Plasticity of upper thermal limits of Australian Paratya spp. (Decapoda, Atyidae) and considerations of climate-change adaptation
Brendan Cox A * , Amanda Reichelt-Brushett
A * , Amanda Reichelt-Brushett  A , Kathryn Taffs
A , Kathryn Taffs  A B and Ross Smith
A B and Ross Smith  A C
A C
A Faculty of Science and Engineering, Southern Cross University, Lismore, NSW, Australia.
B NSW Department of Planning and Environment, Water Science, Ballina, NSW, Australia.
C Hydrobiology Pty Ltd, Brisbane, Qld, Australia.
Marine and Freshwater Research 74(6) 491-499 https://doi.org/10.1071/MF22260
Submitted: 1 December 2022 Accepted: 14 February 2023 Published: 14 March 2023
© 2023 The Author(s) (or their employer(s)). Published by CSIRO Publishing. This is an open access article distributed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND)
Abstract
Context: The ability of ectothermic stream invertebrates to adapt to the predicted increases in mean and extreme stream temperatures is crucial to ensuring they continue to exist.
Aims: To examine the plasticity of thermal limits of Australian Paratya spp. (Decapoda, Atyidae) from streams in eastern New South Wales (NSW). We hypothesised that the upper lethal temperature (ULT, as indicated by the median lethal temperature, LT50) would be higher for warm water-acclimated shrimp individuals than for winter-acclimatised shrimp individuals because of the importance of acclimatisation temperature.
Methods: Controlled experiments were undertaken to determine the ULT by using ramping assays for winter field-acclimatised and warm water laboratory-acclimated Paratya spp.
Key results: Warm water-acclimated shrimp individuals demonstrated a significantly higher LT50 of 36.1°C than did winter-acclimatised shrimp individuals at 34.6°C. Paratya spp. exhibited a limited plasticity for acclimation to warmer temperatures.
Conclusions: Results demonstrated the potential vulnerability of ectothermic stream invertebrates to climate change if stream temperatures increase as predicted and thermal thresholds are exceeded.
Implications: Understanding the ULT of stream invertebrates helps predict their ability to respond to temperature variability and response to climate change. Increasing resilience through target management of resorting riparian vegetation for shade and securing environmental flows may reduce the impacts of stream warming.
Keywords: acclimatisation, climate change, distribution, macroinvertebrates, plasticity, shrimp, stream temperature, thermal tolerance.
Introduction
Changes to thermal regimes linked to climate change may have severe consequences for the structure of in-stream biotic communities and the functions of stream ecosystems. Thermal changes can affect biodiversity, abundance, species distribution and life-history patterns of stream invertebrates (Cox and Rutherford 2000; Narum et al. 2013; Dallas and Rivers-Moore 2018: Bonacina et al. 2023). Stream invertebrates are ectotherms; as such, they are directly affected by alterations in the thermal regime (Narum et al. 2013; Magozzi and Calosi 2015). Mortality may occur as stream temperatures exceed the critical thermal-tolerance limits of individual invertebrate species (Stewart et al. 2013; Pedreros et al. 2020). When temperatures exceed these lethal limits, the number of individuals of a species in a population is reduced until they become absent from the stream community (Dallas and Rivers-Moore 2018). For example, in a study on freshwater mussels, Archambault et al. (2014) found that increasing stream temperature and reduced stream flow directly affect abundance through mortality. Geographical distribution shifts are a potential effect of mortality because of exceedance of thermal limits of stream invertebrates (Verdelhos et al. 2015). In a review of the effects of climate change and water temperature on stream invertebrates, Bonacina et al. (2023) recommended that community-level physiological responses of individuals or populations should be studied in more detail, given that their ecological effects are likely to be enhanced by climate warming. Understanding the upper lethal temperature (ULT), plasticity and response of stream invertebrate species and populations to possible future conditions helps forecast the ecological consequences of climate change (Dallas and Rivers-Moore 2018; Morley et al. 2019; Hidalgo-Galiana et al. 2021).
The seasonal variability of stream thermal regimes has a fundamental influence on the structure of stream invertebrate communities (Pedreros et al. 2020). Examining seasonal variations in thermal thresholds may give some insight into the thermal adaptability of stream invertebrates (Houghton and Shoup 2014). A link between ULT and acclimatisation to seasonal stream temperatures has been studied with varying results. Dallas and Rivers-Moore (2018) showed that the critical thermal limits of two stream invertebrate species significantly differed between late summer and winter; however, those between spring and autumn were less distinct. By contrast, Hidalgo-Galiana et al. (2021) showed that six Coleoptera species had limited acclimation-related plasticity for upper thermal limits and concluded that plasticity of thermal limits is unlikely to buffer the consequences of climate change.
Thermal acclimatisation in response to changing thermal conditions expected under climate change will require organisms to adjust to the changing environmental conditions by plasticity. Physiological plasticity occurs as organisms respond to acclimatisation at a seasonal scale (Gunderson and Stillman 2015). The ability of stream invertebrates to adapt to temperatures outside their current limits is less understood. The impact of climate change on stream invertebrates will depend on the increase in temperature and the rate of increase relative to their adaptive capacity (Palmer et al. 2009). Because climate change leads to increased temperature change, higher average temperatures and more variable temperatures, an ability to adapt will determine the degree of shift of distribution ranges (Verdelhos et al. 2015; Alba-Tercedor et al. 2017; Haase et al. 2019). In eastern Australia this translates specifically to how far the northern limit of specific stream invertebrate species will move south and will be determined by the upper thermal tolerance and plasticity of each species. Because stream invertebrates play a crucial role within aquatic food webs, changes in species’ survival rates will influence community composition and structure, significantly affecting entire stream ecosystems. Change is inevitable, but, how much, is uncertain.
This study aimed to examine the plasticity of thermal limits of a population of Australian Paratya spp. (Decapoda; Atyidae) from streams in eastern New South Wales (NSW). This was achieved through three main objectives. The first was to determine the ULT of winter acclimatised Australian Paratya spp. caught in the field. The second was to determine the ULT on the basis of acclimation to summer stream temperatures simulated in the laboratory. Finally, the ULTs of the two treatments were compared to identify any impacts of acclimation temperature. By examining the acclimated specimens’ responses, the adaptive capacity of Australian Paratya spp. to future warming is considered.
Methods
Study species, collection and acclimatisation
Paratya spp. are the most widespread atyid freshwater shrimp in eastern Australia, found in upland and lowland streams, dams and retention ponds, with distribution from southern Queensland to eastern South Australia (Cook et al. 2006; Rahman et al. 2020). Prior to a reclassification by Suter et al. (2022), it was believed that only a single highly variable Paratya species (Paratya australiensis) occurred in Australia. Since collection and identification of our P. australiensis in 2021 for use in this study, Suter et al (2022) has redescribed and identified 10 distinct linages of Paratya in Australia. Under this new taxonomic classification, our test specimens were re-identified and found to be a combination of P. australiensis and the newly classified species Paratya spinosa sp. nov.
Australian Paratya spp., generally, have a life cycle of 2 years, with females breeding in their second summer (Hancock and Bunn 1997; Williams 1977). Temperature is the most critical factor influencing the breeding season of Australian Paratya spp., with hydrology a secondary influence (Hancock and Bunn 1997). In contrast to the tropical regions, the breeding season of Paratya in temperate and subtropical regions is confined to the warm summer months (Hancock and Bunn 1997; Rahman et al. 2020). Paratya spp. living within subtropical eastern Australia have not been found any closer than 10 km from the start of saline estuarine conditions and can complete their life history in upland rainforest streams (Hancock and Bunn 1997; Williams 1977; Cook et al. 2006).
Specimens of Australian Paratya spp. were collected in July 2021 from a 100-m stretch of Leycester Creek, Barkers Vale (28°33′38.1″S, 153°07′02.7″E), NSW, Australia. This section of the creek is a low-order subtropical stream in the Richmond River catchment. Approximately 520 individuals were collected with a 300-μm sampling net, hand-picked and transported to the laboratory in an aerated bucket. The average stream temperature over the 4 weeks prior to capture was 13°C ± 1.52 (see Supplementary Fig. S1). Water quality at the time of capture was as follows: temperature was 15°C, pH was 8.1 and dissolved oxygen (DO%) saturation was 92.1%. Specimens transported to the laboratory were split between two 200-L aquarium tanks. Each tank contained ~150 L of preconditioned tap water maintained at 15°C. Tap water was preconditioned with Prime Fresh and Saltwater Conditioner to remove chlorine and chloramine and detoxify ammonia, nitrite, nitrate and any heavy metals in the tap water. Water was aerated using large air stones, with water being recirculated through the system at a rate of 400 L h−1 using 750 L h−1 pumps (Model: AQUAPROAP750LV). The first tank contained shrimp used for Assay 1 on winter field-acclimatised shrimp. They were kept at 15°C for 2 days without food before use in Temperature-ramping assay 1. The experiment was conducted 3 days after their collection from the creek.
To examine the ULT of warm water-acclimated shrimp, laboratory acclimation to approximate summer temperatures was required. For Assay 2, ~180 shrimp were laboratory-acclimated to a temperature of 28°C over a 6-week period. The water temperature in the laboratory-acclimation tank was increased from the collection temperature of 15°C at a rate of ~2.7°C week−1 and held at a final temperature of 28°C for 2 weeks. Specimens were fed throughout the acclimation period with algal wafers. Once the acclimation period was complete, the shrimp were transferred to a 200-L holding tank (as above) at 28°C for 2 days without food, before being transferred to experimental apparatuses.
Experimental setup
The apparatus used to examine ULT consisted of four 50-L water baths (Fig. 1). Each water bath contained three small 6.8-L containers suspended in water baths; to avoid any pseudo replication, each small container was filled with preconditioned tap water at a temperature of 15°C for the winter-acclimatised shrimp and 28°C for the warm water-acclimated shrimp and aerated using one large air pump to maintain DO% saturation above 70%. Each small container held 15 shrimp. Shrimp were not fed during the experiment. Three water baths were used as experimental units (nine replicate containers) and heated using thermal circulators. A pump was placed in each water bath to aid in stirring the water, so as to ensure even water temperature throughout the ramping assay. The fourth water bath was held at the control temperature of 15°C for the winter-acclimatised shrimp and 28°C for the warm water-acclimated shrimp for the entirety of the ramping assay. A control (3 replicates each with 15 shrimp) was used to examine potential mortality from factors other than water temperatures (e.g. container effects, fluctuations in DO, water quality and lack of food) (Dallas and Ketley 2011).
Experimental procedure
A ramping assay examined ULT for both winter field-acclimatised and laboratory-acclimated warm-water shrimp. The endpoint criterion was defined as the temperature (°C) that results in the death of an individual (Rezende et al. 2014). Following the acclimation period, shrimp were moved to the experimental apparatus and held at the control temperature of 15°C for field-acclimatised and 28°C for laboratory-acclimated shrimp for 1 h. Following the 1-h experimental in situ acclimation period, the ramping assay was commenced at a heating rate of 2°C h−1, by using thermal circulating heaters. The heating rate was considered to be slow enough to ensure that the core temperature of the shrimp approximated the ambient temperature, but fast enough to avoid acclimation (Ernst et al. 1984; Quinn et al. 1994; Dallas and Ketley 2011). This dynamic assay was chosen not to replicate a specific thermal event, but as a method to examine ULT. Water was heated at this constant rate until the endpoint was observed for all individuals. Throughout the experiment, the temperature was recorded every minute with a data logger (Hobo UA-002-08 temperature- and light-pendant datalogger). Shrimp were continually visually monitored throughout the experiment for mortality. A jet of water from a pipette was used to identify live shrimp from dead shrimp, with lack of agility being the first sign of heat stress. Organisms considered dead were removed and placed in a water tank at the control temperature to confirm mortality. The time of mortality, mortality temperature and size of each shrimp was recorded. Following the ramping assay, organisms were preserved in 70% ethanol and species identification was confirmed. If control survival was above 90%, ULT values were considered valid.
Analysis
To expose the ULT of the shrimp from the two treatments, we estimated median lethal temperature (LT50) and 95% confidence intervals using the PROBIT analysis within IBM SPSS Statistics for Windows (ver. 27.0, IBM Corp., Armonk, NY, USA) by using the total test population response. Outliers were removed from the data; this was only one shrimp per treatment to normalise the data. The resulting regression was used to determine the ULT, which is the temperature that causes mortality of 50% of the test population. Furthermore, to examine thermal thresholds, lethal temperatures for 10 and 90% of the test population (LT10 and LT90 respectively) values were estimated using PROBIT analysis (see Supplementary Tables S1–S2). Dose–response curves were created using the PROBIT results, with proportional survival plotted against temperature. To examine and quantify thermal plasticity in response to acclimatisation and identify significant differences between the two treatments, a 95% confidence interval (CI) upper and lower bound overlap was used.
Results
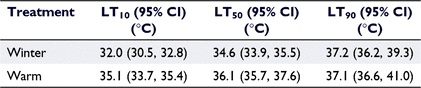
No mortalities occurred in the control population for either treatment. The average mortality temperature for the winter-acclimatised shrimp was 35.4°C (s.d. = 1.58), with 100% mortality occurring at 36.4°C (Fig. 2). The ULT of the winter-acclimatised shrimp by using Ramping-assay 1 had an estimated LT50 value of 34.6°C, and 95% CI (33.9, 35.5) for all individuals tested (Table 1). The calculated window where mortality occurred (LT10–LT90) was 5.2°C (Fig. 2), with most mortality of winter-acclimatised specimens occurring between 32°C and 95% CI (30.5, 32.8) (LT10) and 37.2°C and 95% CI (36.2, 39.3) (LT90). Throughout the experiments, specimens generally remained sedentary, showing limited behavioural changes as temperatures increased. The average size of shrimp tested was 28.03 mm (s.d. = 5.42).
|
The warm water-acclimated shrimp had an estimated ULT (as indicated by LT50) of 36.1°C, 95% CI (35.7, 37.6) (Fig. 2). The average temperature to cause mortality of the warm water-acclimated shrimp was 37.1°C (s.d. = 0.88), with 100% mortality occurring at 37.8°C. The LT10 was 35.1°C, 95% CI (33.7, 35.4), and the LT90 was 37.1°C, 95% CI (36.6, 41.0). The range between LT10 and LT90, where the highest mortality occurred, was 2°C (Fig. 2), being considerably narrower than for the winter-acclimatised shrimp (5.2°C). The average size of warm water-acclimated shrimp tested was 24.76 mm (s.d. = 4.03). Again, specimens generally remained sedentary, showing little behavioural changes as temperatures increased. The ULT of the warm water-acclimated shrimp was 1.5°C higher than that of the winter field-acclimatised shrimp.
In response to acclimatisation, the ULT of warm water-acclimated shrimp was significantly higher (36.1°C, 95% CI (35.7, 37.6)) than that of winter-acclimatised shrimp (34.6°C, 95% CI (33.9, 35.5)). The confidence intervals from the PROBIT analysis had no overlap (Fig. 3) and showed significant differences in the LT50 and LT10 values between warm water-acclimated and winter-acclimatised shrimp specimens. By contrast, the results of the estimated LT90 values were not significantly different between assays shown by the overlap of the confidence intervals. The LT90 for winter-acclimatised shrimp (37.2°C (36.2, 39.3)) is almost identical, being only 0.1°C higher than those of the warm water-acclimated shrimp (37.1°C (36.6, 41.0)).
Discussion
Predicting the consequences of increasing mean and extreme stream water temperatures on the survival and distribution of stream invertebrates is key to understanding the impacts of climate change on stream biota and riverine ecosystems (Stewart et al. 2013; Kim et al. 2017; Bonacina et al. 2023). The ability to acclimatise to changing thermal conditions is expected to be a primary factor that dictates the robustness of stream invertebrates to increasing stream temperatures (Palmer et al. 2009; Rohr et al. 2018). The thermal acclimation potential of Paratya spp. was identified, with the warm water-acclimated shrimp having a higher ULT (+1.52°C) than that of the winter-acclimated shrimp, on the basis of the acclimatisation temperatures of 15 and 28°C respectively, and a rate of temperature increase of 2°C h−1. Gradual temperature ramping was used because it is expected to provide a better ecologically relevant estimate of the impacts of cumulative stream temperature change than is shock (Overgaard et al. 2012; Jørgensen et al. 2019). Because warming leads to mortality, generating thermal tolerance data is vital for understanding how ectothermic stream invertebrates will tolerate increasing average and extreme water temperatures.
The warm water-acclimation temperature mimics the peak summer stream temperatures experienced during hot, dry summers that are becoming more common in eastern NSW (Chessman 2009). Paratya spp. showed some capacity for physiological plasticity to survive higher temperatures in summer. This capacity for physiological plasticity is essential to understanding the impact of climate change and stream warming (Palmer et al. 2009; Morley et al. 2019). Our results highlighted a potential critical temperature of ~37°C for this population of Australian Paratya spp., as demonstrated by the similar LT90 values of 37.2 and 37.1°C, in winter-acclimatised and warm water-acclimated shrimp. An assay using shrimp caught in summer’s height would further clarify extreme upper thermal-tolerance limits. The examination of temperature differences in other Paratya spp. over latitudinal and elevation gradients would also provide insights to physiological plasticity within this genus, with the inclusion of evolutionary responses.
Biological and physiological processes affect thermal tolerance. One of the most critical factors affecting ULT is respiration/metabolic rate. Because metabolic rate increases as temperature rises, which raises biological oxygen demand and therefore requires greater oxygen concentrations to sustain normal bodily functions (Verberk and Calosi 2012; Verberk et al. 2020; Bonacina et al. 2023). We aerated the containers used in the ramping assay, which may have reduced low-oxygen stress in the experiment; however, this might not occur in the natural environment. Once oxygen demand reaches a point where it exceeds the supply, mortality eventuates (Verberk and Calosi 2012). During the assays, as temperature increased, the behaviour of the shrimp was affected, with the initial effects being reduced locomotion, soon followed by death. The size of the shrimp had no bearing on ULT. Similar studies of thermal limits indicated that oxygen limitations may be occurring and are linked to stream invertebrates’ upper thermal tolerance (Magozzi and Calosi 2015; Verberk et al. 2016). Other studies have shown that stream temperature profoundly influences aspects of stream invertebrates’ biology (metabolic rate), physiology and behaviour (Ward and Stanford 1982; Sweeney et al. 1992; Isaak et al. 2012; Semsar-kazerouni and Verberk 2018). Examining oxygen limitation in the form of availability (i.e. dissolved oxygen saturation) rather than the inability of invertebrates to take in enough oxygen to meet biological oxygen demand needs to be further investigated.
Comparisons of the ULT obtained in our study with similar studies of stream invertebrate ULT showed that Australian Paratya spp. have a high tolerance to increased temperatures (Fig. 4). In a review of upper thermal tolerances of stream invertebrates, Stewart et al. (2013) found that upper limits for the major taxonomic groups of stream invertebrates ranged from 22.3°C for Ephemeroptera to 43.4°C for Coleoptera. Compared with this dataset, our Australian Paratya spp. had a higher tolerance to elevated temperatures than most other stream invertebrates except for Odonata and Coleoptera taxa. However, these outcomes are determined from limited data sets, highlighting the urgent need to undertake work in finding the ULT for stream invertebrates.
|
|
Interactions between warming and plasticity
Stream invertebrates are sensitive to water temperature and the thermal regimes of the stream (Ross-Gillespie 2014). To survive changing thermal conditions, stream invertebrates need the ability to adapt through metabolic or behavioural adjustments (Magozzi and Calosi 2015; Pallarés et al. 2021). Stream invertebrates have some physiological plasticity, vital to surviving the range of temperatures experienced over different timescales (González et al. 2010). Although some plasticity in ULT is evident for Australian Paratya spp. from our results, this may not be adequate to keep in step with the increase in mean and extreme stream temperatures predicted under climate-change scenarios. According to CSIRO and Bureau of Meteorology (2020) predictions, by 2040, it is expected that in Australia, mean temperatures will increase to +2.8°C above the pre-industrial temperatures, along with changes in regional patterns of rainfall and increased drought frequency and duration. The lack of any significant difference in LT90 values suggests a limit to the capacity for adaptation to higher stream temperatures. Although some change in ULT is shown in response to acclimatisation, plasticity may be unable to buffer Australian Paratya spp. sufficiently from changing thermal conditions in streams. The lack of buffering indicates that Australian Paratya spp. could be at risk of higher mortality rates associated with an increase in the frequency of extreme events such as heatwaves, low flow events, the rise of the occurrence of high-severity wildfires and the increase in mean temperatures with climate change (Dudgeon 2019; CSIRO and Bureau of Meteorology 2020; Arias Font et al. 2021; Seyedhashemi et al. 2022). Knowledge of thermal limits can be used to delineate guidelines and create trigger points for management action (Gomez Isaza et al. 2022). Species with broad plasticity are more likely to adjust to climate shifts (Pallarés et al. 2021).
The range where increasing water temperatures affect organisms is linked to their ability to survive future warming. The consequences expected will be more intense in tropical and subtropical streams as generally, stream invertebrates in these streams live closer to their physiological thermal limits (Egea-Serrano et al. 2022). We observed a 2°C difference between LT10 and LT90 for warm water-acclimated shrimp and a 5.2°C difference for winter-acclimatised shrimp. Even though mortality of warm-water shrimp occurred at a higher temperature, once temperature exceeded the thermal limits, mortality will occur irrespective of thermal acclimation.
Results from Gunderson and Stillman (2015) indicated that physiological plasticity might not be sufficient to buffer stream invertebrates from increasing stream water temperatures under predicted climate-change conditions. Survival may therefore require an evolutionary response to increasing stream temperatures. Given that plasticity is a trait that can evolve, this may be an adaptive mechanism for stream invertebrates to avoid the impacts of increasing stream temperatures. However, Gunderson and Stillman’s (2015) investigations also suggest that plasticity in thermal tolerance may be evolutionarily constrained. Examining acclimatisation potential and changes in plasticity over multiple generations may give further insights. Our results and research showed that stream invertebrate species with high UTLs will fare best under a warming climate (Palmer et al. 2009).
Ecological consequences
Thermal alteration is associated with an ecological change in freshwater stream ecosystems (Fenoglio et al. 2010; Rivers-Moore et al. 2013; Bruno et al. 2019). Stream temperature alteration may force thermally sensitive species to change their geographical distribution range, negatively affecting stream communities, biodiversity and ecosystems (Fenoglio et al. 2010; Alba-Tercedor et al. 2017; Amundrud and Srivastava 2020). While examining the response of stream invertebrates to changing thermal regimes in South America, Pedreros et al. (2020) suggested that changes in stream temperature could lead to the displacement in altitude and latitude of some stream invertebrates. The distribution range of some Australian Paratya spp. may change and their distribution may be reduced or shifted southward as a response to increasing stream temperatures that exceed their ULT. This may mean a loss of biodiversity if some species of Australian Paratya are restricted to high elevations (Rahman et al. 2020). Field examinations could assess species distribution and predict distribution limits (Chessman 2018; Terblanche and Hoffmann 2020).
As filter feeders and grazers, shrimp play a key role in stream communities (Moulton et al. 2012: Suter et al. 2022). They are essential in controlling periphyton biomass (Moulton et al. 2012) and are a major food source for stream-dwelling fishes such as Mogurnda adspersa and Tandanus tandanus; they are also preyed on by Ornithorhynchus anatinus (platypus) (Bain et al. 2016; Rahman et al. 2020). Their loss may also have stream health impacts because they are consumers of periphyton and, hence, important for ecosystem functioning (Moulton et al. 2012). The loss of this important component from the food web may lead to changes in predator–prey dynamics, disturbing population dynamics of other species with which they interact (Ferris and Wilson 2012). Climate change-associated stream warming disrupts biological communities and severs ecological linkages. Despite this, there are significant gaps in our knowledge of the environmental tolerances, including the ULT of this ecologically significant group (Bonacina et al. 2023).
Maximum stream temperatures are decreased by shading (Johnson 2004; Fuller et al. 2022). Developing resilience in the landscape to reduce in-stream impacts may be important. Thermal impacts could be moderately mitigated through increasing shading by riparian vegetation and ensuring natural environmental flows. Restoring riparian vegetation in inadequately shaded streams may counteract some of the influence of projected warming and drying under climate change (Trimmel et al. 2018; Fuller et al. 2022). However, air temperatures will continue to increase and affect currently well-shaded streams (Fuller et al. 2022).
Conclusions
This study provides new data on winter and summer ULT and the plasticity of Australian Paratya spp. in Australian streams. As hypothesised, the ULT (as indicated by LT50) of warm water-acclimated shrimp was higher than that of winter-acclimatised shrimp. However, the population of Australian Paratya spp. examined in this study had limited plasticity of thermal limits and may be unable to buffer against the influences of climate change on stream temperatures. Australian Paratya spp. showed a vulnerability to rising stream water temperatures under future climate warming, given the limited adaptive response of their UTLs. Climate change-associated stream warming may significantly affect stream invertebrate populations through the contraction of suitable habitat ranges. Furthermore, stream water temperatures influence the growth, feeding, reproduction and, ultimately, survival of ectothermic stream invertebrates. Increasing resilience through target management of resorting riparian vegetation for shade and securing environmental flows may reduce the impacts of stream warming.
Supplementary material
Supplementary material is available online.
Data availability
The data that support this study will be shared upon reasonable request to the corresponding author.
Conflicts of interest
There is no conflicts of interest relating to this article.
Declaration of funding
This research did not receive any specific funding.
Acknowledgements
A huge thanks go to Dr Sue Oeding for assisting with collecting and hand-picking all the shrimp on a cold July day.
References
Alba-Tercedor, J, Sáinz-Bariáin, M, Poquet, JM, and Rodríguez-López, R (2017). Predicting river macroinvertebrate communities distributional shifts under future global change scenarios in the Spanish mediterranean area. PLoS ONE 12, e0167904.| Predicting river macroinvertebrate communities distributional shifts under future global change scenarios in the Spanish mediterranean area.Crossref | GoogleScholarGoogle Scholar |
Amundrud, SL, and Srivastava, DS (2020). Thermal tolerances and species interactions determine the elevational distributions of insects. Global Ecology and Biogeography 29, 1315–1327.
| Thermal tolerances and species interactions determine the elevational distributions of insects.Crossref | GoogleScholarGoogle Scholar |
Archambault, JM, Cope, WG, and Kwak, TJ (2014). Survival and behaviour of juvenile unionid mussels exposed to thermal stress and dewatering in the presence of a sediment temperature gradient. Freshwater Biology 59, 601–613.
| Survival and behaviour of juvenile unionid mussels exposed to thermal stress and dewatering in the presence of a sediment temperature gradient.Crossref | GoogleScholarGoogle Scholar |
Arias Font, R, Khamis, K, Milner, AM, Sambrook Smith, GH, and Ledger, ME (2021). Low flow and heatwaves alter ecosystem functioning in a stream mesocosm experiment. Science of The Total Environment 777, 146067.
| Low flow and heatwaves alter ecosystem functioning in a stream mesocosm experiment.Crossref | GoogleScholarGoogle Scholar |
Bain, PA, Gregg, AL, and Kumar, A (2016). De novo assembly and analysis of changes in the protein-coding transcriptome of the freshwater shrimp Paratya australiensis (Decapoda: Atyidae) in response to acid sulfate drainage water. BMC Genomics 17, 890.
| De novo assembly and analysis of changes in the protein-coding transcriptome of the freshwater shrimp Paratya australiensis (Decapoda: Atyidae) in response to acid sulfate drainage water.Crossref | GoogleScholarGoogle Scholar |
Bonacina, L, Fasano, F, Mezzanotte, V, and Fornaroli, R (2023). Effects of water temperature on freshwater macroinvertebrates: a systematic review. Biological Reviews 98, 191–221.
| Effects of water temperature on freshwater macroinvertebrates: a systematic review.Crossref | GoogleScholarGoogle Scholar |
Bruno, D, Belmar, O, Maire, A, Morel, A, Dumont, B, and Datry, T (2019). Structural and functional responses of invertebrate communities to climate change and flow regulation in alpine catchments. Global Change Biology 25, 1612–1628.
| Structural and functional responses of invertebrate communities to climate change and flow regulation in alpine catchments.Crossref | GoogleScholarGoogle Scholar |
Chessman, BC (2009). Climatic changes and 13-year trends in stream macroinvertebrate assemblages in New South Wales, Australia. Global Change Biology 15, 2791–2802.
| Climatic changes and 13-year trends in stream macroinvertebrate assemblages in New South Wales, Australia.Crossref | GoogleScholarGoogle Scholar |
Chessman, BC (2018). Dissolved-oxygen, current and temperature preferences of stream invertebrates estimated from field distributions: application to assemblage responses to drought. Hydrobiologia 809, 141–153.
| Dissolved-oxygen, current and temperature preferences of stream invertebrates estimated from field distributions: application to assemblage responses to drought.Crossref | GoogleScholarGoogle Scholar |
Cook, BD, Baker, AM, Page, TJ, Grant, SC, Fawcett, JH, Hurwood, DA, and Hughes, JM (2006). Biogeographic history of an Australian freshwater shrimp, Paratya australiensis (Atyidae): the role life history transition in phylogeographic diversification. Molecular Ecology 15, 1083–1093.
| Biogeographic history of an Australian freshwater shrimp, Paratya australiensis (Atyidae): the role life history transition in phylogeographic diversification.Crossref | GoogleScholarGoogle Scholar |
Cox, TJ, and Rutherford, JC (2000). Predicting the effects of time-varying temperatures on stream invertebrate mortality. New Zealand Journal of Marine and Freshwater Research 34, 209–215.
| Predicting the effects of time-varying temperatures on stream invertebrate mortality.Crossref | GoogleScholarGoogle Scholar |
CSIRO and Bureau of Meteorology (2020) State of the climate 2022. (Australian Government: Canberra, ACT, Australia) Available at http://www.bom.gov.au/state-of-the-climate/2022/documents/2022-state-of-the-climate-web.pdf
Dallas, HF, and Ketley, ZA (2011). Upper thermal limits of aquatic macroinvertebrates: comparing critical thermal maxima with 96-LT50 values. Journal of Thermal Biology 36, 322–327.
| Upper thermal limits of aquatic macroinvertebrates: comparing critical thermal maxima with 96-LT50 values.Crossref | GoogleScholarGoogle Scholar |
Dallas, HF, and Rivers-Moore, NA (2018). Temporal thermal refugia and seasonal variation in upper thermal limits of two species of riverine invertebrates: the amphipod, Paramelita nigroculus, and the mayfly, Lestagella penicillata. Aquatic Ecology 52, 333–349.
| Temporal thermal refugia and seasonal variation in upper thermal limits of two species of riverine invertebrates: the amphipod, Paramelita nigroculus, and the mayfly, Lestagella penicillata.Crossref | GoogleScholarGoogle Scholar |
Dudgeon, D (2019). Multiple threats imperil freshwater biodiversity in the anthropocene. Current Biology 29, R960–R967.
| Multiple threats imperil freshwater biodiversity in the anthropocene.Crossref | GoogleScholarGoogle Scholar |
Egea-Serrano, A, Alves, MC, Solé, M, and Tejedo, M (2022). Upper thermal tolerances and vulnerability to global warming in a Brazilian Caatinga fish Astyanax bimaculatus (Linnaeus, 1758) population. Austral Ecology 47, 1157–1161.
| Upper thermal tolerances and vulnerability to global warming in a Brazilian Caatinga fish Astyanax bimaculatus (Linnaeus, 1758) population.Crossref | GoogleScholarGoogle Scholar |
Ernst, MR, Beitinger, TL, and Stewart, KW (1984). Critical thermal maxima of nymphs of three Plecoptera species from an Ozark foothill stream. Freshwater Invertebrate Biology 3, 80–85.
| Critical thermal maxima of nymphs of three Plecoptera species from an Ozark foothill stream.Crossref | GoogleScholarGoogle Scholar |
Fenoglio, S, Bo, T, Cucco, M, Mercalli, L, and Malacarne, G (2010). Effects of global climate change on freshwater biota: a review with special emphasis on the Italian situation. Italian Journal of Zoology 77, 374–383.
| Effects of global climate change on freshwater biota: a review with special emphasis on the Italian situation.Crossref | GoogleScholarGoogle Scholar |
Ferris, R, and Wilson, RS (2012). The physiological arms race: exploring thermal acclimation among interacting species. Journal of Thermal Biology 37, 236–242.
| The physiological arms race: exploring thermal acclimation among interacting species.Crossref | GoogleScholarGoogle Scholar |
Fuller, MR, Leinenbach, P, Detenbeck, NE, Labiosa, R, and Isaak, DJ (2022). Riparian vegetation shade restoration and loss effects on recent and future stream temperatures. Restoration Ecology 30, e13626.
| Riparian vegetation shade restoration and loss effects on recent and future stream temperatures.Crossref | GoogleScholarGoogle Scholar |
Gomez Isaza, DF, Cramp, RL, and Franklin, CE (2022). Fire and rain: a systematic review of the impacts of wildfire and associated runoff on aquatic fauna. Global Change Biology 28, 2578–2595.
| Fire and rain: a systematic review of the impacts of wildfire and associated runoff on aquatic fauna.Crossref | GoogleScholarGoogle Scholar |
González, RA, Díaz, F, Licea, A, Denisse Re, A, Noemí Sánchez, L, and García-Esquivel, Z (2010). Thermal preference, tolerance and oxygen consumption of adult white shrimp Litopenaeus vannamei (Boone) exposed to different acclimation temperatures. Journal of Thermal Biology 35, 218–224.
| Thermal preference, tolerance and oxygen consumption of adult white shrimp Litopenaeus vannamei (Boone) exposed to different acclimation temperatures.Crossref | GoogleScholarGoogle Scholar |
Gunderson, AR, and Stillman, JH (2015). Plasticity in thermal tolerance has limited potential to buffer ectotherms from global warming. Proceedings of the Royal Society of London – B. Biological Sciences 282, 20150401.
Haase, P, Pilotto, F, Li, F, Sundermann, A, Lorenz, AW, Tonkin, JD, and Stoll, S (2019). Moderate warming over the past 25 years has already reorganized stream invertebrate communities. Science of The Total Environment 658, 1531–1538.
| Moderate warming over the past 25 years has already reorganized stream invertebrate communities.Crossref | GoogleScholarGoogle Scholar |
Hancock, MA, and Bunn, SE (1997). Population dynamics and life history of Paratya australiensis Kemp, 1917 (Decapoda: Atyidae) in upland rainforest streams, south-eastern Queensland, Australia. Marine and Freshwater Research 48, 361.
| Population dynamics and life history of Paratya australiensis Kemp, 1917 (Decapoda: Atyidae) in upland rainforest streams, south-eastern Queensland, Australia.Crossref | GoogleScholarGoogle Scholar |
Hidalgo-Galiana, A, Ribera, I, and Terblanche, JS (2021). Geographic variation in acclimation responses of thermal tolerance in South African diving beetles (Dytiscidae: Coleoptera). Comparative Biochemistry and Physiology – A. Molecular & Integrative Physiology 257, 110955.
| Geographic variation in acclimation responses of thermal tolerance in South African diving beetles (Dytiscidae: Coleoptera).Crossref | GoogleScholarGoogle Scholar |
Houghton, DC, and Shoup, L (2014). Seasonal changes in the critical thermal maxima of four species of aquatic insects (Ephemeroptera, Trichoptera). Environmental Entomology , 1059–1066.
| Seasonal changes in the critical thermal maxima of four species of aquatic insects (Ephemeroptera, Trichoptera).Crossref | GoogleScholarGoogle Scholar |
Isaak, DJ, Wollrab, S, Horan, D, and Chandler, G (2012). Climate change effects on stream and river temperatures across the northwest US. From 1980–2009 and implications for salmonid fishes. Climatic Change 113, 499–524.
| Climate change effects on stream and river temperatures across the northwest US. From 1980–2009 and implications for salmonid fishes.Crossref | GoogleScholarGoogle Scholar |
Johnson, SL (2004). Factors influencing stream temperatures in small streams: substrate effects and a shading experiment. Canadian Journal of Fisheries and Aquatic Sciences 61, 913–923.
| Factors influencing stream temperatures in small streams: substrate effects and a shading experiment.Crossref | GoogleScholarGoogle Scholar |
Jørgensen, LB, Malte, H, and Overgaard, J (2019). How to asses Drosophila heat tolerance: unifying static and dynamic tolerance assays to predict heat distribution limits. Functional Ecology 33, 629–642.
| How to asses Drosophila heat tolerance: unifying static and dynamic tolerance assays to predict heat distribution limits.Crossref | GoogleScholarGoogle Scholar |
Kim, KS, Chou, H, Funk, DH, Jackson, JK, Sweeney, BW, and Buchwalter, DB (2017). Physiological responses to short-term thermal stress in mayfly (Neocloeon triangulifer) larvae in relation to upper thermal limits. Journal of Experimental Biology 220, 2598–2605.
| Physiological responses to short-term thermal stress in mayfly (Neocloeon triangulifer) larvae in relation to upper thermal limits.Crossref | GoogleScholarGoogle Scholar |
Magozzi, S, and Calosi, P (2015). Integrating metabolic performance, thermal tolerance, and plasticity enables for more accurate predictions on species vulnerability to acute and chronic effects of global warming. Global Change Biology 21, 181–194.
| Integrating metabolic performance, thermal tolerance, and plasticity enables for more accurate predictions on species vulnerability to acute and chronic effects of global warming.Crossref | GoogleScholarGoogle Scholar |
Morley, SA, Peck, LS, Sunday, JM, Heiser, S, and Bates, AE (2019). Physiological acclimation and persistence of ectothermic species under extreme heat events. Global Ecology and Biogeography 28, 1018–1037.
| Physiological acclimation and persistence of ectothermic species under extreme heat events.Crossref | GoogleScholarGoogle Scholar |
Moulton, TP, Souza, ML, Brito, EF, Braga, MRA, and Bunn, SE (2012). Strong interactions of Paratya australiensis (Decapoda: Atyidae) on periphyton in an Australian subtropical stream. Marine and Freshwater Research 63, 834.
| Strong interactions of Paratya australiensis (Decapoda: Atyidae) on periphyton in an Australian subtropical stream.Crossref | GoogleScholarGoogle Scholar |
Narum, SR, Campbell, NR, Meyer, KA, Miller, MR, and Hardy, RW (2013). Thermal adaptation and acclimation of ectotherms from differing aquatic climates. Molecular Ecology 22, 3090–3097.
| Thermal adaptation and acclimation of ectotherms from differing aquatic climates.Crossref | GoogleScholarGoogle Scholar |
Overgaard, J, Kristensen, TN, and Sørensen, JG (2012). Validity of thermal ramping assays used to assess thermal tolerance in arthropods. PLoS ONE 7, e32758.
| Validity of thermal ramping assays used to assess thermal tolerance in arthropods.Crossref | GoogleScholarGoogle Scholar |
Pallarés, S, Verberk, WCEP, and Bilton, DT (2021). Plasticity of thermal performance curves in a narrow range endemic water beetle. Journal of Thermal Biology 102, 103113.
| Plasticity of thermal performance curves in a narrow range endemic water beetle.Crossref | GoogleScholarGoogle Scholar |
Palmer, MA, Lettenmaier, DP, Poff, NL, Postel, SL, Richter, B, and Warner, R (2009). Climate change and river ecosystems: protection and adaptation options. Environmental Management 44, 1053–1068.
| Climate change and river ecosystems: protection and adaptation options.Crossref | GoogleScholarGoogle Scholar |
Pedreros, P, Guevara-Mora, M, Stehr, A, Araneda, A, and Urrutia, R (2020). Response of macroinvertebrate communities to thermal regime in small Mediterranean streams (southern South America): implications of global warming. Limnologica 81, 125763.
| Response of macroinvertebrate communities to thermal regime in small Mediterranean streams (southern South America): implications of global warming.Crossref | GoogleScholarGoogle Scholar |
Quinn, JM, Steele, GL, Hickey, CW, and Vickers, ML (1994). Upper thermal tolerances of twelve New Zealand stream invertebrate species. New Zealand Journal of Marine and Freshwater Research 28, 391–397.
| Upper thermal tolerances of twelve New Zealand stream invertebrate species.Crossref | GoogleScholarGoogle Scholar |
Rahman, S, Schmidt, D, and Hughes, JM (2020). Genetic structure of Australian glass shrimp, Paratya australiensis, in relation to altitude. PeerJ 8, e8139–e8139.
| Genetic structure of Australian glass shrimp, Paratya australiensis, in relation to altitude.Crossref | GoogleScholarGoogle Scholar |
Rezende, EL, Castañeda, LE, and Santos, M (2014). Tolerance landscapes in thermal ecology. Functional Ecology 28, 799–809.
| Tolerance landscapes in thermal ecology.Crossref | GoogleScholarGoogle Scholar |
Rivers-Moore, NA, Dallas, HF, and Ross-Gillespie, V (2013). Life history does matter in assessing potential ecological impacts of thermal changes on aquatic macroinvertebrates. River Research and Applications 29, 1100–1109.
| Life history does matter in assessing potential ecological impacts of thermal changes on aquatic macroinvertebrates.Crossref | GoogleScholarGoogle Scholar |
Rohr, JR, Civitello, DJ, Cohen, JM, Roznik, EA, Sinervo, B, and Dell, AI (2018). The complex drivers of thermal acclimation and breadth in ectotherms. Ecology Letters 21, 1425–1439.
| The complex drivers of thermal acclimation and breadth in ectotherms.Crossref | GoogleScholarGoogle Scholar |
Ross-Gillespie V (2014) Effects of water temperature on life-history traits of selected South African aquatic insects. PhD thesis, University of Cape Town, Cape Town, South Africa.
Semsar-kazerouni, M, and Verberk, WCEP (2018). It’s about time: linkages between heat tolerance, thermal acclimation and metabolic rate at different temporal scales in the freshwater amphipod Gammarus fossarum Koch, 1836. Journal of Thermal Biology 75, 31–37.
| It’s about time: linkages between heat tolerance, thermal acclimation and metabolic rate at different temporal scales in the freshwater amphipod Gammarus fossarum Koch, 1836.Crossref | GoogleScholarGoogle Scholar |
Seyedhashemi, H, Vidal, J-P, Diamond, JS, Thiéry, D, Monteil, C, Hendrickx, F, Maire, A, and Moatar, F (2022). Regional, multi-decadal analysis on the Loire River Basin reveals that stream temperature increases faster than air temperature. Hydrology and Earth System Sciences 26, 2583–2603.
| Regional, multi-decadal analysis on the Loire River Basin reveals that stream temperature increases faster than air temperature.Crossref | GoogleScholarGoogle Scholar |
Stewart, BA, Close, PG, Cook, PA, and Davies, PM (2013). Upper thermal tolerances of key taxonomic groups of stream invertebrates. Hydrobiologia 718, 131–140.
| Upper thermal tolerances of key taxonomic groups of stream invertebrates.Crossref | GoogleScholarGoogle Scholar |
Suter, PJ, Mynott, JH, and Crump, M (2022). New species of Paratya (Decapoda: Atyidae) from Australian inland waters – linking morphological characters with molecular lineages. Memoirs of Museum Victoria 81, 55–122.
| New species of Paratya (Decapoda: Atyidae) from Australian inland waters – linking morphological characters with molecular lineages.Crossref | GoogleScholarGoogle Scholar |
Sweeney BW, Jackson JK, Newbold JD, Funk DH (1992) Climate change and the life histories and biogeography of aquatic insects in eastern North America. In ‘Global climate change and freshwater ecosystems’. (Eds P Firth, SG Fisher) pp. 143–176. (Springer)
Terblanche, JS, and Hoffmann, AA (2020). Validating measurements of acclimation for climate change adaptation. Current Opinion in Insect Science 41, 7–16.
| Validating measurements of acclimation for climate change adaptation.Crossref | GoogleScholarGoogle Scholar |
Trimmel, H, Weihs, P, Leidinger, D, Formayer, H, Kalny, G, and Melcher, A (2018). Can riparian vegetation shade mitigate the expected rise in stream temperatures due to climate change during heat waves in a human-impacted pre-alpine river? Hydrology and Earth System Sciences 22, 437–461.
| Can riparian vegetation shade mitigate the expected rise in stream temperatures due to climate change during heat waves in a human-impacted pre-alpine river?Crossref | GoogleScholarGoogle Scholar |
Verberk, WCEP, and Calosi, P (2012). Oxygen limits heat tolerance and drives heat hardening in the aquatic nymphs of the gill breathing damselfly Calopteryx virgo (Linnaeus, 1758). Journal of Thermal Biology 37, 224–229.
| Oxygen limits heat tolerance and drives heat hardening in the aquatic nymphs of the gill breathing damselfly Calopteryx virgo (Linnaeus, 1758).Crossref | GoogleScholarGoogle Scholar |
Verberk, WCEP, Overgaard, J, Ern, R, Bayley, M, Wang, T, Boardman, L, and Terblanche, JS (2016). Does oxygen limit thermal tolerance in arthropods? A critical review of current evidence. Comparative Biochemistry and Physiology – A. Molecular & Integrative Physiology 192, 64–78.
| Does oxygen limit thermal tolerance in arthropods? A critical review of current evidence.Crossref | GoogleScholarGoogle Scholar |
Verberk, WCEP, Buchwalter, DB, and Kefford, BJ (2020). Energetics as a lens to understanding aquatic insect’s responses to changing temperature, dissolved oxygen and salinity regimes. Current Opinion in Insect Science 41, 46–53.
| Energetics as a lens to understanding aquatic insect’s responses to changing temperature, dissolved oxygen and salinity regimes.Crossref | GoogleScholarGoogle Scholar |
Verdelhos, T, Marques, JC, and Anastácio, P (2015). Behavioral and mortality responses of the bivalves Scrobicularia plana and Cerastoderma edule to temperature, as indicator of climate change’s potential impacts. Ecological Indicators 58, 95–103.
| Behavioral and mortality responses of the bivalves Scrobicularia plana and Cerastoderma edule to temperature, as indicator of climate change’s potential impacts.Crossref | GoogleScholarGoogle Scholar |
Ward, JV, and Stanford, JA (1982). Thermal responses in the evolutionary ecology of aquatic insects. Annual review of entomology 27, 97–117.
| Thermal responses in the evolutionary ecology of aquatic insects.Crossref | GoogleScholarGoogle Scholar |
Williams, W (1977). Some aspects of the ecology of Paratya australiensis (Crustacea: Decapoda: Atyidae). Marine and Freshwater Research 28, 403–415.
| Some aspects of the ecology of Paratya australiensis (Crustacea: Decapoda: Atyidae).Crossref | GoogleScholarGoogle Scholar |


