Cropping practices influence incidence of herbicide resistance in annual ryegrass (Lolium rigidum) in Australia
J. C. Broster A C , J. E. Pratley
A C , J. E. Pratley  A , R. H. L. Ip B , L. Ang A B and K. P. Seng A B
A , R. H. L. Ip B , L. Ang A B and K. P. Seng A B
A Graham Centre for Agricultural Innovation, Charles Sturt University, Locked Bag 588, Wagga Wagga, NSW 2678, Australia.
B School of Computing and Mathematics, Charles Sturt University, Locked Bag 588, Wagga Wagga, NSW 2678, Australia.
C Corresponding author. Email: jbroster@csu.edu.au
Crop and Pasture Science 70(1) 77-84 https://doi.org/10.1071/CP18355
Submitted: 24 July 2018 Accepted: 19 November 2018 Published: 24 December 2018
Abstract
Herbicide resistance is a common occurrence in southern Australia. The evolution of herbicide resistance is influenced by the selection pressure placed on the weed species controlled by that herbicide. Results from resistance screening of ~4500 annual ryegrass (Lolium rigidum Gaud.) samples were entered in a GIS database, together with several agricultural parameters used in the Australian Bureau of Statistics Agricultural Surveys. This allowed a study of the associations between mode of action of resistance, geographic distribution of resistance across southern Australia, and farming practices employed in particular regions.
Cultivation was negatively associated with resistances in acetyl-CoA carboxylase (ACCase)-inhibiting cyclohexanedione and acetolactate synthase (ALS)-inhibiting herbicides. Higher proportions of wheat sown were associated with higher incidences of resistance. ACCase-inhibiting aryloxyphenoxypropionate and cyclohexanedione and ALS-inhibiting resistances were higher in those shires where soils were predominantly acidic. This study demonstrates the association between farm practice and the evolution of herbicide resistance. The analysis provides reinforcement to the principle of rotating chemical modes of action with non-chemical weed control measures to minimise the risk of herbicide resistance evolution in any farming system.
Additional keywords: crop residue, soil pH, tillage.
Introduction
Herbicide resistance is a common occurrence in weeds of winter crops in southern Australia. In particular, annual ryegrass (Lolium rigidum Gaud.), one of the most prevalent weed species across the southern Australian wheat-cropping zone, has evolved resistance to most herbicide modes of action used for its control. These modes of action, as classified by Australian Pesticides and Veterinary Medicines Authority (APVMA), Herbicide Resistance Action Committee (HRAC) and Weed Science Society of America (WSSA), include: APVMA Group A (HRAC Group A, WSSA Group 1), acetyl-CoA carboxylase (ACCase) inhibitors—‘fops’ (aryloxyphenoxypropionates), ‘dims’ (cyclohexanediones) and ‘dens’ (phenylpyrazoles) (Heap and Knight 1982; Heap and Knight 1986; Boutsalis et al. 2012); APVMA Group B (HRAC Group B, WSSA Group 2), acetolactate synthase (ALS) inhibitors—‘SUs’ (sulfonylureas) and ‘imis’ (imidazolinones) (Matthews et al. 1990; Powles and Howat 1990; Christopher et al. 1991); APVMA Group C (HRAC Group C1, WSSA Group 5) inhibitors of photosynthesis at PSII—triazines (Burnet et al. 1991; Pratley et al. 1993b); APVMA Group D (HRAC Group K1, WSSA Group 3), inhibitors of tubulin formation—dinitroanilines (Pratley et al. 1993b; McAlister et al. 1995); and APVMA Group M (HRAC Group G, WSSA Group 9), inhibitors of EPSP synthase—glycines (Pratley et al. 1996, 1999; Powles et al. 1998). Additionally, as a result of diversity in both its genetics and the management practices used across the southern cropping region, several different patterns of cross-resistance and multiple resistances occur in annual ryegrass (Broster and Pratley 2006).
The efficacy of a particular herbicide to control on-farm populations of annual ryegrass can be determined by a herbicide-resistance test. Long-term data collected through the herbicide-resistance testing service at Charles Sturt University (CSU), Wagga Wagga, have shown extensive variation in the incidence of resistance in annual ryegrass among locations across south-eastern Australia (Broster and Pratley 2006). Similarly, random surveys conducted since 2003 in several states of Australia have shown differences in the incidence of resistance, both between states and between regions within states (Broster et al. 2011, 2012, 2013; Boutsalis et al. 2012; Owen et al. 2014). Compared with the initial surveys in many of the same regions (Pratley et al. 1993a; Henskens et al. 1996; Nietschke et al. 1996; Llewellyn and Powles 2001), the most recent surveys have recorded changes in the incidence and patterns of herbicide resistance. Although samples for the herbicide-resistance testing service at CSU are obtained directly from farms or farm advisers when resistance is suspected, a comparison with a previous Western Australian random survey (Owen et al. 2007) showed good agreement in the frequency of resistance between the two data sources.
The evolution of herbicide resistance is influenced by the selection pressure placed on the weed species controlled by that herbicide. The nature of each combination of herbicide, weed population and resistance status determines the practices that govern the use of different herbicides, thereby influencing the selection pressure exerted by each herbicide (Christoffers 1999). Soil type, management practices and the type of crop grown all influence herbicide use. The persistence of some chemicals can be extended if applied to soils that are acidic (e.g. imidazolinones) or alkaline (e.g. sulfonylureas), thereby limiting crop-rotation options (Black et al. 1999; Kennedy 2003). Reduced tillage can result in increased soil acidity, enhancing the persistence of some herbicides or limiting crop rotations through the pH level itself (Blevins et al. 1983; Locke and Bryson 1997).
Likewise, the requirement of some herbicides for incorporation after application limits their suitability in reduced-cultivation systems (Parsons 1995). Increased levels of crop residue on the soil surface can also reduce the activity of some herbicides because they bind to the plant residue, physically separating them from the soil where they would be activated, reducing their capacity for use in this situation (Locke and Bryson 1997). Additionally, depending upon the herbicide, persistence can either be increased or decreased in reduced-tillage systems, thereby influencing herbicide carryover effects on subsequent crop rotations (Locke and Bryson 1997).
Cultivation can be used for attaining some weed control and reducing the reliance on herbicides (Llewellyn et al. 2004); therefore, the adoption of no-till farming was perceived by farmers in South and Western Australia to lead to increases in herbicide dependence and resistance (D’Emden and Llewellyn 2006).
Regional differences occur across the southern Australian cropping region with respect to crop species grown, rotations adopted and management practices used (ABS 2018). Such differences are due to climatic constraints, soil types, weed problems and availability of suitable cultivars, and are often influenced by regional practices. It is expected, therefore, that management practices would influence the range of herbicides used and thereby indirectly influence the likelihood of herbicide resistance. This paper evaluates the association between the incidence of herbicide resistances across southern Australia and the farming practices adopted on a regional basis.
Materials and methods
Charles Sturt University receives annual ryegrass samples from across the southern cereal-cropping region from November to March each year for resistance testing. The methodology used for testing both post-emergent and pre-emergent herbicides has been described in detail in Broster and Pratley (2006). Briefly, samples received are threshed, cleaned and stored at room temperature until tested. Just before testing, samples are placed in an incubator for 1 week at 2−5°C to break any dormancy and ensure evenness of germination. Seeds for post-emergent testing are planted into a 50 : 50 peat moss–sand mix, and those for pre-emergence testing are planted into soil. Herbicides are applied at the recommended growth stages by using an automated cabinet sprayer applying volumes equivalent to 83 L ha–1 from a flat-fan nozzle at 300 kPa pressure. Rates of 0.5, 1.0 and 2.0 times the recommended label rate are applied to three replicates, and each batch contains known resistant and susceptible biotypes as standards. The results for all sample tests are entered into a geographical information system (GIS) to allow the identification of regional trends.
Agricultural survey data based on each shire were obtained from the Australian Bureau of Statistics (ABS 2018). The data comprised winter crops grown (2001–07 and 2010), amount of cultivation (2001–02 and 2010), and stubble management (2001–02 and 2010). The predominant soil pH for the shire was calculated by using data from the CSIRO Soil Atlas to determine the pH rating (acidic, neutral or alkaline) that held the largest proportion of the shire area (Northcote 1979; McKenzie and Hook 1992). Total shire area and estimated arable area (cropping area plus improved pastures) were also provided.
An analysis was undertaken to evaluate whether the incidence of herbicide resistance could be associated with cropping practices and soil pH. Shires were based on 2015 boundaries and were included only if they fulfilled the conditions of: (i) annual mean area of winter crop >3000 ha; and (ii) minimum of four samples tested for resistance between 2001 and 2015.
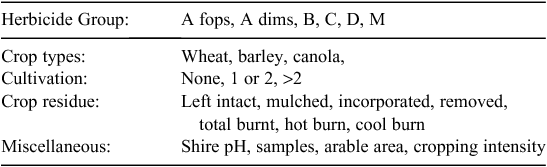
The data were processed in two stages to determine annual means for all categories. First, means were determined for the 2001–07 (crops grown) or 2001–02 (cultivation and stubble management) data, and second, the overall mean was determined by the average of this and the 2010 data. The classes of data available for analysis are shown in Table 1. In total, 4539 samples were received from the 173 shires. After removing shires that did not fulfil the criteria, 122 shires remained to provide samples. Only one shire from Tasmania fulfilled the above criteria, and this was also removed from the dataset because, in later analyses, state was considered as a factor. Thus, the final dataset contained 121 shires.
|
Because the exact characteristics of the farms from which the samples came were unknown, it was assumed that farms matched the predominant characteristics of the corresponding shires. Hence, instead of using the numerical values of the variables, these variables were categorised and indicator variables were introduced for model fitting (Table 2). Some classes of the variables that rarely appeared in the dataset were combined to avoid potential biases.

|
Statistical analyses
The association between farming practices and incidence of herbicide resistance was analysed by using auto-binomial models (Besag 1974, 1975; Johansson 2001), which include the usual logistic regression model as a special case. The usage of auto-binomial models allowed the study of spatial effects along all other variables. The model is briefly described below. The methodology and reasoning behind the statistical procedures chosen are further explained in Ip et al. (2018).
In each shire i, it was considered that the number of resistant samples ki followed a conditional binomial distribution with ‘number of trials’ ni, the number of samples tested, and the ‘probability of success’ p(xi, kj), where xi represented the set of covariates (Table 2) of shire i, and kj represented the number of resistant samples in the ‘neighbourhood’. Beside the characteristics of the shire, the number of herbicide-resistant incidences in shires nearby was also used to model the probability of resistant incidence for that shire. For this, the latitude and longitude at the approximate central point of each shire were obtained. Shires within a distance of 750 km were considered as neighbours with Ni defined as the set containing the neighbours of shire i.
Mathematically, the logarithm-of-odds ratio (OR) under the auto-binomial model is:
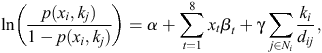
where α is the overall intercept, β is the vector of coefficients associated with the covariates, γ is the coefficient of the ‘spatial effect’, and dij is the distance between shires i and j. This model reduces to the ordinary logistic regression model when γ is zero.
Estimation of the model parameters was done by using the maximum pseudo-likelihood approach (Johansson 2001), and the standard errors of the parameters were estimated by using delete-one jackknife resampling (Friedl and Stampfer 2002). The covariates in the final model were selected by using backward selection. Specifically, all covariates were included in the model at the beginning. After each iteration, the covariate with the greatest P-value was removed if the P-value was >0.2. The process was repeated until all P-values were <0.2. Previous studies have shown that such a cut-off point would prevent elimination of some important covariates (Mickey and Greenland 1989; Maldonado and Greenland 1993).
Of the six herbicide groups considered for analysis, only the Group A dims showed a significant spatial effect in relation to incidence of resistance. Therefore, the resistance incidence of the other herbicide groups was analysed by using the ordinary logistic regression model. All estimations were carried out using R (R Core Team 2017). We refer readers to Ip et al. (2018) for further details of the statistical modelling.
Based on the final models obtained, the probability of each type of herbicide resistance was predicted for each shire. These predicted probabilities were then used to calculate the correlations. Based on how the indicator variables were introduced, a ‘baseline’ shire was a shire in Western Australia, predominantly alkaline, with crops other than wheat predominantly grown, predominantly with no cultivation, and crop residues predominantly managed other than left intact or incorporated.
Results
Note that some findings may appear significant, but the analysis indicates otherwise. This is because the statistical method considers more than one variable during the analysis. For example, differences between states may seem significant, but such differences result from different management practices rather than state per se.
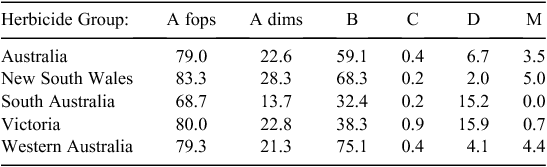
The highest incidences of resistance were found to Groups A and B herbicides, with 79% of samples resistant to Group A fop herbicides (3360 tests), 23% to Group A dim herbicides (4738 tests, with some samples tested to more than one dim), 80% to Group A dens (188 tests), 57% to Group B SUs (3111 tests) and 69% to Group B imi herbicides (232 tests). Resistance incidences were much lower for the other main herbicide groups, with 7% resistant to Group D (3962 tests) and 3% to Group M (2279 tests). Resistance to Group C herbicides was <1% (3928 tests) (Table 3).
|
Correlations between herbicides
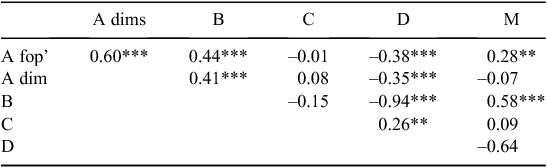
Significant correlations were observed between different herbicide groups and the incidence of resistance. Shires with a higher incidence of Group A fop resistance also had higher levels of Group A dim (P < 0.001), Group B (P < 0.001) and Group M (P < 0.01) resistance (Table 4). Increased Group A dim resistance was also correlated with increased Group B resistance (P < 0.001), and increased Group B resistance was correlated and increased Group M resistance (P < 0.001). Group D resistance was negatively correlated with all other herbicide groups (P < 0.001) except Group C, where a positive correlation (P < 0.01) was reported (Table 4).
|
Association between farm practice and herbicide resistance
Soil pH
Of the 121 shires across four states that fulfilled the criteria for analysis, 86 had soil types classed as acidic (71%) and 35 as alkaline (29%). Ten of the ‘alkaline’ shires were in South Australia (50% of South Australian shires that met the criteria), 17 in Western Australia (33%), six in Victoria (35%) and two in New South Wales (6%) (Fig. 1).

|
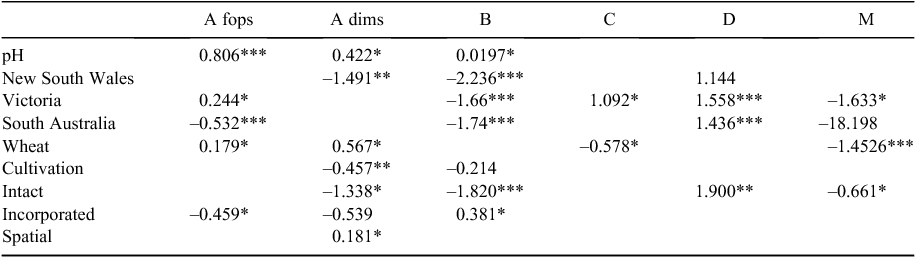
Compared with the base shire (alkaline), the odds of resistances evolving to Group A fop, Group A dim and Group B herbicides were estimated to be, respectively, 2.24 (95% confidence interval (CI) 1.86–2.69), 1.53 (95% CI 1.06–2.19) and 1.22 (95% CI 1.01–1.47) times higher in an ‘acidic’ shire (Table 5). No significant associations were found between soil pH and resistance to Groups C, D and M.
|
States
There were significant differences between states in the incidence of resistance to the different herbicide groups (Table 5) after taking into account all other variables. Compared with Western Australia, resistance to Group A fop herbicides was more likely in Victoria (OR 1.28, 95% CI 1.00–1.62) but less likely in South Australia (OR 0.59, 95% CI 0.47–0.73). Additionally, Group A fop resistance was found to be more likely in Victoria than South Australia (OR 2.17, 95% CI 1.67–2.83).
Where results were available following data analysis, resistances to both Group A dim and B herbicides were more likely to occur in Western Australia, with the odds of Group A dim resistance in a New South Wales shire 0.23 (95% CI 0.08–0.67) times that of a Western Australian shire. The odds of Group B resistances in New South Wales, Victorian and South Australian shires were, respectively, 0.11 (95% CI 0.06–0.20), 0.19 (95% CI 0.15–0.24) and 0.18 (95% CI 0.14–0.23) times that in Western Australia (Table 5).
Resistance to Group D herbicides was, however, more likely in states other than Western Australia, with the odds of Group D resistance in Victoria and South Australia being, respectively, 4.75 (95% CI 3.41–6.62) and 4.20 (95% CI 3.02–5.85) times that in Western Australia (Table 5). Group M resistance was less likely to occur in Victoria than in Western Australia (OR 0.20, 95% CI 0.05–0.81).
Winter crop
Shires that grew more wheat were more likely to have resistance to Group A fop (OR 1.20, 95% CI 1.06–1.92) and Group A dim (OR 1.76, 95% CI 1.03–3.01) herbicides than shires growing a higher proportion of other crops. Conversely, shires that grew more wheat were less likely to have resistance to Group C (OR 0.56, 95% CI 0.10–0.99) and Group M (OR 0.23, 95% CI 0.13–0.42) herbicides than shires growing a higher percentage of other crops (Table 5).
Cultivation
Only for the Group A dim herbicides was a significant difference due to the number of cultivations identified after data analysis. The odds of Group A dim resistance in shires that predominantly have at least one cultivation was 0.63 (95% CI 0.45–0.89) times that in shires with predominantly no cultivation (Table 5).
Crop residue management
Compared with the baseline shire (crop residue managed other than by leaving intact or incorporating), the odds of resistance evolving to Group A dim, Group B and Group M were lower—0.26 (95% CI 0.09–0.78), 0.16 (95% CI 0.09–0.29) and 0.52 (95% CI 0.31–0.87) times, respectively—in those shires where the crop residue was more commonly left intact. Conversely the likelihood of Group D resistance was much higher (OR 6.69, 95% CI 1.80–24.8) where crop residue was left intact (Table 5). When increased proportions of crop residues were incorporated, the likelihood of Group A fop resistance was reduced (OR 0.63, 95% CI 0.44–0.92) while resistance to the Group B herbicides was more likely (OR 1.46, 95% CI 1.00–2.14) than in shires using methods other than left intact or incorporation (Table 5).
Spatial effect
The spatial dependence parameter was found to be significantly positive for Group A dim resistance, meaning that incidences of dim resistance in neighbouring shires tended to be positively correlated.
Discussion
The significant correlation (P < 0.001) between Group A fop and dim herbicides reflects both their widespread use in winter crops and their control of annual ryegrass through inhibition of the same enzyme, acetyl-CoA carboxylase (Owen 1991). Group B herbicide resistance is also widespread and occurs over much of the same regions as for the Group A herbicides. This is shown by its positive association with both Group A fop and Group A dim herbicides, as was reported in an earlier study of New South Wales and Victorian regions (Broster and Pratley 2008).
The negative correlation between Group D and Group A fop, Group A dim and Group B herbicides is indicative of the differing application requirements. The Group D herbicides require soil incorporation immediately after application, whereas Group B herbicides are incorporated by sowing and Group A herbicides provide post-emergent, selective weed control. The Group D requirement for incorporation is largely incompatible with the direct-drilling system, whereas Groups A and B herbicides are routine options for direct drilling.
Resistances to Groups A and B herbicides were shown to be more likely where soils were acidic, whereas Group D resistance was more likely in the states with a high proportion of shires with alkaline soils (South Australia and Victoria) (Fig. 1, Tables 3 and 5). Both the Group A and Group B herbicides have high efficacy, which leads to their rapid uptake but also makes them prone to rapid development of herbicide resistance (Maxwell and Mortimer 1994; Saari et al. 1994). The pre-emergent Group B SU herbicides have limited incorporation requirement compared with many other pre-emergent herbicides, making them eminently suitable for minimum-tillage systems. However, the intensity of SU herbicide use in alkaline soils has historically been influenced by lengthy plant-back periods limiting rotational options, with Group D herbicides a common alternative (Black et al. 1999; Boutsalis et al. 2006; Nufarm Australia 2018a). This has potentially resulted in the following: higher herbicide resistance in acidic soils due to reduced cultivation associated with Group B herbicide use; lower Group A resistance in alkaline soils because some weed control can be obtained by the cultivation needed for incorporation; and increased Group D resistance in the alkaline soils due to greater selection pressure (Boutsalis et al. 2006). As a consequence of high incidence of resistances to Group A and B herbicides, and a greater understanding of application techniques for minimum-tillage systems, the use of Group D herbicides may increase in acidic soils, meaning increased selection pressure for resistance to this herbicide group.
The associations of crop-residue management and herbicide-resistance incidence are more speculative, in that the analysis does not indicate whether the practice is linked directly with the establishment of the particular crop, management of the crop residue, management of the resistant weeds or some combination. The emphasis is likely to differ depending on the crop species and location.
The positive association between Group D resistance and retaining crop residue appears counter-intuitive. The major Group D herbicide used in Australia, trifluralin, is highly volatile and requires soil incorporation. It has low water solubility and may bind to any crop residue before reaching the soil surface (Chauhan et al. 2006; Borger et al. 2015; Lewis et al. 2016). The trifluralin label (for example, TriflurX 480 g a.i. L–1) states that crop residue coverage >40–50% will limit weed control (Nufarm Australia 2018b), and this should limit its use where crop residue remains intact. However, reduced-tillage and crop-residue-retention systems have greater dependence on herbicides for weed control (D’Emden and Llewellyn 2006), and with increasing incidence of Groups A and B herbicide resistance (Table 3), Group D herbicides are used more often for pre-emergent ryegrass control because of the lack of alternative herbicide options. This has meant high selection pressure for resistance to Group D herbicides.
Shires growing a greater proportion of wheat had higher levels of resistance to Group A fop and dim herbicides. The lower risk of Group C resistance in these shires demonstrates the lower frequency of use of this group, limited to other crop species. This provides evidence of the importance of species diversity in crop rotations in enabling herbicide rotation, thereby reducing the likelihood of resistance by lowering selection pressure with respect to any herbicide groups commonly used in these rotations.
Many papers have investigated management practices for herbicide-resistant weeds (Powles and Matthews 1992; Gill 1996; Valverde et al. 2000; Beckie 2007; Moss et al. 2007; Walsh and Powles 2007; Norsworthy et al. 2012), and have also stated that there is a lack of weed control diversity before resistance developing (O’Connell and Allard 2004; Norsworthy et al. 2012). There is, however, limited knowledge of the influence of each of these management practices on resistance evolution (Beckie 2006). Beckie et al. (2004) reported that Group B resistance was higher in reduced-tillage systems, whereas Stanton et al. (2008) linked the frequency of glyphosate use and amount of soil disturbance to the risk of resistance developing.
The findings of this study indicate the potential impact of farm practice on the evolution of herbicide resistance. Continued use of herbicides with the same mode of action predisposes the farm to an economic resistance problem. Cultural practices including the choice of crop species, soil pH, cultivation and crop residue retention influence the choice and frequency of use of herbicides and thus directly or indirectly influence resistance build-up over time. This study reinforces the principle of the need to rotate chemical modes of action to minimise the build-up of resistant weed populations.
Conflicts of interest
The authors declare no conflicts of interest.
Acknowledgements
The assistance of Neil Clark and Associates in provision of data is acknowledged. The authors acknowledge the contributions from the following in aspects of the work presented in this paper: P Baines, R Graham, A Leys, J Ingrey. The following herbicide companies contributed chemicals to support the testing service; Bayer CropScience, BASF Australia, CropCare Australasia, Dow AgroSciences, DuPont, Monsanto, Nufarm, Sumitomo Chemicals and Syngenta Crop Protection.
References
ABS (2018) Agricultural commodities, Australia, 2010–11. Australian Bureau of Statistics, Canberra, ACT. Available at: http://www.abs.gov.au/AUSSTATS/abs@.nsf/DetailsPage/7121.02010-11?OpenDocument (accessed 2 October 2018).Beckie HJ (2006) Herbicide-resistant weeds: management tactics and practices. Weed Technology 20, 793–814.
| Herbicide-resistant weeds: management tactics and practices.Crossref | GoogleScholarGoogle Scholar |
Beckie HJ (2007) Beneficial management practices to combat herbicide-resistant grass weeds in the Northern Great Plains. Weed Technology 21, 290–299.
| Beneficial management practices to combat herbicide-resistant grass weeds in the Northern Great Plains.Crossref | GoogleScholarGoogle Scholar |
Beckie HJ, Hall LM, Meers S, Laslo JJ, Stevenson FC (2004) Management practices influencing herbicide resistance in wild oat. Weed Technology 18, 853–859.
| Management practices influencing herbicide resistance in wild oat.Crossref | GoogleScholarGoogle Scholar |
Besag J (1974) Spatial interaction and the statistical analysis of lattice systems. Journal of the Royal Statistical Society. Series B. Methodological 36, 192–236.
Besag J (1975) Statistical analysis of non-lattice data. The Statistician 24, 179–195.
| Statistical analysis of non-lattice data.Crossref | GoogleScholarGoogle Scholar |
Black ID, Pederson RN, Flynn A, Moerkerk MR, Dyson CB, Kookana R, Wilhelm N (1999) Mobility and persistence of three sulfonylurea herbicides in alkaline cropping soils of south-eastern Australia. Australian Journal of Experimental Agriculture 39, 465–472.
| Mobility and persistence of three sulfonylurea herbicides in alkaline cropping soils of south-eastern Australia.Crossref | GoogleScholarGoogle Scholar |
Blevins RL, Thomas GW, Smith MS, Frye WW, Cornelius PL (1983) Changes in soil properties after 10 years continuous non-tilled and conventionally tilled corn. Soil & Tillage Research 3, 135–146.
| Changes in soil properties after 10 years continuous non-tilled and conventionally tilled corn.Crossref | GoogleScholarGoogle Scholar |
Borger CPD, Riethmuller GP, Ashworth M, Minkey D, Hashem A (2015) Carrier volume is more likely to impact trifluralin efficiency than crop residue. Weed Technology 29, 63–70.
| Carrier volume is more likely to impact trifluralin efficiency than crop residue.Crossref | GoogleScholarGoogle Scholar |
Boutsalis P, Preston C, Broster JC (2006) Management of trifluralin resistance in annual ryegrass (Lolium rigidum Gaudin) in southern Australia. In ‘Proceedings 15th Australian Weeds Conference’. Adelaide, S. Aust. (Eds C Preston, JH Watts, ND Crossman) pp. 507–510. (Weed Management Society of South Australia: Adelaide, S. Aust.)
Boutsalis P, Gill GS, Preston C (2012) Incidence of herbicide resistance in rigid ryegrass (Lolium rigidum) across southeastern Australia. Weed Technology 26, 391–398.
| Incidence of herbicide resistance in rigid ryegrass (Lolium rigidum) across southeastern Australia.Crossref | GoogleScholarGoogle Scholar |
Broster JC, Pratley JE (2006) A decade of monitoring herbicide resistance in Lolium rigidum in Australia. Australian Journal of Experimental Agriculture 46, 1151–1160.
| A decade of monitoring herbicide resistance in Lolium rigidum in Australia.Crossref | GoogleScholarGoogle Scholar |
Broster JC, Pratley JE (2008) Incidence of herbicide resistance in relation to cropping practices of south-eastern Australia. In ‘Proceedings 16th Australian Weeds Conference’. Cairns, Qld. (Eds RD van Klinken, VA Osten, FD Panetta, JC Scanlan) pp. 84–86. (The Weed Society of Queensland: Brisbane, Qld)
Broster JC, Koetz EA, Wu H (2011) Herbicide resistance levels in annual ryegrass (Lolium rigidum Gaud.) in southern New South Wales. Plant Protection Quarterly 26, 22–28.
Broster JC, Koetz EA, Wu H (2012) Herbicide resistance frequencies in ryegrass (Lolium spp.) and other grass species in Tasmania. Plant Protection Quarterly 27, 36–42.
Broster JC, Koetz EA, Wu H (2013) Herbicide resistance levels in annual ryegrass (Lolium rigidum Gaud.) and wild oats (Avena spp.) in south-western New South Wales. Plant Protection Quarterly 28, 126–132.
Burnet MWM, Hildebrand OB, Holtum JAM, Powles SB (1991) Amitrole, triazine, substituted urea, and metribuzin resistance in a biotype of rigid ryegrass (Lolium rigidum). Weed Science 39, 317–323.
Chauhan BS, Gill G, Preston C (2006) Tillage systems affect trifluralin bioavailability in soil. Weed Science 54, 941–947.
| Tillage systems affect trifluralin bioavailability in soil.Crossref | GoogleScholarGoogle Scholar |
Christoffers MJ (1999) Genetic aspects of herbicide-resistant weed management. Weed Technology 13, 647–652.
| Genetic aspects of herbicide-resistant weed management.Crossref | GoogleScholarGoogle Scholar |
Christopher JT, Powles SB, Liljegren DR, Holtum JAM (1991) Cross-resistance to herbicides in annual ryegrass (Lolium rigidum). II. Chlorosulfuron resistance involves a wheat-like detoxification system. Plant Physiology 95, 1036–1043.
| Cross-resistance to herbicides in annual ryegrass (Lolium rigidum). II. Chlorosulfuron resistance involves a wheat-like detoxification system.Crossref | GoogleScholarGoogle Scholar |
D’Emden FH, Llewellyn RS (2006) No-tillage adoption decisions in southern Australian cropping and the role of weed management. Australian Journal of Experimental Agriculture 46, 563–569.
| No-tillage adoption decisions in southern Australian cropping and the role of weed management.Crossref | GoogleScholarGoogle Scholar |
Friedl H, Stampfer E (2002) Jackknife resampling. In ‘Encyclopedia of environmetrics’. Vol. 2. (Eds A El-Shaarawi, WE Piegorsch) pp. 1089–1098. (John Wiley & Sons: Hoboken, NJ, USA)
Gill GS (1996) Management of herbicide resistant ryegrass in Western Australia - research and its adoption. In ‘Proceedings 11th Australian Weeds Conference’. Melbourne, Vic. (Ed. RCH Shepherd) pp. 542–545. (Weed Science Society of Victoria: Melbourne, Vic.)
Heap JW, Knight R (1982) A population of ryegrass tolerant to the herbicide diclofop-methyl. Journal of the Australian Institute of Agricultural Science 48, 156–157.
Heap IM, Knight R (1986) The occurence of herbicide cross-resistance in a population of annual ryegrass, Lolium rigidum, resistant to diclofop-methyl. Australian Journal of Agricultural Research 37, 149–156.
| The occurence of herbicide cross-resistance in a population of annual ryegrass, Lolium rigidum, resistant to diclofop-methyl.Crossref | GoogleScholarGoogle Scholar |
Henskens FLF, Miller R, Walsh MJ, Davidson R (1996) Survey of the occurrence of herbicide resistant ryegrass in Victoria. In ‘Proceedings 11th Australian Weeds Conference’. Melbourne, Vic. (Ed. RCH Shepherd) pp. 124–125. (Weed Science Society of Victoria: Melbourne, Vic.)
Ip RHL, Ang L, Seng KP, Broster JC, Pratley JE (2018) Big data and machine learning for crop protection. Computers and Electronics in Agriculture 151, 376–383.
| Big data and machine learning for crop protection.Crossref | GoogleScholarGoogle Scholar |
Johansson J (2001) Parameter-estimation in the auto-binomial model using the coding-and pseudo-likelihood method approached with simulated annealing and numerical optimization. Pattern Recognition Letters 22, 1233–1246.
| Parameter-estimation in the auto-binomial model using the coding-and pseudo-likelihood method approached with simulated annealing and numerical optimization.Crossref | GoogleScholarGoogle Scholar |
Kennedy ML (2003) The adsorptive and degradative behaviour of imazethapyr (Spinnaker) in some south-eastern Australian soils. PhD Thesis, Charles Sturt University, NSW, Australia.
Lewis KA, Tzilivakis J, Warner D, Green A (2016) An international database for pesticide risk assessments and management. Human and Ecological Risk Assessment 22, 1050–1064.
| An international database for pesticide risk assessments and management.Crossref | GoogleScholarGoogle Scholar |
Llewellyn RS, Powles SB (2001) High levels of herbicide resistance in rigid ryegrass (Lolium rigidum) in the wheat belt of Western Australia. Weed Technology 15, 242–248.
| High levels of herbicide resistance in rigid ryegrass (Lolium rigidum) in the wheat belt of Western Australia.Crossref | GoogleScholarGoogle Scholar |
Llewellyn RS, Lindner RK, Pannell DJ, Powles SB (2004) Grain grower perceptions and the use of integrated weed management. Australian Journal of Experimental Agriculture 44, 993–1001.
| Grain grower perceptions and the use of integrated weed management.Crossref | GoogleScholarGoogle Scholar |
Locke MA, Bryson CT (1997) Herbicide-soil interactions in reduced tillage and plant residue management systems. Weed Science 45, 307–320.
Maldonado G, Greenland S (1993) Simulation study of confounder-selection strategies. American Journal of Epidemiology 138, 923–936.
| Simulation study of confounder-selection strategies.Crossref | GoogleScholarGoogle Scholar |
Matthews JM, Holtum JAM, Liljegren DR, Furness B, Powles SB (1990) Cross-resistance to herbicides in annual ryegrass (Lolium rigidum) I. Properties of the herbicide target enzymes acetyl-coenzyme A carboxylase and acetolactate synthase. Plant Physiology 94, 1180–1186.
| Cross-resistance to herbicides in annual ryegrass (Lolium rigidum) I. Properties of the herbicide target enzymes acetyl-coenzyme A carboxylase and acetolactate synthase.Crossref | GoogleScholarGoogle Scholar |
Maxwell BD, Mortimer AM (1994) Selection for herbicide resistance. In ‘Herbicide resistance in plants—biology and biochemistry’. (Eds SB Powles, JAM Holtum) pp. 1–25. (Lewis: Boca Raton, FL, USA)
McAlister FM, Holtum JAM, Powles SB (1995) Dintroaniline herbicide resistance in rigid ryegrass (Lolium rigidum). Weed Science 43, 55–62.
McKenzie NJ, Hook J (1992) Interpretations of the Atlas of Australian Soils. Consulting Report to the Environmental Resources Information Network (ERIN). Technical Report 94/1992, CSIRO Division of Soils.
Mickey RM, Greenland S (1989) The impact of confounder selection criteria on effect estimation. American Journal of Epidemiology 129, 125–137.
| The impact of confounder selection criteria on effect estimation.Crossref | GoogleScholarGoogle Scholar |
Moss SR, Perryman SAM, Tatnell LV (2007) Managing herbicide-resistant blackgrass (Alopecurus myosuroides): theory and practice. Weed Technology 21, 300–309.
| Managing herbicide-resistant blackgrass (Alopecurus myosuroides): theory and practice.Crossref | GoogleScholarGoogle Scholar |
Nietschke BS, Llewellyn RS, Reeves TG, Matthews JM, Powles SB (1996) Herbicide resistance in wild oats and annual ryegrass. In ‘Proceedings 8th Australian Agronomy Conference’. Toowoomba, Qld. (Ed. M Asghar) p. 691. (Australian Society of Agronomy)
Norsworthy JK, Ward SM, Shaw DR, Llewellyn RS, Nichols RL, Webster TM, Bradley KW, Frisvold GB, Powles SB, Burgos NR, Witt WW, Barrett M (2012) Reducing the risks of herbicide resistance: best management practices and recommendations. Weed Science 60, 31–62.
| Reducing the risks of herbicide resistance: best management practices and recommendations.Crossref | GoogleScholarGoogle Scholar |
Northcote KH (1979) ‘A factual key for the recognition of Australian soils.’ 4th edn (Rellim: Adelaide, S. Aust.)
Nufarm Australia (2018a) Lusta herbicide. Nufarm, Melbourne. Available at: https://www2.nufarm.com/au/product/lusta/ (accessed 6 December 2018)
Nufarm Australia (2018b) TriflurX herbicide label. Nufarm, Melbourne. Available at: https://cdn.nufarm.com/wp-content/uploads/sites/22/2018/05/06182526/TriflurX231115.pdf (accessed 5 July 2018)
O’Connell PJ, Allard J (2004) The management of annual ryegrass (Lolium rigidum Gaud.) in southern Australian broadacre farming systems is lacking in diversity. In ‘Proceedings 14th Australian Weeds Conference’. Wagga Wagga, NSW. (Eds BM Sindel, SB Johnson) pp. 615–618. (Weed Society of New South Wales: Wagga Wagga, NSW)
Owen WJ (1991) Differential inhibition of plant acetyl co-A carboxylase—the biochemical basis for the selectivity of the aryloxyphenoxypropionate and cyclohexanedione herbicides. In ‘Herbicide resistance in weeds and crops’. (Eds JC Caseley, GW Cussans, RK Atkin) pp. 199–212. (Butterworth Heinemann: Oxford, UK)
Owen MJ, Walsh MJ, Llewellyn RS, Powles SB (2007) Widespread occurrence of multiple herbicide resistance in Western Australian annual ryegrass (Lolium rigidum) populations. Australian Journal of Agricultural Research 58, 711–718.
| Widespread occurrence of multiple herbicide resistance in Western Australian annual ryegrass (Lolium rigidum) populations.Crossref | GoogleScholarGoogle Scholar |
Owen MJ, Martinez NJ, Powles SB (2014) Multiple herbicide-resistant Lolium rigidum (annual ryegrass) now dominates across the Western Australian grain belt. Weed Research 54, 314–324.
| Multiple herbicide-resistant Lolium rigidum (annual ryegrass) now dominates across the Western Australian grain belt.Crossref | GoogleScholarGoogle Scholar |
Parsons JM (Ed.) (1995) ‘Australian weed control handbook.’ 10th edn (Inkata Press: Melbourne)
Powles SB, Howat PD (1990) Herbicide-resistant weeds in Australia. Weed Technology 4, 178–185.
| Herbicide-resistant weeds in Australia.Crossref | GoogleScholarGoogle Scholar |
Powles SB, Matthews JM (1992) Multiple herbicide resistance in annual ryegrass (Lolium rigidum): a driving force for the adoption of integrated weed management. In ‘Resistance ’91: achievements and developments in combating pesticide resistance’. (Eds I Denholm, AL Devonshire, DW Holloman) (Elsevier Applied Science: London)
Powles SB, Lorraine-Colwill DB, Dellow JJ, Preston C (1998) Evolved resistance to glyphosate in rigid ryegrass (Lolium rigidum) in Australia. Weed Science 46, 604–607.
Pratley JE, Graham RJ, Leys AR (1993a) Determination of the extent of herbicide resistance in Southern NSW. In ‘Proceedings 10th Australian and 14th Asian-Pacific Weeds Conference’. Brisbane, Qld. pp. 286–288. (Weed Society of Qld: Brisbane, Qld)
Pratley JE, Ingrey JD, Graham RJ (1993b) Spread of herbicide resistance through seed retail outlets. In ‘Proceedings 7th Australian Agronomy Conference’. Adelaide, SA. (Eds GK McDonald, WD Belloti) pp. 151–153. (Australian Society of Agronomy)
Pratley J, Baines P, Eberbach P, Incerti M, Broster J (1996) Glyphosate resistance in annual ryegrass. In ‘Proceedings 11th Annual Conference of the Grassland Society of New South Wales’. p. 122. (Grassland Society of New South Wales: Orange, NSW)
Pratley JE, Urwin N, Stanton RA, Baines PR, Broster JC, Cullis K, Schafer D, Bohn J, Kruger R (1999) Resistance to glyphosate in Lolium rigidum. I. Bioevaluation. Weed Science 47, 405–411.
R Core Team (2017) ‘A language and environment for statistical computing.’ (R Foundation for Statistical Computing: Vienna) Available at: https://www.R-project.org/
Saari LL, Cotterman JC, Thill DC (1994) Resistance to acetolactate synthase inhibiting herbicides. In ‘Herbicide resistance in plants’. (Eds SB Powles, JAM Holtum) pp. 83–140. (CRC Press: Boca Raton, FL, USA)
Stanton RA, Pratley JE, Hudson D, Dill GM (2008) A risk calculator for glyphosate resistance in Lolium rigidum (Gaud.). Pest Management Science 64, 402–408.
| A risk calculator for glyphosate resistance in Lolium rigidum (Gaud.).Crossref | GoogleScholarGoogle Scholar |
Valverde BE, Riches CR, Caseley JC (2000) ‘Prevention and management of herbicide resistant weeds in rice: experiences from Central America with Echinochloa colona.’ (Camara de Insumos Agropecurios de Costa Rica: San José, Costa Rica)
Walsh MJ, Powles SB (2007) Management strategies for herbicide-resistant weed populations in Australian dryland crop production systems. Weed Technology 21, 332–338.
| Management strategies for herbicide-resistant weed populations in Australian dryland crop production systems.Crossref | GoogleScholarGoogle Scholar |


