Fast identification of wheat 1BL.1RS translocation by reversed-phase ultra-performance liquid chromatography (RP-UPLC)
Jianwen Zhou A C , Caixia Han A C , Hui Cao A , Shoumin Zhen A , Zitong Yu A , Xiaohui Li A , Wujun Ma B and Yueming Yan A DA College of Life Science, Capital Normal University, Beijing 100048, China.
B Centre for comparative Genomics, Murdoch University and Australian Export Grain Innovation Centre, Perth, WA 6150, Australia.
C These authors contributed equally to this work.
D Corresponding author. Email: yanym@cnu.edu.cn
Crop and Pasture Science 64(9) 865-873 https://doi.org/10.1071/CP13246
Submitted: 10 July 2013 Accepted: 12 September 2013 Published: 6 November 2013
Journal Compilation © CSIRO Publishing 2013 Open Access CC BY-NC-ND
Abstract
The 1BL.1RS chromosomal translocation in wheat is the result of replacement of the short arm of chromosome 1B of wheat by the short arm of chromosome 1R of rye, which had been widely used as a parental line in worldwide wheat breeding, resulting in a high percentage of wheat cultivars containing this translocation. A fast and reliable approach to identify this translocation is highly desirable in modern wheat breeding. This study compared reversed-phase ultra-performance liquid chromatography (RP-UPLC), acidic polyacrylamide gel electrophoresis (A-PAGE), liquid chromatography coupled to tandem mass spectrometry (LC-MS/MS), allelic-specific PCR, and reversed-phase high-performance liquid chromatography (RP-HPLC) approaches to identify the 1BL.1RS translocation in 76 bread wheat cultivars. Two gliadin bands in the Gli-B1 region of A-PAGE separation were confirmed by LC-MS/MS to be omega secalins from the 1BL.1RS translocation, and they can be used as reliable protein markers for identifying the translocation. A few specific minor peaks eluted at 12–13 min on the RP-UPLC patterns can readily differentiate the 1BL.1RS translocation. Of the 76 wheat cultivars tested, 40 were identified as carrying the 1BL.1RS translocation by RP-UPLC, which was consistent with the results of A-PAGE, HPLC, and PCR. Compared with other established methods, RP-UPLC showed a clear advantage in fast identification of the 1BL.1RS translocation with higher reliability and lower costs, and it is therefore ideal for large-scale screening of the 1BL.1RS translocation in wheat breeding.
Additional keywords: wheat, 1BL.1RS translocation, A-PAGE, HPLC, PCR, UPLC.
Introduction
Wheat (Triticum aestivum L.) is one of the most important cereal crops and is the main food source for >40% of the world population. The 1BL.1RS translocation in wheat resulted from replacing the short arm of the chromosome 1B of wheat by the short arm of the chromosome 1R of rye (Secale cereale L.). In the past few decades, this translocation had been widely used in wheat breeding for improving disease resistance and yield performance (Zhao et al. 2012). Thus, the short arm of rye chromosome 1R is one of the most widely utilised alien chromatins in wheat breeding (Baum and Appels 1991). To date, ~300 cultivars and breeding lines carrying 1BL.1RS have been released worldwide, mostly since 1970 (Graybosch 2001). About 50% of bread wheat cultivars released in China (Zhou et al. 2003), 55% in Bulgaria (Landjeva et al. 2006), and 53% in Hungary (between 1978 and 1999; Kőszegi et al. 2000) carry the 1BL.1RS translocation. The translocation is also found in cultivars or germplasm produced in USA (Lukaszewski 1990), and Eastern Europe and Mexico (Villareal et al. 1997).
The 1BL.1RS translocation was initially designed to improve wheat resistance to several pathogens such as Puccinia recondita, Puccinia graminis, Puccinia striiformis, and Blumeria graminis induced by the genes Sr31, Lr26, Yr8, and Pm8 located on 1RS (Zeller 1973). Cultivars carrying this translocation have also shown other agronomic benefits, including higher yield and better yield stability (Zeller and Hsam 1983; Lelley et al. 2004). However, the 1BL.1RS translocation has been found to have detrimental effects on wheat end-use qualities, such as reduced gluten strength (diminished mixing tolerance) and loaf volume per unit protein, and increased dough stickiness during mixing (Dhaliwal et al. 1988; Milovanovic et al. 1998). These detrimental effects result from the introgression of ω-secalins encoded by Sec-1 locus on the short arm of rye chromosome 1RS, and the loss of low molecular weight glutenin subunits (LMW-GS) encoded by Glu-B3 and gliadins encoded by Gli-B2 on the short arm of wheat chromosome 1BS (Javornik et al. 1991). Due to the negative effects on quality, breeders now seek to reduce the size of translocation in cultivars. A cost-effective and high-throughput approach is highly desirable for parental line selection and early-generation screening during quality wheat breeding.
So far, various methods have been developed to identify the wheat 1BL.1RS translocation, and the most widely used method is acidic polyacrylamide gel electrophoresis (A-PAGE; Koebner and Shepherd 1986). Isoelectric focusing (Chojecki et al. 1983), two-dimensional electrophoresis (2-DE, Gobaa et al. 2007), disease resistance tests (Heun and Fischbeck 1987; Friebe et al. 1989), and morphological observation of chromosomes (Zeller 1973; Mettin et al. 1973; Bennett and Smith 1975; Lukaszewski and Gustafson 1983; Rayburn and Carver 1988) are also among the methods for the identification of this translocation. However, these methods usually have disadvantages such as being time-consuming and having high costs, and therefore are not suitable for large-scale identification and screening in the early generations of wheat breeding. More recently, diagnostic techniques were developed such as rye-specific DNA probes (Gustafson et al. 1988; Heslop-Harrison et al. 1990), monoclonal antibodies (Howes et al. 1989), reversed-phase high-performance liquid chromatography (RP-HPLC, Lookhart et al. 1991), and high performance capillary electrophoresis (HPCE; Lookhart et al. 1996). However, these methodologies are sophisticated, with higher analysis costs, and therefore have not been used widely.
Reversed-phase ultra-performance liquid chromatography (RP-UPLC) is a new technology for the separation and characterisation of proteins based on the differences in surface hydrophobicity. It was developed based on RP-HPLC (Wu et al. 2006). In this system, small-diameter columns (e.g. 1.7 μm) can be used and high column performance (up to 100 000–300 000 theoretical plates/m) can be produced. Compared with RP-HPLC, RP-UPLC analysis is nine times faster, two times higher in resolution, and three times more sensitive (Swartz 2005). Our recent study also showed that RP-UPLC is capable of separating wheat water-soluble proteins and LWM-GS with higher efficiency and resolution than traditional RP-HPLC (Yu et al. 2013a, 2013b).
In this study, an RP-UPLC method for rapid identifying the wheat 1BL.1RS translocations was developed. It was compared with A-PAGE, RP-HPLC, PCR, and liquid chromatography coupled to tandem mass spectrometry (LC-MS/MS) methods to aid the development of the RP-UPLC method. Our results demonstrate that RP-UPLC is capable of fast separation and identification of the secalins translocated from rye with high resolution and reproducibility and lower costs, and thus has the potential for wide application in wheat breeding.
Materials and methods
Wheat materials
Seventy-six common wheat cultivars (Triticum aestivum L., 2n = 6x = 42, AABBDD) and one rye cultivar (Secale cereale L., 2n = 2x = 14, RR), which were mostly released and cultivated in China in the past two decades, were used in the current study (Supplementary Materials Table 1, as available on journal’s website). All germplasm was collected from the National Wheat Germplasm Bank in the Institute of Crop Science, Chinese Academy of Agricultural Sciences (CAAS), China.

|
Protein extraction
Gliadins were extracted from wheat flour by 70% ethanol. About 100 mg of flour was incubated in 400 μL of 70% ethanol and oscillated at least 1 h, and then centrifuged at 13000 rpm for 15 min. The supernatant was transferred to two new 1.5-mL tubes, and loading buffer with 80% glycerol and 0.02% methyl chloride was added to one tube for A-PAGE. The other tube was centrifuged at 4°C and 13 000 rpm for 15 min, then the supernatant was transferred to a sample tube for RP-UPLC analysis.
A-PAGE
The A-PAGE was performed by a modified method according to Yan et al. (2003), with 10% running gel and 5% stacking gel on Hoefer vertical electrophoretic apparatus (Bio-Rad Laboratories Inc., Hercules, CA, USA). Electrophoresis was carried out at a constant voltage of 300 V for 30 min, followed by 500 V for 4 h, at a constant temperature of 14°C. After electrophoresis, gels were stained with a mixture of 12.5 mL of 10% Coomassie Blue R dissolved in ethanol and 500 mL of 10% trichloroacetic acid solution, and destained with distilled water for 12 h.
LC-MS/MS
The expected bands were excised from A-PAGE gels and put in microcentrifuge tubes, and then washed with 200–400 μL of 100 mm NH4HCO3 and 30% acetonitrile (ACN). This step was repeated two or three times until the pellets were colourless, then the destaining buffer was removed and lyophilised for 30 min until the white particles became agglomerated. The dried particles were digested with 7 μL of diluted trypsin solvent (Promega, Madison, WI, USA), diluted with 25 mm NH4HCO3 to a final concentration of 15 ng/μL, and incubated at 37°C for at least 20 h. The peptides were extracted three times with 5% trifluoroacetic acid (TFA), 50% ACN, and 45% water at 37°C. Extracts were pooled and lyophilised, and stored at −80°C before mass spectrometric analysis.
The lyophilised, trypsin-digested peptides were dissolved in 0.1% TFA and mixed with 1 μL of TFA, 500 μL of ACN solution, and 499 μL of water. The peptide mixtures were analysed using reversed-phase capillary liquid chromatography (LC) coupled with electrospray ionisation quadrupole time-of-flight (ESI-Q-TOF) tandem mass spectrometry (MS/MS) (LTQ VELOS; Thermo Finnigan, San Jose, CA, USA). The peptides were subsequently eluted onto a 0.15 mm by 150 mm analytical RP-C18 column (Column Technology Inc., Lombard, IL, USA) and separated at 1 μL/min with an increasing ACN gradient from 4% to 50% from 0 to 30 min and from 50% to 100% at 34 min, then maintained to 40 min. The mobile phases A and B were 0.1% formic acid in water and 0.1% formic acid in ACN, respectively. The mass spectrometer was operated in a positive ion mode with a source temperature of 200°C and a cone gas flow of 10 L/h. All MS/MS spectra were processed using Mass Lynx 4.0 Protein Lynx (Waters Corporation, Milford, MA, USA) to generate PKL files. The MS/MS spectra were searched in the NCBI (www.ncbi.nlm.nih.gov/) non-redundant green plant database (updated to 30/7/2012) using MASCOT version 2.1. The peptide tolerance was set to 100 ppm and fragment mass tolerance to 0.2 Da, two missed cleavages were allowed. Carbamidomethyl (Cys) and oxidation (Met) were specified as variable modifications. Cross-correlation scores of singly, doubly, and triply charged peptides >1.9, 2.2 and 3.75, respectively, were fixed for protein identification.
DNA extraction, AS-PCR amplification, and cloning of secalin genes
Genomic DNA was extracted from dry seeds with a previously established cetyltrimethylammonium bromide (CTAB) protocol (Murray and Thompson 1980). Genomic DNA was extracted from dry seeds by use of CTAB protocol (Murray and Thompson 1980). The ω-secalin complete gene sequences (accessions FJ561478, FJ816038, FJ823443, X60294, AF000227, and FJ561465) from GenBank (www.ncbi.nlm.nih.gov/) were used for designing allelic specific PCR (AS-PCR) primers according to Wang et al. (2012a) named as forward Sec1 (5ʹ-ATGAAGACCTTACTCATGCTTG-3ʹ) and reverse Sec2 (5ʹ-CTATACCACTACTA CAAATGGT-3ʹ). The AS-PCR amplifications were performed in a total volume of 50 μL containing 2.5 U LA Taq polymerase (TaKaRa), 60 ng of template DNA, 25 μL 2X GC buffer I (MgCl2 plus), 0.4 mm dNTP, 0.5 μm of each primer, and double-distilled H2O. The PCR reaction was carried out in a S1000TM thermal cycler (Bio-Rad Laboratories Inc.) with the following program: an initial step of 94°C for 4 min; 34 cycles of 94°C for 45 s, 58°C for 1 min, and 72°C for 70 s; and a final step of 10 min at 72°C. The PCR products were analysed by agarose electrophoresis with 1% gel in Tris–acetic acid–EDTA buffer. The PCR fragments with expected sizes were purified from the gel using the Gel Extraction Kit (Omega Bio-Tek Inc., Norcross, GA, USA). The purified products were then ligated into pGEM-T Easy vector (Tiangen, Beijing, China) and transformed into cells of Escherichia coli TOP 10. The DNA sequencing from three clones of each PCR fragment was carried out by the Beijing Genomic Institute.
Identification of 1BL.1RS translocation by PCR-based markers
Previously reported locus-specific primers (Chai et al. 2006) were used in the identification of the 1BL.1RS translocation. PCR amplifications were performed in a total volume of 20 μL containing 1 U LA Taq polymerase (TaKaRa), 24 ng of template DNA, 10 μL 2X GC buffer I (MgCl2 plus), 0.16 mm dNTP, 0.2 μm of each primer, and double-distilled H2O.
RP-HPLC
The RP-HPLC analysis was performed on an Agilent 1100 instrument with reversed-phased column (ZORBAX 300SB-C18 Stable Band Analytical 4.6 by 250 mm, 5-μm) (Agilent Technologies, Santa Clara, CA, USA). The major analytical parameters were set as 60°C for column temperature, 1.00 mL/min for flow rate, 20 µL for sample volume, and eluting gradient and concentrations of ACN with 0.06% TFA gradually increasing from 21% to 47% (v/v) in 55 min. Column washing time between two adjacent samples was 15 min. The proteins were detected by measuring UV at 210 nm.
RP-UPLC
The RP-UPLC was performed according to Yu et al. (2013a, 2013b) with some modifications on an Acquity UPLC™ (Waters Corporation) with a Waters 300SB C18 column (1.7 μm). Four solutions of elution buffers were used: solution A (ultra-pure water with 0.06% TFA), solution B (ACN with 0.06% TFA), solution C (ultra-pure water), and solution D (methanol). The column was balanced by increasing the concentration of solution B from 21% to 47% within 45 min. The ratios of solution A to B for weak washing and strong washing for needles were 79 : 21% and 53 : 47%, respectively. Sample washing was conducted with solution A from 95% to 5% and solution B from 5% to 95% in 5 min. Final washing was completed with solution C from 90% to 10%, and solution D from 10% to 90% three times within 30 min. At the end, 10 μL of solution D was injected into the column.
Results
Identification of 1BL.1RS translocations by A-PAGE
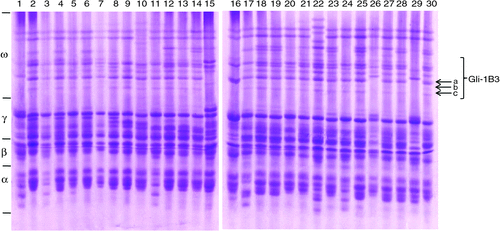
Gliadins, which are traditionally classified into three groups (α/β-, γ-, and ω-gliadins) on the basis of their electrophoretic mobilities on A-PAGE gel (Woychik et al. 1961), were extracted from wheat cultivars by A-PAGE (Fig. 1). The omega secalins are encoded by Sec-1 locus located on the short arm of chromosome 1RS of rye (Shewry et al. 1984; Clarke et al. 1996). According to Sozinov et al. (1987), the gliadin Gli-1B3 area in the ω-region can be used as 1BL.1RS translocation marker loci, in which the protein bands marked as a, b, and c are most likely to be omega secalins from rye (Fig. 1). Based on the A-PAGE results, 50 cultivars were found to have the Gli-1B3 site, probably containing the 1BL.1RS translocation, while 25 cultivars had no Gli-1B3 (see Supplementary Materials, Table 1).
|
Characterisation of omega secalins from the 1BL.1RS translocation by LC-MS/MS
In order to confirm whether the three gliadin bands on the A-PAGE gels indicated in Fig. 1 belong to omega secalins from 1RS of rye, each band was excised from gels and purified. After digesting with trypsin, three proteins named as a, b, and c were further identified by LC-MS/MS and the results are shown in Table 1. According to the molecular weight and cover percentage of the peptide (Table 1 and Supplementary Materials Fig. 1), bands a and c were identified as omega secalins, whereas band b was proved to be wheat gliadin. The A-PAGE analysis also found that some cultivars such as Zhongyou 9507 contained band b (Fig. 1). As shown in Table 1, protein band a was well matched with the omega secalin ACQ83636.1, and two characteristic peptides (R.PFGQQPEQIISQR.P and R.QLNPSEQELQSPQQPVPK.E) were identified, which located at the positions 20–39 and 256–270, respectively. For band c, one characteristic peptide (R.QLNPSEQELQSPQQPVPK.E) well matched with omega secalin ACN96898.1 and located at the positions 20–39 (Table 1 and Supplementary Materials Fig. 1).
The omega secalin genes at the Sec-1 locus from 10 cultivars that were identified to contain bands a and c were amplified and cloned by AS-PCR, and 20 non-redundant ω-secalin genes were amplified. The deduced amino acid sequences of six representative genes (TaSec-NX1, TaSec-NX12, TaSec-NX13 from Neixiang 188, and TaSec-JD1, TaSec-JD2, TaSec-JD3 from Jingdong 8) were compared with previously characterised omega secalin genes (Supplementary Materials Fig. 2), and typical structural features of omega secalin genes of rye were found in all of these genes, including a conserved signal peptide of 19 amino acid residues started with RQL, a short N-terminal region of 12 amino acids, several repetitive domains (PQQP), and a short C-terminal region ending with four valine amino acid residues (De Vita et al. 2012). The two characteristic peptides of omega secalin identified by LC-MS/MS were present in conserved positions of all cloned genes (Supplementary Materials Fig. 2). These results demonstrated that the gliadin bands a and c are omega secalins resulting from the translocation of rye 1RS and can be used as reliable protein markers for the identification of 1BL.1RS translocations.
Identification of 1BL.1RS translocation by PCR-based markers
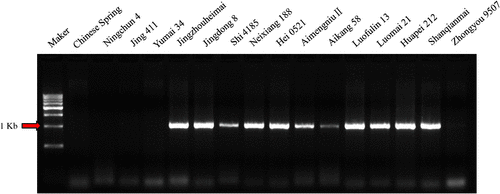
Molecular markers are considered reliable and effective tools to identify different genotypes and can be used in any period of plant development (Bagge et al. 2007). As expected, AS-PCR by primer pair ω-sec-P1 and ω-sec-P2 resulted in a single 1.1 kb fragment and 40 cultivars were confirmed to carry the 1BL.1RS translocation, whereas no fragments were amplified from the 36 non-translocation cultivars (Fig. 2). The results were consistent with those by A-PAGE and LC-MS/MS as well as previous report (Chai et al. 2006).
|
Identification of 1BL.RS translocation by RP-HPLC
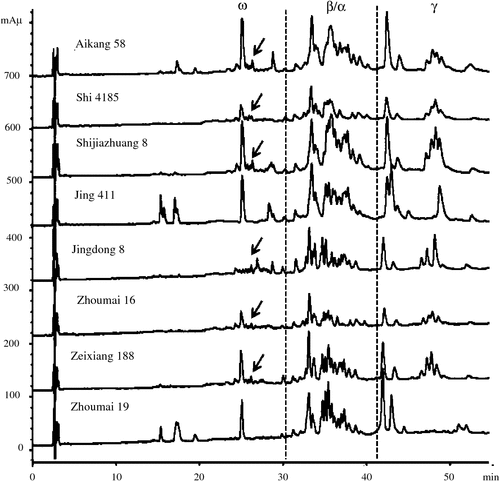
Previous reports showed that wheat gliadins can be clearly separated into three regions based on their surface hydrophobicity and elution order, corresponding to ω-, β/α-, and γ-gliadins (Lookhart and Bean 1995a, 1995b; Wang et al. 2012b). The RP-HPLC analysis (Fig. 3) showed that the sample separation time was ~55 min, and three gliadin areas, corresponding to ω-gliadins (14–28 min), α/β-gliadins (28–40 min), and γ-gliadins (40–54 min) could be clearly identified. In particular, a few minor peaks were present at 25–27 min in the ω-gliadin area in the cultivars carrying the 1BL.1RS translocation, such as Aikang 58, Jingdong 8, and Neixiang 188 (Fig. 3), which were ω-secalins by comparison with the cultivars without the 1BL.1RS translocation. Of the 76 wheat cultivars, 40 contained the 1BL.1RS translocation, consistent with the results of A-PAGE, LC-MS/MS, and PCR.
|
Fast identification of 1BL.RS translocation by RP-UPLC
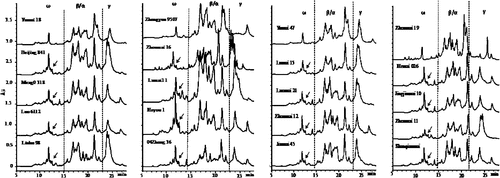
All wheat cultivars identified by A-PAGE, PCR, and RP-HPLC were further subjected to RP-UPLC analysis. The results showed that fast, highly reproducible, and clear separation of seed gliadins could be achieved by gradually increasing the eluting gradient from 21% to 47% over 30 min at a flow rate of 0.5 mL/min and separation temperature of 55°C (Supplementary Materials Table 2 and Fig. 3). The sample separation could be completed in ~27 min, much faster than traditional RP-HPLC method (Fig. 3). In order to test the reproducibility of RP-UPLC separation, 12 consecutive runs were performed for the separation of gliadins from Neixiang 188 under the optimised conditions. The averages, standard deviations, and relative standard deviations (RSD%) of migration time, peak height, and peak area of eight representative peaks are listed in Supplementary Table 2. Results indicated that a high level of reproducibility of RP-UPLC separation was achieved under the optimised conditions, with RSD% values being <0.1 for migration time, <4.0 for peak height, and <5.0 for peak area.
Similar to the RP-HPLC pattern, three gliadin areas were also well separated by RP-UPLC, in which ω-gliadins eluted at 10–15 min, α/β-gliadins at 15–23 min, and γ-gliadins at 23–27 min. All cultivars that were identified to carry the 1BL.1RS translocation by A-PAGE, RP-HPLC, and PCR showed a few minor peaks at 12–13 min in the ω-gliadin area, which corresponded to ω-secalins, making it differentiable between the 1BL.1RS translocation and non-translocation cultivars (Fig. 4). As for the results of A-PAGE, RP-HPLC, and PCR, 40 bread wheat cultivars were identified to have the 1BL.1RS translocation, indicating that a higher percentage of 1BL.1RS translocation is present in the recently released bread wheat cultivars in China.
|
Discussion
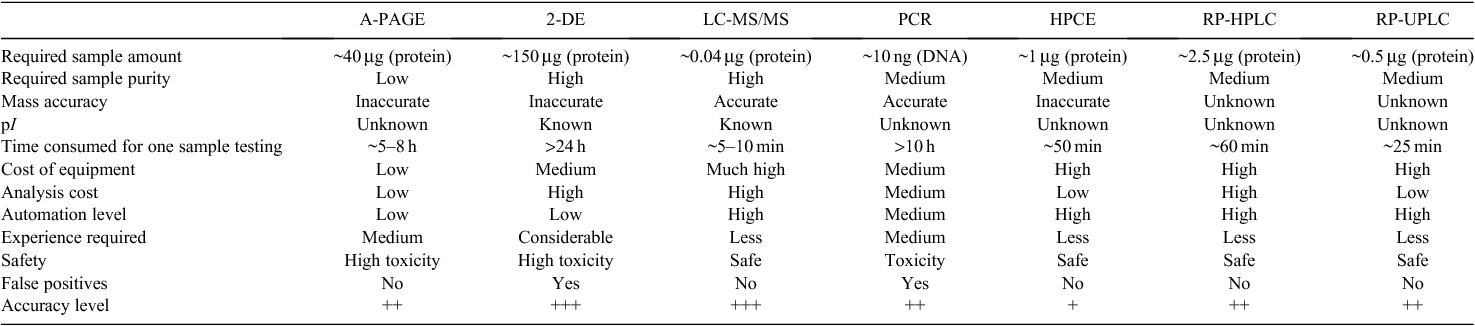
The 1RS chromosome arm is one of the most intensively used rye chromosome segments as the form of a 1BL.1RS translocation in wheat breeding (Rabinovich 1998; Graybosch 2001). So far, numerous methods at the morphological, cell, protein, and molecular levels have been developed to identify this translocation. However, although each method has its own advantages, disadvantages are obviously present (Table 2). Thus, better identification techniques are still required by wheat breeders.
|
Since electrophoretic methods are simple and easy to perform in most laboratories, they are suitable for large-scale and high-throughput 1BL.1RS translocation screening in breeding programs. Thus, A-PAGE has become a widely used method for gliadin analysis, by which 1BL.1RS translocation identification can be achieved (Fig. 1). However, its disadvantages are also obvious, including use of toxic reagents and a slow, labour-intensive procedure with relatively poor resolution and reproducibility. Use of 2-DE and LC-MS/MS can obtain a much high reliability for protein identification, but the sophisticated operation procedures and expensive equipment as well as high analysis costs limited their wide application.
Molecular markers have shown to be an effective tool for identifying desirable genes and genotypes in plant breeding programs (Bagge et al. 2007). The current study (Fig. 2) and a previous report (Chai et al. 2006) demonstrated that PCR-based markers readily differentiate the cultivars carrying the 1BL.1RS translocation, with a throughput and accuracy similar to A-PAGE. Its main drawbacks include that it is highly time-consuming and costly for DNA extraction, PCR reaction, and electrophoretic separation of the amplification products, and false positives are possible.
Various capillary electrophoresis and liquid chromatography methods can realise automatic protein separation with high resolution and reproducibility (Yan et al. 2003; Dong et al. 2009; Gao et al. 2010). For example, both RP-HPLC and HPCE can separate and identify wheat storage proteins with the features of easy use and flexible operation along with stable machine performance (Bietz 1983; Yan et al. 2003). However, RP-HPLC generally needs a long separation time and large amount of analytical reagents. Although HPCE can differentiate between 1AL.1RS and 1BL.1RS translocations (Lookhart et al. 1996), it is difficult to distinguish 1BL.1RS translocation and non-translocation cultivars.
Our results showed that, although both RP-HPLC and RP-UPLC can clearly identify the 1BL.1RS translocation in wheat cultivars based on a few specific peaks in the ω-gliadin region (Figs 3 and 4), RP-UPLC displayed a much faster separation and a significantly lower sample and analytical reagent requirement (Table 2). Therefore, RP-UPLC represents a more efficient way for identifying the wheat 1BL.1RS translocation with lower costs, and it is expected to become a powerful tool for wheat cultivar differentiation and germplasm screening.
In summary, for the identification of the 1BL.1RS translocation in wheat improvement programs, fast gliadin separation with low costs, high throughput, and reliability are particularly important. For rapid identification and large-scale screening of the 1BL.1RS translocation in wheat quality breeding, RP-UPLC is expected to be an appropriate and alternative method.
Acknowledgements
This research was financially supported in part by grants from the National Natural Science Foundation of China (31271703, 31101145), the Chinese Ministry of Science and Technology (2009CB118303) and the National Key Project for Transgenic Crops of China (2011ZX08009-003-004).
References
Bagge M, Xia XC, Lubberstedt T (2007) Functional markers in wheat. Current Opinion in Plant Biology 10, 211–216.| Functional markers in wheat.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXitl2gsrc%3D&md5=5e11fc8f3b1f6586ad5a0f7565fc7081CAS | 17292659PubMed |
Baum M, Appels R (1991) The cytogenetics and molecular architecture of chromosome 1R—one of the most widely utilized source of alien chromatin in wheat varieties. Chromosoma 101, 1–10.
| The cytogenetics and molecular architecture of chromosome 1R—one of the most widely utilized source of alien chromatin in wheat varieties.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2MXhsVOjtQ%3D%3D&md5=14e670f8fa3e49592b487ecda9035023CAS | 1769268PubMed |
Bennett MD, Smith JB (1975) Confirmation of the identification of the rye chromosome in 1B.1R wheat-rye chromosome substitution and translocation lines. Canadian Journal of Genetics and Cytology 17, 117–120.
Bietz JA (1983) Separation of cereal proteins by reversed-phase high-performance liquid chromatography. Journal of Chromatography. A 255, 219–238.
| Separation of cereal proteins by reversed-phase high-performance liquid chromatography.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL3sXmvVagtA%3D%3D&md5=48dee081ac9fb0b3124b438852ac5cb7CAS |
Chai JF, Zhou RH, Jia JZ, Liu X (2006) Development and application of a new codominant PCR marker for detecting 1BL·1RS wheat–rye chromosome translocations. Plant Breeding 125, 302–304.
| Development and application of a new codominant PCR marker for detecting 1BL·1RS wheat–rye chromosome translocations.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XntlWqsr0%3D&md5=cca3388016b1a107c82b2e7c1f5fe90fCAS |
Chojecki AJS, Gale MD, Holt LM, Payne PI (1983) The intrachromosomal mapping of a glucose phosphate isomerase structural gene, using allelic variation among stocks of Chinese Spring wheat. Genetical Research 41, 221–226.
| The intrachromosomal mapping of a glucose phosphate isomerase structural gene, using allelic variation among stocks of Chinese Spring wheat.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL3sXmtV2qtL0%3D&md5=489efefba5861b43c2949b7360cfeb50CAS |
Clarke BC, Mukai Y, Appels R (1996) The Sec-1 locus on the short arm of chromosome 1R of rye (Secale cereale). Chromosoma 105, 269–275.
De Vita P, Ficco DBM, Luciani A, Vincentini O, Pettoello-Mantovani M, Silano M, Maiuri L, Cattivelli L (2012) A-secalin contained decamer shows a celiac disease prevention activity. Journal of Cereal Science 55, 234–242.
| A-secalin contained decamer shows a celiac disease prevention activity.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38Xis1antbY%3D&md5=8a0fdd19013681831d3825ca63add8cfCAS |
Dhaliwal AS, Mares DJ, Skeritt JH, Marshall DR (1988) Protein composition and pentosan content in relation to dough stickiness of 1B/1R translocation wheats. Cereal Chemistry 62, 143–149.
Dong K, Hao CY, Wang AL, Cai MH, Yan YM (2009) Characterization of HMW glutenin subunits in bread and tetraploid wheats by reversed-phase high-performance liquid chromatography. Cereal Research Communications 37, 65–73.
| Characterization of HMW glutenin subunits in bread and tetraploid wheats by reversed-phase high-performance liquid chromatography.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXkvVGqsbg%3D&md5=219099fcabbc5caba2e363c2ff6a2a4eCAS |
Friebe B, Heun M, Bushuk W (1989) Cytological characterization, powdery mildew resistance and storage protein composition of tetraploid and hexaploid 1BL/IRS wheat-rye translocation lines. Theoretical and Applied Genetics 78, 425–432.
| Cytological characterization, powdery mildew resistance and storage protein composition of tetraploid and hexaploid 1BL/IRS wheat-rye translocation lines.Crossref | GoogleScholarGoogle Scholar |
Gao L, Ma W, Chen J, Wang K, Li J, Wang S, Bekes F, Apples R, Yan Y (2010) Characterization and comparative analysis of wheat high molecular weight glutenin subunits by SDS-PAGE, RP-HPLC, HPCE, and MALDI-TOF-MS. Journal of Agricultural and Food Chemistry 58, 2777–2786.
| Characterization and comparative analysis of wheat high molecular weight glutenin subunits by SDS-PAGE, RP-HPLC, HPCE, and MALDI-TOF-MS.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXhslShsb8%3D&md5=57ae2587690c40b3db771161ae724f1eCAS | 20146422PubMed |
Gobaa S, Bancle E, Kleiger G, Stamp P, Branlard G (2007) Effect of the 1BL.1RS translocation on the wheat endosperm, as revealed by proteomic analysis. Proteomics 7, 4349–4357.
| Effect of the 1BL.1RS translocation on the wheat endosperm, as revealed by proteomic analysis.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXhsVOqsL%2FP&md5=94658882ae366b9116ef0e16caed0709CAS | 17973293PubMed |
Graybosch RA (2001) Uneasy unions: quality effects of rye chromatin transfers to wheat. Journal of Cereal Science 33, 3–16.
| Uneasy unions: quality effects of rye chromatin transfers to wheat.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXlt1Oksw%3D%3D&md5=b5984885621f79a0a752544c08ddb906CAS |
Gustafson JP, Dera AR, Petrovic S (1988) Expression of modified rye ribosomal RNA genes in wheat. Proceedings of the National Academy of Sciences of the United States of America 85, 3943–3945.
| Expression of modified rye ribosomal RNA genes in wheat.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC3cnivFGmtw%3D%3D&md5=696bfd7dcff256bd65266045bb8e7fccCAS | 16593938PubMed |
Heslop-Harrison JS, Leitch AR, Schwarzacher T, Anamthawat-Jónsson K (1990) Detection and characterization of 1B/1R translocations in hexaploid wheat. Heredity 65, 385–392.
| Detection and characterization of 1B/1R translocations in hexaploid wheat.Crossref | GoogleScholarGoogle Scholar |
Heun M, Fischbeck G (1987) Identification of wheat powdery mildew resistance genes by analysing host-pathogen interactions. Plant Breeding 98, 124–129.
| Identification of wheat powdery mildew resistance genes by analysing host-pathogen interactions.Crossref | GoogleScholarGoogle Scholar |
Howes NK, Lukow OM, Dawood MR, Bushuk W (1989) Rapid detection of the 1BL.1RS chromosome translocation in hexaploid wheats by monoclonal antibodies. Journal of Cereal Science 10, 1–4.
| Rapid detection of the 1BL.1RS chromosome translocation in hexaploid wheats by monoclonal antibodies.Crossref | GoogleScholarGoogle Scholar |
Javornik B, Sikovié T, Vapa L, Koebner RMD, Rogers WJ (1991) A comparison of methods for identifying and surveying the presence of 1BL.1RS translocations in bread wheat. Euphytica 54, 45–53.
| A comparison of methods for identifying and surveying the presence of 1BL.1RS translocations in bread wheat.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK3MXmt1ymsLY%3D&md5=bab2b090557b05cb20a6628980204f15CAS |
Koebner RMD, Shepherd KW (1986) Controlled introgression to wheat of genes from rye chromosome arm IRS by induction of allosyndesis. Theoretical and Applied Genetics 73, 197–208.
| Controlled introgression to wheat of genes from rye chromosome arm IRS by induction of allosyndesis.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL2sXotFOjtQ%3D%3D&md5=864c556ba8fd7f5a2484b3bfd1b3d53cCAS |
Kőszegi B, Linc G, Juhasz L, Lang L, Molnar-Lang M (2000) Occurrence of the 1RS/1BL wheat-rye translocation in hungarian wheat varieties. Acta Agronomica Hungarica 48, 227–236.
| Occurrence of the 1RS/1BL wheat-rye translocation in hungarian wheat varieties.Crossref | GoogleScholarGoogle Scholar |
Landjeva S, Korzun V, Tsanev V, Vladova R, Ganeva G (2006) Distribution of the wheat-rye translocation 1RS.1BL among bread wheat varieties of Bulgaria. Plant Breeding 125, 102–104.
| Distribution of the wheat-rye translocation 1RS.1BL among bread wheat varieties of Bulgaria.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XjsFOksL4%3D&md5=3caccf57192d21c434ddddf5854852f4CAS |
Lelley T, Eder C, Grausgruber H (2004) Influence of 1BL.1RS wheat-rye chromosome translocation on genotype by environment interaction. Journal of Cereal Science 39, 313–320.
| Influence of 1BL.1RS wheat-rye chromosome translocation on genotype by environment interaction.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXksVantLw%3D&md5=f52d2c67d4d8f6ed11073a4056058665CAS |
Lookhart GL, Bean SR (1995a) A fast method for wheat cultivar differentiation using capillary zone electrophoresis. Cereal Chemistry 72, 42–47.
Lookhart GL, Bean SR (1995b) Rapid differentiation of rice cultivars and of oat cultivars by capillary zone electrophoresis. Cereal Chemistry 72, 312–316.
Lookhart GL, Graybosch R, Peterson J, Lukaszewski A (1991) Identification of wheat lines containing the 1BL/1RS translocation by high performance liquid chromatography. Cereal Chemistry 68, 312–316.
Lookhart GL, Bean SR, Graybosch R, Chung OK, Morean-Sevilla B, Baenziger S (1996) Identification by high-performance capillary electrophoresis of wheat lines containing the 1AL.1RS and the 1BL.1RS translocation. Cereal Chemistry 73, 547–550.
Lukaszewski AJ (1990) Frequency of 1RS.1AL and 1RS.1BL translocations in United States wheats. Crop Science 30, 1151–1153.
| Frequency of 1RS.1AL and 1RS.1BL translocations in United States wheats.Crossref | GoogleScholarGoogle Scholar |
Lukaszewski AJ, Gustafson JP (1983) Translocations and modifications of chromosomes in triticale × wheat hybrids. Theoretical and Applied Genetics 64, 239–248.
| Translocations and modifications of chromosomes in triticale × wheat hybrids.Crossref | GoogleScholarGoogle Scholar |
Mettin D, Bliithner WD, Schlegel G (1973) Additional evidence on spontaneous 1B/1R wheat-rye substitutions and translocations. In ‘Proceedings 4th International Wheat Genetics Symposium’. Columbia, Record Number 19751625247. (Eds ER Sears, LMS Sears) pp. 179–184. (Agricultural Experiment Station, College of Agriculture, University of Missouri: Columbia, MO)
Milovanovic M, Perovic D, Saric M, Prodanovic S, Pavlovic K, Jestrovic Z, Protic R (1998) The influence of 1BL.1RS translocation on technological quality of winter wheat. Cereal Research Communications 26, 321–328.
Murray MG, Thompson WF (1980) Rapid isolation of high molecular weight plant DNA. Nucleic Acids Research 8, 4321–4325.
Rabinovich SV (1998) Importance of wheat-rye translocations for breeding modern cultivars of Triticum aestivum L. Euphytica 100, 323–340.
| Importance of wheat-rye translocations for breeding modern cultivars of Triticum aestivum L.Crossref | GoogleScholarGoogle Scholar |
Rayburn AL, Carver BF (1988) Cytological identification of 1B/1R wheat-rye translocation in winter wheat breeding lines. Euphytica 38, 237–240.
| Cytological identification of 1B/1R wheat-rye translocation in winter wheat breeding lines.Crossref | GoogleScholarGoogle Scholar |
Shewry PR, Bradberry D, Franklin J, White RP (1984) The chromosomal locations linkage relationships of the structural genes for the prolamin storage proteins (secalins) of rye. Theoretical and Applied Genetics 69, 36–69.
| The chromosomal locations linkage relationships of the structural genes for the prolamin storage proteins (secalins) of rye.Crossref | GoogleScholarGoogle Scholar |
Sozinov AA, Novosel’skaya AY, Lushnikova AA, Bogdanov YF (1987) Cytological and biochemical analysis of bread wheat varieties with 1B/1R substitutions and translocation in the karyotype. Tsitologiia i Genetika 21, 256–261.
Swartz ME (2005) UPLC: An introduction and review. Journal of Liquid Chromatography & Related Technologies 28, 1253–1263.
| UPLC: An introduction and review.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXjsF2lsr8%3D&md5=98ad515da204c0271fb9cdd9d88902d4CAS |
Villareal RL, Bañuelos O, Mujeed-Kazi A (1997) Agronomic performance of related durum wheat (Triticum turgidum L.) stocks possessing the chromosome substitution T1BL.1RS. Crop Science 37, 1735–1740.
| Agronomic performance of related durum wheat (Triticum turgidum L.) stocks possessing the chromosome substitution T1BL.1RS.Crossref | GoogleScholarGoogle Scholar |
Wang SL, Chen D, Guo G, Zhang T, Jiang S, Shen X, Perovic D, Prodanovic S, Yan Y (2012a) Molecular characterization of LMW-GS genes from C, N, U and Ss genomes among Aegilops species. Cereal Research Communications 40, 542–551.
| Molecular characterization of LMW-GS genes from C, N, U and Ss genomes among Aegilops species.Crossref | GoogleScholarGoogle Scholar |
Wang SL, Shen XX, Ge P, Li J, Saminathan S, Li XH, Yan YM (2012b) Molecular characterization and dynamic expression patterns of two types of γ-gliadin genes from Aegilops and Triticum species. Theoretical and Applied Genetics 125, 1371–1384.
| Molecular characterization and dynamic expression patterns of two types of γ-gliadin genes from Aegilops and Triticum species.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XhsFWisbnM&md5=d63969db5c6b2ae49e34e498d7cec52aCAS |
Woychik JH, Boundy JA, Dimler RJ (1961) Starch gel electrophoresis of wheat gluten proteins with concentrated urea. Archives of Biochemistry and Biophysics 94, 477–482.
| Starch gel electrophoresis of wheat gluten proteins with concentrated urea.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaF38XhvFymtA%3D%3D&md5=8d7c399bdcdc836598d626ac1c2cde95CAS | 13786728PubMed |
Wu Y, John R, William E, Hobbins WB (2006) Ultra performance liquid chromatography (UPLC) further improves hydrogen/deuterium exchange mass spectrometry. Journal of the American Society for Mass Spectrometry 17, 163–167.
| Ultra performance liquid chromatography (UPLC) further improves hydrogen/deuterium exchange mass spectrometry.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XhtFyitbY%3D&md5=f97bb23d3765ae764a578bfc99ff482cCAS | 16406808PubMed |
Yan YM, Hsam SLK, Yu JZ, Jiang Y, Zeller FJ (2003) Genetic polymorphisms at Gli-Dt gliadin loci in Aegilops tauschii as revealed by acid polyacrylamide gel and capillary electrophoresis. Plant Breeding 122, 120–124.
| Genetic polymorphisms at Gli-Dt gliadin loci in Aegilops tauschii as revealed by acid polyacrylamide gel and capillary electrophoresis.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXkvFGktLk%3D&md5=afcbf95fb9db39dd8b72dacaba5cb4a2CAS |
Yu ZT, Han CX, Wang S, Lv DW, Chen GX, Li XH, Jiang GL, Yan YM (2013a) Fast separation and characterization of water-soluble proteins in wheat grains by reversed-phase ultra-performance liquid chromatography (RP-UPLC). Journal of Cereal Science 57, 288–294.
| Fast separation and characterization of water-soluble proteins in wheat grains by reversed-phase ultra-performance liquid chromatography (RP-UPLC).Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXhtFKgs78%3D&md5=bc7772dfca57e41568a0353f20710089CAS |
Yu ZT, Han CX, Yan X, Li X, Jiang GL, Yan YM (2013b) Rapid characterization of wheat low molecular weight glutenin subunits by ultra performance liquid chromatography (UPLC). Journal of Agricultural and Food Chemistry 61, 4026–4034.
| Rapid characterization of wheat low molecular weight glutenin subunits by ultra performance liquid chromatography (UPLC).Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXlsVertb8%3D&md5=c521ff07a54566792d181f6083d5877bCAS |
Zeller FJ (1973) 1B/1R wheat-rye chromosome substitutions and translocations. In ‘Proceedings of the 4th International Wheat Genetics Symposium’. Record Number: 19751625251. (Eds ER Sears, LMS Sears) pp. 209–221. (Agricultural Experiment Station, College of Agriculture, University of Missouri: Columbia, MO)
Zeller FJ, Hsam SLK (1983) Broadening the genetic variability of cultivated wheat by utilizing rye chromatin. In ‘Proceedings of the 6th International Wheat Genetics Symposium’. (Ed. S Sakamoto) pp. 161–173. (Faculty of Agriculture: Kyoto, Japan)
Zhao CH, Cui F, Wang XQ, Shan SC, Li XF, Bao XG, Wang HG (2012) Effects of 1BL/1RS translocation in wheat on agronomic performance and quality characteristics. Field Crops Research 127, 79–84.
| Effects of 1BL/1RS translocation in wheat on agronomic performance and quality characteristics.Crossref | GoogleScholarGoogle Scholar |
Zhou Y, He ZH, Liu JJ, Liu L (2003) Distribution of 1BL/1RS translocation in Chinese winter wheat and its effect on noodle quality. In ‘Proceedings of 10th International Wheat Genetics Symposium’. (Eds NE Pogna, M Romano, G Galterio). Paestum 3, 1419–1421.


