
Australian Journal of Chemistry
Volume 67 Number 7 2014
The Chemistry of Guanidine, Guanidinium, and Guanidinate Compounds
CH14384The Chemistry of Guanidine, Guanidinium, and Guanidinate Compounds
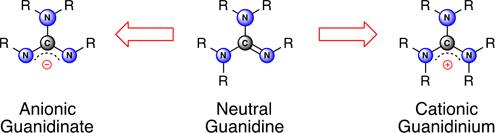
This special issue of the Journal is dedicated to the chemistry of compounds derived from the guanidine functionality. It describes compounds containing this group in its neutral (guanidine), cationic (guanidinium) and anionic (guanidinate) forms. The diversity associated with this functional group is reflected in the contributions from all of the traditional areas of chemical research, including organic, inorganic, physical, and medicinal chemistry.
CH14043Guanidine Motif in Biologically Active Peptides
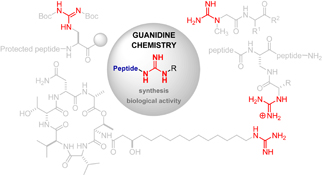
Guanidine is a structural motif present in a large number of biologically active compounds such as peptides or peptide mimetics that exhibit appealing properties and play important roles in medicinal chemistry.
CH13694A Magic Equation: Delta Bonds Plus Bicyclic Guanidinates Equals Strong Reducing Agents
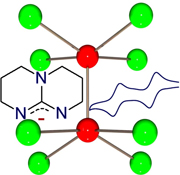
An account is provided of how the destabilization of a delta bond in quadruple bonded species by bicyclic guanidinate ligands leads to record low ionization energies of thermally stable compounds and concomitant production of very strong reducing agents. This paper commemorates the 50th anniversary of the seminal paper published on September 18, 1964 in Science describing how interactions between d-orbitals lead to a quadruple bond in species with idealized D4h symmetry.
CH14125Capture and Fixation of CO2 Promoted by Guanidine Derivatives
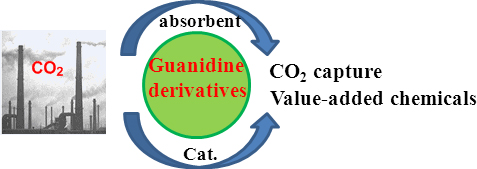
The latest advances on CO2 capture using guanidine and their derivatives as absorbents are summarized. In particular, guanidine-catalyzed transformation of CO2 to a series of value-added chemicals with mechanistic consideration on a molecular level will be particularly elaborated in this article.
CH14172Recent Advances Using Guanidinate Ligands for Chemical Vapour Deposition (CVD) and Atomic Layer Deposition (ALD) Applications
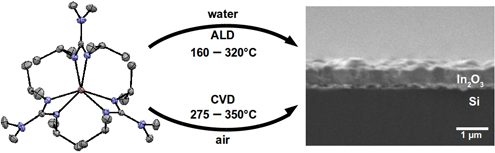
The use of guanidinate ligands for chemical vapour deposition (CVD) and atomic layer deposition (ALD) of thin films over the past 5 years is reviewed. Design trends for metal complexes, promising precursor candidates, and the future outlook for guanidinates in ALD and CVD are reported.
CH13590The Synthesis of Enantiopure α-Fluoro and α,α-Difluoro-β3-Arginine Derivatives
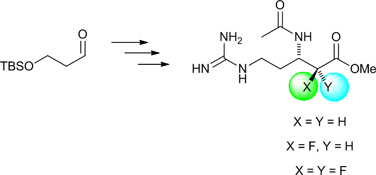
Two N-acetylated α-fluoro and α,α-difluoro-β3-arginine esters, as well as their non-fluorinated analogue, were prepared in enantiomerically pure form from a common aldehyde precursor. The ability of these β3-arginine derivatives to inhibit bovine trypsin was examined.
CH14053Crystallisation-Driven Self-Sorting of H-Bonded Guanidinium or Sodium Sulfonate Constitutional Molecular Frameworks
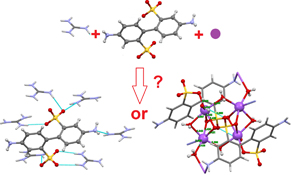
Competitive crystallisation of guanidinium and sodium sulfonates may be done with high selectivity.
CH14157Low-valent Iron Complexes Stabilised by a Bulky Guanidinate Ligand: Synthesis and Reactivity Studies
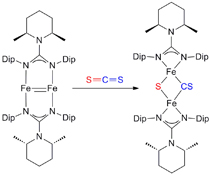
Reactivity of a FeI–FeI multiply bonded species towards a range of unsaturated small molecule substrates was investigated. Depending on the substrate involved, either iron coordination, reductive disproportionation, reductive cleavage, or reductive coupling processes occurred.
CH14144Development of Novel Guanidine–Bisurea Bifunctional Organocatalysts and their Application to Asymmetric α-Hydroxylation of Tetralone-derived β-Keto Esters
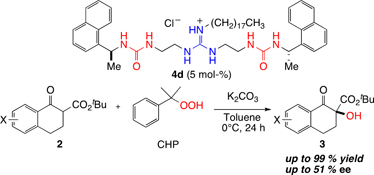
A series of guanidine-bisurea bifunctional organocatalysts, in which the chiral centres are located outside the urea groups, catalyzed the α-hydroxylation of tetralone-derived β-keto esters in up to 99 % yield with up to 51 % enantiomeric excess.
CH14134Synthesis and Structural Characterization of a Series of Group 11 2,2-Dialkyl-1,3-dicyclohexylguanidinate Complexes
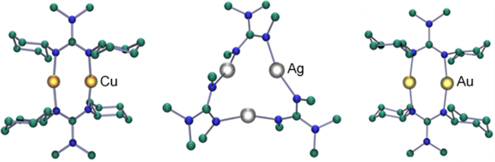
One equivalent of lithium 2,2-dialkyl-1,3-dicyclohexylguanidinate was reacted with one equivalent of Group 11 halide (CuCl, AgBr, and AuCl) to generate oligonuclear complexes with the general formula {M[CyNC(NR2)NCy]}n where {M, n} = {Cu, 2}, {Ag, 3}, and {Au, 2}.
CH14170Formation of an Unusual Bis(diguanidinate) Ligand via Nucleophilic Attack of a Guanidinate onto a Carbodiimide
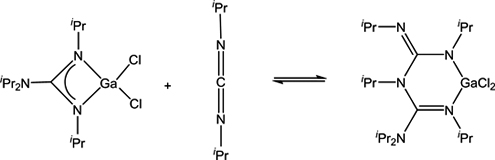
An unexpected new guanidinate expansion reaction was uncovered in which a GaIII gaunidinate 2 adds to the carbodiimide, iPrN=C=NiPr, to give [iPrN{C(NiPr)=NiPr}{C(=NiPr)NiPr2}GaCl2] (4), containing an unusual bis(diguanidinate) ligand.
CH14060Synthesis and Characteristics of Energetic Materials Based on 1,2-Dinitroguanidine
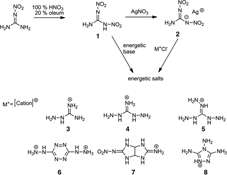
A series of new energetic salts based on 1,2-dinitroguanidine were successfully synthesised and fully characterised.
CH14175Stabilization of Complexes of Redox-Active Guanidino-Functionalized Aromatic Compounds (GFAs) by Hydrogen-Bonding
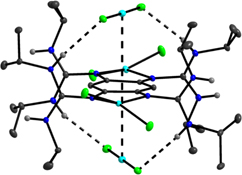
The redox-active guanidine-functionalized 1,2,4,5-tetrakis(N,N’-diisopropylguanidino)benzene was recently shown to be a redox-active switch, and reversibly forms hydrogen-bond aggregates upon two-electron oxidation. In this work, the hydrogen-bond motifs in the first coordination compounds with the neutral and oxidized guanidine as ligand are reported.
CH14182Effect of Intramolecular Hydrogen Bonds on the Gas-Phase Basicity of Guanidines
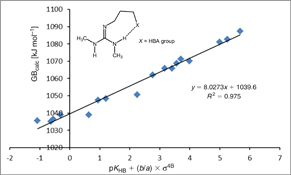
Predicting acid/base properties of unknown molecules using data which can be tabulated is an everlasting goal in chemistry. In this sense, molecules containing intramolecular hydrogen bond are especially problematic. Our results show very good correlation of GB against pKHB and newly introduced σ4B parameter for a series of guanidine bases with intramolecular hydrogen bond.
CH14181Preparation and Structural Studies of Non-Symmetric Guanidinate-Supported Zirconium Complexes
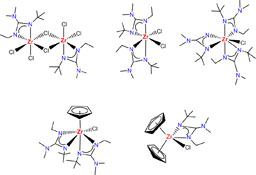
Asymmetric guanidinate-supported zirconium complexes with different degrees of substitution can be easily prepared by reaction of the simple precursors ZrCl4 or [ZrCl2(η5-C5H5)2] with the corresponding lithium guanidinate salt, or by reaction of [Zr{κ2N,N′-(NEt)(N-t-Bu)CNMe2}2Cl2], obtained by insertion of carbodiimide into a Zr–amido bond, with NaC5H5.
CH14255Tin and Mercury Compounds Supported by a Bulky Organometallic Ligand Incorporating a Pendant Guanidine Functionality
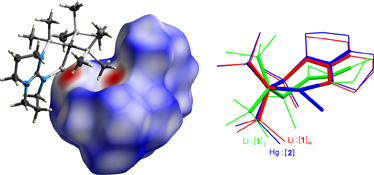
The structures of four compounds containing the bidentate ligand C(SiMe3)2(SiMe2{hpp}) (R, where hppH = 1,3,4,6,7,8,-hexahydro-2H-pyrimido[1,2-a]pyrimidine) are described. The lithium derivative LiR (1), which shows Li···H3C interactions in the solid-state, may be converted into the mercury compound HgRCl (2) and the tin derivatives SnRCl (3), and SnR(P{H}Ar*) (4, Ar* = 2,4,6-tBu3C6H2) with a rare terminal-phosphanide ligand.
CH14271Towards the Synthesis of Guanidinate- and Amidinate-Bridged Dimers of Mn and Ni
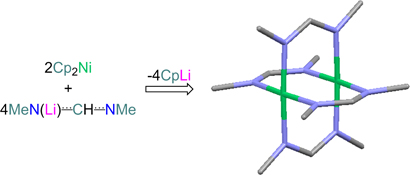
The reaction of monolithiated amidines and guanidines with Cp2M (Cp = cyclopentadienyl; M = Mn, Ni) yields a variety of new organometallic complexes; for the use of Cp2Ni, kinetically unprotected paddle-wheel homodimers of formulation Ni2L4 (L = amidinate, guanidinate) are obtained.
CH14376Cyanobiphenyl versus Alkoxybiphenyl: Which Mesogenic Unit Governs the Mesomorphic Properties of Guanidinium Ionic Liquid Crystals?
Decyloxybiphenyl guanidinium ionic liquid crystals self-assemble into smectic A (SmA) phases regardless of the substitution pattern of the phenylguanidinium head group, but a minimum of C6 alkyl spacer length is required. The cyanobiphenyl analogues are surprisingly non-mesogenic.
CH14340Asymmetric Michael Addition Using Bifunctional Bicyclic Guanidine Organocatalyst: A Theoretical Perspective
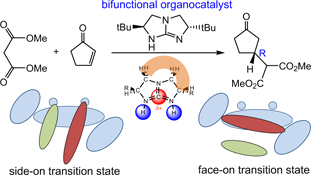
The general principle of asymmetric organocatalysis using chiral bicyclic guanidine as a bifunctional catalyst is illustrated in a density functional theory study of [5,5]-bicyclic guanidine-catalyzed Michael addition of dimethyl malonate to cyclopentenone. Two types of bifunctional activation modes were examined. The calculated enantioselectivity is in excellent accord with experiment result.
CH14228The First Aziridinylguanidinates: New Precursors for Potentially Volatile Metal Guanidinates
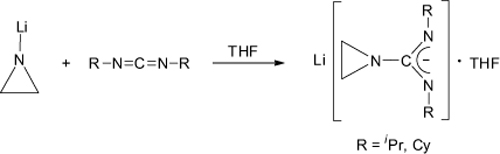
The first lithium-aziridinylamidinates, Li[(C2H4N)C(NR)2]·THF (R = iPr, Cy; iPr = isopropyl, Cy = cyclohexyl), have been prepared by addition of N-aziridinyllithium, C2H4NLi, to either N,N′-diisopropylcarbodiimide or N,N′-dicyclohexylcarbodiimide. In the solid state, these lithium-aziridinylamidinates comprise ladder-type dimeric molecular structures.
CH14179Novel Tartrate-Based Guanidines for Enantioselective Fluorination of 1,3-Dicarbonyl and α-Cyano Carbonyl Compounds
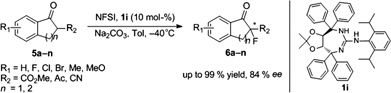
A novel tartrate-based guanidine molecule was identified as a suitable promoter for the enantioselective fluorination of 1,3-dicarbonyl and α-cyano carbonyl compounds to furnish the fluorinated product with up to 84 % ee and 99 % yield using N-fluorobenzenesulfonimide as the fluorinating agent.
CH14187Chiral Bicyclic Guanidine-Catalysed Conjugate Addition of α-Fluoro-β-Ketoesters to Cyclic Enones
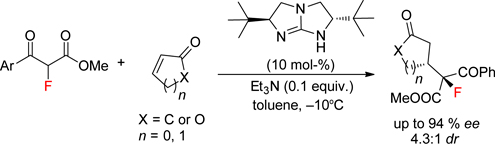
By utilising a chiral bicyclic guanidine as catalyst, the first asymmetric Michael addition of α-fluoro-β-ketoesters to various cyclic enones has been successfully developed, affording a variety of Michael adducts with potential synthetic utilities with satisfactory stereoselectivity (up to 94 % ee and 4.3 : 1 dr).
CH14195Synthesis of Bulky Aryl Group-substituted Chiral Bis(guanidino)iminophosphoranes as Uncharged Chiral Organosuperbase Catalysts
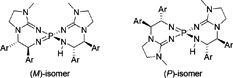
Uncharged chiral bis(guanidino)iminophosphorane organosuperbase catalysts substituted by bulky aryl groups were synthesized and evaluated as chiral organosuperbase catalysts in the enantioselective amination of 2-methyltetralone.
CH14233The Synthesis and Structural Characterization of Peralkylated Triguanide Superbases

A synthetic approach towards peralkylated triguanide superbases based on a coupling reaction between a Vilsmeier salt and monosubstituted guanidines is described. Single crystal X-ray diffraction enabled the first structural characterization of benzyloctamethyl derivative, which was also evaluated as a potential organocatalyst in the transesterification reaction of vegetable oil.
CH14197Copper(I) Iodide-Catalyzed Synthesis of N,N′-Disubstituted Guanidines from N-Substituted Cyanamides
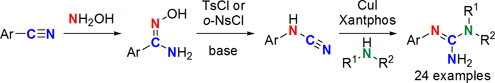
The reaction of N-arylcyanamides, obtained from corresponding nitriles via Tiemann rearrangement, with various primary and secondary alkylamines under the catalysis of copper(I) iodide and Xantphos in DMF afforded a wide variety of N-alkyl-N′-arylguanidine derivatives.



