Variation in flowering time and flowering date stability within a cultivar of French serradella
Rebecca E. Haling A * , Laura Goward
A * , Laura Goward  A B , Adam Stefanski A and Richard J. Simpson
A B , Adam Stefanski A and Richard J. Simpson  A
A
A CSIRO Agriculture and Food, GPO Box 1700, Canberra, ACT 2601, Australia.
B Tasmanian Institute of Agriculture, University of Tasmania, Private Bag 1375, Launceston, Tas. 7250, Australia.
Crop & Pasture Science - https://doi.org/10.1071/CP22222
Submitted: 27 June 2022 Accepted: 29 November 2022 Published online: 23 December 2022
© 2022 The Author(s) (or their employer(s)). Published by CSIRO Publishing. This is an open access article distributed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND)
Abstract
Context: Opportunity exists to expand serradella (Ornithopus spp.) use onto heavier, duplex soil types in permanent pasture environments of south-eastern Australia. This requires cultivars with appropriate flowering times and flowering-date stability (i.e. flowering at the same date in spring regardless of timing of the autumn break).
Aim: This work examined evidence of variation in flowering date and flowering-date stability in the NSW southern tablelands for a widely-used French serradella (O. sativus) cv. Margurita.
Methods: Seed (sourced from a commercial supplier) was sown 21 March 2019 (Canberra, ACT) and 231 individual plants were monitored for time to first flower. A subset of plants exhibiting ‘early’ or ‘late’ flowering were identified and their seeds collected. In 2020, seed from ∼15 plants from each selection was sown in Canberra in late March and early May to represent an ‘early’ and a ‘later’ break of season (n = 3).
Key results: In the early-sown treatment, ‘early-flowering’ selections typically reached the median date of first flower (50%-flowering) from mid- to late August, while ‘late-flowering’ selections reached 50%-flowering early- to mid-September. When sown later, the ‘early-flowering’ selections began flowering from mid- to late September, while the ‘late-flowering’ selections flowered mid-September to early October. The ‘early’ selections exhibited greater flowering-date instability than ‘late’ selections and flowered particularly early when sown early. This indicated diversity within cv. Margurita for flowering-time control (e.g. vernalisation and/or photoperiod requirements).
Implications: Evaluating cultivars of serradellas for flowering date and flowering date stability in their target environment(s) is essential to ensure cultivars are suitably adapted to these environments.
Keywords: flowering, flowering stability, Ornithopus compressus, Ornithopus sativus, pasture legumes, subterranean clover, Trifolium subterraneum, yellow serradella.
Introduction
The most widely grown legume in permanent pasture systems of south-eastern Australia is subterranean clover (Trifolium subterraneum L.). However, there is increasing interest in the use of serradellas (Ornithopus spp.) as alternative legumes for these systems to provide a more botanically-diverse pasture base, a lower requirement for phosphorus fertiliser (Sandral et al. 2019), low oestrogenic activity, and forage with no known reports of bloat (Gladstones and McKeown 1977; Freebairn 1990; Lattimore and McCormick 2012). In Australia, serradellas were initially used on sandy, acid soils in Western Australia (WA) and north-western New South Wales (NSW) (Nichols et al. 2007). More recently, they have also found a role as a legume forage crop on sandy and loamy, acid soils of the NSW Riverina (Hackney et al. 2015). Field experiments on duplex soil types in the Riverina and southern tablelands of NSW demonstrate that both French (O. sativus Brot.) and yellow serradellas (O. compressus L.) have the potential to be as productive as subterranean clover (Dear et al. 2002; Sandral et al. 2019). However, serradellas have been observed to have poor persistence in tablelands environments (Hayes et al. 2015; Hayes et al. 2023).
The persistence of an annual legume depends on the plant successfully completing its lifecycle between the opening rainfall in autumn (the break of season) and the onset of warmer, drier conditions that typically occur towards the end of spring or in early summer. Flowering must occur at a time that ensures sufficient seed is set each year for the legume to regenerate and persist in subsequent years. The optimum date for flowering ideally needs to occur shortly after the risk of frost (which damages flowers and developing seeds) has diminished, to ensure there will be sufficient time for seed growth and maturation to occur before the summer drought (Donald 1970; Aitken 1974; Norman et al. 2005). Local climatic conditions determine the optimum flowering dates across southern Australia. These will, consequently, be predictable but regionally specific. In contrast, germination dates in pastures are highly variable because they are determined by the timing of the break of season. In south-eastern Australia, for example, opening rainfall may typically occur at any time between February (late summer) and June (early winter). Consequently, annual pasture species need to achieve a relatively stable flowering date irrespective of their germination date.
A flowering time assessment of subterranean clover, yellow serradella and French serradella cultivars grown in Perth, Tamworth, Cowra and Canberra (Boschma et al. 2019) has demonstrated that subterranean clovers can exhibit highly stable flowering dates. When sown in mid-March (representing an early break of season) and in early May (a later break of season), the early- and late-sown clover cultivars flowered within only 1–2 days (difference in median flowering dates; average among five cultivars) in Perth, Tamworth and Cowra. In Canberra, the most elevated and coolest site, the average median flowering date of the five cultivars varied by 7 days with the earlier sown material flowering sooner. In comparison, the serradella species in this study exhibited greater flowering date instability than subterranean clovers at all locations (Boschma et al. 2019). Early sown serradellas flowered between 10 and 42 days earlier than the late-sown serradellas. The tendency for earlier flowering when early-sown, was particularly pronounced among the cultivars of French serradella. In addition, three of the French serradella cultivars (cv. Cadiz, Margurita and Eliza) also exhibited a particularly wide range in flowering dates among the plants that represented each cultivar when they were sown early at the Canberra location. For example, plants within the early-sown cohort representing French serradella cv. Margurita commenced flowering over a 3-month period from early June to early September. In contrast, plants representing a locally adapted subterranean clover cultivar (cv. Goulburn) commenced flowering over a two-week period between early- and mid-October.
As outlined above, flowering date instability such as this may have adverse implications for seed set and cultivar persistence. However, it is also undesirable for forage production (cultivars are usually selected to flower close to the optimum flowering date to also maximise the vegetative forage production period), pasture feeding value (digestibility often declines when plants transition from vegetative to reproductive growth), commercial seed production and pasture management (guidelines for management of aerial seeding species emphasise the need to provide relief from grazing during seed filling to support the persistence of annual species but this becomes impossible to implement if flowering time is irregular and unpredictable) (Donald 1970; Aitken 1974; Dear et al. 2008; Thomas et al. 2021).
This paper reports a detailed study of variation in flowering time and flowering date stability of a widely-used French serradella cultivar (cv. Margurita) when grown in the NSW southern tablelands environment. Cultivar Margurita has exhibited significant flowering date instability in this environment (Boschma et al. 2019). This is usually expected to be a reflection of a cultivar’s vernalisation and photoperiod requirements for flowering time control (Goward et al. 2023). However, after an early germination event, the flowering date range of plants representing the cultivar was also very broad by comparison with other pasture legumes (Boschma et al. 2019). We hypothesised that this might indicate genetic diversity within the cultivar for flowering time control. The objective of the present work was: (i) to confirm the existence of flowering date instability in cv. Margurita, and (ii) to assess whether this was likely to be associated with genetic variation for flowering time control within the cultivar.
Materials and methods
Selection of ‘early’ and ‘late’ flowering plants of French serradella cv. Margurita (2019)
A site at the CSIRO Ginninderra Experiment Station, Canberra, Australian Capital Territory (ACT) (−35.20204, 149.08413) was prepared during March 2019. The site, which had not previously been sown to serradella, was cultivated, rolled and irrigated prior to a jute-based weed mat being laid out and pinned down. Two separate areas were prepared adjacent to each other. The first area was sown to create separate nurseries of individual plants derived from seed obtained as a commercial seed lot of French serradella cv. Margurita (source: Eastern Districts Seed Cleaning Company, Kellerberrin, WA) and a commercial seed lot of French serradella cv. Erica (Paish and Wilson Seeds, Boothendarra, WA). The cv. Margurita was the primary cultivar of interest. However, cv. Erica was also included because it is a sister selection to that of cv. Margurita, both having been derived from French serradella cv. Cadiz (Nutt 2004a, 2004b). The cv. Erica was therefore included to assess whether it exhibited the same flowering date instability as cv. Margurita. Holes (25 mm diameter; 0.5 m spacing) were cut into the weed mat and three seeds were sown into each hole (21 March 2019). After emergence (1 April), the plants were thinned to one plant per hole. Some plant losses occurred during the establishment phase and, after taking this into account, cv. Margurita was represented by 231 individual plants and cv. Erica by 33 individual plants. The large discrepancy in representation of the two cultivars was due to very poor emergence by cv. Erica. This was attributed to poor seed quality because the seed lot had been harvested about a decade before. This seed lot was the only known commercial seed of cv. Erica available in Australia at the time of the study.
The second area was sown to replicated strips of pasture legumes, representing a range of maturity types, to provide flowering time controls against which to benchmark the variation in flowering time and flowering date stability of cv. Margurita and Erica. The control genotypes were subterranean clover cv. Izmir (early-season), Seaton Park (mid-season) and Leura (late-season); yellow serradella cv. King (early-season); and French serradella cv. Serratas (late-season) (Lattimore and McCormick 2012; Boschma et al. 2019). These varieties were sourced from commercial suppliers. The control cultivars were sown in replicated strips (n = 3), each comprising 20 ‘clumps’ of plants. Each strip of plants was constructed by cutting 20 holes (25 mm diameter) in the weed mat (two parallel rows of 10 holes, 0.1 m apart, with holes offset and spaced at 0.2 m along each row). The strips were arranged in lanes within a randomised block experiment design. The spacing between strips was 1 m along the lanes, with 0.9 m between the lanes. On 22 March 2019, three seeds were sown into the 20 holes of each strip.
At sowing, all seeds were covered with a scoop of pasteurised potting mix and hand watered. The plots received a combination of hand watering and rainfall during the initial establishment phase. Serradella plants were inoculated with Group S Rhizobium (strain WSM471) and subterranean clovers were inoculated with Group C Rhizobium (WSM1325) on 18 April 2019 by watering with a slurry of peat-based Rhizobium inoculum (NewEdge Microbials, Albury, NSW).
All cultivars had begun to emerge by 1 April 2019. The controls were not thinned and the configuration of the plants is, consequently, referred to as clumps. However, when flowering dates were being assessed, only flowering by the first plant to flower in each clump was recorded; the clumps effectively represented single plants.
On 8–9 April 2019, individual plants (in the case of cv. Margurita and Erica), or clumps of plants (in the case of the control cultivars) were marked with thin bamboo pins. The marker pins: (i) ensured that any late emerging plants were recognised as such and were not assessed (thus late emergence did not confound measures of flowering date), and (ii) assisted accurate monitoring of flowering dates (pins were removed when a plant was recorded as having flowered to avoid double counting). The areas were managed for the duration of the season to reduce incursion of weeds and pests, and irrigated as required.
From 28 June onwards plants were inspected three times per week to monitor for flowering. The date at which the first petals had emerged by 1–2 mm from a calyx was recorded. In the population of plants representing cv. Margurita, the first 14 plants (10 July to 14 August 2019) and the final 38 plants (16 to 27 September 2019) to commence flowering were tagged and were assigned individual line numbers prefixed ‘E’ for early and ‘L’ for late flowering plants, respectively. These plants were individually covered with netting to exclude pollinating insects. The netting was installed on 4 October 2019 and 11 October 2019 for the early and late flowering lines, respectively. Seed was harvested from these plants on 19 Dec 2019.
Cumulative flowering percentage was plotted for all cultivars. For the control cultivars, median time to flowering (i.e. when 50% of plants had commenced flowering) was determined as the 50% intercept of the relationship between date and cumulative flowering percentage based on an average determined for the three replicates.
Assessment of flowering time and flowering date stability among various pasture legumes including ‘early’ and ‘late’ flowering lines from French serradella cv. Margurita (2020)
A second site at the CSIRO Ginninderra Experiment Station, Canberra, ACT (−35.18123, 149.06369) was prepared during March 2020. This site also had never been sown previously to serradella. The site was sprayed with herbicide to control weeds, cultivated, rolled and basal nutrients applied (250 kg/ha single superphosphate). Green plastic weed mat was laid and pinned down in rows 600 mm apart running in a north to south direction. Strips of 20 holes were burned into the weed mat in the same pattern used for the control cultivars in 2019 (i.e. two offset, parallel rows of 10 holes).
Ten cultivars of subterranean clover and five cultivars of serradella, and the ‘early’ and ‘late’ flowering selections from French serradella cv. Margurita were assessed for flowering time and flowering date stability. The cultivars of subterranean clover and serradella ranged from early- through to late-season maturity types, and were selected as flowering time controls and/or to investigate evidence of unstable flowering in these cultivars (Table 1). Thirteen ‘early’ and 15 ‘late’ flowering lines selected from French serradella cv. Margurita in 2019 were included. This subset was selected based on extremes in flowering time combined with good vigour.
Some additional accessions of French serradellas from the Australian Pastures Genebank and some novel selections of French serradella from the Tasmanian Institute of Agriculture (University of Tasmania) were also included as part of the experimental design for the purpose of assessing the flowering time of some diverse lines (Supplementary Information Table S1). While the flowering results of these additional lines are not relevant to the aims of this paper, the results are reported in the Supplementary information for completeness and to facilitate future reference.
All genotypes were sown on 24–25 March 2020 (this date represents an early break of season), and on 5–6 May 2020 (representing a later break of season). Hereafter, these sowing date treatments are referred to as the ‘early-sown’ and ‘late-sown’ treatments. Approximately three germinable seeds were sown into each weed mat hole. The experiment was a randomised block design with three replicates.
Plots were established and managed as described for the control cultivars in 2019. Serradellas were inoculated with Group S Rhizobium (strain WSM471) and subterranean clovers with Group C Rhizobium (WSM1325) on 8 April and 4 June 2020, respectively. The first flower was observed on 7 July 2020 and, thereafter, plots were inspected three times per week to record flowering.
Autumn–winter vigour of the early-sown treatment was scored on 24 July 2020 using a visual score of 1–5 (low to high). A relatively high incidence of individual subterranean clover plants with red leaves and stunting was observed among the clover cultivars; these plants were not scored for flowering time.
For all genotypes, cumulative flowering percentages were determined for each of the three replicates. Median time to flowering (i.e. when 50% of plants have commenced flowering) was determined for each replicate as the 50% intercept of the relationship between date and the cumulative flowering percentage. An average ‘median flowering date’ was determined using the three replicate values. The effect of genotype and sowing date on flowering parameters was determined by analysis of variance in GenStat 20th Edition (VSN International, UK). Data were analysed as number of days to flower from sowing date but is presented in date format to allow interpretation of flowering date stability. Amongst cultivars and seed lines, the least significant difference (l.s.d.) in flowering date is reported at P < 0.05.
Table 2 reports the rainfall and average monthly minimum and maximum air temperatures measured at Ginninderra Experiment Station in 2019 and 2020 relative to longer-term averages.

|
Results
Selection of ‘early’ and ‘late’ flowering plants of French serradella cv. Margurita (2019)
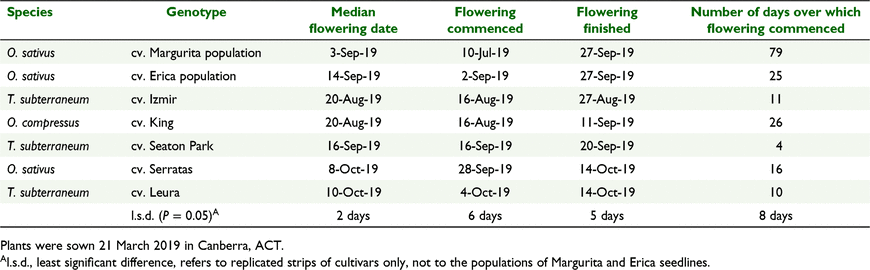
In 2019, the flowering times observed for the control cultivars were consistent with expectations based on their published maturity types. Subterranean clover cv. Izmir and yellow serradella cv. King reached 50% flowering early in the season (20 August), subterranean clover cv. Seaton Park in the mid-season (16 September) and French serradella cv. Serratas and subterranean clover cv. Leura in the late-season range (8 October and 10 October, respectively) (Fig. 1a, Table 3). In most cases, all of the plants representing the control cultivars began flowering within a relatively short period (Table 3). The shortest duration among this cohort was that of cv. Seaton Park (4 days) and longest cv. Serratas (16 days). The exception among the controls was cv. King which initiated flowering over a 26-day period.
Plants representing cv. Margurita commenced flowering over a 79-day period between 10 July and 27 September (Fig. 1a, b, Table 3). The median flowering date for cv. Margurita lay within the early to mid-season maturity-type range of the control cultivars (3 September). The tail ends of the plant population representing cv. Margurita flowered from very early in the season (i.e. a month before cv. King and cv. Izmir had commenced flowering), through to late September. Plants representing cultivar Erica commenced flowering over a 25-day period from 2–27 September with a median flowering date that was indicative of mid-season maturity (14 September), as indicated by comparison with flowering among the control cultivars (Fig. 1a, Table 3).
Assessment of flowering time and flowering date stability among various pasture legumes including ‘early’ and ‘late’ flowering lines from French serradella cv. Margurita (2020)
In 2020, the control cultivars ranked as expected based on their published maturity types (Table 1). When sown late (i.e. early May), subterranean clover cv. Izmir, Geraldton and Dwalganup reached 50%-flowering in early September (early maturity types); Seaton Park, Woogenullup, Mt Barker, Coolamon and Goulburn flowered in mid–late September (mid-late maturity types), and Leura flowered in early–mid October (late maturity type) (Fig. 2a, Table 4). French serradella cultivars Erica and Margurita reached 50% flowering in mid-late September (mid-late maturity) while yellow serradella cv. Yellotas and French serradella cv. Rosa reached 50% flowering in late September to early October (mid-late maturity) (Fig. 2a, Table 4).
When sown early (i.e. 24–25 March 2020), the cultivars maintained a similar relative ranking for 50%-flowering as per the late-sown treatment; 50%-flowering dates observed after early and late sowing were highly correlated (Fig. 2a). However, there were changes in the date at which 50%-flowering was reached by all of the serradella cultivars and by a number of the subterranean clovers (particularly early maturing cultivars) indicating flowering date instability among these cultivars. This is indicated in Fig. 2a when there is a departure of a cultivar’s datapoint from the dashed line of equality. For example, when sown early, subterranean clover cv. Izmir reached 50%-flowering 41 days earlier (in late July), and cv. Geraldton, Dwalganup and Trikkala reached 50%-flowering 19–21 days earlier (in late August) than their median flowering dates after late sowing. Among the serradellas, median flowering dates occurred from 11 (cv. Erica) to 29 days earlier (cv. Margurita) when the cultivars were sown early. In contrast, the mid- to late-season cultivars of subterranean clover (Seaton Park, Woogenellup, Coolamon, Mt Barker, Goulburn, Leura) displayed stable flowering dates (i.e. no differences) when sown early.
In 2020, the selections of French serradella cv. Margurita that had been made in 2019 for ‘early’ and ‘late’ flowering maintained a similar ranking of ‘early’ versus ‘late’ maturity. For the May-sown treatment, ‘early’ flowering selections initiated flowering from mid- to late September, while ‘late’ flowering selections flowered mid-September to early October. When sown early, the ‘early’ selections reached 50% flowering 17–54 days earlier (typically mid- to late August) compared with their respective late sowing treatment. In contrast, when sown early, the ‘late’ selections reached 50% flowering 12–28 days earlier (typically in early–mid September) compared with their respective late sowing treatment. Unlike the flowering date outcomes in 2019, where the ‘early’ and ‘late’ selections had distinctly separate flowering times (Fig. 1b), in 2020, there was some overlap in flowering time among some of the ‘early’ and ‘late’ selections (Figs 2b and 3).
Relative vigour during autumn–winter was assessed for the early-sown treatment (Table 4). For the cultivars of subterranean clover, the vigour score ranged from 1.7 to 4.7 with most cultivars exhibiting a vigour of ≥3. A novel cultivar of French serradella (Rosa) also exhibited good vigour with a score of 3.7. Vigour of the French serradella cultivar Margurita was 2.3 while ‘early’ and ‘late’ selections from this cultivar ranged from 1.7 to 3.3. There was no relationship between the vigour of the ‘early’ or ‘late’ lines selected from cv. Margurita and their median flowering date, nor was there a relationship between vigour and flowering date for the cultivars of subterranean clover or serradellas (relationships not shown).
Discussion
Flowering date stability, maturity type and growth environment
A primary defining feature of an annual pasture legume cultivar is its maturity type. Cultivars are selected by this criterion to match the length of growing season so that they achieve a long period of vegetative forage production that is balanced against the need to produce seed reliably for pasture regeneration (Donald 1970). Flowering date stability is an equally important flowering trait because it delivers these outcomes, irrespective of the timing of the break of season (i.e. germination date). In the Canberra region (average annual rainfall ∼700 mm), late-season cultivars are an appropriate fit to the growing season length (Lattimore and McCormick 2012) and the present work confirmed that late-season subterranean clover cultivars (Goulburn, Leura) display late flowering combined with a stable flowering date in this environment (Fig. 2a). Indeed, among the subterranean clover cultivars, exceptional flowering date stability was observed for all late- and many mid-season cultivars.
In contrast, the early maturing clover cultivars (Trikkala, Geraldton, Dwalganup and Izmir) displayed poor flowering date stability in the Canberra environment. For experimental reasons this is a useful observation. However, these cultivars flower too early for the growing season length at Canberra and are not recommended for use in this region. For their use in pasture systems it is important, therefore, to determine whether their flowering dates are stable under the climatic conditions associated with shorter growing seasons. For example, flowering dates for cv. Izmir were stable when it was grown in warmer environments at Perth, Cowra and Tamworth (Boschma et al. 2019). Collectively, these observations demonstrated that flowering date stability is, in part, a product of the climatic conditions under which a cultivar is grown and that the attainment of flowering date stability should be checked in the environment where use of a cultivar is intended.
In contrast to the subterranean clover cultivars, all of the mid- and late-season serradella cultivars grown in 2020 displayed considerable flowering date instability (Fig. 2a). This is not a characteristic of all serradella varieties. The very late-season yellow serradella (cv. Avila) and French serradella (cv. Serratas) display similar high flowering date stability to late-season subterranean clovers when grown in Canberra (Boschma et al. 2019), but it was clear from the present work that flowering by many serradella cultivars is less stable than subterranean clovers of equivalent maturity type. Although it has been observed previously that later maturing cultivars often display improved flowering date stability (Boschma et al. 2019), the results from the present work demonstrate that the prevalence of flowering date stability is not inextricably linked to the maturity type of a pasture legume.
Maturity type is primarily a consequence of the interaction between the vernalisation and photoperiod (long day) requirements of the cultivar (Goward et al. 2023). Early maturing cultivars of subterranean clover, French serradella and yellow serradella have low responses to vernalisation and/or photoperiod; i.e. flower without the need for prolonged exposure to cold temperatures and/or long days. In contrast, late maturity cultivars of these species have a higher requirement for vernalisation and/or photoperiod (long days) to flower, and demonstrate strong vernalisation–photoperiod interactions (i.e. long days can substitute the need for vernalisation and vice versa; Goward et al. 2023). Flowering date stability is surmised to result from a combination of these factors; e.g. a vernalisation requirement matched to the cool autumn and winter months through which the plant grows, an interaction between the flowering response to lengthening days that overrides and cancels any vernalisation requirement remaining after winter, and a small component of the response to vernalisation that is not eliminated by long daylengths alone to prevent premature flowering if the cultivar germinates during late summer/early autumn (the ‘persistent vernalisation requirement’; Goward et al. 2023). The present work indicated that maturity type is expressed more reliably among subterranean clovers and serradellas than flowering date stability. It is not yet clear how vernalisation requirement, photoperiod response and persistent vernalisation requirement must be combined to ensure cultivars always achieve a stable and characteristic flowering date.
The flowering response(s) of French serradella cv. Margurita
We specifically investigated the flowering behaviour of cv. Margurita because it displayed notably unstable flowering in the Canberra environment in comparison with subterranean clover cultivars of equivalent maturity type that display highly stable flowering (Fig. 2a). In 2019, a very wide range in flowering date was observed among individual seed lines derived from a single, commercial seed lot of cv. Margurita, when they were sown at a time that reflected an early break of season. This was consistent with previous observations by Boschma et al. (2019) who reported that cv. Margurita displayed an unstable flowering date combined with an unusually wide range in the flowering date when sown early in the Canberra environment. Indeed, this was the case for cv. Margurita when grown in all of the southern Australian climatic environments they examined (i.e. Canberra, Perth, Cowra and Tamworth). In 2020, the selected ‘early’ and ‘late’ flowering lines of cv. Margurita retained ‘early’ and ‘late’ flowering dates, respectively (Fig. 2b). This supports the hypothesis that there was genetic diversity within cv. Margurita for flowering time control. The cultivar Margurita was developed from a small number of hardseeded lines selected out of the softseeded cv. Cadiz (Nutt 2004b) and, although primarily self-fertile, is capable of up to 25% outcrossing (Nutt et al. 2021). This rate of cross-pollination is considered large enough to be useful for genetic introgression in a breeding program (Nutt et al. 2021).
In 2020, there was overlap in flowering dates of the ‘early’ and ‘late’ selections (Fig. 3), which was not observed when the initial selections were made in 2019 (Fig. 1b). The overlap in flowering responses of the selection may be due to differences in climatic conditions between 2019 and 2020. The year 2019 was relatively dry (202 mm of autumn–winter rainfall) with a warmer autumn and spring than 2020, which was a relatively wet year (447 mm of autumn–winter rainfall) (Table 2).
In addition to variation in flowering date, it was apparent that the ‘early’ and ‘late’ flowering selections from cv. Margurita also exhibited flowering date instability (i.e. they flowered earlier when sown earlier as shown by the vertical deviation of data points from the line of equality in Fig. 2b). Interestingly, the ‘late’ selections differed mainly in their flowering date (i.e. many of the ‘late’ selections were distributed along the x-axis of Fig. 2b) and displayed similar flowering date instability (i.e. the data points had a similar vertical displacement from the line of equality). In contrast, many ‘early’ selections did not differ in their flowering date, but did so in flowering date instability; i.e. early flowering variants had greater flowering date instability than late flowering variants (Fig. 2b). It is also notable that the average flowering date and flowering date instability over all of the selected lines was similar to that observed for the cv. Margurita population from which they had been sourced. Interestingly, the French serradella cv. Erica, which was also selected from cv. Cadiz in work that paralleled the selection of cv. Margurita (Nutt 2004a), has mid-season maturity and exhibits an unstable flowering date similar to that of the late selections we made from within cv. Margurita. However, cv. Erica displays a substantially more uniform distribution of flowering dates than cv. Margurita when sown early (Table 3, Boschma et al. 2019) and, consequently, we surmise that cv. Erica is a result of selections that were more genetically uniform for flowering time than either its parent (cv. Cadiz) or its sibling (cv. Margurita).
A vigour assessment was undertaken to assess whether flowering dates of the ‘early’ and ‘late’ flowering selections from cv. Margurita was likely to be related to the relative vigour of the lines. Both the ‘early’ and ‘late’ selections exhibited a similar range in vigour, and vigour was not correlated with time to flowering. We concluded that the flowering date assessments of the lines selected from cv. Margurita were not inadvertently confounded by their relative vigour. Overall, French serradella cv. Margurita had poorer vigour than anticipated based on previous performance of this cultivar at experimental sites in the NSW southern tablelands (e.g. Sandral et al. 2019). This may be associated with the relatively wet conditions experienced at the experimental site in autumn–winter 2020 (Table 2).
Overall, the results suggest that there is genetic diversity within cv. Margurita for flowering time control. It also appears that the component lines lack an adequate requirement for vernalisation and/or photoperiod (long days) to ensure a common flowering date and flowering date stability in regions with a climate that is similar to that of Canberra. We hypothesise that this is more likely to be due to differences in vernalisation requirement among the component lines. We envisage ‘early’ flowering lines within cv. Margurita have lower vernalisation requirement(s) than ‘later’ flowering lines. The apparent diversity in flowering date control within cv. Margurita appears to be mirrored by cv. Cadiz from which cv. Margurita was developed. Both cultivars flower earlier and over a relatively long period when sown early in Canberra (Boschma et al. 2019). The flowering dates of cvs. Cadiz and Margurita are also not stable in Perth, but they do not flower over the long period observed in Canberra when sown early in the Perth environment (Boschma et al. 2019). This suggests that phenotypic expression of genetic diversity for flowering control may not be as obvious in Perth where these cultivars were developed. The climatic conditions in Perth and Canberra differ substantially. Perth is relatively warm with low capacity to accrue vernal time and relatively rapid accrual of thermal time. Canberra is colder; vernal time is accumulated rapidly during winter and thermal time is accumulated comparatively slowly.
In conclusion, the results of the present work indicates that it may be possible to select earlier and later flowering variants from within cv. Margurita. However, while this may lead to variants that apparently have a more uniform maturity type due to being more genetically uniform for flowering time, all selections exhibited flowering date instability and there is little evidence that a genotype with ‘better’ stability than what currently exists can be selected from within this population. The work, therefore, highlights the need to understand the factors controlling flowering date and flowering date stability in serradella species to facilitate development of cultivars that have flowering dates and flowering date stability suited to their target environment, and especially for developing more cultivar options for colder climates. Until this is achieved, we recommend that new cultivars of serradellas be evaluated for flowering date and flowering date stability in their target environment(s) to assess whether the cultivars are suitably adapted to these environments. Cultivars adapted to their target environment are essential to maximise vegetative forage production, prolong pasture feeding value and facilitate effective grazing management.
Supplementary material
Supplementary material is available online.
Data availability
The data that support this study will be shared upon reasonable request to the corresponding author.
Conflicts of interest
The authors declare no conflicts of interest.
Declaration of funding
This work was supported by funding from the Australian Government Department of Agriculture and Water Resources as part of its Rural R&D for Profit program for project RnD4P-15-02-016 Phosphorus Efficient Pastures and from Meat and Livestock Australia (MLA), Dairy Australia and Australian Wool Innovation Limited. Data analysis continued under MLA project B.PAS.0361 ‘Serradellas for new environments’. L. Goward was supported by an AW Howard Memorial Trust Research Fellowship and Australian Sustainable Agriculture Scholarships (University of Tasmania and CSIRO).
Acknowledgements
The authors acknowledge Scott McDonald, Michela Battisti and Olivia Brunton for technical assistance. The authors acknowledge Rowan Smith and the Tasmanian Institute of Agriculture (University of Tasmania) for supplying novel germplasm of French serradella.
References
Aitken Y (1974) ‘Flowering time, climate and genotype: the adaptation of agricultural species to climate through flowering.’ (Melbourne University Press: Carlton, Vic., Australia)Aitken Y, Drake FR (1941) Studies of the varieties of subterranean clover. Proceedings of the Royal Society of Victoria 53, 342–393.
Boschma S, Kidd D, Newell M, Stefanski A, Haling R, Hayes R, Ryan M, Simpson R (2019) Flowering time responses of serradella cultivars. In ‘Proceedings of the 2019 Agronomy Australia Conference’. 25–29 August 2019, Wagga Wagga, Australia. (Australian Society of Agronomy). Available at www.agronomyaustraliaproceedings.org
Dear BS, Wilson BCD, Rodham CA, McCaskie P, Sandral GA (2002) Productivity and persistence of Trifolium hirtum, T. michelianum, T. glanduliferum and Ornithopus sativus sown as monocultures or in mixtures with T. subterraneum in the south-eastern Australian wheat belt. Australian Journal of Experimental Agriculture 42, 549–556.
| Productivity and persistence of Trifolium hirtum, T. michelianum, T. glanduliferum and Ornithopus sativus sown as monocultures or in mixtures with T. subterraneum in the south-eastern Australian wheat belt.Crossref | GoogleScholarGoogle Scholar |
Dear BS, Hackney BF, Dyce GM, Rodham CA (2008) Effect of timing of forage conservation on forage yield and quality, seed yield and seedling regeneration of four subterranean clover (Trifolium subterraneum) cultivars. Australian Journal of Experimental Agriculture 48, 1133–1142.
| Effect of timing of forage conservation on forage yield and quality, seed yield and seedling regeneration of four subterranean clover (Trifolium subterraneum) cultivars.Crossref | GoogleScholarGoogle Scholar |
Donald CM (1970) Temperate pasture species. In ‘Australian grasslands’. (Ed. RM Moore) pp. 303–320. (Australian National University Press: Canberra, ACT)
Evans PM, Lawn RJ, Watkinson AR (1992) Use of linear models to predict the date of flowering in cultivars of subterranean clover (Trifolium subterraneum L.). Australian Journal of Agricultural Research 43, 1547–1548.
| Use of linear models to predict the date of flowering in cultivars of subterranean clover (Trifolium subterraneum L.).Crossref | GoogleScholarGoogle Scholar |
Freebairn RD (1990) ‘The history of serradella (Ornithopus spp.) in NSW: a miracle pasture for light soils.’ (NSW Government Printing Service: Dubbo, NSW)
Gladstones JS, McKeown NR (1977) Serradella – a pasture legume for sandy soils. Journal of the Department of Agriculture Western Australia 18, 11–14.
Goward LE, Haling RE, Smith RW, Penrose B, Simpson RJ (2023) Flowering responses of serradella (Ornithopus spp.) and subterranean clover (Trifolium subterraneum L.) to vernalisation and photoperiod and their role in maturity type determination and flowering date stability. Crop & Pasture Science 74,
| Flowering responses of serradella (Ornithopus spp.) and subterranean clover (Trifolium subterraneum L.) to vernalisation and photoperiod and their role in maturity type determination and flowering date stability.Crossref | GoogleScholarGoogle Scholar |
Hackney B, Nutt B, Loi A, Yates R, Quinn J, Piltz J, Jenkins J, Weston L, O’Hare M, Butcher A, Butcher C, Wolfe T, Howieson J (2015) “On-demand” hardseeded pasture legumes – a paradigm shift in crop-pasture rotations for southern Australian mixed farming systems. In ‘Proceedings of the 17th Agronomy Australia Conference’, 20–24 September 2015, Hobart, Australia. (Australian Society of Agronomy). Available at www.agronomyaustraliaproceedings.org
Hayes R, Sandral G, Simpson R, Price A, Stefanksi A, Newell M (2015) A preliminary evaluation of alternative annual legume species under grazing on the Southern Tablelands of NSW. In ‘Proceedings of the 17th Agronomy Australia Conference’, 20–24 September 2015, Hobart, Australia. (Australian Society of Agronomy). Available at www.agronomyaustraliaproceedings.org
Hayes RC, Newell MT, Haling RE, Harris CA, Culvenor RA, Li GD, Badgery WB, Munday N, Price A, Stutz RE, Simpson RJ (2023) Pasture legume persistence in Tableland environments of south-eastern Australia. Crop & Pasture Science 74,
| Pasture legume persistence in Tableland environments of south-eastern Australia.Crossref | GoogleScholarGoogle Scholar |
Lattimore M, McCormick L (2012) Pasture varieties used in New South Wales 2012–2013, NSW Department of Primary Industries and Grassland Society of NSW Inc., NSW, Australia
Nichols PGH, Loi A, Nutt BJ, Evans PM, Craig AD, Pengelly BC, Dear BS, Lloyd DL, Revell CK, Nair RM, Ewing MA, Howieson JG, Auricht GA, Howie JH, Sandral GA, Carr SJ, de Koning CT, Hackney BF, Crocker GJ, Snowball R, Hughes EJ, Hall EJ, Foster KJ, Skinner PW, Barbetti MJ, You MP (2007) New annual and short-lived perennial pasture legumes for Australian agriculture – 15 years of revolution. Field Crops Research 104, 10–23.
| New annual and short-lived perennial pasture legumes for Australian agriculture – 15 years of revolution.Crossref | GoogleScholarGoogle Scholar |
Nichols PGH, Foster KJ, Piano E, Pecetti L, Kaur P, Ghamkhar K, Collins WJ (2013) Genetic improvement of subterranean clover (Trifolium subterraneum L.). 1. Germplasm, traits and future prospects. Crop & Pasture Science 64, 312–346.
| Genetic improvement of subterranean clover (Trifolium subterraneum L.). 1. Germplasm, traits and future prospects.Crossref | GoogleScholarGoogle Scholar |
Norman HC, Cocks PS, Galwey NW (2005) Annual clovers (Trifolium spp.) have different reproductive strategies to achieve persistence in Mediterranean-type climates. Australian Journal of Agricultural Research 56, 33–43.
| Annual clovers (Trifolium spp.) have different reproductive strategies to achieve persistence in Mediterranean-type climates.Crossref | GoogleScholarGoogle Scholar |
NSW Department of Primary Industries (2022a) Serradella. Available at www.dpi.nsw.gov.au/agriculture/pastures-and-rangelands/species-varieties/serradella/part-b [accessed 21 June 2022]
NSW Department of Primary Industries (2022b) Subterranean clover. Available at https://www.dpi.nsw.gov.au/agriculture/pastures-and-rangelands/establishment-mgmt/establishment/clover/part-e [accessed 21 June 2022]
Nutt B (2004a) French serradella – Erica. Plant Varieties Journal 17, 313–315.
Nutt B (2004b) French serradella – Margurita. Plant Varieties Journal 17, 310–312.
Nutt BJ, Harrison RJ, McComb JA, Howieson JG (2021) The breeding system of Ornithopus sativus Brot. subsp. sativus. Grass and Forage Science 76, 3–9.
| The breeding system of Ornithopus sativus Brot. subsp. sativus.Crossref | GoogleScholarGoogle Scholar |
Sandral GA, Price A, Hildebrand S, Fuller CG, Haling RE, Stefanski A, Yang Z, Culvenor RA, Ryan MH, Kidd DR, Diffey S, Lambers H, Simpson RJ (2019) Field benchmarking of the critical external phosphorus requirements of pasture legumes for southern Australia. Crop & Pasture Science 70, 1080–1096.
| Field benchmarking of the critical external phosphorus requirements of pasture legumes for southern Australia.Crossref | GoogleScholarGoogle Scholar |
Thomas DT, Flohr BM, Monjardino M, Loi A, Llewellyn RS, Lawes RA, Norman HC (2021) Selecting higher nutritive value annual pasture legumes increases the profitability of sheep production. Agricultural Systems 194, 103272
| Selecting higher nutritive value annual pasture legumes increases the profitability of sheep production.Crossref | GoogleScholarGoogle Scholar |
Young RR, Morthorpe KJ, Nicol HI, Croft PH (1994) Effects of sowing time and grazing on the dry matter yield, phenology, seed yield, and hardseed levels of annual pasture legumes in western New South Wales. Australian Journal of Experimental Agriculture 34, 189–204.
| Effects of sowing time and grazing on the dry matter yield, phenology, seed yield, and hardseed levels of annual pasture legumes in western New South Wales.Crossref | GoogleScholarGoogle Scholar |