Indirect selection for potential yield in early-generation, spaced plantings of wheat and other small-grain cereals: a review
R. A. Fischer A B and G. J. Rebetzke AA CSIRO Agriculture and Food, GPO Box 1700, Canberra, ACT 2601, Australia.
B Corresponding author. Email: tony.fischer@csiro.au
Crop and Pasture Science 69(5) 439-459 https://doi.org/10.1071/CP17409
Submitted: 10 November 2017 Accepted: 10 January 2018 Published: 16 April 2018
Journal compilation © CSIRO 2018 Open Access CC BY-NC-ND
Abstract
Early-generation (e.g. F2–F4) selection for grain yield itself is frustrated in particular by the small amounts of seed available. However, there has long been an interest in traits related to yield and reasonably faithfully expressed in spaced planting arrangements using little seed; these are potentially useful as indirect selection criteria for yield, with the view to increasing genetic progress per unit cost. This subject is revisited in this review, targeting potential yield (yield in the absence of abiotic and biotic stresses) of small-grain cereals.
A brief assessment of current breeding systems for self-pollinated crops such as wheat reveals that all have some stage during which selection among visually acceptable spaced plants has to, or could, be practiced. The relative performance of different genotypes in such spaced plantings is then explored, highlighting interactions arising from intergenotypic competition as well as from the extra space itself. The theory of indirect selection is presented, along with some practical examples. After a brief survey of possible selection traits and developments in high-throughput measurement, harvest index, fruiting efficiency and stomatal conductance (and its surrogates) are chosen for in-depth review. All three traits show promise, especially in the light of possible new ways of reducing the cost of their measurement in early generations. Remote sensing of foliage temperature for the detection of genotypic differences in stomatal conductance makes this clearly the most promising trait for thorough testing in commercial breeding populations. Such traits could be used directly or they could complement genomic selection in early generations.
Additional keywords: leaf permeability, foliage temperature, molecular markers.
Introduction
Yield progress from breeding, at least measured in relative terms, is slowing as yields rise (Fischer et al. 2014). Early-generation selection (EGS) for yield and other commercial traits aims to improve breeding efficiency by reducing the number of genotypes to be tested in subsequent, expensive yield trials, thereby increasing genetic gain per unit cost. EGS, in particular for yield1 in small-grain cereals, is the subject of this review, being especially relevant in crosses involving elite × elite genetic material common in most productive breeding programs. New high-speed phenotyping techniques make this a worthwhile subject to revisit. In a self-pollinated crop such as wheat, practicing EGS for yield in conventional breeding systems is hampered by three major issues: (1) a lack of seed in early generations (F2–F4), (2) allele segregation and recombination in the subsequent generations before homozygosity is reached, and (3) genotype × environment (G × E) interactions and reduced repeatability across years before plot yield-testing begins.
Because of lack of seed, breeders resort to spaced plants (spaced in both dimensions, usually ~20 cm by 20 cm or more, meaning <25 plants m−2), or bulks of sib lines in the form of unreplicated spaced-hill plots, single or double rows, widely spaced or otherwise, and microplots (three or four short rows, <2–3 m2). Issue 1 above, a lack of seed in early generations, relates to the difficulty of selecting for yield based on plant phenotype under these conditions. Grain weight per plant or microplot in these plantings does not faithfully reflect, even for identical genotypes and environments, the yield in larger plots, which probably need to be >4 m2 so as to be harvestable free of plot-edge effects. At 200 plants m−2, a single 4-m2 plot would need at least 40 g of seed (100 kg ha–1 to give ~200 plants m−2). In the other-mentioned plantings where interplant spacing is much larger in all dimensions than in a normal crop, or where many plants are partly exposed to extra space (edge effects in hill, row and microplots), the phenotypic values of many yield-related traits and grain yield itself is biased. This is because genotypes respond differently to the extra space per plant, and/or because of intergenotypic2 competition between plants. Donald and Hamblin (1976) called such early generation plantings either ‘isolation’ environments (no interplant or intergenotypic competition) or ‘competitive’ environments (with such competition). They pointed out that neither situation represents the environment of intense intragenetic competition seen as a ‘crop’ environment (e.g. yield plots). Intergenotypic competitive planting arrangements lead to selection bias towards traits favouring competitiveness. In spaced plantings (at least 20 cm by 20 cm), even if there were zero interplant competition, there would still be the possibility of bias towards traits favouring the occupation of space. Biases lead to genotype × planting arrangement interactions for many, but hopefully not all, yield-related traits.
Issue 2, allele segregation and recombination in the subsequent generations, arises because, depending on the background and relationships between inbred parents in any cross, the expectation of residual heterozygosity can be as high as 50%, 25% and 12.5% in individual F2, F3 and F4 plants3, respectively. Therefore, many progeny plants need to be retained from F2 onwards to include as many favourable gene and chromosomal recombinations as possible (Sneep 1977; Bos and Caligari 1995). As generations are advanced there is allelic segregation at a locus, although Bernardo (2003) showed that theoretically, if there is no dominant gene action, the genetic correlation between performance of an Fx-derived Fy line4 and a descendent homozygous line is quite high (e.g. for F2-derived lines 0.71, for F3-derived 0.87 and for F4-derived 0.94). Dominance gene action reduces this somewhat in earlier generations, while error variance reduces the equivalent phenotypic correlation more-or-less in inverse proportion to the broad-sense heritability. Bernardo (2003) concluded that, from a genetic standpoint, EGS is expected to be effective particularly in self-pollinated crops including small grains, partly because they have only low levels of dominance gene action. In a comprehensive theoretical analysis, Yang (2009) revisited Bernardo’s calculations, including non-additive gene action, both additive × additive (epistatic) gene action and dominance, and chromosomal linkage effects. He concluded that EGS could be used where non-additive effects were low and heritabilities high; coupling linkage also helped. The problem of heterozygosity in selected plants obviously becomes less the later the generation of selection, something that becomes relevant in bulk-selection methods (see later). The weakened selection response due to issue 2 is of course quite independent of effects arising from issue 1.
The final and perhaps a lesser complication with EGS, at least in managed breeding environments (e.g. irrigated), is issue 3, the more commonly recognised G × E interactions. Even in the same testing location, successive breeding generations are inevitably grown under different weather conditions leading to the possibility of interference in selection during the breeding generations quite independent of issues 1 and 2 above. Of course, conditions will commonly be different from those of the target farm environment(s), and the G × E interactions arising from this may create multiple breeding targets, but this is a problem for all breeding systems moving beyond their first cycle of proper yield testing and not discussed further here.
It follows from the above that direct EGS for yield by using grain production per plant or microplot has generally not been successful (see references cited later and in Bernardo 2003; Yang 2009; Clement et al. 2015). However, the possibility exists of indirect EGS for yield, based on traits related to yield, more faithfully expressed in early-generation planting arrangements and having reasonable heritability and freedom from dominance gene effects. Examples would be flowering date and plant height, visual selection criteria widely used by experienced breeders to ‘tidy up’ segregating populations. Grain weight (GW, weight per grain) is another such highly heritable trait but it is usually unrelated to yield (Bhatt 1980). The search for additional EGS criteria, the subject of this review, assumes that there are certain traits that affect yield, not because of chance genetic linkage but because of fundamental underlying physiological relationships, complex or otherwise. Such traits become essential but not sufficient conditions for higher yield. A complex trait with this property could be harvest index (HI), the partitioning of total carbon to grain. Leaf photosynthetic activity probably essential for greater biomass could be a second example.
The challenge of indirect EGS for yield, especially among spaced plants, is therefore our main subject. The focus is on potential yield, or yield in the absence of abiotic and biotic stresses (Fischer 2015), rather than yield under water shortage (water-limited potential yield). Potential yield is likely to be an easier target around which to explore EGS, and progress in potential yield in wheat usually spills over to give equal relative progress in all but strongly water-limited environments (e.g. for vintage cultivar studies, Araus et al. 2002; for a population study, Olivares-Villegas et al. 2007).
This review is considered timely because research to support breeding has become largely dominated by the search for molecular markers and understanding gene function. To date, this has had little impact on yield selection (Bernardo 2016), and there has been little attention by breeders to the integrated physiology closely underpinning yield progress. For this reason, most references cited here on indirect EGS for yield are not very recent, including many mentioned in a valuable review by Bhatt (1980). A useful recent paper is Clement et al. (2015), albeit involving selection in cotton. Frequent reference is made to the comprehensive text of Bos and Caligari (1995) on selection methods in plant breeding, and to wheat breeding at the International Maize and Wheat Improvement Center (CIMMYT) as a breeding program with which the authors have some familiarity. The review begins with a summary of breeding methods in wheat, using CIMMYT methods over past five decades as a framework. This is followed by a more detailed discussion of differential genotypic responses to spaced plantings, and of the theory of indirect EGS, using some key published examples. Potential traits for indirect yield selection are briefly surveyed, and the remainder of the review concentrates on traits deemed most promising: HI, fruiting efficiency (FE) and stomatal conductance (gs).
Background to breeding for grain yield in small-grain cereals
Breeders have adopted one of several strategies in selecting for yield and other necessary traits in self-pollinated elite × elite genetic materials (Allard 1960):
-
Pedigree breeding. This system involves spaced plantings of F2 plants, in which there is culling among plants of those that clearly carry obvious defects or negative traits such as inappropriate height or maturity, leaf necrosis, sterility and/or disease susceptibility (often revealed through the creation of artificial epidemics). As well, there can be visual selection of plants that exhibit desirable morphological traits suggested by experienced breeders as being reasonably independent of planting density and possibly related to yield (e.g. synchronous tillering, erect leaves, presence or absence of awns). Typically, there could be hundreds of F2 plants per cross, of which only 1–10% are selected. In this classic pedigree breeding system, the next generation (F3) and even those following are also space-planted and selected as in the F2 before final selection at around the F4 or later generation. At this time, desirable plants are identified and are bulked-up separately over one or two generations for seed production and then yield testing in a plot trial at normal plant densities, needing at least 40 g seed per plot.
-
Bulk-population breeding. This became the major alternative to pedigree breeding, distinguished in its classic form by the planting of F2 generations in small plots at commercial density, and harvesting in bulk for planting the next generation under the same conditions. This process may continue with limited visual selection against obviously undesirable phenotypes until a high level of homozygosity is reached (e.g. F5 generation), when single-plant selections are made, bulked-up and evaluated for yield as in pedigree breeding at that same generation. Clearly, this method saves resources relative to pedigree breeding.
-
Modified bulk method, selected bulk method. Alternatives to the classic bulk method have since arisen, such as the modified bulk method, to which the CIMMYT wheat-breeding program moved in the mid-1980s (Singh et al. 1998). In this, seeds from selected F2 plants were separately planted at moderately high density (10 cm spacing), such as in short rows, and bulks of these F2-derived lines moved through F4 and F5 generations. Visual selection for disease and good agronomic features was practiced, but there is no yield measurement. From the F6 sowing, desirable individual plants were selected and advanced for yield testing as pure lines in plots as in the pedigree method. Thus, the system was one of pedigree selection in the F2 and again in the F6. This system was further streamlined in the late 1990s to give a selected bulk method to save land without apparent loss of genetic gain (Singh et al. 1998; Wang et al. 2003). Desirable F2 plants were harvested and not grown separately but bulked together, and then in F3–F5 they were grown at normal high density (5 cm spacing) without selection until individual plants were selected in the F6 as in the modified bulk. This system used pedigree selection only at the F6, leading to further savings of resources while retaining more genetic diversity.
-
Cross or family selection. Sometimes breeders select at the level of the whole cross by measuring yield in early-generation (e.g. F2) bulk plots before retaining higher yielding plots (essentially families5, one derived from each cross), and then selecting within crosses with high yield. Such plots can be replicated but are often not; sometimes hill plots or short rows are used for this initial yield measurement. Such cross or family selection, with replication and inclusion of check cultivars, was strongly advocated by Simmonds (1996). However, early-generation family testing for yield has not been widely practiced in wheat or barley. This is probably because it is not clear that the higher yielding F2–F4 bulks (essentially competing mixtures of differing sib lines) are more likely to produce higher yielding pure lines than the lower yielding bulks (Hamblin and Rosielle 1978)6, and even assuming that there is no small-plot yield bias (see later). Clearly, all of the bulk methods (methods 2–4) risk undesirable genetic drift in the highly competitive bulked generations.7
-
Single-seed descent (SSD) method, doubled-haploid (DH) methods. In the last 30 years or so, two newer selection methods have gained considerable acceptance among breeders. They involve ways of rapidly advancing progeny from crossing to homozygosity, using either fast-generation cycles in the SSD method (Brim 1966) or DH methods. Self-fertilisation under SSD allows further recombination, whereas with DH methods there is usually only one generation (F2) of recombination before homozygosity is reached. Population sizes therefore need to be large but greater costs usually preclude this, resulting in progeny assessment in numbers that are small relative to the other methods. The SSD and DH methods are increasingly used in cereals, but they face the same phenotypic selection challenges as other breeding methods, although without the problem of residual heterozygosity and with more opportunities for marker-aided selection. Most practitioners of SSD avoid the special challenge of selection during inbreeding because the unusual environmental conditions employed (seed vernalisation, very long days, warm temperatures, intergenotypic competition) are not at all representative of the field environment. It is unlikely that useful indirect yield selection can be practiced under these conditions. However, ‘speed breeding’, achieved by markedly increasing the levels of photosynthetically active radiation under the extended photoperiods, and by adhering to only moderate temperatures, has produced relatively ‘normal’ plants of spring wheat and barley, albeit with fewer smaller spikes per plant, in less than 6 weeks to flowering. A brief cold period for the early-harvested seed completes the generation in 8 weeks or so (Watson et al. 2018). These plants show promise as targets for the selection of certain traits.
All of the breeding methods described have a generation, or several, of spaced plantings, which opens up the possibility for efficiency gains through indirect selection for yield in these plantings, whether they be in early generations (as in pedigree and bulk) or later ones (as in modified bulk, SSD and DH). The bulk methods have the added problem of carrying out the initial, individual plant selection from among crowded bulk sowings in which plants are subject to strong intergenotypic competition.
Responses to spaced planting and related arrangements
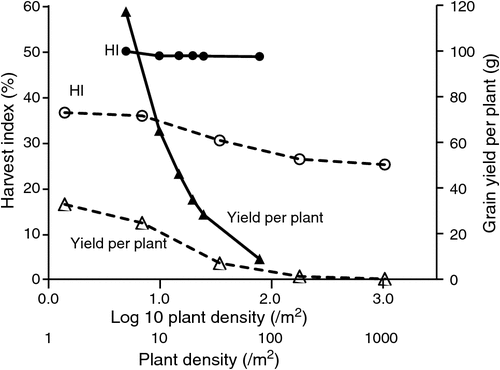
Referring initially to the wider plant spacings of early generations in the pedigree method (and the F2 in the modified bulk method), long experience has shown that indirect yield selection for the obvious numerical components of yield (e.g. spikes per plant, grains per spike, GW) and for grain weight per plant itself8 is usually not effective for predicting yield (e.g. Donald and Hamblin 1976; Bhatt 1980), but there are exceptions (e.g. Thakare and Qualset 1978). Some have argued that for success, such selection needs a planting density so low such that interplant competition is very much reduced or totally avoided (e.g. Fasoulas 1981; Bos and Caligari 1995; Wallace and Yan 2000). Just how low density must be to avoid aboveground competition in wheat is itself an interesting question, also of relevance later in this review. Experience suggests that the density must be <~4 plants m−2. For example, with a 116-cm-tall spring cultivar (when in a normal plot), Puckridge and Donald (1967) in South Australia reported that 38 cm by 38 cm spacings suffered interplant light competition, whereas 85 cm by 85 cm did not (Fig. 1). With irrigated, semi-dwarf spring-wheat cultivars in north-west Mexico, in experiments described in Fischer et al. (2005) it was observed that competition ceased between 40 cm by 15 cm and 40 cm by 30 cm for a short cultivar (75 cm height), and between 40 cm by 30 cm and 60 cm by 40 cm for a taller line (98 cm). The latter result was corroborated by Moreno-Ramos et al. (2004) (also shown in Fig. 1). In Fig. 1, only at 1.5 plants m−2 does yield per plant from Puckridge and Donald (1967) suggest that a plateau in plant size is being reached, illustrating the remarkable plasticity of such cereals under good growing conditions.
|
Under the hexagonal honeycomb design promoted by Fasoulas (1981), the critical minimal inter-plant distance for cereals for zero interplant light competition seems to be ~50 cm; anything less leads to undesirable intergenotypic competition according to the proponents of this design (e.g. Mitchell et al. 1982)9. Although Bos and Caligari (1995) reported several cases of yield per plant at wide spacing being positively correlated with yield, these cases appear to be unusual and rarely found in wheat (e.g. Donald and Hamblin 1976). However, two more recent wheat studies employing the honeycomb method (100 cm by 100 cm spacing), and selection among hundreds of genetic variants that were surprisingly found within released cultivars, did report strong plant ‘yield’ vs plot yield correlations (Fasoula 1990; Tokatlidis et al. 2006). Fischer (1978) noted that spaced plants without interplant competition are in a sense competing with (or responding to) space for extra light, an ability likely to be favoured by tall, late genotypes with floppy leaves and spreading tillers, and equally unlikely to favour crop performance10.
For wheat crops in which close-to-equidistant spacing is employed, the minimum density for maximum yield is ~16–24 plant m–2, at least with autumn-sown, irrigated spring wheat in north-west Mexico (Moreno-Ramos et al. 2004; Fischer et al. 2005). However, most plot experiments adopt row spacings of 15–25 cm, with plants randomly spaced in the rows. Possibly because of the high rectangularity of this plant arrangement and the severe intra-row competition, there is good evidence that it needs more plants for maximum yield (~80 m–2). Even so, above that number, there is little yield response to increased density and little genotype × density interaction, e.g. in Mexico (Fischer et al. 1976), or elsewhere at low latitudes. When segregating populations in both early and later generations are sown at densities producing interplant competition (any spacing <~30 cm by 30 cm or density of sowing >10–12 plants m−2 as per previous paragraph), selection of individuals with superior pure-stand yield is further complicated by the bias caused from the heightened intergenotypic competition. Additionally, it is not helped by the physical difficulty of separating individual plants from their neighbours at higher densities. Initially, intergenotypic competitiveness is driven by seeding-emergence time and early vigour, and by the influence of proximity to one’s neighbours, a random effect at higher density plantings (Bos and Caligari 1995). Later in development, plant height and leaf size become important (Jennings and Herrera 1968; Hamblin and Donald 1974; Kawano et al. 1974). Actually, Spitters and Kramer (1984) noted that in closely spaced competition, relative differences in initial plant size tended to be maintained until maturity, whereas under wide, non-competitive spacing, relative differences were only initially related to differences in size, deviating notably from this pattern by maturity. Thus, there are many examples of the consequent poor relationship of plant yield under such competition to plot yield from wheat and barley studies (Hamblin and Donald 1974; Bhatt 1980; Bos and Caligari 1995; Rebetzke et al. 2013).
This complication from competition could be worse than it first seems, not only weakening relationships but potentially also reversing them. Previous landmark review papers on competition in crops by Professor Colin Donald of University of Adelaide (Donald 1963, 1968, 1981) argued that traits favouring performance among genetically different competing plants were actually often likely to prejudice yield in genetically uniform crops (i.e. yield per plant negatively related to plot yield!)11. This was illustrated by subsequent studies with high-density segregating populations of rice (Kawano and Tanaka 1967; Jennings and Herrera 1968), barley (Hamblin and Donald 1974) and wheat (Reynolds et al. 1994). Donald (1968) proposed that the highest yielding genotypes in crops would be non-competitive ‘communal’ plants, in the form of an ideotype that he described, something which may offer clues to more effective indirect trait selection in spaced plantings for yield advance.
Since Donald’s ideas were first promulgated and supported in barley and rice, there has been, however, limited support for his non-competitive ideotype (Marshall 1991). Nonetheless, Donald’s notion of an ideotype has influenced the thinking of breeders, and particularly the steady but rather modest stream of studies trying to validate EGS criteria for yield in spaced plantings. In the meantime, two reports from studies in irrigated north-west Mexico have supported the Donald notion that higher yielding wheat varieties are seemingly less competitive, being less responsive to extra space artificially created at flag leaf emergence in the growing crop (Reynolds et al. 1994) or available to edge rows well separated from adjacent plots (Sukumaran et al. 2015). In addition, under favourable conditions in South Australia, a breeding-era assessment of 13 older and modern wheat cultivars (Sadras and Lawson 2011) reported the responsiveness in grain yield of plot edge rows to be strongly inversely related to plot yield measured on the inner rows.
Breeders have tried to avoid the problem of selecting individual plants, with or without the intergenotypic competition indicated above, by testing for yield itself in smaller plots among families as early as F2:3. Less seed than the 40 g plot–1 for a proper yield plot is needed for hill plots, single-row plots, single-row plots with a common border cultivar, or untrimmed 2–4-row short (<3 m) plots. However, all of these ‘microplots’ carry the risk of bias due to inter-plot competition and/or differential responses to space (e.g. from untrimmed ends and paths), characteristics that, as described above, are likely of little relevance to true crop performance.12
Professor Ken Frey at Iowa State University pioneered the use of hill plots in his spring-oat breeding program (‘hill’ is a misnomer, hills were simply clumps of 32 seeds sown on flat land on the points of a 30.5 cm by 30.5 cm grid13). Frey claimed that hill plot yield predicted larger plot yield, but this was never convincingly established (Donald and Hamblin 1976), although many useful papers on selection strategies were published by the Iowa team (see later). Canadian spring-wheat breeders also confronted the issue by sowing unreplicated, F2:3 yield microplots, each derived from a random F2-spaced plant. For example, in DePauw and Shebetski (1973), each F3 plot was 5 m by 0.45 m (3 rows, 2.25 m2) in size and was bordered by similar control microplots sown to a common wheat cultivar. Those workers found weak but significant correlations between F3 yield and later generation yields in replicated plots when all yields were expressed as a percentage of the adjacent common control plot (rp = 0.59** with F4 and 0.56* with F5)14. Even so, follow-up work from Canadian breeders concluded that these early-generation microplots carried too much error and involved too much effort (Knott 1979).
Dr C. J. Spitters and colleagues at Wageningen University (as reported in Bos and Caligari 1995) thoroughly explored this question of apparent yield in small plots vs true yield, also using spring-sown spring wheat cultivars. For example, Kramer et al. (1982) assessed 16 cultivars in 2-m-long plots (without end trimming) with 21 cm between rows and no paths, all sown in four replicates in a single year (to avoid confounding from genotype × year (G × Y) interactions). They reported weak phenotypic correlations (rp) between single-row plot yield and ‘true’ yield (from 9-m2 plots), being rp = 0.33 and 0.56* (single-row plots spaced at 21 cm and at 42 cm, respectively). However, removal of border rows and harvesting of the central row of 3-row plots gave a correlation of 0.74** with yield. For the central 4 rows of a 6-row plot, a high correlation of 0.88** was achieved, and for the whole 6-row plot also a high correlation of 0.89** (the greater area of the 6 rows apparently counterbalancing the greater noise from inclusion of the edge rows affected by inter-plot competition).
There is reason to believe that the above studies, all involving spring-sown, spring cereals, represent favourable agroecological situations for competitiveness, as seen, for example, in over-yielding edge rows, to be positively reflected in true yield. This is because, under the long days normally encountered in such environments, development is accelerated relative to growth, and yield becomes more closely linked to vigour and total pre-anthesis growth. Then, true yield relationships are likely to favour competitiveness more strongly, and to differ from those found with lower latitude, autumn sowing of spring-habit cereals, which is the primary interest here. Indeed, in Kramer et al. (1982), yield in the ‘true’ yield plots was closely related to final biomass (rp = 0.72**). Thus, although methodologically valuable, the above studies are not so relevant to the main situation of interest in this review, namely autumn-sown cereals15, where it is even less likely that high-yielding F2–F5 microplots, biased by edge and end effects, will deliver high-yielding pure lines.16
All of the above considerations have led to ongoing interest in traits that can be confidently used in EGS for yield, preferably in the initial space-planted generations with low seed requirements per entry. This interest has recently been heightened by the big advances in the efficiency of phenotyping, essentially reducing the cost of measuring or estimating plant traits; this falls under the banner of high-throughput precision phenotyping (HTPP; Fiorani and Schurr 2013). Phenotyping of spaced plantings by utilising various types of remote sensing has already been automated in specially designed, expensive, indoor phenotyping facilities. Applying the techniques to field plantings by using remote mobile or airborne sensors promises to be less expensive and to allow sampling of more representative field environments (e.g. Araus and Cairns 2014). Furthermore, rapid remote field phenotyping reduces the potential for problems related to weather variation during measurement within a replicate, thereby increasing the precision of heritability of measurements. Thus, HTPP seems poised to greatly increase the speed and reduce the cost of assessing potentially useful traits for EGS, and to facilitate their incorporation at low cost into regular breeding if deemed worthwhile.
Theory and examples of indirect EGS for yield
Useful indirect EGS for grain yield would permit culling based on heritable traits so that the numbers of progeny enriched for yield-enhanced genes and reaching the expensive plot-yield testing stage of breeding are reduced, while minimising the risk of loss of higher yielding progeny. The reduction in population size needs to be substantial to cover the costs of the actual trait measurement (e.g. halving the 1–10% that breeders would normally select in a typical F2 population). One approach used widely in the past by oat breeder Frey to computing the correlated response to such culling is introduced here, following the useful model paper by Takeda and Frey (1985), which appears to bring together all of Frey’s earlier experiences.
The genetic gain relative to the population mean from direct selection for yield (ΔGy) is given by:

where hy2 is the narrow-sense heritability of yield, sy is the phenotypic standard deviation of yield, and k is the selection differential in standard deviation units.17
The genetic gain for indirect yield selection via a trait i, ΔGi.y, in the same population is given by:

where hi and hy are the square roots of the narrow-sense heritabilities of the selected trait (i) and the responding or target trait (y, yield in this case), respectively; rg is the genetic correlation (strictly speaking the additive genetic correlation) between the selection and target traits; and sy is the standard deviation of the target trait.
The ratio between ΔGi,y and ΔGi is often known as the relative selection efficiency (RSE) of indirect vs direct selection for yield and is given by dividing Eqn 2 by Eqn 1:

where the ks cancel if the selection intensity is the same in each strategy, as do the sys.
This is the equation for RSE as popularised by Falconer and Mackay (1997). For indirect selection, it is desirable for RSE to be >1, meaning that rg and hi must be high and/or hy low, but costs of indirect and direct selection must also be considered. In the case of Takeda and Frey (1985) and most other studies from that group, and including Falconer and Mackay (1997), the situation was overly simplified relative to that for EGS, as we shall see.
The validation in Takeda and Frey (1985) of indirect selection with a population of 1200 F8 oat lines is valuable as an example. It was based on several promising traits from earlier studies, namely HI, vegetative growth rate (GR = straw weight ÷ days to heading), and unit straw weight (USW = straw weight ÷ height), all measured on the whole hill plot. Traits (and yield) were measured in one year in hill plots (two replicates) as defined, and predictions were based on Eqn 1, and on Eqn 2 for single indirect traits, and on elaborations of this latter equation for two indirect traits (a selection index). They were tested against actual yield per hill for the same lines and hill plots the following year, always with an overall selection intensity of 2%. Heritabilities and genotypic correlations for the predictions were computed from variances and covariances obtained from the hill-plot values in the selection year. Predictions of population yield gain across the range of indirect selection traits tended to be higher than the realised gains, even for grain yield selection itself, and even though there was no gene segregation or changed planting arrangement to complicate the prediction; thus, there was some G × Y interaction. Also in the selection year, grain yield showed only a weak genetic correlation with HI (rg = 0.22) compared with correlations for GR (0.81) and USW (0.74), meaning the yield differences were largely driven by vigour differences. This brings additional complications due to inter-hill competition (randomisations were presumably different each year), which could have added to normal noise arising from the G × Y interaction. The best yield advance was with a selection system taking the best 25% for HI first, then the best 8% based on GR or USW, although the actual progress (12% over the population mean) was not significantly greater than selecting the best 2% on yield alone. There are many other selection strategies where multiple traits are involved (e.g. independent stratified culling, index selection and tandem selection; Hazel and Lush 1942) and restricted indices for genetically negatively correlated traits, but here, for simplicity, only selection using single traits will be discussed further.
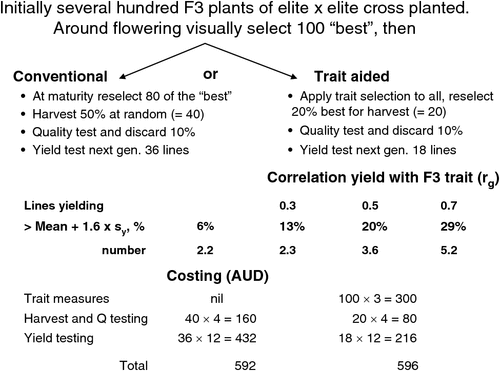
Independent of the work in Iowa, Yonezawa (1983) in Japan calculated the improvement with trait selection in the proportion of desirable progeny (yielding greater than the population mean by some defined selection differential) by using the same trait components of heritability, phenotypic standard deviation and genetic correlations. For example, Fig. 2 shows how the proportion of desirable individuals selected improves as rg increases for a given selection intensity, and in the example chosen, for the assumed costs and a given total cost, rg needs only to exceed 0.3 to be more effective than the conventional strategy with no indirect trait selection. Obviously, the outcome is very dependent on the costs we have chosen for illustration purposes, particularly the relative costs of trait and yield plot measurements. Yonezawa (1983) tabulated many combinations of selection intensity and rg (which can be readily calculated from the normal distribution curve). He pointed to the diminishing returns with increasing rg values (or, if there are multiple selection traits in an index, increasing √R2), arguing that increasing the number of selection units (population size) was generally a more efficient approach than increasing the effort in multiple-trait assessment on individuals.
|
In summary, this discussion reveals several hurdles that must be overcome if EGS for yield is to be successful:
-
There must be a moderate to strong additive genetic correlation between the indirect selection trait and yield across the target environment for the population under study.
-
The selection trait needs to be measured on individuals and to be unaffected by reduced planting density and possible remnant interplant competition seen in normal selection environments, and more broadly must be robust to changes in environment (i.e. little G × E, including G × Y, interaction).
-
The trait should not be greatly affected by allele segregation occurring between the selection generation and homozygosity, meaning the trait is under strong additive genetic control and shows a high narrow-sense heritability.
-
The trait must be simple and inexpensive to measure.
These are strong barriers for indirect trait selection to overcome, and these have rarely been assessed in segregating breeding populations. Thus, the study by Quail et al. (1989) deserves mention here because it attempted just that. Measurements were made in individual-spaced F3 plants (n = 60) from a population derived from a composite, diverse 16-parent spring-wheat cross, and grain yield (potential yield) was later measured in these F3-derived F7 and F8 plots under irrigation in southern New South Wales. After this, homozygous progeny were remeasured as spaced plants. Taking two contrasting F3 traits, plant HI and plant height (HT), they noted that HI failed under their conditions as an indirect yield selection trait largely because F3-spaced-plant HI did not correlate closely with plot HI (even though the latter correlated strongly with plot yield). In addition, HT failed as an indirect trait because although F3 HT was closely correlated with plot HT, the latter was only weakly related to plot yield. Regrowing the progeny as spaced plants in the F8 generation, those authors were able to conclude that G × E interaction was likely a more important factor than segregation in the poor F3 plant vs plot HI relationship (see later). Incidentally, key F3 grain quality traits in this study were highly predictive of the same traits in F7–F8 plots, indicating the feasibility of EGS for such highly heritable traits (Fischer et al. 1989), something often practiced by breeders.
Finally, it is worthwhile mentioning that since the pioneering studies above leading to Eqn 3, several more inadequacies in the assumptions behind the calculations have been recognised in realistic breeding situations (e.g. Piepho and Möhring 2007). These arise because in today’s testing designs, datasets are usually unbalanced and genotypic effects are not independent, such that unbiased heritability estimations from variance components cannot be readily computed. Simulation-based approaches using today’s enhanced modern computing capacity have become an alternative to the traditional approach. Here it is assumed that such issues do not lessen the potential value of EGS for yield, nor the critical value of the genetic correlation between selection traits and yield, but they complicate the prediction of selection progress based on these variance and covariances, and place even greater value on the validation of predictions in real breeding programs.
Possible indirect yield selection traits
As pointed out, the emphasis in this review is on EGS for potential yield, in other words yield under optimal agronomy and without greater than mild shortages of manageable inputs including water. A second criterion has been a focus on lower latitude, autumn sowing of spring-habit cereals, with mention of a few studies of autumn-sown winter wheats. Studies of spring-sown, spring cereals have been valuable methodologically, especially those mentioned above from Iowa, Canada and Netherlands, but consideration of the relationships derived are generally excluded henceforth for the reason already mentioned.
Clearly, the ideal study would relate spaced-plant traits measured in early-generation (F2–F3) segregating populations to plot yield in the F4–F6 from elite × elite crosses. Yield plots must be adequate in size and harvest arrangements (border trimming, etc.; Rebetzke et al. 2013). Field environments need to be managed to avoid stress as required for potential yield determination. In examining the published literature, such studies are scarce. We have had to compromise in order to garner relevant data. Thus, some rainfed studies with no more than moderate water stress are included. In addition, many studies involve homozygous lines, either cultivars or random inbred lines, whereas practically no studies consider spaced plantings wide enough to eliminate intergenotypic competition and some are compromised by narrow spacing and high levels of such competition. Some studies save seed so that several generations can be grown side-by-side, thereby escaping the inevitable noise due to G × Y interactions that breeders must address but providing other useful information. The recording of days to flowering and plant height is always desirable, and the most useful studies work within a constrained range in plant height (70–110 cm, measured to spike tip) and flowering date (±7 days), means and ranges deemed from experience to be appropriate for maximum potential yield in most environments.
Over time, a long list of possible selection criteria for potential yield, and for which there is known genetic variation in wheat, has accumulated (e.g. Bhatt 1980; Pask et al. 2012; Reynolds et al. 2012). In many early studies, genetic variation for yield was dominated by variation in vintage, which could mean that many underlying genetic changes could be operating. Thus, the relevance of only a few traits has been fully validated (e.g. with population or near-isogenic studies), but others have become widely accepted anyhow (e.g. erect leaves in wheat and barley may be one example given their predominance in modern cultivars). Because yield is determined by the complex interactions of many crop processes, many traits can be expected to have only very small effects (e.g. awns on wheat, Rebetzke et al. 2016), or to have effects in only some environments, or only when other traits are appropriate (epistasis). Some traits will likely have trade-offs (e.g. large grains accompanied by fewer grains per spike). Recently, new physiologically derived traits linked to yield have become of interest in gene-to-phenotype analyses (e.g. Wang and van Eeuwijk 2014).
This is all acknowledged, but this review is primarily concerned with only three traits considered most promising and amenable to relatively rapid and inexpensive phenotyping. These traits, as outlined in the Introduction, comprise two partitioning traits, harvest index (HI) and fruiting efficiency (FE), along with a physiological trait, leaf stomatal conductance (gs) and its surrogates. Where appropriate, comparisons with selection for plant height, days to heading or anthesis, and grain yield per plant are also made.
It should be noted that lodging resistance, a trait of paramount importance for potential yield in wheat, in particular autumn-sown spring wheat, is not considered because its possibilities of EGS appear not to have been reported until recently, and although breeders are alert to natural lodging when it occurs, such events are rare in spaced plants. Two other traits showing considerable promise for yield in certain, but not all, environments and likely amenable to EGS, i.e. early plant vigour via increased specific leaf area and flag leaf stay-green, are discussed by Rebetzke et al. (2004) and Christopher et al. (2014), respectively.
Finally, it has been explicit in most genetic studies of traits vs yield that these are simple linear relationships, revealed by linear regression, even when several traits are combined in an index. In reality, single-trait effects may be curvilinear, or when multiple traits are considered, relationships with yield may be more complex. These are not considered here but should not be overlooked given the ease with which complex predictive relationships (e.g. random forest models) can now be explored.
Harvest index
Harvest index is the ratio of grain weight to total aboveground biomass or dry matter at maturity, and is commonly expressed as a proportion or percentage. The remarkable results of Syme (1972) highlighted the possible use of HI as a selection criterion. He showed that, across 49 CIMMYT-derived spring-wheat cultivars (tall and more recent semi-dwarf ones), HI measured on single plants in 1.5-L pots in the glasshouse in South East Queensland predicted average yield measured across multiple global sites (rp = 0.85**). Days to ear emergence and plant height in the glasshouse were also good predictors (rp = –0.75** and –0.38**, respectively), and these traits correlated closely with the same trait measured globally (rp = 0.94** and 0.90**, respectively). Other traits also contributed collectively to a multiple regression predicting yield (rate of leaf appearance, grain weight), but HI remained the dominant trait.
Soon afterwards, Hamblin and Donald (1974) published John Hamblin’s 1971 PhD thesis work on EGS in barley, in which the F3 measurements were performed on closely spaced F3 plants (8 cm by 8 cm), undoubtedly suffering severe intergenotypic competition, and F3 HI was not a strong yield predictor. Following this, however, the potential role of HI in selection was extensively reviewed by Donald and Hamblin (1976). These authors pointed to the relative stability of HI across changing planting densities (decreasing only slightly from isolated plants to commercial densities). Another example, under irrigation in Mexico, Fischer et al. (1977) had shown that HI responded only very modestly to density; between seed sowing densities of 20 and 240 kg ha–1, HI averaged across many semi-dwarf genotypes decreased linearly from 42% to 39%. Other examples with even lower plant densities are seen in Fig. 1. The Puckridge and Donald (1967) data cover almost a 1000-fold range of density, using an old, moderately tall cultivar, whereas Moreno-Ramos et al. (2004) used a modern semi-dwarf durum wheat cultivar, the HI of which was almost unchanged as density increased 16-fold and yield per plant decreased 93% (Fig. 1).
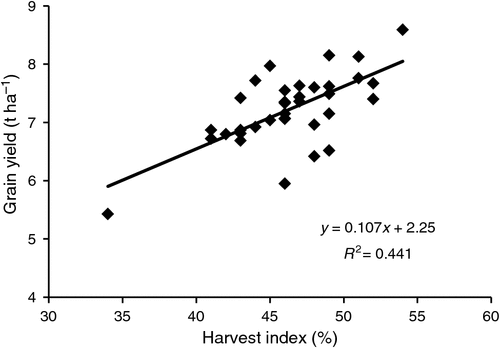
Donald and Hamblin (1976), as well as noting the stability of HI with respect to planting density, pointed to its consistently positive relationship with yield progress from breeding, concluding that HI may have a role as an EGS criterion in spaced plantings. They were much influenced by Syme (1972), and work in CIMMYT (Fischer and Kertesz 1976). The CIMMYT study under irrigation in north-west Mexico investigated 36 wheat genotypes in spaced planting (60 cm by 60 cm) and in bordered plots, all under optimum management. Across genotypes in plots, yield ranged from 5.2 to 8.2 t ha–1; this variation was not related to plant height, days to flowering or biomass, but was related to plot HI (rp = 0.65**). The best spaced-plant predictor of plot yield was the HI of a central culm (rp = 0.66**, Fig. 3), followed by plant HI (rp = 0.56**). Grain yield per plant showed only a weak relationship (rp = 0.33*), despite its large range (74–196 g plant–1). All numerical components of yield measured on the spaced plants gave even smaller correlations, often not significant, although they were good predictors of the same trait in plots. Greater plant height and less erect leaves (both measures of competitiveness) were favourable for yield in the spaced planting but not in the plots. Interestingly, across all genotypes, spaced-plant HI averaged 43.8% (46.3% in the central culms) compared with 41.5% in the plots sown at 100 kg seed ha–1 (R. A. Fischer, unpubl. data). A following year’s data confirmed that HI in spaced plants was a better plot-yield predictor than grain yield per spaced plant (rp = 0.50** vs 0.15 n.s., respectively).
|
After Donald’s early HI papers, a steady stream of publications followed on the possible role of HI in EGS. In particular, in 1980, John Hamblin and Arnold Rosielle18 thoroughly reviewed the subject of criteria for EGS and urged more studies on the use of HI in spaced plants of early-generation segregating populations (rather than fixed lines used in the above-mentioned studies and at risk from vintage bias), while always staying within acceptable height and maturity ranges. However, as mentioned, much of the early work with cereals was in Canada and Iowa (e.g. Nass 1980; Takeda and Frey 1985) with spring-sown spring cultivars and populations; HI showed variable promise, but this body of work is excluded. Studies in Australia, and a few elsewhere, were more relevant to the issue of the value of HI in autumn-planted spring wheat as an EGS criterion, although very few fulfilled all the criteria as required above.
Whan et al. (1981, 1982) reported a major test of HI in EGS across two crosses under moderate rainfall in South Australia, where Colin Donald himself was still active. Random F3 and F4 lines derived from F2 and F3 plants, respectively, were grown in small plots of 2 rows (18 cm apart, 30 cm paths) by 2 m at one site where yield and HI were measured, and a random subset of these lines was grown the following year in wider (4 rows) plots at two sites. HI correlated between F3 and F4 in the first year (r = 0.68**) but HI did not correlate with yield in either F3 or F4 plots of each cross, possibly because HI was negatively, and yield positively, related to heading date; unfortunately no attempt was made to unravel the interrelationships. Obviously, correlations with yield in later generations (across years and sites) were not statistically significant. The fact that plot yield correlated positively with plant height as well as heading date (ranges not specified) suggests that edge effects and interplot competition may have confounded yield measurements in these small, untrimmed plots. The plot size in the second year was slightly larger, but the poor correlation of yield across years (and sites) pointed to strong G × Y interactions. Whan et al. (1981) did show that when generations were grown side-by-side, associations of yield between consecutive generations improved with later generations, something they ascribed to the increase in genotype homozygosity.
Siddique and Whan (1994) proposed that the ratio of spike (‘ear’) weight to stem weight, measured at anthesis, may be a more stable predictor of yield than HI in environments where moisture stress during grain-filling is common. Their detailed study of the trait in rainfed conditions in Western Australia identified that this trait showed strong correlations with yield across random F2-derived, F4 and F5 lines in plots from several crosses and seasons, but the relationships were no stronger than between HI and yield. The authors also showed that spike : stem ratio correlated well across populations when measured in F2 and F3 plants (spacing 20 cm by 6 cm) and again in F4 and F5 plots, but curiously, they did not report the correlation of the trait in F2 and F3 plants with plot yield of the F2-derived F4 and F5 lines. The trait may capture desirable partitioning at anthesis but is difficult to measure, although results from Mexico suggest that the ratio is relatively insensitive to timing over the first week or so after first flowering, the growing weight of grains in the spike being balanced by continuing growth in the rest of the plant (R. A. Fischer, unpubl. data).
Working at Narrabri, New South Wales, and following promising F3 responses to HI selection in the F2 (Bhatt 1977), Bhatt and Derera (1978) measured HI of 153 near-homozygous F5–F7 advanced breeding lines already selected for visual traits such as desirable plant height and maturity and grown in replicated hill plots (20 seeds plot–1, hills spaced 50 cm by 50 cm). High, medium and low HI lines (five each) were sown in a rainfed, replicated large plot trial the following season. The phenotypic correlation of hill and plot HI was 0.99**, of hill HI and plot yield 0.89**, and of GW per hill and plot yield 0.35n.s. Plot yield ranged among genotypes from 1.7 to 3.4 t ha–1 and was closely related to plot HI (rp = 0.90**) but not biomass (rp = 0.10n.s.). Despite assessment under rainfed conditions, these were very promising results in demonstrating that HI in a spaced planting is a strong predictor of plot yield, at least for near-homozygous lines, but constrained for variation in plant height and flowering time. HI selection was followed up in the main Narrabri breeding program by Ellison et al. (1985). Again, it was investigated only in advanced lines already selected for desirable maturity and height. By using 113 lines, HI in hill plots (100 cm by 100 cm) correlated closely with yield in adjacent large plots, under both dryland and irrigated conditions in Narrabri (mean genotypic correlation 0.98**). However, in contrast to Bhatt and Derera (1978), the hill-plot grain weight also correlated with large-plot yield (mean genotypic correlation 0.74**) and positively correlated with large-plot biomass. The average genotypic correlation between Narrabri hill-plot HI and large-plot yield across 13 regional dryland trials in the same year was 0.39 ± 0.06 (±s.e.); these trials showed moderately large genotype × location interaction for yield. The authors recommended initial screening for HI followed by replicated hill plots at multiple sites.
Intrigued by the promise of some of the above results, the winter-wheat breeding program at Oklahoma State University undertook a detailed study of HI in the 1980s. Sharma and Smith (1986) sowed 96 random, F2-derived F3 lines from three crosses of mostly elite lines in adjacent single rows 30 cm apart to give a plant spacing of 30 cm by 5 cm. Yield and biomass per plant were measured on a central 60-cm segment of each row from which HI was calculated. Selecting 15 highest and lowest lines for HI, these F4 lines were sown the following year in single rows, 30 cm apart, but in three replicates at two locations, from which yield and biomass per row and HI were determined. HI was strongly correlated across generations in each population (rp = 0.77** to 0.91**), indicating that the realised heritability was moderate–high (0.44–0.60). However, F4 grain yield was only weakly related to HI, height and heading date in F4, but strongly related to biomass (0.80** < r <0.91**) as it was in the F3. Thus, only in one population and at one location did divergent selection for HI increase grain yield, when F3 HI vs F4 yield correlation was 0.52**. In this same situation, F3 grain yield and biomass also correlated with F4 grain yield (rp = 0.67** and 0.43**, respectively). Because F4 yield varied from only 1.3 to 2.6 t ha–1, water stress was obviously a major constraint in this study and inter-row (intergenetic) competition for soil water would have been severe and probably explained the dominance of the yield–biomass correlations. A parallel study with cultivars tended to confirm the importance of biomass compared with HI in the rainfed Oklahoma environment (Sharma and Smith 1987; Sharma et al. 1987), whereas a study of the combining ability for HI among Oklahoma winter-wheat cultivars (Sharma et al. 1991) showed good general combining ability and some correlations between HI and small-plot yield in F2 bulks. However, the latter results were probably explained by the strong negative correlation between HI and days to heading.
Quail et al. (1989) was mentioned previously because it was one study testing HI that did comply with all of the above conditions. HI was one EGS criterion tested in their population of random lines grown in the F3 singly in pots spaced at 25 cm by 50 cm in the glasshouse. After discarding excessively tall and late lines, F7 and F8 plot yield was only weakly correlated with F3 HI, the best being HI of a central culm (rp = 0.33*, n = 44), whereas partial correlations at constant height were not significant. Disappointingly, HI itself did not correlate well between the glasshouse and field sowings, even for the 16 non-segregating parents of the crosses. The authors concluded that the glasshouse conditions for assessing the spaced plants were too artificial, being characterised by heavily tillered, luxuriant plants producing on average 100 g grain per plant. For only one of the yield tests did retrospective selection for F3 HI increase the population mean yield (selecting the top 25% in the F3 raised F8 yield on average 9% above the population mean).
Until the 1980s, much of the progress in wheat potential yield from breeding was associated with shorter stature and, at lower latitudes, with earlier flowering, both factors increasing HI. An optimum of 75–90 cm was recognised for plant height, even in the absence of lodging, and possibly independent of the dwarfing genes needed to achieve this stature (e.g. Fischer and Quail 1990; Richards 1992). For flowering date, the optimum was largely determined by the climate from one month before, to one month after, flowering and hence depended on the location, but was largely independent of sowing date and duration from sowing to flowering. Working now within the height and flowering date range deemed optimal had weakened the likelihood that HI could be a useful selection criterion for potential yield. Besides, Austin (1980) had speculated that there was an upper limit to HI of ~0.62. Given that the latest wheat varieties, especially winter wheats in the United Kingdom, were in the range 0.50–0.55 and spring wheats were ~0.45–0.50, there would seem little scope to raise HI further. These developments and the disappointing results, for example, of Quail et al. (1989) and others above, may explain why few HI selection studies appeared in the years since that paper.
One exception was a relatively thorough study of HI selection conducted by Borghi et al. (1998) for their bread-wheat breeding program under favourable moisture conditions in northern Italy. Here, F2 plants from nine crosses were spaced-planted (10 cm by 25 cm) and the best 10% selected for visual yield-related traits (appropriate height and maturity, disease resistance, stay-green, large spike), resulting in ~1400 plants finally harvested for yield per plant and HI (of main culm). In the F3 (single 20-cm-spaced head rows, bordered by single rows of a short check cultivar), F4 (single 3-m2 microplot) and F5 (single 10-m2 plot), yield and HI were measured and divergent selection was practiced for top and bottom values for each trait. Correlations across generations (and years) of either trait varied with cross, but for HI correlations were generally low (rp < 0.5) as were those for plot yields. Divergent selection in F2 and F3 for HI or plant yield had little effect on yield in later generations, but when followed by divergent selection in F4 and F5, selection significantly increased plot yield in an F6 replicated plot trial, whether it was direct selection for high and low yield (114.4% of check yield vs 89.6%, respectively) or indirect via high and low HI (105.3% of check yield vs 96.4%, respectively). The high-yielding lines from yield selection alone had lower HI (45%) than those from HI selection (49%) and were several cm taller. It was not a promising result for selection based on HI (or on yield per F2 plant or F3 row), perhaps explained by large G × Y interactions arising from variation in environmental factors, but also probably because of intergenotypic competition in the F2 and F3, and possibly F4.
A recent re-examination of single-plant traits and plot yield was conducted with elite, short (70–79 cm), fixed durum wheat lines (n = 13) at a single location–year near Lleida, Spain (Pedró et al. 2012b). Plants were spaced at 20 cm by 30 cm and adjacent plots were sown at a high density (400 plants m−2); conditions during grain filling were dry, with plot yield ranging from 4 to 8 t ha–1. Many traits were measured in both plantings (except days to anthesis!), but spaced-plant HI did not correlate phenotypically with plot yield (rp = 0.07n.s.); plot yield was closely related to plot HI (rp = 0.76**) and no. of grains per m2 (GN, rp = 0.87**) and, surprisingly, most strongly to plot biomass (rp = 0.95**). Without anthesis dates and more detail on soil water, it is possible that variation in development and the timing of soil-water capture and use was contributing to the large yield variation. Pedró et al. (2012b) measured another partitioning ratio, spike : total biomass ratio, at anthesis in the spaced planting. The ratio, sometimes known as anthesis HI, correlated strongly with GN in the adjacent plots (rp = 0.75**) and moderately with plot yield (rp = 0.56*)19, despite the poor predictions with yield from spaced-plant HI. The fact that this was a rainfed situation with possible grain-filling drought may in part explain the odd results and vindicate the above notion of Siddique and Whan (1994) that, under such conditions, partitioning to spikes at anthesis may be a more reliable predictor of potential yield than HI itself.
Overall, this review has not delivered particularly encouraging results for the use of HI for EGS for potential yield. In summary:
-
Even when fixed genotypes were used and the spaced-plant and plot environments were the same, most studies found much poorer relationships than seen by Syme (1972) and Fischer and Kertesz (1976). Strong relationships at Narrabri (based on hill-plot HI) diminished when other yield trials (and environments) were considered (but these were rainfed environments as were most of the other examples). Plant height varied less in all of these later studies, and this had clearly reinforced HI correlations in the earlier two studies.
-
The four studies working with spaced segregating populations in successive seasons (Whan et al. 1982; Sharma and Smith 1986; Quail et al. 1989; Borghi et al. 1998) faced the added challenges of greater G × E interactions and of segregation between generations, plus prior visual selection for acceptable height and maturity narrowing the yield range. Only Quail et al. (1989) worked with irrigated plants and plots and, maybe for this reason, was the only study to find EGS for HI to be a significant predictor of plot yield (but only in one of four trials as already mentioned).
Do these results suggest that HI be abandoned as a possible EGS criterion for potential yield? There are several issues bearing on this question: (1) were the reported studies definitive; (2) has elite material reached ranges of height and maturity, levels of HI, and physiological regions of possible biomass trade-off that suggest insufficient scope for further raising HI; and (3) can the cost of HI measurement be reduced?
With respect to point 1, whether the studies were definitive, the review indicates that there has not been a study of HI that meets all of the conditions named at the outset, i.e. segregating populations of elite × elite, clearly spaced plants in the early generation (minimising intergenetic competition), and yield testing in bordered plots under potential yield conditions (ideally irrigation). Quail et al. (1989) was closest, but their F3 conditions for HI measurement, i.e. luxuriant spaced pots in the glasshouse (25 cm by 50 cm), were perhaps too unnatural and also may have led to significant intergenotypic competition. The spacing needed to avoid confounding effects of such competition on HI in early generations is an important consideration. Hamblin and Donald (1974), working at 8 cm by 8 cm spacing, found HI to be of no predictive value, not surprising because competition would have been severe. All other studies reported here used spacing <1600 cm2 plant–1 (<40 cm by 40 cm, approx.), with the exceptions of the Narrabri work (hills at 50 cm by 50 cm), that of Fischer in the Yaqui Valley, Mexico (60 cm by 60 cm in year 1, 40 cm by 40 cm in year 2), and probably that of Syme in South East Queensland (although he did not report plant spacing). The very modest change in HI with increasing planting density of given genotypes does not tell us much about what happens with density increases when genotypes are mixed as in segregating populations. This question needs to be resolved by testing repeatability and covariances at spacings from ~20 cm by 20 cm up to as wide as 50 cm by 50 cm; wider spacing may increase too much the influence of soil variation and, of course, the land required. Even so, the proponents of the honeycomb design insist on 100 cm × 100 cm!
Point 2, insufficient scope for further raising HI, is certainly more relevant today than decades ago when HI studies were first reported. Elite spring and winter wheat cultivars approach HI values of ≥0.50. As well, more detailed calculations of the investment needed in stem structure to support a given yield suggest that the maximum possible HI to avoid intolerable lodging risk is closer to 0.55 than the 0.62 proposed earlier (Berry et al. 2007). Finally, the latest study of spring wheat at CIMMYT (Aisawi et al. 2015) found no relationship between yield progress (1966–2009) and HI with genotype ranges of 6.3–7.9 t ha–1 for yield and 0.41–0.47 for HI. The value of HI selection will depend on what proportion of progeny in any cross, after any culling for excessive height and lateness, falls below a value of HI that is incompatible with superior potential yield (e.g. <0.40). It also depends on whether there are easier ways to cull such lower yielding progeny in early generations.
Point 3, whether the cost of HI measurement be reduced, is beyond the scope of this review. Suffice to suggest that HI may be a challenge for proximate remote-sensing techniques, but one recent report claims significant predictive value in using normalised difference vegetation index (NDVI) in rice to estimate HI (Tanger et al. 2017). It is suggested here that more realistic scope exists for the development of robotics to speed and cheapen the measurement of HI (and related traits), automating the steps described for the subsample hand-harvesting and processing method described by Pietragalla and Pask (2012). Any robotic method would determine plant biomass at the same time as HI, and although its correlation with yield in some of the above studies may reflect intergenotypic competition in the small-yield plots, its value for EGS could also to be readily tested.
Fruiting efficiency
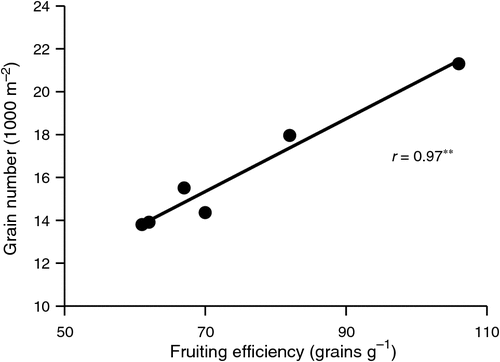
Fruiting efficiency has been defined as the ratio of the number of grains per spike to the dry weight of the spike at anthesis (SDWa; Acreche et al. 2008), and its possible role in yield selection reviewed and promoted (Slafer et al. 2015). In a sense, it is another partitioning-efficiency trait like HI, but its link to yield is obviously via GN. Earlier studies had found this ratio to be unaffected by the major dwarfing genes in spring wheat, the yield advantage they confer deriving from more efficient partitioning at anthesis of dry matter to spikes (Fischer and Stockman 1986; Slafer and Andrade 1993). More recently, the group from Balcarce, Argentina (Abbate et al. 1998) related the increased yield across modern Argentinian wheat cultivars (1984–95 vintages) to GN (rp = 0.89*), which in turn was related to FE (rp = 0.97**, Fig. 4); the relationships were strong even considering the positive bias they may contain arising from how GN and FE were calculated. Those authors termed the ratio ‘spike fertility’ (and Fischer (2011) ‘spike fertility index’), but here the later defined term FE of Slafer et al. (2015) will be used to avoid confusion. Obviously, calculation of FE will be affected by the weight of awns, if present (to date all studies have included the awns), and is quite sensitive to the exact stage when spikes are harvested and to whether the tiny grains present within a few days of any floret being fertilised are removed to determine correct spike structural weight. Fischer (2011) reported that at a mean temperature of 16.4°C, grain weight as a percentage of non-grain spike weight increases 3.6% day–1 from the second to the ninth day after first anthesis in a spike.
|
Abbate et al. (2013) subsequently showed strong phenotypic relationships in plots between FE calculated on the measured spike dry weight (without grains) at 7 and 15 days after anthesis, and a surrogate measured by dividing GN (no. of grains m–2) by chaff dry weight (g m–2), determined with much less effort and greater certainty at crop maturity. The latter correlation (rp) was 0.88** but the slope was <1 because spike (chaff) weight at maturity tended to exceed spike weight at anthesis by 20–40%, both observations also confirmed by Slafer et al. (2015)20. For clarity, FE thus measured at maturity is here designated FEm.
Quail et al. (1989) had included FEm in their selection study some years earlier (they simply called it grains per unit chaff weight and measured it on a main-shoot spike). The correlation between F3 values, which showed high broad-sense heritability (87%) and a wide genotypic range (44–132 g–1), and yield in F7/F8 plots was moderate at one site (rp = 0.35**). This was undoubtedly due to the good correlation between this trait in F3 plants and the same trait in the yield plots (rp = 0.52**), and between the latter and GN in plots (rp = 0.75**), and in turn between GN and yield in plots (rp = 0.81**). However, the predictive value of F3 values of FEm for mean F7/F8 yields across all four sowings was only weakly significant (rp = 0.28, P < 0.10, n = 44).
Mirabella et al. (2016) reviewed all early FE studies in Argentina that used fixed lines (largely done around the humid, wheat-growing region of Balcarce) and then studied the inheritance of FEm in 20 crosses among modern Argentina cultivars. In the former case, genotypic variance was always greater than G × E variance and usually greater than environmental variance. The crosses revealed moderate narrow-sense heritability (h2 = 0.63) in single-row plantings of parent and F1s, and evidence of polygenic control in F2 planting at 20 cm by 5 cm spacing. From each of two of these crosses, Martino et al. (2015) had taken 200 random, F2-derived F3 lines, which were grown at two sites the following year in single-row plots with normal within-row plant density, each bordered by a common cultivar. The FEm values showed significant moderate correlations between F2 and F3 sowings for the two crosses (rp = 0.42** and 0.44**) such that the top 25% in the F2 gave a realised heritability in F3 of 0.28 and 0.30 in the respective crosses. The 28% average increase in FEm in the selected groups was associated with a 12% increase in number of grains per spike, and 13% and 5% decreases in chaff weight and GW, respectively. Negative consequences for yield per unit area due to such trade-offs, such as between FE and spike dry weight at SDWa or GW, were not anticipated. The Argentine work to date concluded with a detailed study of FE for both main shoots and tillers in plots of three contrasting cultivars across agronomic treatments (mostly nitrogen levels) in Pergamino, central Argentina (Terrile et al. 2017); overall cultivar differences were consistent if abiotic stresses were low around flowering, but SDWa and GW trade-offs with high FE were evident. Elía et al. (2016) performed a similar study in irrigated plots in north-east Spain with cultivars and nitrogen levels; across cultivars, the GN vs FE relationship was quite robust (rp = 0.68**) and the GW trade-offs did not seem strong. The authors claimed that FEm showed rank changes relative to FE, but with rp = 0.57** between them, this is not so poor as to rule out the consideration of FEm.
Despite this compelling evidence, contradictory reports on the strength of GN vs FE relationships exist with some datasets. For example, in Mexico, Aisawi et al. (2015) reported that FE was not related to yield or to year of cultivar release (1966–2009). However, García et al. (2014) reported that GN in a random population of ~100 recombinant inbred lines from the cross of elite Mexican semi-dwarf spring wheats from c. 1990, Bacanora and Weebil, was correlated with FE (rp = 0.58** for sowing in Argentina and 0.29** for Mexico). Previously García et al. (2013) had identified strong correlations between potential yield and GN in this population.
Finally, Pedró et al. (2012a) and Slafer et al. (2015) claimed that selection for another FE surrogate in early generations of the spaced planting of a mutated population from the durum cultivar Cham 1 in spaced planting (30 cm by 18 cm) of M2–M5 generations was effective in raising plot yield to control levels. The surrogate was actually number of grains per unit stem length, and selection merely served to raise the level of grain set and spike fertility (and presumably FE) from the low levels in the M2 generation (as expected after mutagenesis) up to levels in M6 similar to those of the control cultivar. It serves to show that where there is large variation in sterility among progeny, selection for grain number per spike in spaced plants can be effective, something of which breeders are well aware.
Most of the above studies worked with FE in plots, and none vindicate the use of FE as an EGS criterion for yield, although Quail et al. (1989) had some success with FEm. At this stage, thorough studies with appropriate segregating populations from elite crosses (as attempted so far only by Martino et al. 2015) are needed, along with attention to the possibility that selection for high FE, while likely to increase GN, may reduce GW. Selection studies will be feasible only if chaff weight can be assumed to be an adequate denominator for calculating a surrogate FE (i.e. FEm), which does appear to be the case so far. FEm could be measured quickly and inexpensively by its incorporation into the robotic determination of HI referred to above. A related and totally ignored partitioning trait, namely grain weight per unit spike weight at maturity, a type of return on dry matter invested in reproductive structure at flowering, could also readily be tested in FEm studies, and would avoid the issue of trade-offs with GW. This ratio, which could be considered the spike harvest index, can reach 6 g g–1 in modern awnless winter wheats in the UK.
Stomatal conductance (and surrogates)
The remarkable and unwitting increase in leaf stomatal conductance (gs) in wheat, and indeed other C3 crops, with yield improvement through breeding has recently been highlighted by Roche (2015). Table 1 lists wheat examples from the Roche (2015) review along with some more information. All of the correlations shown are between the genotype means across environments for potential yield and the various traits, thereby greatly reducing environmental noise. Sometimes the potential yield vs gs correlation varied with stage of development (e.g. Zheng et al. 2011). Stomatal conductance was usually significantly associated with variation in photosynthetic activity, measured in high light and at ambient CO2 (Pmax), which in turn was sometimes significantly related to the potential yield variation.
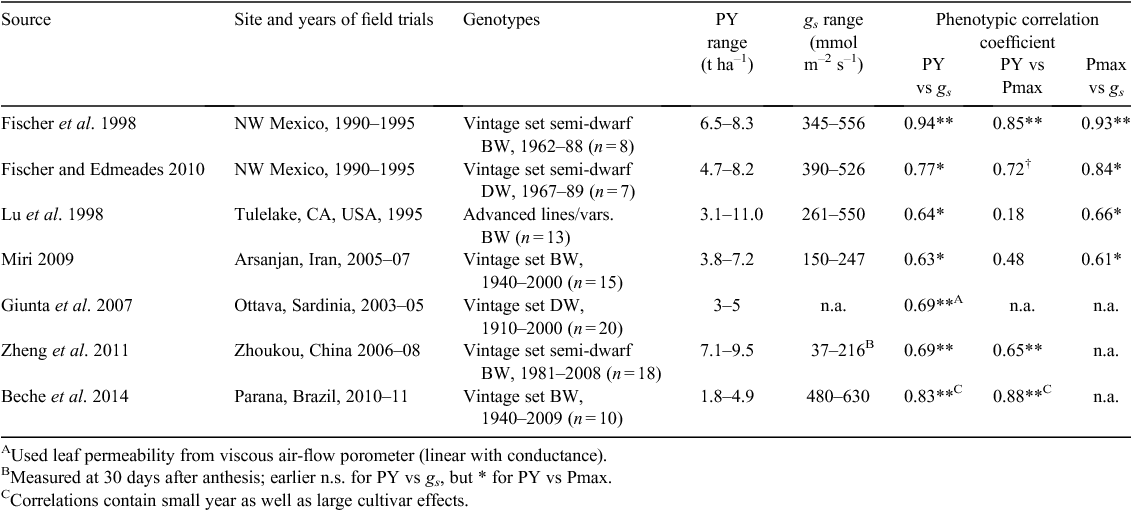
|
The first report of a grain yield–stomatal aperture (and photosynthetic rate) relationship under irrigation appears to come from work with spring-wheat cultivars, largely semi-dwarfs of Mexican and local origin, in Israel (experiments done in 1970–72)21. The experiments used the surrogate leaf permeability (LP), measured with a viscous air-flow porometer; this is an indirect measure of stomatal conductance in amphistomatous leaves (Fischer et al. 1977; Rebetzke et al. 2000). Shimshi and Ephrat (1975) recognised the potential of LP for indirect yield selection. Inspired by these remarkable results, in the 1974–75 growing season in north-west Mexico, access to a cumbersome but portable airflow porometer permitted rapid field measurements of LP (Fischer et al. 1976). Across 33 adapted, short cultivars in a spaced-planting and plot arrangement similar to that used in the previous season by Fischer and Kertesz (1976) mentioned under ‘HI’, Fischer et al. (1981) showed that mean LP of genotypes as spaced plants correlated with that of plots (rp = 0.87**) as well as with plot yield (rp = 0.48**). The latter case exceeded the predictive value of spaced-plant HI (rp = 0.40*). Pmax and gs were also measured repeatedly with other methods but only in the plots and only on the adaxial leaf surfaces, revealing moderate phenotypic correlations between LP, Pmax and gs, and complex relationships to plot grain yield (Fischer et al. 1981).
The early LP results at CIMMYT spawned a unique EGS program for yield conducted there by Dr Patrick Wall (CIMMYT 1977; Reynolds et al. 1999). Selection for LP measured on elite F2-spaced plants in 1975–76 was a useful criterion for advancing population potential yield as measured in the following year in F4 yield plots (n = 411, a single LP measure during F2 grain filling showed a phenotypic correlation with F4 yield of 0.41**, and around anthesis of 0.22*). Selection of the best 40% of F2 lines based on LP delivered a 6% increase in median yield and all 16 (of 411) of the top-yielding F4 lines (CIMMYT 1977)! With F5 yield plots the following year, the phenotypic correlation (rp) of F2 LP with yield was 0.25** for the LP measured at around anthesis, and 0.32** for that ~3 weeks later (CIMMYT 1978). Following on from the results of Wall at CIMMYT, the Quail et al. (1989) study, mentioned earlier, included several LP measurements on the 44 F3 spaced genotypes. However, there were no significant correlations with mean F7 and F8 plot yield, even though LP in the plots did correlate with yield (mean rp = 0.53**), and in one of the four yield plantings, F3 LP and yield plot LP correlated moderately well (rp = 0.50**).
The interest in LP and other indirect surrogates for leaf photosynthesis lagged until faster and more robust instruments became available. A custom-built, fast viscous air-flow porometer permitted a leaf measurement in 4 s while showing a strong correlation with gs in wheat (Rebetzke et al. 2000). More portable instruments were also becoming available for increasingly accurate (but significantly slower) measurement of leaf diffusive or stomatal conductance, often combined with the measurement of Pmax. Finally, a breakthrough came with the hand-held infrared thermometer for instantaneous measurement of leaf and canopy temperature (CT), which is closely but inversely related to canopy or leaf stomatal conductance, other things equal (in particular air temperature, vapour pressure deficit (VPD), wind speed and incident solar radiation; Jackson et al. 1977). Blum et al. (1982), again in Israel, were probably the first to realise the potential value for rapid screening of wheat genotypes by using canopy temperature measurements with an early infrared thermometer, but in the context of drought avoidance.
Use of such convenient, hand-held instruments in north-west Mexico in the late 1980s for rapid measurement demonstrated clearly that genetic progress between 1962 and 1988 in potential yield of semi-dwarf, spring bread wheat, measured over several years between 1990 to 1995, was associated with greater LP, Pmax and gs, as well as cooler canopies (lower CT; Fischer et al. 1998). Associations were seen both before and after anthesis and were generally stronger with afternoon measurements (yield vs gs phenotypic correlation reached 0.94**, Table 1). At the same time, a similar relationship between gs and potential yield progress in spring durum wheat was found, and eventually published (Fischer and Edmeades 2010).
Of special relevance to EGS, CT measurements in the 1992–93 season on spaced plantings of the historic series of semi-dwarf bread wheats in Mexico showed a variable but usually significant negative phenotypic correlation with yield, as high as –0.90** and averaging –0.81*, which was as high as the plot CT–yield correlation that year (–0.78*; Rees et al. 1993). At the same time, CT measurements among reasonably well-adapted lines at an irrigated, hot site in southern Mexico was seen to correlate closely (negatively) with plot yield at that site and, remarkably, with yield at other hot sites around the world (e.g. Amani et al. 1996).
Contemporaneous to the work in Mexico, Clarke (1997) was investigating gs in a high × low stomatal-conductance spring durum wheat cross in Swift Current, Saskatchewan. Over irrigated, unreplicated, random F3 progeny rows, adaxial leaf surface gs was not significantly phenotypically correlated with the same measure on spaced parent F2 plants, and narrow-sense heritability in the F3 was only 0.30, probably because the measurements were taken on only one date each generation and errors were likely high. However, measurements on replicated F4 single-row plots, selected in the F3 for high and low gs, gave a realised heritability of 0.40** and some evidence for a single dominant gene for high gs. This group appears not to have published on yield vs gs relationships.
Related work was undertaken in Canberra, Australia, following the discovery of the relationship between carbon isotope discrimination (CID) and transpiration efficiency in wheat (TE) (Farquhar and Richards 1984). The causal link here is the ratio of leaf intracellular and atmospheric partial pressures of CO2, which, if low, result in low CID and high TE. Differences in CID thus depend on the balance between stomatal and mesophyll conductances, whereas Pmax depends on the sum of the two conductances. Subsequent studies of genetic variation in wheat tended to find positive yield relationships to CID, to gs and to Pmax (e.g. Condon et al. 1987; Fischer et al. 1998). However, this was not always the case. For example, reduced CID was suggested as an EGS criterion for yield for dry conditions after Rebetzke et al. (2002) showed that CID had a high narrow-sense heritability and that divergent selection for CID in a high × low CID BC3 population delivered notable yield responses (low CID giving higher yield under dry conditions, presumably because of higher TE). These yield differences were confirmed separately in a comparison of low CID cultivar, Drysdale, with high CID parent, Hartog, in multiple field trials across Australia (G. Rebetzke, pers. comm.). Although it was shown that CID measured in spaced plants correlated closely with values from plots (Condon et al. 1987), the ambivalence of its relation to Pmax and to yield, even under well-watered conditions, and the expense of the CID measurement, limited further studies of CID as a breeding tool (but see below). Instead, the Canberra group pursued less-expensive, direct measurement of stomatal conductance.
In a separate inheritance study, Rebetzke et al. (2003) phenotyped conductance by using the fast viscous-flow porometer across three populations, each containing ~400 progeny in a structured (Cavalli) genetic design. Populations were developed from crosses and subsequent backcrosses between high- and low-stomatal-conductance parents, and measurements undertaken pre-anthesis on multiple days on well-watered, spaced-hill plots (75 cm by 75 cm). Genetic control was largely additive with some additive × additive epistasis, and family-mean heritabilities varied from 0.06 to 0.70, depending on the population and time of sampling. Interestingly, all progeny were similarly high for LP early in the morning, diverging into low- and high-conductance groups towards midday when VPD was greatest. Low-conductance groups largely contained the low-conductance parent and its backcross derivatives, whereas the high groups contained the high-conductance parents and their backcross derivatives. There was a strong suggestion that genotypic differences in gs were mediated by environmental signals throughout the day. No yield data were collected from these sowings.
The Canberra group did eventually conclude a substantial study of yield relationships with stomatal traits (LP, CT) in large, DH-derived mapping populations (n = 161–190) from three commercial-based crosses grown in bordered plots (4 m2) in a multiple augmented design of one or two replicates (Rebetzke et al. 2014). Grain yield and GN were consistently related to LP (rg values of 0.35**–0.74**) across the three crosses, and for one cross, there was a strong post-anthesis CT relationship (rg = –0.81** and –0.82**, respectively, for yield and GN), but no relationship with pre-anthesis CT. The measures of LP showed high levels of sampling error that could be managed only by repeated-measurements; depending on population, there were totals of 9–25 separate plot-measurement events (each comprising six leaves) per genotype over several dates, leading to narrow-sense heritabilities of 51–88% on a genotype-mean basis. The ground-based CT measurement was clearly faster (~10 s per plot scan) than LP measurements and achieved comparable narrow-sense heritabilities with fewer measurement events, but was found to be affected by microclimatic effects arising from plot canopy-height differences. Use of canopy height as a covariate markedly improved CT vs LP relationships (reaching rg values of –0.69** to –0.89**) and changed the CT vs yield relationships to those reported above. These canopy-height effects and their relationships with yield have been reported by other researchers in wheat and other crops (e.g. Giunta et al. 2008; Jones and Vaughan 2010). They may be solely due to interplot interference in small plots, or may also reflect the lower aerodynamic resistance of taller crops, regardless of plot size.
CIMMYT’s interest in gs vs yield relationships revived with funding from the Australian Centre for International Agricultural Research (ACIAR) to test such traits (and CID) as selection criteria within its spring bread wheat breeding program (van Ginkel et al. 2004; Condon et al. 2008). Thus, the studies, again in north-west Mexico, included only progeny within optimum height and maturity ranges. The first-mentioned paper, of studies in the field in 1999–2001, concluded that CT was highly correlated with yield across hundreds of progeny lines from five crosses (rg = 0.74**) and that CT measurements were very useful in augmenting visual selection by the breeder, but results varied with crosses. Condon et al. (2008) followed (in 2002–04) and took 48–62 F3-derived lines from each of five different elite × elite bread wheat crosses sampled at random after extremes of height and anthesis date had been removed. The lines were grown as bulks in small plots and adjacent large plots over three successive years as F5, F6 and F6 (repeated). The small plots, matching the way breeders handled F3 bulks, were 1.6 m2 in area, comprising two 2-m rows on a single 80-cm-spaced bed, and the large plots, two 5-m rows on each of two adjacent beds. Rows were spaced 20 cm apart on the beds, leaving 60 cm between rows across the furrow. Both plot sizes were replicated twice and were free of biotic stress but only moderately fertilised in order to avoid lodging. The average yield was 5.5 t ha–1. Segregation was not a large factor in this study because traits and yield were measured in all generations, traits on the small plots, and yield on both plot sizes, and correlations were sought between the three-year trait means (two years for some traits and only one year for grain CID), and the three-year large-plot mean yields.
In the 2002–04 Mexico study, CID and LP, and CT measured during grain filling, were always significantly correlated with large-plot yield (on average rg = 0.50**, 0.62** and –0.68**, respectively). Measurements before booting (CID only) or at booting tended to give marginally smaller correlations (rg = 0.51**, 0.55** and –0.62**, respectively). Only one population did not show significant relationships with yield, and this was only for LP. Divergent selection based on the top and bottom 25% for each trait generated mean yield differences of 5–8% of the overall mean large-plot yield. Using the breeder’s ‘ideotype’ score of the small plots generated a difference of 7%, whereas using the small-plot yields gave difference of 10%. On the basis of an economist’s costs per plot, i.e. CID (US$10), LP and CT ($0.20–0.30 each), visual score ($0.45, high because of the skilled observer involved) and yield ($12), the stomatal traits (LP and CT) looked promising for use in breeding (Brennan et al. 2007). The Condon–Brennan study is valuable because it introduces the important question of economics, but it was not a perfect simulation of the breeding process, noting that the selection generations were not spaced-planted and therefore would need reselection in the F4 or F5, and that three years of data were averaged to obtain the genetic correlations. In addition, trait values were not averaged (e.g. pre-plus post-anthesis), which should have improved relationships for LP and CT, as seen, for example, in Fischer et al. (1998).
CIMMYT has published widely on the value of CT as a selection criterion under conditions of water and heat stress, where ancillary measurements suggest it is a short-cut method to detect deeper or more extensive root systems during soil drying cycles (e.g. Olivares-Villegas et al. 2007; Lopes and Reynolds 2011). However, since Condon et al. (2008), there appear to be no further reports on the use of stomatal traits in EGS for potential yield. However, highly significant (negative) relationships between airborne-sensed CT and plot yield across yield trials with advanced lines in the breeding program have been reported, first by Reynolds et al. (1999) and recently by Rutkoski et al. (2016). In Australia, Rebetzke et al. (2013) genetically mapped LP and CT and elucidated relationships with yield in plots of each of three large populations of homozygous DHs, but breeding applications have not ensued. In the meantime, more reports have emerged of recent yield progress being associated with biomass as well as, or instead of, HI in winter wheats in the UK (Shearman et al. 2005), and in spring durum cultivars (Fischer and Edmeades 2010) and spring bread wheats (Aisawi et al. 2015), both in Mexico. Fischer and Edmeades reported yield correlations with gs (rp = 0.89**) as did Aisawi et al. (gs pre-anthesis rp = 0.56**, but post-anthesis only 0.22n.s.), supported by CT post-anthesis (rp = –0.94**) but not pre-anthesis (rp = –0.47n.s.).
Since around 2000, selection for gs has become of interest to breeders of other crops, but mostly in the context of the sensitivity of gs to VPD in warm-season crops (e.g. maize, soybean, sorghum, millet, groundnut). For these crops, greater stomatal sensitivity to rising VPD is postulated as a useful trait in rainfed situations, and special facilities have been constructed for its rapid, but not inexpensive, determination (e.g. Vadez et al. 2014). It is not clear whether genetic variation exists for this trait in wheat, nor whether it would be related to wheat potential yield. Although sugarcane is vegetatively propagated, early selection in the growth cycles has efficiency advantages. Interestingly, gs assessed in yield plots across 131 different clones at only 6 months after set planting out (or 6 months after harvest for ratoon crops) showed strong genetic effects and a moderately strong genotypic correlation (range –0.21 to 0.94, depending on sampling date) with cane yield measured about another 6 months later (Basnayake et al. 2015). Canopy conductance (gs × leaf area) showed generally even stronger genotypic correlations with cane yield.
In summary, it seems that the positive relationships of stomatal-conductance traits with potential yield in elite wheat populations are now becoming commonplace, as emphasised by Roche (2015). Indeed, relationships may have been reinforced as yield progress shifts more towards associations with biomass increase in generally high-HI cultivars of optimal height and flowering date. The conductance vs yield associations are likely due to the less well-demonstrated links between gs and leaf photosynthesis, but causality is not the critical issue. What matters is that there have been only a few studies of stomatal traits in EGS for potential yield, particularly in spaced plantings. Further studies are required to confirm the suggestions above that genotypic interactions due to spacing and segregation may be tolerable, and to follow up properly the exciting results of Wall within the CIMMYT bread-wheat breeding program almost 40 years ago! A special advantage of gs, or its surrogates, is that it appears the relationships hold for measurements made before flowering, although how much earlier is unclear, and that valid measurements may be possible in spaced plants. Following the cogent advocacy of Simmonds (1996), it might be possible for measurements early in the life cycle to be used to cull whole families in F2 and F3 microplots, thus allowing later measurements to concentrate on identifying superior progeny among more spaced plants of the retained families. A second advantage relative to the other traits discussed here is that the prospects for remote and very rapid sensing are good, meaning cost per genotype could be very low.
Final comments
The review has focused on three traits for indirect yield selection considered promising for further attention by pre-breeder–breeder–physiologist teams. We recognise that the history of such trait breeding approaches has not been one of great successes, and we note the possibilities opening up with molecular markers. These were recently reviewed by Bernardo (2016). Molecular markers can be readily applied non-destructively in early generations and there are no issues with growth conditions because even seeds can be used, but further segregation in heterozygous lines is not avoided, meaning problems can arise if population size is small (Bonnett et al. 2005). Quantitative trait locus (QTL) markers offer big advantages for commercial properties influenced by few genes of reasonably large effect (e.g. disease resistance genes, or quality proteins), but still have many limitations when it comes to polygenic quantitative attributes such as yield (Bernardo 2016; Crossa et al. 2017). Genomic selection (GS) is the most recent marker-based technique, and Bernardo concluded that ‘genomic selection should work reasonably well on average when it is applied routinely, particularly in those stages of a breeding program or in breeding situations in which phenotypic selection is non-existent or ineffective’. Bernardo thus does not rule out phenotypic selection, and it was probably not his intention to offer GS as an alternative to phenotypic selection. In fact, many have argued that GS and trait-based selection could complement each another, not only animal breeders who pioneered GS (Pszczola et al. 2013) but also crop breeders (e.g. Cooper et al. 2014; Ghanem et al. 2015; Reynolds and Langridge 2016; Crossa et al. 2017).
Genomic selection is in reality another form of indirect selection for yield, relying on the strength of the association between a breeding line’s genomic signature (as measured with thousands of single nucleotide polymorphism (SNP) markers saturating the whole genome) and its plot yield, as measured in so-called training populations of related genetic material. GS brings the possibility of speeding the breeding cycle, for sampling part of the seed means there is no need to grow and test the entire population in order to select and generate new lines for further crossing leading to the development of new populations enriched for target alleles. However, GS is limited by the need to update algorithms continually as germplasm changes, by inevitable noise in allele–yield relationships arising from error and non-additive gene action, and by the low likelihood of ever detecting rare beneficial alleles. In addition, GS is currently limited by the cost per progeny line for the mapping, something that Crossa et al. (2017) admit may preclude the use of GS in early generations as we have defined them here. The combining of phenotyping and GS that has been attempted to date has come in later generations as seen in the earlier cited work of Rutkoski et al. (2016). Those authors found that the accuracy of potential yield prediction across advanced wheat lines (likely F5+) in north-west Mexico was improved from a quite poor 0.29 with GS alone to 0.54 (+86%) when GS data of a training population was supplemented with multiple readings of plot canopy temperature and spectral reflectance NDVI data (in which yield relationships were clearly dominated by the grainfill CT readings). We conclude that there remains a major potential role for trait-based or phenotypic selection in early generations, assisted by focusing on traits that integrate many physiological processes and appear to be close to yield itself, if not revealing essential conditions for high yield, and which are cheap to measure. In addition, trait-based selection remains critical for selection of parents, especially the potential new sources of genetic variation, because GS cannot of itself generate useful new alleles.
It is for these reasons that this review has explored the landscape for EGS for yield in spaced plantings as described in pedigree and modified bulk breeding above, but as well has considered such selection in at least the first generation after predominantly homozygous plants are handled individually in any bulk-breeding system. In addition, better trait measurement in the field will improve QTL and other mapping analyses of the genetic bases of trait variation. The case for further testing of HI, FE and gs and its surrogates as indirect yield-selection criteria in breeding streams has been made here as strongly as possible. Much is based on work decades ago, ‘simple’ crop research swept aside by the fashionable tidal wave of quantitative and molecular genetics. EGS research needs to be repeated with populations derived from modern cultivars, today’s improved statistics and high-throughput phenotyping methods, at surely much less cost that the vast sums going into molecular work, which to date has delivered little for yield improvement. As urged by Jackson et al. (1996) and many others, it is essential that phenotypic trait validation take place within actual breeding programs, with the full collaboration of breeders and physiologists.
Conflicts of interest
The authors declare no conflicts of interest.
Acknowledgements
This review builds on a lifelong struggle with the subject and an unpublished 1998 paper (RA Fischer: Note on validating physiological selection criteria. Food Legume Newsletter, ACIAR, Canberra) prepared to guide selection in ACIAR projects on wheat, chickpea and groundnut breeding. At the time, it was greatly influenced by Jackson et al. (1996) who rightly challenged physiologists to face the real issues in breeding for yield, and by valuable exchanges with Dr Bob Baker of Saskatoon, who insisted on the need for adequate replication when comparing among breeding methods. However, what is being explored here is not a fully fledged comparison of different breeding methods, which is likely impossible as lamented by Baker, but the search for promising early-generation traits for further rigorous testing. The careful scrutiny of this paper by Phil Jackson is gratefully acknowledged.
References
Abbate PE, Andrade FH, Lazaro L, Bariffi JH, Berardocco HG, Inza VH, Marturano F (1998) Grain yield increase in recent Argentine wheat cultivars. Crop Science 38, 1203–1209.| Grain yield increase in recent Argentine wheat cultivars.Crossref | GoogleScholarGoogle Scholar |
Abbate P, Pontaroli A, Lazaro L, Gutheim F (2013) A method of screening for spike fertility in wheat. The Journal of Agricultural Science 151, 322–330.
| A method of screening for spike fertility in wheat.Crossref | GoogleScholarGoogle Scholar |
Acreche MM, Briceño-Felix G, Sanchez JAM, Slafer GA (2008) Physiological bases of genetic gains in Mediterranean bread wheat yield in Spain. European Journal of Agronomy 28, 162–170.
| Physiological bases of genetic gains in Mediterranean bread wheat yield in Spain.Crossref | GoogleScholarGoogle Scholar |
Aisawi KAB, Reynolds MP, Singh RP, Foulkes MJ (2015) The physiological basis of the genetic progress in yield potential of CIMMYT spring wheat cultivars from 1966 to 2009. Crop Science 55, 1749–1764.
| The physiological basis of the genetic progress in yield potential of CIMMYT spring wheat cultivars from 1966 to 2009.Crossref | GoogleScholarGoogle Scholar |
Allard RW (1960) ‘Principles of plant breeding.’ (John Wiley & Sons: New York)
Amani I, Fischer RA, Reynolds MP (1996) Canopy temperature depression associated with yield of spring wheat cultivars in a hot climate. Journal of Agronomy & Crop Science 176, 119–129.
| Canopy temperature depression associated with yield of spring wheat cultivars in a hot climate.Crossref | GoogleScholarGoogle Scholar |
Araus JL, Cairns JE (2014) Field high-throughput phenotyping: the new crop breeding frontier. Trends in Plant Science 19, 52–61.
| Field high-throughput phenotyping: the new crop breeding frontier.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXhs1CrtbjL&md5=280628e0fdc824e32e3213d18f78e6b5CAS |
Araus JL, Slafer GA, Reynolds MP, Royo C (2002) Plant breeding and drought in C3 cereals: What should we breed for? Annals of Botany 89, 925–940.
| Plant breeding and drought in C3 cereals: What should we breed for?Crossref | GoogleScholarGoogle Scholar |
Austin RB (1980) Physiological limitations to cereal yields and ways of reducing them by breeding. In ‘Opportunities for increasing crop yields’. (Eds RG Hurd, PV Biscoe, C Dennis) pp. 3–19. (Pitman: London)
Basnayake J, Jackson PA, Inman-Bamber NG, Lakshmanan P (2015) Sugarcane for water-limited environments. Variation in stomatal conductance and its genetic correlation with crop productivity. Journal of Experimental Botany 66, 3945–3958.
| Sugarcane for water-limited environments. Variation in stomatal conductance and its genetic correlation with crop productivity.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC28Xkt1KlsL0%3D&md5=4c27fbcb3b879f6b0853328b9dc6993dCAS |
Beche E, Benin G, Lemes da Silva C, Munaro LB, Marchese JA (2014) Genetic gain in yield and changes associated with physiological traits in Brazilian wheat during the 20th century. European Journal of Agronomy 61, 49–59.
| Genetic gain in yield and changes associated with physiological traits in Brazilian wheat during the 20th century.Crossref | GoogleScholarGoogle Scholar |
Bernardo R (2003) On the effectiveness of early generation selection in self-pollinated crops. Crop Science 43, 1558–1560.
| On the effectiveness of early generation selection in self-pollinated crops.Crossref | GoogleScholarGoogle Scholar |
Bernardo R (2016) Bandwagons I, too, have known. Theoretical and Applied Genetics 129, 2323–2332.
| Bandwagons I, too, have known.Crossref | GoogleScholarGoogle Scholar |
Berry PM, Sylvester-Bradley R, Berry S (2007) Ideotype design for lodging-proof wheat. Euphytica 154, 165–179.
| Ideotype design for lodging-proof wheat.Crossref | GoogleScholarGoogle Scholar |
Bhatt GM (1977) Response to two-way selection for harvest index in two wheat (Triticum aestivum L.) crosses. Australian Journal of Agricultural Research 28, 29–36.
| Response to two-way selection for harvest index in two wheat (Triticum aestivum L.) crosses.Crossref | GoogleScholarGoogle Scholar |
Bhatt GM (1980) Early generation criteria for yield selection in wheat. Journal of the Australian Institute of Agricultural Science 46, 14–22.
Bhatt GM, Derera NF (1978) Selection for harvest index among near-homozygous lines of wheat. Journal of the Australian Institute of Agricultural Science 44, 111–112.
Blum A, Mayer J, Gozlan G (1982) Infrared thermal sensing of plant canopies as a screening technique for dehydration avoidance in wheat. Field Crops Research 5, 137–146.
| Infrared thermal sensing of plant canopies as a screening technique for dehydration avoidance in wheat.Crossref | GoogleScholarGoogle Scholar |
Bonnett DG, Rebetzke GJ, Spielmeyer W (2005) Strategies for efficient implementation of molecular markers in wheat breeding. Molecular Breeding 15, 75–85.
| Strategies for efficient implementation of molecular markers in wheat breeding.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXhslyrsrs%3D&md5=9bf60855a5db53e45fece75ad1b2eee6CAS |
Borghi B, Accerbi M, Corbellini M (1998) Response to early generation selection for grain yield and harvest index in bread wheat (T. aestivum L.). Plant Breeding 117, 13–18.
| Response to early generation selection for grain yield and harvest index in bread wheat (T. aestivum L.).Crossref | GoogleScholarGoogle Scholar |
Bos I, Caligari P (1995) ‘Selection methods in plant breeding.’ (Chapman Hall: London)
Brennan JP, Condon AG, Van Ginkel M, Reynolds MP (2007) An economic assessment of the use of physiological selection for stomatal aperture-related traits in the CIMMYT wheat breeding programme. The Journal of Agricultural Science 145, 187–194.
| An economic assessment of the use of physiological selection for stomatal aperture-related traits in the CIMMYT wheat breeding programme.Crossref | GoogleScholarGoogle Scholar |
Brim CA (1966) A modified pedigree method of selection in soybeans. Crop Science 6, 220
| A modified pedigree method of selection in soybeans.Crossref | GoogleScholarGoogle Scholar |
Christopher JT, Veyradier M, Borrell AK, Harvey G, Fletcher S, Chenu K (2014) Phenotyping novel stay-green traits to capture genetic variation in senescence dynamics. Functional Plant Biology 41, 1035–1048.
| Phenotyping novel stay-green traits to capture genetic variation in senescence dynamics.Crossref | GoogleScholarGoogle Scholar |
CIMMYT (1977) Physiology. CIMMYT Report on Wheat Improvement. pp. 106–118. CIMMYT, Mexico, DF.
CIMMYT (1978) Physiology. CIMMYT Report on Wheat Improvement. pp. 117–128. CIMMYT, Mexico, DF.
Clarke JM (1997) Inheritance of stomatal conductance in a durum wheat cross. Canadian Journal of Plant Science 77, 623–625.
| Inheritance of stomatal conductance in a durum wheat cross.Crossref | GoogleScholarGoogle Scholar |
Clement JD, Constable GA, Stiller WN, Liu SM (2015) Early generation selection strategies for breeding better combinations of cotton yield and fibre quality. Field Crops Research 172, 145–152.
| Early generation selection strategies for breeding better combinations of cotton yield and fibre quality.Crossref | GoogleScholarGoogle Scholar |
Condon AG, Richards RA, Farquhar GD (1987) Carbon isotope discrimination is positively correlated with grain yield and dry matter production in field-grown wheat. Crop Science 27, 996–1001.
| Carbon isotope discrimination is positively correlated with grain yield and dry matter production in field-grown wheat.Crossref | GoogleScholarGoogle Scholar |
Condon AG, Reynolds MP, Brennan J, van Ginkel M, Trethowan R, Rebetzke GJ, Bonnett DG, Richards RA, Farquhar GD (2008) Stomatal aperture related traits and yield potential in bread wheat. In ‘Proceedings CIMMYT Wheat Yield Potential Symposium’. (Eds MP Reynolds, PR Hobbs, R Ortiz, J Pietragalla, HJ Braun) pp. 126–133. (CIMMYT: Mexico, DF)
Cooper M, Messina CD, Podlich D, Totir LR, Baumgarten A, Hausmann NJ, Wright D, Graham G (2014) Predicting the future of plant breeding: complementing empirical evaluation with genetic prediction. Crop & Pasture Science 65, 311–336.
| Predicting the future of plant breeding: complementing empirical evaluation with genetic prediction.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXotVKnsbk%3D&md5=7ec300b8e9583c880cda7e7a7d2d03d8CAS |
Crossa J, Pérez-Rodriguez P, Cuevas J, Montesinos-López O, Jarquín D, De Los Campos G, Burgueño J, Comacho-González JM, Pérez-Elizalde S, Beyene Y, Dreisigacker S, Singh R, Zhang X, Gowda M, Roorkiwal M, Rutkoski J, Varshney RK (2017) Genomic selection in plant breeding: Methods, models, and perspectives. Trends in Plant Science 22, 961–975.
DePauw RM, Shebetski LH (1973) An evaluation of an early generation yield testing procedure in Triticum aestivum. Canadian Journal of Plant Science 53, 465–470.
| An evaluation of an early generation yield testing procedure in Triticum aestivum.Crossref | GoogleScholarGoogle Scholar |
Donald CM (1963) Competition among crop and pasture plants. Advances in Agronomy 15, 1–118.
| Competition among crop and pasture plants.Crossref | GoogleScholarGoogle Scholar |
Donald CM (1968) The breeding of crop ideotypes. Euphytica 17, 385–403.
| The breeding of crop ideotypes.Crossref | GoogleScholarGoogle Scholar |
Donald CM (1981) Competitive plants, communal plants and yield in wheat crops. In ‘Wheat science – today and tomorrow’. (Eds LT Evans, WJ Peacock) pp. 223–247. (Cambridge University Press: Cambridge, UK)
Donald CM, Hamblin J (1976) The biological yield and harvest index of cereals as agronomic and plant breeding criteria. Advances in Agronomy 28, 361–405.
| The biological yield and harvest index of cereals as agronomic and plant breeding criteria.Crossref | GoogleScholarGoogle Scholar |
Elía M, Savin R, Slafer GA (2016) Fruiting efficiency in wheat: physiological aspects and genetic variation among modern cultivars. Field Crops Research 191, 83–90.
| Fruiting efficiency in wheat: physiological aspects and genetic variation among modern cultivars.Crossref | GoogleScholarGoogle Scholar |
Ellison FW, Latter BDH, Anttonen T (1985) Optimum regimnes for selection for grain yield and harvest index in spring wheat. Euphytica 34, 625–640.
| Optimum regimnes for selection for grain yield and harvest index in spring wheat.Crossref | GoogleScholarGoogle Scholar |
Falconer DS, Mackay TFC (1997) ‘Introduction to quantitative genetics.’ 4th edn (Addison Wesley Longman: Harlow, UK)
Farquhar GD, Richards RA (1984) Isotopic composition of plant carbon correlates with water-use efficiency. Australian Journal of Plant Physiology 11, 539–552.
| Isotopic composition of plant carbon correlates with water-use efficiency.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL2MXhtFSju7w%3D&md5=25eb169595c9295df4b6c0fc2e874f88CAS |
Fasoula DA (1990) Correlations between auto-, allo- and nil-competition and their implications in plant breeding. Euphytica 50, 57–62.
| Correlations between auto-, allo- and nil-competition and their implications in plant breeding.Crossref | GoogleScholarGoogle Scholar |
Fasoulas A (1981) ‘Principles and methods of plant breeding.’ Publication No. 11. (Aristotle University: Thessaloniki, Greece)
Fiorani F, Schurr U (2013) Future scenarios for plant phenotyping. Annual Review of Plant Biology 64, 267–291.
| Future scenarios for plant phenotyping.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXosFSktLw%3D&md5=55f5687aba95a890aab5fb92ca49fef4CAS |
Fischer RA (1978) Are your results confounded by intergenotypic competition? In ‘Proceedings 5th International Wheat Genetics Symposium’. Vol. II. (Ed. S Ramanjam) pp. 767–777. (Indian Society of Genetics and Plant Breeding: New Delhi)
Fischer RA (2011) Wheat physiology: a review of recent developments. Crop & Pasture Science 62, 95–114.
| Wheat physiology: a review of recent developments.Crossref | GoogleScholarGoogle Scholar |
Fischer RA (2015) Definitions and determination of crop yield, yield gaps and of rates of change. Field Crops Research 182, 9–18.
| Definitions and determination of crop yield, yield gaps and of rates of change.Crossref | GoogleScholarGoogle Scholar |
Fischer RA, Edmeades GO (2010) Breeding and cereal yield progress. Crop Science 50, S85–S98.
| Breeding and cereal yield progress.Crossref | GoogleScholarGoogle Scholar |
Fischer RA, Kertesz Z (1976) Harvest index in spaced plant populations and grain weight in microplots as indicators of yielding ability in spring wheat. Crop Science 16, 55–59.
| Harvest index in spaced plant populations and grain weight in microplots as indicators of yielding ability in spring wheat.Crossref | GoogleScholarGoogle Scholar |
Fischer RA, Quail KJ (1990) The effect of major dwarfing genes on yield potential in spring wheats. Euphytica 46, 51–56.
| The effect of major dwarfing genes on yield potential in spring wheats.Crossref | GoogleScholarGoogle Scholar |
Fischer RA, Stockman YM (1986) Increased kernel number in Norin10-derived dwarf wheat: evaluation of the cause. Australian Journal of Plant Physiology 13, 767–784.
| Increased kernel number in Norin10-derived dwarf wheat: evaluation of the cause.Crossref | GoogleScholarGoogle Scholar |
Fischer RA, Aguilar I, Maurer R, Rivas S (1976) Density and row spacing effects on irrigated short wheats at low latitude. The Journal of Agricultural Science 87, 137–147.
| Density and row spacing effects on irrigated short wheats at low latitude.Crossref | GoogleScholarGoogle Scholar |
Fischer RA, Sanchez M, Syme JR (1977) Pressure chamber and air flow porometer for rapid field indication of water status and stomatal condition in wheat. Experimental Agriculture 13, 341–352.
| Pressure chamber and air flow porometer for rapid field indication of water status and stomatal condition in wheat.Crossref | GoogleScholarGoogle Scholar |
Fischer RA, Bidinger F, Syme JR, Wall PC (1981) Leaf photosynthesis, leaf permeability, crop growth, and yield of short spring wheat genotypes under irrigation. Crop Science 21, 367–373.
| Leaf photosynthesis, leaf permeability, crop growth, and yield of short spring wheat genotypes under irrigation.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL3MXksFyhu7g%3D&md5=d3fd10b0a2edd2e33cfe134495272d2aCAS |
Fischer RA, O’Brien L, Quail KJ (1989) Early generation selection in wheat. II Grain quality. Australian Journal of Agricultural Research 40, 1135–1142.
| Early generation selection in wheat. II Grain quality.Crossref | GoogleScholarGoogle Scholar |
Fischer RA, Rees D, Sayre KD, Lu Z, Condon AG, Larque Saavedra A (1998) Wheat yield progress is associated with higher stomatal conductance and photosynthetic rate, and cooler canopies. Crop Science 38, 1467–1475.
| Wheat yield progress is associated with higher stomatal conductance and photosynthetic rate, and cooler canopies.Crossref | GoogleScholarGoogle Scholar |
Fischer RA, Sayre KD, Ortiz Monasterio I (2005) The effect of raised bed planting on irrigated wheat yield as influenced by variety and row spacing. In ‘Evaluation and performance of permanent raised bed cropping systems in Asia, Australia and Mexico’. ACIAR Proceedings No. PR121. (Eds CA Roth, RA Fischer, CA Meisner) pp. 1–11. (ACIAR: Canberra, ACT)
Fischer RA, Byerlee D, Edmeades GO (2014) ‘Crop yields and global food security: will yield increase continue to feed the world?’ Monograph No. 158. (Australian Centre of International Agricultural Research: Canberra) Available at: http://aciar.gov.au/publication/mn158
Frey KJ (1965) The utility of hill plots in oat research. Euphytica 14, 196–208.
| The utility of hill plots in oat research.Crossref | GoogleScholarGoogle Scholar |
García GA, Hasan AK, Puhl LE, Reynolds MP, Calderini DF, Miralles DJ (2013) Grain yield potential strategies in an elite wheat double-haploid population grown in contrasting environments. Crop Science 53, 2577–2587.
| Grain yield potential strategies in an elite wheat double-haploid population grown in contrasting environments.Crossref | GoogleScholarGoogle Scholar |
García GA, Serrago RA, Gonzalez FG, Slafer GA, Reynolds MP, Miralles DJ (2014) Wheat grain number: Identification of favourable physiological traits in an elite doubled-haploid population. Field Crops Research 168, 126–134.
| Wheat grain number: Identification of favourable physiological traits in an elite doubled-haploid population.Crossref | GoogleScholarGoogle Scholar |
Ghanem ME, Marrou H, Sinclair TR (2015) Physiological phenotyping of plants for crop improvement. Trends in Plant Science 20, 139–144.
| Physiological phenotyping of plants for crop improvement.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXitVeis7bO&md5=73c55f40cffc0367e224694b3da9d97eCAS |
Giunta F, Motzo R, Pruneddu G (2007) Trends since 1900 in yield potential of Italian-bred durum wheat cultivars. European Journal of Agronomy 27, 12–24.
| Trends since 1900 in yield potential of Italian-bred durum wheat cultivars.Crossref | GoogleScholarGoogle Scholar |
Giunta F, Motzo R, Pruneddu G (2008) Has long-term selection for yield in durum wheat also induced changes in leaf and canopy traits. Field Crops Research 106, 68–76.
| Has long-term selection for yield in durum wheat also induced changes in leaf and canopy traits.Crossref | GoogleScholarGoogle Scholar |
Hamblin J, Donald CM (1974) The relationships between plant form, competitive ability and grain yield in a barley cross. Euphytica 23, 535–542.
| The relationships between plant form, competitive ability and grain yield in a barley cross.Crossref | GoogleScholarGoogle Scholar |
Hamblin J, Rosielle AA (1978) Effect of intergenotypic competition on genetic parameter estimation. Crop Science 18, 51–54.
| Effect of intergenotypic competition on genetic parameter estimation.Crossref | GoogleScholarGoogle Scholar |
Hazel LN, Lush JN (1942) The efficiency of three methods of selection. The Journal of Heredity 33, 393–399.
| The efficiency of three methods of selection.Crossref | GoogleScholarGoogle Scholar |
Jackson P, McRae TA (2001) Selection of sugarcane clones in small plots: effects of plot size and selection criteria. Crop Science 41, 315–322.
| Selection of sugarcane clones in small plots: effects of plot size and selection criteria.Crossref | GoogleScholarGoogle Scholar |
Jackson P, Robertson M, Cooper M, Hammer G (1996) The role of physiological understanding in plant breeding; from a breeding perspective. Field Crops Research 49, 11–37.
| The role of physiological understanding in plant breeding; from a breeding perspective.Crossref | GoogleScholarGoogle Scholar |
Jackson RD, Reginato RJ, Idso SB (1977) Wheat canopy temperature–practical tool for evaluating water requirements. Water Resources Research 13, 651–656.
| Wheat canopy temperature–practical tool for evaluating water requirements.Crossref | GoogleScholarGoogle Scholar |
Jennings PR, Herrera RM (1968) Studies on competition in rice 2. Competition in segregating populations. Evolution 22, 332–336.
| Studies on competition in rice 2. Competition in segregating populations.Crossref | GoogleScholarGoogle Scholar |
Jones HG, Vaughan RA (2010) ‘Remote sensing of vegetation.’ (Oxford University Press: Oxford, UK)
Kawano K, Tanaka A (1967) Studies on the competitive ability of rice plant in population. Journal of the Faculty of Agriculture, Hokkaido University 55, 339–361.
Kawano K, Gonzalez H, Lucena M (1974) Intraspecific competition, competition with weeds, and spacing response in rice. Crop Science 14, 841–845.
| Intraspecific competition, competition with weeds, and spacing response in rice.Crossref | GoogleScholarGoogle Scholar |
Knott DRK (1979) Selection for yield in wheat breeding. Euphytica 28, 37–40.
| Selection for yield in wheat breeding.Crossref | GoogleScholarGoogle Scholar |
Kramer Th, van Ooijen JW, Spitters CJT (1982) Selection for yield in small plots of spring wheat. Euphytica 31, 549–564.
| Selection for yield in small plots of spring wheat.Crossref | GoogleScholarGoogle Scholar |
Lawrence MJ, Senadhira D (1998) Quantitative genetics of rice IV. A breeding strategy. Field Crops Research 55, 275–281.
| Quantitative genetics of rice IV. A breeding strategy.Crossref | GoogleScholarGoogle Scholar |
Lopes MS, Reynolds MP (2011) Drought adaptive traits and wide adaptation in elite lines derived from resynthesized hexaploid wheat. Crop Science 51, 1617–1626.
| Drought adaptive traits and wide adaptation in elite lines derived from resynthesized hexaploid wheat.Crossref | GoogleScholarGoogle Scholar |
Lu ZM, Percy RG, Qualset CO, Zeiger E (1998) Stomatal conductance predicts yields in irrigated cotton and bread wheat grown at high temperatures. Journal of Experimental Botany 49, 453–460.
| Stomatal conductance predicts yields in irrigated cotton and bread wheat grown at high temperatures.Crossref | GoogleScholarGoogle Scholar |
Marshall DR (1991) Alternative approaches and perspectives in breeding for higher yields. Field Crops Research 26, 171–190.
| Alternative approaches and perspectives in breeding for higher yields.Crossref | GoogleScholarGoogle Scholar |
Martino DL, Abbate PE, Cendoya MG, Gutheim F, Mirabella NE, Pontaroli AC (2015) Wheat spike fertility: inheritance and relationship with spike yield components in early generations. Plant Breeding 134, 264–270.
| Wheat spike fertility: inheritance and relationship with spike yield components in early generations.Crossref | GoogleScholarGoogle Scholar |
Mirabella NE, Abbate PE, Ramirez IA, Pontaroli AC (2016) Genetic variation for wheat spike fertility and early breeding materials. The Journal of Agricultural Science 154, 13–22.
| Genetic variation for wheat spike fertility and early breeding materials.Crossref | GoogleScholarGoogle Scholar |
Miri HR (2009) Grain yield and morpho-physiological changes from 60 years of genetic improvement of wheat in Iran. Experimental Agriculture 45, 149–163.
| Grain yield and morpho-physiological changes from 60 years of genetic improvement of wheat in Iran.Crossref | GoogleScholarGoogle Scholar |
Mitchell JW, Baker RJ, Knott DR (1982) Evaluation of honeycomb selection for single plant yield in durum wheat. Crop Science 22, 840–843.
| Evaluation of honeycomb selection for single plant yield in durum wheat.Crossref | GoogleScholarGoogle Scholar |
Moreno-Ramos OH, Rodriguez-Casas J, Johnson D, Thompson TL (2004) Wheat response to population and bed spacing in northwest Mexico. Cereal Research Communications 32, 273–279.
Nass HG (1980) Harvest index as a selection criterion for grain yield in 2 spring wheat crosses grown at 2 population densities. Canadian Journal of Plant Science 60, 1141–1146.
Olivares-Villegas JJ, Reynolds MP, McDonald GK (2007) Drought-adaptive traits in the Seri/Babax hexaploid wheat population. Functional Plant Biology 34, 189–203.
| Drought-adaptive traits in the Seri/Babax hexaploid wheat population.Crossref | GoogleScholarGoogle Scholar |
Pask AJD, Pietragalla J, Mullan DM, Reynolds MP (Eds) (2012) ‘Physiological breeding Vol. II: A field guide to wheat phenotyping.’ (CIMMYT: Mexico, DF)
Pedró A, Savin R, Parry MAJ, Slafer GA (2012a) Selection for high grain number per unit stem length through four generations from mutants in a durum wheat population to increase yields of individual plants and crops. Field Crops Research 129, 59–70.
| Selection for high grain number per unit stem length through four generations from mutants in a durum wheat population to increase yields of individual plants and crops.Crossref | GoogleScholarGoogle Scholar |
Pedró A, Savin R, Slafer GA (2012b) Crop productivity as related to single-plant traits at key phenological stages in durum wheat. Field Crops Research 138, 42–51.
| Crop productivity as related to single-plant traits at key phenological stages in durum wheat.Crossref | GoogleScholarGoogle Scholar |
Piepho H-P, Möhring J (2007) Computing heritability and selection response from unbalanced plant breeding trials. Genetics 177, 1881–1888.
| Computing heritability and selection response from unbalanced plant breeding trials.Crossref | GoogleScholarGoogle Scholar |
Pietragalla J, Pask A (2012) Grain yield and yield components. In ‘Physiological breeding Vol II: A field guide to wheat phenotyping’. Ch. 18. (Eds AJD Pask, J Pietragalla, DM Mullan, MP Reynolds) pp. 95–103. (CIMMYT: Mexico, DF)
Pszczola M, Veerkamp RF, de Haas Y, Wall E, Strabel T, Calus MPL (2013) Effect of predictor traits on accuracy of genomic breeding values for feed intake based on a limited cow reference population. Animal 7, 1759–1768.
| Effect of predictor traits on accuracy of genomic breeding values for feed intake based on a limited cow reference population.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXhsFOisb%2FE&md5=6e8d9af96d617d5794d0677df96846f2CAS |
Puckridge DW, Donald CM (1967) Competition among wheat plants sown at a wide range of densities. Australian Journal of Agricultural Research 18, 193–211.
| Competition among wheat plants sown at a wide range of densities.Crossref | GoogleScholarGoogle Scholar |
Quail KJ, Fischer RA, Woods JT (1989) Early generation selection in wheat. I. Yield potential. Australian Journal of Agricultural Research 40, 1117–1133.
| Early generation selection in wheat. I. Yield potential.Crossref | GoogleScholarGoogle Scholar |
Rebetzke GJ, Read JJ, Barbour MM, Condon AG, Rawson HM (2000) A hand-held porometer for rapid assessment of leaf conductance in wheat. Crop Science 40, 277–280.
| A hand-held porometer for rapid assessment of leaf conductance in wheat.Crossref | GoogleScholarGoogle Scholar |
Rebetzke GJ, Condon AG, Richards RA, Farquhar GD (2002) Selection for reduced carbon isotope discrimination increases aerial biomass and grain yield of rainfed bread wheat. Crop Science 42, 739–745.
| Selection for reduced carbon isotope discrimination increases aerial biomass and grain yield of rainfed bread wheat.Crossref | GoogleScholarGoogle Scholar |
Rebetzke GJ, Condon AG, Richards RA, Farquhar GD (2003) Gene action for leaf conductance in three wheat crosses. Australian Journal of Agricultural Research 54, 381–397.
| Gene action for leaf conductance in three wheat crosses.Crossref | GoogleScholarGoogle Scholar |
Rebetzke GJ, Botwright TL, Moore CS, Richards RA, Condon AG (2004) Genotypic variation in specific leaf area for genetic improvement of early vigour in wheat. Field Crops Research 88, 179–189.
| Genotypic variation in specific leaf area for genetic improvement of early vigour in wheat.Crossref | GoogleScholarGoogle Scholar |
Rebetzke GJ, Rattey AR, Farquhar GD, Richards RA, Condon AG (2013) Genomic regions for canopy temperature and their genetic association with stomatal conductance and grain yield in wheat. Functional Plant Biology 40, 14–33.
| Genomic regions for canopy temperature and their genetic association with stomatal conductance and grain yield in wheat.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XhvVertLnI&md5=d10f2ccd2a030bf00171f4423c8d9c89CAS |
Rebetzke GJ, Fischer RA, van Herwaarden AF, Bonnett DG, Chenu K, Rattey AR, Fettell NA (2014) Plot size matters: interference from intergenotypic competition in plant phenotyping studies. Functional Plant Biology 41, 107–118.
| Plot size matters: interference from intergenotypic competition in plant phenotyping studies.Crossref | GoogleScholarGoogle Scholar |
Rebetzke GJ, Boonee DG, Reynolds MP (2016) Awns reduce grain number to increase grain size and harvestable yield in irrigated and rainfed spring wheat. Journal of Experimental Botany 67, 2573–2586.
| Awns reduce grain number to increase grain size and harvestable yield in irrigated and rainfed spring wheat.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC28XhsF2ntbbI&md5=289edd89a0632c7692309a6504636fa3CAS |
Rees D, Sayre KD, Acevedo E, Nava Sanchez T, Lu Z, Zeiger E, Limon A (1993) Canopy temperature in wheat: relationships of yield and potential as a technique for early generation selection. Wheat Special Report No. 10. CIMMYT, Mexico, DF.
Reynolds M, Langridge P (2016) Physiological breeding. Current Opinion in Plant Biology 31, 162–171.
| Physiological breeding.Crossref | GoogleScholarGoogle Scholar |
Reynolds M, Acevedo E, Sayre K, Fischer R (1994) Yield potential in modern wheat varieties: Its association with a less competitive ideotype. Field Crops Research 37, 149–160.
| Yield potential in modern wheat varieties: Its association with a less competitive ideotype.Crossref | GoogleScholarGoogle Scholar |
Reynolds MP, Rajaram S, Sayre KD (1999) Physiological and genetic changes in irrigated wheat in post-Green Revolution period and approaches for meeting projected global demand. Crop Science 39, 1611–1621.
| Physiological and genetic changes in irrigated wheat in post-Green Revolution period and approaches for meeting projected global demand.Crossref | GoogleScholarGoogle Scholar |
Reynolds MP, Pask AJD, Mullan DM (Eds) (2012) ‘Physiological breeding Vol I: Interdisciplinary approaches to improve crop adaptation.’ (CIMMYT: Mexico, DF)
Richards RA (1992) The effect of dwarfing genes in spring wheat in dry environments. I. Agronomic characteristics. Australian Journal of Agricultural Research 43, 517–527.
| The effect of dwarfing genes in spring wheat in dry environments. I. Agronomic characteristics.Crossref | GoogleScholarGoogle Scholar |
Roche D (2015) Stomatal conductance is essential for higher yield potential. Critical Reviews in Plant Sciences 34, 429–453.
| Stomatal conductance is essential for higher yield potential.Crossref | GoogleScholarGoogle Scholar |
Rutkoski J, Poland J, Mondal S, Autrique E, Gonzalez Perez L, Crossa J, Reynolds MP, Singh R (2016) Canopy temperature and vegetation indices from high-throughput phenotyping improve accuracy of pedigree and genomic selection for grain yield in wheat. G3–Genes Genomes Genetics 6, 2799
| Canopy temperature and vegetation indices from high-throughput phenotyping improve accuracy of pedigree and genomic selection for grain yield in wheat.Crossref | GoogleScholarGoogle Scholar |
Sadras VO, Lawson C (2011) Genetic gain in yield and associated changes in phenotype, trait plasticity and competitive ability of South Australian wheat varieties released between 1958 and 2007. Crop & Pasture Science 62, 533–549.
| Genetic gain in yield and associated changes in phenotype, trait plasticity and competitive ability of South Australian wheat varieties released between 1958 and 2007.Crossref | GoogleScholarGoogle Scholar |
Sharma RC, Smith EL (1986) Selection for high and low harvest index in three winter wheat populations. Crop Science 26, 1147–1150.
| Selection for high and low harvest index in three winter wheat populations.Crossref | GoogleScholarGoogle Scholar |
Sharma RC, Smith EL (1987) Effect of seeding rates on harvest index, grain yield, and biomass yield in winter cereals. Crop Science 27, 528–531.
| Effect of seeding rates on harvest index, grain yield, and biomass yield in winter cereals.Crossref | GoogleScholarGoogle Scholar |
Sharma RC, Smith EL, McNew RW (1987) Stability of harvest index and grain yield in wheat. Crop Science 27, 104–108.
| Stability of harvest index and grain yield in wheat.Crossref | GoogleScholarGoogle Scholar |
Sharma RC, Smith EL, McNew RW (1991) Combining ability analysis for harvest index in winter wheat. Euphytica 55, 229–234.
| Combining ability analysis for harvest index in winter wheat.Crossref | GoogleScholarGoogle Scholar |
Shearman VJ, Sylvester-Bradley R, Scott RK, Foulkes MJ (2005) Physiological processes associated with yield progress in the UK. Crop Science 45, 175–185.
Shimshi D, Ephrat E (1975) Stomatal behaviour of wheat cultivars in relation to their transpiration, photosynthesis and yield. Agronomy Journal 67, 326–331.
| Stomatal behaviour of wheat cultivars in relation to their transpiration, photosynthesis and yield.Crossref | GoogleScholarGoogle Scholar |
Siddique KHM, Whan BR (1994) Ear:stem ratios in breeding populations of wheat: significance for yield improvement. Euphytica 73, 241–254.
| Ear:stem ratios in breeding populations of wheat: significance for yield improvement.Crossref | GoogleScholarGoogle Scholar |
Simmonds NW (1996) Family selection in plant breeding. Euphytica 90, 201–208.
| Family selection in plant breeding.Crossref | GoogleScholarGoogle Scholar |
Singh RP, Rajaram S, Miranda A, Huerta-Espinoza J, Autrique E (1998) Comparison of two crossing and four selection schemes for yield, yield traits, and slow rusting resistance to leaf rust in wheat. Euphytica 100, 35–43.
| Comparison of two crossing and four selection schemes for yield, yield traits, and slow rusting resistance to leaf rust in wheat.Crossref | GoogleScholarGoogle Scholar |
Slafer GA, Andrade F (1993) Physiological attributes related to the generation of grain-yield in bread wheat cultivars released at different eras. Field Crops Research 31, 351–367.
| Physiological attributes related to the generation of grain-yield in bread wheat cultivars released at different eras.Crossref | GoogleScholarGoogle Scholar |
Slafer GA, Elia M, Savin R, Garcia GA, Terrile II, Ferrante A, Miralles DJ, Gonzalez FG (2015) Fruiting efficiency: an alternative trait to further rise wheat yield. Food and Energy Security 4, 92–109.
| Fruiting efficiency: an alternative trait to further rise wheat yield.Crossref | GoogleScholarGoogle Scholar |
Sneep J (1977) Selection for yield in early generations of self-fertilizing crops. Euphytica 26, 27–30.
| Selection for yield in early generations of self-fertilizing crops.Crossref | GoogleScholarGoogle Scholar |
Spitters CJ, Kramer T (1984) Changes in relative growth rate with plant ontogeny in spring wheat genotypes grown as isolated plants. Euphytica 43, 833–847.
Sukumaran S, Reynolds MP, Lopes MS, Crossa J (2015) Genome-wide association study for adaptation to agronomic plant density: A component of high yield potential in spring wheat. Crop Science 55, 2609–2619.
| Genome-wide association study for adaptation to agronomic plant density: A component of high yield potential in spring wheat.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC28XnvVKksb0%3D&md5=1b2ee4c3378bd61401b7b8e12064ffdbCAS |
Syme JR (1972) Single plant characters as a measure of field plot performance of wheat cultivars. Australian Journal of Agricultural Research 23, 753–760.
| Single plant characters as a measure of field plot performance of wheat cultivars.Crossref | GoogleScholarGoogle Scholar |
Takeda K, Frey KJ (1985) Increasing grain yield of oats by independent culling for harvest index and vegetative growth index or unit straw weight. Euphytica 34, 33–41.
| Increasing grain yield of oats by independent culling for harvest index and vegetative growth index or unit straw weight.Crossref | GoogleScholarGoogle Scholar |
Tanger P, Klassen S, Mojica JP, Lovell JT, Moyers BT, Baraoidan M, Naredo MEB, McNally KL, Poland J, Bush DR, Leung H, Leach JE, McKay JK (2017) Field-based high throughput phenotyping rapidly identifies genomic regions controlling yield components in rice. Scientific Reports 7, 42839
| Field-based high throughput phenotyping rapidly identifies genomic regions controlling yield components in rice.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2sXjtlygsLY%3D&md5=f147156462924d7ce255e93f0baf97e7CAS |
Terrile II, Miralles DJ, Gonzalez FC (2017) Fruiting efficiency in wheat (Triticum aestivum L.): Trait response to different growing conditions and its relation to spike dry weight at anthesis and grain weight at harvest. Field Crops Research 201, 86–96.
| Fruiting efficiency in wheat (Triticum aestivum L.): Trait response to different growing conditions and its relation to spike dry weight at anthesis and grain weight at harvest.Crossref | GoogleScholarGoogle Scholar |
Thakare RB, Qualset CO (1978) Empirical evaluations of single-plant and family selection strategies in wheat. Crop Science 18, 115–118.
| Empirical evaluations of single-plant and family selection strategies in wheat.Crossref | GoogleScholarGoogle Scholar |
Tokatlidis IS, Xynias IN, Tsialtas JT, Papadopoulos II (2006) Single-plant selection at ultra-low density to improve stability of a bread wheat cultivar. Crop Science 46, 90–97.
| Single-plant selection at ultra-low density to improve stability of a bread wheat cultivar.Crossref | GoogleScholarGoogle Scholar |
Vadez V, Kholova J, Medina S, Kakkera A, Anderberg H (2014) Transpiration efficiency: new insights into an old story. Journal of Experimental Botany 65, 6141–6153.
| Transpiration efficiency: new insights into an old story.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2MXitl2gsb8%3D&md5=7c0d0eeea8a7e1685759dd0e470bfe95CAS |
van Ginkel M, Ortiz R (2017) Cross the best with the best, and select the best: HELP in breeding selfing crops. Crop Science 58, 1–14.
van Ginkel M, Reynolds MP, Trethowan RT, Hernandez E (2004) Can canopy temperature depression measurements help breeders in selecting for yield in wheat under irrigated production conditions? In ‘New directions for a diverse planet. Proceedings 4th International Crop Science Congress’. (Eds RA Fischer et al.) pp. 3.4.6. (The Regional Institute: Gosford, NSW)
Wallace DH, Yan W (2000) ‘Plant breeding and whole-system crop physiology.’ (CAB International: Wallingford, UK)
Wang H, van Eeuwijk FA (2014) A new method to infer causal phenotype networks using QTL and phenotypic information. PLoS One 9, e103997
| A new method to infer causal phenotype networks using QTL and phenotypic information.Crossref | GoogleScholarGoogle Scholar |
Wang J, van Ginkel M, Podlich D, Ye G, Trethowan R, Pfeiffer W, DeLacy IH, Cooper M, Rajaram S (2003) Comparison of two breeding strategies by computer simulation. Crop Science 43, 1764–1773.
| Comparison of two breeding strategies by computer simulation.Crossref | GoogleScholarGoogle Scholar |
Watson A, Ghosh S, Williams MJ, Cuddy WS, Simmonds J, Rey MD, Md Hatta MA, Hinchliffe A, Steed A, Reynolds D, et al (2018) Speed breeding is a powerful tool to accelerate crop research and breeding. Nature Plants 4, 23–29.
| Speed breeding is a powerful tool to accelerate crop research and breeding.Crossref | GoogleScholarGoogle Scholar |
Whan BR, Rathjen AJ, Knight R (1981) The relation between wheat lines derived from the F2, F3, F4 and F5 generations for grain yield and harvest index. Euphytica 30, 419–430.
| The relation between wheat lines derived from the F2, F3, F4 and F5 generations for grain yield and harvest index.Crossref | GoogleScholarGoogle Scholar |
Whan BR, Knight R, Rathjen AJ (1982) Response to selection for grain yield and harvest index in F2, F3, and F4 derived lines of two wheat crosses. Euphytica 31, 139–150.
| Response to selection for grain yield and harvest index in F2, F3, and F4 derived lines of two wheat crosses.Crossref | GoogleScholarGoogle Scholar |
Yang R-C (2009) When is early generation effective in self-pollinated crops? Crop Science 49, 2065–2079.
| When is early generation effective in self-pollinated crops?Crossref | GoogleScholarGoogle Scholar |
Yonezawa K (1983) Practical implications of improving the precision of genotype assessment in selection – a theory. Euphytica 32, 543–555.
| Practical implications of improving the precision of genotype assessment in selection – a theory.Crossref | GoogleScholarGoogle Scholar |
Zheng TC, Zhang XK, Yin GH, Wang LN, Han YL, Chen L, Huang F, Tang JW, Xia XC, He ZH (2011) Genetic gains in grain yield, net photosynthesis and stomatal conductance achieved in Henan Province of China between 1981 and 2008. Field Crops Research 122, 225–233.
| Genetic gains in grain yield, net photosynthesis and stomatal conductance achieved in Henan Province of China between 1981 and 2008.Crossref | GoogleScholarGoogle Scholar |
1Here yield refers solely to grain produced on an area basis (kg ha–1 or g m–2) in an adequately bordered, dense monoculture of genetically uniform material (conditions of iso-competition according to Bos and Caligari 1995).
2‘Genotypic’ is used here throughout, but ‘genetic’ can be used if plants are related, as in for example sib lines.
3Alternatively, the inbreeding coefficient is 0.50, 0.75 or 0.875 or higher, respectively.
4The expressions Fx-derived Fy lines refer to sib lines arising from a selected single plant in the xth generation (after two inbreds are crossed) and bulked thereafter until the yth testing generation, as in F2-derived F6 lines (abbreviated to F2:6), which could be encountered in the modified bulk method (see later). The later the generation of derivation, the more closely related are the sib lines. Also, it is implied that there has been little or no selection within each line, but whole lines could have been discarded entirely in generation advance.
5The term ‘family’ can be applied to any group of different but related sib lines, in this case all from one cross; the term ‘families’ can also be applied to sibs derived from a single early-generation plant.
6A similar strategy was proposed for transplanted paddy rice by Lawrence and Senadhira (1998), a situation favoured by the normally low plant densities for such rice in farmers’ fields (~ 20 plants m−2), permitting small, bordered yield plots with very little seed. They suggest strong selection of crosses based on the mean yield performance of 30–40 random F2-derived F3 families per cross, using replicated plots with only 10 transplanted plants per plot. Selected high-yielding F3 plants are then advanced without selection to F6, when they enter another round of small-plot yield testing. This seems never to have been thoroughly followed up at the International Rice Research Institute (IRRI), where the last author (Senadhira) later worked as a breeder.
7Recently, van Ginkel and Ortiz (2017) proposed plot-yield testing of F1s, followed by propagation of highest yielding crosses using double haploidy, and further yield testing. There is no genetic drift, only onerous F1 seed production, and again, traits for indirect EGS could be useful.
8Such selection fits the general definition of indirect selection for yield, even when the trait upon which selection is based is grain yield per plant (or, as we shall see later, yield m–2 in a microplot).
9After some initial interest, use of the elegant but complicated and space-demanding honeycomb design of single plants seems to have now become rare.
10Kawano et al. (1974) showed that amongst rice cultivars, responsiveness to space was related to competitive ability, and both were positively related to early vigour and plant height, with long duration adding to responsiveness, and all negatively related to yield.
11At the time, this contradicted the general notion that the survivors in bulk hybrid populations contained many genotypes that were well adapted and superior agriculturally; for example, this was the general experience then with a large barley composite population at UC Davis, California (Allard 1960).
12Jackson and McRae (2001) is an excellent analysis of the effect of bias due to interplot competition, albeit with clonally reproduced sugarcane. This bias substantially reduced the genetic correlation between cane yield in single-row plots and that in the inner rows of adjacent, multi-row plots but did not much affect the correlation of CCS (sugar concentration) between the two situations, such that single-row SCC was the better predictor of large-plot sugar yield (= cane yield × CCS).
13The actual dimensions of the seed spread are not specified, but Frey (1965) suggested that the method of hand-sowing into a scape made by a hoe was adopted throughout, presumably placing all seeds within a few cm of each other.
14Significance is designated throughout as n.s. for P > 0.10, † for 0.10 > P > 0.05, * for 0.05 > P >0.01, and ** for P < 0.01; rp refers to the phenotypic correlation coefficient.
15One such study in north-west Mexico looked at adjacent small and large plots replicated five times. The small plot was 3 rows by 20 cm by 3 m, with a skip row between plots and trimming to 2 m just before harvest; thus, there was interference only from interplot competition; the 36 cultivars were nested in three height groups. The small plot vs large plot yield phenotypic correlation was 0.68** (R. A. Fischer, unpubl. data).
16Of course, breeders may choose to ignore edge-effect bias in the interests of more efficient deployment of their resources, but given the small yield gains being sought these days, this warrants thorough analysis.
17Thus, selecting the top 50% of a population has a selection differential in standard units of 0.67, or a mean that is (0.67 × sy where sy is the standard deviation) above the mean of the total population, whereas for the top 10% of the population the superiority of the mean is (1.28 × sy). Note sy × k refers to the mean of the selected group, not the cut-off trait value for selection, and that others may define k as selection intensity, the proportion of the population selected (0.5 and 0.1, respectively, in this example); standard tables in plant breeding books relate the selection intensity to k (and to the cut-off point).
18This paper was drafted as a book chapter, never to be published because of the untimely death of the editor. RAF received in 1981 the unpublished 45-page manuscript, entitled ‘Genetic and physiological approaches to breeding for crop yield’. It is a uniquely clear and comprehensive review of the subject.
19Spaced-plant specific leaf weight produced the highest correlation (0.61*) with plot yield.
20Abbate et al. (2013) actually found that the increase in the weight of the spike structure from anthesis to maturity in crops was positively related to the estimated source : sink ratio during grain filling.
21Actually, the results were first seen in an unpublished 1973 report ‘Stomatal behaviour of wheat cultivars in relation to their transpiration, photosynthesis and yield’ submitted to Ford Foundation by Shimshi and Ephrat of the Agricultural Research Organisation, Bet-Dagan, Israel.


