Tagasaste silvopastures in steep-hill country. 1. Tagasaste edible dry-matter production and nutritive value
Katherine Tozer A * , Grant Douglas B , Emma Noakes C , Rose Greenfield A and Catherine Cameron A
A * , Grant Douglas B , Emma Noakes C , Rose Greenfield A and Catherine Cameron A
A AgResearch, Private Bag 3123, Hamilton 3240, New Zealand.
B GBDScience, Church Street, Palmerston North 4410, New Zealand.
C AgResearch, Private Bag 11008, Palmerston North 4410, New Zealand.
Crop & Pasture Science 74(9) 871-887 https://doi.org/10.1071/CP22221
Submitted: 27 June 2022 Accepted: 7 January 2023 Published: 7 March 2023
© 2023 The Author(s) (or their employer(s)). Published by CSIRO Publishing. This is an open access article distributed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND)
Abstract
Context: Tagasaste (Cytisus proliferus) has potential to supplement pasture production in steep-hill country and increase pasture resilience.
Aims: To (1) quantify production of edible dry matter (EDM) of pruned 2-year-old tagasaste shrubs and branches from unpruned 10-year-old tagasaste trees, and (2) determine the effect of proximity of the 10-year-old tagasaste trees on selected pasture species established as spaced transplants.
Methods: A site was established on the eastern coast of the North Island of New Zealand on steep-hill country (>20° slope). Tagasaste and pasture species production was measured over 3 years.
Results: Tagasaste shrubs produced an average of 2.7 kg EDM shrub−1 year−1 and a tree branch produced 129 g EDM year−1. The metabolisable energy content of tagasaste branches averaged 10.0 MJ kg EDM−1, the crude protein content ranged from 18% to 27% and neutral detergent fibre content averaged 39%. Branch diameter and length were poor predictors of EDM branch−1. There was no effect of increasing proximity to tagasaste on DM production of the resident pasture. Effects of increasing proximity to tagasaste on the growth and survival of selected pasture species are reported in a companion paper.
Conclusions: Edible-DM production was much greater from shrubs than tree branches and it was not possible to predict branch EDM from branch diameter or length.
Implications: A tagasaste silvopasture is likely to be more productive if tagasaste is managed for grazing in situ than if using mature trees for harvesting of branches for browse.
Keywords: browse feed, forage shrubs, forage trees, hill country, pasture–tree system, tagasaste silvopasture, tree lucerne, woody forages.
Introduction
Given the increasing frequency of drought in many countries, forage options are required for hill-country pastoral systems that mitigate feed deficits and increase farm-system resilience to climatic extremes. There is unexplored potential for woody species such as tagasaste (Cytisus proliferus L. f. var. palmensis Christ) to supplement the production from pasture during drought and increase farm-system resilience (Mackay-Smith et al. 2021; Tozer et al. 2021).
Tagasaste is a deep-rooted, fast-growing, drought-tolerant, leguminous tree that originated from the Canary Islands. It thrives in hot climates with well drained soils and can reach a height of 5 m (Snook 1982, 1986; Francisco-Ortega et al. 1991; Dann and Trimmer 2003). Tagasaste produces edible forage with a high digestibility (e.g. 71–78%) and crude protein content (e.g. 17–26%; Borens and Poppi 1990), which can increase livestock productivity. For example, supplementing annual pastures with tagasaste in Western Australia increased the reproductive performance, liveweight gains and wool production of sheep (Oldham and Moore 1989a, 1989b). However, livestock performance may be less than would be expected on the basis of chemical tests because of the presence of phenolic compounds that inhibit intake (Oldham et al. 1991; Edwards et al. 1997).
Although tagasaste is drought tolerant and requires well drained soils, tagasaste total and edible dry-matter (EDM) production increase with an increasing rainfall (Lefroy 2002). In Mediterranean climates with lower rainfall and annual pasture systems, tagasaste can produce more EDM than do pastures and make a substantial contribution to feed supply and livestock growth (Snook 1984; Lefroy et al. 1992; Lefroy 2002). In higher-rainfall regions with perennial pasture systems, which occur in Australia and New Zealand, tagasaste is less productive than is herbaceous pasture, but provides additional forage to supplement pastures in times of feed deficit, maintaining livestock production and adding resilience to farming systems (Lefroy 2002; Tozer et al. 2021).
Research on the establishment and management of tagasaste planted as blocks or spaced trees in perennial pastures has been conducted on flat and low-sloping land (e.g. Townsend and Radcliffe 1987, 1990; Douglas et al. 1996) and there has been some selection for tagasaste lines with desirable growth characteristics (Douglas et al. 1998). On flat and low-sloping land, EDM production of tagasaste is highly variable, ranging from 1.7 to 17.9 t DM ha−1 year−1, depending on the site, plant age and tree density (Townsend and Radcliffe 1987, 1990; Woodfield and Forde 1987; Douglas et al. 1996, 1998).
Herbage production on steep slopes is generally lower than on flat or low-sloping land, owing to reduced concentrations of nitrogen and carbon and a lower water-holding capacity on steeper slopes (e.g. Ledgard et al. 1982). Therefore, it cannot be assumed that tagasaste production data collected from low-sloping land is directly transferable to tagasaste in hill country; research is required to quantify this. In New Zealand, farmed hill country, with slopes greater than 15° and altitudes up to 1000 m, comprises 18% of the total land area (Kerr 2016). In these environments, tagasaste can provide multiple ecosystems benefits in addition to forage production, such as nitrogen fixation (Douglas et al. 2004), soil stabilisation (Andrews 1998), carbon sequestration (Wochesländer et al. 2016), provision of fodder for bees (Dann and Trimmer 1986) and shelter for livestock.
Tagasaste can be directly grazed, in which case shrubs need to be managed to maximise the production of green leafy material within reach of livestock. Most tagasaste production data are from tagasaste stands managed using this approach (e.g. Eastham et al. 1993; Douglas et al. 1996). An alternative is a ‘cut and carry’ or ‘cut and drop’ approach, in which branches from larger tagasaste trees are harvested and made available to foraging livestock. However, no data are available on the EDM of tagasaste branches from established trees. It is also not known whether tagasaste branch or trunk characteristics are related to EDM, as occurs for poplar (Populus spp.) and willow (Salix spp.; e.g. Kemp et al. 2001). Identifying such relationships is useful, to estimate the EDM available for livestock.
There is also no data available on how key temperate perennial pasture species, such as perennial ryegrass (Lolium perenne L.) or cocksfoot (Dactylis glomerata L.), are affected by the presence of tagasaste established for browse forage in hill-country pastures, hereafter referred to as a ‘tagasaste silvopasture’.
A study was conducted between August 2018 and June 2021 on a steep hill-country site to (1) quantify the EDM and nutritive values of entire tagasaste shrubs and branches of tagasaste trees; (2) determine whether there was any relationship between tagasaste branch characteristics and EDM; and (3) quantify the effect of increasing proximity to tagasaste on the growth and survival of selected pasture species.
In a companion paper by Tozer et al. (2023), we report on tagasaste EDM production and nutritive value, relationships between tagasaste and branch characteristics, and provide details on site characteristics, including solar radiation levels, soil moisture content and nutrient status, and herbage production and botanical composition of the resident pasture. In a companion paper by Tozer et al. (2023), we report on the growth and survival of selected grasses and legumes grown in increasing proximity to tagasaste.
Materials and methods
Site establishment and measurements
The field site was situated on a typical beef and sheep farm near Wairoa on the eastern coast of the North Island of New Zealand (39°01′11″S, 177°34′17.69″E, 60 asl, Waituku Station).
The area has a temperate maritime climate with average maximum temperatures in the mid-twenties in summer (December–February) and average minimum temperatures of approximately 6°C in winter (June–August). Most of the rain typically falls in winter (long-term average: 508 mm) and least rain falls in summer (long-term average: 275 mm). For the experimental period (August 2018–June 2021), total monthly rainfall was often lower than the long-term average monthly rainfall. This resulted in summer droughts in 2019–2020 and 2020–2021 and a much lower total annual rainfall in 2018 (1171 mm) and 2019 (1160 mm) than the 10-year average annual rainfall (2005–2014, 1510 mm; Fig. 1). Data were obtained from the New Zealand National Climate Database, using the nearest meteorological station as a proxy, and interpolated for the field site according to (Tait et al. 2006).
|
|
The soils were Mahoenui steepland soils (Rijkse 1979) and were classified as Orthic raw soils according to the New Zealand soil classification system (Hewitt 2010). They occur where tephra has been eroded from steep slopes and are susceptible to erosion.
The field site was on a north-west-facing hillside, with an average slope of 30°. The pasture on the hillside had not been sown or oversown for at least 30 years prior to the study.
The field site comprised two adjacent plantings of tagasaste: the first comprised 2-year-old tagasaste trees (hereafter referred to as ‘shrubs’) suitable for direct grazing, and the second a 10-year-old tagasaste silvopasture, in which tagasaste branches could be harvested from mature trees and made available to livestock in situ.
Shrub (2-year-old) silvopasture
The site was a fenced 0.05 ha planting of tagasaste, established in 2018 from transplants, at spacings of 3.5 m, which was equivalent to 816 shrubs ha−1. The slope averaged 22 ± 6° (average ± s.e.m.). The pasture was dominated by Yorkshire fog grass (Holcus lanatus L.), cocksfoot (Dactylis glomerata L.), browntop (Agrostis capillaris L.), paspalum (Paspalum dilatatum Poir.) and microlaena (Microlaena stipoides (Labill.) R.Br.).
In June 2020, 10 of the shrubs were selected for use in this study on the basis of similarity in size. The shrubs had an average height of 3.1 ± 0.5 m, canopy width of 2.7 ± 0.1 m, and root-collar diameter (diameter of the trunk at ground level) of 7.3 ± 0.7 cm (±s.e.m.). Shrubs were multi-stemmed with an average of 4 ± 0.4 stems shrub−1, emanating from 31 ± 1.8 cm above the ground surface.
Measurements
The production of EDM was assessed on four occasions, at approximately 3-monthly intervals between 1 July 2020 and 30 June 2021. On the first occasion, shrubs were pruned to remove approximately 80% of green leaf and stem on the basis of a visual estimate. This equated to pruning the shrubs to an average height of 1.5 ± 0.02 m and canopy width of 0.9 ± 0.03 m. Shrubs were pruned to the same height and canopy width thereafter. Edible (leaf and stem ≤5 mm in diameter) and inedible (stem >5 mm in diameter) portions were separated and oven dried at 65°C, for approximately 48 h, to a constant weight.
Tagasaste nutritive value was quantified in September 2020 and December 2020. A handful of leaf and edible stem (100–200 g, ≤5 mm in diameter) was obtained from each shrub and bulked over all shrubs, which equated to ≈50 g of EDM per replicate block. Vegetation was snap-frozen in dry ice, stored at −20°C, freeze dried, ground to a fine powder (sieve size <1 mm, Cyclone sample mill; UDY, Fort Collins, CO, USA), and sent to Hill Laboratories (Hamilton, New Zealand) for estimation of metabolisable energy (Kaiser et al. 2005), crude protein (AOAC 2000a) and ash (Thiex et al. 2012).
Site management
Livestock were excluded from the shrub planting for the study duration. The pasture was trimmed to approximately 10 cm residual height with a brush cutter (FS 85 R, Stihl, New Zealand) at 3-monthly intervals during the 12-month measurement period.
Tree (10-year-old) silvopasture
We quantified the relationship between branch characteristics and EDM of tagasaste trees, and the impact of increasing proximity to tagasaste on pasture species growth and survival, in a 10-year-old 0.6 ha tagasaste plantation.
Tagasaste was planted at spacings of 4 m × 4 m in 2010. Trees were thinned to spacings of 8 m × 8 m in 2019, equating to a spacing of 156 trees ha−1. Tagasaste trees had an average height of 4.3 ± 0.2 m, canopy width of 6.1 ± 0.4 m, trunk diameter (at the first branch) of 24.3 ± 0.9 cm and root-collar diameter of 25.7 ± 4.1 cm, with an average of 7.1 ± 0.9 branches per tree greater than 2.5 cm in diameter, when measured in November 2018 (average ± s.e.m., n = 7).
The shade study was established in the tagasaste silvopasture as a split-plot randomised complete-block design, with seven replicates positioned across the hill within the tagasaste plantation. Shade treatments (‘heavy shade’, ‘light shade’, and ‘open pasture’) were randomly applied to 150 cm × 60 cm main plots. Within each main plot, nine pasture species treatments (reported in Tozer et al. 2023) and a bare ground treatment were randomly allocated to 30 cm × 30 cm split-plots, which were arranged into two adjacent rows of five split-plots perpendicular to the hill slope within each main plot. The bare ground treatment allowed us to quantify emergence from the soil seedbank. The slope of each main plot was similar across all shade treatments, averaging 21° (ranging from 18.9° to 23.5° ± 1.6° (±s.e.m.)).
‘Heavy shade’ was defined as plots established within 1 m of a tagasaste trunk, ‘light shade’ between 1 m and 2 m of a tagasaste trunk and the ‘open pasture’ treatment as between trees and not directly under the canopy of a tagasaste tree. The open pasture treatment could not be regarded as being uninfluenced by adjacent tagasaste trees, above or below ground.
Measurements
Characteristics of the tagasaste trees, soil nutrients, micro-climatic variables, resident pasture production and sown pasture species were measured at intervals of 4–7 weeks from 22 August 2018 to 9 June 2021 (Table 1). There were four measurements in 2018, seven in 2019, eight in 2020 and four in 2021. Measurement frequency was lower in summer when pasture growth was slower. There were fewer visits than envisaged throughout autumn 2020 because of COVID-19-related travel restrictions. Measurements were completed within 48 h.

|
To quantify the EDM portion of tagasaste branches and determine whether there was a relationship between tagasaste branch diameter or branch length and EDM, one branch was randomly selected from six randomly selected trees, on seven occasions (3 December 2018, and 15 January, 14 March, 17 May, 16 July, 29 September and 4 December 2019). Trees neighbouring the shade treatments were avoided. The branch was removed from the tree at the junction of the branch and main trunk and the branch length and diameter were recorded. Edible and inedible portions were separated and oven dried at 65°C, for approximately 48 h, to a constant weight.
To quantify tagasaste nutritive value and mineral content, a handful (100–200 g) of edible forage was stripped from five to seven branches from seven randomly selected trees within each of the seven replicate blocks and bulked for each replicate block. This equated to ≈50 g of EDM per replicate block. Vegetation was snap-frozen in dry ice, stored at −20°C, freeze dried, ground to a fine powder (sieve size <1 mm, Cyclone sample mill; UDY, Fort Collins, CO, USA), and sent to Foragelab, Waynesboro, Pennsylvania, USA for wet-chemistry analyses and estimation of metabolisable energy, crude protein (AOAC 2000a), neutral detergent fibre (NDF; Van Soest et al. 1991), acid detergent fibre (ADF; Van Soest et al. 1991; AOAC 2000b), lignin (Goering and Van Soest 1970) and ash (Thiex et al. 2012).
Litter-accumulation and -composition data were collected to determine whether growth of pasture species was likely to be affected by litter cover. This was quantified using litter traps installed in the ground such that the top was level with the ground surface. Traps comprised PVC pipe sections with a depth of 100 mm and an internal diameter of 100 mm. The base comprised shade-cloth attached by glue and rubber-bands. Traps were positioned either adjacent to or downslope from the centre of each of the 21 main plots. Litter in each trap was collected and stored chilled until separation into tagasaste leaf, twig and reproductive material (flowers, pods), oven dried at 95°C for 48 h and the components were weighed. Dry weights for each component were summed to derive seasonal and annual total litter production.
Tagasaste canopy cover was quantified for each shade treatment on 16 July 2019, 13 March and 12 November 2020 and 28 January and 9 June 2021, by using a DSLR Nikon D5100 camera with a Sigma 4.5 mm f/2.8 circular fisheye lens held on a tripod (Zhang et al. 2005). Three photos were taken from random positions along the longitudinal mid-line of each plot, from 50 cm above the ground surface, between 0800 hours and 1000 hours. The digital hemispherical images were analysed using image J software (National Institutes of Health, USA) to quantify the percentage of the total number of pixels that comprised tagasaste canopy (Schneider et al. 2012).
Herbage production and botanical composition of resident pasture was estimated from pasture cuts in fixed quadrats (25 cm × 40 cm). Quadrats were protected by a cage (35 cm wide × 45 cm long × 27 cm high) to exclude livestock. Electric shears (12-VS Shearing Handpiece, Heiniger, New Zealand) were used to harvest the pasture within the quadrats to 3 cm above ground level. There was little pasture growing in heavy shade and often insufficient to harvest; thus, herbage production was not assessed for the heavy-shade treatment because it was considered that data collected would be unreliable. Harvested vegetation was kept chilled (4°C). A subsample was dissected into perennial ryegrass, cocksfoot, microlaena, other grasses, white clover (Trifolium repens L.), red clover (Trifolium pratense L.), subterranean clover (Trifolium subterraneum L.), broadleaf species and dead vegetation. All components and the remainder of the whole sample were oven dried at 65°C for 48 h and weighed to estimate the contribution of the different vegetation types to total dry matter (DM).
Emergence from the seedbank was quantified by counting and removing all seedlings that emerged from the bare ground treatment. Seedlings were categorised as grasses, legumes, broadleaf species or tagasaste.
Total solar radiation was measured 30 cm above the ground surface at four random locations along the longitudinal mid-line of each plot, within 1 h of solar noon, using the Photometer app (Photometer ver. 4.10.1. © 2017–2021, Przemek Pardel) installed on a Samsung Galaxy A51 smartphone.
Photosynthetically active radiation (PAR (400–700 nm waveband, μmol of photons m−2 s−1)) was measured at the same time and by the same sampling method as for total solar radiation, with a LI-250A light meter fitted with a LI-190R quantum sensor (LI-COR Biosciences, NE, USA). The first measurement was on 15 November 2018, after which equipment was not available until 26 September 2019. Thereafter, PAR data were collected on each measurement occasion.
Soil-surface temperature was logged hourly using a Thermochron® 1-Wire® iButton (iButtonLink Technology, WI, USA) positioned 0.5 cm below the soil surface in ziplock bags adjacent to each plot. Air temperature was also measured hourly with the same device enclosed within a radiation shield on a stand 30 cm above the ground and located adjacent to each plot.
Soil moisture content was measured at a depth of 0–12 cm by using a portable time domain reflectometry (TDR) instrument (Hydrosense II; Campbell Scientific), calibrated for the field site by using gravimetric soil moisture values. For the calibration, seven cores removed to a depth of 12 cm were bulked for each of three locations (which ranged in soil moisture levels) on every second or third measurement occasion (n = 16). Seven TDR readings were also obtained for each of the three locations. Soil samples were stored chilled, fresh weights obtained, oven dried at 105°C for 48 h and re-weighed to obtain the gravimetric soil moisture content. The TDR data (y) were plotted against the gravimetric soil moisture content data (x) to obtain the regression equation (y = 1.093x + 1.65, R2 = 0.92).
Soil nutrient tests were conducted on 22 August 2018 and 13 November 2021. In each plot, a 2.5-cm-diameter soil core was taken to a depth of 7.5 cm from adjacent to each surviving pasture species and bulked for each plot. Each core hole was filled with soil collected from adjacent to the plot. Soil was kept chilled at 4°C in a sealed bag until processing at Hill Laboratories, Hamilton, within 2 weeks of collection.
Site management
Livestock were excluded from the tagasaste silvopasture during establishment between August and October 2018. The plantation was grazed approximately every 6 weeks between October 2018 and June 2021, including on two occasions in 2018, seven in 2019, eight in 2020 and four in 2021, with Coopworth ewes and lambs or hoggets, at an average stocking rate of 254 ± 31 stock units ha−1 (±s.e.m.). The definition of stock units was based on industry guidelines in which one ewe is ascribed a value of one stock unit (SU) and a hogget 0.7 stock units (table 2.3 in Beef + Lamb New Zealand 2018). The number and class of livestock for each grazing depended on pasture availability and farm management constraints. Each grazing event lasted from 1 to 4 days (average of 2.4 ± 0.2 days (±s.e.m.)), depending on the class and number of livestock available for grazing the plantation. Herbage mass before grazing averaged 2400 ± 70 kg DM ha−1 (±s.e.m.) and the residual herbage mass after grazing averaged 1700 ± 50 kg DM ha−1 (±s.e.m.) on the basis of visual estimates by the farm manager.
Within 24 h before grazing, plots were covered with a plastic-mesh sheet (with 1 cm in diameter cells) pinned to the ground. This prevented stock from grazing and trampling the test plants while still allowing sunlight to reach the plants. The mesh sheet was removed within 48 h after stock were removed from the plantation.
Statistical analysis
Data for EDM of the tagasaste shrubs were summarised to obtain means and their standard errors. All data from the tagasaste silvopasture were analysed by randomised-block analysis of variance using Genstat, 21st edition (Genstat 2021). Treatments fitted in the analyses were three shade levels (heavy, light, open). Mean separation was assessed by Fisher’s protected least significant difference (l.s.d.). Data for total solar radiation and PAR were log-transformed to normalise the variance.
Results
Shrub silvopasture
EDM production
A 2-year-old tagasaste shrub produced ≈2.7 kg EDM year−1, with the lowest production occurring in autumn (Fig. 2). Edible dry matter comprised 78% of total annual DM. The trimming regime largely prevented the shrubs from producing flowers (in winter) or seeds (in summer); reproductive material (flowers and seeds) comprised 0.33% of the total annual EDM.
Nutritive values
The content of metabolisable energy in tagasaste EDM averaged 11.7 and 10.6 MJ (kg DM)−1, total crude protein 24% and 21%, neutral detergent fibre 24% and 36%, acid detergent fibre 17% and 21%, lignin 8% and 7%, and ash 4.9% and 6.2%, in September and December 2020 respectively (Table 2).

|
Tree silvopasture
EDM production
Edible dry matter of branches harvested from 10-year-old trees averaged 129 g branch−1 (range: 47–295 g). Branch length averaged 219 cm (range: 124–370 cm) and diameter averaged 2.5 cm (range: 1.4–3.8 cm).
In December 2018, there was a strong association between EDM and branch diameter (BD), with EDM = 173 × BD – 97 (R2 = 0.995, n = 7 branches, P < 0.05), but there was little association between branch diameter and yield attributes at any of the other individual harvests, as indicated by low R2 values. Across all harvests, the relationship between EDM and branch diameter was described by the following equation: EDM = 73 × BD + 31, with R2 value of 0.208 (n = 43 branches, P = 0.002).
There was no relationship between branch length and edible or inedible DM for any of the individual harvests or across all harvests (data not shown, P > 0.05).
Nutritive values and mineral content
The content of metabolisable energy in tagasaste EDM averaged 10.0 MJ (kg DM)−1, crude protein 20%, neutral detergent fibre 38%, acid detergent fibre 27%, lignin 8%, and ash 5.4% (Table 2).
The range in the content of metabolisable energy of tagasaste EDM was 9.2–10.7 MJ (kg DM)−1, crude protein 17–27%, neutral detergent fibre 28–44%, acid detergent fibre 22–33%, lignin 7–11%, 2.2–4.1% and ash 3.7–7.5% (Table 2).
The range in the content of calcium in total EDM was 0.33–0.83%, phosphorus: 0.15–0.28%, magnesium: 0.15–0.28%, magnesium: 0.21–0.44%, potassium: 1.02–1.64%, sodium: 0.09–0.23%, iron: 82–280 PPM, manganese: 105–218 PPM, zinc: 28–50 and copper: 5–15 PPM (Table 3).

|
Tagasaste litter accumulation and composition
There were significant (P < 0.05) differences among the shade treatments in litter mass for 7 of the 11 seasons (Fig. 3). The weight of litter was greater in heavy shade and/or light shade than in open pasture for all seven seasons, and in most seasons there was no significant difference between the light- and heavy-shade treatments in litter weight (P > 0.05). Tagasaste seedpods comprised 53–59% of total DM, twig (of any diameter) comprised 9–17% of total DM and leaf 30–36% of total DM depending on the year, when averaged over all shade treatments.
Tagasaste canopy cover
Canopy cover in the heavy- and light-shade treatments was similar and at least double that in the open treatment when measured on five occasions between July 2019 and July 2021 (P < 0.01, Fig. 4). Although the canopies of tagasaste trees were not directly over open-pasture treatment locations, test pasture species received some shading from neighbouring trees and the steep hillside.
Total solar radiation, photosynthetically active radiation and temperature
Solar radiation was lower in the heavy shade than in open pasture for all 17 measurement occasions, with radiation levels in light shade being intermediate but not always significantly different from either (P < 0.05, Fig. 5a). Trends for PAR were similar to those of total solar radiation, with significant differences on 13 of 14 measurement occasions (P < 0.05, Fig. 5c). Air temperature 30 cm above the ground surface was similar in all treatments (Fig. 5b) but there was more variation in the soil temperature, with a trend towards higher temperatures in the open treatment in summer (Fig. 5d). There was no difference between the shade treatments in the annual average, average minimum and average maximum air and soil temperatures (P > 0.05, Table 4).

|
Soil moisture
Soil moisture content was higher in open-pasture than in the heavy- and/or light-shade treatments on 5 of 19 occasions when sampled from under live plants (P < 0.05, Fig. 6).
Soil nutrients
The soil pH was lower in the heavy- and light-shade treatments than in the open pasture (averaging 5.2 for heavy and light vs 5.7 for open pasture, P < 0.05). Potassium concentrations were higher in soil from the heavy-shade treatment than from open pasture (1.13 vs 0.79 meq 100 g−1, P < 0.05), with concentrations in soil from beneath the light-shade treatment being intermediate (0.97 meq 100 g−1) but not significantly different from either. There were no other differences among shade treatments in measured soil parameters in 2019 or 2020 (P < 0.05, Table 5).

|
Herbage production and botanical composition of resident pasture
Herbage production (kg DM ha−1) of the resident pasture between the spaced tagasaste trees was greater in open pasture than in light shade in spring 2020 (P < 0.01, Table 6). There was also a trend towards greater herbage production in open than in light shade in 2019–2020 in three of the seasons, and for total annual herbage production, but differences were not significant (P > 0.05).
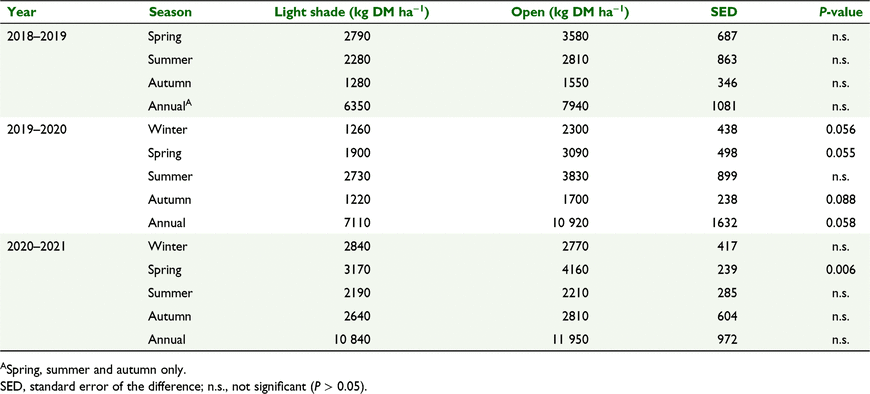
|
Botanical composition was similar in the light-shade and open-pasture treatments for most species on most measurement dates (P > 0.05, data not shown). Microlaena, followed by cocksfoot, were the most dominant species at the field site, with perennial ryegrass contributing an average of 10% of total DM (Fig. 7). The percentage of total DM was greater in open pasture than light shade for other grasses on eight occasions (P < 0.05, Table 7), cocksfoot on one occasion (P < 0.05), microlaena on two occasions (P < 0.05) and broadleaved species on one occasion (P < 0.05). The percentage of dead vegetation in total DM was greater in light shade than open pasture on two occasions (P < 0.05, Table 7).

|
Emergence from the seedbank
Seedling emergence of broadleaf species was greater from soil under open pasture than heavy shade in 2018–2019 (440 vs 130 seedlings m−2, P < 0.05, Table 8). There was a similar trend in 2020–2021, with greater emergence of broadleaf species from soil under open pasture than heavy shade and light shade, with differences approaching significance (240 vs an average of 47 seedlings m−2, P = 0.051).

|
Discussion
EDM production of tagasaste
The total annual EDM of a tagasaste shrub, averaging 2.7 kg EDM shrub−1 year−1, was within the range reported by Woodfield and Forde (1987) of 1.3–5.5 kg EDM shrub−1 year−1 for 2-year-old tagasaste shrubs, grown on flat land in the central North Island. The value obtained here was double the average of 1.3 kg EDM shrub−1 year−1, over a 3-year period for shrubs established on low-sloping land in Canterbury in the South Island of New Zealand (Townsend and Radcliffe 1987). Therefore, results indicated that tagasaste shrubs can be as productive in North Island hill country as on gently-sloping land. The higher rainfall of our North Island site and greater plant age may have contributed to the higher production than at the site in Canterbury, which received less than 550 mm per annum.
Assuming that a tagasaste shrub produces 2.7 kg EDM shrub−1 year−1, then when planted at a density of 816 shrubs ha−1, approximately 2.2 t EDM ha−1 would be produced. These estimates of tagasaste EDM per hectare are plausible given the values for tagasaste EDM per hectare reported in other studies, although planting densities and topographies vary among studies. For example, tagasaste in Australia produced 2.0 t EDM ha−1 year−1 at planting densities of 8000 trees ha−1 in a 640 mm average annual rainfall (AAR) zone in New South Wales and produced 7.9 t EDM ha−1 year−1 at planting densities of 20 000 trees ha−1 in a 1100 mm AAR zone in Victoria (Lefroy 2002).
The EDM of tagasaste branches was low. On the basis of an average of 129 g EDM branch−1, eight branches would be required to provide 1 kg of EDM. Further, the general lack of relationships between the branch and yield attributes would make it difficult to estimate the forage yield of tagasaste branches. The trees were closely spaced and gnarled, which would lead to differences between trees and branches in the amount of PAR received and plant growth. Possibly wider spacing and straighter branch growth would be required for relationships between branch characteristics and EDM to develop. Given these issues, and that manual harvesting is labour intensive, forage supplied from regular harvesting of branches is not a viable option for the provision of large quantities of feed. A better option would be direct grazing of tagasaste shrubs.
Nutritive value and mineral content
Key nutritive values of 10-year-old tagasaste EDM were similar to those found in the literature from a range of sites. For example, the metabolisable energy content of 10.0 MJ ME (kg DM)−1 and a crude protein content ranging from 17% to 27% of 10-year-old tagasaste trees were within the range obtained for metabolisable energy (9–12.5 MJ ME (kg DM)−1) and crude protein (14–30%) of tagasaste plantings in Western Australia (Tudor et al. 2001) and crude protein (17–26%) in Canterbury, New Zealand (Borens and Poppi 1990). The metabolisable energy and crude protein contents of the EDM harvested from 2-year-old shrubs were also within these cited ranges. Nutritive values of EDM from 2-year-old shrubs tended to be higher than those obtained from the 10-year-old trees (e.g. metabolisable energy, crude protein), and the fibre contents lower (e.g. NDF, ADF), possibly reflecting higher-quality regrowth after trimming. There were no published data available on the effect of tree age and defoliation on the quality of EDM; research is required to investigate this.
Increasing levels of neutral detergent fibre (NDF) are associated with a reduction in voluntary livestock intake (Ball et al. 2001). The values obtained for NDF in our study (30% and 38% of total DM respectively in 2-year-old and 10-year-old tagasaste trees) was similar to that of tagasaste grown in the central North Island of New Zealand (35% of total DM, Douglas et al. 1996). The content of NDF was also within ranges reported for lucerne (Medicago sativa L.) and red clover, and less than 50%, above which livestock growth can be suppressed (Ball et al. 2001).
The benefits of tagasaste forage for livestock performance may be overestimated if based solely on the nutritive-value data provided here. Tagasaste contains condensed tannins and alkaloids (Assefa et al. 2008), which can reduce palatability, animal intake, digestibility and absorption of protein (Edwards et al. 1997; Tudor et al. 2001; Assefa et al. 2008). In Australia, concentrations of condensed tannins have ranged from 0.5% to 5.0% in the cooler winter–spring period to 10–12% in the hot, dry late summer–autumn (Edwards et al. 1997; Wiley 2006). Increases in the concentration of condensed tannins during late summer–autumn have been associated with lower intake and growth rates in beef cattle (Edwards et al. 1997; Wiley 2006). The concentrations of phenolic compounds were not assessed here and so it is not possible to comment on implications for livestock productivity in this study.
The foliage of tagasaste contains many minerals, such as calcium and other minerals required in low amounts, which exceed concentrations required for lactating ewes with a single lamb (NRC 1975; Grace 1983). The sodium concentration of leaves was low, as was also found by Douglas et al. (1996) in a moist environment on silt loam soils, and concentrations of phosphorus and sulfur were marginal to meet animal requirements in Canterbury, New Zealand (Borens and Poppi 1990).
Tagasaste litter contributes nitrogen to the soil, which can be used to support the growth of neighbouring plants. In a coppiced tagasaste plantation in Western Australia with a density of 2330 trees ha−1, 18–25 kg DM of leaf litter per tree was produced with a N content of 1.5–2.0% of total DM (Unkovich et al. 2000). This corresponded to the production of 447 kg N ha−1 through litterfall alone. Litter production was much greater in the Western Australian study than in our study (<6 kg DM tree−1 year−1 in the heavy-shade treatment) and this is most likely to reflect differences in how the tagasaste was managed as well as how litter was measured. In the flat Western Australian site, the trees were coppiced to maintain a higher green leaf content. To measure litter fall, 1 m2 quadrats were used, which would assist in scaling the figures. On our steep-hill country site, small litter traps were used because it was not possible to assess litter on the uneven and steep topography by using large quadrats. Trees also flowered and produced pods each year, which comprised approximately 50% of the canopy litter in our study averaged over all shade treatments, most of which fell in spring and summer. The presence of litter in the open-pasture treatment demonstrated how tagasaste leaves, twigs and pods were carried by wind, water, stock or other dispersal mechanisms into the open spaces between the trees on the steep hill slope.
The extent to which the trees flowered and produced pollen and nectar would also be influenced by tagasaste defoliation management. Although capturing the effect of management on flowering and pollen and nectar provision was beyond the scope of this study, it should be recognised that provision of pollen and nectar is a critical ecosystem service. Tagasaste pollen and nectar provide a rich food source for bees (Webb and Shand 1985) and birds (Norton and Miller 2000).
Microclimate
An increase in tagasaste canopy cover resulted in a decline in solar radiation and PAR beneath the canopy, as expected. This suggests that growth responses were likely to be strongly related to differences in shading. Negative effects of shading on plant growth under controlled conditions have been attributed to reductions in PAR and changes in light quality (e.g. Devkota et al. 1997; Dodd et al. 2005).
Shade studies under controlled conditions are used as a first step to screen plants for their suitability in silvopastures (e.g. Gist and Mott 1957; Lin et al. 1998; Mauromicale et al. 2010; Ehret et al. 2015; Pang et al. 2019). Although these studies are valuable in determining whether plants are shade tolerant, care must be taken when extrapolating results to silvopastures where multiple interacting and confounding factors affect microclimatic conditions and plant growth (Dodd et al. 2005). For example, Dodd et al. (2005) used shade cloth with different levels of light interception to apply shade treatments. They found that increased shading was associated with increasing soil moisture content, most likely because of reduced solar radiation reaching the soil surface and a reduction in evaporation. Conversely, in our study, increased shading was associated with reduced soil moisture content, particularly in winter. This may be related to increased root competition and water use by tagasaste, leading to a reduction in soil moisture close to the tree. Trees may also intercept the rainfall and reduce the amount of rainfall reaching the understorey when compared with open pasture (Benavides et al. 2009). Increased tagasaste litter fall and breakdown may also affect pH and soil chemistry when compared with open pasture. The contribution of tagasaste to N fixation was not assessed in this study, but presumably N fixation would increase with proximity to a tagasaste tree. A study design such as ours does not enable us to identify key mechanisms leading to changes in the growth of understorey pasture species, given the many confounding factors. However, it provides real-world data in a variable environment, which is reflected by the large error terms in the statistical analyses of the measured parameters. This study demonstrated how results from field studies involving multiple factors can differ significantly from results obtained under controlled conditions, and enabled hypothesis development regarding mechanisms for future testing.
Resident pasture production and emergence from the seedbank
Shading had little effect on herbage production or botanical composition of resident pasture, which was dominated by microlaena and cocksfoot. Results are in contrast to those of controlled studies where increased shading has been shown to significantly reduce net herbage production by up to 80%, and to lead to a decline in legume content (e.g. Devkota et al. 1998; Dodd et al. 2005). The lack of a shade effect on herbage production of resident pasture in our study was contrary to expectations and may have reflected the patchy herbage growth on the steep hillside. Larger sample sizes and more samples may be required to detect differences. It may also reflect differences in how species respond to shading. Cocksfoot is less affected by shading than are many pasture species and microlaena is tolerant of shading (Magcalemacandog and Whalley 1991; Devkota et al. 1998). In the study of Dodd et al. (2005), the most prevalent species were perennial ryegrass and Yorkshire fog grass, both of which were suppressed by shading. There was little legume present at our site, so it is not possible to comment on the shade effect on legume content.
Emergence data from the seedbank suggested that broadleaved weed abundance would be greater in shade, but this was not reflected in the pasture botanical composition. Differences in plant functional-group abundance above and below ground have occurred in other hill-country studies. For example, broadleaved species were prevalent in the seedbank but only comprised a minor component of the percentage of total pasture DM when sampled in spring from under Canterbury beef and sheep pastures (Tozer et al. 2010). Both our study and that of Tozer et al. (2010) demonstrated how establishment from natural reseeding into a hill-country sward is generally low, and that the botanical composition does not necessarily reflect the composition of the seedbank (e.g. Hume and Barker 1991).
Conclusions
This study has shown the potential for tagasaste to provide nutritious browse forage for livestock in the eastern coast of the North Island of New Zealand, to supplement hill-country pastures when forage supply is constrained.
Shrubs, established and managed for direct grazing on sloping land, produced 2.7 kg EDM shrub−1 year−1 and it is estimated that they can make a significant biological contribution to the overall feed supply at a spacing of 816 shrubs ha−1 in steep, hill country of low productivity. In contrast, single tagasaste branches produced an average of 129 g EDM branch−1, necessitating eight branches to produce 1 kg of EDM. Given this, and the lack of ability to predict the forage supply from branch characteristics, it is recommended that tagasaste be established for direct grazing, if wanting to maximise the contribution of tagasaste forage in a silvopastoral system. However, tagasaste browse provided from branches will provide valuable minerals that are essential for livestock growth.
Data availability
The data that support this study will be shared upon reasonable request to the corresponding author.
Conflicts of interest
The authors declare no conflicts of interest.
Declaration of funding
We acknowledge the financial support of the Ministry of Primary Industries in this Sustainable Farming Funded project (405641), with co-funding and in-kind involvement of Beef + Lamb New Zealand, Ballance Agri-Nutrients and Hawke’s Bay Regional Council.
Acknowledgements
The leadership and involvement of experienced eastern coast farmers has also been invaluable. The authors thank Tony Craven, Caitlyn Dawbin, Craig Traill, Elizabeth North, Tracy Dale, Wai Aparau for assistance with field work and laboratory analyses. Thanks also go to Peter Manson (Hawke’s Bay Regional Council) for his expert advice throughout the study, Ian Tarbotton for providing strategic direction, and Robyn Dynes (AgResearch) and Cara Brosnahan (Beef + Lamb New Zealand) for their constructive comments on the manuscript.
References
Andrews G (1998) The use of trees and shrubs for livestock production. Australian Biologist 11,AOAC (2000a) ‘Protein (crude) in animal feed (990.03). Official methods of analysis.’ (Association of Official Analytical Chemists: MD, USA)
AOAC (2000b) ‘Fiber (acid detergent) and lignin in animal feed (973.18). Official methods of analysis.’ (Association of Official Analytical Chemists: MD, USA)
Assefa G, Sonder K, Wink M, Kijora C, Steinmueller N, Peters KJ (2008) Effect of variety and harvesting management on the concentration of tannins and alkaloids in tagasaste (Chamaecytisus palmensis). Animal Feed Science and Technology 144, 242–256.
| Effect of variety and harvesting management on the concentration of tannins and alkaloids in tagasaste (Chamaecytisus palmensis).Crossref | GoogleScholarGoogle Scholar |
Ball DM, Collins M, Lacefield G, Martin N, Mertens D, Olson K, Putnam D, Undersander D, Wolf M (2001) ‘Understanding forage quality.’ American Farm Bureau Federation Publication 1. pp. 1–17.
Beef + Lamb New Zealand (2018) ‘A guide to feed planning for sheep farmers.’ (Beef + Lamb New Zealand: Wellington, New Zealand)
Benavides R, Douglas GB, Osoro K (2009) Silvopastoralism in New Zealand: review of effects of evergreen and deciduous trees on pasture dynamics. Agroforestry Systems 76, 327–350.
| Silvopastoralism in New Zealand: review of effects of evergreen and deciduous trees on pasture dynamics.Crossref | GoogleScholarGoogle Scholar |
Borens FMP, Poppi DP (1990) The nutritive value for ruminants of tagasaste (Chamaecytisus palmensis), a leguminous tree. Animal Feed Science and Technology 28, 275–292.
| The nutritive value for ruminants of tagasaste (Chamaecytisus palmensis), a leguminous tree.Crossref | GoogleScholarGoogle Scholar |
Dann P, Trimmer B (1986) ‘Tagasaste (tree lucerne).’ Agfact P2.1.7. 1st edn. (NSW Agriculture: Sydney, NSW, Australia)
Dann P, Trimmer B (2003) ‘Tagasaste (tree lucerne).’ Agfact P2.1.7. Reviewed May 2003, Brendan George. (NSW Agriculture: Sydney, NSW, Australia)
Devkota NR, Kemp PD, Hodgson JG (1997) Screening pasture species for shade tolerance. Proceedings of the Agronomy Society of New Zealand 27, 119–128.
Devkota NR, Kemp PD, Valentine I, Hodgson J (1998) Performance of perennial ryegrass and cocksfoot cultivars under tree shade. Proceedings of the Agronomy Society of New Zealand 28, 129–135.
Dodd MB, McGowan AW, Power IL, Thorrold BS (2005) Effects of variation in shade level, shade duration and light quality on perennial pastures. New Zealand Journal of Agricultural Research 48, 531–543.
| Effects of variation in shade level, shade duration and light quality on perennial pastures.Crossref | GoogleScholarGoogle Scholar |
Douglas GB, Bulloch BT, Foote AG (1996) Cutting management of willows (Salix spp.) and leguminous shrubs for forage during summer. New Zealand Journal of Agricultural Research 39, 175–184.
| Cutting management of willows (Salix spp.) and leguminous shrubs for forage during summer.Crossref | GoogleScholarGoogle Scholar |
Douglas GB, Woodfield DR, Foote AG (1998) Elite selection of tagasaste (Chamaecytisus palmensis) for drought-prone sites. Proceedings of the New Zealand Grassland Association 60, 181–186.
| Elite selection of tagasaste (Chamaecytisus palmensis) for drought-prone sites.Crossref | GoogleScholarGoogle Scholar |
Douglas GB, Gadgil RL, Ede FJ, Kimberlery MO, Sandberg AM, Lowe AT, Foote AG (2004) Relative performance of 18 nitrogen-fixing plant species at three unstable coastal sand dune sites in New Zealand. New Zealand Journal of Forestry Science 34, 219–237.
Eastham J, Scott PR, Steckis RA, Barton AFM, Hunter LJ, Sudmeyer RJ (1993) Survival, growth and productivity of tree species under evaluation for agroforestry to control salinity in the Western Australian wheatbelt. Agroforestry Systems 21, 223–237.
| Survival, growth and productivity of tree species under evaluation for agroforestry to control salinity in the Western Australian wheatbelt.Crossref | GoogleScholarGoogle Scholar |
Edwards NJ, Mailey JC, McNeill DM, Lowry JB, McSweeney CS, Henry D, Oldham CM (1997) The effect of intake, palatability and digestibility of phenolic compounds in tagasaste (Chamaecytisus proliferus). In ‘Proceedings of the XVIII International Grassland Congress’, 8–19 June, Winnipeg and Saskatoon, Canada. Vol. 1. (Eds J Buchanan-Smith, L Bailey, P McCaughey) pp. 17–18. (International Grassland Society)
Ehret M, Graß R, Wachendorf M (2015) The effect of shade and shade material on white clover/perennial ryegrass mixtures for temperate agroforestry systems. Agroforestry Systems 89, 557–570.
| The effect of shade and shade material on white clover/perennial ryegrass mixtures for temperate agroforestry systems.Crossref | GoogleScholarGoogle Scholar |
Francisco-Ortega J, Fernandez-Galvan M, Santos-Guerra A (1991) A literature survey (1696–1991) on the fodder shrubs tagasaste and escobon (Chamaecytisus proliferus (L.fil) Link sensu lato) (Fabaceae: Genisteae). New Zealand Journal of Agricultural Research 34, 471–488.
| A literature survey (1696–1991) on the fodder shrubs tagasaste and escobon (Chamaecytisus proliferus (L.fil) Link sensu lato) (Fabaceae: Genisteae).Crossref | GoogleScholarGoogle Scholar |
Genstat (2021) ‘Genstat for Windows.’ 21st edn. (VSN International: Hemel Hempstead, UK)
Gist GR, Mott GO (1957) Some effects of light intensity, temperature, and soil moisture on the growth of alfalfa, red clover and birdsfoot trefoil seedlings. Agronomy Journal 49, 33–36.
| Some effects of light intensity, temperature, and soil moisture on the growth of alfalfa, red clover and birdsfoot trefoil seedlings.Crossref | GoogleScholarGoogle Scholar |
Goering HK, Van Soest PJ (1970) ‘Forage fiber analysis.’ USDA Agricultural Research Service. Handbook number 379. (US Department of Agriculture, US Government Printing Office: Washington, DC, USA)
Grace N (1983) ‘The mineral requirements of grazing ruminants.’ Occasional publication no. 9. (New Zealand Society of Animal Production: New Zealand)
Hewitt AE (2010) ‘New Zealand Soil Classification.’ Landcare Research Science Series No. 1. (Manaaki Whenua Press: Lincoln, New Zealand)
Hume DE, Barker DJ (1991) Natural reseeding of five grass species in summer dry hill country. Proceedings of the New Zealand Grassland Association 53, 97–104.
| Natural reseeding of five grass species in summer dry hill country.Crossref | GoogleScholarGoogle Scholar |
Kaiser A, Freer M, Flinn P, Black J (2005) Prediction of metabolisable energy content of forages and concentrates from measurements of digestibility. In ‘The AFIA Quality Evaluation Committee Meeting’, 17 February 2005, Adelaide, SA, Australia. (Australian Fodder Industry Association: Adelaide, Australia)
Kemp PD, Mackay AD, Matheson LA, Timmins ME (2001) The forage value of poplars and willows. Proceedings of the New Zealand Grassland Association 63, 115–119.
| The forage value of poplars and willows.Crossref | GoogleScholarGoogle Scholar |
Kerr GA (2016) Why a hill country symposium? New Zealand Grassland Association Research and Practice Series 16, 9–11.
| Why a hill country symposium?Crossref | GoogleScholarGoogle Scholar |
Ledgard SF, Sheath GW, Gillingham AG (1982) Influence of some soil and pasture components on the growth of hill country pastures 1. Winter and spring production. New Zealand Journal of Experimental Agriculture 10, 239–244.
| Influence of some soil and pasture components on the growth of hill country pastures 1. Winter and spring production.Crossref | GoogleScholarGoogle Scholar |
Lefroy EC (2002) ‘Forage Trees and Shrubs in Australia: their current use and future potential.’ Publication No. 02/039. (CSIRO Sustainable Ecosystems, Australia: ACT, Australia)
Lefroy EC, Dann PR, Wildin JH, Wesley-Smith RN, McGowan AA (1992) Trees and shrubs as sources of fodder in Australia. Agroforestry Systems 20, 117–139.
| Trees and shrubs as sources of fodder in Australia.Crossref | GoogleScholarGoogle Scholar |
Lin CH, McGraw RL, George MF, Garrett HE (1998) Shade effects on forage crops with potential in temperate agroforestry practices. Agroforestry Systems 44, 109–119.
| Shade effects on forage crops with potential in temperate agroforestry practices.Crossref | GoogleScholarGoogle Scholar |
Mackay-Smith TH, Burkitt L, Reid J, López IF, Phillips C (2021) A framework for reviewing silvopastoralism: a New Zealand hill country case study. Land 10, 1386
| A framework for reviewing silvopastoralism: a New Zealand hill country case study.Crossref | GoogleScholarGoogle Scholar |
Magcalemacandog DB, Whalley RDB (1991) Distribution of Microlaena stipoides and its association with introduced perennial grasses in a permanent pasture on the Northern Tablelands of New South Wales. Australian Journal of Botany 39, 295–303.
| Distribution of Microlaena stipoides and its association with introduced perennial grasses in a permanent pasture on the Northern Tablelands of New South Wales.Crossref | GoogleScholarGoogle Scholar |
Mauromicale G, Occhipinti A, Mauro RP (2010) Selection of shade-adapted subterranean clover species for cover cropping in orchards. Agronomy for Sustainable Development 30, 473–480.
| Selection of shade-adapted subterranean clover species for cover cropping in orchards.Crossref | GoogleScholarGoogle Scholar |
Norton BDA, Miller CJ (2000) Some issues and options for the conservation of native biodiversity in rural New Zealand. Ecological Management & Restoration 1, 26–34.
| Some issues and options for the conservation of native biodiversity in rural New Zealand.Crossref | GoogleScholarGoogle Scholar |
NRC (1975) ‘Nutrient requirements of sheep.’ (National Research Council, National Academy of Sciences: Washington, DC, USA)
Oldham CM, Moore PM (1989a) Tagasaste Chamaecytisus palmensis an evergreen fodder tree, in grazing systems of Mediterranean type climates; 1. Feed value for reproduction when grazed by merino ewes at joining. In ‘Proceedings of the XVI international grasslands congress’, Nice, France. (Ed. R Desroches) pp. 1251–1252. (Association Française pour la Production Fourragère (The French Grassland Society))
Oldham CM, Moore PM (1989b) Tagasaste Chamaecytisus palmensis an evergreen fodder tree, in grazing systems of Mediterranean type climates. 2. The feeding value for wool production when grazed by young merino ewes over summer and autumn. In ‘Proceedings of the international grasslands congress XVI’, Nice, France. (Ed. R Desroches) pp. 1253–1254. (Association Française pour la Production Fourragère (The French Grassland Society))
Oldham C, Allen G, Moore P, Mattinson B (1991) Animal production from tagasaste growing in deep sands in a 450 mm winter rainfall zone. Journal of the Department of Agriculture, Western Australia 32, 24–30.
Pang K, Van Sambeek JW, Navarrete-Tindall NE, Lin C-H, Jose S, Garrett HE (2019) Responses of legumes and grasses to non-, moderate, and dense shade in Missouri, USA. I. Forage yield and its species-level plasticity. Agroforestry Systems 93, 11–24.
| Responses of legumes and grasses to non-, moderate, and dense shade in Missouri, USA. I. Forage yield and its species-level plasticity.Crossref | GoogleScholarGoogle Scholar |
Rijkse WC (1979) Soils of part Urewera-Waikaremoana area, North Island, New Zealand. (New Zealand Soil Bureau, Department of Scientific and Industrial Research: Wellington, New Zealand)
Schneider CA, Rasband WS, Eliceiri KW (2012) NIH Image to ImageJ: 25 years of image analysis. Nature Methods 9, 671–675.
| NIH Image to ImageJ: 25 years of image analysis.Crossref | GoogleScholarGoogle Scholar |
Snook LC (1982) Tagasaste (Tree Lucerne) Chamaecytisus palmensis: a shrub with high potential as a productive fodder crop (Australia). Journal of the Australian Institute of Agricultural Science 48, 209–213.
Snook LC (1984) Tagasaste (tree lucerne): Chamaecytisus palmensis, a browse shrub which will increase production from grazing animals. Proceedings of the Australian Society of Animal Production 15, 589–592.
Snook LC (1986) ‘Tagasaste (tree lucerne), high production fodder crop.’ (Night Owl Publishers: Shepparton, Vic., Australia)
Tait A, Henderson R, Turner R, Zheng Z (2006) Thin plate smoothing interpolation of daily rainfall for New Zealand using a climatological rainfall surface. International Journal of Climatology 26, 2097–2115.
Thiex N, Novotny L, Crawford A (2012) Determination of ash in animal feed: AOAC official method 942.05 revisited. Journal of AOAC INTERNATIONAL 95, 1392–1397.
| Determination of ash in animal feed: AOAC official method 942.05 revisited.Crossref | GoogleScholarGoogle Scholar |
Townsend RJ, Radcliffe JE (1987) Establishment and management of tagasaste. Proceedings of the New Zealand Grassland Association 48, 109–113.
| Establishment and management of tagasaste.Crossref | GoogleScholarGoogle Scholar |
Townsend RJ, Radcliffe JE (1990) Tagasaste forage production systems. New Zealand Journal of Agricultural Research 33, 627–634.
| Tagasaste forage production systems.Crossref | GoogleScholarGoogle Scholar |
Tozer KN, Barker GM, Cameron CA, James TK (2010) Relationship between seedbank and aboveground botanical composition during spring. New Zealand Plant Protection 63, 90–95.
| Relationship between seedbank and aboveground botanical composition during spring.Crossref | GoogleScholarGoogle Scholar |
Tozer K, Douglas G, Dodd M, Müller K (2021) Vegetation options for increasing resilience in pastoral hill country. Frontiers in Sustainable Food Systems 5, 550334
| Vegetation options for increasing resilience in pastoral hill country.Crossref | GoogleScholarGoogle Scholar |
Tozer K, Noakes E, Douglas G, Greenfield R, Cameron C (2023) Tagasaste silvopastures in steep-hill country. 2. Effect of increasing proximity to tagasaste on the growth and survival of companion pasture species. Crop & Pasture Science 74,
Tudor G, Costa N, Taylor E, Edwards N (2001) Tagasaste. Final report. Meat & Livestock Australia, Department of Agriculture, Sydney, NSW, Australia.
Unkovich MJ, Pate JS, Lefroy EC, Arthur DJ (2000) Nitrogen isotope fractionation in the fodder tree tagasaste (Chamaecytisus proliferus) and assessment of N2 fixation inputs in deep sandy soils of Western Australia. Australian Journal of Plant Physiology 27, 921–929.
| Nitrogen isotope fractionation in the fodder tree tagasaste (Chamaecytisus proliferus) and assessment of N2 fixation inputs in deep sandy soils of Western Australia.Crossref | GoogleScholarGoogle Scholar |
Van Soest PJ, Robertson JB, Lewis BA (1991) Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. Journal of Dairy Science 74, 3583–3597.
| Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition.Crossref | GoogleScholarGoogle Scholar |
Webb CJ, Shand JE (1985) Reproductive biology of tree lucerne (Chamaecytisus palmensis, Leguminosae). New Zealand Journal of Botany 23, 597–606.
| Reproductive biology of tree lucerne (Chamaecytisus palmensis, Leguminosae).Crossref | GoogleScholarGoogle Scholar |
Wiley T (2006) Chapter 8: Fodder shrubs. In ‘Perennial pastures for Western Australia’. Bulletin 4690. (Eds G Moore, P Sanford, T Wiley) pp. 191–202. (Department of Primary Industries and Regional Development: Perth, WA, Australia)
Wochesländer R, Harper RJ, Sochacki SR, Ward PR, Revell C (2016) Tagasaste (Cytisus proliferus Link.) reforestation as an option for carbon mitigation in dryland farming systems. Ecological Engineering 97, 610–618.
| Tagasaste (Cytisus proliferus Link.) reforestation as an option for carbon mitigation in dryland farming systems.Crossref | GoogleScholarGoogle Scholar |
Woodfield DR, Forde MB (1987) Genetic variability within tagasaste. Proceedings of the New Zealand Grassland Association 48, 103–108.
| Genetic variability within tagasaste.Crossref | GoogleScholarGoogle Scholar |
Zhang Y, Chen JM, Miller JR (2005) Determining digital hemispherical photograph exposure for leaf area index estimation. Agricultural and Forest Meteorology 133, 166–181.
| Determining digital hemispherical photograph exposure for leaf area index estimation.Crossref | GoogleScholarGoogle Scholar |


