Spotlight on Varietal Thiols and Precursors in Grapes and Wines*
David W. JefferyThe Australian Research Council Training Centre for Innovative Wine Production, School of Agriculture, Food, and Wine, and Waite Research Institute, The University of Adelaide (UA), PMB 1, Glen Osmond, SA 5064, Australia. Email: david.jeffery@adelaide.edu.au
Australian Journal of Chemistry 69(12) 1323-1330 https://doi.org/10.1071/CH16296
Submitted: 16 May 2016 Accepted: 2 July 2016 Published: 20 July 2016
Abstract
Wine is an amazingly complex natural product that requires dedicated scientists to resolve many of its mysteries. Traditional synthetic organic chemistry and modern analytical techniques are powerful tools at the disposal of wine chemists who tackle the complexities of wine in order to improve scientific understanding and provide practical solutions to industry. Part of this quest for knowledge relates to maintaining or improving wine quality, which underpins consumer acceptance and links to the competitiveness of wineries in a global market. Wine aroma is an important aspect of wine quality and garners much attention from researchers. Grape-derived aroma compounds are one area of particular importance owing to their distinctiveness and ability to impart ‘varietal aromas’ to wines. Varietal thiols imparting tropical and citrus notes that are characteristic of wines such as Sauvignon Blanc have emerged, along with their grape-derived precursors, as an area of interest over the past two decades. These compounds have also caught our attention and we have made some important contributions to this field, including identifying new precursors, developing novel analytical methods, and conducting studies that provide unique insights into the biochemical transformations occurring in grape berries and juice, and during fermentation.
Introduction
Wine is a fascinating natural product containing a multitude of chemical constituents.[1] Apart from water and ethanol, components include sugars, higher alcohols and other volatiles (e.g. esters, acids, carbonyls, phenols, heterocycles), proteins, pigments, amino acids, phenolics, minerals, organic acids, and much more. Although none of these components are unique to wine, it is their particular combinations and concentrations that produce the sensory properties (aroma, flavour, colour, mouthfeel, etc.) that we perceive in the myriad styles of wine available.
The origins of the various wine constituents add to the complexity of wine, with many factors and decisions influencing the composition of the final product. That is, different grape varieties and fermentation and aging processes (influenced by both chemical and microbiological transformations) all have a bearing on wine style and sensory properties[2] and, in turn, wine quality and consumer appeal.[3] Wine aroma is one of the main determinants of wine quality, with odorants described as primary (grape), secondary (fermentation), and tertiary (ageing and storage) based on their origins. The aroma of a wine is thus an expression of the combination of different volatile components of varied origins that are affected by a range of inputs, starting with grape berry development, continuing through the winemaking process, and evolving over time in the bottle. Studying these intricate natural phenomena provides important scientific understanding that leads to innovations and helps drive quality improvements.
Varietal Wine Aromas
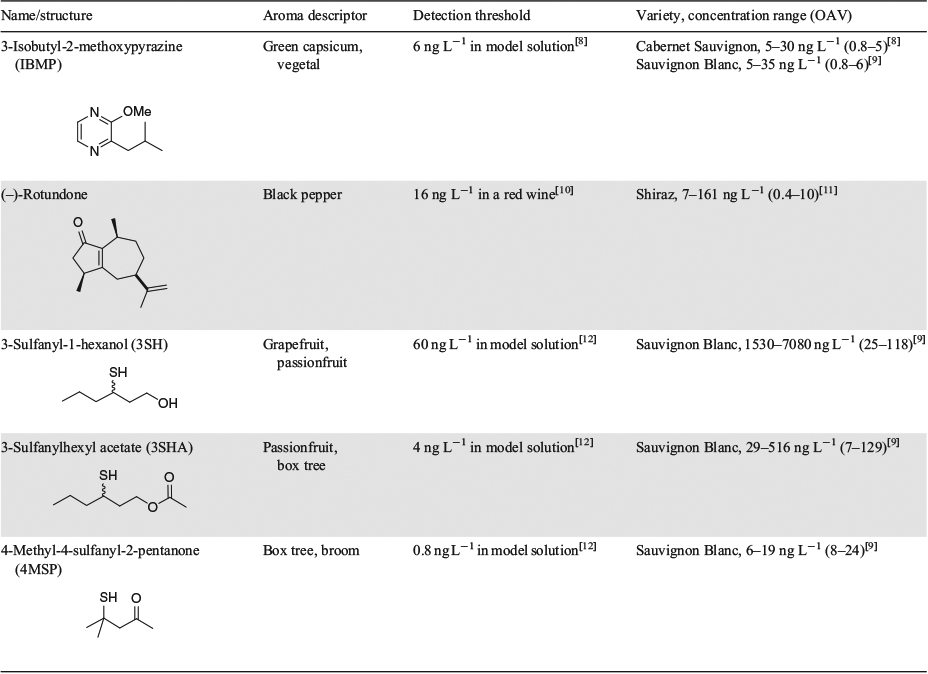
Despite there being over 1000 volatile compounds in wine (and likely many more) at concentrations spanning several orders of magnitude,[4] the aroma of wine (and foods more generally) can be recreated with a much smaller number of key odour-active components.[5] Especially important to this concept are odorants that impart a distinct effect as a result of being present at suprathreshold concentrations (defined in terms of odour activity value, OAV = concentration/aroma detection threshold, with OAV > 1) and compounds having characteristic aroma qualities that may define a particular varietal wine. Based on aroma detection thresholds (which differ depending on the matrix, such as neutral white or red wine, or hydroalcoholic model solution), an impact odorant may reach OAV > 1 either because it is present at a high concentration and surpasses a high aroma detection threshold, or because it is found at trace levels and has a very low detection threshold. The latter is often the case for potent varietal (primary) aroma compounds that are also characteristic of certain grape varieties, such as 3-isobutyl-2-methoxypyrazine (IBMP) in Cabernet Sauvignon wines, rotundone in Shiraz wines, and the so-called varietal thiols 3-sulfanyl-1-hexanol (3SH), its O-acetate 3-sulfanylhexyl acetate (3SHA), and 4-methyl-4-sulfanyl-2-pentanone (4MSP) (along with IBMP) in Sauvignon Blanc wines (Table 1). Note the presence of a chiral centre in 3SH and 3SHA leads to enantiomers, which are studied far less but have been reported to possess slightly different aroma qualities and detection thresholds.[6] The low abundances and array of chemistries (e.g. pyrazine, bicyclic sesquiterpenoid, polyfunctional thiol) of such compounds pose particular analytical challenges, but these can be addressed with a combination of organic synthesis and analytical approaches as discussed later.
|
Interestingly, whereas some grape-derived aroma compounds exist in grape berries as free volatiles that can be extracted into wine unchanged (e.g. rotundone, IBMP), others such as varietal thiols are present in non-volatile precursor forms that require liberation through yeast enzyme activity during fermentation. Owing to their major impact on varietal characters of Sauvignon Blanc, among other grape varieties, the varietal thiols 3SH, 3SHA, and 4MSP and their precursors have garnered a good deal of attention from grape and wine researchers over the past two decades,[7] including contributions from the present author and his colleagues.
Identification of Varietal Thiol Precursors in Grapes
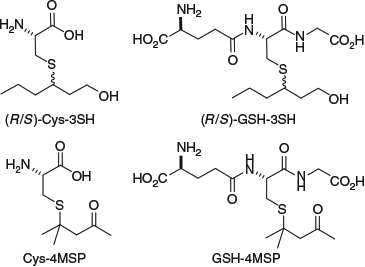
Varietal thiols are essentially absent from grapes, although we have shown the presence of low concentrations (~100 ng L–1) of 3SH in ripening grapes berries.[13] Instead, the characteristic passionfruit, grapefruit, and box-tree (cat urine) aromas of Sauvignon Blanc are expressed as a result of fermentation, which prompted the initial investigation of this phenomenon. Non-volatile glycosidic aroma precursors in grapes that lead to odorants in wine (e.g. monoterpenoids such as linalool and geraniol; C13-norisoprenoids such as β-damascenone) were identified in the 1980s[14] but it took almost another two decades before the presence of l-cysteine (Cys) and l-glutathione (GSH) conjugates of 3SH and 4MSP was determined in Sauvignon Blanc grapes (Fig. 1).[15] These conjugates constituted a new type of flavour precursor, leading to an array of activity aimed at understanding how they are formed and their influence on wine aroma. Notably, no conjugated forms of 3SHA have been identified in grape berries; instead, 3SHA arises from 3SH during fermentation under the action of yeast alcohol acetyltransferase, Atf1p.[16]
|
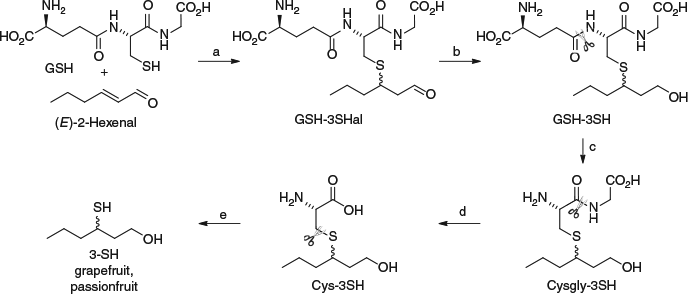
Thiol conjugates were investigated in a range of studies in the ensuing years to examine aspects such as their prevalence and localisation in grape berries, and transformation during fermentation.[17] In 2009, we identified the GSH conjugate of 4MSP (Fig. 1), which was hypothesised to be present based on knowledge of GSH detoxification pathways (i.e. conjugation and deactivation of electrophiles with nucleophilic GSH) and on the existence of the related cysteine conjugate Cys-4MSP, as well as the presence of both GSH- and Cys-3SH.[18] Highlighting the natural synergy of organic synthesis and analytical chemistry in our work, chemical synthesis was used to produce authentic GSH-4MSP in a Michael addition between GSH and mesityl oxide (Scheme 1a). The authentic compound was purified and characterised, and used to confirm the identity of GSH-4MSP in a grape juice extract (obtained by low-pressure C18 chromatography) with HPLC-tandem mass spectrometry (MS/MS) and co-injection experiments.
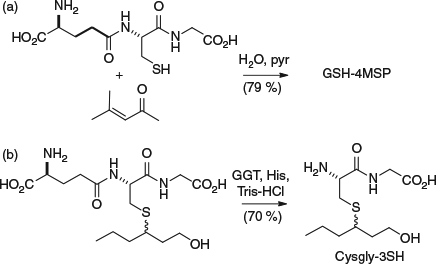
|
The identification of GSH-4MSP resolved one issue, but there were still missing pieces of the puzzle in terms of GSH-3SH formation and conversion to Cys-3MH in grape berries. The tripeptide GSH (and its conjugates) is naturally broken down to Cys (and corresponding conjugates) in biological systems, proceeding via l-cysteinylglycine (Cysgly) or γ-glutamylcysteine as dipeptide intermediates. Plants may utilise either of these pathways and the predominant enzymatic degradation routes remain to be determined.[19] However, mass spectral evidence for formation of Cysgly-3SH on exogenous enzyme treatment of a Sauvignon Blanc juice[15b] led to the proposition that Cysgly-3SH should be present in grape juices (and/or during fermentation), although its existence had not been formally identified in grapes. In 2011, we had undertaken a study on the effect of grape transportation on precursors (described later) and the elevated concentrations of GSH- and Cys-3SH in that work piqued our interest in identifying Cysgly-3SH.[20] Rather than undertaking a synthesis from the constituent amino acids, the authentic compound was derived by treating GSH-3SH (already on hand) with crude γ-glutamyltranspeptidase (GGT) (Scheme 1b), the enzyme responsible for transferring γ-glutamate to an acceptor amino acid in the formation of Cysgly from GSH. Reaction conditions were assessed to optimise the yield of Cysgly-3SH (10 equivalents of histidine as acceptor amino acid, 24-h incubation time) and the product was purified and fully characterised. As with the identification of GSH-4MSP, HPLC-MS/MS was used along with co-injection experiments of the authentic compound to verify the presence of Cysgly-3SH in Sauvignon Blanc grape juice. This provided a link between GSH- and Cys-3SH, with the apparent lack of accumulation of Cysgly-3SH helping to explain why its presence had not been verified before. However, this work did not probe the alternative pathway for GSH-3SH degradation via the γ-glutamylcysteine conjugate as a result of vacuolar carboxypeptidases or cytosolic phytochelatin synthases,[19] and these aspects require further study.
Analysis of Diastereomeric Precursors of 3SH in Grape Juice and Wine
To begin exploring the effects of grape processing and winemaking, and to link precursor concentrations in juice with varietal thiols in wine, in 2010, we developed a stable isotope dilution analysis (SIDA) method to analyse the diastereomers of Cys- and GSH-3MH using HPLC-MS/MS with multiple reaction monitoring (MRM).[21] Previous methods examined only the Cys conjugate, often by gas chromatography (GC)-MS after derivatisation, and usually without resolving the diastereomers. HPLC with electrospray-ionisation MS was deemed more suitable for the analysis of these non-volatile, water-soluble conjugates, and deuterated internal standards (obtained through synthesis) were employed for reliable quantitation by SIDA (a common theme throughout the present paper). All parameters were optimised and the method was validated for analytes isolated from grape juice or wine by solid-phase extraction (SPE).
The 3SH precursor diastereomer profile was evaluated for a range of grape varieties, providing the first comprehensive assessment of both 3SH precursors in juice (Table 2) and wine (to a lesser extent). This work revealed the predominance of GSH-3SH over its Cys-3SH counterpart and the prevalence of the (S)-diastereomer (referring to the alkyl chain stereocentre), especially for the GSH conjugate. The presence of sizable quantities of precursors in varieties other than Sauvignon Blanc was also identified, as well as the existence of precursors in bottled commercial wines. These results provided the basis for further studies and also pointed to a relationship between GSH diastereomer ratios and grape variety, as well as indicating the potential stereospecific formation of GSH-3SH in grape berries. In addition, on identifying Cysgly-3SH (described earlier), this analyte was included in the precursor method and validated using deuterated Cys-3SH as internal standard.[20]

|
Conversion of 3SH Precursors During Fermentation – Stereochemical Outcomes
Around the same time as developing the precursor analytical method, model fermentation studies were conducted with a pure diastereomer of GSH-3SH, prepared as shown in Scheme 2, and subsequently determined to be the (R)-configuration.[22] That paper also reported the synthesis and full characterisation of a range of thiol conjugates and their deuterated analogues required as internal standards for HPLC-MS/MS analysis.
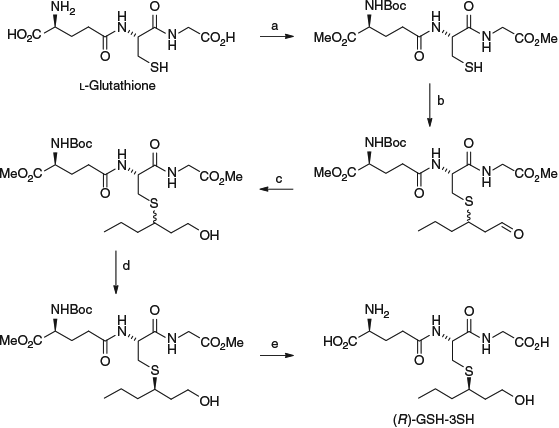
|
Moreover, the study showed that fermentation of a single diastereomer of GSH-3SH yielded not only the related Cys-3SH diastereomer (ascertained by HPLC-MS/MS and verified separately using enzymatic degradation with GGT), but also (R)-3SH as a single enantiomer (determined by chiral GC-MS analysis). This work revealed for the first time that fermentation of a GSH conjugate could release the corresponding varietal thiol, as well as highlighting the relationship between precursor and free thiol stereochemistry, and the ability of yeast to degrade GSH-3SH into Cys-3SH. Furthermore, based on conversion yields, it appeared that Cys-3SH was more easily utilised by yeast in the release of 3SH[23] – a substantially lower conversion efficiency for GSH-3SH has since been shown[24] due to the need for yeast to first convert the GSH precursor to its Cys counterpart.[25]
3SH Precursor Formation and Degradation
With reference to GSH detoxification pathways mentioned earlier, the formation of GSH-conjugated thiol precursors appears to be a natural response by grapevines for dealing with oxidative stress or berry damage. Breakdown of the GSH conjugates then leads to Cysgly intermediates and ultimately to Cys conjugates. Although the pathway to formation of mesityl oxide is unclear, (E)-2-hexenal is formed from linolenic acid owing to oxidative stress or berry damage via the lipoxygenase–hydroperoxide lyase (LOX/HPL) pathway,[26] whereas GSH is a natural antioxidant (and nucleophile) present in grapes.[27] It seemed obvious that these two components are necessary to form GSH-3SH and it is expected that glutathione S-transferases (GSTs) play a role in the conjugation.[27,28] However, the first step in the conjugation of (E)-2-hexenal should lead to an aldehyde, not the primary alcohol present in GSH-3SH – this fact was seemingly ignored in the literature regarding varietal thiol precursors.
In 2011, we set about deciphering aspects of conjugation of GSH to (E)-2-hexenal by conducting experiments with deuterated (E)-2-hexenal as well as by inhibiting enzymatic reactions, and evaluating the results with HPLC-MS/MS.[27] Enzyme inhibition involved freezing whole grape bunches in liquid nitrogen and grinding them to a fine powder before adding methanol/chloroform (2 : 1 v/v). The aqueous layer was recovered and an aliquot was prepared for precursor analysis, revealing that whereas Cys-3SH concentration was relatively unaffected, there was a dramatic decrease in GSH-3SH concentration. This suggested that GSH-3SH could be formed during processing as a result of berry damage, and pointed to the role of enzymatic conjugation. We proposed that Cys-3SH, as a breakdown product of GSH-3SH, was extracted from the grape berries and mostly unaffected by the processing step (i.e. grape crushing) whereas most of the GSH-3SH found in grape juice arose as a result of processing (i.e. on harvest or through post-harvest operations). In addition, crushing grape berries in the presence of d8-(E)-2-hexenal (synthesised from d10-1-butanol) led to formation of the labelled, conjugated aldehyde (i.e. d8-GSH-3SHal) whose natural (unlabelled) analogue had so far been ignored. Analysis by HPLC-MS/MS identified both labelled GSH-3SHal and the usual precursor, GSH-3SH (as the d8-isotopologue), whose presence can be explained on the basis of aldehyde reduction due to enzymes belonging to the alcohol dehydrogenase (ADH) or aldo-keto reductase (AKR) families.[27] Separately, we revealed the impact of sample preparation method on precursor concentrations, and showed that freezing grape juice did not significantly influence the results, but freezing whole grape berries led to a drastic increase in GSH-3SH concentrations.[13] This outcome reinforced the notion that berry damage (as a result of freezing in this case) leads to post-harvest GSH-3SH formation, and in a practical sense, showed that freezing juice for later precursor analysis was much preferred over freezing grapes.
These results, in conjunction with our work identifying Cysgly-3SH and relating GSH-3SH to free thiol 3SH, and the processing studies described later, provide a more complete story for GSH-3SH formation and degradation in grape berries and during fermentation (Scheme 3). It remains to be clarified how the diastereomeric ratio of GSH-3SH precursors eventuates, and the extent of chemical versus enzymatic conjugation needs to be assessed – these factors may well be linked.
Considering that Cys-3SH is more efficiently utilised by yeast whereas the major precursor in grape juices is GSH-3SH, the degradation of GSH-3SH into Cys-3SH has important implications for maximising the yield of varietal thiols during fermentation. We undertook several studies to investigate the effects of grape ripening (including an assessment of different Sauvignon Blanc clones) and the impact of processing operations, consisting of a comparison of hand and mechanical harvesting, and evaluation of the effects of transporting (included treatments with antioxidants SO2 and ascorbic acid) or storing (included analysis of GSH and C6 compounds) mechanically harvested Sauvignon Blanc grapes.[13,20,23,27,28] A summary of results and explanations of the findings appear in Table 3.

|
Analysis of Varietal Thiols in Juice and Wine
Measurement of varietal thiols complements the precursor studies and provides a link between grape berry constituents and wine aroma outcomes. Ideally, it would be useful to predict wine thiol concentrations by measuring precursors in juice. The analysis of varietal thiols is complicated by their extremely low abundance (in the nanogram per litre range, Table 1) and the reactivity of sulfur (e.g. potential for oxidation and binding to surfaces).[13,29] Several GC-MS methods were available but they were not overly attractive owing to requiring the use of mercury complexes or elaborate procedures for thiol isolation, or less common instrumentation having chemical ionisation functionality.[7] Aiming to avoid some of these drawbacks, in 2011, we developed a method for 3SH to be able to relate our precursor results to winemaking outcomes.[13] We chose thiol derivatisation with pentafluorobenzyl bromide (PFBBr), already in use in several other studies, and optimised a SIDA method for a common benchtop GC-MS with electron ionisation (EI), using d10-3SH as internal standard. The procedure involved spiking wine with internal standard, adding EDTA (to prevent thiol oxidation) and NaCl, extracting with pentane, washing extracts with bicarbonate solution (to remove interferences), back-extracting 3SH into cold sodium hydroxide solution (exploiting the pKa of thiols), and in situ derivatisation with PFBBr before neutralisation with tartaric acid and headspace analysis using solid-phase microextraction (SPME).
Each step was fully optimised and the method, which can be used to quantify 3SH at 40 ng L–1 (i.e. below its aroma detection threshold), was used to survey a range of commercial wines. Precursor analysis of commercial wines and juices was also undertaken using our HPLC-MS/MS method. Again, the presence of precursors in bottled wine was demonstrated, which may have implications for in-mouth flavour release as a result of oral microflora.[30] The fate of these precursors in the bottle is also worthy of follow-up studies to determine whether they constitute a latent source of varietal thiols. Significantly, this study was the first to attempt to link 3SH precursors in juice to 3SH concentrations in the corresponding wine on a commercial scale, using Chardonnay, Pinot Gris, Sauvignon Blanc, and Riesling juices (Table 4). Precursor concentrations tended to be lower after fermentation but there was apparently no relationship between precursor concentrations in juice and 3SH content in the wines on a molar basis, after accounting for remaining precursors in the wines. On reflection, this was not too surprising given the commercial nature of the samples and the variability that entails. Recent work by others has examined the lability of thiol precursors in an attempt to account for their decrease during fermentation, but apart from releasing varietal thiols, alternative fates of the precursors were unresolved.[31]
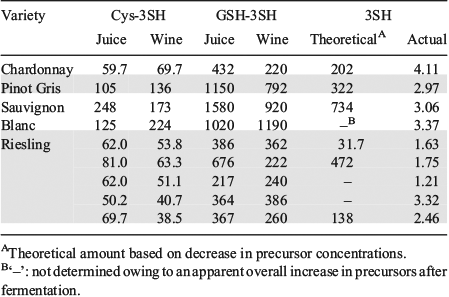
|
The lack of correlation between free thiols and precursors has since been verified by others[32] and highlights the need for more understanding about varietal thiol biogenesis in wines. Although grape-derived precursors are certainly a source of varietal thiols in wine, a lack of correlation may come down to several factors, such as not accounting for thiol losses as a result of volatilisation or adsorption during fermentation, or not currently measuring all precursor forms. For instance, the existence of GSH-3SHal (see Scheme 3) that we proposed with our studies using d8-(E)-2-hexenal has since been verified, along with its bisulfite adduct GSH-3SH-SO3 (bisulfite is used as an antioxidant during winemaking) (Fig. 2); these compounds can be converted to 3SH during fermentation (requiring liberation of the aldehyde and enzymatic carbonyl reduction by yeast), albeit in low yield.[33] In addition, sulfonic acid derivatives of (E)-2-hexenal and mesityl oxide have recently been synthesised as proposed alternative precursors to 3SH and 4MSP[34] (Fig. 2). Formation of free thiols would likely require enzymatic reduction of the sulfonate but fermentation studies confirming this have not been reported as yet. Incidentally, H2S produced as a by-product of fermentation via enzymatic reduction of sulfite can potentially combine with (E)-2-hexenal or mesityl oxide to produce varietal thiols 3SH (after aldehyde reduction by yeast) and 4MSP more directly, although this appears to be a minor pathway.[35]
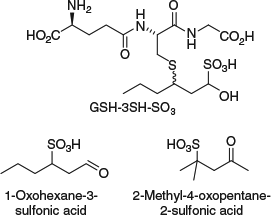
|
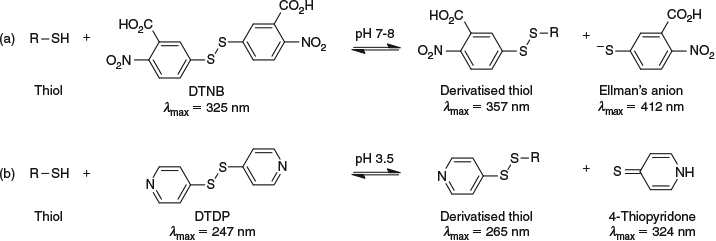
Despite our GC-MS method serving its purpose at the time, we were not satisfied with only being able to measure 3SH and continued to evaluate other options. Ideas revolved around derivatisation and analysis by HPLC-MS/MS, and some consideration was given to Ellman’s reagent (5,5′-dithiobis(2-nitrobenzoic acid), DTNB), which has been used to spectrophotometrically assess biological thiols such as Cys and GSH by monitoring Ellman’s anion at 412 nm (Scheme 4). It has also been employed to evaluate the concentration of varietal thiol stock solutions but its optimum pH is ~7–8, which is far from wine pH at 3–4. In 2015, we identified 4,4′-dithiodipyridine (DTDP) as a suitable alternative to DTNB that was much more compatible with the pH of wine (Scheme 4). However, rather than determine thiol concentrations spectrophotometrically (trace levels of varietal thiols would be swamped by other thiols present), we used it in conjunction with HPLC-MS/MS analysis.[29]
We optimised the full procedure, which involved spiking wine with labelled internal standards (another SIDA method), adding EDTA, 50 % acetaldehyde solution, and DTDP reagent, isolating derivatives by SPE, and analysing them by HPLC-MS/MS with MRM. Acetaldehyde was added to bind bisulfite, which we found would otherwise consume all of the derivatising reagent and lead to poor detection of varietal thiols. This method was not only developed for 3SH, 3SHA, and 4MSP, but also for two other highly potent wine aroma compounds, benzenemethanethiol (smoky) and 2-furanmethanethiol (roasted coffee), derived from wine aging and oak storage. This required the synthesis of labelled analogues of the two additional analytes. Our new method performed as well as the most advanced method available for measuring these five thiols (derivatisation and analysis with negative chemical ionisation GC-MS),[36] but was far less convoluted in its sample preparation. Although the ultimate dream would be to take wine, add labelled standard and derivatising agent, and then analyse directly by HPLC-MS/MS – an approach that could work for 3SH given its greater abundance – we are more than satisfied with what we have been able to develop and it is now used routinely for our studies on varietal thiols.
Conclusion
Wine is an amazing natural product and understanding its complexity is a serious undertaking. Synthetic organic chemistry and modern analytical techniques are powerful tools for wine chemists who aim to unravel the complexities of wine to improve scientific knowledge and provide practical solutions to winemakers. Varietal thiols and precursors have emerged as an area of interest over the past two decades and we have made some important contributions in this field. These include identifying new precursors, developing novel analytical methods, and conducting studies to provide unique insight into the biochemical transformations occurring in grape berries and juice, and during fermentation.
Although the present work has addressed many questions, new ones have arisen that remain unanswered. One aspect is the notion of post-harvest flavour modification in relation to grape-derived constituents, which we have observed for varietal thiol precursors but the concept may extend further. Other studies can be envisaged that address the stereoisomeric distribution of precursors and free thiols, and tease apart the role of biotic and abiotic formation of precursors. In addition, continued research is needed to identify other precursors or routes to thiol biogenesis that more fully account for free thiols in wine. Finally, the methods we have developed are simply the tools we require – it is the application of analytical methods that will lead to ground-breaking outcomes, whether that is in the development of new yeast strains, for example, or in the optimisation of grape-growing and winemaking practises.
* The author is the winner of the 2015 RACI Peter W. Alexander Medal.
Acknowledgements
I acknowledge my past and present colleagues and coauthors named in the references who have contributed to the work presented in this article. In particular, I am grateful to Dr Dimitra Capone from the Australian Wine Research Institute for her substantial contributions to this body of research.
References
[1] D. W. Jeffery, K. L. Wilkinson, in The Oxford Handbook of Food Fermentations (Eds C. W. Bamforth, R. E. Ward) 2014, pp. 54–147 (Oxford University Press: New York, NY).[2] See pp. 349–386 in: R. S. Jackson, Wine Tasting: A Professional Handbook 2009 (Academic Press: San Diego, CA).
[3] See pp. 387–426 in: R. S. Jackson, Wine Tasting: A Professional Handbook 2009 (Academic Press: San Diego, CA).
[4] P. Polášková, J. Herszage, S. E. Ebeler, Chem. Soc. Rev. 2008, 37, 2478.
| Crossref | GoogleScholarGoogle Scholar | 18949121PubMed |
[5] A. Dunkel, M. Steinhaus, M. Kotthoff, B. Nowak, D. Krautwurst, P. Schieberle, T Hofmann, Angew. Chem. Int. Ed. 2014, 53, 7124.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXhtVShurjM&md5=aa0b3fda2901ddbea49f27798aff57caCAS |
[6] T. Tominaga, Y. Niclass, E. Frerot, D. Dubourdieu, J. Agric. Food Chem. 2006, 54, 7251.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28Xot1Kgurk%3D&md5=a7b06dcabd53b5502d0c1c43702536daCAS | 16968090PubMed |
[7] A. Roland, R. Schneider, A. Razungles, F. Cavelier, Chem. Rev. 2011, 111, 7355.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXptlSmsrw%3D&md5=179c57fd273f19a03ba7fa025f34cf83CAS | 21780789PubMed |
[8] D. Roujou de Boubee, C. Van Leeuwen, D. Dubourdieu, J. Agric. Food Chem. 2000, 48, 4830.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3cXmtVGqsbc%3D&md5=95c20bf4308879d1dfb221ec504ea6dfCAS | 11052741PubMed |
[9] F. Benkwitz, T. Tominaga, P. A. Kilmartin, C. Lund, M. Wohlers, L. Nicolau, Am. J. Enol. Vitic. 2012, 63, 62.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XlvFSht7k%3D&md5=6ee0cb3ab80a28dd749235b07859b643CAS |
[10] C. Wood, T. E. Siebert, M. Parker, D. L. Capone, G. M. Elsey, A. P. Pollnitz, M. Eggers, M. Meier, T. Vössing, S. Widder, G. Krammer, M. A. Sefton, M. J. Herderich, J. Agric. Food Chem. 2008, 56, 3738.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXlslanu7o%3D&md5=8e351393515d2d39d717785e709b7d31CAS | 18461961PubMed |
[11] D. Jeffery, T. Siebert, D. Capone, K. Pardon, K. van Leeuwen, M. Solomon, Aust. Wine Res. Inst. Tech. Rev. 2009, 180, 11.
[12] T. Tominaga, R. Baltenweck-Guyot, C. Peyrot des Gachons, D. Dubourdieu, Am. J. Enol. Vitic. 2000, 51, 178.
| 1:CAS:528:DC%2BD3cXmtlSmsb4%3D&md5=eb4ca62b6a0ce857c38ef6679267494fCAS |
[13] D. L. Capone, M. A. Sefton, D. W. Jeffery, J. Agric. Food Chem. 2011, 59, 4649.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXktFemsrs%3D&md5=80c1c0f5b0924b946a69abb0494477abCAS | 21456560PubMed |
[14] A. K. Hjelmeland, S. E. Ebeler, Am. J. Enol. Vitic. 2015, 66, 1.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2MXkvFWns7k%3D&md5=e8a33090f9100afc3266293f7a110c08CAS |
[15] (a) T. Tominaga, C. Peyrot des Gachons, D. Dubourdieu, J. Agric. Food Chem. 1998, 46, 5215.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1cXntV2ltL4%3D&md5=4a30fa890ef10e66553372de215c8918CAS |
(b) C. Peyrot des Gachons, T. Tominaga, D. Dubourdieu, J. Agric. Food Chem. 2002, 50, 4076.
| Crossref | GoogleScholarGoogle Scholar |
[16] J. H. Swiegers, R. Willmott, A. Hill-Ling, D. L. Capone, K. H. Pardon, G. M. Elsey, K. S. Howell, M. A. de Barros Lopes, M. A. Sefton, M. Lilly, I. S. Pretorius, in Developments in Food Science (Eds W. L. P. Bredie, M. A. Petersen) 2006, pp. 113–116 (Elsevier: Amsterdam).
[17] A. Peña-Gallego, P. Hernández-Orte, J. Cacho, V. Ferreira, Food Chem. 2012, 131, 1.
| Crossref | GoogleScholarGoogle Scholar |
[18] B. Fedrizzi, K. H. Pardon, M. A. Sefton, G. M. Elsey, D. W. Jeffery, J. Agric. Food Chem. 2009, 57, 991.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXisVajtA%3D%3D&md5=bb7311e14fa6ea6d443feb2c82729f5bCAS | 19125618PubMed |
[19] (a) G. Noctor, A. Mhamdi, S. Chaouch, Y. Han, J. Neukermans, B. Marquez-Garcia, G Queval, C. H. Foyer, Plant Cell Environ. 2012, 35, 454.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XjtVKnt7w%3D&md5=bdcf4e17fd975867e4380edba8586431CAS | 21777251PubMed |
(b) I. Cummins, D. P. Dixon, S. Freitag-Pohl, M. Skipsey, R. Edwards, Drug Metab. Rev. 2011, 43, 266.
| Crossref | GoogleScholarGoogle Scholar |
[20] D. L. Capone, K. H. Pardon, A. G. Cordente, D. W. Jeffery, J. Agric. Food Chem. 2011, 59, 11204.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXht1WksLrP&md5=dc69d1f6917ef93b8372717f86c47b9dCAS | 21942856PubMed |
[21] D. L. Capone, M. A. Sefton, Y. Hayasaka, D. W. Jeffery, J. Agric. Food Chem. 2010, 58, 1390.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXmslWruw%3D%3D&md5=ace01c1667de9daf1e3da307bc62caa2CAS | 20078076PubMed |
[22] P. A. Grant-Preece, K. H. Pardon, D. L. Capone, A. G. Cordente, M. A. Sefton, D. W. Jeffery, G. M. Elsey, J. Agric. Food Chem. 2010, 58, 1383.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXmslWlsA%3D%3D&md5=e05dea86e97990e6b6089144db24d977CAS | 20078075PubMed |
[23] D. L. Capone, M. A. Sefton, D. W. Jeffery, in Flavor Chemistry of Wine and Other Alcoholic Beverages (Eds M. C. Qian, T. Shellhammer) 2012, pp. 15–35 (American Chemical Society: Washington, DC).
[24] G. Winter, T. Van Der Westhuizen, V. J. Higgins, C. Curtin, M. Ugliano, Aust. J. Grape Wine Res. 2011, 17, 285.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXpsFGms74%3D&md5=e7c691a2d9d1e43be7104034f1208762CAS |
[25] A. Cordente, D. Capone, C. Curtin, Appl. Microbiol. Biotechnol. 2015, 99, 9709.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2MXht1Kmu73P&md5=5ad925c10c2df00f1c68ebda9e95f2b1CAS | 26227410PubMed |
[26] C. M. Kalua, P. K. Boss, Aust. J. Grape Wine Res. 2010, 16, 337.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXptlWksL4%3D&md5=5c88fc3e4b5177c36a46389031c721b6CAS |
[27] D. L. Capone, D. W. Jeffery, J. Agric. Food Chem. 2011, 59, 4659.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXjvF2qs7k%3D&md5=43f02cc840db5349e9c92b777bb47b10CAS | 21449563PubMed |
[28] D. L. Capone, C. A. Black, D. W. Jeffery, J. Agric. Food Chem. 2012, 60, 3515.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38Xkt1Olsb4%3D&md5=282c9bac315c7c50e36a186dbff3113dCAS | 22435800PubMed |
[29] D. L. Capone, R. Ristic, K. H. Pardon, D. W. Jeffery, Anal. Chem. 2015, 87, 1226.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2MXks1KntA%3D%3D&md5=96c160d549abfb0b9f874350f9869298CAS | 25562625PubMed |
[30] C. Starkenmann, B. Le Calve, Y. Niclass, I. Cayeux, S. Beccucci, M. Troccaz, J. Agric. Food Chem. 2008, 56, 9575.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXhtFGntrzL&md5=d721db9d4ca7753c46815d7690599565CAS | 18811169PubMed |
[31] B. Concejero, P. Hernandez-Orte, J. Astrain, B. Lacau, C. Baron, V. Ferreira, LWT – Food Sci. Technol. 2016, 65, 770.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2MXhsV2ntb%2FK&md5=3da59e1195a6992e199935dbe729a8d4CAS |
[32] F. R. Pinu, S. Jouanneau, L. Nicolau, R. C. Gardner, S. G. Villas-Boas, Am. J. Enol. Vitic. 2012, 63, 407.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XhsFSisLrJ&md5=1fccd2e5fb37afee7b1f8dc3e78b2a84CAS |
[33] C. Thibon, C. Böcker, S. Shinkaruk, V. Moine, P. Darriet, D. Dubourdieu, Food Chem. 2016, 199, 711.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2MXitVyksrrP&md5=97afe9ea3e685593298ae5c01a236533CAS | 26776028PubMed |
[34] N. Duhamel, F. Piano, S. J. Davidson, R. Larcher, B. Fedrizzi, D. Barker, Tetrahedron Lett. 2015, 56, 1728.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2MXjsFCqurk%3D&md5=2de9368a02538bae13e7f0d0359b425aCAS |
[35] R. Schneider, F. Charrier, A. Razungles, R. Baumes, Anal. Chim. Acta 2006, 563, 58.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XivVOhtrw%3D&md5=a354acb498199060494dbb21973ab459CAS |
[36] L. Mateo-Vivaracho, J. Zapata, J. Cacho, V. Ferreira, J. Agric. Food Chem. 2010, 58, 10184.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXhtVelur%2FE&md5=b58c3713492da831c29642ca04301423CAS | 20718418PubMed |