Cobalt oxide-based catalysts for acidic oxygen evolution reactions
Huihui Li A , Shuhao Wang
A , Shuhao Wang  A and Chuan Zhao
A and Chuan Zhao  A *
A *
A

Huihui Li received her MEng degree from Beijing Normal University in 2021 and BSc degree from Beijing University of Technology in 2018. Now she is a PhD candidate in the School of Chemistry at the University of New South Wales (UNSW), Sydney, under the supervision of Prof. Chuan Zhao. Her research focuses on the electrocatalysis of water splitting in acidic media, with an emphasis on catalyst stability and mechanisms under industrial conditions. Her research interests focus on material synthesis, electrochemical testing and advanced characterisation techniques. |

Shuhao Wang is a postdoctoral research fellow at UNSW, Sydney. He received his Bachelor’s and Master’s degrees in materials science and engineering from Fuzhou University and completed his PhD at UNSW in 2024. His research focuses on computational electrochemistry, integrating density functional theory and molecular dynamics to design and understand advanced electrocatalysts for energy conversion. He has extensive experience in high-performance computing and the application of machine learning for mechanistic studies and catalyst optimisation. |

Chuan Zhao is a professor at the School of Chemistry at UNSW, Sydney. He is currently the deputy director of the Australian Research Council (ARC) Training Centre for the Global Hydrogen Economy, and the deputy research chair and flagship program director of the ARC Centre of Excellence on Green Electrochemical Transformation of Carbon Dioxide. He is interested in discovering novel electrochemical methodologies and nanomaterials for energy applications, including water splitting, hydrogen fuel cells, CO2 and N2 reduction, batteries, and sensors. |
Abstract
Proton exchange membrane water electrolysis (PEMWE) is a cornerstone technology for green hydrogen production, yet the sluggish oxygen evolution reaction (OER) in acidic media remains a major bottleneck. Noble metal oxides such as IrO2 and RuO2 are effective but suffer from high cost and scarcity. As a non-precious alternative, spinel cobalt oxide (Co3O4) has attracted attention due to its promising performance results from its mixed-valence structure, tunable electronic properties and catalytic potential. However, its practical application is challenged by poor conductivity, moderate activity and instability under acidic conditions due to proton attack and lattice degradation. This review summarises recent advances in Co3O4-based electrocatalysts for acidic OER. We first introduce three key OER pathways: adsorbate evolution mechanism (AEM), lattice oxygen mechanism (LOM) and oxide path mechanism (OPM) and their relevance to Co3O4 performance. Then, we introduce the structural and electronic characteristics of Co3O4 that influence its catalytic behaviour. Next, we review a range of engineering strategies, including element doping, heterostructure construction, surface modification and defect engineering, all aimed at enhancing the activity and durability of Co3O4. Finally, we highlight critical challenges and offer perspectives for advancing Co3O4 as a viable acidic OER catalyst.
Keywords: acidic water electrolysis, activity and stability, cobalt oxide, electronic structure design, non-noble metal catalysts, oxygen evolution reaction, proton exchange membrane electrolyser, renewable energy.
Introduction
Hydrogen has emerged as a promising clean energy carrier due to its high gravimetric energy density (142 MJ kg−1), zero carbon emissions upon utilisation and broad applicability across sectors such as transportation, chemical manufacturing and energy storage.1–3 Electrochemical water splitting offers an environmentally friendly and scalable approach for producing green hydrogen using renewable electricity.4–7 Compared with alkaline water electrolysis, proton exchange membrane water electrolysis (PEMWE) features high efficiency, superior current densities, high hydrogen purity and a more compact system architecture, making it more suitable for intermittent renewable energy sources and industrial-scale deployment.8 However, the sluggish kinetics and harsh operating environment of the oxygen evolution reaction (OER) at the anode impose significant challenges, necessitating the development of efficient and durable electrocatalysts under acidic conditions.9,10 To date, noble metal oxides such as IrO2 remain the benchmark acidic OER catalysts due to their exceptional electrochemical performance.11 IrO2 is well known for its outstanding stability under harsh acidic conditions but suffers from limited intrinsic activity and extremely high cost (~US$140 g−1).12 By contrast, RuO2 exhibits higher OER activity and relatively lower cost, making it a highly promising candidate for acidic water electrolysis.13–17 However, its poor durability has significantly restricted its practical application. Despite the progress achieved for enhancing its stability,18 ruthenium is still a precious metal with limited availability and high price (~US$16 g−1),12 which continues to pose economic challenges for large-scale deployment of PEMWE systems. These limitations have prompted increasing interest in developing alternative non-noble metal-based catalysts, which could potentially deliver both high performance and economic feasibility in acidic OER environments.19–21
Among various non-precious metal oxides, cobalt oxide (Co3O4) with a spinel structure has emerged as a promising candidate for acidic OER electrocatalysis. The spinel Co3O4 consists of Co2+ ions at the tetrahedral site and Co3+ ions located at the octahedral sites, forming a mixed-valence system that enables favourable redox properties and fast charge transfer capability. In addition, its tunable electronic structure and flexible coordination environment offer opportunities for modulating catalytic activity through doping or other structural engineering approaches.22 These intrinsic features have attracted widespread attention for developing Co3O4-based acidic OER catalysts.23–25 However, despite its advantages, Co3O4 suffers from limited stability in acidic media due to issues such as proton-induced dissolution, phase reconstruction and the loss of active sites under harsh oxidative potentials.26 To address these challenges, various strategies have been explored to enhance both the activity and stability of Co3O4, encompassing but not limited to heteroatom doping, heterostructure construction, surface coatings and lattice defect engineering.22 For example, Chong et al. developed a La- and Mn-co doped Co3O4 catalyst derived from a zeolitic imidazolate framework with an overpotential of 353 mV at 10 mA cm−2 and excellent stability over 360 h in acid electrolyte, which enabled up to 4000 mA cm−2 in PEMWE with minimal degradation, demonstrating the practical promise of doped Co3O4 for acidic OER.27 Apart from doping approaches, Ram et al. demonstrated that delamination of cobalt tungstate creates lattice vacancies that trap water and hydroxide ions, stabilising the catalyst in acidic conditions. Their catalyst achieved a current density of 1.8 A cm−2 at 2.0 V and operated stably at 1 A cm−2 for over 600 h at 80°C in a PEMWE system, representing a threefold improvement in activity compared to previous catalysts.28 Given the growing interest in Co3O4-based catalysts for acidic OER and the wide range of structural and electronic modifications being pursued, a review is timely to consolidate current knowledge, identify key challenges and guide future research directions.
In this review, we provide a comprehensive overview of the recent advances in cobalt oxide-based catalysts for the OER under acidic conditions, with a particular focus on their structural design, performance enhancement strategies and stability challenges. We begin by discussing the current understanding of the acidic OER mechanism, including the adsorption evolution mechanism (AEM), the lattice oxygen mechanism (LOM) and the emerging oxide path mechanism (OPM), as all of these pathways are potentially involved in Co3O4 and are crucial for understanding their activity and stability. Then we summarise the intrinsic structural and electronic properties of spinel Co3O4 relevant to its OER activity and degradation behaviour in acid. Subsequently, we highlight recent progress in engineering strategies such as doping, heterostructure construction, surface modification, defect engineering and lattice regulation. Finally, we outline key challenges and offer future perspectives for the development of Co3O4-based electrocatalysts for practical application in acidic water electrolysis.
OER mechanism in acids
A fundamental understanding of the reaction mechanisms is crucial for the design of Co3O4-based catalysts for acidic OER, which generally involves three representative pathways: adsorbate evolution mechanism (AEM), lattice oxygen mechanism (LOM) and oxide path mechanism (OPM). These three mechanisms are schematically illustrated in Fig. 1.
Schematic illustration of three OER mechanisms in acidic media. (a) adsorbate evolution mechanism (AEM); (b) lattice oxygen mechanism (LOM); (c) oxide path mechanism (OPM).
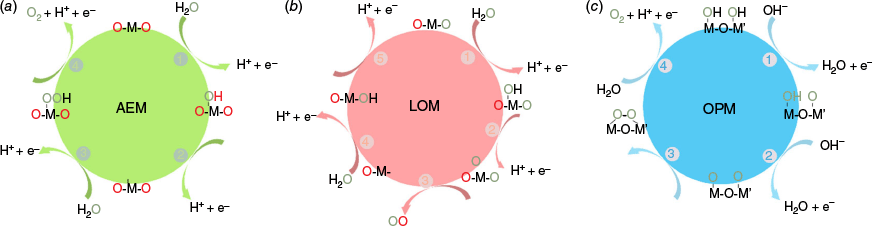
Adsorption evolution mechanism (AEM)
The conventional AEM pathway has been extensively studied in various metal oxide catalysts, including Co3O4. In acidic OER, Co3O4 typically exhibits the classical four-step proton-electron transfer process involving adsorbed intermediates such as OH*, O* and OOH* on Co active sites. The process can be described as follows: firstly, the adsorbed water molecule discharges and forms OH* intermediates, which then undergo a deprotonation process to generate O*. A subsequent nucleophilic attack by H2O on O* leads to the formation of OOH* species, followed by the release of O2 molecules (Fig. 1a, Eqn 1–4).
The AEM follows a scaling relationship among intermediates, which is the binding energy difference of HO* and HOO* and is constant (ΔGHOO∗ − ΔGHO∗ = 3.2 ± 0.2 eV).29 Therefore, this results in a minimum theoretical overpotential of 0.37 V. In situ electrochemical attenuated total reflection Fourier transform infrared spectroscopy (in situ FT-IR) is used to capture the OOH* species and then identify the AEM pathway.30–32 Another distinctive characteristic of AEM-driven catalysts is the involvement of concerted proton-electron transfer (CPET) steps,33 wherein the proton and electron are transferred simultaneously at each elementary step. This CPET behaviour results in a negligible pH dependence of the OER activity when plotted on the reversible hydrogen electrode (RHE) scale, leading to a ρRHE value (ρRHE = ∂(log j)/∂pH) close to zero. By contrast, non-concerted mechanisms such as the lattice oxygen mechanism (LOM) and oxide path mechanism (OPM) involve decoupled proton and electron transfer processes and thus typically exhibit larger ρRHE values, indicating a stronger pH dependence.34 Therefore, combined analysis of intermediate species using spectroscopic techniques and kinetic pH dependence offers a powerful approach to distinguish between AEM and non-AEM pathways in acidic OER catalysis.
Lattice oxygen mechanism (LOM)
The initial two steps of LOM are the same as AEM, which are the formation of OH* and O* species. The O* species can then combine with oxygen from the catalyst lattice, resulting in the release of O2 and the formation of abundant oxygen vacancies in the catalyst lattice structure (Eqn 5–7).35 The oxygen vacancies can be refilled by water molecules and form OH* species. Finally, through a one-electron oxidation step, the proton from OH* is removed to form the O* species again (Fig. 1b). Compared to traditional AEM, LOM circumvents the high theoretical overpotentials caused by the scaling relationship due to the formation of OOH*. Therefore, this mechanism provides a novel route to designing efficient OER catalysts.
Notably, it has also been shown that at the beginning of the reaction, O2 can be formed directly between the lattice oxygen atoms in the oxide by direct coupling (Eqn 8)36:
Several in situ techniques including X-ray absorption spectroscopy (XAS), surface-enhanced Raman spectroscopy (SERS), differential electrochemical mass spectroscopy (DEMS) and computational simulations have been used to explore and verify the feasibility of LOM pathways.8 Although the enhancement of activity by LOM has been researched widely, the critical challenge of LOM based electrocatalysts is the unsatisfactory durability. This is because of the bulk oxygen diffusion and structural reconstruction due to the continuous formation of oxygen vacancies and the dissolution of metal ions in the lattice structure of the catalysts. To enhance the stability, the participation of lattice oxygen should be completely or partially inhibited. Alternatively, to keep the promising activity of the LOM pathway, timely refilling of lattice oxygen vacancies through interaction with water molecules not only maintains active sites but also enhances catalyst stability. This refilling process, where oxygen atoms from water replenish vacancies, followed by deprotonation and removal of adsorbed hydrogen to regenerate clean active sites, has been increasingly reported in noble metal catalysts37,38 and alkaline OER systems.39 However, such studies remain scarce for Co3O4 in acidic OER, highlighting a valuable and promising direction for future research.
Oxide path mechanism (OPM)
The OPM pathway, characterised by the direct coupling of adjacent metal–oxygen (M–O) intermediates (Fig. 1c) without the formation of OOH* or oxygen vacancies, offers a pathway to exceed the theoretical limits of activity and stability.9,10 The OPM pathway has the most stringent requirements for the catalyst structure as it needs a special geometric configuration and electronic structure of metal active sites. Specifically, the dual metal active sites with appropriate atomic distance are expected to be beneficial for the O–O coupling. In that case, dual-atom catalysts are attractive because they consist of two adjacent homo- or hetero-metal centres. For example, Wang et al. reported that doping Ba cations into a Co3O4 framework to form Co3−xBaxO4 promotes the OPM and simultaneously improves activity as well as the stability in acidic electrolytes.40 The Ba doping contributed to the shorter metal–metal distance and increased adsorbed OH, which results in the O–O radical coupling and open coordination sites for O–O band formation.40 The research developing OPM based acidic OER catalysts is still at an early stage.12 In addition, Cui et al. demonstrated that asymmetric dual Cooct sites in spinel Co3O4, especially those bridging oxygen-deficient and pristine Cooct centres, efficiently promote direct O–O coupling via the DOM pathway.41 The degree of asymmetry between these dual sites was found to correlate with the reaction free energy of the rate-determining step, revealing a volcano-type relationship that offers a quantitative descriptor for catalyst optimisation. Experimentally, plasma-treated Co3O4 exhibited excellent performance in 0.5 M of H2SO4, achieving 10 and 1000 mA cm−2 at low overpotentials of 287 and 420 mV respectively. This study provides a mechanistic basis for engineering asymmetric coordination environments to activate O–O bond formation, offering valuable insights into oxide-pathway mechanisms in acidic OER.
Importantly, these mechanisms are not mutually exclusive. In practical catalytic systems, the OER processes rarely follow a single isolated mechanism. Instead, many catalysts exhibit a transition or coexistence of multiple pathways. Understanding and controlling the mechanistic crossover is therefore essential for the rational design of highly active and durable OER catalysts.
Structural and catalytic characteristics of Co3O4 in acidic OER
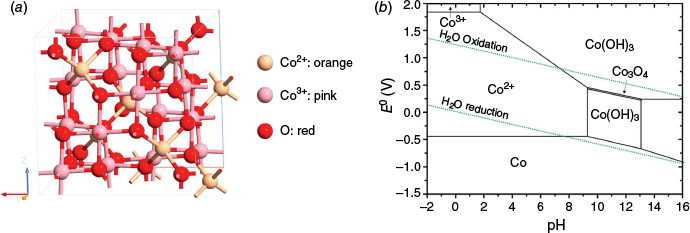
Spinel Co3O4 is a widely reported cobalt oxide with a formula of AB2O4, where the A site (tetrahedral) is occupied by Co2+ and the B site (octahedral) is occupied by Co3+ species (Fig. 2a).42 This mixed-valence structure offers multiple redox-active Co sites and facilitates charge transfer by intervalence electron transport between Co2+ and Co3+, which is particularly beneficial for mediating the multi-electron transfer processes, including OER.43–45 In acidic environments, where catalyst dissolution is a critical issue, the Pourbaix diagram of cobalt (Fig. 2b) indicates that Co3O4 is prone to corrosion under anodic potentials. This suggests that, although Co3O4 is thermodynamically metastable, its stability can be enhanced kinetically through various surface modifications or suitable dopant incorporation strategies. These features make Co3O4 a promising, though still challenging, candidate for acidic OER catalysis.
(a) Structure of Co3O4 normal spinel. Orange, Co2+; pink, Co3+; red, O.46 (b) Pourbaix diagram (potential/pH) calculated for the cobalt/water system.47
Until now, the noble metal modification of Co3O4 has been extensively studied; the development of noble-metal-free Co3O4-based catalysts under acidic conditions remains both critically important and reasonably scarce. To provide insights into this emerging direction, we summarise representative noble-metal-free Co3O4-based OER catalysts in acidic media in Table 1.
| Catalysts | Electrolyte | Overpotential (mV) @ 10 mA cm−2 | Stability (h) @ 10 mA cm−2 | References | |
|---|---|---|---|---|---|
| Nd-doped Co3O4 | 0.5 M of H2SO4 | 304 | 18 | 48 | |
| 3-D Co3O4/NC-250 | 0.5 M of H2SO4 | 225 | 80 | 30 | |
| F-Co3O4/CP | 0.5 M of H2SO4 | 350 | 80 | 49 | |
| Co3–xBaxO4 | 0.5 M of H2SO4 | 278 | 110 | 40 | |
| HfO2/Co3O4/FTO | 1 M of H2SO4 | 470 | 42 | 50 | |
| Co3O4/CeO2 | 0.5 M of H2SO4 | 423 ± 8 | 50 | 51 | |
| Co3O4−xFx | 0.5 M of H2SO4 | 349 | / | 52 | |
| 13.4% Ni-Co3O4 NFs | 0.5 M of H2SO4 | 330 | 80 | 53 | |
| CoO/Co3O4 | 0.5 M of H2SO4 | 396 | 145 | 54 | |
| Co3O4@C-GS | H2SO4 (pH = 1) | 350 | 20 | 55 | |
| Ce-Co3O4 | 0.5 M of H2SO4 | 348 | 25 | 56 | |
| V−CoP2 | 0.5 M of H2SO4 | 91 | / | 57 |
Forward slashes indicate that the corresponding articles did not report stability data at a current density of 10 mA cm−2.
For acidic OER, the electronic configuration and spatial arrangement of cobalt ions in Co3O4 play a pivotal role in determining catalytic activity. It is widely reported that the Co3+ at the octahedral sites serve as the actual active sites, whereas the Co2+ at the tetrahedral sites exhibit negligible activity.46 The coexistence of Co2+ and Co3+ provides distinct coordination environments, which facilitates electron transfer. This dual-valence structure enables multiple electron transfer pathways, showing great potential for catalytic processes such as OER. In particular, a moderate presence of Co2+ can promote electronic delocalisation, enhancing electron transport across the spinel lattice. Co2+ can facilitate oxygen vacancy formation under oxidative potentials, enhancing lattice oxygen mobility and promoting the LOM mechanism to accelerate OER kinetics. However, excessive Co2+ content may reduce the density of catalytically active Co3+ sites and cause structural instability, especially under acidic conditions where Co2+ is prone to dissolution. Therefore, the Co2+/Co3+ ratio critically influences both the number of active sites and the catalyst’s electronic structure. Tailoring this ratio through heteroatom doping, defect engineering, cation exchange or controlled annealing treatments presents a viable strategy for optimising the OER performance of Co3O4-based materials.
One of the most explored strategies to enhance the OER performance of Co3O4 under acidic conditions is morphology engineering. Co3O4 can be synthesised in diverse morphologies, such as spheres, cubes, sheets and wires, which modulate surface area, porosity and mass transport properties. In addition to morphological tuning, controlling the exposed crystal facet has emerged as a powerful strategy to modulate surface energetics and active site distribution, which directly influence the adsorption energies of OER intermediates and thus the kinetics of surface electrochemistry. For example, Zhao et al. systematically investigated the facet-dependent acidic OER performance of Co3O4. They found that Co3O4 with mixed (111) and (110) facets exhibited superior activity and stability compared to those exposing only (111) or (100) planes.58 Further insights into the facet-dependent stability mechanism were provided by Sheng et al.,59 who tracked the facet evolution of spinel Co3O4 nanosheets during acidic OER. Their study revealed that low-index facets such as (110), (100) and (211) are especially susceptible to dissolution, whereas high-index facets like (311) and (402) tend to emerge during electrolysis. In addition, the etching of the moderately active yet structurally unstable (110) facets was found to accelerate the degradation of both the highly active (100) and the structurally stable (111) facets located nearby.
Moreover, the electronic configuration of cobalt centres, particularly the position of the d-band centre, also plays a vital role in determining the intrinsic catalytic behaviour of Co3O4. Modulation of the d-band centre represents a crucial electronic strategy to optimise the adsorption-desorption behaviour of OER intermediates (*OH, *O, *OOH) and enhance catalytic activity. A moderate downshift of the d-band centre can weaken the binding of *OOH, promoting its desorption and facilitating the O2 evolution step, while avoiding catalyst poisoning caused by overly strong intermediate binding.60 Conversely, an upshift may aid in initial *OH adsorption but hinder product release. Therefore, achieving a balanced d-band centre is crucial to ensuring both high activity and durability. The d band centre of the Co active sites can be effectively tailored through various approaches, including aliovalent cation doping, strain engineering, defect engineering and heterostructure construction. These methods influence the local coordination environment and electronic structure of cobalt sites, thereby fine-tuning the binding energies of key reaction intermediates. These strategies not only enhance the intrinsic activity but also help address stability issues under harsh acidic conditions. The detailed implementation and effects of these engineering strategies will be systematically discussed in next section.
Although Co3O4 is considered a promising non-noble metal catalyst due to its tunable electronic structure and abundance, its practical application under acidic conditions remains hindered by several inherent drawbacks. These include low electrical conductivity, a limited density of catalytically active sites and modest intrinsic OER activity, all of which compromise its overall performance. Structurally, Co3O4 also suffers under harsh oxidative conditions: Co3+ sites, though catalytically active, tend to be further oxidised to unstable Co4+ species (e.g. soluble CoO2), which initiate catalyst dissolution.58 This eventually leads to the dissolution of Co into thermodynamically favoured Co2+ species, as predicted by the Pourbaix diagram (Fig. 2b), raising concerns about its long-term stability. However, a recent study by Priamushko et al. reveals that the dissolution kinetics of Co3O4 are reasonably sluggish, and much of the degradation is transient and surface-localised processes.26 This finding suggests that, despite its unfavourable thermodynamics, Co3O4 still holds great promise as a durable acidic OER catalyst, given that appropriate surface modification and electrochemical regulation strategies are employed.
Strategies for enhancing Co3O4 stability
Heteroatom doping
Heteroatom doping offers a powerful strategy to enhance the stability and activity of Co3O4 for acidic OER. By introducing heteroatoms (noble metals,23–25,61 transition metals,20,21,31,32,53,62–65 rare-earth elements66,67 or nonmetal elements49,52) into the lattice, key properties such as electronic structure, oxygen binding strength and resistance to acid corrosion can be finely tuned. The following sections summarise recent advances in Co3O4 doping strategies based on dopant categories.
Noble metal oxides such as RuO2 and IrO2 remain the benchmark catalysts for PEMWE due to their exceptional activity and stability, but their high cost limits large-scale application. Reducing their content while simultaneously enhancing activity and stability is therefore essential for the practical deployment of PEMWE. To this end, anchoring single atoms or highly dispersed clusters of noble metals onto acid-resistant supports has emerged as a promising approach, as it allows for precise modulation of the electronic structure and local coordination environment of the active sites.68–71 Recent studies have demonstrated that noble metal atoms anchored on Co3O4 can significantly enhance both OER activity and stability under acidic conditions. In particular, atomically dispersed Ir or Ru on Co3O4 surfaces not only maximises atomic utilisation efficiency but also creates strong metal–support interactions that modulate the d-band centre of the active sites, thereby optimising the adsorption energies of OER intermediates. These interactions can also suppress the dissolution or aggregation of noble metal species during long-term operation, leading to improved durability. For example, Zhang et al. systematically investigated the impact of interatomic distance (Fig. 3a–i) between Ir single atoms embedded within the Co3O4 spinel lattice on the catalyst’s acid stability.24 They proved that the introduction of Ir single atoms into spinel Co3O4 significantly enhances catalyst stability by increasing the migration barrier of neighbouring Co atoms and that the stabilising effect becomes more pronounced as the Ir–Ir distance decreases, reaching optimal performance when Co atoms are sandwiched between Ir atoms at ~0.6-nm spacing. The optimal catalyst lead to minimal Co dissolution and only ~20-mV degradation after 60 h at 10 mA cm−2 under acidic OER conditions.
HAADF-STEM images of Ir1/Cu0.3Co2.7O4 with different Ir–Ir distances. Ir1/Cu0.3Co2.7O4 with d = 1.1 nm (a), d = 0.8 nm (b) and d = 0.6 nm (c). Distance distribution of Ir single atoms (d) and intensity profile (e) of atoms located at the square frame in (a). Distance distribution of Ir single atoms (f) and intensity profile (g) of atoms located at the square frame in (b). Distance distribution of Ir single atoms (h) and intensity profile (i) of atoms located at the square frame in (c).24 (j) Schematic illustration of the formation process of Ru-Co3O4. (k) HAADF-STEM image of Ru-Co3O4. Inset in (k) shows a schematic illustration and simulated structure of the single atom Ru-Co3O4 model for the selected orange square area. The Ru single atom is highlighted within the dotted yellow circle in (k). (l) 3-D atom-overlapping Gaussian function fitting mapping of the selected yellow squares area in (k). (m) Intensity profiles along the green dashed rectangle regions in (k). (n) Schematic illustration of the creation of local strains induced by Ru single atom decoration in Ru-Co3O4. The light green and red arrows indicate the local strain induced by a Ru single atom.25
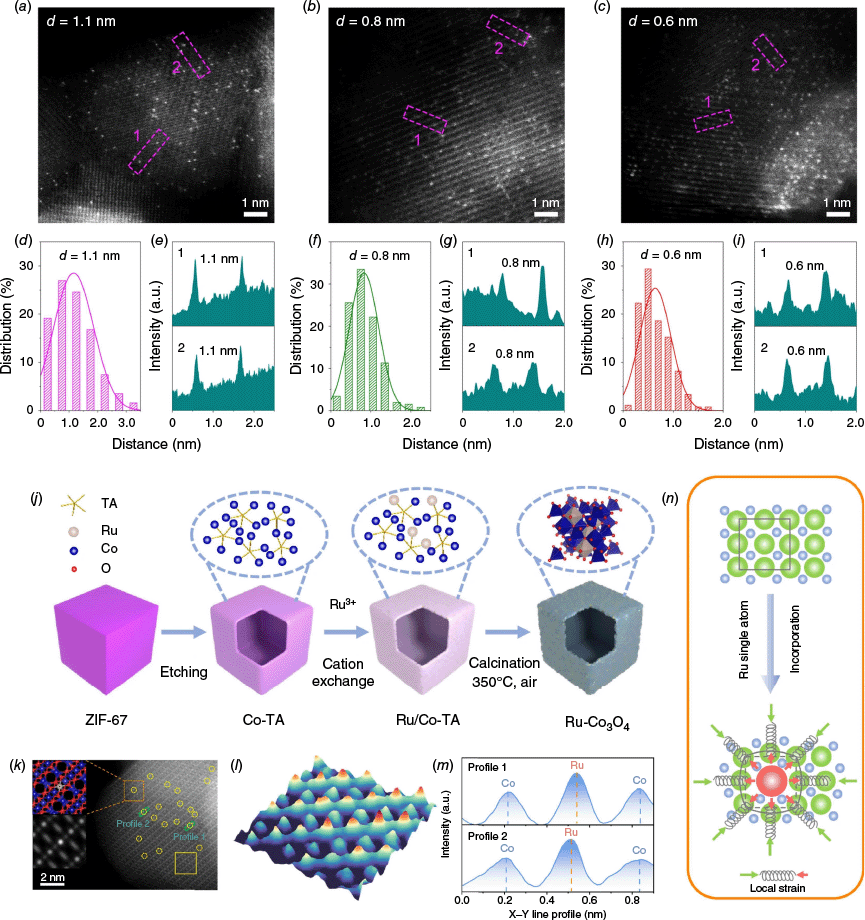
Furthermore, the incorporation of noble metal atoms can induce lattice distortion and local strain within the Co3O4 matrix, which further tailors its electronic structure and enhances its intrinsic catalytic performance. For example, Zuo et al. demonstrated that embedding larger-sized Ru single atoms into the Co3O4 lattice generates localised compressive strain, leading to shortened Co–O bonds and enhanced lattice integrity.25 Operando X-ray absorption and theoretical studies confirmed that this local strain effectively suppresses Co dissolution, thereby significantly improving the corrosion resistance and long-term OER durability (Fig. 3n), with the catalyst maintaining stable operation for over 400 h at 30 mA cm−2 in acidic media.
Doping Co3O4 with non-noble transition metals (Fig. 4) represents a cost-effective and versatile strategy to enhance its stability and catalytic performance under acidic OER conditions. Elements such as 3d transition metal elements (Mn18,19 Ni53), 4d elements (Mo,32,62 Zr31) and a 5d element (W63) have been widely explored due to their ability to modulate the electronic structure and defect chemistry of the host lattice, thereby improving both corrosion resistance and redox kinetics. Because of the high flexibility of electrons in 3d metals,72 3d dopants primarily modulate the local electronic structure and promote active site generation through lattice distortion and defects. Among 3d transition metals, manganese (Mn) has garnered particular attention due to its unique ability to improve both the acid stability and catalytic activity of Co3O4.64,65 Notably, Li et al. demonstrated that incorporating Mn into the spinel lattice of Co3O4 to form Co2MnO4, which markedly enhances the catalyst durability under acidic OER conditions.65 DFT calculations rationalise the enhanced OER activity and long-term durability of Co2MnO4 by demonstrating near-ideal adsorption energies for key reaction intermediates and revealing that the robust Mn-O bonds mitigate lattice oxygen loss, thereby preventing structural degradation. As a result, Co2MnO4 was found to have an activity capable of delivering 1000 mA cm−2 below 2 V (v. RHE) without iR correction and exhibited a remarkable operational stability of over 1500 h at 200 mA cm−2 in pH 1 electrolyte, which is comparable to state-of-the-art Ir-based catalysts.
Compared to 3d metals, 4d/5d elements possess more spatially extended d-electronic wave functions, enabling stronger orbital hybridisation with the 3d element (Co). This interaction tailors the electronic structure to optimise OER intermediates adsorption and modulate both activity and stability, making these elements particularly promising under harsh conditions. For instance, a recent study by Sun et al. demonstrated that doping a small amount of Mo (~0.5 wt%) into spinel Co3O4 creates oxygen vacancies that lead to a stable OPM pathway during acidic OER.32 The resulting Mo-doped oxygen vacancy enriched Co3O4 (VO-MoxCo3−xO4) achieved a current density of 100 mA cm−2 at an overpotential of 490 mV, while maintaining stable performance over extended operation. Detailed experimental evidence and DFT calculations revealed that the successful pathway transition can be attributed to the synergistic effects of oxygen vacancy engineering and electronic structure modulation. Specifically, Mo doping activates lattice oxygen and introduces a controlled concentration of oxygen vacancies, which promotes O–O radical formation and steer the reaction from vacancy-driven LOM to the more stable OPM pathway. The variable valence states of Mo (e.g. Mo4+/Mo6+) help stabilise these vacancies, avoiding excessive structural degradation. At the same time, Mo incorporation modulates the Co 3d-O 2p orbital interaction, enhancing *OH adsorption and deprotonation, which are the key steps in OPM. Spectroscopic identification of *–O–O– and *–O–O intermediates near 1.7 V v. RHE further confirms the OPM pathway.
Another 5d element, W, is known for its tunable chemical states, with flexible oxidation states and coordination numbers enabling versatile interactions, it has also attracted increasing research interests. W not only exhibits high electrical conductivity and positive charge density but also possesses large spin magnetic moments, which contribute to improved charge transfer kinetics and structural stability. For example, Cao et al. demonstrated that atomically dispersing tungsten into the spinel lattice of Co3O4 (W-Co3O4) achieves a remarkably low overpotential of only 251 mV at 10 mA cm−2 and maintains stable performance over 240 h at an industrially relevant current density of 1 A cm−2 in a PEMWE system.63 Combined experimental and DFT studies reveal that single W atoms act as highly active sites, outperforming the intrinsic activity of Co in acidic environments, while simultaneously stabilising surface Co and O atoms against corrosion during the OER process.
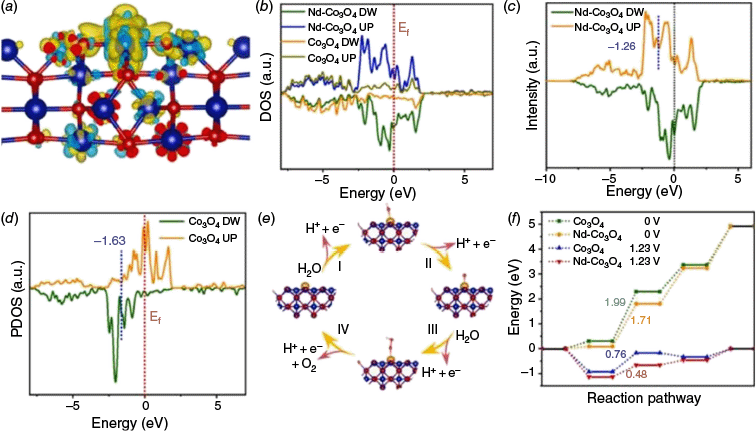
The incorporation of these rare-earth elements (La, Ce, Er, etc.) into Co3O4 has emerged as an effective strategy to boost its electrocatalytic performance.48,56,73,74 Owing to the distinctive 4f electron configurations, variable valence states and reasonably large ionic radii of rare-earth elements, they offer valuable opportunities to tailor the electronic structure and surface chemistry of the host material.66,67 These characteristics enable rare-earth element dopants to modulate the physicochemical environment of Co3O4, facilitating improvements in intrinsic activity and catalytic stability. Mechanistically, rare-earth elements doping can promote the generation of lattice defects, tune the band structure, optimise the adsorption energies of reaction intermediates and enhance the material’s structural robustness under harsh electrochemical conditions. For example, Zhang et al. developed a high-performance Nd-doped Co3O4 catalyst for acidic OER by introducing neodymium into the spinel lattice by hydrothermal and thermal calcination strategies.48 The catalyst exhibited a low overpotential of 304 mV at 10 mA cm−2 in 0.5 M of H2SO4 and demonstrated excellent long-term stability over 24 h in a PEM flow cell, highlighting its potential for practical acidic water electrolysis applications. DFT calculations revealed that Nd incorporation enhanced the electrical conductivity by the electron accumulation around Nd–O bonds (Fig. 5a). In addition, the total density of states (DOS) analysis showed a significant increase in the DOS near the Fermi level (Fig. 5b–d), indicating a higher carrier concentration and increased electronic conductivity. Furthermore, DFT revealed that the Nd doping had optimised the adsorption energy of intermediates and reduced the energy barrier for critical OER steps (Fig. 5e, f). DFT calculations demonstrated a high solubility energy for Nd atoms in Co3O4 (−4.41 eV), suggesting strong thermodynamic stability against leaching and indicating robust structural integrity of the Nd-doped active sites under harsh OER conditions. These results demonstrate that Nd plays a key role in enhancing both catalytic activity and stability.
(a) Charge density difference of the Nd-Co3O4 model (the light green and yellow regions represent the electron accumulation and deletion respectively). (b) DOS and (c, d) Calculated d-band centres of Co sites for Co3O4 and Nd-Co3O4 models. (e) The four electron OER pathway of Nd-Co3O4. (f) Calculated free energy diagram of O adsorption.48
Compared with transition metal doping, non-metal element incorporation offers an alternative and often complementary approach to tuning the electronic structure, surface chemistry and catalytic behaviour of Co3O4. Light non-metal elements can introduce significant local charge redistribution due to their electronegativity differences and unique bonding characteristics. These dopants often promote the formation of oxygen vacancies, modulate M–O covalency and alter the spin or orbital occupancy of Co cations, all of which can substantially influence the OER kinetics. More importantly, non-metal doping typically avoids introducing unstable metal–metal interactions or redox-inactive species, making it particularly attractive for enhancing catalytic activity while maintaining structural integrity under acidic conditions. As such, non-metal doping emerges as a versatile strategy for optimising the performance of Co3O4-based catalysts in PEMWE.
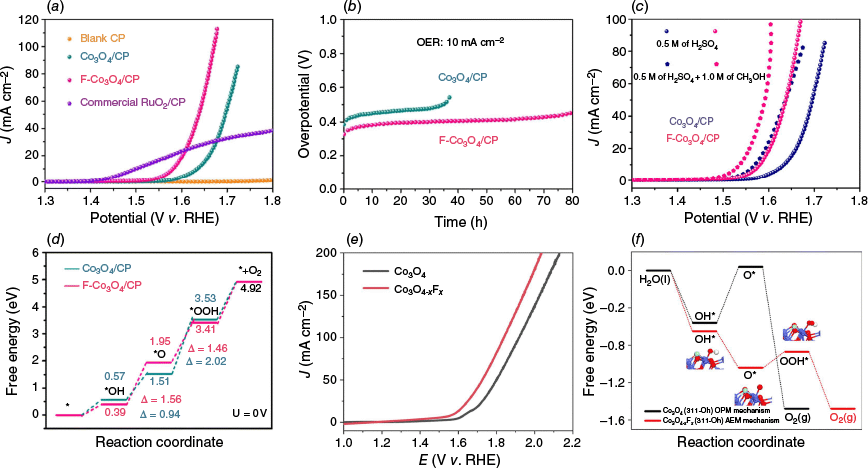
Fluorine (F) has attracted special attention, given its high electronegativity and strong electron-withdrawing nature, it can effectively modulate the local electronic structure and enhance M–O covalency in Co3O4. A recent study by Hao et al. demonstrated that trace amounts of F doped into Co3O4 nanoneedles significantly enhanced both activity and stability in acidic OER. The F-doped Co3O4/CP catalyst exhibited an overpotential of 350 mV at 10 mA cm−2 (Fig. 6a) and maintained stable performance for over 80 h (Fig. 6b).49 Mechanistic investigations revealed that F incorporation promotes OH− coverage on the catalyst surface (Fig. 6c), inhibits acid-induced corrosion and facilitates the formation of OER intermediates such as *OOH. DFT calculations further confirmed that F doping improves electron transfer and lowers the energy barrier for key reaction steps (Fig. 6d). Similarly, Wang et al. reported that introducing F atoms into Co3O4 generates geometrically reconstructed F–Co–O active sites within a Co3O4−xFx phase, enabling pre-activation of cobalt species and altering the OER reaction pathway.52 The resulting catalyst delivered an impressive overpotential of 349 mV at 10 mA cm−2 (Fig. 6e) and demonstrated an exceptional operational durability of 120 h at 100 mA cm−2 in acidic media. Quasi in situ and operando characterisations, coupled with DFT calculation results (Fig. 6f), revealed that F doping facilitates a switch in the rate-determining step (RDS) and modulates the pre-oxidation behaviour of Co sites, thereby boosting the formation of reactive CoIV = O species with faster kinetics.
(a) LSV polarisation curves; (b) stability testing; (c) LSV curves in 0.5 M of H2SO4 with and without 1.0 M of CH3OH; (d) the ΔG diagram of the OER (311) of Co3O4/CP and F-Co3O4/CP.49 (e) LSV polarisation curves of Co3O4−xFx and Co3O4 without iR correction. (f) Free energy diagram for the OER at Oh3+ over pristine Co3O4 and Co3O4−xFx at 1.6 V v. RHE. Oh denotes the octahedral Co sites.52
Heterostructure construction
Constructing heterostructures represents another promising strategy to synergistically integrate the complementary properties of different components, thereby enabling enhanced electronic modulation, interfacial charge transfer and structural robustness in electrocatalysts. For Co3O4-based systems, forming heterojunctions with other functional oxides such as CeO2 or RuO2 has shown great promise in improving OER activity and stability.23–25 For example, Huang et al.51 demonstrated that the introduction of nanocrystalline CeO2 can effectively modify the redox properties and local bonding environment of Co3O4, facilitating the oxidation of Co3+ to catalytically active Co4+ without undergoing surface reconstruction. As a result, the Co3O4/CeO2 nanocomposite exhibited enhanced acidic OER activity, achieving overpotentials of ~423 mV (Fig. 7a) and 347 mV (Fig. 7b) at 10 mA cm−2 on fluorine-doped tin oxide (FTO) and carbon paper electrodes respectively, while maintaining stability comparable to pristine Co3O4. This electronic modulation not only enhances the intrinsic OER activity but also maintains comparable stability to pristine Co3O4, thereby breaking the conventional activity-stability trade-off. Combined mechanistic insights from kinetic isotope effects, pH/temperature dependence and in situ/ex situ spectroscopic analyses further confirmed that CeO2 acts as a redox-active promoter that tunes the local coordination and accelerates the OER kinetics under acidic conditions.
(a) iR-corrected CV curves of both catalysts, the inset shows the overpotential (with error bar) required for each catalyst to reach a geometric catalytic current density of 10 mA cm−2 based on the averages of three individual electrodes. (b) CV curves of Co3O4 and Co3O4/CeO2 catalysts on carbon paper electrodes recorded in 0.5 M of H2SO4 solution, in comparison with the bare carbon paper electrode and the benchmark RuO2 catalyst on carbon paper electrode.51 (c) In situ ATR-IR spectra recorded during the multi-potential steps of Co3O4/RuO2-C. (d) In situ Raman spectrum of Co3O4/RuO2-C.75 (e) Schematic illustration of heterointerface electron migration in acid-treated RuO2/Co3O4. (f) OER pathway in an acidic electrolyte for acid-treated RuO2/Co3O4.76
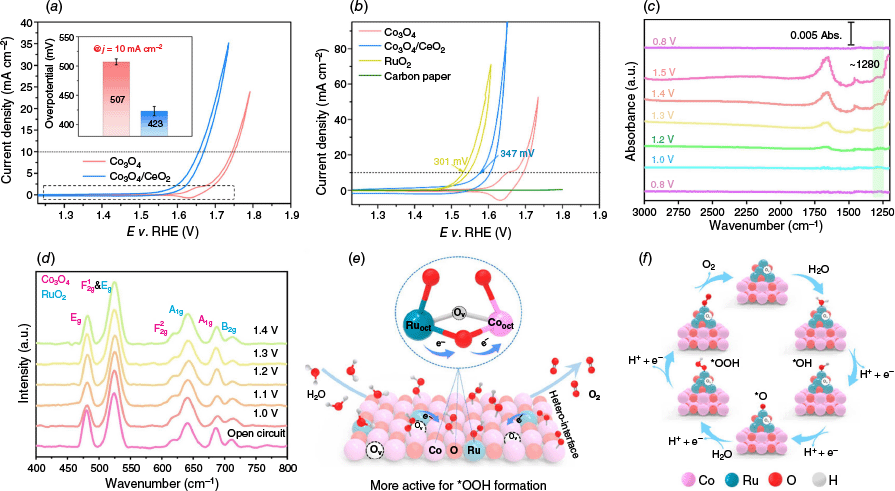
Coupling Co3O4 with highly conductive and catalytically active RuO2 has also been demonstrated for boosting acidic OER performance. The formation of a p–n heterojunction between p-type Co3O4 and n-type RuO2 facilitates efficient charge separation and interfacial electron redistribution, thereby lowering the reaction energy barrier. Zhou et al. developed a Co3O4/RuO2 heterojunction supported on carbon with only 2.74 wt% Ru loading, which achieved an impressively low overpotential of 170 mV at 10 mA cm−2 in 0.1 M of HClO4, along with excellent operational durability.75 The strong coupling at the p–n junction interface facilitated electron transfer from RuO2 to Co3O4, effectively pre-modifying the electronic structure of Ru and promoting the generation of active Ru sites. In situ ATR-IR (Fig. 7c) and in situ Raman spectroscopy (Fig. 7d) further revealed that the OER proceeds via an AEM pathway and that the presence of Co3O4 accelerates the redox dynamics of RuO2. DFT calculations confirmed that the heterojunction structure lowers the energy barriers for OER intermediates, offering a clear mechanistic rationale for the observed high performance. In another study, Huang et al. developed an acid-treated RuO2/Co3O4 nanostructured catalyst featuring a well-defined heterogeneous interface and low Ru loading, which exhibited a remarkable overpotential of 152 mV at 10 mA cm−2 in 0.5 M of H2SO4, far outperforming commercial RuO2 (221 mV).76 Through comprehensive characterisations, they revealed that the electron transfer occured from octahedral Ru (Ruoct) to CooctIII sites by shared oxygen atoms at the interface (Fig. 7e, f), and this interaction was further strengthened by the presence of oxygen vacancies. These vacancies, in synergy with the heterojunction structure, modulate electronic dispersion and optimise the adsorption of key oxygen intermediates such as *OOH, thereby accelerating OER kinetics. The catalyst maintained excellent stability over 150 h with a low degradation rate of 0.67 mV h−1.
Surface modification
Applying a protective layer on the catalyst surface is another promising strategy to increase the corrosion resistance of Co3O4. Both the carbon layer and the metal oxide layer (such as a TiO2 layer with high corrosive resistance) have been widely studied. Yang et al. developed carbon-coated Co3O4 nanoarrays (Co3O4@C/CP) using an electroplating and two-step thermal treatment approach, in which an amorphous carbon layer was formed in situ during vacuum annealing to enhance both the catalyst-substrate interface and surface protection.77 This design enabled Co3O4@C/CP to deliver outstanding acid stability with a lifetime exceeding 86.8 h at 100 mA cm−2 in 0.5 M of H2SO4 and a low overpotential of 370 mV at the same current density.77 The improved durability originates from the robust interfacial contact and the corrosion-resistant carbon shell that prevents catalyst degradation under oxidative potentials. Similarly, Lai et al. reported a one-step synthesis of Co3O4 nanoparticles embedded in a hydrophobic mesoporous carbon matrix (Co/29BC), in which the carbon layer formed during the thermal decomposition process significantly enhanced conductivity and protected the Co3O4 surface from acid corrosion. The resulting catalyst showed a low overpotential of 350 mV at 10 mA cm−2 and sustained stable operation over 50 h in a PEM water electrolyser at 10 mA cm−2.78 Here, the combination of optimised Co3+ active sites, surface oxygen vacancies and a protective carbon coating contributed to both high activity and acid resistance.
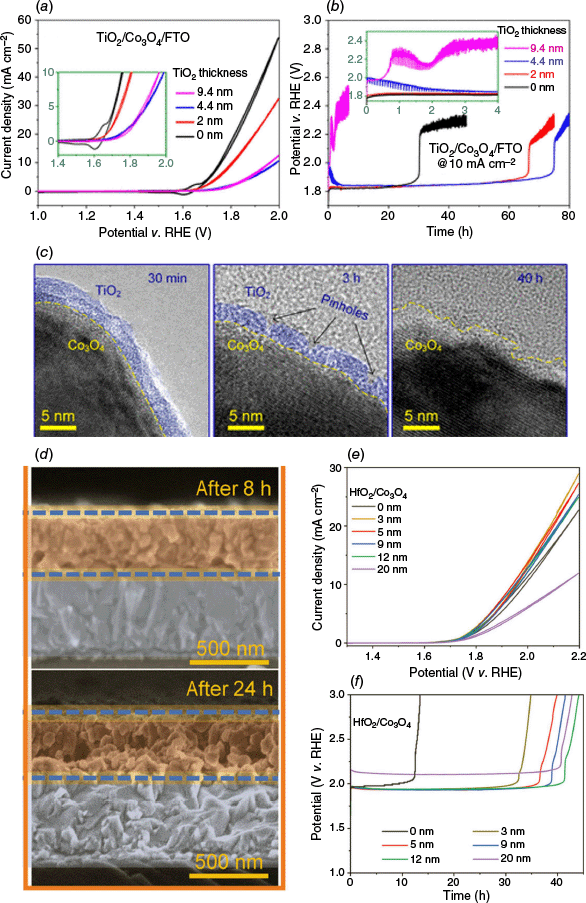
In addition to carbon layers, coating Co3O4 with corrosion-resistant metal oxides has also been demonstrated to be effective. For instance, Tran-Phu et al. investigated the effect of amorphous TiO2 coatings introduced by atomic layer deposition (ALD) on the stability and activity of nanostructured Co3O4 electrocatalysts.79 Their study revealed a strong correlation between TiO2 thickness and OER performance. Specifically, a 4.4-nm TiO2 layer offered an optimal balance between surface protection and catalytic accessibility (Fig. 8a, b). Furthermore, this coating extended the catalyst lifetime by approximately threefold (up to 80 h of continuous operation at near zero pH), while maintaining high activity, as controlled pitting in the thin oxide layer allowed efficient reactant penetration (Fig. 8c). Building upon this insight, Ta et al. conducted a systematic comparison of various dielectric ALD coatings (including Al2O3, SiO2, TiO2, SnO2 and HfO2) on Co3O4 OER anodes.50 Among these, HfO2 was identified as the most effective protective layer. Notably, the HfO2 coating was able to maintain its thickness even after prolonged operation (Fig. 8d). A 12-nm HfO2 coating extended the operational lifetime in 1 M H2SO4 by more than threefold (Fig. 8e, f).50 DFT calculations provided a molecular-level understanding of this performance trend. By simulating the (110) surface of Co3O4 interacting with different oxide coatings by Co–O–M (M is metal) bridges, they found that the bonding energy followed the order: Co–O–Hf (−3.48 eV) > Co–O–Sn (−3.44 eV) > Co–O–Al (−3.34 eV) > Co–O–Ti (−3.04 eV) > Co–O–Si (−2.89 eV). The strongest Co–O–Hf interaction suggests a more robust interface, which underpins the superior structural stabilisation and corrosion resistance observed experimentally.
(a) Cyclic voltammetry (scan rate, v = 0.005 V s−1; 5th recorded scan) recorded for as-prepared Co3O4 (black line) and with various TiO2 thickness (inset shows magnified plot of precatalytic region)79 and (b) corresponding chronopotentiometric curves (inset displaying a magnified plot of initial 4 h of testing).79 (c) TEM images of 4.4-nm TiO2/Co3O4/FTO after 30 min, 3 h and 40 h of chronopotentiometric measurements.79 (d) Cross-sectional SEM images of Co3O4/FTO anodes coated with 3–4 nm thick overlayers of HfO2 after 8 (top) and 24 h (bottom) of galvanostatic OER tests in 1 M of H2SO4 at 10 mA cm−2 (orange, Co3O4 electrocatalyst; blue, FTO; and the blue dashed line: the boundary of pristine Co3O4 layer).50 (e) Quasi-stabilised cyclic voltammetric sweeps (third scan with scan rate 0.005 V s−1) of Co3O4/FTO electrodes coated with different HfO2 thicknesses.50 (f) Chronopotentiometric curves recorded at 10 mA cm−2 in 1 M of H2SO4. Currents are normalised to the geometric surface area.50
Defect engineering and lattice regulation
Defect engineering, particularly the introduction and regulation of lattice vacancies, has emerged as a powerful approach to modulate the catalytic properties of spinel Co3O4. Among the various types of defects, oxygen vacancies play a critical role in enhancing the OER performance and stability under acidic conditions. Oxygen vacancies are known to increase the electronic conductivity of the material and expose more active sites by disrupting the local coordination environment.80 They can also promote the LOM by enhancing oxygen ion mobility and facilitating the formation and desorption of oxygen intermediates.81,82 However, the high reactivity of lattice oxygen in the LOM often leads to structural degradation and dissolution, particularly in acidic media. To address this issue, lattice-level regulation strategies have been developed to precisely tailor the concentration and distribution of oxygen vacancies. For instance, combining defect formation with cation doping (e.g. Mn, Ti, Mo) can create a stabilised defective environment in which the oxygen vacancies are compensated by the presence of high-valence dopants, preventing framework collapse while still enhancing OER activity. Additionally, controlled thermal treatments83 or reducing gases such as H2 or H2–N2 mix84,85 can be employed to induce subsurface oxygen vacancies while maintaining a robust surface structure. However, uncontrolled or excessive oxygen vacancies, especially on the surface, can compromise the structural integrity of Co3O4, particularly in acidic environments where Co2+ species become soluble. This often leads to rapid degradation of the catalyst during operation.
Recent studies have also provided evidence that moderate and well-regulated oxygen vacancies are crucial for achieving the desired balance between activity and durability. For example, Rong et al. reported the design of vacancy-rich Co3O4 hollow nanocubes (Vo–Co3O4 HNCs) that exhibited excellent OER performance under acidic conditions, with a low overpotential of 265 mV at 10 mA cm−2 and remarkable long-term stability over 130 h at 20 mA cm−2, outperforming RuO2 and most noble-metal-based catalysts.86 The catalyst was further tested in a PEMWE device, which achieved a 1 A cm−2 at a voltage corresponding to ~48.8 kWh kg−1 H2. Through a combination of experimental and theoretical analyses, the authors demonstrated that oxygen vacancies not only lowered the energy barriers for intermediate adsorption and desorption, thereby enhancing catalytic kinetics, but also inhibited Co dissolution by modulating the LOM pathway, preserving structural integrity. These results clearly validate that oxygen vacancy concentration must be precisely engineered to avoid over-defectiveness that leads to catalyst degradation.
Additionally, Recent studies have shown that the deliberate introduction of oxygen vacancies, especially in combination with transition metal dopants, can effectively trigger a transition from the conventional AEM to the LOM pathway, thereby enhancing both the intrinsic activity and structural robustness of Co3O4-based catalysts. For example, Deng et al. employed a dual-doping strategy with Mn and Ru, accompanied by a moderate density of oxygen vacancies, to promote LOM participation, resulting in a significantly lowered overpotential (230 mV at 10 mA cm−2) and a 12-fold improvement in operational stability over pristine Co3O4 under acidic conditions.87 DFT calculations in this work further confirmed that such defect–dopant synergy facilitates O–O bond formation by *OH and lattice oxygen, offering a robust route toward high-efficiency acidic OER electrocatalysts.
Conclusion and perspectives
Co3O4 has emerged as a promising non-precious catalyst for acidic OER, owing to its promising performance results from its rich redox chemistry, flexible valence states and structural tunability. Despite extensive efforts to enhance their activity and durability through various structural and electronic modifications, Co3O4-based catalysts in acidic PEMWE still fall short of the performance requirements for industrial applications. In this review, we analysed the fundamental mechanisms underlying OER, including AEM, LOM and OPM and emphasised the importance of mechanistic transitions in optimising both activity and durability. Then we systematically examined the structural and electronic characteristics of Co3O4 and their implications for acidic OER, highlighting the influence of the electronic configuration and spatial arrangement of cobalt ions, surface morphology, as well as the crystal facet exposure. Building on the understanding of these, a broad range of engineering strategies, including heteroatom doping, heterostructure construction, surface modification, defect engineering and lattice regulation, have been employed to enhance the catalytic performance of Co3O4 through electronic tuning and structural stabilisation.
Despite these advances, critical challenges remain, including achieving both high activity and long-term stability, understanding degradation mechanisms under real operating conditions and assessing performance in practical PEM electrolysers. The integration of advanced in situ characterisations, application-specific testing and data-driven design approaches will be essential for future breakthroughs. To this end, these aspects require particular research efforts for achieving active and durable cobalt-based, or in general non-noble-metal-based, electrocatalysts for large-scale green hydrogen production in acidic environments.
Trade-offs between activity and stability
One of the most critical challenges in acidic OER catalysis lies in balancing high intrinsic activity with long-term durability. Although various strategies have successfully enhanced OER kinetics, they may also accelerate structural degradation, particularly under acidic and high-potential conditions. Highly active sites, such as low-coordination metal centres or labile lattice oxygen, are often thermodynamically unstable, making them prone to dissolution or reconstruction. Therefore, future research should focus on identifying design principles that achieve a delicate balance between reactivity and resilience, for instance, by introducing stabilising elements, forming self-healing surface layers or dynamically regulating active phases under operating conditions.
Degradation mechanisms
Despite recent advances, the fundamental understanding of degradation pathways in acidic environments remains limited due to the complexity of solid–liquid–gas interfaces under high oxidative potentials. Traditional ex situ methods fail to capture dynamic changes during OER, highlighting the urgent need for in situ and operando techniques. The use of advanced characterisation tools, such as in situ X-ray absorption spectroscopy (XAS), Raman spectroscopy and electrochemical mass spectrometry (EC-MS), has begun to shed light on the real-time evolution of surface structure, valence states and intermediate species.88,89 Combining these with isotopic labeling and electrochemical kinetic analysis will be key to mapping out degradation mechanisms such as metal dissolution, oxygen vacancy accumulation, phase transformation and active site reconstruction. A more comprehensive mechanistic picture will ultimately guide the rational design of robust and corrosion-resistant electrocatalysts.
Device application
Although the search for efficient acidic OER catalysts has largely focused on intrinsic material properties, it is equally important to evaluate catalyst performance within realistic membrane electrode assembly (MEA) configurations under industrially relevant current densities (≥1 A cm−2). Parameters such as catalyst loading, mass transport, ionic conductivity and interfacial adhesion significantly influence overall device performance.90 Furthermore, application-specific requirements, such as intermittent operation under fluctuating renewable energy input, pose additional challenges for catalyst stability and efficiency. Therefore, a unified evaluation framework that integrates electrochemical metrics (overpotential, Tafel slope, durability), techno-economic indicators (cost per kilowatt) and device-level testing is essential for guiding material selection toward practical implementation in large-scale PEM electrolysers.
AI-guided materials discovery
The immense compositional space of Co3O4 and other transition metal oxides and their derivatives offers vast opportunities for discovering novel OER electrocatalysts, yet traditional trial-and-error approaches are time-consuming and resource-intensive. The integration of high-throughput computation, machine learning and materials informatics provides a promising avenue to accelerate catalyst discovery. AI-guided frameworks can identify key descriptors (M–O covalency, e.g. orbital filling, dissolution potential) from large datasets and predict optimal compositions with tailored activity and stability. Coupling these predictions with experimental automation platforms enables rapid validation and iteration. In the future, closed-loop systems combining in silico modelling, robotic synthesis and adaptive learning may significantly reduce the development cycle of next-generation OER catalysts, facilitating the deployment of efficient, earth-abundant and acid-stable materials in practical water electrolysers.
Data availability
Data sharing is not applicable as no new data were generated or analysed during this study. The data presented in the figures were derived from previous published work, and the relevant references are provided in the corresponding sections of the main text.
Declaration of funding
The study is supported by the Australian Research Council (IC200100023, CE230100017, DP250101509, FL250100099, shared by Shuhao Wang and Chuan Zhao).
References
1 Li W, Ni Z, Akdim O, Liu T, Zhu B, Kuang P, et al. Dual active site engineering in porous NiW bimetallic alloys for enhanced alkaline hydrogen evolution reaction. Adv Mater 2025; 37: 2503742.
| Crossref | Google Scholar | PubMed |
2 Periasamy P, Mohanta YK. Chapter 3. Global energy crisis: need for energy conversion and storage. In: I. Chakrabartty, K. R. Hakeem, editors. Green nanomaterials in energy conversion and storage applications. Apple Academic Press; 2024. pp. 1–29. doi:10.1201/9781003398578-3
3 Zhang W, Yuan W, Zhang X, Ke Y, Wu Y, Bai Y, et al. Functional high-entropy alloys: promising catalysts for high-performance water splitting. J Mater Chem A 2024; 12(30): 18705-18732.
| Crossref | Google Scholar |
4 Sharma SK, Wu C, Malone N, Titheridge LJ, Zhao C, Gupta P, et al. Ru-based catalysts for proton exchange membrane water electrolysers: the need to look beyond just another catalyst. Int J Hydrog Energy 2025; 102: 1461-1479.
| Crossref | Google Scholar |
5 Kwofie F, He Z, Zeng W, Gao M, Li Y, Zhang J, et al. Metallic ruthenium and ruthenium oxide heterojunctions boost acidic oxygen evolution reaction activity and durability. Electrochim Acta 2025; 512: 145442.
| Crossref | Google Scholar |
6 Wang S, Dastafkan K, Wu S, Sun Q, Rong C, Yao D, et al. Anionic oxidation activity/stability regulated by transition metals in multimetallic (oxy)hydroxides for alkaline water oxidation. ACS Catal 2025; 15(1): 44-53.
| Crossref | Google Scholar |
7 Baibars IO, Huang H, Xiao Y, Wang S, Nie Y, Jia C, et al. Efficient hydrogen evolution at Ni/CeOx interfaces in anion-exchange membrane water electrolysers. Energy Env Sci 2025; 18(12): 6248-6259.
| Crossref | Google Scholar |
8 Feng W, Chang B, Ren Y, Kong D, Tao HB, Zhi L, et al. Proton exchange membrane water splitting: advances in electrode structure and mass-charge transport optimization. Adv Mater 2025; 37: 2416012.
| Crossref | Google Scholar | PubMed |
9 Song Y, Chen H, Wang X, Weng C, Zou K, Wang C, et al. Engineering Ir-based catalysts for high current density applications in proton exchange membrane water electrolyzers. Energy Environ Sci 2025; 18(1): 130-154.
| Crossref | Google Scholar |
10 Liu Z, Wang X, Xie G, Ge J. Acid oxygen evolution reaction: mechanisms, design principles, and prospects for application in membrane electrodes. Chem Eng J 2024; 499: 155901.
| Crossref | Google Scholar |
11 Zhang D, Wu Q, Wu L, Cheng L, Huang K, Chen J, et al. Optimal electrocatalyst design strategies for acidic oxygen evolution. Adv Sci 2024; 11(38): 2401975.
| Crossref | Google Scholar | PubMed |
12 Liu H, Zhang Z, Fang J, Li M, Sendeku MG, Wang X, et al. Eliminating over-oxidation of ruthenium oxides by niobium for highly stable electrocatalytic oxygen evolution in acidic media. Joule 2023; 7(3): 558-573.
| Crossref | Google Scholar |
13 Li W, Wang C, Lu X. Breaking the bottleneck of activity and stability of RuO2-based electrocatalysts for acidic oxygen evolution. Nano Lett 2024; 24(38): 11779-11792.
| Crossref | Google Scholar | PubMed |
14 Zi Y, Zhang C, Zhao J, Cheng Y, Yuan J, Hu J. Research progress in structure evolution and durability modulation of Ir- and Ru-based OER catalysts under acidic conditions. Small 2024; 20(50): 2406657.
| Crossref | Google Scholar | PubMed |
15 Lu Q, Liu J, Zou X, Huang B, Wu W, Yin J, et al. Breaking the activity-stability trade-off of RuO2 via metallic ru bilateral regulation for acidic oxygen evolution reaction. Angew Chem Int Ed 2025; 64(22): e202503733.
| Crossref | Google Scholar | PubMed |
16 Chen G, Lu R, Ma C, Zhang X, Wang Z, Xiong Y, et al. A long-range disordered RuO2 catalyst for highly efficient acidic oxygen evolution electrocatalysis. Angew Chem Int Ed 2024; 63(50): e202411603.
| Crossref | Google Scholar | PubMed |
17 Liu Y, Wang Y, Li H, Kim MG, Duan Z, Talat K, et al. Effectiveness of strain and dopants on breaking the activity-stability trade-off of RuO2 acidic oxygen evolution electrocatalysts. Nat Commun 2025; 16(1): 1717.
| Crossref | Google Scholar | PubMed |
18 Zhang J, Fu X, Kwon S, Chen K, Liu X, Yang J, et al. Tantalum-stabilized ruthenium oxide electrocatalysts for industrial water electrolysis. Science 2025; 387(6729): 48-55.
| Crossref | Google Scholar | PubMed |
19 Ni J, Shi Z, Wang Y, Yang J, Wu H, Wang P, et al. Development of noble metal-free electrocatalysts towards acidic water oxidation: from fundamental understanding to state-of-the-art catalysts. eScience 2024; 5: 100295.
| Crossref | Google Scholar |
20 Li J, Tian W, Li Q, Zhao S. Acidic oxygen evolution reaction: fundamental understanding and electrocatalysts design. ChemSusChem 2024; 17(15): e202400239.
| Crossref | Google Scholar | PubMed |
21 Dong Z, Li B, Zhu Y. Noble-metal-free metal oxides for catalyzing acidic oxygen and hydrogen evolution reactions: recent developments and future perspectives. Energy Fuels 2024; 38(14): 12387-12408.
| Crossref | Google Scholar |
22 Wang C, Deng R, Guo M, Zhang Q. Recent progress of advanced Co3O4-based materials for electrocatalytic oxygen evolution reaction in acid: from rational screening to efficient design. Int J Hydrog Energy 2023; 48: 31920-31942.
| Crossref | Google Scholar |
23 Nguyen KN, Hoang Nguyen LB, Bui TK, Nguyen KQ, Pham VV. Review of water splitting electrolysis over cobalt oxide nanomaterials. ACS Appl Nano Mater 2025; 8(7): 3254-3271.
| Crossref | Google Scholar |
24 Zhang Z, Jia C, Ma P, Feng C, Yang J, Huang J, et al. Distance effect of single atoms on stability of cobalt oxide catalysts for acidic oxygen evolution. Nat Commun 2024; 15(1): 1767.
| Crossref | Google Scholar | PubMed |
25 Zuo S, Wu ZP, Xu D, Ahmad R, Zheng L, Zhang J, et al. Local compressive strain-induced anti-corrosion over isolated Ru-decorated Co3O4 for efficient acidic oxygen evolution. Nat Commun 2024; 15(1): 9514.
| Crossref | Google Scholar | PubMed |
26 Priamushko T, Franz E, Logar A, Bijelić L, Guggenberger P, Escalera-López D, et al. Be aware of transient dissolution processes in Co3O4 acidic oxygen evolution reaction electrocatalysts. J Am Chem Soc 2025; 147(4): 3517-3528.
| Crossref | Google Scholar | PubMed |
27 Chong L, Gao G, Wen J, Li H, Xu H, Green Z, et al. La- and Mn-doped cobalt spinel oxygen evolution catalyst for proton exchange membrane electrolysis. Science 2023; 380(6645): 609-616.
| Crossref | Google Scholar | PubMed |
28 Ram R, Xia L, Benzidi H, Guha A, Golovanova V, Garzón Manjón A, et al. Water-hydroxide trapping in cobalt tungstate for proton exchange membrane water electrolysis. Science 2024; 384(6702): 1373-1380.
| Crossref | Google Scholar | PubMed |
29 Man IC, Su HY, Calle-Vallejo F, Hansen HA, Martínez JI, Inoglu NG, et al. Universality in oxygen evolution electrocatalysis on oxide surfaces. ChemCatChem 2011; 3(7): 1159-1165.
| Crossref | Google Scholar |
30 Yang X, Cheng J, Li H, Xu Y, Tu W, Zhou J. Self-supported N-doped hierarchical Co3O4 electrocatalyst with abundant oxygen vacancies for acidic water oxidation. Chem Eng J 2023; 465: 142745.
| Crossref | Google Scholar |
31 Zhao X, Shao Y, Cai J, Yue X, Huang S. Zirconium-doped Co3O4 for enhancing the acidic oxygen evolution reaction by triggering the lattice oxygen-mediated mechanism. ACS Appl Mater Interfaces 2025; 17: 36698-36705.
| Crossref | Google Scholar |
32 Sun L, Feng M, Peng Y, Zhao X, Shao Y, Yue X, et al. Constructing oxygen vacancies by doping Mo into spinel Co3O4 to trigger a fast oxide path mechanism for acidic oxygen evolution reaction. J Mater Chem A 2024; 12(15): 8796-8804.
| Crossref | Google Scholar |
33 Lewis NB, Bisbey RP, Westendorff KS, Soudackov AV, Surendranath Y. A molecular-level mechanistic framework for interfacial proton-coupled electron transfer kinetics. Nat Chem 2024; 16(3): 343-352.
| Crossref | Google Scholar | PubMed |
34 Ren X, Zhai Y, Yang N, Wang B, Liu S (Frank). Lattice oxygen redox mechanisms in the alkaline oxygen evolution reaction. Adv Funct Mater 2024; 34(32): 2401610.
| Crossref | Google Scholar |
35 Xie Y, Luo F, Yang Z. Acidic oxygen evolution reaction via lattice oxygen oxidation mechanism: progress and challenges. Energy Mater 2025; 5(3): 500026.
| Crossref | Google Scholar |
36 Diaz-Morales O, Calle-Vallejo F, de Munck C, Koper MTM. Electrochemical water splitting by gold: evidence for an oxide decomposition mechanism. Chem Sci 2013; 4(6): 2334-2343.
| Crossref | Google Scholar |
37 Qi M, Du X, Shi X, Wang S, Lu B, Chen J, et al. Single-atom Ru-triggered lattice oxygen redox mechanism for enhanced acidic water oxidation. J Am Chem Soc 2025; 147(21): 18295-18306.
| Crossref | Google Scholar | PubMed |
38 Cao J, Liang X, Gao W, Yin D, Bu X, Yang S, et al. Reversible lattice oxygen participation in Ru1−xO2−x for superior acidic oxygen evolution reaction. J Mater Chem A 2025; 13(22): 16807-16815.
| Crossref | Google Scholar |
39 Lei X, Jiang C, Han Q, Zhang X, Zhao K, Yan N, et al. Unraveling the oxygen vacancy site mechanism of a self-assembly hybrid catalyst for efficient alkaline water oxidation. ACS Catal 2024; 14(7): 4523-4535.
| Crossref | Google Scholar |
40 Wang N, Ou P, Miao RK, Chang Y, Wang Z, Hung SF, et al. Doping shortens the metal/metal distance and promotes OH coverage in non-noble acidic oxygen evolution reaction catalysts. J Am Chem Soc 2023; 145(14): 7829-7836.
| Crossref | Google Scholar | PubMed |
41 Cui M, Guo R, Zhou Y, Zhao W, Liu Y, Luo W, et al. Asymmetric site-enabled O–O coupling in Co3O4 for oxygen evolution reaction. ACS Catal 2024; 14(21): 16353-16362.
| Crossref | Google Scholar |
42 Luo W, Tian H, Li Q, Meng G, Chang Z, Chen C, et al. Controllable electron distribution reconstruction of spinel NiCo2O4 boosting glycerol oxidation at elevated current density. Adv Funct Mater 2024; 34(3): 2306995.
| Crossref | Google Scholar |
43 Tahira A, Padervand M, Dawi E, Aftab U, Ghasemi S, Vigolo B, et al. Co3O4 Hybrid electrocatalysts; materials description and mechanistic aspects toward hydrogen production, oxygen evolution-reduction, and CO2 reduction reactions. Chem Rec 2025; 25(3): e202400166.
| Crossref | Google Scholar | PubMed |
44 Mannu P, Dharman RK, Nga TTT, Mariappan A, Shao YC, Ishii H, et al. Tuning of oxygen vacancies in Co3O4 electrocatalyst for effectiveness in urea oxidation and water splitting. Small 2024; 21(4): 2403744.
| Crossref | Google Scholar | PubMed |
45 Zhou W, Chen M, Zhao D, Zhu C, Wang N, Lei W, et al. Engineering spin state in spinel Co3O4 nanosheets by V-doping for bidirectional catalysis of polysulfides in lithium–sulfur batteries. Adv Funct Mater 2024; 34(37): 2402114.
| Crossref | Google Scholar |
46 Zhou D, Yu J, Tang J, Li XY, Ou P. Octahedral Co2+-O-Co3+ in mixed cobalt spinel promotes active and stable acidic oxygen evolution. Adv Energy Mater 2025; 15(10): 2404007.
| Crossref | Google Scholar |
47 Li A, Sun Y, Yao T, Han H. Earth-abundant transition-metal-based electrocatalysts for water electrolysis to produce renewable hydrogen. Chem Eur J 2018; 24(69): 18334-18355.
| Crossref | Google Scholar | PubMed |
48 Zhang X, Zhang M, Zhang S, Baizhumanova TS, Tungatarova SA, Zhang H, et al. Rare-earth neodymium doping boosts the catalytic performance of Co3O4 for acidic water oxidation. J Alloy Compd 2025; 1031: 181103.
| Crossref | Google Scholar |
49 Hao G, Zhao T, Fang Q, Jia Y, Li D, Zhong D, et al. Trace F-doped Co3O4 nanoneedles for enhanced acidic water oxidation activity via promoting OH coverage. Green Chem 2024; 26(18): 9782-9790.
| Crossref | Google Scholar |
50 Ta XMC, Trần-Phú T, Yuwono JA, Nguyen TKA, Bui AD, Truong TN, et al. Optimal coatings of Co3O4 anodes for acidic water electrooxidation. Small 20: 2304650.
| Crossref | Google Scholar | PubMed |
51 Huang J, Sheng H, Ross RD, Han J, Wang X, Song B, et al. Modifying redox properties and local bonding of Co3O4 by CeO2 enhances oxygen evolution catalysis in acid. Nat Commun 2021; 12(1): 3036.
| Crossref | Google Scholar | PubMed |
52 Wang Y, Guo P, Zhou J, Bai B, Li Y, Li M, et al. Tuning the Co pre-oxidation process of Co3O4 via geometrically reconstructed F–Co–O active sites for boosting acidic water oxidation. Energy Env Sci 2024; 17(22): 8820-8828.
| Crossref | Google Scholar |
53 Wang T, Shi Y, Fei J, Zhu J, Song L, Li C, et al. Three-dimensional interconnected nanofibers consisting of ultra-small Ni-doped Co3O4 nanoparticles for acidic overall water splitting at high current density. Appl Catal B Environ Energy 2024; 358: 124367.
| Crossref | Google Scholar |
54 Zhang D, Zhang D, Cao Y, Chen Y, Liu B. Heterogeneous CoO/Co3O4 nanostructures as efficient anodes for hydrogen generation via acidic water electrolysis. ACS Appl Nano Mater 2025; 8(13): 6615-6625.
| Crossref | Google Scholar |
55 Liu Z, Ji Q, Li N, Tang B, Lv L, Liu Y, et al. Interface engineering a high content of Co3+ sites on Co3O4 nanoparticles to boost acidic oxygen evolution. Langmuir 2023; 39(46): 16415-16421.
| Crossref | Google Scholar | PubMed |
56 Zhao W, Xu F, Yang J, Hu X, Weng B. Ce Single-atom incorporation enhances the oxygen evolution reaction of Co3O4 in acid. Inorg Chem 2024; 63(4): 1947-1953.
| Crossref | Google Scholar | PubMed |
57 Wang Y, Jiao Y, Yan H, Yang G, Tian C, Wu A, et al. Vanadium-incorporated CoP2 with lattice expansion for highly efficient acidic overall water splitting. Angew Chem 2022; 134(12): e202116233.
| Crossref | Google Scholar |
58 Zhao M, Liang H. Crystal facet regulation and Ru incorporation of Co3O4 for acidic oxygen evolution reaction electrocatalysis. ACS Nanosci Au 2024; 4(6): 409-415.
| Crossref | Google Scholar | PubMed |
59 Sheng Z, Wang S, Jiang Q, Ni Y, Zhang C, Ahmad A, et al. Crystal facet evolution of spinel Co3O4 nanosheets in acidic oxygen evolution catalysis. Catal Sci Technol 2023; 13: 4542-4549.
| Crossref | Google Scholar |
60 Du Y, Xie F, Lu M, Lv R, Liu W, Yan Y, et al. Continuous strain tuning of oxygen evolution catalysts with anisotropic thermal expansion. Nat Commun 2024; 15(1): 1780.
| Crossref | Google Scholar | PubMed |
61 Zhu W, Yao F, Cheng K, Zhao M, Yang CJ, Dong CL, et al. Direct dioxygen radical coupling driven by octahedral ruthenium–oxygen–cobalt collaborative coordination for acidic oxygen evolution reaction. J Am Chem Soc 2023; 145: 17995-18006.
| Crossref | Google Scholar | PubMed |
62 Liu Z, Shen W, Liang X, Liang J, Xu K, Ge X, et al. Dual-regulation engineering of spinel Co3O4 via Mo electron buffering and asymmetric sites coupling for acidic oxygen evolution reaction. ACS Catal 2025; 15(11): 8943-8953.
| Crossref | Google Scholar |
63 Cao J, Zhang D, Ren B, Song P, Xu W. Tungsten single atoms incorporated in cobalt spinel oxide for highly efficient electrocatalytic oxygen evolution in acid. Energy Environ Sci 2024; 17(16): 5911-5921.
| Crossref | Google Scholar |
64 Zhu S, Yang R, Li H, Huang S, Wang H, Liu Y, et al. Reconstructing hydrogen-bond network for efficient acidic oxygen evolution. Angew Chem Int Ed 2024; 63: e202319462.
| Crossref | Google Scholar | PubMed |
65 Li A, Kong S, Guo C, Ooka H, Adachi K, Hashizume D, et al. Enhancing the stability of cobalt spinel oxide towards sustainable oxygen evolution in acid. Nat Catal 2022; 5(2): 109-118.
| Crossref | Google Scholar |
66 Wang X, Hu J, Lu T, Wang H, Sun D, Tang Y, et al. Importing atomic rare-earth sites to activate lattice oxygen of spinel oxides for electrocatalytic oxygen evolution. Angew Chem Int Ed 2025; 64(3): e202415306.
| Crossref | Google Scholar | PubMed |
67 Ajmal S, Rasheed A, Sheng W, Dastgeer G, Nguyen QAT, Wang P, et al. Synergetic modulation of electronic properties of cobalt oxide via “Tb” single atom for uphill urea and water electrolysis. Adv Mater 2025; 37(1): 2412173.
| Crossref | Google Scholar | PubMed |
68 Zhou Y, Mao Y, Ye C, Wang Z, Wei S, Kennedy JV, et al. Ru Single atoms anchored on Co3O4 nanorods for efficient overall water splitting under pH-universal conditions. Adv Energy Mater 15: 2500700.
| Crossref | Google Scholar |
69 Zeng Y, Yan L, Tian S, Sun X. Loading IrOx clusters on MnO2 boosts acidic water oxidation via metal–support interaction. ACS Appl Mater Interfaces 2023; 15(40): 47103-47110.
| Crossref | Google Scholar | PubMed |
70 Li L, Wang P, Shao Q, Huang X. Recent progress in advanced electrocatalyst design for acidic oxygen evolution reaction. Adv Mater 2021; 33(50): 2004243.
| Crossref | Google Scholar | PubMed |
71 Chen L, Liu Y, Cong H, Ge Q, Zhao W, Jiang N, et al. Recent progress and perspective for oxygen evolution reaction under acidic environments. Mater Chem Front 2024; 8(4): 986-1014.
| Crossref | Google Scholar |
72 Wang S, Liu X, Chen X, Dastafkan K, Fu ZH, Tan X, et al. Super-exchange effect induced by early 3d metal doping on NiFe2O4(0 0 1) surface for oxygen evolution reaction. J Energy Chem 2023; 78: 21-29.
| Crossref | Google Scholar |
73 Pan S, Li H, Wang T, Fu Y, Wang S, Xie Z, et al. Er-Doping enhances the oxygen evolution performance of cobalt oxide in acidic medium. ACS Catal 2024; 14(18): 13814-13824.
| Crossref | Google Scholar |
74 Zheng Z, Li J, Zhang T, Wang G, Jiang K, Hou X, et al. LaMn-doped cobalt spinel catalysts for enhanced oxygen evolution performance in acidic media. Int J Hydrog Energy 2024; 83: 682-689.
| Crossref | Google Scholar |
75 Zhou F, Chen J, Yang Y, Ke X, Liu X, Zhang L, et al. Exceptionally active and stable RuO2 by constructing p-n heterojunction between Co3O4 and RuO2 for acidic water oxidation. Appl Surf Sci 2023; 641: 158508.
| Crossref | Google Scholar |
76 Huang X, Lee C, Li Y, Xu J, Liu D. Acid-treated RuO2/Co3O4 nanostructures for acidic oxygen evolution reaction electrocatalysis. ACS Appl Nano Mater 2024; 7(8): 9244-9251.
| Crossref | Google Scholar |
77 Yang X, Li H, Lu A-Y, Min S, Idriss Z, Hedhili MN, et al. Highly acid-durable carbon coated Co3O4 nanoarrays as efficient oxygen evolution electrocatalysts. Nano Energy 2016; 25: 42-50.
| Crossref | Google Scholar |
78 Lai Q, Vediyappan V, Aguey-Zinsou KF, Matsumoto H. One-step synthesis of carbon-protected Co3O4 nanoparticles toward long-term water oxidation in acidic media. Adv Energy Sustain Res 2021; 2(11): 2100086.
| Crossref | Google Scholar |
79 Tran-Phu T, Chen H, Daiyan R, Chatti M, Liu B, Amal R, et al. Nanoscale TiO2 coatings improve the stability of an earth-abundant cobalt oxide catalyst during acidic water oxidation. ACS Appl Mater Interfaces 2022; 14(29): 33130-33140.
| Crossref | Google Scholar | PubMed |
80 Badreldin A, Abusrafa AE, Abdel-Wahab A. Oxygen-deficient cobalt-based oxides for electrocatalytic water splitting. ChemSusChem 2021; 14(1): 10-32.
| Crossref | Google Scholar | PubMed |
81 Wang YH, Li L, Shi J, Xie MY, Nie J, Huang GF, et al. Oxygen defect engineering promotes synergy between adsorbate evolution and single lattice oxygen mechanisms of OER in transition metal-based (oxy)hydroxide. Adv Sci 2023; 10(32): 2303321.
| Crossref | Google Scholar | PubMed |
82 Liu H, Li X, Peng C, Zhu L, Zhang Y, Cheng H, et al. Activating the lattice oxygen in (Bi0.5Co0.5)2O3 by vacancy modulation for efficient electrochemical water oxidation. J Mater Chem A 2020; 8(26): 13150-13159.
| Crossref | Google Scholar |
83 Hu J, Zeng X, Wang G, Qian B, Liu Y, Hu X, et al. Modulating mesoporous Co3O4 hollow nanospheres with oxygen vacancies for highly efficient peroxymonosulfate activation. Chem Eng J 2020; 400: 125869.
| Crossref | Google Scholar |
84 Liu Y, Zhang H, Wan H, Zhang W, Jiang N, Huang G, et al. Tuning lithium storage properties of cubic Co3O4 crystallites: the effect of oxygen vacancies. J Alloy Compd 2019; 787: 720-727.
| Crossref | Google Scholar |
85 Zhu B, Yang G, Gao D, Wu Y, Zhao J, Fu Y, et al. Special atmosphere annealed Co3O4 porous nanoclusters with oxygen defects and high proportion of Co2+ for oxygen evolution reaction. J Alloy Compd 2019; 806: 163-169.
| Crossref | Google Scholar |
86 Rong C, Wang S, Shen X, Jia C, Sun Q, Zhang Q, et al. Defect-balanced active and stable Co3O4−x for proton exchange membrane water electrolysis at ampere-level current density. Energy Environ Sci 2024; 17: 4196-4204.
| Crossref | Google Scholar |
87 Deng Q, Li H, Pei K, Wong LW, Zheng X, Tsang CS, et al. Strategic design for high-efficiency oxygen evolution reaction (OER) catalysts by triggering lattice oxygen oxidation in cobalt spinel oxides. ACS Nano 2024; 18(49): 33718-33728.
| Crossref | Google Scholar | PubMed |
88 Hong SY, Yao ZC, Cheng X, Jiang Z, Tang T, Hu JS. Advanced in situ spectroscopic techniques for probing the acidic oxygen evolution reaction. J Phys Chem C 2024; 128(41): 17219-17239.
| Crossref | Google Scholar |
89 Chen Z, Fan Q, Zhou J, Wang X, Huang M, Jiang H, et al. Toward understanding the formation mechanism and OER catalytic mechanism of hydroxides by in situ and operando techniques. Angew Chem Int Ed 2023; 62(51): e202309293.
| Crossref | Google Scholar | PubMed |
90 Tao HB, Liu H, Lao K, Pan Y, Tao Y, Wen L, Zheng N. The gap between academic research on proton exchange membrane water electrolysers and industrial demands. Nature Nanotechnology 2024; 19(8): 1074-1076.
| Crossref | Google Scholar | PubMed |
 Huihui Li received her MEng degree from Beijing Normal University in 2021 and BSc degree from Beijing University of Technology in 2018. Now she is a PhD candidate in the School of Chemistry at the University of New South Wales (UNSW), Sydney, under the supervision of Prof. Chuan Zhao. Her research focuses on the electrocatalysis of water splitting in acidic media, with an emphasis on catalyst stability and mechanisms under industrial conditions. Her research interests focus on material synthesis, electrochemical testing and advanced characterisation techniques. |
 Shuhao Wang is a postdoctoral research fellow at UNSW, Sydney. He received his Bachelor’s and Master’s degrees in materials science and engineering from Fuzhou University and completed his PhD at UNSW in 2024. His research focuses on computational electrochemistry, integrating density functional theory and molecular dynamics to design and understand advanced electrocatalysts for energy conversion. He has extensive experience in high-performance computing and the application of machine learning for mechanistic studies and catalyst optimisation. |
 Chuan Zhao is a professor at the School of Chemistry at UNSW, Sydney. He is currently the deputy director of the Australian Research Council (ARC) Training Centre for the Global Hydrogen Economy, and the deputy research chair and flagship program director of the ARC Centre of Excellence on Green Electrochemical Transformation of Carbon Dioxide. He is interested in discovering novel electrochemical methodologies and nanomaterials for energy applications, including water splitting, hydrogen fuel cells, CO2 and N2 reduction, batteries, and sensors. |