Diagnosis and management of halo blight in Australian mungbeans: a review
Thomas J. Noble A , Anthony J. Young B , Colin A. Douglas C , Brett Williams A and Sagadevan Mundree A D
A , Anthony J. Young B , Colin A. Douglas C , Brett Williams A and Sagadevan Mundree A D
A Centre for Tropical Crops and Bio Commodities (CTCB), Queensland University of Technology, Brisbane City, Qld 4000, Australia.
B School of Agriculture and Food Sciences, The University of Queensland, Gatton, Qld 4343, Australia.
C Department of Agriculture and Fisheries (DAF), Hermitage Research Facility, Warwick, Qld 4370, Australia.
D Corresponding author. Email: sagadevan.mundree@qut.edu.au
Crop and Pasture Science 70(3) 195-203 https://doi.org/10.1071/CP18541
Submitted: 29 November 2018 Accepted: 8 February 2019 Published: 18 March 2019
Journal Compilation © CSIRO 2019 Open Access CC BY-NC-ND
Abstract
Mungbean (Vigna radiata L. Wilczek var. radiata) is an important food crop cultivated on over 6 Mha throughout the world. Its short duration of 55–70 days, capacity to fix atmospheric nitrogen, and exceptional grain nutritional profile makes the crop a staple for smallholder and subsistence farmers. In Australia, mungbean is grown as a high-value export crop and established as a main summer rotation for dryland farmers. A major threat to the integrity of the industry is halo blight, a bacterial disease leading to necrotic lesions surrounded by a chlorotic halo that stunts and ultimately kills the plant. Caused by Pseudomonas savastanoi pv. phaseolicola, this seed-borne disease is extremely difficult to control, resulting in significant yield loss and production volatility. The challenge of managing halo blight is exacerbated by a wide host range that includes many legume and weed species, and the presence of multiple epidemiologically significant strains. Molecular technologies could play a pivotal role in addressing these issues. This review synthesises current and emerging technologies to develop improved management strategies for the control of halo blight in mungbean.
Additional keywords: bacterial pathogen, disease management, host–pathogen interactions, molecular characterisation.
Introduction
Mungbean (Vigna radiata L. Wilczek var. radiata) is a vital component of global nutrition and food security (Day 2013). With the capacity to fix atmospheric nitrogen and short duration of 55–70 days, mungbean is a leading choice for double and intercropping farming systems, particularly between cereals (Senaratne and Gunasekera 1994; Yaqub et al. 2010). Consumption of mungbean worldwide has increased 60% over the last three decades with over 6 Mha of farming land being used for cultivation, concentrated mainly in Southeast Asia (Kim et al. 2015). This growing demand for mungbean requires innovative approaches to stabilise and increase production of the crop.
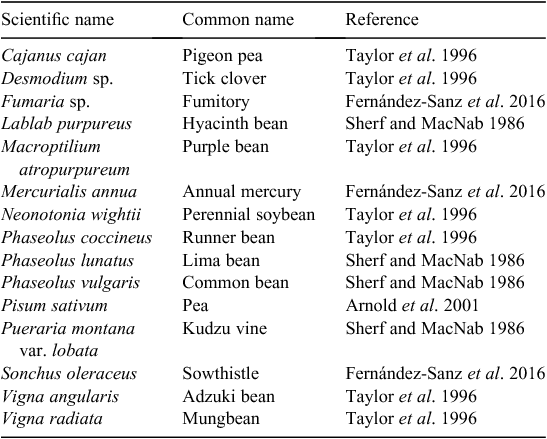
One of the foremost risk factors to the stability of the Australian mungbean industry is the seed-borne bacterial disease halo blight (Ryley and Tatnell 2011; AMA 2015). Identified in 1931 on French bean (common bean, Phaseolus vulgaris L.) in Queensland, Australia, it was not until the mid-1980s that halo blight was found infecting mungbeans (Ryley et al. 2010). At the same time, during 1983–84, the first recorded study of mungbeans affected by halo blight was reported in Pakistan (Akhtar 1988). Recently halo blight has been found in mungbeans in China, the world’s second highest producer (Sun et al. 2017). Given its broad host range (Table 1) and known geographical distribution, halo blight is suspected to be present in many mungbean-growing areas, but has not yet been documented.
|
Significance of halo blight to mungbean
Halo blight is caused by a group of bacterial strains belonging to Pseudomonas savastanoi pv. phaseolicola, closely related to a range of fluorescent pseudomonads that cause disease in a broad range of plant hosts (Arnold et al. 2011). Characterised by Burkholder (1926) as Phytomonas medicaginis variant phaseolicola, it was revised to Pseudomonas medicaginis variant phaseolicola by Dowson (1943) and then later to P. syringae pathovar phaseolicola (Young et al. 1978). Most recently P. syringae was regrouped into nine discrete genomospecies with P. savastanoi including the pathovars phaseolicola, savastanoi, glycinea and tabaci (Gardan et al. 1992). While it is still common to see these pathovars referred to as Pseudomonas syringae, the nomenclature P. savastanoi pv. phaseolicola is used throughout this review in light of the most recent taxonomic work.
Although the mechanisms of infection are yet to be fully elucidated, the key pathogenicity determinants have been identified. One of the main factors is the production of phaseolotoxin by some strains, which are referred to as toxigenic (Prosen et al. 1993). Characteristic symptoms of toxigenic strains are round, water-soaked lesions surrounded by a chlorotic halo of greenish yellow (Fig. 1) (Taylor et al. 1979a). Symptoms are most evident on leaf tissue, where a bacterial ooze can be seen exuding from the infection site. A slightly different symptom is observed when non-toxigenic strains are involved in infection. These strains proliferate within the plant, causing water-soaked lesions without the characteristic chlorotic halos (Patil et al. 1974; González et al. 2003). Yield loss directly attributable to halo blight is inherently problematic to determine through controlled experiments because it is difficult to exclude the pathogen from control plots as well as achieve consistent inoculation and symptom expression of trial plots. Taking into consideration these factors, a field study in Australia of mungbeans inoculated with a mixture of P. savastanoi pv. phaseolicola isolates found yield losses up to 75% and severe stunting (Ryley et al. 2010). A survey of mungbean fields throughout China between 2009 and 2014 reported similar yield reductions of 30–50% and total crop failure in extremely infected fields (Sun et al. 2017).

|
Environmental factors of disease development
Epidemics of halo blight are facilitated by cool (18–23°C), wet and windy conditions, which facilitate bacterial dispersal and infection (Taylor et al. 1979b). One study showed that the bacteria could infect plants situated 26 m away (Walker and Patel 1964). Marques and Samson (2016b) investigated the epiphytic life cycle of P. savastanoi pv. phaseolicola, confirming that asymptomatic dispersal precedes symptoms and that infection is dependent on weather conditions, developmental phase of the crop and strain of bacterium. This has large implications for crop management.
Growth of P. savastanoi pv. phaseolicola and production of phaseolotoxin are tightly regulated by temperature (Aguilera et al. 2017). Therefore, environmental effects such as location, sowing time and growing conditions can have significant impacts on establishment and severity of disease symptoms. The ideal temperature range for growth of P. savastanoi. pv. phaseolicola is 25–28°C and temperatures >49°C are lethal (Burkholder 1926; Nüske and Fritsche 1989; Aguilera et al. 2017), whereas phaseolotoxin production is highest at 18–20°C and ceases at temperatures >28°C (Nüske and Fritsche 1989; Aguilera et al. 2017). Temperatures of 18–28oC coincide with the ideal conditions for mungbean plants growing in warm and humid subtropical regions. Thus, in Australia, the most severe infections are generally found in southern Queensland and northern New South Wales. Disease epidemics are substantially less prevalent and severity of infection is lower in the central Queensland region, located in the semi-arid tropics. From a management perspective, this makes central Queensland a good option for clean seed production, which is a crucial factor in the control of a seed-borne disease.
Epidemiology of Pseudomonas savastanoi pv. phaseolicola
Pseudomonas savastanoi pv. phaseolicola comprises a broad array of distinct strains that vary in their epidemiology. By using disease reactions among a differential set of eight genotypes of common bean, nine distinct races were identified within a broad international collection of strains isolated from multiple legume species (Taylor et al. 1996). These differential reactions were attributed to the interactions of five pairs of avirulence (avr) and resistance (R) genes (Taylor et al. 1996). Races 2 and 7 were identified in Australia, isolated from purple bean (Macroptilium atropurpureum (DC.) Urb.) and perennial soybean (Neonotonia wightii (Wight&Arn.) J.A.Lackey) (Taylor et al. 1996). Race 7 has been further characterised in Australia infecting mungbeans, based on 30 isolates inoculated on the common bean differential set (Taylor et al. 1996; Ryley et al. 2010). Although currently there is no definitive mungbean differential set for halo blight, differences in pathogenicity among those isolates have been observed (Ryley et al. 2010). Twelve putative races have been identified, of which two strains are the most prevalent. These are referred to as the ‘T’ and ‘K’ strains, designated by the origin of the isolates initially characterised: T11544, isolated from mungbeans in Toowoomba in 2005; and K4287, isolated from mungbeans in Kingaroy in 2013. T11544 has been used in conventional resistance breeding, with some mungbean genotypes expressing moderate resistance (Ryley et al. 2010), whereas K4287 apparently overcomes all known resistance (Kelly 2016).
Seed-borne infections are recognised as the primary source of inoculum and play a major role in the long-distance dispersal of P. savastanoi pv. phaseolicola (Grogan and Kimble 1967; Taylor et al. 1979a). Seedlots with as little as 0.01% infected seed can lead to outbreaks of halo blight (Taylor 1970), alongside the capacity to transmit disease for up to 4 years in uncontrolled storage, and 6–10 years in controlled grain-storage facilities (7–10°C, 45–50% relative humidity) (Taylor et al. 1979a). The persistence of infection in seeds makes halo blight particularly difficult to manage (Taylor 1970). The provision of disease-free seed stocks and the use of sensitive diagnostic techniques are imperative to eliminating infected seed and reducing transmission.
Host–pathogen interactions
Although seed-borne inoculum is the main source of epidemics, bacteria readily enter through natural openings such as stomata and wounds (Taylor et al. 1979b). The bacteria then move into and proliferate in the apoplast (Melotto et al. 2008; Rufián et al. 2017). At this juncture, plants launch their first line of molecular defence, the innate immunity of each plant cell. Specialised receptors on the plant cell surface recognise molecular structures essential for a pathogen survival, such as flagellin, peptidoglycan, elongation factor and lipopolysaccharide. These are known as microbe-associated molecular patterns (MAMPs) (Jones and Dangl 2006; Newman et al. 2013). Surprisingly, little is known about MAMPs, their range and diversity, although molecular tools are rapidly advancing our knowledge. Further research into this area has been suggested with the aim to reveal novel antimicrobial agents through identification of MAMPs and their signalling pathways (Vidaver and Lambrecht 2004; McCann et al. 2012).
Many Gram-negative bacteria use quorum-sensing signalling molecules to overcome host defences. Using N-acyl homoserine lactones (AHLs) as signalling molecules, individual cells can sense population density before entering through external surfaces and releasing virulence factors (Cha et al. 1998). This has been shown to be a key virulence factor allowing bacteria to coordinate attacks and maximise the likelihood of success (von Bodman et al. 2003). A novel transcriptional regulator aefRNPS3121 identified in a mutant P. savastanoi pv. phaseolicola strain has been shown to regulate the synthesis of AHL and the induction of type III secretion system (T3SS) genes in response to cell density (Deng et al. 2009). The T3SS is an essential protein complex that facilitates transport of pathogenicity determinants such as pectinases, cellulases and proteases from the bacterium into the host. Thus, the quorum-sensing associated signalling and effector molecules directly affect the ability of the bacterium to infect its host.
Early genetic work on P. savastanoi pv. phaseolicola demonstrated many basic mechanisms of virulence. Transposon mutagenesis helped to define the role of hrp (hypersensitive reaction and pathogenicity) genes (Lindgren et al. 1986), which have since been extensively characterised as major contributors to the T3SS (Alfano and Collmer 1997). The capacity for P. savastanoi pv. phaseolicola to gain entry to its host and interfere with plant defences is dependent on the use of bacterial T3SS translocating effector proteins to the host cells (Vencato et al. 2006; Cunnac et al. 2009). Susceptibly in a host is caused by pathogen effectors mimicking hormones that induce the host to cease defence too early or produce the incorrect defence signals (Gimenez-Ibanez et al. 2014; Ma et al. 2015). Effector-triggered immunity results when a host plant recognises a bacterial effector through resistance (R) genes, commonly stimulating a rapid and amplified defence mechanism leading to localised cell death, known as the ‘hypersensitive response’ (Dangl and Jones 2001). A minor change in an avr gene producing the effectors, or the R genes recognising them, can have a profound effect on the interaction between a particular bacterial strain and plant species (Flor 1971; Collinge and Slusarenko 1987; Jackson and Taylor 1996). Identifying the avr genes present in the P. savastanoi pv. phaseolicola population infecting mungbeans and their associated R genes will be a valuable step towards controlling halo blight.
Plant pathogenic bacteria have a remarkable ability to manipulate their genomes to avoid host defence systems. They move DNA within and between bacterial genomes by means of mobile genetic elements such as plasmids, bacteriophages, integrons and transposons (Frost et al. 2005). Non-pathogenic strains of P. savastanoi pv. phaseolicola can gain virulence capabilities through persistent contact with pathogenic strains, as revealed by confocal microscopy (Rufián et al. 2017). Thus, continual monitoring of changes in the pathogen’s repertoire of effectors, and mining new sources for resistance, will be necessary to stay ahead of the rapidly evolving pathogen population.
Diagnosis of the halo blight pathogen
In Australia, mungbean seed-crop production currently relies heavily on the absence of observed symptoms in the field. The use of diagnostic assays to screen diseased mungbean material and seed for pathogenic bacteria has not yet been implemented. Investigating molecular technologies to detect P. savastanoi pv. phaseolicola in mungbean seed samples will have a major economic benefit to the industry, providing cleaner seed and stability in production.
In the later stages of infection, halo blight disease symptoms can be particularly difficult to identify on a visual basis. Lesions become indistinguishable from other necrotic leaf spots or natural senescence as the water-soaked lesions turn a dry papery brown and the yellow halo dissipates (Burkholder 1930). Serology, plate culture, microscopy and molecular diagnostics are used globally throughout the bean industry and overcome the disadvantages of visual identification (Guthrie et al. 1965; Vuurde et al. 1991; Prosen et al. 1993; Félix-Gastélum et al. 2016). The differing characteristics of the strains of P. savastanoi pv. phaseolicola offer several targets for specific diagnostic tools.
The primary target for molecular diagnostics is PCR amplification of the phaseolotoxin gene cluster (Prosen et al. 1993). However, phaseolotoxin-negative strains, such as are present in Spain, are undetectable by PCR targeting the phaseolotoxin gene cluster (Rico et al. 2003). Two genetic lineages, those with the tox gene cluster (tox+) and those without (tox–), have been definitively separated (Oguiza et al. 2004), and a multiplex PCR now capable of detecting both tox– and tox+ strains was developed (Rico et al. 2006).
Quantitative detection of plant pathogens through qPCR is a powerful technique using highly specific primers and fluorescent probes (Schaad and Frederick 2002). A hydrolysis-probe-based qPCR assay was designed to amplify both tox– and tox+ by targeting the cytochrome o ubiquinol oxidase subunit II gene. This had a reported detection limit of 4.5 × 103 colony forming units (CFU) mL–1 (Xu and Tambong 2011). Because this is a single-copy gene, it is equal to four and a half cells per reaction when 1 µL template is used. A similar assay, targeting a site-specific recombinase gene, reported a detection limit of 7 CFU per reaction (Seok Cho et al. 2010).
An alternative diagnostic assay that can be deployed in the field is loop-mediated isothermal amplification (LAMP). By using a combination of six specially designed primers, amplification takes place under isothermal conditions (Notomi et al. 2000). A LAMP protocol has been established to detect P. savastanoi pv. phaseolicola and could serve as a rapid protocol for identification and detection of the bacteria in samples away from the laboratory (Li et al. 2009).
Digital droplet PCR (ddPCR) is a recent technology that provides both detection and quantification. Unlike other methods, ddPCR does not require inclusion of standards of known concentration to achieve quantification (Huggett and Whale 2013). The technology is also reported to deal better with environmental samples and inhibitors than qPCR, making it an ideal choice for seed testing (Dingle et al. 2013). Although there are no reported ddPCR primers for P. savastanoi pv. phaseolicola, the technology has been used to detect and provide quantification of other important Gram-negative plant bacterial pathogens such as Erwinia amylovora and Ralstonia solanacearum (Dreo et al. 2014). Current qPCR primers specific to P. savastanoi pv. phaseolicola are expected to be compatible with the ddPCR platform. A comprehensive set of reported PCR primers specific to P. savastanoi. pv. phaseolicola (Table 2) could be trialled to test their usefulness in identifying strains infecting Australian mungbeans.

|
Management strategies
Bacterial seed-borne diseases continue to have outbreaks causing significant economic loss due to limited management options. It is extremely difficult to control bacterial pathogens such as P. savastanoi. pv. phaseolicola that have a wide host range encompassing the majority of the Fabaceae family, including weed species (Table 1). To develop an effective strategy to manage bacterial disease, an integrated approach is essential, taking into consideration conducive conditions, cultural practices, chemical options, seed source and crop susceptibility. Although halo blight inoculum is primarily introduced though infected seed, cultural practices greatly influence the development and spread of the disease. Crop rotation, removal of crop debris and volunteers, weed control, tilling, restricting movement though paddocks especially during wet conditions, as well as thoroughly washing and disinfecting machinery play important roles in controlling disease outbreaks (Hall and Nasser 1996). Movement through paddocks should be carefully considered because crops that appear symptomless may harbour large populations of epiphytically infected plants, after which mechanical damage will allow these epiphytes to enter freely and cause disease (Marques and Samson 2016a).
Streptomycin, kanamycin and copper oxychloride have previously been used as foliar sprays and seed treatments by the American bean industry, but are not a viable option in Australia owing to regulations prohibiting their use on plant crops, poor efficacy and uneconomical application regimes (Taylor 1972; Taylor and Dudley 1977; Sundin et al. 1994). Thermotherapy using hot air or water to kill pathogens is an unexplored area in Australia; however, reported premature germination and reduced shelf life of planting seed are adverse effects to be considered (Grondeau et al. 2011). Effective management of halo blight will involve a greater emphasis on cultural practices, a better understanding of infection pathways from alternative hosts, production and maintenance of certified disease-free seed and ultimately production of varieties that have increased resistance to the disease (Taylor 1970; Taylor et al. 1979b; Bastas and Sahin 2017).
Strict seed-production protocols are the first line of defence against seed-borne bacterial diseases. Seed crops should be grown in climates and locations non-conducive to pathogens, under drip irrigation to limit the amount of free moisture, and far removed from commercial crops (Grogan and Kimble 1967; Webster et al. 1983; Gitaitis and Walcott 2007). Confirming the presence of pathogens in planting seed by using diagnostic assays such as serology, culturing and PCR further reduces the risk of epidemics (Vuurde et al. 1991; Prosen et al. 1993; Marques et al. 2000; Rico et al. 2006; Xu and Tambong 2011). Ultimately, managing the transmission of seed-borne diseases relies on precise identification of the target pathogens in planting seed and the development of targeted resistance to the pathogens within the host species (Bastas and Sahin 2017). Ensuring that seed is of the highest quality and free of disease through a rigorously upheld seed scheme is a key factor to reducing the impact of bacterial disease (Gitaitis and Walcott 2007).
A seed scheme implemented in 1998 by the Australian Mungbean Association requires all seed crops to be visually inspected for disease by a third-party seed inspector (AMA 2015). However, it is unlikely that the current implementation of this strategy is having the desired impact on disease prevention, with severe epidemics seen in Australia on a yearly basis (Ryley et al. 2010). Sole reliance on visual field inspections to produce clean seed can lead to epidemics, owing to fundamental characteristics of the halo blight pathogen. As an asymptomatic epiphyte able to survive on the outer surface of a plant, P. savastanoi. pv. phaseolicola becomes pathogenic only under favourable environmental conditions (Grogan and Kimble 1967; Legard and Schwartz 1987; Niknejad Kazempour 2002; Marques and Samson 2016b). Rainfall volume and intensity is thought to induce disease symptoms by driving the bacterium into the apoplast of the leaves where it has the optimum environment and nutrients available for growth (Marques and Samson 2016b). This potential for latent infections severely compromises the efficacy of certification schemes based only on visual symptoms.
For most of its cultivated history, mungbean has been grown by subsistence agriculture relying on conventional plant-breeding techniques (Fernandez et al. 1988). These techniques are still employed today in both developing and developed countries, although the transition towards molecular-based breeding is moving at a fast pace as technologies mature and costs fall (Chen et al. 2013; Schafleitner et al. 2015; Noble et al. 2018). With the increased adoption of genomic technologies to characterise and select germplasm, researchers are discovering untapped resources of genetically diverse material that may contain unique alleles for disease resistance (Lawn and Rebetzke 2006; Schafleitner et al. 2015; Noble et al. 2018).
The gene-for-gene interactions between P. savastanoi. pv. phaseolicola and its hosts make it ideal for marker-assisted selection, because resistance is commonly conferred by a single, large-effect quantitative trait locus (QTL) (Jenner et al. 1991; Stevens et al. 1998; Tsiamis et al. 2000). Establishing these marker associations in mungbean will be accomplished through genome-wide association studies comprising large, diverse mungbean mapping populations representative of worldwide germplasm (Schafleitner et al. 2015; Noble et al. 2018). To limit the effect of major R genes breaking down over time, implementation of genomic selection in breeding programs will help to ensure that small-effect genes are also incorporated into new cultivars (Jannink et al. 2010). This will accelerate the breeding cycle by replacing lengthy phenotypic evaluation with models able to predict the breeding value of lines by incorporating phenotypic data and high-density markers (Nair et al. 2012; Chen et al. 2013; Dhole and Reddy 2013).
Conclusions and recommendations
Halo blight is a destructive disease in mungbean crops throughout Australia and is emerging globally in other production areas. Eliminating infected seed and developing resistant commercial cultivars are the key strategies required to control halo blight. Adoption of the methods discussed in this review will have a substantial impact on reducing the incidence and severity of halo blight in mungbean-cropping regions.
In the short term, P. savastanoi pv. phaseolicola isolated from mungbean plants across a broad range of Australian growing regions should be screened against the PCR assays listed in Table 2. This would provide foundational molecular tools to identify and screen for the disease. Development of a diagnostic assay for seedlots would have a beneficial effect on monitoring and controlling the spread of halo blight. Whole-genome sequencing of unique strains will reveal unique targets to develop diagnostic assays able to differentiate between strains endemic to a particular region. These assays would provide further sensitivity in identification, and surveillance of population dynamics. Understanding of which strains are present on particular mungbean genotypes in particular regions and years, and under which specific environmental conditions, will inform and direct pathology research.
Development and implementation of genomic tools will be required to support sustainable resistance breeding in the long term. Genome-wide association studies will identify regions of the mungbean genome related to disease resistance. Once these associations have been made, the identified markers could be used to guide introgression of disease resistance into genetically favourable backgrounds. Having the means to edit genomes directly with breakthrough technologies such as CRISPR will further reduce the timeframe from gene discovery to cultivars possessing traits of interest (Dangl et al. 2013). Sequencing collections of mungbean accessions in combination with genome-editing tools could see stable resistance introduced into mungbean with greater accuracy and speed.
No single strategy will be the answer. An integrated approach that continues to address all of these areas will be needed to overcome halo blight in mungbeans.
Conflicts of interest
The authors declare that there are no conflicts of interest.
Acknowledgements
This work was supported by an Australian Mungbean Association PhD scholarship (2017000885). The authors acknowledge the Centre for Tropical Crops and Biocommodities, Queensland University of Technology, Brisbane, Qld, and the Australian Mungbean Association for providing funding to complete this work under the PhD scholarship.
References
Aguilera S, Alvarez-Morales A, Murillo J, Hernandez-Flores JL, Bravo J, De la Torre-Zavala S (2017) Temperature-mediated biosynthesis of the phytotoxin phaseolotoxin by Pseudomonassyringae pv. phaseolicola depends on the autoregulated expression of the phtABC genes. PLoS One 12, e0178441| Temperature-mediated biosynthesis of the phytotoxin phaseolotoxin by Pseudomonassyringae pv. phaseolicola depends on the autoregulated expression of the phtABC genes.Crossref | GoogleScholarGoogle Scholar | 28570637PubMed |
Akhtar MA (1988) Pseudomonas syringae pv. phaseolicola on mungbean. Pakistan Journal of Agricultural Research 9, 424–426.
Alfano JR, Collmer A (1997) The type III (Hrp) secretion pathway of plant pathogenic bacteria: trafficking harpins, Avr proteins, and death. Journal of Bacteriology 179, 5655–5662.
| The type III (Hrp) secretion pathway of plant pathogenic bacteria: trafficking harpins, Avr proteins, and death.Crossref | GoogleScholarGoogle Scholar | 9294418PubMed |
AMA (2015) About us. Australian Mungbean Association. Available at: http://www.mungbean.org.au/about-us.html#industry-size-and-value (accessed 15 October 2018).
Arnold DL, Gibbon MJ, Jackson RW, Wood JR, Brown J, Mansfield JW, Taylor JD, Vivian A (2001) Molecular characterization of avrPphD, a widely-distributed gene from Pseudomonas syringae pv. phaseolicola involved in non-host recognition by pea (Pisum sativum). Physiological and Molecular Plant Pathology 58, 55–62.
| Molecular characterization of avrPphD, a widely-distributed gene from Pseudomonas syringae pv. phaseolicola involved in non-host recognition by pea (Pisum sativum).Crossref | GoogleScholarGoogle Scholar |
Arnold DL, Lovell HC, Jackson RW, Mansfield JW (2011) Pseudomonas syringae pv. phaseolicola: from ‘has bean’ to supermodel. Molecular Plant Pathology 12, 617–627.
| Pseudomonas syringae pv. phaseolicola: from ‘has bean’ to supermodel.Crossref | GoogleScholarGoogle Scholar | 21726364PubMed |
Audy P, Braat CE, Saindon G, Huang HC, Laroche A (1996) A rapid and sensitive PCR-based assay for concurrent detection of bacteria causing common and halo blights in bean seed. Phytopathology 86, 361–366.
| A rapid and sensitive PCR-based assay for concurrent detection of bacteria causing common and halo blights in bean seed.Crossref | GoogleScholarGoogle Scholar |
Bastas KK, Sahin F (2017) Evaluation of seedborne bacterial pathogens on common bean cultivars grown in central Anatolia region, Turkey. European Journal of Plant Pathology 147, 239–253.
| Evaluation of seedborne bacterial pathogens on common bean cultivars grown in central Anatolia region, Turkey.Crossref | GoogleScholarGoogle Scholar |
Burkholder WH (1926) A new bacterial disease of the bean. Phytopathology 16, 915–927.
Burkholder WH (1930) The genus Phytomonas. Phytopathology 20, 1–23.
Cha C, Gao P, Chen Y-C, Shaw PD, Farrand SK (1998) Production of acyl-homoserine lactone quorum-sensing signals by gram-negative plant-associated bacteria. Molecular Plant-Microbe Interactions 11, 1119–1129.
| Production of acyl-homoserine lactone quorum-sensing signals by gram-negative plant-associated bacteria.Crossref | GoogleScholarGoogle Scholar | 9805399PubMed |
Chen H-M, Ku H-M, Schafleitner R, Bains TS, George Kuo C, Liu C-A, Nair RM (2013) The major quantitative trait locus for mungbean yellow mosaic Indian virus resistance is tightly linked in repulsion phase to the major bruchid resistance locus in a cross between mungbean [Vigna radiata (L.) Wilczek] and its wild relative Vigna radiata ssp. sublobata. Euphytica 192, 205–216.
| The major quantitative trait locus for mungbean yellow mosaic Indian virus resistance is tightly linked in repulsion phase to the major bruchid resistance locus in a cross between mungbean [Vigna radiata (L.) Wilczek] and its wild relative Vigna radiata ssp. sublobata.Crossref | GoogleScholarGoogle Scholar |
Collinge DB, Slusarenko AJ (1987) Plant gene expression in response to pathogens. Plant Molecular Biology 9, 389–410.
| Plant gene expression in response to pathogens.Crossref | GoogleScholarGoogle Scholar | 24277091PubMed |
Cunnac S, Lindeberg M, Collmer A (2009) Pseudomonas syringae type III secretion system effectors: repertoires in search of functions. Current Opinion in Microbiology 12, 53–60.
| Pseudomonas syringae type III secretion system effectors: repertoires in search of functions.Crossref | GoogleScholarGoogle Scholar | 19168384PubMed |
Dangl JL, Jones JDG (2001) Plant pathogens and integrated defence responses to infection. Nature 411, 826–833.
| Plant pathogens and integrated defence responses to infection.Crossref | GoogleScholarGoogle Scholar | 11459065PubMed |
Dangl JL, Horvath DM, Staskawicz BJ (2013) Pivoting the plant immune system from dissection to deployment. Science 341, 746–751.
| Pivoting the plant immune system from dissection to deployment.Crossref | GoogleScholarGoogle Scholar | 23950531PubMed |
Day L (2013) Proteins from land plants—potential resources for human nutrition and food security. Trends in Food Science & Technology 32, 25–42.
| Proteins from land plants—potential resources for human nutrition and food security.Crossref | GoogleScholarGoogle Scholar |
Deng X, Xiao Y, Lan L, Zhou J-M, Tang X (2009) Pseudomonas syringae pv. phaseolicola mutants compromised for type III secretion system gene induction. Molecular Plant-Microbe Interactions 22, 964–976.
| Pseudomonas syringae pv. phaseolicola mutants compromised for type III secretion system gene induction.Crossref | GoogleScholarGoogle Scholar | 19589072PubMed |
Dhole VJ, Reddy KS (2013) Development of a SCAR marker linked with a MYMV resistance gene in mungbean (Vigna radiata L. Wilczek). Plant Breeding 132, 127–132.
| Development of a SCAR marker linked with a MYMV resistance gene in mungbean (Vigna radiata L. Wilczek).Crossref | GoogleScholarGoogle Scholar |
Dingle TC, Sedlak RH, Cook L, Jerome KR (2013) Tolerance of droplet-digital PCR vs real-time quantitative PCR to inhibitory substances. Clinical Chemistry 59, 1670–1672.
| Tolerance of droplet-digital PCR vs real-time quantitative PCR to inhibitory substances.Crossref | GoogleScholarGoogle Scholar | 24003063PubMed |
Dowson WJ (1943) On the generic names Pseudomonas, Xanthomonas and Bacterium for certain bacterial plant pathogens. Transactions of the British Mycological Society 26, 4–14.
| On the generic names Pseudomonas, Xanthomonas and Bacterium for certain bacterial plant pathogens.Crossref | GoogleScholarGoogle Scholar |
Dreo T, Pirc M, Ramšak Ž, Pavšič J, Milavec M, Žel J, Gruden K (2014) Optimising droplet digital PCR analysis approaches for detection and quantification of bacteria: a case study of fire blight and potato brown rot. Analytical and Bioanalytical Chemistry 406, 6513–6528.
| Optimising droplet digital PCR analysis approaches for detection and quantification of bacteria: a case study of fire blight and potato brown rot.Crossref | GoogleScholarGoogle Scholar | 25173868PubMed |
Félix-Gastélum R, Maldonado-Mendoza IE, Navarrete-Maya R, Olivas-Peraza NG, Brito-Vega H, Acosta-Gallegos JA (2016) Identification of Pseudomonas syringae pv. phaseolicola as the causal agent of halo blight in yellow beans in northern Sinaloa, Mexico. Phytoparasitica 44, 369–378.
| Identification of Pseudomonas syringae pv. phaseolicola as the causal agent of halo blight in yellow beans in northern Sinaloa, Mexico.Crossref | GoogleScholarGoogle Scholar |
Fernandez G, Shanmugasundaram S, McLean B (1988) The AVRDC mungbean improvement program: the past, present and future. In ‘Mungbean. Proceedings Second International Symposium’. Bangkok. (Asian Vegetable Research and Development Center: Tainan, Taiwan)
Fernández-Sanz AM, Rodicio MR, González AJ (2016) Pseudomonas syringae pv. phaseolicola isolated from weeds in bean crop fields. Letters in Applied Microbiology 62, 344–348.
| Pseudomonas syringae pv. phaseolicola isolated from weeds in bean crop fields.Crossref | GoogleScholarGoogle Scholar | 26880144PubMed |
Flor HH (1971) Current status of the gene-for-gene concept. Annual Review of Phytopathology 9, 275–296.
| Current status of the gene-for-gene concept.Crossref | GoogleScholarGoogle Scholar |
Frost LS, Leplae R, Summers AO, Toussaint A (2005) Mobile genetic elements: the agents of open source evolution. Nature Reviews. Microbiology 3, 722
| Mobile genetic elements: the agents of open source evolution.Crossref | GoogleScholarGoogle Scholar | 16138100PubMed |
Gardan L, Bollet C, Abu Ghorrah M, Grimont F, Grimont PAD (1992) DNA relatedness among the pathovar strains of Pseudomonas syringae subsp. savastanoi Janse (1982) and proposal of Pseudomonas savastanoi sp. nov. International Journal of Systematic Bacteriology 42, 606–612.
| DNA relatedness among the pathovar strains of Pseudomonas syringae subsp. savastanoi Janse (1982) and proposal of Pseudomonas savastanoi sp. nov.Crossref | GoogleScholarGoogle Scholar |
Gimenez-Ibanez S, Boter M, Fernández-Barbero G, Chini A, Rathjen JP, Solano R (2014) The bacterial effector HopX1 targets JAZ transcriptional repressors to activate jasmonate signaling and promote infection in Arabidopsis. PLoS Biology 12, e1001792
| The bacterial effector HopX1 targets JAZ transcriptional repressors to activate jasmonate signaling and promote infection in Arabidopsis.Crossref | GoogleScholarGoogle Scholar | 24558350PubMed |
Gitaitis R, Walcott R (2007) The epidemiology and management of seedborne bacterial diseases. Annual Review of Phytopathology 45, 371–397.
| The epidemiology and management of seedborne bacterial diseases.Crossref | GoogleScholarGoogle Scholar | 17474875PubMed |
González AI, De la Vega MP, Ruiz ML, Polanco C (2003) Analysis of the argK-tox gene cluster in nontoxigenic strains of Pseudomonas syringae pv. phaseolicola. Applied and Environmental Microbiology 69, 4979–4982.
| Analysis of the argK-tox gene cluster in nontoxigenic strains of Pseudomonas syringae pv. phaseolicola.Crossref | GoogleScholarGoogle Scholar | 12902295PubMed |
Grogan RG, Kimble KA (1967) The role of seed contamination in the transmission of Pseudomonas phaseolicola in Phaseolus vulgaris. Phytopathology 57, 28–31.
Grondeau C, Samson R, Sands DDC (2011) A review of thermotherapy to free plant materials from pathogens, especially seeds from bacteria. Critical Reviews in Plant Sciences 13, 57–75.
| A review of thermotherapy to free plant materials from pathogens, especially seeds from bacteria.Crossref | GoogleScholarGoogle Scholar |
Guthrie JW, Huber DM, Fenwick HS (1965) Serological detection of halo blight. The Plant Disease Reporter 49, 297–299.
Hall R, Nasser LCB (1996) Practice and precept in cultural management of bean diseases. Canadian Journal of Plant Pathology 18, 176–185.
| Practice and precept in cultural management of bean diseases.Crossref | GoogleScholarGoogle Scholar |
Huggett JF, Whale A (2013) Digital PCR as a novel technology and its potential implications for molecular diagnostics. Clinical Chemistry 59, 1691–1693.
| Digital PCR as a novel technology and its potential implications for molecular diagnostics.Crossref | GoogleScholarGoogle Scholar | 24100808PubMed |
Jackson AO, Taylor CB (1996) Plant–microbe interactions: life and death at the interface. The Plant Cell 8, 1651–1668.
| Plant–microbe interactions: life and death at the interface.Crossref | GoogleScholarGoogle Scholar | 12239356PubMed |
Jannink J-L, Lorenz AJ, Iwata H (2010) Genomic selection in plant breeding: from theory to practice. Briefings in Functional Genomics 9, 166–177.
| Genomic selection in plant breeding: from theory to practice.Crossref | GoogleScholarGoogle Scholar | 20156985PubMed |
Jenner C, Hitchin E, Mansfield J, Walters K, Betteridge P, Teverson D, Taylor J (1991) Gene-for-gene interactions between Pseudomonas syringae pv. phaseolicola and Phaseolus. Molecular Plant-Microbe Interactions 4, 553–562.
| Gene-for-gene interactions between Pseudomonas syringae pv. phaseolicola and Phaseolus.Crossref | GoogleScholarGoogle Scholar | 1666524PubMed |
Jones J, Dangl J (2006) The plant immune system. Nature 444, 323–329.
| The plant immune system.Crossref | GoogleScholarGoogle Scholar | 17108957PubMed |
Kelly L (2016) Management of the major mungbean diseases in Australia. GRDC Update Papers, 21 June 2016. Available at: https://grdc.com.au/resources-and-publications/grdc-update-papers/tab-content/grdc-update-papers/2016/06/management-of-the-major-mungbean-diseases-in-australia (accessed 19 February 2018).
Kim SK, Nair RM, Lee J, Lee SH (2015) Genomic resources in mungbean for future breeding programs. Frontiers of Plant Science 6, 626
| Genomic resources in mungbean for future breeding programs.Crossref | GoogleScholarGoogle Scholar |
Lawn RJ, Rebetzke GJ (2006) Variation among Australian accessions of the wild mungbean (Vigna radiata ssp. sublobata) for traits of agronomic, adaptive, or taxonomic interest. Australian Journal of Agricultural Research 57, 119–132.
| Variation among Australian accessions of the wild mungbean (Vigna radiata ssp. sublobata) for traits of agronomic, adaptive, or taxonomic interest.Crossref | GoogleScholarGoogle Scholar |
Legard D, Schwartz H (1987) Sources and management of Pseudomonas syringae pv. phaseolicola and Pseudomonas syringae pv. syringae epiphytes on dry beans in Colorado. Phytopathology 77, 1503–1509.
| Sources and management of Pseudomonas syringae pv. phaseolicola and Pseudomonas syringae pv. syringae epiphytes on dry beans in Colorado.Crossref | GoogleScholarGoogle Scholar |
Li X, Nie J, Ward L, Madani M, Hsiang T, Zhao Y, De Boer SH (2009) Comparative genomics-guided loop-mediated isothermal amplification for characterization of Pseudomonas syringae pv. phaseolicola. Journal of Applied Microbiology 107, 717–726.
| Comparative genomics-guided loop-mediated isothermal amplification for characterization of Pseudomonas syringae pv. phaseolicola.Crossref | GoogleScholarGoogle Scholar | 19486391PubMed |
Lindgren PB, Peet RC, Panopoulos NJ (1986) Gene cluster of Pseudomonas syringae pv. ‘phaseolicola’ controls pathogenicity of bean plants and hypersensitivity on nonhost plants. Journal of Bacteriology 168, 512–522.
| Gene cluster of Pseudomonas syringae pv. ‘phaseolicola’ controls pathogenicity of bean plants and hypersensitivity on nonhost plants.Crossref | GoogleScholarGoogle Scholar | 3023280PubMed |
Ma KW, Jiang S, Hawara E, Lee D, Pan S, Coaker G, Song J, Ma W (2015) Two serine residues in Pseudomonas syringae effector HopZ1a are required for acetyltransferase activity and association with the host co-factor. New Phytologist 208, 1157–1168.
| Two serine residues in Pseudomonas syringae effector HopZ1a are required for acetyltransferase activity and association with the host co-factor.Crossref | GoogleScholarGoogle Scholar | 26103463PubMed |
Marques ASdA, Samson R (2016a) Population dynamics of Pseudomonas savastanoi pv. phaseolicola in bean, throughout the epiphytic and pathogenic phases. Pesquisa Agropecuária Brasileira 51, 623–630.
| Population dynamics of Pseudomonas savastanoi pv. phaseolicola in bean, throughout the epiphytic and pathogenic phases.Crossref | GoogleScholarGoogle Scholar |
Marques ASdA, Samson R (2016b) Population dynamics of Pseudomonas savastanoi pv. phaseolicola in bean, throughout the epiphytic and pathogenic phases. Pesquisa Agropecuária Brasileira 51, 623–630.
| Population dynamics of Pseudomonas savastanoi pv. phaseolicola in bean, throughout the epiphytic and pathogenic phases.Crossref | GoogleScholarGoogle Scholar |
Marques ASdA, Corbière R, Gardan L, Tourte C, Manceau C, Taylor JD, Samson R (2000) Multiphasic approach for the identification of the different classification levels of Pseudomonas savastanoi pv. phaseolicola. European Journal of Plant Pathology 106, 715–734.
| Multiphasic approach for the identification of the different classification levels of Pseudomonas savastanoi pv. phaseolicola.Crossref | GoogleScholarGoogle Scholar |
McCann HC, Nahal H, Thakur S, Guttman DS (2012) Identification of innate immunity elicitors using molecular signatures of natural selection. Proceedings of the National Academy of Sciences of the United States of America 109, 4215–4220.
| Identification of innate immunity elicitors using molecular signatures of natural selection.Crossref | GoogleScholarGoogle Scholar | 22323605PubMed |
Melotto M, Underwood W, Sheng YH (2008) Role of stomata in plant innate immunity and foliar bacterial diseases. Annual Review of Phytopathology 46, 101–122.
| Role of stomata in plant innate immunity and foliar bacterial diseases.Crossref | GoogleScholarGoogle Scholar | 18422426PubMed |
Nair RM, Schafleitner R, Kenyon L, Srinivasan R, Easdown W, Ebert AW, Hanson P (2012) Genetic improvement of mungbean. SABRAO Journal of Breeding and Genetics 44, 177–190.
Newman M-A, Sundelin T, Nielsen JT, Erbs G (2013) MAMP (microbe-associated molecular pattern) triggered immunity in plants. Frontiers of Plant Science 4, 139
| MAMP (microbe-associated molecular pattern) triggered immunity in plants.Crossref | GoogleScholarGoogle Scholar |
Niknejad Kazempour M (2002) Study of the impact of some virulence genes on the epiphytic fitness. Journal of Water and Soil Science 6, 219–229.
Noble TJ, Tao Y, Mace ES, Williams B, Jordan DR, Douglas CA, Mundree SG (2018) Characterization of linkage disequilibrium and population structure in a mungbean diversity panel. Frontiers of Plant Science 8, 2102
| Characterization of linkage disequilibrium and population structure in a mungbean diversity panel.Crossref | GoogleScholarGoogle Scholar |
Notomi T, Okayama H, Masubuchi H, Yonekawa T, Watanabe K, Amino N, Hase T (2000) Loop-mediated isothermal amplification of DNA. Nucleic Acids Research 28, e63
| Loop-mediated isothermal amplification of DNA.Crossref | GoogleScholarGoogle Scholar | 10871386PubMed |
Nüske J, Fritsche W (1989) Phaseolotoxin production by Pseudomonas syringae pv. phaseolicola: The influence of temperature. Journal of Basic Microbiology 29, 441–447.
| Phaseolotoxin production by Pseudomonas syringae pv. phaseolicola: The influence of temperature.Crossref | GoogleScholarGoogle Scholar | 2600779PubMed |
Oguiza JA, Rico A, Rivas LA, Sutra L, Vivian A, Murillo J (2004) Pseudomonas syringae pv. phaseolicola can be separated into two genetic lineages distinguished by the possession of the phaseolotoxin biosynthetic cluster. Microbiology 150, 473–482.
| Pseudomonas syringae pv. phaseolicola can be separated into two genetic lineages distinguished by the possession of the phaseolotoxin biosynthetic cluster.Crossref | GoogleScholarGoogle Scholar | 14766926PubMed |
Patil S, Hayward A, Emmons R (1974) An ultraviolet-induced nontoxigenic mutant of Pseudomonas phaseolicola of altered pathogenicity. Phytopathology 64, 590
| An ultraviolet-induced nontoxigenic mutant of Pseudomonas phaseolicola of altered pathogenicity.Crossref | GoogleScholarGoogle Scholar |
Prosen D, Hatziloukas E, Schaad NW, Panopoulos NJ (1993) Specific detection Pseudomonas syringae DNA in bean seed by PCR-based amplification of a phaseolotoxin gene region. Phytopathology 83, 965–970.
| Specific detection Pseudomonas syringae DNA in bean seed by PCR-based amplification of a phaseolotoxin gene region.Crossref | GoogleScholarGoogle Scholar |
Rico A, López R, Asensio C, Aizpún MT, Asensio-S-Manzanera MC, Murillo J (2003) Nontoxigenic strains of Pseudomonas syringae pv. phaseolicola are a main cause of halo blight of beans in Spain and escape current detection methods. Phytopathology 93, 1553–1559.
| Nontoxigenic strains of Pseudomonas syringae pv. phaseolicola are a main cause of halo blight of beans in Spain and escape current detection methods.Crossref | GoogleScholarGoogle Scholar | 18943619PubMed |
Rico A, Erdozáin M, Ortiz-Barredo A, Ruiz De Galarreta JI, Murillo J (2006) Short communication. Detection by multiplex PCR and characterization of nontoxigenic strains of Pseudomonas syringae pv. phaseolicola from different places in Spain. Spanish Journal of Agricultural Research 4, 261–267.
| Short communication. Detection by multiplex PCR and characterization of nontoxigenic strains of Pseudomonas syringae pv. phaseolicola from different places in Spain.Crossref | GoogleScholarGoogle Scholar |
Rufián JS, Macho AP, Corry DS, Mansfield JW, Ruiz-Albert J, Arnold DL, Beuzón CR (2017) Confocal microscopy reveals in planta dynamic interactions between pathogenic, avirulent and non-pathogenic Pseudomonas syringae strains. Molecular Plant Pathology 19, 537–551.
| Confocal microscopy reveals in planta dynamic interactions between pathogenic, avirulent and non-pathogenic Pseudomonas syringae strains.Crossref | GoogleScholarGoogle Scholar | 28120374PubMed |
Ryley M, Tatnell J (2011) Management of the major foliar diseases of mungbeans and peanuts in Australia. In ‘Proceedings 4th Asian Conference on Plant Pathology and 18th Biennial Australasian Plant Pathology Society Conference’. 26–29 April, Darwin, NT. (Australasian Plant Pathology Society) Available at: https://eprints.usq.edu.au/25323/1/APPS2011.pdf (accessed 13 September 2017)
Ryley M, Douglas C, Ryan M, Tatnell J, Martin W, King K, Keller L (2010) Integrated management of foliar pathogens of mungbean in Australia. In ‘Proceedings 1st Australian Summer Grains Conference’. Gold Coast, Qld. pp. 1–9. (Grains Research and Development Corporation: Canberra, ACT)
Schaad NW, Frederick RD (2002) Real-time PCR and its application for rapid plant disease diagnostics. Canadian Journal of Plant Pathology 24, 250–258.
| Real-time PCR and its application for rapid plant disease diagnostics.Crossref | GoogleScholarGoogle Scholar |
Schaad NW, Cheong SS, Tamaki S, Hatziloukas E, Panopoulos NJ (1995) A combined biological and enzymatic amplification (BIO-PCR) technique to detect Pseudomonas syringae pv. phaseolicola in bean seed extracts. Phytopathology 85, 243–248.
| A combined biological and enzymatic amplification (BIO-PCR) technique to detect Pseudomonas syringae pv. phaseolicola in bean seed extracts.Crossref | GoogleScholarGoogle Scholar |
Schaad NW, Berthier-Schaad Y, Knorr D (2007) A high throughput membrane BIO-PCR technique for ultra-sensitive detection of Pseudomonas syringae pv. phaseolicola. Plant Pathology 56, 1–8.
| A high throughput membrane BIO-PCR technique for ultra-sensitive detection of Pseudomonas syringae pv. phaseolicola.Crossref | GoogleScholarGoogle Scholar |
Schafleitner R, Nair RM, Rathore A, Wang YW, Lin CY, Chu SH, Lin PY, Chang JC, Ebert AW (2015) The AVRDC—The World Vegetable Center mungbean (Vigna radiata) core and mini core collections. BMC Genomics 16, 344
| The AVRDC—The World Vegetable Center mungbean (Vigna radiata) core and mini core collections.Crossref | GoogleScholarGoogle Scholar | 25925106PubMed |
Senaratne R, Gunasekera MTK (1994) Nitrogen-fixation, growth and yield of intercropped mungbean (Vigna radiata L.) and groundnut (Arachis hypogaea L.) as affected by the genotype. Journal of Agronomy & Crop Science 173, 53–60.
| Nitrogen-fixation, growth and yield of intercropped mungbean (Vigna radiata L.) and groundnut (Arachis hypogaea L.) as affected by the genotype.Crossref | GoogleScholarGoogle Scholar |
Seok Cho M, Jeon YH, Jung Kang M, Ahn HI, Baek HJ, Wang Na Y, Mi Choi Y, San Kim T, Suk Park D (2010) Sensitive and specific detection of phaseolotoxigenic and nontoxigenic strains of Pseudomonas syringae pv. phaseolicola by TaqMan real-time PCR using site-specific recombinase gene sequences. Microbiological Research 165, 565–572.
| Sensitive and specific detection of phaseolotoxigenic and nontoxigenic strains of Pseudomonas syringae pv. phaseolicola by TaqMan real-time PCR using site-specific recombinase gene sequences.Crossref | GoogleScholarGoogle Scholar |
Sherf AF, MacNab AA (1986) ‘Vegetable diseases and their control.’ (John Wiley & Sons: New York)
Stevens C, Bennett MA, Athanassopoulos E, Tsiamis G, Taylor JD, Mansfield JW (1998) Sequence variations in alleles of the avirulence gene avrPphE.R2 from Pseudomonas syringae pv. phaseolicola lead to loss of recognition of the AvrPphE protein within bean cells and a gain in cultivar-specific virulence. Molecular Microbiology 29, 165–177.
| Sequence variations in alleles of the avirulence gene avrPphE.R2 from Pseudomonas syringae pv. phaseolicola lead to loss of recognition of the AvrPphE protein within bean cells and a gain in cultivar-specific virulence.Crossref | GoogleScholarGoogle Scholar | 9701811PubMed |
Sun S, Zhi Y, Zhu Z, Jin J, Duan C, Wu X, Xiaoming W (2017) An emerging disease caused by Pseudomonas syringae pv. phaseolicola threatens mung bean production in China. Plant Disease 101, 95–102.
| An emerging disease caused by Pseudomonas syringae pv. phaseolicola threatens mung bean production in China.Crossref | GoogleScholarGoogle Scholar | 30682319PubMed |
Sundin GW, Demezas DH, Bender CL (1994) Genetic and plasmid diversity within natural populations of Pseudomonas syringae with various exposures to copper and streptomycin bactericides. Applied and Environmental Microbiology 60, 4421–4431.
Taylor JD (1970) The quantitative estimation of the infection of bean seed with Pseudomonas phaseolicola (Burkh.) Dowson. Annals of Applied Biology 66, 29–36.
| The quantitative estimation of the infection of bean seed with Pseudomonas phaseolicola (Burkh.) Dowson.Crossref | GoogleScholarGoogle Scholar |
Taylor JD (1972) Field studies on halo-blight of beans (Pseudomonas phaseolicola) and its control by foliar sprays. Annals of Applied Biology 70, 191–197.
| Field studies on halo-blight of beans (Pseudomonas phaseolicola) and its control by foliar sprays.Crossref | GoogleScholarGoogle Scholar |
Taylor JD, Dudley CL (1977) Seed treatment for the control of halo-blight of beans (Pseudomonas phaseolicola). Annals of Applied Biology 85, 223–232.
| Seed treatment for the control of halo-blight of beans (Pseudomonas phaseolicola).Crossref | GoogleScholarGoogle Scholar |
Taylor JD, Dudley CL, Presly L (1979a) Studies of halo blight seed infection and disease transmission in dwarf beans. Annals of Applied Biology 93, 267–277.
| Studies of halo blight seed infection and disease transmission in dwarf beans.Crossref | GoogleScholarGoogle Scholar |
Taylor JD, Phelps K, Dudley CL (1979b) Epidemiology and strategy for the control of halo-blight of beans. Annals of Applied Biology 93, 167–172.
| Epidemiology and strategy for the control of halo-blight of beans.Crossref | GoogleScholarGoogle Scholar |
Taylor JD, Teverson DM, Allen DJ, Pastor Corrales MA (1996) Identification and origin of races of Pseudomonas syringae pv. phaseolicola from Africa and other bean growing areas. Plant Pathology 45, 469–478.
| Identification and origin of races of Pseudomonas syringae pv. phaseolicola from Africa and other bean growing areas.Crossref | GoogleScholarGoogle Scholar |
Tsiamis G, Mansfield JW, Hockenhull R, Jackson RW, Sesma A, Athanassopoulos E, Bennett MA, Stevens C, Vivian A, Taylor JD, Murillo J (2000) Cultivar-specific avirulence and virulence functions assigned to avrPphF in Pseudomonas syringae pv. phaseolicola, the cause of bean halo-blight disease. The EMBO Journal 19, 3204–3214.
| Cultivar-specific avirulence and virulence functions assigned to avrPphF in Pseudomonas syringae pv. phaseolicola, the cause of bean halo-blight disease.Crossref | GoogleScholarGoogle Scholar | 10880434PubMed |
Vencato M, Tian F, Alfano JR, Buell CR, Cartinhour S, DeClerck GA, Guttman DS, Stavrinides J, Joardar V, Lindeberg M, Bronstein PA, Mansfield JW, Myers CR, Collmer A, Schneider DJ (2006) Bioinformatics-enabled identification of the HrpL regulon and type III secretion system effector proteins of Pseudomonas syringae pv. phaseolicola 1448A. Molecular Plant-Microbe Interactions 19, 1193–1206.
| Bioinformatics-enabled identification of the HrpL regulon and type III secretion system effector proteins of Pseudomonas syringae pv. phaseolicola 1448A.Crossref | GoogleScholarGoogle Scholar | 17073302PubMed |
Versalovic J, Koeuth T, Lupski R (1991) Distribution of repetitive DNA sequences in eubacteria and application to fingerprinting of bacterial genomes. Nucleic Acids Research 19, 6823–6831.
| Distribution of repetitive DNA sequences in eubacteria and application to fingerprinting of bacterial genomes.Crossref | GoogleScholarGoogle Scholar | 1762913PubMed |
Versalovic J, Schneider M, Bruijn FJd, Lupski JR (1994) Genomic fingerprint of bacteria using repetitive sequence-based polymerase chain reaction. Methods in Molecular and Cellular Biology 5, 25–40.
Vidaver AK, Lambrecht PA (2004) Bacteria as plant pathogens. The Plant Health Instructor.
von Bodman SB, Bauer WD, Coplin DL (2003) Quorum sensing in plant-pathogenic bacteria. Annual Review of Phytopathology 41, 455–482.
| Quorum sensing in plant-pathogenic bacteria.Crossref | GoogleScholarGoogle Scholar | 12730390PubMed |
Vuurde JWL, Franken AAJM, Birnbaum Y, Jochems G (1991) Characteristics of immunofluorescence microscopy and of dilution-plating to detect Pseudomonas syringae pv. phaseolicola in bean seed lots and for risk assessment of field incidence of halo blight. Netherlands Journal of Plant Pathology 97, 233–244.
| Characteristics of immunofluorescence microscopy and of dilution-plating to detect Pseudomonas syringae pv. phaseolicola in bean seed lots and for risk assessment of field incidence of halo blight.Crossref | GoogleScholarGoogle Scholar |
Walker JC, Patel PN (1964) Splash dispersal and wind as factors in epidemiology of halo blight of bean. Phytopathology 54, 140–141.
Webster DM, Atkin JD, Cross JE (1983) Bacterial blights of snap beans and their control. Plant Disease 67, 935–940.
| Bacterial blights of snap beans and their control.Crossref | GoogleScholarGoogle Scholar |
Xu R, Tambong JT (2011) A TaqMan real-time PCR assay targeting the cytochrome o ubiquinol oxidase subunit II gene for detection of several pathovars of Pseudomonas syringae. Canadian Journal of Plant Pathology 33, 318–331.
| A TaqMan real-time PCR assay targeting the cytochrome o ubiquinol oxidase subunit II gene for detection of several pathovars of Pseudomonas syringae.Crossref | GoogleScholarGoogle Scholar |
Yaqub M, Mahmood T, Akhtar M, Iqbal MM, Ali S (2010) Induction of mungbean [Vigna radiata (L.) Wilczek] as a grain legume in the annual rice-wheat double cropping system. Pakistan Journal of Botany 42, 3125–3135.
Young JM, Dye DW, Bradbury JF, Panagopoulos CG, Robbs CF (1978) A proposed nomenclature and classification for plant pathogenic bacteria. New Zealand Journal of Agricultural Research 21, 153–177.
| A proposed nomenclature and classification for plant pathogenic bacteria.Crossref | GoogleScholarGoogle Scholar |


