Contrasting natal origin and movement history informs recovery pathways for three lowland river species following a mass fish kill
Jason D. Thiem A B F , Lee J. Baumgartner
A B F , Lee J. Baumgartner  B , Ben Fanson C , Aleksey Sadekov D , Zeb Tonkin C and Brenton P. Zampatti
B , Ben Fanson C , Aleksey Sadekov D , Zeb Tonkin C and Brenton P. Zampatti  E
E
A Department of Primary Industries, Narrandera Fisheries Centre, Narrandera, NSW 2700, Australia.
B Institute for Land, Water and Society, Charles Sturt University, Albury, NSW 2640, Australia.
C Arthur Rylah Institute for Environmental Research, Department of Environment, Land, Water and Planning, 123 Brown Street, Heidelberg, Vic. 3084, Australia.
D Ocean Graduate School, The ARC Centre of Excellence for Coral Reef Studies, The University of Western Australia, Perth, WA 6009, Australia.
E Commonwealth Scientific and Industrial Research Organisation (CSIRO), Locked Bag 2, Glen Osmond, SA 5064, Australia.
F Corresponding author. Email: jason.thiem@dpi.nsw.gov.au
Marine and Freshwater Research 73(2) 237-246 https://doi.org/10.1071/MF20349
Submitted: 7 December 2020 Accepted: 3 May 2021 Published: 4 June 2021
Journal Compilation © CSIRO 2022 Open Access CC BY-NC
Abstract
Understanding the spatial and temporal scales over which key population processes occur is fundamental to effective fisheries management, especially when informing recovery actions following extreme events. In 2018–19, hypoxia-induced fish kills occurred in the lower Darling River, south-eastern Australia. We collected carcasses of three potamodromous species that perished during these events to reconstruct their lifetime movements and identify potential recovery mechanisms. Golden perch Macquaria ambigua, Murray cod Maccullochella peelii and silver perch Bidyanus bidyanus otolith 87Sr/86Sr profiles were compared with water 87Sr/86Sr ratios to better understand natal provenance and movement history, and to identify the scale at which migration influences population processes. Golden perch were predominantly locally spawned (Darling River), although we found some evidence of emigration into the nearby Murray River early in life and return movements into the Darling River. Murray cod were mainly locally spawned and thereafter lifelong residents, with some evidence of stocking supplementing populations. Silver perch were mostly immigrants, with the Murray River (>500 km away) the principal source of fish. For recovery of native fish populations to be effective in the Darling River, recovery actions are required that incorporate knowledge on the relevant spatial and temporal scales over which life history processes occur.
Keywords: hypoxia, river regulation, freshwater fish, otolith microchemistry, strontium isotope.
Introduction
Human-facilitated river development is affecting freshwater ecosystems at an alarming rate, with an estimated 50% of available freshwater run-off captured for human use (Jackson et al. 2001). A global boom in irrigation expansion and hydropower development will see an unprecedented level of additional water infrastructure construction over the next two decades (Neumann et al. 2011). Indeed, it is estimated that by 2050 irrigated agriculture will expand by 140% globally (Caldera and Breyer 2019). Such development is significantly altering aquatic ecosystems from their natural state (Ormerod et al. 2010) and promoting a global decline in freshwater biodiversity (Strayer and Dudgeon 2010).
River development adversely affects native freshwater fish through changes to the seasonal timing and volume of river flows (Zeiringer et al. 2018), obstruction of movement pathways (Baumgartner et al. 2014a) and habitat alteration (Seliger and Zeiringer 2018). Cumulatively, these factors act in concert and over many years, which can have substantial implications for populations and community composition. For instance, flow regulation may decrease river discharge and subsequently reduce natural cues for fish to move and spawn (Krabbenhoft et al. 2014). In years when cues do occur, barriers to migration may prevent longitudinal movements required for individuals to complete their life cycle (Harris et al. 2017). And, if spawning is successful, then the altered timing, quantity or quality of river flows can affect the availability or suitability of both in-channel and off-channel nursery habitats, leading to reduced growth (Spurgeon and Pegg 2017), dispersal (Robinson et al. 1998) and survival of recruits (Tonkin et al. 2021). Understanding the spatial and temporal scales of these interactions is critical to effectively control and manage the negative effects of river development.
The degree of interruption to these natural riverine processes caused by human development, and the associated ecological impacts, are not static. Rivers are dynamic entities and are predicted to experience more severe climate extremes, specifically increased frequencies and duration of drought interspaced with extreme floods (Arnell and Gosling 2013; Du et al. 2014). Therefore, fish populations may be subject to rapid fluctuations in the availability of suitable habitats, with access to these habitats limited by migration barriers, such as dams and weirs (e.g. Mueller et al. 2011), as well as the frequency and duration of connection events (e.g. Lyon et al. 2010). These fundamental changes in the quantity and connectivity among critical habitats affect the structure and function of fish populations (Koster et al. 2020) and favour species with tolerances to flow extremes, flexible movement strategies and generalist habitat needs (Koehn 2004).
Drought and flooding are natural features of dryland rivers (Walker et al. 1995; Baumgartner et al. 2017). Correspondingly, many riverine fish species have evolved complex life history strategies to exploit specific habitat and flow conditions across different spatial and temporal scales (Lange et al. 2018; Zeug and Winemiller 2008). For example, some species have eggs and larvae that passively drift or actively move hundreds of kilometres (Copp et al. 2002; Stuart and Sharpe 2020). Others have ontogenetic habitat preferences that transition from nursery habitats as juveniles to feeding and spawning habitats as adults (Rosenberger and Angermeier 2003). The migration requirements of many species also change ontogenetically, and small-scale movements at juvenile stages can be replaced by larger, system-scale, movements as fish grow into adulthood (Kynard et al. 2002). For long-lived species, these processes act over decades. Thus, understanding the spatial and temporal nature of critical life history needs is essential to the design and implementation of effective recovery programs.
In multispecies fish communities, there is usually a range of life history strategies across species. More often than not, different species have contrasting needs at the egg, larval, juvenile and adult stages (Van Winkle et al. 1993); this is despite experiencing the same environmental conditions. These requirements can be difficult to disentangle without an adequate understanding of the life history for each species over its entire lifetime (Baumgartner et al. 2014b). There is then the significant challenge of designing recovery programs for altered river systems that provide for the ecological needs of each species while also accounting for the increasing challenges presented by climate change (Daufresne and Boët 2007). As such, effective fisheries management requires the development of a robust recovery program that incorporates knowledge of the spatial and temporal scales over which key population processes occur, and is dynamic enough to account for extreme events and the required recovery actions associated with these (Lyon and O’Connor 2008; Koehn and Todd 2012; Thiem et al. 2017).
The Barwon–Darling River (hereafter referred to as the Darling River), located in south-eastern Australia, is symptomatic of a large dryland river affected by human modification and a changing climate (Mallen-Cooper and Zampatti 2020). The highly modified river hydrology has had a chronic detrimental effect on native fish populations (Gehrke et al. 1995; Gehrke and Harris 2000). In 2018–19, overabstraction and drought induced an extended cease-to-flow period. Concurrent high water temperatures resulting from climate extremes culminated in a series of hypoxia-induced fish kills that occurred over a constrained reach of the lower Darling River, near Menindee in New South Wales (NSW; Jackson and Head 2020). The death of significant numbers of native fish sparked national and international outrage, prompting widespread calls for a significant government response to facilitate recovery (Vertessy et al. 2019). However, for this recovery to be effective, empirical data on the spatial and temporal scales over which key population processes occur are required to guide investment.
We investigated the age structure, provenance and movement history of three species collected in the region of the fish kill using otolith-based approaches. Specifically, we collected otoliths from the carcasses of golden perch Macquaria ambigua, Murray cod Maccullochella peelii and silver perch Bidyanus bidyanus. These species are native to the Murray–Darling Basin (MDB), are relatively long lived (maximum age >20 years) and are considered potamodromous (Lintermans 2007). Long-distance riverine movements of all three species have previously been documented, particularly during periods of high river discharge and flooding (e.g. Reynolds 1983; Llewellyn 2014; Thiem et al. 2020). These large movements are often associated with periodic spawning in adult golden perch and silver perch, and the subsequent long-distance dispersal of eggs, larvae and juveniles (Koster et al. 2021; Stuart and Sharpe 2020). By contrast, long-distance movements of adult Murray cod appear facultative and are not required for reproduction, with predictable annual spawning occurring under a range of river discharges and the well-developed larvae of this nesting species able to actively settle out in favourable, localised habitats (Reynolds 1983; Kopf et al. 2014).
Otoliths were used to assess age structure and generate stable isotope (87Sr/86Sr) profiles. This information was used to elucidate whether these species complete their life cycle within the Darling River (lifelong ‘local’ residents) or whether immigration and emigration influence population demography, as has been demonstrated in other parts of the southern MDB for both golden perch and silver perch (Zampatti et al. 2018). Using 87Sr/86Sr as a geochemical marker is useful because: (1) the stable isotope ratio of dissolved Sr in water reflects the age and composition of the underlying soil and bedrock, which typically varies between sub-basins within catchments; and (2) it is readily substituted for Ca at an approximate 1:1 ratio in the otolith structure. Thus, changes in the 87Sr/86Sr ratio can represent movements between isotopically distinct rivers or regions within rivers (Humston et al. 2017; Kennedy et al. 2000). We hypothesised that, although occupying similar habitats as adults, the three species would display contrasting lifetime movements that extended beyond the direct fish kill area. If true, recovery actions would need to be applied across larger spatial and temporal scales than the immediate fish kill location.
Materials and methods
Study area and fish kill context
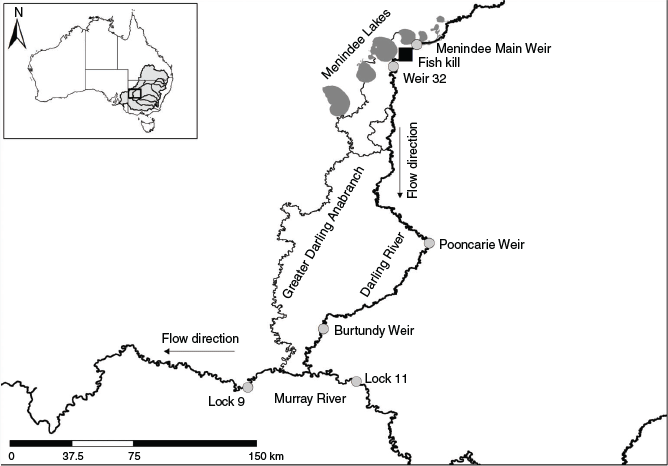
The Darling River is 2740 km long and has a catchment area of 650 000 km2 that drains the north-western section of the MDB in south-eastern Australia (Fig. 1, inset; Thoms and Sheldon 2000). The hydrology of the river and its tributaries is characterised by extreme climatic variability that has been exacerbated by river regulation and water abstraction (Mallen-Cooper and Zampatti 2020). For example, capture of rainfall by large dams in the headwaters and the subsequent diversion of flows for irrigated agriculture has resulted in reductions in annual run-off. This has brought about a loss of periodicity in small floods, as well as changes to seasonal components of the flow regime (Thoms and Sheldon 2000). End-of-system flows from the Darling River into the Murray River are now largely regulated by releases from the Menindee Lakes, a series of naturally occurring, but modified, ephemeral lakes ~500 km upstream of the junction of the two rivers (Fig. 1). The lake system was converted for water storage and subsequent re-regulation of Darling River flows in the 1960s.
|
In late 2018, the combination of extended low river flows (Fig. 2) resulting from upstream water abstraction and drought and high water temperatures led to persistent thermal stratification of the lower Darling River downstream of Menindee Main Weir near the town of Menindee in NSW (Fig. 1). Hypoxic conditions occurred in the lower water layers, particularly in the ~40 km of weir pool upstream of Weir 32 (Fig. 1). On three occasions in the summer of 2018–19, localised weather patterns were suspected to have caused sudden destratification of the water column upstream of Weir 32, resulting in hypoxic conditions in the whole water column and subsequent fish kill events (Australian Academy of Science 2019; NSW Department of Primary Industries 2019; Vertessy et al. 2019).

|
Fish collection and otolith analyses
In December 2018 and January 2019, golden perch (n = 39), Murray cod (n = 40) and silver perch (n = 41) carcasses were opportunistically collected from the fish kill reach. Where possible, biological information, including length (mm) and weight (g), were recorded from all specimens, although in some instances the advanced state of decomposition meant that weight could not be reliably obtained. Sagittal otolith pairs were removed from each individual, and these were cleaned, dried and stored in individually labelled vials.
To determine the annual age of each individual, and subsequent birth year, the right sagittal otolith from each fish was prepared as a transverse section based on established protocols that have previously been used for these species (Anderson et al. 1992; Mallen-Cooper and Stuart 2003; Wright et al. 2020). Estimated age was assigned based on a combination of annulus count and edge type in relation to capture date, and all individuals were assigned a nominal birth date of 1 October (Anderson et al. 1992). Otoliths were aged, independent of any knowledge of fish length, by two experienced readers.
The left sagittal otolith was used to retrospectively determine the provenance and movement history of each individual. Otoliths were first embedded in epoxy resin and a transverse section through the primordium of ~400 µm was obtained. Thin sections were polished using 9-µm lapping film, excess resin was trimmed and sections were placed with the side closest to the primordium facing upwards. Otolith sections were fixed by a thin layer of epoxy resin onto master slides, with 20–50 otolith sections per slide (depending on otolith size) and set in a drying oven at 50°C for 4 h. Master slides were rinsed and sonicated in Milli-Q water (Millipore) and air-dried overnight in a Class 100 laminar flow cabinet at ambient temperature.
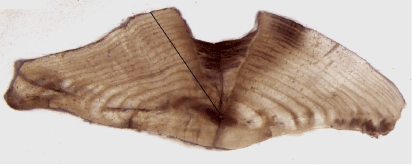
Laser ablation–inductively coupled plasma mass spectrometry (LA/ICP-MS) was used to measure 87Sr/86Sr in the otoliths of each individual. Analysis was undertaken by the Advanced Geochemical Facility for Indian Ocean Research at the University of Western Australia. The system consisted of a Thermo Scientific NEPTUNE Plus multicollector ICP-MS coupled to a Teledyne Analyte G2 excimer LA system. Master slides were placed in the sample cell and the primordium of each otolith was located visually with a 400× magnification objective and a video imaging system. The intended LA path on each sample was then digitally plotted using an image analysis system calibrated to the laser. Otoliths were ablated along a transect, from the primordium (core) to the proximal margin (edge; Fig. 3), using a 25 × 100-μm rectangular laser slit. The laser was pulsed at 10 Hz and scanned at 10 μm s–1 across the sample. Standardisation and Rb correction were undertaken following the methods detailed by Woodhead et al. (2005), and external reproducibility was assessed using three in-house carbonate standards with mean (±2 s.d.) 87Sr/86Sr ratios of 0.709176 ± 0.000025 (n = 20), 0.70592 ± 0.00012 (n = 21) and 0.713017 ± 0.000028 (n = 20).
|
All research was undertaken in accordance with Fisheries NSW Scientific Collection Permit P01/0059(A)-2.0.
Natal origin assignment
To match otolith-derived 87Sr/86Sr ratios with location-specific water 87Sr/86Sr ratios, we used existing water 87Sr/86Sr sample collections that were obtained across the mainly contiguous MDB spanning 2012–18 (Zampatti 2019; Zampatti et al. 2018, 2019; Ye et al. 2020). Because the Menindee Main Weir is 12 m in height and forms an impassable barrier to upstream fish movement, except in major flood years, we compared 87Sr/86Sr ratios from water samples of the lower Darling River to connected rivers and tributaries of the southern MDB. The lower Darling River exhibits a temporally stable unique 87Sr/86Sr, rendering this approach robust (Zampatti et al. 2019; see Fig. S1 of the Supplementary material). We acknowledge that under some circumstances (i.e. predominantly during flooding) downstream movement of individuals between the mid and lower Darling River may occur (e.g. Stuart and Sharpe 2020), although we are unable to resolve these movements with the approach used in this study.
Using the unique 87Sr/86Sr of the lower Darling River (mean ± s.d. 0.70766 ± 0.00014, based on 61 samples collected over 7 years; Zampatti et al. 2019) and Murray River (87Sr/86Sr all above 0.710; Fig. S1), we first assessed the probability of the sample coming from the lower Darling River. To do this, we assumed that the lower Darling River 87Sr/86Sr was normally distributed with a mean (±s.d.) of 0.70766 ± 0.00028 (based on smoothed 87Sr/86Sr profiles from resident fish in the lower Darling River). We then took the average of all 87Sr/86Sr reads at <100 µm from the core and estimated the probability of obtaining that 87Sr/86Sr or higher (one-sided approach). Next, we used a total error rate of 0.5% across the 120 fish. With this error rate, we calculated a threshold probability (0.00013) for each fish. If fish had a higher probability than the threshold, it was classified as lower Darling River origin, otherwise it was classified as being from the Murray River (immigrant). Individuals originating from the Murray River were further classified as coming from the lower Murray or mid-Murray River (Fig. S2–S4 of the Supplementary material) using the threshold 87Sr/86Sr of 0.717 or higher for the mid-Murray. We acknowledge, however, temporal variability in water 87Sr/86Sr ratios. This is particularly evident for the lower Murray River based on variable input from the Darling River (Fig. S1). As such, for the purpose of this study we grouped different regions of the Murray River and simply defined inter-regional movement as movement between the lower Darling and Murray rivers, regardless of direction and occurring at least once during a lifetime.
A complication is that some golden perch and Murray cod, but not silver perch, were stocked as fingerlings (~60 days of age) into the lower Darling River in several years (Table S1 of the Supplementary material). The origin of these fish is from hatcheries that use water sourced from the Murrumbidgee River (a large tributary of the Murray River in the southern MDB). To elucidate hatchery origin for the Murray River-assigned fish, we used the same approach as for the lower Darling River except using the known 87Sr/86Sr ratio (mean = 0.715) and deviation (0.00028) from the Murrumbidgee River. Stocked fish in the lower Darling River also show an abrupt change in the Sr profile between 100 and 800 µm, reflecting the transition from hatchery water to lower Darling River water when fish are released as fingerlings. Therefore, fish were classified as stocked if they showed this transition and had an indicative hatchery 87Sr/86Sr.
Results
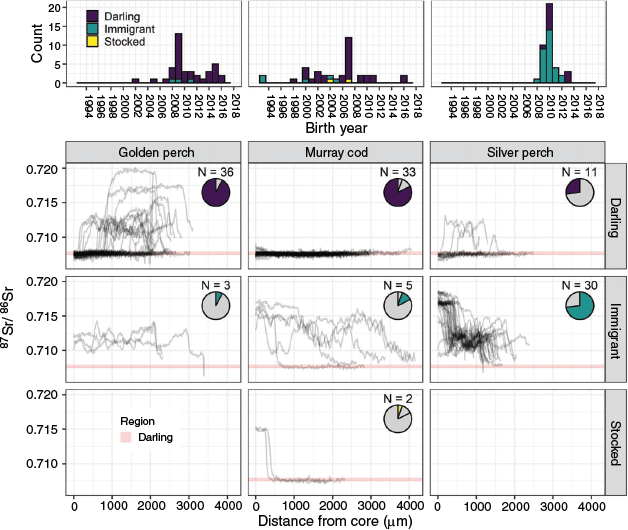
Golden perch of a wide range of sizes and ages were collected, with a modal age of 9+ years equating to the nominal birth year of 2009 (Table 1; Fig. 4). Similarly, Murray cod spanned a large size and age range (up to 1270 mm long and 26 years old, equating to a birth year of 1992), with a modal age of 11+ years equating to a nominal birth year of 2007 (Table 1; Fig. 4). By contrast, silver perch had a narrow range of sizes, ages and associated nominal birth years, with a modal age of 8+ years equating to a nominal birth year of 2010 (Table 1; Fig. 4).

|
|
In general, 87Sr/86Sr profiles were indicative of contrasting lifetime movement patterns among the three species in the lower Darling River study reach (Fig. 4) and could be summarised as follows:
-
Murray cod: predominantly lower Darling River natal source with limited inter-regional movements.
-
Golden perch: predominantly lower Darling River natal source with some inter-regional movements.
-
Silver perch: predominantly Murray River natal source with substantial inter-regional movements.
An assessment of individual fish 87Sr/86Sr profiles (Fig. S2–S4) revealed the lower Darling River was the predominant natal origin of both golden perch (92%) and Murray cod (83%), although it accounted for only 27% of silver perch (Fig. 4). The Murray River was the predominant (73%) natal source of silver perch in our sample and for a small proportion of golden perch (8%) and Murray cod (12%). Stocking also contributed a small proportion of Murray cod (5%) to the lower Darling River. No golden perch or silver perch were of stocked origin. Although all the lower Darling River natal origin Murray cod exhibited lifelong residency within the lower Darling River, golden perch and, to a lesser extent, silver perch exhibited two contrasting lifetime movement patterns. Specifically, of the 36 golden perch with a lower Darling River natal origin, 56% were lifelong residents, whereas 44% moved into the nearby Murray River early in life and subsequently returned to the lower Darling River (Fig. 4). And, of the 11 silver perch with a lower Darling River natal origin, 64% were lifelong residents, whereas 36% moved into the nearby Murray River at various stages in their life and subsequently returned.
Discussion
Our assessment of three species of lowland river fish that died during hypoxia-induced fish kills indicates support for our hypothesis that, in a short reach of the lower Darling River, individuals spanned a range of ages and exhibited contrasting lifetime movement patterns and natal origins. Otolith stable isotope analysis suggests that localised spawning and recruitment of all three species had occurred, but that movement (both emigration and immigration) among regions varies in relative importance for the different species and is contrastingly influencing population dynamics. Understanding the intricacies of these results at a species level, in the context of managing fish populations in modified rivers, is essential to determine the variable recovery pathways that may be required to restore riverine fishes (Zeug and Winemiller 2008; Baumgartner et al. 2014b). More generally, it highlights the importance of maintaining connectivity between habitats to ensure population function (Radinger and Wolter 2014).
Life history and movement among species
For fishes inhabiting regulated rivers, the frequency and extent of large-scale movements has been affected by the interaction between regulating structures, water abstraction and diversion and the often-exacerbated low-flow conditions they create, resulting in serial disconnection (Barbarossa et al. 2020; Mallen-Cooper and Zampatti 2020). As a result, extensive global population declines of migratory species have ensued (Wilcove and Wikelski 2008; Limburg and Waldman 2009). The present study validates spawning and recruitment of Murray cod, golden perch and silver perch in the lower Darling River, as has been documented previously (e.g. Sharpe and Stuart 2018; Zampatti 2019), but indicates variable influence of inter-regional movements on population structure. For example, most Murray cod collected in this study spent their entire lives (up to 26 years) in the lower Darling River, indicating limited reliance on inter-regional movements for population function. Although large-scale movements of adults have been documented, particularly in response to elevated river flows (e.g. Llewellyn 2014; Thiem et al. 2020), both juveniles and adults are largely considered site attached (Jones and Stuart 2007; Koehn and Nicol 2016; Thiem et al. 2018), a result generally supported by the findings of the present study.
Although inter-regional movement was rare for Murray cod in our sample, it was common for silver perch and, to a lesser extent, golden perch. Immigration from the Murray River into the lower Darling River was the dominant movement type exhibited by silver perch that were affected by the fish kill event. For golden perch, movements were predominantly characterised as return migrations following emigration out of the Darling River early in life, consistent with the findings of Zampatti (2019). Both species have been documented to exhibit large bidirectional movements over a range of life stages, often in response to elevated flows (Reynolds 1983; Baumgartner et al. 2014a; Llewellyn 2014; Thiem et al. 2020), and these movements appear important to support population function and resilience of both species in the lower Darling River.
Silver perch have suffered substantial reductions in abundance and distribution throughout their range, including in the Murray River, as result of the negative effects of river regulation including reduced river discharge, altered seasonal timing of river flows, barriers to migration and cold water pollution (Lintermans 2007; Mallen-Cooper and Brand 2007). Despite this, spawning and recruitment of silver perch in the remaining lotic sections of the Murray River are regularly observed (e.g. Tonkin et al. 2007, 2019; King et al. 2016). Tonkin et al. (2019) identified substantial interannual variation in silver perch recruitment strength in the Murray River, attributing strong year classes to high river flows the year after spawning, with these high flows promoting growth and dispersal. Several studies have documented movement of Murray River silver perch into other connected habitats (e.g. Zampatti et al. 2018; Koster et al. 2021). Further, Zampatti et al. (2018) identified that some silver perch captured in the lower Murray River exhibited a Darling River natal origin. It is likely that large-scale (hundreds of kilometres), longitudinally intact and perennial lotic habitats of the mid-Murray River and Darling River support the spawning and early stage recruitment of silver perch (Mallen-Cooper and Zampatti 2018, 2020; Tonkin et al. 2019). At times, the lower Murray River also supports spawning and recruitment of silver perch (Zampatti et al. 2018). Individuals may remain within these regions or migrate into other regions at a range of life stages. Thus, the results of this study and others highlight the importance of inter-regional connectivity that enables the movement of silver perch as a fundamental process to maintain resilient populations in all three regions.
In contrast to silver perch, golden perch collected after the fish kill in the lower Darling River predominantly exhibited a Darling River natal origin. Two movement modes were observed from these fish: (1) life-long residency; and (2) downstream emigration to the Murray River followed by return migration to the Darling River. Both movement types were represented in similar proportions. Spawning and recruitment of golden perch within the Darling River have been identified in other studies (e.g. Ebner et al. 2009; Sharpe 2011). In turn, high river discharge, including overbank flood events, has been shown to influence the dispersal of eggs, larvae and juveniles that may span substantial distances and can include the downstream movement of individuals from the mid to lower Darling River past Menindee Main Weir (Stuart and Sharpe 2020). Using the approach presented in this study, we were unable to resolve potential downstream movement of golden perch from the mid to lower Darling River, thus potentially underestimating the spatial scale of movements. Nevertheless, regardless of the specific origin in the Darling River, in some years spawning of golden perch in the Darling River contributes to population contingents beyond the Darling River, including the lower and mid-Murray River (Zampatti et al. 2015, 2018, 2019). As such, provision of elevated flows at the local scale, which are generally required for spawning, recruitment and dispersal, can have regional-scale benefits if connectivity with the Murray River is facilitated.
Recovery and management
Contrasting lifetime movement and natal origin among the three fish species collected after the fish kill suggests a range of fisheries management actions may be required to promote population recovery and persistence in the lower Darling River, particularly in the reach between Weir 32 and Menindee Main Weir. For Murray cod, limited inter-regional movement means interventions can be considered at the local scale. In the first instance, this should include protecting the remnant population in unaffected reaches of the lower Darling River to ensure that localised spawning and recruitment combined with small-scale movements can contribute to repopulation (Koehn and Todd 2012). Murray cod spawn annually, although the presence of gaps in numerous age classes suggests that recruitment in the lower Darling River is variable. To rebuild populations, recruitment needs to be supported by the provision of hydraulically complex, flowing water habitats at key times to support growth and survival (Tonkin et al. 2017, 2021; Mallen-Cooper and Zampatti 2018; Stuart et al. 2019). Indeed, Murray cod are often a focal species for environmental water allocation within the MDB, and the storage and subsequent reregulation of water in the Menindee Lakes means that targeted environmental flows can be delivered to the lower Darling River to support population restoration (Koehn et al. 2014; Sharpe and Stuart 2018).
In contrast, age structure data from the fish-kill reach suggest golden perch and silver perch recruitment is more episodic and that population structure may be highly reliant on inter-regional movements between the lower Darling and Murray rivers. Thus, management of these populations needs to consider regional processes and bidirectional connectivity. For silver perch, contiguous, large-scale lotic habitats in the mid-Murray and lower Darling rivers are associated with spawning and downstream larval drift, with both spawning and recruitment able to be supported through the provision of environmental flows (King et al. 2016; Tonkin et al. 2019).
For golden perch, promoting local spawning and recruitment in the Darling River represents a critical first step. This can be achieved by protecting or augmenting key hydrological characteristics, particularly spring–summer within-channel or overbank flows (Sharpe 2011; Zampatti 2019). Following this, maintaining bidirectional passage past weirs is essential (Baumgartner et al. 2014a). This requires both the installation and maintenance of effective fishways, the provision of longitudinal hydrological connectivity and suitable hydraulic conditions to disperse early life stages and juveniles, and the promotion of immigration into the Darling River (Baumgartner et al. 2006, 2014a; Koehn et al. 2014). Indeed, in other regions of the MDB, immigration has been demonstrated to promote the recovery of golden perch populations following fish kills (Thiem et al. 2017).
Conclusion
Effective and efficient management of fish population recovery following catastrophic events requires an understanding of potential recovery pathways and processes (Lyon and O’Connor 2008; Thiem et al. 2017). Our results demonstrate the contrasting spatial scales of movement exhibited by individuals of three moderate- to long-lived potamodromous species throughout their lifetime in a regulated reach of the lower Darling River. In particular, the importance of inter-regional movements in structuring populations varied among species. Native fish populations in the lower Darling River, already under stress from a range of human-induced stressors, were locally depleted following severe fish kills in 2018–19. The results from this study emphasise the need for complementary local and regional-scale management that will differ in relative importance for each species. Protection of remnant fish populations in unaffected reaches of the lower Darling River is paramount. Further, pairing flow restoration at the local scale to promote spawning, growth and recruitment with regional processes including the provision of end-of-system flows for enhanced connectivity and effective fish passage represents viable restoration activities that will have measurable local and regional benefits.
Conflicts of interest
Lee J. Baumgartner is an Associate Editor of Marine and Freshwater Research. Despite this relationship, he took no part in the review and acceptance of this manuscript, in line with the publishing policy. The authors declare that they have no further conflicts of interest.
Declaration of funding
Funding was provided by the Murray–Darling Basin Authority (Contract number MD004886; Recovering the lower Darling) and the Department of Primary Industries – Fisheries.
Acknowledgements
Iain Ellis, Rob Gregory, Paul Grosse, Clinton Hann, Peter Heath, Graeme McCrabb, Maree McCrabb, Jarryd McGowan, Debbie Newport, Nick O’Brien, Rohan Rehwinkel, Josh Shead, Chris Smith, Wayne Smith, Arron Strawbridge, Ian Wooden and Daniel Wright assisted with data collection. Comments from three anonymous reviewers substantially improved the manuscript.
References
Anderson, J. R., Morison, A. K., and Ray, D. J. (1992). Age and growth of Murray cod, Maccullochella peelii (Perciformes: Percichthyidae), in the lower Murray–Darling Basin, Australia, from thin-sectioned otoliths. Marine and Freshwater Research 43, 983–1013.| Age and growth of Murray cod, Maccullochella peelii (Perciformes: Percichthyidae), in the lower Murray–Darling Basin, Australia, from thin-sectioned otoliths.Crossref | GoogleScholarGoogle Scholar |
Arnell, N. W., and Gosling, S. N. (2013). The impacts of climate change on river flow regimes at the global scale. Journal of Hydrology 486, 351–364.
| The impacts of climate change on river flow regimes at the global scale.Crossref | GoogleScholarGoogle Scholar |
Australian Academy of Science (2019). Investigation of the causes of mass fish kills in the Menindee region NSW over the summer of 2018–2019. (AAS: Canberra, ACT, Australia.) Available at https://www.science.org.au/supporting-science/science-policy-and-sector-analysis/reports-and-publications/fish-kills-report
Barbarossa, V., Schmitt, R. J. P., Huijbregts, M. A. J., Zarfl, C., King, H., and Schipper, A. M. (2020). Impacts of current and future large dams on the geographic range connectivity of freshwater fish worldwide. Proceedings of the National Academy of Sciences of the United States of America 117, 3648–3655.
| Impacts of current and future large dams on the geographic range connectivity of freshwater fish worldwide.Crossref | GoogleScholarGoogle Scholar | 32015125PubMed |
Baumgartner, L. J., Reynoldson, N., and Gilligan, D. M. (2006). Mortality of larval Murray cod (Maccullochella peelii peelii) and golden perch (Macquaria ambigua) associated with passage through two types of low-head weirs. Marine and Freshwater Research 57, 187–191.
| Mortality of larval Murray cod (Maccullochella peelii peelii) and golden perch (Macquaria ambigua) associated with passage through two types of low-head weirs.Crossref | GoogleScholarGoogle Scholar |
Baumgartner, L., Zampatti, B., Jones, M. J., Stuart, I., and Mallen-Cooper, M. (2014a). Fish passage in the Murray–Darling Basin, Australia: not just an upstream battle. Ecological Management & Restoration 15, 28–39.
| Fish passage in the Murray–Darling Basin, Australia: not just an upstream battle.Crossref | GoogleScholarGoogle Scholar |
Baumgartner, L. J., Conallin, J., Wooden, I., Campbell, B., Gee, R., Robinson, W. A., and Mallen-Cooper, M. (2014b). Using flow guilds of freshwater fish in an adaptive management framework to simplify environmental flow delivery for semi-arid riverine systems. Fish and Fisheries 15, 410–427.
| Using flow guilds of freshwater fish in an adaptive management framework to simplify environmental flow delivery for semi-arid riverine systems.Crossref | GoogleScholarGoogle Scholar |
Baumgartner, L. J., Wooden, I. J., Conallin, J., Robinson, W., and Thiem, J. D. (2017). Managing native fish communities during a long-term drought. Ecohydrology 10, e1820.
| Managing native fish communities during a long-term drought.Crossref | GoogleScholarGoogle Scholar |
Caldera, U., and Breyer, C. (2019). Assessing the potential for renewable energy powered desalination for the global irrigation sector. The Science of the Total Environment 694, 133598.
| Assessing the potential for renewable energy powered desalination for the global irrigation sector.Crossref | GoogleScholarGoogle Scholar | 31401507PubMed |
Copp, G. H., Faulkner, H., Doherty, S., Watkins, M. S., and Majecki, J. (2002). Diel drift behaviour of fish eggs and larvae, in particular barbel, Barbus barbus (L.), in an English chalk stream. Fisheries Management and Ecology 9, 95–103.
| Diel drift behaviour of fish eggs and larvae, in particular barbel, Barbus barbus (L.), in an English chalk stream.Crossref | GoogleScholarGoogle Scholar |
Daufresne, M., and Boët, P. (2007). Climate change impacts on structure and diversity of fish communities in rivers. Global Change Biology 13, 2467–2478.
| Climate change impacts on structure and diversity of fish communities in rivers.Crossref | GoogleScholarGoogle Scholar |
Du, H., Xia, J., Zeng, S., She, D., and Liu, J. (2014). Variations and statistical probability characteristic analysis of extreme precipitation events under climate change in Haihe River Basin, China. Hydrological Processes 28, 913–925.
| Variations and statistical probability characteristic analysis of extreme precipitation events under climate change in Haihe River Basin, China.Crossref | GoogleScholarGoogle Scholar |
Ebner, B. C., Scholz, O., and Gawne, B. (2009). Golden perch Macquaria ambigua are flexible spawners in the Darling River, Australia. New Zealand Journal of Marine and Freshwater Research 43, 571–578.
| Golden perch Macquaria ambigua are flexible spawners in the Darling River, Australia.Crossref | GoogleScholarGoogle Scholar |
Gehrke, P. C., and Harris, J. H. (2000). Large-scale patterns in species richness and composition of temperate riverine fish communities, south-eastern Australia. Marine and Freshwater Research 51, 165–182.
| Large-scale patterns in species richness and composition of temperate riverine fish communities, south-eastern Australia.Crossref | GoogleScholarGoogle Scholar |
Gehrke, P. C., Brown, P., Schiller, C. B., Moffatt, D. B., and Bruce, A. M. (1995). River regulation and fish communities in the Murray–Darling river system, Australia. Regulated Rivers 11, 363–375.
| River regulation and fish communities in the Murray–Darling river system, Australia.Crossref | GoogleScholarGoogle Scholar |
Harris, J. H., Kingsford, R. T., Peirson, W., and Baumgartner, L. J. (2017). Mitigating the effects of barriers to freshwater fish migrations: the Australian experience. Marine and Freshwater Research 68, 614–628.
| Mitigating the effects of barriers to freshwater fish migrations: the Australian experience.Crossref | GoogleScholarGoogle Scholar |
Humston, R., Doss, S. S., Wass, C., Hollenbeck, C., Thorrold, S. R., Smith, S., and Bataille, C. P. (2017). Isotope geochemistry reveals ontogeny of dispersal and exchange between main-river and tributary habitats in smallmouth bass Micropterus dolomieu. Journal of Fish Biology 90, 528–548.
| Isotope geochemistry reveals ontogeny of dispersal and exchange between main-river and tributary habitats in smallmouth bass Micropterus dolomieu.Crossref | GoogleScholarGoogle Scholar | 27615608PubMed |
Jackson, S., and Head, L. (2020). Australia’s mass fish kills as a crisis of modern water: understanding hydrosocial change in the Murray–Darling Basin. Geoforum 109, 44–56.
| Australia’s mass fish kills as a crisis of modern water: understanding hydrosocial change in the Murray–Darling Basin.Crossref | GoogleScholarGoogle Scholar |
Jackson, R. B., Carpenter, S. R., Dahm, C. N., McKnight, D. M., Naiman, R. J., Postel, S. L., and Running, S. W. (2001). Water in a changing world. Ecological Applications 11, 1027–1045.
| Water in a changing world.Crossref | GoogleScholarGoogle Scholar |
Jones, M. J., and Stuart, I. G. (2007). Movements and habitat use of common carp (Cyprinus carpio) and Murray cod (Maccullochella peelii peelii) juveniles in a large lowland Australian river. Ecology Freshwater Fish 16, 210–220.
| Movements and habitat use of common carp (Cyprinus carpio) and Murray cod (Maccullochella peelii peelii) juveniles in a large lowland Australian river.Crossref | GoogleScholarGoogle Scholar |
Kennedy, B. P., Blum, J. D., Folt, C. L., and Nislow, K. H. (2000). Using natural strontium isotopic signatures as fish markers: methodology and application. Canadian Journal of Fisheries and Aquatic Sciences 57, 2280–2292.
| Using natural strontium isotopic signatures as fish markers: methodology and application.Crossref | GoogleScholarGoogle Scholar |
King, A. J., Gwinn, D. C., Tonkin, Z., Mahoney, J., Raymond, S., and Beesley, L. (2016). Using abiotic drivers of fish spawning to inform environmental flow management. Journal of Applied Ecology 53, 34–43.
| Using abiotic drivers of fish spawning to inform environmental flow management.Crossref | GoogleScholarGoogle Scholar |
Koehn, J. D. (2004). Carp (Cyprinus carpio) as a powerful invader in Australian waterways. Freshwater Biology 49, 882–894.
| Carp (Cyprinus carpio) as a powerful invader in Australian waterways.Crossref | GoogleScholarGoogle Scholar |
Koehn, J. D., and Nicol, S. J. (2016). Comparative movements of four large fish species in a lowland river. Journal of Fish Biology 88, 1350–1368.
| Comparative movements of four large fish species in a lowland river.Crossref | GoogleScholarGoogle Scholar | 26919062PubMed |
Koehn, J. D., and Todd, C. R. (2012). Balancing conservation and recreational fishery objectives for a threatened fish species, the Murray cod, Maccullochella peelii. Fisheries Management and Ecology 19, 410–425.
| Balancing conservation and recreational fishery objectives for a threatened fish species, the Murray cod, Maccullochella peelii.Crossref | GoogleScholarGoogle Scholar |
Koehn, J. D., King, A. J., Beesley, L., Copeland, C., Zampatti, B. P., and Mallen-Cooper, M. (2014). Flows for native fish in the Murray–Darling Basin: lessons and considerations for future management. Ecological Management & Restoration 15, 40–50.
| Flows for native fish in the Murray–Darling Basin: lessons and considerations for future management.Crossref | GoogleScholarGoogle Scholar |
Kopf, S. M., Humphries, P., and Watts, R. J. (2014). Ontogeny of critical and prolonged swimming performance for the larvae of six Australian freshwater fish species. Journal of Fish Biology 84, 1820–1841.
| Ontogeny of critical and prolonged swimming performance for the larvae of six Australian freshwater fish species.Crossref | GoogleScholarGoogle Scholar | 24814314PubMed |
Koster, W. M., Dawson, D. R., Kitchingman, A., Moloney, P. D., and Hale, R. (2020). Habitat use, movement and activity of two large-bodied native riverine fishes in a regulated lowland weir pool. Journal of Fish Biology 96, 782–794.
| Habitat use, movement and activity of two large-bodied native riverine fishes in a regulated lowland weir pool.Crossref | GoogleScholarGoogle Scholar | 32017088PubMed |
Koster, W. M., Stuart, I., Tonkin, Z., Dawson, D., and Fanson, B. (2021). Environmental influences on migration patterns and pathways of a threatened potamodromous fish in a regulated lowland river network. Ecohydrology 14, e2260.
| Environmental influences on migration patterns and pathways of a threatened potamodromous fish in a regulated lowland river network.Crossref | GoogleScholarGoogle Scholar |
Krabbenhoft, T. J., Platania, S. P., and Turner, T. F. (2014). Interannual variation in reproductive phenology in a riverine fish assemblage: implications for predicting the effects of climate change and altered flow regimes. Freshwater Biology 59, 1744–1754.
| Interannual variation in reproductive phenology in a riverine fish assemblage: implications for predicting the effects of climate change and altered flow regimes.Crossref | GoogleScholarGoogle Scholar |
Kynard, B., Zhuang, P., Zhang, L., Zhang, T., and Zhang, Z. (2002). Ontogenetic behavior and migration of Volga River Russian sturgeon, Acipenser gueldenstaedtii, with a note on adaptive significance of body color. Environmental Biology of Fishes 65, 411–421.
| Ontogenetic behavior and migration of Volga River Russian sturgeon, Acipenser gueldenstaedtii, with a note on adaptive significance of body color.Crossref | GoogleScholarGoogle Scholar |
Lange, K., Meier, P., Trautwein, C., Schmid, M., Robinson, C. T., Weber, C., and Brodersen, J. (2018). Basin-scale effects of small hydropower on biodiversity dynamics. Frontiers in Ecology and the Environment 16, 397–404.
| Basin-scale effects of small hydropower on biodiversity dynamics.Crossref | GoogleScholarGoogle Scholar |
Limburg, K. E., and Waldman, J. R. (2009). Dramatic declines in North Atlantic diadromous fishes. Bioscience 59, 955–965.
| Dramatic declines in North Atlantic diadromous fishes.Crossref | GoogleScholarGoogle Scholar |
Lintermans, M. (2007). ‘Fishes of the Murray–Darling Basin: an Introductory Guide.’ (Murray–Darling Basin Commission: Canberra, ACT, Australia.)
Llewellyn, L. C. (2014). Movements of Golden Perch Macquaria ambigua (Richardson) in the mid Murray and lower Murrumbidgee Rivers (New South Wales) with notes on other species. Australian Zoologist 37, 139–156.
| Movements of Golden Perch Macquaria ambigua (Richardson) in the mid Murray and lower Murrumbidgee Rivers (New South Wales) with notes on other species.Crossref | GoogleScholarGoogle Scholar |
Lyon, J. P., and O’Connor, J. P. (2008). Smoke on the water: can riverine fish populations recover following a catastrophic fire-related sediment slug? Austral Ecology 33, 794–806.
| Smoke on the water: can riverine fish populations recover following a catastrophic fire-related sediment slug?Crossref | GoogleScholarGoogle Scholar |
Lyon, J., Stuart, I., Ramsey, D., and O’Mahony, J. (2010). The effect of water level on lateral movements of fish between river and off-channel habitats and implications for management. Marine and Freshwater Research 61, 271–278.
| The effect of water level on lateral movements of fish between river and off-channel habitats and implications for management.Crossref | GoogleScholarGoogle Scholar |
Mallen-Cooper, M., and Brand, D. A. (2007). Non-salmonids in a salmonid fishway: what do 50 years of data tell us about past and future fish passage? Fisheries Management and Ecology 14, 319–332.
| Non-salmonids in a salmonid fishway: what do 50 years of data tell us about past and future fish passage?Crossref | GoogleScholarGoogle Scholar |
Mallen-Cooper, M., and Stuart, I. G. (2003). Age, growth and non-flood recruitment of two potamodromous fishes in a large semi-arid/temperate river system. River Research and Applications 19, 697–719.
| Age, growth and non-flood recruitment of two potamodromous fishes in a large semi-arid/temperate river system.Crossref | GoogleScholarGoogle Scholar |
Mallen-Cooper, M., and Zampatti, B. P. (2018). History, hydrology and hydraulics: rethinking the ecological management of large rivers. Ecohydrology 11, e1965.
| History, hydrology and hydraulics: rethinking the ecological management of large rivers.Crossref | GoogleScholarGoogle Scholar |
Mallen-Cooper, M., and Zampatti, B. P. (2020). Restoring the ecological integrity of a dryland river: why low flows in the Barwon–Darling River must flow. Ecological Management & Restoration 21, 218–228.
| Restoring the ecological integrity of a dryland river: why low flows in the Barwon–Darling River must flow.Crossref | GoogleScholarGoogle Scholar |
Mueller, M., Pander, J., and Geist, J. (2011). The effects of weirs on structural stream habitat and biological communities. Journal of Applied Ecology 48, 1450–1461.
| The effects of weirs on structural stream habitat and biological communities.Crossref | GoogleScholarGoogle Scholar |
Neumann, K., Stehfest, E., Verburg, P. H., Siebert, S., Müller, C., and Veldkamp, T. (2011). Exploring global irrigation patterns: a multilevel modelling approach. Agricultural Systems 104, 703–713.
| Exploring global irrigation patterns: a multilevel modelling approach.Crossref | GoogleScholarGoogle Scholar |
NSW Department of Primary Industries (2019). Fish death interim investigation report: lower Darling River fish death event, Menindee 2018/19. (DPI: Orange, NSW, Australia.) Available at https://www.dpi.nsw.gov.au/fishing/habitat/threats/fish-kills-2019-2020/Fish-death-interim-investigation-report.pdf
Ormerod, S. J., Dobson, M., Hildrew, A. G., and Townsend, C. R. (2010). Multiple stressors in freshwater ecosystems. Freshwater Biology 55, 1–4.
| Multiple stressors in freshwater ecosystems.Crossref | GoogleScholarGoogle Scholar |
Radinger, J., and Wolter, C. (2014). Patterns and predictors of fish dispersal in rivers. Fish and Fisheries 15, 456–473.
| Patterns and predictors of fish dispersal in rivers.Crossref | GoogleScholarGoogle Scholar |
Reynolds, L. F. (1983). Migration patterns of five fish species in the Murray–Darling River system. Marine and Freshwater Research 34, 857–871.
| Migration patterns of five fish species in the Murray–Darling River system.Crossref | GoogleScholarGoogle Scholar |
Robinson, A. T., Clarkson, R. W., and Forrest, R. E. (1998). Dispersal of larval fishes in a regulated river tributary. Transactions of the American Fisheries Society 127, 772–786.
| Dispersal of larval fishes in a regulated river tributary.Crossref | GoogleScholarGoogle Scholar |
Rosenberger, A., and Angermeier, P. L. (2003). Ontogenetic shifts in habitat use by the endangered Roanoke logperch (Percina rex). Freshwater Biology 48, 1563–1577.
| Ontogenetic shifts in habitat use by the endangered Roanoke logperch (Percina rex).Crossref | GoogleScholarGoogle Scholar |
Seliger, C., and Zeiringer, B. (2018). River connectivity, habitat fragmentation and related restoration measures. In ‘Riverine Ecosystem Management’. pp. 171–186. (Springer: Cham, Switzerland.)
Sharpe, C. P. (2011). Spawning and recruitment ecology of golden perch (Macquaria ambigua Richardson 1845) in the Murray and Darling Rivers. Ph.D. Thesis, Griffith University, Brisbane, Qld, Australia.
Sharpe, C., and Stuart, I. (2018). Environmental flows in the Darling River to support native fish populations. (Commonwealth Environmental Water Office: Canberra, ACT, Australia.) Available at https://www.environment.gov.au/water/cewo/publications/environmental-flows-darling-river-fish-2016-17
Spurgeon, J. J., and Pegg, M. A. (2017). Juvenile growth of a macrohabitat generalist tied to hydrological character of large-river system. Freshwater Biology 62, 291–302.
| Juvenile growth of a macrohabitat generalist tied to hydrological character of large-river system.Crossref | GoogleScholarGoogle Scholar |
Strayer, D. L., and Dudgeon, D. (2010). Freshwater biodiversity conservation: recent progress and future challenges. Journal of the North American Benthological Society 29, 344–358.
| Freshwater biodiversity conservation: recent progress and future challenges.Crossref | GoogleScholarGoogle Scholar |
Stuart, I. G., and Sharpe, C. P. (2020). Riverine spawning, long distance larval drift, and floodplain recruitment of a pelagophilic fish: a case study of golden perch (Macquaria ambigua) in the arid Darling River, Australia. Aquatic Conservation 30, 675–690.
| Riverine spawning, long distance larval drift, and floodplain recruitment of a pelagophilic fish: a case study of golden perch (Macquaria ambigua) in the arid Darling River, Australia.Crossref | GoogleScholarGoogle Scholar |
Stuart, I., Sharpe, C., Stanislawski, K., Parker, A., and Mallen-Cooper, M. (2019). From an irrigation system to an ecological asset: adding environmental flows establishes recovery of a threatened fish species. Marine and Freshwater Research 70, 1295–1306.
| From an irrigation system to an ecological asset: adding environmental flows establishes recovery of a threatened fish species.Crossref | GoogleScholarGoogle Scholar |
Thiem, J. D., Wooden, I. J., Baumgartner, L. J., Butler, G. L., Forbes, J. P., and Conallin, J. (2017). Recovery from a fish kill in a semi-arid Australian river: can stocking augment natural recruitment processes? Austral Ecology 42, 218–226.
| Recovery from a fish kill in a semi-arid Australian river: can stocking augment natural recruitment processes?Crossref | GoogleScholarGoogle Scholar |
Thiem, J. D., Wooden, I. J., Baumgartner, L. J., Butler, G. L., Forbes, J., Taylor, M. D., and Watts, R. J. (2018). Abiotic drivers of activity in a large, free-ranging, freshwater teleost, Murray cod (Maccullochella peelii). PLoS One 13, e0198972.
| Abiotic drivers of activity in a large, free-ranging, freshwater teleost, Murray cod (Maccullochella peelii).Crossref | GoogleScholarGoogle Scholar | 29883481PubMed |
Thiem, J. D., Wooden, I. J., Baumgartner, L. J., Butler, G. L., Taylor, M. D., and Watts, R. J. (2020). Hypoxic conditions interrupt flood-response movements of three lowland river fish species: implications for flow restoration in modified landscapes. Ecohydrology 13, e2197.
| Hypoxic conditions interrupt flood-response movements of three lowland river fish species: implications for flow restoration in modified landscapes.Crossref | GoogleScholarGoogle Scholar |
Thoms, M. C., and Sheldon, F. (2000). Water resource development and hydrological change in a large dryland river: the Barwon–Darling River, Australia. Journal of Hydrology 228, 10–21.
| Water resource development and hydrological change in a large dryland river: the Barwon–Darling River, Australia.Crossref | GoogleScholarGoogle Scholar |
Tonkin, Z., King, A., Mahoney, J., and Morrongiello, J. (2007). Diel and spatial drifting patterns of silver perch Bidyanus bidyanus eggs in an Australian lowland river. Journal of Fish Biology 70, 313–317.
| Diel and spatial drifting patterns of silver perch Bidyanus bidyanus eggs in an Australian lowland river.Crossref | GoogleScholarGoogle Scholar |
Tonkin, Z., Kitchingman, A., Lyon, J., Kearns, J., Hackett, G., O’Mahony, J., Moloney, P. D., Krusic-Golub, K., and Bird, T. (2017). Flow magnitude and variability influence growth of two freshwater fish species in a large regulated floodplain river. Hydrobiologia 797, 289–301.
| Flow magnitude and variability influence growth of two freshwater fish species in a large regulated floodplain river.Crossref | GoogleScholarGoogle Scholar |
Tonkin, Z., Stuart, I., Kitchingman, A., Thiem, J. D., Zampatti, B., Hackett, G., Koster, W., Koehn, J., Morrongiello, J., Mallen-Cooper, M., and Lyon, J. (2019). Hydrology and water temperature influence recruitment dynamics of the threatened silver perch Bidyanus bidyanus in a regulated lowland river. Marine and Freshwater Research 70, 1333–1344.
| Hydrology and water temperature influence recruitment dynamics of the threatened silver perch Bidyanus bidyanus in a regulated lowland river.Crossref | GoogleScholarGoogle Scholar |
Tonkin, Z., Yen, J., Lyon, J., Kitchingman, A., Koehn, J. D., Koster, W. M., Lieschke, J., Raymond, S., Sharley, J., Stuart, I., and Todd, C. (2021). Linking flow attributes to recruitment to inform water management for an Australian freshwater fish with an equilibrium life-history strategy. Science of the Total Environment 752, 141863.
| Linking flow attributes to recruitment to inform water management for an Australian freshwater fish with an equilibrium life-history strategy.Crossref | GoogleScholarGoogle Scholar |
Van Winkle, W., Rose, K. A., Winemiller, K. O., Deangelis, D. L., Christensen, S. W., Otto, R. G., and Shuter, B. J. (1993). Linking life history theory, environmental setting, and individual-based modeling to compare responses of different fish species to environmental change. Transactions of the American Fisheries Society 122, 459–466.
| Linking life history theory, environmental setting, and individual-based modeling to compare responses of different fish species to environmental change.Crossref | GoogleScholarGoogle Scholar |
Vertessy, R., Barma, D., Baumgartner, L., Mitrovic, S., Sheldon, F., and Bond, N. (2019). Independent assessment of the 2018–19 fish deaths in the lower Darling. (Australian Government: Canberra, ACT, Australia.) Available at https://www.mdba.gov.au/publications/mdba-reports/response-fish-deaths-lower-darling
Walker, K. F., Sheldon, F., and Puckridge, J. T. (1995). A perspective on dryland river ecosystems. Regulated Rivers 11, 85–104.
| A perspective on dryland river ecosystems.Crossref | GoogleScholarGoogle Scholar |
Wilcove, D. S., and Wikelski, M. (2008). Going, going, gone: is animal migration disappearing. PLoS Biology 6, e188.
| Going, going, gone: is animal migration disappearing.Crossref | GoogleScholarGoogle Scholar | 18666834PubMed |
Woodhead, J., Swearer, S., Hergt, J., and Maas, R. (2005). In situ Sr-isotope analysis of carbonates by LA-MC-ICP-MS: interference corrections, high spatial resolution and an example from otolith studies. Journal of Analytical Atomic Spectrometry 20, 22–27.
| In situ Sr-isotope analysis of carbonates by LA-MC-ICP-MS: interference corrections, high spatial resolution and an example from otolith studies.Crossref | GoogleScholarGoogle Scholar |
Wright, D. W., Zampatti, B. P., Baumgartner, L. J., Brooks, S., Butler, G. L., Crook, D. A., Fanson, B. G., Koster, W., Lyon, J., Strawbridge, A., Tonkin, Z., and Thiem, J. D. (2020). Size, growth and mortality of riverine golden perch (Macquaria ambigua) across a latitudinal gradient. Marine and Freshwater Research 71, 1651–1661.
| Size, growth and mortality of riverine golden perch (Macquaria ambigua) across a latitudinal gradient.Crossref | GoogleScholarGoogle Scholar |
Ye, Q., Giatas, G., Brookes, J., Furst, D., Gibbs, M., Oliver, R., Shiel, R., Zampatti, B. P., Aldridge, K., Bucater, L., Busch, B., Hipsey, M., Lorenz, Z., Maas, R., and Woodhead, J. (2020). Commonwealth Environmental Water Office Long-Term Intervention Monitoring Project 2014–2019: Lower Murray River Technical Report. South Australian Research and Development Institute, Aquatic Sciences, Adelaide.
Zampatti, B. P. (2019). Ecology and population dynamics of golden perch in a fragmented, flow-impacted river: implications for conservation and management. Ph.D. Thesis, The University of Adelaide, Adelaide, SA, Australia.
Zampatti, B. P., Wilson, P. J., Baumgartner, L., Koster, W., Livore, J. P., McCasker, N., Thiem, J. D., Tonkin, Z., and Ye, Q. (2015). Reproduction and recruitment of golden perch (Macquaria ambigua ambigua) in the southern Murray–Darling Basin in 2013–2014: an exploration of river-scale response, connectivity and population dynamics. South Australian Research and Development Institute (Aquatic Sciences), Adelaide, SA, Australia.
Zampatti, B. P., Strawbridge, A., Thiem, J. D., Tonkin, Z., Maas, R., Woodhead, J., and Fredberg, J. (2018). Golden perch (Macquaria ambigua) and silver perch (Bidyanus bidyanus) age demographics, natal origin and migration history in the River Murray, Australia. South Australian Research and Development Institute (Aquatic Sciences), Adelaide, SA, Australia.
Zampatti, B., Fanson, B., Strawbridge, A., Tonkin, Z., Thiem, J., Butler, G., Balcombe, S., Koster, W., King, A., Crook, D., Woods, R., Brooks, S., Lyon, J., Baumgartner, L., and Doyle, K. (2019). Basin-scale population dynamics of Golden Perch and Murray Cod: relating flow to provenance, movement and recruitment in the Murray–Darling Basin. In ‘Murray–Darling Basin Environmental Water Knowledge and Research Project – Fish Theme Research Report’. (Eds A. Price, S. Balcombe, P. Humphries, A. King, and B. Zampatti.) pp. 26–30. (Centre for Freshwater Ecology, La Trobe University: Wodonga, Vic., Australia.)
Zeiringer, B., Seliger, C., Greimel, F., and Schmutz, S. (2018). River hydrology, flow alteration, and environmental flow. In ‘Riverine Ecosystem Management’. pp. 67–89. (Springer: Cham, Switzerland.)
Zeug, S. C., and Winemiller, K. O. (2008). Relationships between hydrology, spatial heterogeneity, and fish recruitment dynamics in a temperate floodplain river. River Research and Applications 24, 90–102.
| Relationships between hydrology, spatial heterogeneity, and fish recruitment dynamics in a temperate floodplain river.Crossref | GoogleScholarGoogle Scholar |


