Retained fishing gear and associated injuries in the east Australian grey nurse sharks (Carcharias taurus): implications for population recovery
C. S. Bansemer A B and M. B. Bennett AA School of Biomedical Sciences, University of Queensland, St Lucia, Qld 4072, Australia.
B Corresponding author. Email: carley.bansemer@uqconnect.uq.edu.au
Marine and Freshwater Research 61(1) 97-103 https://doi.org/10.1071/MF08362
Submitted: 27 December 2008 Accepted: 11 June 2009 Published: 29 January 2010
Abstract
Incidental hooking of Carcharias taurus is a threat to their populations’ recovery on the east coast of Australia. Photo-identification techniques were used to investigate the frequency of hooking at 25 aggregation sites along the east coast of Australia between 2006 and 2008. Of the 673 sharks identified, 113 sharks were identified with signs of 119 incidences of hooking. For sharks with both their left and right flank photographed during a single survey day, up to 29% of females and 52% of males were identified with retained fishing gear or an attributed jaw injury. The largest number of sharks identified (222) were from a year-round aggregation of immature and mature sharks at Fish Rock, New South Wales, Australia. Forty-eight per cent of all sharks identified with retained fishing gear were first identified at this site. Fish Rock, a designated critical habitat for C. taurus, allows most forms of line fishing except fishing with bait or wire trace while anchored or moored. As interactions with fishing gear can result in debilitating disease, morbidity and death, the high incidence of hooked individual C. taurus is considered a key threatening process that is likely to reduce this shark population’s ability to recover.
Introduction
The viability of certain shark populations is of global concern (Robbins et al. 2006; Dulvy et al. 2008; Ferretti et al. 2008). Many shark species that are targeted commercially, caught and harvested as bycatch, or captured incidentally and released by recreational or commercial fishers are at risk of over-exploitation and possible extinction (Baum et al. 2005; Campana et al. 2006; Myers et al. 2007). Significant population declines of the grey nurse shark, Carcharias taurus (Rafinesque 1810), throughout most of its range has resulted in it being listed as Vulnerable on the World Conservation Union’s Red List of threatened species (IUCN 2009) and Critically Endangered on the Australian east coast (Bansemer and Bennett 2008).
Carcharias taurus is highly susceptible to even low levels of human-induced mortality due to a late age-at-maturity and a low reproductive output (Dicken et al. 2007). Demographic studies based on abundance estimates and known anthropogenic mortality rates for C. taurus along the east coast of Australia in 2002 predicted quasi-extinction (where ≤50 females remain in the population) to occur within as little as 45 to 53 years (Otway et al. 2004). Based on concerns about the species’ viability, particularly in relation to significant declines in the Australian east coast population, C. taurus is currently protected (no sharks can be retained, intentionally captured or killed) under the Commonwealth of Australia and state legislation (Bansemer and Bennett 2008).
However, this species tends to aggregate at inshore rocky reefs (Otway and Burke 2004) that provide suitable habitats for many bony fish species that are targeted by fishers. The Australian Recovery Plan for C. taurus recognised that incidental hooking (and escape by or release of the shark) by recreational and commercial fishers was a key threat to the viability of the east coast population (Environment Australia 2002). In response, Commonwealth and state management agencies enacted various levels of protection for C. taurus at some aggregation sites along the east Australian coastline (Bennett and Bansemer 2004). The protection measures range from ‘marine sanctuary zones’ (no-take areas) that vary in size from <500 m to ≥1 km through to grey nurse shark ‘critical habitat areas’ that restrict only the use of wire trace line while anchored or moored (Bennett and Bansemer 2004; NSW Department of Primary Industries and Fisheries 2007).
As C. taurus cannot be retained, the impact of fishers on the east coast population is likely to reflect mortality related to the process of hooking and escape, capture and release or to injuries that result from fishing gear that remains attached to sharks, such as ingested hooks. While there is evidence that indicates the short-term survival rate of released sharks can be high (Gurshin and Szedlmayer 2004; Moyes et al. 2006; Hight et al. 2007), these studies do not address the issue of longer-term mortality that may result from ingested hooks and associated disease. Peritonitis, intra-lesional bacteria and cachexi have been observed in blue sharks, Prionace glauca, with retained hooks (Borucinska et al. 2001, 2002). Hooks were observed embedded in the distal oesophagus and to have perforated the gastric wall and lacerated the liver. Borucinska et al. (2002) concluded that debilitation and death as a result of retained hooks could not be ruled out and that morbidity and mortality of sharks released alive need further examination.
As incidental hooking of C. taurus on the east coast of Australia is a recognised threat to population recovery, protection measures that aimed to reduce incidental hooking and capture were implemented (Environment Australia 2002). The primary aim of the current study was to quantify the incidence of attached fishing gear and gear-related injuries after introduction of measures to reduce fishing activity–grey nurse shark interactions. The study also explored the demography of sharks with retained hooks or fishing-related injuries, and the types of fishing gear involved.
Materials and methods
Photo-identification surveys and study site
Photographs of C. taurus were collected by SCUBA divers or snorkellers from 23 grey nurse shark aggregation sites along the Queensland (Qld) and New South Wales (NSW) coastline between 2004 and 2008. In addition, four photo-identification (PID) surveys, each of 27–48 days duration, centred on the months of February and July were made by the primary author (CSB) between July 2006 and February 2008. These PID surveys covered 25 sites (Fig. 1) and aimed to photograph all grey nurse sharks at each aggregation site as part of a broader project to study the species’ population status, ecology, movements and migrations along the Australian east coast. Individual sharks with attached fishing gear or with jaw or other injuries were not preferentially targeted.

|
The protocol for PID surveys comprised two 20–60-min dives conducted in a single day at a C. taurus aggregation site. Sites with aggregations of <20 sharks generally required a single day’s effort to collect photographs of all of the sharks present at that time. In comparison, sites with aggregations of >20 sharks sometimes required several days to ensure most or all of the sharks present were photographed to allow their individual identities to be determined.
Individual sharks were identified based on the unique spot-patterns on their flanks and photographic ‘recaptures’ of individuals were determined by matching spot-patterns in the initial image with subsequent images (Bansemer and Bennett 2008). For each identification of a shark flank, the maturity (immature and mature) and the presence of retained fishing gear or jaw injuries inflicted by fishing gear were recorded. Maturity was inferred from an estimate of a shark’s total length (Lt) based on published correlations between maturity and Lt (Lucifora et al. 2002; Dicken et al. 2007). Male sharks ≤200 cm Lt and female sharks ≤220 cm Lt were considered immature (Bansemer and Bennett 2009).
Fishing gear and jaw injuries inflicted by fishing gear
An incidence with fishing gear was recorded when a shark was first identified with retained fishing gear or injuries that were considered to have resulted from a previous incidence with fishing gear. Jaw injuries appeared similar to injuries observed on sharks with fishing gear still attached and included tissue necrosis and abscesses that extended around the site of a previously embedded hook, jaw dislocation and tearing, and healed injuries that had left a permanent deformity (see Fig. S1 available online as an Accessory Publication to this paper). Some sharks were hooked on more than one occasion and each clearly different hooking event was recorded as a single incidence with fishing gear. When results include multiple hooking events, they are reported as the total number of incidences with retained fishing gear or jaw injuries rather than the total number of individuals identified with retained fishing gear or jaw injuries. Sharks that had been tail-roped, presumably to enable fishing gear to be removed before their release were included in the counts of sharks with retained fishing gear or associated injuries. These sharks presented either with rope still looped around the caudal peduncle or with abrasions consistent with ‘friction burns’ from struggling when tail-roped. The maturity of a shark when it is first identified with retained fishing gear or related injury is reported. For sharks without retained fishing gear or injuries, the most recent maturity status is reported.
Analyses
To determine whether underwater photographers targeted sharks with retained hooks or resultant jaw injuries, the four PID surveys undertaken by CSB were also analysed separately. Results from PID surveys were compared with results obtained from the total identified population of C. taurus (i.e. includes photos provided by the recreational diving community). Several sharks were identified with the same attached fishing gear or injury at multiple sites. In these cases, an incidence was recorded only for the earliest observation when fishing gear or a gear-related injury was present.
The overall frequencies of occurrence of (1) attached fishing gear or fishing gear + injury, and (2) gear-related injury only were determined for male and female sharks irrespective of site of photographic capture for the PID survey data alone and for the total identified population of C. taurus. PID survey data were also used to investigate the occurrence of fishing gear and jaw injuries in relation to a shark’s maturity status. Results for sharks identified by their contralateral flank were combined with results for sharks identified by their left or right flank only. Additional analysis was undertaken on a sub-sample of sharks that were matched for their left and right flank and had both flanks photographed on a single day during a PID survey period.
Inter-site differences in the frequency of occurrence of attached gear were evaluated based on data from the PID surveys only. The number of identified sharks with gear recorded at a site was expressed as a proportion of the total number of identified sharks seen at that site (irrespective of whether those sharks were seen elsewhere during the PID surveys). Only 15 of the 25 PID surveyed sites were included in this inter-site analysis as there were no sharks or only a single shark identified at the other sites. To test for inter-site differences, a chi-squared analysis was used. The potential for a shark to have been hooked at another location was reduced by only analysing sharks with retained hooks. Chi-squared analyses were also used to test for (1) a difference between the proportion of male and female sharks identified during PID surveys with retained fishing gear or resultant jaw injuries, and (2) whether the proportion of sharks with retained fishing gear or resultant injuries differed between immature and mature sharks.
Results
Jaw injuries
Jaw injuries caused by fishing gear varied and were often progressive (Fig. 2). Retained hooks located in the jaw did not always result in a jaw injury and in some instances relatively small injuries healed completely. More severe jaw injuries were observed on sharks with either small or large hooks. During PID surveys, 25 males and 26 females were recorded with jaw injuries only (no hook or trailing line present) and the severity of the injury and degree of likely permanent deformity varied. Many sharks with retained fishing gear also presented with jaw injuries.
Hook location
The location of attached fishing gear included ‘internal’ where no hook was visible and nylon or wire trace protruded from the mouth, gill slits or cloaca or ‘external’ where the hook was attached and visible in the shark’s jaw or other external attachment points. Thirty-two different occurrences of ‘internally located’ fishing gear and 56 occurrences of ‘externally located’ fishing gear were recorded between 2004 and 2008. The location of a further 19 occurrences with fishing gear could not be confidently determined.
Occurrence of retained fishing gear and jaw injuries in C. taurus
A total of 673 sharks were identified during PID surveys between July 2006 and February 2008 and 119 occurrences of retained fishing gear or injuries consistent with interactions with fishing gear were recorded from 113 different sharks (Table 1). Between 2004 and 2008, 930 individual C. taurus were identified from 23 of the 25 aggregation sites surveyed (Fig. 1) along the NSW and southern Qld coastline; no sharks were identified from North-west Solitary Island or South-west Solitary Island. Overall, 166 occurrences of retained fishing gear or injury that could be attributed to a previous incidence with fishing gear were recorded from 158 identified sharks (Table 1). There was no significant difference between the occurrence of fishing gear and jaw injuries between PID surveys and for all sharks identified between 2004 and 2008 (χ12 = 0.00; P = 0.9859).
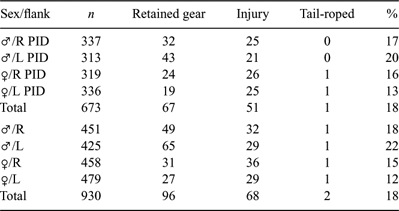
|
A maximum of 23 female and 29 male sharks were matched for their contralateral flank on a single survey day during any of the PID survey periods. The maximum occurrence of retained fishing gear or jaw injuries during an individual survey period for sharks matched for their contralateral flank during a single dive was 29% for females and 52% for males (Table 2).
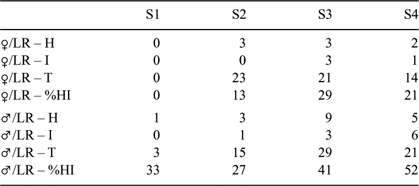
|
In PID surveys and for all sharks identified between 2004 and 2008, males had a higher occurrence of retained fishing gear and jaw injuries than females. However, this difference was not significant for the PID surveys (χ12 = 3.115; P = 0.0776), but was significant across all identifications between 2004 and 2008 (χ12 = 12.742; P = 0.0004) with a higher than expected occurrence in males and significantly lower occurrence in females.
During PID surveys, the occurrence of retained fishing gear and gear-related jaw injuries was significantly higher for mature sharks than immature sharks (χ12 = 5.126; P = 0.0236). However, when the occurrence of retained fishing gear was analysed separately from jaw injuries, there was no significant difference between mature or immature sharks (χ12 = 0.22; P = 0.8825). However, fewer immature sharks had jaw injuries compared with mature sharks (χ12 = 11.33; P = 0.0008) (Table 3).
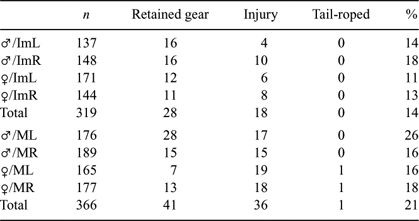
|
The total number of sharks identified at different aggregation sites varied considerably during PID surveys, with more than 30% (222 individuals) identified at Fish Rock (Table 4). During PID surveys, 32 sharks were first identified with retained fishing gear at Fish Rock; the expected value was 21 (based on the combined proportion of sharks with retained gear from the l5 aggregation sites where five or more sharks were identified during PID surveys). Fish Rock was the only site that had a significantly higher number of sharks with retained fishing gear than expected (χ12 = 5.762; P = 0.012) (Table 4).
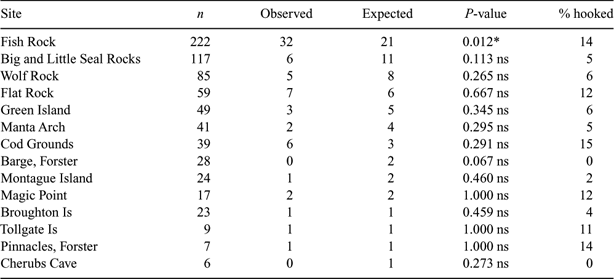
|
The types of fishing gear observed on individual C. taurus between 2004 and 2008 varied considerably (see Fig. S2). For 100 out of 166 occurrences, the retained fishing gear could be further divided into five categories: (1) relatively light fishing gear consistent with that used by recreational fishers (n = 48); (2) gear commonly used by commercial long-line fishers (n = 11); (3) gear often used by fishers when trolling or drifting (n = 4); (4) heavier gear (large hooks) that may have been targeting shark (n = 33); and (5) sharks identified with rope around their caudal peduncle (n = 3).
Discussion
In the current study, up to 52% of males and 29% of females (with their left and right flanks photographed on a single PID survey day) were observed with attached fishing gear or gear-related injuries. Previous visual surveys conducted along the east coast of Australia between 1999 and 2000 reported that 9.55% of C. taurus were observed with fishing gear attached, while in 2003, 29.3% (of 24 tagged sharks) were observed with fishing gear within 12 months of being tagged (Otway and Burke 2004). These results suggest that the proportion of sharks with attached fishing gear has increased, although the methods used to obtain these data vary. Although 20% of males and 15% of females that had only a single flank photographed during PID surveys were observed with attached fishing gear or associated injuries, it is likely that some of these sharks had fishing gear or associated injuries present on the flank not photographed. All sharks that were identified with visible external fishing gear or jaw injuries have survived the hooking event and escaped, or possibly survived a capture-and-release event.
Sharks that swallow hooks are likely to suffer a shortened lifespan and reduced reproductive output as ingested hooks cause serious disease and may result in long-term mortality (Borucinska et al. 2002; Donaldson et al. 2008). In the current study, only 32 sharks (3.4%) showed evidence of internalised fishing gear, with fishing line trailing from the mouth, gill-slits or cloaca. However, necropsies of eight accidentally caught and killed C. taurus found that although there was no external evidence of fishing gear, six sharks (75%) were hooked internally (Otway and Burke 2004). The disparity between these two results suggests that we underestimated the true rate of internalised gear and that the true rate of interaction between fishing activities and C. taurus may be substantially higher than reported here. It appears that fishing activities remain a key threatening process to this species and is likely to decrease the recovery potential for this population.
While the current study does not attempt to quantify the proportion of sharks that are likely to suffer morbidity as a result of interactions with fishing gear, it does provide a rate of hooking of C. taurus along the east coast of Australia. An important assumption of the spatial analysis in this study is that the site of first identification of a shark with attached fishing gear is where the interaction occurred. While interactions could have occurred at other locations, the assumption is supported by multiple photographic recaptures of individual sharks initially without attached gear and subsequently with attached gear at the same site. This was particularly noticeable at Fish Rock, a heavily fished site.
In December 2003, three C. taurus aggregation sites in Queensland waters (Wolf Rock, Cherubs Cave and Henderson Rock) were protected from all forms of line fishing within a 1.2-km radius of a central coordinate. For Flat Rock and Wolf Rock, the proportion of hooked sharks during the scheduled surveys was lower than for all sites combined. Importantly, at Wolf Rock where pregnant sharks remain for up to 10 months during gestation (Bansemer and Bennett 2009), the incidence of gear and injuries was low. In most instances, retained fishing gear was present on individual sharks at the time of their first identification at Wolf Rock and may indicate that these sharks had been hooked before their arrival at Wolf Rock. Across all sites, significantly fewer female sharks were observed with attached fishing gear or jaw injuries compared with male sharks. The behaviour of pregnant C. taurus may inadvertently reduce their exposure to incidental hooking as 67% of all identified mature female C. taurus (161 of 240) may spend up to 10 months (during gestation) every 2 or 3 years within the no-take marine sanctuary zone that surrounds Wolf Rock (Bansemer and Bennett 2009).
Protection measures for C. taurus in Queensland waters appear successful, although illegal fishing within these protected waters is an ongoing problem (Carley Bansemer, unpubl. data). In NSW state waters, the protection measures for C. taurus are varied. In 2003, the NSW Department of Primary Industries and Fisheries (DPI) declared 10 sites ‘critical habitat’ for C. taurus and yet most forms of fishing are still allowed (NSW Department of Primary Industries and Fisheries 2007) (Fig. 1). However, additional protection is provided for C. taurus at several sites through overlapping marine sanctuary zones that are managed by the NSW Marine Park Authority. In this study, Big and Little Seal Rocks combined had the second highest number of sharks identified during the scheduled surveys (123 individuals) of which only six were observed with retained fishing gear. The marine sanctuary zone that surrounds Big and Little Seal Rocks extends by at least 2 km in all directions. Across all sites, significantly fewer immature sharks were observed with fishing gear or jaw injuries than mature sharks and ~16% of all immature sharks were identified at Seal Rocks. Juvenile C. taurus from South African waters are relatively site-attached and remain in nursery areas until they reach sexual maturity (Smale 2002; Dicken et al. 2007). The proportion of immature sharks identified within the no-take waters at Seal Rocks combined with their likely restricted movement patterns should decrease their exposure to fishers and may contribute to the reduced proportion of juvenile C. taurus observed with fishing gear or associated injuries.
In comparison, at Fish Rock off the mid-north NSW coast the ‘grey nurse shark critical habitat’ managed by NSW DPI extends for ~200 m from the high water mark on the rock itself. Within the critical habitat zone, line fishing with wire trace or bait while anchored or moored is prohibited although fishing with fly or artificial lure while anchored or moored, bait while trolling or drifting, and fly or lure with or without wire trace is allowed. A further ‘grey nurse shark critical habitat buffer zone’ extends for ~800 m beyond the critical habitat zone and permits all forms of line fishing except fishing while anchored or moored using a wire trace line. The grey nurse shark Recovery Plan released in 2002 identified that further protection was still required at Fish Rock with anecdotal reports from divers that up to 75% of sharks could be observed with retained fishing gear (Environment Australia 2002).
The results from the current study support the requirement of more stringent protection measures at this site. Fish Rock was the only C. taurus aggregation site that had a significantly higher rate of sharks with retained hooks than was expected. In addition, during scheduled surveys 34% (222 out of 647) of all identified sharks were seen at Fish Rock and 48% of sharks identified across all sites with retained fishing gear were first seen with retained fishing gear at Fish Rock. While NSW DPI acknowledges that all forms of line fishing (even artificial lures if a fish is on line) can harm grey nurse sharks, they consider the use of wire trace for bottom fishing and setlines as the most harmful fishing method and have restricted management efforts to these fishing apparatus (NSW Department of Primary Industries and Fisheries 2007).
The variation in types of fishing gear observed in this study suggests that C. taurus are susceptible to incidental hooking by many fishing methods and types of fishing gear. It is often assumed that trolling and drift fishing are acceptable at C. taurus aggregation sites as sharks are unlikely to come to the surface to feed, especially when bait is not used (e.g. lures). Four sharks were identified trailing lures (three at Fish Rock, one at Flat Rock) in this study and while C. taurus may not typically ascend to the surface to feed, they are likely to target a hooked fish that instinctively retreats to the bottom after being hooked. A relatively large proportion of hooked C. taurus was observed with gear that is likely used to target the capture of sharks and several sharks were observed with gear consistent with commercial long-line fishing activities. While there are no documented reports of C. taurus bycatch from trawler fishers along the east coast of Australia, three sharks were identified during this study with ropes around their caudal peduncles and several more were photographed or observed, but the images were of insufficient quality for identification. The fact that ropes were observed tied around the caudal peduncle of C. taurus does not provide evidence that these sharks were caught and released by trawler fishers although tail ropes are commonly used in commercial trawl fisheries to manoeuvre large sharks caught as bycatch (Shark Assessment Group 2001). While the fishers are likely trying to release the sharks unharmed, the practice is considered to cause physiological stress and may also cause spinal injuries that may lead to eventual mortality (Shark Assessment Group 2001; Department of the Environment and Heritage 2003).
In conclusion, the proportion of sharks that have been hooked at least once has not declined and current protection measures have not succeeded in reducing the hooking rate of the critically endangered grey nurse shark. Fish Rock recorded the highest occurrence of retained fishing gear and sharks of all maturity levels and sex aggregate in consistently large numbers throughout the year at this site. The closure of Fish Rock to all forms of fishing within at least a 1-km radius is justified and would likely result in a significant decrease in the hooking rate of this species along the east coast of Australia and potentially assist with this population’s recovery.
Acknowledgements
We acknowledge and thank the editor and referees (including Dr C. Huveneers) for their time and constructive comments. We thank A. Kilpatrick, D. Harasti, P. and N. Hitchens, D. Biddulp, P. Heuttner, K. Holzheimer, A. Nel, K. and C. Phillips, A. Walsh, W. Roberts, R. Peterlin, P. Simpson, M. and D. Davey, V. Temple, T. Starr, M. Jordan, D. Arthur, D. Siviero, D. Bowden and the numerous other volunteer divers, dive shops and clubs for their support, field assistance and images of C. taurus. Financial support was provided by the Hermon Slade Foundation and the Queensland Government PhD Smart State Initiative. In-kind support was provided by W. Lancaster of ZSPORTS, R. Peterlin and the Jervis Bay Dive Club, Ron Henry and the ANU Scuba Club, Underwater World (Mooloolaba), and Australia Zoo. This research was conducted in accordance with University of Queensland Ethics Approval SBMS/196/04/DEH, SBMS/228/05/DEH, SBMS/560/06/DEH and SBMS/640/07/HSF.
Bansemer, C. S. , and Bennett, M. B. (2008). Multi-year validation of photographic identification of grey nurse sharks, Carcharias taurus, and applications for non-invasive conservation research. Marine and Freshwater Research 59, 322–331.
| Crossref | GoogleScholarGoogle Scholar |
Borucinska, J. , Martin, J. , and Skomal, G. (2001). Peritonitis and pericarditis associated with gastric perforation by a retained fishing hook in a blue shark. Journal of Aquatic Animal Health 13, 347–354.
| Crossref | GoogleScholarGoogle Scholar |
Dicken, M. L. , Booth, A. J. , and Smale, M. J. (2007). Spatial and seasonal distribution patterns of juvenile and adult raggedtooth sharks (Carcharias taurus) tagged off the east coast of South Africa. Marine and Freshwater Research 58, 127–134.
| Crossref | GoogleScholarGoogle Scholar |
Ferretti, F. , Myers, R. A. , Serena, F. , and Lotze, H. K. (2008). Loss of large predatory sharks from the Mediterranean Sea. Conservation Biology 22, 952–964.
| Crossref | GoogleScholarGoogle Scholar | PubMed |
Lucifora, L. O. , Menni, R. C. , and Escalante, A. H. (2002). Reproductive ecology and abundance of the sand tiger shark, Carcharias taurus, from the southwestern Atlantic. ICES Journal of Marine Science 59, 553–561.
| Crossref | GoogleScholarGoogle Scholar |
Otway, N. M. , Bradshaw, C. J. A. , and Harcourt, R. G. (2004). Estimating the rate of quasi-extinction of the Australian grey nurse shark (Carcharias taurus) population using deterministic age-and stage-classified models. Biological Conservation 119, 341–350.
| Crossref | GoogleScholarGoogle Scholar |
Smale, M. J. (2002). Occurrence of Carcharias taurus in nursery areas of the Eastern and Western Cape, South Africa. Marine and Freshwater Research 53, 551–556.
| Crossref | GoogleScholarGoogle Scholar |




