One-pot iterative native chemical ligation–desulfurisation chemistry leveraging a coumarin-based photolabile protecting group for cysteine
Lucas Kambanis A B , Timothy S. Chisholm A , Peter H. G. Egelund A B , Sameer S. Kulkarni A B and Richard J. Payne A B *
A B *
A
B
Abstract
An iterative one-pot peptide native chemical ligation (NCL)–desulfurisation strategy, featuring 7-diethylamino-3-methyl coumarin (DEAMC) as an orthogonal protecting group for cysteine, is described. We show that selective desulfurisation of unprotected cysteine residues can be achieved in the presence of DEAMC-protected cysteine residues, allowing subsequent DEAMC photodeprotection and native chemical ligation to be performed without purification. The efficiency of this approach was exemplified through the one-pot synthesis of a 60-residue mucin-1 peptide.
Keywords: coumarin, cysteine, desulfurisation, native chemical ligation, one-pot synthesis, photolabile, solid-phase peptide synthesis.
Introduction
The development of chemoselective peptide ligation methodologies has revolutionised chemical protein synthesis, providing rapid access to structurally complex polypeptide and protein molecules.1–4 Native chemical ligation (NCL),1,5 which enables fusion of peptide fragments by an amide bond-forming reaction between a peptide bearing an N-terminal cysteine (Cys) residue and a peptide with a C-terminal thioester functionality, remains the gold standard method for protein synthesis (Fig. 1a).6 Although this method initially relied on the availability of a suitably placed Cys (1.8% abundance) at the ligation junction, subsequent introduction of desulfurisation chemistry, which converts Cys to alanine (Ala) at the ligation junction,7–9 along with the development of thiolated analogues of other amino acids, has greatly expanded the scope of NCL.4,10–19
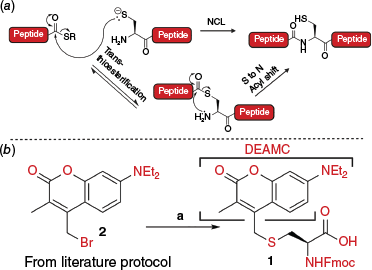
(a) Mechanism of an NCL reaction (b) Synthesis of Fmoc-Cys(DEAMC)-OH building block 1: (a) 2 (prepared as per published protocol31), Fmoc-Cys-OH, DIPEA, THF, rt, 16 h, 65%.
With these impressive advances, large proteins can now be assembled successfully by sequential ligations of multiple fragments. A popular ligation approach involves the ligation of three or more fragments in a bi-directional manner that benefits from the use of bifunctional fragments that feature a thioester at the C-terminus and an N-terminal Cys residue. For these approaches, masking of the Cys residue or thioester during an initial NCL reaction is essential to prevent undesired side reactions, including peptide cyclisation or self-ligation. Several strategies have been developed for performing multi-fragment ligation–desulfurisation protocols in a ‘one-pot’ manner,20 including the use of Cys protecting groups; thiazolidine (Thz),21–24 acetamidomethyl (Acm)25,26 and trifluoromethylacetamidomethyl (TfAcm).27 However, depending on the peptide sequence and modifications, the conditions required for deprotection may be incompatible or necessitate intermediate purification, significantly hampering the speed, efficiency and overall yield of chemical protein synthesis.28
Photolabile protecting groups have emerged as attractive alternatives as they provide the ability to perform iterative ligations in a one-pot manner due to the reagent-free nature of the deprotection steps. However, notable examples, which include nitroveratryl and quinoline-based protecting groups,29,30 require harsh UV irradiation for deprotection, potentially leading to unwanted side reactions on peptide and protein molecules. Capitalising on our previous work on the use of the 7-diethylamino-3-methyl coumarin (DEAMC) protecting group on selenocysteine (Sec) to facilitate one-pot diselenide-selenoester ligation chemistry,31 herein we validate the DEAMC moiety as a suitable protecting group for Cys, which can be cleanly removed under mild conditions by photoirradiation using 450-nm visible light. Importantly, this method enables chemoselective desulfurisation of unprotected Cys residues in the presence of DEAMC-protected Cys residues, thus streamlining the one-pot NCL–desulfurisation protocol.
Results and discussion
The proposed DEAMC-protected cysteine was synthesised in a single step by alkylating Fmoc-Cys-OH with our previously reported DEAMC-Br reagent 2 in the presence of N,N-diisopropylethylamine (DIPEA), furnishing the target building block 1 in 65% yield (Fig. 1b).
In order to assess the photodeprotection efficiency of DEAMC along with its stability during ligation, a model peptide H2N-C(DEAMC)SPGYS-NH₂ (3) was synthesised by Fmoc-strategy solid-phase peptide synthesis (Fmoc SPPS) and purified by reverse-phase HPLC (see ESI for synthetic details). The purified peptide 3 was dissolved in a denaturing buffer comprising 6 M of guanidine·HCl and irradiated with blue light (λ = 450 nm, LED) to test the deprotection of the DEAMC group from the side chain of Cys. Pleasingly, clean and quantitative deprotection was observed within 30 s, affording the corresponding cysteinyl peptide (4) with an unprotected N-terminal cysteine residue (see ESI for details). Next, model peptide 3 was dissolved in the same denaturing buffer, this time with the inclusion of tris(2-carboxyethyl)phosphine (TCEP) and 4-mercaptophenylacetic acid (MPAA), additives typically used for NCL, and incubated at 37°C for 16 h. Importantly, the DEAMC remained intact under these conditions, demonstrating its compatibility under NCL conditions (see the ‘Stability and deprotection of C(DEAMC)SPGYS 3’ section in the Supplementary material for details). These control experiments of model peptide 3 provided confidence in the proposed use of DEAMC to mask N-terminal Cys-containing peptide thioesters for use in iterative ligation-based assembly of large peptides and proteins.
Having optimised conditions for the introduction and deprotection of a DEAMC-protected Cys residue, attention was turned to evaluating its utility in protein assembly using an iterative NCL–desulfurisation strategy. Towards this end, a 60-residue unglycosylated analogue (5) comprising three copies of the extracellular variable number tandem repeat (VNTR) of transmembrane glycoprotein mucin-1 (MUC1) was selected as a target peptide (Fig. 2a). Specifically, a A21C mutant of this 60-residue peptide was selected to showcase selective desulfurisation chemistry of a DEAMC-protected Cys at the ligation junction before carrying out subsequent photodeprotection and ligation steps. Based on the retrosynthetic analysis, this strategy necessitated the synthesis of three differentially functionalised peptides: fragment 6, a peptide bearing an N-terminal cysteine; fragment 7, a bifunctional peptide featuring an N-terminal DEAMC-protected cysteine and a C-terminal alkyl thioester; and fragment 8, a peptide with a C-terminal alkyl thioester. All three peptides were synthesised using Fmoc-SPPS on a hyper acid-labile 2-chlorotrityl chloride (2-CTC) resin. In the case of fragments 7 and 8, the fully elongated peptide was cleaved from resin under mild acidic conditions and subjected to C-terminal thioesterification using 7-azabenzotriazol-1-yloxy)tripyrrolidinophosphonium hexafluorophosphate (PyAOP), DIPEA and the alkyl thiol 3-mercaptoethylpropionate (see Fig. 2b for the synthesis of bifunctional peptide fragment 7, including the coupling of the N-terminal DEAMC-protected Cys building block 1).32 Finally, global acidolytic cleavage and purification by reverse-phase HPLC furnished the suitably functionalised fragments ready for assembly by ligation.
(a) Sequence of target A21C MUC1 60mer 5 (ligation junctions indicated by dotted lines). (b) General Fmoc-SPPS (see ESI), Coupling: Fmoc-Cys(DEAMC)-OH 1 (1.2 eq.), HOAt (2.4 eq.), DIC (2.4 eq.), DMF, rt, 3 h; Boc-protection: (a) General Fmoc deprotection (see ESI), (b) Boc-anhydride (5 eq.), DIPEA (15 eq.), DMF, rt, 1 h; Resin Cleavage: 1,1,1,3,3,3-hexafluoroisopropanol (HFIP)/DCM (30% v/v, 5 mL), rt, 1 h; Thioesterification: ethyl 3-mercaptopropionate (30 eq.), PyAOP (5 eq.), DIPEA (5 eq.), −30°C, 3 h; Acidolytic Cleavage: TFA, iPr3SiH, H2O (18 : 1 : 1 v/v/v), rt, 2 h. Analytical HPLC and inlay of ESI-MS of purified MUC1 fragment 7 (c) Synthesis of MUC1 60mer 5 by one-pot iterative NCL reactions. (d) HPLC trace of crude reaction mixture after one-pot four-step ligation assembly. (e) Analytical HPLC and inlay of ESI-MS of purified MUC1 60mer 5.
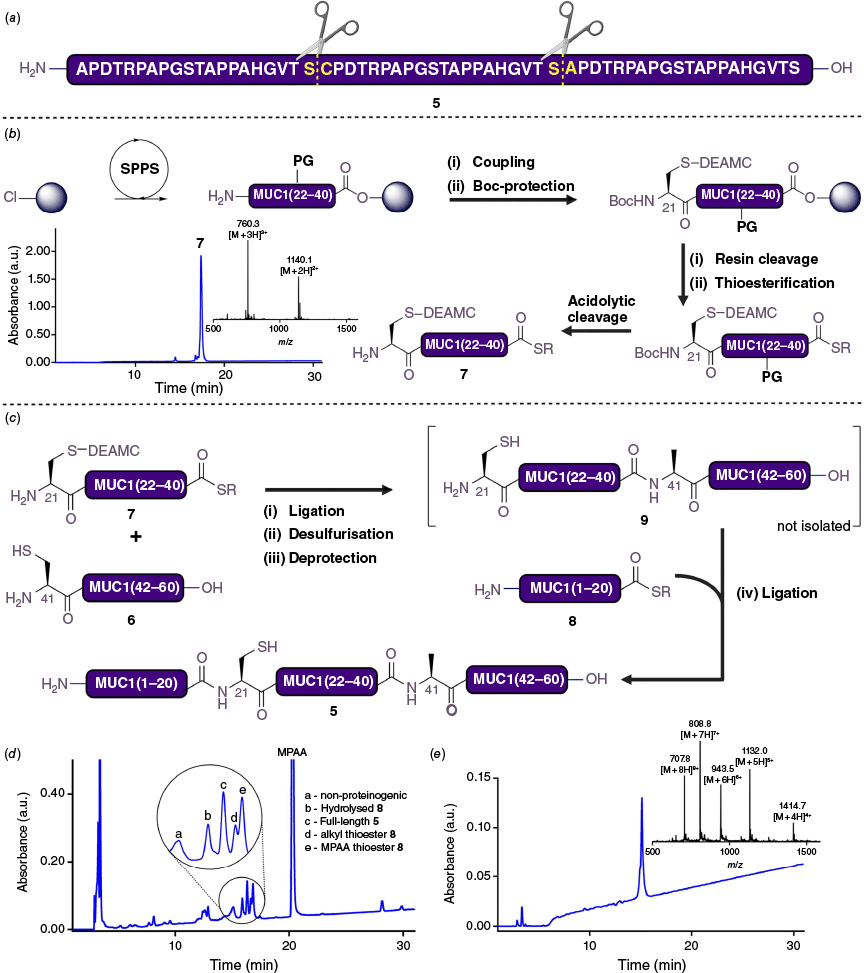
With peptides 6–8 in hand, the synthesis of the A21C MUC1 polypeptide (5) was initiated by reacting equimolar amounts of cysteinyl fragment 6 and bifunctional fragment 7 in the ligation buffer (6 M of guanidine·HCl, 1 M of HEPES, 200 mM of TCEP, 20 mM of MPAA, pH 7.0) at 5-mM peptide concentration (see Fig. 2c). Upon completion of NCL in 3 h (based on UPLC-MS analysis), the reaction mixture was acidified to pH 3.0 with 5 M of HCl, and MPAA was extracted with ethyl acetate.33 This step is crucial since MPAA acts as a radical quencher, blocking the desulfurisation pathway. Although photodesulfurisation chemistry has been previously reported,9,34 it was not pursued here due to concerns regarding the potential instability of the DEAMC group under UV irradiation. Instead, we investigated whether this desulfurisation step could be performed using 2,2′-azobis[2-(2-imidazolin-2-yl)propane] dihydrochloride (VA-044) as a radical initiator. To attempt this, the pH of the reaction mixture was re-adjusted to 7.0 using 5 M of NaOH and it was treated with VA-044 (15 mM) at 37°C for 16 h. Pleasingly, complete desulfurisation of Cys at the ligation junction was observed, whereas the DEAMC-protected Cys residue at the N-terminus remained unaffected. The reaction mixture was centrifuged to remove precipitated TCEP oxide and sulfide byproducts. The pellet was washed and re-suspended in an equivalent volume of ligation buffer (6 M of guanidine·HCl, 1 M of HEPES, 200 mM of TCEP, 200 mM of MPAA, pH 7.0) and recentrifuged. Supernatants were combined and irradiated with 450-nm light for 15 min, leading to the quantitative deprotection of the DEAMC group to afford 9 (now bearing a free N-terminal cysteine) as the major product. Without intermediate purification, 9 was subjected to another NCL reaction with thioester fragment 8 (4.0 eq.), which proceeded to completion within 3 h. After reverse-phase HPLC purification and lyophilisation, the target 60-residue C21A MUC1 polypeptide 5 was isolated in 34% yield over four steps (76% per step), underscoring the utility of this one-pot multi-step reaction manifold with the DEAMC-protected Cys as a key linchpin.
Conclusions
The DEAMC photolabile protecting group was successfully installed on the side chain of Cys, enabling selective desulfurisation of unprotected Cys residues, followed by photodeprotection by LED irradiation at λ = 450 nm. This mild, reagent-free step is fully compatible with an iterative NCL assembly strategy, allowing efficient one-pot synthesis of larger polypeptides and proteins without intermediary purification steps. The utility of this methodology was highlighted through the successful synthesis of a 60-residue A21C MUC1 polypeptide. Future work will involve broader application of DEAMC-protected cysteine in the ligation-based assembly of challenging protein targets, particularly those requiring selective desulfurisation of Cys/thiolated amino acid at the ligation junction in the presence of native cysteine residues.
Experimental section
Photodeprotection of the DEAMC group
A peptide bearing a DEAMC-protected Cys residue was dissolved to a concentration between 2 and 5 mM in ligation buffer (6 M of guanidine·HCl, 1 M of HEPES, pH 6–7 with or without TCEP and MPAA additives). Photodeprotection was performed using a PennOC light reactor with 450-nm light set to 100% intensity for a duration between 0:30 and 15:00 min (extended reaction times did not lead to deleterious by-products based on UPLC-MS analysis).
General NCL protocol
Peptide fragments were dissolved to a concentration of 2–5 mM in ligation buffer (6 M of guanidine·HCl, 1 M of HEPES, 200 mM of TCEP, 200 mM of MPAA, pH 7.0) and incubated at 37°C until completion (confirmed by UPLC-MS analysis of an aliquot of the reaction mixture).
General desulfurisation protocol
After ligation, the reaction mixture was acidified (pH 3.0) and residual MPAA was extracted with ethyl acetate. The pH was readjusted to 7.0 and the ligated product was treated with VA-044 (15 mM) at 37°C for 16 h to achieve complete desulfurisation of Cys at the ligation junction (reaction progress was monitored by UPLC-MS analysis of an aliquot of the reaction mixture).
Data availability
The data that support this study are available in the article and accompanying supplementary material.
Declaration of funding
The authors acknowledge funding from the Australian Research Council Centre of Excellence for Innovations in Peptide and Protein Science (CE200100012, to Richard J. Payne).
Acknowledgements
The authors thank Anthony Ayoub (The University of Sydney) for the technical assistance with mass spectrometry and Dr Wendy Tran (The University of Sydney) for the technical assistance with nuclear magnetic resonance spectroscopy.
Author contributions
The manuscript was written with contributions from all authors and all authors have given approval to the final version of the manuscript.
References
1 Kent SBH. Total chemical synthesis of proteins. Chem Soc Rev 2009; 38(2): 338-351.
| Crossref | Google Scholar | PubMed |
2 Bondalapati S, Jbara M, Brik A. Expanding the chemical toolbox for the synthesis of large and uniquely modified proteins. Nat Chem 2016; 8(5): 407-418.
| Crossref | Google Scholar | PubMed |
3 Kulkarni SS, Sayers J, Premdjee B, Payne RJ. Rapid and efficient protein synthesis through expansion of the native chemical ligation concept. Nat Rev Chem 2018; 2(4): 122.
| Crossref | Google Scholar |
4 Conibear AC, Watson EE, Payne RJ, Becker CF. Native chemical ligation in protein synthesis and semi-synthesis. Chem Soc Rev 2018; 47(24): 9046-9068.
| Crossref | Google Scholar | PubMed |
5 Dawson P, Muir T, Clark-Lewis I, Kent S. Synthesis of proteins by native chemical ligation. Science 1994; 266(5186): 776-779.
| Crossref | Google Scholar | PubMed |
6 Johnson ECB, Kent SBH. Insights into the mechanism and catalysis of the native chemical ligation reaction. J Am Chem Soc 2006; 128(20): 6640-6646.
| Crossref | Google Scholar | PubMed |
7 Wan Q, Danishefsky SJ. Free-radical-based, specific desulfurization of cysteine: a powerful advance in the synthesis of polypeptides and glycopolypeptides. Angew Chem Int Ed 2007; 46(48): 9248-9252.
| Crossref | Google Scholar | PubMed |
8 Haase C, Seitz O. Extending the scope of native chemical peptide coupling. Angew Chem Int Ed 2008; 47(9): 1553-1556.
| Crossref | Google Scholar | PubMed |
9 Chisholm TS, Clayton D, Dowman LJ, Sayers J, Payne RJ. Native chemical ligation–photodesulfurization in flow. J Am Chem Soc 2018; 140(29): 9020-9024.
| Crossref | Google Scholar | PubMed |
10 Malins LR, Payne RJ. Synthetic amino acids for applications in peptide ligation–desulfurization chemistry. Aust J Chem 2015; 68(4): 521-537.
| Google Scholar |
11 Sayers J, Thompson RE, Perry KJ, Malins LR, Payne RJ. Thiazolidine-protected β-thiol asparagine: applications in one-pot ligation–desulfurization chemistry. Org Lett 2015; 17(19): 4902-4905.
| Crossref | Google Scholar | PubMed |
12 Haase C, Rohde H, Seitz O. Native chemical ligation at valine. Angew Chem Int Ed 2008; 47(36): 6807-6810.
| Crossref | Google Scholar | PubMed |
13 Chen J, Wan Q, Yuan Y, Zhu J, Danishefsky SJ. Native chemical ligation at valine: a contribution to peptide and glycopeptide synthesis. Angew Chem Int Ed 2008; 47(44): 8521-8524.
| Crossref | Google Scholar | PubMed |
14 Dawson PE. Native chemical ligation combined with desulfurization and deselenization: a general strategy for chemical protein synthesis. Isr J Chem 2011; 51(8–9): 862-867.
| Crossref | Google Scholar |
15 Thompson RE, Chan B, Radom L, Jolliffe KA, Payne RJ. Chemoselective peptide ligation–desulfurization at aspartate. Angew Chem Int Ed 2013; 52(37): 9723-9727.
| Crossref | Google Scholar | PubMed |
16 Cergol KM, Thompson RE, Malins LR, Turner P, Payne RJ. One-pot peptide ligation–desulfurization at glutamate. Org Lett 2014; 16(1): 290-293.
| Crossref | Google Scholar | PubMed |
17 Malins LR, Cergol KM, Payne RJ. Chemoselective sulfenylation and peptide ligation at tryptophan. Chem Sci 2014; 5(1): 260-266.
| Crossref | Google Scholar |
18 Malins LR, Cergol KM, Payne RJ. Peptide ligation–desulfurization chemistry at arginine. ChemBioChem 2013; 14(5): 559-563.
| Crossref | Google Scholar | PubMed |
19 Malins LR, Giltrap AM, Dowman LJ, Payne RJ. Synthesis of β-thiol phenylalanine for applications in one-pot ligation–desulfurization chemistry. Org Lett 2015; 17(9): 2070-2073.
| Crossref | Google Scholar | PubMed |
20 Liao P, He C. Azole reagents enabled ligation of peptide acyl pyrazoles for chemical protein synthesis. Chem Sci 2024; 15(21): 7965-7974.
| Crossref | Google Scholar | PubMed |
21 Bang D, Kent SBH. A one-pot total synthesis of crambin. Angew Chem Int Ed 2004; 43(19): 2534-2538.
| Crossref | Google Scholar | PubMed |
22 Huang YC, Chen CC, Gao S, Wang YH, Xiao H, Wang F, Tian CL, Li YM. Synthesis of L‐ and D‐ubiquitin by one‐pot ligation and metal‐free desulfurization. Chem Eur J 2016; 22(22): 7623-7628.
| Crossref | Google Scholar | PubMed |
23 Thompson RE, Liu X, Alonso-García N, Pereira PJB, Jolliffe KA, Payne RJ. Trifluoroethanethiol: an additive for efficient one-pot peptide ligation − desulfurization chemistry. J Am Chem Soc 2014; 136(23): 8161-8164.
| Crossref | Google Scholar | PubMed |
24 Siman P, Blatt O, Moyal T, Danieli T, Lebendiker M, Lashuel HA, Friedler A, Brik A. Chemical synthesis and expression of the HIV‐1 Rev protein. ChemBioChem 2011; 12(7): 1097-1104.
| Crossref | Google Scholar | PubMed |
25 Veber D, Milkowski J, Varga S, Denkewalter R, Hirschmann R. Acetamidomethyl. A novel thiol protecting group for cysteine. J Am Chem Soc 1972; 94(15): 5456-5461.
| Crossref | Google Scholar | PubMed |
26 Maity SK, Jbara M, Laps S, Brik A. Efficient palladium‐assisted one‐pot deprotection of (acetamidomethyl) cysteine following native chemical ligation and/or desulfurization to expedite chemical protein synthesis. Angew Chem Int Ed 2016; 55(28): 8108-8112.
| Crossref | Google Scholar | PubMed |
27 Tang S, Si YY, Wang ZP, Mei KR, Chen X, Cheng JY, Zheng JS, Liu L. An efficient one‐pot four‐segment condensation method for protein chemical synthesis. Angew Chem Int Ed 2015; 54(19): 5713-5717.
| Crossref | Google Scholar | PubMed |
28 Spears RJ, McMahon C, Chudasama V. Cysteine protecting groups: applications in peptide and protein science. Chem Soc Rev 2021; 50(19): 11098-11155.
| Crossref | Google Scholar | PubMed |
29 Karas JA, Scanlon DB, Forbes BE, Vetter I, Lewis RJ, Gardiner J, Separovic F, Wade JD, Hossain MA. 2‐Nitroveratryl as a photocleavable thiol‐protecting group for directed disulfide bond formation in the chemical synthesis of insulin. Chem Eur J 2014; 20(31): 9549-9552.
| Crossref | Google Scholar | PubMed |
30 Wang S, Zhou Q, Li Y, Wei B, Liu X, Zhao J, Ye F, Zhou Z, Ding B, Wang P. Quinoline-based photolabile protection strategy facilitates efficient protein assembly. J Am Chem Soc 2022; 144(3): 1232-1242.
| Crossref | Google Scholar | PubMed |
31 Kambanis L, Chisholm TS, Kulkarni SS, Payne RJ. Rapid one-pot iterative diselenide–selenoester ligation using a novel coumarin-based photolabile protecting group. Chem Sci 2021; 12(29): 10014-10021.
| Crossref | Google Scholar | PubMed |
32 Kajihara Y, Yoshihara A, Hirano K, Yamamoto N. Convenient synthesis of a sialylglycopeptide-thioester having an intact and homogeneous complex-type disialyl-oligosaccharide. Carbohydr Res 2006; 341(10): 1333-1340.
| Crossref | Google Scholar | PubMed |
33 Boll E, Drobecq H, Ollivier N, Blanpain A, Raibaut L, Desmet R, Vicogne J, Melnyk O. One-pot chemical synthesis of small ubiquitin-like modifier protein–peptide conjugates using bis (2-sulfanylethyl) amido peptide latent thioester surrogates. Nat Protoc 2015; 10(2): 269-292.
| Crossref | Google Scholar | PubMed |
34 Kambanis L, Ayoub A, Bedding MJ, Egelund PH, Maxwell JW, Franck C, Lambrechts L, Hawkins PM, Chisholm TS, Mackay JP. Expressed protein ligation in flow. J Am Chem Soc 2024; 146(31): 22027-22035.
| Crossref | Google Scholar | PubMed |


