
Australian Journal of Chemistry
Volume 69 Number 8 2016
CH16030Co-Condensation Assisted Preparation of MoVI Schiff Base Modified Mesoporous Silica Catalyst for Enhanced Epoxidation of Olefins
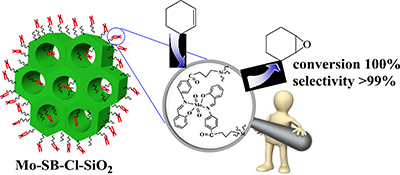
An organic–inorganic catalyst was prepared by the reaction of p-salicylidine aminobenzoic acid with mesoporous silica modified by 3-chloropropyl groups. This involved the hydrolysis and co-condensation of tetraethylorthosilicate and 3-chloropropyltrimethoxysilane and the introduction of MoO2(acac)2 into mesoporous silica functionalized with a Schiff base ligand. The heterogeneous catalyst showed good catalytic activities in the liquid-phase epoxidation of olefins.
CH15640Synthesis, Structural Characterisation, DFT Studies, and Spectroscopic Properties of Copper(I) Complexes with Extended C–H⋅⋅⋅π Interactions
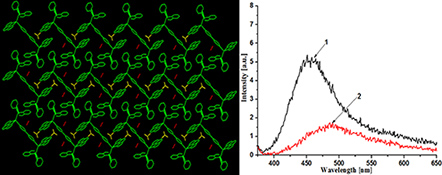
Two copper(i) complexes, [Cu(phen)(PPh2(o-C6H4CHO))2](BF4)2 (1) and [Cu2(4,4′-bipy)(phen)2(PPh2(o-C6H4CHO))2](BF4)2·2CH3CN (2), have been prepared and characterised.
CH15630Effect of Co-Crystal Formers on the Supramolecular Patterns and Luminescence Properties of Co-Crystals Comprising Fenbufen and Diverse N-Heterocycles
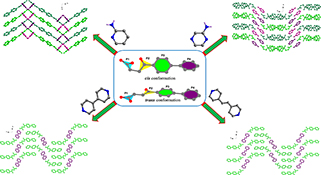
Four co-crystals have been obtained from the reaction of fenbufen and different N-heterocycles by grinding method or solution evaporation. Structural analyses indicate that the conformations of Fbf molecules are highly related to the lone pair···π, C–H···π, or C–H···O interactions, which lead to the formation of diverse supramolecular patterns.
CH15728Structural Diversity and Properties of Six ZnII/CdII Coordination Polymers Based on an O-Bridged Semi-Rigid Bis-pyridyl-bis-amide and Different Dicarboxylates
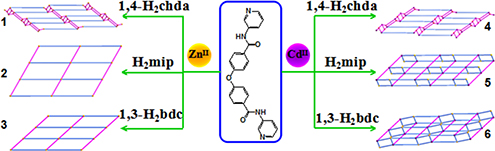
Six new 2D ZnII/CdII coordination polymers have been synthesized via hydrothermal or solvothermal reaction. The photoluminescence properties and photocatalytic activities of the title complexes have been studied.
CH15747A Novel Self-Assembled Hierarchical-Structured Catalyst for the Diffusion of Macromolecules
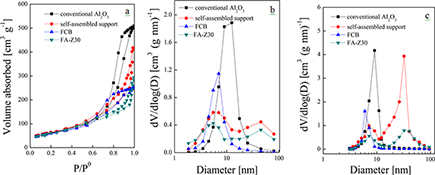
A novel self-assembled hierarchical catalyst, FA-Z30, is prepared and applied to FCC diesel hydrodesulfurization. Average conversion rates per unit volume of HDS, HDN, and HDArs are higher due to the continuous and interconnected porous structure (54% of total pore volume in the range 30–60 nm) and increased sulfidity at 75 %.
CH16017Comparison of Conventional and Microwave Heating for Evaluation of Microwave Effects
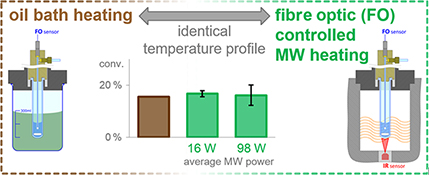
A convenient method is devised to eliminate most of the irreproducibility observed in microwave-heated organic transformations. This approach was applied in the investigation of microwave effects in a reaction of 2-chloropyridine. The observed differences in reaction rates were traced back to thermal differences, while non-thermal effects could not be detected.
CH16101Capto-Dative Stabilization by Thermal Oxidation of 2-Oxo-1,2,3,4-tetrahydropyrimidines
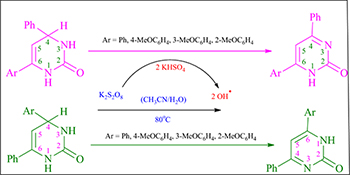
The steric and electronic effects of aryl substitution on the oxidation of various 4,6-diaryl-2-oxo-1,2,3,4-tetrahydropyrimidines were investigated. A capto-dative stabilized radical intermediate involved in the reaction governs the type of product formed.
CH15793Density Functional Computations on 6-Aminouracil: Effect of Amino Group in the 6th Position on the Watson–Crick Base Pair Uridine–Adenosine
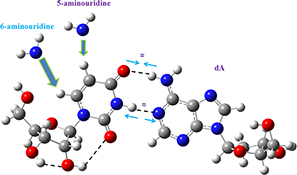
Differences in the interaction of 5-aminouridine and 6-aminouridine with 2′-deoxyadenosine. The Fourier transform infrared and Fourier transform Raman spectra of 6-aminouracil were determined.
CH16051Organic Light-Emitting Diodes Based on a Solution-Processable Benzimidazole-Functionalised Cationic Iridium Complex
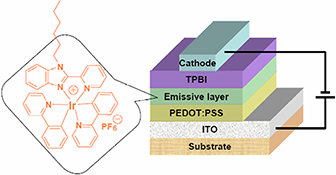
The non-doped electroluminescent device with the configuration ITO/PEDOT : PSS/Ir-complex/TPBI/Ba/Al using cationic iridium complex as emissive layer emits orange light (λmax = 594 nm) with an external quantum efficiency of 5.6 %, a luminance efficiency of 9.3 cd A–1, and a power efficiency of 3 lm W–1.
CH16046Preparation and Characterization of Copper(II) and Nickel(II) Complexes with N-Benzyliminodiacetamide Derivatives
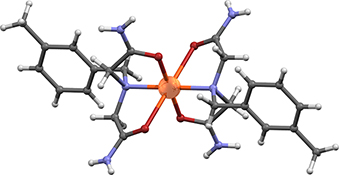
Copper(ii) and nickel(ii) complexes with N-benzyliminodiacetamide derivatives, RN(CH2CONH2)2 (R= ortho-methylbenzyl, meta-methylbenzyl, para-methylbenzyl, ortho-chlorobenzyl, meta-chlorobenzyl, para-chlorobenzyl, para-fluorobenzyl, and para-bromobenzyl), were prepared and characterized by infrared spectroscopy and thermal analysis (thermogravimetric and differential thermal analyses). N-Benzyliminodiacetamide derivatives form stable complexes with copper(ii) and nickel(ii), but not with cobalt(ii), under the experimental conditions described in this paper. The X-ray structural analysis revealed octahedral coordination of nickel(ii) and copper(ii) ions in these complexes.
CH16006Anticancer Evaluation of Tris(triazolyl)triazine Derivatives Generated via Click Chemistry
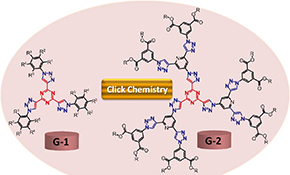
Click chemistry has been utilised for the preparation of seven new dendrimers. The anticancer potential of the new compounds was evaluated against various human cancer cell lines. The results revealed the high anticancer potential of compound G1a with no toxicity on normal cells in comparison with the standard drug doxorubicin.
CH15826A Highly Selective Chemosensor for Naked-Eye Detection of Fluoride and Aluminium(III) Ions Based on a New Schiff Base Derivative
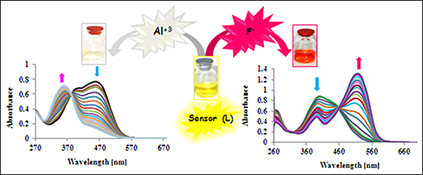
Receptor L proved to be a colorimetric fluoride (F–), dihydrogen phosphate (H2PO4–), and aluminium(iii) sensor. This new chromogenic receptor shows selective coloration for the above ions. The sensor showed a colour change upon addition of fluoride, dihydrogen phosphate, or aluminium(iii) ions.
CH15496Photoinitiated Synthesis of Sulfides in Water
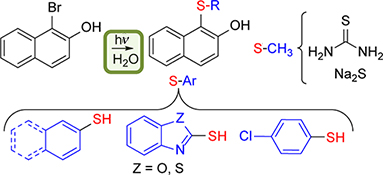
This paper reports a synthetic route to obtain aryl sulfides using inexpensive and non-toxic reactants and water as solvent, that avoids the use of catalysts or heating. The photoinduced reaction between soluble substrates and a series of thiols in alkaline aqueous medium produces the corresponding sulfides in moderate to good yields.
CH16058Flowers in Australia: Phytochemical Studies on the Illawarra Flame Tree and Alstonville

Investigation on the phytochemistry of flowers of the Australian species ‘Illawarra flame tree’ (Brachychiton acerifolius) and the endemic Alstonville (Tibouchina lepidota) revealed the compounds responsible for the intense colour of the flowers. Additionally, known biological activities of the identified compounds from the former species correlated with their use in indigenous medicine.
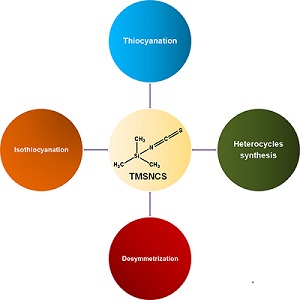
The versatility of trimethylsilyl isothiocyanate (TMSNCS) as a reagent is impressive, especially in heterocycle synthesis. TMSNCS is also commonly used in reactions of thiocyanation and isothiocyanation. The advantages of TMSNCS are moderated toxicity, chemical stability, high tolerance with other functional groups, and ease of handling. This paper highlights a selection of many different and recently reported types of reactions that this reagent is able to promote.



