
Australian Journal of Chemistry
Volume 64 Number 8 2011
RESEARCH FRONTS: 1. 32nd APS; 2. N-Heterocyclic Carbenes
CH11310The Ever Changing Faces of Polymer Science: The 32nd Australasian Polymer Symposium

This research front presents papers from the 32nd Australasian Polymer Symposium, 13–16 February, 2011 at Coffs Harbour, Sydney, NSW, Australia.
CH11113Photochemical Methods for the Preparation of Complex Linear and Cross-linked Macromolecular Structures
Basic strategies dealing with the synthesis of complex linear and cross-linked macromolecular structures by photochemical methods are reported with particular references to recent works conducted in the area.
CH11152End Group Reactions of RAFT-Prepared (Co)Polymers
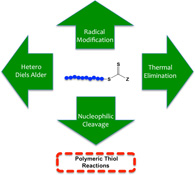
This review highlights approaches and chemistries for end-group removal and functionalization in (co)polymers prepared by reversible addition–fragmentation chain-transfer (RAFT) radical polymerization.
CH11156Progress Toward Robust Polymer Hydrogels
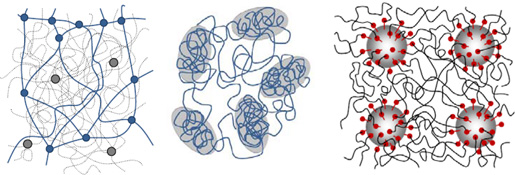
Conventional synthetic hydrogel materials are sometimes limited by their extreme brittleness. We review here recent work illustrating that the control of network topology can greatly improve the toughness of high-swelling hydrogel materials. We also comment on possible toughening mechanisms and introduce mechanical properties charts to graphically illustrate the differences in mechanical behaviour of these new materials.
CH11128Star-shaped Poly(2-oxazoline)s by Dendrimer Endcapping
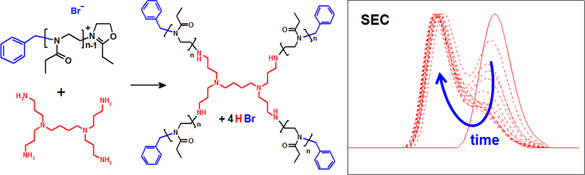
The synthesis of star-shaped poly(2-ethyl-2-oxazoline) is reported by direct end-capping of the living polymer chains with dendritic multiamines. The end-capping kinetics revealed less efficient end-capping with larger poly(2-ethyl-2-oxazoline) chains and increasing dendrimer generation. The solution viscosity and cloud point temperature of the star-shaped polymers were much less affected by chain length compared with their linear analogues.
CH11123Reversible Addition–Fragmentation Chain Transfer (RAFT) Polymerization in Miniemulsion Based on In Situ Surfactant Generation
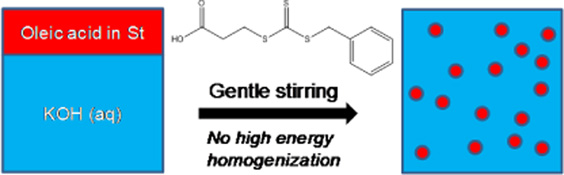
RAFT polymerization of styrene has been implemented in miniemulsion using in situ surfactant generation with oleic acid and potassium hydroxide without high energy mixing. The results demonstrate that a system that proceeds predominantly by a miniemulsion mechanism can be achieved under carefully selected conditions.
CH11131pH-Responsive Chiral Nanostructures
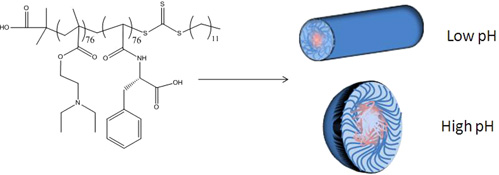
Using reversible addition fragmentation chain transfer (RAFT) polymerization, a responsive poly(vinyl amino acid)amphiphilic diblock copolymer has been prepared. This chiral amino acid diblock copolymer has been assembled at both low and high pH to form both cylindrical and spherical micelles. The characterization and application of these chiral nanostructures is described.
CH11133'Pseudo-star' Copolymers Formed by a Combination of RAFT Polymerization and Isocyanate-Coupling
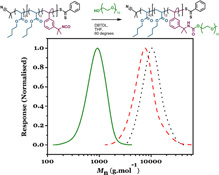
We describe a facile means of synthesizing low-polydispersity block copolymers with site-specific isocyanate functionalities via RAFT polymerization. The isocyanate groups are then exploited to attach polyethylene glycol to the copolymer to yield pseudo-star copolymers. These amphiphilic copolymers are then self-assembled in water to form monodisperse polymer particles.
CH11145Soft Core–Hard Shell Silicone Hybrid Nanoparticles Synthesized by Miniemulsion Polymerization: Effect of Silicone Content and Crosslinking on Latex Film Properties
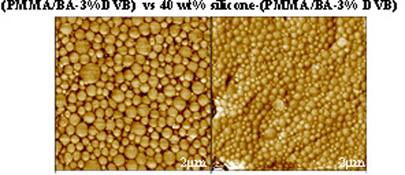
“Soft” silicone core-“hard” polyacrylate shell nanoparticles were prepared via a single-step miniemulsion polymerization. The effect of latex morphology and stiffness of polyacrylate shells (their Tg and crosslinking degrees) on composite film properties were investigated. Hybrid latexes with good thermal and excellent water resistant film properties could be formulated for further use in waterborne coatings.
CH11242Characterization of the Non-uniform Reaction in Chemically Amplified Calix[4]resorcinarene Molecular Resist Thin Films
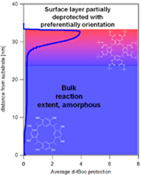
The ccc stereoisomer-purified tert-butoxycarbonyloxy (t-Boc) protected calix[4]resorcinarene molecular resists for next-generation photolithography exhibit a non-uniform photoacid-catalyzed reaction in thin films. The surface displays a reduced reaction extent with preferential orientation, compared with the amorphous bulk.
CH11130Functionalized Nanoporous Membranes from Reactive Triblock Polymers
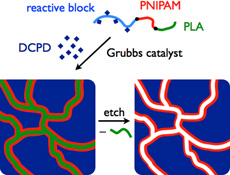
Reactive triblock polymers are used to form functional and stimulus-responsive nanoporous membranes. The development of such ‘smart’ membranes capable of responding to environmental stimuli could greatly expand the scope of membrane applications in, for example, medicine.
CH11117Synthesis of Acyclic, Symmetrical 3,3'-Allyl Dithioethers, from the Alkylation of 3-Mercapto-2-mercaptomethylprop-1-ene in the Presence of Sodium Hydride
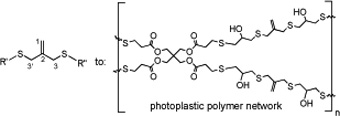
Difunctional monomers with various polymerizable end groups and containing 3,3´-allyl dithioethers in the centre of the chain, have been prepared using two different alkylation methods. These allyl dithioethers can be polymerized directly, or with further chemical reactions transformed into more complex monomers, for photoplastic polymer networks, illustrated by one example.
CH11176Temperature Dependent Stress Relaxation in a Model Diels–Alder Network
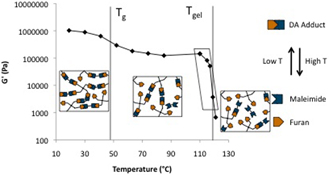
The complex shear modulus (G*(ω)) of a model reversible covalent network formed by the Diels–Alder reaction was shown to depend strongly on temperature near the gel point. Mid- and low-frequency scaling features of G*(ω) over the experimental frequency and temperature range were interpreted to be a result of the network dynamics.
CH11213Synthesis and Self-assembly of Amphiphilic Hetero-arm Molecular Brushes
Amphiphilic hetero-arm molecular brushes (AMBs) were prepared by a combination of ‘grafting from’ and ‘grafting onto’ methodologies. Use was made of the alternating tendency in the copolymerization of styrene and maleic anhydride. The AMBs eventually obtained consist of PEG-substituted styrene and N-alkyl maleimide. Self-assembly of the AMBs was investigated.

This vial contains a few remaining mgs of the original crystalline carbene sample of 1,3-di(1-adamantyl)imidazol-2-ylidene from August 27, 1990. This small amount of material pales in comparison with the quantities of nucleophilic singlet carbenes in use today. A Foreword by A. J. Arduengo opens this issue's Research Front on N-Heterocyclic carbenes.
CH11244Yes They Can: Small-molecule Activation with Stable Diaminocarbenes
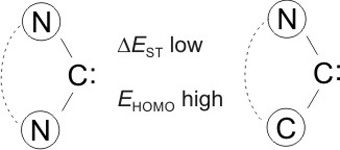
Similarly to (alkyl)(amino)carbenes, stable acyclic and ferrocene-based cyclic diaminocarbenes exhibit high HOMO energies EHOMO and low singlet–triplet gaps ΔEST. These ambiphilic singlet carbenes react with fundamentally important small molecules like CO and NH3. This was hitherto thought to be impossible for N-heterocyclic carbenes and related diaminocarbenes.
CH11265Abnormal N-heterocyclic Carbenes: More than Just Exceptionally Strong Donor Ligands
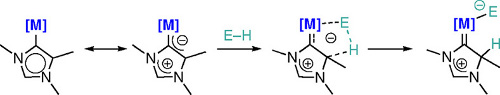
Abnormal carbene ligands – isomeric to classical N-heterocyclic carbenes but comprising a much more pronounced mesoionic character — are proposed to act cooperatively with the metal center to accomplish demanding bond activation processes.
CH111851,3,4-Trisubtituted-1,2,3-Triazol-5-ylidene 'Click' Carbene Ligands: Synthesis, Catalysis and Self-Assembly
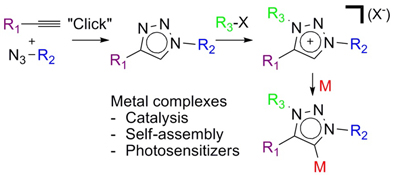
This review examines the use of the mild and modular CuAAC ‘click’ reaction for development of a novel family of abnormal or mesoionic N-heterocyclic carbenes and their corresponding metal complexes. These new ‘click’ carbene metal complexes have been utilised in catalysis, self-assembly and as photosensitizers.
CH11227Metal Complexes of an Ionic Liquid-Derived Carbene
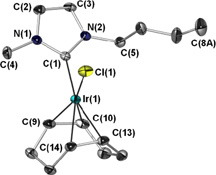
A range of metal carbene complexes containing the ionic liquid-derived N-heterocyclic carbene 1-nbutyl-3-methylimidazol-2-ylidene (IBuMe, 1) have been prepared. The sigma donation and steric properties of 1 are discussed relative to those of common imidazol-2-ylidene ligands.
CH11243Novel Intramolecular CAryl–S Bond Activation by an Electron Rich, Ring-Expanded-NHC-Rh centre: A Combined Experimental and DFT Study
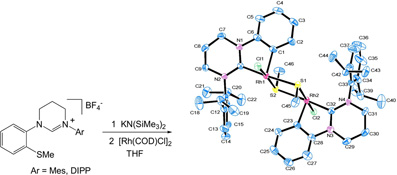
The reaction of highly basic, sterically demanding 1-(2-methylthiophenyl)-tetrahydropyrimid-2-ylidenes with a [Rh(COD)Cl]2 dimer leads to cleavage of the Caryl-SMe bond, metallation at the aryl ring and oxidation of the Rh centre to form a MeS-bridged dimer. The reaction is an example of the unusual chemistry promoted by these novel ligands.
CH11183Enabling the Development of N-Heterocyclic Carbene (NHC) Catalyzed Reactions: Practical Methods for the Preparation of 1-Acyl-2-Alkylcycloalkenes from Cycloalkanones
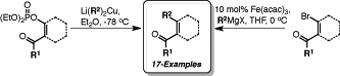
The synthesis of 1-acyl-2-alkylcycloalkenes from a variety of cycloalkanones has been achieved via either β-keto enol phosphates or β-bromoenones. Both methods exploit simple and readily scalable transformations allowing the preparation of the desired compounds to be achieved in three, or four steps, respectively. The utility of these strategies has been examined, resulting in the preparation of 17 disubstituted cycloalkenes.
CH11264N-Heterocyclic Carbene-Promoted Rauhut–Currier Reactions between Vinyl Sulfones and ?,?-Unsaturated Aldehydes
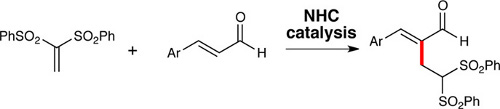
N-heterocyclic carbenes (NHCs) promote the addition of 1,1-bis(phenylsulfonyl)ethylene to the α-position of α,β-unsaturated aldehydes. The proposed reaction pathway for this Rauhut–Currier-type reaction includes an unusual conjugate addition of an NHC to a bis-vinyl sulfone.
CH11246Carbene-Based Lewis Pairs for Hydrogen Activation
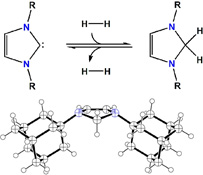
This article examines the scope of acid–base systems for reversible hydrogen activation using bulky imidazol-2-ylidenes and a range of electrophiles. Boranes, carbocations and a transition metal complex were investigated as the acids. In the absence of dihydrogen, abnormal carbene adduct formation was observed in some cases. Hydrogen activation with these systems can result in hydride reduction of the imidazolium cations to 2H-imidazolines (aminals).
CH11193A Neutral Gallium(I) N-Heterocyclic Carbene Analogue: Synthesis, Characterization and Theoretical Analysis
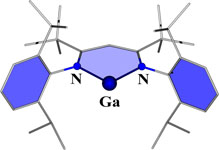
A six-membered N-heterocyclic gallium(I) compound (see picture) has been synthesized, crystallographically characterized, and its electronic properties analyzed using density functional theory.
CH11173NHC-Based Self-Assembled Monolayers on Solid Gold Substrates
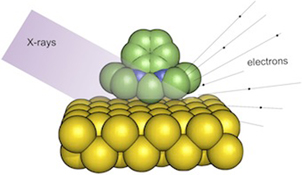
Self-assembled monolayers of the N-heterocyclic carbene 1,3-diethylbenzimidazol-2-ylidene on gold were fabricated by chemisorption from solution, thus establishing a completely new adsorbate system in this field.



