e.g. Gong et al. 2005; Hattori et al. 2005; Li et al. 2009); however, beneficial effects of foliar-applied Si for counteracting these stresses have received less attention (Guével et al. 2007; Hellal et al. 2012).
The present investigation showed that increased activity of antioxidant enzymes such as CAT, POD, APX and SOD occurred to alleviate water-stress-induced adverse effects on wheat plants. Similar findings have been reported by Tari et al. (2004) and Ashraf (2009). Molassiotis et al. (2006) found that ROS-induced oxidative damage may cause oxidation of lipids and proteins. However, in the view of Møller et al. (2007), a balance between ROS generation and the activities of antioxidant enzymes may ensure the extent to which oxidative damage and signalling will take place. In fact, capacity to scavenge ROS may promote drought tolerance in plants (Tsugane et al. 1999).
In the present research, application of Si and SA enhanced the activity of some important enzymes taking part in the oxidative defence system and decreased the levels of H2O2 and MDA in water-stressed plants. Furthermore, in cv. Sirvan, the synergistic effects of Si + SA on activity of antioxidant enzymes were greater than effects of Si or SA applied separately, under water-stress conditions.
Our results are similar to findings reported by other workers demonstrating that Si application to soil is very effective in mitigating the harmful effects of environmental stresses including drought (Ashraf et al. 2009; Li et al. 2009; Parveen and Ashraf 2010; Zhang et al. 2017). A similar mechanism of Si and SA in reducing drought stress is the improvement of antioxidant activity in plants under abiotic stresses (Senaratna et al. 2000; Liang et al. 2007). Our findings also show that exogenously applied Si (e.g. Liang et al. 2007) and SA (e.g. Khodary 2004; Shakirova 2007) modulate the activities of vital antioxidant enzymes such as SOD and POD, and improve plant tolerance to drought stress.
An increase in mineral nutrient ions (K+, Mg2+, Ca2+ and Na+) is believed to be another critical mechanism for plants to resist to stress (Zhu et al. 2005). In the present investigation, accumulation of K+, Ca2+ and Mg2+ took place in the leaves of plants subjected to water-limited conditions, and foliar-applied Si, similar to SA, caused a further enhancement in K+ levels in the leaves of water-stressed wheat plants.
Soluble sugars also generally increase in plant tissues exposed to water-limited conditions and they are potential contributors to osmoregulation (Shao et al. 2006). In our study, soluble sugars (as osmolytes) were considerably enhanced in the wheat leaves exposed to low water supply, more markedly in cv. Sirvan (drought-tolerant), than in cv. Shiraz. Foliar application of SA and Si further increased soluble sugar content and in cv. Sirvan, and the synergistic effect of Si + SA application was greater than of Si or SA alone. Enhanced levels of soluble sugars are believed to have a role in stress tolerance, because soluble sugars are actively involved in protection of enzyme structure, osmoregulation, biological membrane stabilisation and protection against hydroxyl radicals (Shao et al. 2006). Nayyar and Walia (2003) found that stress-resistant plants usually accumulate greater amounts of soluble sugars than stress-sensitive plants.
Leaf water potential is a potential indicator for determining plant water status, and it plays an important role in enhancing plant photosynthetic rate (Endres et al. 2010). In the present investigation, drought-tolerant cv. Sirvan maintained significantly higher Ψw and RWC than drought-sensitive cv. Shiraz under water-limited conditions.
Zhu et al. (2004) believed that Si considerably improves the water status of plant leaves, which in turn helps the plant to mitigate cellular dehydration, and hence lower oxidative stress. Moreover, Gong et al. (2005, 2008) reported that addition of Si to soil can improve leaf Ψw in plants subjected to water-limited regimens. Si-induced improvement in Ψw may be associated with enhanced stomatal conductance and higher RWC. Thus, Si plays an effective role in maintaining water balance in plant tissues, most probably through higher water uptake. Isa et al. (2010) reported that Si supply may improve the rigidity and strength of cell walls, thereby helping to reduce the solute leakage and stabilise the ultrastructure of biological membranes.
Silicon deposition in the cytoplasm of cells is a unique mechanism of Si in reducing abiotic stress in plants.
Nonetheless, the functions of Si and SA in water uptake and osmoregulation in plants under drought are not yet well defined. The results of this research show that osmolyte accumulation in leaves of plants treated with Si and SA under drought stress was more prominent in drought-stressed plants receiving no Si or SA treatment. Thus, supply of Si (Zhang et al. 2017) and SA (Hayat et al. 2010) can enhance the ability of plants to adjust themselves osmotically so as to maintain high water content and leaf water potential.
Conclusion
Foliar application of Si, SA and especially the combination Si + SA, markedly improved grain yield and yield components of the two wheat cultivars under water-deficit. In Si, SA and Si + SA treatments, grain yield was 15.63%, 16.60% and 24.32% higher respectively, than with no foliar application under water stress in cv. Sirvan, and 10.25%, 16.02% and 19.25% higher in cv. Shiraz. The results of the study highlight the role of Si and SA application in regulating water-stress response of wheat, suggesting that Si and SA are involved in physiological activities. These results showed positive effects of Si and SA in terms of increased antioxidant activity as well as relative water content and leaf water potential. In addition, Si and SA stimulated the active accumulation of some osmolytes in leaves of water-stressed wheat plants, which suggests enhanced osmoregulation ability. The synergistic effects of Si + SA application on yield and physiological parameters were greater than of Si or SA applied separately. Therefore, proper application of Si and SA might result in increased production of wheat, particularly in dryland areas.
Conflicts of interest
The authors declare no conflicts of interest.
Acknowledgements
This work was supported by Shiraz University, Iran. The authors are grateful to the reviewers and editorial team for comments that greatly improved the manuscript.
References
Aebi H (1984) Catalase in vitro. Methods in Enzymology 105, 121–126.| Catalase in vitro.Crossref | GoogleScholarGoogle Scholar | 6727660PubMed |
Ahmad I, Basra SMA, Wahid A (2014) Exogenous application of ascorbic acid, salicylic acid and hydrogen peroxide improves the productivity of hybrid maize at low temperature stress. International Journal of Agricultural and Biological Science 16, 825–830.
Ahmed M, Hassen FU, Qadeer U, Aslam MA (2011) Silicon application and drought tolerance mechanism of sorghum. African Journal of Agricultural Research 6, 594–607.
Ali EF, Hassan FAS (2017) Water stress alleviation of roselle plant by silicon treatment through some physiological and biochemical responses. Annual Research & Review in Biology 21, 1–17.
| Water stress alleviation of roselle plant by silicon treatment through some physiological and biochemical responses.Crossref | GoogleScholarGoogle Scholar |
Arora N, Bhardwaj R, Sharma P, Arora HK (2008) 28-Homobrassinolide alleviates oxidative stress in salt treated maize (Zea mays L.) plants. Brazilian Journal of Plant Physiology 20, 153–157.
| 28-Homobrassinolide alleviates oxidative stress in salt treated maize (Zea mays L.) plants.Crossref | GoogleScholarGoogle Scholar |
Ashraf M (2009) Biotechnological approach of improving plant salt tolerance using antioxidants as markers. Biotechnology Advances 27, 84–93.
| Biotechnological approach of improving plant salt tolerance using antioxidants as markers.Crossref | GoogleScholarGoogle Scholar | 18950697PubMed |
Ashraf M, Foolad MR (2007) Roles of glycine betaine and proline in improving plant abiotic stress resistance. Environmental and Experimental Botany 59, 206–216.
| Roles of glycine betaine and proline in improving plant abiotic stress resistance.Crossref | GoogleScholarGoogle Scholar |
Ashraf M, Rahmatullah R, Ahmad M, Afzal M, Tahir A, Kanwal S, Maqsood MA (2009) Potassium and silicon improve yield and juice quality in sugarcane (Saccharum officinarum L.) under salt stress. Journal of Agronomy & Crop Science 195, 284–291.
| Potassium and silicon improve yield and juice quality in sugarcane (Saccharum officinarum L.) under salt stress.Crossref | GoogleScholarGoogle Scholar |
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry 72, 248–254.
| A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding.Crossref | GoogleScholarGoogle Scholar | 942051PubMed |
Broadley M, Brown P, Cakmak I, Ma JF, Rengel Z, Zhao F (2012) Beneficial elements. In ‘Marschner’s mineral nutrition of higher plants’. 3rd edn (Ed. P Marschner) (Academic Press: London, UK)
Cakmak I, Strbac D, Marschner H (1993) Activities of hydrogen peroxide-scavenging enzymes in germinating wheat seeds. Journal of Experimental Botany 44, 127–132.
| Activities of hydrogen peroxide-scavenging enzymes in germinating wheat seeds.Crossref | GoogleScholarGoogle Scholar |
Castillo FJ (1996) Antioxidative protection in the inducible CAM plant Sedum album L. following the imposition of severe water stress and recovery. Oecologia 107, 469–477.
| Antioxidative protection in the inducible CAM plant Sedum album L. following the imposition of severe water stress and recovery.Crossref | GoogleScholarGoogle Scholar | 28307389PubMed |
Chen W, Yao X, Cai K, Chen J (2011) Silicon alleviates drought stress of rice plants by improving plant water status, photosynthesis and mineral nutrient absorption. Biological Trace Element Research 142, 67–76.
| Silicon alleviates drought stress of rice plants by improving plant water status, photosynthesis and mineral nutrient absorption.Crossref | GoogleScholarGoogle Scholar | 20532668PubMed |
Cooke J, Leishman MR (2011) Is plant ecology more siliceous than we realise? Trends in Plant Science 16, 61–68.
| Is plant ecology more siliceous than we realise?Crossref | GoogleScholarGoogle Scholar | 21087891PubMed |
Dhindsa RS, Matow W (1981) Drought tolerance in two mosses: correlated with enzymatic defense against lipid peroxidation. Journal of Experimental Botany 32, 79–91.
| Drought tolerance in two mosses: correlated with enzymatic defense against lipid peroxidation.Crossref | GoogleScholarGoogle Scholar |
Emam Y (2011) ‘Cereal production.’ (Shiraz University Press: Shiraz, Iran)
Endres L, Silva JV, Ferreira VM, Ver G, Barbosa DS (2010) Photosynthesis and water relations in Brazilian sugarcane. The Open Agriculture Journal 4, 31–37.
| Photosynthesis and water relations in Brazilian sugarcane.Crossref | GoogleScholarGoogle Scholar |
Erocli L, Lulli L, Mariotti M, Masoni A, Arduini I (2007) Postanthesis dry matter and nitrogen dynamics in durum wheat. Crop Science 34, 1443–1451.
Gong H, Chen K (2012) The regulatory role of Si on water relations, photosynthetic gas exchange, and carboxylation activities of wheat leaves in field drought conditions. Acta Physiologiae Plantarum 34, 1589–1594.
| The regulatory role of Si on water relations, photosynthetic gas exchange, and carboxylation activities of wheat leaves in field drought conditions.Crossref | GoogleScholarGoogle Scholar |
Gong H, Zhu X, Chen K, Wang S, Zhang C (2005) Silicon alleviates oxidative damage of wheat plants in pots under drought. Plant Science 169, 313–321.
| Silicon alleviates oxidative damage of wheat plants in pots under drought.Crossref | GoogleScholarGoogle Scholar |
Gong HJ, Chen KM, Zhao ZG, Chen GC, Zhou WJ (2008) Effects of silicon on defense of wheat against oxidative stress under drought at different developmental stages. Biologia Plantarum 52, 592–596.
| Effects of silicon on defense of wheat against oxidative stress under drought at different developmental stages.Crossref | GoogleScholarGoogle Scholar |
González A, Bermejo V, Gimeno BS (2010) Effect of different physiological traits on grain yield in barley grown under irrigated and terminal water deficit conditions. The Journal of Agricultural Science 148, 319–328.
| Effect of different physiological traits on grain yield in barley grown under irrigated and terminal water deficit conditions.Crossref | GoogleScholarGoogle Scholar |
Guével MH, Menzies JG, Belanger RR (2007) Effect of root and foliar applications of soluble silicon on powdery mildew control and growth of wheat plants. European Journal of Plant Pathology 119, 429–436.
| Effect of root and foliar applications of soluble silicon on powdery mildew control and growth of wheat plants.Crossref | GoogleScholarGoogle Scholar |
Guttieri MJ, Stark JC, O’Brien K, Souza E (2001) Relative sensitivity of spring wheat grain yield and quality parameters to moisture deficit. Crop Science 41, 327–335.
| Relative sensitivity of spring wheat grain yield and quality parameters to moisture deficit.Crossref | GoogleScholarGoogle Scholar |
Hattori T, Inanagaa S, Arakib H (2005) Application of silicon enhanced drought tolerance in Sorghum bicolor. Physiologia Plantarum 123, 459–466.
| Application of silicon enhanced drought tolerance in Sorghum bicolor.Crossref | GoogleScholarGoogle Scholar |
Hattori T, Sonobe K, Inanaga S (2007) Short term stomatal responses to light intensity changes and osmotic stress in sorghum seedlings raised with and without silicon. Environmental and Experimental Botany 60, 177–182.
| Short term stomatal responses to light intensity changes and osmotic stress in sorghum seedlings raised with and without silicon.Crossref | GoogleScholarGoogle Scholar |
Hayat S, Ahmad A, Mobin M, Hussain A, Faridduddin Q (2000) Photosynthetic rate, growth and yield of mustard plants sprayed with 28 homobrassinolide. Photosynthetica 38, 469–471.
| Photosynthetic rate, growth and yield of mustard plants sprayed with 28 homobrassinolide.Crossref | GoogleScholarGoogle Scholar |
Hayat Q, Hayat S, Irfan M, Ahmad A (2010) Effect of exogenous salicylic acid under changing environment. A review. Environmental and Experimental Botany 68, 14–25.
| Effect of exogenous salicylic acid under changing environment. A review.Crossref | GoogleScholarGoogle Scholar |
Hellal FA, Abdelhameid M, Abo-Basha Doaa M, Zewainy RM (2012) Alleviation of the adverse effects of soil salinity stress by foliar application of silicon on faba bean (Vica faba L.). Journal of Applied Sciences Research 8, 4428–4433.
Hodges DM, De Lond JM, Forney CF, Prange RK (1999) Improving the thiobarbituric acid-reactive substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Planta 207, 604–611.
| Improving the thiobarbituric acid-reactive substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds.Crossref | GoogleScholarGoogle Scholar |
Isa M, Bai S, Yokoyama T, Ma JF, Ishibashi Y, Yuasa T, Iwaya-Inoue M (2010) Silicon enhances growth independent of silica deposition in a low-silica rice mutant. Plant and Soil 331, 361–375.
| Silicon enhances growth independent of silica deposition in a low-silica rice mutant.Crossref | GoogleScholarGoogle Scholar |
Kaydan D, Yagmur M, Okut N (2007) Effects of salicylic acid on the growth and some physiological characters in salt stressed wheat (Triticum aestivum L.). Tarim Bilimleri Dergisi 13, 114–119.
Khan W, Prithiviraj B, Smith D (2003) Photosynthetic responses of corn and soybean to foliar application of salicylates. Journal of Plant Physiology 160, 485–492.
| Photosynthetic responses of corn and soybean to foliar application of salicylates.Crossref | GoogleScholarGoogle Scholar | 12806776PubMed |
Khodary SEA (2004) Effect of salicylic acid on growth, photosynthesis and carbohydrate metabolism in salt-stressed maize plants. International Journal of Agriculture and Biology 6, 5–8.
Li QF, Ma CC, Ji J (2009) Effect of silicon on water metabolism in maize plants under drought stress. Acta Ecologica Sinica 29, 4163–4168.
Liang YC, Sun WC, Zhu YG (2007) Mechanisms of silicon-mediated alleviation of abiotic stresses in higher plants: a review. Environmental Pollution 147, 422–428.
| Mechanisms of silicon-mediated alleviation of abiotic stresses in higher plants: a review.Crossref | GoogleScholarGoogle Scholar |
Liang Y, Zhu J, Li Z, Chu G, Ding Y, Zhang J, Sun W (2008) Role of Si in enhancing resistance to freezing stress in two contrasting winter wheat cultivars. Environmental and Experimental Botany 64, 286–294.
| Role of Si in enhancing resistance to freezing stress in two contrasting winter wheat cultivars.Crossref | GoogleScholarGoogle Scholar |
Ma JF, Takahashi E (2002) Functions of silicon in plant growth. In ‘Soil, fertilizer and plant silicon research in Japan’. (Eds JF Ma, E Takahashi) (Elsevier Science: Amsterdam)
Maghsoudi K, Maghsoudi Moud AA (2008) Analysis of the effects of stomatal frequency and size on transpiration and yield of wheat (Triticum aestivum L.). American-Eurasian Journal of Agricultural & Environmental Sciences 3, 865–872.
Maghsoudi K, Emam Y, Ashraf M (2015) Influence of foliar application of silicon on chlorophyll fluorescence, photosynthetic pigments, and growth in water-stressed wheat cultivars differing in drought tolerance. Turkish Journal of Botany 39, 625–634.
Maghsoudi K, Emam Y, Ashraf M (2016) Foliar application of silicon at different growth stages alters growth and yield of selected wheat cultivars. Journal of Plant Nutrition 39, 1194–1203.
| Foliar application of silicon at different growth stages alters growth and yield of selected wheat cultivars.Crossref | GoogleScholarGoogle Scholar |
Mauad M, Cruscio CAC, Nascente AS, Filho HG, Lima GPP (2016) Effects of silicon and drought stress on biochemical characteristics of leaves of upland rice cultivars. Ciência Agronômica 47, 532–539.
| Effects of silicon and drought stress on biochemical characteristics of leaves of upland rice cultivars.Crossref | GoogleScholarGoogle Scholar |
Misra N, Saxena P (2009) Effect of salicylic acid on proline metabolism in lentil grown under salinity stress. Plant Science 177, 181–189.
| Effect of salicylic acid on proline metabolism in lentil grown under salinity stress.Crossref | GoogleScholarGoogle Scholar |
Molassiotis A, Sotiropoulos T, Tanou G, Diamantidis G, Therios I (2006) Boron-induced oxidative damage and antioxidant and nucleolytic responses in shoot tips culture of the apple rootstock EM9 (Malus domestica Borkh). Environmental and Experimental Botany 56, 54–62.
| Boron-induced oxidative damage and antioxidant and nucleolytic responses in shoot tips culture of the apple rootstock EM9 (Malus domestica Borkh).Crossref | GoogleScholarGoogle Scholar |
Møller IM, Jensen PE, Hansson A (2007) Oxidative modifications to cellular components in plants. Annual Review of Plant Biology 58, 459–481.
| Oxidative modifications to cellular components in plants.Crossref | GoogleScholarGoogle Scholar | 17288534PubMed |
Morris K, MacKerness SA, Page T, John CF, Murphy AM, Carr JP, Buchanan-Wollaston V (2000) Salicylic acid has a role in regulating gene expression during leaf senescence. The Plant Journal 23, 677–685.
| Salicylic acid has a role in regulating gene expression during leaf senescence.Crossref | GoogleScholarGoogle Scholar | 10972893PubMed |
Murmu K, Murmu S, Kundu CK, Bera PS (2017) Exogenous proline and glycine betaine in plants under stress tolerance. International Journal of Current Microbiology and Applied Sciences 6, 901–913.
| Exogenous proline and glycine betaine in plants under stress tolerance.Crossref | GoogleScholarGoogle Scholar |
Nakano Y, Asada K (1981) Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant & Cell Physiology 22, 867–880.
Nayyar H, Walia DP (2003) Water stress induced proline accumulation in contrasting wheat genotypes as affected by calcium and abscisic acid. Biologia Plantarum 46, 275–279.
| Water stress induced proline accumulation in contrasting wheat genotypes as affected by calcium and abscisic acid.Crossref | GoogleScholarGoogle Scholar |
Parveen N, Ashraf M (2010) Role of silicon in mitigating the adverse effects of salt stress on growth and photosynthetic attributes of two maize (Zea mays L.) cultivars grown hydroponically. Pakistan Journal of Botany 42, 1675–1684.
Senaratna T, Touchell D, Bunn E, Dixon K (2000) Acetyl salicylic acid (Aspirin) and salicylic acid induce multiple stress tolerance in bean and tomato plants. Plant Growth Regulation 30, 157–161.
| Acetyl salicylic acid (Aspirin) and salicylic acid induce multiple stress tolerance in bean and tomato plants.Crossref | GoogleScholarGoogle Scholar |
Shakirova FM (2007) Role of hormonal system in the manifestation of growth promoting and anti-stress action of salicylic acid. In ‘Salicylic acid, a plant hormone’. (Eds S Hayat, A Ahmad) (Springer: Dordrecht, The Netherlands)
Shao HB, Liang ZS, Shao MA (2006) Osmotic regulation of 10 wheat (Triticum aestivum L.) genotypes at soil water deficits. Colloids and Surfaces. B, Biointerfaces 47, 132–139.
| Osmotic regulation of 10 wheat (Triticum aestivum L.) genotypes at soil water deficits.Crossref | GoogleScholarGoogle Scholar |
Shen X, Zhou Y, Duan L, Li Z, Eneji AE, Li J (2010) Si effects on photosynthesis and antioxidant parameters of soybean seedlings under drought and ultraviolet-B radiation. Journal of Plant Physiology 167, 1248–1252.
| Si effects on photosynthesis and antioxidant parameters of soybean seedlings under drought and ultraviolet-B radiation.Crossref | GoogleScholarGoogle Scholar | 20713250PubMed |
Sinclair TR (2011) Challenges in breeding for yield increase for drought. Trends in Plant Science 16, 289–293.
| Challenges in breeding for yield increase for drought.Crossref | GoogleScholarGoogle Scholar | 21419688PubMed |
Sonobe K, Hattori T, An P, Tsuji W, Eneji AE, Kobayashi S, Kawamura Y, Tanaka K, Inanaga S (2010) Effect of silicon application on sorghum root responses to water stress. Journal of Plant Nutrition 34, 71–82.
| Effect of silicon application on sorghum root responses to water stress.Crossref | GoogleScholarGoogle Scholar |
Szabados L, Savoure A (2010) Proline: a multifunctional amino acid. Trends in Plant Science 15, 89–97.
| Proline: a multifunctional amino acid.Crossref | GoogleScholarGoogle Scholar | 20036181PubMed |
Tahir MA, Tullah R, Aziz T, Ashraf M, Kanwal S, Maqsood MA (2006) Beneficial effects of silicon in wheat (Triticum aestivum L.) under salinity stress. Pakistan Journal of Botany 38, 1715–1722.
Tari I, Simon LM, Deer KA, Csiszar J, Bajkan S, Kis G, Szepesi A (2004) Influence of salicylic acid on salt stress acclimation of tomato plants: oxidative stress responses and osmotic adaptation. Acta Physiologiae Plantarum 26, 230–237.
Tsugane K, Kobayashi K, Niwa Y, Ohba Y, Wada K, Kobayashi H (1999) A recessive Arabidopsis mutant that grows photoautotrophically under salt stress shows enhanced active oxygen detoxification. The Plant Cell 11, 1195–1206.
| A recessive Arabidopsis mutant that grows photoautotrophically under salt stress shows enhanced active oxygen detoxification.Crossref | GoogleScholarGoogle Scholar | 10402422PubMed |
Tuna AL, Kaya C, Dilkilitas M, Yokas I, Buruni B, Altunlu H (2007) Comparative effects of various salicylic acid derivatives on key growth parameters and some enzyme activities in salinity stressed maize (Zea mays L.) plants. Pakistan Journal of Botany 39, 787–798.
Veljovic-Jovanovic S, Noctor G, Foyer CH (2002) Are leaf hydrogen peroxide concentrations commonly overestimated? The potential influence of artefactual interference by tissue phenolics and ascorbate. Plant Physiology and Biochemistry 40, 501–507.
| Are leaf hydrogen peroxide concentrations commonly overestimated? The potential influence of artefactual interference by tissue phenolics and ascorbate.Crossref | GoogleScholarGoogle Scholar |
Yao XQ, Chu JZ, Wang GY (2009) Effects of drought stress and selenium supply on growth and physiological characteristics of wheat seedlings. Acta Physiologiae Plantarum 5, 1031–1036.
Zamaninejad M, Khorasani SK, Moeini MJ, Heidarian AR (2013) Effect of salicylic acid on morphological characteristics, yield and yield components of corn (Zea mays L.) under drought condition. European Journal of Experimental Biology 3, 153–161.
Zhang ZJ, Li HZ, Zhou WJ, Takeuchi Y, Yoneyama K (2006) Effect of 5-aminolevulinic acid on development and salt tolerance of potato (Solanum tuberosum L.) microtubers in vitro. Plant Growth Regulation 49, 27–34.
Zhang W, Xie Z, Wang L, Li M, Lang D, Zhang X (2017) Silicon alleviates salt and drought stress of Glycyrrhiza uralensis seedling by altering antioxidant metabolism and osmotic adjustment. Journal of Plant Research 130, 611–624.
| Silicon alleviates salt and drought stress of Glycyrrhiza uralensis seedling by altering antioxidant metabolism and osmotic adjustment.Crossref | GoogleScholarGoogle Scholar | 28290079PubMed |
Zhu Z, Wei G, Li J, Qian Q, Yu J (2004) Silicon alleviates salt stress and increases antioxidant enzymes activity in leaves of salt-stressed cucumber (Cucumis sativus L.). Plant Science 167, 527–533.
| Silicon alleviates salt stress and increases antioxidant enzymes activity in leaves of salt-stressed cucumber (Cucumis sativus L.).Crossref | GoogleScholarGoogle Scholar |
Zhu X, Gong H, Chen G, Wang S, Zhang C (2005) Different solute levels in two spring wheat cultivars induced by progressive field water stress at different developmental stages. Journal of Arid Environments 62, 1–14.
| Different solute levels in two spring wheat cultivars induced by progressive field water stress at different developmental stages.Crossref | GoogleScholarGoogle Scholar |