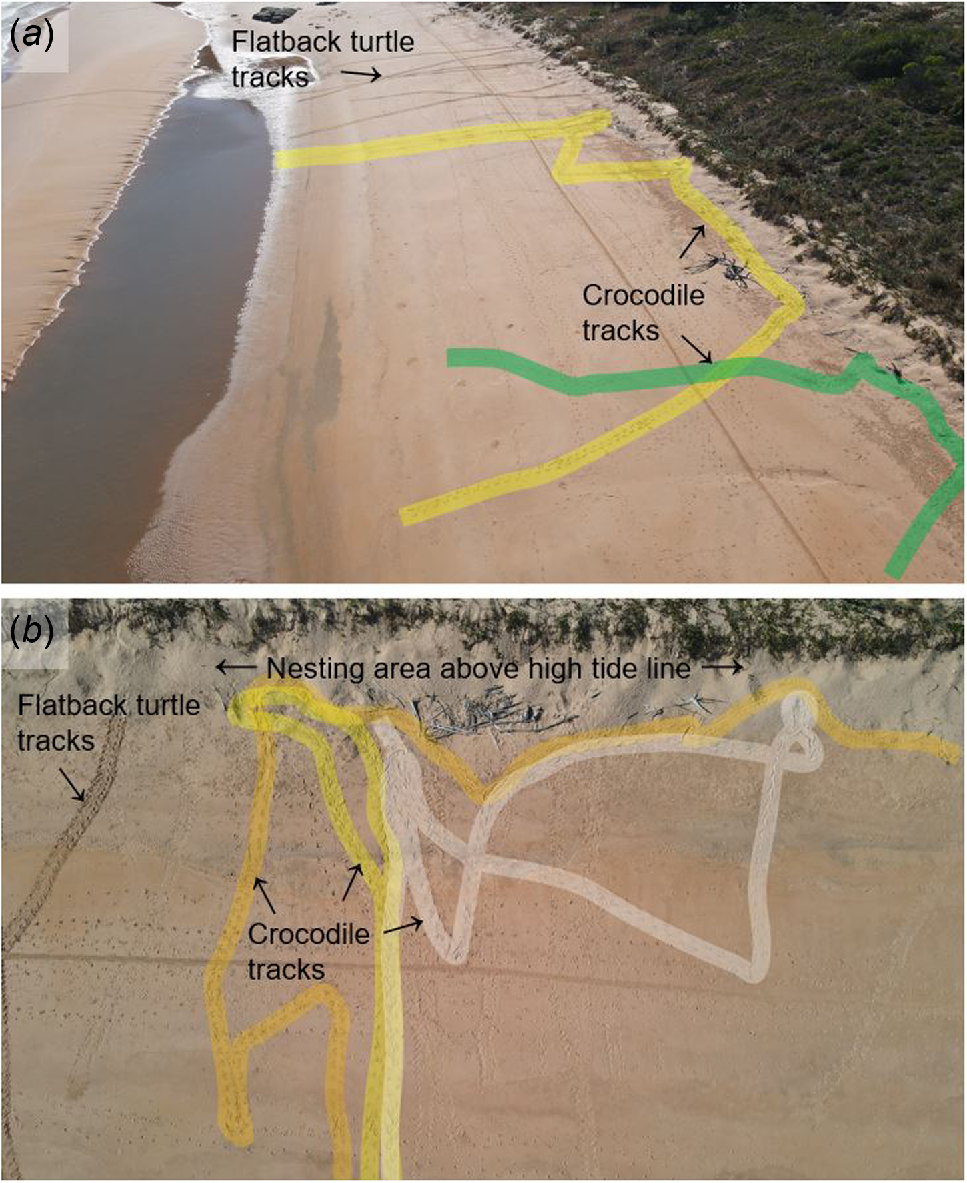
Observations on predation of the flatback turtle Natator depressus by the saltwater crocodile Crocodylus porosus at a major rookery in northern Australia
Casper Avenant A B * and James Gee A
A B * and James Gee A
A
B
Abstract
We examined predation by the saltwater crocodile (Crocodylus porosus) on the flatback turtle (Natator depressus), an Australian endemic, at Cape Domett, a significant nesting site in Cambridge Gulf, northern Australia, where crocodile populations have recovered since legislative protection was implemented in the 1970s.
The investigation aimed to document crocodile predation on flatback turtles across hatchling and adult life stages, assess predation strategies, and evaluate ecological impacts on this genetically distinct turtle stock.
Conducted over three Austral winters (2021–2023), the study utilised infrared videography, unmanned aerial vehicles, and beach patrols to observe predation events in a population of ~3250 nesting female flatback turtles.
Five predation events were recorded, involving juvenile (<2 m) and large (~5 m) crocodiles. Large crocodiles employed ambushing strategies at the waterline or on land, with tracks, remains, and carcasses indicating an estimated predation rate of one adult turtle per week. Juvenile crocodiles targeted hatchlings during emergence, and scavenging of turtle carcasses was documented.
The recovery of C. porosus has likely intensified predation pressure on N. depressus. Infrared cameras and drones proved effective in capturing unbiased diurnal and nocturnal predation interactions, contributing to the limited global literature on crocodilian predation of sea turtles.
These findings position C. porosus as a significant ecological threat to flatback turtles. Comprehensive assessments, including nest inventories and thermal drone surveys, are recommended to refine predation estimates and enhance population viability models, informing conservation strategies for N. depressus amid increasing crocodile populations.
Keywords: drone, flatback turtle, hatchling, infrared videography, marine turtle, predation, saltwater crocodile, scavenging.
Introduction
Predation constitutes a significant ecological pressure across all life stages of sea turtles. While adult and large juvenile sea turtles typically exhibit low predation rates due to their substantial size and effective predator-avoidance strategies (Heithaus 2013), incubating eggs and newly emerged hatchlings on sandy beaches remain highly susceptible to a diverse array of predators, e.g. ghost crabs, goannas, foxes and birds (Lei and Booth 2017; Avenant et al. 2024; King et al. 2024). Among these, the saltwater crocodile (Crocodylus porosus) is hypothesised to represent a notable predator of nesting female turtles and their early life stages. The geographical distribution spans northern Australia to the east coast of India (Patro and Padhi 2019; Fukuda et al. 2022; Lloyd-Jones et al. 2023) and thus C. porosus exhibiting considerable range overlap with several sea turtle species, particularly at mainland and island nesting beaches. The recovery of C. porosus populations in northern Australia, following legislative protection since the 1970s, has restored some populations to near pre-exploitation levels after extensive hunting for their skins between the 1940s and 1970s (Fukuda et al. 2021). This resurgence potentially amplifies predation pressure on co-occurring sea turtle populations.
The predatory efficacy of C. porosus is underpinned by a suite of physiological and behavioural adaptations, including acute sensory capabilities (vision, olfaction, gustation, and hearing), streamlined morphological characteristics facilitating concealment in aquatic environments, and exceptional agility for short-distance pursuits (Caldicott et al. 2005). The dietary breadth of C. porosus is considerable, with adults preying on large vertebrates such as water buffalo, pigs, birds, turtles, and snakes (Adame et al. 2018), and juveniles primarily consuming crustaceans and insects (Isberg 2007). The spatial and temporal predictability of sea turtle nesting may render turtles a significant food resource for crocodiles in regions of sympatry. Nesting turtles, characterised by slow, awkward terrestrial locomotion and a trance-like state during oviposition, are particularly vulnerable to predation by large crocodiles. Conversely, juvenile crocodiles are more likely to target hatchlings during their emergence or excavate nests to consume eggs (Whiting and Whiting 2011).
Although predation of sea turtles by C. porosus, the American crocodile (C. acutus), and the American alligator (Alligator mississippiensis) has been documented (Ortiz et al. 1997; Nifong et al. 2011; Whiting and Whiting 2011), such observations remain infrequent, and the broader ecological impact of crocodilians on sea turtle populations is inadequately elucidated. We aim to provide a detailed examination of predation by C. porosus on the hatchling and adult life stages of the flatback turtle (Natator depressus), an Australian endemic, at a major nesting rookery in northern Australia. Through this investigation, a more comprehensive understanding of crocodilian-sea turtle interactions is sought, with implications for the conservation of N. depressus in the context of recovering crocodile populations.
Materials and methods
Study area
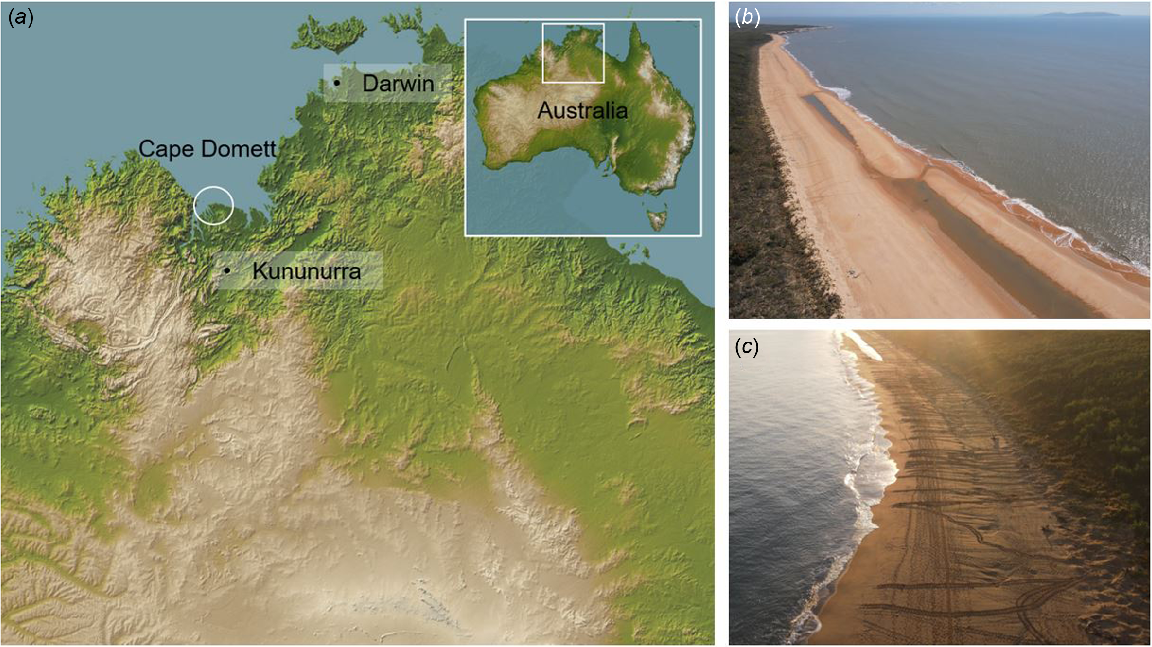
The investigation was conducted at Cape Domett (14°48.1′S, 128°24.5′E), situated at the entrance to Cambridge Gulf, ~110 km north-northwest of Kununurra in the East Kimberley region of tropical northern Australia (Fig. 1a). Characterised by a tidal range exceeding 7 m during spring tides, the area features an extensive intertidal zone and relatively turbid waters. The primary nesting beach at Cape Domett, spanning 1.9 km (Fig. 1b, c), supports high-density nesting of the flatback turtle (N. depressus), a genetically distinct stock comprising ~3250 nesting females (Fitzsimmons et al. 2020). Nesting occurs year-round, with peak activity in August–September, and no other turtle species have been recorded at this site (Whiting et al. 2008). Flatback turtles are medium-sized sea turtles, with a curved carapace length of ~90 cm and a body mass ranging from 75 to 85 kg (Limpus 2007). The region also serves as critical habitat for the saltwater crocodile (C. porosus), with seasonal creeks, tidal mudflats, floodplains, mangrove wetlands, and sandy beaches facilitating their presence within Cambridge Gulf (Cresswell et al. 2011; Semeniuk et al. 2011). C. porosus is recognised as a predator of sea turtles (Whiting and Whiting 2011).
(a) Map of the study area, illustrating the location of the Natator depressus rookery at Cape Domett, northern Australia. (b, c) Panoramic photographs of the ~1.9 km nesting beach where observations were conducted. Map adapted from NASA (2010). Photographs by C. Avenant (b, c).
Data collection
Observations of predation on N. depressus were conducted along the main nesting beach during annual 2-week surveys in August–September, coinciding with the Austral winter, from 2021 to 2023. Daily beach patrols, commencing at 06:00, were undertaken to monitor nesting activity, during which the presence of crocodile tracks, and sightings of crocodiles on the shore or in adjacent waters, were systematically recorded. Predation events were documented through direct observation and, where feasible, captured using smartphones or compact cameras. In 2023, opportunistic aerial surveys were conducted using unmanned aerial vehicles (DJI Mavic Air 2 and Mini Pro 3), selected for their compact size and low noise profiles to minimise disturbance to wildlife, offering reduced impact compared to larger drones employed in prior studies (Bevan et al. 2018; Sellés-Ríos et al. 2022). Drones were operated at an altitude of ~60 m for comprehensive beach coverage, descending to a minimum of 30 m for focused recordings to mitigate potential stress to wildlife. Additionally, pole-mounted infrared video cameras (Dahua Eyeball/Cube Cameras and Mobile Network Video Recorder), as detailed by Avenant et al. (2024), were strategically deployed near nests exhibiting signs of imminent hatchling emergence or bearing visible hatchling or crocodile tracks. These cameras recorded continuously for 5 days to capture predation events. All video footage, encompassing both drone and infrared recordings, was manually analysed to identify turtle hatchling activity, crocodile presence, or evidence of predation.
Animal ethics and permits
All research protocols included in this paper have been approved by the Western Australia Department of Biodiversity, Conservation and Attractions (licence number U10/2020-2022) and the DBCA’s Animal Ethics Committee (2022-09C). The procedures comply with the Animal Welfare Act 2002 (Western Australia) and the requirements of the code for the care and use of animals for scientific purposes.
Results
Observations at Cape Domett revealed frequent presence of saltwater crocodiles (C. porosus), with individuals observed drifting in shallow waters or basking on the beach throughout diurnal periods. Data collected through beach patrols, drone surveys, and infrared videography documented predation and scavenging events involving both hatchling and adult flatback turtles (N. depressus). These methods provided comprehensive evidence of crocodile activity, including direct predation during daytime, scavenging incidents, and nocturnal hatchling predation by juvenile crocodiles.
Over 35 days of observations across three nesting seasons (7, 14, and 14 days in 2021–2023), morning beach patrols recorded an average of 2.4–4.6 crocodile tracks per day, 0.1–0.9 crocodiles basking on the shore, and 0.8–1.1 crocodiles in adjacent waters (Table 1). A notable drone survey on a single morning in 2023, conducted independently of track counts and post-nesting surveys, identified 11 crocodiles (~2.5–5 m in length) basking along the 1.9 km nesting beach, indicating significant crocodile presence.
| Year (# survey days) | Crocodile tracks Mean (s.d., range, n) | Crocodiles sighted on beach Mean (s.d., range, n) | Crocodiles sighted in water Mean (s.d., range, n) | |
|---|---|---|---|---|
| 2021 (7) | 4.4 (2.6, 1–8, 31) | 0.9 (0.9, 0–2, 6) | 1.1 (0.9, 0–3, 8) | |
| 2022 (14) | 2.4 (1.2, 0–4, 33) | 0.1 (0.4, 0–1, 2) | 0.8 (0.7, 0–2, 11) | |
| 2023 (14) | 4.6 (2.5, 1–9, 64) | 0.8 (1.0, 0–3, 11) | 1.1 (1.4, 0–4, 16) |
Five distinct predation and scavenging events were documented (summarised in Table 2).
| Obs. No. | Date | Crocodile life stage | Turtle life stage | Event | Method | |
|---|---|---|---|---|---|---|
| 1 | 10/08/2022 | Adult | Adult | Predation | Direct observation | |
| 2 | 03/08/2022 | Adult | Adult | Predation/scavenging | Direct observation | |
| 3 | 07/08/2023 | Adult | Adult | Predation | Track analysis | |
| 4 | 09/08/2023 | Adult | Adult | Scavenging | Direct observation/Drone camera | |
| 5 | 12/08/2023 | Juvenile | Hatchling | Predation | Direct observation and infrared cameras |
Ambush Predation (10 August 2022): At sunrise, a large crocodile (~5 m) was observed drifting 10 m offshore, evidently targeting a female turtle attempting to nest. No additional turtle tracks were recorded, suggesting a solitary nesting attempt. As the turtle crawled toward the water following an unsuccessful nesting effort, the crocodile approached the shoreline, positioning itself behind breaking waves (Fig. 2a). Upon the turtle’s entry into the water, the crocodile executed a lateral lunge, seizing the turtle’s front left flipper. Minimal resistance was observed, likely due to the turtle’s exhaustion. The crocodile then swam with the turtle beyond a rocky promontory, rendering the outcome (consumption or escape) indeterminate.
(a) Moments preceding an attack by a ~5 m saltwater crocodile (Crocodylus porosus) on a female flatback turtle (Natator depressus) reentering the water following an unsuccessful nesting attempt. (b, c) A ~5 m crocodile adjacent to a turtle carcass. (d) Turtle carcass exhibiting a crushed carapace and multiple puncture marks from crocodile teeth. (e) Tracks and slide marks in the sand indicating the site of a crocodile attack on a turtle. (f) Evidence of the turtle being dragged toward the water. Photographs by J. Gee (a–f).

Carcass Observation (3 August 2022): In the afternoon, a deceased turtle exhibiting a crushed head, carapace, and puncture marks was recorded at low tide (Fig. 2d). A large crocodile (>5 m) was positioned adjacent to the carcass, holding the turtle’s head in its jaws (Fig. 2b). The crocodile retreated to the water upon observer approach, leaving the carcass (Fig. 2c), which was absent by the following morning, likely scavenged or removed by tidal action.
Terrestrial Attack (7 August 2023): Evidence of a terrestrial predation event was identified through converging crocodile and turtle tracks near the beach crest. Disturbed sand, carapace fragments, and turtle entrails indicated a violent encounter (Fig. 2e). Tracks returning to the ocean, accompanied by drag marks from the turtle’s carapace, suggested the crocodile transported the turtle seaward (Fig. 2f). It is unclear whether the crocodile located the turtle by following the track or intercepted the turtle while crawling back to the water.
Prolonged Scavenging (9–14 August 2023): A turtle carcass, initially observed without visible injuries below the high tide line on 9 August, was monitored over 6 days. Dingo (Canis lupus dingo) tracks were noted on 10 August, but no feeding occurred (Fig. 3a). A crocodile (3.5 m in length) was observed adjacent to the carcass by 13 August. It had apparently dismembered the carcass, removing the head and rear flippers, and was recorded exerting force on the posterior section of the carapace, resulting in an audible crunch (see Fig. 3b, c). Subsequent inspections revealed progressive degradation, with only the headless anterior half remaining by 14 August, surrounded by crocodile slide marks (Fig. 3d, e). On 15 August, the same crocodile was observed performing ‘death rolls’ to dislodge flesh, with the carcass reduced to a fragmented carapace attached to the plastron (Fig. 3f). Two additional crocodiles (3 m) were noted nearby, one in the shallows and one at the waterline.
Sequential documentation of a saltwater crocodile (Crocodylus porosus) scavenging a flatback turtle (Natator depressus) carcass over several days. (a) Carcass initially observed without visible external damage. (b, c) Carcass being consumed by a large crocodile days later. (d) Approximately three-quarters of the carcass remaining the following day. (e) Half the carcass remaining, surrounded by crocodile tracks in the sand. (f) Large crocodile performing a ‘death roll’ on the remains, 6 days after initial observation. Photographs by C. Avenant (a–f).

Hatchling Predation (12–14 August 2023): Infrared videography on 12 August captured a juvenile crocodile (<2 m) preying on hatchlings emerging post-midnight, concurrent with night heron activity. The crocodile consumed at least five hatchlings over 1.5 h, moving intermittently while herons maintained their distance (Fig. 4a). On 13 August, a subadult crocodile (~2.5 m) was observed pursuing hatchlings, capturing at least two with head-lifting motions to facilitate swallowing (Fig. 4b). Impressions in wet sand marked termination points of hatchling tracks at the crocodile’s jaws (Fig. 4c). Similar predation by a comparably sized crocodile was noted on another morning, with tracks leading to high-density nesting areas (Fig. 4d). Drone imagery on 14 August revealed extensive crocodile tracks across the beach, indicative of hatchling-targeted hunting, though the number of contributing crocodiles remains uncertain (Fig. 5a, b). It remains uncertain whether the tracks were produced by a single crocodile or multiple crocodiles.
(a) Nocturnal predation of emerging flatback turtle (Natator depressus) hatchlings by a juvenile saltwater crocodile (Crocodylus porosus) and night herons. (b) A crocodile pursuing hatchlings near the water’s edge. (c, d) Converging hatchling tracks and crocodile slide marks on the beach, indicative of predation activity. Photographs by C. Avenant (a) and J. Gee (b–d).

Discussion
The predation of flatback turtles (N. depressus) by saltwater crocodiles (C. porosus) at Cape Domett, as documented in this investigation, provides a comprehensive account of predator-prey dynamics at a high-density flatback turtle nesting beach in northern Australia. These findings contribute to and extend the limited body of literature on crocodilian predation of sea turtles (Table 3; Hirth et al. 1993; Ortiz et al. 1997; Sutherland and Sutherland 2003; Leis 2008; Whiting et al. 2008; Nifong et al. 2011; Whiting and Whiting 2011; Koeyers et al. 2015). The observed frequency of adult turtle predation (approximately one event per week) and hatchling predation during emergence events at Cape Domett aligns with patterns reported at other Australian sites, such as Crab Island and Cape van Diemen, where C. porosus similarly targets N. depressus and L. olivacea adults (Sutherland and Sutherland 2003; Leis 2008; Whiting and Whiting 2011). The integration of direct observations, unmanned aerial vehicle (drone) surveys, and infrared videography elucidated specific behaviours, including stalking, scavenging with ‘death rolls,’ and juvenile crocodile predation on hatchlings triggered by concurrent night heron activity. These observations underscore the opportunistic and ambush strategies of C. porosus (Caldicott et al. 2005), and highlight the heightened vulnerability of turtles during nesting and hatchling emergence phases.
| Location | Crocodilian species | Sea turtle species | Turtle life stage | Description | Source | |
|---|---|---|---|---|---|---|
| New River, South Carolina, USA | A. mississippiensis | n/a | Adult | Flipper tag in crocodile gut contents. | Nifong et al. (2011) | |
| South Ponte Vedra Beach, Florida, USA | A. mississippiensis | C. mydas | Adult | Punctured carapace. | Nifong et al. (2011) | |
| Georgia barrier islands, Georgia, USA | A. mississippiensis | C. caretta | Adult | Scavenged carcasses. | Nifong et al. (2011) | |
| Playa Nancite, Costa Rica | C. acutus | L. olivacea | Adult | Punctured carapace, tracks. ~2 per month. | Ortiz et al. (1997) | |
| Piguwa, Papua New Guinea | C. porosus | D. coriacea | Adult | Turtle’s head bitten off in shallow water. | Hirth et al. (1993) | |
| Crab Island, Cape York Peninsula, Australia | C. porosus | N. depressus | Adult | Intersecting tracks at waterline, blood, turtle pieces. ~1 per week. | Sutherland and Sutherland (2003) | |
| Crab Island, Cape York Peninsula, Australia | C. porosus | N. depressus | Adult | Carcasses, bloody remains, crocodile tracks. ~2 per week. | Leis (2008) | |
| Hatchling | Intersecting tracks, tracks around nests. | |||||
| Cape van Diemen, Tiwi Islands, Australia | C. porosus | L. olivacea, N. depressus | Adult | Attacks during nesting. ~1 per week. ~1% of nesting population. | Whiting and Whiting (2011) | |
| Egg | Dug up nest, scattered egg remains. | |||||
| Cape Domett, Cambridge Gulf, Australia | C. porosus | N. depressus | Adult | Attacks at water’s edge. ~1 per week. | Whiting et al. (2008) and Whiting and Whiting (2011) | |
| Hatchling | Taken at high tide mark. | |||||
| Winyalkin Island, Kimberley coast, Australia | C. porosus | C. mydas | Adult | Attack at water’s edge. | Koeyers et al. (2015) |
Crocodilian predation on sea turtles appears to be geographically widespread, encompassing C. porosus, C. acutus, and A. mississippiensis across regions including Australia, Papua New Guinea, Costa Rica, and the United States (Table 3). Adult turtles are frequently targeted at the water’s edge or during nesting, with evidence such as punctured carapaces, severed limbs, and intersecting tracks corroborating predation events (Ortiz et al. 1997; Sutherland and Sutherland 2003; Nifong et al. 2011). Scavenging, as observed at Cape Domett, is also documented for A. mississippiensis (Nifong et al. 2011), suggesting that crocodilians exploit both live and deceased turtles. Hatchling predation, consistent with findings at Crab Island and Cape van Diemen (Leis 2008; Whiting and Whiting 2011), was frequently recorded at Cape Domett, whereas egg predation, reported solely at Cape van Diemen (Whiting and Whiting 2011), was not observed, potentially due to detection limitations or lower prevalence.
The recovery of C. porosus populations since the cessation of large-scale hunting in the 1970s, facilitated by protective legislation and expansive habitats, has likely intensified predation pressure at Cape Domett, where crocodiles are abundant alongside dense N. depressus nesting (Webb et al. 1978; Fukuda et al. 2021). This trend is consistent with other Australian turtle rookeries (Sutherland and Sutherland 2003; Whiting and Whiting 2011), whereas predation by C. acutus in Costa Rica (approximately two adults per month; Ortiz et al. 1997) and A. mississippiensis in the United States (Nifong et al. 2011) appears less frequent, possibly attributable to lower crocodilian densities or less sea turtle nesting activity.
The ecological implications of crocodilian predation for the Cape Domett N. depressus stock remain unclear due to insufficient quantification of predation rates across egg, hatchling, and adult life stages. While the loss of approximately one adult per week may exert minimal impact on the substantial Cape Domett population, smaller N. depressus rookeries with lower nesting densities could be more susceptible. Hatchling predation, observed during emergence events, likely affects a disproportionate number of individuals given their inherently low survival rates (Limpus 2007). Furthermore, predation by multiple predator species has been widely observed on sea turtle nesting beaches globally (Barton and Roth 2008; King et al. 2024), with nocturnal predation by night herons documented at Cape Domett, alongside evidence of egg predation by dingoes (C. Avenant, unpubl. data), suggesting cumulative pressures and complicating the predation dynamics.
The methodological framework employed in this study, combining beach patrols, infrared videography, and drone surveys, provides a robust approach for predation research, surpassing reliance on indirect evidence such as tracks or remains. Infrared cameras proved particularly effective in capturing unbiased nocturnal behaviours without disturbing wildlife, consistent with prior applications (Avenant et al. 2024; Avenant 2025). Drone surveys facilitated aerial documentation of crocodile tracks and size estimations, reinforcing their utility in behavioural and population studies (Ezat et al. 2018; Thapa et al. 2018). Future research integrating thermal drone imaging (Sellés-Ríos et al. 2022) and satellite tracking (Read et al. 2007; Mascarenhas-Junior et al. 2023) of crocodiles is recommended to further clarify movement patterns and predation strategies, thereby enhancing understanding of the frequency and spatial distribution of crocodile-turtle interactions.
Data availability
The data presented in this study are not publicly available as they are part of ongoing monitoring efforts. Access to the data is restricted to ensure the integrity and continuity of the research process. For further inquiries, please contact the corresponding author.
Declaration of funding
Research was funded by the DBCA through the North West Shelf Flatback Turtle Conservation Program and the Kununurra Office of the East Kimberley Region.
Author contributions
Both authors contributed to the written manuscript, and read and approved the final manuscript.
Acknowledgements
We thank the DBCA’s Parks and Wildlife Service – Kimberley Region (Brad, Toby and Sean) for their support, Miriuwung-Gajerrong DBCA Rangers (Clay, Wayne and Winston) and Traditional Owners (Arthur and Ralph) for their assistance in the field.
References
Adame MF, Jardine TD, Fry B, et al. (2018) Estuarine crocodiles in a tropical coastal floodplain obtain nutrition from terrestrial prey. PLoS ONE 13(6), e0197159.
| Crossref | Google Scholar |
Avenant C (2025) Insights into prey handling and feeding strategies by ghost crabs on sea turtle eggs and hatchlings. Food Webs 43, e00400.
| Crossref | Google Scholar |
Avenant C, Fossette S, Whiting S, et al. (2024) Predation rates on flatback turtle Natator depressus eggs and hatchlings at an island rookery. Marine Biology 171(12), 228.
| Crossref | Google Scholar |
Barton BT, Roth JD (2008) Implications of intraguild predation for sea turtle nest protection. Biological Conservation 141(8), 2139-2145.
| Crossref | Google Scholar |
Bevan E, Whiting S, Tucker T (2018) Measuring behavioral responses of sea turtles, saltwater crocodiles, and crested terns to drone disturbance to define ethical operating thresholds. PLoS ONE 13(3), e0194460.
| Crossref | Google Scholar |
Caldicott DGE, Croser D, Manolis C, et al. (2005) Crocodile attack in Australia: an analysis of its incidence and review of the pathology and management of crocodilian attacks in general. Wilderness & Environmental Medicine 16, 143-159.
| Crossref | Google Scholar |
Cresswell ID, Bridgewater P, Semeniuk V (2011) The coastal habitats and vegetation of the Kimberley region. Journal of the Royal Society of Western Australia 94, 197-206.
| Google Scholar |
Ezat MA, Fritsch CJ, Downs CT (2018) Use of an unmanned aerial vehicle (drone) to survey Nile crocodile populations: a case study at Lake Nyamithi, Ndumo game reserve, South Africa. Biological Conservation 223(May), 76-81.
| Crossref | Google Scholar |
Fitzsimmons NN, Pittard SD, McIntyre N, et al. (2020) Phylogeography, genetic stocks, and conservation implications for an Australian endemic marine turtle. Aquatic Conservation: Marine and Freshwater Ecosystems 30, 440-460.
| Crossref | Google Scholar |
Fukuda Y, Webb G, Edwards G, et al. (2021) Harvesting predators: simulation of population recovery and controlled harvest of saltwater crocodiles Crocodylus porosus. Wildlife Research 48(3), 252-263.
| Crossref | Google Scholar |
Fukuda Y, Moritz C, Jang N, et al. (2022) Environmental resistance and habitat quality influence dispersal of the saltwater crocodile. Molecular Ecology 31(4), 1076-1092.
| Crossref | Google Scholar |
Hirth HF, Kasu J, Mala T (1993) Observations on a leatherback turtle Dermochelys coriacea nesting population near Piguwa, Papua New Guinea. Biological Conservation 65, 77-82.
| Crossref | Google Scholar |
Isberg SR (2007) Nutrition of juvenile saltwater crocodiles (Crocodylus porosus) in commercial production systems. CAB Reviews: Perspectives in Agriculture, Veterinary Science, Nutrition and Natural Resources 2(February), 1-11.
| Crossref | Google Scholar |
King J, Whiting SD, Adams PJ, et al. (2024) Camera traps show foxes are the major predator of flatback turtle nests at the most important mainland western Australian rookery. Wildlife Research 51, WR22109.
| Crossref | Google Scholar |
Koeyers A, Koeyers J, Tucker AD (2015) Chelonia mydas (Green Sea Turtle). Predation. Herpetological Review 46(2), 240.
| Google Scholar |
Lei J, Booth DT (2017) How best to protect the nests of the endangered loggerhead turtle Caretta caretta from monitor lizard predation. Chelonian Conservation and Biology 16(2), 246-249.
| Crossref | Google Scholar |
Lloyd-Jones LR, Brien ML, Feutry P, et al. (2023) Implications of past and present genetic connectivity for management of the saltwater crocodile (Crocodylus porosus). Evolutionary Applications 16, 911-935.
| Crossref | Google Scholar |
Mascarenhas-Junior PB, Sousa Correia JM, Simões PI (2023) Tracking crocodylia: a review of telemetry studies on movements and spatial use. Animal Biotelemetry 11(1), 21.
| Crossref | Google Scholar |
NASA (2010) Australia, shaded relief and colored height. NASA Earth Observatory. Available at https://earthobservatory.nasa.gov/images/5100/australia-shaded-relief-and-colored-height [accessed 3 October 2024]
Nifong JC, Frick MG, Eastman SF (2011) Putative Predation and scavenging of two sea turtle species by the American Alligator, Alligator mississippiensis, in Coastal Southeastern United States. Herpetological Review 42(4), 511-513.
| Google Scholar |
Ortiz RM, Plotkin PT, Owens DW (1997) Predation upon olive ridley sea turtles (Lepidochelys olivacea) by the American crocodile (Crocodylus acutus) at Playa Nancite, Costa Rica. Chelonian Conservation and Biology 2, 585-587 Available at http://www.sidalc.net/cgi-bin/wxis.exe/?IsisScript=OET.xis&method=post&formato=2&cantidad=1&expresion=mfn=017998.
| Google Scholar |
Patro S, Padhi SK (2019) Saltwater crocodile and human conflict around Bhitarkanika National Park, India: a raising concern for determining conservation limits. Ocean & Coastal Management 182(January), 104923.
| Crossref | Google Scholar |
Read MA, Grigg GC, Irwin SR, et al. (2007) Satellite tracking reveals long distance coastal travel and homing by translocated estuarine crocodiles, Crocodylus porosus. PLoS ONE 2(9), e949.
| Crossref | Google Scholar |
Sellés-Ríos B, Flatt E, Ortiz-García J, et al. (2022) Warm beach, warmer turtles: using drone-mounted thermal infrared sensors to monitor sea turtle nesting activity. Frontiers in Conservation Science 3(July), 954791.
| Crossref | Google Scholar |
Semeniuk V, Manolis C, Webb GJW, et al. (2011) The Saltwater Crocodile, Crocodylus porosus Schneider, 1801, in the Kimberley coastal region. Journal of the Royal Society of Western Australia 94, 407-416.
| Google Scholar |
Sutherland RW, Sutherland EG (2003) Status of the flatback turtle (Natator depressus) rookery on Crab Island, Australia with notes on predation by crocodiles. Chelonian Conservation and Biology 4(3), 612-619 Available at https://chelonian.org/wp-content/uploads/file/CCB_Vol_4_Nos1-4(2001-2005)/Sutherland_and_Sutherland_2003.pdf.
| Google Scholar |
Thapa GJ, Thapa K, Thapa R, et al. (2018) Counting crocodiles from the sky: monitoring the critically endangered gharial (Gavialis gangeticus) population with an unmanned aerial vehicle (UAV). Journal of Unmanned Vehicle Systems 6(2), 71-82.
| Crossref | Google Scholar |
Webb GJW, Messel H, Crawford J, et al. (1978) Growth rates of Crocodylus porosus (Reptilia: Crocodilia) from Arnhem Land, Northern Australia. Wildlife Research 5, 385-399.
| Crossref | Google Scholar |
Whiting SD, Whiting AU (2011) Predation by the saltwater crocodile (Crocodylus porosus) on sea turtle adults, eggs, and hatchlings. Chelonian Conservation and Biology 10(2), 198-205.
| Crossref | Google Scholar |
Whiting AU, Thomson A, Chaloupka M, et al. (2008) Seasonality, abundance and breeding biology of one of the largest populations of nesting flatback turtles, Natator depressus: Cape Domett, Western Australia. Australian Journal of Zoology 56(5), 297-303.
| Crossref | Google Scholar |